- 1Department of Anesthesiology, Louisiana State University Health Sciences Center, New Orleans, LA, United States
- 2Department of Surgery, Tulane University School of Medicine, New Orleans, LA, United States
The diagnosis and management of poisoning is essential in critical care medicine. Traditionally, these conditions fall under the category of toxidromes that are the signs and symptoms associated with a particular class of poisons. However, there has been a steady increase in designer drugs and contaminants of recreational drugs themselves. Examples of adulterants in cocaine include the local anesthetic benzocaine and the anti-parasitic levamisole. This paper presents the clinical signs, laboratory findings, and treatment of patients who have been exposed to these substances.
1. Introduction
Admissions to the ICU related to substance abuse is, unfortunately, a common global issue. A recent study revealed that substance abuse was responsible for one quarter of ICU admissions and 23% of total charges (1). Illicit drug use was associated with 13% of these patients (1). Identification of the illicit drug on clinical presentation is often difficult. These substances often fall into multiple categories of toxidromes, the signs and symptoms associated with a particular class of poisons (2). Polysubstance abuse is common.
There has been the increasing use of “adulterants” that are legal substances which are added to these illicit drugs. This process, called “cutting”, is to either further enhance their effects or expand volume, both of which enhance the drug dealers' profits (3, 4). Laboratory tests can confirm the most common illicit substances but often will not identify altered forms (5).
Our experience in a trauma center encompasses patients with substance abuse issues, but we had sparse knowledge of contaminants before caring for the patient in the clinical example below. In this paper we present examples illicit drug contaminants, a little-known subject for critical care physicians.
1.1. Cocaine
Cocaine is one of the most common illegal psychostimulant drugs (4, 6, 7). It is abused as either the water-soluble hydrochloride salt or a water-insoluble base known as “freebase and crack”. Cocaine blocks the transporters for dopamine, norepinephrine, and serotonin (8). With this blockade, there is continued stimulation by monoamines at the pre- and post- synapses to create euphoria that leads to addiction. Symptoms include tachycardia, hypertension, hyperthermia, and agitation (7). The long list of sequelae includes myocardial and cerebral infarctions. Acute kidney injury may be related to decreased renal blood flow from vascular smooth muscle constriction and rhabdomyolysis (9). Cutaneous vasculopathy with rheumatologic features including antineutrophil cytoplasmic antibodies (ANCA) can occur (10).
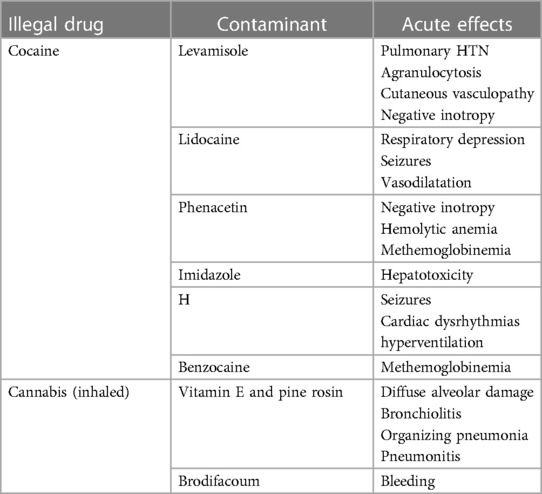
Adulterants in seized cocaine samples include levamisole, phenacetin, lidocaine, imidazole, and caffeine (11–13). Each of these compounds themselves will cause pathologic changes like cocaine making additional diagnoses difficult (Table 1).
Levamisole is an antihelminthic in widespread veterinary use. It had been used as an adjuvant chemotherapy agent but was withdrawn from the US market in 2000 after side effects of agranulocytosis, cutaneous vasculopathy, and leukoencephalopathy were identified (14–16). Levamisole is the most widely used adulterant in up to 88% of cocaine samples to improve its euphoric effects (4, 14, 15). The mechanism for euphoria includes the metabolism to aminorex, an amphetamine-like substance once used as a diet drug. Aminorex was taken off the market secondary to pulmonary hypertension (17). Levamisole increases antibody production to various antigens by functioning as a hapten involved with isoimmune antineutrophil cell membrane antigens. Cutaneous manifestations include purpura, hemorrhagic bullae, and livedo reticularis (10). An immune-mediated mechanism has been suggested for eosinophilic inflammatory coronary artery pathology (18).
