- 1Department of Anesthesia, General Intensive Care Medicine and Pain Medicine, Medical University of Vienna, Vienna, Austria
- 2Outcome Research Consortium, Cleveland, OH, United States
- 3Center for Medical Statistics, Informatics and Intelligent Systems, Medical University of Vienna, Vienna, Austria
- 4IT Systems and Communications, Medical University of Vienna, Vienna, Austria
Background: In relatively healthy middle-aged patients, recent studies have shown that supplemental oxygen did not significantly increase one-year mortality after noncardiac surgery. If supplemental oxygen influences long-term mortality, specifically in elderly patients with cardiovascular risk-factors, remains unknown. Thus, we evaluated the effect of supplemental oxygen on two-year mortality in patients with cardiovascular risk factors undergoing moderate- to high-risk major abdominal surgery.
Methods: This is a follow-up study of a prospective, randomized, double-blinded, clinical trial. Two hundred fifty-eight patients, who were at least 45 years of age and at-risk for cardiovascular complications were randomly assigned to receive 80 vs. 30% oxygen during surgery and for the first two postoperative hours. Vital status was obtained from all patients 2 years after surgery using the national registry. Preoperative and postoperative maximum concentrations of NT-proBNP, Troponin T (TnT), Copeptin, von Willebrand Factor (vWF), static oxidation-reduction potential (sORP) and oxidation-reduction potential capacity (cORP) were tested for association with two-year mortality.
Results: The median age of patients was 74 years (25th-75th percentile 70–78 years). 25.8% (95% CI: 17.3–32.4%) of patients in the 80% oxygen group and 22.3% (95% CI: 14.8–29.1%) in the 30% oxygen group died within 2 years after surgery. No significant difference in two-year mortality was found between patients, who received 80% oxygen concentration, versus patients, who received 30% oxygen concentration (estimated hazard ratio 1.145; 95% CI 0.693–1.893; p = 0.597). Preoperative Copeptin concentrations and postoperative maximum vWF activity were significantly associated with two-year mortality (p < 0.001).
Conclusion: Our results are consistent with previous studies, that showed that supplemental oxygen did not increase long-term mortality. Therefore, it is becoming more evident that supplemental oxygen may not have a significant effect on long-term outcome in patients undergoing major abdominal surgery.
Introduction
Based on the most recent and largest trials it is becoming more evident that supplemental oxygen may not affect postoperative outcome including surgical site infection, pulmonary or cardiovascular complications [1–4]. Several long-term follow-up studies of these randomized controlled trials, evaluating the influence of supplemental oxygen on long-term mortality, have been performed recently but showed inconsistent results [5–7].
The follow-up analysis of the “Effect of High Perioperative Oxygen Fraction on Surgical Site Infection and Pulmonary Complications” (PROXI trial), for example, showed that 80% oxygen during surgery was associated with a significantly increased long-term mortality as compared to 30% oxygen [5]. Interestingly, they found that this effect was present only in patients undergoing cancer surgery but not in patients undergoing noncancer surgery [5]. They further showed that cancer-free survival was significantly shorter in the 80% group leading to the assumption that hyperoxia induces neovascularization [8]. In contrast, the follow-up study by Podolyak et al. found no significant effect of supplemental oxygen on long-term mortality in the overall population, which was also irrespective of a previous cancer diagnosis [6]. The long-term follow-up analysis of the most recent and largest trial, which included over 3,400 patients, also found no significant effect of supplemental oxygen on long-term mortality in the overall population, and also not in cancer and in non-cancer patients [7]. However, all these studies have in common that they included relatively young/middle-aged and healthy patients, who were at low risk for cardiovascular complications [5–7]. In contrast, elderly patients are at increased risk for long-term postoperative complications and have increased postoperative mortality after major abdominal surgery [9], and therefore, a possible disadvantageous effect of supplemental oxygen might be more likely in this patient population. In detail, animal studies showed that hyperoxia might lead to coronary vasoconstriction [10, 11], which might lead to impaired organ perfusion, and subsequently pronounced postoperative long-term complications in cardiac risk patients. Furthermore, an in-vitro study has shown that hyperoxia leads to an increase in oxidative stress [12]. Similar results were found in patients, who had a myocardial infarction and received supplemental oxygen. In fact, the authors found a larger infarct size in patients, who received supplemental oxygen, as compared to patients, who received ambient air [13]. However, the effect of perioperative supplemental oxygen on long-term mortality, specifically in elderly patients with cardiovascular complications, who had major abdominal surgery is still not entirely clarified.
