- Department of Animal Sciences, North Dakota State University, Fargo, ND, United States
Previous studies have reported that nutritional restriction from days 50 to 130 applied in young nulliparous ewes reduces umbilical blood flow (UBF). We hypothesized that during restriction, UBF and fetal and placentome dimensional measurements would decrease compared to adequately fed ewes, but upon realimentation, ewes would have similar UBF as ewes that were not restricted. We also hypothesized that multiparous ewes would be more resilient to nutrient restriction compared to nulliparous ewes. In experiment 1, second-parity Dorset ewes carrying singletons were assigned to an adequate nutrition group (CON, n = 7) or a restricted (60% of CON) group (RES, n = 8), from days 50 to 90 of gestation. In experiment 2, on day 50 of gestation, adult (15-month) nulliparous (NUL; n = 12) and multiparous (MUL; n = 16) Dorset ewes carrying singletons were randomly assigned to receive 100% of NRC recommendations (CON) or 60% of CON (RES). On day 90, all ewes were fed 100% of nutritional recommendations according to body weight. Ewe body weight and conceptus measurements via ultrasonography were recorded every 10 days from days 50 to 130 of gestation. We measured 10 random placentomes, fetal biparietal and abdominal length, and kidney length and width. Doppler mode was used to obtain UBF, pulsatility index (PI), and resistance index (RI). Lamb weight and parturition problems were recorded. In experiment 1, on day 80, UBF decreased (P ≤ 0.05 means separation of unprotected F test), placentome size tended to decrease (P ≤ 0.10), and PI and RI tended to increase in RES vs. CON ewes (P ≤ 0.10). In experiment 2, there were no three-way interactions or main effects of treatments on UBF, PI, RI, and placentome size (P ≥ 0.57). There was a parity-by-day interaction (P < 0.05) for RI, but UBF was not affected by parity or diet. After realimentation, there was no effect of treatment on ultrasound measurements in both experiments. At birth, lambs and placental measurements were not different (P ≥ 0.43). Restriction from days 50 to 90 does not seem to influence umbilical hemodynamics or conceptus growth in adult white face sheep, regardless of parity.
1 Introduction
Environmental factors such as temperature and altitude affect fetal and placental development. Nutrition is the most important among these factors (Wu et al., 2004). In the North American Midwest during times of drought, dietary energy requirements are often not met. Furthermore, ewes may need nutritional supplements either during the period of major placental development (early and mid-gestation) or during the period of major fetal development (last third of gestation).
The majority of placental growth happens in the first two-thirds of pregnancy, with the placenta reaching its maximum weight by day 90 in sheep (Stegeman, 1974). In sheep, abnormal Doppler velocimetry measurements from a variety of fetal blood vessels have been correlated with intrauterine growth restriction, fetal and neonatal mortality, and developmental abnormalities in the offspring (Galan et al., 1998; Rigano et al., 2001; Ferrazzi et al., 2002). Moreover, changes in umbilical blood flow are the earliest Doppler abnormalities detected in sheep intrauterine growth restriction (Rigano et al., 2001; Ferrazzi et al., 2002). Some studies in sheep have shown that a nutrient restriction from days 30 to 80 of gestation does not affect fetal or placental growth (Anthony et al., 2003); however, our laboratory has demonstrated that, in young ewes, a 40% nutrient restriction beginning on day 50 reduces umbilical blood flow (UBF) at day 70 and UBF does not recover to control values until day 130 in which ewes were euthanized (Lemley et al., 2012). Furthermore, impairing placental growth or uteroplacental blood supply affects fetal growth trajectory (Reynolds et al., 2005; Vonnahme et al., 2013). Late-gestation nutrient restriction results in decreased growth and development of the fetus (Redmer et al., 2004). Therefore, low birth weight is a common result of late-gestation nutrient restriction with several studies reporting intrauterine growth restriction (Ferrazzi et al., 2002; Redmer et al., 2004; Lemley et al., 2012). Few studies have been done analyzing the effects of realimentation in pregnant animals. In beef cows, a 40% nutrient restriction applied from days 30 to 140 of gestation did not change the uterine blood flow (Camacho et al., 2014). Upon realimentation (days 140 to 198 of gestation), ipsilateral blood flow was increased in the previously restricted cows, compared to cows that never experienced a nutrient restriction (Camacho et al., 2014).
In several species, parity can influence litter size as well as birth weight. The number of pigs born from multiparous sows is greater than that from primiparous sows (Mahan, 1998; Whitley et al., 2002). In sheep, the conception rate increases and fetal loss decreases as parity increases (Lafi et al., 2009). Similarly, the likelihood of twins increases as the number of parities increases in sheep (Lafi et al., 2009). In cattle, sheep, and mares, birth weight increases as parity increases (Kayisiz et al., 2011; Yakubu et al., 2014; Abdel-Mageed and El-Gawad, 2015; Klewitz et al., 2015; Lv et al., 2015). In mares, the diameter of the uterine artery increases more throughout pregnancy in multiparous (three to eight foalings) compared to first- and second-parity mares (Klewitz et al., 2015). Similarly, blood flow is increased in the uterine artery during the third period of gestation in multiparous mares when compared to first- and second-parity mares (Klewitz et al., 2015). In women, pulsatility index (PI) measurements taken on the uterine artery are greater in primiparous mothers compared to multiparous mothers during mid-gestation (17 to 18 weeks; Suzuki, 2006). Similarly, many studies show that nulliparous women have greater blood pressure, pregnancy-induced hypertension, and greater risk of preeclampsia than multiparous women (Duckitt and Harrington, 2005; Rurangirwa et al., 2012). The pulsatility index, an indirect measurement of blood vessel resistance, is correlated with the resistance index (RI; Suzuki, 2006), and both measurements are usually increased when blood flow is decreased.
A good proportion of the studies analyzing the influence of parity in reproductive traits do not report maternal age (Abdel-Magged and El-Gawad, 2015; Klewitz et al., 2015). To our knowledge, in sheep, only one study has analyzed the effect of maternal age, controlling for parity, in placental development (Borowicz et al., 2005). Several studies in humans and other mammals suggest that there could be an independent effect of parity in the maternal utero-placental physiology that could enhance the reproductive capacity of the multiparous mother (Kelly et al., 1992; Whitley et al., 2002; Wilsher and Allen, 2003; Elliott et al., 2009). Studies in animals and humans show no difference in the reproductive effects of parity after the second parturition (Wilsher and Allen, 2003; Zaborski et al., 2009), suggesting that possible adaptive physiological changes of parity happen after the first pregnancy (Wilsher and Allen, 2003; Zaborski et al., 2009).
There is limited information on how realimentation and parity impact UBF in the ewe. The objective of experiment 1 was to determine if realimenting previously restricted multiparous pregnant ewes would restore UBF to control levels during mid-gestation. The objective of experiment 2 was to investigate if parity, independent of maternal age, influences the effect of nutrition on umbilical blood flow in sheep. We investigated the effects of parity and nutrient availability during mid-gestation on the UBF, PI, RI, body, and placental measurements of the fetus. We hypothesized that during dietary restriction, UBF, and fetal and placentome measurements, would be lower as compared to adequately fed ewes, but upon realimentation, ewes would have similar UBF as ewes that were not restricted. We also hypothesized that the negative effects of nutrient restriction would be worse in nulliparous ewes.
