
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Anim. Sci. , 15 December 2022
Sec. Animal Physiology and Management
Volume 3 - 2022 | https://doi.org/10.3389/fanim.2022.1072712
This article is part of the Research Topic Early Life Nutrition in Poultry View all 3 articles
Many benefits have been found in supplementing essential oils such as oregano oil (EOO) to poultry, including increased body weight gain, antioxidant activity, and better gastrointestinal morphology. However, few studies tested the influence of EOO supplementation on laying hens and reported conflicting results regarding its efficacy in improving their health and performance. Therefore, we aimed to explore the effects of dietary EOO on performance, gastrointestinal (GIT) traits, blood lipid, and antioxidant capacity in laying hens during the rearing phase. A total of 300-day-old Hy-line-Brown chicks were used, and treatment diets consisted of corn-soybean based either without (CON) or with EOO (Ecodiar®, 0.275 g/kg diet). Birds were randomized across treatments with five pens/treatment and 30-birds/pen. Pen weights and feed rejected were recorded every two weeks (1-17 weeks of age), to calculate daily feed intake (ADFI), body weight (BW), and daily weight gain (ADWG). At 11 and 14 weeks of age, blood samples were collected from 3 birds/pen and analyzed for blood lipids and antioxidant levels, and 5-birds/treatment were euthanized, and GIT traits were tested. Differences in measured parameters across weeks and between treatments were assessed using GLMM with Tukey’s Post hoc test applied to significant results in R 3.3.1 (α set at 0.05). Body weights at weeks 3, 11, 13, and 17 were significantly higher in the EOO group compared to the CON group (all P ≤ 0.05), ADWG was significantly higher in EOO birds compared to CON birds at 9 and 13 weeks old (all P ≤ 0.05), while no significant differences in ADFI were observed between treatments across weeks of the trials. At both 11 and 17 weeks old, triglyceride levels were significantly lower, while high-density-lipoprotein levels were higher in EOO (all P ≤ 0.05). Malondialdehyde levels were lower in the EOO group versus CON (p=0.01), while EOO birds had higher glutathione levels (p=0.01) than CON. Finally, at 12 weeks old, the weight of the entire GIT and empty gizzard were higher in the EOO group versus CON (all P ≤ 0.05), while liver and spleen weights were not significantly different between groups. In conclusion, dietary oregano supplementation exerted promoting effects on the performance of Hy-Line Brown pullets.
The earliest public concern about the use of antibiotics in livestock was in Great Britain (Swann, 1969). In the last decade, actual legislative action has occurred in the United States to prevent the use of antibiotics as growth promoters (Lillehoj et al., 2018). Various alternative products have been tested and used on poultry, namely phytogenics (Gadde et al., 2017). An essential oil is a product obtained by hydrodistillation, steam distillation, or dry distillation or by a suitable mechanical process without heating (for Citrus fruits) of a plant or some parts of it (Rubiolo et al., 2010).
Essential oils are composed of a variety of active compounds that provide the oil with its specific properties (Bakkali et al., 2008). These active compounds provide their parent plant with antibacterial, antiviral, antifungal, and aromatic properties (Bakkali et al., 2008). Essential oils have been shown to benefit the health of swine, poultry, and ruminants like cattle and goats (Nehme et al., 2021). Essential oils have various beneficial mechanisms to biological processes, such as the degradation of pathogens by acting as prooxidants and the prevention of mutagens penetrating cell membranes (Bakkali et al., 2008).
Many benefits have been found in supplementing essential oils to poultry. These benefits include increased body weight gain, improved feed conversion ratio, better gastrointestinal morphology, and increased antioxidant activity (Lee et al., 2003; Bozkurt et al., 2009; Hashemipour et al., 2013; Ghazi et al., 2015; Fonseca-García et al., 2017). Others have found decreased bacterial counts on poultry products like table eggs and meat (Migliorini et al., 2019; Denli et al., 2019).
One essential oil studied across poultry species is the essential oil of oregano (EOO). Oregano strains can be found across the world, including in countries like Greece and Turkey (Leyva-López et al., 2017). The two main active ingredients in EOO are carvacrol and thymol, with the percentage of each compound varying between species (Leyva-López et al., 2017). Oregano oil has been shown to have antibacterial, antioxidant, antiviral, antiparasitic, and immunomodulatory effects when fed to poultry (Alagawany et al., 2018).
Commercial laying flocks often live over one year, making them one of the longest types of poultry to be raised and producing (USDA ERS, 2022). Known studies have conflicting results on the efficacy of improving health and performance in poultry when supplementing an essential oil (Wang et al., 2019; Adaszyńska-Skwirzyńska et al., 2021; Ramirez, Peñuela-Sierra, and Ospina, 2021) with an thorough review written by Widodo (2020). Previous studies supplement the essential oil of oregano in conjunction with another substance (Ghazi et al., 2015; Amer et al., 2021; Saleh et al., 2021). Based on these studies, there are conflicting results on the effects of oregano oil on performance parameters, lipid profile, and antioxidant capacity. The studies mentioned above only examined laying hens older than 28 weeks old. Based on our knowledge, there are no studies have looked at the effects of dietary EOO on laying hens younger than 17 weeks old. Health and performance during the pullet phase sets a foundation for the subsequent production and performance during the laying phase (Bouvarel et al., 2011). Therefore, it is imperative to investigate the supplementation to improve health and performance during the pullet phase. We hypothesize that supplementing the essential oil will increase health and performance of pullets, therefore better prepare them for the laying phase. This study aimed to explore the effects of dietary EOO on performance measures, gastrointestinal traits, lipid profile, and antioxidant capacity in laying hens during the rearing phase.
Ethical statement: This experiment was approved by the Clemson University Institutional Animal Care and Use Committee (IACUC protocol AUP2021-0068).
Two hundred and ninety-day-old chicks (Hy-Line® Brown) were obtained from a local commercial hatchery in December 2021. Birds were housed in 10 pens (29 birds per pen) in a controlled ventilation and temperature house at Morgan Poultry Center, Clemson, South Carolina, USA (34˚39’23”N, 82˚50’09”W). Each pen was 5.04 m2 with approximately 3 in. (7.6 cm) clean pine wood shavings covering the floor. For the first 3 weeks, the heat was provided by a focal electric brooder per pen in addition to a gas-fired brooder for the entire house. The temperature was initially set at 95-97˚F at day 0, then progressively reduced by 4-6˚F every week until 3 weeks, as focal brooders were removed at this time. Temperature was reduced weekly until 6 weeks of age to 70 ˚F, then maintained to the end of the rearing phase (17 weeks), following the standard breed guidelines (Hy-Line, 2022). Feed and water were provided ad libitum. From 0 to 3 weeks, the feed was provided in tube feeders, and water was in gallon drinkers. For the first week of life, supplementary feed trays were provided. After 3 weeks, feed was provided in circular hanging feeders, and water was available in automatic cup drinkers.