Local anesthetics are added to cocaine since they have the same analgesic properties and cannot be detected as an adulterant by the drug user. Physicians are familiar with lidocaine used as an antidysrhythmic and local anesthetic controlled by the FDA and in the over-the-counter topical analgesics. What is likely unknown is that 99.9% pure lidocaine and benzocaine powders can be purchased for “research and development” from common online sites without a prescription. The established maximum cumulative dose of intravenous lidocaine during treatment of ventricular dysrhythmias is 3 mg/kg (19). As a local anesthetic injection without epinephrine the maximum dose is 4.5 mg/kg (20). Clearly the drug dealers cutting cocaine with lidocaine greatly exceed this maximum. Overdose results in negative inotropy, vasodilatation, seizures, and respiratory depression (20). Hypercapnia and respiratory acidosis exacerbate central nervous system depression. A report from the United Kingdom indicated that 60% of cocaine was adulterated with benzocaine (21). Benzocaine is rapidly absorbed across mucous membranes. Methemoglobinema results from the oxidation of the iron in hemoglobin to the ferric state. Cyanosis will be manifested after topical 150–300 mg, concentrations of methemoglobin result in metabolic acidosis, convulsions, and coma; levels about 70% are lethal (21).
Caffeine is the most widely used stimulant consumed by over 80% of the population in the world. In moderate amounts of less than 400 mg/day in healthy adults there is nominal toxicity (22). Toxic symptoms occur after injection of 1 gram, hospitalization is needed after 2 g, and ingested doses of 5–50 g are fatal (23). It undergoes acetylation, oxidation, and N-demethylation in the liver (6). Therefore, alcohol and abused drugs in addition to cocaine potentiate the effects of caffeine. Caffeine enhances the reinforcing effects of cocaine and its motivational value (24). The combination of caffeine and cocaine makes users more likely to keep seeking out the drug than they would if they were addicted to cocaine alone.
Phenacetin is an antipyretic and analgesic that is cleaved to form acetaminophen. It was removed from the market because of renal carcinogenicity. It is a negative inotrope, can generate methemoglobinemia through its metabolites, and can cause hemolytic anemia (25–27). Phenacetin has no stimulant properties but is used as a cutting agent to increase the bulk of cocaine.
Imidazole has fungicidal, antiprotozoal, and antihypertensive properties (26). The most common use is as a topic antifungal such as ketoconazole. It is part of the theophylline molecule derived from tea leaves and coffee beans and acts as a central nervous system stimulant. Imidazole itself is hepatotoxic via ATP depletion in cells with mitochondrial damage.
1.2. Cannabis
Cannabis is the most widely used psychoactive substance. Through Δ9 -tetrahydrocannabinol (THC), cannabinoid receptors CB1 in central and peripheral neurons are stimulated. Usually, the effects of decreased locomotor activity, cognitive impairment, analgesia, hypothermia, and appetite stimulation are considered of low toxicity but may be exacerbated when consumed in large doses (28, 29).
Cannabis can also be smoked using E-cigarettes containing Δ8 -tetrahydrocannabinol synthesized from cannabidiol (CBD) (30, 31). Cutting agents in high levels, in addition to heavy metals leaching from the devices, are respiratory irritants (Table 1). Vitamin E acetate is a cutting agent that has been added to marijuana oils and has been associated to vaping-associated lung injury (EVALI) that includes diffuse alveolar damage, bronchiolitis with organizing pneumonia, and granulomatous pneumonitis (32). Pine rosin, a known lung irritant has been identified as an adulterant (31). Lung examination upon presentation does not correlate with the severity of the disease that can include diffuse alveolar damage, pneumonitis, and organizing pneumonia (30).
Synthesized cannabinols are dissolved in alcohol and acetone and sprayed on plant material. These are sold under a variety of names such as “K2” and “Spice”. They are classified as DEA Schedule 1 but compositions are continually modified to circumvent this DEA classification. Intoxication can be severe including psychosis, respiratory depression, cardiac arrest, nephrotoxity, hyperemesis, rhabdomyolysis, hyperthermia, seizures, and cerebral ischemia (33). The most lethal adulterant of synthetic cannabinoids is brodifacoum, a vitamin K-dependent antagonist (34, 35). It is used to enhance the effects because of longer periods of lipid storage, hepatic metabolism, and slow release. Compared to the anticoagulation of warfarin it is 100 times greater and has a longer half-life of 20–130 days (35).
