
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Anesthesiol. , 07 December 2023
Sec. Neuroanesthesiology
Volume 2 - 2023 | https://doi.org/10.3389/fanes.2023.1237981
 Mestet Yibeltal Shiferaw*
Mestet Yibeltal Shiferaw* Tsegazeab Laeke T/Mariam
Tsegazeab Laeke T/Mariam Abenezer Tirsit Aklilu
Abenezer Tirsit Aklilu Kibruyisfaw Zewudie Shumbash
Kibruyisfaw Zewudie Shumbash Yemisirach Bizuneh Akililu
Yemisirach Bizuneh Akililu Bethelhem Yishak Worku
Bethelhem Yishak Worku Mengistu Ayele
Mengistu Ayele
Background: A chronic cranial subdural hematoma arising after post-spinal anesthesia is a rare but serious and life-threatening complication of spinal anesthesia. It usually mimics the typical post-spinal-anesthesia headache or post-dural-puncture headache, potentially masking its detection. Abducens nerve palsy tends to occur in chronic subdural hematoma of post-dural-puncture etiology rather than in cases attributed to other causes of subdural hematoma. Preferential damage to the abducens nerve is frequent and can be attributed to its anatomic course because the abducens nerve runs in the direction of the typical caudad displacement of the brain related to intracranial hypotension.
Observation: Here, we present a report on the clinical presentation, pathogenesis, and management of two cases that developed bilateral abducens nerve palsy following post-spinal anesthesia administered for cesarean sections due to obstetric indications.
Lesson: Post-spinal-anesthesia-induced chronic subdural hematoma, although a rare, life-threatening complication, must be differentiated from post-spinal-anesthesia headache and treated surgically. Cranial nerve palsy (more commonly called abducens nerve palsy) is more common in post-spinal-anesthesia-induced subdural hematoma than subdural hematomas of other etiologies as the cerebrospinal fluid brain cushioning is partly lost. Cranial nerve palsies resolve in most cases if surgery is performed in a timely manner.
Benign complications from spinal anesthesia are relatively high, but only 0.05% of complications are life-threatening (1). Chronic cerebral subdural hematoma (CSDH) is among these rare, serious, and life-threatening complications. The common post-spinal-anesthesia headache or post-dural-puncture headache (PDPH) may mask the detection of a potentially life-threatening cerebral subdural hematoma (2, 3).
While it is relatively uncommon for patients to experience abducens palsy when having chronic subdural hematoma resulting from other causes to a large extent, it is not unusual for post-spinal-anesthesia-related chronic subdural hematoma to present with abducens nerve palsy.
We present the case of a 25-year-old female patient who complained of severe global headache and associated double vision for a duration of 4 weeks following a cesarean section performed with spinal anesthesia due to poor progress in labor. She received multimodal analgesia for the headache, but she showed no clinical response. There was no history of trauma, and she had not used anticoagulants.
Objectively, she exhibited normal vital signs and maintained a normal level of consciousness. Neurologically, she demonstrated intact function, with the exception of bilateral abducens nerve palsy and horizontal nystagmus.
Her complete blood count and coagulation profile were within normal limits. On imaging, she was diagnosed with bilateral chronic subdural hematoma of thicknesses 12 and 6 mm on the right and left sides, respectively, with a tight brain and effaced sulci and fissures (Figure 1).

Figure 1. Preoperative MRI image of the patient showing bilateral hyperintensity on T2–T1 suggesting late sub-acute subdural hematoma with mass effect on the brain.
Written informed consent was obtained, and a unilateral burr hole was drilled at the maximal thickness of the hematoma, and complete evacuation of the hematoma was carried out with significant clinical improvement. After surgery, the headache completely subsided, and the abducens nerve palsy showed partial improvement immediately on the postoperative day and recovered completely within the first month of her postoperative course. A follow-up computed tomography (CT) scan was performed to assess the extent of hematoma evacuation. The scan revealed good evacuation with very minimal pneumocephalus on the side of the hematoma evacuation (Figure 2). There was no expansion of the hematoma on the contralateral side. The effaced brain structures also normalized, as evidenced by the opened Sylvian fissure, basal cistern, and hemispheric sulci. The patient was discharged with improvement on her third postoperative day (Figure 3).
