
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Anim. Sci. , 17 December 2021
Sec. Animal Nutrition
Volume 2 - 2021 | https://doi.org/10.3389/fanim.2021.775345
 Taylor J. Garcia1†
Taylor J. Garcia1† Nichole M. Cherry2
Nichole M. Cherry2 Kimberly A. Guay1
Kimberly A. Guay1 Jeffrey A. Brady2
Jeffrey A. Brady2 James P. Muir2,3
James P. Muir2,3 William B. Smith1*†
William B. Smith1*†The objective of our experiment was to evaluate preservation and revitalization strategies for rumen inoculum anticipating research and veterinary applications. Rumen fluid samples were collected from 12 harvested cattle. Liquid samples were divided into five 500-mL aliquots which were randomly allocated to one of five treatments in a 2 × 2 + 1 augmented factorial design. Factors included preservation method [freezing (FZN) or lyophilization (LYO)] and preservative (glycerol; + or –). A fresh control (CON) was maintained from each sample. Feedstuffs used in this experiment were alfalfa hay, Coastal bermudagrass hay, cracked maize, rice bran, and soybean meal. Reference feedstuffs were subjected to batch culture in vitro true digestibility (IVTD) and in vitro NDF digestibility (IVNDFD) assays using inoculum from each of the five treatments. There was an effect (P < 0.05) of preservation method, preservative, and their interaction for both IVTD and IVNDFD of each of the five references feedstuffs. Freezing or lyophilization of rumen inoculum reduced (P < 0.05) IVTD and IVNDFD of reference feeds relative to the CON. Despite lower degradation of feeds when frozen or lyophilized rumen fluid was used rather than fresh, differences between them in IVTD and IVNDFD suggest that, in the absence of fresh inoculum, preserved rumen fluid may be a viable option for veterinary applications, such as transfaunation, but likely will not be viable for research applications.
One of the principal treatment strategies for animals with acidosis is transfaunation. This refers to transferring microorganisms, including bacteria, protozoa, fungi, and archaea, from a healthy rumen to a sick recipient (DePeters and George, 2014). Healthy microbes and chemical constituents in the rumen fluid play an important role in re-establishing the rumen microbial population, essentially acting as a probiotic. In vitro digestibility using inoculum from cattle and sheep predict in vivo dry matter digestibility (Denek et al., 2006). However, establishment and maintenance of cannulated animals represents an obstacle in the research and veterinary applications.
The demand to identify an alternative inoculum to rumen contents is mainly driven by issues related to the surgical modification of animals and the care required to house these animals throughout the year. Although rumen fluid can be obtained via stomach tubing, avoiding the need for cannulation, such samples often contain saliva; their collection causes tremendous stress on the host animal, and samples may not represent the entire rumen content (Mould et al., 2005).
Using frozen rumen content as an inoculum source can yield lower degradability values when compared to fresh inoculum. However, in the absence of fresh inoculum, frozen rumen liquor has been suggested as a possible alternative (Mohamed et al., 2002). Luchini et al. (1996b) further showed that glycerol addition to rumen inoculum resulted in no change in proteolytic activity of the resulting preserved material. Glycerol acts as a cryoprotectant, often in semen preservation techniques (Tada et al., 1990; Rota et al., 2006), by penetrating the cell membrane of biological organisms and preventing water crystallization and increased electrolyte concentrations (Luchini et al., 1996b). An understanding of viable preservation methods would allow rumen liquor to be stored for later application in research and veterinary applications.
Since all samples were obtained from cattle postmortem, rumen fluid collection for preservation was exempt from oversight of the Tarleton State University Institutional Animal Care and Use Committee.
Our experiment was conducted as a completely randomized design with a 2 × 2 + 1 augmented factorial treatment arrangement (Table 1). Factors in the arrangement were preservation method (n = 2) and preservative (n = 2); each factor (as well as the interaction) were compared to fresh inoculum (CON). Treatments included CON, frozen (−20°C) without cryoprotectant (FZN−), frozen with cryoprotectant (FZN+), lyophilized without cryoprotectant (LYO−), and lyophilized with cryoprotectant (LYO+).
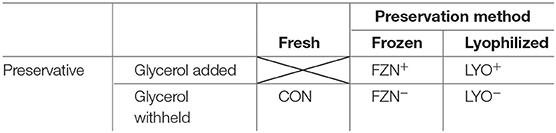
Table 1. Visual representation of the augmented factorial design used in the evaluation of rumen fluid preservation.
