
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Anim. Sci., 21 November 2022
Sec. Animal Physiology and Management
Volume 3 - 2022 | https://doi.org/10.3389/fanim.2022.1040064
Culture environment during in vitro embryo production can affect embryo phenotype and pregnancy outcomes, making culture modifications a logical approach for improving embryo competence. Previously, the addition of the growth factors FGF2, LIF, and IGF1, termed FLI, to the culture medium improved bovine embryo development, and re-expansion following cryopreservation. The objective of this study was to investigate the survival of cryopreserved FLI treated embryos at day 15 of pregnancy and evaluate conceptus transcriptomes. Embryos were produced using in vitro fertilization of abattoir-derived oocytes, cultured to the blastocyst stage in the presence or absence of FLI (+/- FLI), and cryopreserved by slow-rate freezing. Thawed embryos were transferred into non-lactating recipient beef cows and eight days later conceptuses were recovered and analyzed. For a subset of conceptuses whole transcriptome analysis was performed by using the NovaSeq 6000. There was no detectable difference in conceptus recovery or average conceptus length between the two groups. There were 32 differentially expressed transcripts, 23 up-regulated and nine down-regulated in the +FLI group compared to -FLI. Genes were involved in interferon signaling, prostaglandin synthesis, and placental development. This study reveals that embryos cultured with or without FLI and cryopreserved by slow-rate freezing have similar developmental competence up to day 15 of development. Nevertheless, differences in gene expression exhibit an effect of FLI on conceptus signaling during elongation.
Reproductive technologies are becoming a necessary tool to increase the efficiency of food animal production to meet the demands of the growing population. As the need for food increases, producers rely on innovative strategies to capitalize on superior genetics and enhance fertility. One such strategy is the use of in vitro embryo production (IVP) which circumvents issues associated with ovulation and fertilization failure (Hansen, 2020). Furthermore, it allows multiple donors to be fertilized to one straw of semen, it is less reliant on hormonal manipulation, and can make use of a variety of donors (Ferré et al., 2020a). In recent years, advances in the IVP system have led to an increase in the adoption of this technology, but pregnancy rates following embryo transfer are still lower than following artificial insemination (Hansen, 2020; Viana, 2021). Optimization of the system will require greater understanding of embryo viability and factors that drive a successful pregnancy.
Large-scale implementation of IVP relies on ease of transport of genetics and flexible timing of embryo transfer based on recipient availability. Cryopreservation of embryos is the most efficient and practical approach for IVP to reach the industry. There are two commonly used methods of cryopreservation of embryos, slow-rate freezing and vitrification (Ferré et al., 2020b). Cryopreservation by slow-rate freezing is more desirable compared to vitrification because the embryos can be thawed and directly transferred to the female, whereas vitrification requires washing of embryos prior to transfer due to the presence of toxic concentrations of cryoprotectants (Sanches et al., 2016; Hansen, 2020). In addition, vitrification requires additional training and the use of laboratory facilities at the time of transfer (Vajta et al., 1998), making slow-rate freezing the practical choice for on-farm use. Regardless of method, cryopreservation does result in decreased pregnancy success (Hansen, 2020).
In vitro produced embryos, on average, have 20% lower pregnancy rates than their in vivo counterparts (Říha et al., 2002; Lonergan et al., 2003; Block et al., 2010; Sudano et al., 2013; Ealy et al., 2019). Moreover, embryos cryopreserved by slow-rate freezing IVP have approximately 10% lower pregnancy rates compared to fresh IVP, thus negatively impacting the adoption of this technology (Farin et al., 1999; Pontes et al., 2009; Ferré et al., 2020b). Some of the factors that impact survival following cryopreservation include culture environment and embryo selection. Using morphological assessment alone to clearly identify blastocyst stage embryos that have the developmental competence to withstand cryopreservation and survive to term is a challenge.
Culture environment can positively affect embryo phenotype and transcriptome, making culture modifications a logical approach for improving embryo viability following cryopreservation (Enright et al., 2000; Driver et al., 2012). These modifications include additions or substitutions to the culture medium, such as l-carnitine, leukemia inhibitory factor, hyaluronan, or forskolin (Block et al., 2009; Paschoal et al., 2014; Kocyigit and Cevik, 2015; Saraiva et al., 2017; Zolini et al., 2019). Recently, the addition of fibroblast growth factor 2 (FGF2) (40 ng/ml), leukemia inhibitory factor (LIF) (20 ng/ml), and insulin-like growth factor 1 (IGF1) (20 ng/ml), termed FLI, was shown to improve bovine embryo development to the blastocyst stage from 31% to 42%, as well as in vitro re-expansion following slow-rate cryopreservation from 39% to 82% (Stoecklein et al., 2021), thus addition of FLI may promote conceptus survival after embryo transfer.
Frequently, modifications to the culture medium are not explored further than development to the blastocyst stage. Following the first week of development, critical events that dictate the survival of an embryo are taking place. For example, from days 12 -15 of development, the conceptus is undergoing elongation and rapidly growing. The trophectoderm of the elongating conceptus secretes interferon-τ (IFNT2) as the signal for maternal recognition of pregnancy. During this time, IFNT2 acts on the luminal epithelium to inhibit transcription of the estrogen receptor and oxytocin receptor. Thus, preventing the production of luteolytic pulses of PGF2α by the endometrium and ensuring corpus luteum maintenance (Spencer et al., 2004; Robinson et al., 2008; Brooks et al., 2014). Interferon tau also induces interferon stimulated genes that have a role in uterine receptivity and pregnancy establishment through their upregulation in both the luminal and glandular epithelium. Embryo loss has been reported to be as great as twenty percent from days 8-28 of development (Wiltbank et al., 2016). This can result from poor timed elongation, low progesterone following fertilization, and glandular histotroph deficiencies (Clemente et al., 2009; Lonergan, 2011; Ribeiro et al., 2016). As a result, IFNT2 actions are critical for maintaining the corpus luteum and ensure conceptus viability (Brooks et al., 2014).
Embryo survival is easily affected during the dynamic period from hatching to conceptus elongation, and culture medium modifications may help mitigate some of the challenges associated with viability during this critical period. The objective of this study was to determine how the addition of FLI to in vitro culture medium affects embryo elongation following cryopreservation. It was hypothesized that supplementation of FLI to culture medium improves conceptus viability by promoting conceptus development and elongation through increased recovery and length, and inducing transcriptomic differences.
All animal procedures were conducted in accordance with the Guide for the Care and Use of Agriculture Animals in Research and Teaching and approved by the Institutional Animal Care and Use Committee of the University of Missouri and the Institutional Animal Care Use Committee of the Office of Research at The Ohio State University. Females were located at university research facilities and embryo transfers were performed in late spring and early fall.
All embryo production media: oocyte maturation (OMM), fertilization (IVF-TALP), wash (HEPES-TALP), culture (SOF-BE2), and the supplement penicillamine, hypotaurine, and epinephrine (PHE) were prepared in house following recipes previously published (Tríbulo et al., 2019; Stoecklein et al., 2021). Sperm purification gradient Isolate® (Irvine Scientific, Santa Ana, CA, USA) was acquired ready to use. The cytokine cocktail, FLI, was supplemented as, FGF2 (40 ng/ml), LIF (20 ng/ml), and IGF1 (20 ng/ml), to the culture medium (Yuan et al., 2017; Stoecklein et al., 2021).
Embryos were produced in vitro by using abattoir-derived cumulus oocyte complexes (COCs) that were fertilized and cultured by using standard procedures (Ortega et al., 2018; Tríbulo et al., 2019; Stoecklein et al., 2021). In short, COCs were collected from abattoir derived ovaries recovered from Bosc taurus cattle and placed in 500 µl of equilibrated OMM (50 COCs/well) overlaid with 300 µl mineral oil and matured for 22-24 h in a humidified atmosphere containing 5% (v/v) CO2. After the maturation period, the COCs were inseminated with sperm from a single Holstein bull known to have high fertility in vitro (Stoecklein et al., 2021). Fertilization proceeded for 18-20 h in a humidified atmosphere containing 5% (v/v) CO2. At the end of fertilization, putative zygotes (oocytes exposed to sperm) were denuded from their cumulus cells by vortexing for 5 min in 200 µl of hyaluronidase (10,000 units/ml) (Tríbulo et al., 2019). A minimum of 25 and up to 50 putative zygotes were placed in a 5-well dish containing 500 µl of SOF-BE2 overlaid with 300 µl mineral oil. Half of the putative zygotes were supplemented with FLI at the moment they were placed in the culture plate.Embryos were cultured at 38.5°C in a humidified atmosphere containing 5% (v/v) O2 and 5% (v/v) CO2 with the balance N2 for 7 days. Cleavage and development to the blastocyst stage were recorded at days 3 and 7, respectively (Figure 1).