Light was provided by a single 60-watt incandescent overhead lightbulb per pen, and pens were kept on a decreasing lighting schedule starting at 20L:4D cycle at 1 week old and decreased by increments of either 1.5 or 2 hours until 10L:14D from 7 to 17 weeks old (Table 1; Hy-Line, 2022).
The chicks were randomly assigned to 2 treatments (5 pens per treatment); a control group fed basal diet (CON), and an experimental group (EOO) fed basal diet with oregano oil supplementation following a completely randomized design. The essential oil of oregano, EOO (Ecodiar® Powder, Nutrinae, Palo Alto, CA, United States), was given in a dose of 0.275 g/1 kg basal diet per manufacturer’s instructions. Ecodiar® Powder was a commercial product sourced from Origanum vulgare ssp. Hirtum, contains 85-90% carvacrol and 0.5-02% thymol, as provided by the manufacturer.
The basal diet was formulated to meet or exceed requirements (Table 2), following the standard breed guidelines (Hy-Line, 2022). The hens were fed a mash diet throughout the experiment. The starter 1 diet was given from 0-3 weeks old, the starter 2 diet was given from 4-6 weeks old, the grower diet was given from 7-15 weeks old, and the pre-lay diet from 15-17 weeks of age.
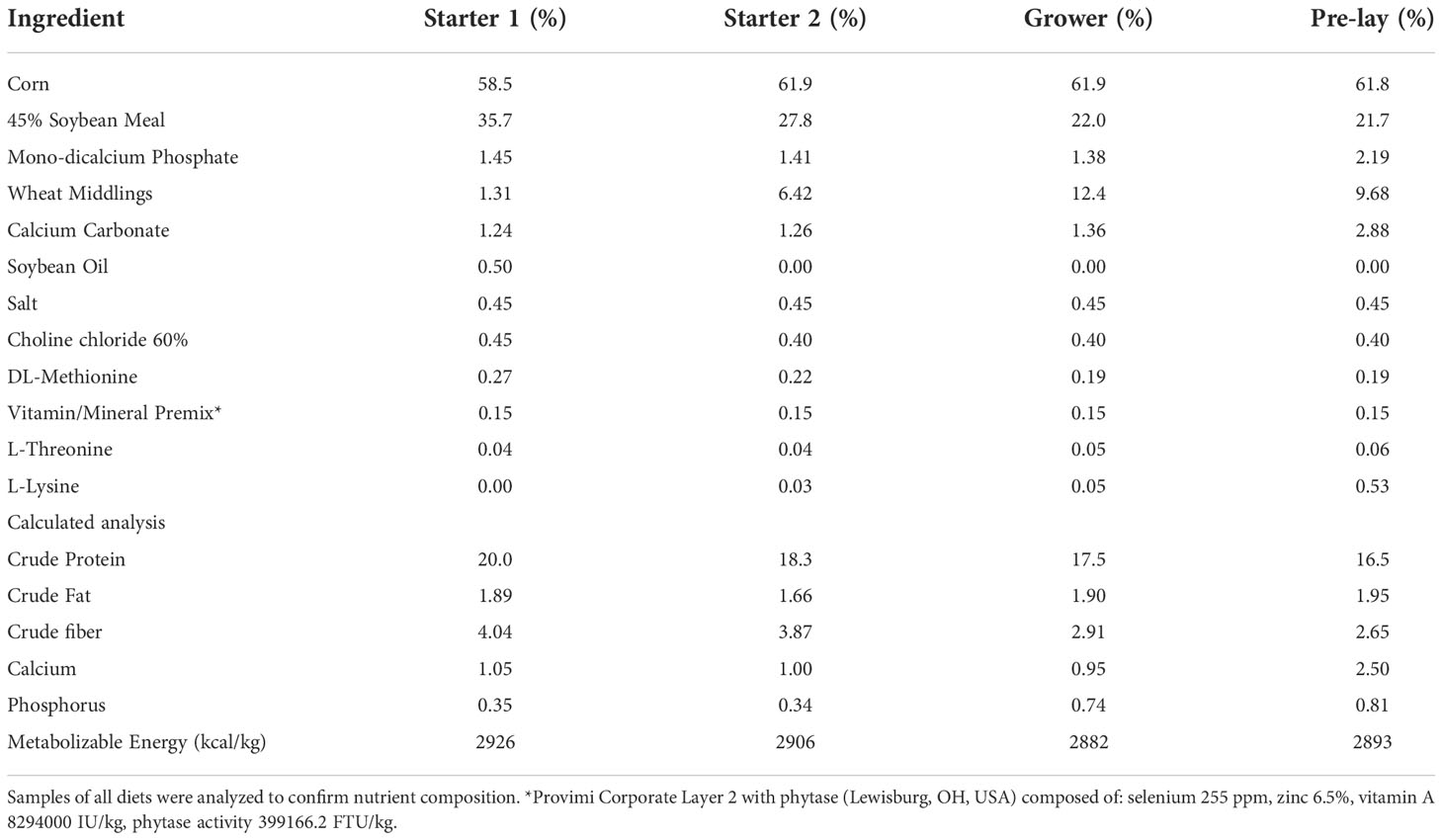
Table 2 Ingredient percentage and calculated nutrient analysis of 4 basal diets used in this experiment.
Body weight (BW) and average daily feed intake per bird (ADFI) were calculated weekly at weeks 1, 3, 5, 7, 9, 11, 13, 15, and week 17. Feed offered and refused were recorded weekly, and ADFI was calculated, similarly, body weight of birds was used to calculate average daily body weight gain per bird (ADWG) using the following formulas.
A total of five birds per treatment (1 bird per pen) were euthanized by cervical dislocation without anesthesia and dissected following Saldaña et al. (2015). A small incision was made at the distal end of the keel bone to access the abdominal cavity. The proximal end of the GIT was cut at the post-crop esophagus. The distal end was cut before the cloaca. The entire tract and accessory organs were excised and weighed as the whole GIT (g). Liver and spleen were removed and weighed separately. The duodenal loop was separated, and the entire length of the GIT was measured in cm. The small intestine length (SI) from the end of the gizzard to the ileocecal junction was measured in cm. Each cecum was measured from the ileocecal junction to the tip of the ceca to the nearest tenth of a millimeter, and the results were presented as the average () of both ceca. The proventriculus (PROV) was removed from the tract and weighed whole (g). The proventriculus was then cut open, and any contents were removed. The proventriculus was then weighed again as proventriculus empty (g). The gizzard was removed from the tract and weighed whole. It was then cut open, and the pH was taken in each lobe by a calibrated handheld pH meter (sympHony, VWR, Pennsylvania, USA). The average () of pH was calculated. Finally, the gizzard contents were removed, and the empty gizzard was weighed (g).