Rumen contents were collected from 12 randomly selected cattle (Bos taurus taurus, B. taurus indicus, or B. taurus indicus × B. taurus taurus) harvested at the Tarleton Meat Laboratory, Stephenville, TX. Samples were collected on four different days: four on 29 March, four on 20 April, one on 4 May, and three on 11 May 2018. Rumen content was collected immediately after inspection of offal. Digestive organs were removed from each harvested animal, placed in an offal barrel, and punctured with a knife. Rumen contents were collected from different locations within the rumen and pooled while transferring into pre-warmed thermoses (39°C). Rumen liquor (~3.5 L) was removed by straining samples through eight layers of cheesecloth. Samples were transported to the laboratory at Texas A&M AgriLife Research and Extension Center at Stephenville, TX (1.6 km). Rumen liquor from each animal was subjected to each of the five treatments (2 × 2 + 1), thus each treatment was replicated 12 times.
Liquid material samples from each animal were divided into five, 500-mL aliquots. Aliquots were randomly allocated to one of five preservation treatments (described in section Experimental Design). A visual illustration of treatments and treatment allocation has been included in Figure 1.
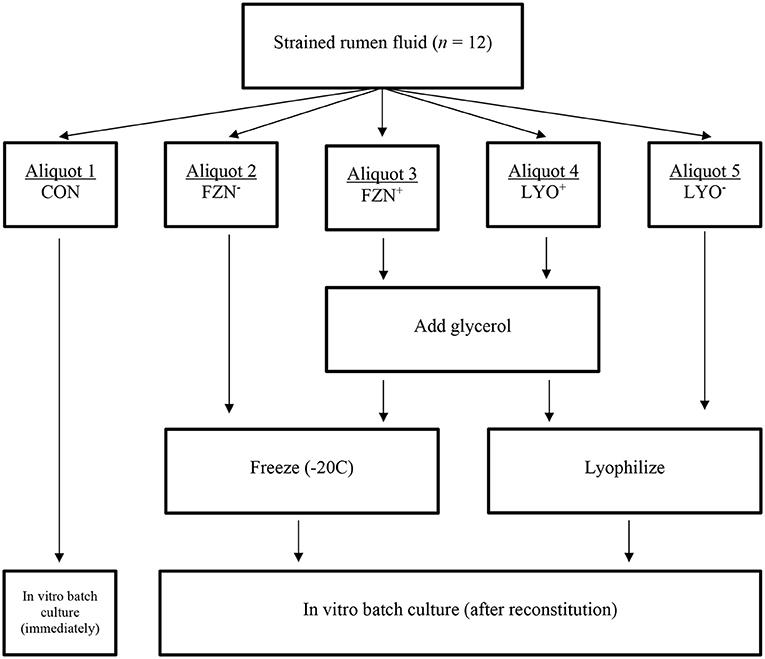
Figure 1. Diagrammatic representation of treatment allocations in the evaluation of rumen fluid preservation techniques.
Aliquots assigned to FZN− were placed inside a plastic container, infused with CO2, and frozen solid at −20°C until ready for use. Glycerol (C3H8O3; VWR BHD Chemicals, Radnor, PA) was used as the cryoproctectant at 5% of total volume in FZN+ and LYO+ treatments (Luchini et al., 1996a). Aliquots assigned to FZN+ received 25 mL of pre-warmed glycerol (39°C), infused with CO2 for 20 min, under anaerobic conditions. At completion of mixing, samples were transferred to a plastic container infused with CO2 and placed inside a freezer (−20°C) until ready for assays. Additional glycerol did not prevent the solid freezing of material. Prior to lyophilization (LYO− and LYO+), samples were centrifuged at 5,000 × g for 30 min at 4°C (Hsu and Fahey, 1990; Luchini et al., 1996a). Separation of each aliquot was maintained throughout the procedure. Following centrifugation, supernatant was discarded, and microbial pellets were recovered and pooled in two 50-mL conical tubes. Aliquots assigned to LYO− were placed directly onto the freeze dryer (Vir Tis bench top, SP Industries, INC, Warminister, PA). Aliquots assigned to LYO+ were placed inside a beaker, glycerol was added, and the sample was vortexed for 20 s. After adding and mixing glycerol, samples were transferred into appropriate tubes and placed on the freeze dryer according to the procedure described for LYO−. Because the addition of glycerol prevented the complete lyophilization of sample material, samples were removed from the freeze dryer and transferred to a freezer when technicians observed no further change in sample desiccation. Samples were left on the freeze dryer until complete lyophilization was achieved. Once samples were successfully lyophilized, samples were transferred into a freezer (−20°C) until ready for assays. Lyophilized samples were reconstituted with pre-warmed (39°C) deionized water at original volume under anaerobic conditions.
