- 1Agriculture and Food, Commonwealth Scientific and Industrial Research Organisation, Armidale, NSW, Australia
- 2School of Environmental and Rural Sciences, University of New England, Armidale, NSW, Australia
Lambs in Australia undergo painful husbandry procedures as part of common husbandry. The magnitude and duration of pain are difficult to assess in lambs. Most currently used methods rely on behavioral expressions and physiological markers that may fail to detect the state of pain an animal experience. This study examined motivation of 12-week-old lambs experiencing chronic pain to self-medicate by consumption of feed containing an analgesic agent as an indicator of pain in lambs. In this study, 36 male Merino lambs were individually penned and acclimated to pelleted feed and two artificial odors: strawberry and banana. Once acclimated to odored feed, lambs were tested for their individual preference for the odors. Lambs were then assigned to one of two groups: Sham—sham handled day 0 and 7 or Ring—Ring castrated day 0 and tail docked day 7. To enable self-medication testing, lambs underwent a conditioning period (day 0–3) followed by the self-medication period (day 7–12). On day 0 lambs were castrated or sham handled, and then offered only medicated feed that contained an odor cue (either strawberry or banana). On day 7, lambs underwent tail-docking or sham handling and were offered both the conditioned medicated feed and non-medicated feed. Amount of each feed consumed was recorded 1 and 12 h after offer each day. Blood samples were taken for cortisol and white blood cell analysis and behavioral observations were recorded for 12 h following treatment. There was no difference in preference for medicated feed between Ring and Sham lambs during the self-medication phase (P = 0.18). Lambs in both groups displayed a significant preference for strawberry cued medicated feed during the self-medicated period when compared to the other testing periods (P = 0.05). Ring lambs displayed more active pain behaviors (mean = 15.1) than Sham (mean = 0.4, P < 0.05). Following castration, Ring lambs had a higher neutrophil/lymphocyte ratio at 6, 24, 48, and 72 h. This study was not able to demonstrate that lambs can self-medicate for a state of pain.
Introduction
In experimental settings, sheep have been shown to have the ability to learn to self-medicate. There has been extensive research conducted previously with sheep on their ability to learn to self-medicate for parasitic infection (Villalba et al., 2010; Fishpool et al., 2012; Juhnke et al., 2012) as well as other internal pain not caused by parasites (Provenza et al., 2000; Villalba and Provenza, 2001; Villalba et al., 2006). If sheep can learn to self-medicate, then their choice to ingest medications that are non-addictive would provide a strong indicator that the animal is motivated to alleviate a negative affective state.
One of the methods used to determine if an animal has learnt to self-medicate is through preference testing. In preference testing, animals are offered choices and their relative choices indicate preferences. For example, the animal is provided with a specific situation/resource (bedding type) and given a variety of options (bare flooring, hay, or woodchips) and they are allowed to essentially “vote with their feet” as to which of these they prefer (Duncan, 1992). In this case, of the offered choices, their preference would be the bedding type that they spend the most time on or interacting with.
Preference testing could also potentially be used to make inferences about an animal's pain state if the animal can associate the preference with pain relief. For instance, in a scenario where an animal selects a non-addictive analgesic when it is given a choice of a normal feed and a feed containing an analgesic, this could be indicative that the animal is in pain. For the animal to have the ability to make such a choice, it must first experience the consequences (whether positive or negative) of the feed options available to it. An individual animal is likely to learn the benefit of a substance through trial-and-error and by pairing the association of the behavior and consequence together (Provenza and Balph, 1987; Villalba and Provenza, 2007). The trial-and-error experience can be created by including a conditioning period prior to the preference test and there is evidence that this conditioning is effective in rats suffering from chronic pain (Colpaert et al., 2001). Conditioning chickens to feed medicated with an NSAID has also been demonstrated to be effective. In the study lame and sound chickens were trained to the medicated feed cued with a color, during preference testing chickens consumed more medicated feed when experiencing lameness (Danbury et al., 2000). There is little work to see if conditioning is effective in situations of chronic pain in lambs following castration and tail-docking (Rennie, 2005).
Rubber ring castration and tail-docking is the most common husbandry procedure used in Australia and causes acute and chronic pain. The use of rubber rings for castration and tail-docking have been shown to cause an acute pain phase lasting up to 4 h (Lester et al., 1996; Kent et al., 2000), and a chronic pain phase that can last for up to 35 days (Mellema et al., 2006; Melches et al., 2007). Several analgesics have been tested for their efficacy at reducing the pain response associated with ring castration and tail-docking in sheep, including bupivacaine (Graham et al., 1997), lignocaine (Small et al., 2020), naloxone (Wood et al., 1991), and non-steroidal anti-inflammatories (NSAID) such as diclofenac have been shown to reduce cortisol response (Graham et al., 1997; Paull et al., 2012) and abnormal behaviors (Molony et al., 1997).
Flunixin is a potent NSAID and has been shown to reduce pain-related behaviors in lambs that have undergone mulesing (Paull et al., 2007) as well as reducing inflammation and pain in lambs that have undergone surgical castration and tail-docking (Marini et al., 2017). When looking at a model of ischemic pain and hyperalgesia in sheep (through application of a tourniquet), flunixin has been shown to be effective at reducing the development of hyperalgesia (Welsh and Nolan, 1994). It is commonly used in veterinary medicine for its anti-inflammatory, analgesic, and antipyretic properties. Like other NSAIDs, it reduces inflammation by inhibiting cyclooxygenase and, in turn, decreasing the production of prostaglandins (Cheng et al., 1998). Flunixin is reported to be more potent as an analgesic than codeine in rats and has been shown to be a comparable analgesic to morphine in primates; unlike codeine and morphine, animals do not develop a tolerance or an addiction to the analgesic action of flunixin (Ciofalo et al., 1977).
The objectives of the present study were to condition lambs to a medicated feed after a painful event and then test whether lambs that subsequently experience acute and chronic pain after ring tail-docking could identify, as indicated through preference testing, a flunixin medicated food.
Materials and Methods
The experiment was undertaken at CSIRO's FD McMaster Laboratory, Armidale, New South Wales (NSW), Australia. The protocol and conduct of the experiment were approved by The CSIRO Chiswick Animal Ethics Committee under the NSW Animal Research Act, 1985 (approval ARA 15/09). Lambs were ear tagged at birth and ewe-lamb pairs allocated to two cohorts, cohort being based on birthdate. Prior to the study, ewes and lambs were exposed to the food pellets that were to be used later in the experiment, through daily feeding for 1 week whilst in the paddock. This was done to allow them to adjust to the change in feed as well as reduce the novelty of the feed.
Acclimation Period
At 7–8 weeks of age 36 male Merino lambs were checked for health, drenched with 2 ml of Zolvix (Novartis Animal Health, Australia), 2 ml of Flukazole C (Virbac, Australia), and vaccinated with Glanvac 6S B12 (Zoetis, Australia) prior to being moved into an animal house with their mothers. In the animal house, the animals became accustomed to indoor housing and to a standard pelleted ration (Ridley Agriproducts, wheat, millrun, and lucerne pellets, Australia; 17% crude protein dry matter; 9.04 MJ/kg dry matter) ad libitum. Throughout the experiment water was available ad libitum.