At 11 and 17 weeks of age, 3 birds per replicate were randomly selected, and blood samples were taken from the brachial wing vein. Whole blood samples were immediately transferred to an EDTA vacutainer and placed on ice to be transported. Blood samples were transferred to a 1.5 mL microcentrifuge tube, and plasma was separated at 6000 rpm for 10 minutes at 4˚C. Plasma samples were analyzed for total triglycerides, cholesterol, and high-density lipoproteins (HDL). Triglycerides and cholesterol were determined using Boehringer Mannheim commercial kits (Baden-Wurttemberg, Germany), while HDL level was determined using Roche commercial kits (Basel, Switzerland).
Serum total antioxidant capacity (TAC) was determined using the Randox total antioxidant status kit (Randox Laboratories Ltd, Crumlin, UK) following Habibi et al. (2014). Determination of malondialdehyde (MDA), glutathione peroxidase (GSH-Px), and superoxide dismutase (SOD) were determined by the colorimetric method using commercial kits from Nanjing Bioengineering Institute (Nanjing, Jiangsu, China). Alanine Transferase (ALT) and aspartate transferase (AST) were determined using commercial kits from Sigma-Aldrich (Burlington, M.A., USA).
Statistical analyses were performed using the R software ‘stats’ package (version 3.3.1, R Core Team, 2013). Descriptive statistics were calculated using the “psych” package. Evaluation of data with a Shapiro-Wilk’s test (p>0.05) using “shapiro.test” package and a visual inspection of histograms using “hist.” package revealed that data from all measurements were normally distributed. Generalized linear mixed models were developed with family set to “Poisson,” using the lme4 package (Bates et al., 2014) to describe the influence of oregano supplementation on performance parameters, GIT traits (weight (g), and percentage (%) as grams of organ per gram of body weight), blood lipid parameters, and antioxidant capacity, across weeks of age, and all possible interactions. Dietary treatment and week of age were included as main effects and unit and individual birds where possible, as random effects, p ≤ 0.05 was considered significant, using the following model:
where Yijklis the dependent variable, µ is the overall mean, Biis the effect of the dietary treatment, Tj is the effect of week of age, BTi j is the interaction effect between Bidietary treatment and Tj week of age,Cklis the effect of individual birds within the unit of Bi, and across Tj weeks of age, and eijkl is the residual error.
Statistically significant effects were further analyzed using Tukey’s honestly significant difference (HSD) multiple comparison procedure using the “multcomp” package (Hothorn et al., 2008). Tukey’s HSD significant differences between pairwise comparisons are indicated in figures or tables by different superscript letters. Data are presented as mean ± standard error of the mean (SEM) with P values of the pairwise comparisons.
Results of production measures are in Table 3. Body weights at weeks 3, 11, 13, and 17 were significantly higher in the EOO group compared to the CON group (p ≤ 0.05). ADWG was significantly higher in EOO birds compared to CON birds at 9 and 13 weeks old (p ≤ 0.05), while no significant differences in ADFI were observed between treatments across weeks of the trials.
Results of gastrointestinal traits of organ weights are presented in Table 4. At 12 weeks old, the weight and percentages (expressed as grams of organ per gram of body weight) of the entire GIT and empty gizzard were significantly higher in the EOO group when compared to the CON group (p≤0.05). At 17 weeks old, the weight and percentages of the empty proventriculus were significantly higher in EOO birds compared to CON birds (p≤0.05). Liver and spleen weights and their respective percentages were not significantly different at both ages across treatments (p>0.05). At 12 weeks old, proventriculus weight and percentage was not significantly different (p>0.05). At 17 weeks old, entire GIT and gizzard weights and their percentages were not significantly different across treatments (p>0.05).
Results of gastrointestinal traits of organ lengths are presented in Table 5. At 12 weeks old, average ceca lengths were significantly higher in the EOO group compared to the CON group (p≤0.05). The percentage of ceca length at 12 weeks old did not differ between groups (p>0.05). At 17 weeks of age, the ceca length and percentage of ceca length to body weight were significantly lower in the EOO treatment compared to CON treatment (p≤0.05). Gizzard pH and whole GIT and SI lengths and their percentages of weight were not significantly different between treatments across ages (p>0.05).
Results of blood lipid profile are presented in Table 6. At both 11 and 17 weeks old, triglyceride levels were significantly lower, and HDL levels were significantly higher in the EOO group (p=0.01). At 11 weeks old, cholesterol levels were significantly higher in the EOO group compared to the CON group (p=0.03). Cholesterol levels did not differ significantly at 17 weeks old.
Results of antioxidant capacity in serum are presented in Table 7. At both 11 and 17 weeks old, TAC levels were significantly higher in the EOO group (p=0.01). MDA levels were significantly lower in the EOO group compared to the CON group (p=0.01). At both ages, birds supplemented with oregano oil had significantly higher GSH-Px concentrations (p=0.01). Finally, concentrations of ALT and AST were significantly lower in EOO birds at both ages (p=0.01).
Dietary supplementation of the essential oil of oregano increased body weights at specific ages. In our study, birds supplemented with EOO had significantly higher body weights at 3, 11, 13, and 17 weeks old. One explanation for this may be the essential oil of oregano promoting digestibility by stimulating enzyme secretion in the gut which is hypothesized by Ghazi et al. (2015) who supplemented broilers with oregano oil under heat stress. Another explanation could be the essential oil of oregano inducing an increase in nutrient metabolism as described by Reyer et al. (2017) who supplemented day-old broilers with a dietary essential oil blend of star anise, rosemary, thyme, and oregano. It is important to note that the BW of the EOO group was within standard guidelines of brown recommendations (Hy-Line, 2022) while at many ages birds of the CON group were underweight. A negative relationship between body weight and efficiency exists during the laying phase, so it is important that birds reach the optimal weight at maturity (Robinson andWilson, 1996).
In the current study, birds supplemented with EOO showed significantly higher average daily weight gain when compared to control birds. One explanation may be pullets supplemented with oregano oil increased digestive health through better nutrient utilization as described by Zhang et al. (2021) who found similar results with a higher ADWG with no difference in ADFI from day 1 to 21 in broilers supplemented with EOO. Consistent with our findings, several studies on broilers supplemented with oregano oil reported higher body weight gain at certain ages (Hashemipour et al., 2013; Ghazi et al., 2015; Tzora et al., 2017; Amer et al., 2021; Zhang et al., 2021).
Feed intake was not impacted by supplementation of the essential oil of oregano across all ages in this study. Previous studies agree with this finding (Zhao et al., 2021; Karadagoglu et al., 2018; Cufadar, 2018; Feng et al., 2021). The composition or inclusion rate of EOO used in this study did not seem to impact the palatability of the experimental diet or influence FI as described by Abo Ghanima et al. (2020) who supplemented carvacrol to layer hens. We expected feed intake to increase, due to the aromatic nature of essential oils but they may not have the same effect on layer hens in relation to feed intake (Abo Ghanima et al., 2020).