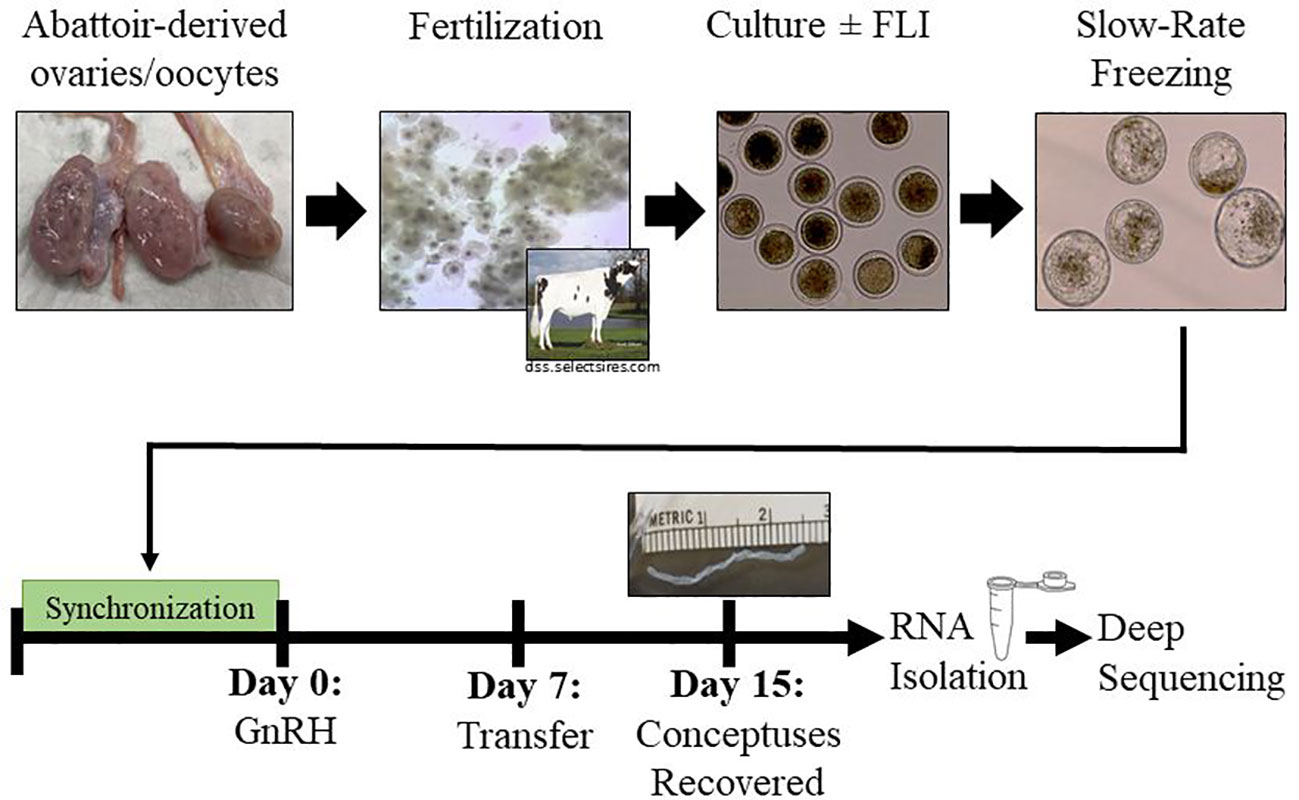
Figure 1 Experimental Design. Abattoir-derived oocytes were collected and matured for 22 hours before fertilization for 16-18 hours. After 7 days in culture (+/-FLI), blastocyst stage embryos were cryopreserved by slow-rate freezing. Groups of five embryos were transferred into recipient females 7 days after the last administration of GnRH of a 5-day CO-Synch plus CIDR® protocol. Conceptuses were recovered on day 15 and collected for transcriptomic analysis.
Seven days post insemination, blastocyst stage (stage 6) embryos of quality grade 1 (International Embryo Technology Society), were cryopreserved using slow-rate freezing as previously described (Stoecklein et al., 2021). Briefly, embryos were loaded into straws (5 embryos per straw) with ethylene glycol and sucrose (BioLife Freeze Medium Ethylene Glycol with Sucrose, AgTech Inc, Manhattan, KS, USA) and placed in the freezing machine (Beltron EFT 3002). Loaded straws were seeded, held at -6°C for 10 min, and then subjected to a temperature gradient of -0.5°C/min until reaching -32°C and kept at this temperature for 10 min (Prather et al., 1987; Block et al., 2011; Zolini et al., 2019). Immediately after, straws were plunged into liquid nitrogen and stored until embryo transfer.
Twenty-six non-lactating recipient beef cows were synchronized using a 5-day CO-Synch plus CIDR® (Bridges et al., 2008). Briefly, all cows received 100µg of gonadotropin releasing hormone (GnRH; Factrel) and a CIDR was inserted on day -5. The CIDR was removed on day 0 and PGF2α (Lutalyse®; 25 mg) was administered. A second injection of PGF2α was administered 8 ± 2 hours later. GnRH was administered 72 hours after CIDR removal. Seven days after the last injection of GnRH the ovaries were evaluated by transrectal ultrasonography to determine presence, size, and location of corpora lutea (CL). Cows with a CL ≥ 18.0 mm were randomized to receive either -FLI or +FLI and embryo transfer was performed. Straws containing embryos were retrieved from the liquid nitrogen container and placed in a water bath set to 28°C for 45 seconds. The straw was then loaded into and embryo transfer gun (ET Gun 17274, WTA, College Station, TX, USA) and embryos were deposited in the uterine horn ipsilateral the CL. A total of 65 -FLI and 65 +FLI embryos were transferred (5 embryos transferred per cow).
Eight days after the transfer of embryos (day 15 of pregnancy), females were euthanized, and reproductive tracts were collected (Figure 1). Tracts labelled with the cow ID were transported to the laboratory for processing. Connective tissue surrounding the uterus (broad ligament) was trimmed off and the ovaries were removed, after recording the relative location of the CL, to allow manipulation of the uterus with ease. In addition, the intercornual ligament was sectioned allowing the uterine horns to be fully separated and extended. To flush, a large hemostat was placed cranial to the internal cervical os to seal the uterine compartment. An Argyle Tomcat Catheter (Penn Veterinary Supply Inc, Lancaster, PA) was then inserted into the oviductal end of the uterine horn contralateral to the CL and 20 ml of flush medium (BioLife Advantage Complete Flush Media, AgTech Inc, Manhattan, KS, USA) were administered. The uterus was massaged allowing the flush medium to move from the contralateral uterine horn into the uterine body and expelled through an incision on the oviductal end of the uterine horn ipsilateral to the CL and into a grid plate. The flush was examined under a stereomicroscope (SZ61, Olympus, Center Valley, PA), conceptuses were recovered, measured, split into two equal pieces, placed in a tube containing the cow number and conceptus length and snap frozen in liquid nitrogen. Samples were stored at -80°C until further analysis.