1.3. Synthetic cathinones
Synthetic cathinones are a group of potent designer drugs, often referred to as “bath salts”. Their effect is like 3,4-methylenedioxymethamphetamine (MDMA; ecstasy) with the blockade of dopamine and norepinephrine uptake (36). Animal studies demonstrated that the synthetic cathinone methylenedioxypyrovalerone (MDPV) has greater potency than cocaine with respect to hyperactivity and cardiovascular stimulation (36). Neurologic symptoms include agitation, paranoia, hallucinations, myoclonus, and psychosis. In addition to hyperthermia, hypertension, and tachycardia liver failure, kidney failure, and compartment syndrome with rhabdomyolysis have been reported (37).
1.4. Xylazine
Xylazine is a veterinary drug used as a sedative, analgesic, and muscle relaxant (38, 39). It has a structure that is similar to phenothiazines, tricyclic antidepressants, and clonidine. As for clonidine, it is a potent central α2-receptor agonist that will decrease the release of norepinephrine and dopamine. The intended use, in addition to cutting, is to enhance the sedation and analgesia of the illicit drug. Xylazine was first identified as a cutting agent in Puerto Rico and has adulterated heroin and cocaine and (38, 39). The contaminant of fentanyl with xylazine has been considered as the deadliest drug threat in the United States (40). More than 90% of illicit drug samples in Philadelphia are positive for xylazine with the street names “tranq”, “tranq dope”, and “zombie drug” (41).
The most noted side effect of xylazine is characteristic necrotic skin ulcers that are likely caused by vasoconstriction and poor skin perfusion (42). Based on case reports, the effects of overdosage include hypotension, bradycardia, hyperglycemia, areflexia, elevated cardiac enzymes, coma, and respiratory failure (43).
2. Management of complications
The acuity of substance abuse patients admitted to the ICU is complex but well within the realm of care addressed by intensive care physicians.
Respiratory embarrassment may be related to the overdose suppression of spontaneous ventilation or pulmonary parenchymal pathology as found with EVALI. The need for tracheal intubation and mechanical ventilation is straight-forward for most of these patients and often performed before the patient arrives in the ICU. Systemic glucocorticoid therapy was shown to be effective in the treatment of EVALI (32).
As many of the patients are polysubstance abusers, treatment of the drug effects, as well as underlying psychiatric issues, may require the use of multiple agents such as quetiapine and benzodiazepines (33, 44). For severe withdrawal, high dose lorazepam alone was ineffective when compared to the synergistic actions of propofol infusions with reduced lorazepam doses (45).
Acute kidney injury treatment is largely supportive (9). Restoration intravascular volume is essential since acute tubular necrosis may be related to hypovolemia resulting from poor intake, diarrhea, and vomiting. The latter is often associated with altered electrolyte levels. CPK's should be monitored to reveal rhabdomyolysis that may not be evident on physical examination. Dialysis may be necessary (9).
Cardiovascular toxicity, especially with cocaine, is the most difficult life-threatening processes requiring ICU care. The evidence for pharmacologic treatment is limited for the management of tachycardia, hypertension, dysrhythmia, and coronary vasospasm in a comprehensive review of the literature (46). Labetalol and carvedilol will control hypertension and tachycardia. Nitroglycerin is recommended for cocaine-associated chest pain and vasospasm with the risk of tachycardia (47). Dexmedetomidine will control hypertension at high doses (1.0 µg/kg) with the possibility of bradycardia (48). Beta blockers will decrease heart rate as expected but are used cautiously to prevent unopposed hypertension. Esmolol is effective but will cause more hypotension when comparted to other beta blockers. The sequelae of hypertension and tachycardia related to hypoxemia from methemoglobinemia require treatment especially for patients with cardiovascular disease or levels of ≥30% by co-oximetry (25, 49). Methylene blue (1%, 1–2 mg/kg) over 5 min with repeat every 30 min is the usual therapy. Hyperbaric oxygen treat has been reported for methemoglobinemia that was refractive to methylene blue. The cardiac effects of caffeine overdose are ameliorated with dialysis.
The extensive skin necrosis and infection related to xylazine is treated with appropriate antibiotics, topical treatment, and surgical debridement if needed.
3. Take home message
The management of patients requiring ICU care for toxicities related to substance abuse is challenging. The clinical pathophysiology may be related to a single drug, multiple drugs, and often adulterated illegal agents. For many of these patients, such as the one described above, supportive critical care is an easily identifiable task but comes with the cost of extensive resource management. It is critical to consider adulterants that would cause unexpected findings such as methemoglobinemia, lidocaine toxicity, necrotic skin lesions, or rhabdomyolysis in the absence of trauma or a compartment syndrome.
