- 1Department of Anesthesiology and Perioperative Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
- 2Surgery Service Line, Veterans Affairs Pittsburgh Healthcare System, Pittsburgh, PA, United States
- 3Department of Surgery, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
- 4Department of Pharmacy, Veterans Affairs Pittsburgh Healthcare System, Pittsburgh, PA, United States
- 5Medicine Service Line, Veterans Affairs Pittsburgh Healthcare System, Pittsburgh, PA, United States
Background: For abdominal surgery involving cephalad surgical trespass (such as sleeve gastrectomy and pancreatectomy), existing intrathecal morphine (ITM) recommendations of ≤150 μg may not achieve meaningful analgesia, potentially leading to side effects of intravenous opioids during or after surgery. This study aimed to present (i) an ITM dosing guideline to improve upon existing dosing guidelines (≤150 µg) and (ii) an analgesic duration predictor derived from the proposed vs. existing dosing guideline.
Methods: We used a mixed-method multi-hypothetical framework to demonstrate that five-drug antiemetic prophylaxis before spinal morphine administration may allow for ≥250 μg doses, which with further refinement may confer meaningful analgesia, downstream opioid sparing, and prevention of nausea/vomiting. A retrospective, case-matched quality improvement initiative was implemented, followed by multiple regression to (i) calculate successful spinal morphine dosing and (ii) predict analgesic duration in our Veteran patient population.
Results: As opposed to the currently recommended dose of ≤150 μg, 250 μg was the start-point for spinal morphine dosing, with adjustments for gender, height, and age. The 250 μg dose (and incremental adjustments) was associated with a 16 h baseline analgesic duration, while the <200 μg dose was associated with only 8 h; the latter analgesic duration (i.e., ≤8 h) was adversely influenced by factors that did not affect the ≥250 μg dose analgesic duration.
Conclusion: We achieved meaningful prophylaxis against nausea/vomiting with the five “keyword” drugs (all five drugs were used in 94% of our patients who received the ≥250 μg morphine dose). This seems to facilitate adherence to oral/enteral non-opioid analgesics after surgery, possibly contributing to analgesic duration. Conversely, avoidance of usual intraoperative (fentanyl, remifentanil, hydromorphone) and postoperative (hydromorphone, oxycodone, hydrocodone) opioids may have prolonged perceived analgesic duration (and avoided nausea) by preventing opioid-induced hyperalgesia and/or tolerance. We presume that the ≥250 μg morphine dose had sufficient “cephalad reach” for various procedures, including those where endoscopic cases were converted to open. This approach may prevent reflexive intraoperative administration of usual intravenous opioids. Five-drug antiemetic prophylaxis may allow for improved analgesic outcomes and systemic opioid reductions, via patient-based parameters of a spinal morphine dose start-point of at least 250 μg, as opposed to the currently recommended dose of ≤150 μg.
1 Introduction
Professional society guidance and/or consensus statements may not reflect recent innovations or important advances in “repurposing” viable therapeutic options, such as intrathecal morphine [ITM (1)]. For example, recommended opioid selection for intraoperative use, in light of the opioid epidemic, may not fully reflect important advances in determining intravenous methadone dose response, such as (i) incorporating a “single intraoperative dose that minimized pain and post-anesthesia care unit (PACU) opioid requirement (2)” separately for patients going home the same day (3) vs. those being planned for a 1-day hospital admission (2) and (ii) acknowledging “concentrations …below the minimum effective concentration, (for which) clinical benefit will not ensue, even with slow…elimination.” (2) ITM likely shares similar threshold-based dose attributes as IV methadone. Recent professional society ITM dose–response guidance (4) seems to have recommended lower doses/concentrations than would still be meaningfully effective for transabdominal/truncal surgery. Another shared attribute of both IV methadone (2, 3) and ITM (1) is potent emetogenic effects in the absence of recently reported (1) multimodal nausea/vomiting prophylaxis. Avoidance of postoperative nausea/vomiting (PONV) now appears to be a 90%–95% achievable goal [on postoperative days (POD) 0–1 (1)] for patients receiving a five-drug combination regimen administered prior to procedural anesthesia, irrespective of conventional (5) PONV risk determination.
The hypotheses generated from this observational manuscript are as follows: (i) ITM (similar to IV methadone) appears to have a threshold dose in adults above which higher-quality analgesia can be achieved, (ii) current published guidelines/recommendations for ITM dosing [100–150 μg (4)] may be insufficient for transabdominal or adjacent surgery, (iii) five-drug PONV prophylaxis presented herein and previously published (1) may have indirect analgesic efficacy by allowing ongoing adherence to an oral/enteral non-opioid analgesic regimen during the ITM’s window of efficacy, and (iv) antiemetic momentum may be sustained further into the hospital stay with “booster” dosing after initial pan-prophylaxis against PONV. The objective of this manuscript is to validate these hypotheses as potential guides for short-term practice adjustments. Moreover, our specific aims include presenting (i) an easily calculable ITM dosing guideline with likely better cephalad spread than the existing guideline doses (of 100–150 µg) and (ii) an analgesic duration predictor, while formulating long-term research agenda items for hypothesis confirmation and further care advances.
