
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Anim. Sci. , 18 November 2021
Sec. Animal Welfare and Policy
Volume 2 - 2021 | https://doi.org/10.3389/fanim.2021.740778
This article is part of the Research Topic Positive Welfare: From Concept to Implementation View all 9 articles
Observations of play in animals have been suggested as a promising indicator of positive emotions and thus of positive animal welfare. However, if play can follow the proposed reward cycle concept where animals estimate and value reward differently in different phases of the cycle (anticipation, consummation and post-consummation) is unclear. To investigate if a reward cycle for play exists in growing pigs, we carried out an exploratory study where pigs were tested when they were naïve to a reward cycle test (first occasion) against when they were accustomed to going through the test after having the access to an open play arena with objects. Forty undocked pigs were housed in a weaner stable with two castrated males and two females per pen. Within each litter, we randomly selected and tested one male and one female test pig, each being tested as naïve or accustomed to the testing environment. The first week the pigs (n = 20) were tested four times and regarded as naïve during the first day. After that they were regarded as accustomed, and were tested twice a week for 3 weeks. We observed the behavior of the tested pairs in three subsequent stages: (1) in a holding pen 3 min, (2) in a play arena 15 min, and (3) in their home pen 10 min. When accustomed, pigs showed more locomotor play, social interactions and standing, and a tendency of more orientation toward the play arena and exploring bars facing the play arena (i.e., reward-seeking behavior) in the holding pen than when they were naïve, suggesting an anticipation to enter the play arena. Performing high numbers of object play in all sessions, and for accustomed pigs more exploration and social interaction, but less locomotor play and walking in the play arena may suggest consumption of play and exploration. Finding more lying and sitting in accustomed pigs, but less standing and walking in the home pen is in line with the previous hypothesis of the post-consummatory behaviors. Our study showed mixed results for the existence of a reward cycle for play in pigs and generated questions for future research.
Positive emotions are suggested to be shown in a rewarding environment, which elicit a cognitive appraisal in the central nervous system that is followed by physiological and behavioral reactions (Boissy et al., 2007). Yeates and Main (2008) indicate that it is important to consider both the animals positive feeling, i.e., what an animal “likes,” and which resources the animals are motivated to obtain, i.e., what an animal “wants.” If animals' likes to perform play behavior it may be possible to investigate if the animals will show behaviors indicating that it wants to play. Paul and Mendl (2018) discuss the problem that there is not one uniform definition of positive emotions, but there are two types of definitions. The descriptive definitions, that are many and varying, are used more to tell “this is what I am going to talk about.” The prescriptive definitions can be grouped into three categories; (1) emotional building blocks (for ex., Anderson and Adolphs, 2014), (2) emotions as states elicited by instrumental reinforces (for ex., Rolls, 2014), and (3) emotions as states that mediate goal directed learning (for ex., Dickinson and Balleine, 2009). If animals through repeated exposures are conditioned to expect or anticipate that a certain procedure will lead to the possibilities to perform play behaviors we may be able to identify behaviors that indicate this anticipation. Lawrence et al. (2019) have created an interpretation of the inter-relationship between the four elements of Positive Animal Welfare (PAW) and the scientific literature. The elements are: (1) positive emotions (the capacity of animals to experience positive emotions), (2) positive affective engagement (seeks to create a link between positive emotions and behaviors animals are motivated to engage in), (3) Quality of Life (QoL, acts to give PAW a role in defining an appropriate balance of positive over negative emotions) and (4) Happiness (brings a full life perspective to PAW). Play behaviors may induce positive emotions that animals are motivated to engage in, and in the process of playing increase QoL and bring happiness to animals.
There is a limited knowledge on how positive emotions in animals are expressed behaviorally, but among certain affiliative behaviors and vocalization, observations of play have been proposed as a promising indicator of positive emotions and good animal welfare (Boissy et al., 2007; Yeates and Main, 2008). If engaging in play is experienced as positive, it seems reasonable that animals would anticipate performing such behavior (Fraser and Duncan, 1998). Such a finding would also render support to the concept of a “reward cycle” or a “successful cycle of reward acquisition” as proposed by Keeling et al. (2008) and Mendl et al. (2010), respectively.
These cycles involve a wanting (anticipation) phase, a consummation phase and a post-consummation (satisfactory) phase (Keeling et al., 2008). Accompanying behavioral responses (e.g., anticipation, consumption, satisfaction) have also been suggested to be associated with positive emotions (Spruijt et al., 2001). Anticipatory behavior is commonly regarded as the behavioral response that animals show from the time when they receive a stimulus signaling that a forthcoming reward will be presented until the reward is presented (Anderson et al., 2020). The animal has learned during repeated exposures to the stimulus the connection between the stimulus and the reward, i.e., it has been conditioned to the stimulus. During anticipatory periods mink and horses have shown an increased activity (Hansen and Jeppesen, 2006; Peters et al., 2012) and mink, laying hens and calves have shown a proximity to where the reward will be presented (Vinke et al., 2006; Wichman et al., 2012; Neave et al., 2021). In a previous study of the reward cycle in lambs, Chapagain et al. (2014) found that lambs performed an increasing number of recordings sniffing the pen and standing facing the play arena over the 4 weeks of testing. Anderson et al. (2015) found that lambs receiving a palatable food or opportunities to play in an arena had a higher frequency of behavioral transitions (i.e., number of times the animal changes between different behaviors), more walking of longer duration and more exploration of shorter duration. Calves were found to show an increased frequency of behavioral transitions and decreased latency to access the reward arena (a larger enriched pen) when being housed in smaller basic pens compared to calves being housed in larger enriched pens (Neave et al., 2021). In a critical review about anticipatory behaviors in animals Anderson et al. (2020) discuss that many studies use the frequency of behavioral transitions as a measure of positive emotions, but raise the problems that these responses may relate more to frustration than to a positive emotional state.
In pigs, it has been found that during anticipation for a positive reward they showed more behavior toward the door from which they entered into the anticipation box (Reimert et al., 2013), made less high-frequency vocalizations and had a shorter latency to approach the anticipated event (Imfeld-Mueller et al., 2011) and showed a higher proportion of activity (sitting, standing or walking), (Imfeld-Mueller and Hillmann, 2012).
When the reward is presented for the animal the consumption of the reward follows. This consists of either eating food in humans (Kringelbach et al., 2012), dust bathing in hens (Zimmerman et al., 2011) or performing play behaviors (Anderson et al., 2015), which was the subject of this study. Such behaviors are performed during the consummation phase. In humans, the consumption phase has been called the liking phase (Kringelbach et al., 2012). When testing the reward cycle for play in pairs of lambs in two studies following each other, the consumption was performance of social, locomotor and object play (Chapagain et al., 2014; Anderson et al., 2015), and when offering a palatable food reward it was eating (Anderson et al., 2015).
After consumption of the reward, the animal is expected to show satisfaction or relaxation such as resting and eating. These behaviors are associated with the post-consummatory phase (Keeling et al., 2008). To our knowledge, there are only few studies that have investigated how this phase would express itself behaviorally following a reward, but it seems plausible that this phase will be linked to the previously accustomed reward. Kringelbach et al. (2012) show in a food pleasure cycle for humans how consummation is followed by a satiety or learning phase, where one learns and updates predictions for the reward. However, in a previous study of the reward cycle for play in lambs eating was the main behavior during the post-consumption phase after having been in a play arena (Chapagain et al., 2014). During the first min the lambs were standing and walking more, but from the second min eating had the highest number of recordings (Chapagain et al., 2014).
The aim of this study was to explore whether a reward cycle for play in pigs exist by comparing naïve pigs going through a play arena test for the first time with accustomed pigs going through the same test after repeated exposures to the play arena. To investigate this we used tentative behaviors found in previous studies on other species. We predicted that the accustomed pigs would show more walking, exploration and orientation toward the play arena during the anticipation phase, more object, locomotor and social play behaviors in the arena during the consumption phase and more resting and eating during the post-consumption phase than the naïve pigs. Finding a positive correlation between those behaviors being more common in the experienced pigs than in the naïve pigs during the anticipation phase and play in the arena and between play in the arena and resting in the home pen would support our hypothesis that the pigs were anticipating play, and that consumption of more play resulted in more relaxation.
The Swedish Ethical Committee of Experimental Animals in Uppsala approved this study (Drn: C 34/12).
