
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Anim. Sci. , 10 November 2022
Sec. Animal Nutrition
Volume 3 - 2022 | https://doi.org/10.3389/fanim.2022.1026958
This article is part of the Research Topic Early Life Nutrition in Poultry View all 3 articles
This study evaluated the effectiveness of a new probiotic product developed to reduce the effect of Salmonella infections and compared it to the efficacy of commercial probiotics in broiler chicken. Based on the in vitro assessment of the growth characteristics and safety to human health, four bacterial isolates were isolated, characterized, and identified as excellent candidates for the development of commercial probiotic feed additives for poultry. Compatibility and interactions among the four selected strains were investigated. After that, a preliminary study was conducted in which the selected isolates were evaluated individually in vivo with three different methods of application (water, feed, and oral gavage). The cycle included N = 312 chicks, which were divided into 13 groups, including control, distributed into four batteries, with 78 broiler chickens in each battery. There were eight replicates with 24 chicks in each replicate, and the analysis was randomly done in triplicate. The intentional parameters were growth performance, microbial analysis and humoral immune response. The results of the preliminary study assisted in formulating the new probiotic product. Then In vivo evaluations for the newly formulated product were performed with the comparison with two imported commercial products (Alterion and Galli pro fit) used in poultry farms in Kuwait. The second cycle included N = 96 chicks that were divided into four groups, including control. Each group has three replicates and each replicate has eight chicks, and the analysis was randomly done in triplicate. The results showed that although antibiotics were not used, all the growth parameters were similar and sometimes better than the control. The new product inhibited the growth of salmonella as a control and all chickens in different treatment gained a high mass of meat. The statistical analysis showed that no differences were observed in bird weight, weight gain, feed consumption, and feed efficiency between bacterial strains p>0.05. Also, the different probiotic treatments did not affect the total antibody IgM titers significantly in the broilers (P > 0.05). Thus, the newly formulated product was effective in reducing the salmonella.
Poultry is reared in an environment that supports bacterial contamination from dust, farm personnel, and rodents. These microorganisms usually reside on their feathers and skin and in their digestive system. Microorganisms present in poultry are the primary source of cross-contaminated poultry products. When the animals are slaughtered, most of these microorganisms are eliminated (Huis in ‘t Veld et al., 1993). However, subsequent contamination is possible at any stage of production, which includes de-feathering, evisceration, washing, and storage by cooling or freezing. Most of the microbial flora of poultry colonize the lower gastrointestinal tract, especially the ceca and cloaca (Beery et al., 1988). The ceca are two blind-ended tubes where fermentation occurs. In the ceca, the undigested food particles are broken down by microorganisms. The ceca of poultry normally contain mustard to dark-brown froth, which is excreted about once every day. The cloaca is a common chamber in the digestive system that is used to remove feces and urine and lay eggs.
The bacterial populations in different parts of the gastrointestinal (GI) tract have high diversity, and the population density increases from the proximal to the distal GI tract (Richards et al., 2005). The microbial profile in each region of the GI tract is unique, and the community becomes more complex as the chickens grow older (Yegani and Korver, 2008). Various factors associated with the age, diet, and environmental conditions of the chicken can affect the balance among the microbial communities in the gut. The composition of the microbial community changes greatly within the first 2–3 weeks of hatching and stabilizes when they are 5–6 weeks old (Torok et al., 2009).
Lactic Acid Bacteria (LAB) are a large group of microorganisms in the gastrointestinal tract of humans and birds. These are gram-positive, non-spore-forming, rod-shaped bacilli or cocci that are used as probiotic supplements to improve the health of the host. LAB are facultative anaerobic bacteria that can grow in both the presence or absence of oxygen. The benefits range from improving the gut ecosystem to producing antagonistic effects against the pathogens in the GI tract (Ljungh and Wadstöm, 2009). LAB exert their antagonistic effects against GI pathogens either by increasing resistance against enteric pathogens and reducing their colonization or by producing antimicrobial substances. Thus, these two characteristics were prioritized when selecting LAB as a probiotic candidate (Taheri et al., 2009).
The GI tract in broiler chickens contains different types of microorganisms. These microorganisms are mainly bacteria that can be divided into potentially pathogenic and beneficial groups (Gabriel et al., 2006). Harmful bacteria might cause localized or systemic infections, intestinal putrefaction, and toxin formation. The beneficial bacteria might stimulate the immune system and inhibit the growth and establishment of harmful microbial groups, and are generally known as probiotics (Jeurissen et al., 2002). The oral administration of fermentative bacteria, known as probiotics, is performed to spread the beneficial bacteria in the intestinal tract.
Different studies have defined probiotics in several ways. Probiotics are live microbial feed supplements, which beneficially affect the host by improving its intestinal microbial balance (Fuller, 1989); “a live microbial feed that is beneficial to health” (Salminen et al., 1998); “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” (FAO/WHO, 2001). These live microorganisms include strains of Lactobacillus, Bifidobacterium, and yeasts.
The development of the poultry industry and the increasing demand for poultry and poultry products can lead to overcrowding of cages and negatively affect the health of the birds, especially when several diseases appear. Their immune system weakens, making them more susceptible to various pathogenic bacteria, such as E. coli, Salmonella spp., Clostridium perfringens, and Campylobacter spp. In such situations, the use of antimicrobial growth promoters is increased to enhance gut health and prevent the development and propagation of diseases. The overuse of antibiotics might cause bacteria to develop resistance to these antibiotics. Additionally, when humans consume antibiotic-ingested poultry, the antibiotic residue enters their system and causes the bacteria in humans to develop resistance to those antibiotics (Yong Ha et al., 2016). Therefore, scientists are investigating alternative products to treat these diseases in poultry and improve their immunity. These products must be safe for both poultry and humans and need to be accepted by consumers.
Probiotic preparations can be administered to poultry and farm animals after birth when they are highly susceptible to diseases. These preparations can be mixed in food for preventative or curative purposes; they can also be administered orally or added to water for continuous feed. The use of probiotics in poultry and farm animals is expected to increase due to stricter regulations controlling antibiotic growth promoters in animal feed. Additionally, the ban on growth-promoting antibiotics in the European Union (EU) will eventually affect every poultry exporting country because poultry products that contain antibiotic residues of EU-banned products, or those that harbor multi-drug resistant pathogenic bacterial species belonging to Salmonella, Staphylococcus, Listeria, Enterococcus, and Campylobacter (Edens, 2003), are inhibited from entering the EU.
This study was approved by the department committee of the Environment and Life Sciences Research Center in Kuwait Institute for Scientific Research under Project No. FB114C (2019). The procedures and protocols followed the official animal welfare guidelines and regulations (Reference No. PMO/PV/RP/032/2017). The protocol recommended the humane treatment of experimental animals with no pain, stress, or harm.
The isolation, characterization, and identification of Lactic Acid Bacteria were performed in a previous study (phase 1), where 89 presumptive LAB were isolated from chicken samples collected from three different poultry farms across four seasons. The preliminary biochemical identification of these isolates using the Analytical Profile Index (API) and further confirmation by deoxyribonucleic acid (DNA) sequencing showed that these LAB strains belonged to 27 strains of Lactobacillus and Pediococcus. Eleven representative strains of these isolates were then screened for their probiotic potential through the in vitro assessment of their tolerance to low pH, bile salts, and antibiotics, ability to aggregate, co-aggregate, and produce bacteriocins, antagonistic activity against selected enteric pathogens, hydrophobicity, and attachment to the tissues in the cecum and ileum. Based on the in vitro assessment of the growth characteristics and safety to human health, four isolates were identified as potential candidates for the development of poultry probiotics (Balba et al., 2012); these isolates included Lactobacillus plantarum, Lactobacillus parabuchneri, Lactobacillus brevis, and Pediococcus pentosaccus.