2 Materials and Methods
Animal care and use for both experiments were according to protocols approved by the North Dakota State University Animal Care and Use Committee (#A15076).
2.1 Animals and Experimental Design
2.1.1 Experiment 1
Forty-two second-parity Dorset ewes were used (BW = 63.11 ± 1.96 kg). Estrus was synchronized using progesterone containing controlled internal drug release (CIDR) devices. After synchronization, ewes were fed a pelleted diet (Table 1) and hay for ad libitum intake. All ewes were bred to one ram, and breeding dates were recorded. Pregnancy diagnosis and fetal enumeration were performed on day 30 of gestation via ultrasonography (Aloka ProSound Alpha 6, Seattle, WA). Fifteen singleton-carrying ewes were randomly divided into two treatment groups: control (CON group; n = 7) and restricted (RES group; n = 8), and placed in individual pens. All ewes received a pelleted diet once daily from days 30 to 50 at 100% of NRC recommendations (NRC, 1985). On day 50, CON ewes continued to receive 100% of NRC recommendations throughout the duration of the study while RES ewes received 60% of requirements from day 50 to day 90 of gestation. On day 91, ewes were realimented to 100% of NRC requirements. Body weight and ultrasonography scans were performed every 10 days from day 50 until day 110 or 130 of gestation. Diets were adjusted every 10 days based on body weight. After samples were obtained on day 130 of gestation, ewes were group-housed and received hay and water for ad libitum intake. Birth was monitored, and placentas were collected. Lamb birth weight, number of cotyledons, and placental fetal membrane and cotyledon weights were recorded.
2.1.2 Experiment 2
Ninety-one multiparous and 37 adult nulliparous (approximately 1.5 years of age; BW = 76.70 ± 1.99 kg) Dorset ewes were used. Estrus was synchronized using progesterone-containing CIDR devices. After synchronization, all ewes were bred to three different rams and breeding dates were recorded via use of rams with chest markers. Thirty-eight ewes were rebred by the same rams 17 days later. Pregnancy diagnosis and fetal enumeration were performed from day 30 to day 40 of gestation via ultrasonography (Aloka ProSound Alpha 6, Seattle, WA). After pregnancy was confirmed, 12 singleton-carrying nulliparous ewes and 16 singleton-carrying multiparous ewes (one to three previous parities) were housed in individual pens. At ANPC, ewes were fed a pelleted diet (Table 1) and hay for ad libitum intake for 5 days. After this period, all ewes were fed a pelleted diet once daily at 100% of NRC recommendations until day 50 of gestation. On day 50 of gestation, 12 nulliparous (NUL) ewes were randomly divided into two treatment groups: Control (CON; n = 6) and restricted (RES; n = 6) and 16 multiparous (MUL) ewes were randomly divided into two treatment groups: control (CON; n = 8) and restricted (RES; n = 8). Control ewes continued to receive 100% of NRC recommendations throughout the duration of the study while RES ewes received 60% of requirements from day 50 to day 90 of gestation. On day 91, RES ewes were realimented to 100% of NRC requirements. Body weight and ultrasonography scans were performed every 10 days from day 50 until day 110 or 130 of gestation. Diets were adjusted every 10 days based on body weight. After samples were obtained on day 130 of gestation, ewes were housed and received hay and water for ad libitum intake. Birth was monitored, and lamb birth weights and parturition problems (dystocia, placental retention, lamb vitality, and early lamb mortality) were recorded.
2.2 Gestational Measurements
Beginning on day 50, and every 10 days until day 110, ewes were restrained during the ultrasound procedure so that conceptus measurements and umbilical hemodynamics could be obtained. All measurements were obtained before feeding. Conceptus measurements included the length and width from 10 random placentomes. Fetal biparietal and abdominal lengths and kidney length and width in duplicate were collected every 10 days. For umbilical hemodynamic measurements, Doppler mode was used to obtain UBF, PI, and resistance index (RI) as previously described (Lemley et al., 2012).
2.3 Statistical analyses
2.3.1 Experiment 1
Data were analyzed as a completely randomized design. Repeated data were analyzed using the MIXED procedure of SAS (SAS software version 9.4, SAS Institute, Cary, NC). Ewe was treated as a random independent variable; treatment and day were treated as fixed effects. UBF, PI, RI, placentome area, fetal biparietal and abdominal lengths, kidney length and width, and ewe body weight were the dependent variables. Ewe, treatment, and day were included in the class statement; day was included in the repeated statement, dependent variables (treatment, day, and their interaction) were included in the model statement, and LSmeans were separated using the PDIFF option of the LSMEANS statement. Birth data were analyzed using the GLM procedure; the class statement included treatment, and the model statement included placental weight, cotyledon number, cotyledon weight, fetal membrane, and birth weight; and means were separated using the PDIFF option. P values ≤ 0.05 are considered significant. Tendencies are described when P values are >0.05 but ≤0.10.
2.3.2 Experiment 2
The research was conducted as a completely randomized design with a two-by-two factorial arrangement of treatments and repeated measures. Data were analyzed using the MIXED procedure of SAS (SAS software version 9.4, SAS Institute, Cary, NC). Ewe was treated as a random independent variable; parity (NUL or MUL) was modeled as factor 1, and diet (CON or RES) was modeled as factor 2. Both factors and day were treated as fixed effects. Umbilical blood flow, PI, RI, placentome area, fetal biparietal and abdominal lengths, kidney width and length, and ewe body weight were the dependent variables. Ewe, ram, parity, diet, and day of gestation were included in the class statement; the model statement included parity, diet, day of gestation, and all their interactions. Day of gestation was included in the repeated statement; LSmeans were separated using the PDIFF option of the LSMEANS statement. Ewe initial body weight was added as a covariate for all the dependent variables and had a significant effect on ewe body weight, and kidney length and width. For these variables, the covariate was included in the model. Birth data were analyzed using the GLM procedure; the class statement included parity and diet; and the model statement included birth weight, placental retention, dystocia, low lamb viability, and lamb mortality. Means were separated using the LSMEANS option. P values ≤ 0.05 were considered significant. P values > 0.05 but ≤ 0.10 were considered as a tendency.
3 Results
3.1 Gestational Measurements
3.1.1 Experiment 1
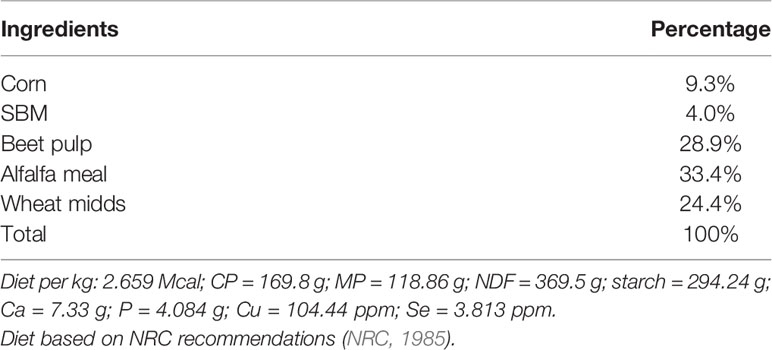
On day 50 of gestation, both treatment groups had similar ewe body weight (62.08 ± 1.88 kg; P = 0.45; Figure 1A) and fetal ultrasonography measurements (Figures 1B–D). By day 70, RES ewes were lighter and remained lighter than CON ewes throughout the experiment (P < 0.05; Figure 1). There were no treatment-by-day treatment effects on fetal abdominal girth, biparital distance, or kidney lengths and width (P ≥ 0.15; Figure 1). As expected, there was a day effect (P < 0.01) where all measurements increased as gestation advanced (Figures 1A–D).
