
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Anesthesiol. , 12 February 2024
Sec. Neuroanesthesiology
Volume 3 - 2024 | https://doi.org/10.3389/fanes.2024.1351698
 Alisha Sachdev†
Alisha Sachdev† Daniel Torrez
Daniel Torrez Sarah Sun
Sarah Sun George Michapoulos
George Michapoulos Nicholas C. Rigler
Nicholas C. Rigler Alexandra L. Feldner
Alexandra L. Feldner Young Soo Hong
Young Soo Hong Robert J. McCarthy*†
Robert J. McCarthy*†
Introduction: National representative estimates on in-hospital delirium after acute ischemic stroke are not well established and there is limited data on the impact of delirium on clinical outcomes following mechanical thrombectomy. We evaluated risk factors for delirium and the impact on outcomes following mechanical thrombectomy for acute ischemic stroke.
Methods: This is a retrospective study of patients who underwent mechanical thrombectomy for acute ischemic stroke at a single tertiary comprehensive stroke center between April 2011 and December 2019. Delirium was assessed using the Confusion Assessment Method for the Intensive Care Unit. Patient characteristics, comorbidities, laboratory data, elapsed times, tissue plasminogen activator use, duration of the procedure, type of anesthesia, National Institute of Health stroke scores (NIHSS), sedation scores, reperfusion grades, complications, length of hospital stay, discharge disposition, and 90-day mortality were evaluated.
Results: Five hundred and two patients were evaluated, and post-procedural delirium was identified in 24/467 (5.1%) patients. Thirty-five patients could not be assessed for delirium due to excessive sedation. The incidence of delirium in white vs. non-white patients <65 years was 5/137 (3.6%) compared to 0/91 (0%), and 7/176 (4.0%) compared to 12/63 (19%) in patients ≥65 years, P = 0.006. Bias reduction multi-variable analysis identified low postprocedural hemoglobin level odds ratio of 0.76 (95% CI 0.61–0.92, P = 0.006), greater age (odds ratio 1.04, 95% CI 1.01–1.009, P = 0.024), and non-white race odds ratio of 2.52 (95% CI 1.06–6.38, P = 0.030) as factors associated with delirium [Brier score = 0.045, C-index = 0.800, and Akaike Information Criterion (AIC) = 174]. General anesthesia was not associated with an increased delirium risk. NIHSS at 24 and 48 h and discharge, length of stay, and 90-day mortality were not different between delirium and no-delirium groups. Delirium patients had a reduced odds ratio of 0.13 (05% CI 0.01–1.00, P = 0.02) for home discharge.
Discussion: Delirium following mechanical thrombectomy for acute ischemic stroke primarily affected older patients and was associated with reduced odds of home discharge following hospitalization. Changes in NIHSS during hospitalization and 90-day mortality were not adversely affected by the presence of delirium. General anesthesia was not associated with an increased delirium risk following mechanical thrombectomy.
Almost 800,000 individuals suffer from a stroke in the United States annually (1). Despite attempts at public education on early recognition of stroke symptoms to improve chances of timely care, patient knowledge remains poor in the US population (2). Upon presentation, initial neurologic assessment for acute ischemic stroke (AIS), stabilization, and resuscitation are essential to optimize perfusion to affected areas of the brain (3). IV tissue-type plasminogen activator (tPA) has proven to be a beneficial treatment for select patients with AIS up to 4.5 h after the onset of symptoms (4). The greatest benefits, including reduced in-hospital mortality, reduced symptomatic intracerebral hemorrhage, and increased independent ambulation at discharge, occur when the drug is administered early after stroke onset, and decline as length of time to administration increases (2). The DAWN trial demonstrated that mechanical thrombectomy beyond this initial window of 6–24 h beyond last known well time also had improved disability outcomes at 90 days in patients with clinical deficits who were disproportionately severe relative to the infarct volume (5).