The compatibility and interactions among the four selected strains (Lactobacillus plantarum, Lactobacillus parabuchneri, Pediococcus pentosaceus, and Lactobacillus brevis) were investigated using two different experimental methods, which included the production and excretion of antimicrobial compounds [agar diffusion method (Guo et al., 2010)] and the coexistence and growth in solid media (cross-streak method (Pederson and Tannock, 1989; Ripamonti et al., 2011).
The broiler chickens were raised in battery cages instead of floor pens to reduce operational costs and improve production efficiency. Cobb broiler chicks (1 d old) (Kuwait United Poultry Company, Kuwait), were used in this study. The building in which the chicks were reared had a partly artificial and natural environment. Because Kuwait has extreme weather conditions in summer and winter that might affefct the environment inside a poultry house, cooling pads and fans were used for ventilation. The battery cage temperature was adjusted according to the age of the chicks as follows: 23–30°C in the first week, 28–30°C in the second week, 26–28°C in the third week, 24–26°C in the fourth week, and 22–24°C in the last week. The humidity level was kept below 50% during the study. The broiler chicks were fed a starter diet from the day of hatching till they were 7 d old (one week), a grower diet from 8 to 21 d of age (2–3 weeks), and a finisher diet from 22 to 35 d of age (4–5 weeks). The diet was corn and/or soy-based and met the rules and regulations of the National Research Council (NRC). The evaluation test was performed in two cycles, and the result of the first cycle helped in formulating the final probiotic product and planning the second cycle.
The first cycle of this study consisted of the evaluation of the selected isolates in vivo with three different applications (water, feed, and oral gavage or beak). The chicks (N = 312; 1 d old) were divided into 13 groups (n = 24 chicks/battery), including control, and handled separately. Each battery consisted of three levels, and an area of 0.85 m2 was provided to each bird. The broiler chicks were vaccinated according to the protocol followed for poultry farms in Kuwait, as shown in Table 1. In our experiments, only the control chicks were vaccinated following the protocol, whereas the chicks in the remaining 12 groups were only provided the lactic acid bacteria selected from our isolates (Lactobacillus plantarum, Lactobacillus parabuchneri, Pediococcus pentosaceus, and Lactobacillus brevis). The classification of the 13 groups based on the bacterial strain types and the methods of application are presented in Table 2.
Three methods were used to investigate the probiotic efficiency and included feed delivery, drinking water, and oral delivery (gavage).
The probiotic candidates were mixed in the feed for the first three days of the week. Each strain was grown in the MRS broth overnight or for 48 h (at 37°C) and harvested by centrifugation at 8,000 rpm for 15 min. Then, they were re-suspended in PBS (pH 7.4) and added to a premix with the basal diet for 10 min using a miniature mixer. This pre-mixture, containing the product and the feed (1 kg), was then transferred into a larger mixer (total capacity: 300 kg), where the final volume of the weekly batch of feed was prepared.
For the first three days of the week, drinking water was supplied through pipes (nipples drinker installed) connected to a 20-L drum. A small pump was installed to agitate the water constantly. The water containing the probiotic was prepared daily and supplied for the first three days of the week in the probiotic water treatment groups.
Probiotic cultures were re-suspended in PBS solution (pH 7.4) that contained approximately 108 CFU/mL. Each bird received 1 mL of the PBS mixed solution on days 1, 2, and 3 of the weeks; the birds in the negative control group received 1 mL of PBS solution (pH 7.4) on the same days.
Based on the results of the in vivo test in cycle 1 of the selected isolates, the new product was developed in equal percentages from the four isolates. The second test was conducted for the new probiotic product, which was administered in feed, and compared to two commercial products (Alterion and Galli pro fit). The commercial products were selected specifically for their ability to inhibit the proliferation of pathogenic organisms and improve the digestibility of the feed. Selected chicks (N = 96; 1 d old) were divided into four groups, including the control group (n = 24 chicks/group), and handled separately. The chicks were vaccinated according to the protocol only in the control group, while the chicks in the remaining groups were given probiotic products, as shown in Table 3.
The mean bird growth rate, feed intake, feed conversion ratio, and mortality were determined for each feeding stage. Three birds (randomly picked from each pen) were killed when they were 30 d old. The caeca of the birds were dissected, and a bacterial enumeration of the cecal extract was made. The total number of Lactic acid bacteria, Salmonella, and E. coli was counted.
Microbial analysis for Lactic acid bacteria (LAB), Escherichia coli (E. coli), and Salmonella was conducted by extracting the cecal substance following standard microbiological methods (Schoeni and Doyle, 1992). The samples were analyzed by applying spreading technology. From each group, three chicken samples (30 d old) were collected and slaughtered on the farm and transferred to the laboratory under refrigerated conditions for further analysis. In the laboratory, the collected chicken samples were prepared according to the protocol described by Al-Khalaifa and Al-Nasser (Al-Khalaifa et al., 2019). Each chicken was first weighed and washed with diluted disinfectants at a ratio of 1:2. The abdominal area was de-feathered and sprayed with 70% ethanol before dissection to ensure that the area was sterile. Then, the skin was cut using a pair of sterile scissors and removed from the abdomen area with a pair of sterile forceps. The covering membrane was cut carefully to reach the digestive system of the chicken. The lower intestine was surgically exposed, following which the caeca were removed aseptically and weighed. To isolate the LAB, Salmonella, and E. coli from the caeca, the contents were extracted following a method described by Schoeni and Doyle (1992). The cecal content was squeezed in a sterile Petri dish, and then the caeca were cut longitudinally with a sterile scalpel and rinsed in a 0.85% (w/v) sterile NaCl solution (1:9 v/v) to remove the contents. Any residual cecal content was removed by gently scraping the cecal epithelium. The crude extracts of the caeca were transferred into a sterile stomacher bag and homogenized for 3 min. The collected crude extracts were used directly for microbial analysis. The LAB, E. coli, and Salmonella counts were determined using standard microbiological methods, as described by Lorch (1995) and Al-Khalaifah et al. (2021), and the samples were analyzed by applying the spreading technique. The E. coli and Salmonella count experiments were conducted using the Brilliance E. coli selective and the Xylose-Lysine-Desoxycholate agar media (Oxoid), respectively, while the LAB experiments were conducted using the de Man, Rogosa, Sharpe (MRS) medium (oxoid). Serial dilution was performed using the crude samples with saline, and 0.1 mL of the prepared sample was spread onto the surface of the medium with a sterile spreader. The plates were then incubated aerobically for 24 h at 37°C for both E. coli and Salmonella, whereas, for LAB, the plates were incubated anaerobically for 48 h at 30°C. The colonies were counted at the end of the incubation period. The colony counts were transformed into log values.
