- Department of Animal and Dairy Sciences, University of Wisconsin, Madison, WI, United States
Farm animals routinely undergo painful husbandry procedures early in life, including disbudding and castration in calves and goat kids, tail docking and castration in piglets and lambs, and beak trimming in chicks. In rodents, inflammatory events soon after birth, when physiological systems are developing and sensitive to perturbation, can profoundly alter phenotypic outcomes later in life. This review summarizes the current state of research on long-term phenotypic consequences of neonatal painful procedures in rodents and farm animals, and discusses the implications for farm animal welfare. Rodents exposed to early life inflammation show a hypo-/hyper-responsive profile to pain-, fear-, and anxiety-inducing stimuli, manifesting as an initial attenuation in responses that transitions into hyperresponsivity with increasing age or cumulative stress. Neonatal inflammation also predisposes rodents to cognitive, social, and reproductive deficits, and there is some evidence that adverse effects may be passed to offspring. The outcomes of neonatal inflammation are modulated by injury etiology, age at the time of injury and time of testing, sex, pain management, and rearing environment. Equivalent research examining long-term phenotypic consequences of early life painful procedures in farm animals is greatly lacking, despite obvious implications for welfare and performance. Improved understanding of how these procedures shape phenotypes will inform efforts to mitigate negative outcomes through reduction, replacement, and refinement of current practices.
Introduction
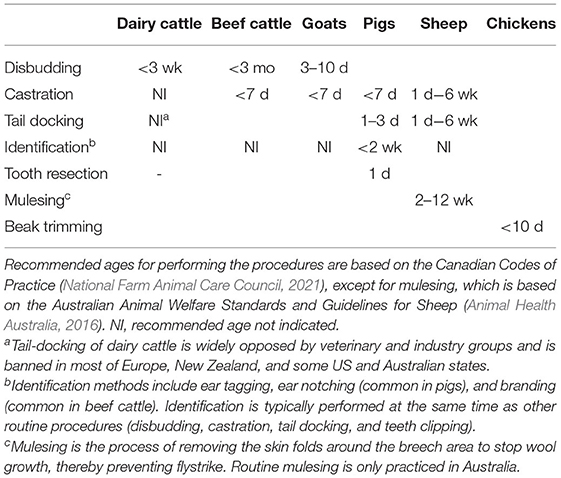
Animals in intensive farming systems routinely undergo painful procedures in early life that involve some form of anatomical modification. These procedures are often done to prevent an animal welfare issue (e.g., beak trimming to reduce injurious pecking in laying hens), but are themselves harmful due to the pain, inflammation, and mutilation they cause. Painful procedures are among the most emotive of public concerns about animal welfare, and are subject to increasing voluntary standards or legislation depending on country (Phillips et al., 2009; Lundmark et al., 2013). Many of these regulations recommend or mandate that mutilations are performed at the youngest age possible, in many cases within the 1st week of life, to minimize tissue damage and improve handling ease (Table 1). For example, removing the horn buds on calves or goat kids when they are free-floating under the skin (disbudding) is less invasive than removing horns that have attached to the skull (dehorning; Stafford and Mellor, 2011).
Although regulations often include analgesic provisions for certain procedures in mammals (i.e., disbudding, castration, tail docking, and mulesing), many only require pain control above a certain age (e.g., Council of Europe, 1988; New Zealand National Animal Welfare Advisory Committee, 2018; Animal Health Australia, 2020), despite empirical evidence that neonates feel pain (Mellor and Stafford, 2004; Mellor and Diesch, 2006). In addition, compliance with voluntary or mandated standards may be limited (Cozzi et al., 2015; De Briyne et al., 2016; Winder et al., 2018; Saraceni et al., 2021) and when they are followed, pain often persists far longer than the duration of action of available drugs. Thus, the vast majority of painful procedures are performed with inadequate or no pain relief, affecting many millions of animals each year.
Untreated or undertreated early life pain has been shown to disrupt brain development and corresponding behavioral functions across the life span in humans and rodents (Schwaller and Fitzgerald, 2014; Victoria and Murphy, 2016a,b; Grunau, 2020; Oldenburg et al., 2020; Williams and Lascelles, 2020). These long-lasting disruptions are mainly observed when inflammatory injuries occur early in life, when developing physiological systems are highly plastic and sensitive to perturbations, as mature animals treated in a similar manner do not develop these deficits to the same extent (Oldenburg et al., 2020). Thus, early life painful procedures in farm animals may alter the animal's developmental trajectory, with long-lasting behavioral implications. To date, the potential role of early life painful procedures on phenotypic development has been largely overlooked by animal welfare scientists and policy-makers.
The purpose of this narrative review is to summarize the current state of research on the long-term behavioral consequences of early life painful procedures. To this end, I review the literature on rat and mouse models (herein referred to as rodents), for which there is extensive knowledge in this area, and for common farm animals (cattle, pigs, goats, sheep, and chickens), for which few studies exist. I then discuss factors that modulate the relationship, and conclude with implications for animal welfare and priorities for future research.
Long-Term Consequences of Neonatal Inflammation in Rodents
Research on the long-term consequences of neonatal inflammation has been driven, at least in part, by the increasing number of babies admitted to neonatal intensive care units. These babies routinely undergo multiple painful procedures that trigger local inflammation and pain lasting for several hours or days (Anand, 1998; Britto et al., 2014). Inflammation during early human brain development has been associated with an increased risk of developing long-lasting cognitive and motor deficits, schizophrenia, autism, depression, anxiety, attention-deficit disorders, and altered pain sensitivity (Sarkar et al., 2019; Grunau, 2020; Williams and Lascelles, 2020).
Despite a clear relationship between neonatal painful procedures in the intensive care unit and later behavioral function, causality is difficult to determine due to the confounding co-morbidities necessitating this care. Therefore, a variety of rodent models have been developed. These models typically involve subjecting a neonate to an inflammatory injury in the 1st or 2nd weeks of life, which is roughly equivalent to a preterm or term human baby, respectively (Semple et al., 2013). The plantar incision model and repetitive needle prick model have been used to mimic surgical injuries and repeated heel lances, respectively, which are commonly performed in human neonates in intensive care. Other models simulate immune challenges by injecting an inflammatory agent such as carrageenan, complete Freund's adjuvant, or lipopolysaccharide at a single or multiple time points in early life. Following exposure to neonatal inflammation, animals undergo behavioral tests as juveniles (~20–34 days of age), adolescents (~35–59 days of age), or adults (~60 days of age on).
Compared to uninjured animals or animals that are injured later in life, rodents exposed to neonatal inflammation exhibit alterations in pain sensitivity, fear, anxiety, stress coping, spatial cognition, social behavior, and reproductive development that persist into or arise during adulthood and can have repercussions for the next generation. I discuss these consequences in the following subsections.
Although a comprehensive review of the evolutionary mechanism behind these inflammation-induced developmental changes is beyond the scope of this paper, life history theories have proposed that early life stress accelerates the individual's developmental trajectory in ways that confer a survival advantage (Callaghan and Tottenham, 2016). Specifically, it may be adaptive to rely less on the parent and achieve self-sufficiency earlier in an unpredictable or dangerous environment. However, while accelerated maturation may confer benefits in environments where long-term survival is uncertain, there are likely latent costs to this developmental trajectory that may manifest as greater vulnerability to stress-related disorders in later life (Callaghan and Tottenham, 2016).
The proximate mechanisms underlying the long-term phenotypic consequences of early life inflammation are still unclear, but may be due to developmental changes in a variety of neurobiological pathways, including the hypothalamic-pituitary-adrenal axis, central and autonomic nervous systems, immune system, cardiovascular system, sleep and circadian system, oxidative stress, gut microbiome, microglial activity, epigenetics, and brain structure and function. These physiological mechanisms are beyond the scope of this paper and have been reviewed elsewhere (Schwaller and Fitzgerald, 2014; Victoria and Murphy, 2016a,b; Agorastos et al., 2019; Grunau, 2020; Oldenburg et al., 2020; Williams and Lascelles, 2020).
Pain Sensitivity
Persistent alterations in pain sensitivity following early life painful procedures are well-documented [reviewed in Beggs (2015), Victoria and Murphy (2016a), Walker (2019), and Williams and Lascelles (2020)]. This effect manifests as a hypo-/hyper-sensitive profile, in which neonatally-injured animals are less sensitive to brief mechanical or thermal noxious stimulation compared to controls, but more sensitive to severe or persistent injury experienced later in life (Moriarty et al., 2019; Walker, 2019; van den Hoogen et al., 2020). This is consistent with the cumulative stress (multi-hit) hypothesis, which proposes that individuals exposed to early life stress are more susceptible to subsequent stressors (Walker et al., 2009a; Peña et al., 2019; Sarkar et al., 2019). Age may also be a vulnerability factor for hyper-sensitivity, as stress accumulates over an animal's lifetime. In agreement, neonatal inflammation in humans is associated with reduced basal sensitivity in childhood that transitions to enhanced pain sensitivity in adolescence and adulthood (Williams and Lascelles, 2020). While it is generally accepted that early life inflammation is a risk factor for heightened acute pain later in life, its influence on chronic painful conditions remains unknown (Williams and Lascelles, 2020).
Fear
Fear motivates behaviors that occur on exposure to an explicit threat in the animal's immediate environment. Neonatal inflammation effects on fear processing have been assessed using fear conditioning paradigms, which involve measuring an animal's learned association between a foot shock and a particular context (e.g., a room) or cue (e.g., auditory tone). If the animal learns this association, they will respond to the conditioned stimulus (context or cue) with a characteristic freezing posture. Neonatal inflammation attenuated conditioned freezing in adolescence and early adulthood, suggesting an impaired memory for fear stimuli (Davis et al., 2018, 2020, 2021; Xia et al., 2020). This effect appears to reverse with age, as older adults that experienced inflammation early in life showed sustained fear responses to a conditioned cue (Doenni et al., 2017) or context (Tishkina et al., 2016). Similarly, middle-aged rats exposed to repetitive hot-plate stimuli as neonates learned an active avoidance task faster than controls (Bernardi et al., 1986). Cumulative stress may also induce enhanced fear memory, as neonatal rats subjected to repetitive needle pricks in conjunction with maternal separation, a potent early life stressor, froze more to a conditioned stimulus in adolescence compared to undisturbed controls (Davis and Burman, 2020).
This finding—an initial attenuation in fear behavior following neonatal inflammation that is reversed by age and/or cumulative stress—parallels the hypo-/hyper-responsive profile observed for pain sensitivity discussed above. Alternatively, this effect could be due to differences in pain sensitivity to the foot shock. As previously discussed, neonatal inflammation can reduce or heighten future pain sensitivity, which could lead to attenuated or enhanced responses to a conditioned fear stimulus, respectively. However, this does not appear to be the case in studies that tested for this possibility (Bernardi et al., 1986; Tishkina et al., 2016; Doenni et al., 2017).
Anxiety
Anxiety motivates behavioral responses to potential, signaled, or ambiguous threat. Anxiety behaviors in rodents are commonly assessed using the elevated plus/zero maze and open field tests. These tests are based on rodents' aversion to open and elevated spaces, with animals that spend less time on the open sections of an elevated maze, or in the center of an open field, interpreted as being more anxious. Neonatal inflammation in the 1st postnatal week decreased anxiety in juvenile and adolescent rodents (Anseloni et al., 2005; Rico et al., 2010; Lima et al., 2014; Chen et al., 2016; Bukhari et al., 2018; Zuke et al., 2019). This effect reverses in adulthood, with neonatally-injured rodents exhibiting increased anxiety compared to controls (Anand et al., 1999; Walker et al., 2004, 2009a, 2011, 2012; Roizenblatt et al., 2010; Negrigo et al., 2011; Sominsky et al., 2012b, 2013; Butkevich et al., 2017; Berkiks et al., 2018; Custódio et al., 2018). Some exceptions have been reported, with adult rodents exposed to early life inflammation exhibiting no change (Comim et al., 2016; Bukhari et al., 2018; Ranger et al., 2019; Mooney-Leber and Brummelte, 2020) or a reduction in anxiety behavior (Victoria et al., 2013, 2015).