Reference feeds were selected to represent not only common feeds found in ruminant diets, but also feeds that varied in nutrient content (Table 2). Soybean [Glycine max (L.) Merr.] meal represented a high-protein, low-fiber concentrate. Cracked maize (Zea mays L.) was selected to represent a feedstuff high in energy, low in protein, and low in fiber. Rice (Oryza sativa L.) bran represented low-protein, low-energy, and high-fiber feed. The roughage sources were chosen because they are commonly used in the southeastern United States. ‘Coastal’ bermudagrass hay [Cynodon dactylon (L.) Pers.] represented low protein and high fiber, while alfalfa (Medicago sativa L.) hay represented high protein and high fiber. Reference feeds were dried in a forced-air gravity convection oven to a constant weight at 55°C. Samples were ground to pass through a 2-mm screen using a Wiley mill (Arthur H. Thomas Co., Philadelphia, PA), and a subsample was ground to pass through a 1-mm screen.
Neutral detergent fiber and ADF were measured sequentially using the ANKOM200 Fiber Analyzer (ANKOM Technology Corporation, Fairport, NY; Vogel et al., 1999). The procedure included sodium sulfite and α-amylase, and values were expressed inclusive of residual ash. Acid detergent lignin was determined using the sulfuric acid method (Method 973.18; AOAC, 2000). Nitrogen and carbon were measured using the Dumas total combustion method (Elementar Americas, Mt. Laurel, NJ; Method 990.09; AOAC, 2000), and CP was calculated based on the nitrogen content of each sample (N × 6.25).
In vitro true digestibility (IVTD) and in vitro NDF digestibility (IVNDFD) were determined by the DAISYII Incubator (ANKOM Technology Corporation, Fairport, NY). ANKOM F57 filter bags (ANKOM Technology Corporation, Fairport, NY) were pre-rinsed in acetone for 5 min and completely air-dried. Weight from each F57 filter bag was recorded. Representative 0.5-g samples (ground through 2-mm screen) were placed into filter bags and heat-sealed. Each of the five reference feedstuffs (replicated four times), plus blank bags (22 bags in total), were placed in the DaisyII Incubator containing buffer and preserved inoculum in separate incubation jars (5 treatments × 12 animals = 60 jars). The blank bag was used to calculate a correction factor that adjusted for weight loss or gain from the filter bags. Samples were incubated for 48 h in a rumen fluid/buffer solution (ANKOM Technology, 2017). For CON, incubations were performed as soon as possible after rumen fluid collection, thereby representing a “standard” of immediate incubation following collection. For the remaining treatments, each run (4 jars per incubator) was performed with thawed or reconstituted material from a single animal at a time.
At completion of incubation, jars were removed and fluid drained. Samples were removed, rinsed with tap water, boiled in an NDF solution using an ANKOM200 fiber analyzer to remove microbial debris and any remaining soluble fractions, and dried under forced air at 105°C for 24 h. Post in vitro NDF residue weight was recorded. In vitro true digestibility was calculated on DM basis according to
where W1 = bag tare weight, W2 = sample weight, W3 = final bag weight after in vitro and NDF treatment, C1 = blank bag correction (final oven dried weight divided by original weight). In vitro NDF digestibility was calculated with the same equation but instead of multiplying W2 by DM, W2 was multiplied by NDF weight.
Data were analyzed as an augmented factorial using PROC GLIMMIX in SAS® v. 9.4 (SAS Institute, Cary, NC). The model equation was described as
where yijklm was the dependent response variable, μ was the overall mean, Mi was the fixed effect of preservation method, Pj was the fixed effect of preservative, MPij was the fixed interaction of preservation method and preservative, sk was the random effect of rumen source, rl was the random effect of batch-culture run, and εijklm was the random residual error.