At 9–10 weeks old, ewe-lamb pairs were moved into individual pens (2.5 × 1.5 m), which allowed interaction with adjacent animals. During this time, lambs were exposed to two artificial odors, strawberry and banana (IMCD, Australia) that were later used as cues. The odors were sprayed onto the pellets (10 ml per kg of feed, diluted with water to 0.15%), the odor that was added to the feed was alternated between days. The lambs were weaned from their mothers at 10–11 weeks of age.
Odor Preference Test
After weaning, lambs were kept in individual pens and tested for their preference of the two odors during the week prior to experimental treatments. This was done to determine their base preference to be used for comparison of their choice during the self-medication test. Feed was placed into an 11 L feed bucket which was labeled to indicate which odor it contained. Lambs were given two buckets each containing 800 g of pellets and 200 g of chaff. The feed was sprayed with a 10 ml volume of an allocated odor (diluted with water to 0.15%). Lambs were offered both feed buckets simultaneously between 08:00 and 09:00 h for 4 days. The location (left or right) of the odors (strawberry and banana) was alternated each day, and the feed was removed 12 h after offer and weighed. During this period, lambs were also handled for 2 min once a day to reduce subsequent handling stress. Four of the lambs were removed from the study due to ill health or reactive temperaments, hence 32 of the 36 lambs from the odor preference test were used for the self-medication testing.
Self-Medication Testing
Conditioning Period
Lambs were either ring castrated, or sham handled as if they were to be castrated on day 0, and the treatments were spaced to occur every 2 min. The two treatments were:
• Ring castrated day 0 and tail-docked day 7 (Ring).
• Sham castrated day 0 and sham tail-docked day 7 (Sham).
The treatments were then allocated to subsequent subgroups, ensuring that the odor used for the medicated feed was evenly divided between the treatment groups, and assigned randomly to each lamb (Figure 1). During this phase, lambs were only presented with one bucket of feed which contained the medicated feed with their assigned odor cue. Feed was prepared in the bucket with which the animals were presented and contained 600 g of pellets and 200 g of chaff mixed together. The amount of feed on offer was just below recommended requirements to ensure lambs could consume all feed on offer. Lambs were weighed the day before castration to calculate a correct medication dosage of flunixin based on 4.0 mg/kg. The dose of flunixin in this study was doubled due to the effect of the rumen in reducing bioavailability and this approach has been implemented in previous studies in lambs to mitigate this effect (Marini et al., 2016, 2017). Liquid flunixin (BOVA, Australia) was then applied directly to the pellets using a syringe and mixed through the feed, incorporation being identified by the change in pellet color. The odor corresponding to the allocated treatment (Fluxinin + cue odor) was then added to the feed; using a spray bottle, 10 ml was applied and mixed through. At time of treatment application, lambs were lifted from the pens and were held on their backs on the pen railing, Ring lambs were castrated using an elastrator. Sham lambs were handled for 1 min as if they were being castrated. At 30 min after castration lambs were offered the bucket of feed. This procedure also occurred on days 1–3 post-treatment. The feed bucket was placed in the same location each day, with location (L or R) alternated between pens. On days 4–6, lambs were given pellets and chaff without odor or flunixin to ensure that they would not have therapeutic levels of flunixin (Marini et al., 2015) when they were tail docked.
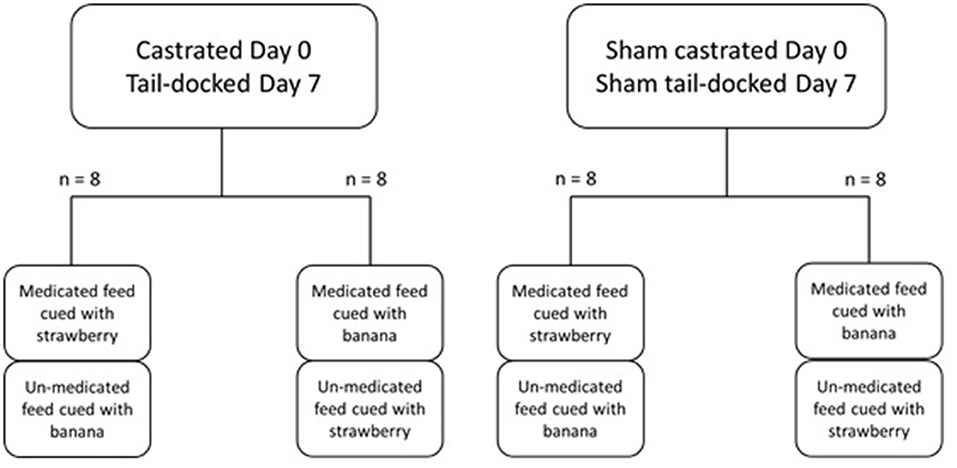
Figure 1. Diagrammatic representation of the experimental design indicating the allocation of groups and sub-groups during the experiment.
Self-Medication Period
On the treatment day for tail-docking (day 7), lambs were weighed so the dosage of flunixin could be adjusted. Lambs that were not to be tail docked (Sham) were handled as if they were to be tail-docked and the treated lambs had rings placed on the third palpable joint of the tail (Ring), treatments were again spaced to occur every 2 min. At this stage lambs still had necrotic scrotal tissue attached.
Thirty minutes following treatment all lambs were then offered a choice of both the feeds with the odors (medicated 1,200 g and non-medicated 1,200 g). The amount of feed made available in each container was above the lambs' full daily allowance and the amount offered had to be more than recommended requirements as lambs readily consumed 1,000 g of feed. The bucket containing the medicated feed was placed in the same location in the pen as during the conditioning phase.
To receive their full dose of flunixin, lambs had to consume all their allowance from the medicated feed container. Feed was prepared as described for the conditioning period, making sure the medicated feed contained the odor to which the lambs had been conditioned. For 5 consecutive days following tail-docking, the feed was weighed at 1 and at 12 h after offering to obtain individual intakes of both feeds. Both feed troughs were removed at 12 h, nothing else was offered.
After the self-medication test, lambs were treated with CLiKZiN (Novartis, Australia) for fly control and returned to the paddock. After 5 weeks, lambs re-entered the animal house and were retested for their preference of each odor (without flunixin) for 5 days using the same method as in the odor preference test (section Odor Preference Test). The amount of feed offered to lambs was increased to 1,400 g per bucket (1,200 g of pellets + 200 g of chaff) to account for their higher bodyweight. The location of the feed with the cue for medicated feed was again placed in the same location as during conditioning and the self-medication test.
Measurements
Behaviors
Video cameras were used to continuously record the behavior of lambs in the study. Five cameras were mounted on roofing rafters to record 3–4 of the pens. Each camera provided a view of the entire area available to the lambs. The cameras were connected to digital video recorders and captured by IVMS4200 software from Hangzhou Hikvision Digital Technology Co., Ltd. (Hangzhou, China). The behavior of the lambs in their pens on day 0–3 and day 7–10 were collated from the digital video records by observation of a replay of the video using The Observer software (Noldus, The Netherlands). The behaviors recorded (Table 1) have been validated for ring castration (Dinniss et al., 1999; Archer et al., 2004; Paull et al., 2012; Jongman et al., 2016). Behavioral assessment was undertaken by a single operator, who was blinded to the animals' treatment. The pain avoidance behavior assessment took place every 5 min for 1 min duration during the first 90 min post treatment (day 0 and day 7). Postural behavior assessment took place every 15 min for 12 h on the days of treatment as well as on days 1–3 and days 8–10. Observation time points were synchronized to each lamb's individual treatment time.