Therefore, we evaluated in this follow-up study of a prospective randomized clinical trial, which investigated the effect of perioperative 80 vs. 30% oxygen on postoperative maximum NT-proBNP concentrations, the effect of the perioperative administration of supplemental oxygen on two-year all-cause mortality in patients at-risk for cardiovascular complications undergoing moderate- to high-risk major abdominal surgery. Furthermore, we evaluated the association between our study specific laboratory biomarkers including NT-proBNP, Troponin T, Copeptin, oxidation-reduction potential and vWF and two-year mortality.
Materials and methods
Study design
This is a follow-up study of a prospective, randomized, double-blinded, single-center clinical trial conducted at the Medical University of Vienna, which investigated the effect of supplemental perioperative oxygen on postoperative maximum NT-proBNP concentrations [3]. In detail, patients of at least 45 years of age with at least one cardiovascular risk factor undergoing moderate- to high-risk major abdominal surgery were randomized randomly to receive 80 vs. 30% oxygen for the duration of surgery and the first two postoperative hours [14]. The original trial was approved by the Ethics Committee of the Medical University of Vienna (Ethikkommission Medizinische Universität Wien; Borschkegasse 8b/6, 1090, Vienna, Austria; EK-Number 1744/2017) on the 13th of November 2017. Written informed consent was obtained of all patients participating in the study prior to randomization for the original study. This follow-up study was approved on the 1st of June 2022 by the same Ethics Committee with a waived requirement for informed consent (EK-Number 1213/2021). The original trial was registered before enrolment of the first patient at ClinicalTrials.gov (NCT03366857; https://clinicaltrials.gov/ct2/show/NCT03366857) and the European Trial Database (EudraCT 2017-003714-68) and was conducted according to the Declaration of Helsinki and Good Clinical Practice. This manuscript adheres to the applicable CONSORT guidelines. The results of the primary outcome and several sub-analyses from the original trial have been published previously [3, 15–17].
Participants
Eligible patients were over 45 years of age and underwent moderate- to high-risk major abdominal surgery under general anesthesia. Patients had to meet one of the following criteria for inclusion: (1) History of coronary artery disease, (2) History of peripheral artery disease, (3) History of stroke or (4) Any three of the following six criteria: (a) age over 70 years, (b) undergoing major surgery, (c) history of congestive heart failure, (d) history of transient ischemic attack, (e) diabetes and currently taking oral hypoglycemic agent or insulin, (f) history of hypertension. Patients were randomized to receive either 80 or 30% inspired oxygen concentration for the duration of surgery and the first two postoperative hours. The protocol for anesthesiologic and hemodynamic management was published previously [14]. Vital status and cause of death was identified for all patients using the database of the Medical University of Vienna (Sterbedatenabgleich Medizinische Universität Wien), which is based on the national registry, on the 15th of March 2022. Our study specific biomarkers of the original trial included NT-proBNP, Troponin T (TnT), Copeptin, von Willebrand Factor (vWF), static oxidation-reduction potential (sORP) and oxidation-reduction potential capacity (cORP) and were assessed shortly before induction of anesthesia, within 2 h after surgery and on the first and third postoperative days [14]. Copeptin was measured for a smaller number of patients, because it was amended after the inclusion of 87 patients. All preoperative and postoperative laboratory measurements were included in this follow-up study.
Statistical analysis
Continuous variables were summarized using mean, standard deviation (SD), median, quartiles [25th percentile; 75th percentile] as well as minimum and maximum values. Descriptive statistics are given for both groups separately. Categorical variables were summarized using absolute and percent values. To evaluate associations of perioperative oxygen concentration, preoperative baseline variables including comorbidities, ASA physical status, as well as preoperative and postoperative maximum cardiovascular and stress biomarkers and MINS on two-year mortality in the overall study population, we first performed univariable Cox proportional hazard models. Factors being significant in the univariable models (p < 0.05) were further analyzed in multivariable Cox proportional hazard models with backward model selection. Kaplan-Meier estimates were calculated for both study groups as well as for patients with ASA physical status I or II vs. III or IV. For all patients, two-year follow-up data was available. All analyses were performed using R version 4.1.1.