In order to appropriately test the augmented factorial, orthogonal contrasts (rather than F-tests in a standard ANOVA) were constructed to test the effect of preservation method, the effect of preservative, and the effect of the interaction of preservation method and preservative. Post hoc means separations were determined using the Dunnett's procedure (comparisons with a control), where CON served as the control. All means were reported as least squares means.
Multiple α levels were defined for this experiment. The first was defined as α1 = 0.01, such that strong differences among responses would be declared when P < 0.01. The second was defined as α2 = 0.05, such that differences among responses would be declared when 0.01 ≤ P < 0.05. The third was defined as α3 = 0.10, such that tendencies for differences among responses would be declared when 0.05 ≤ P < 0.10.
In vitro true digestibility values of reference feedstuffs are presented in Table 3. Using the Dunnett's test, FZN+, LYO+, and LYO− differed (P < 0.01) from CON for alfalfa, bermudagrass, and rice bran, and FZN− differed (P < 0.01) from CON for bermudagrass and rice bran. In these instances of differences, IVTD values from preserved rumen inoculum were less than those from CON. Using contrasts, there was an interaction of preservation method and preservative (P < 0.01) for alfalfa, bermudagrass, and rice bran. Glycerol addition decreased IVTD more in the frozen treatments than in the lyophilized treatments. There was an effect of preservative (P < 0.01) on IVTD of both rice bran and soybean meal. In both cases, the addition of glycerol decreased IVTD relative to its exclusion.
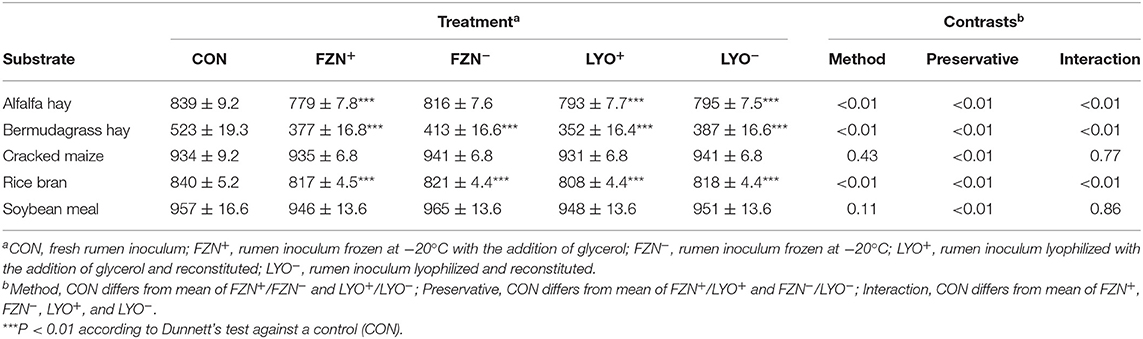
Table 3. In vitro true digestibility (g kg−1 DM; mean ± standard error of the mean) of feed ingredients subjected to digestion with rumen inoculum preserved with or without the addition of glycerol as a cryoprotectant.
In vitro NDF digestibility values of reference feedstuffs are presented in Table 4. Using the Dunnett's test, FZN+, LYO+, and LYO− differed (P < 0.01) from CON for alfalfa, bermudagrass, and rice bran, and FZN− differed (P < 0.01) from CON for bermudagrass. In these instances of differences, IVNDFD values from preserved rumen inoculum were less than those from CON. Using contrasts, there was an interaction of preservation method and preservative (P ≤ 0.01) for alfalfa, cracked maize, and rice bran. Glycerol addition decreased IVNDFD more in the frozen treatments than in the lyophilized treatments. There was an effect of preservative (P < 0.01) on IVNDFD of both bermudagrass and soybean meal. In both cases, the addition of glycerol decreased IVNDFD relative to its exclusion.