A total of four filamentous conceptuses per treatment, from four different cows, were used for transcriptomic analysis. Each sample was one half of a conceptus without an embryonic disc. The extremes in length were not chosen, meaning, each conceptus submitted for sequencing was within 2.5 cm of the average length. RNA was isolated by using the trizol/chloroform method. Briefly, conceptus samples were homogenized in 500 μl trizol combined with 150 μl chloroform. The mixed trizol homogenate and chloroform were transferred to a phase lock tube (Andwin Scientific, Schaumburg, IL, USA) and centrifuged for 10 min at 14,000g at 4°C. The top aqueous phase was transferred to a new tube, mixed with an equal volume of 100% ethanol, and transferred to a spin column tube (Epoch Life Science Inc, Missouri City, TX, USA). After centrifugation of this mixture, DNAse digestion by using the RNase-Free DNAse Set (Qiagen, Hilden, Germany) proceeded for 15 min at room temperature. After an RNA prewash and two RNA washes, the column was transferred to a collection tube and the tissue sample was eluted in 75 μl of RNase-free water. Concentration of the sample was measured by using a nanodrop, and the sample was stored at -80°C.
For all samples submitted for sequencing RNA concentration and integrity were recorded. With the assistance of the University of Missouri Genomics Technology Core, amplification and reverse transcription were performed by using the mRNA Stranded Library Preparation (poly A enrichment). Libraries of cDNA were sequenced on a single NovaSeq S4 - PE100 (NovaSeq 6000) Flow Cell (NGS platform of Illumina). Sequencing was done at a depth of 50 million reads per sample. After assessment for quality, the samples transcriptomes were aligned to the cow genome using Hisat2 and FeatureCounts was used to determine the read counts per gene (Liao et al., 2014; Kim et al., 2015). EdgeR was used to identify differentially expressed genes (FDR < 0.05). Gene ontology (GO) enrichment analysis [ShinyGO 0.76 (Ge et al., 2020)] was used to identify molecular functions and biological processes associated with the differentially expressed genes.
A power analysis conducted using the PWR package of RStudio (Desktop 1.4.1106) indicated that 126 embryos needed to be transferred in this experiment (63 per treatment) to achieve statistical soundness. Data for embryo length and average embryos recovered both by cow and by treatment were analyzed by ANOVA by using the GLM procedure of the Statistical Analysis System (SAS) version 9.4. Data for embryo recovery rate were analyzed by using a logistic regression model of the Glimmix procedure of SAS. Treatment was modeled as a fixed factor, and cow was modeled as a random factor. Significance was determined as P < 0.05.
Out of the 26 cows receiving embryos, the overall pregnancy rate (at least one conceptus recovered) for this study was 76.9%. The pregnancy rate was not different (P > 0.05) between the -FLI (control) and +FLI groups (Figure 2). At the day 15 conceptus recovery (Figure 3), 33.8 ± 5.87% of the -FLI and 32.3 ± 5.8% of the + FLI embryos were recovered (P > 0.05; Figure 3). Per pregnant cow, 2.00 ± 0.32 and 2.33 ± 0.32 conceptuses were recovered in the -FLI and +FLI groups, respectively (Table 1, P > 0.05).

Figure 2 Survival at day 15 of bovine embryos supplemented with and without FLI during culture. At embryo transfer, recipient females (n = 26) received embryos treated with or without FLI. On day 15, females were identified as pregnant or open (non-pregnant) based on the presence of at least one conceptus (P = 0.37). Values are presented as least squares means ± SEM.

Figure 3 Conceptus Recovery. Of the embryos transferred (n = 65 per treatment), there was no difference (P = 0.85) in embryo recovery between the –FLI (22 conceptuses recovered) and +FLI groups (21 conceptuses recovered). Values are presented as least squares means ± SEM.
Overall, the range in length varied from 0.1 cm to 13 cm. Each conceptus was classified as either ovoid (0.1 – 0.4 cm), tubular (0.5 – 1.9 cm), or filamentous (> 2.0 cm) (Ribeiro et al., 2016). There was no difference (P > 0.05) in average conceptus length between the -FLI (2.87 ± 0.68 cm) and +FLI (3.54 ± 0.70 cm) groups (Table 2). The average length of the filamentous conceptuses was 3.33 ± 0.73 cm (-FLI) and 4.18 ± 0.75 cm (+FLI), as shown in Figure 4. Table 2 shows the classification of the recovered conceptuses. Of the 22 -FLI conceptuses recovered, 13 were filamentous, six were tubular, and three was ovoid. Of the 21 +FLI treated conceptuses recovered, 14 were filamentous, three were tubular, and four were ovoid.
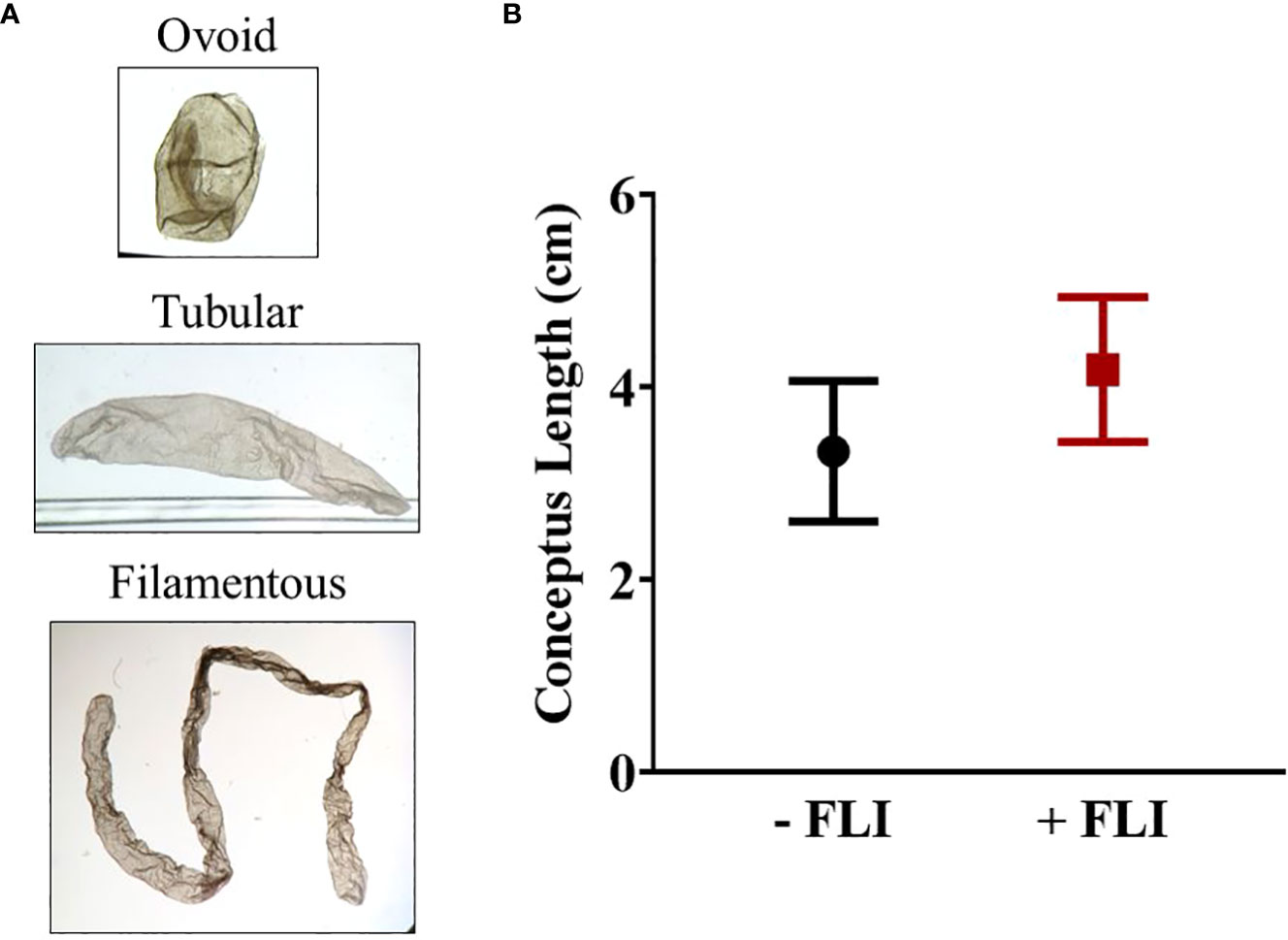
Figure 4 (A) Representative images of each category of conceptus. (B) Average length of -FLI and +FLI conceptuses that were classified as filamentous (> 2.0 cm). Results are reported as least square means ± standard error. P = 0.42.