Ten broiler chickens of four weeks of age from each treatment were applied to test the humoral immune response. Antibody titers were measured using sheep red blood cells (RBC). The chickens were injected with 1 ml of diluted sheep RBC solution (7% v/v in 0.9% NaCl). After a week of injection, blood serum samples were collected using centrifugation methods, and differential antibody titers were measured using commercial ELISA kits. 50 µl of the respective standards were added to each well in the 96-well tray. Then 40 µl of each sample was added to the sample wells, followed by 10 µl of biotin-conjugated anti-chicken antibody. Then, 50 µl of streptavidin-HRP was added to each sample, neatly avoiding the blank control wells, and reagents were mixed completely. The plate was covered with a sealer and incubated for 60 min at 37°C. After incubation, the sealer was detached, and the plate was washed with wash buffer five times; the wells were overfilled and soaked for at least 30 sec to 1 min (Oguz et al., 2018). After each washing, paper towels were used to blot the plates. Then 50 µl substrate solution A was added to each well followed by 50 µl of substrate solution B (care was taken not to expose the substrate solution B to light as it is light sensitive). The plate was sealed with another sealer and incubated for 10 min in the dark at 37°C. Simultaneously, after adding 50 µl of stop solution to each well, the blue solution instantly turned yellow. Finally, the optical density (OD) value was measured at 40 nm within 30 min, after adding a stop solution using a microplate reader (Oguz et al., 2018; Al-Khalaifah and Uddin, 2022; Al-Khalaifah et al., 2022).
The study contains two sets of experiments (“Cycle 1” & “Cycle 2”).
Cycle 1 consists of a five-by-three factorial design. Three hundred twelve (N=312) one day old chicks were selected, at random, from Kuwait United Poultry Company (KUPCO). A sample number was randomly assigned to each chicken. A combination of factor “Lab Strain” and factor “Delivery System” were randomly assigned to each sample number. Out of the 312 total chickens, only thirty-nine (n=39) samples were analyzed.
Cycle 2 consists of a completely randomized single factor design. The overall sample number was reduced to ninety-six (N=96) chickens randomly selected from KUPCO. A sample number was randomly assigned to each chicken. Probiotic products were randomly assigned to the sample number. Out of the 96 total chickens, only twelve (n=12) samples were analyzed.
The statistical analysis was done through the R Statistical Software Package for response variables body weight, weight gain, feed efficiency, and feed consumption. Following data import, checks of the normality, of each response variable, was conducted visually through histograms and statistically through Shapiro-Wilk tests. Outlier detection was done visually through boxplots. The two-way Type III ANOVA was then conducted.
The overall statistical model for Cycle 1 was (Response Variable = LAB Strain + Delivery System + LAB Strain: Delivery System) and for Cycle 2 (Response Variable = Probiotic Product) was then tested for each response variable. Each of the models was then visually checked for normality, independence, equal variance, and factor effects. Pairwise comparisons were done through Tukey’s honest significance test when needed. The differences between the treatment means were considered significant at p<0.05.
For the remaining response variables, a one-way ANOVA and the general linear model method were used to analyze the overall differences between treatments, and the analysis was performed using the Minitab software (Minitab Inc., State College, PA). The differences between the treatment means were considered to be significant at P < 0.05, based on parametric studies and Bonferroni tests. Some data were arcsine transformed to achieve normality. For non-parametric cases, medians were used, and the Kruskal-Walli’s test was performed.
All combinations of cell-free neutralized and non-neutralized supernatants and strains assayed using the agar diffusion method and the cross-streak method showed that the inhibition zones for the four isolates were absent. The tested strains showed that they were compatible in vitro.
Growth performance of the in vivo evaluation experiments for the selected strains showed that the selected strains were effective in keeping the chickens healthy and preventing deaths even when antibiotics were not administered. Moreover, all birds in the different treatment groups gained a high mass of meat (Tables 4–6). Although the weight of the birds was high in all treatments, the water delivery application for all bacterial strains was optimal. Feed consumption was better than expected, and the birds in all treatments consumed a substantial quantity of food during the experiment. The results showed that the body weight of the birds that were administered lactic acid bacteria was more with Pediococus pentosaceus and Lactobacillus parabuchneri. The feed consumption and the feed efficiency for all treatments, including control, were similar; however, water intake treatments showed higher feed efficiency.
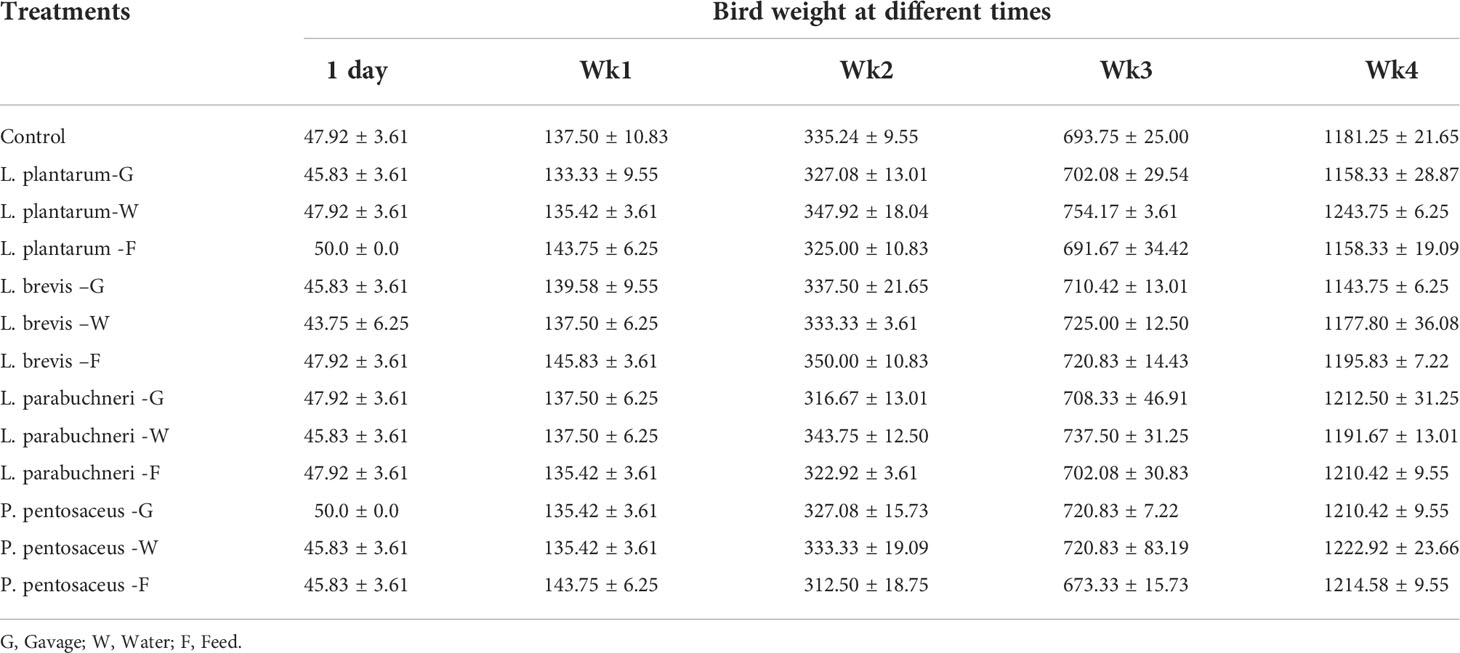
Table 4 The weight of the birds for the total duration of the experiment and different treatments (Cycle 1).
