
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Anesthesiol. , 30 March 2023
Sec. Perioperative Medicine
Volume 2 - 2023 | https://doi.org/10.3389/fanes.2023.1172018
Obesity and anemia are increasing prevalence around the globe. They can interplay and are both associated with high morbidity and poorer clinical outcomes. Inflammation and iron deficiency are important contributors to anemia in obese patients. Furthermore, obesity surgery can trigger high blood loss, increased demand for blood transfusions and long-term nutrient deficiency. Patient blood management programs have been crucial in improving patients' clinical results whilst minimizing costs in many different settings, such as orthopedic surgery, cardiovascular surgery, pregnancy and intensive care unit patients. In this mini-review, we will discuss applications of patient blood management principles in caring of obese individuals during the patient journey.
The World Health Organization (WHO) describes obesity as one of the most blatantly visible, yet most neglected, public-health problems that threatens to overwhelm both more and less developed countries (1). Overweight and obese, defined by body mass index (BMI) between 25 and 30 kg/m2 and over 30 kg/m2 respectively, are present in over 40% of the adult population and around 20% among children and adolescents in many countries of the globe (1–3). This disease is associated with increased morbidity and mortality, mainly secondary to cardiovascular disease, diabetes type 2, osteoarthritis and cancer (1–4). High BMI is among the top five risk factors in terms of attributable deaths and disability-adjusted life-years (4). Although new insights have contributed to the understanding of the complex underlying mechanisms of the disease, its prevention and treatment are still an immense challenge to health systems (1, 5).
In a similar way, anemia is also a world-wide problem, affecting 1.95–2.36 billion people. Anemia is associated with reduced work productivity, impaired neurocognitive development, diminished quality of life, and increased adult and child morbidity and mortality (6). In the pre-operative and in-hospital settings, anemia rates can reach up to 40% and 74% of the patients respectively, and it is associated with worse clinical outcomes, such as increased risk of blood transfusions, intensive care admissions, length of hospital stay, morbidity and mortality (6–8).
The objectives of this article are to discuss the points in the patient journey where obese patient can be found to be anemic, the causes of anemia, strategies to minimize the risk of anemia development, and currently available treatments.
According to the WHO definition, women with hemoglobin (Hb) < 12.0 g/dl and men with Hb < 13.0 g/dl are considered anemic (9). However, some argue that a new definition of anemia is needed in the context of high-blood-loss surgery, because preoperative Hb below 13 g/dl may increase morbidity, mortality and transfusion, regardless of sex (8).
Iron deficiency is the most common cause of anemia, followed by chronic inflammation and renal disorders (7).
Iron deficiency anemia (IDA) can be secondary to high demand or reduced intake of this nutrient. (Table 1) Iron deficiency is most commonly defined by a serum ferritin <30 µg/L. Previous studies have shown that this cut-off is associated with high sensitivity (92%) and specificity (98%). However, ferritin is an acute-phase reactant which means it can be elevated in the presence of inflammation (8). If there are increased inflammatory markers, such as C-reactive protein, a ferritin of 30 to 100 µg/L and a transferrin saturation <20% can still be used to diagnose IDA (10).
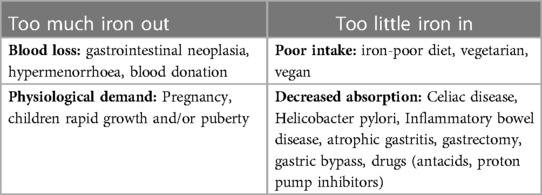
Table 1. Common causes of anemia. Adapted from Lin et al. (7).
Anemia of chronic inflammation (ACI) is regarded as the most frequent anemia in hospitalized and critically ill patients (11). Many disorders are associated with this condition, including infection, autoimmune diseases, cancer, and various chronic diseases of the kidney, heart, and lung.(11) One possible underlying mechanism is a combination of interleukin-induced hepcidin blockage of duodenal iron absorption, and iron accumulation in the reticuloendothelial cells as a result of shortened erythrocyte life-span plus inflammation-suppressed erythropoiesis (11–13).