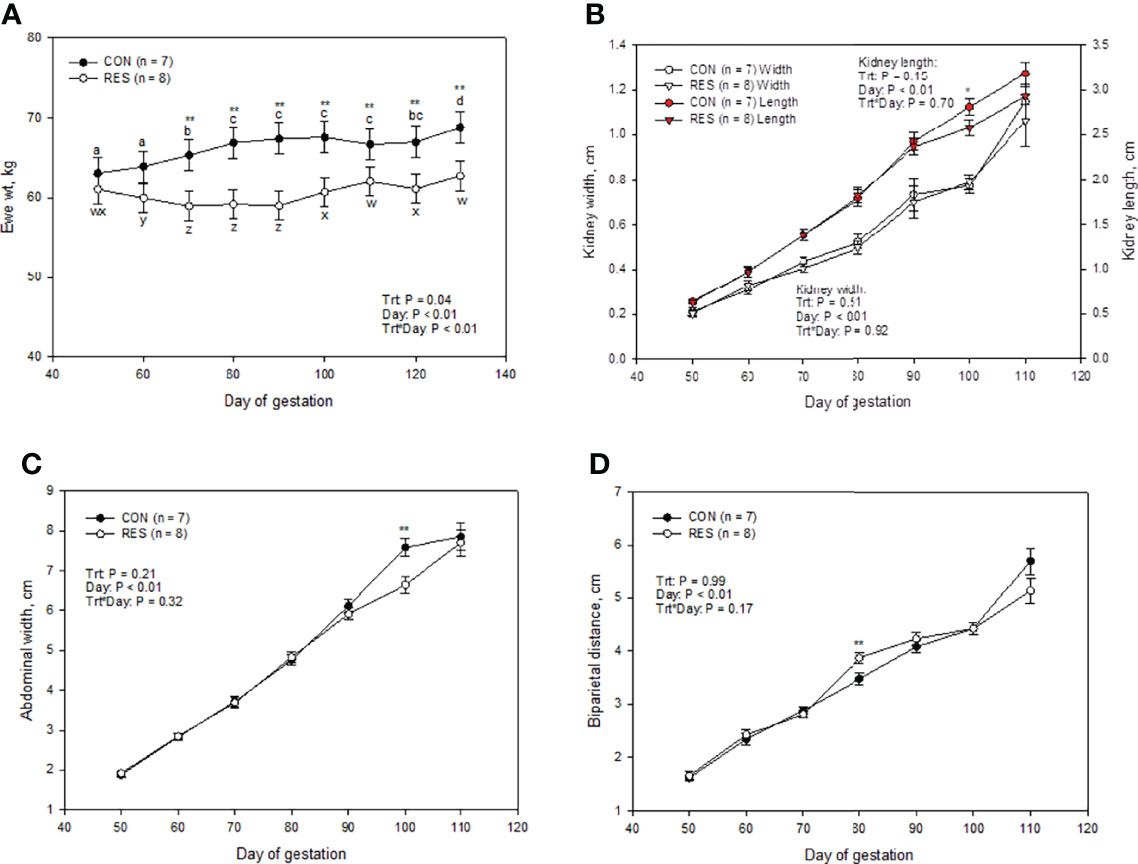
Figure 1 Impacts of maternal nutrition on ewe weight (A), fetal kidney length and width (B), abdominal width (C), and biparietal distance (D) from d 50 to 110 of gestation. CON = 100% of NRC recommendations. RES = 60% of CON from day 50 to 90 of gestation. abcdLSMEANS ± SEM between day differ P ≤ 0.05. Differences between CON and RES are denoted by **P ≤ 0.05, *P ≤ 0.10 within a day.
There was no interaction of treatment and day on placentome area (P = 0.49). There were no treatment-by-day interactions or main effects of treatment (P > 0.19; Figures 2B–D) for any measurements obtained in UBF, PI, and RI. On day 80, UBF decreased (P ≤ 0.05; Figure 2D), placentome area tended to decrease (P ≤ 0.10; Figure 2A), and PI and RI (P ≤ 0.10; Figures 2B, C) tended to increase in RES compared to CON. On day 90, all these measurements were similar to CON (Figure 2).
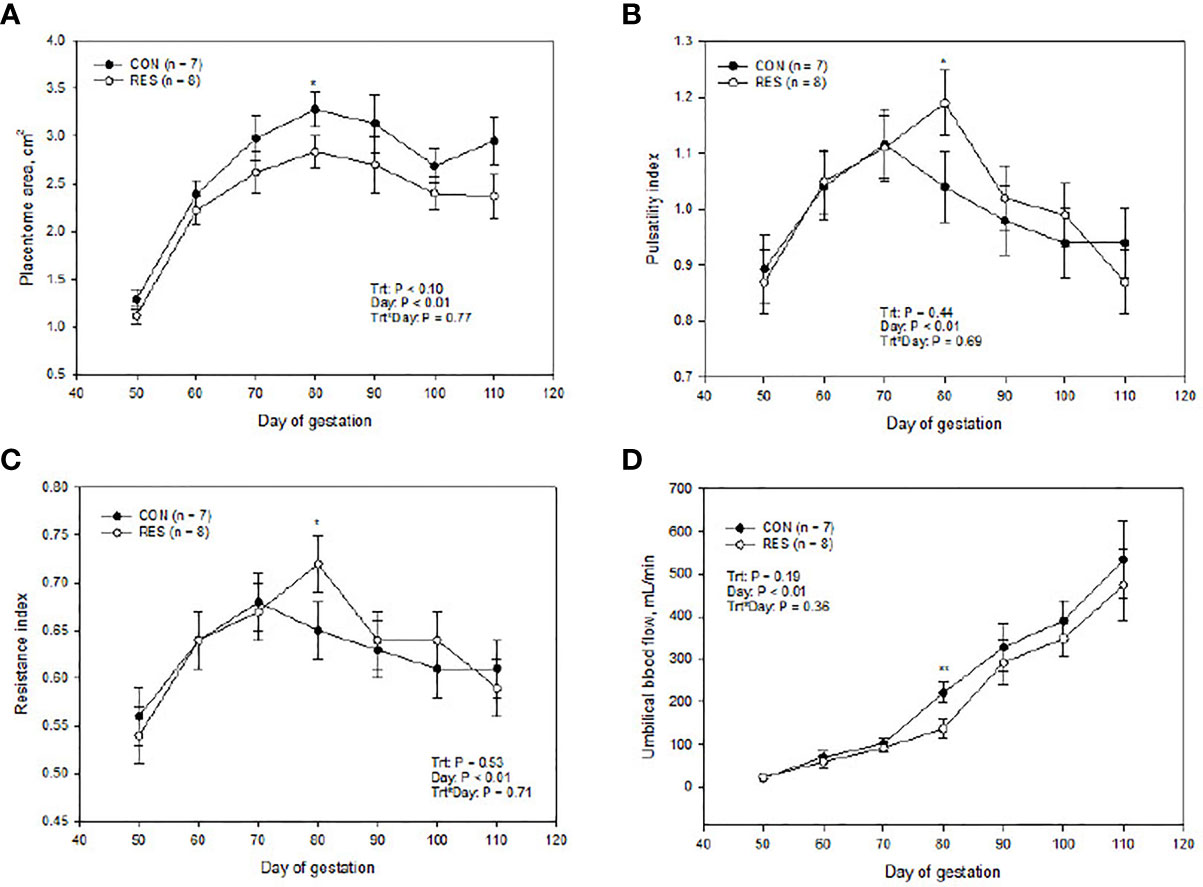
Figure 2 Impacts of maternal nutrition on placentome area (A), umbilical pulsatility index (B), umbilical resistance index (C), and umbilical blood flow (D) from d 50 to 110 of gestation. CON = 100% of NRC recommendations. RES = 60% of CON from days 50 to 90 of gestation. Differences between CON and RES are denoted by **P ≤ 0.05, *P ≤ 0.10 within a day.