2 Materials and methods
2.1 Ethics approval
This report was an IRB-approved quality improvement initiative and was exempt from patient research consent above and beyond clinical surgery and anesthesia consent. Institutional approval (approval/exemption 1670098-1, VA Pittsburgh Healthcare System, Pittsburgh, PA, USA) allowed for tracking and external reporting of outcome data, entailing general anesthesia (GA)/ITM data and GA (without ITM) case-matched controls.
2.2 External reporting precedents and guidance, patient identification/selection, and data collation
Following STROBE guidelines, we previously disclosed that our five-drug antiemetic prophylaxis was declared as the institutional standard of care for ITM recipients soon after our group's first case series was accepted for publication (which focused on addressing joint replacement surgery under spinal anesthesia including ITM) (6). In our subsequent publication (1), patients who received both ITM and GA were then case-matched as a second cohort as follows. We retrospectively reviewed and prospectively collected quality improvement data from a single US Veterans hospital. Institutional approval enabled our tracking and external reporting of GA patients receiving ITM and GA patients without ITM as case-matched controls. Case matching was based on the type of procedure (including Current Procedure Terminology codes, as applicable), procedure duration, gender, body mass index (BMI), and American Society of Anesthesiologists' Physical Status Classification. Our first (1) case queries included consecutive cases of non-smoking Veterans undergoing bariatric surgery (n = 26) and non-consecutive non-bariatric surgery cases (n = 109, with different smoking statuses not tracked in real time). Case-matched historical or concurrent controls of GA cases (1) (n = 135 without ITM) were identified and analyzed, entailing cases occurring from 31 May 2016 to 31 August 2022. After these cases were analyzed and reported (1), 5 further retrospective unmatched GA/ITM cases (16 November 2021–11 July 2022) and 89 further prospective GA/ITM cases (9 September 2022–14 June 2023, unmatched) were added to the data pool to enhance outcome transparency; the latter 89 cases routinely included (i) antiemetic booster dosing (detailed later) and (ii) postoperative oral non-opioid analgesics such as acetaminophen, a COX-2 inhibitor, and dextromethorphan (7). Overall, 94 cases were incorporated and analyzed into the previously (1) published dataset for this current report.
2.3 Anesthesia procedure
All ITM doses were diluted in 2.5–3.0 ml of sterile water at the time of the procedure, instead of the previous uncited guidance (4) restricting intrathecal injectate to <2 ml volume [possibly driven (4) by concerns of co-administered local anesthetic and “high spinal”]. The ITM procedures in the present report were performed in a monitored block room, as previously reported (8, 9). Analgesic durations from the ITM procedures were determined from electronic medical records using methods previously reported (10–13). Hands-on anesthesia providers and/or attending anesthesiologists in the operating room were asked to not administer IV opioids (fentanyl, remifentanil, hydromorphone) during surgery when ITM was used, but this request was neither enforced nor enforceable. The presence and absence of such doses were tracked and analyzed.
2.4 Setting and participants, study size, and bias
The setting was a single US Veterans hospital, with matched cases having occurred no earlier than 31 May 2016 and prospective cases having occurred no later than 14 June 2023. All patients whose data were reviewed were either known in advance of surgery to be admitted to the hospital after surgery or were already hospitalized inpatients presenting for surgery. All prospective patients were ITM recipients. In other words, patients who refused the ITM procedure were not included in any data analysis (i.e., no one who refused the ITM procedure was analyzed as a “case–control”). The total case number reached was a convenience sample with no a priori power calculations. The absence of a further query of 94 additional control cases was related to no available further research support. No further efforts were made to address potential sources of bias other than those listed above.
2.5 Quantitative variables, and statistical methods
As reported (1, 6), the chief distinction in categorizing PONV prophylaxis was having received five categories of the described antiemetics, or less than five. The five antiemetics were perphenazine (as antidopaminergic), diphenhydramine (an antihistamine), dexamethasone, aprepitant (as neurokinin-1 antagonist), and either palonosetron or a minimum of 8 mg ondansetron (as 5-hydroxytryptamine type 3 antagonist). For the 89 latest (unmatched) cases (September 2022–June 2023), (i) palonosetron was exclusively used (not ondansetron) for prophylaxis, and (ii) all five prophylaxis drugs were given before patients were transported to the operating room.
Supplemental data (Supplementary Tables S1–S6) are serially presented as descriptive statistics and multivariable regression analyses, modeled to address this manuscript's objectives related to (i) ITM dose–response observations, (ii) verification of the potential value of the five-drug PONV prophylaxis, and (iii) augmentation of non-opioid analgesic-associated success, while putting forth efforts to avoid parenteral/oral opioids downstream from the ≥250 µg ITM dose, which is acknowledged as higher than currently (4) recommended (Table 1). Multivariable analyses were utilized to adjust for potential confounders driven by clinical judgment, to develop and strengthen the dose calculator and analgesic duration regression models, and to identify potentially independent associated predictors of outcome (for future research).

Table 1. Potential effects of (i) intrathecal morphine (ITM), (ii) potentially synergistic five-drug PONV prophylaxis, and (iii) potentially antagonistic (i.e., net hyperalgesic) intravenous opioids given during GA, when ITM is present at a ≥250 µg minimum dose.