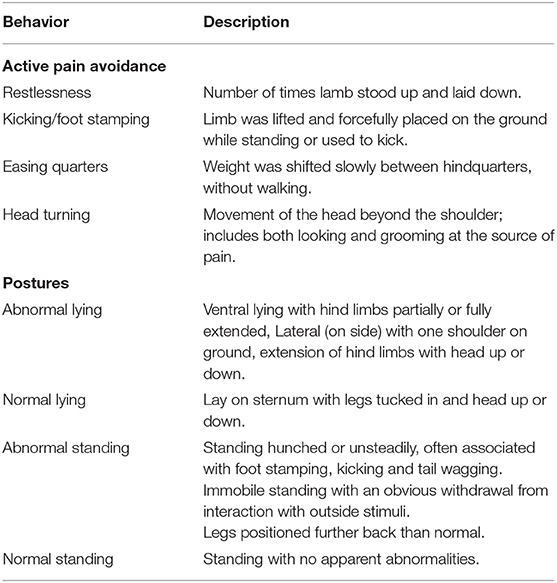
Table 1. Ethogram used for behavioral observations of lamb's sham castrated and tail-docked, or castrated and tail-docked with rubber rings.
Physiology
Blood was collected via the jugular vein using 21-gauge needles into 10 ml vacutainers containing EDTA. Individual blood samples were collected at 0 h, 30 min, 6 h, and 12 h on the day of treatment (day 0 and day 7) and then every morning (08:00–09:00 h) up to 72 h post-treatment. Neutrophil and lymphocyte count in whole blood were determined with an automated hematology analyzer (Cell Dyn 3500R, Abbott Diagnostics, Illinois U.S.A). The blood samples were then centrifuged at 2,000 × g for 15 min at 5°C and plasma were separated into three aliquots which were then stored at −20°C until assayed for cortisol concentration. Plasma cortisol concentrations were determined using a commercial radioimmunoassay (Plasma Cortisol RIA, MP Biomedical, Australia) which has been previously adapted and validated for ovine plasma (Paull et al., 2007). Coefficients of variation on the quality control plasma samples (50.3, 101.1, 211.7 nmol/L cortisol) were 8.48, 9.85, 7.28% for intra-assay and 11.2, 10.46, and 10.35% for inter-assay, respectively.
At the time of blood sampling (from 30 min onwards) the lambs also had their wounds palpated as previously described (Melches et al., 2007) and their behavioral response recorded as a score from 0 = no response, 1 = wincing to 2 = struggling, attempt at escape. The lambs were weighed on Day 1, 7, 14, 21, 28, and 35 relative to day 0 (day of castration).
Statistics
All data were analyzed using R (R Development Core Team, Boston, Massachusetts) and the packages nlme (Pinheiro et al., 2018) and pscl (Zeileis et al., 2008) were used. Data were tested for normality using the Shapiro-Wilk test and visual inspection of residual plots. In all instances P < 0.05 was considered statistically significant and 0.1 > P > 0.05 was considered a statistical tendency, results are presented as mean ± SEM. Lamb 8454 from the Ring group was removed from analysis for the self-medication test and excluded from week 5 preference testing as he was removed during the self-medication test due to being lethargic and not eating.
Active pain avoidance behaviors had to be combined and required the use of zero inflated Poisson model due to a high number of zeros in the data. Postural behavior (as a proportion) was analyzed using a repeated measures analysis, fitting the main effects of treatment, time, and potential interactions, and fitting lamb as a random variable. Postural data analyzed following castration were normal lying, abnormal lying, and total standing. Total standing was analyzed using a Kruskal-Wallis test. For tail docking, postural behavior was analyzed for abnormal lying, normal lying, and normal standing.
Bodyweight, cortisol, and neutrophil/lymphocyte ratio were analyzed using a repeated measures analysis, fitting the main effects of treatment, time, and potential interactions. The bodyweight of castrated animals was also analyzed separately to test the effect of medicated feed preference on weight gain. For neutrophil/lymphocyte ratio and cortisol, pre-treatment values (time 0 h) were fitted as a covariate when significant and animal was fitted as a random effect. Wound palpation scores were analyzed using a GLM with a Poisson distribution with the frequency counts as the outcome. Neutrophil/lymphocyte ratio required a log transformation and cortisol required a square root transformation.
Feed Preference Analysis
Feed intake was recorded daily at 12 h (20:00–21:00 h) after lambs were first offered their feed and during the self-medication phase feed intake was also recorded at 1 h after being offered (9:00–10:00 h). The calculation for feed preference used in this study was described by Bell (1959). Preference Indices (PI) are more sensitive than the individual intake of lambs (as intake can vary across days) and PI have been used in several studies (Mirza and Provenza, 1990; Provenza et al., 1990; Ngwa et al., 2000; Omokanye et al., 2001). However, the animal's intake (g) was also analyzed. To allow for calculation, 5 g of feed was included to the PI calculation for instances where lambs did not consume any of the feed, 5 g was chosen as it was lower than the lowest intake recorded (12 g). The preference was calculated as:
Lamb feed choice (tested as feed intake and preference index) were analyzed as a repeated measures analysis using a non-linear mixed effects model. The main effects that were fitted, where appropriate, were treatment (Ring or Sham), feed type (medicated or un-medicated), the location of the feed bucket (left or right), odor cue (strawberry or banana), and day. When significant, the preference for the medicated cue prior to castration was fitted as a covariate for the self-medication phase and week 5 data and lamb was fitted as a random effect. Feed intake data obtained during the self-medication phase in the first hour and for week 5 was not able to be normalized and was analyzed using a Kruskal-Wallis test. Intake of flunixin (mg) was also calculated for each gram of feed consumed.
Results
The lambs' weight gain was not affected by treatment (Ring or Sham, P = 0.28). For the animals that were castrated there was no effect of their PI for medicated feed on weight gain (P = 0.50). During the preference test there was no odor (banana vs. strawberry, P = 0.58) or location (left or right, P = 0.76) or day (P = 0.96) effect on the consumption of feed. When analyzing the PI of the cue to be used for medicated feed there was no effect of odor (P = 0.63), location (P = 0.65), or day (P = 0.43). Although there were no overall odor preferences, two lambs indicated a stronger preference for strawberry (PI < 0.50) and five lambs indicated a stronger preference for banana (PI > 0.50), however their average preference did not fall outside two standard deviations from the mean (Mean = 0.51 ± 0.17).
Conditioning Period
All lambs consumed the feed on offer throughout the week, except for one lamb who on day 2 only consumed 200 g. In the 90 min following treatment, lambs that were castrated displayed more active pain behaviors (mean =15.1) than sham lambs (mean = 0.4, P < 0.05).