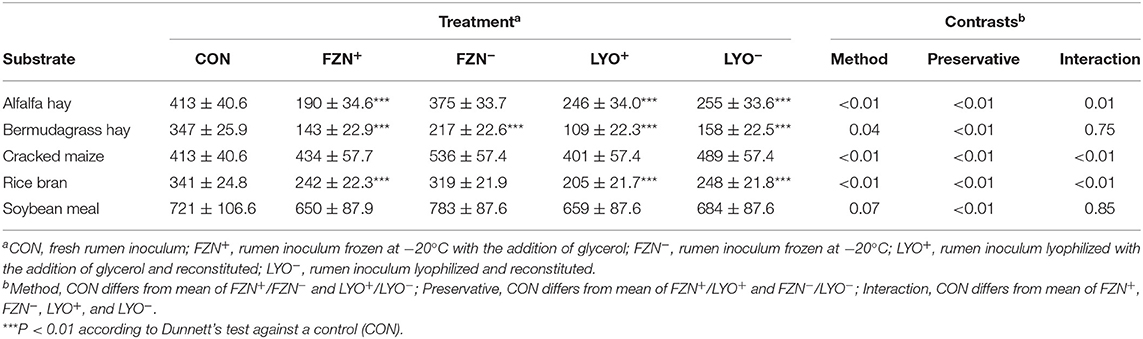
Table 4. In vitro NDF digestibility (g kg−1 DM; mean ± standard error of the mean) of feed ingredients subjected to digestion with rumen inoculum preserved with or without the addition of glycerol as a cryoprotectant.
Holden (1999) compared 10 feeds with different CP, NDF, and ADF values and their in vitro digestion with rumen liquor from donor cows consuming either grass hay or a haylage and silage-based TMR. Results showed that source of inoculum affected IVTD for grass pasture, TMR, alfalfa hay, grass hay, steam flaked maize, and dry ground maize. Other studies have shown that source of inoculum affect feed IVDMD (Church and Peterson, 1960; Bezeau, 1965). Although incubating high-starch grains with lower quality forages in the same digestion vessel might influence IVTD, Holden (1999) showed it to be inconsequential.
Preservation method (freezing or lyophilization) of rumen fluid increased the IVNDFD of cracked maize (Table 4); similar results were observed for soybean meal. These results can possibly be attributed to the two “selections” applied to the microbial material. First, filtering through cheesecloth selected a liquid fraction, thereby eliminating most microbes that would be attached to fiber and associated with the solid material. This level of selection was applied equally to all five treatment combinations. Secondly, treatments applied selected for microbes that could withstand preservation. A different set of microbes present in CON and any treated material could explain increased IVNDFD.
The collection of rumen fluid from slaughtered cattle poses challenges due to transportation, exposure to oxygen, and unknown donor health, all of which affect the microbial population found within the rumen; however, where access to fresh rumen fluid is restricted, slaughter cattle rumen contents may be a viable option. Mohamed et al. (2002) examined in vitro degradability using a range of inocula produced by fresh or stored (−20°C up to 10 wk) rumen contents recovered from slaughter; results from their study indicated the degradability was reduced with freezing at all points throughout a 96-h incubation. Results from our study coincide with those results, with FZN− exhibiting the least decrease in IVTD among the treatments.
Luchini et al. (1996a) compared the proteolytic activity of lyophilized and frozen ruminal microorganisms with glycerol and reported that glycerol addition had no effect on proteolytic activity of the preserved microorganisms. In our study, all IVTD and IVNDFD values were higher for treatments without addition of glycerol; however, larger differences were observed in IVNDFD values. Other studies found 5% DMSO to be a more effective cryprotectant when compared to glycerol (Nsabimana et al., 2003; Denek and Can, 2007). It should be noted, however, that glycerol has been shown to be fermented by ruminal microbes (Ferraro et al., 2009; Del Bianco Benedeti et al., 2016) and, thus, could cause some confounding effects in our observations.
Potential for decreased in vitro digestibility values of reference feedstuffs relative to in vivo values has been associated with gas accumulation in either in vitro or in situ bags (Nocek et al., 1979; Marinucci et al., 1992). This could explain the decreased digestibility values observed in our experiment, especially if a biofilm were present in preserved rumen fluid (FRZ or LYO) that was not present in CON, though one would need in vivo comparisons to fully flesh out this possibility. Another explanation for lower degradability of roughage material may be length of incubation. An incubation period of 48 h is usually insufficient for low-quality roughage sources (Barnes, 1967; Grant et al., 1974; Can et al., 2009). An increase in incubation to 72 h may improve fermentation estimates when alternatives to fresh rumen fluid are utilized as inoculum sources. Denek et al. (2010) suggested that a 72-h incubation period should be adequate for determining IVDMD of roughages when frozen rumen fluid was used; however, the authors did not specify when samples of roughages and concentrates are used. Because the roughage samples in our experiment were only incubated for 48 h, this may offer one explanation as to why IVTD and IVNDFD showed lower vales from preserved rumen fluid when compared to fresh rumen inoculum.
