- 1School of Anesthesia, College of Health Science and Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia
- 2School of Medicine, College of Health Science and Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia
- 3School of Public Health, College of Health Science and Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia
Background: Brachial plexus block is used as a surgical anesthesia and analgesia for postoperative pain. Recently, the use of local anesthetics for regional nerve block has been enhanced by mixing them with a different class of drugs as adjuvants. These adjuvants of local anesthetics improved the quality and duration of nerve block and reduced the dose-dependent side effects of local anesthetics. However, the effectiveness of these adjuvants varies depending on the nature of the nerve block and the type of local anesthetics used. Therefore, we aimed to compare the postoperative analgesic effectiveness of dexamethasone vs. tramadol when used as adjuvants to bupivacaine for ultrasound-guided supraclavicular block in upper extremity surgery.
Methods: Utilizing a prospective cohort study design, 126 consecutive patients who were undergoing upper extremity surgery with ultrasound-guided supraclavicular block were included. Patients were divided into three groups based on the preference of the responsible anesthetist to use adjuvants with bupivacaine for the block. The dexamethasone group (n = 42) were given 30 ml of 0.25% bupivacaine with 8 mg dexamethasone, the tramadol group (n = 42) were given 30 ml of 0.25% bupivacaine with 100 mg tramadol, and the non-adjuvant group (n = 42) were given 30 ml of 0.25% bupivacaine alone. The primary outcomes evaluated were postoperative pain severity using the numerical pain rating scale (NRS), the duration of analgesia, and the total postoperative analgesic consumption. Secondary outcomes included the incidence of postoperative complications.
Result: A total of 126 patients were recruited and analyzed. There was no statistical difference in the demographic data among the groups. The postoperative NRS score was significantly reduced in the dexamethasone and tramadol group compared with the non-adjuvant group (p < 0.001). The NRS score in the dexamethasone group at 18 and 24 h was statistically much lower than in the tramadol and non-adjuvant group. The postoperative duration of analgesia was significantly prolonged in the dexamethasone (1,069 ± 316.99 min) group compared with the tramadol (617.02 ± 214.05 min) and non-adjuvant (434.17 ± 111.23 min) groups (p < 0.001). Patients in the non-adjuvant group had a significantly higher total analgesic consumption over 24 h. The dexamethasone group experienced significantly fewer incidences of nausea, with no differences in other complications among the groups.
Conclusion: The addition of dexamethasone as an adjuvant to bupivacaine for ultrasound-guided supraclavicular block improves postoperative analgesia. We recommend the integration of dexamethasone as an adjuvant to local anesthetics during nerve blocks to enhance postoperative pain management after surgery.
Introduction
Brachial plexus block (BPB) is now widely practiced as a single anesthesia technique and/or as part of multimodal analgesia in upper extremity surgeries (1, 2). Supraclavicular block is an effective technique for BPB in surgeries in all portions of the upper extremity below the shoulder joint, e.g., the elbow, forearm, and hand. It is carried out at the division of the brachial plexus and is traditionally called the “spinal of the upper extremities” (1–4).
Local anesthetics for nerve block are given at higher doses, possibly resulting in a dose-dependent side effect on the cardiovascular and nervous systems. In addition, the limitation of the use of local anesthetics for nerve blocks is that the analgesic effect of the block lasts only a few hours, resulting in early moderate to severe pain; therefore, there is a need for adjuvant therapies (5–7).
Several adjuvants have been used to attempt to prolong the analgesia provided by peripheral nerve block (PNB), including perineural dexamethasone and tramadol (8–10). Dexamethasone is a steroid that can reduce pain and the inflammatory response to tissue damage after surgery. Its analgesic mechanism as an adjuvant is not clearly known but is believed to be due to a decrease in inflammation by inhibiting the release of phospholipase α2 and also by blocking the transmission of pain through the nociceptive C-fibers (11, 12). Tramadol is a μ-agonist opioid that inhibits the reuptake of norepinephrine and serotonin. These neurotransmitters are involved in descending inhibitory pain pathways associated with pain relief (13, 14).