Dietary essential oil of oregano supplementation increased entire GIT, empty gizzard, and proventriculus weights and percentages of body weights, while no differences were recorded in liver and spleen weights. This study found birds with dietary essential oregano oil had heavier GIT weights compared to the control. This is in conjunction with overall heavier birds supplemented with EOO at 17 weeks old. To date, no previous studies investigated the influence of EOO supplementation on GIT weights. With a larger proportion of GIT to body weight, this could potentially lead to a greater capacity for digestion as it has been shown in the past that oregano oil has increased specific digestive enzyme activities, but further research is needed (Jang et al., 2007; Krishan andNarang, 2014).
In the current study, gizzard weights were significantly heavier in the EOO group at 12 weeks old. One potential reason for the increase in gizzard weight in our study may be due to the EOO supplementation as described by Irawan et al. (2020) who attributed increased gizzard weight in broilers to essential oil supplementation. Irawan et al. (2020) states that few studies have looked at the effects of essential oils on young broilers, similar to our study.
The proventriculus may be considered the “true stomach” of the laying hen as it secretes mucous, hydrochloric acid, and pepsinogen (Svihus, 2014). Proventriculus weights were significantly higher in the group supplemented with oregano oil at 17 weeks old. The increase in proventriculus weights may be due to an increase in specific enzymatic activity due to essential oil supplementation as described by Hashemipour et al. (2013) who supplemented dietary thymol and carvacrol to broilers. However, food passes quickly through this organ and is not used for food storage, which may explain why no differences were found at a younger age in our study (Nitsan et al., 1991; Svihus, 2014).
In birds supplemented with EOO, we found an increase in average ceca length at 12 weeks and a decrease in average ceca length at 17 weeks. Similar to the current study, Akyurek and Yel (2011) found a significant increase in ceca length in broilers supplemented with thymol and carvacrol. The ceca are considered an adaptable organ that can change based on the diet (Clench and Mathias, 1995). The ceca have many functions such as electrolyte and water absorption and fermentation (Svihus et al., 2013). The mechanism and relationship between essential oils and ceca length is unclear, and histological work is needed which is beyond the scope of this study (Clench andMathias, 1995).
The liver is the main organ responsible for lipid metabolism (Gyamfi et al., 2019). It is well known cholesterol, triglycerides, and HDL levels are components of lipid metabolism. Triglycerides are often absorbed in the intestines from the diet and can be stored in the liver, with high levels of triglycerides possibly indicating hepatocyte injury (Gyamfi et al., 2019; Semova andBiddinger, 2021). The liver can synthesize cholesterol or take up excess cholesterol from the blood (Chan et al., 2015). High-density lipoproteins can be synthesized in the liver and transport excess cholesterol from circulation and tissue to the liver (Ouimet et al., 2019). High HDL levels may have a protective effect on cardiovascular health as described by Poernama et al. (1992).
Cholesterol levels were significantly higher in the EOO group at 12 weeks of age, while no significant differences were seen in cholesterol at 17 weeks old. One possible explanation may be the active ingredients in oregano oil influencing the mechanisms of lipid metabolism as Abo Ghanima et al. (2020) attributes one of the active compounds in oregano essential oil, thymol, to the reduction of cholesterol synthesis. Cholesterol levels are important for laying hens as it provides the backbone for sex hormones, which is crucial during the laying phase (Naber, 1976). However, our study and previous studies are inconsistent on the impact of EOO on cholesterol levels in laying hens (Bozkurt et al., 2016; Gul et al., 2019; Migliorini et al., 2019; Saleh et al., 2021). Differences in cholesterol levels between our study and previous studies may be due to different inclusion rates of the essential oil, feeding schedule, or preparation method of the essential oil (Lim et al., 2006).
Plasma triglycerides were significantly lower in birds supplemented with EOO. One explanation could be interrupting the mechanisms involved in lipogenesis as described by Saleh et al. (2021) who supplemented laying hens with dietary oregano and sage oil. Another possible explanation could be the influence of essential oils on inhibiting enzymes utilized in the process of lipogenesis as described by Dehghani et al., 2019 who supplemented dietary thyme essential oil to quail.
It is assumed that HDL synthesis and secretion pathway is similar to very low-density lipoproteins in poultry (Hermier, 1997). Birds with dietary EOO had significantly higher plasma HDL concentrations at both ages compared to control birds. Like other essential oils, EOO contains other chemical compounds called flavonoids (Moghrovyan et al., 2019). One possible explanation for an increase in HDL is the presence of flavonoids from EOO increasing the production of apolipoprotein A1 as described by Haryanto et al. (2016) who supplemented broilers with banana peel meal.
While a working knowledge of lipid metabolism exists in poultry, but there is there is little known on the effects of essential oils on lipid metabolism in chickens (Griffin & Hermier, 1988; Brenes & Roura, 2010; Cherian, 2015). EOO has a clear positive impact on HDL, triglycerides, and cholesterol levels in pullets, but more research is needed to understand the underlying mechanisms.
Antioxidants are vital to biological systems, as they stabilize and often scavenge for free radicals, thus protecting from oxidative stress (Shinde et al., 2012). Compounds like carvacrol and thymol, which are found in the essential oil of oregano, have strong antioxidant properties and can potentially increase the animal’s defense against oxidative stress (Park et al., 2014). TAC, SOD, and GSH-Px are well-known antioxidant enzymes as indicators of antioxidant activity in poultry (Yu et al., 2019). Like SOD, GSH-Px is a natural scavenger and enzyme that catalyzes the reduction of hydrogen peroxide and peroxide radicals into harmless molecules (Fattman et al., 2003; Fanucchi, 2014). MDA has been used as a biomarker for lipid peroxidation (Esterbauer and Cheeseman, 1990). AST and ALT are enzymes used as indicators for hepatocellular injury rather than human liver function (Telega, 2018). In this current study, TAC, SOD, and GSH-Px levels were significantly higher in EOO hens compared to CON hens across both ages. MDA, ALT, and AST concentrations were significantly lower in EOO pens compared to CON pens across both groups.
The importance of measuring TAC is the result of a single number that provides insight into all antioxidants in the animal (Cecchini and Fazio, 2020). TAC can be reflective of the overall antioxidant defense system in the layer hen with higher TAC levels meaning better antioxidant defense (Yu et al., 2019). In this study, TAC levels were significantly higher in birds supplemented with oregano oil possibly due to the active ingredients in EOO as described by Gadde et al. (2017) who states it is well known that phytogenics- specifically the active ingredients in essential oils- have antioxidant properties. Like this study, Previous studies found an increase in TAC concentrations in supplementing EOO to broilers (Hashemipour et al., 2013; Reshadi et al., 2020; Zhang et al., 2021).