For the dose calculator, only ITM doses of >200 μg (n = 166) were incorporated into the model, while for the analgesic duration predictor, two separate duration models were created for >200 μg (n = 166) vs. ≤200 mcg (n = 59). For the described multivariable linear regression models, the variables considered were those listed in Supplementary Tables S1–S6. No sensitivity analyses were performed. All statistical analyses utilized IBM SPSS statistical software (version 29, IBM Corporation, Armonk, NY, USA).
2.6 Anatomic rationale for ITM dosing increase above existing recommendations
Acknowledging the detailed clinical factors presented in the Supplementary Material, the specific aim of this manuscript is to incorporate the described Supplementary Material findings into (i) a practical ITM dose calculator for transabdominal/truncal surgery and (ii) an analgesic ITM duration predictor model for the institution of origin, which could be applied and modified as needed for other patient populations. Both of these models, when implemented into practice, may offset the disadvantages of existing (4) ITM dose guidelines (of only 100–150 µg) that may render patients as undertreated, until further confirmatory or refinement studies are available. The present authors' disagreement with the 100–150 µg recommendation of Gustafsson et al. (2019) is further based on long-established (2008) ITM work in joint replacement orthopedic surgery which declared 200 µg as an optimal dose for knee replacement surgery (14), providing comparable knee analgesia as 300 µg ITM and superior analgesia over 100 µg ITM. Knee arthroplasty analgesia entails the L1–S3 nerve distribution and an intrathecal insertion typically at L2 or caudad. Anatomically, it seems irrational to use a potentially underdosed (100–150 µg) amount of ITM for abdominal surgery with expectations of any meaningful analgesia as cephalad as T6, while creating usual procedural risks of ITM (bleeding, infection, nerve damage) in the process. Respiratory depression-related outcomes were tracked in real time using an open-ended “comments” field that was included in the cumulative data set for ITM cases (but not for historical controls).
3 Results
3.1 Participants and descriptive data
As itemized and detailed in Supplementary Tables S1–S6, the number of patients per analyzed observational condition is summarized in Table 2. To maximize data inclusion in the single-institution setting of only moderate-size caseloads, we present the available sample sizes (in CONSORT-like diagrams) associated with each analysis demonstrated in Figure 1, Table 3, and Supplementary Tables S1–S6. There were no missing data among the 225 cases that received ITM (Supplementary Tables S2, S3), 58 of which were sub-analyzed as bariatric cases (Supplementary Tables S1, S4, S5). For the bariatric cases particularly in Supplementary Table S1, 37 historical control cases were retained and used from our previous (1) report (i.e., cases that did not receive ITM), for purposes of the described regression analysis. Although Roux-en-Y gastric bypass cases seem to generate more of a postoperative pain response than do sleeve gastrectomy cases (15), we opted to combine these bariatric case types for a more meaningful analysis of postoperative pain and analgesic duration prediction (while excluding more variable general colorectal and genitourinary cancer cases, from a postoperative pain perspective; Supplementary Table S1). Further following similar logic in bariatric case analysis, leading to our PONV-specific analysis (Supplementary Table S6), we retained all previously reported (1) bariatric sleeve gastrectomy cases, both ITM (n = 18) and historical controls (n = 20), and added newly available ITM-utilized sleeve gastrectomy case data (n = 18), based on the generally accepted clinical observation that sleeve gastrectomy patients experience more PONV (15) than those who underwent other types of gastric bypass procedures (i.e., which had been included in the pain/analgesia analyses of Supplementary Tables S4–S5).
