
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Anesthesiol. , 08 September 2023
Sec. Cardiothoracic Anesthesiology
Volume 2 - 2023 | https://doi.org/10.3389/fanes.2023.1190383
Introduction: For patients undergoing cardiac surgery and catheterization procedures, severe post-operative nausea and vomiting (PONV) can occur despite standard anti-emetic interventions. Aprepitant, a neurokinin-1 (NK-1) receptor blocker, is safe and effective at preventing PONV resistant to standard therapies.
Methods: Patients with a history of severe PONV presenting for cardiac surgery or catheterization procedures from January 1, 2018 to January 6, 2021 were identified. After pharmacist approval, patients received aprepitant pre-operatively (Dose: 80 mg for weight >50 kg, 40 mg for weight 30–50 kg). A retrospective chart review was performed. Primary outcomes of the incidence of PONV and PONV-related complications were evaluated.
Results: Seventeen patients were included with a mean age of 16.0 years at the time of their initial procedure, which acted as the “control” procedure, and 17.5 years when they received aprepitant. After the control procedure 64.7% of patients required rescue anti-emetics. When this group of patients received aprepitant pre-operatively at their subsequent procedure, only 17.6% required rescue medication (p = 0.005). Similarly, 64.7% of patients suffered at least one PONV-related complication after the control procedure. With aprepitant use pre-operatively, 5.9% of the same patients experienced a PONV-complication (p = 0.0003). Specifically, unplanned ICU admission due to severe PONV after catheterization procedures decreased from 55.6% (5/9) in the control group to 0 after these patients were treated pre-emptively with aprepitant (p = 0.01). For surgical patients, there were significant decreases in PONV-related complications including delayed oral intake and delayed ambulation (p = 0.04) in the aprepitant group compared to the control group.
Discussion: This small, retrospective study supports the conclusion that preoperative aprepitant administration in patients undergoing cardiac catheterization or cardiac surgery with a history of congenital heart disease and severe PONV significantly reduces the incidence of PONV and PONV-related complications. Decreasing these complications will likely improve the surgical experience for patients and families while also decreasing hospital costs and improving efficiency.
Postoperative nausea and vomiting (PONV) remains a common and distressing complication following surgery (1, 2). When poorly controlled, PONV can lead to significant dehydration, electrolyte derangements, declining mental status, delayed ambulation, and subsequent delayed discharge (3). These symptoms are a major reason for unplanned hospital admission, increased hospital cost, and often make patients less willing to seek treatment in the future (4). For a subset of patients with congenital heart disease (CHD) undergoing cardiac surgery and catheterization (cath) procedures, severe PONV occurs despite intra-operative prophylaxis with dexamethasone and ondansetron. The length of most cardiac surgeries combined with intra-operative and postoperative narcotic requirements for patients undergoing sternotomy can increase the risk of PONV significantly, leading to delayed recovery. While narcotic requirements are much less, cardiac cath is associated with several unique PONV-related complications, such as bleeding and hematoma formation at vascular access sites and prolonged “flat time.” Thus, finding new antiemetic strategies for PONV in this patient population is important in order to improve postoperative morbidity and patient satisfaction.
A combination of agents that block neurotransmitter activity such as serotonin 5-HT3 receptor antagonists, dopamine receptor antagonists, corticosteroids, and neurokinin-1 (NK1) receptor antagonists are often employed to prevent the undesired nausea and vomiting that results from anesthesia and chemotherapeutics (5). More specifically, NK1 receptor antagonists have gained traction as a viable way to preemptively treat PONV and PONV-related symptoms due to their nonstandard mechanism of action when compared to other available antiemetics. NK1 receptor antagonists act by blocking the binding of Substance P to NK1 receptors in both the brainstem emetic center and in the GI tract. Aprepitant, a high-affinity NK1 receptor antagonist, has been used most commonly to prevent nausea and vomiting in cancer patients undergoing highly emetogenic chemotherapy and in non-cardiac surgical patients (6–8). Previous studies have demonstrated a reduced incidence of PONV-related complications following peri-procedure aprepitant administration when compared to other conventional antiemetics (5, 8, 9). Aprepitant is also a part of the most recent American Society of Enhanced Recovery and Society for Ambulatory Anesthesia Consensus Guidelines for the Management of Post-operative Nausea and Vomiting, though the guidance regarding its use in pediatric patients is much more vague than for adults (10).