Results of in vitro digestibility comparisons suggest that, as the digestibility of a feedstuff increases, the treatment of the rumen inoculum has less influence on the potential digestive capability. In nearly all cases, the addition of glycerol as a preservative to frozen or lyophilized rumen fluid resulted in additional decreases in IVTD and IVNDFD. However, in two different instances, frozen rumen fluid exhibited similar IVTD and IVNDFD to fresh rumen inoculum. These results indicate that the preservation of rumen fluid for later use may not be suitable for research purposes. However, despite lower feed degradation when inoculating with frozen or lyophilized rumen fluid, there may be a place for preserved rumen inoculum in veterinary medical applications. In the absence of fresh inoculum, preserved rumen fluid may be a viable option for transfaunation procedures. However, additional research is required to optimize preserved rumen fluid as an inoculum.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the animal study because, since all samples were obtained from cattle postmortem, rumen fluid collection for preservation was exempt from oversight of the Tarleton State University Institutional Animal Care and Use Committee.
TG: conceptualization and writing—original draft preparation. WS: methodology, formal analysis, supervision, and project administration. TG and NC: investigation. NC and JM: resources. TG and WS: data curation and funding acquisition. KG, JB, JM, and WS: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
This research was funded, in part, by Tarleton State University's Office of Research and Innovation, Student Research Grant 18105.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This work was completed in partial fulfillment of the requirements for the degree of Master of Science at Tarleton State University.
ANKOM Technology (2017). In vitro True Digestibility Using the DaisyII Incubator. Available online at: https://www.ankom.com/sites/default/files/document-files/Method_3_Invitro_D200_D200I.pdf (accessed February 28, 2018).
AOAC (2000). Official Methods of Analysis of the Association of Official Analytical Chemists International, 17th ed. Gaithersburg, MD: AOAC International.
Barnes, R. E. (1967). Collaborative in vitro rumen fermentation studies on forage substrates. J. Anim. Sci. 26, 1120–1130. doi: 10.2527/jas1967.2651120x
Bezeau, L. M. (1965). Effect of source of inoculum on digestibility of substrate in in vitro digestion trials. J. Anim. Sci. 24, 823–825. doi: 10.2527/jas1965.243823x
Can, A., Hummel, J., Mobashar, M., Boeser, U., and Sudekum, K. H. (2009). Comparison of sheep ruminal fluid with sheep and horse faeces as inoculum for in vitro gas production measurements. J. Appl. Anim. Res. 35, 143–148. doi: 10.1080/09712119.2009.9707004
Church, D. C., and Peterson, R. G. (1960). Effect of several variables on in vitro rumen fermentation. J. Dairy Sci. 43, 81–92. doi: 10.3168/jds.S0022-0302(60)90114-4
Del Bianco Benedeti, P., Paulino, P. V. R., Marcondes, M. I., Maciel, I. F. S., da Silva, M. C., and Faciola, A. P. (2016). Partial replacement of ground corn with glycerol in beef cattle diets: Intake, digestibility, performance, and carcass characteristics. PLoS ONE 11:e0148224. doi: 10.1371/journal.pone.0148224
Denek, N., and Can, A. (2007). Use of faecal fluid for dry matter digestibility of ruminant feeds. J. Appl. Anim. Res. 31, 29–32. doi: 10.1080/09712119.2007.9706624
Denek, N., Can, A., and Avci, M. (2010). Frozen rumen fluid as microbial inoculum in the two-stage in vitro digestibility assay of ruminant feeds. S. Afr. J. Anim. Sci. 40, 251–256.
Denek, N., Can, A., and Koncagual, S. (2006). Usage of slaughtered animal rumen fluid for dry matter digestibility of ruminant feeds. J. Anim. Vet. Advan. 5, 459–461.