Intriguingly, these age-dependent effects mirror the hypo-/hyper-responsive profile for pain sensitivity and fear described above. Stress, which accumulates with age, may precipitate anxiety in neonatally-injured animals, a hypothesis which is supported by several findings. For example, a noxious or stressful experience in adulthood unmasked anxiety behavior following early life inflammation (Walker et al., 2009a; Low and Fitzgerald, 2012). Similarly, while neonatal inflammation decreased anxiety in male adult rats, this pattern reversed after food deprivation or induction of periodontal disease (Breivik et al., 2002). The expression of anxiety behavior following neonatal inflammation also depends on the behavioral paradigm used (Tishkina et al., 2016) and whether the animal is from a low- or high-anxiety line (Claypoole et al., 2017). The 1st postnatal week appears to be a critical period for persistent effects in rodents, as injuries incurred in the 2nd week resulted in a transient increase in anxiety that resolved by adulthood (Schellinck et al., 2003; Anseloni et al., 2005; Spencer et al., 2005; Dinel et al., 2014; Doenni et al., 2017). However, few studies have examined whether inflammation incurred beyond the 2nd postnatal week influences anxiety behavior, and it is possible that critical windows of plasticity emerge later in development (Reh et al., 2020).
Stress Coping
The forced swim test is widely used for assessing stress coping strategies in rodents. When rodents are placed in water without an escape route, they adopt either an active coping strategy of climbing and swimming, or a passive strategy of floating, the latter of which has been linked to stress-related disorders like depression and autism (Commons et al., 2017). Neonatal inflammation in male rodents did not alter behavior in the forced swim test during development (Rico et al., 2010; Dinel et al., 2014; Tishkina et al., 2016), but increased passive coping in adulthood (Doosti et al., 2013; Dinel et al., 2014; Comim et al., 2016; Tishkina et al., 2016; Butkevich et al., 2017; Berkiks et al., 2018; Custódio et al., 2018). Only 2 of these studies included females, with different results between species; neonatal inflammation increased passive coping in adult female rats (Doosti et al., 2013) but not mice (Custódio et al., 2018). A few studies have reported more active coping in male and female rats exposed to neonatal carrageenan (Anseloni et al., 2005; Victoria et al., 2013, 2015). However, chronic stress reversed this effect, consistent with the cumulative stress hypothesis (Victoria et al., 2015). Overall, these results suggest that neonatal inflammation may be a risk factor for altered stress coping in adulthood.
Spatial Cognition
Several studies have examined how neonatal inflammation affects spatial cognition using a water maze task, in which the animal is placed in a pool of water and must rely on spatial cues to find a hidden escape platform. Rodents that were exposed to neonatal inflammation learned the location of the platform at the same rate (Williamson and Bilbo, 2014; Henderson et al., 2015; Ranger et al., 2019) or faster (Mooney-Leber and Brummelte, 2020) than controls. However, they were less able to remember the platform's location for long periods of time (Harré et al., 2008; Henderson et al., 2015; Mikhailenko et al., 2015; Nuseir et al., 2015, 2017; Chen et al., 2016; Peng et al., 2019; Ranger et al., 2019; Mooney-Leber and Brummelte, 2020; Butkevich et al., 2021). Long-lasting spatial memory deficits in neonatally-injured rats have also been observed in other behavioral paradigms [radial 8-arm maze: Anand et al. (2007), object-in-place memory test: MacRae et al. (2015b), and context-object discrimination: Bukhari et al. (2018)].
Some exceptions to this pattern have been observed. A few studies reported no effect of neonatal inflammation on spatial memory in a water maze (Schellinck et al., 2003; Stolp et al., 2011; Lee et al., 2016) or Y-maze task (Dinel et al., 2014). Other have found that spatial memory deficits associated with early life inflammation are only revealed after rats are exposed to a second immune challenge in adulthood (Bilbo et al., 2005a,b, 2006), consistent with the cumulative stress hypothesis. Importantly, an immune challenge in adulthood did not impair memory in rats that did not experience neonatal inflammation, demonstrating the role of the early developmental period in modifying adult behavior. In further support of the cumulative stress hypothesis, chronic stress accelerated the onset of memory deficits in adult rats exposed to neonatal carrageenan (Henderson et al., 2015). Overall, there is compelling evidence that neonatal inflammation impairs memory, but not initial acquisition, of a spatial task during development and adulthood, and that this deficit is unmasked or amplified by stressors in later life.
Social Behavior
Early life stressors cause long-lasting social deficits (Spencer, 2017), and painful procedures are no exception. Neonatal inflammation reduced social interactions in male and female rodents during development (MacRae et al., 2015a,b; Doenni et al., 2016; Lee et al., 2016; Burke and Trang, 2017; Kentner et al., 2018) and adulthood (Breivik et al., 2002; Carlezon et al., 2019; Smith et al., 2020), and heightened social reactivity and lowered aggression in adolescent male mice (Granger et al., 2001). Conspecifics directed less contact toward neonatally-injured animals, suggesting that early life inflammation may not necessarily decrease social interest directly, but rather increase the likelihood of social rejection (MacRae et al., 2015a,b). Sex-specific deficits in social discrimination have also been observed; neonatally-injured adult females have a harder time discriminating between a novel and familiar conspecific (Smith et al., 2020), whereas the opposite effect is reported in adult males (Anand et al., 1999).
While these studies show a clear link between early life painful procedures and altered social development, only staged dyadic interactions have been evaluated to date. More ecologically relevant tests that assess how well animals cope in a group are needed to better understand the long-lasting social implications of early life painful procedures.
Reproductive Development
In male rats, lipopolysaccharide injected in early life delays sexual maturation (Mayila et al., 2018) and reduces sperm presence (Walker et al., 2011). In females, neonatal lipopolysaccharide has been reported to both delay (Knox et al., 2009; Walker et al., 2011; Wu et al., 2011) and accelerate (Sominsky et al., 2012a; Kentner et al., 2018) puberty, as indicated by vaginal opening and first estrus. Regardless of whether puberty was delayed or accelerated, however, neonatally-injured females had fewer follicles and greater incidence of irregular estrus cycling compared to saline-treated controls, suggesting lower reproductive capacity (Wu et al., 2011; Sominsky et al., 2012a).
Inflammation during early life can also compromise adult sexual behaviors, with females displaying less receptive behavior and more rejections toward males and receiving fewer mounts (Walker et al., 2011). Neonatally-injured males made fewer mounting attempts toward females (Walker et al., 2011; Mayila et al., 2018) and spent less time investigating a swab laced with the scent of an estrus female (Carlezon et al., 2019).
Intergenerational Effects
Early life painful procedures not only alter behavioral development, but can have long-lasting repercussions for their offspring. Higher mortality and morbidity have been observed in litters born to neonatally-injured mothers (Sominsky et al., 2012a). Female offspring in these litters displayed increased corticosterone at 2 weeks of age, increased catch-up growth and delayed emergence of the first estrus cycle (Sominsky et al., 2012a). Catch-up growth during critical developmental periods increases the long-term risk of diseases, such as obesity, type-2 diabetes and cardiovascular diseases (Singhal, 2017). Poor maternal care may account, at least in part, for the impaired growth and development of female offspring. Indeed, rat dams that experienced neonatal inflammation engage in less maternal care than saline-treated dams (Walker et al., 2012).
Further, pups of parents exposed to neonatal inflammation are more anxious and sensitive to pain compared to control litters (Walker et al., 2012). This effect is partly due to deficits in maternal care, but is also transferred through the paternal line, suggesting that epigenetic mechanisms play a role in the intergenerational transmission of neonatal inflammation-induced phenotypes.
Long-Term Consequences of Early Life Painful Procedures in Farm Animals
Unlike rodent models of neonatal inflammation, painful procedures in young farm animals involve permanent anatomical alterations, including the full removal of horn buds or testes, and partial removal or mutilation of tails, ears, skin, teeth, and beaks within the first few months of life. In the following subsections, I summarize our current understanding of pain associated with these procedures, and the limited research on persistent behavioral changes in later life.
Disbudding
Disbudding, the destruction of horn-growing tissue to prevent horn growth, is routinely practiced on dairy cattle and goat farms, and to a lesser extent in beef herds, to avoid horn injuries to animals and humans and reduce space requirements. Disbudding is performed by applying a heated iron or caustic paste to the horn bud, resulting in a third-degree thermal or chemical burn, respectively. Both methods cause acute behavioral and physiological pain responses in calves and goat kids (Stock et al., 2013; Winder et al., 2017; Herskin and Nielsen, 2018; Hempstead et al., 2020; Reedman et al., 2020). Burns from hot-iron disbudding take 6–13 weeks to re-epithelialize in calves (Adcock and Tucker, 2018a; Adcock et al., 2019) and 5–9 weeks in kids (Alvarez et al., 2019). The wounds remain sensitive to mechanical stimulation throughout this time, if not longer (Adcock and Tucker, 2018a; Alvarez et al., 2019; Casoni et al., 2019). Calves also experience ongoing pain (i.e., pain in the absence of stimulation) for at least 3 weeks after disbudding (Adcock and Tucker, 2020a; Adcock et al., 2020).
Calves increase use of a shelter for at least 3 days after disbudding, which may reflect reduced sociability (Gingerich et al., 2020). Three weeks after disbudding, calves were more likely to prioritize suckling over risk avoidance compared to non-disbudded controls (Adcock and Tucker, 2021). These motivational changes likely arise from the pain and inflammation of still healing wounds. However, it is intriguing that these results mirror the reductions in social interactions and anxiety seen in adolescent rodents exposed to neonatal inflammation. It would be interesting to explore whether these effects persist or how they might evolve beyond the healing period.
A few studies have evaluated behavioral consequences of disbudding after the wounds have healed. Disbudded calves initiated more agonistic body contacts as adults than horned cattle (Knierim et al., 2015; Lutz et al., 2019), although the opposite pattern was reported during a food competition test (Reiche et al., 2020a). Compared to horned cattle, calves disbudded around 2 months of age had lower stress responses to an adrenocorticotropic hormone challenge at 11 months (Reiche et al., 2020b), and spent more time inactive at 9–12 months of age (Reiche et al., 2020a). Disbudding also resulted in sex-dependent changes in exploration of a novel object at 10–11 months of age (Reiche et al., 2020a). These behavioral differences were attributed to the presence/absence of horns, but the possibility that nociceptive input from disbudding may have also influenced development of these behaviors deserves consideration.
Only one study has evaluated whether age at the time of disbudding affects behavior in later life. Heifers disbudded at 3 or 35 days of age had a greater cardiac, but not behavioral, response to vaccine injections at 11 months of age compared to heifers disbudded at 56 days of age (Adcock and Tucker, 2020b). This finding provides preliminary evidence that disbudding at earlier ages may increase pain sensitivity in later life. However, the lack of a non-disbudded control (e.g., naturally polled animal) to serve as a baseline means it cannot be ruled out that heifers disbudded at 56 days of age may have been hyporesponsive to the injections.
Castration
Male farm animals are routinely castrated to reduce aggressive and sexual behavior and to achieve more desirable carcass characteristics. Physical methods include surgically removing the testicles, applying a rubber ring at the base of the scrotum, or crushing the spermatic cords with a clamp. Regardless of the method used, castration causes acute pain in calves, piglets, lambs, and goat kids (Rault et al., 2011; Coetzee, 2013; Stafford, 2017; Graves et al., 2020; Prunier et al., 2020). Surgical incisions take between 4 and 11 weeks to heal in beef calves, and behavioral and physiological changes indicative of pain are present for several weeks, with the most persistent changes seen after the rubber-ring method [reviewed in Adcock and Tucker (2018b)]. Calves castrated in the 1st week of life heal faster, but have more prolonged inflammation and are more sensitive around the wound site compared to calves castrated at 10–11 weeks of age (Norring et al., 2017). Studies of longer-term pain and healing after castration are lacking in pigs, lambs, and goat kids. Neuromas, which are abnormal growths of nerve tissue that may be associated with chronic pain (pain that persists beyond healing), have been reported at the site of castration in horses (Bengtsdotter et al., 2019), but have yet to be confirmed in other species.