The study was conducted at the Swedish National Livestock Research Center Lövsta, Uppsala during April-May 2012. 10 L from the second farrowing batch of Specific Pathogen Free (SPF) Yorkshire sows were used. Five sows were inseminated by Yorkshire boars and five sows by Landrace boars resulting in five litters of pure-bred Yorkshire (YxY) and 5 L of half Yorkshire and half Landrace (YxL). Piglets were undocked and weaned at a mean age of 33 days by removal of the sow. Half of the litters (both YxY and YxL) had been subjected to one enrichment object per week, i.e., a knotted rope, a ball or a tire, during the 3 weeks before weaning in order to test which enrichment activated the piglets the most. For the first 6 days post-weaning, all three enrichment objects were placed in the five pens, which had objects before weaning, while the other five pens continued to have no objects (see Lidfors et al., 2020). Pigs were weighed three times during the study; at weaning (mean ± SE: 15.47 ± 0.64 kg), 2 weeks after weaning (28.12 ± 0.93 kg) and 4 weeks after weaning (42.95 ± 1.26 kg).
At day 11 post-weaning, 4 growing pigs from each of the 10 L (two females and two castrated males of similar weight) were moved to a weaner stable and housed together with their littermates in 10 separate pens. The pigs were given an acclimatization period in the weaner stable of 5 days before the observations begun. Each pen had a total area of 6.5 m2 (3.25 × 2 m) with concrete solid floor along the feeding trough and the resting area and elevated slatted floor for the manure area and water nipple. The walls were constructed of galvanized steel bars. These pens were henceforth called “home pen(s).” The humidity was adjusted at 80% and the temperature ranged from 21.3–27.3°C. Water was provided ad libitum through a water nipple. The growing pigs were fed a commercial weaner feed (SOLO 331 P BK, Lantmännen) in a stainless steel feeder trough. Feeding was provided manually three times a day at 8:00 a.m. (1 h before observations), 12:00 a.m. and 15:00 p.m. (1 h after finishing the observations). Pigs also received 0.5 to 1 kg long straw (wheat) twice daily. Feces were removed from the home pens in the morning and around 12 a.m.
To investigate the reward cycle in pigs, in one end of the testing room (Figure 1), a “play arena” (1.3 ×4.5 m) for the consumption of play and two holding pens for measuring if pigs would show anticipation were created. The play arena (Figure 1), where we aimed to stimulate play behaviors had concrete floor and was covered with long-cut straw. In the arena, two of each of a single welly, brushes, traffic cones, rubber pipes, balls and knotted ropes were presented. The knotted ropes were hanging from the wall, and all other objects were placed on the floor. Half of the pigs (5 of the 10 pens) had previous experience of knotted ropes, balls and rubber rings hanging in their pens before and the first 6 days after weaning (Lidfors et al., 2020). Adjacent to the arena, a pen (1.3 × 1.55 m) was built, containing a gate toward the home pen corridor and the gate entering into the arena. This pen was henceforth called “holding pen.” The gate between the play arena and the holding pen consisted of bars so that pigs could see through. Due to lack of space in the room and to avoid having pigs using up their energy on walking too far to get to the holding pen we built up the holding pen on the right side of the play arena when testing pigs from the five pens on the right side. The holding pen was build up to the left side of the play arena when testing the pigs on the left side. When one holding pen was used, the other holding pen was deconstructed in order to increase the space in the play arena.
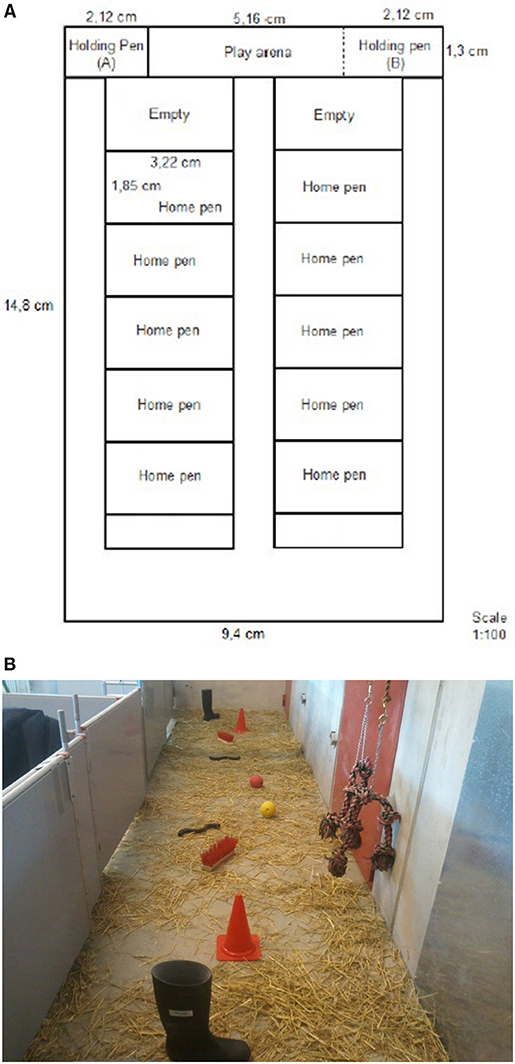
Figure 1. Drawing of the stable. The room had 10 home pens, a play arena and the holding pen for testing pig behavior. The holding pen was moved between the right and left sides so that pigs did not have to walk so far to enter the holding pen (A) vs. (B).
In each of the home pens, one female and one castrated male pig were randomly chosen as focal animals and marked with pig marking spray. The two remaining pigs were not used for behavioral observations. Information on previous experiences of objects was blind to the observers. The first day of behavioral observations the pigs were naïve to the procedure, and they are hereafter called “naïve pigs.” Pigs were always tested in the same pair and the other two pigs remained in the home pen. The procedure was carried out in three steps:
(1) A pair of pigs was led into the holding pen, where they remained for 3 min.
(2) The gate into the play arena was opened and pigs entered into the play arena where they were kept for 15 min.
(3) The pair was led back to their home pen where they were observed for 10 min.
During the first week of the observations each of the ten pairs underwent the procedure four times, and only during the first day pigs were regarded as naïve to the procedure. During this week, the naïve pigs were expected to learn to associate entering into the holding pen was something that preceded entering into the presumably rewarding play arena, thus being accustomed for the coming weeks of testing. In the three coming weeks, each of the 10 pairs underwent this procedure twice per week with a 2 day gap in between test sessions, which is hereafter called “week 2, 3 and 4” of testing. After each test session, faces were removed from the arena and objects were re-placed on the same place. Objects were washed at the end of each week and not after each test session or day.
A pilot study was carried out where four pigs were tested in an identical experimental set-up to test for practical limitations, to construct an ethogram and to standardize observations between the observers. Standardization between observers was done by watching the same videos individually and scoring behavior, and thereafter comparing the recordings and fine tuning recordings. No test of inter-observer reliability was carried out. Two observers, one for each pig, observed the behavior of the focal pigs in each session. Three observers carried out observations throughout the study, one observer was the same in each session, whereas one of the other observers carried out observations during weeks 1 and 3 and the other during weeks 2 and 4. It was not possible to keep the observers blind to if pigs were accustomed or not to the procedures. Behavioral observations were recorded in the holding pen, the arena and the home pen (see previous explanation). Recordings were conducted during the first session in week 1 (naïve pigs) on all pigs during the same day, and during all sessions during the three study weeks (accustomed pigs). The pens were tested in order of placement and initial pair each day alternated.
Three protocols were prepared for each phase of the test. In each protocol, focal pigs were observed using both instantaneous scan sampling every 30 s (for behaviors with long durations, Table 1) and continuous frequency sampling within every 30 s (for behaviors with short durations, Table 1). These observations were performed during the complete periods in each pen (3 + 15 + 10 min). A stopwatch gave a sound signal every 30 s to facilitate these recordings.