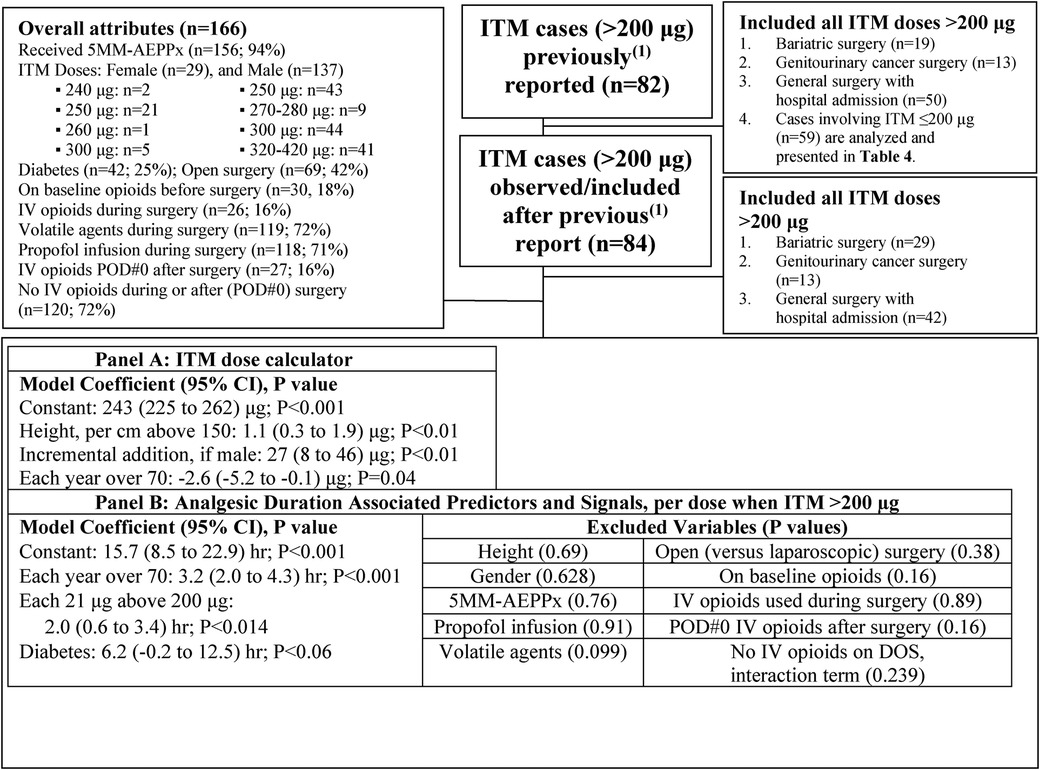
Figure 1. Case selection diagram (for Panels A and B), to derive an ITM dose calculation formula (A) incorporating the ≥250 μg start-point dose for ITM, aiming for both clinically meaningful analgesic duration, and further opioid avoidance into postoperative day (POD) 1 (B). Linear relationships are not assumed outside the listed ITM dose range (240–420 μg). ITM, intrathecal morphine; 5MM-AEPPx, five-drug multimodal antiemetic prophylaxis with palonosetron, perphenazine, aprepitant, diphenhydramine, and dexamethasone; POD, postoperative day; CI, confidence interval; DOS, day of surgery; PONV, postoperative nausea/vomiting.
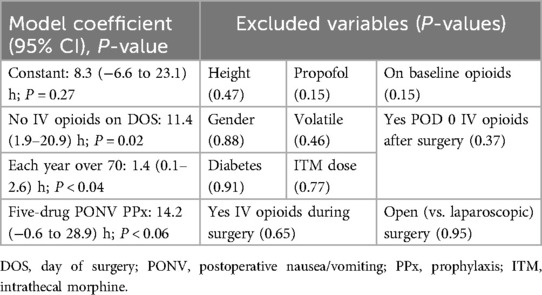
Table 3. Linear regression: analgesic duration predictors or signals, when ITM was dosed at ≤200 µg (n = 59).
3.2 Outcome data and main results
Case selection and other collated attributes are presented in Figure 1, wherein Panel A presents the suggested ITM dose calculator starting at a lower threshold of ∼250 µg for non-orthopedic surgery, while Panel B presents associated analgesic duration predictors (and “signals” with P < 0.1), when the ITM dose was >200 µg.
Based on 166 GA/ITM cases with doses >200 μg, with ITM dosing aiming to minimize exposure to downstream opioids, a practical dosing formula that the authors recommend starts with ∼250 μg, adding a further 25 μg for men, then adding ∼10 μg for each ∼9 cm above 150 cm in height (or ∼10 μg for each ∼3 inches of height above 5 feet tall; Figure 1A). An ITM duration prediction model was also generated for these n = 166 cases (Figure 1B), showing (i) an ∼16 h duration constant, (ii) each year over an age of 70 being associated with 3 h of ITM/multimodal duration, and (iii) each ∼20 μg above 200 μg being associated with an additional 2 h of ITM/multimodal duration. One signal of note, warranting future study, was a trend of an additional 6 h duration in patients with diabetes (P < 0.06). Therefore, if there are dosing concerns from a safety perspective, elderly patients and/or diabetic patients may not necessarily be harmed (from the perspective of analgesic duration) with slight ITM dose reductions from the proposed equation (Figure 1A), as indicated, if other potential comorbidities (e.g., ability to extubate at the end of a case) are being considered.
When analyzing the lower-dose GA/ITM cases of ≤200 μg, the prediction model (Table 3) generated an analgesic duration coefficient of ∼8 h (as opposed to 16 h above in Figure 1B), with an additional 11 h of duration observed in patients that did not receive IV opioids either during surgery or after the operating room on POD 0 (i.e., an interaction term, perhaps indicating that the ≤200 μg dose was, in fact, sufficient for some patients). With lower (≤200 μg) ITM doses, the age-based effect on duration above (i.e., as in Figure 1B) was not as robust. Meanwhile, having received five-drug PONV prophylaxis (with palonosetron, perphenazine, aprepitant, diphenhydramine, and dexamethasone) was an associated signal (P < 0.06) of 14 h analgesic prolongation, perhaps related to improved non-opioid oral/enteral non-opioid regimen adherence, while lower-dose (≤200 μg) ITM was still effective. Ten other factors (Table 3) appeared to be non-contributory but may have been underpowered.
3.3 Available data on respiratory depression, and underlying non-ITM probable causes
Four cases were annotated in real time as having respiratory depression issues prompting delayed extubation and/or early reintubation and forcing an intensive care unit (ICU) stay that seems likely to not have otherwise occurred.