Anaemia and iron deficiency are common, so all obese patients should be evaluated for them, with particular attention to those undergoing surgery (13–16). In the specific setting of pre-bariatric surgery, the prevalence of anemia ranges from 6.1% to 22% (12). Gowanlock et al. showed that just before weight-loss surgery, 12% had anemia and 19% had iron deficiency (17), though it may be counterintuitive that an obese individual can develop a nutritional deficiency involving iron. The precise relationship between these obesity and iron remains unclear. Zhao et al. performed a meta-analysis of 13,393 overweight/obese and 26,621 non-overweight participants. The obese patients had lower serum iron concentrations, lower transferrin saturation, and significantly increased risk of iron deficiency (OR: 1.31) (14). Farhangi et al. reported that obese females (BMI > 30 kg/m2) had lower Hb (12.5 ± 1.0 g/dl vs. 13.0 ± 0.9 g/dl, p = 0.02) than non-obese counterparts (15). On the other hand, Arshad et al. found no correlation between severely obese patients (BMI > 40 kg/m2) and anemia (16).
Anemia can be due to other causes. Chronic, mild systemic inflammation is a feature of obesity. Excessive adipocyte tissue promotes immune activation, macrophage infiltration, release of pro-inflammatory cytokines, reduction in anti-inflammatory mediators, and dysregulation of free fatty acid release. Activation of the inflammatory response stimulates the synthesis and release of acute phase mediators, such as C-reactive protein, interleukin (IL)-6, IL-1, IL-8 and tumour necrosis factor-α, leading to hepcidin imbalance on duodenal iron absorption and sequestration in macrophages (12, 13).
Obesity treatment is complex and multidisciplinary. The aims include reduction of health risks and promotion of weight loss, while preventing weight regain. Possible interventions are lifestyle modification, dietary change, medication and surgery (18, 19).
Surgery has clear benefits but can increase the likelihood of anemia in this already at-risk population. Surgery is the most effective treatment for morbid obesity in terms of long-term weight loss, improvement of co-morbidities and quality of life, and decrease in mortality (18, 19). As a result, there has been a steep increase in the number of bariatric procedures performed worldwide. In 2018, approximately 252,000 bariatric procedures were performed in the USA (18). Several techniques have been developed, with sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB) being the most frequent (18, 20). The adjustable gastric band (AGB) and biliopancreatic diversion (BP) are less commonly employed. Kizy et al. reported on a large number of bariatric surgeries performed in the USA from 2012 to 2015, SG accounted for 63% of procedures compared to 30% and 2% for RYGB and AGB, respectively (21). The preference for SG is related to lower complication rates, whilst achieving similar weight-loss to other procedures (21). According to the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) data registry, SG had approximately half of the risk-adjusted odds of mortality, serious morbidity, and leak in the first 30 days compared with RYGB (20).
After metabolic surgery, nutrient deficiency and anemia are frequently reported. According to a recent report from the American Society of Haematology, people who have undergone bariatric procedures show the highest risk for anemia, with 33%-49% presenting with anemia within 2 years after the surgery (22). Among those patients, the ones having SG and RYGB, were diagnosed with post-operative anemia in around 17% and 45%, respectively (23).
Nutritional anemia resulting from bariatric procedures can involve deficiencies in vitamin B12, folate, and iron, with the latter responsible for most cases. Around 30%–43% of patients will develop iron or vitamin B12 deficiency after SG and RYGB (17, 24). Moreover, 19%–35% of the patients develop vitamin B12 deficiency in 5 years after RYGB. Compared with the SG group, the odds ratio for postoperative vitamin B12 deficiency in the RYGB patients was 3.55 (p < 0.001) (25).
Nutrient deficiency can occur quite a long time after surgery, with a median reported interval of 42.5 to 51 months (26). For example, one Canadian study of 388 patients showed that 6 months after surgery, 17% and 3% of the patients presented with iron deficiency and iron deficient anemia (IDA), respectively. After a mean follow-up of 2.5 years, 43% had developed iron deficiency and 16% IDA (17). The delayed presentation of anemia has led bariatric surgery guidelines to recommend monitoring iron studies every 3 to 6 months in the first postoperative year and annually thereafter (20, 27).
Blood loss during surgical procedures is independently associated with an increased risk of poorer clinical outcome (27). Initiatives such as hemostatic agents, and minimally invasive surgical procedures can minimize bleeding and reduce the risk of subsequent anemia and transfusion (8).
