
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Anim. Sci., 18 February 2022
Sec. Animal Physiology and Management
Volume 3 - 2022 | https://doi.org/10.3389/fanim.2022.807267
This article is part of the Research TopicMinimally Invasive Monitoring of Stress in Farm Animals, Volume IView all 8 articles
 Matthew L. Livingston1
Matthew L. Livingston1 Anthony Pokoo-Aikins1
Anthony Pokoo-Aikins1 Thomas Frost1
Thomas Frost1 Lisa Laprade1
Lisa Laprade1 Vy Hoang2
Vy Hoang2 Bartek Nogal2
Bartek Nogal2 Chelsea Phillips1
Chelsea Phillips1 Aaron J. Cowieson3*
Aaron J. Cowieson3*Environmental heat stress creates a detriment to the welfare and performance in broiler chickens. While there are some dietary mineral and vitamin supplements that mitigate this condition, a rapid, plasma-based detection method would improve management response and broaden the scientific understanding of heat stress. A total of 960 broilers were used to determine the effect of heat stress and dietary electrolyte balance on blood biochemistry. Sex sorted chicks were allocated to 48 pens with 20 chicks per pen creating 6 treatments (3 diets x 2 house environments) with eight replicates and fed one of three dietary treatments: a control containing primarily sodium chloride (NaCl), a heat stress formulation containing bicarbonate (NaHCO3), or heat stress fortified with 200 ppm vitamin C and E (NaHCO3 Fortified). Birds were housed in two different temperature-controlled environments either a thermoneutral (Control) or heat stressed (Heat Stress) environment. At day 28, 35 and 42 venous blood was collected and analyzed using rapid detection methods followed by post-mortem veterinary evaluations. Performance was measured at weekly intervals. Mortality was significantly higher in broilers exposed to heat stress as compared to thermoneutral, while broilers that received dietary sodium chloride also had higher mortality than bicarbonate fed birds. Heat stress significantly impacted potassium, hematocrit, uric acid, total protein, globulin, hematocrit, lymphocytes, sodium, and glucose. This study demonstrates that blood biochemistry of broiler chickens is influenced by dietary intervention and changing environmental conditions. This pattern suggests a blood biomarker footprint of sub-optimal nutrition or poor environmental conditions that may provide valuable information into physiological changes in response to dietary electrolytes, vitamins, and heat stress. Furthermore, this footprint may potentiate the development of diagnostic tools, combining biomarkers to determine nutrition and health status of individual broiler flocks, for nutritionists, veterinarians, and live production managers to manage flocks for environmental, humane, and productive purposes.
Heat stress has been a great concern in the broiler industry for many years due to its negative impact on production performance and the health status of poultry (Lara and Rostagno, 2013). Reduced feed consumption, increased morbidity, poor meat quality, as well as economic losses are typical effects of mild heat or acute stress, while mortality can be associated with chronic stress. Many studies showed that broilers raised in prolonged heat stress environments experience reduced feed intake, lowered body weight and higher feed conversion ratio at market/processing age. Previous calculations estimate the total economic losses to be $128 to $165 million to the US poultry industry (St-Pierre et al., 2003). The importance of mitigating the negative effects associated with heat stress may continue to grow as a result of ongoing global climate changes (Nardone et al., 2010).
Heat stress is known to cause changes in multiple blood parameters, for example an increase in heterophil to lymphocyte (H:L) ratio and a decrease in thiobarbituric acid reactive substances (TBARS) (Altan et al., 2003; Borges et al., 2004; Zulkifli et al., 2009; Tan et al., 2010). Some investigations have shown movement in blood minerals and glucose concentration (Borges et al., 2004; Mujahid et al., 2009; Olanrewaju et al., 2010). Other studies have shown changes in serum concentrations of uric acid, aspartate aminotransferase (AST), creatine kinase, total protein, albumin, and globulin associated with short or prolonged heat stress (Akşit et al., 2006; Liu et al., 2016; Attia et al., 2017; Luo et al., 2018). A recent study found a decrease in plasma CO2, HCO3, potassium, and an increase in pH and glucose when broilers were exposed to increased environmental temperatures over 33°C for a minimum of 5 h (Beckford et al., 2020).
Supplementation with some feed additives, minerals, and vitamins into broiler diets has shown to partially mitigate the negative effects of heat stress including mortality. Multiple studies have shown supplementation of vitamin E and vitamin C reduced the adverse effects of chronic heat stress on performance (Tawfeek et al., 2014; Attia et al., 2017). The use of non-chloride salts, such as sodium bicarbonate, have also been shown to reduce the negative effects of heat stress through an improved balance of electrolytes (Borges et al., 2004; Ahmad and Sarwar, 2006; Ahmad et al., 2006).
Recently rapid detection methods using Point-of-Care (POC) devices, such as the i-STAT® Handheld Clinical Analyzer, Zoetis Vetscan VS2, and iCheck™ Carotene, have been utilized in animal care and research (Martin et al., 2010; Hoppes et al., 2015; Lindholm and Altimiras, 2016; Raila et al., 2017; Livingston et al., 2020). These devices are portable bench top or handheld analytical devices used for rapid multivariable analysis of blood chemistry (gases, minerals and other parameters). Moreover, these devices are beginning to be used to correlate various blood values or markers with abnormal conditions or diseases in broilers (Stinefelt et al., 2005; Martin et al., 2010; Lindholm and Altimiras, 2016; Celi et al., 2019; Livingston et al., 2019; Cowieson et al., 2020). These POC devices allow for rapid detection of multiple metabolites that have not typically been the focus of heat stress markers in broilers. Given the use of POC devices in various other disease challenge models (Celi et al., 2019; Cowieson et al., 2020), their analysis may be helpful in determining the onset of heat stress indicators in broilers.
The possibility of continued environmental change has created a need for further research into broiler heat stress. The unique blood physiology that occurs under heat stress conditions has been documented, however, there are few studies that investigated the blood physiology in broilers while under dietary mitigating factors such as vitamin C, vitamin E, non-chloride salts, and other non-nutritional feed additives. Also, it is necessary to understand the physiological principals supporting additives that can improve performance under heat stress conditions. Understanding these principles may further the ability to treat heat stress and help in the development of cost-effective solutions. This study sought to investigate the blood physiology (using rapid POC devices) of broilers fed dietary sodium chloride, a balanced electrolyte formula with bicarbonate, vitamin E, and vitamin C while being exposed to normal or heat stressed environments; and the utilization of simple to operate POC devices to rapidly identify and communicate these conditions in order to mount a rapid humane, environmental, and economic response.