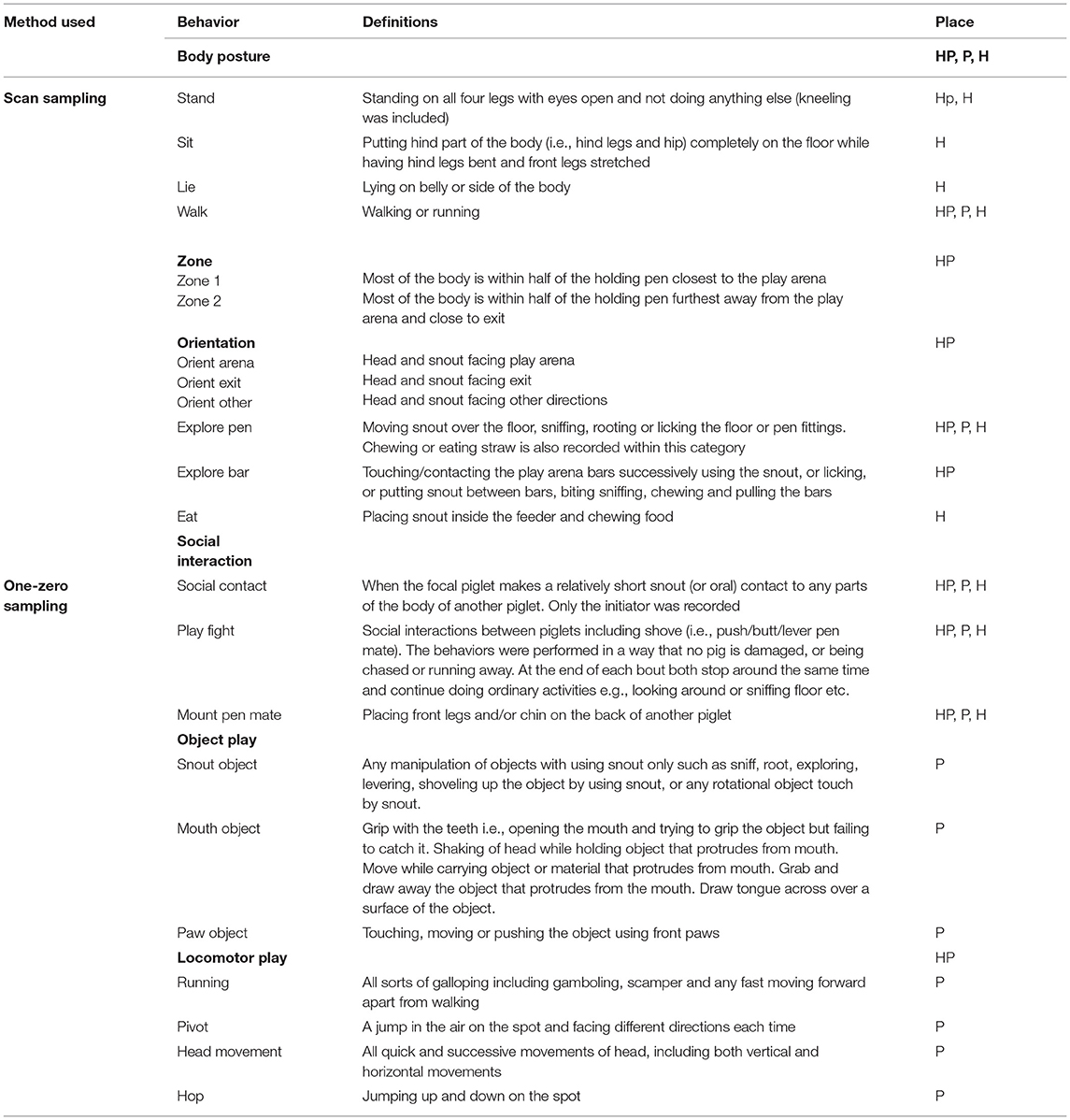
Table 1. Behavioral recording methods, behaviors, their definitions and place of observation (HP, Holding pen; P, Play arena; H, Home pen).
Statistical analysis was performed in SAS Software version 9.3 (Statistical Analysis Systems, SAS Institute, Cary, NC, USA). A non-parametric GLIMMIX procedure (Generalized Linear Model for Mixed procedure) was utilized for dependent variables taking into account a Binomial distribution for all investigated variables. Statistical analysis was done separately for the holding pen, the play arena and the home pen. During week 1 we only used the data set of 1 day when the pigs were naïve to the procedure and during week 2, 3, and 4 we used 2 days per week. Pen was used as a random effect. For the purpose of multiple comparisons, we used a Studentised Maximum Modulus, SMM method, and differences within effects were tested with a t-test. The model tested if there were significant effects of the following factors; week (Week 1 = naïve, Weeks 2, 3, 4 = accustomed), sex (females, castrated males), time (holding pen;1, 2 vs. 3 min, play arena; 0–5 min, 5–10 min, 10–15 min; home pen; first 5 vs. last 5 min), previous experience (1 = if a pig had objects before and after weaning or 2 = not). The effect of time was excluded from the model for variable “walk” in the holding pen and the effect previous object experience from the model for variable “standing” in the home pen since data did not convert. The number of instantaneous recordings of “stand” in the play arena was insufficient to run the analysis. In order to show the results means per pen and pig within each week were first calculated, and then medians with 95% confidence intervals (CL) were calculated. All reported p-values are 2-tailed, and significance level was set at p < 0.05 and tendencies at p < 0.1.
Spearman correlation coefficients using procedure CORR was used to assess if there were positive correlations between those behaviors being more common in the experienced pigs than in the naïve pigs during the anticipation phase and play in the arena. Additionally, procedure CORR was used to assess if there were any positive correlations between play in the arena and resting and eating in the home pen that could support our hypotheses about the reward cycle for play. This was done only for week 4 as we wanted to make sure that the pigs had been completely accustomed to the procedures of being moved from the holding pen, to the play arena and back to the home pen at this point. Tests were done on a pen level (n = 10). Similar correlations were performed in other animal studies investigating the reward cycle or individual stages of the cycle (Chapagain et al., 2014; Anderson et al., 2015).
The significant differences in the performance of behaviors in the three stages of the reward cycle when considering the main fixed effects are summarized in Table 2. The most significant effects were week of testing and time. Regarding sex and experience with objects, some results were found in the holding pen and home pen. In the holding pen, castrated male pigs had a higher number of recordings of “explore pen” than female pigs. Pigs that had experience with objects before and after weaning had a higher number of recordings of “orient arena” than pigs without the experience. In the home pen, castrated males had a higher number of recordings of “lie” than females, but a lower number of recordings of “stand,” “walk” and “eat.”
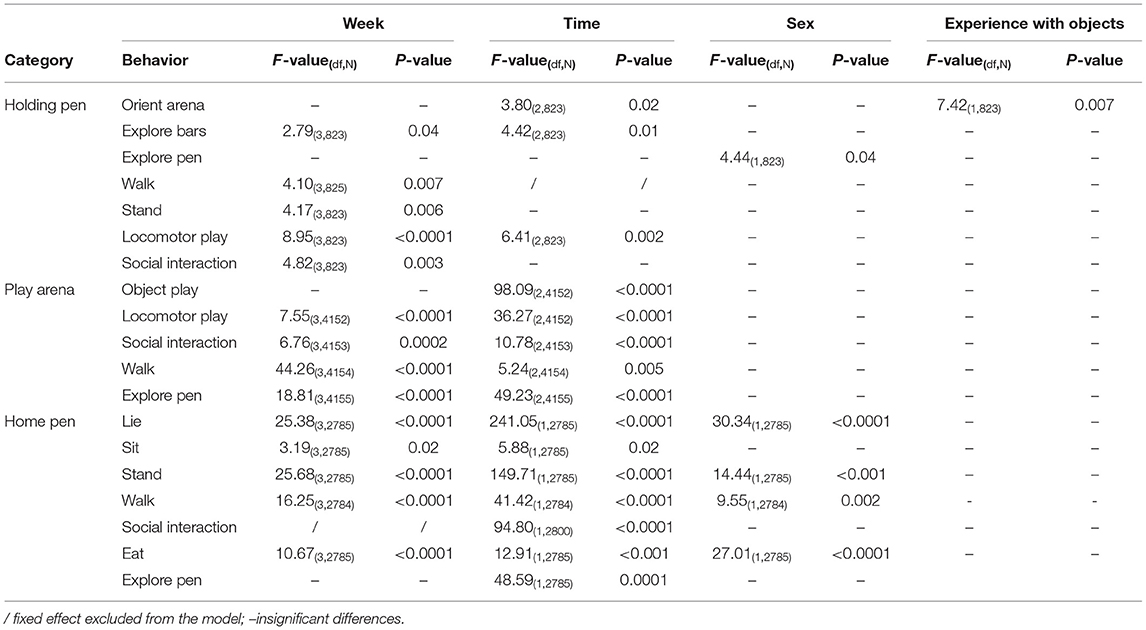
Table 2. Behavioral observations in different stages of a reward cycle with significant differences across weeks, time, sex, and experience with objects.