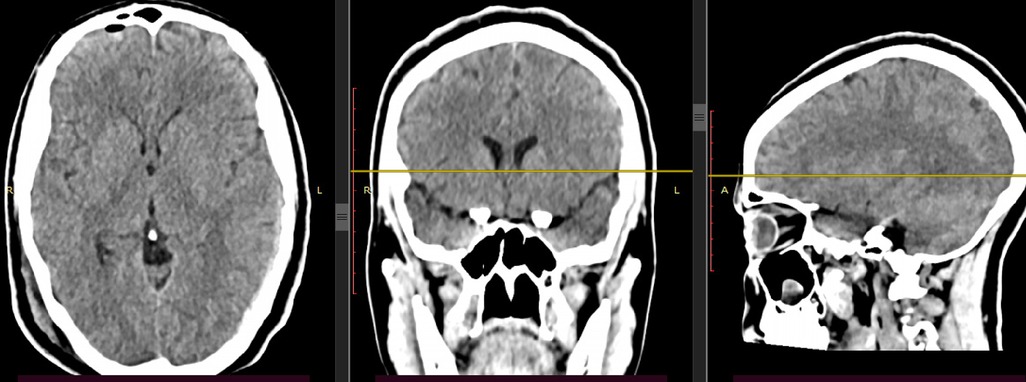
Figure 2. Control CT scan of the patient after right-side burr hole and complete hematoma evacuation and no significant hematoma on the left side.

Figure 3. Patient on her first postoperative day with good clinical status with the subdural drain in situ.
We present the case of a 28-year-old female patient who complained of severe global headache and associated double vision persisting for 3 weeks following a cesarean section performed under spinal anesthesia due to obstructed labor. She received multimodal analgesia, but she showed no clinical response. There was no history of trauma, and she denied using anticoagulants.
Objectively, she exhibited normal vital signs and maintained a normal level of consciousness. Neurologically, she showed intact function, with the exception of bilateral abducens nerve palsy and horizontal nystagmus (Figure 4).

Figure 4. Patient with bilateral abducens nerve palsy following a spinal-anesthesia-induced bilateral chronic subdural hematoma.
Her complete blood count and coagulation profile were within normal limits. On imaging, she was diagnosed with bilateral chronic subdural hematoma of thickness 10 mm on both the right and left sides, with a tight brain and effaced sulci and fissures (Figure 5).
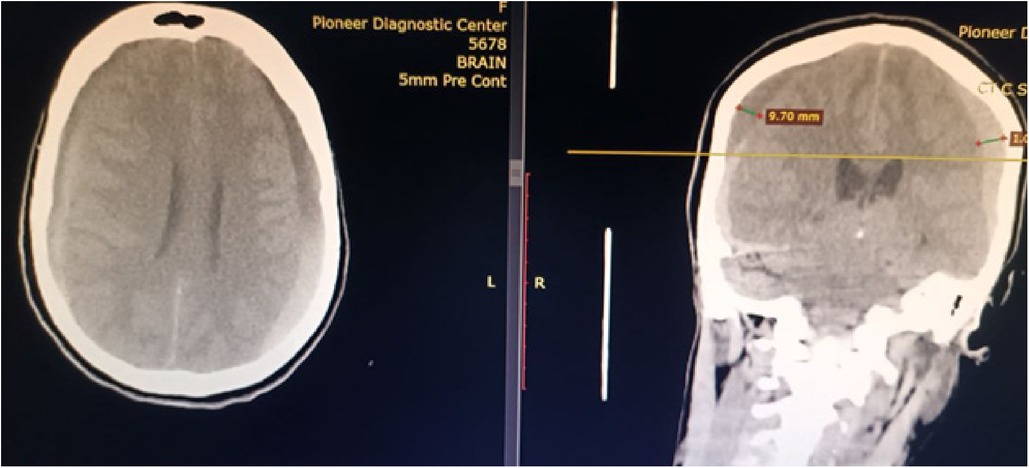
Figure 5. CT scan of a patient showing bilateral sub-acute subdural hematoma with mass effect on the brain.
The patient was treated by drilling a bilateral burr hole and evacuating the hematoma as in the first case, and she too was discharged with improvement on her third postoperative day. Her abducens nerve palsy was resolved completely by the third week post-surgery.
Headaches and backaches are the dominant symptoms that develop after an accidental dural puncture. Approximately 90% of headaches occur within 3 days of the procedure. Headaches may present immediately after a dural puncture, which is consistent with our cases. However, this is rare, and its occurrence should alert the physician to consider alternative causes. Approximately 72% of headaches resolve within 7 days, and 87% resolve in 6 months. Post-dural puncture headaches can be successfully treated with an epidural blood patch. However, it is noteworthy that an epidural blood patch has no role in the treatment of post-spinal-anesthesia-induced chronic subdural hematomas and cranial nerve palsies (4–8). Recently, administering a minimally invasive sphenopalatine ganglion block has been shown to provide significant symptomatic improvement for post-spinal-dural-puncture headache as it effectively blocks the parasympathetic-induced vasodilation and alleviates the associated headache (9).