However, the use of aprepitant for the prevention of PONV and PONV-related complications in patients with CHD is not well described. The purpose of this study is to evaluate the incidence of PONV and PONV-related complications after administration of pre-operative oral aprepitant to patients with CHD and history of severe PONV undergoing cardiac surgery or cath procedures. The authors hypothesized that patients undergoing cardiac surgery or cath would experience significantly less PONV and fewer PONV-related complications after aprepitant treatment compared to their prior anesthetics.
This study was approved by our institutional review board (COMIRB #21-4280). Patients presenting for cardiac surgery or cardiac cath procedures between January 1, 2018 and January 6, 2021 with a history of severe PONV were identified either by documentation in the electronic medical record or by self-identification by the patient or the patient's family. Oral aprepitant was given pre-operatively with dosing of 40 mg for weight 30–50 kg and 80 mg for weight >50 kg. Though choice of maintenance anesthetic was left to the discretion of the attending pediatric cardiac anesthesiologist, the practice of the group is relatively uniform in this regard. Inhaled isofluorane, dexmedetomidine infusion, and opioid boluses were used for maintenance of anesthesia during cardiac surgery with standard PONV prophylaxis of dexamethasone (0.1 mg/kg iv) and ondenastron (0.1 mg/kg iv) given after separation from cardiopulmonary bypass (CPB). An anesthetic consisting of isoflurane combined with low dose propofol infusion (∼50 mcg/kg/min), opioid boluses, and standard PONV prophylaxis was used during cardiac cath procedures.
Data was collected both for the “control procedure” during which severe PONV occurred and for the “aprepitant procedure” prior to which the patient received aprepitant. The control procedure for three patients (two cardiac surgeries and one cardiac catheterization) occurred at an outside hospital. Information was documented based on history obtained from the patient and family but details including anesthetic drugs used, narcotics received and procedural length were not available. The following data were collected: patient age (years), patient weight (kg), diagnosis, procedure performed, procedure location, pre-op sedation medications, aprepitant dose, length of procedure (min), airway management technique (natural, LMA, endotracheal tube), induction medications, anti-emetics given intraoperatively, maintenance of anesthesia, documentation of PONV in notes, anti-emetics given within first 24 h after extubation, narcotics given post-operatively, and PONV-related complications. Primary outcomes were presence of PONV and incidence of PONV-related complications. Specific complications assessed in patients who underwent cardiac catheterization included: hematoma, bleeding from cath site, prolonged flat time, unplanned inpatient admission and unplanned ICU admission, while complications assessed in patients who underwent cardiac surgery included: delayed oral intake, delayed extubation, delayed ambulation, and prolonged ICU stay defined as PONV listed as primary contributor to patient's inability to leave ICU for home or lower level of inpatient care for that calendar day.
Continuous variables are shown as median and interquartile range while categorical groups are shown as counts (percents). Wilcoxon rank sum tests are used to compare continuous variables between the apretitant and control group and either Pearson chis-square or Fisher's exact tests are evaluated to examine differences in proportions between groups. For pre-post analyses within the apretitant group, we conducted Wilcoxon signed rank tests. All analysis were two-sided and p-values <0.05 were considered statistically significant Analyses were conducted using SAS 9.4 Copyright (c) 2002–2012 by SAS Institute Inc., Cary, NC, USA.