3.3.1 First case (2021)
This male Veteran, age 50, BMI = 42 kg/m2, received (i) 200 µg ITM, (ii) all five medications for PONV prophylaxis, and (iii) coinciding lidocaine- (0.5 mg/kg/h; 180 mg total) and propofol-only total intravenous anesthesia (5 g in total, encompassing induction and maintenance for a 160 min open hemicolectomy, with bispectral index values of 42–58 intraoperatively). Dexmedetomidine 10 µg IV was given by the hands-on anesthesia provider without consulting with the attending anesthesiologist 8 min before sugammadex reversal (400 mg). Absence of pain (from ITM) may have inhibited prompt emergence, and increments of naloxone to a total of 0.6 mg did not render the ITM effect as reversed, which shifted the differential diagnosis toward respiratory acidosis that had rapidly developed after the uninformed dexmedetomidine dose, soon after mechanical ventilation was converted to bag ventilation in a super-morbidly obese patient. The core temperature upon emergence was 35.9°. This patient was extubated the following morning in the ICU, reporting a pain score of zero and ultimately benefitting from an estimated ITM analgesic duration of 32 h.
3.3.2 Second case (2022)
This male Veteran, age 61, BMI = 36 kg/m2, received (i) 300 µg ITM, (ii) all five medications for PONV prophylaxis, and (iii) a balanced volatile (sevoflurane, end-tidal 0.2%–0.6%)—propofol anesthetic (2 g in total), encompassing induction and maintenance for a 210 min laparoscopic cholecystectomy with planned hospital admission, exiting the operating room late into the evening (∼11:30 p.m.). Bispectral index values were lower than above, ranging from 22 to 50 intraoperatively. Dexmedetomidine 10 µg IV was given 15 min before sugammadex reversal (400 mg). The absence of pain (from ITM) may again have inhibited prompt emergence, and increments of naloxone to a total of 0.2 mg did not render the ITM effect as reversed, This again seems to have shifted the differential diagnosis toward respiratory acidosis that had rapidly developed after the dexmedetomidine dose, soon after mechanical ventilation was converted to bag ventilation in a morbidly obese patient. This patient was extubated less than 3 h after arrival to the ICU.
3.3.3 Third case (2022)
This male Veteran, age 73, BMI = 28 kg/m2, received (i) 250 µg ITM, (ii) three of five medications for PONV prophylaxis (neither dexamethasone nor diphenhydramine), and (iii) balanced sevoflurane/propofol for anesthetic maintenance (for a nearly 13 h laparoscopic-converted to open Whipple procedure). Extubation immediately after the case was neither considered nor attempted but was accomplished uneventfully 12 h after ∼9:30 p.m. ICU arrival.
3.3.4 Fourth case (2023)
This male Veteran, age 75, BMI = 30 kg/m2, received (i) 320 µg ITM, (ii) all five medications for PONV prophylaxis, and (iii) balanced sevoflurane (end-tidal 0.9%–1.1%) and propofol anesthetic [1.4 g in total, encompassing induction and maintenance for an 8 h robotic sigmoid colectomy and colorectal anastomosis (for symptomatic vesicointestinal fistula), with bispectral index values of 48–60 intraoperatively]. Sugammadex reversal (300 mg) was not preceded by any end-of-case dexmedetomidine. However, the recorded core body temperature for 2 h before end-of-case and initial extubation was 33.9°. The patient was reintubated ∼40 min later, presumably related to hypothermia (since no naloxone was used by the care team at the time), and was uneventfully extubated 12 h after the described reintubation the previous evening.
4 Discussion
The specific aims, using multivariable regression analyses, were to present (i) a practical ITM dose calculator for transabdominal/truncal surgery and (ii) an associated analgesic duration predictor. Such procedures included relatively cephalad trespass sites in the context of abdominal/axial anatomy, including bariatric surgery, nephrectomy, and pancreatic surgery. We were motivated to do so based on the existing guidelines (4) that seem to underdose ITM (100–150 μg recommendation), with the downstream potential of acute opioid escalation sequelae that include the development of tolerance, hyperalgesia, and/or PONV. Our models showed a ∼250 μg start-point ITM dose to be seemingly rational, with adjustments available (based on both presented models, Figure 1 and Table 3) related to age (decrease ITM dose if over 70), gender (increase ITM dose if male), height (incremental ITM dose increase for achieving dermatomal objectives), and possibly diabetes (allowing for decrease of ITM dose if desired, in a diabetic patient). The 250 μg baseline dose (with associated incremental increases) was associated with a 16 h duration starting coefficient before other statistical adjustments, while the ≤200 μg dose was associated with only an 8 h duration starting coefficient, while being influenced by associated factors that did not influence the ≥250 μg dose duration.
Meanwhile, it seems probable that the five-drug PONV prophylaxis (palonosetron, perphenazine, aprepitant, diphenhydramine, and dexamethasone) may allow for better adherence to an oral/enteral non-opioid analgesic regimen, based on this latter (<200 μg) model. However, further study is needed to address both ITM dosing categories.