Common to both cases in this study was that both patients underwent cesarean sections under spinal anesthesia induced using 18-gauge spinal needles, which are relatively large and have the potential to cause significant cerebrospinal leakage. Ideally, fine spinal needles of 25–27 gauge should have been used to minimize cerebrospinal fluid leakage. Initially, the onset of the two patients’ headaches was also characteristic of post-dural-puncture headaches; however, they became persistent headaches after some days due to the development of a subdural hematoma. In addition, both patients had developed abducens nerve palsy with no prior history of a clinically significant headache.
Following spinal anesthesia, there is a potential leakage of cerebrospinal fluid (CSF), which causes the brain to descend caudad in an upright posture, as the CSF cushion for the brain is displaced downward. This downward traction can damage some of the cranial nerves that anchor the brain in the skull (5–8).
Although other cranial nerve palsies can occur after a lumbar puncture, the abducens nerve (cranial nerve VI) is affected in the majority of cases (92%–95%) (3, 10). Nearly 80% of the cases are unilateral (3). Abducens palsy can coexist with oculomotor (cranial nerve III) or trochlear (cranial nerve IV) nerve palsies. Multiple coexisting cranial nerve palsies can be masked by a large esotropia, making the exact diagnosis of these cranial nerve palsies difficult (11).
Preferential damage to the abducens nerve can be explained by its anatomic course. As the nerve emerges into the subarachnoid space from the caudal pons, it immediately ascends the clivus, crosses the branches of the basilar artery, and pierces the dura mater. Then, the nerve bends at nearly a right angle over the petrous apex of the temporal bone (12, 13). The abducens nerve runs in the direction of the typical caudad displacement of the brain with intracranial hypotension. As a result, the traction associated with changes in intracranial pressure is fully transmitted to the nerve. The nerve can be stretched by caudal displacement of the pons, and it also may be compressed at the dura, petrous apex, or basilar artery if (1) the penetration aperture for the nerve in the dura or petrous apex is sharply edged or (2) the branches of the basilar artery are well developed.
In other words, cranial nerve palsies from intracranial hypotension can occur even in the absence of the classic PDPH. The most common cranial nerve to be affected by intracranial hypotension is the abducens nerve (14), which is most sensitive to intracranial hypotension not because of its longer course, as was earlier believed, but due to three acute angulation points between the dural entrance point and its anastomosis with the periarterial sympathetic plexus (15).
There is a good margin of recovery of the physiological activity of the sixth cranial nerve, the total recovery rate can reach up to 73% within 6 months when the CSDH is unilateral, and the mean recovery time is 3 months (16). The duration of the course (of weeks to months) for the cranial nerve palsies with good prognosis suggests neurapraxia (focal segmental demyelination) or axonotmesis (axonal interruption and Wallerian degeneration with preservation of supporting tissue framework) as a potential pathological mechanism (17).
Because intracranial hypotension associated with CSF leakage seems to play a major role in the pathogenesis, minimizing CSF leakage with smaller, pencil-point needles is recommended (18, 19).
The limitation of this case study was that further follow-up imaging was not possible as it could not be obtained.
Post-spinal-anesthesia-induced chronic subdural hematomas, although rare, are a life-threatening complication and must be differentiated from post-spinal-anesthesia headaches and treated surgically. Cranial nerve palsy (more commonly known as abducens nerve palsy) is more common in post-spinal-anesthesia-induced subdural hematomas than subdural hematomas of other etiologies as the CSF brain cushioning is partly lost. The cranial nerve palsies recover in the majority of cases if surgery is performed in a timely manner.
The datasets presented in this article are not readily available because only the uploaded information will be publicized. Requests to access the datasets should be directed toeWliZWx0YWxtZXN0ZXRAZ21haWwuY29t.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Conceptualization: MS and MA. Case narration: MS, MA, YA, BW, TT, AA, and KS. Writing – review & editing: MS, MA YA, BW, TT, AA, and KS. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CSF, cerebrospinal fluid; CSDH, chronic cerebral subdural hematoma; PDPH, post-dural puncture headache.