This trial was conducted at AHPharma, INC. Maryland, USA between the months of August through October of 2019. Protocol and oversight for this study was approved by the local institutional animal care and use committee (IACUC).
Hubbard-Cobb chicks (960) obtained from a local commercial hatchery were visually vent-sex sorted by trained hatchery employees and allocated into 48 floor type pens. There were three dietary treatments formulated with sodium chloride, sodium bicarbonate, or sodium bicarbonate fortified with additional vitamin C and E (NaCl, NaHCO3, NaHCO3 Plus) in starter (d 0–14), grower (day 15–28), and finisher (d 29–42) phases (Table 1). There were 10 male and 10 female broilers per pen with a density of ~924 cm2 per bird. The broiler house was split into two temperatures controlled, tunnel ventilated environments destined for a thermoneutral or heat challenged condition (Control, Heat Stress), creating a 3 x 2 split plot design with 8 replicates. Each floor pen was constructed of PCV framework and plastic mesh netting, supplied with nipple water drinking lines, feeders, and bedded with fresh pine shavings (15 cm deep). Broilers received ~23 h of continuous light for the first 5 days at 10.7 to 13.9 lux and 21 h of continuous light from day 6–11. From day 12 to day 42 birds received 20 h of continuous light at 2.15 to 3.23 lux.
Housing temperatures at day 0 were 34°C for the first 12 h, 32°C from 12 to 24 h followed by 30 ± 2°C. Thereafter, temperature was decreased by ~0.5°C per day until a target of ~21–22°C and 60% relative humidity were reached and remained until day 21. Half of the house remained like this for the duration of the study (Control) while an adaptation, maintenance, and final period were established for the heat stress treatment which was separated by an insulated divided wall, essentially creating two rooms within the same house of equal air pressure, volume, and wind speed. From d 21–42 the remaining half of the house was programed to reach a high temperature adaptation period (21–25 day old). This consisted of a day cycle of 6 h at 30°C and 60% relative humidity from 10 a.m. to 4 p.m. The maintenance period consisted of a day cycle of 6 h at 35°C and 60% of relative humidity from 10 a.m. to 4 p.m. and an evening cycle of 18 h at 25°C and 60% of relative humidity 4 p.m. to 10 a.m. from day 26–36. The final period was 8 h at 35°C and 60% of relative humidity, from 8 a.m. to 4 p.m. and a night cycle of 16 h at 25°C and 60% relative humidity from 4 p.m. to 8 a.m. Cross-house ventilation system with sidewall and or ceiling fans were used to control house temperature.
Diets were formulated to meet or exceed NRC (NRC, 1994) requirements. Sodium chloride diets were formulated with 0.4–0.5% sodium chloride, while bicarbonate and bicarbonate fortified diets contained only 0.28–0.30% sodium chloride and 0.18–0.28% sodium bicarbonate. Bicarbonate fortified diets were formulated with the addition of 200 ppm vitamin C (ROVIMIX® STAY-C®35, DSM) and vitamin E (ROVIMIX® E50, DSM) (Table 1).
Total broiler pen body weight (BW), feed consumption, and mortality were recorded at 1, 7, 14, 21, 28, 35, and 42 d of age and feed conversion ratio (FCR) calculated. BW and feed consumption per bird were calculated using total pen body weight and feed consumption divided by total pen bird number. Observations for mortality were performed twice daily, recorded, and calculations were performed as needed on a per pen basis. At 28, 35, and 42 d of age two male broilers per pen were selected for venous blood analysis; females were not utilized for blood analysis to reduce variance that exists between sexes (Livingston et al., 2020). Approximately 4–5 mL of venous blood was initially drawn and allocated into three blood collection tubules as 0.5 ml Heparinized, 1+ ml EDTA treated, and 3 ml serum collection tubules. A systematic approach to catching broilers and drawing of blood was developed to assure uniform collection and analysis of samples.
Heparinized blood (~0.2 ml) was analyzed in the i-Stat® Alinity V handheld blood analyzer fitted with a Chem8+ cartridge (Abbott Point of Care Inc., Princeton, NJ), which measured hematocrit (HCT), hemoglobin blood urea nitrogen creatinine ionized calcium (iCa), glucose (iGlu), chloride (iCl) sodium (iNa), potassium (iK), total carbon dioxide (TCO2), and anion gap (iAnGap).
Remaining heparinized blood (0.1 ml) was analyzed in the Vetscan® VS2 Chemistry Analyzer (Abaxis, Inc.) using the Avian/Reptilian Profile Plus cartridge (Abbott Point of Care Inc., Princeton, NJ). This resulted in aspartate aminotransferase (AST), creatine kinase (CK), uric acid (UA), glucose (vGLU), calcium (vCa), phosphorus (vP), total protein (TP), albumin (ALB), albumin/globulin (GLOB), potassium (vK) and sodium (vNa).
Whole blood (3 ml) was spun and serum removed and stored on wet ice. 0.40 ml serum was used for total carotenoids (mg/kg) using the iCheck® carotene photometer device and test kit (BioAnalyt GmbH, Potsdam, Germany) (Kawashima et al., 2010). Precisely 1.0 ml of EDTA blood was mixed with 0.20 ml cellular fixant (Transfix®, MBL International), stored and shipped on wet ice to Cayman Analytical Laboratories (Ann Arbor, MI) for Heterophil to Lymphocyte ratio analysis using Flow Cytometry (Lentfer et al., 2015; Bílková et al., 2017). The remaining serum was frozen on dry ice and shipped to Cayman Analytical Laboratories (Ann Arbor, MI) for the measurement of thiobarbituric acid reactive substances (TBARS). This method uses the reaction of malondialdehyde (MDA) and Thiobarbituric acid (TBA) in the glacial acetic acid medium.
Broilers subject to blood draw were immediately euthanized and evaluated for veterinary health status. Gizzard erosion, food pad lesion scores and duodenum, middle gut, and cecum lesion scores were all recorded on a 0–3 scale of measure. A score of 0 indicates no detectable erosion or lesions, while a score of 1, 2, or 3 indicated a mild, moderate, or severe presence (respectively).