When pigs had become accustomed to enter the play arena, they had higher number of recordings of “orient arena,” “explore bars,” “stand,” “locomotor play” and “social interaction,” and fewer number of recordings of “walk” than when they were naïve to the play arena (Figure 2A). Over 4 weeks of testing, changes in the number of recordings of “walk” showed a gradual decrease while “locomotor play” and “social interaction” was significantly higher in accustomed pigs compared to the naïve pigs in each of the tested weeks (Figure 2A).
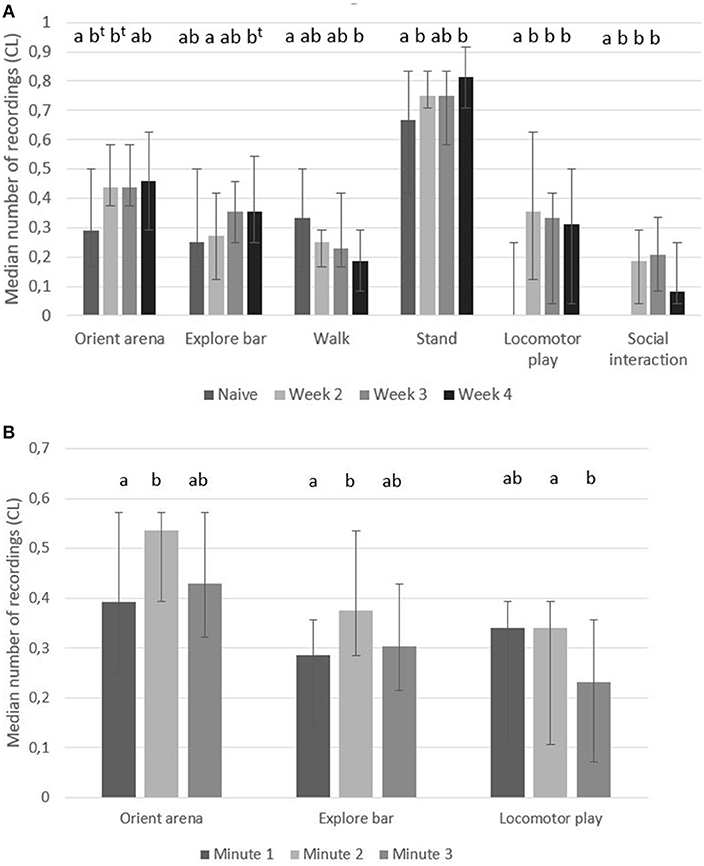
Figure 2. Holding pen behavior. Median number of recordings (95% CL) of behaviors in pairs of pigs when kept in a holding pen for 3 min before being let into a play arena; (A) when they were naïve (first time in the arena, week 1) or accustomed (week 2–4 in the arena) and (B) for each of the 3 min separately (week 1–4). Significant differences between weeks or times are indicated by different superscripts (p < 0.05, t when p < 0.1).
In the 3 min in the holding pen, there was a significant effect of time in the behaviors “orient arena,” “explore bar” and “locomotor play” (Figure 2B). In the second min pigs had a higher number of recordings of “orient arena” and “explore bar” than during the first min while in the third min pig had had a lower number of recordings of “locomotor play” compared to the first 2 min (Figure 2B).
Object play was the most commonly recorded behavior in the play arena, but it did not differ between accustomed pigs and naïve pigs (p = 0.17, F = 1.673, 4,168). In the play arena, accustomed pigs had a higher number of recordings of “explore pen” and “social interaction” than naïve pigs, but a lower number of recordings of “locomotor play” and “walk” (Figure 3A).
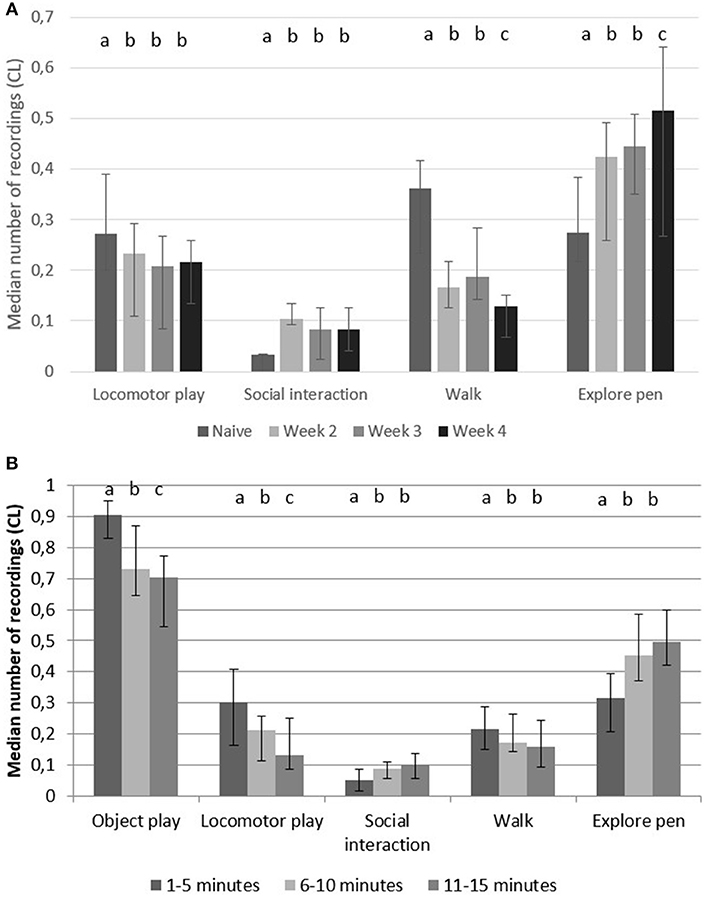
Figure 3. Play arena behaviors. Median number of recordings (95% CL) of object play, locomotor play, social interactions, walk and explore pen in pairs of pigs (A) during 15 min in a play arena when they were naïve (first time in arena) and when they were accustomed (week 2–4 in arena) vs. (B) during 5 min periods during all 4 weeks (n = 10 pairs). Significant difference between weeks vs. time in play arena are indicated by different superscripts (p < 0.05).
When dividing the 15 min of testing into three 5 min periods, the time periods had a significant effect on “object play,” “locomotor play,” “social interaction,” “walk” and “explore pen” (Table 2). “Object play,” “locomotor play” and “walk” occurred more during the first 5 min compared to the following 10 min, but “social interaction” and “explore pen” occurred more in the final 10 min (Figure 3B).
There were no significant effects of previous experience to objects or sex of pigs. Regarding the six tested objects, pigs had the highest number of interactions (mouth, sniff, paw) in the following order; brush (148.4 ± 6.68, mean ± SE, n = 10 pens), traffic cone (129.5 ± 11.48), rubber pipe (129.4 ± 21.33), knotted rope (100 ± 7.31), welly (97 ± 8.42) and ball (52.4 ± 4.03).
After finishing the testing in the play arena and returning back to their home pen, the most frequently recorded behavior was “lie” in accustomed pigs, whereas naïve pigs had similar number of recordings of “lie” and “stand” (Figure 4A). On the same figure it is illustrated that accustomed pigs performed “sit,” but naïve pigs did not. Accustomed pigs had a lower number of recordings of “stand” and “walk” than naïve pigs. The number of recordings of “eat” was low, but accustomed pigs had a higher number of recordings of “eat” during week 3 and a tendency for higher numbers during week 2 and 4 compared to naïve pigs (Figure 4A). It was noted that the two remaining pigs in the home pen often made social contact with the returning two pigs, including pushing them up from a lying position.