In the first hour following castration lambs in the Ring group displayed more acute pain related behaviors compared to Sham lambs (Table 2). With Ring lambs displaying more restless hindquarters (P = 0.01) and kicking/foot stamping (P = 0.001). Ring lambs displayed a higher proportion of abnormal lying on the day of treatment (day 0, 34 ± 3%) compared with day 2 and 3 (17 ± 4%, P < 0.05). Sham lambs displayed fewer abnormal behaviors (12 ± 4%) than Ring lambs on the day of treatment (24 ± 4%, P = 0.02). For normal lying only a day effect was seen (P < 0.001), Ring lambs exhibited normal lying more often on days 1 (27 ± 4%), 2 (35 ± 4%), and 3 (32 ± 4%) compared with the treatment day (16 ± 3%). Treatment affected the display of normal standing (P = 0.04). Lambs in the Ring group tended to stand less [H(1) = 4.01, P = 0.04] than Sham lambs (difference = 13.0, the critical difference was 13.09).
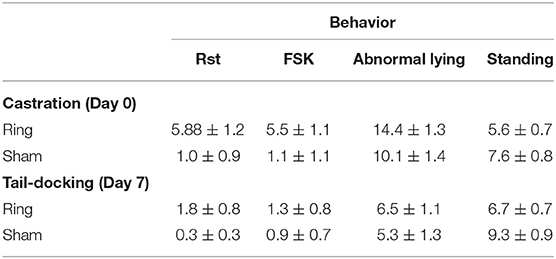
Table 2. Mean count (mean ± SEM) of acute pain and postural behaviors related to pain for lambs in the Sham (n =15) and Ring (n =16) treatment groups following castration and tail-docking.
Following castration there was a time by treatment effect (P = 0.02) for the neutrophil/lymphocyte ratio between Ring and Sham lambs. There was also a time effect, with Ring lambs having a higher neutrophil/lymphocyte ratio at 6, 24, 48, and 72 h compared to their baseline (P < 0.05). Sham lambs also had an increase in neutrophil/lymphocyte ratio at 12 and 48 h compared to baseline (P < 0.05). At 72 h post treatment, Ring lambs still had a higher mean neutrophil/lymphocyte ratio than Sham lambs (Figure 2).
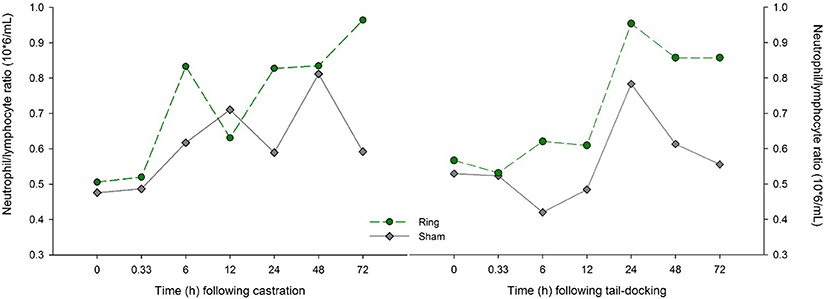
Figure 2. Raw data of the mean neutrophil lymphocyte ratios for lambs in the Sham (n =16) and Ring (n =16) treatment groups following castration and tail-docking.
There was a tendency for the interaction between time and treatment on cortisol concentration to be significant (P = 0.053) and a significant time effect (P < 0.001). Thirty minutes following treatment, Ring lambs had an increase in cortisol concentration (transformed mean = 3.5, back transformed = 12.25 nmol/L, P < 0.001) compared to baseline. Ring lambs had higher cortisol concentrations at 30 min compared to Sham lambs (transformed mean = 8.3 back transformed = 68.89 vs. transformed mean = 7.25 back transformed = 56.64 nmol/L, respectively, P < 0.001). At 6, 12, 24 h, Ring lambs' cortisol concentrations returned to baseline, but they experienced a significant rise again and were higher than baseline concentrations at 48 h (P = 0.02) and 72 h (P = 0.001). Sham lambs experienced no change in cortisol concentration (Figure 3).
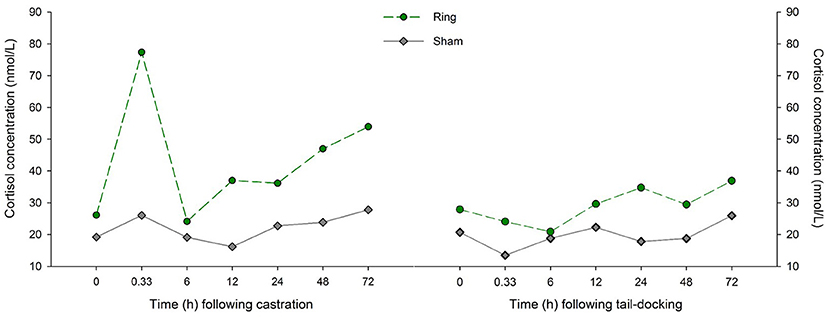
Figure 3. Raw data of the mean cortisol concentrations (nmol/L) for lambs in the Sham (n =16) and Ring (n =16) treatment groups following castration and tail-docking.
Treatment affected the lambs' reaction to palpation (P = 0.001) with more Ring lambs reacting (mean = 5) compared to Sham lambs (mean = 2). There was no time effect (P = 0.16).
Self-Medication Period
In the 90 min following tail-docking, Ring lambs showed more active pain behaviors than sham lambs (P < 0.001, Table 2). There was no overall treatment difference in the display of abnormal lying (P = 0.44), however, there was a time by treatment effect on the display of abnormal lying (P = 0.02), with lambs in the Sham group displaying more abnormal lying on day 8 (24%) and 9 (23%) compared with day 7 (13%). There was no difference between treatment groups for normal lying (P = 0.20) and normal standing (P = 0.12).
In the first hour of being offered the feed, Ring lambs consumed on average 121.3 ± 13.5 g and Sham lambs consumed 109.5 ± 16.5 g of medicated feed (P = 0.59). Ring lambs consumed 161.6 ± 19.9 g and Sham lambs 168.3 ± 15.9 g of un-medicated feed. The average intake of medicated feed in Ring lambs corresponded to an intake of approximately 10 ± 1% of the lambs flunixin dose in the first hour and 43 ± 3% in 12 h. Only one lamb consumed 1% of their flunixin dose on average in the first hour (Table 3). There was no difference in PI between Ring and Sham lambs in the first hour (P = 0.18).

Table 3. Average approximate percentage dose of flunixin consumed by lambs in the ring group (n = 15) at 1 and 12 h during the self-medication phase.
For the 12 h feed intake, there was no effect of feed location (P = 0.75), or day (P = 0.75) and there was no difference between Ring and Sham lambs' intake of feed (P = 0.61). The cue odor strawberry influenced feed intake (P < 0.05), with strawberry cued feed having 149.2 ± 25.5 g extra pellets consumed. At 12 h, Ring lambs consumed on average 516.7 ± 44.6 g and Sham lambs consumed 489.7 ± 48.9 g of medicated feed. Ring lambs consumed 469.7 ± 48.6 g and Sham lambs 518.5 ± 40.0 g of un-medicated feed. Ring lambs on average consumed 43 ± 3% of their flunixin dose (Table 3). There was also no overall difference (P = 0.57) between the Sham and Ring treatment in the choice of medicated or non-medicated feed.
For neutrophil/lymphocyte ratio following tail-docking, there was a significant time by treatment interaction (P = 0.02) and the effect of treatment approached significance (P = 0.064). Sham lambs experienced an increase in neutrophil/lymphocyte ratio (transformed = 3.49, back-transformed = 1.25, P = 0.02) at 24 h compared with baseline, while Ring lambs had a significantly higher (P < 0.05) neutrophil/lymphocyte ratio compared with baseline at 24, 48, and 72 h following treatment (Figure 2). There was no effect of time (P = 0.37), treatment (P = 0.33), or their interactions (P = 0.75) on cortisol concentrations for both Ring and Sham lambs (Figure 3). For the palpation scores, there was a treatment effect (P < 0.001); more lambs in the Ring group reacted to palpation (mean = 8.3) compared to Sham (mean = 1.5).