The average total transcript reads for the control conceptuses were 90,543,416.5 with an alignment to the bovine genome of 97.54%. For the +FLI conceptuses, the average total transcript reads were 87,699,659.75 with an alignment to the bovine genome of 97.67% (Table 3). Between the two groups, 32 genes were found to be differentially expressed (Figure 5, FDR < 0.05). All genes are presented in (Supplemental Dataset 1). Twenty-three of these genes were increased and nine decreased in the +FLI group compared to the -FLI group. GO enrichment analysis for biological processes associated with the differentially expressed genes revealed 29 terms that were overrepresented between the two groups (Supplemental Dataset 2). Among these terms were regulation of response to stress, gonad development, metabolic processes, and defense responses. GO analysis for molecular function revealed 26 terms overrepresented between the two groups (Supplemental Dataset 3). Figure 6 depicts the top 20 pathways overrepresented in the +FLI group in each pathway database. These terms included amino acid binding, aromatase activity, oxidoreductase activity, and NADPH activity.

Table 3 Summary of the total transcript reads and aligned proportions to the bovine genome for embryos treated with or without FLI.
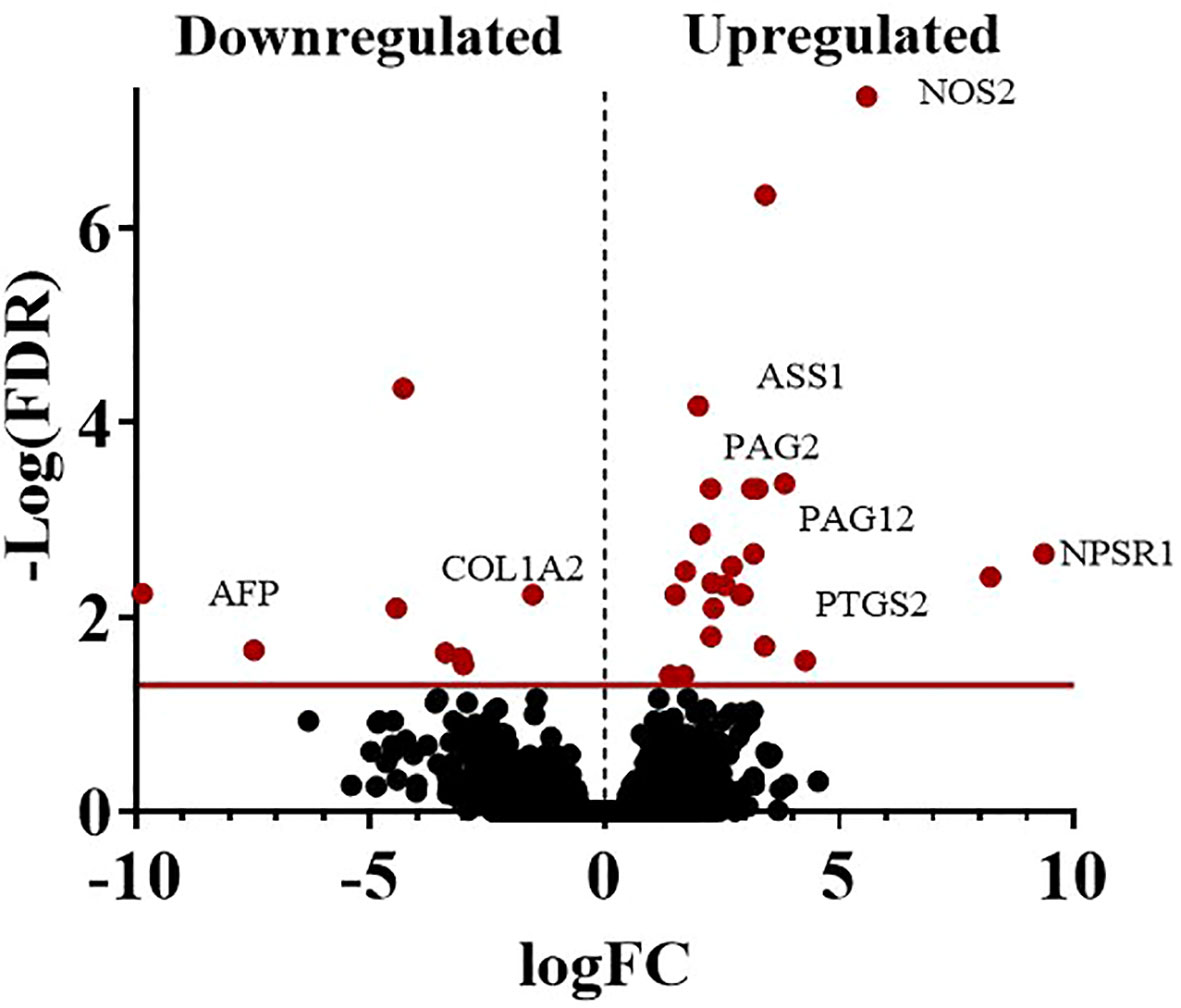
Figure 5 Volcano plot representing the number of DEGs when comparing conceptuses derived from +FLI or -FLI treated embryos. At a sequencing depth of 50 million reads per sample, 32 genes were differentially expressed. Nine genes were down-regulated, and 23 genes were up-regulated in the +FLI group. FDR < 0.05.
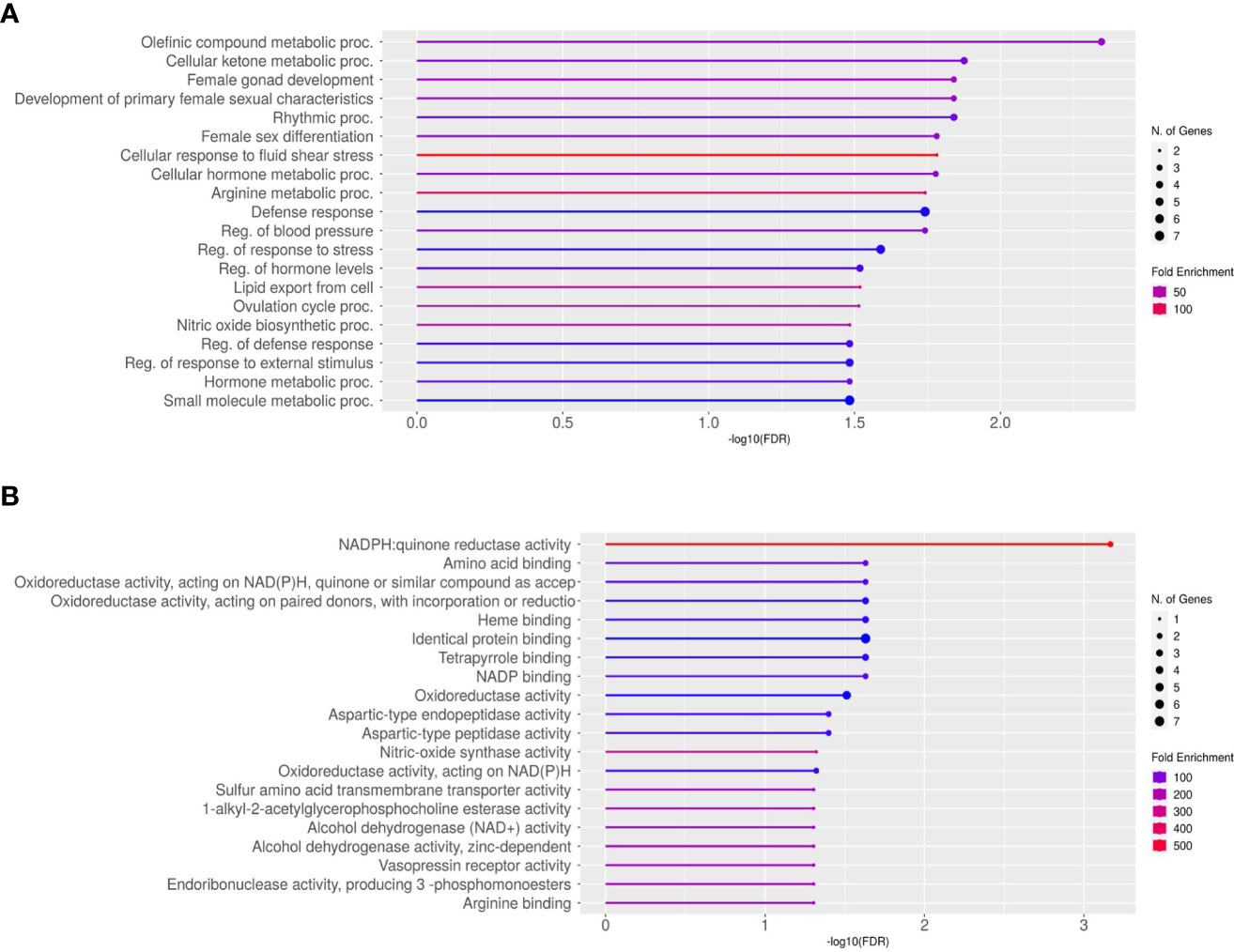
Figure 6 Lollipop Chart of gene ontology enrichment for the top 20 overrepresented pathways for (A) Biological Processes and (B) Molecular Function. FDR < 0.05.