Delirium can frequently occur in patients after AIS, with occurrence rates ranging from 3.5% according to a retrospective review of the Nationwide Inpatient Sample to 25% according to a meta-analysis of 32 cross-sectional and cohort clinical trials (6, 7). Patients with delirium following stroke have higher odds of in-hospital mortality, longer length of stay, higher proportion of being discharged to a skilled nursing facility, and long-term care of hospice. Delirium following AIS has also been associated with speech disorders, leukoencephalopathy, chronic obstructive pulmonary disease, and early use of physical restraints (8). Delirium has also been associated with worse outcomes after AIS, including increased hospital mortality, longer length of stay, and decreased incidence of discharge home (6). Despite tPA or intraarterial thrombolysis therapy, Pan et al. reported that up to 54% of patients experience at least one episode of delirium following AIS treatment with tPA or intraarterial thrombolytic therapy (9). There is limited data on the impact of delirium on clinical outcomes following MT with or without IV tPA administration.
We hypothesized that patients with incident delirium following MT would have poorer outcomes, less improvement in NIHSS during hospitalization, less favorable discharge disposition, and greater 90-day mortality compared to patients who did not exhibit delirium.
This study was approved by the Institutional Review Board of Rush University (20030509-IRB01, approval date 31 March 2020). This manuscript was prepared using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The study was granted a waiver of informed consent because it evaluated existing records, was not greater than minimal risk, and was deemed to be Health Insurance Portability and Accountability Act compliant because safeguards were in place to protect the personal health information of the subjects. The study design was a retrospective cohort of consecutive adult patients who underwent mechanical thrombectomy for ischemic stroke at Rush Medical Center between 1 January 2011 and 31 December 2019.
The stroke neurology team at Rush University Medical Center maintains a log of all patients undergoing this procedure. The data captured for each patient includes the date of procedure, sex, age, body mass index, allergies, smoking history, alcohol use, history of hypertension, dyslipidemia, congestive heart failure, atrial fibrillation, diabetes, location of tPA administration (prior to or after arrival at Rush), initial hospital status, initial National Institute of Health stroke score (NIHSS), modified Rankin scores (mRS), thrombolysis in cerebral infarction (TICI) reperfusion grade, presence of periprocedural hemorrhagic, time from hospital arrival to operating room, time from hospital arrival to arterial puncture, time from last known well (LKW) status to puncture, time from LKW to recanalization, NIHSS at 24 and 48 h after the procedure and at discharge, postoperative length of stay, in-hospital complications, discharge disposition, mRS at admission and at 90 days after the procedure, and any rehospitalizations during this time.
In addition, the following information was obtained from the patient's electronic medical record: American Society of Anesthesiologists (ASA) status, total operating room time, type of anesthesia received for the procedure, use of fentanyl, propofol, midazolam, and dexmedetomidine during the procedure, reason for intubation if applicable, use of inhaled anesthetic, episodes of hypertension (systolic blood pressure >220) and hypotension (systolic blood pressure <160), and procedural complications. Clinical laboratory data including blood urea nitrogen, serum creatinine, glucose, hemoglobin, hematocrit, platelet counts, glycated hemoglobin (Hgb A1C), sodium, total cholesterol, high-density lipoprotein, low-density lipoprotein, and triglyceride level were obtained. An estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation (2021) (10).
Delirium assessments were made using the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) over the first 72 h after the procedure, and the corresponding Richmond Agitation–Sedation Scale (RASS) scores were recorded. Post-MT patients were admitted to the Neurological Sciences Intensive Care Unit and groin checks, vital signs, and full neurological exams were regularly evaluated per protocol. Neurological exams were performed by ICU nurses trained in performing a complete NIHSS stroke assessment. This stroke assessment was also performed by the stroke team and the ICU team covering the patient. All physicians and advanced practice providers on each of these teams have special training from the institution in completing neurological assessments. RASS scores obtained 24 h around the CAM-ICU assessment were used to classify delirium motor subtypes: hypoactive delirium was a positive CAM-ICU and all RASS of 0 to −3 values, hyperactive delirium was a positive CAM-ICU and all RASS of +1 to +4 values, and mixed was a positive CAM-ICU and both positive and negative RASS values (11).