When various reactive oxygen species attack certain molecules like proteins, lipids, and DNA, by-products like MDA are formed (Tsikas, 2017). MDA is considered the best indicator of lipid peroxidation, and a lower concentration of this compound translates to fewer reactions occurring, thus decreasing oxidative stress (Abo Ghanima et al., 2020). In the current study, MDA concentrations were significantly lower in the group supplemented with EOO. One implication is the components of oregano oil, carvacrol and thymol, protect lipids and slow lipid peroxidation reactions from occurring as described by Botsoglou et al. (2002) who supplemented broilers with dietary EOO. The finding in this study is in agreement with previous studies supplementing oregano oil on laying hens (Gul et al., 2019; Abo Ghanima et al., 2020; Büyükkılıç Beyzi et al., 2020).
Superoxide dismutase catalyzes the breakdown of a superoxide anion into water and oxygen (Weisiger and Fridovich, 1973). Superoxide anions are just one of many reactive oxygen species (ROS) that, when this concentration surpasses the concentration of antioxidants, the biological system is in oxidative stress (Haida & Hakiman, 2019). This study found SOD concentrations to increase from EOO supplementation. One possible explanation could be the phenolic compounds in EOO scavenging radicals, like the superoxide anion, further increasing antioxidant activity as described by Yanishlieva et al. (1999) who examined antioxidant activity of thymol and carvacrol in two types of fats. Like this current stud, studies in the past found an increase in SOD levels when supplementing laying hens with an essential oil (Zhang et al., 2021; Yu et al., 2019).
GSH-Px is known to have an important part in antioxidant defense, but its role in other biological systems are still being explored (Surai et al., 2018). The current findings show supplementation of EOO increased serum GSH-Px levels. The presence of phenolic compounds like carvacrol and thymol in the oregano plant may explain increased GSH-Px activity as described by Shan et al. (2005) who examined the antioxidant capacity of various extracts. Other studies found similar increases in GSH-Px in laying hens and quail, respectively (Herve et al., 2018; Yu et al., 2019).
Our current findings suggest EOO supplementation lowered AST and ALT concentrations. One explanation may be due to the antioxidant properties of the oregano oil that protect cells from DNA damage as described by Abo Ghanima et al. (2020) who supplemented laying hens with thymol, carvacrol, and euganol. Similar studies in both broilers and quail found similar results to our study (Herve et al., 2018; Herve et al., 2019; Oladokun et al., 2021).
This study explored the effects of the dietary essential oil of oregano on performance, GIT traits, blood lipid profile, and antioxidant capacity in layer pullets. The essential oil of oregano has a significant positive impact on performance and some physiological measures in pullets, but the underlying mechanisms need further research. Overall, the essential oil of oregano supplementation may be beneficial to the health of Hy-Line Brown pullets.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by the Clemson University Institutional Animal Care and Use Committee.
All authors contributed to the experimental design, sampling and preparation, review, and editing of this article and approved the submitted version. All authors contributed to the article and approved the submitted version.
This study was supported by the United Sorghum Checkoff Program (project # RG002-21) and from the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch project #NC1029.
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture. We would like to thank Rafael Cabrera for donation of the essential oil of oregano. We would like to thank the employees and student workers at the Morgan Poultry Center for their help in daily care and troubleshooting. We would also like to thank our undergraduate volunteers- especially Bailey Ward, Christianna Hoshko, and Isabella Raymond- for their help during the trial.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abo Ghanima M. M., Alagawany M., Abd El-Hack M. E., Taha A., Elnesr S. S., Ajarem J., et al. (2020). Consequences of various housing systems and dietary supplementation of thymol, carvacrol, and euganol on performance, egg quality, blood chemistry, and antioxidant parameters. Poultry Sci. 99 (9), 4384–4397. doi: 10.1016/j.psj.2020.05.028
Adaszyńska-Skwirzyńska M., Szczerbińska D., Zych S. (2021). The Use of Lavender (Lavandula angustifolia) Essential Oil as an Additive to Drinking Water for Broiler Chickens and Its In Vitro Reaction with Enrofloxacin. Animals, 11(6), 1535. doi: 10.3390/ani11061535
Akyurek H., Yel A. (2011). Influence of dietary thymol and carvacrol preparation and/or an organic acid blend on growth performance, digestive organs and intestinal microbiota of broiler chickens. Afr. J. Microbiol. Res. 5 (8), 979–984. doi: 10.5897/AJMR10.203
Alagawany M., ABD EL-HACK M. E., FARAG M. R., SHAHEEN H. M., ABDEL-LATIF M. A., NORELDIN A. E., et al. (2018). The usefulness of oregano and its derivatives in poultry nutrition. World's poultry Sci. J. 74 (3), 463–474. doi: 10.1017/S0043933918000454
Amer S. A., Tolba S. A., Al Sadek D. M. M., Abdel Fattah D. M., Hassan A. M., Metwally A. E. (2021). Effect of supplemental glycerol monolaurate and oregano essential oil blend on the growth performance, intestinal morphology, and amino acid digestibility of broiler chickens. BMC Veterinary Res. 17 (1), 312. doi: 10.1186/s12917-021-03022-5
Bakkali F., Averbeck S., Averbeck D., Idaomar M. (2008). Biological effects of essential oils – A review. Food Chem. Toxicol. 46 (2), 446–475. doi: 10.1016/j.fct.2007.09.106
Bates D., Mächler M., Bolker B., Walker S. (2014). Fitting linear mixed-effects models using lme4. J. Stat. Software 67. doi: 10.48550/arXiv.1406.5823
Botsoglou N. A., Florou-Paneri P., Christaki E., Fletouris D. J., Spais A. B. (2002). Effect of dietary oregano essential oil on performance of chickens and on iron-induced lipid oxidation of breast, thigh and abdominal fat tissues. Br. poultry Sci. 43 (2), 223–230. doi: 10.1080/00071660120121436
Bouvarel I., Nys Y., Lescoat P. (2011). “12 - Hen nutrition for sustained egg quality,” in Improving the Safety and Quality of Eggs and Egg Products. Eds. Nys Y., Bain M., Van Immerseel F. (UK: Woodhead Publishing), 261–299.