Results
260 patients were included from December 2017 to December 2019. Two patients in the 80% oxygen group were excluded after randomization because surgery was postponed. The analysis included all patients surviving at least 3 days after surgery such that the maximum/minimum of laboratory parameters over the first 3 days could be calculated. One patient in the 80% oxygen group died on the third postoperative day and was therefore excluded from the dataset. Therefore, 257 patients were included in this follow-up analysis. Baseline characteristics and demographic data were balanced between both groups (Table 1). Although the inclusion criteria was > 45 years of age, our actual median age of patients included was 74 years (25th percentile−75th percentile 70–78 years). Thirty-two patients were between 45 and 64 years (median age 60 years [55; 63]), 209 patients were between 65 and 85 years of age (median age 74 years [71; 77]) and 15 patients were over 85 years (median age 86 years [85; 87]).
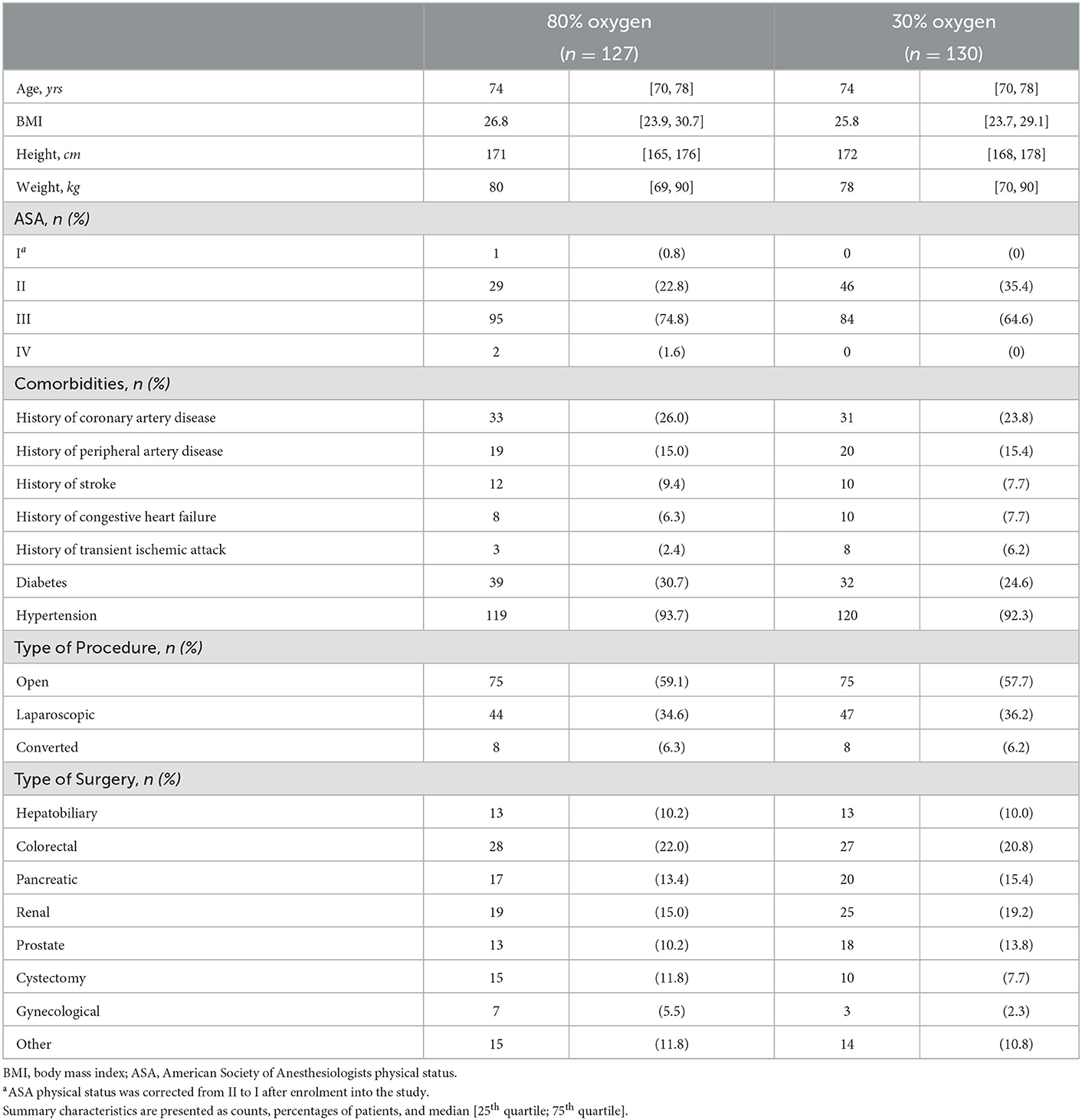
Overall, we observed a two-year mortality of 23.7% (95% confidence interval (CI): 18.4% to 28.8%) in our study population. After 2 years 32 of 128 patients (25.8%; 95% CI: 17.3–32.4%) had died in the 80% oxygen group compared to 29 of 130 patients (22.3%; 95% CI: 14.8–29.1%) in the 30% oxygen group. There was no significant difference in two-year mortality between patients, who received 80% oxygen concentration, vs. patients, who received 30% oxygen concentration (estimated hazard ratio 1.145; 95% CI 0.693–1.893; p = 0.597) (Figure 1). Detailed descriptive statistics for continuous and categorical variables, as well as separately for