The primary outcome was the hospital discharge disposition and 90-day mortality following thrombectomy for AIC. The primary variable of interest was a diagnosis of delirium recorded during hospitalization. The primary outcome was compared between patients who had a delirium episode following thrombectomy and those without a delirium episode using Fisher's exact test. Differences in proportions and 95% confidence intervals for the difference were calculated using the Clopper–Pearson method.
Secondary outcomes included the difference in the incidence of delirium between patients who received general anesthesia compared with sedation, changes in NIH stroke scores between initial presentation and discharge, and multivariable assessment of factors associated with post-procedural diagnosis of delirium. Univariable associations between patient characteristics, history, tPA administration, procedural times, revascularization grade, initial stroke NIH stroke scores, hypotensive episodes during the procedure, and post-procedural laboratory values between patients exhibiting delirium and those that did not using a chi-squared test or the Mann–Whitney U-test. Changes in NIH stroke scores from presentation through hospital discharge were evaluated using a generalized estimation equation with patient ID as a subject variable, time (baseline, 24 h, 48 h, and discharge) as a within-subject variable, delirium as a factor, and age as a covariate, using an exchangeable covariance matrix. Estimated marginal means were compared pairwise and adjusted for multiple comparisons using the Bonferroni method.
Variables that demonstrated an effect size >0.2 on univariable analysis were entered into a generalized linear model and forward and backward elimination was performed based on minimization of the Akaike information criteria. Multicollinearity was assessed by evaluating the variance inflation factor for variables in the final model. Multi-variable adjusted estimates of the odds of a delirium episode for variables in the model were made using a bias reduction generalized linear model logistic model based on an adjusted scores approach to bias reduction (12). The effect size of the variables in the final equation is expressed as the exponent of beta with 95% confidence intervals of the estimates.
The sample for this study was a convenience sample of consecutive subjects who underwent MT for AIC during the study period. Based on an evaluation of the mechanical thrombectomy procedural schedules, we estimated that 8 years of data would result in approximately 500 cases. If 15% of the patients exhibited a delirium episode, a sample of 522 subjects would have a power of 0.8 at an alpha of 0.5 to detect an odd ratio of 3.0 for the primary outcome of 90-day mortality in patients that had a delirium episode compared to patients that did not.
A P < 0.05 was required to reject the null hypothesis. Statistical tests were two-sided. Missing data were excluded case-wise. The number of cases with missing data is shown in the tables. Statistical analysis was performed using RStudio version 2023.09.1 Build 494, Posit Software, PBC., Boston, MA; URL: http://www.posit.co/ and R version 4.3.2, release date 31-Oct-2023 (The R Foundation for Statistical Computing, Vienna, Austria; URL: https://cran.r-project.org). Sample size analysis was performed using PASS 15.0.13, release date 10-Feb-2020 (NCSS, LLC, Kaysville UT).
Five hundred and two patients underwent an MT for an AIC in the study period and were included in the study. The flow of subjects through the study is shown in Figure 1. Twenty-four of 467 (5.1%) patients were identified with delirium in the post-procedural period. Two patients (4%) had mixed delirium and 22/24 (96%) had hypoactive delirium. Thirty-five patients did not have a CAM ICU assessment performed in the post-procedural period because their level of sedation or agitation precluded assessment (Figure 2).
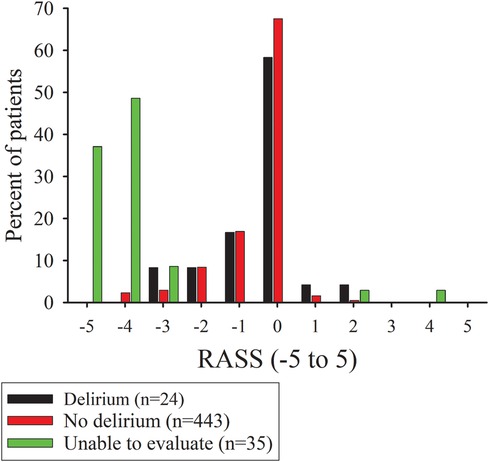
Figure 2. Bar graphs of the percentage of subjects with Richmond agitation-sedation scores (RASS) at the time around the confusion assessment method for the intensive care unit (CAM-ICU) assessment for patients with delirium or RASS value at first assessment for no delirium or unable to evaluate patients.