Several studies have demonstrated a prolonged duration of analgesia associated with PNB when adjuvants were added to local anesthetics (8, 10, 15). Evidence on the effectiveness of adjuvants in resource-limited areas is scarce. Although catheter insertion and patient-controlled analgesia are viable in high-income settings, they are often impractical in low-resource environments due to costs, catheter shortages, and a lack of trained staff. Using adjuvants in nerve blocks is a practical approach to ensure effective postoperative pain management in upper extremity surgery. Studying the effectiveness of adjuvants with local anesthetics in these settings is crucial in assessing feasibility, safety, and benefits. We hypothesized that the use of dexamethasone as an adjuvant with bupivacaine for supraclavicular blocks will provide better postoperative analgesia than tramadol. Therefore, this study evaluated the postoperative analgesic effectiveness of dexamethasone vs. tramadol given as an adjuvant to bupivacaine for ultrasound-guided supraclavicular block in patients undergoing upper extremity surgery.
Materials and methods
Study design and patients
The study was conducted at Sodo Christian General Hospital (SCGH) over 6 months, from 5 March to 5 September 2020, and ethical clearance was obtained from the Institutional Review Board (IRB) of Wolaita Sodo University, College of Health Sciences and Medicine (decision number 1091/1090/12) on 2 March 2020. Written informed consent was obtained from each participant. The study was registered in a research registry with the unique identification number of researchregistry7977, which can be found at https://www.researchregistry.com/browse-the-registry#home/. The study was reported in line with Strengthening the Reporting of Cohort, Cross-Sectional, and Case-Control Studies (STROCSS) criteria: www.strocssguideline.com (16).
A prospective cohort study was conducted on patients aged >18 years who had an American Society of Anesthesiologists (ASA) physical status of I and II, were scheduled for elective upper extremity surgery, and required supraclavicular block. Patients with a history of chronic pain illness and untreated preoperative pain from any source, with a failed or partial block, given additional blocks to supraclavicular block, who were uncooperative, and/or had any contraindications to block or known allergies to medications were excluded from the study.
Sample size
Although no previous studies had been conducted in this research area, the results of a study conducted in India were used as base data to calculate the sample size. From this study, the mean duration of analgesia (1,023.87 ± 161.01, 454.47 ± 44.29), which provides a larger sample size than other outcome variables, was used to calculate the sample size (17). Based on this information, the sample size was calculated with the statistical software used for previous power analyses (G power version 3.1.9.4). After inputting a substituting power of 80%, a type I alpha error of 0.05, and an effect size of 0.8 into the software, a sample size of 105 was obtained. After adding a 10% contingency and considering an equal allocation ratio of one to one, the total sample size became 126. A consecutive sampling technique was used to select study participants, and all patients who met the inclusion criteria were included until the sample size was achieved.
Data collection procedure
On the morning of surgery, a trained anesthesia professional evaluated the patients for eligibility and collected preoperative data by history taking and chart reviewing after obtaining informed written consent. In addition, patients were instructed on how to self-report pain in the numeric pain rating scale (NRS) score.
Upon patient arrival in the operating room, routine standard monitoring was applied, with the measurement of baseline vital signs—heart rate (HR), blood pressure (BP), and oxygen saturation (SpO2)—throughout the procedure. Then, patients were placed in a suitable position under all aseptic conditions and ultrasound-guided supraclavicular block was administered as an anesthesia technique for upper extremity surgery. As our institution has not yet allowed randomized control trials (RCTs), patients were not randomized but were instead classified into dexamethasone (n = 42), tramadol (n = 42), and non-adjuvant (n = 42) groups depending on the choice and decision of the anesthetist in charge to use adjuvants for local anesthetics during nerve block. To improve the quality and duration of supraclavicular block, our institution used dexamethasone (8 mg) or tramadol (100 mg) as adjuvants to 30 ml of 0.25% bupivacaine. Following the block, patients were classified into a dexamethasone group (given 8 mg of dexamethasone as an adjuvant to 30 ml of 0.25% bupivacaine), tramadol group (given 100 mg of tramadol as an adjuvant to 30 ml of 0.25% bupivacaine), or non-adjuvant group (given 30 ml of 0.25% bupivacaine without adjuvants) (Figure 1). Then, the time of the block performed was recorded and the onset of the block was checked up to 30 min. The block was considered to have failed when sensation and pain were felt upon a traumatic pinprick with a blunt needle at the blocked site after 30 min.