Evaluating effects of castration in later life is difficult due to the confounding reduction in testosterone, which regulates the expression of many behaviors (Rault et al., 2011). In addition, castrated males live relatively short lives compared to their species' lifespan, with castrated pigs, sheep, and goats typically slaughtered under 1 year of age, and beef steers by 2 years of age. Surgical castration resulted in lower slaughter weights in pigs compared to immunocastration, a nonsurgical method that consists of a 2-dose administration of anti-gonadotrophin-releasing hormone vaccine (Morgan et al., 2019). Lower slaughter weight may be associated with behavioral differences, but this was not examined. There is some evidence that castrating soon after birth increases sensitivity to noxious stimuli later in life; lambs castrated at 1 day of age had greater pain responses to tail docking 1 month later than those castrated at 10 days of age (McCracken et al., 2010).
Tail Docking
Partial amputation of the tail with a cold knife, cautery knife, or rubber band is routinely performed in piglets and lambs, but for different reasons. Piglets' tails are docked to prevent tail biting, an injurious and undesirable behavior. In lambs, tails are docked to reduce the risk of flystrike, a painful condition, although there is sparse evidence to support this rationale (Sutherland and Tucker, 2011; Orihuela and Ungerfeld, 2019). Tail docking is also performed on some dairy cattle operations because it is thought to improve udder cleanliness. However, this purported benefit has been widely disproven and legislative and non-legislative initiatives have resulted in the practice being discontinued on dairies in many countries (Sutherland and Tucker, 2011).
Tail docking is an acutely painful procedure that may induce chronic pain (Sutherland and Tucker, 2011). Neuromas have been observed in docked tails of lambs (French and Morgan, 1992), piglets (Simonsen et al., 1991; Herskin et al., 2015; Sandercock et al., 2016), and heifers (Eicher et al., 2006). Tail-docked pigs and cattle have long-term sensitivity in the tail stump (Di Giminiani et al., 2017; Troncoso et al., 2018). Further, genes involved in inflammatory and neuropathic pain were upregulated for at least 16 weeks following tail docking in piglets (Sandercock et al., 2019).
Cows that were docked as adults at 18 months of age responded similarly to an adrenocorticotropic hormone challenge at 5 years of age as cows with intact tails (Phipps et al., 1995), but long-term effects of docking young calves has not been evaluated. Ewes that were tail-docked at 3- to 4-d of age showed more behaviors indicative of pain during parturition as adults than undocked ewes (Clark et al., 2014). Further, rams preferred tailed ewes as sexual partners over ewes that were docked at 60 days of age (Orihuela et al., 2018). However, in these studies, potential long-term effects of the tail docking procedure itself cannot be disentangled from effects due to the presence/absence of a full-length tail, which may serve important behavioral functions.
Identification
A variety of methods are used to identify animals or ownership, including tagging, notching, or tattooing ears soon after birth (cattle, sheep, goats, and pigs), or by hot-iron or freeze branding at older ages (cattle). All methods are performed without anesthesia or analgesia across species, despite causing pain of varying severity and duration (Lay et al., 1992a,b,c; Schwartzkopf-Genswein et al., 1997a,b,c; Leslie et al., 2010; Numberger et al., 2016; Lomax et al., 2017). Ear tags may result in local inflammation and severe and persistent ear lesions in sheep (Edwards et al., 2001) and pigs (Bergqvist et al., 2015). Hot-iron cattle brands take longer than 10 weeks to heal and are sensitive during this time (Tucker et al., 2014a,b). Radio frequency identification methods that involve sensors implanted in the skin, peritoneum, or rumen are expected to cause less tissue damage and milder pain than other methods, but studies on this technique are scarce (Prunier et al., 2020). No studies have evaluated whether identification methods influence behaviors later in life.
Tooth Resection
Piglets have 8 needle teeth that may be clipped or ground shortly after birth to limit lesions on siblings and the sow's teats. Tooth resection causes acute behavioral and physiological changes (Sutherland, 2015; Prunier et al., 2020), a temporary reduction in weight gain (Weary and Fraser, 1999), as well as mouth lesions, including hemorrhages, abscesses, pulp cavity openings, and fractures, that have been observed for at least 7 weeks (Hay et al., 2004), with effects most marked after clipping compared to grinding. Piglets spent more time lying and less time suckling, playing, and fighting on the 1st day after teeth clipping compared to intact piglets, but no behavioral changes were observed at later points during lactation (Fu et al., 2019). The possibility of behavioral changes after weaning has not been investigated.
Mulesing
In Australian Merino sheep, the wrinkled, wooly skin around the perineum is commonly removed to reduce the incidence of flystrike. This procedure, called mulesing, results in smooth scar tissue that does not grow wool, thus reducing the collection of urine and fecal matter which attracts blowflies. Physiological and behavioral changes, including hunched standing and reduced feeding and activity, occur in the hours after the procedure (Edwards et al., 2011; Fisher, 2011). Wounds take at least 4 weeks to fully contract (Lomax et al., 2008), and altered gait, growth, and behaviors for up to 3 weeks have been reported (Hemsworth et al., 2009). Behavioral effects beyond the healing period have not been explored.
Beak Trimming
Beak trimming is performed in the egg industry to reduce the incidence of cannibalism and injurious pecking. Trimming was traditionally done by removing the tip of the beak with a heated blade, but this method is increasingly replaced by infrared beak treatment, which consists of applying a non-contact infrared energy source to the beak tissue of day-old chicks, causing the tip to fall off after a couple weeks. Signs of acute pain have been observed after beak trimming in day-old chicks, but there is little evidence for prolonged pain (Gentle, 2011). In older birds, however, behavioral changes consistent with pain, including reduced activity, feeding, and preening, are observed for at least 6 weeks after trimming with a heated blade (Gentle, 2011; Glatz and Underwood, 2021). Neuromas, possible evidence of chronic pain, were observed in beaks trimmed with a heated blade (Breward and Gentle, 1985; Lunam et al., 1996), but were absent in infrared-treated beaks (McKeegan and Philbey, 2012; Struthers et al., 2019). Neuromas eventually resolved in beaks trimmed within the 1st week of life, but persisted in older birds (Glatz and Underwood, 2021). Thus, there is clear evidence that trimming soon after hatching reduces the risk of long-lasting pain compared to trimming at older ages. Whether beak trimming in young birds alters behavioral development is unknown.
Modulating Factors
Injury Etiology
The inflammatory response is triggered by endogenous molecules released by physical trauma (damage-associated molecular patterns; DAMPs) or molecules from foreign microbes (pathogen-associated molecular patterns; PAMPs; Gong et al., 2020). In rodents, neonatal inflammation is modeled by physical trauma (e.g., repetitive needle stick, plantar incision, and formalin injection) or injection of PAMPs (e.g., lipopolysaccharide, complete Freund's adjuvant; Tizard, 2021). These models elicit unique inflammatory pathways with potentially disparate developmental effects (Walker, 2013). Painful procedures in farm animals involve physical trauma, and so provoke a DAMP-mediated inflammatory response, that likely differs depending on the type of injury (e.g., burn vs. hemorhage: Pantalone et al., 2021). Pathogen exposure at the time of injury or later during wound healing, eliciting PAMP-mediated inflammation, is also possible.
The severity or repetitiveness of physical trauma, or dosing protocol of foreign substance, is also important, as sustained or multiple episodes of inflammation have more marked effects on development than a mild or single episode in humans and rodents (Oldenburg et al., 2020). Painful procedures in farm animals are often severe enough to generate prolonged inflammation and even symptoms of chronic pain, as discussed in the previous section. In addition, multiple painful procedures are often done concurrently. For example, piglets routinely undergo tail docking, tooth resection, ear notching/tagging, and castration (if males) on the same day. Thus, in general, the inflammatory response elicited by painful procedures in farm animals is likely much more severe than most rodent models, suggesting that adverse developmental consequences could be more pronounced. In addition, painful procedures in farm animals involve physical modification, which could influence behavioral development independent of inflammation.
Age at Injury
Many physiological systems are functionally immature at birth and are shaped by experiences during critical periods of development, allowing the animal to uniquely adapt to their environment. Thus, individuals are vulnerable during this time to stressors that may permanently alter adult phenotypes. In rodents, the 1st postnatal week is a critical period in which noxious stimulation can affect behavioral development, with fewer and less pronounced effects observed when injuries were incurred in the 2nd week (Walker et al., 2009b). However, the plasticity of physiological systems peaks at different times during development, resulting in a staggered emergence of critical windows of vulnerability beginning in the prenatal period through adolescence (Reh et al., 2020). For example, rats that were injected with lipopolysaccharide at 14 or 21 days, but not at 7 or 28 days, had attenuated fever responses to an immune challenge as adults, suggesting that different aspects of the adult phenotype are affected by injury at distinct critical time points (Spencer et al., 2006).
Farms animals are born or hatched in a more mature developmental state and undergo less neurologic development postnatally compared to humans and rodents (Wood et al., 2003), and thus may be less vulnerable to long-term developmental programming as neonates. However, the few studies conducted in precocial species (e.g., sheep, squid) found that early life painful procedures altered adult phenotypes, suggesting the presence of critical sensitive periods during development (Clark et al., 2014; Howard et al., 2019). Indeed, the importance of the early postnatal environment for adult performance and welfare is increasingly recognized in farm animals (Reynolds and Vonnahme, 2017). As physiological systems peak in plasticity at different time points (Reh et al., 2020), assessing multiple long-term outcomes of painful procedures performed at ages spanning birth through adolescence is needed to understand the timing of critical periods during development and implications for welfare.
Age at Testing
Rodent studies consistently suggest that behavioral outcomes following neonatal inflammation change over the lifespan. Opposite effects are often observed in adolescence and adulthood, reflecting the transition from stress hyporesponsiveness to hyperresponsiveness (e.g., Chen et al., 2016). In other cases, disruptions appear in adolescence and resolve by adulthood, while others manifest only after puberty (e.g., Walker et al., 2004; Fan et al., 2011; Dinel et al., 2014; MacRae et al., 2015b). The mechanisms behind this time-dependent emergence of behavioral effects are unclear, but indicate the importance of including assessment points throughout the lifespan to fully understand the long-term consequences of painful procedures.
Sex
Some, but not all, rodent studies evaluating males and females report sex-specific effects of early life inflammation on behavior later in life. However, it is difficult to draw conclusions about which sex is more susceptible, as effects are often contradictory (Negrigo et al., 2011; Walker et al., 2012; Lee et al., 2016; Smith et al., 2020). Some of this variability could reflect fluctuation in gonadal hormone levels across the estrous cycle, which modulates behavior, and is often cited as a reason for excluding females (Walf and Frye, 2006; Chari et al., 2020). Indeed, the rodent literature on early life inflammation has focused heavily on males, although this is changing as funding agencies increasingly mandate the inclusion of sex as a biological variable in preclinical studies (Mogil, 2020).
Animal production systems are typically female-skewed, as females produce milk and eggs, and more females than males are needed to breed the next generation. Many males are slaughtered at an early age or culled immediately after birth (e.g., unwanted male chicks in the egg industry), and only a small proportion are kept for breeding. Thus, long-term effects of painful procedures may be particularly relevant for female farm animals as they live longer lives, on average, than their male counterparts. Given sex differences observed in rodents, studies should include females whenever possible and use caution when extrapolating results between sexes.