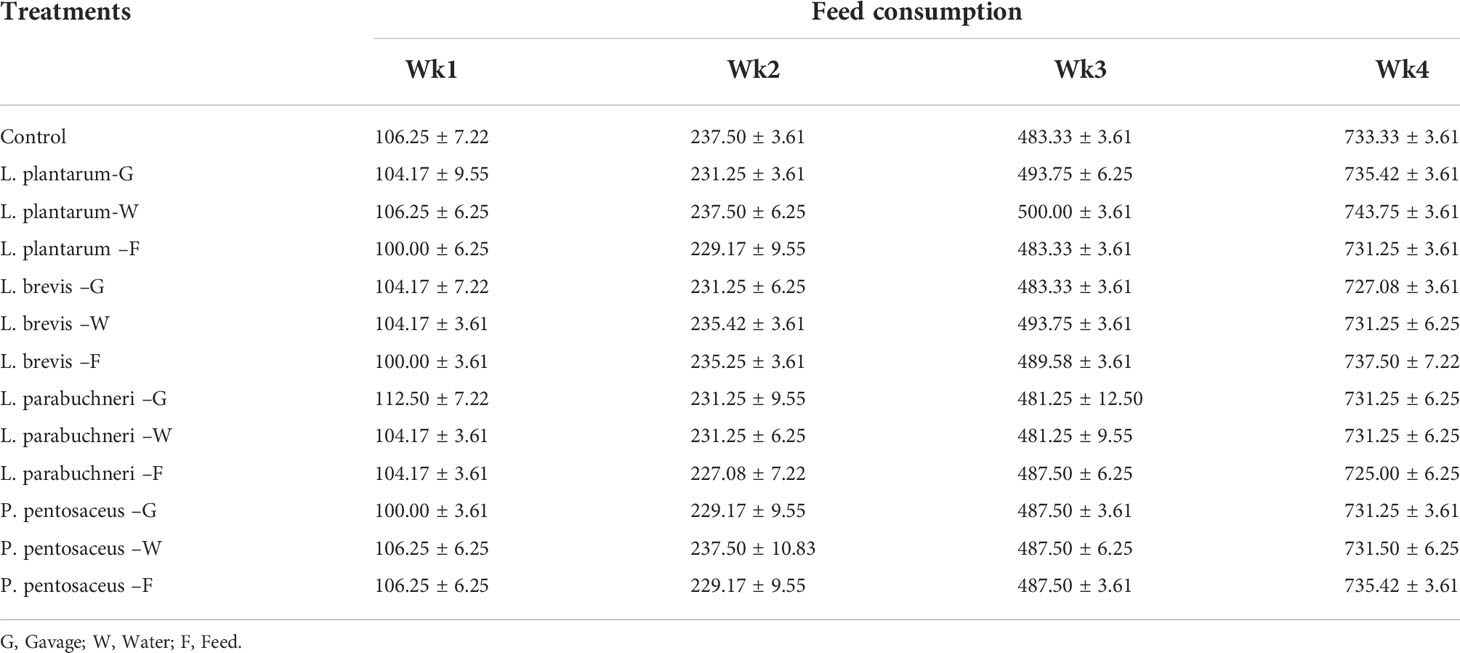
Table 5 Feed consumption for the total duration of the experiment and different treatments (Cycle 1).
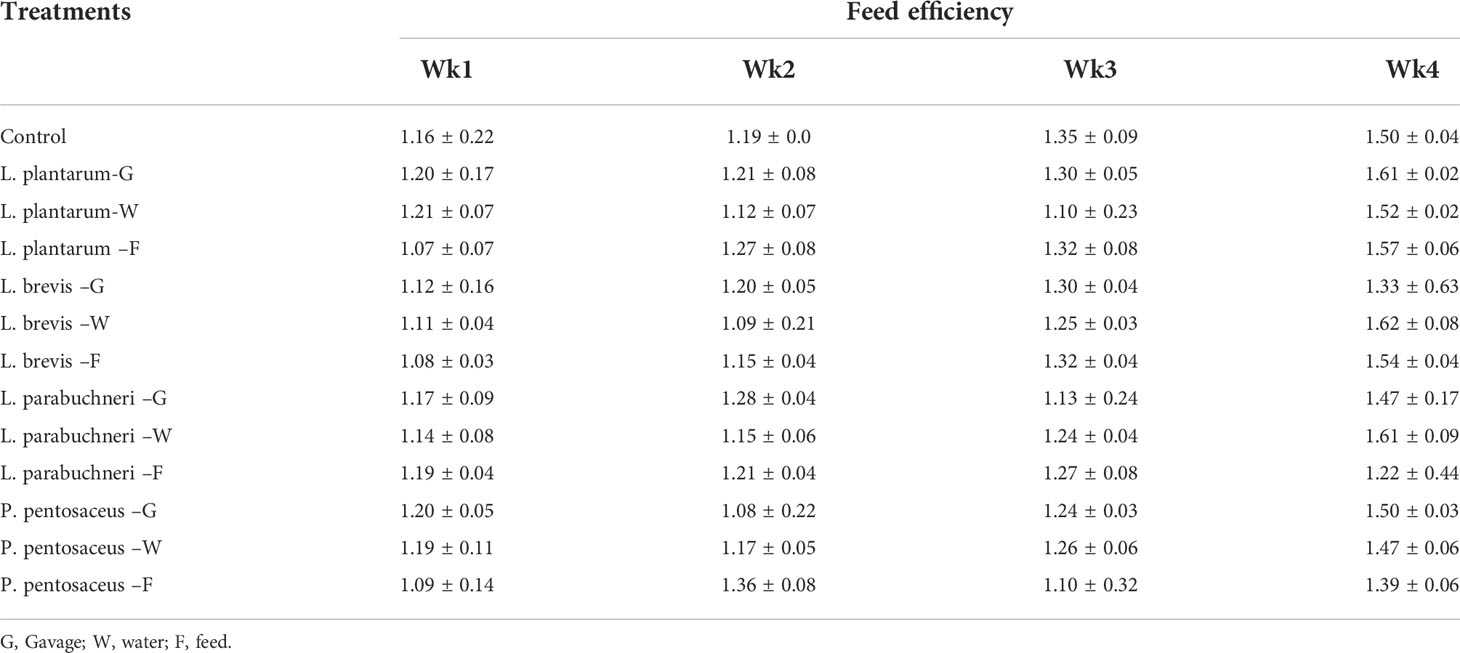
Table 6 Feed efficiency for the total duration of the experiment and different treatments (Cycle 1).
The main effect of bacteria and the interaction between the type of bacteria and application were significant p<0.05 for bird weight, weight gain, & feed efficiency. However, pairwise comparisons revealed no significant mean difference between the control group and the bacterial strains used. But this situation is different by comparison between Pediococcus pentosaceus and control; a significant increase in weight, weight gain, & feed efficiency was observed.
The microbial count for the in vivo test after four weeks of treatment is shown in Table 7. The control samples were treated with antibiotics as mentioned in the vaccine protocol; the Salmonella counts were almost zero. The addition of Lactobacillus isolates in all other treatments did not inhibit the growth of Salmonella and E. coli. However, the physical property including food consumption and effectiveness, is not involved. All the probiotic treatments affected the count of LAB and E. coli until the end of the experiment, i.e., their counts were similar beyond treatments. The Salmonella counts differed significantly and were absent only in the control group that was administered antibiotics following the protocol.