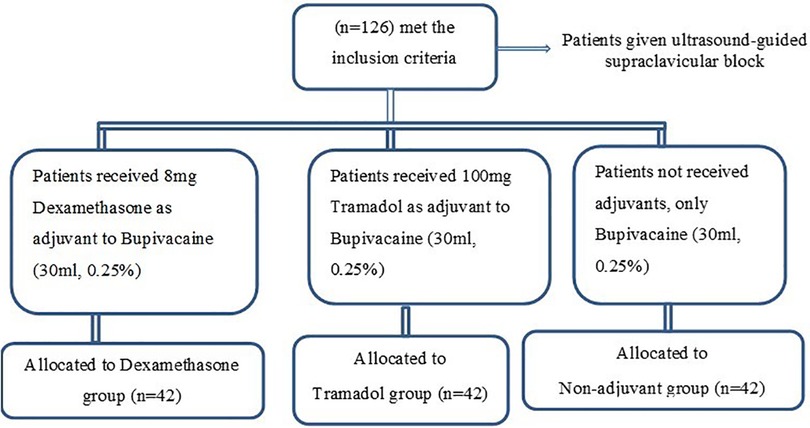
Figure 1. Patient allocation and enrollment chart of patients scheduled for upper extremity surgery under supraclavicular block.
After surgery, patients were discharged from the operating room to the post-anesthesia care unit (PACU), where postoperative pain severity, the duration of analgesia, total postoperative analgesic consumption, and the incidence of postoperative complications were assessed and recorded up to 24 h by data collectors who were unaware of the group allocation.
The primary outcome measures included postoperative pain severity, the mean duration of analgesia, and total postoperative analgesic consumption, which were obtained when patients arrived in the PACU 6, 12, 18, and 24 h postoperatively. Patients were asked to report their postoperative pain using a standardized 11-point NRS from 0 (no pain) to 10 (worst imaginable pain). In addition, the time of the first analgesic request and total analgesic consumption were recorded on the patient's chart. Secondary outcomes, such as the incidence of postoperative complications related to the adjuvants (nausea, vomiting, shivering, hypotension, bradycardia, and respiratory complications), were recorded and informed treatment as per the hospital protocols. Patients who experienced nausea were treated with 10 mg of metoclopramide intravenously (IV).
The quality of data maintained through a questionnaire was pre-tested on 5% of the sample size at Wolaita Sodo University Comprehensive Specialized Hospital, and necessary modifications were made based on the nature of the gaps identified in the questionnaire. In addition, training was provided to the data collectors and supervisor. Patients were closely supervised and followed-up during the data collection, and the collected data were cross-checked for their completeness, clarity, and consistency.
Operational definitions
Duration of analgesia
The time in minutes from the onset of blockage to the first time analgesia was administered.
Total consumption of analgesia
The total dose of analgesic medication administered in milligrams within the first 24 h after the onset of nerve block.
Failed block
When patients perceive a pinprick sensation after 30 min of the block procedure.
Onset of nerve block
No sensation and pain are felt upon a traumatic pinprick with a blunt needle at a blocked site.
Numerical pain rating scale
A valid pain assessment tool in which patients are asked to score their pain ratings on a scale of 0–10, where 0 = no pain, 1–3 = mild pain, 4–6 = moderate pain, 7–9 = severe pain, and 10 = worst possible pain.
Data analysis and interpretation
After manual checks for data completeness, the data were entered into EPI-info 7 and transferred for analysis to SPSS version 25. Data were tested for normality using a histogram, Q-Q plot inspection, and the Shapiro–Wilk test. After checking homogeneity of variance by Levene's test normally distributed and continuous data were analyzed using one-way analysis of variance (ANOVA) with post hoc analysis for multiple test and non-normally distributed data were analyzed using Kruskal-Wallis H rank test. Comparisons of categorical variables were analyzed using Pearson's chi-squared test or Fisher's exact test with post hoc as required. Results are presented as mean ± SD for symmetric data and median ± IQR for asymmetric data, whereas categorical data are reported as counts and percentages. P- < 0.05 was considered statistically significant.
Results
A total of 126 patients were recruited during the study period and analyzed based on whether they received dexamethasone, tramadol, or a non-adjuvant to bupivacaine for ultrasound-guided supraclavicular block in upper extremity surgery. The patient demographic characteristics age, sex, and BMI were comparable between groups (Table 1). Most of the patients were in ASA status I and were treated by senior orthopedic specialists, but there were no significant differences between the three groups. Patient characteristics, such as mean vital signs, intraoperative blood loss, intraoperative fluid balance, and the duration of surgery, were also comparable between the groups, with a p > 0.05, as shown in Table 2.