Data were analyzed using JMP Pro 14 (SAS Institute, 2018). Growth performance and health status data were analyzed as a two-way ANOVA (Diet*Environment) by the age of the bird. Blood physiology data at 28, 35, and 42 d was analyzed as a three-way ANOVA (Diet*Environment*Day). Means were separated with Tukey's adjustment for multiple comparisons test (P < 0.05). Pen was considered the individual experimental unit for growth performance parameters, while individual blood draw and health status was considered the experimental unit (n = 20) for blood physiology parameters. H:L ratio data was log transformed where necessary. Linear response of blood physiology to time was also determined negating the effect of Diet or Temperature.
Effects of Diet and Temperature on overall BW, FCR, feed consumption, mortality, and health status are presented in Table 2, while weekly results are summarized in the subsequent paragraphs for the purposes of brevity. Broilers fed the bicarbonate fortified diet had greater BW from day 7–28 when compared to birds fed sodium chloride or bicarbonate alone (P < 0.01), while broilers fed bicarbonate and bicarbonate fortified diets produced superior BW compared to sodium chloride fed birds at 35 d (P < 0.01). Sodium chloride fed broilers had reduced BW from 7 to 35 d when compared to either bicarbonate or bicarbonate fortified fed birds. Final BW was not significantly different between diets at 42 d. Broilers raised under a thermoneutral environment (Control)—had greater BW than heat stressed broilers only at 28 d (P = 0.015).
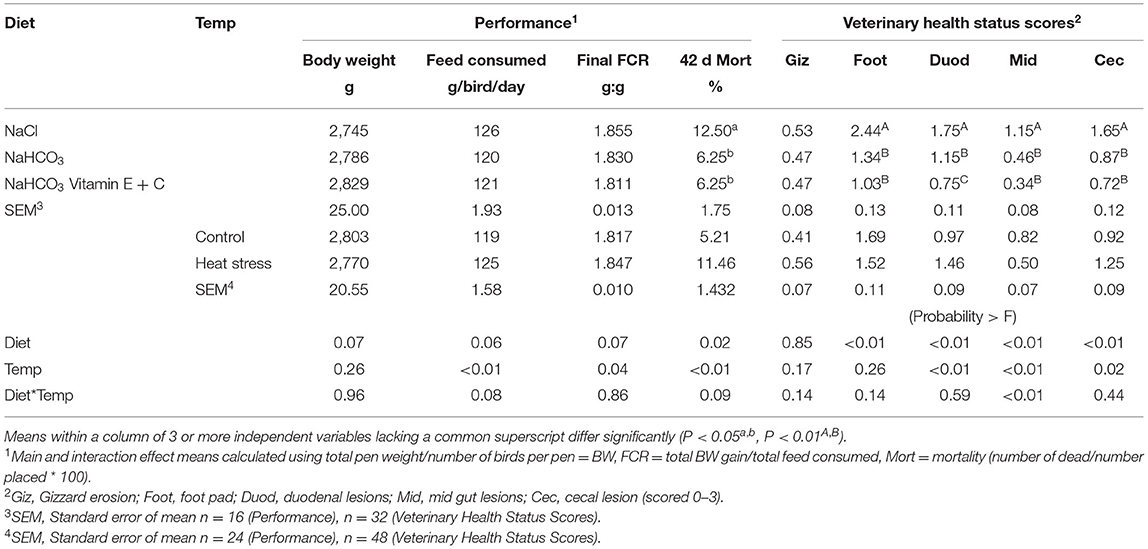
Table 2. Effect of diet and temperature (control or heat stress) on final broiler performance and veterinary health status.
Broilers fed bicarbonate fortified diets had significantly lower FCR when compared to fed sodium chloride at 7–35 d (P < 0.01). Heat stressed broilers had increased feed intake (P < 0.04) and higher FCR when compared with thermoneutral birds from 22 to 42 d (P < 0.04). Bicarbonate fortified fed broilers consumed more feed from 0–14 d than sodium chloride or bicarbonate fed birds, while sodium chloride fed broilers consumed less feed from 15–28 d compared to bicarbonate or bicarbonate fortified diets (P < 0.01).
Thermo manipulation increased weekly mortality from 22 to 42 d, as well as final mortality when compared to those raised in a thermoneutral environment (P < 0.01). Mortality from 29 to 42 d and final mortality was increased in broilers fed sodium chloride when compared to those fed bicarbonate and bicarbonate fortified diets (P < 0.05). Broilers fed sodium chloride or subjected to thermo manipulation had significantly greater mortality than any other treatments, however a significant interaction was only observed from 29 to 25 d (P < 0.05).
Veterinary observations indicated that broilers fed bicarbonate diets had significantly reduced foot pad scores, while fortification of bicarbonate diets reduced these even more (P < 0.01); however, heat stress had no significant effect on foot pad scores. Salt diets and heat stress significantly increased most intestinal lesions (P < 0.02) when compared to bicarbonate diets, while fortification improved duodenal lesions (P < 0.01).
Effect of broiler diet and thermo manipulation on blood mineral composition over time are presented in Table 3. Age resulted in a significant effect (P < 0.01) on all blood mineral composition except for vK and a linear effect on vNa, iNa, iK, iCl, and iCa was observed. At 35 d there was a significant increase in blood Vet scan phosphorus (vPHOS) and vCa, and a decrease in iNa, iCa, and iAnGap when compared to 28 or 42 d broilers. While 28 d blood vNa, iNa and iCl was significantly greater compared to 35 or 42 d.
Birds fed the diet based on sodium bicarbonate had a greater iNa than birds fed the diet based on sodium chloride (P < 0.05). Birds fed sodium chloride diets had greater iK compared to birds fed bicarbonate or bicarbonate fortified diets (P < 0.01). Broilers fed sodium chloride diets had greater levels of iCl than broilers fed the bicarbonate fortified diets (P < 0.01).
Environmental temperature had a significant effect on iNa and vK, with heat stressed broilers having increased blood iNa and decreased blood iK levels (P < 0.05).
Broilers subjected to heat stress had significantly greater iNa than thermoneutral housed broilers at 42 d resulting in an age*temperature interaction (P < 0.01). Sodium chloride fed broilers had significantly reduced blood iNa at 42 d compared to either bicarbonate or bicarbonate fortified diets resulting in an age*diet interaction (P < 0.05). Broilers subjected to heat stress had significantly reduced iK at 42 d when compared to all other treatments, this resulted in an age*temperature interaction (P < 0.05). Broilers housed under heat stress had significantly greater vPHOS at 35 d when compared to other treatments, this resulted in a significant age*temperature interaction for vPHOS (P < 0.05). A similar age*temperature interaction was observed with heat stressed broilers at 35 d regarding an increased vCa (P < 0.05).