Pain Management
Long-term behavioral consequences following early life inflammation in rodents have been prevented or mitigated by peri-operative analgesic treatment with morphine (Bhutta et al., 2001; Henderson et al., 2015; Victoria et al., 2015), sucrose (Nuseir et al., 2015, 2017), melatonin (Berkiks et al., 2018), indomethacin (Lee et al., 2016), or ketamine (Anand et al., 2007). A sufficient duration of analgesia is critical for modulating long-term effects, as treating intra-operative, but not post-operative pain, still results in behavioral disruptions later in life (Walker et al., 2009b; Burke and Trang, 2017; Ririe et al., 2021).
These findings have important implications for pain management in farm animals, as early life procedures are often done with inadequate or no pain relief. Painful procedures in farm animals typically generate prolonged inflammation and pain, such that analgesic treatment at the time of the procedure may not be enough to mitigate long-term effects. Indeed, local anesthesia and analgesia given at the time of disbudding ameliorates acute pain (Herskin and Nielsen, 2018), but does not abolish behavioral changes and pain sensitivity in the following days and weeks (Adcock and Tucker, 2018a, 2020a, 2021; Casoni et al., 2019; Gingerich et al., 2020). Currently, there are no strategies for managing long-term pain in farm animals, which may reflect under-recognition of the issue, lack of long-acting drugs, and impracticalities of a multiple-dosing protocol. Developing practical and safe analgesic therapies that relieve pain for its entire duration could have welfare benefits that persist throughout the lifespan.
Environment
Maternal care may protect against adverse consequences of early life inflammation (Walker et al., 2003). Rodent dams showed a transient increase in maternal care, spending more time on the nest and nursing, when their pups were exposed to inflammatory pain compared to dams of control litters (Hood et al., 2003; Davis et al., 2020). Pups that received more maternal care in the immediate hours after inflammation were less likely to exhibit social impairments as adults (Hood et al., 2003). However, dams were more likely to neglect their pups on subsequent days, indicating a trade-off to the initial enhancement in maternal care (Hood et al., 2003; Anseloni et al., 2005; Roizenblatt et al., 2010). Environmental enrichment also appears to buffer against social deficits induced by early life inflammation (MacRae et al., 2015b).
The young of farm animals are often raised without maternal contact (e.g., dairy calves, chicks), or are weaned from their mother at an early age (e.g., piglets, lambs, and goat kids). The rearing environment may also be characterized by nutritional stress, due to early weaning or restricted milk/feed allowances. Maternal and social deprivation, nutritional restriction, and early weaning considerably alter brain development, with long-term adverse behavioral consequences (Cantor et al., 2019; Costa et al., 2019). It is possible that these developmental disruptions could mask or exacerbate long-term effects of painful procedures. However, inflammation and social stress may influence development through different mechanisms that do not necessarily exacerbate each other (e.g., Mooney-Leber and Brummelte, 2020), although this requires further study.
Welfare Implications
Evidence from rodents suggests that early life painful procedures predispose animals to heightened pain sensitivity, greater expression of fear, anxiety, and passive coping behaviors, and impaired spatial memory in adulthood, particularly following additional stress exposure. Long-lasting impairments in social behavior and reproductive development are also observed. Discrepancies in this pattern may reflect differences in etiology, age at the time of injury and time of testing, sex, pain management, or rearing environment.
Equivalent research in farm animals is lacking, despite obvious potential consequences for animal welfare and productivity. To date, research shows that early life painful procedures in farm animals produce prolonged pain, which may develop into a chronic state that persists after healing. There is some preliminary evidence that these procedures can influence the animal's developmental trajectory in similar ways as rodents (e.g., McCracken et al., 2010; Clark et al., 2014; Adcock and Tucker, 2020b). However, results can be difficult to interpret due to confounding anatomical modifications or potential chronic pain that accompany these procedures. Studies that compare the long-term effects of performing a procedure at different ages are needed to disassociate effects due to developmental programming from anatomical changes. In addition, studies that compare long-term behavioral and physiological outcomes in animals receiving different pharmacological interventions (e.g., short- vs. long-lasting analgesia) will provide insight into causal mechanisms.
The role of neonatal experiences in shaping adult phenotypes is increasingly recognized in farm animals. A growing body of literature indicates that stress during early life has long-lasting consequences for welfare and performance; however, to date, studies have focused on stressors such as maternal or social deprivation, nutritional restriction, and thermal stress (Roland et al., 2016; Cantor et al., 2019), and the long-term effects of early life painful procedures have not been considered. Painful procedures have been justified on the grounds that their benefits outweigh the costs, the latter of which is typically characterized in terms of acute pain. Re-evaluating these costs and benefits, in light of the long-term effects of early life inflammation in rodents, is critical to making informed decisions that maximize animal welfare. To fully understand their welfare implications, studies on painful procedures should consider behavioral outcomes throughout the lifespan (e.g., pain sensitivity, anxiety, fear, stress coping, cognition, social behavior, and reproductive development, among other outcomes), as well as health and performance correlates.
In the long-term, painful procedures are not socially sustainable, as public concern for farm animal welfare continues to grow (Fernandes et al., 2021). Ultimately, the goal is to phase out painful procedures through the development of improved housing, management, and breeding practices (e.g., Knierim et al., 2015; Scheper et al., 2016; Nicol, 2018; Yunes et al., 2019; Brien et al., 2021; Gascoigne et al., 2021). In the short-term, continued research on strategies to refine current practices is needed, including greater analgesic coverage, modifications to the rearing environment such as increasing maternal and social contact and nutritional allowances, and performing the procedure with the most appropriate method and at an age that is least detrimental to short- and long-term welfare. Providing practical support to farmers through extension efforts will be critical for successfully adopting sustainable practices.
Conclusion
Neonatal painful procedures in rodents have detrimental sensory, affective, cognitive, social, and reproductive effects throughout the lifespan, which may persist into the next generation. Although painful procedures are a key welfare issue in farm animals, most studies have focused on the short-term consequences, and potential long-lasting developmental outcomes of early life inflammation are generally not considered. Future research on the effects of neonatal painful procedures on behavior in later life, as well as the underlying mechanisms and modulating factors, will be critical for generating best practice recommendations that safeguard animal welfare.
Author Contributions
SA conceived the review, performed the literature search, analyzed and interpreted the findings, and wrote the manuscript.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adcock, S. J. J., Cruz, D. M., and Tucker, C. B. (2020). Behavioral changes in calves 11 days after cautery disbudding: effect of local anesthesia. J. Dairy Sci. 103, 8518–8525. doi: 10.3168/jds.2020-18337
Adcock, S. J. J., and Tucker, C. B. (2018a). The effect of disbudding age on healing and pain sensitivity in dairy calves. J. Dairy Sci. 101, 10361–10373. doi: 10.3168/jds.2018-14987
Adcock, S. J. J., and Tucker, C. B. (2018b). “Painful procedures: when and what should we be measuring?,” in Advances in Cattle Welfare, eds C. B. Tucker (Duxford: Woodhead Publishing), 157–198. doi: 10.1016/B978-0-08-100938-3.00008-5
Adcock, S. J. J., and Tucker, C. B. (2020a). Conditioned place preference reveals ongoing pain in calves 3 weeks after disbudding. Sci. Rep. 10:3849. doi: 10.1038/s41598-020-60260-7
Adcock, S. J. J., and Tucker, C. B. (2020b). The effect of early burn injury on sensitivity to future painful stimuli in dairy heifers. PLoS ONE 15:e0233711. doi: 10.1371/journal.pone.0233711
Adcock, S. J. J., and Tucker, C. B. (2021). Injury alters motivational trade-offs in calves during the healing period. Sci. Rep. 11:6888. doi: 10.1038/s41598-021-86313-z
Adcock, S. J. J., Vieira, S. K., Alvarez, L., and Tucker, C. B. (2019). Iron and laterality effects on healing of cautery disbudding wounds in dairy calves. J. Dairy Sci. 102, 10163–10172. doi: 10.3168/jds.2018-16121
Agorastos, A., Pervanidou, P., Chrousos, G. P., and Baker, D. G. (2019). Developmental trajectories of early life stress and trauma: a narrative review on neurobiological aspects beyond stress system dysregulation. Front. Psychiatry 10:118. doi: 10.3389/fpsyt.2019.00118
Alvarez, L., Adcock, S. J. J., and Tucker, C. B. (2019). Sensitivity and wound healing after hot-iron disbudding in goat kids. J. Dairy Sci. 102, 10152–10162. doi: 10.3168/jds.2018-16062
Anand, K. J. S. (1998). Clinical importance of pain and stress in preterm neonates. Neonatology 73, 1–9. doi: 10.1159/000013953
Anand, K. J. S., Coskun, V., Thrivikraman, K. V., Nemeroff, C. B., and Plotsky, P. M. (1999). Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol. Behav. 66, 627–637. doi: 10.1016/S0031-9384(98)00338-2
Anand, K. J. S., Garg, S., Rovnaghi, C. R., Narsinghani, U., Bhutta, A. T., and Hall, R. W. (2007). Ketamine reduces the cell death following inflammatory pain in newborn rat brain. Pediatr. Res. 62, 283–290. doi: 10.1203/PDR.0b013e3180986d2f
Animal Health Australia (2016). Australian Animal Welfare Standards and Guidelines for Sheep. Available online at: http://www.animalwelfarestandards.net.au/files/2011/01/Sheep-Standards-and-Guidelines-for-Endorsed-Jan-2016-061017.pdf (accessed August 13, 2021).
Animal Health Australia (2020). Australian Animal Welfare Standards and Guidelines. Available online at: http://www.animalwelfarestandards.net.au/ (accessed August 13, 2021).