Some of these hemostatic agents, such as antifibrinolytics have been used since the 1960s for menstrual bleeding and hereditary bleeding disorders. Recently, it has been studied in acute trauma, postpartum hemorrhage, gastrointestinal bleeding and intraoperative. Tranexamic acid (TXA), currently the most used antifibrinolytic agent, is a synthetic lysine analog that inhibits the activation of plasminogen to plasmin thereby stabilizing the clot. It has a terminal half-life of around 2 h and it is excreted in the urine. In many clinical situations, the risk of thromboembolic events is not increased, but its use should be avoided in patients with hematuria, active intravascular clotting and thromboembolic disease (28). Although the causality and pathophysiological mechanism are unclear, TXA use is associated with improvement in clinical outcomes in acute trauma, postpartum hemorrhage and orthopedic surgery (29–31). In metabolic surgery, around 2%–4% of patients experience bleeding, with 3% requiring reoperation (32). Tranexamic acid has been reported to reduce the incidence of bleeding, surgery duration, hospital length of stay, without increasing thromboembolic events (32–34). A meta-analysis suggested that a dose of 1 g of TXA given at anesthetic induction might be as effective as higher doses (35). Therefore, TXA may be used in patients who have documented fibrinolysis based on viscoelastic testing. The appropriate dose is unclear and needs to be evaluated.
A variety of bariatric procedures are available, and detailed comparison between them is beyond the scope of this article. Briefly, it has been demonstrated that less invasive techniques such as laparoscopic procedures, can improve clinical outcomes and be performed safely (36). Laparoscopic bariatric surgery has been associated with lower morbidity, mortality, and time to discharge (37). The safety profile with maintained weight-loss efficacy has boosted the frequency of laparoscopic bariatric procedures from 63% in 2002 to over 90% in 2008 (37). Therefore, laparoscopic techniques should be the preferred approach in the weight-loss surgery context.
To minimize the consequences of anemia, treatment can be given to directly increase the red cells, such as transfusion, iron therapy, and B12/folate supplementation. Treatment can also be tailored to the underlying mechanism of the disease. These approaches will be discussed separately.
RBC transfusions can be life-saving, but they should be used judiciously and unnecessary transfusion is to be avoided because of potential infectious and noninfectious adverse events (38). Defining when transfusion is clinically appropriate has been the focus of efforts around the globe. Some use a combination of measured parameters, such as hemoglobin level, clinical signs and symptoms, the availability of other anemia therapies, and the presence of risk factors for hemorrhage (39). Several studies have looked at liberal (transfusion threshold Hb < 9.0 g/dl) vs. restrictive (transfusion threshold Hb < 7–8.0 g/dl) transfusion policies in diverse clinical settings (39–49). Systematic reviews and meta-analyses of randomized controlled trials found that the restrictive approach has a similar or superior outcome compared to the liberal transfusion strategy (39, 50). Consequently, restrictive strategy is supported by current guidelines (38, 51) (Table 2).
Oral iron is the preferred treatment in the majority of patients with IDA (7, 18). The recommended daily dose for adults is 100 to 200 mg of elemental iron, and for children 3 to 6 mg/kg of body weight (18, 52, 53). It should be taken on an empty stomach and with agent to enhance absorption, such as vitamin C (7, 52). However, ingestion of the medication without a previous food consumption may increase its side effects, including nausea, epigastric discomfort, abdominal cramps, and constipation (54).
Since the majority of patients experience adverse events from oral iron, adherence is a critical issue. Compared to conventional daily dosing, alternate-day dosing is associated with fewer gastrointestinal side effects and reduced hepcidin levels which promote iron absorption (53, 55). Conditions associated with iron malabsorption such as chronic use of proton pump inhibitors, celiac disease, atrophic gastritis and bariatric surgery may make patients unsuitable for oral iron (52).
Some patients having bariatric surgery have impaired iron absorption because of anatomical changes, blood loss and red meat intolerance, however, around 65% can still benefit from oral iron formulations (56).
For patients where IV iron is the preferred alternative because of lack of tolerance or effectiveness of oral iron, many products are available. Current formulations are well tolerated and effective, but users should be aware that iron content, maximum dose per infusion and infusion rate differ between products (57).