Bozkurt M., Alçiçek A., Çabuk M., Küçükyilmaz K., Çatli A. U. (2009). Effect of an herbal essential oil mixture on growth, laying traits, and egg hatching characteristics of broiler breeders. Poultry Sci. 88 (11), 2368–2374. doi: 10.3382/ps.2009-00048
Bozkurt M., Bintaş E., Kırkan Ş, Akşit H., Küçükyılmaz K., Erbaş G., et al. (2016). Comparative evaluation of dietary supplementation with mannan oligosaccharide and oregano essential oil in forced molted and fully fed laying hens between 82 and 106 weeks of age. Poultry Sci. 95 (11), 2576–2591. doi: 10.3382/ps/pew140
Brenes A., Roura E. (2010). Essential oils in poultry nutrition: Main effects and modes of action. Anim. Feed Sci. Technol. 158 (1), 1–14. doi: 10.1016/j.anifeedsci.2010.03.007
Büyükkılıç Beyzi S., Konca Y., Kaliber M., Sarıözkan S., Kocaoğlu Güçlü B., Aktuğ E., et al. (2020). Effects of thyme essential oil and A, C, and E vitamin combinations to diets on performance, egg quality, MDA, and 8-OHdG of laying hens under heat stress. J. Appl. Anim. Res. 48 (1), 126–132. doi: 10.1080/09712119.2020.1746662
Cecchini S., Fazio F. (2020). Assessment of Total Antioxidant Capacity in Serum of Heathy and Stressed Hens. Animals 10(11). doi: 10. Doi: 10.3390/ani10112019
Chan J., Karere G. M., Cox L. A., VandeBerg J. L. (2015). “Animal Models of Diet-induced Hypercholesterolemia,” in Hypercholesterolemia. Ed. Kumar S. A. (London: IntechOpen). doi: 10.5772/59610
Cherian G. (2015). Nutrition and metabolism in poultry: role of lipids in early diet. J. Anim. Sci. Biotechnol. 6 (1), 28. doi: 10.1186/s40104-015-0029-9
Clench M. H., Mathias J. R. (1995). The avian cecum: a review. Wilson Bull. 107 (1), 93–121. https://www.jstor.org/stable/4163516.
Cufadar Y. (2018). Effects of Dietary Oregano Essential Oil Supplementation on Performance and Eggshell Quality in Laying Hens. Selcuk J. Agric. Food Sci. 32 (2), 158–161. doi: 10.15316/SJAFS.2018.79
Dehghani N., Afsharmanesh M., Salarmoini M., Ebrahimnejad H. (2019). In vitro and in vivo evaluation of thyme (Thymus vulgaris) essential oil as an alternative for antibiotic in quail diet1. J. Anim. Sci. 97 (7), 2901–2913. doi: 10.1093/jas/skz179
Denli M., Vural A., Alp S. Y. (2019). The Influence Of Oregano Essential Oil On Egg Quality And Egg Shell Contamination Of Laying Hens Kept In Furnished Cages. Sci. Papers-Series D-Animal Sci. 62 (2), 48–52.
Esterbauer H., Cheeseman K. H. (1990). [42] Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods enzymology 186, 407–421. doi: 10.1016/0076-6879(90)86134-H
Fanucchi M. V. (2014). “Chapter 11 - Development of Antioxidant and Xenobiotic Metabolizing Enzyme Systems,” in The Lung, 2nd ed.. Eds. Harding R., Pinkerton K. E. (Boston: Academic Press), 223–231.
Fattman C. L., Chang L., Termin T. A., Petersen L., Enghild J. J., Oury T. D. (2003). Enhanced bleomycin-induced pulmonary damage in mice lacking extracellular superoxide dismutase. Free Radical Biol. Med. 35 (7), 763–771. doi: 10.1016/S0891-5849(03)00402-7
Feng J., Lu M., Wang J., Zhang H., Qiu K., Qi G., et al. (2021). Dietary oregano essential oil supplementation improves intestinal functions and alters gut microbiota in late-phase laying hens. J. Anim. Sci. Biotechnol. 12 (1), 72. doi: 10.1186/s40104-021-00600-3
Fonseca-García I., Escalera-Valente F., Martínez-González S., Carmona-Gasca C. A., Gutiérrez-Arenas ,. D. A., Ávila-Ramos F. (2017). Effect of oregano oil dietary supplementation on production parameters, height of intestinal villi and the antioxidant capacity in the breast of broiler. Austral J. veterinary Sci. 49 (2), 83–89. doi: 10.4067/S0719-81322017000200083
Gadde U., Kim W. H., Oh S. T., Lillehoj H. S. (2017). Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Health Res. Rev. 18 (1), 26–45. doi: 10.1017/S1466252316000207
Ghazi S., Amjadian T., Norouzi S. (2015). Single and combined effects of vitamin C and oregano essential oil in diet, on growth performance, and blood parameters of broiler chicks reared under heat stress condition. Int. J. Biometeorol 59 (8), 1019–1024. doi: 10.1007/s00484-014-0915-4
Griffin H., Hermier D. (1988). “Chapter 16 - Plasma lipoprotein metabolism and fattening in poultry,” in Leanness in Domestic Birds. Eds. Leclercq B., Whitehead C. C. (UK: Butterworth-Heinemann), 175–201. doi: 10.1016/B978-0-408-01036-8.50020-8
Gul M., Yılmaz E., Apaydin B., Sezmiş G., Kaya A., Timurkaan S., et al. (2019). Effects of oregano essential oil (Origanum syriacum L.) on performance, egg quality, intestinal morphology and oxidative stress in laying hens. Eur. Poultry Sci. 83, 1612–9199. doi: 10.1399/eps.2019.290
Gyamfi D., Ofori Awuah E., Owusu S. (2019). “Chapter 2 - Lipid Metabolism: An Overview,” in The Molecular Nutrition of Fats. Ed. Patel V. B. (Cambridge, MA, USA: Academic Press), 17–32.
Habibi R., Sadeghi G., Karimi A. (2014). Effect of different concentrations of ginger root powder and its essential oil on growth performance, serum metabolites and antioxidant status in broiler chicks under heat stress. Br. Poultry Sci. 55 (2), 228–237. doi: 10.1080/00071668.2014.887830
Haida Z., Hakiman M. (2019). A comprehensive review on the determination of enzymatic assay and nonenzymatic antioxidant activities. Food Sci. Nutr. 7 (5), 1555–1563. doi: 10.1002/fsn3.1012
Haryanto A., Miharja K., Wijayanti N. (2016). Effects of Banana Peel Meal on the Feed Conversion Ratio and Blood Lipid Profile of Broiler Chickens. Int. J. Poultry Sci. 15 no 15, 27–34. doi: 10.3923/ijps.2016.27.34
Hashemipour H., Kermanshahi H., Golian A., Veldkamp T. (2013). Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poultry Sci. 92 (8), 2059–2069. doi: 10.3382/ps.2012-02685
Hermier D. (1997). Lipoprotein Metabolism and Fattening in Poultry. J. Nutr. 127 (5), 805S–808S. doi: 10.1093/jn/127.5.805S
Herve T., Raphaël K. J., Ferdinand N., Laurine Vitrice F. T., Gaye A., Outman M. M., et al. (2018). Growth Performance, Serum Biochemical Profile, Oxidative Status, and Fertility Traits in Male Japanese Quail Fed on Ginger (Zingiber officinale, Roscoe) Essential Oil. Veterinary Med. Int. 2018, 7682060. doi: 10.1155/2018/7682060
Herve T., Raphaël K. J., Ferdinand N., Victor Herman N., Willy Marvel N. M., Cyril D'Alex T., et al. (2019). Effects of Ginger (Zingiber officinale, Roscoe) Essential Oil on Growth and Laying Performances, Serum Metabolites, and Egg Yolk Antioxidant and Cholesterol Status in Laying Japanese Quail. J. veterinary Med. 2019, 7857504. doi: 10.1155/2019/7857504
Hothorn T., Bretz F., Westfall P. (2008). Simultaneous inference in general parametric models. Biom. J. 50 (2008), 346–363. doi: 10.1002/bimj.200810425
Hyline (2022). Brown Commercial Layers Management Guide (USA: Hyline International). Available at: https://www.hyline.com/varieties/brown.