Anseloni, V. C., He, F., Novikova, S. I., Turnbach Robbins, M., Lidow, I. A., Ennis, M., et al. (2005). Alterations in stress-associated behaviors and neurochemical markers in adult rats after neonatal short-lasting local inflammatory insult. Neuroscience 131, 635–645. doi: 10.1016/j.neuroscience.2004.11.039
Beggs, S. (2015). Long-term consequences of neonatal injury. Can. J. Psychiatry 60, 176–180. doi: 10.1177/070674371506000404
Bengtsdotter, E. A., Ekman, S., and Andersen, P. H. (2019). Neuromas at the castration site in geldings. Acta Vet. Scand. 61:43. doi: 10.1186/s13028-019-0479-8
Bergqvist, A.-S., Forsberg, F., Eliasson, C., and Wallenbeck, A. (2015). Individual identification of pigs during rearing and at slaughter using microchips. Livest. Sci. 180, 233–236. doi: 10.1016/j.livsci.2015.06.025
Berkiks, I., Benmhammed, H., Mesfioui, A., Ouichou, A., El hasnaoui, A., Mouden, S., et al. (2018). Postnatal melatonin treatment protects against affective disorders induced by early-life immune stimulation by reducing the microglia cell activation and oxidative stress. Int. J. Neurosci. 128, 495–504. doi: 10.1080/00207454.2017.1398156
Bernardi, M., Genedani, S., and Bertolini, A. (1986). Behavioral activity and active avoidance learning and retention in rats neonatally exposed to painful stimuli. Physiol. Behav. 36, 553–555. doi: 10.1016/0031-9384(86)90330-6
Bhutta, A. T., Rovnaghi, C., Simpson, P. M., Gossett, J. M., Scalzo, F. M., and Anand, K. J. S. (2001). Interactions of inflammatory pain and morphine in infant rats: long-term behavioral effects. Physiol. Behav. 73, 51–58. doi: 10.1016/S0031-9384(01)00432-2
Bilbo, S. D., Biedenkapp, J. C., Der-Avakian, A., Watkins, L. R., Rudy, J. W., and Maier, S. F. (2005a). Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J. Neurosci. 25, 8000. doi: 10.1523/JNEUROSCI.1748-05.2005
Bilbo, S. D., Levkoff, L. H., Mahoney, J. H., Watkins, L. R., Rudy, J. W., and Maier, S. F. (2005b). Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav. Neurosci. 119, 293–301. doi: 10.1037/0735-7044.119.1.293
Bilbo, S. D., Rudy, J. W., Watkins, L. R., and Maier, S. F. (2006). A behavioural characterization of neonatal infection-facilitated memory impairment in adult rats. Behav. Brain Res. 169, 39–47. doi: 10.1016/j.bbr.2005.12.002
Breivik, T., Stephan, M., Brabant, G. E., Straub, R. H., Pabst, R., and von Hörsten, S. (2002). Postnatal lipopolysaccharide-induced illness predisposes to periodontal disease in adulthood. Brain Behav. Immun. 16, 421–438. doi: 10.1006/brbi.2001.0642
Breward, J., and Gentle, M. J. (1985). Neuroma formation and abnormal afferent nerve discharges after partial beak amputation (beak trimming) in poultry. Experientia 41, 1132–1134. doi: 10.1007/BF01951693
Brien, F. D., Walkom, S. F., Swan, A. A., and Brown, D. J. (2021). Substantial genetic gains in reducing breech flystrike and in improving productivity traits are achievable in Merino sheep by using index selection. Anim. Prod. Sci. 61, 345–362. doi: 10.1071/AN20248
Britto, C. D., Rao Pn, S., Nesargi, S., Nair, S., Rao, S., Thilagavathy, T., et al. (2014). PAIN—perception and assessment of painful procedures in the NICU. J. Trop. Pediatr. 60, 422–427. doi: 10.1093/tropej/fmu039
Bukhari, S. H. F., Clark, O. E., and Williamson, L. L. (2018). Maternal high fructose diet and neonatal immune challenge alter offspring anxiety-like behavior and inflammation across the lifespan. Life Sci. 197, 114–121. doi: 10.1016/j.lfs.2018.02.010
Burke, N. N., and Trang, T. (2017). Neonatal injury results in sex-dependent nociceptive hypersensitivity and social behavioral deficits during adolescence, without altering morphine response. J. Pain 18, 1384–1396. doi: 10.1016/j.jpain.2017.07.003
Butkevich, I. P., Mikhailenko, V. A., Vershinina, E. A., Aloisi, A. M., and Barr, G. A. (2017). Long-term effects of chronic buspirone during adolescence reduce the adverse influences of neonatal inflammatory pain and stress on adaptive behavior in adult male rats. Front. Behav. Neurosci. 11:11. doi: 10.3389/fnbeh.2017.00011
Butkevich, I. P., Mikhailenko, V. A., Vershinina, E. A., and Barr, G. A. (2021). The long-term effects of neonatal inflammatory pain on cognitive function and stress hormones depend on the heterogeneity of the adolescent period of development in male and female rats. Front. Behav. Neurosci. 15:150. doi: 10.3389/fnbeh.2021.691578
Callaghan, B. L., and Tottenham, N. (2016). The stress acceleration hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci. 7, 76–81. doi: 10.1016/j.cobeha.2015.11.018
Cantor, M. C., Neave, H. W., and Costa, J. H. C. (2019). Current perspectives on the short- and long-term effects of conventional dairy calf raising systems: a comparison with the natural environment. Transl. Anim. Sci. 3, 549–563. doi: 10.1093/tas/txy144
Carlezon, W. A., Kim, W., Missig, G., Finger, B. C., Landino, S. M., Alexander, A. J., et al. (2019). Maternal and early postnatal immune activation produce sex-specific effects on autism-like behaviors and neuroimmune function in mice. Sci. Rep. 9:16928. doi: 10.1038/s41598-019-53294-z
Casoni, D., Mirra, A., Suter, M. R., Gutzwiller, A., and Spadavecchia, C. (2019). Can disbudding of calves (1 vs. 4 weeks of age) induce chronic pain? Physiol. Behav. 199, 47–55. doi: 10.1016/j.physbeh.2018.11.010
Chari, T., Griswold, S., Andrews, N. A., and Fagiolini, M. (2020). The stage of the estrus cycle is critical for interpretation of female mouse social interaction behavior. Front. Behav. Neurosci. 14:113. doi: 10.3389/fnbeh.2020.00113
Chen, M., Xia, D., Min, C., Zhao, X., Chen, Y., Liu, L., et al. (2016). Neonatal repetitive pain in rats leads to impaired spatial learning and dysregulated hypothalamic-pituitary-adrenal axis function in later life. Sci. Rep. 6:39159. doi: 10.1038/srep39159
Clark, C., Murrell, J., Fernyhough, M., O'Rourke, T., and Mendl, M. (2014). Long-term and trans-generational effects of neonatal experience on sheep behaviour. Biol. Lett. 10:20140273. doi: 10.1098/rsbl.2014.0273
Claypoole, L. D., Zimmerberg, B., and Williamson, L. L. (2017). Neonatal lipopolysaccharide treatment alters hippocampal neuroinflammation, microglia morphology and anxiety-like behavior in rats selectively bred for an infantile trait. Brain Behav. Immun. 59, 135–146. doi: 10.1016/j.bbi.2016.08.017
Coetzee, J. F. (2013). Assessment and management of pain associated with castration in cattle. Vet. Clin. North Am. Food Anim. Pract. 29, 75–101. doi: 10.1016/j.cvfa.2012.11.002
Comim, C. M., Bussmann, R. M., Simão, S. R., Ventura, L., Freiberger, V., Patrício, J. J., et al. (2016). Experimental neonatal sepsis causes long-term cognitive impairment. Mol. Neurobiol. 53, 5928–5934. doi: 10.1007/s12035-015-9495-5
Commons, K. G., Cholanians, A. B., Babb, J. A., and Ehlinger, D. G. (2017). The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem. Neurosci. 8, 955–960. doi: 10.1021/acschemneuro.7b00042
Costa, J. H. C., Cantor, M. C., Adderley, N. A., and Neave, H. W. (2019). Key animal welfare issues in commercially raised dairy calves: Social environment, nutrition, and painful procedures. Can. J. Anim. Sci. 99, 649–660. doi: 10.1139/cjas-2019-0031
Council of Europe (1988). Recommendation Concerning Cattle. Available online at: http://www.coe.int/t/e/legal_affairs/legal_co-operation/biological_safety_and_use_of_animals/farming/Rec%20cattle%20E.asp (accessed September 5 2016).
Cozzi, G., Gottardo, F., Brscic, M., Contiero, B., Irrgang, N., Knierim, U., et al. (2015). Dehorning of cattle in the EU Member States: a quantitative survey of the current practices. Livest. Sci. 179, 4–11. doi: 10.1016/j.livsci.2015.05.011
Custódio, C. S., Mello, B. S. F., Filho, A. J. M. C., de Carvalho Lima, C. N., Cordeiro, R. C., Miyajima, F., et al. (2018). Neonatal immune challenge with lipopolysaccharide triggers long-lasting sex- and age-related behavioral and immune/neurotrophic alterations in mice: relevance to autism spectrum disorders. Mol. Neurobiol. 55, 3775–3788. doi: 10.1007/s12035-017-0616-1
Davis, S. M., and Burman, M. A. (2020). Maternal separation with neonatal pain influences later-life fear conditioning and somatosenation in male and female rats. Stress 77, 1–10. doi: 10.1080/10253890.2020.1825674
Davis, S. M., Rice, M., and Burman, M. A. (2020). Inflammatory neonatal pain disrupts maternal behavior and subsequent fear conditioning in a rodent model. Dev. Psychobiol. 62, 88–98. doi: 10.1002/dev.21889
Davis, S. M., Rice, M., Rudlong, J., Eaton, V., King, T., and Burman, M. A. (2018). Neonatal pain and stress disrupts later-life pavlovian fear conditioning and sensory function in rats: evidence for a two-hit model. Dev. Psychobiol. 60, 520–533. doi: 10.1002/dev.21632
Davis, S. M., Zuke, J. T., Berchulski, M. R., and Burman, M. A. (2021). Amygdalar corticotropin-releasing factor signaling is required for later-life behavioral dysfunction following neonatal pain. Front. Physiol. 12:660792. doi: 10.3389/fphys.2021.660792
De Briyne, N., Berg, C., Blaha, T., and Temple, D. (2016). Pig castration: will the EU manage to ban pig castration by 2018? Porc. Health Manag. 2:29. doi: 10.1186/s40813-016-0046-x
Di Giminiani, P., Edwards, S. A., Malcolm, E. M., Leach, M. C., Herskin, M. S., and Sandercock, D. A. (2017). Characterization of short- and long-term mechanical sensitisation following surgical tail amputation in pigs. Sci. Rep. 7:4827. doi: 10.1038/s41598-017-05404-y
Dinel, A. L., Joffre, C., Trifilieff, P., Aubert, A., Foury, A., Le Ruyet, P., et al. (2014). Inflammation early in life is a vulnerability factor for emotional behavior at adolescence and for lipopolysaccharide-induced spatial memory and neurogenesis alteration at adulthood. J. Neuroinflammation 11:155. doi: 10.1186/s12974-014-0155-x
Doenni, V. M., Gray, J. M., Song, C. M., Patel, S., Hill, M. N., and Pittman, Q. J. (2016). Deficient adolescent social behavior following early-life inflammation is ameliorated by augmentation of anandamide signaling. Brain Behav. Immun. 58, 237–247. doi: 10.1016/j.bbi.2016.07.152
Doenni, V. M., Song, C. M., Hill, M. N., and Pittman, Q. J. (2017). Early-life inflammation with LPS delays fear extinction in adult rodents. Brain Behav. Immun. 63, 176–185. doi: 10.1016/j.bbi.2016.11.022
Doosti, M.-H., Bakhtiari, A., Zare, P., Amani, M., Majidi-Zolbanin, N., Babri, S., et al. (2013). Impacts of early intervention with fluoxetine following early neonatal immune activation on depression-like behaviors and body weight in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 43, 55–65. doi: 10.1016/j.pnpbp.2012.12.003
Edwards, D. S., Johnston, A. M., and Pfeiffer, D. U. (2001). A comparison of commonly used ear tags on the ear damage of sheep. Anim. Welf. 10, 141–151.