The median (1st and 3rd quartile) age of the patients in the delirium group was 75 years (67, 82) which was greater than the no delirium group, with a difference of 10y (95% CI 2–12, P < 0.008) (Table 1). The risk ratio for delirium in a patient >65 years was 3.6 (95% CI 1.4–9.5, P = 0.006). There was a significant interaction between race and age among patients <65 and ≥65 years, where the incidence of delirium was 5/137 (3.6%) and 7/176 (4.0%) in white patients compared with 0/91 (0%) and 12/63 (19%) in non-white patients, P = 0.006. There were no other significant differences in patient characteristics, risk factors, medical history, initial NIH stroke scores, modified Rankin scores at presentation, and IV tPA administration in patients with and without delirium.
Procedural data and post-procedural laboratory data are shown in Table 2. The median LKW to procedure start time was 55 min (95% CI −100 to −8, P = 0.028) shorter in the delirium group. Post-procedural clinical chemistry values demonstrated a lower median HgB (difference of 1.7 g/dl) (95% CI −2.9 to −0.6, P = 0.005), and lower median platelet count (difference −22 103/mm) (−47 to −3, P = 0.048), in the delirium group.
General anesthesia was administered to 2/24 (8.3%) of the patients that had delirium compared with 97/443 (21.8%) of the no-delirium group, with a difference of −13.5 (95% CI, −27–0.3, P = 0.184). Drugs administered during the procedure are shown in Table 3. There was no difference in the proportions of patients receiving midazolam, vasopressors, nicardipine, or platelet inhibitors or in the agents administered for sedation or general anesthesia between delirium and no-delirium groups. Multi-variable analysis identified low hemoglobin level odds ratio of 0.76 (95% CI 0.61–0.92, P = 0.006), greater age (odds ratio 1.04, 95% CI 1.01–1.009, P = 0.024) and non-white race odds ratio of 2.52 (95% CI 1.06–6.38, P = 0.030) as factors associated with delirium: Brier score = 0.045, C-index = 0.800, and AIC = 174 (Table 4).
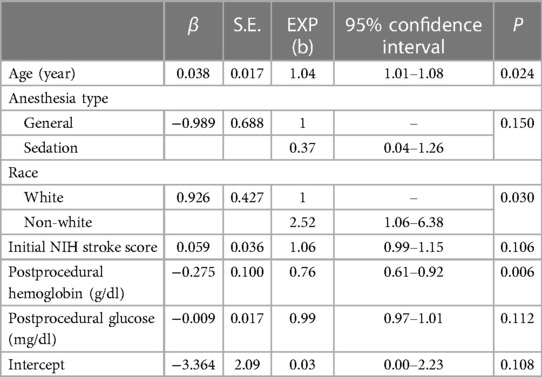
Table 4. Bias-reduced logistic regression of variables associated with delirium following mechanical thrombectomy for acute ischemic stroke.
Patient outcomes are shown in Table 5. Estimated mean NIH stroke scores were reduced from initial values by 5.4 (95% CI 4.1–6.7, P < 0.001), 7.5 (95% CI 6.2–8.9, P < 0.001), and 9.4 (95% CI 8.2–10.7, P < 0.001) in the no-delirium group and by 6.7 (95% CI 2.8–10.5, P < 0.001), 9.9 (95% CI 5.9–13.8, P < 0.001), and 12.6 (95% CI 8.4–16.8, P < 0.001) in the delirium group at 24h, 48h, and at discharge, respectively. There was no group by time difference in NIH stroke scores (P = 0.16). The length of stay was similar between the groups. The likelihood of a home discharge was reduced in the delirium group compared to the no-delirium group, with an odds ratio of 0.13 (95% CI 0.01–1.00, P = 0.02). Mortality and mRS at 90 days were not significantly different between groups.
The important findings of this study are the relatively low incidence of post-procedural delirium in patients undergoing MT for AIS. Although post-procedural delirium was associated with a reduced likelihood of home discharge, it did not adversely affect the change in NIHSS or 90-day mortality from initial presentation through hospital discharge. Risk factors for delirium in our study were a lower post-procedural Hgb, advanced age, and non-white race. There were no associations between the type of anesthesia administered, general vs. sedation, or the anesthetic agents received during the procedure with delirium in the post-procedural period.