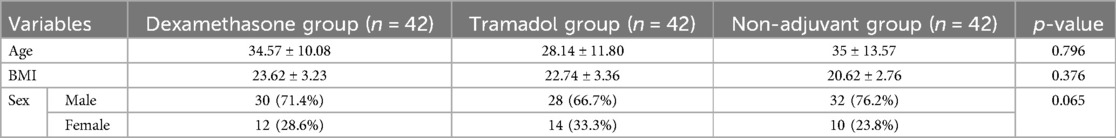
Table 1. Demographic characteristics of the patient groups who underwent upper extremity surgery under ultrasound-guided supraclavicular block.
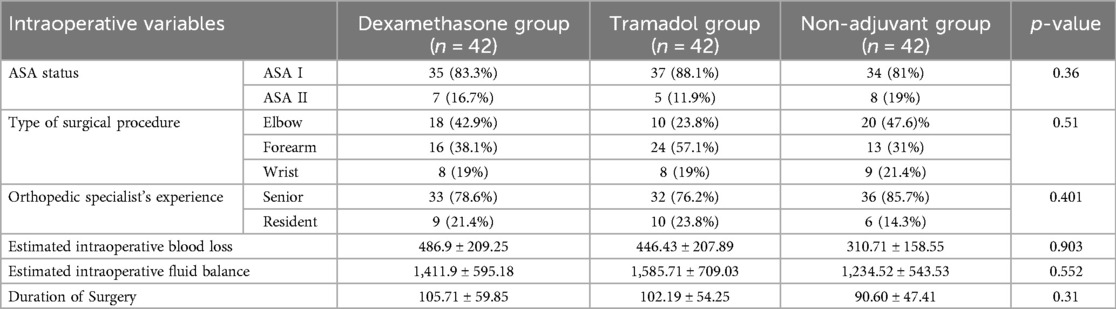
Table 2. Perioperative characteristics of the patient groups who underwent upper extremity surgery under ultrasound-guided supraclavicular block.
Postoperative pain severity on the NRS was significantly lower in patients in the dexamethasone group at 12, 18, and 24 h postoperatively [H = 57.43 (2, N = 126), p < 0.001, η2 = 0.454]. The proportion of variability in the NRS score ranked by the dexamethasone and tramadol groups was 0.454, indicating a strong relationship between dexamethasone and tramadol and the change in the NRS score. Post-hoc analysis showed a significant reduction in the NRS score between dexamethasone and the non-adjuvant group, with an adjusted p < 0.001. In addition, there was a reduction in the NRS score between the tramadol and non-adjuvant groups, with an adjusted p <0.001 at the 12th, 18th and 24th hours. However, a significant difference in the NRS score between the dexamethasone and tramadol groups was detected only at the 18th and 24th hours (Table 3, Figure 2).
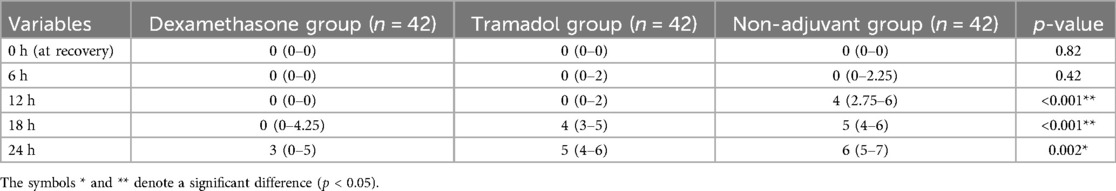
Table 3. Comparison of the pain severity score (NRS) between the dexamethasone, tramadol, and non-exposed groups, who underwent upper extremity surgery under ultrasound-guided supraclavicular block.
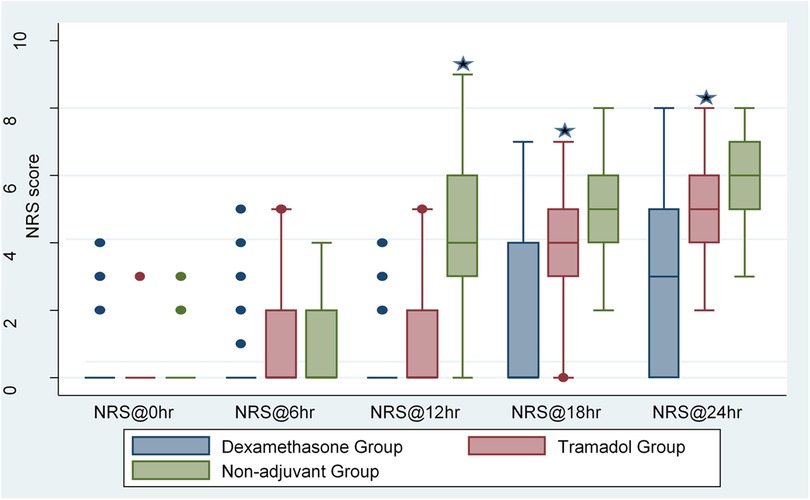
Figure 2. Comparisons of pain intensity by NRS score between the dexamethasone, tramadol, and non-adjuvant groups who underwent upper extremity surgery under ultrasound-guided supraclavicular block.