Edwards, L. E., Arnold, N. A., Butler, K. L., and Hemsworth, P. H. (2011). Acute effects of mulesing and alternative procedures to mulesing on lamb behaviour. Appl. Anim. Behav. Sci. 133, 169–174. doi: 10.1016/j.applanim.2011.05.014
Eicher, S. D., Cheng, H. W., Sorrells, A. D., and Schutz, M. M. (2006). Short communication: behavioral and physiological indicators of sensitivity or chronic pain following tail docking. J. Dairy Sci. 89, 3047–3051. doi: 10.3168/jds.S0022-0302(06)72578-4
Fan, L.-W., Tien, L.-T., Zheng, B., Pang, Y., Lin, R. C. S., Simpson, K. L., et al. (2011). Dopaminergic neuronal injury in the adult rat brain following neonatal exposure to lipopolysaccharide and the silent neurotoxicity. Brain Behav. Immun. 25, 286–297. doi: 10.1016/j.bbi.2010.09.020
Fernandes, J. N., Hemsworth, P. H., Coleman, G. J., and Tilbrook, A. J. (2021). Costs and benefits of improving farm animal welfare. Agriculture 11:104. doi: 10.3390/agriculture11020104
Fisher, A. D. (2011). Addressing pain caused by mulesing in sheep. Appl. Anim. Behav. Sci. 135, 232–240. doi: 10.1016/j.applanim.2011.10.019
French, N. P., and Morgan, K. L. (1992). Neuromata in docked lambs' tails. Res. Vet. Sci. 52, 389–390. doi: 10.1016/0034-5288(92)90045-4
Fu, L.-,l., Zhou, B., Li, H.-,z., Liang, T.-,t., Chu, Q.-,p., Schinckel, A. P., et al. (2019). Effects of tail docking and/or teeth clipping on behavior, lesions, and physiological indicators of sows and their piglets. Anim. Sci. J. 90, 1320–1332. doi: 10.1111/asj.13275
Gascoigne, E., Mouland, C., and Lovatt, F. (2021). Considering the 3Rs for castration and tail docking in sheep. Practice 43, 152–162. doi: 10.1002/inpr.29
Gentle, M. J. (2011). Pain issues in poultry. Appl. Anim. Behav. Sci. 135, 252–258. doi: 10.1016/j.applanim.2011.10.023
Gingerich, K. N., Choulet, V., and Miller-Cushon, E. K. (2020). Disbudding affects use of a shelter provided to group-housed dairy calves. J. Dairy Sci. 103, 10519–10529. doi: 10.3168/jds.2020-18267
Glatz, P. C., and Underwood, G. (2021). Current methods and techniques of beak trimming laying hens, welfare issues and alternative approaches. Anim. Prod. Sci. 61, 968–989. doi: 10.1071/AN19673
Gong, T., Liu, L., Jiang, W., and Zhou, R. (2020). DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 20, 95–112. doi: 10.1038/s41577-019-0215-7
Granger, D. A., Hood, K. E., Dreschel, N. A., Sergeant, E., and Likos, A. (2001). Developmental effects of early immune stress on aggressive, socially reactive, and inhibited behaviors. Dev. Psychopathol. 13, 599–610. doi: 10.1017/S0954579401003108
Graves, M. T., Schneider, L., Cox, S., Caldwell, M., Krawczel, P., Lee, A., et al. (2020). Evaluation of the pharmacokinetics and efficacy of transdermal flunixin for pain mitigation following castration in goats. Transl. Anim. Sci. 4:txaa198. doi: 10.1093/tas/txaa198
Grunau, R. E. (2020). Personal perspectives: infant pain—a multidisciplinary journey. Paediatr. Neonatal Pain 2, 50–57. doi: 10.1002/pne2.12017
Harré, E. M., Galic, M. A., Mouihate, A., Noorbakhsh, F., and Pittman, Q. J. (2008). Neonatal inflammation produces selective behavioural deficits and alters N-methyl-d-aspartate receptor subunit mRNA in the adult rat brain. Eur. J. Neurosci. 27, 644–653. doi: 10.1111/j.1460-9568.2008.06031.x
Hay, M., Rue, J., Sansac, C., Brunel, G., and Prunier, A. (2004). Long-term detrimental effects of tooth clipping or grinding in piglets: a histological approach. Anim. Welf. 13, 27–32.
Hempstead, M. N., Waas, J. R., Stewart, M., and Sutherland, M. A. (2020). Goat kids are not small calves: species comparisons in relation to disbudding. Anim. Welf. 29, 293–312. doi: 10.7120/09627286.29.3.293
Hemsworth, P. H., Barnett, J. L., Karlen, G. M., Fisher, A. D., Butler, K. L., and Arnold, N. A. (2009). Effects of mulesing and alternative procedures to mulesing on the behaviour and physiology of lambs. Appl. Anim. Behav. Sci. 117, 20–27. doi: 10.1016/j.applanim.2008.12.007
Henderson, Y. O., Victoria, N. C., Inoue, K., Murphy, A. Z., and Parent, M. B. (2015). Early life inflammatory pain induces long-lasting deficits in hippocampal-dependent spatial memory in male and female rats. Neurobiol. Learn. Mem. 118, 30–41. doi: 10.1016/j.nlm.2014.10.010
Herskin, M. S., and Nielsen, B. H. (2018). Welfare effects of the use of a combination of local anesthesia and NSAID for disbudding analgesia in dairy calves-reviewed across different welfare concerns. Front. Vet. Sci. 5:117. doi: 10.3389/fvets.2018.00117
Herskin, M. S., Thodberg, K., and Jensen, H. E. (2015). Effects of tail docking and docking length on neuroanatomical changes in healed tail tips of pigs. Animal 9, 677–681. doi: 10.1017/S1751731114002857
Hood, K. E., Dreschel, N. A., and Granger, D. A. (2003). Maternal behavior changes after immune challenge of neonates with developmental effects on adult social behavior. Dev. Psychobiol. 42, 17–34. doi: 10.1002/dev.10076
Howard, R. B., Lopes, L. N., Lardie, C. R., Perez, P. P., and Crook, R. J. (2019). Early-life injury produces lifelong neural hyperexcitability, cognitive deficit and altered defensive behaviour in the squid Euprymna scolopes. Philos. Trans. R. Soc. B 374:20190281. doi: 10.1098/rstb.2019.0281
Kentner, A. C., Scalia, S., Shin, J., Migliore, M. M., and Rondon-Ortiz, A. N. (2018). Targeted sensory enrichment interventions protect against behavioral and neuroendocrine consequences of early life stress. Psychoneuroendocrinology 98, 74–85. doi: 10.1016/j.psyneuen.2018.07.029
Knierim, U., Irrgang, N., and Roth, B. A. (2015). To be or not to be horned - consequences in cattle. Livest. Sci. 179, 29–37. doi: 10.1016/j.livsci.2015.05.014
Knox, A. M. I., Li, X. F., Kinsey-Jones, J. S., Wilkinson, E. S., Wu, X. Q., Cheng, Y. S., et al. (2009). Neonatal lipopolysaccharide exposure delays puberty and alters hypothalamic Kiss1 and Kiss1r mRNA expression in the female rat. J. Neuroendocrinol. 21, 683–689. doi: 10.1111/j.1365-2826.2009.01885.x
Lay, D., Friend, T., Randel, R., Bowers, C., Grissom, K., and Jenkins, O. (1992a). Behavioral and physiological effects of freeze or hot-iron branding on crossbred cattle. J. Anim. Sci. 70, 330–336. doi: 10.2527/1992.702330x
Lay, D. C., Friend, T. H., Bowers, C. L., Grissom, K. K., and Jenkins, O. C. (1992b). A comparative physiological and behavioral study of freeze and hot-iron branding using dairy cows. J. Anim. Sci. 70, 1121–1125. doi: 10.2527/1992.7041121x
Lay, D. C., Friend, T. H., Grissom, K. K., Bowers, C. L., and Mal, M. E. (1992c). Effects of freeze or hot-iron branding of Angus calves on some physiological and behavioral indicators of stress. Appl. Anim. Behav. Sci. 33, 137–147. doi: 10.1016/S0168-1591(05)80003-6
Lee, J. H., Espinera, A. R., Chen, D., Choi, K.-E., Caslin, A. Y., Won, S., et al. (2016). Neonatal inflammatory pain and systemic inflammatory responses as possible environmental factors in the development of autism spectrum disorder of juvenile rats. J. Neuroinflammation 13:109. doi: 10.1186/s12974-016-0575-x
Leslie, E., Hernández-Jover, M., Newman, R., and Holyoake, P. (2010). Assessment of acute pain experienced by piglets from ear tagging, ear notching and intraperitoneal injectable transponders. Appl. Anim. Behav. Sci. 127, 86–95. doi: 10.1016/j.applanim.2010.09.006
Lima, M., Malheiros, J., Negrigo, A., Tescarollo, F., Medeiros, M., Suchecki, D., et al. (2014). Sex-related long-term behavioral and hippocampal cellular alterations after nociceptive stimulation throughout postnatal development in rats. Neuropharmacology 77, 268–276. doi: 10.1016/j.neuropharm.2013.10.007
Lomax, S., Sheil, M., and Windsor, P. A. (2008). Impact of topical anaesthesia on pain alleviation and wound healing in lambs after mulesing. Aust. Vet. J. 86, 159–168. doi: 10.1111/j.1751-0813.2008.00285.x
Lomax, S., Witenden, E., Windsor, P., and White, P. (2017). Effect of topical vapocoolant spray on perioperative pain response of unweaned calves to ear tagging and ear notching. Vet. Anaesth. Analg. 44, 163–172. doi: 10.1111/vaa.12384
Low, L. A., and Fitzgerald, M. (2012). Acute pain and a motivational pathway in adult rats: Influence of early life pain experience. PLoS ONE 7:e34316. doi: 10.1371/journal.pone.0034316
Lunam, C. A., Glatz, P. C., and Hsu, Y. J. (1996). The absence of neuromas in beaks of adult hens after conservative trimming at hatch. Aust. Vet. J. 74, 46–49. doi: 10.1111/j.1751-0813.1996.tb13734.x
Lundmark, F., Berg, C., and Röcklinsberg, H. (2013). “‘Unnecessary suffering’ as a concept in animal welfare legislation and standards,” in The Ethics of Consumption: The Citizen, the Market and the Law, eds H. Röcklinsberg and P. Sandin (Wageningen: Wageningen Academic Publishers), 114–119. doi: 10.3920/978-90-8686-784-4_18
Lutz, J., Burla, J.-B., Gygax, L., Wechsler, B., Würbel, H., and Friedli, K. (2019). Horned and dehorned dairy cows differ in the pattern of agonistic interactions investigated under different space allowances. Appl. Anim. Behav. Sci. 218:104819. doi: 10.1016/j.applanim.2019.05.008
MacRae, M., Kenkel, W. M., and Kentner, A. C. (2015a). Social rejection following neonatal inflammation is mediated by olfactory scent cues. Brain Behav. Immun. 49, 43–48. doi: 10.1016/j.bbi.2015.02.026
MacRae, M., Macrina, T., Khoury, A., Migliore, M. M., and Kentner, A. C. (2015b). Tracing the trajectory of behavioral impairments and oxidative stress in an animal model of neonatal inflammation. Neuroscience 298, 455–466. doi: 10.1016/j.neuroscience.2015.04.048
Mayila, Y., Matsuzaki, T., Iwasa, T., Tungalagsuvd, A., Munkhzaya, M., Yano, K., et al. (2018). The reduction in sexual behavior induced by neonatal immune stress is not related to androgen levels in male rats. Int. J. Dev. Neurosci. 71, 163–171. doi: 10.1016/j.ijdevneu.2018.08.003
McCracken, L., Waran, N., Mitchinson, S., and Johnson, C. B. (2010). Effect of age at castration on behavioural response to subsequent tail docking in lambs. Vet. Anaesth. Analg. 37, 375–381. doi: 10.1111/j.1467-2995.2010.00547.x
McKeegan, D. E. F., and Philbey, A. W. (2012). Chronic neurophysiological and anatomical changes associated with infrared beak treatment and their implications for laying hen welfare. Anim. Welf. 21, 207–217. doi: 10.7120/09627286.21.2.207
Mellor, D. J., and Diesch, T. J. (2006). Onset of sentience: the potential for suffering in fetal and newborn farm animals. Appl. Anim. Behav. Sci. 100, 48–57. doi: 10.1016/j.applanim.2006.04.012
Mellor, D. J., and Stafford, K. J. (2004). Animal welfare implications of neonatal mortality and morbidity in farm animals. Vet. J. 168, 118–133. doi: 10.1016/j.tvjl.2003.08.004
Mikhailenko, V. A., Butkevich, I. P., Vershinina, E. A., and Ulanova, N. A. (2015). Long-term changes in adaptive behavior of rats after inflammatory pain stimulation during neonatal development. J. Evol. Biochem. Physiol. 51, 122–130. doi: 10.1134/S0022093015020052
Mogil, J. S. (2020). Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat. Rev. Neurosci. 21, 353–365. doi: 10.1038/s41583-020-0310-6
Mooney-Leber, S. M., and Brummelte, S. (2020). Neonatal pain and reduced maternal care alter adult behavior and hypothalamic–pituitary–adrenal axis reactivity in a sex-specific manner. Dev. Psychobiol. 62, 631–643. doi: 10.1002/dev.21941
Morgan, L., Itin-Shwartz, B., Koren, L., Meyer, J. S., Matas, D., Younis, A., et al. (2019). Physiological and economic benefits of abandoning invasive surgical procedures and enhancing animal welfare in swine production. Sci. Rep. 9:16093. doi: 10.1038/s41598-019-52677-6
Moriarty, O., Tu, Y., Sengar, A. S., Salter, M. W., Beggs, S., and Walker, S. M. (2019). Priming of adult incision response by early-life injury: neonatal microglial inhibition has persistent but sexually dimorphic effects in adult rats. J. Neurosci. 39, 3081–3093. doi: 10.1523/JNEUROSCI.1786-18.2019
National Farm Animal Care Council (2021). Codes of Practice for the Care and Handling of Farm Animals. Available online at: https://www.nfacc.ca/codes-of-practice (accessed August 12, 2021).