Modifications to the in vitro embryo culture system are a logical approach for increasing embryo competency to develop to the blastocyst stage and pregnancy establishment (Block et al., 2009; Block et al., 2011). Previously, it was shown that the addition of three cytokines (FGF2, LIF, and IGF1) increased bovine embryo development to the blastocyst stage and survival following cryopreservation (Stoecklein et al., 2021). While the morphological quality of the blastocyst stage embryo is often indicative of developmental competence, there are numerous examples of improved development to the blastocyst stage that do not result in improved pregnancy rates or development to term (Spate et al., 2012; Redel et al., 2016). Thus, the present study investigated the effect of supplementation of FGF2, LIF, and IGF1 (FLI) during embryo culture on day 15 conceptus recovery, length, and transcriptome.
The period of conceptus elongation is a critical time of development, and pregnancy loss can be exceedingly high during this period (Wiltbank et al., 2016; Diskin et al., 2016). Additionally, frozen in vitro produced embryos are more susceptible to pregnancy failure compared to their in vivo counter parts (Farin and Farin, 1995; Ealy et al., 2019; Hansen, 2020). Embryo loss could be due to poor embryo viability, a compromised maternal environment, and/or a miscommunication between the conceptus and maternal system (Wiltbank et al., 2016; Ealy et al., 2019). The in vitro system fails to adequately mimic the maternal environment and thus the efficiency is low (Farin et al., 2001). Here, it is reported that the addition of FLI, during the embryo culture period in vitro did not result in an increase in conceptus recovery at day 15 when five morphologically normal blastocyst stage embryos were cryopreserved and transferred per recipient. The addition of FLI to the culture medium did increase the number of embryos that reach the blastocyst stage and are eligible for cryopreservation, but it is possible that beyond said increase, the competency to establish pregnancy is not affected. Additionally, FLI supplementation improved the proportion of blastocysts that survived slow-rate freezing when analyzed by in vitro hatching rates (Stoecklein et al., 2021), however, the same was not observed in vivo. It is possible that the dynamic maternal system adequately supports hatching of both embryos treated with and without FLI whereas the static in vitro system does not (Hansen et al., 2014).
Conceptus length has been shown to be different between AI-derived or in vivo produced and in vitro produced embryos and has been suggested as an indicator of conceptus survival (Lonergan, 2011; Barnwell et al., 2015). In this study there was no difference in conceptus length between the two groups, despite large variation in conceptus length. Furthermore, there was no difference in the number of ovoid, tubular, and filamentous conceptuses between treatment groups. Differences between in vivo and in vitro produced embryos have been reported at day 13, however, those differences were no longer apparent at day 16 (Bertolini et al., 2002; Lonergan et al., 2007; Barnwell et al., 2015). In vitro produced embryos that successfully undergo hatching from the zona pellucida and early elongation events, may have the same viability as their in vivo counterparts even if those early events are delayed. Thus, is possible that phenotypic differences between conceptuses in the +FLI and -FLI groups are no longer apparent by day 15 and viability of the two groups remain similar. Another possible explanation is that the two groups are developmentally similar up to this day 15 timepoint and deviations may arise later in development. For example, cloned embryos have similar day 30 pregnancy rates when compared to IVP embryos, however, nearly half of these are lost between days 30 and 60 (Wiltbank et al., 2016).
Using RNA Sequencing to analyze a subset of filamentous conceptuses in each group revealed differences in transcriptome between the control and FLI treated groups. While the two groups appeared developmentally similar at day 15, differences in gene expression were observed. Among the differentially detected transcripts were PTGS2, CYP19A1, NOS2, ASS1, and TLR2, products of which are involved in biological processes such as cellular response to stress, arginine and nitric oxide metabolism, and inflammatory response. Prostaglandin synthase 2 (PTGS2) is of particular interest as it is part of the signaling mechanism between the conceptus and the maternal endometrium that is critical to CL maintenance and embryo survival. Through the Janus Kinase (JAK)-signal pathway, trophectoderm-derived IFNT acts on the endometrium to regulate PTGS2 expression (Thatcher et al., 2001). Studies in the bovine have shown that IFNT increases PTGS2 expression in the endometrium and drives Prostaglandin E2 (PGE2) production (Arosh et al., 2004). It is evident that PGE inhibits the actions of Prostaglandin F2α on the CL but the mechanism is not completely understood (Ochoa et al., 2018). Interestingly, PTGS2 expression was increased in the +FLI group compared to the -FLI group. Given that PTGS2 drives an increase in luteoprotective PGE2, it is reasonable to conclude that the conceptus is regulating maternal signaling to strengthen its survival and development (Emond et al., 2004). Although conceptus length was not different between the two groups, the difference in PTGS2 expression may indicate a greater chance of preventing luteolysis and subsequently increased survival later in pregnancy.
Aromatase or CYP19A1, a key enzyme in the biosynthesis of estrogens, was found to be upregulated in the +FLI group. In porcine, CYP19A1 null embryos cannot not survive beyond 28 days, but these embryos can be rescued when co-transferred with embryos derived by in vitro fertilization (Meyer et al., 2019). Little is known about CYP19A1 in the bovine embryo and elongating conceptus, but one study showed that CYP19A1 was highly expressed in bovine conceptuses classified as long on day 15 compared to those classified as short (Barnwell et al., 2016). An increase in CYP19A could indicate increased signaling in the estrogen biosynthesis chain and thus be promoting conceptus derived estrogen secretion later in gestation. However, little is known about the role bovine conceptus estrogen secretion and its role in pregnancy maintenance.
Nitric-oxide synthase 2 (NOS2) was also upregulated in the +FLI compared to the -FLI group. NOS2 is the inducible form of nitric oxide synthase that produces nitric oxide and has a variety of molecular functions throughout the body, which include amino acid binding, oxidoreductase activity, heme binding, and NADP binding. Additionally, NOS2 has a large role in many metabolic and immune response processes. Expression and localization of NOS2 has been characterized in the bovine oviduct and its role in oocyte and embryo development have been explored, but little is known about NOS2 in the bovine conceptus (Ulbrich et al., 2006; Schwarz et al., 2010). Uterine histotroph contains increased levels of arginine, leucine, glutamine, and glucose during the period of elongation in response to actions by progesterone and IFNT (Bazer et al., 2012). In sheep, it is proposed that glucose and arginine increase NOS2, and arginine increases IFNT. Furthermore, metabolism of arginine to nitric oxide by NOS stimulates the proliferation of ovine trophectoderm cells (Bazer et al., 2012). Given this, increased expression of NOS2 could indicate an increase in arginine metabolism leading to greater trophectoderm growth and increased conceptus survival.