The mean duration of analgesia was greater in the dexamethasone group (1,069 ± 316.99) than in the tramadol and non-adjuvant groups (p < 0.001). A post-hoc analysis showed the duration of analgesia was significantly prolonged between the dexamethasone and tramadol groups, the dexamethasone and non-adjuvant groups, and the tramadol and non-adjuvant groups (Table 4).

Table 4. Comparison of the duration of analgesia between the dexamethasone, tramadol, and non-exposed groups, who underwent upper extremity surgery under ultrasound-guided supraclavicular block.
Total diclofenac consumption was significantly different between the groups [H = 26.65 (2, N = 126) p < 0.001, η2 = 0.206]. The medium effect size of 0.206 indicates that the dexamethasone and tramadol groups had a proportionate variation in median total diclofenac consumption. Furthermore, post-hoc comparison showed no statistically significant differences in total analgesic consumption between the dexamethasone and tramadol groups. The cumulative consumption of diclofenac at different intervals between groups was also significantly different. Cumulative diclofenac consumption showed a significant difference between dexamethasone and non-adjuvant at the 12th hour with an adjusted p-value <0.01. However, there were no statistically significant differences in cumulative diclofenac consumption between tramadol and non-adjuvant at all-time intervals for 24 h except at the 18th hour (Table 5).
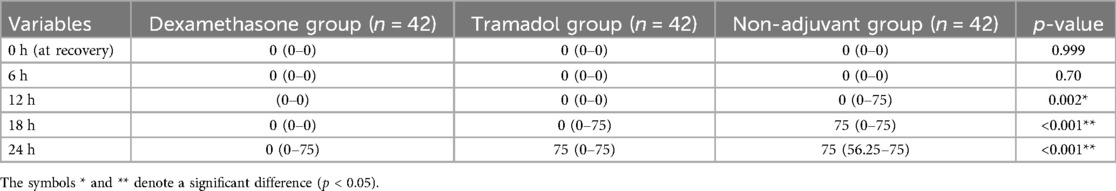
Table 5. Comparison of analgesic consumption between groups, who underwent upper extremity surgery under ultrasound-guided supraclavicular block.
With regard to postoperative complications, the overall incidence of nausea and vomiting was 19.84% over 24 h. However, the proportion of patients experiencing nausea was significantly lower in the dexamethasone group (3.17%) than in the tramadol (7.14%) and non-adjuvant (9.52%) groups [χ2 (2, N = 126) = 3.76, p = 0.026]. However, there was no difference in the incidence of sedation and hypotension between the groups. There were no recorded complications such as pneumothorax, systemic local anesthetic toxicity, failed block, or hand weakness between groups (Figure 3).
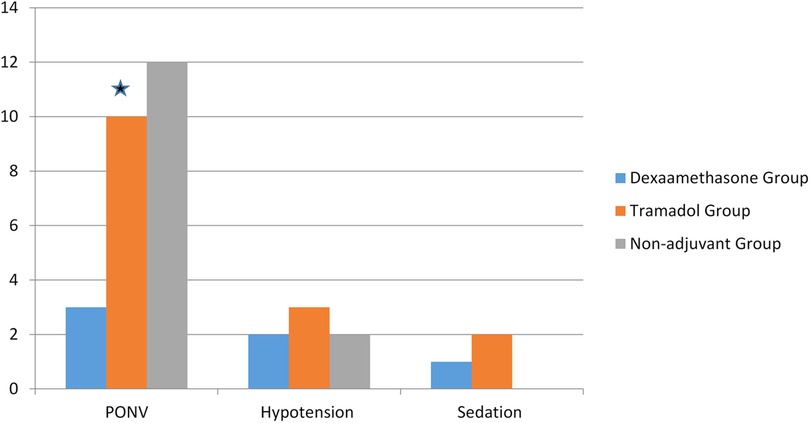
Figure 3. The incidence of postoperative complications between groups who underwent upper extremity surgery under ultrasound-guided supraclavicular block.