Irawan A., Hidayat C., Jayanegara A., Ratriyanto A. (2020). Essential oils as growth-promoting additives on performance, nutrient digestibility, cecal microbes, and serum metabolites of broiler chickens: a meta-analysis. Anim. Biosci. 34 (9), 1499–1513. doi: 10.5713/ab.20.0668
Jang I. S., Ko Y. H., Kang S. Y., Lee C. Y. (2007). Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens. Anim. Feed Sci. Technol. 134 (3), 304–315. doi: 10.1016/j.anifeedsci.2006.06.009
Karadagoglu O., Ozsoy B., Olmez M., Aydin O. D., Sahin T. (2018). The Effects of Drinking Water Supplemented with Essential Oils on Performance, Egg Quality and Egg Yolk Fatty Acid Composition in Laying Hens. İstanbul Üniversitesi Veteriner Fakültesi dergisi 44 (2), 85. doi: 10.26650/actavet.2018.410397
Krishan G., Narang A. (2014). Use of essential oils in poultry nutrition: A new approach. J. Advanced Veterinary Anim. Res. 1 (4), 156–162. doi: 10.5455/javar.2014.a36
Lee K.-., Everts H., Kappert H. J., Frehner M., Losa R., Beynen A. C. (2003). Effects of dietary essential oil components on growth performance, digestive enzymes and lipid metabolism in female broiler chickens. Br. Poultry Sci. 44 (3), 450–457. doi: 10.1080/0007166031000085508
Leyva-López N., Gutiérrez-Grijalva E., Vazquez-Olivo G., Heredia J. B. (2017). Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules (Basel Switzerland) 22 (6), 989. doi: 10.3390/molecules22060989
Lillehoj H., Liu Y., Calsamiglia S., Fernandez-Miyakawa M., Chi F., Cravens R. L., et al. (2018). Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Veterinary Res. 49 (1), 76. doi: 10.1186/s13567-018-0562-6
Lim K. S., You S. J., An B. K., Kang C. W. (2006). Effects of Dietary Garlic Powder and Copper on Cholesterol Content and Quality Characteristics of Chicken Eggs. Asian-Australasian J. Anim. Sci., 582–586. doi: 10.5713/ajas.2006.582
Migliorini M. J., Boiago M. M., Stefani L. M., Zampar A., Roza L. F., Barreta M., et al. (2019). Oregano essential oil in the diet of laying hens in winter reduces lipid peroxidation in yolks and increases shelf life in eggs. J. thermal Biol. 85, 102409. doi: 10.1016/j.jtherbio.2019.102409
Moghrovyan A., Sahakyan N., Babayan A., Chichoyan N., Petrosyan M., Trchounian A. (2019). Essential Oil and Ethanol Extract of Oregano (Origanum vulgare L.) from Armenian Flora as a Natural Source of Terpenes, Flavonoids and other Phytochemicals with Antiradical, Antioxidant, Metal Chelating, Tyrosinase Inhibitory and Antibacterial Activity. Curr. Pharm. design 25 (16), 1809–1816. doi: 10.2174/1381612825666190702095612
Naber E. C. (1976). The Cholesterol Problem, the Egg and Lipid Metabolism in the Laying Hen*. Poultry Sci. 55 (1), 14–30. doi: 10.3382/ps.0550014
Nehme R., Andrés S., Pereira R. B., Ben Jemaa M., Bouhallab S., Ceciliani F., et al. (2021). Essential Oils in Livestock: From Health to Food Quality. Antioxidants (Basel Switzerland) 10 (2), 330. doi: 10.3390/antiox10020330
Nitsan Z., Dunnington E. A., Siegel P. B. (1991). Organ Growth and Digestive Enzyme Levels to Fifteen Days of Age in Lines of Chickens Differing in Body Weight. Poultry Sci. 70 (10), 2040–2048. doi: 10.3382/ps.0702040
Oladokun S., MacIsaac J., Rathgeber B., Adewole D. (2021). Essential Oil Delivery Route: Effect on Broiler Chicken's Growth Performance, Blood Biochemistry, Intestinal Morphology, Immune, and Antioxidant Status. Animals 11, 3386. doi: 10.3390/ani11123386
Ouimet M., Barrett T. J., Fisher E. A. (2019). HDL and Reverse Cholesterol Transport. Circ. Res. 124 (10), 1505–1518. doi: 10.1161/CIRCRESAHA.119.312617
Park J. H., Kang S. N., Shin D., Shim K. S. (2014). Antioxidant Enzyme Activity and Meat Quality of Meat Type Ducks Fed with Dried Oregano (Origanum vulgare L.) Powder. Asian-Australas J. Anim. Sci. 28 (1), 79–85. doi: 10.5713/ajas.14.0313
Poernama F., Subramanian R., Cook M. E., Attie A. D. (1992). High density lipoprotein deficiency syndrome in chickens is not associated with an increased susceptibility to atherosclerosis. Arterioscler. Thromb. J. Vasc. Biol. 12 (5), 601–607. doi: 10.1161/01.ATV.12.5.601
Ramirez S. Y., Peñuela-Sierra L. M., Ospina M. A. (2021). Effects of oregano ( Lippia origanoides ) essential oil supplementation on the performance, egg quality, and intestinal morphometry of Isa Brown laying hens. Veterinary World 14 (3), 595–602. doi: 10.14202/vetworld.2021.595-602
Reshadi H., Torki M., Mohammadi H. (2020). Changes in performance, egg quality and blood parameters of laying hens fed selenium and oregano oil. Anim. Production Sci. 60 (13), 1620–1629. doi: 10.1071/AN19319
Reyer H., Zentek J., Männer K., Youssef I. M. I., Aumiller T., Weghuber J., et al. (2017). Possible Molecular Mechanisms by Which an Essential Oil Blend from Star Anise, Rosemary, Thyme, and Oregano and Saponins Increase the Performance and Ileal Protein Digestibility of Growing Broilers. J. Agric. Food Chem. 65 (32), 6821–6830. doi: 10.1021/acs.jafc.7b01925
Robinson F. E., Wilson J. L. (1996). Reproductive failure in overweight male and female broiler breeders. Anim. Feed Sci. Technol. 58 (1), 143–150. doi: 10.1016/0377-8401(95)00880-2
Rubiolo P., Sgorbini B., Liberto E., Cordero C., Bicchi C. (2010). Essential oils and volatiles: sample preparation and analysis. A review. Flavour Fragrance J. 25 (5), 282–290. doi: 10.1002/ffj.1984