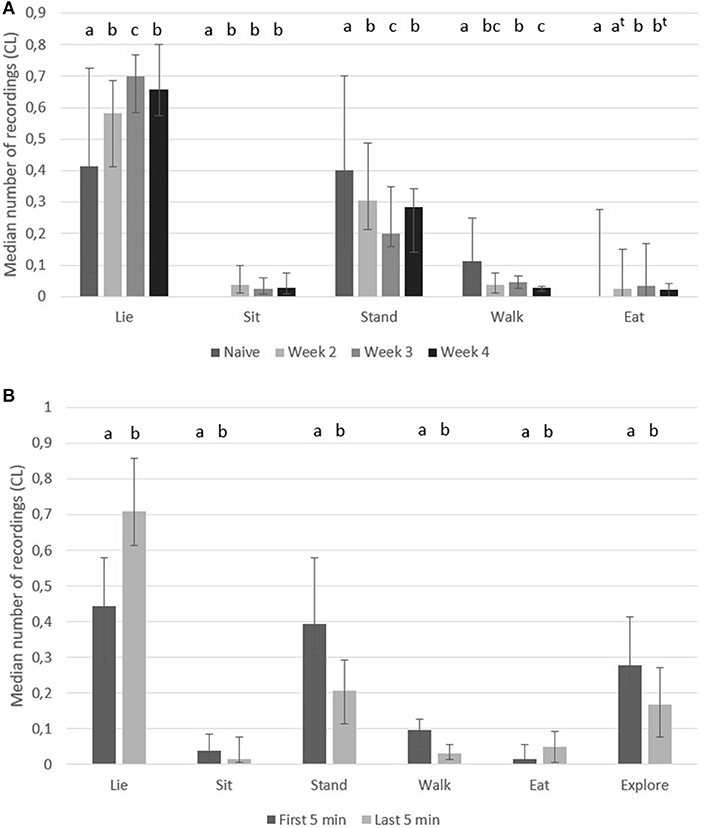
Figure 4. Home pen behavior. Median number of recordings (95% CL) of body postures in pigs (A) in the first 10 min after returning to their home pen from the play arena when they were naïve (first time in arena) and when they were accustomed (week 2–4 in arena), and (B) during the first 5 min and the last 5 min (n = 10 pairs). Significant difference between weeks vs. time are indicated by different superscripts (p < 0.05, t when p < 0.1).
In the first 5 min, pigs performed a higher number of recordings of “stand,” “walk,” “sit,” and “explore pen,” but performed a lower number of recordings of “lie” and “eat” (Figure 4B).
In order to explore if the results from this study indicates the existence of a reward cycle for play in commercial pigs we have put together the results where naïve pigs differed significantly from the accustomed pigs (Figure 5). We tested four of the behaviors in the holding pen that had increased from naïve pigs to experienced pigs against object play in the play arena during week 4. Further, we tested the total amount of play (“total play” = object play + locomotor play + social interactions) in the play arena against “social interaction” in the holding pen and “lie” and “eat” in the home pen during week 4. The predictions of a positive correlation between these behaviors in the holding pen and play behavior in the play arena could not be confirmed. There was only one tendency of a positive correlation between “social interactions” in the holding pen and “total play” in the play arena (r = 0.57, p = 0.088, n = 10). The following results were found when comparing the holding pen and the play arena; “social interactions”–“object play” (r = 0.45, p = 0.19, n = 10), “orient arena”–“object play” (r = 0.25, p = 0.48, n = 10), “explore bar”–“object play” (r = 0.17, p = 0.63, n = 10), and “locomotor play”–“object play” (r = 0.23, p = 0.53, n = 10). When comparing the play arena and the home pen the following results were found; “total play”–“lie” (r = −0.30, p = 0.40) and “total play”–“eat” (r = 0.097, p = 0.79).
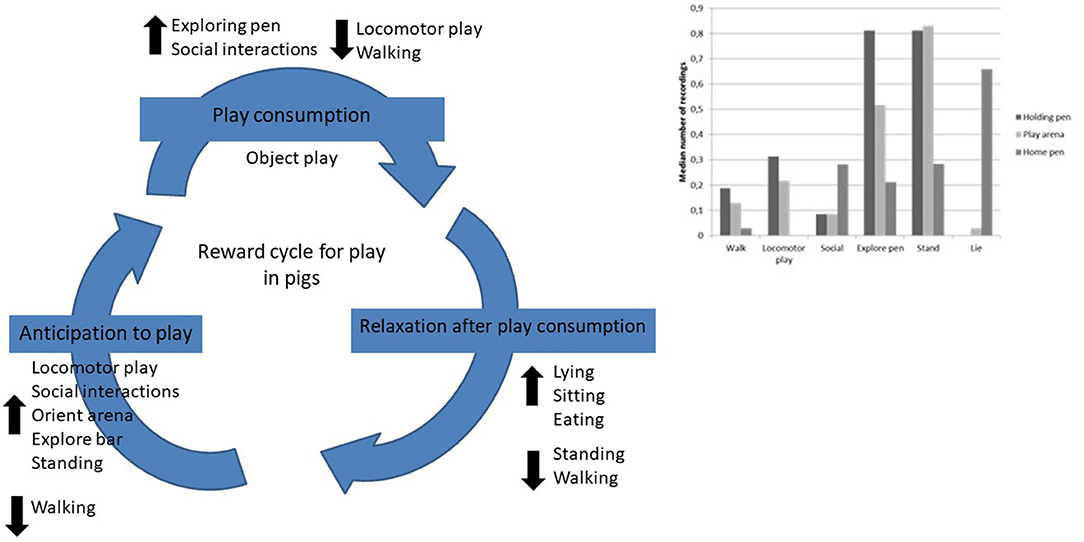
Figure 5. Reward cycle proposal for play in pigs based on the their behaviors in the holding pen, play arena and home pen.
In regard to the results of this study, a reward cycle for play in the 7–10 weeks old pigs showed mixed results. The main findings that could support the existence of a reward cycle was that pigs accustomed to enter the play arena showed more “locomotor play,” “social interactions,” “explore bar,” “stand,” and tended to show more “orient arena,” but showed less “walk” in the holding pen than naïve pigs and these behaviors could indicate that they anticipated to access the play arena. Once in the play arena accustomed pigs showed more “social interactions” and “explore pen,” but less “locomotor play” and “walk” than the naïve pigs. Regardless of whether pigs were naïve or accustomed they showed a large amount of “object play,” and together with the performance of locomotor play and social interactions the pigs could be regarded to have shown consumption of play in the play arena. When returning to their home pen accustomed pigs showed more “lie,” “sit” and “eat,” whereas naïve pigs showed more “stand” and “walk” indicating that accustomed pigs were more relaxed after their session in the play arena. However, the test of correlations between behaviors in the holding pen and play arena vs. in the play arena and home pen did not show any associations between the pigs performance in the different areas of the test.
Increased locomotor play and social interactions including some elements of social play performed in the holding pen indicated that the accustomed pigs had learned to associate between being in the holding pen and being let into the play arena where they would perform different types of play behaviors. During the 3 min in the holding pen accustomed pigs tended to orient toward the play arena and explored the bars facing the play arena more than the naïve pigs. This suggest that the pigs had learned that they would be let into the play arena soon. Dudink et al. (2006) found that when pigs were offered positive stimuli, e.g., extra space, food, or straw, they were orientated more often toward the location in which the reward was offered. Also silver foxes spent more time in the front of their cages where reward was offered by a person (Moe et al., 2006). Our set-up was so that the pigs could see all the objects placed on the floor when they were looking through the bars from the holding pen. Thus, we cannot separate between if the pigs saw the objects and just wanted to access them or if they remembered the interactions with the objects from the previous sessions. To separate these things we could have used a full wall, which was done in a similar study with lambs (Anderson et al., 2015).
Classical conditioning was used to induce learning in the pigs in this study. In order for the pigs to associate being in the holding pen (unconditioned stimuli) with an unconditioned response (being let into the play arena), they had four repetitions of the procedure before they were tested as accustomed pigs during two repetitions per week for 3 weeks. Over time the learning was expected to develop so that the holding pen became a conditioned stimuli and entering the play arena became a conditioned response. The time in the holding pen was the same throughout the study as was done in previous studies (van der Harst et al., 2003; Chapagain et al., 2014; Anderson et al., 2015), but other studies have increased the time to allow animals to show more anticipatory behaviors (van den Bos et al., 2003). We only used the first test day of the four training days, and called the pigs naïve during this day, but it could have been interesting to also show how the learning developed day by day, as done by Anderson (2016). There are many ways of studying how learning through classical condition takes place, but that was not the main focus of this study.
Higher frequency of locomotor play and social interactions in the accustomed pigs are forms of play behaviors which indicate that they were preparing themselves for the coming play (i.e., anticipating to play, Dudink et al., 2006) and can be a sign of finding play a rewarding experience. This is in agreement with our first prediction, i.e., accustomed pigs would show more play than naïve pigs which was based on other studies in pigs showing higher activity level and play behavior when anticipating environmental enrichment (Dudink et al., 2006) and increased level of activity and running while anticipating food reward (Haskell et al., 1996).