Week 5 Preference
Overall, there was no difference in the intake of medicated or un-medicated feed (P = 0.96), there was no prior treatment (P = 0.41), odor (P = 0.80), or location effect (P = 0.45) on lambs' feed intake. There was a day effect with lambs consuming less feed on day 46 compared to days 47–50 (α = 0.05, difference = 40.20). There was no difference between treatment groups and PI of the medicated odor cue (P = 0.95).
The results of feed intake were reflected in the overall PI. When looking at feed PI across the difference periods of the experiment (preference, self-medication phase, and week 5) there was no effect of treatment (P = 0.74) or period (P = 0.95) but there was an effect of the odor (P = 0.04) and an interaction between odor and period (P = 0.05). Lambs in both the Ring and Sham group showed a higher preference for strawberry cued feed during the self-medication phase (P = 0.05, Figure 4). This preference did not occur at any other period.
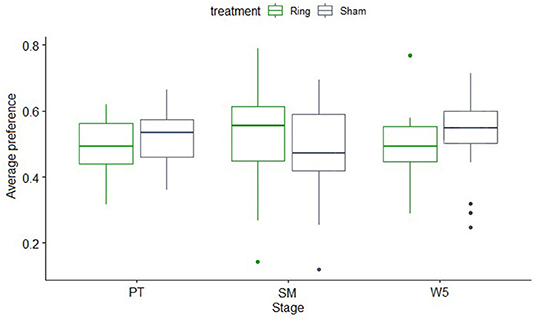
Figure 4. Box and whiskers plot showing the median (line), 25–75% quartiles, maximum and minimum of the average preference indices for the Ring (n = 15) and Sham (n = 16) treatment groups across the different periods of the experiment. PT, preference test; SM, self-medication phase; W5, preference test 5 weeks post tail-docking.
Discussion
Ring animals experienced pain in response to both castration and tail-docking as indicated by the increased amount of active pain avoidance behaviors and increased reaction to wound palpation in comparison to Sham lambs. Lambs in the Ring group also experienced inflammation associated with the treatments as shown by the increase in neutrophil/lymphocyte ratio in both castrated and tail-docked lambs. However, Ring lambs did not show an increase in abnormal postures following ring tail-docking and did not experience a peak in cortisol concentration following treatment as they did for castration. There was also no indication that the lambs in the Ring group were able to self-medicate for pain through preference for medicated feed after tail docking.
It is known that ring castration and tail-docking causes acute pain within the first few hours following application (Lester et al., 1996; Kent et al., 2000), with acute behaviors observable within 5–10 min (Small et al., 2021). As lambs that were in the Ring group displayed more active pain behaviors than Sham lambs following castration, it can be assumed that they were experiencing some pain before they were given access to feed containing flunixin. During the conditioning phase, all lambs consumed their entire dose of flunixin (except for one lamb on day 2) so we would have expected that all lambs could have experienced pain alleviation. However, the lack of change in reaction to palpation following castration indicates that the lambs in the Ring group were still experiencing hyperalgesia, which we would have expected to reduce if lambs were effectively medicated. Providing lambs with free access to feed medicated with flunixin prior to surgical castration and tail-docking has been shown to effectively alleviate the pain associated with the procedures (Marini et al., 2017) and may be a more practical method of allowing animals to self-medicate.
Previous work has indicated that lambs would be required to eat at least 22 g of the medicated feed in 10 min for flunixin to be present in their blood, and continued consumption of medicated feed would lead to therapeutic concentrations in their blood within 2 h (Marini et al., 2016). During the self-medication phase, lambs consumed on average just over 100 g in the first hour (approximately 10% of their flunixin dose) and so there may have been a delay in obtaining pain relief. To reach and to maintain therapeutic levels lambs would have had to have eaten most of their medicated feed in the 8–12 h following tail-docking (Marini et al., 2016). In the study by Marini et al. (2016), ewes that consumed their full dose of flunixin (4.0 mg/kg) over 12 h reached slightly higher than inferred therapeutic concentrations (1.78 ± 0.48 μg/ml). There is no work on the therapeutic concentration of flunixin in sheep however therapeutic effects are seen in horses when plasma concentrations reach 0.2–0.9 μg/ml (Toutain et al., 1994). Lambs in the Ring group consumed on average 43% of their flunixin dose in the 12 h that they had access to the medicated feed; this suggests that lambs may not have reached therapeutic concentrations of flunixin. The information from the pharmacokinetics study Marini et al. (2016) should be applicable to these 12–13 week old lambs, as they would have functioning rumens (Boda et al., 1962). After removal of feed at 12 h, flunixin concentrations in the blood would have declined and lambs would have needed to continue to select the medicated feed on the following days to achieve and maintain therapeutic concentrations. While plasma was collected in this study, the drug concentrations in the plasma were not analyzed, so we can only use our previous work to assume that some lambs would not have achieved therapeutic concentrations of flunixin in their blood.
A majority of the studies that have reported self-medication in animals including sheep is in response to a parasitic infection (Villalba et al., 2010; Fishpool et al., 2012; Juhnke et al., 2012). It is suggested that the animals learn which substance will improve a negative state (whether it be caused by parasitic infection or pain) by trial and error; the animal develops preferences through the interactions of the foods characteristics (odor, flavor, and texture) and post-ingestive feedback (Provenza et al., 1992, 2006). In this current study we ensured that lambs were experiencing pain that was induced by ring castration before giving them the opportunity to learn the benefits of a medicated feed. To help the animals learn through post-ingestive feedback the medicated feed was cued with an odor, and the location of the feed bucket which contained the medicated feed was kept in the same location throughout the conditioning period and the self-medication phase. However, the lambs in this study did not display self-medication in response to the painful procedure of tail docking. Perhaps the mechanisms for learning to self-medicate for pain may be different to other negative states such as nutritional deficit and parasitism. The information on flavor and odor that an animal receives from post-ingestive feedback is processed predominantly via the limbic system (Provenza et al., 1992), whereas information that would be received from a painful stimulus such as tail-docking would be received through a multitude of pathways including both direct stimulation of nociceptive fibers and release of inflammatory mediators that in turn affect a range of strictures and processed within the central nervous system (Viñuela-Fernández et al., 2007; Gaynor and Muir, 2009). The complexity of the pain associated with ring castration and tail-docking as well as the limited acute ischemic pain phase may have also impacted the ability of flunixin in providing effective pain relief in this trial. During husbandry procedures such as castration and tail-docking it is often recommended that a multimodal analgesia strategy be implemented to cover the various pain phases and to take advantage of the fast uptake and short duration of local anesthetics and the slow uptake and long action of NSAIDs. Flunixin has been shown to reduce cortisol response and decrease pain avoidance behaviors following ring castration (Paull et al., 2012), however NSAIDS effectiveness at ameliorating the pain associated with ring castration in lambs and cattle is limited (Petherick et al., 2014; Paull et al., 2015).