Discussion
Our study demonstrated that dexamethasone is a more effective adjuvant to bupivacaine than tramadol for enhancing postoperative analgesia in ultrasound-guided supraclavicular blocks. It significantly reduces postoperative pain, prolongs analgesia duration, and decreases total analgesic consumption after upper extremity surgery. In addition, tramadol is an effective adjunct compared with bupivacaine alone for postoperative pain management in this context. This is in line with other studies of upper extremity surgeries (14, 18–21).
The median (interquartile range) NRS score of the dexamethasone group after 18 h postoperatively, in our case, was 0 (0–4.25), which was comparable with the study in India, 2.73 and 1.96 in Visual Analogue Scale (VAS) at 12 h and 24 h, respectively (22). This finding was also comparable with previous studies in which dexamethasone and tramadol decreased postoperative pain and the requirement for analgesia, and prolonged the duration of analgesia in upper extremity surgery (14, 18–21).
This study shows that tramadol decreases pain intensity for the first 12 h and analgesic consumption for up to 24 h during the postoperative period. This result was in line with a study by Khosa et al. in Dera Ghazi Khan, in 2015, which compared the tramadol group with a non-exposed group, that showed a lower VAS score in the tramadol group (23). In addition, previous studies that have examined the analgesic effects of tramadol in patients undergoing an open cholecystectomy and upper extremity procedure reported comparable results (12, 14, 24–26).
In this study, the mean time for the duration of analgesia by one-way ANOVA was significantly prolonged in the dexamethasone group [mean time, 1,069 ± 316.99 min vs. the tramadol group; mean time: 617.02 ± 214.05 min (p < 0.001)]. In a study by Shrestha et al., the mean time to the first analgesic request in the dexamethasone group for upper extremity surgery was comparable with our study (1,028.17 ± 194.51 min). This finding was also supported by other studies. However, a study conducted by Kiran showed that the mean time to the first analgesic request in the dexamethasone group was 20.66 min. This could be due to the difference in surgery and block types (open cholecystectomy with oblique subcostal transversus abdominis plane block), the use of different local anesthetics with a short duration of action (ropivacaine), or the hospital pain management protocol (22).
In our study, the mean time to the first analgesic request in the tramadol group for upper extremity surgery was 617.02 min (p < 0.001). In contrast, the study by Kumar et al. reported a time to the first analgesic request of 12.8 ± 0.86 h. This could be due to the difference in the local anesthesia drug (levobupivacaine) (24). Another study by Soulioti et al. reported that the time to the first analgesic request in the tramadol group was 13 ± 2.3 h. One reason for this difference in the duration of time compared with our study may be the technique used (supraclavicular block vs. interscalene block) (13).
In addition, we found that the total amount of analgesic consumption during the 24-h postoperative period was lower. The median diclofenac consumption was 0 (0–75) and 75 (0–75) mg in the dexamethasone and tramadol groups, respectively. This result contrasted with a previous study by Acharya et al. in which the total diclofenac consumption of the dexamethasone group was 100 (100–200); this could be due to the short duration of analgesia compared with the present study (27).
The current study has certain limitations, including a lack of randomization and an outcome that was only assessed up to 24 h postoperatively, thus potentially missing longer-term effects or complications. It was conducted at a single institution, which may restrict the generalizability of the findings to other settings with different patient populations and surgical practices.
In conclusion, the use of dexamethasone as an adjuvant to bupivacaine for ultrasound-guided supraclavicular block improves postoperative analgesia for upper extremity surgery. This study showed a prolonged duration of analgesia, a reduced postoperative pain score, and reduced total analgesic consumption. We recommend the integration of dexamethasone as an adjuvant to local anesthetics for nerve blocks to enhance postoperative pain management after surgery.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Wolaita Sodo University, College of Health Sciences and Medicine Institutional Review Board (IRB). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HT: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AS: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing. GD: Formal Analysis, Methodology, Supervision, Validation, Visualization, Writing – review & editing. MT: Formal Analysis, Methodology, Supervision, Validation, Visualization, Writing – review & editing. EH: Formal Analysis, Methodology, Supervision, Validation, Visualization, Writing – review & editing. AA: Formal Analysis, Methodology, Supervision, Validation, Visualization, Writing – review & editing. MA: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank Wolaita Sodo University, Sodo Christian Hospital, the study participants, and the data collectors for their cooperation and support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bruce BG, Green A, Blaine TA, Wesner LV. Brachial plexus blocks for upper extremity orthopaedic surgery. JAAOS, Journal of the American Academy of Orthopaedic Surgeons. (2012) 20(1):38–47. doi: 10.5435/00124635-201201000-00005
2. Pester JM, Varacallo M. Brachial Plexus Block Techniques. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright 2022, StatPearls Publishing LLC. (2022).