Eighteen patients received aprepitant prior to cardiac procedures between January 1, 2018 and January 6, 2021. One patient was excluded who required cannulation onto ECMO at the conclusion of cardiac surgery. Seventeen patients were included with a mean age of 16.0 years (range, 8–27 years) at the time of “control procedure” and mean age of 17.5 years (range, 10–28 years) at the time of “aprepitant procedure.” For the control procedure, four patients underwent cardiac surgery, three underwent non-cardiac surgery, one underwent general anesthesia for cardiac MRI, and nine underwent cardiac catheterization. Three of the 17 patients had their control procedure performed at an outside hospital. In the “aprepitant group,” five patients received aprepitant prior to cardiac surgery, while twelve patients received aprepitant prior to cardiac catheterization. Cardiac diagnoses include (patients may have multiple): history of heart transplantation (3), arrhythmia including heart block (6), repaired tetralogy of fallot with pulmonary valve and/or artery abnormality (3), aortic valve stenosis/left ventricular outflow track obstruction/aorta abnormalities (6), intramural coronary artery (1), pericardial effusion (1). Procedural and patient characteristics, found in Table 1, include: patient age, weight, and sex; procedure type and length; narcotics received intra-operatively and narcotics received in the first 24 h post operatively. No significant differences were found in procedural variables between the “control group” and the “aprepitant group.”
When patients served as their own controls, 94.1% of patients experienced PONV with 64.7% requiring rescue anti-emetics after the “control procedure.” The patient who did not experience PONV had a history of severe chemotherapy-induced nausea and vomiting prior to his “aprepitant procedure.” When this group of patients received aprepitant pre-operatively prior to their subsequent cardiac procedure, only 11.8% experienced PONV (p = 0.0002) with 17.6% receiving rescue medication (p = 0.005). 64.7% of patients suffered at least one PONV-related complication after the control procedure. With aprepitant use pre-operatively, 5.9% of the same patients experienced a PONV related complication (Table 2). Tables 3, 4 provide more detailed breakdown of PONV complications.
The groups were then divided based on procedure into “surgery” (cardiac and non-cardiac) and “cardiac catheterization” groups and outcomes were compared again. Of note, for this comparison, the patients were not necessarily their own controls. PONV related complications after cardiac cath decreased from 55.6% in the control group to 0% in the aprepitant group (p = 0.003). Most significantly unplanned ICU admission due to severe PONV decreased from 44.4% in the control group to 0% in the aprepitant group (p = 0.01, Table 3).
In patients undergoing surgical procedures, the incidence of PONV related complications also decreased significantly from 57.1% to 0% (p = 0.04). Specifically, delayed oral intake and delayed ambulation decreased from 57.1% to 0% (p = 0.04, Table 4). Other complications experienced by patients in the control group included work-up for ischemia as a cause of severe PONV on POD 1 (echocardiogram, troponin) and 7 kg weight loss in the post-operative period a patient with normal BMI due to protracted PONV.
The results of this study suggest that preoperative aprepitant administration in patients with CHD and a history of severe PONV undergoing either cardiac cath or surgery significantly reduces the incidence of PONV-related complications and decreases the need for a rescue antiemetic medications. These results may be explained in part by the novel mechanism of action of aprepitant.
There are a number of perioperative factors that can contribute to the incidence of PONV including use of volatile anesthetics, perioperative opioid administration, and prolonged length of surgery. Patient-specific characteristics such as history of motion sickness, history of PONV and female sex also increase risk for PONV.
Multiple neurotransmitters, including serotonin, dopamine, and substance P, play a role in the pathophysiology of nausea and vomiting. Serotonin is released by enterochromaffin cells in the GI tract in response to physiologic abnormalities. It binds to 5-HT3 peripheral receptors located on vagal afferents and to central receptors in the chemoreceptor trigger zone (CTZ) within the area postrema located at the floor of the fourth ventricle, an area outside the blood-brain barrier. Activation of these central chemoreceptors appears to play an important role in initiation of the vomiting reflex (11). Opioids administration also results in dopamine release and subsequent activation of the CTZ via dopamine-2 (D2) receptors. Similarly, the antiemetic properties in glucocorticoids, though incompletely understood, may be due to interactions with serotonin, blocking inflammation which triggers stimulation of vagal afferents or by direct central action at the nucleus tractus solitaries (12, 13). These processes help us understand how the most commonly employed antiemetics work including: 5-HT3 receptor antagonists (ondansetron), dopamine receptor antagonists (metoclopramide), and corticosteroids (dexamethasone). However, the neurokinin-1 receptor blocker aprepitant acts by blocking the binding of Substance P to NK1 receptors in the brainstem emetic center and the GI tract, which may be more effective as prophylaxis against PONV when compared to other available antiemetics (6–8).