Toll-like receptors (TLRs) are membrane receptors that recognize foreign molecules and signal the immune system (Akira and Kiyoshi, 2004). TLRs have been characterized in the endometrium of many livestock species, however little is known about conceptus expression of these genes (Davies et al., 2008; Ruiz-González et al., 2015; Yoo et al., 2019). In the ovine, TLRs have a role in maternal recognition of pregnancy and specifically, TLR2 has been detected in trophoblast cells at day 13 (Ruiz-González et al., 2015; Kaya et al., 2017). Here, toll-like receptor 2 (TLR2) was downregulated in the +FLI group compared to the -FLI group. Given that TLR2 is expressed in innate immune cells and given the role of type one interferons in the innate immune system, it is possible that TLRs and IFNT could have interplay with each other and influence the maternal conceptus signaling mechanism. The abundance of message for two pregnancy-associated glycoproteins (PAG2, PAG12) and a trophoblast Kunitz domain (TKDP4) were found to be upregulated in the +FLI compared to the -FLI group. TKDP4 has an established role in placental development and trophoblast proliferation (Betsha et al., 2013) and has a predicted interaction with closely related PAG2 and PAG12. Both PAG2 and PAG12 are expressed in the trophectoderm cells, and interestingly, both are expressed in the mononucleate and invasive binucleate cells of the placenta (Xie et al., 1997). The roles of these ancient PAGs have yet to be described but it is possible that PAG2, PAG12, and TKDP4 play a role in conceptus elongation and placental-uterine interaction (Touzard et al., 2013). Previous studies have shown that an increased concentration of PAGs is associated with decreased pregnancy loss (Wallace et al., 2015; Pohler et al., 2016). There is conflicting evidence that the supplementation of cytokines to the IVP system increases circulating PAG concentrations after embryo transfer, and one explanation for this could be the individual effect of each cytokine additive (Vailes et al., 2019; Seekford et al., 2021). It is not assumed that each added cytokine has the same effect on the developing embryo which may suggest the benefit to adding a cytokine cocktail. Given that some PAGs were increased in the +FLI group, this may support the idea that embryos treated with a cytokine combination, such as FLI, may have increased levels of PAGs later in gestation resulting in reduced pregnancy loss.
In conclusion, data from this work indicates that day 15 conceptuses from IVP embryos produced with or without FLI supplementation during culture are phenotypically similar. While there was no difference detected in conceptus recovery or length, transcriptomic analysis revealed differences in molecular and biological functions related to inflammatory response, metabolic processes, sex differentiation, and placental development, and indicate an effect of FLI on conceptus development and signaling during the elongation period. Further research will reveal survival past day 15 and will help elucidate the mechanisms that drive in vitro embryo viability.
The datasets presented in this study can be found in online repositories. The name of the repository and accession number can be found below: NCBI; GSE215089.
The animal study was reviewed and approved by ACUC Committee, University of Missouri.
KS, MO, RP, BD, and AG-G participated in study execution, analysis, manuscript drafting, and critical discussion. All authors contributed to the article and approved the submitted version.
This research is supported by the Clifton Murphy Scholarship Fund, USDA National Needs Fellowship funded by USDA NIFA Grant 2019-38420-28972, USDA NIFA Predoctoral Fellowship Grant 2022-67011-36568, and University of Missouri CAFNR Dissertation Research Improvement Grant Fund, and USDA National Institute of Food and Agriculture, Multi-State Hatch project 1023183 (OHO01496-MRF).
The authors would also like acknowledge Dr. Bo Harstine from Select Sires, Inc. (Plain City, Ohio) for the donation of bull semen used for this project and Missouri Prime Beef Packers (Pleasant Hope, MO) for the bovine ovaries used for oocyte collection. Thanks, are also extended to Lee Spate of the Prather Laboratory for preparing the FLI supplement and to Dr. Jessica Drum for the transferring embryos at the University of Missouri BRTF. The authors would like to thank The Ohio State University for use of animals and laboratory facilities.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2022.1040064/full#supplementary-material
Supplementary Data Sheet 1 | Overall gene expression for -FLI and +FLI groups.
Supplementary Data Sheet 2 | GO Terms for biological processes associated with the differentially expressed genes between the +FLI and -FLI groups.
Supplementary Data Sheet 3 | GO Terms for molecular function associated with the differentially expressed genes between the +FLI and -FLI groups.
Akira S., Kiyoshi T. (2004). Toll-like receptor signalling. Nat. Rev. Immunol. 4 (7), 499–511. doi: 10.1038/nri1391
Arosh J. A., Banu S. K., Kimmins S., Chapdelaine P., MacLaren L. A., Fortier M. A. (2004). Effect of interferon-τ on prostaglandin biosynthesis, transport, and signaling at the time of maternal recognition of pregnancy in cattle: Evidence of polycrine actions of prostaglandin E2. Endocrinology 145 (11), 5280–5293. doi: 10.1210/en.2004-0587
Barnwell C. V., Farin P. W., Whisnant C. S., Alexander J. E., Farin C. E. (2015). Maternal serum progesterone concentration and early conceptus development of bovine embryos produced in vivo or in vitro. Domest. Anim. Endocrinol. 52, 75–81. doi: 10.1016/j.domaniend.2015.03.004
Barnwell C. V., Farin P. W., Ashwell C. M., Farmer W. T., Galphin S. P., Farin C. E. (2016). Differences in MRNA Populations of Short and Long Bovine Conceptuses on Day 15 of Gestation. Molecul. Reproduction Dev. 83 (5), 424–441. doi: 10.1002/mrd.22640
Bazer F. W., Kim J., Gwonhwa S., Hakhyun Ka, Tekwe C. D., Guoyao Wu. (2012). Select nutrients, progesterone, and interferon tau affect conceptus metabolism and development. Ann. New York Acad. Sci. 1271 (1), 88–96. doi: 10.1111/j.1749-6632.2012.06741.x
Bertolini M., Beam S. W., Shim H., Bertolini L. R., Moyer A. L., Famula T. R., et al. (2002). Growth, development, and gene expression by in vivo- and in vitro-produced day 7 and 16 bovine embryos. Mol. Reprod. Dev. 63 (3), 318–285. doi: 10.1002/mrd.90015
Betsha S., Hoelker M., Salilew-Wondim D., Held E., Rings F., Große-Brinkhause C., et al. (2013). Transcriptome profile of bovine elongated conceptus obtained from SCNT and IVP pregnancies. Mol. Reprod. Dev. 80 (4), 315–333. doi: 10.1002/mrd.22165
Block J., Bonilla L., Hansen P. J. (2009). Effect of addition of hyaluronan to embryo culture medium on survival of bovine embryos in vitro following vitrification and establishment of pregnancy after transfer to recipients. Theriogenology 71 (7), 1063–1071. doi: 10.1016/j.theriogenology.2008.11.007
Block J., Bonilla L., Hansen P. J. (2010). Efficacy of in vitro embryo transfer in lactating dairy cows using fresh or vitrified embryos produced in a novel embryo culture Medium1. J. Dairy Sci. 93 (11), 5234–5242. doi: 10.3168/jds.2010-3443
Block J., Hansen P. J., Loureiro B., Bonilla L. (2011). Improving post-transfer survival of bovine embryos produced in vitro: Actions of insulin-like growth factor-1, colony stimulating factor-2 and hyaluronan. Theriogenology 76 (9), 1602–1609. doi: 10.1016/j.theriogenology.2011.07.025