Pigs are known for their exploratory and foraging nature (Van de Weerd et al., 2003), and the exploratory nature of pigs has a crucial role in pigs' “survival” (Studnitz et al., 2007). Therefore, the finding in this study that the accustomed pigs explored the bars facing the play arena more than the naïve pigs may be a sign that they were anticipating to enter the play arena. In previous studies of anticipation in rats they were found to have higher levels of exploration (Spruijt et al., 2001).
Accustomed pigs showed more standing than naïve pigs, but less walking. However, both naïve and accustomed pigs never showed sitting or lying positions in the holding pen. In previous studies, horses increased their time spent standing and walking as a result of food anticipation (Peters et al., 2012), rats increased their locomotor behavior in anticipation for some form of reward (van den Bos et al., 2003), and silver foxes and mice increased their activity when anticipating both feed and non-feed related rewards (Moe et al., 2006; Luuk et al., 2012). The difference in our study compared to the previous ones is that we found a reduction in walking in the accustomed pigs compared to when they were naïve to the test. However, as locomotor play was absent in naïve pigs and performed to a relatively high degree in the accustomed pigs adding locomotor play and walking together would give an increase in general locomotion in the pigs in our study. Thus, our results agree with previous studies that general activity level increased in pigs accustomed to anticipate the reward.
It is possible that a phase of anticipation that becomes too long can lead to inactivity or frustration in animals (Bloomsmith and Lambeth, 1995). The risk of causing frustration in animals when studying anticipation was thoroughly discussed by Anderson et al. (2020). It was noted during the pilot study that when pigs had to spend longer time in the holding pen (e.g., 4–6 min), they attempted to jump over the exit gate leading back to their home pens. In this study “locomotor play” was significantly lower, and “orient arena” and “explore bar” was slightly lower during the third min. Thus, in future studies an anticipation time of 2 min may be enough, but definitely no longer than 3 min.
As predicted, the pigs performed all three types of play (i.e., object, locomotor and social play) in the play arena. Object play was the most performed type of play during the play arena observation. This may be due to that the pigs had the least experience with these objects compared with other substrates in the environment (i.e., straw, play partners, etc.). Since pigs are curious, exploratory and foraging (Van de Weerd et al., 2003), they investigated all new objects in their environment. It is likely that pigs would lose interest in the objects with time if they would have continuous access to them. Trickett et al. (2009) reported this, where pigs with continuous access to rope interacted more with it the first week and at the time after changing it to a fresh one. Van de Weerd et al. (2003) reported similar results when within 5 days a significant habituation to most objects occurred and object interaction decreased significantly by time. In this study, there were no significant effects of having previous experience with objects before weaning and during 6 days after weaning. This may be due to that they then had three objects (a hanging rope, a rubber ball and a rubber tire) permanently hanging in their pen (Lidfors et al., 2020). Since then some time has passed without having access to any objects, so some novelty effect was probably occurring in this test. In the arena, two of each of a single welly, brushes, traffic cones, rubber pipes, balls and knotted ropes were presented. The knotted ropes and the balls were the same as half of the pigs had access to before and after weaning. The most popular objects were brushes, traffic cones and rubber pipes, none of which pigs had previous experience with.
Locomotor play was higher in the naïve pigs compared to the accustomed pigs. This can be the result of lower weight in the pigs at that age that made it easier to move faster or perform physically active behaviors such as running, pivoting etc. The temperature in the pig room was increasing during the study and went up to 27.3°C at some point, which may also have affected their level of movements over time. Huynh et al. (2005) found decreased activity levels in growing pigs when temperatures exceeded 24.2°C. Locomotor play was highest during the first 5 min, which can be due to the access to a bigger space after being in a holding pen that induced locomotor activity during the first min after entrance to the larger play arena. These results are in agreement with Jensen and Kyhn (2000) who found that dairy calves performed more locomotor play after they were introduced to a larger space. This phenomenon is called a rebound effect and is a response of animals to having been kept in a space that did not allow for locomotor activity (Jensen and Kyhn, 2000). Blackshaw et al. (1997) noted that even if locomotor play in pigs increased with age it reached a constant level at the age of 26–30 days. Pigs in this study had an age of 49–77 days, thus being considerably older than in that study. However, we believe that by bringing them to the play arena we stimulated play to levels higher than could be expected if they had stayed in their home pen.
Social interactions, including social play, was the least performed play type in the play arena and it increased over 15 min of observation and was significantly higher during the last 5 min of the observation. According to Jensen et al. (1998) social play and locomotor play usually are connected to each other. However, in this study we noted that sometimes when one pig started to run the other pig started to follow but this locomotor play did not lead to play fight or social interaction; rather they ended it by going back to pen exploration or object interaction. That social interaction including social play was performed the least of all three types of play during all sessions, can have to do with the novelty effect. By that we mean that the play partners were the pen mates and siblings, so they were not new to each other and therefore no novelty effect was involved in social interactions. In previous studies on male lambs being let into a play arena after 5 min in a holding pen social play was the most common play behavior (Chapagain et al., 2014; Anderson et al., 2015). The difference between studies may be that the lambs only had three objects (tunnel, chain and ball or platform and two balls) and a larger area.
The most observed body posture in the play arena was standing, followed by walking. Both standing and walking can suggest that animals were alert/active and curios to explore the arena. The naïve pigs performed more walking than the accustomed pigs, and one can interpret this as an attempt to discover the new environment. However, exploration was lowest in the naïve pigs and increased over the second, third and fourth week of testing as well as being higher during the last 10 min of the time in the play arena compared to the first 5 min. Špinka et al. (2001) proposed that exploration has associations with play in many ways and Blackshaw et al. (1997) proposed that exploration can be the first movement toward play initiation. Therefore, higher exploration of the pen during both the last week and the last 5 min of the observations can be interpreted as habituation to the objects and a decrease of the novelty effect of these objects (Van de Weerd et al., 2003; Trickett et al., 2009). So, it could be that they compensated their novelty seeking motivation with higher performance of exploratory behaviors toward the pen instead.
Accustomed pigs were lying more, while standing and walking less than naïve pigs during their first 10 min in the home pen, and they also showed small amounts of sitting which naïve pigs did not. This indicates that they needed to relax after their active period away from the home pen, and supports our predictions that pigs would show relaxation after having been offered access to a play arena. Similar results were found with humans showing a satiety phase after consumption of food (Kringelbach et al., 2012). Lying was higher during the second 5 min than during the first 5 min in the home pen, thus indicating that it took some time for the pigs to relax. Similar finding were reported by Chapagain et al. (2014) where lambs were still in an activated stage during the first min after returning to their home pen after having spent 20 min in a play arena. However, that study found that lambs performed higher amounts of eating silage when returning to their home pen than lying down, indicating that they needed to restore their energy loss after the play session. In this study accustomed pigs also performed more eating of concentrate when returning to their home pen than accustomed pigs, but the levels of eating was relatively small compared to the lying. In the study by Haskell et al. (1996), investigating the persistence of foraging behavior during the post-consumption phase of a feed reward, the pigs with larger reward had more persistent feeding motivation which disappeared over time and by learning. The impression from our study was that pigs were quite exhausted after the session in the play arena. This is based on the observations that they tried to lie down quite soon after returning, but were disturbed by the other pigs that pushed them up many times. Some pigs even lied down in the play arena at the end of the session toward the end of the study. Their high growth rate and the temperature being a bit higher than optimal in the barn may have contributed to this. Thus, eating may have been post-poned to after the 10 min of observation time that we used in this study.
When the pigs returned to the home pen the two remaining companion pigs that were never moved to the play arena initiated social interactions with the returning pigs. This may have been performed as a form of reunion-related behaviors with non-play littermates especially since it occurred more often in the first 5 min of the observation. Reimert et al. (2017) found that when two pigs were moved from their home pen to a test room the pigs remaining in the home pen performed more nose-nose and nose-body contact toward the returning pigs when they had received positive treatment compared to negative treatment in the test room. In this study the pigs had only received positive treatment in the play arena. Boissy and Le Neindre (1997) found that heifers initiated licking and sniffing of conspecifics at reunion with them following a period of separation. Having both two play and two companion non-play pigs in the same pen affected both naïve and accustomed pigs as the non-play pigs disturbed the behavior of the play pigs' performance of relaxation-related behaviors.
According to Zimmerman et al. (2011), comfort behavior in domestic fowl was reported as an indicator of relaxation and positive emotional states. There were only four recordings of comfort behaviors performed by the pigs in this study, thus they were too few to be able to carry out any statistical analysis.