1. Auroy Y, Narchi P, Messiah A, Litt L, Rouvier B, Samii K. Serious complications related to regional anesthesia: results of a prospective survey in France. Anesthesiology. (1997) 87(3):479–86. doi: 10.1097/00000542-199709000-00005
2. Acharya R, Chhabra SS, Ratra M, Sehgal AD. Cranial subdural haematoma after spinal anaesthesia. Br J Anaesth. (2001) 86(6):893–5. doi: 10.1093/bja/86.6.893
3. Thorsen G. Neurological complications after spinal anesthesia. Acta Chir Scand. (1947) 95:1–272.20295686
4. Turnbull DK, Shepherd DB. Post-dural puncture headache: pathogenesis, prevention and treatment. Br J Anaesth. (2003) 91(5):718–29. doi: 10.1093/bja/aeg231
5. Ferrante E, Savino A, Brioschi A, Marazzi R, Donato MF, Riva M. Transient oculomotor cranial nerves palsy in spontaneous intracranial hypotension. J Neurosurg Sci. (1998) 42:177–9.10192060
6. Mokri B. Spontaneous intracranial hypotension. Curr Neurol Neurosci Rep. (2001) 1:109–17. doi: 10.1007/s11910-001-0005-y
7. Brady-McCreery KM, Speidel S, Hussein MA, Coats DK. Spontaneous intracranial hypotension with unique strabismus due to third and fourth cranial neuropathies. Binocul Vis Strabismus Q. (2002) 17:43–8.11874382
8. Nishio I, Williams BA, Williams JP. Diplopia: a complication of dural puncture. Anesthesiology (2004) 100:158–64. doi: 10.1097/00000542-200401000-00025
9. Puthenveettil N, Rajan S, Mohan A, Paul J, Kumar L. Sphenopalatine ganglion block for treatment of post-dural puncture headache in obstetric patients: an observational study. Indian J Anaesth. (2018) 62(12):972–7. doi: 10.4103/ija.IJA_443_18
10. Hayman IR, Wood PM. Abducens nerve paralysis following spinal anesthesia. Ann Surg. (1942) 115:864–8. doi: 10.1097/00000658-194205000-00016
11. King RA, Calhoun JH. Fourth cranial nerve palsy following spinal anesthesia: a case report. J Clin Neuroophthalmol. (1987) 7:20–2.2952674
12. Bennett JL, Pelak VS. Palsies of the third, fourth, and sixth cranial nerves. Ophthalmol Clin North Am. (2001) 14:169–83.11370565
13. Sowka J. Neurogenic diplopia: paralysis of cranial nerves III, IV, and VI. Optom Clin. (1996) 5:53–6.8972509
14. Wardhan R, Wrazidlo SM. Iatrogenic dural puncture-induced abducens nerve palsy: treatment with epidural blood patch. Cureus. (2020) 12(1):e6622. doi: 10.7759/cureus.6622
15. Cain RB, Patel NP, Hoxworth JM, Lal D. Abducens palsy after lumbar drain placement: a rare complication in endoscopic skull base surgery. Laryngoscope. (2013) 123:2633–8. doi: 10.1002/lary.24177
16. Salunke P, Savardekar A, Sura S. Delayed-onset bilateral abducens paresis after head trauma. Indian J Ophthalmol. (2012) 60(02):149–50. doi: 10.4103/0301-4738.90491
18. Vandam LD, Dripps RD. Long-term follow-up of patient who received 10,098 spinal anesthetics. JAMA. (1965) 16:586–91.
Keywords: abducens palsy, chronic subdural hematoma, post-dural puncture headache, spinal anesthesia, sphenopalatine ganglion block, subdural hematoma abducens palsy, subdural hematoma
Citation: Shiferaw MY, T/Mariam TL, Aklilu AT, Shumbash KZ, Akililu YB, Worku BY and Ayele M (2023) Bilateral abducens nerve palsy from post-spinal-anesthesia-induced bilateral chronic subdural hematoma: case report. Front. Anesthesiol. 2:1237981. doi: 10.3389/fanes.2023.1237981
Received: 16 June 2023; Accepted: 21 November 2023;
Published: 7 December 2023.
Edited by:
Muh-Shi Lin, Kuang Tien General Hospital, TaiwanReviewed by:
Edvin Zekaj, Galeazzi Orthopedic Institute (IRCCS), Italy© 2023 Shiferaw, T/Mariam, Aklilu, Shumbash, Akililu, Worku and Ayele. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mestet Yibeltal Shiferaw eWliZWx0YWxtZXN0ZXRAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.