patients surviving or dying within two-year of follow up are presented in Supplementary Table 1. Detailed results on survival probabilities within 2 years for the overall population are presented in Table 2a, are presented separately for the 80 and 30% oxygen groups in Table 2b, and are presented separately for patients with ASA physical status I or II vs. III or IV in Table 2c.
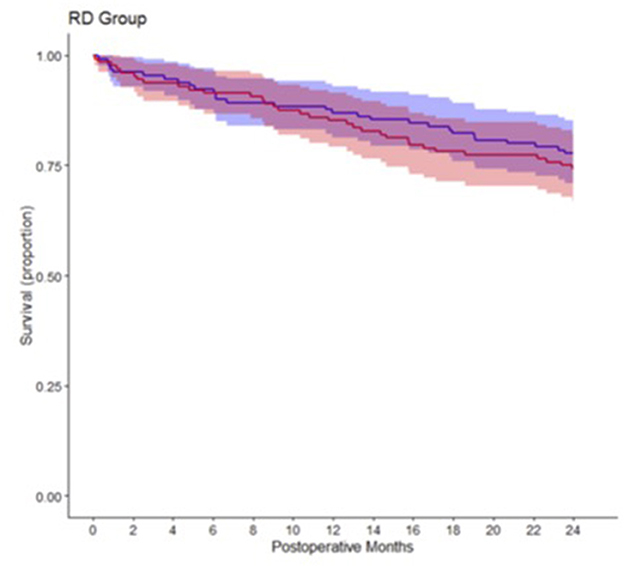
Figure 1. Kaplan-Meier survival estimate curves comparing patients, who received 80% intraoperative oxygen (red) and patients, who received 30% intraoperative oxygen (blue).
Table 2. Detailed survival for 6, 12, 18, and 24 months after surgery for (a) All patients, (b) Separately for both study groups and (c) Separately for patients with ASA physical status I or II vs. III or IV.
Fifty patients (82%) died because of cancer-related complications, 5 patients (8%) died because of cardiovascular events and 4 patients (7%) died because of unspecified events. In two patients the cause of death was unknown. Entity of complications was not specified.
Univariable analysis
In the univariable analysis only ASA physical status III and IV (p = 0.045) and age (p = 0.002) were significantly associated with increased two-year mortality (Table 2). Figure 2 shows Kaplan-Meier estimates separately for patients with ASA I or II vs. III or IV physical status. Of the assessed laboratory biomarkers only preoperative vWF activity (p < 0.001), sORP and cORP values (p = 0.004; p = 0.010), Copeptin concentrations (p < 0.001) and postoperative maximum vWF activity and minimum cORP values were significantly associated with two-year mortality in the univariable analysis (Table 3).