The four cases of unplanned inability to immediately extubate, or requirement to soon reintubate, indicate that there may be value to adding specific ITM-focused points above usual vigilance in intraoperative care. These include (i) avoiding unnecessary sedatives administered near the time of extubation, (ii) intraoperative euthermia, and (iii) acknowledging possible intraoperative acid–base shifts during IV fluid shortages, leading to normal saline default use and subsequent hyperchloremic metabolic acidosis that may lead to post-emergence early susceptibility to respiratory depression and rapidly mixed acidosis. The third point is based on personal observation (lead author BAW) in cases having occurred since the timeline of this collected data set. Another potentially useful consideration, when the described doses of ITM are used, is the delaying of switching the ventilator from mechanical mode to “bag mode” in the commonplace effort to stimulate respiratory effort via hypercarbia (and untreated pain). Recalling that pain is a potent stimulus to breathe, assisting emergence from anesthesia, the corollary is that the necessary ITM doses described to potentially affect the trajectory of the opioid epidemic (related to usual exposure to usual opioids) will commonly block the pain for many hours. As a result, the hands-on anesthesia provider at the bedside may do well to treat ITM patients quite differently to achieve prompt emergence. This could occur by allowing for extubation and PACU transfer to occur before any post-ITM opioid dosing in the OR; in contrast with the default of employing “usual” IV opioids and/or co-administered sedatives. In other words, delaying dexmedetomidine (e.g., for post-traumatic stress disorder-related emergence agitation risks) until PACU (i.e., after extubation) may be useful in ITM cases, particularly in those at risk for (or diagnosed with) obstructive sleep apnea. This was described in the first case and the second case above.
“Right-sized” ITM dosing compared to previous (4) recommendations seems to also involve preferable avoidance of probably unnecessary IV opioids during surgery [which could be potentially replaced by infused esmolol (16) or low-dose lidocaine, and delay the decision for further opioids to postoperative recovery after GA emergence]. Another factor potentially helpful for “right-sized” ITM dosing may incorporate the previously reported (1) and observationally successful five-drug PONV prophylaxis. Reticence to escalate ITM dosing to achieve desired analgesic effects (including considerations for relatively cephalad surgical sites) may be based, in part, on PONV avoidance. Therefore, to counteract PONV concerns, the five-drug (1) PONV prophylaxis seems to have the potential to allow for escalating ITM doses beyond current (4) recommendations. Based on recent observational work addressing IV opioid escalation intraoperatively (17), initial ITM underdosing that leads to reactive IV opioid supplementation intraoperatively may undermine ITM duration benefits (compared with ITM avoiding other intraoperative IV opioids such as fentanyl, remifentanil, and hydromorphone, as per Supplementary Tables S1–S5). Further research could explore whether supplemental intraoperative IV methadone, esmolol (16), or lidocaine (instead of fentanyl/remifentanil/hydromorphone) may be protective of ITM duration effects, when compared with our observations of possibly undermined ITM duration effects associated with intraoperative fentanyl/remifentanil/hydromorphone use.
5 Limitations
Veterans may be considered a low-risk population for PONV. However, Veterans have not been specifically studied in presented PONV consensus guidelines, nor have patients specifically receiving ITM. Therefore, forecasts of the five-drug PONV prophylaxis success in ITM cases may differ in the non-Veteran (with or without ITM) population. As a result, testing the five-medication technique may potentially offset some enthusiasm with the described combined strategies of ITM up-dosing before operating room entry. To offset this limitation, we allowed for signals (with P ≥ 0.05 but ≤0.1) into our final regression equations, often representing non-traditional but potentially interacting risk factors, to query for potential epidemiologic influence (or “bedside habit” influence) beyond traditional PONV risk factors.
Next, because postoperative bladder catheters were fairly ubiquitous in this postoperative patient population, issues regarding postoperative urinary retention will need to be separately addressed in future research. Importantly, our data did not account for potentially useful intrathecal adjuvants (e.g., preservative-free magnesium sulfate) or co-administered local anesthetics such as bupivacaine, including very low doses that might not be sufficient to be a self-sufficient anesthetic, but possibly being useful for meaningful antinociception.
Furthermore, we acknowledge that we were unable to derive and present expected a priori sample size determinations and power analyses. Our observations are limited to a single-center Veterans population, which may not generalize to broader, diverse patient populations. For this observational study, we could only “case-match” cases that were performed and assess similar historical control cases performed at the same institution in the described time periods as comparators. It is difficult to expand an observational sample size (with limited resource support) to cases that are neither present in one's institution nor present in the institution's medical record archive. Future work expanding the cohort and including non-Veteran populations are needed to enhance applicability. Using the described consecutive caseload, our goal was to provide preliminary data for external researchers to create their own cohorts (such as in non-Veteran populations) or prospective randomized study groups incorporating the described paradigm shifts, as soon as the significant findings and signals were noted in the analysis.
Finally, incorporating all described multimodal processes into an overarching enhanced recovery protocol (including the described five-medication multimodal antiemetic, and otherwise complete opioid avoidance until after extubation, and being stationed in PACU) may create a useful start-point for prospective study, which could include a head-to-head comparison of ITM 150 µg vs. either (i) ITM 250 µg or (ii) ITM dosed per the formula in Figure 1A.