Negrigo, A., Medeiros, M., Guinsburg, R., and Covolan, L. (2011). Long-term gender behavioral vulnerability after nociceptive neonatal formalin stimulation in rats. Neurosci. Lett. 490, 196–199. doi: 10.1016/j.neulet.2010.12.050
New Zealand National Animal Welfare Advisory Committee (2018). Code of Welfare: Painful Husbandry Procedures. Available online at: https://www.mpi.govt.nz/dmsdocument/46045-Code-of-Welfare-Painful-husbandry-procedures (accessed August 13, 2021).
Nicol, C. (2018). “Feather pecking and cannibalism: can we really stop beak trimming?,” in Advances in Poultry Welfare, eds J. A. Mench (Duxford: Woodhead Publishing), 175–197. doi: 10.1016/B978-0-08-100915-4.00009-9
Norring, M., Mintline, E. M., and Tucker, C. B. (2017). The age of surgical castration affects the healing process in beef calves. Transl. Anim. Sci. 1, 358–366. doi: 10.2527/tas2017.0044
Numberger, J., Ritzmann, M., Übel, N., Eddicks, M., Reese, S., and Zöls, S. (2016). Ear tagging in piglets: the cortisol response with and without analgesia in comparison with castration and tail docking. Animal 10, 1864–1870. doi: 10.1017/S1751731116000811
Nuseir, K. Q., Alzoubi, K. H., Alabwaini, J., Khabour, O. F., and Kassab, M. I. (2015). Sucrose-induced analgesia during early life modulates adulthood learning and memory formation. Physiol. Behav. 145, 84–90. doi: 10.1016/j.physbeh.2015.04.002
Nuseir, K. Q., Alzoubi, K. H., Alhusban, A., Bawaane, A., Al-Azzani, M., and Khabour, O. F. (2017). Sucrose and naltrexone prevent increased pain sensitivity and impaired long-term memory induced by repetitive neonatal noxious stimulation: role of BDNF and β-endorphin. Physiol. Behav. 179, 213–219. doi: 10.1016/j.physbeh.2017.06.015
Oldenburg, K. S., O'Shea, T. M., and Fry, R. C. (2020). Genetic and epigenetic factors and early life inflammation as predictors of neurodevelopmental outcomes. Semin. Fetal Neonatal Med. 25:101115. doi: 10.1016/j.siny.2020.101115
Orihuela, A., and Ungerfeld, R. (2019). Tail docking in sheep (Ovis aries): a review on the arguments for and against the procedure, advantages/disadvantages, methods, and new evidence to revisit the topic. Livest. Sci. 230:103837. doi: 10.1016/j.livsci.2019.103837
Orihuela, A., Ungerfeld, R., Fierros-García, A., Pedernera, M., and Aguirre, V. (2018). Rams prefer tailed than docked ewes as sexual partners. Reprod. Domest. Anim. 53, 1473–1477. doi: 10.1111/rda.13287
Pantalone, D., Bergamini, C., Martellucci, J., Alemanno, G., Bruscino, A., Maltinti, G., et al. (2021). The role of DAMPS in burns and hemorrhagic shock immune response: pathophysiology and clinical issues. Review. Int. J. Mol. Sci. 22:7020. doi: 10.3390/ijms22137020
Peña, C. J., Nestler, E. J., and Bagot, R. C. (2019). Environmental programming of susceptibility and resilience to stress in adulthood in male mice. Front. Behav. Neurosci. 13:40. doi: 10.3389/fnbeh.2019.00040
Peng, L., Zhu, M., Yang, Y., Weng, Y., Zou, W., Zhu, X., et al. (2019). Neonatal lipopolysaccharide challenge induces long-lasting spatial cognitive impairment and dysregulation of hippocampal histone acetylation in mice. Neuroscience 398, 76–87. doi: 10.1016/j.neuroscience.2018.12.001
Phillips, C. J. C., Wojciechowska, J., Meng, J., and Cross, N. (2009). Perceptions of the importance of different welfare issues in livestock production. Animal 3, 1152–1166. doi: 10.1017/S1751731109004479
Phipps, A. M., Matthews, L. R., and Verkerk, G. A. (1995). Tail docked dairy cattle: fly induced behaviour and adrenal responsiveness to ACTH. Proc. NZ Soc. Anim. Prod. 55, 61–63.
Prunier, A., Devillers, N., Herskin, M. S., Sandercock, D. A., Sinclair, A. R. L., Tallet, C., et al. (2020). “Husbandry interventions in suckling piglets, painful consequences and mitigation,” in The Suckling and Weaned Piglet, ed C. Farmer (Wageningen: Wageningen Academic Publishers), 107–138. doi: 10.3920/978-90-8686-894-0_4
Ranger, M., Tremblay, S., Chau, C. M. Y., Holsti, L., Grunau, R. E., and Goldowitz, D. (2019). Adverse behavioral changes in adult mice following neonatal repeated exposure to pain and sucrose. Front. Psychol. 9:2394. doi: 10.3389/fpsyg.2018.02394
Rault, J.-L., Lay, D. C., and Marchant-Forde, J. N. (2011). Castration induced pain in pigs and other livestock. Appl. Anim. Behav. Sci. 135, 214–225. doi: 10.1016/j.applanim.2011.10.017
Reedman, C. N., Duffield, T. F., DeVries, T. J., Lissemore, K. D., Karrow, N. A., Li, Z., et al. (2020). Randomized control trial assessing the efficacy of pain control strategies for caustic paste disbudding in dairy calves younger than 9 days of age. J. Dairy Sci. 103, 7339–7350. doi: 10.3168/jds.2019-18118
Reh, R. K., Dias, B. G., Nelson, C. A., Kaufer, D., Werker, J. F., Kolb, B., et al. (2020). Critical period regulation across multiple timescales. Proc. Natl. Acad. Sci. U. S. A. 117:23242. doi: 10.1073/pnas.1820836117
Reiche, A.-M., Dohme-Meier, F., and Claudia Terlouw, E. M. (2020a). Effects of horn status on behaviour in fattening cattle in the field and during reactivity tests. Appl. Anim. Behav. Sci. 231:105081. doi: 10.1016/j.applanim.2020.105081
Reiche, A. M., Hankele, A. K., Hess, H. D., Dohme-Meier, F., and Ulbrich, S. E. (2020b). The ACTH challenge and its repeatability in fattening bulls—influences of physiological state, challenge time standardization, and horn status. Domest. Anim. Endocrinol. 72:106360. doi: 10.1016/j.domaniend.2019.03.001
Reynolds, L. P., and Vonnahme, K. A. (2017). Livestock as models for developmental programming. Animal Frontiers 7, 12–17. doi: 10.2527/af.2017-0123
Rico, J. L. R., Ferraz, D. B., Ramalho-Pinto, F. J., and Morato, S. (2010). Neonatal exposure to LPS leads to heightened exploratory activity in adolescent rats. Behav. Brain Res. 215, 102–109. doi: 10.1016/j.bbr.2010.07.001
Ririe, D. G., Eisenach, J. C., and Martin, T. J. (2021). A painful beginning: early life surgery produces long-term behavioral disruption in the rat. Front. Behav. Neurosci. 15:630889. doi: 10.3389/fnbeh.2021.630889
Roizenblatt, S., Andersen, M. L., Bignotto, M., D'Almeida, V., Martins, P. J. F., and Tufik, S. (2010). Neonatal arthritis disturbs sleep and behaviour of adult rat offspring and their dams. Eur. J. Pain 14, 985–991. doi: 10.1016/j.ejpain.2010.03.008
Roland, L., Drillich, M., Klein-Jöbstl, D., and Iwersen, M. (2016). Invited review: influence of climatic conditions on the development, performance, and health of calves. J. Dairy Sci. 99, 2438–2452. doi: 10.3168/jds.2015-9901
Sandercock, D. A., Barnett, M. W., Coe, J. E., Downing, A. C., Nirmal, A. J., Di Giminiani, P., et al. (2019). Transcriptomics analysis of porcine caudal dorsal root ganglia in tail amputated pigs shows long-term effects on many pain-associated genes. Front. Vet. Sci. 6:314. doi: 10.3389/fvets.2019.00314
Sandercock, D. A., Smith, S. H., Di Giminiani, P., and Edwards, S. A. (2016). Histopathological characterization of tail injury and traumatic neuroma development after tail docking in piglets. J. Comp. Pathol. 155, 40–49. doi: 10.1016/j.jcpa.2016.05.003
Saraceni, J., Winder, C. B., Renaud, D. L., Miltenburg, C., Nelson, E., and Van Os, J. M. C. (2021). Disbudding and dehorning practices for preweaned dairy calves by farmers in Wisconsin, USA. J. Dairy Sci. 104, 11995–12008. doi: 10.3168/jds.2021-20411
Sarkar, T., Patro, N., and Patro, I. K. (2019). Cumulative multiple early life hits- a potent threat leading to neurological disorders. Brain Res. Bull. 147, 58–68. doi: 10.1016/j.brainresbull.2019.02.005
Schellinck, H. M., Stanford, L., and Darrah, M. (2003). Repetitive acute pain in infancy increases anxiety but does not alter spatial learning ability in juvenile mice. Behav. Brain Res. 142, 157–165. doi: 10.1016/S0166-4328(02)00406-0
Scheper, C., Wensch-Dorendorf, M., Yin, T., Dressel, H., Swalve, H., and König, S. (2016). Evaluation of breeding strategies for polledness in dairy cattle using a newly developed simulation framework for quantitative and Mendelian traits. Genet. Sel. Evol. 48:50. doi: 10.1186/s12711-016-0228-7
Schwaller, F., and Fitzgerald, M. (2014). The consequences of pain in early life: Injury-induced plasticity in developing pain pathways. Eur. J. Neurosci. 39, 344–352. doi: 10.1111/ejn.12414
Schwartzkopf-Genswein, K. S., Stookey, J. M., de Passillé, A. M., and Rushen, J. (1997a). Comparison of hot-iron and freeze branding on cortisol levels and pain sensitivity in beef cattle. Can. J. Anim. Sci. 77, 369–374. doi: 10.4141/A96-127
Schwartzkopf-Genswein, K. S., Stookey, J. M., Janzen, E. D., and McKinnon, J. (1997b). Effects of branding on weight gain, antibiotic treatment rates and subsequent handling ease in feedlot cattle. Can. J. Anim. Sci. 77, 361–367. doi: 10.4141/A96-104
Schwartzkopf-Genswein, K. S., Stookey, J. M., and Welford, R. (1997c). Behavior of cattle during hot-iron and freeze branding and the effects of subsequent handling ease. J. Anim. Sci. 75, 2064–2072. doi: 10.2527/1997.7582064x
Semple, B. D., Blomgren, K., Gimlin, K., Ferriero, D. M., and Noble-Haeusslein, L. J. (2013). Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 106–107, 1–16. doi: 10.1016/j.pneurobio.2013.04.001
Simonsen, H. B., Klinken, L., and Bindseil, E. (1991). Histopathology of intact and docked pigtails. Br. Vet. J. 147, 407–412. doi: 10.1016/0007-1935(91)90082-X
Singhal, A. (2017). Long-term adverse effects of early growth acceleration or catch-up growth. Ann. Nutr. Metab. 70, 236–240. doi: 10.1159/000464302
Smith, C. J., Kingsbury, M. A., Dziabis, J. E., Hanamsagar, R., Malacon, K. E., Tran, J. N., et al. (2020). Neonatal immune challenge induces female-specific changes in social behavior and somatostatin cell number. Brain Behav. Immun. 90, 332–345. doi: 10.1016/j.bbi.2020.08.013
Sominsky, L., Fuller, E. A., Bondarenko, E., Ong, L. K., Averell, L., Nalivaiko, E., et al. (2013). Functional programming of the autonomic nervous system by early life immune exposure: Implications for anxiety. PLoS ONE 8:e57700. doi: 10.1371/journal.pone.0057700