Occasionally adverse events are seen with the IV products. Hypophosphatemia is a common problem, especially with iron polymaltose and ferric carboximaltose (57). As clinical symptoms of hypophosphatemia are not frequent, serum phosphate levels should be monitored (57).
The optimal dosage of IV iron can be calculated with Ganzoni's equation (58):
(Units: actual body weight kg; actual Hb g/dl; the “500” refers to iron stores, in mg, for individuals with a body weight greater than or equal to 35 kg).
Specific product documentation can provide alternatives doses and schedules.
Anemia in the preoperative setting is considered a relative contraindication to elective surgery (51, 59). Therefore, when possible, anemia identification and treatment before the surgery is considered the standard of practice (59). When IDA is diagnosed, oral iron should be the first option when there is enough time available for improvement until the surgery (>6 weeks) (7). However, when the anemia is severe and/or there is a shorter time until the surgery, IV iron might be helpful (7).
In a systematic review of perioperative IV iron, an increase in hemoglobin was noted in 11 of 17 studies and a decrease in transfusion was noted in 8 of 13 studies with the strongest evidence for preoperative use (60). However, the clinical impact of this increase on an improvement of patient's long-term outcomes is uncertain (7, 61). Recent studies have shown conflicting evidence. In a meta-analysis evaluating 936 patients with anemia in the preoperative setting for cardiovascular surgery, IV iron did not reduce the transfusion requirements but was associated with decreased mortality (62). On the other hand, the PREVENT trial, found no difference between groups in terms of red cell transfusion and 30-day mortality, despite observing a beneficial effect on the Hb in the iron group. The design of the trial could explain some of the contradictory results. PREVENTT randomised 487 patients with anemia, who were scheduled to undergo major elective abdominal surgery (63). There was a relatively short time from iron infusion to surgery (median of 15 days) meaning the benefit of IV iron was not fully realised. Furthermore, including patients with anemia (due to any cause) rather than just iron deficiency anemia would lead to a heterogeneous group with some who would not be expected to respond to iron.
It appears that IV iron is effective in improving anemia, but its long term impact on clinical outcomes is still unclear and should be addressed in the future trials.
Vitamin B12 absorption depends on an acidic environment and gastric parietal cell-derived intrinsic factor which are altered after SG and RYGB. The average storage of Vitamin B12 is enough to maintain reasonable levels for up to two years, thereby delaying presentation of deficiency. Vitamin B12 supplementation is recommended as prophylaxis after the surgery and also as treatment when deficiency is established. When deficiency and neurological symptoms are present vitamin B12 1 mg intramuscularly on alternate days should be given until there is no further improvement, then administered every 2 months. In individuals without neurological symptoms, vitamin B12 1 mg intramuscularly three times per week for 2 weeks should be given. After the initial treatment, patients should receive a maintenance dose of 1 mg vitamin B12 every 2–3 months (18).
Folic acid absorption takes place in the small bowel, so it is most affected by RYGB and duodenal switch procedures. Folic acid deficiency can also occur after SG, because of non-adherence with the multivitamin and mineral supplements, or other causes of malabsorption. Treatment consists of oral folic acid 5 mg per day for 4 months (18).
Anemia of Inflammation (AI) has variable origins and treatment should target the underlying condition and its effects on iron metabolism and erythropoiesis. A couple of treatment options can be considered.
Recombinant erythropoiesis-stimulating agents (ESAs) have been used successfully for the treatment of AI, stimulating the red blood cell production by the bone marrow, especially in patients with cancer or renal failure. Widespread use is not recommended because of the increased morbidity reported in some studies. Nonetheless, ESAs remains approved for certain indications, including anemic patients undergoing major non-cardiac surgery (11).
Iron replacement can be useful when there is absolute (combined AI and IDA) or relative iron deficiency (iron sequestered in the reticuloendothelial system). Oral iron can be effective in combined anemia. On the other hand, IV iron can be a better choice in specific situations as chronic heart failure and inflammatory bowel disease (11). Although obesity associated-AI can be similar to previously described diseases associated AI, the optimal strategy for managing it, is still based on the treatment of obesity itself, as there are no clinical trials exploring the ESAs and iron replacement in this specific group of patients.