This study looked at aprepitant use in a specific population: patients with CHD and a history of severe PONV undergoing cardiac procedures. In this group of patients at extremely high risk for PONV, the use of aprepitant was associated with significant reductions in all PONV-related parameters: incidence of PONV, use of rescue medications, and complications regardless of whether the patients were grouped so that procedure type was matched (patients were not necessarily their own controls though all patients shared history of severe PONV) or with patients serving as their own controls (in this case, procedure type does not necessarily match).
Analysis of patients when divided into groups based on procedure type revealed that no patients undergoing cardiac catheterization experienced any PONV-related complication after their “aprepitant procedure” when compared to 55.6% of patients following their control procedure (p = 0.003). When specific complications were analyzed separately, there was a significant decrease in unplanned ICU admissions in the aprepitant group (p = 0.01). Similarly, a significant decrease in complications was also observed in the cardiac surgery patients with no patients experiencing a PONV-related complication (delayed ambulation and delayed po intake) after their “aprepitant procedure” compared to 57.1% of patients following their control procedure (p = 0.04) (Table 3).
Analysis of PONV incidence and complication rates when patients served as their own controls yielded similar results. Pre-operative aprepitant administration was associated with statistically significant decreases in incidence of PONV, post procedure antiemetic requirement and incidence of PONV-related complications (Table 2). While many factors beyond the administration of aprepitant have the potential to impact PONV incidence, such as procedure type, procedure length, and intraoperative/postoperative narcotic administration, no significant differences were found in any of these variables between the control and aprepitant groups in this study (Table 1).
Given that ERAS (Enhanced Recovery After Surgery) protocols designed to “fast track” patients post-operatively have been shown to improve outcomes in adult and pediatric subspecialties (14, 15), there is a strong desire to develop such protocols for the pediatric cardiac surgical population (16). As pediatric cardiac centers work to develop ERAS, aprepitant should be considered as a useful part of these protocols for patients at high risk for PONV.
Cost is also an important parameter to consider, as is hospital efficiency in the era of increasingly common staffing shortages. Per Children's Hospital Colorado chargemaster, the “self-pay” charge for aprepitant is between $400-$450, depending on dose. However, this is substantially less than the cost of 1 h of “Phase 1 Recovery” ($1,014) or room/board for an unplanned admission: ∼$5,600 for inpatient admission and $6,800 for ICU admission (17) (Table 5). Even though these numbers are gross overestimates due to insurance contracts paying ∼20%–30% of the price list cost, the relative percentages are the same. These costs also do not include physician billing, medications, and laboratory tests. The economic effect of PONV in CHD patients may also be confounded by bundled payments and this warrants further study. However, monetary cost does not take into account decreased staffing burden, increased hospital efficiency, increased patient comfort, and alignment with the hospital's commitment to provide the best care possible provided by effective PONV prophylaxis.
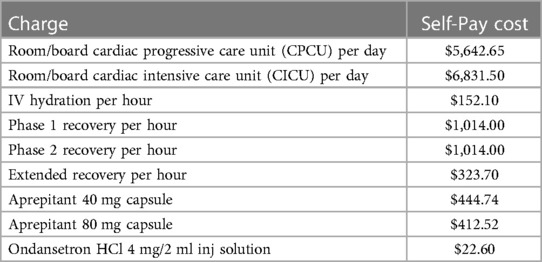
Table 5. Children's hospital Colorado costs (17).
There are two important drug interactions associated with aprepitant that must be noted. First, aprepitant use may result in decreased INR in patients anticoagulated with coumadin via CYP2C9 interactions. Aprepitant may also decrease the effectiveness of hormonal contraceptives for up to 28 days following the last dose (18). Therefore, women using hormonal contraceptives should be advised to use alternative methods of contraception following treatment with aprepitant.