The differences in these feedback systems, the differences in central processing of that feedback combined with the limited action of flunixin may have impacted on the ability of the animals in this study to learn from the single castration event that the feed they consumed that contained flunixin was linked to the relief from pain that they experienced. It may also be possible that the lambs experienced some level of conditioned place aversion which led to the higher preference for only one of the odor cues during the self-medication phase. Conditioned place aversion in response to pain has been demonstrated in calves, where calves that have been disbudded and either received no pain relief or pain relief that did not last for the entire conditioning period, led those animals to avoid the areas that the painful treatment occurred (Ede et al., 2019a,b). Although there was no previous preference displayed by lambs in this study between the banana and strawberry cues it is possible that the negative experience of castration and handling may have caused lambs to pair the negative experience with the banana cue.
Ring lambs displayed active pain avoidance behaviors and increased reaction to wound palpation after both castration and tail docking. However, following tail-docking Ring lambs did not display postural behaviors indicative of pain. The procedure of tail-docking with rings is known to cause pain in lambs, with lambs displaying more postural behaviors indicative of pain than those who have received pain-relief (Graham et al., 1997; Kent et al., 1998) although the responses to tail docking are much less than to castration (Small et al., 2020). The difference seen in the display of abnormal postures in the current study may have been due to age, with the studies by Graham et al. (1997) and Kent et al. (1998) using lambs aged 5–8 days and 3 weeks old, respectively. Lambs in the current study were aged between 12 and 13 weeks when tail docked. A previous study by McCracken et al. (2010) that tail-docked lambs at a separate time to castration reported that the age the lambs were castrated had an effect on the reaction to tail-docking, with lambs castrated at 1 day old reacting more to subsequent tail-docking than lambs castrated at 10 days of age. It has been suggested that that early life pain experiences increases hyperalgesia in later life (Pattinson and Fitzgerald, 2004). Although lambs in the current study experienced ear-tagging within a few hours of birth, the level of pain produced by that procedure is low in comparison to castration (Grant, 2004). The reduced response to tail-docking in the current study is possibly due to castration and tail-docking occurring at an older age in these lambs with no prior exposure to severe pain.
Following tail-docking, the lambs in the current study also did not show a significant elevation in cortisol compared with the control treatment. Tail-docking alone has been shown to result in increased cortisol concentrations that remains elevated for 1–2 h (Mellor and Murray, 1989; Graham et al., 1997; Kent et al., 1998). Kent et al. (1998) did report a lower cortisol increase in lambs that were only tail-docked (60 nmol/L) compared with lambs that were only castrated (120 nmol/L). In this present study lambs did not display elevated cortisol after tail-docking, with Ring lambs having an average cortisol concentration below 25 nmol/L at 30 min following treatment, which was no different to Sham lambs.
Sex has also been shown to effect lambs' response to tail-docking, with male lambs reported to have lower cortisol response to tail-docking at 8 weeks of age compared with female lambs of the same age (Turner et al., 2006). It is known that the response to pain in lambs can vary depending on factors such as age and sex (Molony et al., 1993; Turner et al., 2006; Guesgen et al., 2011). The reduced response to the pain of tail-docking compared with that from castration in Ring lambs may have affected the lambs' choice to consume medicated feed.
It has been previously shown that sheep have the ability to learn to self-medicate for parasitism, but this study was not able to demonstrate that lambs can self-medicate for a state of pain. There may have been several possibilities as to why lambs were unable to learn to medicate for pain. The learning mechanism required to link the experience of pain relief following the consumption of a medicated feed may be far more complicated than the post-ingestive feedback systems associated with animals gaining nutritional wisdom. The conditioning paradigm may have been inappropriate for the lambs to associate flavor cues and pain relief. There may also be the possibility that lambs in the Ring group may not have been experiencing a sufficient level of pain, as indicated by their lack of cortisol response and postural behaviors indicative of pain, that would have motivated them to preferentially select the medicated feed. Future studies looking at self-medication in livestock should consider alternative training applications, such as inclusion of color coded feed as well as alternative models of pain such as lameness (Danbury et al., 2000) or surgical castration and tail-docking (Marini et al., 2017).
Conclusions
This study used rubber ring castration as a model of chronic pain to condition lambs to medicated feed cued with a novel flavor. Through testing preference for medicated and un-medicated feed, we tested whether lambs could self-medicate following the subsequent painful procedure of tail docking. Although lambs that underwent ring castration consumed all their medicated feed during the conditioning phase of the trial, they did not display self-medicative behavior following subsequent ring tail-docking. It is possible that the conditioning paradigm may have been inadequate to train lambs to associate flavor cues with pain relief. Alternatively, the lack of cortisol and postural behavior changes may indicate that pain responses to tail-docking were insufficient to motivate lambs to preferentially selected the medicated feed. It is also possible that the learning mechanism to link the experience of pain relief following the consumption of a medicated feed may be far more complicated than learning feed aversions. A preference for lambs to consume medicated feed rather than un-medicated feed following a painful procedure could not be demonstrated in this study.
Data Availability Statement
The data that support this study will be shared upon reasonable request to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the CSIRO Chiswick Animal Ethics Committee under the NSW Animal Research Act, 1985 (approval ARA 15/09).
Author Contributions
DM, CL, and IC: conceptualization. DM: writing—original draft preparation. CL and IC: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by CSIRO; the University of New England; and funding contributors, Meat and Livestock Australia; and the Commonwealth Government.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Sue Belson and Sam Robinson for their assistance in animal care and handling. We also thank Carol Petherick, Geoff Hinch, and Alison Small for comments and revision of the paper. CSIRO acknowledges the Traditional Owners of the lands that we live and work on across Australia and pays its respect to Elders past and present. CSIRO recognizes that Aboriginal and Torres Strait Islander peoples have made and will continue to make extraordinary contributions to all aspects of Australian life including culture, economy and science.
References
Archer, N., Johnston, A. M., and Khalid, M. (2004). Differences in the acute pain responses of two breeds of lamb following castration and tail docking with the rubber ring method. Anim. Welfare 13, 135–141.
Bell, R. (1959). Preference thresholds for taste discrimination in goats. J. Agric. Sci. 52, 125–128. doi: 10.1017/S0021859600035759
Boda, J. M., Riley, P., and Wegner, T. (1962). Tissue glycogen levels in relation to age and some parameters of rumen development in lambs. J. Anim. Sci. 21, 252–257. doi: 10.2527/jas1962.212252x
Cheng, Z., Nolan, A. M., and McKellar, Q. A. (1998). Measurement of cyclooxygenase inhibition in vivo: a study of two non-steroidal anti-inflammatory drugs in sheep. Inflammation 22, 353–366. doi: 10.1023/A:1022364731126
Ciofalo, V. B., Latranyi, M. B., Patel, J. B., and Taber, R. I. (1977). Flunixin meglumine: a non-narcotic analgesic. J. Pharmacol. Exp. Therapeut. 200, 501–507.