Effect of broiler diets and environmental conditions on blood proteins and other metabolites over time are presented in Table 4. Age resulted in a significant effect on all parameters when accounting for diet and temperature with a pronounced increase in blood carotene, AST, CK, TP, ALB and GLOB as broilers aged (P < 0.01), while UA significantly decreased with age (P < 0.01). This also resulted in a significant linear effect (P < 0.01) over time.
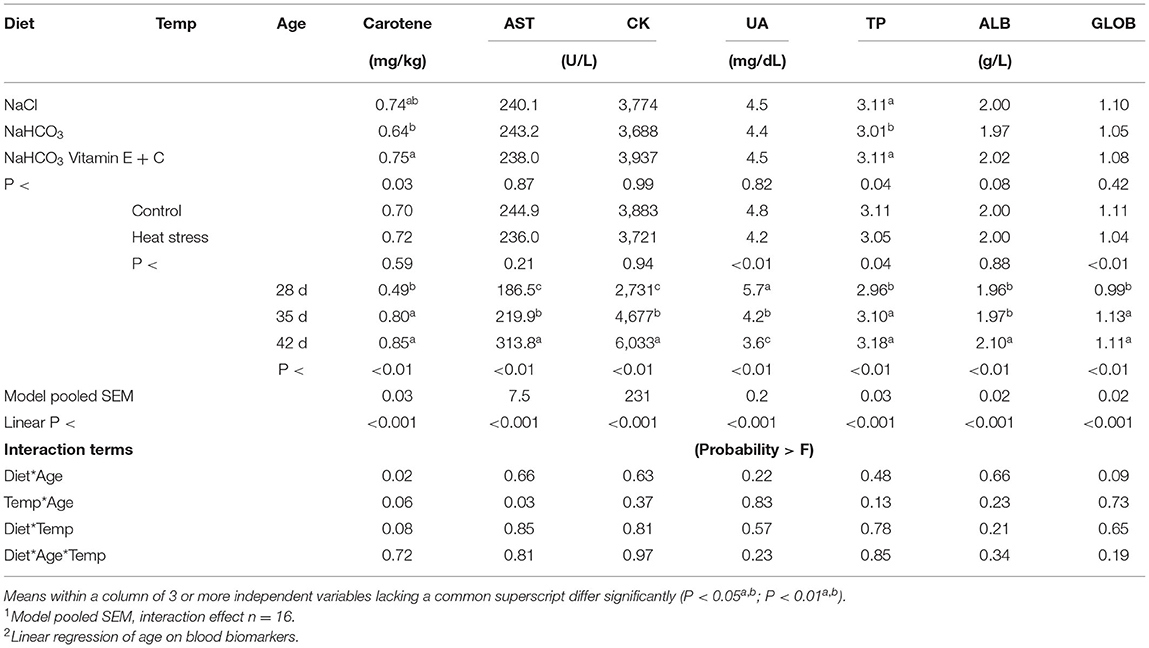
Table 4. Effects of diet, temp and age on broiler blood carotene, aspartate aminotransferase (AST), creatine kinase (CK), bile acids (BA), uric acid (UA), total protein (TP), albumin (ALB), globulin (GLOB).
The main effect of temperature resulted in reduced levels of carotene and TP in bicarbonate fed birds when compared to sodium chloride or bicarbonate fortified fed broilers (P < 0.04). As age increased, sodium chloride and bicarbonate fortified fed broilers displayed increased carotene levels, while bicarbonate fed broilers did not display an increased carotene level resulting in a diet*age interaction (P < 0.05). The main effect of temperature led to reduced levels of UA, TP and GLOB in heat stressed broilers when compared to thermoneutral housed birds (P < 0.05).
Effects of diet and temperature on remaining plasma metabolites over time are presented in Table 5. Age resulted in a significant effect on all parameters except for TCO2. As age increased a significant decrease in blood vGlu, iGlu, HCT, and an increase in lymphocytes (L) was observed; while MDA, heterophil (H), and H:L ratio was greatest at 35 d (P < 0.01). This also resulted in a significant linear effect (P < 0.01) over time for all parameters except for TCO2.
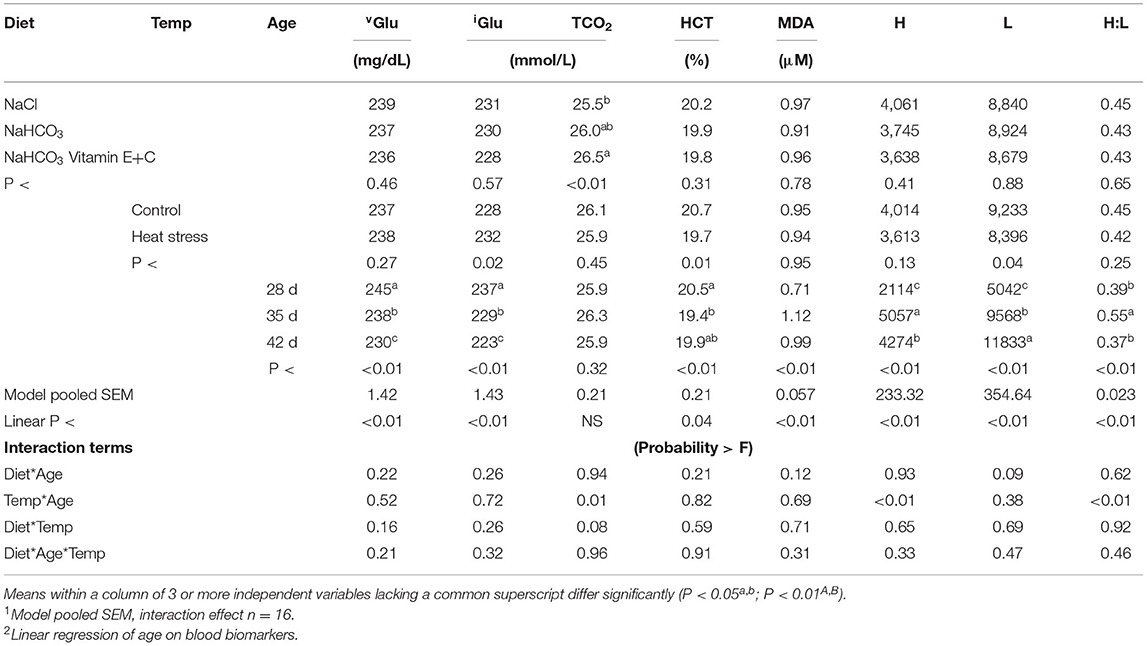
Table 5. Effect of diet and temperature and age on blood protein composition and other metabolites over time.