The incidence of post-stroke delirium varies widely and depends upon the method of assessment but is estimated to be around 23% (95% CI, 17%–28%) (13). Silva et al. found that the hypoactive motor subtype of delirium (CAM-ICU positive plus RASS of 0 to −3) is the most prevalent motor subtype following a stroke and was associated with increased risk of death and functional dependence at 30 and 90 days and higher 90-day mortality (14). In their study, only 40% of the patients underwent thrombolysis treatment, and the lack of thrombolytic therapy was also an independent risk factor for poor post-stroke outcomes. Like the aforementioned study, we predominantly observed the hypoactive motor subtype of delirium post-MT.
Older age has consistently been associated with delirium, and the incidence of delirium within 72 h of a stroke in patients >65 years has been found to be 25% (15). We also observed an increase in the risk ratio for the occurrence of delirium in subjects >65 years. Race has not been found to be an independent predictor of delirium; however, like in our study, significant race-by-age interactions have been found to exist for incident delirium (16). Younger African-American subjects (<65) have lower rates of delirium compared to age-matched white patients, but the co-morbidity and socioeconomic-adjusted rates are similar in patients ≥65 (16). In our study, low HgB levels postprocedural were associated with an increased risk of delirium. Anemia has been shown to be associated with an increased risk of delirium in older patients (17). Unmeasured differences in co-morbidities and socioeconomic differences may account for the differences seen in the delirium rate in older subjects in our study.
In our study, 83% of the patients have successful recanalization, comparable to randomized controlled trials, indicating that successful recanalization can be achieved in 59%–88% of patients who undergo MT in the setting of an acute stroke (18). Prior studies have examined the association of reperfusion therapies on the incidence of post-procedural delirium and long-term outcomes. Ahmad et al. found that patients with delirium after reperfusion therapy had no difference in 90-day good outcomes despite having longer hospital admissions and being less likely to be discharged home (19). The incidence of delirium in the study was 52/179 (55.3%), but only 28% of the patients had an MT. Rollo et al. identified delirium in 30% of patients following AIS and found delirium to negatively impact survival at 90 days; however, only 33% of the patients in this study were treated with MT (20). Unlike these studies, all of our patients underwent MT alone or in combination with tPA, which may account for our reduced incidence of delirium.
The optimal anesthetic for mechanical thrombectomy remains under investigation. Advantages of general anesthesia include decreased aspiration risk with a secure airway and decreased patient movement during the procedure, which may shorten the time to recanalization. Benefits of sedation include the ability to perform direct neurological assessments, a shorter time to initiation of the procedure, and fewer hemodynamic fluctuations due to anesthetic medications (2). Initial retrospective, cohort studies suggested that general anesthesia resulted in worse neurological outcomes and mortality when compared to conscious sedation (21–23). Subsequent single-center randomized trials comparing these anesthetics and their effects concluded that functional outcomes were either the same or improved in the patients undergoing general anesthesia vs. sedation (24–27). General anesthesia did tend to increase the time from arrival till groin puncture but did not impact door-to-reperfusion time or duration of the thrombectomy procedure (28). A meta-analysis of 1,379 patients in 2018 and 4,802 patients in 2021 undergoing MT using either GA or sedation found that reperfusion rates were comparable and there were no significant differences in 90-day functional outcomes (29–31).
The impact of anesthesia choice on the incidence of postprocedural delirium for mechanical thrombectomy has not been extensively studied. A recent trial that evaluated anesthesia choice on outcomes following mechanical thrombectomy in 640 subjects listed postprocedural delirium among the secondary outcomes, but results have not been reported to date (32). In our study, we did not observe an adverse effect of anesthesia type or specific pharmacological therapies on the number of patients with delirium following MT.