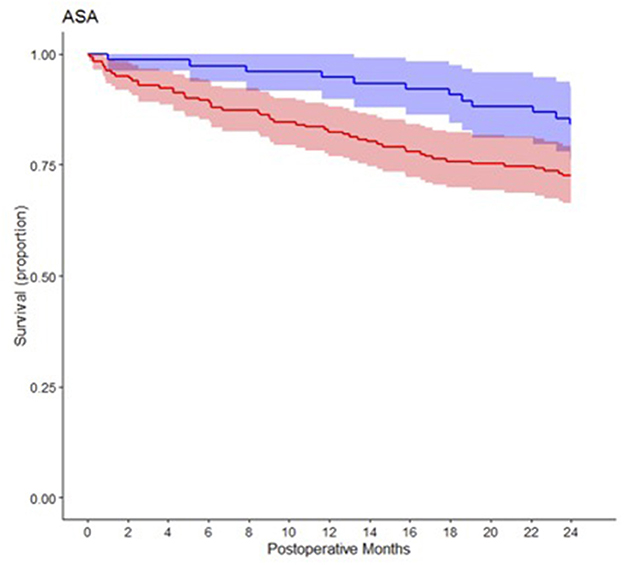
Figure 2. Kaplan-Meier survival estimate curves comparing patients with ASA status 1 or 2 (blue) and patients with ASA status 3 and 4 (red).
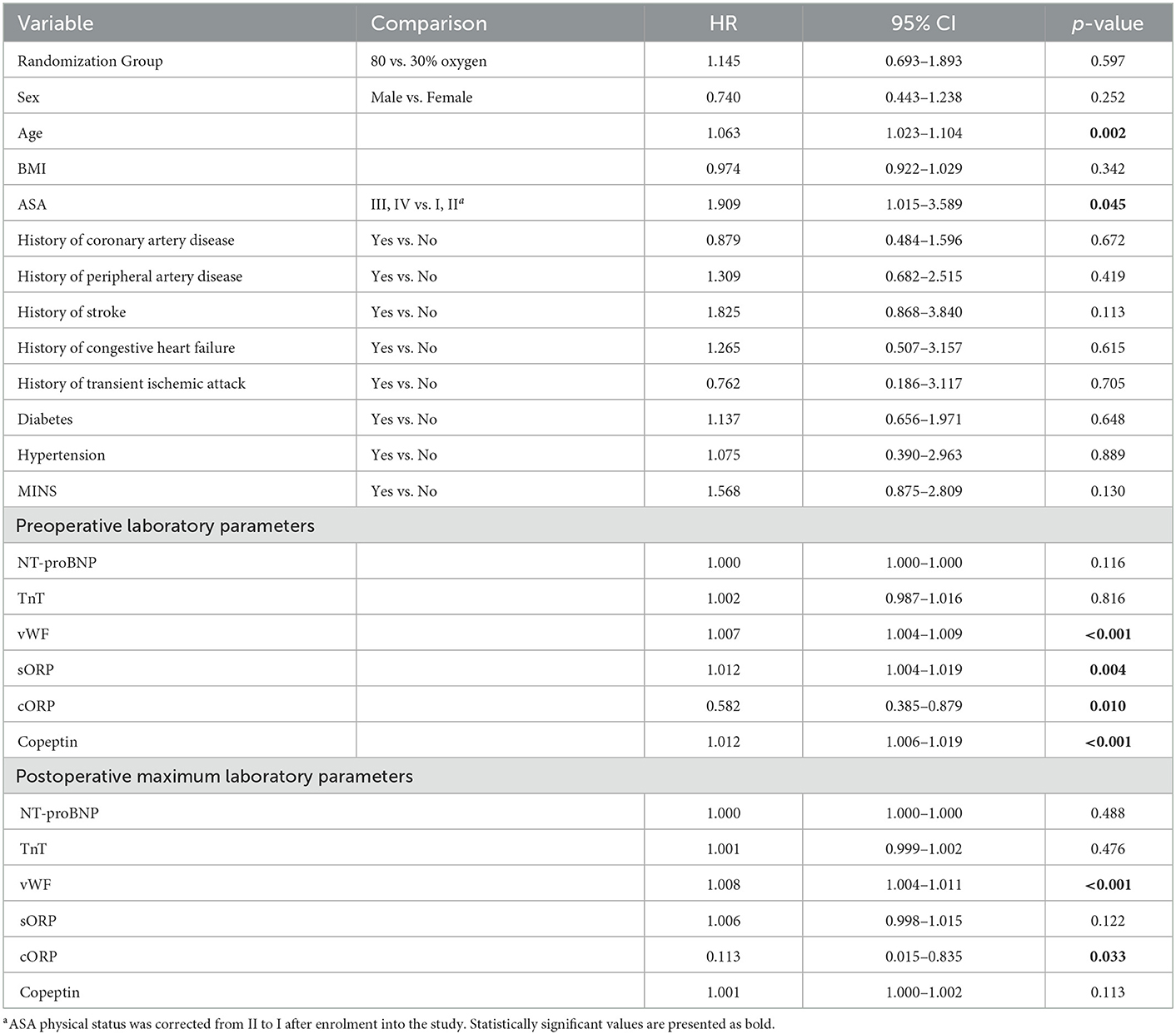
Table 3. Univariable Cox regression model for baseline characteristics and preoperative laboratory parameters and two-year mortality (HR, hazard ratio; CL, confidence limit).
Multivariable analysis
Since preoperative Copeptin concentrations were missing in the first 87 patients, we calculated two multivariable models with backward model selection. In detail, one only included age, ASA physical status, preoperative vWF activity, sORP and cORP values as well as postoperative maximum vWF-values and a second one additionally included preoperative Copeptin concentrations leading to a reduction of the sample size due to the missing values of Copeptin. In the multivariable model including Copeptin, only age (p = 0.005), postoperative maximum vWF activity (p < 0.001) and preoperative Copeptin concentrations (p < 0.001) were significantly associated with increased two-year mortality (Supplementary Table 2b). In the multivariable analysis including all patients, only age (p = 0.013) and preoperative vWF activity (p < 0.001) were significantly associated with increased two-year mortality (Supplementary Table 2a).
Discussion
We observed in this follow-up analysis no significant effect of perioperative administration of 80 vs. 30% oxygen concentration on two-year mortality in patients at-risk for cardiovascular complications undergoing moderate- to high-risk abdominal surgery. There was a significant association between preoperative Copeptin concentrations and vWF activity and 2-year mortality.