The main effect of temperature resulted in reduced L, HCT, and increased iGlu in thermo manipulated broilers (P < 0.04). While birds fed sodium chloride had reduced TCO2 when compared to bicarbonate fortified fed broilers (P < 0.01). Finally, heat stressed broilers had significantly greater TCO2 levels at 35 compared to 28 or 42 d, resulting in a temp*age interaction (P = 0.01).
The experiment that is reported herein was primarily designed to investigate the varying blood physiology of chicks exposed to heat stress conditions; and to identify, using simply to use “point of care devices,” those markers that can be manipulated using dietary changes that have shown to reduce performance and welfare deficiencies. Only males were selected for blood physiology in order to eliminate the variation that has been reported between sexes (Livingston et al., 2020). In this trial broiler performance of the control group was consistent with breeder guidelines when accounting for a decrease in male to female population. Heat stress combined with salt containing diets increased mortality and decreased performance and veterinary health status, which was the design of this trial (Table 2). When compared to diets containing sodium chloride, bicarbonate containing diets were highly effective at improving BW from 14 to 35 d, while the added vitamins E and C also improved FCR from 7 to 35 d. Heat stress was successful at reducing BW only at 28 d, which was directly after the initial week of heat stress. Broiler FCR remained poorer in broilers under heat stress for the duration of the trial. Mortality was increased with the addition of heat stress throughout the trial, while diets containing sodium chloride also contributed to increased mortality, as was expected. Broilers under heat stress and fed diets containing sodium chloride had the greatest mortality, however this was directly associated with the main effects of both salt and heat, resulting in a lack of significant interaction between Diet and Environment (P = 0.09).
Blood biomarkers using the rapid detection methods known as Point-of-Care (POC) devices, which may include the i-STAT® Handheld Clinical Analyzer, Zoetis Vetscan VS2, and iCheck™ Carotene, are becoming more commonly used as diagnostic tools in animal health care and research (Martin et al., 2010; Hoppes et al., 2015; Lindholm and Altimiras, 2016; Raila et al., 2017; Cowieson et al., 2020; Livingston et al., 2020). However, the rapid growth rate of the modern broiler has shown that age has a significant influence on the values of these biomarkers (Livingston et al., 2019, 2020; Cowieson et al., 2020). This study had similar results as indicated by the linear response to age on most all measured biomarkers from 28 to 42 d. However, the influence of heat stress was noted on iNa, iK, UA, TP, GLOB, iGLU, HCT, and L; while Diet influenced iNa, iK, iCl, Carotene, TP, and TCO2.
Heat Stress has been shown to reduce the partial pressure of blood CO2 (due to excessive panting) which results in increased plasma pH and bicarbonate, a condition known as respiratory alkalosis (Borges et al., 2004, 2007; Wang et al., 2018; Beckford et al., 2020). In this study total CO2, traditionally calculated TCO2 = HCO3 + 0.03 PCO2 (Maren, 1967), was measured and found to be relatively similar in all broilers except for increased levels of 35 d heat stressed birds (temp*age P = 0.01). However, TCO2 in this study was measured using an analyte metrologically traceable to the International Federation of Clinical Chemistry (IFCC) TCO2 reference method rather than being calculated from partial pressure of blood CO2 and pH. This may contribute to the lack of significant TCO2 values in heat stress compared to thermoneutral raised broilers, as a decrease in PCO2 could be offset by an increase in blood HCO3 resulting in similar or bias TCO2 values.
Plasma levels of K and Na have been reported to decrease while broilers are exposed to heat stress (Belay and Teeter, 1993; Borges et al., 2004, 2007; Beckford et al., 2020). In this study plasma iK and iNa were reduced during the first week of thermo manipulation, while plasma iK continued to be lower through the remainder of the trial. This concurs with previously published studies (Borges et al., 2004; Ahmad and Sarwar, 2006; Olanrewaju et al., 2010; Beckford et al., 2020). However, in this trial iNa values increased in heat stressed broilers during the period of rapid growth (28–42 d).
The reported K and Na reduction in heat stressed broilers has been attributed to increased water consumption, which was speculated to have caused hemodilution (Borges et al., 2004). In the present study there are some indications of this phenomenon as suggested by the reduction in HCT of heat stressed broilers compared thermoneutral housed broilers (19.7 vs. 20.7, P = 0.01), and the previously reported reduction in iK (5.34 vs. 5.53, P = 0.03); however, vK and vNa (Vetscan® VS2 Chemistry Analyzer) were not measurably different between treatments. The reduction of plasma iK in both bicarbonate diets is most likely due to the increase in iNa and response of the aldosterone system (Subramanya and Ellison, 2014; Terker et al., 2015).
There was a significant increase in plasma vPHOS and vCa at 35 d. However, in heat stressed broilers this was significantly more pronounced, as indicated by the temp*age interaction (P = 0.02). This occurred even while dietary Ca and available phosphorus were being reduced from grower to finisher phase. These age-specific changes may be related to the convergence of high environmental temperature at a time when the bird is also experiencing rapid skeletal muscle growth. Such extreme metabolic demands have been previously shown to significantly influence acid-base balance (Hamm and Simon, 1987; Olanrewaju et al., 2010).
Values from these devices, although rapid and consistent, do vary when compared to more traditional and published methods such as the COBAS (Ruiz-Jimenez et al., 2021), requiring investigations into their relationship to disorders such as heat stress and dietary changes. In this study some discrepancies were observed where both I-STAT Alinity and VetScan VS2 machines measured similar biomarkers glucose, calcium, potassium, and sodium. It should be noted that the I-STAT Alinity uses ion-selective electrode potentiometry to measure sodium, potassium, and ionized calcium; while the Vetscan VS2 uses colorimetric and enzymatic methods to measure sodium and potassium and Arsenazo III to measure total calcium. However, glucose was measured using similar methods and values correlated with an r2 = 0.95 (data not shown for brevity).