Author contributions
PM and RP equally contributed to the research, writing, and editing of this manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The author RP declared that he was an editorial board member of Frontiers at the time of submission. This had no impact on the peer review process and the final decision. The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Westerhausen D, Perkins AJ, Conley J, Khan BA, Farber M. Burden of substance abuse-related admissions to the medical ICU. Chest. (2020) 157:61–6. doi: 10.1016/j.chest.2019.08.2180
2. Holstege CP, Borek HA. Toxidromes. Crit Care Clin. (2012) 28:479–98. doi: 10.1016/j.ccc.2012.07.008
3. Arango-Meriño L, Quevedo-Castro C, Mancera-Barros J, Sarmiento-Gutiérrez A, Arana VA, Granados-Reyes J. Cutting agents in cocaine: a temporal study of the period 2015–2017 in the northern region of Columbia. Forensic Sci Int. (2021) 327:110911. doi: 10.1016/j.forsciint.2021.110911
4. Kudlacek O, Hofmaier T, Luf A, Mayer FP, Stockner T, Nagy C, et al. Cocaine adulteration. J Chem Neuroanat. (2017) 83–84:75–81. doi: 10.1016/j.jchemneu.2017.06.001
5. Schwarz DA, George MP, Bluth MH. Toxicology in addiction medicine. Clin Lab Med. (2016) 36:685–92. doi: 10.1016/j.cll.2016.07.009
6. Bravo RR, Faria AC, Brito-da-Costa AM, Carmo H, Mladenka P, DaSilva DD, et al. Cocaine: an updated overview on chemistry, detection, biokinetics, and pharmacotoxicological aspects including abuse pattern. Toxins (Basel). (2022) 14:278. doi: 10.3390/toxins14040278
7. Zimmerman JL. Poisonings. In: Parillo JE, Dellinger P, editors. Critical care medicine. 5th ed. Philadelphia, PA, USA: Elsevier (2019). p. 1093–110.
8. Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, et al. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev. (2011) 63:585–640. doi: 10.1124/pr.108.000869
9. Pendergraft WF, Herlitz LC, Thornley-Brown D, Rosner M, Niles JL. Nephrotoxic effects of common and emerging drugs of abuse. Clin J Am Soc Nephrol. (2014) 9:1996–2005. doi: 10.2215/CJN.00360114
10. Milman N, Smith CD. Cutaneous vasculopathy associated with cocaine use. Arthritis Care Res. (2011) 63:1195–202. doi: 10.1002/acr.20483
11. Broséus J, Gentile N, Bonadio F, Garcia L, Gasté L, Esseiva P. Qualitative, quantitative and temporal study of cutting agents for cocaine and heroin over 9 years. Forensic Sci Int. (2015) 257:307–13. doi: 10.1016/j.forsciint.2015.09.014
12. Schneider S, Meys F. Analysis of illicit cocaine and heroin samples seized in Luxembourg from 2005 to 2010. Forensic Sci Int. (2011) 212:242–6. doi: 10.1016/j.forsciint.2011.06.027
13. Zacca J, Botelho É, Vieira M, Almeida F, Ferreira L, Maldaner A. Brazilian federal police drug chemical profiling—the PeQui project. Sci Justice. (2014) 54:300–6. doi: 10.1016/j.scijus.2014.02.008
14. Auffenberg C, Rosenthal LJ, Dresner N. Levamisole: a common cocaine adulterant with life-threatening side effects. Psychosomatics. (2013) 54:590–3. doi: 10.1016/j.psym.2013.02.012
15. Buchanan JA, Lavonas EJ. Agranulocytosis and other consequences due to the use of illicit cocaine contaminated with levamisole. Curr Opin Hematol. (2021) 19:27–31. doi: 10.1097/MOH.0b013e32834da9ef
16. Midthun KM, Nelson LS, Logan BK. Levamisole-a toxic adulterant in illicit drug preparations: a review. Ther Drug Monit. (2021) 43:221–8. doi: 10.1097/FTD.0000000000000851
17. Hofmaier T, Luf A, Seddik A, Stockner T, Holy M, Freissmuth M, et al. Aminorex, a metabolite of the cocaine adulterant levamisole, exerts amphetamine like actions at monoamine transporters. Neurochem Int. (2014) 73:32–41. doi: 10.1016/j.neuint.2013.11.010
18. Michaud K, Grfabherr S, Shiferaw K, Doenz F, Augsburger M, Mangin P. Acute coronary syndrome after levamisole-adultered cocaine abuse. J Forensic Leg Med. (2014) 21:48–52. doi: 10.1016/j.jflm.2013.10.015
19. Panchal AR, Berg KM, Kudenchuk PJ, Del Rios M, Hirsch KG, Link MS, et al. 2018 American heart association focused update on advanced cardiovascular life support use of antiarrhythmic drugs during and immediately after cardiac arrest: an update to the American heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. (2018) 138:e740–9. doi: 10.1161/CIR.0000000000000613
20. Lirk P, Berde CB. Local anesthetics. In: Parillo JE, Dellinger P, editors. Critical care medicine. 5th ed. Philadelphia, PA, USA: Elsevier (2019). p. 865–90.