Humoral immune response of different bacterial isolates (Lactobacillus plantarum, Pediococcus pentosaceus, L. brevis, and L. parabuchneri) on chicken antibodies obtained from sera of broiler chickens (three weeks old) is presented in Table 8. The chickens were administered bacterial isolates through gavage (G), water (W), and feed (F). There was no significant (P > 0.05) effect on the total antibody titers (IgA and IgM) in the broiler chickens across treatments. Also the effect of different bacterial isolates (Lactobacillus plantarum, Pediococcus pentosaceus, L. brevis, and L. parabuchneri) on chicken antibodies obtained from the sera of broiler chickens (four weeks old) is presented in Table 9. There was no significant (P > 0.05) effect on the total antibody titers (IgA and IgM) in the broiler chickens across treatments.
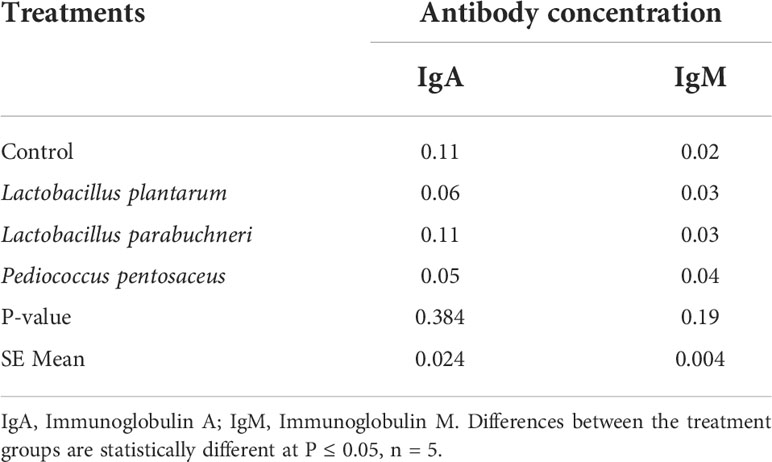
Table 8 The effect of different bacterial isolates on antibody-mediated immune responses in three-week-old chickens (Cycle 1).
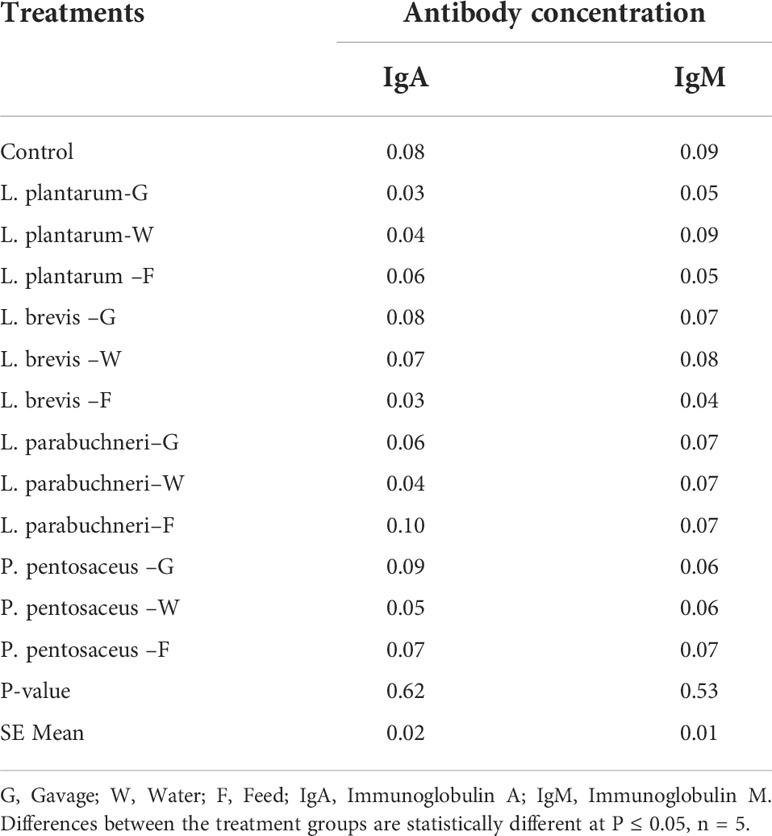
Table 9 The effect of different bacterial isolates on antibody-mediated immune responses in four-week-old chickens (Cycle 1).
Growth performance of the in vivo evaluation experiments showed that the formulated probiotic was successful in conserving the birds in the good physical condition and eliminated mortality in the absence of antibiotic administration. Additionally, all growth parameters measured were positive and promising for this product (Table 10). The weight of the birds in all treatment groups was high. The activity of the formulated product was similar to that of the two commercially available products that were used. No differences were observed for bird weight, weight gain, feed consumption, and feed efficiency between bacterial strains p>0.05. The body weight, weight gain, feed consumption, and feed efficiency for different probiotic products, including control, were similar.
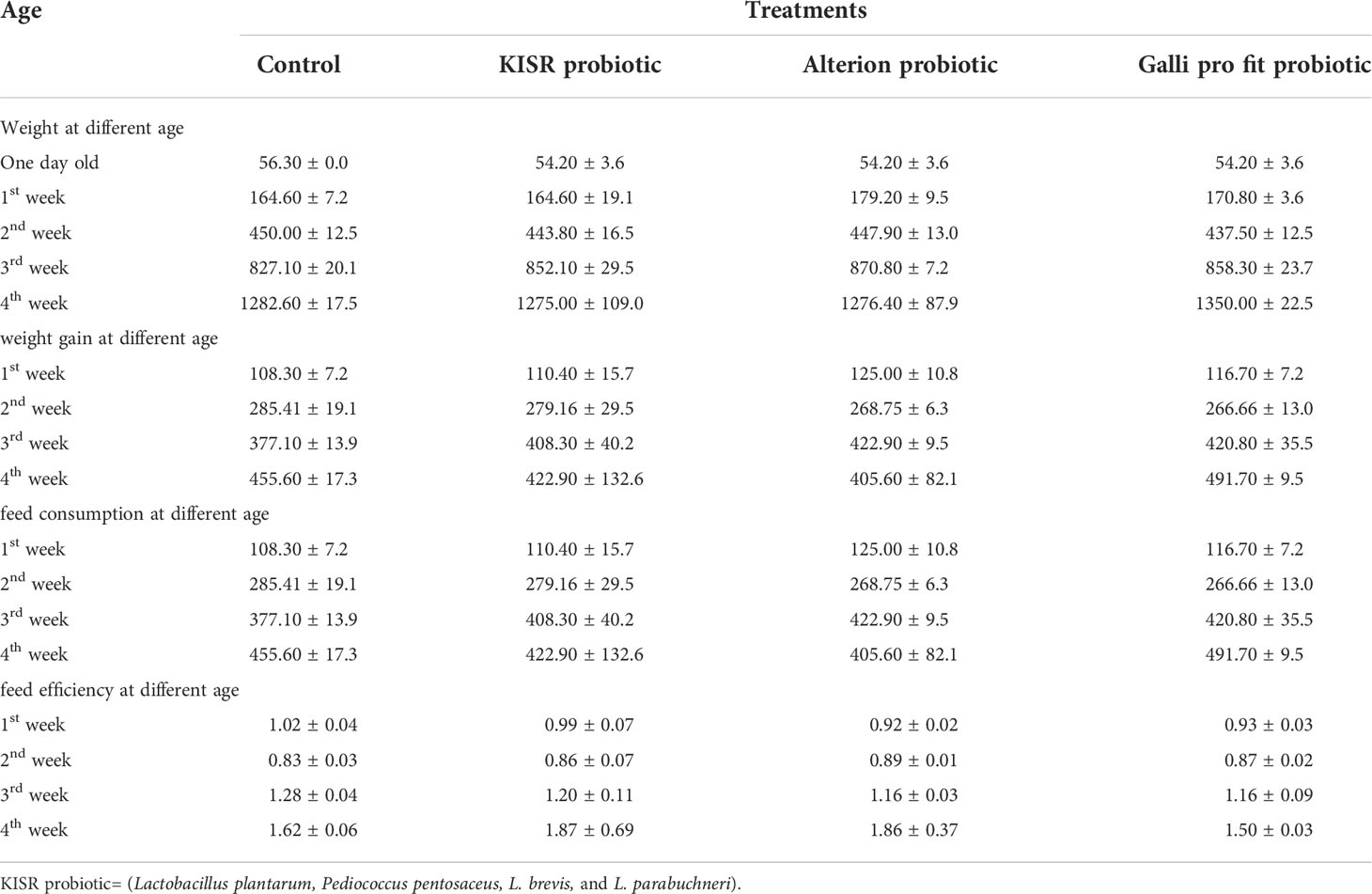
Table 10 The effect of the application of different types of probiotics on the body weight, body weight gains, feed consumption and feed efficiency of the broilers in Kuwait (Cycle 2).