Despite increased understanding of the pathophysiology, the prevalence of obesity and its negative impact on patient outcomes are continuously arousing concern worldwide, both at the level of the individual and the broader health service. In this review, we discussed briefly how patient blood management strategies could be used to help obese patients achieve better clinical outcomes. Initiatives like anemia screening and its early treatment, active use of haemostatic agents, and less invasive surgical approaches, can transform high risk clinical scenarios into ones with low morbidity and mortality.
However, what might be the optimal treatment(s) remains to be an open question. In fact, it is still challenging to treat obesity-associated anemia of inflammation, where iron replacement can improve the results, but frequently it is not enough to solve the problem. Research is needed to better understand the roles of pro-inflammatory agents and iron hemostasis in this setting to inform development of new drugs. Another outstanding issue is lack of non-invasive surgical procedures for morbid obesity patients. Currently available procedures can lead to high weight loss but carry significant morbidity over the short and/or long term. Research is much needed to develop novel non-invasive tools to improve effectiveness and safety profile for the patients in need. Improved understanding of the disease process will be crucial to achieve this.
GD, GC and WW conceived the idea of the study. GD and GC wrote the initial draft. GD, GC, WW revised it critically and added important clinical insights. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Haslam DW, James WPT. Obesity. Lancet. (2005) 366(9492):1197–209. doi: 10.1016/S0140-6736(05)67483-1
2. de Lorenzo A, Romano L, di Renzo L, di Lorenzo N, Cenname G, Gualtieri P. Obesity: a preventable, treatable, but relapsing disease. Nutrition. (2020) 71:110615. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0899900719301984 doi: 10.1016/j.nut.2019.110615.31864969
4. Jaacks LM, Vandevijvere S, Pan A, McGowan CJ, Wallace C, Imamura F, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. (2019) 7:231–40. doi: 10.1016/S2213-8587(19)30026-9
5. World Health Organization (WHO). Obesity: Preventing and managing the global epidemic—WHO technical report series. Geneva, Switzerland: WHO (2000).
6. Hofmann A, Shander A, Blumberg N, Hamdorf JM, Isbister JP. Gross I. Patient blood management: improving outcomes for millions while saving billions. What is holding it up? Anesth Analg. (2022) 135(3):511–23. doi: 10.1213/ANE.0000000000006138
7. Lin Y. Preoperative anemia-screening clinics. Hematology (United States). (2019) 1:570–76. doi: 10.1182/hematology.2019000061
8. Muñoz M, Gómez-Ramírez S, Kozek-Langeneker S, Shander A, Richards T, Pavía J, et al. “Fit to fly”: overcoming barriers to preoperative haemoglobin optimization in surgical patients. Br J Anaesth. (2015) 115:15–24 doi: 10.1093/bja/aev165
9. Who CM. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva, Switzerland: World Health Organization (2011).
10. Muñoz M, Acheson AG, Auerbach M, Besser M, Habler O, Kehlet H, et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia. (2017) 72(2):233–47. doi: 10.1111/anae.13773
11. Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. (2019) 133:40–50 doi: 10.1182/blood-2018-06-856500
12. Benotti PN, Wood GC, Kaberi-Otarod J, Still CD, Gerhard GS, Bistrian BR. New concepts in the diagnosis and management approach to iron deficiency in candidates for metabolic surgery: should we change our practice? Surg Obes Relat Dis. (2020) 16:2074–81. doi: 10.1016/j.soard.2020.08.018
13. Purdy JC, Shatzel JJ. The hematologic consequences of obesity. Eur J Haematol. (2021) 106:306–19. doi: 10.1111/ejh.13560