DePeters, E. J., and George, L. W. (2014). Rumen transfaunation. Immunol. Let. 162, 69–76. doi: 10.1016/j.imlet.2014.05.009
Ferraro, S. M., Mendoza, G. D., Miranda, L. A., and Gutiérrez, C. G. (2009). In vitro gas production and ruminal fermentation of glycerol, propylene glycol and molasses. Anim. Feed Sci. Tech. 154, 112–118. doi: 10.1016/j.anifeedsci.2009.07.009
Grant, R. J., Van Soest, P. J., and McDowell, R. E. (1974). Influence of rumen fluid source and fermentation time on in vitro true dry matter digestibility. J. Dairy Sci. 57, 1201–1205. doi: 10.3168/jds.S0022-0302(74)85037-X
Holden, L. A. (1999). Comparison of methods of in vitro dry matter digestibility for ten feeds. J. Dairy Sci. 82, 1791–1794. doi: 10.3168/jds.S0022-0302(99)75409-3
Hsu, J. T., and Fahey, G. C. (1990). Effects of centrifugation speed and freezing on composition of ruminal bacterial samples collected from defaunated sheep. J. Dairy Sci. 73, 149–152. doi: 10.3168/jds.S0022-0302(90)78658-4
Luchini, N. D., Broderick, G. A., and Combs, D. K. (1996a). In vitro determination of ruminal protein degradation using freeze-stored ruminal microorganism. J. Anim. Sci. 74, 2488–2499. doi: 10.2527/1996.74102488x
Luchini, N. D., Broderick, G. A., and Combs, D. K. (1996b). Preservation of ruminal microorganisms for in vitro determination of ruminal protein degradation. J. Anim. Sci. 74, 1134–1143. doi: 10.2527/1996.7451134x
Marinucci, M. T., Dehority, B. A., and Loerch, S. C. (1992). In vitro and in vivo studies of factors affecting digestion of feeds in synthetic fiber bags. J. Anim. Sci. 70, 296–307. doi: 10.2527/1992.701296x
Mohamed, R., Chaudry, A. S., and Rowlinson, P. (2002). Fresh or frozen rumen contents as sources of inocula to estimate in vitro degradation of ruminant feeds. Proc. Br. Soc. Anim. Sci. 164, 265–273. doi: 10.1017/S1752756200008206
Mould, F. L., Kliem, K. E., Morgan, R., and Mauricio, R. M. (2005). In vitro microbial inoculum: a review of its function and properties. Anim. Feed Sci. Technol. 123, 31–50. doi: 10.1016/j.anifeedsci.2005.04.028
Nocek, J. E., Cummins, K. A., and Polan, C. E. (1979). Ruminal disappearance of crude protein and dry matter in feeds and combined effects in formulated rations. J. Dairy Sci. 62, 1587–1598. doi: 10.3168/jds.S0022-0302(79)83466-9
Nsabimana, E., Kišidayov,á, S., Macheboeuf, D., Newbold, C. J., and Jouany, J. P. (2003). Two-step freezing procedure for cryopreservation of rumen ciliates an effective tool for creation of a rumen protozoa bank. Appl. Environ. Microbiol. 69, 3826–3832. doi: 10.1128/AEM.69.7.3826-3832.2003
Rota, A., Milani, C., Cabianca, G., and Martini, M. (2006). Comparison between glycerol and ethylene glycol for dog semen cryopreservation. Theriogenology 65, 1848–1858. doi: 10.1016/j.theriogenology.2005.10.015
Tada, N., Sato, M., Yamanoi, J., Mizorogi, T., Kasai, K., and Ogawa, S. (1990). Cryopreservation of mouse spermatozoa in the presence of raffinose and glycerol. J. Reprod. Fert. 89, 511–516. doi: 10.1530/jrf.0.0890511
Keywords: rumen content, in vitro digestibility, inoculum, preservation, preserved rumen fluid, transfaunation
Citation: Garcia TJ, Cherry NM, Guay KA, Brady JA, Muir JP and Smith WB (2021) Preservation Method of Rumen Fluid Collected From Harvested Cattle Alters in vitro Digestibility of Reference Feedstuffs. Front. Anim. Sci. 2:775345. doi: 10.3389/fanim.2021.775345
Received: 13 September 2021; Accepted: 30 November 2021;
Published: 17 December 2021.
Edited by:
Uchenna Anele, North Carolina Agricultural and Technical State University, United StatesReviewed by:
Peter Erickson, University of New Hampshire, United StatesCopyright © 2021 Garcia, Cherry, Guay, Brady, Muir and Smith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: William B. Smith, d2JzMDAwMUBhdWJ1cm4uZWR1
†Present address: Taylor J. Garcia, Department of Animal and Food Sciences, Oklahoma State University, Stillwater, OK, United States
William B. Smith, Department of Animal Sciences, Auburn University, Auburn, AL, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.