The microbial count after four weeks reflected their diet, as shown in Table 11. The addition of newly formulated probiotic products inhibited the growth of Salmonella, whereas LAB and E. coli counts did not change. The commercial product Alterion could not inhibit Salmonella growth.
Humoral immune response found that the effect of different probiotics (KISR Probiotic, Alterion Probiotic, and Galli pro fit Probiotic) on chicken antibodies obtained from the sera of broiler chickens (four weeks old) is presented in Table 12. The different probiotic treatments did not affect the total antibody IgM titers significantly in the broilers (P > 0.05). However, the IgA antibody titers of the four-week-old broiler chickens were affected significantly by the probiotic (P = 0.044). The results showed that P3F contributed to the highest IgA titers followed by P1F. The IgA titers for control.
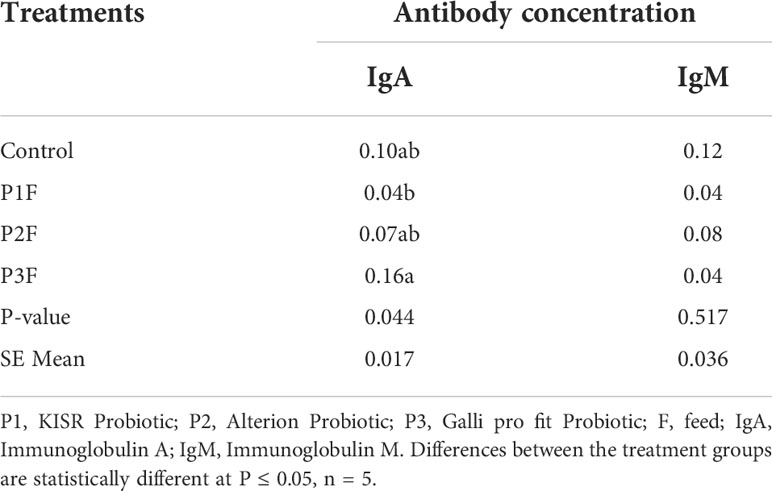
Table 12 The effect of different bacterial isolates on antibody-mediated immune responses of four-week-old chickens (Cycle 2).
This study aimed to evaluate the effectiveness of a newly developed probiotic product to reduce the incidence of Salmonella infections as well as to compare the newly formulated product with the commercial probiotics used in the farms. Four LAB strains were successfully isolated from phase 1 (L. plantarum, L. parabuchneri, L. brevis, and Pediococcus pentosaccus). The isolates were characterized, and identified as excellent candidates for the development of commercial probiotic feed additives for poultry production in Kuwait. In the current study, a compatibility assessment for the selected isolates has been done and a new multistrain probiotic was developed to be used against probiotics available in the market. To determine the compatibility between these isolates and the effectiveness of the final probiotic formulation, we evaluated the efficiency of the selected isolates strains. Some studies have suggested that the positive effects of multi-strain probiotics with different strain characteristics might create an anaerobic probiotic region that can enhance the colonization and survival of probiotic strains at the expense of pathogens (Timmerman et al.) (Timmerman et al., 2004). Mono-strain probiotic effects, however, are limited to strain-specific properties and survival. Multi-strain probiotics show a lower feed-conversion ratio and number of damaged eggs (Balevi, et al.) (Balevi et al., 2001). Additionally, multi-strain probiotics enhance performance more than single-strain products (Balevi et al., 2001; Gardiner et al., 2004; Timmerman et al., 2004). Only a few studies have investigated multi-strain probiotic products composed of LAB strains with selected functions, such as immunomodulation activity, adherence to host intestinal epithelium, and inhibition of host invasion by pathogenic bacteria.
Interestingly, some studies have shown that using multi-strain probiotics (MSP) as feed additives improved the bird performance. For example, Avishek Biswas (Biswas et al., 2022) reported that using a mixture of Bacillus coagulans Unique IS2 + Bacillus subtillis UBBS14 + Saccharomyces boulardii Unique 28 positively effect on bird health. The supplementation of multi strains probiotics at 107 CFU/g diet improved the status of total of 256 broiler chicks and showed significant effects with improved performance, immune response, gut morphology and expression of nutrient transporter genes. Thus, the MSP could be a suitable alternative to antibiotic growth promoters in chicken diets. Mohammed, jiang (Mohammed et al., 2019) also studied the effects of using symbiotic (4 microbial strains of probiotic and FOS) in broiler chicks reared under heat stress. They observed that the symbiotic improved the antioxidant status and inhibited the harmful effects of heat stress on broilers. In addition, the study of Uraisha Ramlucken (Ramlucken et al., 2020) on growth performance and gut health in male Ross 308 broiler chickens challenged with Clostridium perfringens Type A.
The multi-strain Bacillus probiotic product improved growth performance and generally had a positive effect on C. perfringens challenged-broiler well-being, indicated by gut and liver health observations.
Further, substantiate the attractiveness of multi-strain Bacillus probiotics as a replacement for other undesirable in-feed growth promoting and antibacterial additives. Furthermore, Rine Christopher (Reuben et al., 2022) aimed in his study to evaluate the effects of novel mono- and multi-strain probiotics on the growth performance, intestinal microbiota and haemato-biochemical parameters of broilers. Rine concluded that the supplementation with novel multi-strain probiotics improved growth, intestinal health and haemato-biochemical parameters in broilers and could be used as suitable antibiotic alternatives.
The results of the current investigation agree with the previously reported literature. The mechanism of creating a probiotic product based on multi-strains is existing. However, the compatibility and interactions among these four selected lactic acid bacteria strains are novel. LAB has been documented to produce antimicrobial agents to inhibit some types of bacteria. These antimicrobials agents, such as bacteriocins and organic acids can also inhibit bacteria from the same group (LAB group). In the current study, the bacterial cultures’ coexistence was tested to confirm their viability in the same environment. The gut microflora of young birds is unstable and can easily be affected by infection through pathogens. Therefore, maintaining an optimal gut microflora strongly affects the health and growth of the bird. The addition of probiotics can inhibit the growth of pathogens and boost the microbial balance of the host. As a dietary supplement, probiotics can also improve the growth of broiler chickens (Yang et al., 2008).
The first cycle showed that the delivery of the probiotic for all bacterial strains and different application methods; via drinking water, feed, or oral gavage improved the performance of the birds with minor differences between them in weight. In contrast, in the second cycle, all the applied treatments including the newly formulated probiotic gain similar weight with control.