3.1.2 Experiment 2
There was no three-way interaction or main effect of parity in ewe body weights throughout the experiment (P = 0.46; Figure 3A). However, nutrient restriction, day of gestation, and their interaction influenced ewe weight (Figure 3A). On day 50 of gestation, the MUL and NUL, CON and RES groups had similar body weights (P ≥ 0.94). By day 70, MUL- and NUL-restricted (MUL-RES, NUL-RES) ewes had decreased body weights when compared to MUL and NUL control (MUL-CON, NUL-CON) ewes (P ≤ 0.04; Figure 3). Restricted ewes maintained lesser body weights until the end of the experiment (Figure 3A). Multiparous-RES animals had decreased (P < 0.01) weights compared to MUL-CON ewes by day 60 and maintained this difference throughout the experiment (Figure 3A), whereas NUL-RES ewes were lighter (P = 0.04) than NUL-CON by day 70 and only tended to be lighter on day 80 (P = 0.07; Figure 3A). For the remaining days of the experiment, NUL-RES ewes were lighter than NUL-CON.
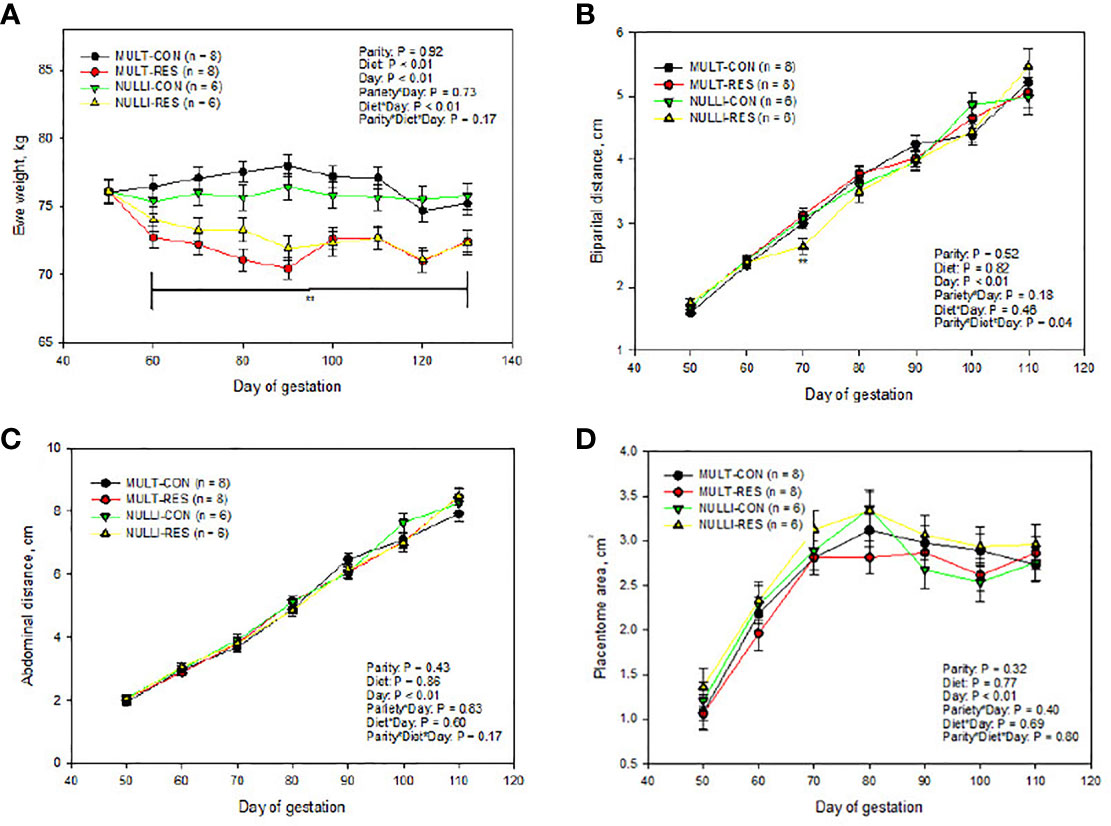
Figure 3 Impacts of maternal nutrition on ewe weight (A), fetal biparietal distance (B), fetal abdominal distance (C), and placentome area (D) from days 50 to 110 of gestation in multiparous (MULT) and nulliparous (NULL) ewes. CON = 100% of NRC recommendations. RES = 60% of CON from days 50 to 90 of gestation. Differences between CON and RES are denoted by **P ≤ 0.05, *P ≤ 0.10 within a day.
There were no three-way interaction or main effects of parity and diet for UBF, PI, and RI (Figures 4B–D). Multiparous-CON animals had similar UBF, PI, and RI values compared to MUL-RES (Figures 4B–D). Similarly, NUL-CON were not different than NUL-RES (Figure 4). An unprotected mean separation of UBF showed that MUL-CON ewes had similar values to NUL-CON during the length of the study (P ≥ 0.42). However, UBF of NUL-RES ewes tended to be greater (P = 0.09) than that of MUL-RES animals on day 90 and was greater on day 110 (P = 0.01). The resistance index showed an interaction effect of parity by day (Figure 4B). The pulsatility index and RI means, respectively, were greater (P = 0.04) and tended to be greater (P = 0.10) in MUL-CON vs. NUL-CON on days 50 and 100. The pulsatility index tended to be greater (P = 0.08) on NUL-CON vs. MUL-CON on day 60. The resistance index and PI respectively were greater (P = 0.02) and tended to be greater (P = 0.07) in NUL-RES vs. MUL-RES on day 70 of gestation (Figure 4). On the other hand, PI and RI were decreased (P =0.03) in NUL-RES vs. MUL-RES on day 90. Day influenced (P < 0.01) all three Doppler US measurements, with UBF values increasing as gestation advanced, and PI and RI reaching their peak on day 80 of gestation (Figures 4A–C).