Further investigations in this area would be beneficial to find out more about the behaviors connected to each phase of the reward cycle namely appetitive/anticipatory, consumption and post-consumption/relaxation. Some suggestions for improvements of the current study are presented separately for the holding pen, play arena and the home pen.
The holding pen should preferably have four walls around it so that the pigs do not see the arena, i.e., rewards, as done in a previous study on lambs (Anderson et al., 2015). The time pigs are left in the holding pen could be reduced to 2 min to reduce the risk of frustration, which has been suggest in previous research (Moe et al., 2009; Zimmerman et al., 2011; Peters et al., 2012). Another design where the time is very short in the beginning and then step by step prolonged is another suggested improvement that was used for lambs by Anderson (2016). The pigs should preferably be tested in another room, as the pigs to be tested later and the non-play pigs in the home pens seemed to be affected by hearing the play pigs' activities in the holding pen and play arena. This was done in previous studies on pigs by for example Reimert et al. (2013, 2017). In future studies, testing pigs from different housing conditions to see if their anticipatory behavior is different would be very interesting (see review by Anderson et al., 2020; Neave et al., 2021).
The play arena could be improved in future studies by making it larger, as it seemed it was a bit too small for the pigs to perform full locomotor play. Earlier studies by Forkman et al. (2007) suggest that the size of the arena is important and that it should be adjusted according to the size of the animals. However, in this study we used all space available in the room for the play arena, and even build up and removed the holding pen on each side to allow the pigs' maximum space. It is also important to secure that the floor is not slippery as this will affect the pigs' possibilities to run and show locomotor play. We had to add some straw to reduce the slipperiness during the training week of the pigs. The heat in the room is also important to keep below 24°C, as too warm environment may lead to reduced activity level in the pigs, shown by Huynh et al. (2005).
In the home pen it should be avoided to have other pigs that were not allowed to play meeting the returning pigs, as this disturbed their possibilities to show behaviors indicating relaxation. The social re-union behaviors from the non-play pigs has also been shown before by Reimert et al. (2017). There is need for more studies on pigs after they have experienced access to a play arena as a reward, as there are very few studies on this in other species (Chapagain et al., 2014). This is important in order to show if there exist a reward cycle for play in pigs.
In conclusion, this study gave a very mixed evidence for if there exist an anticipation–consumption–post-consumption reward cycle for play in pigs. The behaviors indicating anticipation to enter a play arena shown by accustomed pigs in the holding pen were increased frequencies of locomotor play and social interactions, tendencies of increased frequencies of orientation toward the arena, exploring the bars dividing the holding pen and arena, and standing, as well as decreased frequencies of walking. The behaviors indicating consumption of play in the play arena were a high frequency of interactions with the objects during all session, and that accustomed pigs showed higher frequencies of exploring the pen and social interactions, but lower frequencies of locomotor play and walking. The behaviors indicating relaxation in the home pen in the accustomed pigs were higher frequencies of lying and sitting, and tendencies of higher frequencies of eating, but lower frequencies of standing and walking. There is a need for more research on a higher number of pigs to find out if a reward cycle exist for play in pigs.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by the Swedish Ethical Committee of Experimental Animals in Uppsala approved this study (Drn: C 34/12).
LL initiated the study, supervised the MSc student, performed some of the behavioral observations, performed some of the statistical analysis, and wrote the paper together with the co-authors. NF did the study as her MSc thesis, in that work planned data collection, carried out a pilot study, performed most of the behavioral recordings, put all the data in Excel, and wrote the first outlines of the paper. CA was involved in this study during the beginning of his PhD, then carried out many behavioral observations, handled the pigs during the study, as well as wrote introduction, and has been involved in writing the paper. MZ was involved in planning the study, carried out the statistical analysis, and has been writing on the paper.
This study was conducted within the Center of Excellence in Animal Welfare Sciences, a research collaborative environment supported by Formas (Svenska Forskningsrådet Formas, Grant Number 221-2010-35). The Swedish University of Agricultural Sciences was funding the Master thesis in Animal Science.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Grateful acknowledgment goes to advice and assistance by Anna Wallenbeck and Eva Norling and all personnel at the pig barn of the Swedish National Livestock Research Center, Uppsala, Sweden.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2021.740778/full#supplementary-material
Anderson, A. J., and Adolphs, R. (2014). A framework for studying emotions across species. Cell 157, 187–200. doi: 10.1016/j.cell.2014.03.003
Anderson, C. (2016). Investigating anticipatory behaviors in lambs (Dissertation thesis), Acta Universitatis Agriculturae Sueciae, Swedish University of Agricultural Sciences. Uppsala, Sweden.
Anderson, C., Von Keyserlingk, M. A. G., Lidfors, L. M., and Weary, D. M. (2020). Anticipatory behaviour in animals: a critical review. Anim. Welf. 29, 231–238. doi: 10.7120/09627286.29.3.231
Anderson, C., Yngvesson, J., Boissy, A., Uvnäs-Moberg, K., and Lidfors, L. (2015). Behavioural expression of positive anticipation for food or opportunity to play in lambs. Behav. Proc. 113, 152–158. doi: 10.1016/j.beproc.2015.02.003
Blackshaw, J. K., Swain, A. J., Blackshaw, A. W., Thomas, F. J. M., and Gillies, K. J. (1997). The development of playful behaviour in piglets from birth to weaning in three farrowing environments. Appl. Anim. Behav. Sci. 55, 37–49. doi: 10.1016/S0168-1591(97)00034-8
Bloomsmith, M. A., and Lambeth, S. P. (1995). Effects of predictable vs. unpredictable feeding schedules on chimpanzee behavior. Appl. Anim. Behav. Sci. 44, 65–74. doi: 10.1016/0168-1591(95)00570-I
Boissy, A., and Le Neindre, P. (1997). Behavioral, cardiac and cortisol responses to brief peer separation and reunion in cattle. Physiol. Behav. 61, 693–699.
Boissy, A., Manteuffel, G., Bak Jensen, M., Oppermann Moe, R., Spruijt, B., Keeling, L. J., et al. (2007). Assessment of positive emotions in animals to improve their welfare. Phys. Behav. 92, 375–397. doi: 10.1016/j.physbeh.2007.02.003
Chapagain, D., Uvnäs-Moberg, K., and Lidfors, L. M. (2014). Investigation the motivation to play in lambs. Appl. Anim. Behav. Sci. 160, 64-74. doi: 10.1016/j.applanim.2014.08.004
Dickinson, A., and Balleine, B. (2009). “Hedonics: the cognitive-motivational interface,” in Pleasures of the Brain, eds M. L. Kringelbach, and K. C. Berridge (Oxford: Oxford University Press), 74–84.
Dudink, S., Simonse, H., Marks, I., de Jonge, F. H., and Spruijt, B. M. (2006). Announcing the arrival of enrichment increases play behaviour and reduces weaning-stress-induced behaviors of piglets directly after weaning. Appl. Anim. Behav. Sci. 101, 86–101. doi: 10.1016/j.applanim.2005.12.008
Forkman, B., Boissy, A., Meunier-Salaün, M.-C., Canali, E., and Jones, R. B. (2007). A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 92, 340–374. doi: 10.1016/j.physbeh.2007.03.016
Fraser, D., and Duncan, I. J. H. (1998). “Pleasures,” “pains” and animal welfare: toward a natural history of affect. Anim. Welf. 7, 383–396.