Several studies have indicated the beneficial effects of probiotic bacteria on the growth of broiler chickens, the composition of the gut microbiota, and the development of the immune system. Lactobacillus is the most popular example since they have immunomodulatory properties and intestinal health benefits. One study investigated the effects of various doses of a multi-strain lactobacilli mixture (Lactobacillus salivarius, L. reuteri, L. crispatus, and L. johnsonii) on the innate and adaptive immune responses in broiler chickens. The variations in antibody titers were not significant (Ding et al., 2019; Alizadeh et al., 2020). On the other hand, when Wang (Wang et al., 2019) conducted a study to investigate the effects of a Lactobacillus plantarum strain as a probiotic along with oxygen levels on the immune response of chickens at high altitudes, they found that L. plantarum significantly increased the levels of IgA and anti-BSA antibodies. Pediococcus pentosaceus, a strain of lactic acid bacteria, is widely used in the food industry as it can produce antimicrobial agents and thus, also functions as a probiotic. However, only a few strains have been isolated, which is a limiting factor for conducting analysis. Few such isolates have been detected in poultry and ducks’ gastrointestinal tracts (GI) (Jiang et al., 2020). In our study, the different probiotic treatments did not affect the total antibody IgM titers significantly in the broilers (P > 0.05). However, the IgA antibody titers of the four-week-old broiler chickens were affected significantly by the probiotic type. Wang (Wang et al., 2018) conducted a study to evaluate the effects of microencapsulated probiotics and prebiotics (microencapsulated Enterococcus faecium, microencapsulated Lactobacillus plantarum, Bacillus subtilis, β-mannose, and fructo-oligosaccharide) on broiler chickens. They found that the serum IgA levels were higher than those of the control and other treatment groups.
The conclusion of the in vivo evaluation experiments of the selected strains showed that these strains, that were initially selected in phase 1 of the project, were practical in maintaining the birds healthy and preventing deaths even in the absence of the use of antibiotics. A very promising results, suggesting that the selected strains have good potential for utilization or the development of probiotic as feed additive to control Salmonella in poultry production.
During the current study, we demonstrated that the isolates are compatible to each other and can be mixed to produce one probiotic product. A new product was developed due to the successful of the preliminary study in vivo test of the selected isolates. The product is a mixture of all the four isolates with a similar ratio.
The evaluation test was performed for the new probiotic and compared to two commercially available products (Alterion and Galli pro fit). The results showed that the product was effective in keeping the birds healthy and preventing deaths even in the absence of the use of antibiotics. Additionally, the growth parameters were high, chicken gained a high mass of meat and succussed in inhibiting salmonella.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by The procedures and protocols followed the official animal welfare guidelines and regulations (Reference No. PMO/PV/RP/032/2017).
Study participants worked with me to analyze samples, compile and tabulate results. For example, HA-K was one of the active participants in terms of preparing the place for the experiment, preparing the birds and following up on the workers on the farm. MK had a role in participating in choosing some alternative ways to work with live poultry when facing some obstacles. He was also responsible for providing the required percentages of isolates for each bacterial strain to work in the field. HA-M worked to provide us with the required quantities of the product using the fermentation device. The role of HA-S, HS, and AA-M was to grow the bacteria in the laboratory and to ensure its purity and effectiveness with periodic examination during the experiment. All authors contributed to the article and approved the submitted version.
The authors would like to extend their sincere thanks to the management of the Kuwait Institute for Scientific Research (KISR), Kuwait United Poultry Company (KUPCO) and the Kuwait Foundation for the Advancement of Sciences (KFAS) for their technical and financial support toward the execution of this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2022.1026958/full#supplementary-material
Alizadeh M., Shojadoost B., Astill J., Taha-Abdelaziz K., Karimi S. H., Bavananthasivam J., et al. (2020). Effects of in ovo inoculation of multi-strain lactobacilli on cytokine gene expression and antibody-mediated immune responses in chickens. Front. veterinary Sci. 7, 105. doi: 10.3389/fvets.2020.00105
Al-Khalaifa H., Al-Nasser A., Al-Surayee T., Al-Kandari S., Al-Enzi N., Al-Sharrah T., et al. (2019). Effect of dietary probiotics and prebiotics on the performance of broiler chickens. Poultry Science. 98 (10), 4465–4479. doi: 10.3382/ps/pez282
Al-Khalaifah H., Al-Nasser A., Al-Surrayai T. (2021). Dietary polyunsaturated fatty acids and cellular immune response in broiler chickens. Book chapter in Prime Archives in Immunology Book: 2nd Edition. Editors: Ajmal K., Al-harrasi A.. Hyderabad, India: Vide Leaf. 2021. doi: 10.37247/PAIMMU2ED.2.2021.6
AL-Khalaifah H. S., AL-Nasser A., Surrayai T. (2022). Effects From Dietary Addition of Sargassum sp., Spirulina sp., or Gracilaria sp. Powder on Immune Status in Broiler Chickens. Front Vet Sci 9, 928235. doi: 10.3389/fvets.2022.928235
AL-Khalaifah H., Uddin S. J. S. (2022). Assessment of sargassum sp., spirulina sp., and gracilaria sp. as poultry feed supplements: Feasibility and environmental implications 14, 8968. doi: 10.3390/su14148968
Balba M. T., Al-Zenki S., Yateem A., Al-Surrayai T., Al-Daher R., Isolation Y. (2012). Characterization and evaluation of lactic acid bacteria for development of poultry probiotics (Phase i); report no. KISR11219 (Kuwait: Kuwait Institute for Scientific Research).
Balevi T., Ucan U. S., Coskun B., Kurtoglu V., Cetingul I. S. (2001). Effect of dietary probiotic on performance and humoral immune response in layer hens. Br. Poultry Sci. 42, 456–461. doi: 10.1080/00071660120073133
Beery J. T., Hugdahl M. B., Doyle M. P. (1988). Colonization of gastrointestinal tract of chicks by campylobacter jejuni. Appl. Environ. Microbiol. 54 (10), 2365–2370. doi: 10.1128/aem.54.10.2365-2370.1988
Biswas A., Dev K., Tyagi P. K., Mandal A. (2022). The effect of multi-strain probiotics as feed additives on performance, immunity, expression of nutrient transporter genes and gut morphometry in broiler chickens. Anim. Bioscience 35 (1), 64–74. doi: 10.5713/ab.20.0749
Ding S., Wang Y., Yan W., Li A., Jiang H., Fang J. (2019). Correction: Effects of lactobacillus plantarum 15–1 and fructooligosaccharides on the response of broilers to pathogenic escherichia coli O78 challenge. PloS One 14 (9), e0222877. doi: 10.1371/journal.pone.0222877
Edens F. W. (2003). An alternative for antibiotic use in poultry: Probioticsprobiotics. Rev. Brasiliera Ciec. Avícola 5 (2), 75–97.
FAO/WHO (2001). Health and nutritional probiotics in food including powder milk with live lactic acid bacteria (Cordoba, Argentinea: Joint FAO/WHO expert consultation).