Bridges G. A., Helser L. A., Grum D. E., Mussard M. L., Gasser C. L., Day M. L. (2008). Decreasing the interval between GnRH and PGF2α from 7 to 5 days and lengthening proestrus increases timed-AI pregnancy rates in beef cows. Theriogenology 69 (7), 843–851. doi: 10.1016/j.theriogenology.2007.12.011
Brooks K., Burns G., Spencer T. E. (2014). Conceptus elongation in ruminants: Roles of progesterone, prostaglandin, interferon tau and cortisol. J. Anim. Sci. Biotechnol. 5 (1), 535. doi: 10.1186/2049-1891-5-53
Clemente M., de la Fuente J., Fair T., Al Naib A., Gutierrez-Adan A., Roche J. F., et al. (2009). Progesterone and conceptus elongation in cattle: A direct effect on the embryo or an indirect effect via the endometrium? Reproduction 138 (3), 507–517. doi: 10.1530/REP-09-0152
Davies D., Meade K. G., Herath S., Eckersall P.D., Gonzalez D., White J. O., et al. (2008). Toll-like receptor and antimicrobial peptide expression in the bovine endometrium. Reprod. Biol. Endocrinol. 6 (1), 535. doi: 10.1186/1477-7827-6-53
Diskin M. G., Waters S. M., Parr M. H., Kenny D. A. (2016). “Pregnancy losses in cattle: Potential for improvement”. Reproduction Fertility Dev. 28 (1–2), 83–93. doi: 10.1071/RD15366
Driver A. M., Peñagaricano F., Huang W., Ahmad K. R., Hackbart K. S., Wiltbank M. C., et al. (2012). RNA-Seq analysis uncovers transcriptomic variations between morphologically similar in vivo- and in vitro-derived bovine blastocysts. BMC Genomics 13 (1), 1185. doi: 10.1186/1471-2164-13-118
Ealy A., Wooldridge L., McCoski S. (2019). Post-transfer consequences of in vitro-produced embryos in cattle. J. Anim. Sci. 97:2555–2568. doi: 10.1093/jas/skz116
Emond V., MacLaren L. A., Kimmins S., Arosh J. A., Fortier M. A., Lambert R. D. (2004). Expression of cyclooxygenase-2 and granulocyte-macrophage colony-stimulating factor in the endometrial epithelium of the cow is up-regulated during early pregnancy and in response to intrauterine infusions of interferon-1. Biol. Reprod. 70 (1), 54–645. doi: 10.1095/biolreprod.103.018689
Enright B. P., Lonergan P., Dinnyes A., Fair T., Ward F. A., Yang X., et al. (2000). Culture of in vitro produced bovine zygotes in vitro vs in vivo: Implications for early embryo development and quality. Theriogenology 54 (5), 659–673. doi: 10.1016/S0093-691X(00)00381-2
Farin P. W., Crosier A. E., Farin C. E. (2001). Influence of in vitro systems on embryo survival and fetal development in cattle. Theriogenology 55 (1), 151–170. doi: 10.1016/s0093-691x(00)00452-0
Farin P. W., Farin C. E. (1995). Transfer of bovine embryos produced in vivo or in vitro: Survival and fetal development. Biol. Reprod. 52 (3), 676–682. doi: 10.1095/biolreprod52.3.676
Farin P. W., Slenning B. D., Britt J. H. (1999). Estimates of pregnancy outcomes based on selection of bovine embryos produced in vivo or in vitro. Theriogenology 52 (4), 659–670. doi: 10.1016/S0093-691X(99)00160-0
Ferré L. B., Kjelland M. E., Strøbech L. B., Hyttel P., Mermillod P., Ross P. J. (2020a). Review: Recent advances in bovine in vitro embryo production: Reproductive biotechnology history and methods. Animal 14 (5), 991–1004. doi: 10.1017/S1751731119002775
Ferré L. B., Kjelland M. E., Taiyeb A. M., Campos-Chillon F., J. Ross P. (2020b). Recent progress in bovine in vitro-derived embryo cryotolerance: Impact of in vitro culture systems, advances in cryopreservation and future considerations. Reprod. Domest. Anim. 55 (6), 659–765. doi: 10.1111/rda.13667
Ge S. X., Jung D., Yao R. (2020). ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 36 (8), 2628–2295. doi: 10.1093/bioinformatics/btz931
Hansen P. J. (2020). The incompletely fulfilled promise of embryo transfer in cattle–why aren’t pregnancy rates greater and what can we do about it? J. Anim. Sci. 98 (11):1-20. doi: 10.1093/jas/skaa288
Hansen P. J., Denicol A. C., Dobbs K. B. (2014). Maternal embryokines that regulate development of the bovine preimplantation embryo. Turk J. Vet. Anim. Sci. 10:589-598. doi: 10.3906/vet-1405-96
Kaya M. S., Kose M., Guzeloglu A., Kıyma Z., Atli M. O. (2017). Early pregnancy-related changes in toll-like receptors expression in ovine trophoblasts and peripheral blood leukocytes. Theriogenology 9340–45. doi: 10.1016/j.theriogenology.2017.01.031
Kim D., Langmead B., Salzberg S. L. (2015). HISAT: A fast spliced aligner with low memory requirements”. Nat. Methods 12 (4), 357–605. doi: 10.1038/nmeth.3317
Kocyigit A., Cevik M. (2015). Effects of leukemia inhibitory factor and insulin-like growth factor-I on the cell allocation and cryotolerance of bovine blastocysts. Cryobiology 71 (1), 64–695. doi: 10.1016/j.cryobiol.2015.05.068
Liao Y., Smyth G. K., Shi W. (2014). FeatureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30 (7), 923–305. doi: 10.1093/bioinformatics/btt656
Lonergan P. (2011). Influence of progesterone on oocyte quality and embryo development in cows. Theriogenology 76 (9), 1594–1601. doi: 10.1016/j.theriogenology.2011.06.012
Lonergan P., Rizos D., Gutierrez-Adán A., Moreira P. M., Pintado B., de la Fuente J., et al. (2003). Temporal divergence in the pattern of messenger RNA expression in bovine embryos cultured from the zygote to blastocyst stage in vitro or in vivo. Biol. Reprod. 69 (4), 1424–1431. doi: 10.1095/biolreprod.103.018168
Lonergan P., Woods A., Fair T., Carter F., Rizos D., Ward F., et al. (2007). Effect of embryo source and recipient progesterone environment on embryo development in cattle. Reproduction Fertility Dev. 19 (7), 861–868. doi: 10.1071/RD07089
Meyer A. E., Pfeiffer C. A., Brooks K. E., Spate L. D., Benne J. A., Cecil R., et al. (2019). New perspective on conceptus estrogens in maternal recognition and pregnancy establishment in the pig†. Biol. Reprod. 101 (1), 148–161. doi: 10.1093/biolre/ioz058
Ortega M.S., João G. N., Moraes D. J., Patterson M. F., Smith S. K., Behura S. P., et al. (2018). Influences of sire conception rate on pregnancy establishment in dairy cattle. Biol. Reprod. 99 (6), 1244–1545. doi: 10.1093/biolre/ioy141
Paschoal D. M., Sudano M. José, Guastali M. D., Dias Maziero RosiáraRosária, Crocomo LetíciaF., Oña Magalhães L. C., et al. (2014). Forskolin effect on the cryosurvival of in vitro-produced bovine embryos in the presence or absence of fetal calf serum”. Zygote 22 (2), 146–575. doi: 10.1017/S0967199412000354
Pohler K. G., Pereira M. H.C., Lopes F. R., Lawrence J. C., Keisler D. H., Smith M. F., et al. (2016). Circulating Concentrations of Bovine Pregnancy-Associated Glycoproteins and Late Embryonic Mortality in Lactating Dairy Herds. J. Dairy Sci. 99 (2), 1584–94. doi: 10.3168/jds.2015-10192
Pontes J. H. F., Nonato-Junior I., Sanches B. V., Ereno-Junior J. C., Uvo S., Barreiros T. R. R., et al. (2009). Comparison of embryo yield and pregnancy rate between in vivo and in vitro methods in the same nelore (Bos indicus) donor cows. Theriogenology 71 (4), 690–697. doi: 10.1016/j.theriogenology.2008.09.031
Prather R. S., Spire M. F., Schalles R. R. (1987). Evaluation of cryopreservation techniques for bovine embryos. Theriogenology 28 (2), 195–204. doi: 10.1016/0093-691X(87)90266-4
Redel B. K., Spate L. D., Lee K., Mao J., Whitworth K. M., Prather. R. S. (2016). Glycine supplementation in vitro enhances porcine preimplantation embryo cell number and decreases apoptosis but does not lead to live births. Mol. Reprod. Dev. 83 (3), 246–585. doi: 10.1002/mrd.22618
Ribeiro E. S., Greco L. F., Bisinotto R. S., Lima FábioS., Thatcher W. W., Santos JoséE. (2016). Biology of preimplantation conceptus at the onset of elongation in dairy Cows1. Biol. Reprod. 94 (4), 1–18. doi: 10.1095/biolreprod.115.134908
Říha J., Machatková M., Pavlok A. (2002). Viability of fresh and frozen transferred IVP bovine embryos. Czech J. Anim. Sci. 47 (7), 261–267.