14. Zhao L, Zhang X, Shen Y, Fang X, Wang Y, Wang F. Obesity and iron deficiency: a quantitative meta-analysis. Obes Rev. (2015) 16:1081–93. doi: 10.1111/obr.12323
15. Farhangi MA, Keshavarz SA, Eshraghian M, Ostadrahimi A, Saboor-Yaraghi DA. White blood cell count in women: relation to inflammatory biomarkers, haematological profiles, visceral adiposity, and other cardiovascular risk factors. J Health Popul Nutr. (2013) 31(1):58–64. doi: 10.3329/jhpn.v31i1.14749
16. Arshad M, Jaberian S, Pazouki A, Riazi S, Rangraz MA, Mokhber S. Iron deficiency anemia and megaloblastic anemia in obese patients. Rom J Intern Med. (2017) 55(1):3–7. doi: 10.1515/rjim-2016-0046
17. Gowanlock Z, Lezhanska A, Conroy M, Crowther M, Tiboni M, Mbuagbaw L, et al. Iron deficiency following bariatric surgery: a retrospective cohort study. Blood Adv. (2020) 4(15):3639–47. doi: 10.1182/bloodadvances.2020001880
18. O’Kane M, Parretti HM, Pinkney J, Welbourn R, Hughes CA, Mok J, et al. British Obesity and metabolic surgery society guidelines on perioperative and postoperative biochemical monitoring and micronutrient replacement for patients undergoing bariatric surgery—2020 update. Obes Rev. (2020) 21:e13087. doi: 10.1111/obr.13087
19. Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, et al. European Guidelines for obesity management in adults. Obes Facts. (2015) 8(6):402–24. doi: 10.1159/000442721
20. Mechanick JI, Apovian C, Brethauer S, Garvey WT, Joffe AM, Kim J, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures—2019 update. Am College Endocrinol. (2019) 25(12):1346–59. doi: 10.4158/GL-2019-0406
21. Kizy S, Jahansouz C, Downey MC, Hevelone N, Ikramuddin S, Leslie D. National trends in bariatric surgery 2012–2015: demographics, procedure selection, readmissions, and cost. Obes Surg. (2017) 27(11):2933–39. doi: 10.1007/s11695-017-2719-1
22. American Society of Hematology. Iron-Deficiency Anemia (2023). Available at: https://www.hematology.org/education/patients/anemia/iron-deficiency.
23. Lupoli R, Lembo E, Saldalamacchia G, Avola CK, Angrisani L, Capaldo B. Bariatric surgery and long-term nutritional issues. World J Diabetes. (2017) 8(11):464–74. doi: 10.4239/wjd.v8.i11.464
24. Alexandrou A, Armeni E, Kouskouni E, Tsoka E, Diamantis T, Lambrinoudaki I. Cross-sectional long-term micronutrient deficiencies after sleeve gastrectomy versus roux-en-Y gastric bypass: a pilot study. Surg Obes Relat Dis. (2014) 10(2):262–68. doi: 10.1016/j.soard.2013.07.014
25. Kwon Y, Kim HJ, lo Menzo E, Park S, Szomstein S, Rosenthal RJ. Anemia, iron and vitamin B12 deficiencies after sleeve gastrectomy compared to Roux-en-Y gastric bypass: a meta-analysis. Surg Obes Relat Dis. (2014) 10(4):589–97. doi: 10.1016/j.soard.2013.12.005
26. Kotkiewicz A, Donaldson K, Dye C, Rogers AM, Mauger D, Kong L, et al. Anemia and the need for intravenous iron infusion after roux-en-Y gastric bypass. Clin Med Insights Blood Disord. (2015) 8:9–17. doi: 10.4137/CMBD.S21825
27. Busetto L, Dicker D, Azran C, Batterham RL, Farpour-Lambert N, Fried M, et al. Practical recommendations of the obesity management task force of the European association for the study of obesity for the post-bariatric surgery medical management. Obes Facts. (2018) 10:597–632. doi: 10.1159/000481825
28. Patel PA, Wyrobek JA, Butwick AJ, Pivalizza EG, Hare GMT, Mazer CD, et al. Update on applications and limitations of perioperative tranexamic acid. Anesth Analg. (2022) 135(3):460–73. doi: 10.1213/ANE.0000000000006039
29. Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. (2013) 17(10):1–79. Available at: https://www.journalslibrary.nihr.ac.uk/hta/hta17100/ doi: 10.3310/hta17100.