Fuller R. (1989). Probiotics in man and animals. J. Appl. Bacteriology 66, 365–378. doi: 10.1111/j.1365-2672.1989.tb05105.x
Gabriel I., Lessire M., Mallet S., Guillot J. F. (2006). Microflora of the digestive tract: Critical factors and consequence for poultry. Worlds Poultry Sci. J. 62, 499–511. doi: 10.1079/wps2006111
Gardiner D. M., Cozijnsen A. J., Wilson L. M., Pedras M. S. C., Howlett B. J. (2004). The sirodesmin biosynthetic gene cluster of the plant pathogenic fungus leptosphaeria maculans. Mol. Microbiol. 53, 1307–1318. doi: 10.1111/j.1365-2958.2004.04215.x
Guo X. H., Kim J. M., Nam H. M., Park S. Y., Kim J. M. (2010). Screening lactic acid bacteria from swine origins for multistrain probiotics based on In vitro functional properties. Anaerobe. 16 (4), 321–326. doi: 10.1016/j.anaerobe.2010.03.006
Huis in ‘t Veld J. H. J., Mulder R. W. A. W., Snijders J. M. A. (1993). Impact of animal husbandry and slaughter technologies on microbial contamination of meat: Monitoring and control. Meat Sci. 36 (1994), 123–154. doi: 10.1016/0309-1740(94)90038-8
Jeurissen S. H., Lewis F., van der Klis J. D., Mroz Z., Rebel J. M., ter Huurne A. A. (2002). Parameters and techniques to determine intestinal health of poultry as conatituted by immunity, integrity and functionality. Curr. Issues Intestinal Microbiol. 3, 1–14.
Jiang J., Yang B., Ross R. P., Stanton C., Zhao J., Zhang H., et al. (2020). Comparative genomics of pediococcus pentosaceus isolated from different niches reveals genetic diversity in carbohydrate metabolism and immune system. Front. Microbiol. 11, 253. doi: 10.3389/fmicb.2020.00253
Ljungh A., Wadstöm T. (2009). Lactobacillus molecular biology: From genomic to probiotic (Sweden: Horizon Scientific Press).
Lorch H. (1995). Basic methods for counting microorganisms in soil and water. Methods Appl. Soil Microbiol. Biochem., 146–161.
Mohammed A., Jiang S., Jacobs J., Cheng H. W. (2019). Effect of a synbiotic supplement on cecal microbial ecology, antioxidant status, and immune response of broiler chickens reared under heat stress. Poultry science. 98 (10), 4408–4415. doi: 10.3382/ps/pez246
Oguz E. K., Arihan O., Oguz A. R. (2018). Oxidative and genotoxic effects of bisphenol a on primary gill cell culture of lake van fish (Alburnus tarichi güldenstädt, 1814). Chem. Ecol. 34, 914–924. doi: 10.1080/02757540.2018.1520846
Pederson K., Tannock G. W. (1989). Colonization of the porcine gastrointestinal tract by lactobacillus. Appl. Environ. Microbiol. 55, 279–283. doi: 10.1128/aem.55.2.279-283.1989
Ramlucken U., O.Ramchuran S., Moonsamy G., Lalloo R., S.Thantsha M., Jansen C., et al. (2020). A novel bacillus based multi-strain probiotic improves growth performance and intestinal properties of clostridium perfringens challenged broilers. Poultry Sci. 99 (1), 331–341. doi: 10.3382/ps/pez496
Reuben R. C., Sarkar S. L., Ibnat H., Roy P. C., Jahid I. K. (2022) Novel mono- and multi-strain probiotics supplementation modulates growth, intestinal microflora composition and haemato-biochemical parameters in broiler chickens. First published: Veterinary medicine and Science 8(2), 668–68011. doi: 10.1002/vms3.709
Richards J. D., Gong J., deLange C. F. M. (2005). The gastrointestinal microbiota and its role in monogastric nutrition and health with emphasis on pigs: Current understanding, possible modulations and new technologies for ecological studies. Can. J. Anim. Sci. 85, 421–435. doi: 10.4141/A05-049
Ripamonti B., Agazzi A., Bersani C., De Dea P., Pecorini C., Pirani S., et al. (2011). Screening of species-specific lactic acid bacteria for veal calves multi-strain probiotic adjuncts. Anaerobe J. 17 (3), 97–105. doi: 10.1016/j.anaerobe.2011.05.001
Salminen E., Ouehand A. C., Isolauri E. (1998). Clinical application of probiotic bacteria. Int. Dairy J. 8, 563–572. doi: 10.1016/S0958-6946(98)00077-6
Schoeni J. L., Doyle M. P. (1992). Reduction of campylobacter jejuni colonization of chicks by cecum colonizing bacteria producing anti-c. jejuni metabolites. Appl. Environ. Microbiol. 85 (2), 664–670. doi: 10.1128/aem.58.2.664-670.1992
Taheri H., Tabandeh F., Moravej H., Zaghari M., Shivazad M., Shariati P. (2009). Potential probiotic of lactobacillus johnsonii LT171 for chicken nutrition. Afr. J. Biotechnol. 8 (21), 5833–5837. doi: 10.5897/AJB09.1062
Timmerman H. M., Koning C. J., Mulder L., Rombouts F. M., Beynen A. C. (2004). Monostrain, multistrain and multispecies journal of dairy science vol. 90 no. 6, 2007 probiotics–a comparison of functionality and efficacy. Int. J. Food Microbiol. 96, 219–233. doi: 10.1016/j.ijfoodmicro.2004.05.012
Torok V. A., Hughes R. J., Ophel-Keller K., Ali M., Macalpine R. (2009). Influence of different litter materials on caecal microbiota colonization in broiler chickens. Poultry Sci. 88 (12), 2474–2481. doi: 10.3382/ps.2008-00381
Wang Y., Dong Z., Song D., Zhou H., Wang W., Miao H., et al. (2018). Effects of microencapsulated probiotics and prebiotics on growth performance, antioxidative abilities, immune functions, and caecal microflora in broiler chickens. Food Agric. Immunol. 29, 859–869. doi: 10.1080/09540105.2018.1463972
Wang L., Fu G., Liu S., Li L., Zhao X. (2019). Effects of oxygen levels and a lactobacillus plantarum strain on mortality and immune response of chickens at high altitude. Sci. Rep. 9, 1–9. doi: 10.1038/s41598-019-52514-w
Yang Y., Iji P., Kocher A., Mikkelsen L., Choct M. (2008). Effects of mannanoligosaccharide and fructooligosaccharide on the response of broilers to pathogenic escherichia coli challenge. Br. poultry science. 49 (5), 550–559. doi: 10.1080/00071660802290408
Yegani M., Korver D. R. (2008). Factors affecting intestinal health in poultry. Poultry Sci. 87, 2052–2063. doi: 10.3382/ps.2008-00091
Keywords: Salmonella, broiler chicken, antibiotics, compatibility, Kuwait
Citation: Al-Surrayai T, Al-Khalaifah H, Al-Mansour H, Kishk M, Al-Mutairi A, Sultan H and Al-Saleem H (2022) Evaluation of the lactic acid bacteria based formulated probiotic product for poultry. Front. Anim. Sci. 3:1026958. doi: 10.3389/fanim.2022.1026958
Received: 24 August 2022; Accepted: 19 October 2022;
Published: 10 November 2022.
Edited by:
Olajide Mark Sogunle, Federal University of Agriculture, Abeokuta, NigeriaReviewed by:
Santosh Dulal, Center for Molecular Dynamics, NepalCopyright © 2022 Al-Surrayai, Al-Khalaifah, Al-Mansour, Kishk, Al-Mutairi, Sultan and Al-Saleem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: T. Al-Surrayai, dHN1cmFpYUBraXNyLmVkdS5rdw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.