21. Chakladar A, Willers JW, Pereskokova E, Beaumont P, Uncles DR. White powder, blue patients: methaemoglobinaemia associated with benzocaine-adulterated cocaine. Resuscitation. (2010) 81:138–9. doi: 10.1016/j.resuscitation.2009.10.019
22. Willson C. The clinical toxicology of caffeine: a review and case study. Toxicol Rep. (2018) 5:1140–52. doi: 10.1016/j.toxrep.2018.11.002
23. Bonsignore A, Sblano S, Pozzi F, Ventura F, Dell’Erba A, Palmiere C. A case of suicide by ingestion of caffeine. Forensic Sci Med Pathol. (2014) 10:448–51. doi: 10.1007/s12024-014-9571-6
24. Prieto JP, Scorza C, Serra GP, Perra V, Galvalisi M, Abin-Carriquiry JA, et al. Caffeine, a common active adulterant of cocaine, enhances the reinforcing effect of cocaine and its motivational value. Psychoparmacology. (2016) 233:2879–89. doi: 10.1007/s00213-016-4320-z
25. Hunter L, Gordge L, Dargan PI, Wood DM. Methaemoglobinaemia associated with the use of cocaine and volatile nitrites as recreational drugs: a review. Br J Clin Pharmacol. (2011) 72:18–28. doi: 10.1111/j.1365-2125.2011.03950.x
26. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Pharmaceuticals. IARC Monographs on the evaluation of carcinogenic risks to humans, No.100A.PHENACETIN. Lyon (FR): International Agency for Research on Cancer (2012). p. 377–98. Available at: https://www.ncbi.nlm.nih.gov/books/NBK304337/#:∼:text=IARC%20Working%20Group%20on,nih.gov/books/NBK304337/
27. Haegler P, Joerin L, Krähenbühl S, Bouibir J. Hepatocellular toxicology of imidazole and triazole antimicotic agents. Toxicol Sci. (2017) 157:183–95. doi: 10.1093/toxsci/kfx029
28. Schmid Y, Scholz I, Mueller L, Exadaktylos AK, Ceschi A, Liechti ME, et al. Emergency department presentations related to acute toxicity following recreational use of cannabis products in Switzerland. Drug Alcohol Depend. (2020) 206:107726. doi: 10.1016/j.drugalcdep.2019.107726
29. Fong K, Hsueh A, Kendric K, Kunihira K, Siddiqi N, Phan TH, et al. Unwitting adult marijuana poisoning: a case series. Clin Toxicol. (2021) 59:913–7. doi: 10.1080/15563650.2021.1891241
30. Cobb NK, Solanki JN. E-cigarettes, vaping devices, and acute lung injury. Respir Care. (2020) 65:713–8. doi: 10.4187/respcare.07733
31. Meehan-Atrash J, Rahman I. Novel Δ8-tetrahydrocannabinol vaporizers contain unlabeled adulterants, unintended byproducts of chemical synthesis, and heavy metals. Chem Res Toxicol. (2022) 35:73–6. doi: 10.1021/acs.chemrestox.1c00388
32. Layden JE, Ghinai I, Pray I, Kimball A, Layer M, Tenforde MW, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin—final report. N Engl J Med. (2020) 382:903–16. doi: 10.1056/NEJMoa1911614
33. Cooper ZD. Adverse effects of synthetic cannabinoids: management of acute toxicity and withdrawal. Curr Psychiatry Rep. (2016) 18:52. doi: 10.1007/s11920-016-0694-1
34. Kumar S, Bhagia G. Brodifacoum-laced synthetic marijuana toxicity: a fight against time. Am J Case Rep. (2020) 21:e927111. doi: 10.12659/AJCR.927111
35. Ross CH, Singh P, Simon EL. Hemorrhagic soft tissue upper airway obstruction from brodifacoum-contaminated synthetic cannabinoid. J Emerg Med. (2019) 57:47–50. doi: 10.1016/j.jemermed.2019.03.007
36. Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone(MDPV), a principal constituent of psychoactive “bath salts” products. Neuropsychopharmacology. (2013) 38:552–62. doi: 10.1038/npp.2012.204