Colpaert, F. C., Tarayre, J. P., Alliaga, M., Bruins Slot, L. A., Attal, N., and Koek, W. (2001). Opiate self-administration as a measure of chronic nociceptive pain in arthritic rats. Pain 91, 33–45. doi: 10.1016/S0304-3959(00)00413-9
Danbury, T. C., Weeks, C. A., Waterman-Pearson, A. E., Kestin, S. C., and Chambers, J. P. (2000). Self-selection of the analgesic drug carprofen by lame broiler chickens. Vet. Rec. 146, 307–311. doi: 10.1136/vr.146.11.307
Dinniss, A. S., Stafford, K. J., Mellor, D. J., Bruce, R. A., and Ward, R. N. (1999). The behaviour pattern of lambs after castration using a rubber ring and/or castrating clamp with or without local anaesthetic. N. Z. Vet. J. 47, 198–203. doi: 10.1080/00480169.1999.36143
Duncan, I. J. H. (1992). Measuring preferences and the strength of preferences. Poult. Sci. 71, 658–663. doi: 10.3382/ps.0710658
Ede, T., Lecorps, B., von Keyserlingk, M. A. G., and Weary, D. M. (2019a). Calf aversion to hot-iron disbudding. Sci. Rep. 9, 5344. doi: 10.1038/s41598-019-41798-7
Ede, T., von Keyserlingk, M. A. G., and Weary, D. M. (2019b). Assessing the affective component of pain, and the efficacy of pain control, using conditioned place aversion in calves. Biol. Lett. 15, 20190642. doi: 10.1098/rsbl.2019.0642
Fishpool, F. J., Kahn, L. P., Tucker, D. J., Nolan, J. V., and Leng, R. A. (2012). Voluntary intake of a medicated feed block by grazing sheep is increased by gastrointestinal nematode infection. Anim. Product. Sci. 52, 1136–1141. doi: 10.1071/AN12104
Gaynor, J. S., and Muir, W. W. (2009). Handbook of Veterinary Pain Managmement. St. Louis, MI: Elsevier Health Sciences.
Graham, M. J., Kent, J. E., and Molony, V. (1997). Effects of four analgesic treatments on the behavioural and cortisol responses of 3-week-old lambs to tail docking. Vet. J. 153, 87–97. doi: 10.1016/S1090-0233(97)80013-5
Grant, C. (2004). Behavioural responses of lambs to common painful husbandry procedures. Appl. Anim. Behav. Sci. 87, 255–273. doi: 10.1016/j.applanim.2004.01.011
Guesgen, M. J., Beausoleil, N. J., Minot, E. O., Stewart, M., Jones, G., and Stafford, K. J. (2011). The effects of age and sex on pain sensitivity in young lambs. Appl. Anim. Behav. Sci. 135:51–56. doi: 10.1016/j.applanim.2011.09.008
Jongman, E., Hemsworth, P., and Campbell, A. (2016). Assessment of Pain Responses Following Castration of Lambs Using the Elastrator Ring With and Without Midline Injections of Lignocaine. Meat and Livestock Australia: Sydney. 19.
Juhnke, J., Miller, J., Hall, J. O., Provenza, F. D., and Villalba, J. J. (2012). Preference for condensed tannins by sheep in response to challenge infection with Haemonchus contortus. Vet. Parasitol. 188, 104–114. doi: 10.1016/j.vetpar.2012.02.015
Kent, J. E., Jackson, R. E., Molony, V., and Hosie, B. D. (2000). Effects of acute pain reduction methods on the chronic inflammatory lesions and behaviour of lambs castrated and tail docked with rubber rings at less than two days of age. Vet. J. 160, 33–41. doi: 10.1053/tvjl.2000.0465
Kent, J. E., Molony, V., and Graham, M. J. (1998). Comparison of methods for the reduction of acute painproduced by rubber ring castration or tail docking of week-old lambs. Vet. J. 155, 39–51. doi: 10.1016/S1090-0233(98)80033-6
Lester, S. J., Mellor, D. J., Holmes, R. J., Ward, R. N., and Stafford, K. J. (1996). Behavioural and cortisol responses of lambs to castration and tailing using different methods. N. Z. Vet. J. 44, 45–54. doi: 10.1080/00480169.1996.35933
Marini, D., Colditz, I. G., Hinch, G., Petherick, J. C., and Lee, C. (2017). Self-administration by consumption of flunixin in feed alleviates the pain and inflammation associated with castration and tail docking of lambs. Appl. Anim. Behav. Sci. 188, 26–33. doi: 10.1016/j.applanim.2016.12.008
Marini, D., Pippia, J., Colditz, I., Hinch, G., Petherick, J., and Lee, C. (2015). Randomised trial of the bioavailability and efficacy of orally administered flunixin, carprofen and ketoprofen in a pain model in sheep. Aust. Vet. J. 93, 265–270. doi: 10.1111/avj.12351
Marini, D., Pippia, J., Colditz, I. G., Hinch, G. N., Petherick, C. J., and Lee, C. (2016). Palatability and pharmacokinetics of flunixin when administered to sheep through feed. PeerJ 4, e1800. doi: 10.7717/peerj.1800
McCracken, L., Waran, N., Mitchinson, S., and Johnson, C. B. (2010). Effect of age at castration on behavioural response to subsequent tail docking in lambs. Vet. Anaesth. Analg. 37, 375–381. doi: 10.1111/j.1467-2995.2010.00547.x
Melches, S., Mellema, S. C., Doherr, M. G., Wechsler, B., and Steiner, A. (2007). Castration of lambs: A welfare comparison of different castration techniques in lambs over 10 weeks of age. Vet. J. 173, 554–563. doi: 10.1016/j.tvjl.2006.01.006
Mellema, S. C., Doherr, M. G., Wechsler, B., Thueer, S., and Steiner, A. (2006). Influence of local anaesthesia on pain and distress induced by two bloodless castration methods in young lambs. Vet. J. 172, 274–283. doi: 10.1016/j.tvjl.2005.06.002
Mellor, D. J., and Murray, L. (1989). Effects of tail docking and castration on behaviour and plasma cortisol concentrations in young lambs. Res. Vet. Sci. 46, 387–391. doi: 10.1016/S0034-5288(18)31185-8
Mirza, S. N., and Provenza, F. D. (1990). Preference of the mother affects selection and avoidance of foods by lambs differing in age. Appl. Anim. Behav. Sci. 28, 255–263. doi: 10.1016/0168-1591(90)90104-L
Molony, V., Kent, J. E., Hosie, B. D., and Graham, M. J. (1997). Reduction in pain suffered by lambs at castration. Vet. J. 153, 205–213. doi: 10.1016/S1090-0233(97)80041-X
Molony, V., Kent, J. E., and Robertson, I. S. (1993). Behavioural responses of lambs of three ages in the first three hours after three methods of castration and tail docking. Res. Vet. Sci. 55, 236–245. doi: 10.1016/0034-5288(93)90087-V
Ngwa, A. T., Pone, D. K., and Mafeni, J. M. (2000). Feed selection and dietary preferences of forage by small ruminants grazing natural pastures in the Sahelian zone of Cameroon. Anim. Feed Sci. Technol. 88, 253–266. doi: 10.1016/S0377-8401(00)00215-7
Omokanye, A. T., Balogun, R. O., Onifade, O. S., Afolayan, R. A., and Olayemi, M. E. (2001). Assessment of preference and intake of browse species by Yankasa sheep at Shika, Nigeria. Small Rumin. Res. 42, 201–208. doi: 10.1016/S0921-4488(01)00250-4
Pattinson, D., and Fitzgerald, M. (2004). The neurobiology of infant pain: development of excitatory and inhibitory neurotransmission in the spinal dorsal horn. Reg. Anesth. Pain Med. 29, 36–44. doi: 10.1097/00115550-200401000-00009
Paull, D., Lee, C., Colditz, I., Atkinson, S., and Fisher, A. (2007). The effect of a topical anaesthetic formulation, systemic flunixin and carprofen, singly or in combination, on cortisol and behavioural responses of Merino lambs to mulesing. Aust. Vet. J. 85, 98–106. doi: 10.1111/j.1751-0813.2007.00115.x
Paull, D., Small, A., Lee, C., Labeur, L., and Colditz, I. (2015). Effect of local infusion of NSAID analgesics administered alone or in combination on the pain associated with band castration in calves. Aust. Vet. J. 93, 271–277. doi: 10.1111/avj.12348
Paull, D. R., Small, A. H., Lee, C., Palladin, P., and Colditz, I. G. (2012). Evaluating a novel analgesic strategy for ring castration of ram lambs. Vet. Anaesth. Analg. 39, 539–549. doi: 10.1111/j.1467-2995.2012.00716.x
Petherick, J. C., Small, A. H., Mayer, D. G., Colditz, I. G., Ferguson, D. M., and Stafford, K. J. (2014). A comparison of welfare outcomes for weaner and mature Bos indicus bulls surgically or tension band castrated with or without analgesia: 1. Behavioural responses. Appl. Anim. Behav. Sci. 157, 23–34. doi: 10.1016/j.applanim.2014.05.003
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., and R Core Team. (2018). nlme: Linear and Nonlinear Mixed Effects Models. R package version 3. 1–118. Vienna: R Foundation.