The strengths of our study were the inclusion of all cases of MT following AIS during the study period and the relative homogeneity in clinical characteristics between the delirium and no-delirium groups. The findings of our study may be generalizable to stroke centers primarily treating moderate to high-severity ischemic strokes, such as those in our cohort. Nevertheless, the results of our study should only be interpreted in the context of its limitations. The study design was retrospective; the study included data from a single center. We utilized CAM-ICU and RASS assessments made primarily by nurses in the stroke unit and ICU, and the application of other delirium assessment scales could result in differences compared to those found in our study. We observed only 24 cases of delirium and only one subject that experienced delirium died within 90 days of the procedure; therefore, our sample lacks sufficient power to detect a difference between groups at the low rate of delirium observed. The study period was long (9 years), and changes in patient management during this period could have affected the results of our study. Although we were able to ascertain mortality data on all patients, we were unable to obtain sufficient Rankin scores at 90 days to assess functional impairment with high certainty between the delirium and no-delirium groups. There is a known association between LKW duration and outcomes following MT, such that for every 15 min less time from LKW to treatment, there is an increase in independent ambulation, function independence at discharge, lower mortality, and hospital discharge (33). In our study, LWK was 55 min shorter in the delirium compared to the no-delirium group which could have impacted our outcomes compared with the no-delirium group. Despite these limitations, our findings support the growing body of evidence that delirium following MT affects discharge status but does not affect 90-day outcomes.
In conclusion, we observed a low rate of delirium following MT following an acute AIS. Delirium primarily affected older patients and was associated with less home discharge. Changes in stroke scores during hospitalization and 90-day mortality were not adversely affected by the presence of delirium. The type of anesthesia during the MT procedure was not associated with an increase in the number of patients that exhibited delirium post-procedure.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were approved by the Institutional Review Board of Rush University IRB01. The studies were conducted in accordance with the local legislation and institutional requirements. The study was granted a waiver of informed consent because it evaluated existing records, was not greater than minimal risk, and was deemed to be HIPAA compliant because safeguards were in place to protect the personal health information of the subjects.
AS: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing, Validation. DT: Methodology, Supervision, Writing – original draft, Writing – review & editing, Data curation, Investigation. SS: Data curation, Investigation, Writing – original draft, Validation. GM: Data curation, Investigation, Validation, Writing – original draft. NR: Data curation, Investigation, Validation, Writing – original draft. AF: Data curation, Investigation, Validation, Writing – original draft. YH: Data curation, Investigation, Validation, Writing – original draft. RM: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by University Anesthesiologists, S.C., Chicago, IL.
The authors would like to thank Mario Moric, M.S. in statistics for his consultation on the statistics in this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2020 update: a report from the American heart association. Circulation. (2020) 141:e139–596. doi: 10.1161/CIR.0000000000000757
2. Bhatia A, Businger J. Perioperative management of the acute stroke patient. From door to needle to NeuroICU. Anesthesiol Clin. (2023) 41:27–38. doi: 10.1016/j.anclin.2022.11.001
3. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke. A guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
4. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3–4.5 h after acute ischemic stroke. N Engl J Med. (2008) 359:1317–29. doi: 10.1056/NEJMoa0804656
5. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6–24 h after stroke with a mismatch between deficit and infarct. N Engl J Med. (2018) 378:11–21. doi: 10.1056/NEJMoa1706442
6. Pujara D, Kamal H, Mir O, Reddy S, Parsha K, Patil S, et al. Impact of in-hospital delirium on outcomes of acute ischemic stroke. J Neurointerv Surg. (2021) 13(Supplement 1):A41–2. (Abstract P-029). doi: 10.1136/neurintsurg-2021-SNIS.65
7. Shaw RC, Walker G, Elliott E, Quinn TJ. Occurrence rate of delirium in acute stroke settings. Stroke. (2019) 50:3028–36. doi: 10.1161/STROKEAHA.119.025015
8. Rollo E, Callea A, Brunetti V, Vollono C, Marotta J, Imperatori C, et al. Delirium in acute stroke: a prospective, cross-sectional, cohort study. Eur J Neurol. (2021) 28:1590–600. doi: 10.1111/ene.14749
9. Pan AP, Agarwal K, Taffet GE, Jones SL, Potter T, Bako A, et al. Delirium leads to poor in-hospital and 90-day outcomes among patients with acute ischemic stroke with and without intravenous thrombolysis or intraarterial therapy. Stroke Vasc Interv Neurol. (2022) 2:e000338. doi: 10.1161/SVIN.122.000338
10. National Kidney Foundation. CKD-EPI Creatinine Equation. (2021). Available online at: https://www.kidney.org/professionals/kdoqi/gfr_calculator/formula (accessed August 24, 2022).