37. German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci. (2014) 97:2–8. doi: 10.1016/j.lfs.2013.07.023
38. Ruiz-Colón K, Chavez-Arias C, Diaz-Alcalà JE, Martínez MA. Xylazine intoxication in humans and its importance as an emerging adulterant in abused drugs: a comprehensive review of the literature. Forensic Sci Int. (2014) 240:1–8. doi: 10.1016/j.forsciint.2014.03.015
39. Ayub S, Parnia S, Poddar K, Bachu AK, Sullivan A, Khan AM, et al. Xylazine in the opioid epidemic: a systematic review of case reports and clinical implications. Cureus. (2023) 15:e36864. doi: 10.7759/cureus.36864
40. United States Drug Enforcement Administration. DEA Reports Widespread Threat of Fentanyl Mixed with Xylazine [undated]. https://www.dea.gov/alert/dea-reports-widespread-threat-fentanyl-mixed-xylazine
41. Hoffman J. Tranq Dope: Animal Sedative Mixed With Fentanyl Brings Fresh Horror to U.S. Drug Zones. (2023) The New York Times. https://www.nytimes.com/2023/01/07/health/fentanyl-xylazine-drug.html
42. Malayala SV, Papudesia BN, Bobb R, Wimbush A. Xylazine-induced skin ulcers in a person who injects drugs in Philadelphia, Pennsylvania, USA. Cureus. (2022) 19:e28160. doi: 10.7759/cureus.28160
43. Spoerke DG, Grimes MJ, Honea BN. Human overdose with the veterinary tranquilizer xylazine. Am J Emerg Med. (1986) 4:222–4. doi: 10.1016/0735-6757(86)90070-7
44. Connors NJ, Alsakha A, Larocque A, Hoffman RS, Landry T, Gosselin S. Antipsychotics for the treatment of sympathomimetic toxicity: a systemic review. Am J Emerg Med. (2019) 37:1880–90. doi: 10.1016/j.ajem.2019.01.001
45. Subramanian K, Gowda RM, Jani K, Zewedie W, Ute R. Propofol combined with lorazepam for severe poly substance misuse and withdrawal states in intensive care unit: a case series and review. Emerg Med J. (2004) 21:632–4. doi: 10.1136/emj.2003.007526
46. Richards JR, Garber D, Laurin EG, Albertson TE, Derlet RW, Amsterdam EA, et al. Treatment of cocaine cardiovascular toxicity: a systemic review. Clin Toxicol. (2016) 54:345–64. doi: 10.3109/15563650.2016.1142090
47. Amsterdam EA, Wenge NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, et al. AHA/ACC guideline for the management of patients with non-ST segment elevation acute coronary syndromes: a report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. (2014) 64:e139–228. doi: 10.1016/j.jacc.2014.09.017
48. Kontak AC, Victor RG, Vongpatanasin W. Dexmedetomidine as a novel countermeasure for cocaine-induced central sympathoexcitation in cocaine-addicted humans. Hypertension. (2013) 61:388–94. doi: 10.1161/HYPERTENSIONAHA.112.203554
Keywords: adulterants, cocaine, toxicity, cathinones, cannabis, illicit drugs, xylazine
Citation: Pino RM and McGrew PR (2023) Diagnosis and management of the patient with contaminated illicit drug poisoning. Front. Anesthesiol. 2:1234567. doi: 10.3389/fanes.2023.1234567
Received: 4 June 2023; Accepted: 29 September 2023;
Published: 12 October 2023.
Edited by:
Marco Fiore, Università degli Studi della Campania “Luigi Vanvitelli”, ItalyReviewed by:
Etrusca Brogi, University of Pisa, ItalyJuan Carlos Lopez-Delgado, Hospital Clinic of Barcelona, Spain
© 2023 Pino and McGrew. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richard M. Pino cnBpbm8xQGxzdWhzYy5lZHU=
 Richard M. Pino
Richard M. Pino Patrick R. McGrew2
Patrick R. McGrew2