30. Roberts I, Shakur-Still H, Aeron-Thomas A, Beaumont D, Belli A, Brenner A, et al. Tranexamic acid to reduce head injury death in people with traumatic brain injury: the CRASH-3 international RCT. Health Technol Assess (Rockv). (2021) 25(26):1–76. doi: 10.3310/hta25260
31. Shakur H, Roberts I, Fawole B, Chaudhri R, El-Sheikh M, Akintan A, et al. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. (2017) 389(10084):2105–16. doi: 10.1016/S0140-6736(17)30638-4
32. Lech P, Michalik M, Waczyński K, Osowiecka K, Dowgiałło-Gornowicz N. Effectiveness of prophylactic doses of tranexamic acid in reducing hemorrhagic events in sleeve gastrectomy. Langenbecks Arch Surg. (2022) 407(7):2733–7. doi: 10.1007/s00423-022-02630-5
33. Chakravartty S, Sarma DR, Chang A, Patel AG. Staple line bleeding in sleeve gastrectomy—a simple and cost-effective solution. Obes Surg. (2016) 26(7):1422–8. doi: 10.1007/s11695-015-1986-y
34. Brito Rd, Oliveira Cd, Moura ECR, Campelo GP, Lima RC, Fe CdM, et al. Tranexamic acid effects in postoperative bleeding outcomes in laparoscopic sleeve gastrectomy: a controlled study. Acta Cir Bras. (2022) 37(7):e370702. doi: 10.1590/acb370702
35. Ker K, Prieto-Merino D, Roberts I. Systematic review, meta-analysis and meta-regression of the effect of tranexamic acid on surgical blood loss. Br J Surg. (2013) 100:1271–79. doi: 10.1002/bjs.9193
36. Wittgrove AC, Clark GW. Laparoscopic gastric bypass, roux en-Y-500 patients: technique and results, with 3-60 month follow-up. Obes Surg. (2000) 10(3):233–39. doi: 10.1381/096089200321643511
37. Sundbom M. Laparoscopic revolution in bariatric surgery. World J Gastroenterol. (2014) 20(41):15135–43. doi: 10.3748/wjg.v20.i41.15135
38. Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA. (2016) 316:2025–35. doi: 10.1001/jama.2016.9185
39. Mazer CD, Whitlock RP, Fergusson DA, Hall J, Belley-Cote E, Connolly K, et al. Restrictive or liberal red-cell transfusion for cardiac surgery. N Engl J Med. (2017) 377(22):2133–44. doi: 10.1056/NEJMoa1711818
40. Carson JL, Sieber F, Cook DR, Hoover DR, Noveck H, Chaitman BR, et al. Liberal versus restrictive blood transfusion strategy: 3-year survival and cause of death results from the FOCUS randomised controlled trial. Lancet. (2015) 385(9974):1183–9. doi: 10.1016/S0140-6736(14)62286-8
41. Carson JL, Stanworth SJ, Dennis JA, Trivella M, Roubinian N, Fergusson DA, et al. Transfusion thresholds for guiding red blood cell transfusion. Cochrane Database Syst Rev. (2021) 12(12):CD002042. doi: 10.1002/14651858.CD002042.pub5
42. Cooper HA, Rao Sv, Greenberg MD, Rumsey MP, McKenzie M, Alcorn KW, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT randomized pilot study). Am J Cardiol. (2011) 108(8):1108–11. doi: 10.1016/j.amjcard.2011.06.014
43. Fan YX, Liu FF, Jia M, Yang JJ, Shen JC, Zhu GM, et al. Comparison of restrictive and liberal transfusion strategy on postoperative delirium in aged patients following total hip replacement: a preliminary study. Arch Gerontol Geriatr. (2014) 59(1):181–5. doi: 10.1016/j.archger.2014.03.009
44. Foss NB, Kristensen MT, Jensen PS, Palm H, Krasheninnikoff M, Kehlet H. The effects of liberal versus restrictive transfusion thresholds on ambulation after hip fracture surgery. Transfusion (Paris). (2009) 49(2):227–34. doi: 10.1111/j.1537-2995.2008.01967.x
45. Gregersen M, Borris C, Damsgaard Marie E. Postoperative blood transfusion strategy in frail, anemic elderly patients with hip fracture: the TRIFE randomized controlled trial. Acta Orthop. (2015) 86(3):363–72. doi: 10.3109/17453674.2015.1006980
46. Gregersen M, Borris LC, Damsgaard EM. Blood transfusion and overall quality of life after hip fracture in frail elderly patients-the transfusion requirements in frail elderly randomized controlled trial. J Am Med Dir Assoc. (2015) 16(9):762–66. doi: 10.1016/j.jamda.2015.03.022