6 Conclusions
We postulated, then validated, a 250 μg (as opposed to 100–150 μg) dose as a potentially viable and observationally tested start-point for ITM dosing, with additional dose adjustment opportunities based on gender, height, and age. The 250 μg start-point ITM dose (and its incremental adjustments) was associated with an unadjusted 16 h duration coefficient. Meanwhile, ITM doses <200 μg [such as those recommended (4) presently] were associated with only an unadjusted 8 h duration coefficient and were influenced by factors that did not affect the ≥250+ μg duration coefficient. PONV prophylaxis with the five described drugs seems to (i) allow for a more confident PONV-free postoperative course, (ii) be augmented by daily oral booster dosing of the two oral antiemetics (perphenazine and aprepitant), and (iii) allow for better patient adherence to oral/enteral non-opioid analgesics on a schedule [particularly agents such as dextromethorphan (18, 19) and celecoxib (20)], while the ITM is providing efficacious analgesia. There may be value in considering 24 and 48 h downstream effects on both pain and PONV recovery parameters when considering the merits of higher ITM doses recommended herein. Specifically, ITM in tandem with five-drug PONV prophylaxis, would be central to the preoperative phase. Thereafter, PONV oral booster dosing with perphenazine and aprepitant on POD 1 (as shown in Supplementary Table S5), in tandem with postoperative and proactive, enterally absorbed non-opioid analgesics such as acetaminophen, COX-2 inhibitors, and dextromethorphan, would seem central to the postoperative phase (instead of commonplace reactive dosing). Integrating these pre- and intra-/post operative maneuvers is intended to avoid potentially any exposure to downstream opioids. Our observed downstream opioid avoidance success presently approximates 17% with the described strategy (as shown in Supplementary Table S4), that perhaps other non-opioid analgesic/antinociceptive maneuvers (e.g., IV esmolol, IV lidocaine, and/or intrathecal magnesium) may improve, based on future research and/or clinical observation. Finally, “force of habit” and “unconscious biases” at the bedside in the operating room may need to be actively addressed preventatively, since the “margin for error” may significantly differ with proposed vs. past-recommended ITM doses (or no ITM at all).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
This quality improvement report involving human participants was reviewed by the VA Pittsburgh Healthcare System Institutional Review Board, and was defined as an exempt protocol (1670098). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
BW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing. DH: Investigation, Methodology, Writing – review & editing. CD: Conceptualization, Investigation, Methodology, Writing – review & editing. KG: Conceptualization, Investigation, Methodology, Writing – review & editing. JL: Conceptualization, Investigation, Methodology, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors are grateful to our Veterans for their service and that their understanding of the described maneuvers as a means to offset the risks of the opioid epidemic sufficiently resonated to receive the described procedures as part of our local recommended standard of care. We are also grateful for the clinical support of our chief anesthesiologist, James W. Ibinson MD, PhD, and our local surgeons, Drs. Ramesh Ramanathan (bariatric surgery), Gregory Watson, Robert Tessler, Robert Handzel, Christine Leeper, Brian Zuckerbraun, and Kelly McCoy (general surgery) and Amir Toussi (urology). Finally, we are grateful for the coordinating efforts of local general surgery physician assistants Megan White and Benjamin Purvis. This manuscript is dedicated to the contemporaneous Executive Director of the United States Veterans Administration National Surgery Office, Mark A. Wilson, MD, PhD.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanes.2025.1521409/full#supplementary-material
References
1. Williams BA, Holder-Murray JM, Nettrour JF, Ibinson JW, DeRenzo JS, Dalessandro C, et al. Aim for zero: prevention of postoperative nausea and vomiting using an off-patent five-drug multimodal approach. Br J Anaesth. (2023) 131(1):e1–4. doi: 10.1016/j.bja.2023.01.005
2. Kharasch ED, Brunt LM, Blood J, Komen H. Intraoperative methadone in next-day discharge outpatient surgery: a randomized, double-blinded, dose-finding pilot study. Anesthesiology. (2023) 139:405–19. doi: 10.1097/ALN.0000000000004663
3. Komen H, Brunt LM, Deych E, Blood J, Kharasch ED. Intraoperative methadone in same-day ambulatory surgery: a randomized, double-blinded, dose-finding pilot study. Anesth Analg. (2019) 128:802–10. doi: 10.1213/ANE.0000000000003464
4. Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations: 2018. World J Surg. (2019) 43:659–95. doi: 10.1007/s00268-018-4844-y