3. D’Souza RS, Johnson RL. Supraclavicular Block. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright 2022, StatPearls Publishing LLC. (2022).
4. Koscielniak-Nielsen ZJ. Ultrasound-guided peripheral nerve blocks: what are the benefits? Acta Anaesthesiol Scand. (2008) 52(6):727–37. doi: 10.1111/j.1399-6576.2008.01666.x
5. Suzuki S, Gerner P, Lirk P. 20 - Local anesthetics. In: Hemmings HC, Egan TT, editors. Pharmacology and Physiology for Anesthesia (Second Edition). Philadelphia: Elsevier (2019). p. 390–411.
6. Ketonis C, Ilyas AM, Liss F. Pain management strategies in hand surgery. Orthopedic Clinics of North America. (2015) 46(3):399–408. doi: 10.1016/j.ocl.2015.02.008
7. Brattwall M, Jildenstl P, Stomberg M, Jakobsson J. Upper extremity nerve block: how can benefit, duration, and safety be improved? An update. F1000Res. (2016) 5:907. doi: 10.12688/f1000research.7292.1
8. Bailard NS, Ortiz J, Flores RA. Additives to local anesthetics for peripheral nerve blocks: evidence, limitations, and recommendations. Am J Health-Syst Pharm. (2014) 71(5):373–85. doi: 10.2146/ajhp130336
9. Edinoff AN, Houk GM, Patil S, Bangalore Siddaiah H, Kaye AJ, Iyengar PS, et al. Adjuvant drugs for peripheral nerve blocks: the role of alpha-2 agonists, dexamethasone, midazolam, and non-steroidal anti-inflammatory drugs. Anesth Pain Med. (2021) 11(3):e117197. doi: 10.5812/aapm.117197
10. Patacsil JA, McAuliffe MS, Feyh LS, Sigmon LL. Local anesthetic adjuvants providing the longest duration of analgesia for single-injection peripheral nerve blocks in orthopedic surgery: a review of the literature. AANA J. (2016) 84(2):95–103.27311150
11. Pehora C, Pearson AM, Kaushal A, Crawford MW, Johnston B. Dexamethasone as an adjuvant to peripheral nerve block. Cochrane Database Syst Rev. (2017) 11(11):Cd011770. doi: 10.1002/14651858.CD011770.pub2
12. Rasmussen SB, Saied NN, Bowens C Jr, Mercaldo ND, Schildcrout JS, Malchow RJ. The duration of peripheral nerve blockade in the upper and lower extremities is prolonged with dexamethasone when added to ropivacaine: a retrospective database analysis. Pain Med. (2013) 14(8):1239–47. doi: 10.1111/pme.12150
13. Soulioti E, Tsaroucha A, Makris A, Koutsaki M, Sklika E, Mela A, et al. Addition of 100 mg of tramadol to 40 ml of 0.5% ropivacaine for interscalene brachial plexus block improves postoperative analgesia in patients undergoing shoulder surgery compared to ropivacaine alone-A randomized controlled study. Medicina (Kaunas). (2019) 55(7):399. doi: 10.3390/medicina55070399
14. Regmi N, Subba S, Sharma U. A comparative clinical evaluation of the efficacy of tramadol as an adjuvant to bupivacaine en brachial plexus block for upper limb surgery. J Nepalgunj Med Coll. (2017) 13:13. doi: 10.3126/jngmc.v13i2.16535
15. Weinstein EJ, Levene JL, Cohen MS, Andreae DA, Chao JY, Johnson M, et al. Local anesthetics and regional anesthesia versus conventional analgesia for preventing persistent postoperative pain in adults and children. Cochrane Database Syst Rev. (2018) 4(4):Cd007105. doi: 10.1002/14651858.CD007105.pub3
16. Mathew G, Agha R, Albrecht J, Goel P, Mukherjee I, Pai P, et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional, and case-control studies in surgery. Int J Surg. (2021)(96):106165. doi: 10.1016/j.ijsu.2021.106165
17. Shrestha BR, Maharjan SK, Shrestha S, Gautam B, Thapa C, Thapa PB, et al. Comparative study between tramadol and dexamethasone as an admixture to bupivacaine in a supraclavicular brachial plexus block. JNMA J Nepal Med Assoc. (2007) 46(168):158–64.18340366