The inclusion of vitamins E and C has been utilized in many studies to reduce the negative impact of heat stress (Farooqi et al., 2005; Habibian et al., 2014; Hajati et al., 2015; Attia et al., 2017). While the effects of both vitamins E and C in this trial were positive regarding broiler performance, there were no interaction effects on Diet*Temp. However, the main effect of including bicarbonate and vitamin E and C in this trial did increase the carotene and TP values when compared to the bicarbonate only diet. This may indicate that some negative impacts of adding bicarbonate can be mitigated with the addition of these vitamins to increase carotene absorption in the enterocyte and improve plasma TP.
Heat stress had a significant negative impact on plasma HCT (P < 0.01), L (P < 0.05), UA (P < 0.01), TP (P < 0.05), and GLOB (P < 0.01) and a significant increase on GLU (P < 0.05). The decrease of TP and GLOB are consistent with previous studies (Liu et al., 2016; Xue et al., 2017). While those studies found an increase in UA production while broilers underwent heat stress, the results in this study differed. This may be due to the lack of oxidative stress induced by this model of heat stress, which would also explain the similarities in MDA expression. While the increase in GLU was consistent with previous research that indicates a release of glucocorticoids under heat stress increasing GLU (Kutlu and Forbes, 1993; Borges et al., 2007; Beckford et al., 2020).
The use of the rapid detection devices to measure markers associated with animal health is well-established (Meluzzi et al., 1992; Martin et al., 2010; Livingston et al., 2020). While this study aimed to determine the effect of heat stress and dietary electrolyte balance on broiler blood physiology, the effect of heat stress was more pronounced when dietary chloride was incorporated as was expected. Nonetheless, the effect of such data suggests an opportunity to monitor physiological and nutritional changes from broiler blood data. Figure 1 illustrates how formulated and analyzed dietary differences can be detected by evaluating broiler blood physiology. For example: analyzed sodium values were relatively lower than formulated and this was detected by a statistical drop in broiler blood iNa levels (Figure 1A). A similar trend can be identified as blood iCl levels followed a statistical decrease as dietary chloride levels are reduced (Figure 1B). Analyzed potassium values were ~20% greater than formulated which potentially explains why plasma iK of broilers in this study were greater than previous reported studies (Livingston et al., 2020). Regardless of formulation, the ultimate physiological response found in broiler plasma was that of the true, analyzed diets. Using this, the results of these rapid detection devices may allow further measurements of unidentified milling, formulation, ingredient, or management inaccuracies.
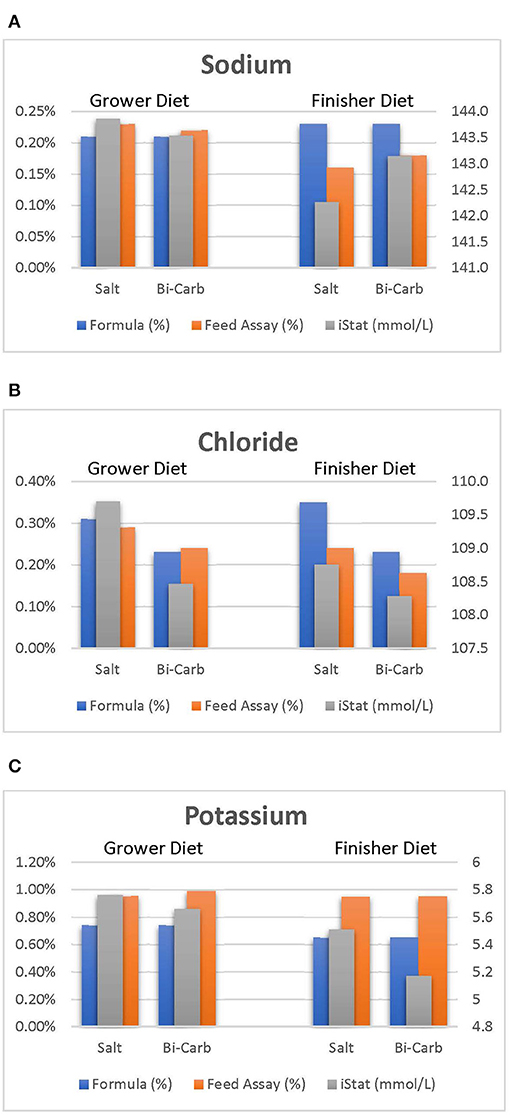
Figure 1. Sodium (A), chloride (B), and potassium (C) values of blood (mmol/L), formulated, and assayed feed during grower and starter periods.
A negative impact of unbalanced dietary electrolyte levels and a transient negative influence of heat stress was noted in broilers chickens fed traditional corn/soy diets, with confirmation of stress noted in mortality percentage and postmortem inspections. It can be concluded that balancing electrolytes with the addition of sodium bicarbonate was successful at improving overall performance from 14 to 35 d of age regardless of heat stress, and the addition of vitamins C and E was highly beneficial. Many blood biomarkers proved to be responsive to these challenges. Age also effected most biomarkers, especially during the period of rapid growth rate. The primary markers associated with higher sodium chloride containing diets include iNa, iK, iCl, carotene, TP, and TCO2. The primary markers associated with heat stress are iNa, iK, UA, TP, GLOB, iGlu, HCT, and L. The effect of age on these blood biomarkers contributes significantly to their values and should continue to be considered when investigating their impact from treatment effects. The data suggest there is a potential for a blood biochemical “fingerprint” of dietary imbalances and environmental conditions that could be used to achieve their early diagnosis. Furthermore, this suggests that nutritionists, veterinarians, and live production managers could monitor and evaluate broiler performance, welfare and overall health status using rapid blood detection devices and biomarkers that may interface nutrition and physiology from a perspective not previously available.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by Institutional Animal Care and Use Committee - AHPharma, Inc.
ML, TF, and AC developed the experiment design. ML oversaw the animal experiment. VH performed the relevant literature review. AP-A, ML, BN, and CP performed data analysis. ML drafted the manuscript. AP-A, CP, LL, TF, VH, BN, and AC revised and reviewed all drafts. All authors contributed to the article and approved the submitted version.
This work was supported by DSM Nutritional Products, Kaiseraugst, Switzerland. The funder had the following involvement with the study—experimental design, data analysis and interpretation, and manuscript preparation.
VH and BN are employed by the company InsideTracker, while ML, AP-A, TF, LL, CP, and AC are employed by DSM.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank the staff at AHPharma, Inc., for their efforts in animal husbandry and management of this trial.