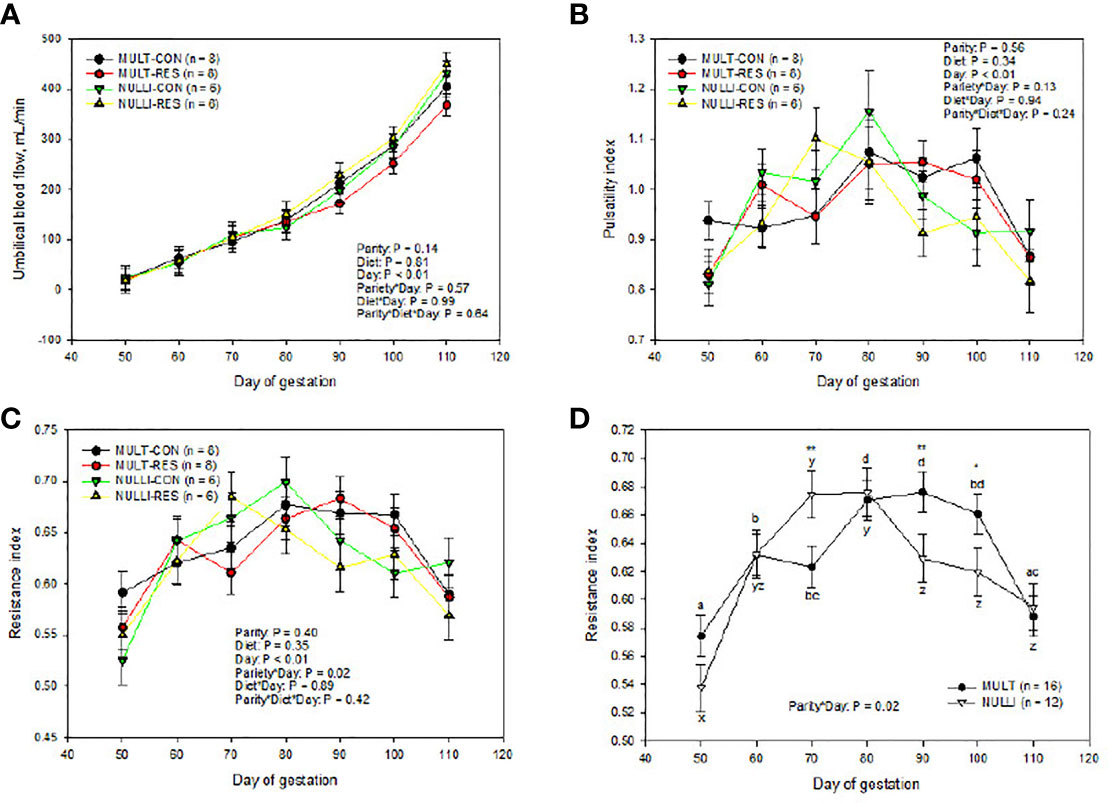
Figure 4 Impacts of maternal nutrition on fetal umbilical blood flow (A), umbilical pulsatility index distance (B), and umbilical resistance index (C, D) from days 50 to 110 of gestation in multiparous (MULT) and nulliparous (NULL) ewes. CON = 100% of NRC recommendations. RES = 60% of CON from days 50 to 90 of gestation. abcdLSMEANS ± SEM between days differ P ≤ 0.05. Differences between CON and RES are denoted by **P ≤ 0.05, *P ≤ 0.10 within a day.
3.2 Lamb Birth and Placental Weights
3.2.1 Experiment 1
At birth, lambs and placental measurements were similar between the treatment groups (P > 0.43, Table 2).
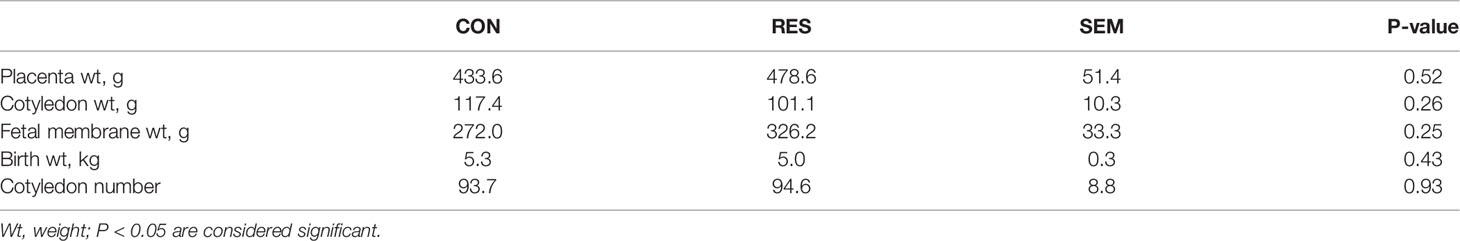
Table 2 Placental weight, cotyledon weight, birth weight, fetal membrane weight, and cotyledon number.
3.2.2 Experiment 2
No differences in birth weight were observed among treatment groups (Table 3). Maternal and lamb birth problems [placental retention, dystocia, low lamb viability, and/or early lamb mortality (first 24 hours)] were analyzed. No differences were found among treatment groups in birth problems (Table 3). An LSMEAN separation (data not shown) demonstrates that NUL-RES animals showed a near tendency (P = 0.11) to have greater birth problems when compared to MUL-RES and MUL-CON (Table 3).
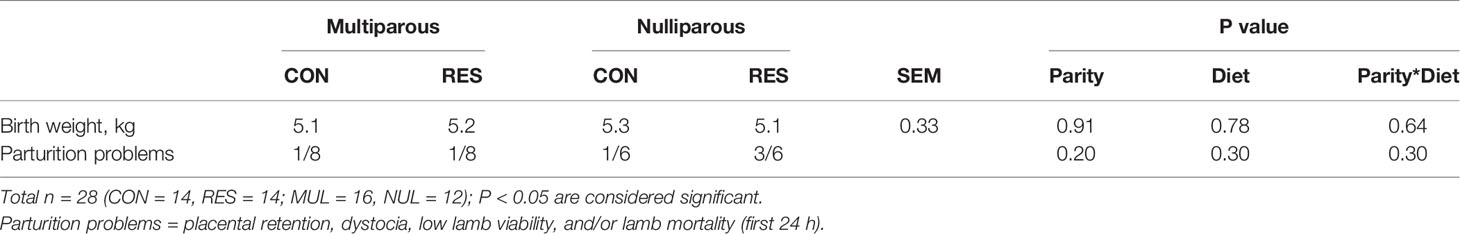
Table 3 Birth weight means (kg) and number of birth-related problems (number of ewes and/or lambs per treatment).