18. Baloda R, Bhupal JP, Kumar P, Gandhi GS. Supraclavicular brachial plexus block with or without dexamethasone as adjuvant to 0.5% levobupivacaine: a comparative study. J Clin Diag Res. (2016) 10(6):Uc09–12. doi: 10.7860/JCDR/2016/18325.8048
19. Kim YJ, Lee GY, Kim DY, Kim CH, Baik HJ, Heo S. Dexamathasone added to levobupivacaine improves postoperative analgesia in ultrasound guided interscalene brachial plexus blockade for arthroscopic shoulder surgery. Korean J Anesthesiol. (2012) 62(2):130–4. doi: 10.4097/kjae.2012.62.2.130
20. Movafegh A, Razazian M, Hajimaohamadi F, Meysamie A. Dexamethasone added to lidocaine prolongs the blockage of the axillary brachial plexus. Anesth Analg. (2006) 102(1):263–7. doi: 10.1213/01.ane.0000189055.06729.0a
21. Vieira PA, Pulai I, Tsao GC, Manikantan P, Keller B, Connelly NR. Dexamethasone with bupivacaine increases the duration of analgesia in ultrasound-guided interscalene brachial plexus blockade. European J Anesthesiol. (2010) 27(3):285–8. doi: 10.1097/EJA.0b013e3283350c38
22. Bala R, Kumar K. Comparison of the analgesic efficacy of dexamethasone versus tramadol as an adjuvant to ropivacaine for blockage of the oblique subcostal transversus abdominis plane in open cholecystectomy. Indian J Pain. (2019) 33:141. doi: 10.4103/ijpn.ijpn_62_19
23. Khosa AH, Asad N, Durrani H. Does the addition of tramadol to the local anesthetic mixture improve the quality of axillary brachial plexus block: a comparative study in the teaching hospital, Dera Ghazi Khan. Age (Years). (2015) 34(13.9):34.87–9.9. ISSN: 0094-6354.
24. B TA. Comparison of efficacy of ropivacine alone with tramadol-ropivacaine combination in supraclavicular brachial plexus block for upper limb surgery. Biomedica. (2015) 31(4):281–3. ISSN: 1992-4852.
25. Kirksey MA, Haskins SC, Cheng J, Liu SS. Local anesthetic peripheral nerve block adjuvants for prolongation of analgesia: a systematic qualitative review. PLoS One. (2015) 10(9):e0137312. doi: 10.1371/journal.pone.0137312
26. Kumari A, Chhabra H, Gupta R, Kaur H. Comparative study of the effectiveness of tramadol and butorphanol as adjuvants to levobupivacaine for supraclavicular brachial Plexus block. Anesthesia, Essays Res. (2019) 13(3):446–51. doi: 10.4103/aer.AER_110_19
Keywords: postoperative analgesia, ultrasound-guided supraclavicular block, adjuvants, bupivacaine, dexamethasone, tramadol, upper extremity surgery
Citation: Tadesse H, Sintayhu A, Dendir G, Tila M, Habtu E, Alemu A and Alemayehu M (2024) The role of adjuvants in regional anesthesia: the postoperative analgesic effectiveness of dexamethasone vs. tramadol given as adjuvants to bupivacaine for ultrasound-guided supraclavicular block for upper extremity surgery—a prospective cohort study. Front. Anesthesiol. 3:1423919. doi: 10.3389/fanes.2024.1423919
Received: 26 April 2024; Accepted: 13 September 2024;
Published: 8 October 2024.
Edited by:
Thomas Schricker, McGill University, CanadaReviewed by:
Amaresh Vydyanathan, Albert Einstein College of Medicine, United StatesLalit Gupta, University of Delhi, India
Bahadir Ciftci, Istanbul Medipol University, Türkiye
Copyright: © 2024 Tadesse, Sintayhu, Dendir, Tila, Habtu, Alemu and Alemayehu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashagrie Sintayhu, YXNoYWdyaWUyMEBnbWFpbC5jb20=
 Haregewoin Tadesse1
Haregewoin Tadesse1 Ashagrie Sintayhu
Ashagrie Sintayhu Getahun Dendir
Getahun Dendir Mebratu Tila
Mebratu Tila Afewerk Alemu
Afewerk Alemu Mihiretu Alemayehu
Mihiretu Alemayehu