Sominsky, L., Meehan, C. L., Walker, A. K., Bobrovskaya, L., McLaughlin, E. A., and Hodgson, D. M. (2012a). Neonatal immune challenge alters reproductive development in the female rat. Horm. Behav. 62, 345–355. doi: 10.1016/j.yhbeh.2012.02.005
Sominsky, L., Walker, A. K., Ong, L. K., Tynan, R. J., Walker, F. R., and Hodgson, D. M. (2012b). Increased microglial activation in the rat brain following neonatal exposure to a bacterial mimetic. Behav. Brain Res. 226, 351–356. doi: 10.1016/j.bbr.2011.08.038
Spencer, K. A. (2017). Developmental stress and social phenotypes: integrating neuroendocrine, behavioural and evolutionary perspectives. Philos. Trans. R. Soc. B 372:20160242. doi: 10.1098/rstb.2016.0242
Spencer, S. J., Heida, J. G., and Pittman, Q. J. (2005). Early life immune challenge—effects on behavioural indices of adult rat fear and anxiety. Behav. Brain Res. 164, 231–238. doi: 10.1016/j.bbr.2005.06.032
Spencer, S. J., Martin, S., Mouihate, A., and Pittman, Q. J. (2006). Early-life immune challenge: defining a critical window for effects on adult responses to immune challenge. Neuropsychopharmacology 31, 1910–1918. doi: 10.1038/sj.npp.1301004
Stafford, K. (2017). “Husbandry procedures,” in Advances in Sheep Welfare, eds D. M. Ferguson, C. Lee, and A. Fisher (Duxford: Woodhead Publishing), 211–226. doi: 10.1016/B978-0-08-100718-1.00011-X
Stafford, K. J., and Mellor, D. J. (2011). Addressing the pain associated with disbudding and dehorning in cattle. Appl. Anim. Behav. Sci. 135, 226–231. doi: 10.1016/j.applanim.2011.10.018
Stock, M. L., Baldridge, S. L., Griffin, D., and Coetzee, J. F. (2013). Bovine dehorning: assessing pain and providing analgesic management. Vet. Clin. North Am. Food Anim. Pract. 29, 103–133. doi: 10.1016/j.cvfa.2012.11.001
Stolp, H. B., Johansson, P. A., Habgood, M. D., Dziegielewska, K. M., Saunders, N. R., and Ek, C. J. (2011). Effects of neonatal systemic inflammation on blood-brain barrier permeability and behaviour in juvenile and adult rats. Cardiovasc. Psychiatry Neurol. 2011:469046. doi: 10.1155/2011/469046
Struthers, S., Gupta, A., Gomis, S., Herwig, E., and Schwean-Lardner, K. (2019). Understanding how infrared beak treatment affects the beak tissue and the healing response of brown and white feathered layer pullets. Animals 9:665. doi: 10.3390/ani9090665
Sutherland, M. A. (2015). Welfare implications of invasive piglet husbandry procedures, methods of alleviation and alternatives: a review. NZ Vet. J. 63, 52–57. doi: 10.1080/00480169.2014.961990
Sutherland, M. A., and Tucker, C. B. (2011). The long and short of it: a review of tail docking in farm animals. Appl. Anim. Behav. Sci. 135, 179–191. doi: 10.1016/j.applanim.2011.10.015
Tishkina, A., Stepanichev, M., Kudryashova, I., Freiman, S., Onufriev, M., Lazareva, N., et al. (2016). Neonatal proinflammatory challenge in male Wistar rats: effects on behavior, synaptic plasticity, and adrenocortical stress response. Behav. Brain Res. 304, 1–10. doi: 10.1016/j.bbr.2016.02.001
Tizard, I. R. (2021). "Adjuvants and adjuvanticity. Vaccines Veterinarians. 2021:75–86.e71. doi: 10.1016/B978-0-323-68299-2.00016-2
Troncoso, R. J., Herzberg, D. E., Meneses, C. S., Müller, H. Y., Werner, M. P., and Bustamante, H. (2018). Mechanical/thermal sensitivity and superficial temperature in the stump of long-term tail-docked dairy cows. PeerJ 6:e5213. doi: 10.7717/peerj.5213
Tucker, C. B., Mintline, E. M., Banuelos, J., Walker, K. A., Hoar, B., Drake, D., et al. (2014a). Effect of a cooling gel on pain sensitivity and healing of hot-iron cattle brands. J. Anim. Sci. 92, 5666–5673. doi: 10.2527/jas.2014-7860
Tucker, C. B., Mintline, E. M., Banuelos, J., Walker, K. A., Hoar, B., Varga, A., et al. (2014b). Pain sensitivity and healing of hot-iron cattle brands. J. Anim. Sci. 92, 5674–5682. doi: 10.2527/jas.2014-7887
van den Hoogen, N. J., Patijn, J., Tibboel, D., and Joosten, E. A. (2020). Repetitive noxious stimuli during early development affect acute and long-term mechanical sensitivity in rats. Pediatr. Res. 87, 26–31. doi: 10.1038/s41390-019-0420-x
Victoria, N. C., Inoue, K., Young, L. J., and Murphy, A. Z. (2013). A single neonatal injury induces life-long deficits in response to stress. Dev. Neurosci. 35, 326–337. doi: 10.1159/000351121
Victoria, N. C., Karom, M. C., and Murphy, A. Z. (2015). Analgesia for early-life pain prevents deficits in adult anxiety and stress in rats. Dev. Neurosci. 37, 1–13. doi: 10.1159/000366273
Victoria, N. C., and Murphy, A. Z. (2016a). Exposure to early life pain: long term consequences and contributing mechanisms. Curr. Opin. Behav. Sci. 7, 61–68. doi: 10.1016/j.cobeha.2015.11.015
Victoria, N. C., and Murphy, A. Z. (2016b). The long-term impact of early life pain on adult responses to anxiety and stress: historical perspectives and empirical evidence. Exp. Neurol. 275, 261–273. doi: 10.1016/j.expneurol.2015.07.017
Walf, A. A., and Frye, C. A. (2006). A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology 31, 1097–1111. doi: 10.1038/sj.npp.1301067
Walker, A. K., Hawkins, G., Sominsky, L., and Hodgson, D. M. (2012). Transgenerational transmission of anxiety induced by neonatal exposure to lipopolysaccharide: implications for male and female germ lines. Psychoneuroendocrinology 37, 1320–1335. doi: 10.1016/j.psyneuen.2012.01.005
Walker, A. K., Hiles, S. A., Sominsky, L., McLaughlin, E. A., and Hodgson, D. M. (2011). Neonatal lipopolysaccharide exposure impairs sexual development and reproductive success in the Wistar rat. Brain Behav. Immun. 25, 674–684. doi: 10.1016/j.bbi.2011.01.004
Walker, A. K., Nakamura, T., Byrne, R. J., Naicker, S., Tynan, R. J., Hunter, M., et al. (2009a). Neonatal lipopolysaccharide and adult stress exposure predisposes rats to anxiety-like behaviour and blunted corticosterone responses: implications for the double-hit hypothesis. Psychoneuroendocrinology 34, 1515–1525. doi: 10.1016/j.psyneuen.2009.05.010
Walker, C.-D., Kudreikis, K., Sherrard, A., and Johnston, C. C. (2003). Repeated neonatal pain influences maternal behavior, but not stress responsiveness in rat offspring. Dev. Brain Res. 140, 253–261. doi: 10.1016/S0165-3806(02)00611-9
Walker, F. R., March, J., and Hodgson, D. M. (2004). Endotoxin exposure in early life alters the development of anxiety-like behaviour in the Fischer 344 rat. Behav. Brain Res. 154, 63–69. doi: 10.1016/j.bbr.2004.01.019
Walker, S. M. (2013). “Long-term effects of early pain and injury: animal models,” in Oxford Textbook of Paediatric Pain, eds P. J. McGrath, B. J. Stevens, S. M. Walker, and W. T. Zempsky (Oxford: Oxford University Press), 20–29. doi: 10.1093/med/9780199642656.003.0003
Walker, S. M. (2019). Long-term effects of neonatal pain. Semin. Fetal Neonatal. Med. 24:101005. doi: 10.1016/j.siny.2019.04.005
Walker, S. M., Tochiki, K. K., and Fitzgerald, M. (2009b). Hindpaw incision in early life increases the hyperalgesic response to repeat surgical injury: critical period and dependence on initial afferent activity. Pain 147, 99–106. doi: 10.1016/j.pain.2009.08.017
Weary, D. M., and Fraser, D. (1999). Partial tooth-clipping of suckling pigs: effects on neonatal competition and facial injuries. Appl. Anim. Behav. Sci. 65, 21–27. doi: 10.1016/S0168-1591(99)00052-0
Williams, M. D., and Lascelles, B. D. X. (2020). Early neonatal pain—a review of clinical and experimental implications on painful conditions later in life. Front. Pediatr. 8:30. doi: 10.3389/fped.2020.00030
Williamson, L. L., and Bilbo, S. D. (2014). Neonatal infection modulates behavioral flexibility and hippocampal activation on a Morris Water Maze task. Physiol. Behav. 129, 152–159. doi: 10.1016/j.physbeh.2014.02.033
Winder, C. B., Bauman, C. A., Duffield, T. F., Barkema, H. W., Keefe, G. P., Dubuc, J., et al. (2018). Canadian National Dairy Study: heifer calf management. J. Dairy Sci. 101, 10565–10579. doi: 10.3168/jds.2018-14680
Winder, C. B., LeBlanc, S. J., Haley, D. B., Lissemore, K. D., Godkin, M. A., and Duffield, T. F. (2017). Clinical trial of local anesthetic protocols for acute pain associated with caustic paste disbudding in dairy calves. J. Dairy Sci. 100, 6429–6441. doi: 10.3168/jds.2017-12724
Wood, S. L., Beyer, B. K., and Cappon, G. D. (2003). Species comparison of postnatal CNS development: functional measures. Birth Defects Res. B Dev. Reprod. Toxicol. 68, 391–407. doi: 10.1002/bdrb.10037
Wu, X.-Q., Li, X.-F., Ye, B., Popat, N., Milligan, S. R., Lightman, S. L., et al. (2011). Neonatal programming by immunological challenge: effects on ovarian function in the adult rat. Reproduction 141, 241–248. doi: 10.1530/REP-10-0252
Xia, D., Min, C., Chen, Y., Ling, R., Chen, M., and Li, X. (2020). Repetitive pain in neonatal male rats impairs hippocampus-dependent fear memory later in life. Front. Neurosci. 14:722. doi: 10.3389/fnins.2020.00722
Yunes, M. C., Teixeira, D. L., von Keyserlingk, M. A. G., and Hötzel, M. J. (2019). Is gene editing an acceptable alternative to castration in pigs? PLoS ONE 14:e0218176. doi: 10.1371/journal.pone.0218176
Keywords: neonatal pain, developmental stress, inflammation, behavior, livestock
Citation: Adcock SJJ (2021) Early Life Painful Procedures: Long-Term Consequences and Implications for Farm Animal Welfare. Front. Anim. Sci. 2:759522. doi: 10.3389/fanim.2021.759522
Received: 16 August 2021; Accepted: 15 November 2021;
Published: 10 December 2021.
Edited by:
Pascale Chavatte-Palmer, INRA UMR 1198 Biologie du Développement et Reproduction, FranceReviewed by:
Mette S. Herskin, Aarhus University, DenmarkDaniel M. Weary, University of British Columbia, Canada
Copyright © 2021 Adcock. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah J. J. Adcock, c2FyYWguYWRjb2NrQHdpc2MuZWR1
 Sarah J. J. Adcock
Sarah J. J. Adcock