Provenza, F. D., and Balph, D. F. (1987). Diet learning by domestic ruminants: theory, evidence and practical implications. Appl. Anim. Behav. Sci. 18, 211–232. doi: 10.1016/0168-1591(87)90218-8
Provenza, F. D., Burritt, E. A., Clausen, T. P., Bryant, J. P., Reichardt, P. B., and Distel, R. A. (1990). Conditioned flavor aversion: a mechanism for goats to avoid condensed tannins in blackbrush. Am. Nat. 136, 810–828. doi: 10.1086/285133
Provenza, F. D., Burritt, E. A., Perevolotsky, A., and Silanikove, N. (2000). Self-regulation of intake of polyethylene glycol by sheep fed diets varying in tannin concentrations. J. Anim. Sci. 78, 1206–1212. doi: 10.2527/2000.7851206x
Provenza, F. D., Pfister, J. A., and Cheney, C. D. (1992). Mechanisms of learning in diet selection with reference to phytotoxicosis in herbivores. J. Range Manage. 45, 36–45. doi: 10.2307/4002523
Provenza, F. D., Villalba, J. J., and Bels, V. (2006). “Foraging in domestic herbivores: linking the internal and external milieux,” in Feeding in Domestic Vertebrates: From Structure to Behaviour, ed V. Bels (Oxfordshire: CABI Publ.), 210–240. doi: 10.1079/9781845930639.0210
Rennie, A. E. (2005). Studies of Chronic Inflammatory Pain in Lambs After Rubber Ring Castration and Tail-Docking: Self-Administration of Analgesic and Neurohistochemistry to Validate Behavioural Assessment. Doctor of Philosophy, University of Edinburgh.
Small, A., Marini, D., and Colditz, I. (2021). Local anesthetic delivered with a dual action ring and injection applicator reduces the acute pain response of lambs during tail docking. Animals 11, 2242. doi: 10.3390/ani11082242
Small, A. H., Jongman, E. C., Niemeyer, D., Lee, C., and Colditz, I. G. (2020). Efficacy of precisely injected single local bolus of lignocaine for alleviation of behavioural responses to pain during tail docking and castration of lambs with rubber rings. Res. Vet. Sci. 133:210–218. doi: 10.1016/j.rvsc.2020.09.025
Toutain, P. L., Autefage, A., Legrand, C., and Alvinerie, M. (1994). Plasma concentrations and therapeutic efficacy of phenylbutazone and flunixin meglumine in the horse: pharmacokinetic/pharmacodynamic modelling. J. Vet. Pharmacol. Therap. 17, 459–469. doi: 10.1111/j.1365-2885.1994.tb00278.x
Turner, A. I., Hosking, B. J., Parr, R. A., and Tilbrook, A. J. (2006). A sex difference in the cortisol response to tail docking and ACTH develops between 1 and 8 weeks of age in lambs. J. Endocrinol. 188, 443–449. doi: 10.1677/joe.1.06328
Villalba, J. J., and Provenza, F. D. (2001). Preference for polyethylene glycol by sheep fed a quebracho tannin diet. J. Anim. Sci. 79, 2066–2074. doi: 10.2527/2001.7982066x
Villalba, J. J., and Provenza, F. D. (2007). Self-medication and homeostatic behaviour in herbivores: learning about the benefits of nature's pharmacy. Animal 1, 1360–1370. doi: 10.1017/S1751731107000134
Villalba, J. J., Provenza, F. D., Hall, J. O., and Lisonbee, L. D. (2010). Selection of tannins by sheep in response to gastrointestinal nematode infection. J. Anim. Sci. 88, 2189–2198. doi: 10.2527/jas.2009-2272
Villalba, J. J., Provenza, F. D., and Shaw, R. (2006). Sheep self-medicate when challenged with illness-inducing foods. Anim. Behav. 71, 1131–1139. doi: 10.1016/j.anbehav.2005.09.012
Viñuela-Fernández, I., Jones, E., Welsh, E. M., and Fleetwood-Walker, S. M. (2007). Pain mechanisms and their implication for the management of pain in farm and companion animals. Vet. J. 174, 227–239. doi: 10.1016/j.tvjl.2007.02.002
Welsh, E. M., and Nolan, A. M. (1994). Effects of nonsteroidal antiinflammatory drugs on the hyperalgesia to noxious mechanical stimulation-induced by the application of a tourniquet to a forelimb of sheep. Res. Vet. Sci. 57, 285–291. doi: 10.1016/0034-5288(94)90119-8
Wood, G. N., Molony, V., Fleetwoodwalker, S. M., Hodgson, J. C., and Mellor, D. J. (1991). Effects of local-anesthesia and intravenous naloxone on the changes in behavior and plasma-concentrations of cortisol produced by castration and tail docking with tight rubber rings in young lambs. Res. Vet. Sci. 51, 193–199. doi: 10.1016/0034-5288(91)90013-E
Keywords: self-medication, pain relief, animal welfare, husbandry procedures, sheep
Citation: Marini D, Colditz IG and Lee C (2021) Can Lambs in Pain Identify Medicated Feed? Front. Anim. Sci. 2:741631. doi: 10.3389/fanim.2021.741631
Received: 15 July 2021; Accepted: 27 September 2021;
Published: 22 October 2021.
Edited by:
Janice Swanson, Michigan State University, United StatesReviewed by:
Sabrina Lomax, The University of Sydney, AustraliaE. Tobias Krause, Friedrich-Loeffler-Institute, Germany
Copyright © 2021 Marini, Colditz and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danila Marini, ZGFuaWxhLm1hcmluaUBjc2lyby5hdQ==
 Danila Marini
Danila Marini Ian G. Colditz
Ian G. Colditz Caroline Lee
Caroline Lee