This small, retrospective study has several limitations. First, when the patients were their own controls, the control procedure did not necessarily match the aprepitant procedure. Some patients who underwent cardiac catheterization as their control procedure went on to undergo cardiac surgery for their aprepitant procedure, and vice versa. Though we identified no significant differences between procedural times and narcotic administration between the groups the inherent differences between these two types of procedures (amount of anesthesia required, invasiveness) that cannot be controlled for could impact the incidence of PONV and PONV-related complications. Additionally, these data points from three patients whose control procedure occurred at an outside hospital were not available and history provided by patient and family was used to categorize complications. This is especially important when comparing narcotic administration as two of these three patients underwent cardiac surgery at an OSH as their control procedure and likely received a substantial amount of opioids. The overall small study sample size combined with the large variance in opioid exposure among patients creates the possibility of a Type 2 error. The nature of the OSH control procedures only amplifies this. Thus, there could be a difference in opioid use between the groups that was not detected.
Additionally, for the sub-analysis where groups were formed based on procedure type, patients did not serve as their own controls if the control procedure type differed from the “aprepitant procedure.” The specific timing of PONV in the early postoperative period was also not assessed.
Lack of availability of the liquid preparation of aprepitant at our institution at the time of the study also limited the weight range of eligible patients. Aprepitant is approved for use in children over 6 months of age. Our study population was limited to patients weighing more than 30 kg because a 40 mg tablet was the lowest available dose. However, the study population of adolescents and young adults does include two high risk populations for PONV based on age.
Finally, the study was limited in its ability to assess the effect of aprepitant use on PACU time and “time to discharge” for same-day procedures, such as cardiac catheterization for transplant surveillance or electrophysiology studies. Patients have a mandatory “flat time” of either two or four hours following these procedures followed by a mandatory two-hour observation period. Thus, PACU time for this group of patients may not reflect how PONV, or lack thereof, affected their recovery time.
Though our study is small and has many limitations its results are clearly in favour of pre-operative aprepitant use in pediatric patients undergoing cardiac catheterization or cardiac surgery with a history of congenital heart disease and severe PONV. The incidence of PONV and PONV-related complications were significantly reduced and. decreasing these complications will likely improve the surgical experience for patients and families while also decreasing hospital costs and improving efficiency.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by University of Colorado School of Medicine Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
JB: student at University of Colorado School of Medicine who acquired and analyzed the data and wrote large portions of the manuscript. MT: director of Cardiac Anesthesia at Children's Hospital Colorado who helped with study design, editing, and mentorship of senior author. KK: pharmacist at Children's Hospital Colorado, directly involved in determining each patient's eligibility to receive aprepitant. LS: statistician who performed statistical analysis of the data. RC: designed study, helped with data acquisition, and wrote the manuscript with JB. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Navari RM. Pharmacological management of chemotherapy-induced nausea and vomiting: focus on recent developments. Drugs. (2009) 69(5):515–33. doi: 10.2165/00003495-200969050-00002
2. Cao X, White PF, Hong MA. An update on the management of postoperative nausea and vomiting. J Anesth. (2017) 31(4):617–26. doi: 10.1007/s00540-017-2363-x
3. Sweis I, Yegiyants SS, Cohen M. The management of postoperative nausea and vomiting: current thoughts and protocols. Aesthetic Plast Surg. (2013) 37(3):625–33. doi: 10.1007/s00266-013-0067-7