Ahmad, T., Mushtaq, T., Mahr-Un-Nisa Sarwar, M., Hooge, D. M., and Mirza, M. A. (2006). Effect of different non-chloride sodium sources on the performance of heat-stressed broiler chickens. Br. Poult. Sci. 47, 249–256. doi: 10.1080/00071660600753342
Ahmad, T., and Sarwar, M. (2006). Dietary electrolyte balance: implications in heat stressed broilers. World's Poult. Sci. J. 62, 638–653. doi: 10.1017/S0043933906001188
Akşit, M., Yalcin, S., Özkan, S., Metin, K., and Özdemir, D. (2006). Effects of temperature during rearing and crating on stress parameters and meat quality of broilers. Poult. Sci. 85, 1867–1874. doi: 10.1093/ps/85.11.1867
Altan, Ö. Z. G. E., Pabuçcuoglu, A., Altan, A., Konyalioglu, S., and Bayraktar, H. (2003). Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult. Sci. 44, 545–550. doi: 10.1080/00071660310001618334
Attia, Y. A., Al-Harthi, M. A., El-Shafey, A. S., Rehab, Y. A., and Kim, W. K. (2017). Enhancing tolerance of broiler chickens to heat stress by supplementation with vitamin E, vitamin C and/or probiotics. Ann. Anim. Sci. 17, 1155. doi: 10.1515/aoas-2017-0012
Beckford, R. C., Ellestad, L. E., Proszkowiec-Weglarz, M., Farley, L., Brady, K., Angel, R., et al. (2020). Effects of heat stress on performance, blood chemistry, and hypothalamic and pituitary mRNA expression in broiler chickens. Poult. Sci. 99, 6317–6325. doi: 10.1016/j.psj.2020.09.052
Belay, T., and Teeter, R. G. (1993). Broiler water balance and thermobalance during thermoneutral and high ambient temperature exposure. Poult. Sci. 72, 116–124. doi: 10.3382/ps.0720116
Bílková, B., Bainová, Z., Janda, J., Zita, L., and Vinkler, M. (2017). Different breeds, different blood: Cytometric analysis of whole blood cellular composition in chicken breeds. Vet. Immunol. Immunopathol. 188, 71–77. doi: 10.1016/j.vetimm.2017.05.001
Borges, S. A., Da Silva, A. F., and Maiorka, A. (2007). Acid-base balance in broilers. World's Poult. Sci. J. 63, 73–81. doi: 10.1017/S0043933907001286
Borges, S. A., Da Silva, A. F., Majorka, A., Hooge, D. M., and Cummings, K. R. (2004). Physiological responses of broiler chickens to heat stress and dietary electrolyte balance (sodium plus potassium minus chloride, milliequivalents per kilogram). Poult. Sci. 83, 1551–1558. doi: 10.1093/ps/83.9.1551
Celi, P., Verlhac, V., Calvo, E. P., Schmeisser, J., and Kluenter, A. M. (2019). Biomarkers of gastrointestinal functionality in animal nutrition and health. Anim. Feed Sci. Technol. 250, 9–31. doi: 10.1016/j.anifeedsci.2018.07.012
Cowieson, A. J., Livingston, M. L., Nogal, B., Hoang, V., Crespo, R., and Livingston, K. A. (2020). Effect of coccidial challenge and vaccination on the performance, veterinary postmortem scores, and blood biochemistry of broiler chickens. Poult. Sci. 99, 3831–3840. doi: 10.1016/j.psj.2020.05.018
Farooqi, H. A. G., Khan, M. S., Khan, M. A., Rabbani, M., Pervez, K., and Khan, J. A. (2005). Evaluation of betaine and vitamin C in alleviation of heat stress in broilers. Int. J. Agric. Biol. 5, 744–746.
Habibian, M., Ghazi, S., Moeini, M. M., and Abdolmohammadi, A. (2014). Effects of dietary selenium and vitamin E on immune response and biological blood parameters of broilers reared under thermoneutral or heat stress conditions. Int. J. Biometeorol. 58, 741–752. doi: 10.1007/s00484-013-0654-y
Hajati, H., Hassanabadi, A., Golian, A., Nassiri-Moghaddam, H., and Nassiri, M. R. (2015). The effect of grape seed extract and vitamin C feed supplementation on some blood parameters and HSP70 gene expression of broiler chickens suffering from chronic heat stress. Ital. J. Anim. Sci. 14, 3273. doi: 10.4081/ijas.2015.3273
Hamm, L. L., and Simon, E. E. (1987). Roles and mechanisms of urinary buffer excretion. Am. J. Physiol. 253, F595–F605. doi: 10.1152/ajprenal.1987.253.4.F595
Hoppes, S. M., Boyd, J. D., and Brightsmith, D. J. (2015). Impact of delayed analysis in avian blood biochemical values measured with the Abaxis VetScan VS2. J. Avian Med. Surg. 200–209. doi: 10.1647/2014-033
Kawashima, C., Nagashima, S., Sawada, K., Schweigert, F. J., Miyamoto, A., and Kida, K. (2010). Effect of β-carotene supply during close-up dry period on the onset of first postpartum luteal activity in dairy cows. Reprod. Domest. Anim. 45, e282–e287. doi: 10.1111/j.1439-0531.2009.01558.x
Kutlu, H. R., and Forbes, J. M. (1993). Changes in growth and blood parameters in heat-stressed broiler chicks in response to dietary ascorbic acid. Livest. Prod. Sci. 36, 335–350. doi: 10.1016/0301-6226(93)90050-R
Lara, L. J., and Rostagno, M. H. (2013). Impact of heat stress on poultry production. Animals 3, 356–369. doi: 10.3390/ani3020356
Lentfer, T. L., Pendl, H., Gebhardt-Henrich, S. G., Fröhlich, E. K. F., and Von Borell, E. (2015). H/L ratio as a measurement of stress in laying hens–methodology and reliability. British Poult. Sci. 56, 157–163. doi: 10.1080/00071668.2015.1008993
Lindholm, C., and Altimiras, J. (2016). Point-of-care devices for physiological measurements in field conditions. A smorgasbord of instruments and validation procedures. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 202, 99–111. doi: 10.1016/j.cbpa.2016.04.009
Liu, Q. W., Feng, J. H., Chao, Z., Chen, Y., Wei, L. M., Wang, F., et al. (2016). The influences of ambient temperature and crude protein levels on performance and serum biochemical parameters in broilers. J. Anim. Physiol. Anim. Nutr. 100, 301–308. doi: 10.1111/jpn.12368