47. Grover M, Talwalkar S, Casbard A, Boralessa H, Contreras M, Boralessa H, et al. Silent myocardial ischaemia and haemoglobin concentration: a randomized controlled trial of transfusion strategy in lower limb arthroplasty. Vox Sang. (2006) 90(2):105–12. doi: 10.1111/j.1423-0410.2006.00730.x
48. Hajjar LA, Vincent JL, Galas FRBG, Nakamura RE, Silva CMP, Santos MH, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. (2010) 304(14):1559–67. doi: 10.1001/jama.2010.1446
49. Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion requirements in critical care investigators, Canadian critical care trials group. N Engl J Med. (1999) 340(6):409–17. doi: 10.1056/NEJM199902113400601
50. Shander A, Hardy JF, Ozawa S, Farmer SL, Hofmann A, Frank SM, et al. A global definition of patient blood management. Anesth Analg. (2022) 135(3):476–88. doi: 10.1213/ANE.0000000000005873
51. Mueller MM, van Remoortel H, Meybohm P, Aranko K, Aubron C, Burger R, et al. Patient blood management: recommendations from the 2018 Frankfurt consensus conference. JAMA. (2019) 321(10):983–97. doi: 10.1001/jama.2019.0554
52. Camaschella C. Iron-Deficiency Anemia. N Engl J Med. (2015) 372(19):1832–43. doi: 10.1056/NEJMra1401038
53. Pasricha SR, Tye-Din J, Muckenthaler MU, Swinkels DW. Iron deficiency. Lancet. (2021) 397:233–48 doi: 10.1016/S0140-6736(20)32594-0
54. Love AL, Billett HH. Obesity, bariatric surgery, and iron deficiency: true, true, true and related. Am J Hematol. (2008) 83:403–9. doi: 10.1002/ajh.21106
55. Stoffel NU, Cercamondi CI, Brittenham G, Zeder C, Geurts-Moespot AJ, Swinkels DW, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. (2017) 4(11):e524–33. doi: 10.1016/S2352-3026(17)30182-5
56. Steenackers N, van der Schueren B, Mertens A, Lannoo M, Grauwet T, Augustijns P, et al. Iron deficiency after bariatric surgery: what is the real problem? Proc Nutr Soc. (201877(4):445–55. doi: 10.1017/S0029665118000149
57. Auerbach M, Gafter-Gvili A, Macdougall IC. Intravenous iron: a framework for changing the management of iron deficiency. Lancet Haematol. (2020) 7:e342–50. doi: 10.1016/S2352-3026(19)30264-9
58. Koch TA, Myers J, Goodnough LT. Intravenous iron therapy in patients with iron deficiency Anemia: dosing considerations. Anemia. (2015) 2015:763576. doi: 10.1155/2015/763576
59. ABIM Foundation. Society for the Advancement of Patient Blood Management (2023). Available at: https://www.choosingwisely.org/societies/society-for-the-advancement-of-blood-management/.
60. Peters F, Ellermann I, Steinbicker AU. Intravenous iron for treatment of Anemia in the 3 perisurgical phases: a review and analysis of the current literature. Anesth Analg. (2018) 126:1268–82. doi: 10.1213/ANE.0000000000002591
61. Ganzoni AM. Eisen-Dextran intravenös: therapeutische und experimentelle möglichkeiten. Schweiz Med Wochenschr. (1970) 100(7):301–3. 5413918.
62. Liu HM, Tang Xs, Yu H, Yu H. The efficacy of intravenous iron for treatment of anemia before cardiac surgery: an updated systematic review and meta-analysis with trial sequential analysis. J Cardiothorac Surg. (2023) 18(1):16. doi: 10.1186/s13019-023-02119-2
Keywords: anemia, bariatric surgery, obesity, patient blood management, weight loss
Citation: Duarte GC, Wei W and Cho G (2023) A patient blood management perspective on Anemia in the obese patient journey. Front. Anesthesiol. 2:1172018. doi: 10.3389/fanes.2023.1172018
Received: 22 February 2023; Accepted: 17 March 2023;
Published: 30 March 2023.
Edited by:
Bruno Benites, University of Campinas (UNICAMP), BrazilReviewed by:
Uzung Yoon, Jefferson University Hospitals, United States© 2023 Duarte, Wei and Cho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gustavo de Carvalho Duarte Z3VzdGF2by5kdWFydGVAbnpibG9vZC5jby5ueg==
Specialty Section: This article was submitted to Perioperative Medicine, a section of the journal Frontiers in Anesthesiology
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.