4. Kovac AL. Postoperative nausea and vomiting in pediatric patients. Paediatr Drugs. (2021) 23(1):11–37. doi: 10.1007/s40272-020-00424-0
5. Curran MP, Robinson DM. Aprepitant: a review of its use in the prevention of nausea and vomiting. Drugs. (2009) 69(13):1853–78. doi: 10.2165/11203680-000000000-00000
6. Schoffelen R, Lankheet AG, van Herpen CML, van der Hoeven JJM, Desar IME, Kramers C. Drug-drug interactions with aprepitant in antiemetic prophylaxis for chemotherapy. Neth J Med. (2018) 76(3):109–14.29667586
7. Zhang L, Lu S, Feng J, Dechaphunkul A, Chang J, Wang D, et al. A randomized phase III study evaluating the efficacy of single dose NEPA, a fixed antiemetic combination of netupitant and palonsetron, versus an aprepitant regiment for prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving highly emetogenic chemotherapy (HEC). Ann Oncol. (2018) 29(2):452–258. doi: 10.1093/annonc/mdx698
8. Weibel S, Rucker G, Eberhart LH, Pace NL, Hartl HM, Jordan OL, et al. Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis. Cochrane Database Syst Rev. (2020) 10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2
9. Singh PM, Borle A, Rewari V, Makkar JK, Trikha A, Sinha AC, et al. Aprepitant for postoperative nausea and vomiting: a systematic review and meta-analysis. Postgrad Med J. (2016) 92(1084):87–98. doi: 10.1136/postgradmedj-2015-133515
10. Gan TJ, Belani KG, Bergese S, Chung F, Diemunsch P, Habib AS, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. (2020) 131(2):411–48. doi: 10.1213/ANE.0000000000004833
11. Butterworth JF IV, Mackey DC, Wasnick JD, editors. Morgan & mikhail’s clinical anesthesiology. 5th ed. New York, NY: McGraw Hill (2013).
12. Maitra S, Som A, Baidya DK, Bhattacharjee S. Comparison of ondansetron and dexamethasone for prophylaxis of postoperative nausea and vomiting in patients undergoing laparoscopic surgeries: a meta-analysis of randomized controlled trials. Anesthesiol Res Pract. (2016) 2016:7089454. doi: 10.1155/2016/7089454
13. Chu CC, Hsing CH, Sheih JP, Chien CC, Ho CM, Wang JJ. The cellular mechanism of the antiemetic action of dexamethasone and related glucocorticoids against vomiting. Eur J Pharmacol. (2014) 722:48–54. doi: 10.1016/j.ejphar.2013.10.008
14. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. (2017) 152(3):292–8. doi: 10.1001/jamasurg.2016.4952
15. Rove KO, Brockel MA, Saltzman AF, Dönmez MI, Brodie KE, Chalmers DJ, et al. Prospective study of enhanced recovery after surgery protocol in children undergoing reconstructive operations. J Pediatr Urol. (2018) 14(3):252.e1–e9. doi: 10.1016/j.jpurol.2018.01.001
16. Fuller S, Kumar SR, Roy N, Mahle WT, Romano JC, Nelson JS, et al. The American association for thoracic surgery congenital cardiac surgery working group 2021 consensus document on a comprehensive perioperative approach to enhanced recovery after pediatric cardiac surgery. J Thorac Cardiovasc Surg. (2021) 162(3):931–54. doi: 10.1016/j.jtcvs.2021.04.072
17. Children's Hospital Colorado. Price Transparency at Children's Hospital Colorado (undated). https://www.childrenscolorado.org/your-visit/insurance-financial-resources/price-transparency/ (Accessed February 6, 2022).
18. FDA. Aprepitant Prescribing Information. (undated) Ref. 9985512. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021549s017lbl.pdf
Keywords: PONV (postoperative nausea and vomiting), aprepitant, congenital heart disease, cardiac catheterization, congenital cardiac surgery, congenital cardiac anesthesia
Citation: Belk JW, Twite MD, Klockau KS, Silveira LJ and Clopton RG (2023) Effects of aprepitant on post-operative nausea and vomiting in patients with congenital heart disease undergoing cardiac surgery or catheterization procedures: a retrospective study with subjects as their own historical control. Front. Anesthesiol. 2:1190383. doi: 10.3389/fanes.2023.1190383
Received: 20 March 2023; Accepted: 22 August 2023;
Published: 8 September 2023.
Edited by:
Alessandro Belletti, IRCCS San Raffaele Scientific Institute, ItalyReviewed by:
Nishant Kumar, University of Delhi, India© 2023 Belk, Twite, Klockau, Silveira and Clopton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel G. Clopton cmFjaGVsLmNsb3B0b25AY2hpbGRyZW5zY29sb3JhZG8ub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.