5. Gan TJ, Belani KG, Bergese S, Chung F, Diemunsch P, Habib AS, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. (2020) 131:411–48. doi: 10.1213/ANE.0000000000004833
6. Williams BA, Ibinson JW, Cellurale M, Nalepka T, Becker DB. Same-day and next-day pain and nausea parameters after intrathecal morphine for abdominal panniculectomy and mastectomy post-bariatric surgery. Pain Med. (2021) 22:3114–6. doi: 10.1093/pm/pnab171
7. King MR, Ladha KS, Gelineau AM, Anderson TA. Perioperative dextromethorphan as an adjunct for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. (2016) 124:696–705. doi: 10.1097/ALN.0000000000000950
8. Williams BA, Ibinson JW, Ritter ME, Ezaru CS, Rakesh HR, Paiste HJ, et al. Extended perineural analgesia after hip and knee replacement when buprenorphine-clonidine-dexamethasone is added to bupivacaine: preliminary report from a randomized clinical trial. Pain Med. (2020) 21:2893–902. doi: 10.1093/pm/pnaa229
9. Williams BA, Ibinson JW, Mikolic JM, Boudreaux-Kelly MY, Paiste HJ, Gilbert KL, et al. Day-one pain reductions after hip and knee replacement when buprenorphine-clonidine-dexamethasone is added to bupivacaine nerve/plexus blocks: a randomized clinical trial. Pain Med. (2022) 23:57–66. doi: 10.1093/pm/pnab325
10. Williams BA, Ibinson JW, Mangione MP, Modrak RT, Tonarelli EJ, Rakesh H, et al. Research priorities regarding multimodal peripheral nerve blocks for postoperative analgesia and anesthesia based on hospital quality data extracted from over 1,300 cases (2011–2014). Pain Med. (2015) 16:7–12. doi: 10.1111/pme.12609
11. Williams BA, Dalessandro JH, Kennedy PJ, Ezaru CS, Ritter ME, Maerz DA, et al. Institutional benchmarks of joint replacement analgesia and mobilization after intrathecal morphine vs multimodal peripheral nerve/plexus blocks. Pain Med. (2021) 22:3116–9. doi: 10.1093/pm/pnab193
12. Williams BA, Dalessandro JH. Letter to the editor-same-day pain and rehabilitation parameters after knee replacement with multimodal, mepivacaine-based, perineural analgesia-a case report of bilateral procedures one year apart. Pain Med. (2021) 22:765–6. doi: 10.1093/pm/pnaa257
13. Ritter ME, Williams BA. Rare respiratory depression after adjuvant perineural buprenorphine dual lower-extremity peripheral nerve blocks. Pain Med. (2022) 23:1194–7. doi: 10.1093/pm/pnac012
14. Hassett P, Ansari B, Gnanamoorthy P, Kinirons B, Laffey JG. Determination of the efficacy and side-effect profile of lower doses of intrathecal morphine in patients undergoing total knee arthroplasty. BMC Anesthesiol. (2008) 8:1–9. doi: 10.1186/1471-2253-8-5
15. Schumann R, Ziemann-Gimmel P, Sultana A, Eldawlatly AA, Kothari SN, Shah S, et al. Postoperative nausea and vomiting in bariatric surgery: a position statement endorsed by the ASMBS and the ISPCOP. Surg Obes Relat Dis. (2021) 17:1829–33. doi: 10.1016/j.soard.2021.08.005
16. Bahr MP, Williams BA. Esmolol, antinociception, and its potential opioid-sparing role in routine anesthesia care. Reg Anesth Pain Med. (2018) 43:815–8. doi: 10.1097/AAP.0000000000000873
17. Curry CS, Craig WY, Richard JM, Ward DS. Increasing intraoperative hydromorphone does not decrease postoperative pain: a retrospective observational study. Br J Anaesth. (2021) 126(3):e95–7. doi: 10.1016/j.bja.2020.11.026
18. Henderson DJ, Withington BS, Wilson JA, Morrison LM. Perioperative dextromethorphan reduces postoperative pain after hysterectomy. Anesth Analg. (1999) 89:399–402. doi: 10.1097/00000539-199908000-00028
19. Chau-In W, Sukmuan B, Ngamsangsirisapt K, Jirarareungsak W. Efficacy of pre- and postoperative oral dextromethorphan for reduction of intra- and 24-hour postoperative morphine consumption for transabdominal hysterectomy. Pain Med. (2007) 8:462–7. doi: 10.1111/j.1526-4637.2006.00226.x
Keywords: palonosetron, perphenazine, aprepitant, diphenhydramine, dexamethasone, intrathecal morphine, postoperative nausea and vomiting
Citation: Williams BA, Hall DE, Dalessandro C, Garbelotti KE and Ludden JM (2025) Patient-centered intrathecal morphine dose response in major abdominal surgeries when augmented by innovative five-drug antiemetic prophylaxis. Front. Anesthesiol. 4:1521409. doi: 10.3389/fanes.2025.1521409
Received: 1 November 2024; Accepted: 23 January 2025;
Published: 18 February 2025.
Edited by:
Wael Saasouh, Wayne State University, United StatesReviewed by:
Firoozeh Madadi, Shahid Beheshti University of Medical Sciences, IranRaghuraman M. Sethuraman, Sree Balaji Medical College and Hospital, India
Copyright: © 2025 Williams, Hall, Dalessandro, Garbelotti and Ludden. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brian A. Williams, d2lsbGlhbXNiYUBhbmVzLnVwbWMuZWR1; YnJpYW4ud2lsbGlhbXM2QHZhLmdvdg==; d2lsbGlhbXNiYUBnbWFpbC5jb20=
 Brian A. Williams
Brian A. Williams Daniel E. Hall1,2,3
Daniel E. Hall1,2,3 Kelly E. Garbelotti
Kelly E. Garbelotti