Hansen, S. W., and Jeppesen, L. L. (2006). Temperament, stereotypies and anticipatory behaviour as measures of welfare in mink. Appl. Anim. Behav. Sci. 99, 172–182. doi: 10.1016/j.applanim.2005.10.005
Haskell, M. J., Terlouw, E. M. C., Lawrence, A. B., and Deans, L. A. (1996). The post-feeding responses of sows to the daily presentation of food rewards in a test arena. Appl. Anim. Behav. Sci. 49, 125–135. doi: 10.1016/0168-1591(96)01043-X
Huynh, T. T. T., Aarnink, A. J. A., Gerrits, W. J. J., Heetkamp, M. J. H., Canh, T. T., Spoolder, H. A. M., et al. (2005). Thermal behaviour of growing pigs in response to high temperature and humidity. Appl. Anim. Behav. Sci. 91, 1–16. doi: 10.1016/j.applanim.2004.10.020
Imfeld-Mueller, S., and Hillmann, E. (2012). Anticipation of a food ball increases short-term activity levels in growing pigs. Appl. Anim. Behav. Sci. 137, 23–29. doi: 10.1016/j.applanim.2012.01.012
Imfeld-Mueller, S., van Wezemael, L., Stauffacher, M., Gygax, L., and Hillmann, E. (2011). Do pigs distinguish between situations of different emotional valences during anticipation? Appl. Anim. Behav. Sci. 131, 86–93. doi: 10.1016/j.applanim.2011.02.009
Jensen, M. B., and Kyhn, R. (2000). Play behaviour in group-housed dairy calves, the effect of space allowance. Appl. Anim. Behav. Sci. 67, 35-46. doi: 10.1016/S0168-1591(99)00113-6
Jensen, M. B., Vestergaard, K. S., and Krohn, C. C. (1998). Play behaviour in dairy calves kept in pens: the effect of social contact and space allowance. Appl. Anim. Behav. Sci. 56, 97–108. doi: 10.1016/S0168-1591(97)00106-8
Keeling, L., Algers, B., Blokhuis, H., Boissy, A., Lidfors, L., Mendl, M., et al. (2008). “Looking on the bright side of life: reward, positive emotions and animal welfare,” in Proceedings of the 42th Congress of the ISAE, International Society for Applied Ethology (Dublin), 5-9.
Kringelbach, M. L., Stein, A., and van Hartevelt, T. J. (2012). The functional human neuroanatomy of food pleasure cycles. Physiol. Behav. 106, 307–316. doi: 10.1016/j.physbeh.2012.03.023
Lawrence, A. B., Vigors, B., and Sandoe, P. (2019). What is so positive about positive animal welfare? A critical review of the literature. Animals 9:783. doi: 10.3390/ani9100783
Lidfors, L., Hultman, P., and Zupan, M. (2020). Providing additional objects to straw reduces piglets' redirected behaviour post-weaning but influences weight gain pre-weaning negatively. Acta Agric. Scand., Sec. A—Anim. Sci. 69, 239–248. doi: 10.1080/09064702.2020.1775286
Luuk, H., Fahrenkrug, J., and Hannibal, J. (2012). Circadian rhythms and food anticipatory behavior in Wfs1-deficient mice. Biochem. Biophys. Res. Com. 424, 717–723. doi: 10.1016/j.bbrc.2012.07.017
Mendl, M., Burman, O. H. P., and Paul, E. S. (2010). An integrative and functional framework for the study of animal emotion and mood. Proc. Royal Soc. B. 277, 2895–2904. doi: 10.1098/rspb.2010.0303
Moe, R. O., Bakken, M., Kittilsen, S., Kingsley-Smith, H., and Spruijt, B. M. (2006). A note on reward-related behaviour and emotional expressions in farmed silver foxes (Vulpes vulpes)—basis for a novel tool to study animal welfare. Appl. Anim. Behav. Sci. 101, 362–368. doi: 10.1016/j.applanim.2006.02.004
Moe, R. O., Nordgreen, J., Janczak, A. M., Spruijt, B. M., Zanella, A. J., and Bakken, M. (2009). Trace classical conditioning as an approach to the study of reward-related behaviour in laying hens: a methodological study. Appl. Anim. Behav. Sci. 121, 171–178. doi: 10.1016/j.applanim.2009.10.002
Neave, H. W., Webster, J. R., and Zobel, G. (2021). Anticipatory behaviour as an indicator of the welfare of dairy calves in different housing environments. PLoS ONE 16:e0245742. doi: 10.1371/journal.pone.0245742
Paul, E. S., and Mendl, M. T. (2018). Animal emotion: descriptive and prescriptive definitions and their implications for a comparative perspective. Appl. Anim. Behav. Sci. 205, 202–209. doi: 10.1016/j.applanim.2018.01.008
Peters, S. M., Bleijenberg, E. H., van Dierendonck, M. C., van der Harst, J. E., and Spruijt, B. M. (2012). Characterization of anticipatory behaviour in domesticated horses (Equus caballus). Appl. Anim. Behav. Sci. 138, 60–69. doi: 10.1016/j.applanim.2012.01.018
Reimert, I., Bolhuis, J. E., Kemp, B., and Rodenburg, T. B. (2013). Indicators of positive and negative emotions and emotional contagion in pigs. Physiol. Behav. 109, 42–50. doi: 10.1016/j.physbeh.2012.11.002
Reimert, I., Fong, S., Rodenburg, T. B., and Bolhuis, J. E. (2017). Emotional states and emotional contagion in pigs after exposure to a positive and negative treatment. Appl. Anim. Behav. Sci. 193, 37-42. doi: 10.1016/j.applanim.2017.03.009
Rolls, E. T. (2014). Emotion and Decision-Making Explained. Oxford: Oxford University Press. doi: 10.1093/acprof:oso/9780199659890.001.0001
Špinka, M., and Newberry, R. C., and Bekoff, M. (2001). Mammalian play: training for the unexpected. Quart. Rev. Biol. 76, 141–168. doi: 10.1086/393866
Spruijt, B. M., van den Bos, R., and Pijlman, F. T. A. (2001). A concept of welfare based on reward evaluating mechanisms in the brain; anticipatory behaviour as an indicator for the state of reward systems. Appl. Anim. Behav. Sci. 72, 145–171. doi: 10.1016/S0168-1591(00)00204-5
Studnitz, M., Jensen, M. B., and Pedersen, L. J. (2007). Why do pigs root and in what will they root? A review on the exploratory behaviour of pigs in relation to environmental enrichment. Appl. Anim. Behav. Sci. 107, 183–197. doi: 10.1016/j.applanim.2006.11.013
Trickett, S. L., Guy, J. H., and Edwards, S. A. (2009). The role of novelty in environmental enrichment for the weaned pig. Appl. Anim. Behav. Sci. 116, 45–51. doi: 10.1016/j.applanim.2008.07.007
Van de Weerd, H. A., Docking, C. M., Day, J. E., Avery, P. J., and Edwards, S. A. (2003). A systematic approach toward developing environmental enrichment for pigs. Appl. Anim. Behav. Sci. 84, 101–118. doi: 10.1016/S0168-1591(03)00150-3
van den Bos, R., Meijer, M. K., van Renselaar, J. P., van der Harst, J. E., and Spruijt, B. M. (2003). Anticipation is differently expressed in rats (Rattus norvegicus) and domestic cats (Felis silvestris catus) in the same Pavlovian conditioning paradigm. Behav. Brain Res. 141, 83–89. doi: 10.1016/S0166-4328(02)00318-2
van der Harst, J. E., Baars, A. M., and Spruijt, B. M. (2003). Standard housed rats are more sensitive to rewards than enriched housed rats as reflected by their anticipatory behaviour. Behav. Brain Res. 142, 151–156. doi: 10.1016/s0166-4328(02)00403-5
Vinke, C. M., Houx, B. B., van den Bos, R., and Spruijt, B. M. (2006). Anticipatory behaviour and stereotypical behaviour in farmed mink (Mustela vision) in the presence, absence and after removal of swimming water. Appl. Anim. Behav. Sci. 96, 129–142. doi: 10.1016/j.applanim.2005.04.022
Wichman, A., Keeling, L. J., and Forkman, B. (2012). Cognitive bias and anticipatory behaviour of laying hens housed in basic and enriched pens. Appl. Anim. Behav. Sci. 140, 62–69. doi: 10.1016/j.applanim.2012.05.006
Yeates, J. W., and Main, D. C. J. (2008). Assessment of positive welfare: a review. Vet. J. 175, 293–300. doi: 10.1016/j.tvjl.2007.05.009
Keywords: fattening pigs, anticipation, consumption, relaxation, locomotor play, object play, social interactions, exploration
Citation: Lidfors LM, Farhadi N, Anderson C and Zupan Šemrov M (2021) Investigating the Reward Cycle of Play in Pigs (Sus scrofa). Front. Anim. Sci. 2:740778. doi: 10.3389/fanim.2021.740778
Received: 13 July 2021; Accepted: 19 October 2021;
Published: 18 November 2021.
Edited by:
Rafael Freire, Charles Sturt University, AustraliaReviewed by:
Tamara Alejandra Tadich, Austral University of Chile, ChileCopyright © 2021 Lidfors, Farhadi, Anderson and Zupan Šemrov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lena M. Lidfors, bGVuYS5saWRmb3JzQHNsdS5zZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.