4 Discussion
The placenta is essential for endocrine production and nutrient exchange between the dam and fetus. In ruminants, including sheep, the fetal–maternal exchange occurs in structures called the placentome. Placentome size has been previously used as an indirect measurement of nutrient delivery to the fetus (Redmer et al., 2004). The umbilical cord is the structure that transports the nutrients and oxygen from the placentomes to the fetal circulatory system and transports carbon dioxide and waste products from the fetus to the placentomes. Assessment of UBF is used as an index of nutrient delivery (Kiserud, 2005; Lemley et al., 2012). In experiment 1, on day 80, RES ewes had smaller placentomes, reduced UBF, and increased measurements of resistance (i.e., PI and RI). These measurements suggest that UBF and nutrient exchange might have been compromised on day 80. However, these measurements were similar prior to realimentation; therefore, the recovery in blood flow parameters was not an effect of realimentation.
A previous study performed in our laboratory reported that in primiparous young ewes a 40% nutrient restriction from days 50 to 130 of gestation resulted in decreased UBF starting on day 80 of gestation (Lemley et al., 2012). Experiment 1 was done with multiparous ewes, and we preserved the same housing conditions, breed, and experimental protocol of Lemley et al. (2012). Therefore, we hypothesized that in Lemley et al. (2012) parity had an aggravating effect, additionally to nutrient restriction, on blood flow. The effects of parity on uterine microanatomy and UBF in sheep are not well characterized. Therefore, we tested the effects of nutrient restriction and parity during mid pregnancy in ewes in experiment 2.
In experiment 2, we hypothesized that parity could have a protective effect on blood flow parameters and that nulliparous ewes would experience the detrimental effects of nutrient restriction on umbilical blood flow. However, none of the effects of nutrient restriction were observed in this experiment. Moreover, NUL-RES animals tended to have and had a greater UBF on days 90 and 110, respectively, when compared to MUL-RES. We also found that PI, RI, and UBF did not respond to treatment similarly. On days 50 and 100, in which PI and RI were greater and tended to be greater in MUL-CON vs. NUL-CON, UBF was not different between them. Similarly, on day 70, RI and PI, respectively, were greater and tended to be greater when comparing NUL-RES vs. MUL-RES and had a similar UBF. The only day in which PI and RI values responded the same as UBF was on day 90, in the comparison between NUL-RES vs. MUL-RES. These findings suggest that the generalized idea of PI and RI being inversely related to UBF is not always true (Elmetwally et al., 2016; Beltrame et al., 2017; Elmetwally and Meinecke-Tillmann, 2018). There could be other factors such as tissue-specific vessel properties that influence these results.
Experiment 1 showed that a 40% nutrient restriction in mid and mid-to-late gestation does not influence kidney measurements. These results were corroborated in experiment 2; therefore, we conclude that there was no effect of any of the treatments in the kidney size of the developing fetus. The kidneys, unlike other organs of the developing fetus (e.g., small intestine, spleen), seem not to be affected by a lower nutrient availability in mid to late gestation (50 to 130 days of gestation; Osgerby et al., 2002; Lemley et al., 2012). In experiment 2, we saw a three-way interaction in biparietal distance. This interaction effect was probably driven by the lesser biparietal distance in NUL-RES ewes on day 70 of gestation. Placental growth occurs during the first two-thirds of pregnancy (Redmer et al., 2004), with fetal growth being exponential during the last third of pregnancy (Redmer et al., 2004). Nevertheless, some studies have shown that when a severe nutrient restriction is applied during mid-gestation, and the fetuses are collected at the end of the restriction period (before the last period of gestation; no realimentation), fetal weight can be affected (Murdoch et al., 2003; Zhou et al., 2008; Ma et al., 2011). Abdominal width, however, as well as the remaining days of biparietal width measurements were not affected by nutritional treatment. Similarly, these results are analogous to what we saw in experiment 1 and seems to demonstrate that fetal growth in adult white face ewes seems to be protected against a 40% nutrient restriction during mid-gestation.
In both experiments, we found that a 40% nutrient restriction during mid-gestation did not affect birth weights. Furthermore, others have demonstrated that moderate to severe nutrient restriction applied during early-to-mid and mid-to-late gestation periods have also shown no effects on birth weight (Gilbert et al., 2005; Sebert et al., 2008; Kotsampasi et al., 2009; Sharkey et al., 2009; Lekatz et al., 2013). These results corroborate that the majority of the fetal development occurs during the last third of pregnancy (Redmer et al., 2004). Studies in cows, mares, and sheep have shown an influence of parity in birth weight (Kayisiz et al., 2011; Yakubu et al., 2014; Abdel-Mageed and El-Gawad, 2015; Klewitz et al., 2015; Lv et al., 2015). In our study, parity did not have an effect on birth weight. This is opposite to another study done in cross-bred sheep in which nulliparous dam lambs had lower birth weights than multiparous animals (Yakubu et al., 2007). Breed differences could be influencing the dissimilar results.
Although the number of animals in experiment 2 is not enough to make inferences between birth-related problems and treatments, it is interesting that NUL animals seemed, at least numerically, to have more parturition problems than MUL animals and that NUL-RES animals seemed to be more prone to these problems than any of the other treatment groups. In sheep, some studies show higher lambing difficulty and early lamb mortality in nulliparous ewes (Kelly et al., 1992; Southey et al., 2004; McHugh et al., 2016), while others show no difference between nulliparous and multiparous animals (Leontides et al., 2000).
Nutrient restriction during mid-gestation in multiparous cows showed that uterine blood flow did not decrease during the restriction period; however, it increased upon realimentation (Camacho et al., 2014). In this study, the detrimental effects of a 40% nutrient restriction on ewe body weights were seen in both experiments. However, our findings from both experiments suggest that UBF in adult white-face sheep is resistant to changes with a 40% mid-gestation nutrient restriction, and there is not an increase in UBF upon realimentation. The decreased UBF observed in restricted adolescent nulliparous ewes (Lemley et al., 2012) might be therefore an effect of age at first gestation. In the pregnant adult sheep, nutrient partitioning prioritizes the placenta and fetus (Redmer et al., 2004). However, the hierarchy of nutrient partitioning in adolescent pregnancy has a higher priority for maternal tissue growth and fat deposition (Redmer et al., 2004). Adolescent pregnant ewes compete for nutrient resources with the developing fetus (Redmer et al., 2004). Additionally, nulliparous adult animals have greater fetal, cotyledonary, and caruncular weights as well as a greater cotyledonary angiogenic factor expression than nulliparous adolescent ewes (Borowicz et al., 2005). Nutrient restriction during mid-pregnancy could exacerbate these differences. Furthermore, mid-gestation nutrient-restriction effects seen in other studies in sheep vary in breed, period of restriction, and severity of the restriction (Kelly et al., 1992; Reynolds et al., 2005). More research is needed to further examine the effects of age and nutrient restriction on fetal development.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by corresponding author under reasonable request.
Ethics Statement
Animal care and use for both experiments were according to protocols approved by the North Dakota State University Animal Care and Use Committee (#A15076).
Author Contributions
MV-H performed and supervised the experiment, collected the samples, and analyzed and interpreted the data. KS assisted with the data and tissue collection, conceptualization, and supervision. KV supervised the data and tissue collection, conceptualization, funding acquisition, formal analysis, project administration, and writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This project was supported in part by the Agriculture and Food Research Initiative Competitive Grant no. 2016-67016-24884 from the USDA National Institute of Food and Agriculture.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
MH-V was supported by a scholarship from the Secretariat of Higher Education, Science, Technology and Innovation of the Republic of Ecuador. The authors would also like to thank Terry Skunberg and Justin Gilbertson of the NDSU Animal Nutrition & Physiology Center for assistance with animal care, and Jim Kirsch, Sheri Dorsam, and all graduate and undergraduate students that helped with data and tissue collection.