Livingston, M. L., Cowieson, A. J., Crespo, R., Hoang, V., Nogal, B., Browning, M., et al. (2020). Effect of broiler genetics, age, and gender on performance and blood chemistry. Heliyon 6, e04400. doi: 10.1016/j.heliyon.2020.e04400
Livingston, M. L., Ferket, P. R., Brake, J., and Livingston, K. A. (2019). Dietary amino acids under hypoxic conditions exacerbates muscle myopathies including wooden breast and white stripping. Poult. Sci. 98, 1517–1527. doi: 10.3382/ps/pey463
Luo, J., Song, J., Liu, L., Xue, B., Tian, G., and Yang, Y. (2018). Effect of epigallocatechin gallate on growth performance and serum biochemical metabolites in heat-stressed broilers. Poult. Sci. 97, 599–606 doi: 10.3382/ps/pex353
Maren, T. H.. (1967). Carbonic anhydrase: chemistry, physiology, and inhibition. J. Physiol. Rev. 47, 595–781. doi: 10.1152/physrev.1967.47.4.595
Martin, M. P., Wineland, M., and Barnes, H. J. (2010). Selected blood chemistry and gas reference ranges for broiler breeders using the i-STAT® handheld clinical analyzer. Avian Dis. 54, 1016–1020. doi: 10.1637/9223-122209-Reg.1
Meluzzi, A., Primiceri, G., Giordani, R., and Fabris, G. (1992). Determination of blood constituents reference values in broilers. Poult. Sci. 71, 337–345. doi: 10.3382/ps.0710337
Mujahid, A., Akiba, Y., and Toyomizu, M. (2009). Progressive changes in the physiological responses of heat-stressed broiler chickens. J. Poult. Sci. 46, 163–167. doi: 10.2141/jpsa.46.163
Nardone, A., Ronchi, B., Lacetera, N., Ranieri, M. S., and Bernabucci, U. (2010). Effects of climate changes on animal production and sustainability of livestock systems. Livest. Sci. 130, 57–69. doi: 10.1016/j.livsci.2010.02.011
NRC (1994). National Research Council. Nutrient Requirements of Poultry, 8th Edn. Washington, DC: National Academic Press.
Olanrewaju, H. A., Purswell, J. L., Collier, S. D., and Branton, S. L. (2010). Effect of ambient temperature and light intensity on physiological reactions of heavy broiler chickens. Poult. Sci. 89, 2668–2677. doi: 10.3382/ps.2010-00806
Raila, J., Kawashima, C., Sauerwein, H., Hülsmann, N., Knorr, C., Myamoto, A., et al. (2017). Validation of blood vitamin A concentrations in cattle: comparison of a new cow-side test (iCheck™ FLUORO) with high-performance liquid chromatography (HPLC). BMC Vet. Res. 13, 1–6. doi: 10.1186/s12917-017-1042-3
Ruiz-Jimenez, F., Gruber, R., Correa, M., and Crespo, R. (2021). Comparison of portable and conventional laboratory analyzers for biochemical tests in chickens. Poult. Sci. 100, 746–754. doi: 10.1016/j.psj.2020.11.060
Stinefelt, B., Leonard, S. S., Blemings, K. P., Shi, X., and Klandorf, H. (2005). Free radical scavenging, DNA protection, and inhibition of lipid peroxidation mediated by uric acid. Ann. Clin. Lab. Sci. 35, 37–45.
St-Pierre, N. R., Cobanov, B., and Schnitkey, G. (2003). Economic losses from heat stress by US livestock industries. J. Dairy Sci. 86, E52–E77. doi: 10.3168/jds.S0022-0302(03)74040-5
Subramanya, A. R., and Ellison, D. H. (2014). Distal convoluted tubule. Clin. J. Am. Soc. Nephrol. 9, 2147–2163. doi: 10.2215/CJN.05920613
Tan, G. Y., Yang, L., Fu, Y. Q., Feng, J. H., and Zhang, M. H. (2010). Effects of different acute high ambient temperatures on function of hepatic mitochondrial respiration, antioxidative enzymes, and oxidative injury in broiler chickens. Poult. Sci. 89, 115–122. doi: 10.3382/ps.2009-00318
Tawfeek, S. S., Hassanin, K. M. A., and Youssef, I. M. I. (2014). The effect of dietary supplementation of some antioxidants on performance, oxidative stress, and blood parameters in broilers under natural summer conditions. J. World's Poult. Res. 4, 10–19.
Terker, A. S., Zhang, C., McCormick, J. A., Lazelle, R. A., Zhang, C., Meermeier, N. P., et al. (2015). Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 21, 39–50. doi: 10.1016/j.cmet.2014.12.006
Wang, Y., Saelao, P., Chanthavixay, K., Gallardo, R., Bunn, D., Lamont, S. J., et al. (2018). Physiological responses to heat stress in two genetically distinct chicken inbred lines. Poult. Sci. 97, 770–780. doi: 10.3382/ps/pex363
Xue, B., Song, J., Liu, L., Luo, J., Tian, G., and Yang, Y. (2017). Effect of epigallocatechin gallate on growth performance and antioxidant capacity in heat-stressed broilers. Arch. Anim. Nutr. 71, 362–372. doi: 10.1080/1745039X.2017.1355129
Keywords: broiler, biomarker, blood physiology, heat stress, vitamins, sodium bicarbonate
Citation: Livingston ML, Pokoo-Aikins A, Frost T, Laprade L, Hoang V, Nogal B, Phillips C and Cowieson AJ (2022) Effect of Heat Stress, Dietary Electrolytes, and Vitamins E and C on Growth Performance and Blood Biochemistry of the Broiler Chicken. Front. Anim. Sci. 3:807267. doi: 10.3389/fanim.2022.807267
Received: 01 November 2021; Accepted: 24 January 2022;
Published: 18 February 2022.
Edited by:
Edward Narayan, The University of Queensland, AustraliaReviewed by:
Kyung-Woo Lee, Konkuk University, South KoreaCopyright © 2022 Livingston, Pokoo-Aikins, Frost, Laprade, Hoang, Nogal, Phillips and Cowieson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aaron J. Cowieson, YWFyb24uY293aWVzb25AZHNtLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.