11. Robinson TN, Raeburn CD, Tran ZV, Brenner LA, Moss M. Motor subtypes of postoperative delirium in older adults. Arch Surg. (2011) 146:295–300. doi: 10.1001/archsurg.2011.14
12. Kosmidis I, Firth D. Jeffreys-prior penalty, finiteness and shrinkage in binomial-response generalized linear models. Biometrika. (2021) 108:71–82. doi: 10.1093/biomet/asaa052
13. Shaw RC, Walker G, Elliott E, Quinn TJ. Occurrence rate of delirium in acute stroke settings: systematic review and meta-analysis. Stroke. (2019) 50:3028–36. doi: 10.1161/STROKEAHA.119.025015
14. Fialho Silva IT, Assis Lopes P, Timotio Almeida T, Ramos SC, Caliman Fontes AT, Guimarães Silva D, et al. Impact of delirium and its motor subtypes on stroke outcomes. Stroke. (2021) 52:1322–29. doi: 10.1161/STROKEAHA.120.026425
15. Sheng AZ, Shen Q, Cordato D, Zhang YY, Yin Chan DK. Delirium within three days of stroke in a cohort of elderly patients. J Am Geriatr Soc. (2006) 54:1192–8. doi: 10.1111/j.1532-5415.2006.00806.x
16. Khan BA, Perkins A, Hui SL, Gao S, Campbell NL, Farber MO, et al. Relationship between African-American race and delirium in the ICU. Crit Care Med. (2016) 44:1727–34. doi: 10.1097/CCM.0000000000001813
17. Joosten E, Lemiengre J, Nelis T, Verbeke G, Milisen K. Is anaemia a risk factor for delirium in an acute geriatric population? Gerontology. (2006) 52:382–5. doi: 10.1159/000095126
18. Papanagiotou P, Ntaios G. Endovascular thrombectomy in acute ischemic stroke. Circ Cardiovasc Interv. (2018) 11:e005362. doi: 10.1161/CIRCINTERVENTIONS.117.005362
19. Ahmad MJ, Anjum S, Kumar A, Sebaugh J, Joseph M, Williams B, et al. Abstract TMPO57: acute ischemic stroke patients receiving reperfusion therapy do not have worse 90-day outcomes after delirium. Stroke. (2022) 53:ATMP57. doi: 10.1161/str.53.suppl_1.TMP57
20. Rollo E, Brunetti V, Scala I, Callea A, Marotta J, Vollono C, et al. Impact of delirium on the outcome of stroke: a prospective, observational, cohort study. J Neurol. (2022) 269:6467–75. doi: 10.1007/s00415-022-11309-2
21. Abou-Chebl A, Lin R, Hussain MS, Jovin TG, Levy EI, Liebeskind DS, et al. Conscious sedation versus general anesthesia during endovascular therapy for acute anterior circulation stroke: preliminary results from a retrospective, multicenter study. Stroke. (2010) 41:1175–9. doi: 10.1161/STROKEAHA.109.574129
22. Abou-Chebl A, Yeatts SD, Yan B, Cockroft K, Goyal M, Jovin T, et al. Impact of general anesthesia on safety and outcomes in the endovascular arm of interventional management of stroke (IMS) III trial. Stroke. (2015) 46:2142–8. doi: 10.1161/STROKEAHA.115.008761
23. Campbell BCV, van Zwam WH, Goyal M, Menon BK, Dippel DWJ, Demchuk AM, et al. Effect of general anaesthesia on functional outcome in patients with anterior circulation ischaemic stroke having endovascular thrombectomy versus standard care: a meta-analysis of individual patient data. Lancet Neurol. (2018) 17:47–53. doi: 10.1016/s1474-4422(17)30407-6
24. Schönenberger S, Uhlmann L, Hacke W, Schieber S, Mundiyanapurath S, Purrucker JC, et al. Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA. (2016) 316:1986–96. doi: 10.1001/jama.2016.16623