References
Abdel-Mageed I., El-Gawad M. A. (2015). Effects of Breed, Parity and Post-Mating Nutrition on Reproductive Wastage and Pregnancy Outcomes of Egyptian Sheep. Small Rumin. Res. 130, 171–177. doi: 10.1016/j.smallrumres.2015.06.009
Anthony R., Scheaffer A., Wright C., Regnault T. (2003). Ruminant Models of Prenatal Growth Restriction. Reprod Suppl. 61, 183–194. doi: 10.1530/biosciprocs.5.014
Beltrame R. T., Covre C., Littig L. B., de Martins A. B., Quirino C. R., Junior A. B., et al. (2017). Transrectal Doppler Sonography of Uterine Blood Flow in Ewes During Pregnancy. Theriogenology 91, 55–61. doi: 10.1016/j.theriogenology.2016.12.026
Borowicz P., Vonnahme K., Grazul-Bilska A., Redmer D., Johnson M., Reynolds L. (2005). The Effect of Maternal Age (Age at First Pregnancy) on Placental Expression of the Major Angiogenic Factors and Their Receptors. Pproc. J. Soc Gynecol. Investig. 12, 327A–328A.
Camacho L., Lemley C., Prezotto L., Bauer M., Freetly H., Swanson K., et al. (2014). Effects of Maternal Nutrient Restriction Followed by Realimentation During Midgestation on Uterine Blood Flow in Beef Cows. Theriogenology 81 (9), 1248–1256.e1243. doi: 10.1016/j.theriogenology.2014.02.006
Duckitt K., Harrington D. (2005). Risk Factors for Pre-Eclampsia at Antenatal Booking: Systematic Review of Controlled Studies. Br. Med. J. 330, 565. doi: 10.1136/bmj.38380.674340.E0
Elliott C., Morton J., Chopin J. (2009). Factors Affecting Foal Birth Weight in Thoroughbred Horses. Theriogenology 71 (4), 683–689. doi: 10.1016/j.theriogenology.2008.09.041
Elmetwally M. A., Meinecke-Tillmann S. (2018). Simultaneous Umbilical Blood Flow During Normal Pregnancy in Sheep and Goat Foetuses Using non-Invasive Colour Doppler Ultrasound. Anim. Reprod. 15 (2), 148. doi: 10.21451/1984-3143-AR2017-976
Elmetwally M., Rohn K., Meinecke-Tillmann S. (2016). Noninvasive Color Doppler Sonography of Uterine Blood Flow Throughout Pregnancy in Sheep and Goats. Theriogenology 85 (6), 1070–1079. doi: 10.1016/j.theriogenology.2015.11.018
Ferrazzi E., Bozzo M., Rigano S., Bellotti M., Morabito A., Pardi G., et al. (2002). Temporal Sequence of Abnormal Doppler Changes in the Peripheral and Central Circulatory Systems of the Severely Growth‐Restricted Fetus. Ultrasound Obstet. Gynecol. 19 (2), 140–146. doi: 10.1046/j.0960-7692.2002.00627.x
Galan H. L., Hussey M. J., Chung M., Chyu J. K., Hobbins J. C., Battaglia F. C. (1998). Doppler Velocimetry of Growth-Restricted Fetuses in an Ovine Model of Placental Insufficiency. Am. J. Obstet. Gynecol. 178 (3), 451–456. doi: 10.1016/S0002-9378(98)70419-3
Gilbert J. S., Lang A. L., Grant A. R., Nijland M. J. (2005). Maternal Nutrient Restriction in Sheep: Hypertension and Decreased Nephron Number in Offspring at 9 Months of Age. J. Physiol. 565 (1), 137–147. doi: 10.1113/jphysiol.2005.084202
Kaygisiz A., Bakir G., Yilmaz I., Vanli Y. (2011). Estimation of Variance Components and Genetic Parameters for Direct and Maternal Effects on Birth Weight in Brown Swiss Cattle. Pak. Vet. J. 31 (1), 70–74.
Kelly R., Speijers E., Ralph I., Newnham J. (1992). Lambing Performances and Wool Production of Maiden and Adult Merino Ewes Fed Different Amounts of Lupin Seed in Mid-Pregnancy. Crop Pasture Sci. 43 (2), 339–354. doi: 10.1071/AR9920339
Kiserud T. (2005). Physiology of the Fetal Circulation. Proc. Semin. Fetal. Neonatal. Med. 10, 493–503. doi: 10.1016/j.siny.2005.08.007
Klewitz J., Struebing C., Rohn K., Goergens A., Martinsson G., Orgies F., et al. (2015). Effects of Age, Parity, and Pregnancy Abnormalities on Foal Birth Weight and Uterine Blood Flow in the Mare. Theriogenology 83 (4), 721–729. doi: 10.1016/j.theriogenology.2014.11.007
Kotsampasi B., Balaskas C., Papadomichelakis G., Chadio S. (2009). Reduced Sertoli Cell Number and Altered Pituitary Responsiveness in Male Lambs Undernourished In Utero. Anim. Reprod. Sci. 114 (1), 135–147. doi: 10.1016/j.anireprosci.2008.08.017
Lafi S., Talafha A., Giadinis N., Kalaitzakis E., Pourliotis K., Panousis N. (2009). Factors Affecting the Reproductive Performance of Awassi Sheep Flocks in North-East of Jordan: An Epidemiological Study. Trop. Anim. Health Prod. 41 (8), 1755–1764. doi: 10.1007/s11250-009-9374-z
Lekatz L., Luther J., Caton J., Vonnahme K. (2013). Impacts of Maternal Nutritional Plane on Umbilical Artery Hemodynamics, Fetal and Placentome Growth in Sheep. Anim. Reprod. 10 (2), 99–105.
Lemley C. O., Meyer A. M., Camacho L. E., Neville T. L., Newman D. J., Caton J. S., et al. (2012). Melatonin Supplementation Alters Uteroplacental Hemodynamics and Fetal Development in an Ovine Model of Intrauterine Growth Restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302 (4), R454–R467. doi: 10.1152/ajpregu.00407.2011
Leontides L., Fthenakis G. C., Amiridis G. S., Saratsis P. (2000). A Matched Case-Control Study of Factors Associated With Retention of Fetal Membranes in Dairy Ewes in Southern Greece. Prev. Vet. Med. 44 (1-2), 113–120. doi: 10.1016/S0167-5877(99)00115-4
Lv S.-J., Yang Y., Dwyer C., Li F. K. (2015). Pen Size and Parity Effects on Maternal Behaviour of Small-Tail Han Sheep. Animal 9 (07), 1195–1202. doi: 10.1017/S175173111500052X
Mahan D. (1998). Relationship of Gestation Protein and Feed Intake Level Over a Five-Parity Period Using a High-Producing Sow Genotype. J. Anim. Sci. 76 (2), 533–541. doi: 10.2527/1998.762533x
Ma Y., Zhu M. J., Uthlaut A. B., Nijland M. J., Nathanielsz P. W., Hess B. W., et al. (2011). Upregulation of Growth Signaling and Nutrient Transporters in Cotyledons of Early to Mid-Gestational Nutrient Restricted Ewes. Placenta 32 (3), 255–263. doi: 10.1016/j.placenta.2011.01.007
McHugh N., Berry D. P., Pabiou T. (2016). Risk Factors Associated With Lambing Traits. Animal 10 (1), 89–95. doi: 10.1017/S1751731115001664
Murdoch W. J., Kirk E.A.V., Vonnahme K. A., Ford S. P. (2003). Ovarian Responses to Undernutrition in Pregnant Ewes, USA. Reprod. Biol. Endocrinol. 1 (6), 1–8. doi: 10.1186/1477-7827-1-6
National Research Council, Subcommittee on Sheep Nutrition, Committee on Animal Nutrition, Board on Agriculture (1985). Nutrient Requirements of Sheep. Sixth Revised Edition (Washington, D.C: National Academy Press).