Robinson Rs, Hammond Aj, Wathes Dc, Hunter Mg, Mann Ge (2008). “Corpus luteum–Endometrium–Embryo interactions in the dairy cow: Underlying mechanisms and clinical relevance. Reprod. Domest. Anim. 43 (s2), 104–112. doi: 10.1111/j.1439-0531.2008.01149.x
Ruiz-González I., Minten M., Wang X., Dunlap K. A., Bazer F. W. (2015). Involvement of TLR7 and TLR8 in conceptus development and establishment of pregnancy in sheep. Reprod. (Cambridge England) 149 (4), 305–165. doi: 10.1530/REP-14-0537
Sanches B. V., Lunardelli P. A., Tannura J. H., Cardoso B. L., Colombo Pereira M. H., Gaitkoski D., et al. (2016). “A new direct transfer protocol for cryopreserved IVF embryos”. Theriogenology 85 (6), 1147–1515. doi: 10.1016/j.theriogenology.2015.11.029
Saraiva H., Batista R., Alfradique V., Pinto P., Ribeiro L., Oliveira C. S., et al. (2017). L-carnitine supplementation during vitrification or warming of in vivo-produced ovine embryos does not affect embryonic survival rates, but alters CrAT and PRDX1 expression. Theriogenology 105 (September):150-157. doi: 10.1016/j.theriogenology.2017.09.022
Schwarz K., Pires P., Bem T. De, Adona Pr, Leal C. (2010). Consequences of nitric oxide synthase inhibition during bovine oocyte maturation on meiosis and embryo development. Reprod. Domest. Anim. 45 (1), 75–805. doi: 10.1111/j.1439-0531.2008.01242.x
Seekford Z. K., Wooldridge L. K., Dias N. W., Timlin C. L., Sales ÁlvaroF., Speckhart S. L., et al. (2021). Interleukin-6 supplementation improves post-transfer embryonic and fetal development of in vitro-produced bovine embryos”. Theriogenology 170 (August), 15–22. doi: 10.1016/j.theriogenology.2021.04.004
Spate L. D., Redel B. K., Brown A. N., Murphy C. N., Prather R. S. (2012). Replacement of bovine serum albumin with n-Methyl-D-Aspartic acid and homocysteine improves development, but not live birth. Mol. Reprod. Dev. 79 (5), 310. doi: 10.1002/mrd.22032
Spencer T. E., Burghardt R. C., Johnson G. A., Bazer F. W. (2004). “Conceptus signals for establishment and maintenance of pregnancy”. Anim. Reprod. Sci. 82–83:537–550. doi: 10.1016/j.anireprosci.2004.04.014
Stoecklein K. S., Ortega M.S., Spate L. D., Murphy C. N., Prather R. S. (2021). Improved cryopreservation of in vitro produced bovine embryos using FGF2, LIF, and IGF1. PloS One 16 (2), e02437275. doi: 10.1371/journal.pone.0243727
Sudano M. J., Paschoal D. M., Maziero R. R. D., Rascado T. S., Guastali M. D., Crocomo L. F., et al. (2013). Improving postcryopreservation survival capacity: An embryo-focused approach,”. Anim. Reprod. 10 (n3), 160–167.
Thatcher W. W., Guzeloglu A., Mattos R., Binelli M., Hansen T. R., Pru J. K. (2001). Uterine-conceptus interactions and reproductive failure in cattle. Theriogenology 56 (9), 1435–1450. doi: 10.1016/S0093-691X(01)00645-8
Touzard E., Reinaud P., Dubois O., Guyader-Joly C., Humblot P., Ponsart C., et al. (2013). Specific expression patterns and cell distribution of ancient and modern PAG in bovine placenta during pregnancy. Reproduction 146 (4), 347–625. doi: 10.1530/REP-:13-0143
Tríbulo P., Rivera RocíoM., Ortega Obando M. S., Jannaman E. A., Hansen P. J. (2019). “Production and culture of the bovine embryo”,” in Comparative embryo culture. Ed. Herrick J. R. (New York, NY: Springer New York) (2006) pp. 115–129. doi: 10.1007/978-1-4939-9566-0_8
Ulbrich S. E., Rehfeld S., Bauersachs S., Wolf E., Rottmayer R., Hiendleder S., et al. (2006). Region-specific expression of nitric oxide synthases in the bovine oviduct during the oestrous cycle and in vitro. J. Endocrinol. 188 (2), 205–213. doi: 10.1677/joe.1.06526
Vailes M. T., McCoski S. R., Wooldridge L. K., Reese S. T., Pohler K., Roper D. A., et al. (2019). Post-transfer outcomes in cultured bovine embryos supplemented with epidermal growth factor, fibroblast growth factor 2, and insulin-like growth factor 1. Theriogenology 124, 1–8. doi: 10.1016/j.theriogenology.2018.09.023
Vajta G., Holm P., Kuwayama M., Booth P.j., Jacobsen H., Greve T., et al. (1998). Open pulled straw (OPS) vitrification: A new way to reduce cryoinjuries of bovine ova and embryos”. Mol. Reprod. Dev. 51 (1), 53–585. doi: 10.1002/(SICI)1098-2795(199809)51:1<53::AID-MRD6>3.0.CO;2-V
Viana J. H. M. (2021). 2020 statistics of embryo production and transfer in domestic farm animals. Embryo Technol. Newslett. 39:1-14.
Wallace R. M., Pohler K. G., Smith M. F., Green J. A. (2015). Placental PAGs: Gene Origins, Expression Patterns, and Use as Markers of Pregnancy. Reproduction 149 (3), R115–26. doi: 10.1530/REP-14-0485
Wiltbank M. C., Baez G. M., Garcia-Guerra A., Toledo M. Z., Monteiro P. L.J., Melo L. F., et al. (2016). Pivotal periods for pregnancy loss during the first trimester of gestation in lactating dairy cows. Theriogenology 86 (1), 239–535. doi: 10.1016/j.theriogenology.2016.04.037
Xie S., Green J., Bixby J. B., Szafranska B., DeMartini J. C., Hecht S., et al. (1997). The diversity and evolutionary relationships of the pregnancy-associated glycoproteins, an aspartic proteinase subfamily consisting of many trophoblast-Expressed Genes. Proc. Natl. Acad. Sci. 94 (24), 12809–12165. doi: 10.1073/pnas.94.24.12809
Yoo I., Han J., Lee S., Jung W., Kim Ji H., Kim Y. W., et al. (2019). Analysis of stage-specific expression of the toll-like receptor family in the porcine endometrium throughout the estrous cycle and pregnancy. Theriogenology 125 (February), 173–183. doi: 10.1016/j.theriogenology.2018.11.003
Yuan Ye, Spate L. D., Redel B. K., Tian Y., Zhou J., Prather R. S., et al. (2017). Quadrupling efficiency in production of genetically modified pigs through improved oocyte maturation. Proc. Natl. Acad. Sci. 114 (29), E57965. doi: 10.1073/pnas.1703998114
Zolini A. M., Carrascal-Triana E., de King A. R., Hansen P. J., Alves Torres C. A., Block J. (2019). Effect of addition of l-carnitine to media for oocyte maturation and embryo culture on development and cryotolerance of bovine embryos produced in vitro. Theriogenology 133 (July), 135–143. doi: 10.1016/j.theriogenology.2019.05.005
Keywords: in vitro fertilization, bovine, conceptus elongation, FGF2, LIF, IGF1, FLI
Citation: Stoecklein KS, Garcia-Guerra A, Duran BJ, Prather RS and Ortega MS (2022) Actions of FGF2, LIF, and IGF1 on bovine embryo survival and conceptus elongation following slow-rate freezing. Front. Anim. Sci. 3:1040064. doi: 10.3389/fanim.2022.1040064
Received: 08 September 2022; Accepted: 27 October 2022;
Published: 21 November 2022.
Edited by:
Graham Cliff Lamb, Texas A&M University College Station, United StatesReviewed by:
Pat Lonergan, University College Dublin, IrelandCopyright © 2022 Stoecklein, Garcia-Guerra, Duran, Prather and Ortega. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Sofia Ortega, c29maWEub3J0ZWdhQHdpc2MuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.