25. Löwhagen Hendén P, Rentzos A, Karlsson JE, Rosengren L, Leiram B, Sundeman H, et al. General anesthesia versus conscious sedation for endovascular treatment of acute ischemic stroke: the AnStroke trial (anesthesia during stroke). Stroke. (2017) 48:1601–7. doi: 10.1161/STROKEAHA.117.016554
26. Simonsen CZ, Yoo AJ, Sørensen LH, Juul N, Johnsen SP, Andersen G, et al. Effect of general anesthesia and conscious sedation during endovascular therapy on infarct growth and clinical outcomes in acute ischemic stroke: a randomized clinical trial. JAMA Neurol. (2018) 75:470–7. doi: 10.1001/jamaneurol.2017.4474
27. Sun J, Liang F, Wu Y, Zhao Y, Miao Z, Zhang L, et al. Choice of ANesthesia for EndoVAScular treatment of acute ischemic stroke (CANVAS): results of the CANVAS pilot randomized controlled trial. J Neurosurg Anesthesiol. (2020) 32:41–7. doi: 10.1097/ANA.0000000000000567
28. Schönenberger S, Hendén PL, Simonsen CZ, Uhlmann L, Klose C, Pfaff JAR, et al. Association of general anesthesia vs procedural sedation with functional outcome among patients with acute ischemic stroke undergoing thrombectomy: a systematic review and meta-analysis. JAMA. (2019) 322:1283–93. doi: 10.1001/jama.2019.11455
29. Ilyas A, Chen CJ, Ding D, Foreman PM, Buell TJ, Ironside N, et al. Endovascular mechanical thrombectomy for acute ischemic stroke under general anesthesia versus conscious sedation: a systematic review and meta-analysis. World Neurosurg. (2018) 112:e355–67. doi: 10.1016/j.wneu.2018.01.049
30. Shen H, Ma X, Wu Z, Shao X, Cui J, Zhang B, et al. Conscious sedation compared to general anesthesia for intracranial mechanical thrombectomy: a meta-analysis. Brain Behav. (2021) 11:e02161. doi: 10.1002/brb3.2161
31. Kogan E, Twyman K, Heap J, Milentijevic D, Lin JH, Alberts M. Assessing stroke severity using electronic health record data: a machine learning approach. BMC Med Inform Decis Mak. (2020) 20:8. doi: 10.1186/s12911-019-1010-x
32. Impact of Anesthesia Type on Outcome in Patients with Acute Ischemic Stroke (AIS) Undergoing Endovascular Treatment (CANVAS). NCT02677415. ClinicalTrials.gov (2016). Available online at: https://clinicaltrials.gov/study/NCT02677415 (accessed January 01, 2024).
Keywords: delirium, mechanical thrombectomy, anesthesia, outcomes, 90-day mortality
Citation: Sachdev A, Torrez D, Sun S, Michapoulos G, Rigler NC, Feldner AL, Hong YS and McCarthy RJ (2024) Postprocedural delirium following mechanical thrombectomy for acute ischemic stroke: a retrospective study. Front. Anesthesiol. 3:1351698. doi: 10.3389/fanes.2024.1351698
Received: 6 December 2023; Accepted: 29 January 2024;
Published: 12 February 2024.
Edited by:
Carlos Darcy Alves Bersot, Federal University of São Paulo, BrazilReviewed by:
José Eduardo Guimarães Pereira, Hospital Central do Exercito, Brazil© 2024 Sachdev, Torrez, Sun, Michapoulos, Rigler, Feldner, Hong and McCarthy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert J. McCarthy cm9iZXJ0X2pfbWNjYXJ0aHlAcnVzaC5lZHU=
†ORCID Alisha Sachdev orcid.org/0000-0001-6501-1390 Robert J. McCarthy orcid.org/0000-0002-0966-5311
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.