Osgerby J., Wathes D., Howard D., Gadd T. (2002). The Effect of Maternal Undernutrition on Ovine Fetal Growth. J. Endocrinol. 173 (1), 131–141. doi: 10.1677/joe.0.1730131
Redmer D., Wallace J., Reynolds L. (2004). Effect of Nutrient Intake During Pregnancy on Fetal and Placental Growth and Vascular Development. Domest. Anim. Endocrinol. 27 (3), 199–217. doi: 10.1016/j.domaniend.2004.06.006
Reynolds L. P., Borowicz P. P., Vonnahme K. A., Johnson M. L., Grazul‐Bilska A. T., Redmer D. A., et al. (2005). Placental Angiogenesis in Sheep Models of Compromised Pregnancy. J. Physiol. 565 (1), 43–58. doi: 10.1113/jphysiol.2004.081745
Rigano S., Bozzo M., Ferrazzi E., Bellotti M., Battaglia F. C., Galan H. L. (2001). Early and Persistent Reduction in Umbilical Vein Blood Flow in the Growth-Restricted Fetus: A Longitudinal Study. Am. J. Obstet. Gynecol. 185 (4), 834–838. doi: 10.1067/mob.2001.117356
Rurangirwa A. A., Gaillard R., Steegers E. A., Hofman A., Jaddoe V. W. (2012). Hemodynamic Adaptations in Different Trimesters Among Nulliparous and Multiparous Pregnant Women; the Generation R Study. Am. J. Hypertens. 25 (8), 892–899. doi: 10.1038/ajh.2012.57
Sebert S., Hyatt M., Chan L., Patel N., Bell R., Keisler D., et al. (2009). Maternal Nutrient Restriction Between Early and Midgestation and its Impact Upon Appetite Regulation After Juvenile Obesity. Endocrinology 150 (2), 634–641. doi: 10.1210/en.2008-0542
Sharkey D., Gardner D. S., Symonds M. E., Budge H. (2009). Maternal Nutrient Restriction During Early Fetal Kidney Development Attenuates the Renal Innate Inflammatory Response in Obese Young Adult Offspring. Am. J. Physiol. Renal Physiol. 297 (5), F1199–F1207. doi: 10.1152/ajprenal.00303.2009
Southey B., Rodriguez-Zas S., Leymaster K. (2004). Competing Risks Analysis of Lamb Mortality in a Terminal Sire Composite Population. J. Anim. Sci. 82 (10), 2892–2899. doi: 10.2527/2004.82102892x
Stegeman J. H. (1974). Placental Development in the Sheep and its Relation to Fetal Development. A Qualitative and Quantitative Anatomic and Histologic Study. Bijdr. Dierkd. 44 (1), 3–72. doi: 10.1163/26660644-04401002
Suzuki S. (2006). Influence of Parity on Second-Trimester Uterine Artery Doppler Waveforms in Twin Pregnancy. J. Matern. Fetal. Neonatal. Med. 19 (3), 193–194. doi: 10.1080/14767050600587850
Vonnahme K., Lemley C., Shukla P., O’Rourke S. (2013). 2011 and 2012 Early Careers Achievement Awards: Placental Programming: How the Maternal Environment can Impact Placental Function. J. Anim. Sci. 91 (6), 2467–2480. doi: 10.2527/jas.2012-5929
Whitley N. C., Thomas M., Ramirez J., Moore A., Cox N. (2002). Influences of Parity and Level of Feed Intake on Reproductive Response to Insulin Administration After Weaning in Sows. J. Anim. Sci. 80 (4), 1038–1043. doi: 10.2527/2002.8041038x
Wilsher S., Allen W. (2003). The Effects of Maternal Age and Parity on Placental and Fetal Development in the Mare. Equine Vet. J. 35 (5), 476–483. doi: 10.2746/042516403775600550
Wu G., Bazer F. W., Cudd T. A., Meininger C. J., Spencer T. E. (2004). Maternal Nutrition and Fetal Development. J. Nutr. 134 (9), 2169–2172. doi: 10.1093/jn/134.9.2169
Yakubu H., Barje P., Iyeghe-Erakpotobor G. (2014). Influence of Calf Parity Number, Season of Calving and Period of Calving on Birth and Weaning Weights of Friesian-Bunaji Calves. World J. Life Sci. Med. Res. 3 (2), 59.
Yakubu D., Mostyn A., Wilson V., Pearce S., Alves-Guerra M., Pecqueur C., et al. (2007). Different Effects of Maternal Parity, Cold Exposure and Nutrient Restriction in Late Pregnancy on the Abundance of Mitochondrial Proteins in the Kidney, Liver and Lung of Postnatal Sheep. Reproduction 133 (6), 1241–1252. doi: 10.1530/REP-06-0211
Zaborski D., Grzesiak W., Szatkowska I., Dybus A., Muszynska M., Jedrzejczak M.. (2009). Factors Affecting Dystocia in Cattle. Reprod. Domest. Anim. 44 (3), 540–551. doi: 10.1111/j.1439-0531.2008.01123.x
Keywords: blood flow, sheep, pregnancy, refeeding, undernutrition
Citation: Vasquez-Hidalgo MA, Swanson KC and Vonnahme KA (2022) Effects of Mid-Gestation Nutrient Restriction, Realimentation, and Parity on the Umbilical Hemodynamics of the Pregnant Ewe. Front. Anim. Sci. 3:855345. doi: 10.3389/fanim.2022.855345
Received: 15 January 2022; Accepted: 13 May 2022;
Published: 17 June 2022.
Edited by:
Sarita Bonagurio Gallo, University of São Paulo, BrazilReviewed by:
Jon Schoonmaker, Purdue University, United StatesPaulo Roberto Leme, University of São Paulo, Brazil
Copyright © 2022 Vasquez-Hidalgo, Swanson and Vonnahme. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manuel A. Vasquez-Hidalgo, bWFudWVsLnZzcXVlemhpZGFsZ29AbmR1cy5lZHU=
 Manuel A. Vasquez-Hidalgo
Manuel A. Vasquez-Hidalgo Kendall C. Swanson
Kendall C. Swanson Kimberly A. Vonnahme
Kimberly A. Vonnahme