Saldaña B., Guzmán P., Cámara L., García J., Mateos G. G. (2015). Feed form and energy concentration of the diet affect growth performance and digestive tract traits of brown-egg laying pullets from hatching to 17 weeks of age1 1Financial support was provided by Ministerio de Economía y competitividad (Project AGL2011–27004 and Project AGL 2014-56139-R). Poultry Sci. 94 (8), 1879–1893. doi: 10.3382/ps/pev145
Saleh A. A., Hamed S., Hassan A. M., Amber K., Awad W., Alzawqari M. H., et al. (2021). Productive Performance, Ovarian Follicular Development, Lipid Peroxidation, Antioxidative Status, and Egg Quality in Laying Hens Fed Diets Supplemented with Salvia officinalis and Origanum majorana Powder Levels. Animals 11 (22), 3513. doi: 10.3390/ani11123513
Semova I., Biddinger S. B. (2021). Triglycerides in Nonalcoholic Fatty Liver Disease: Guilty Until Proven Innocent. Trends Pharmacol. Sci. 42 (3), 183–190. doi: 10.1016/j.tips.2020.12.001
Shan B., Cai Y. Z., Sun M., Corke H. (2005). Antioxidant Capacity of 26 Spice Extracts and Characterization of Their Phenolic Constituents. J. Agric. Food Chem. 53 (20), 7749–7759. doi: 10.1021/jf051513y
Shinde A., Ganu J., Naik P. (2012). Effect of Free Radicals & Antioxidants on Oxidative Stress: A Review. J. Dental Allied Sci. 1 (2), 63. doi: 10.4103/2277-4696.159144
Surai P. F., Kochish I. I., Fishinin V. I. (2018). Glutathione peroxidases in poultry biology: Part 2. Modulation enzymatic activities. world's poultry Sci. J. 74 (2), 239–250. doi: 10.1017/S0043933918000260
Svihus B. (2014). Function of the digestive system1 1Presented as a part of the Informal Nutrition Symposium “From Research Measurements to Application: Bridging the Gap” at the Poultry Science Association's annual meeting in San Diego, California, July 22–25, 2013. J. Appl. Poultry Res. 23 (2), 306–314. doi: 10.3382/japr.2014-00937
Svihus B., Choct M., Classen H. L. (2013). Function and nutritional roles of the avian caeca: a review. World's Poultry Sci. J. 69 (2), 249–264. doi: 10.1017/S0043933913000287
Swann M. (1969). Report of Joint Committee on the Use of Antibiotics in Animal Husbandry and Veterinary Medicine (London: HMSO).
Telega G. W. (2018). “Jaundice,” in Nelson Pediatric Symptom-Based Diagnosis. Eds. Kliegman R. M., Lye P. S., Bordini B. J., Toth H., Basel D. (PA, USA: Elsevier, Philadelphia), 255–274.e1.
Tsikas D. (2017). Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Analytical Biochem. 524, 13–30. doi: 10.1016/j.ab.2016.10.021
Tzora A., Giannenas I., Karamoutsios A., Papaioannou N., Papanastasiou D., Bonos E., et al. (2017). Effects of Oregano, Attapulgite, Benzoic Acid and their Blend on Chicken Performance, Intestinal Microbiology and Intestinal Morphology. J. poultry Sci. 54 (3), 218–227. doi: 10.2141/jpsa.0160071
USDA ERS (2022) Poultry Sector at a Glance. Available at: https://www.ers.usda.gov/topics/animal-products/poultry-eggs/sector-at-a-glance/#:~:text=Table%2Degg%2Dtype%20chickens%20begin,optional%20after%20a%20molting%20period.
Wang H., Liang S., Li X., Yang X., Long F., Yang X. (2019). Effects of encapsulated essential oils and organic acids on laying performance, egg quality, intestinal morphology, barrier function, and microflora count of hens during the early laying period. Poultry Sci. 98 (12), 6751–6760. doi: 10.3382/ps/pez391
Weisiger R. A., Fridovich I. (1973). Superoxide dismutase: organelle specificity. J. Biol. Chem. 248 (10), 3582–3592. doi: 10.1016/S0021-9258(19)43969-0
Widodo E. (2020). The Prospective Use of Essential Oil from Herbs as Feed Additive for Laying Poultry: A Review. IOP Conf. Series.Earth Environ. Sci. 478 (1), 1–5. doi: 10.1088/1755-1315/478/1/012003
Yanishlieva N. V., Marinova E. M., Gordon M. H., Raneva V. G. (1999). Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 64 (1), 59–66. doi: 10.1016/S0308-8146(98)00086-7
Yu C., Guo Y., Yang Z., Yang ,. W., Jiang ,. S. (2019). Effects of star anise (Illicium verum Hook.f.) essential oil on nutrient and energy utilization of laying hens. Anim. Sci. J. 90 (7), 880–886. doi: 10.1111/asj.13221
Zhang L. Y., Peng Q. Y., Liu Y. R., Ma Q. G., Zhang J. Y., Guo Y. P., et al. (2021). Effects of oregano essential oil as an antibiotic growth promoter alternative on growth performance, antioxidant status, and intestinal health of broilers. Poultry Sci. 100 (7), 101163. doi: 10.1016/j.psj.2021.101163
Keywords: oregano oil, laying hen, pullet, performance, lipid profile, antioxidant capacity, gastrointestinal traits
Citation: Johnson AM, Anderson G, Arguelles-Ramos M and Ali AAB (2022) Effect of dietary essential oil of oregano on performance parameters, gastrointestinal traits, blood lipid profile, and antioxidant capacity of laying hens during the pullet phase. Front. Anim. Sci. 3:1072712. doi: 10.3389/fanim.2022.1072712
Received: 17 October 2022; Accepted: 22 November 2022;
Published: 15 December 2022.
Edited by:
Vishwajit S. Chowdhury, Kyushu University, JapanReviewed by:
Guofeng Han, Jiangsu Academy of Agricultural Sciences (JAAS), ChinaCopyright © 2022 Johnson, Anderson, Arguelles-Ramos and Ali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed A. B. Ali, QWxpOUBjbGVtc29uLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.