In contrast to the follow-up analysis of the PROXI trial, but consistent with all other follow-up studies, we found no significant influence of perioperative hyperoxia on long-term mortality even in our older and sicker patient population [5–7]. The results of the PROXI follow-up analysis showed a significantly higher mortality rate in patients undergoing cancer surgery, and a significantly shorter cancer-free survival rate in the 80% oxygen group [5, 8]. The authors hypothesized that perioperative hyperoxia might lead to increased neovascularization of the remaining tumor tissue, which might affect long-term mortality [4, 5]. In contrast, all other randomized trials showed that a FiO2 of 0.8 has no harmful effect on postoperative complications including cardiovascular events, surgical site infection, and pulmonary complications [1–3, 18]. A large study in the emergency setting tested the effect of supplemental oxygen in patients, who had myocardial infarction on one-year all-cause mortality [19]. They randomized 6,629 patients to 6 liters oxygen per minute for 6–12 h within the acute event vs. ambient air [19]. They also observed no significant difference in one-year mortality between both groups [19]. Thus, it seems reasonable that oxygen does not influence long-term mortality in the perioperative setting as well, which was described also in our study and in most other follow-up analyses [6, 7]. The underlying confounder for these contrasting results are still not entirely cleared.
Nevertheless, we observed a considerably higher overall mortality rate of 23% as compared to previous studies [5–7]. This might be because we mainly included mostly elderly patients with many cardiovascular comorbidities. In fact, the median age of our patient population was 74 years whereas patients in other trials were approximately 10 to 20 years younger [5–7]. Furthermore, over 90% of our patients had a history of hypertension requiring anti-hypertensive treatment, approximately 30% had diabetes and over 20% had coronary artery disease [3]. In previous trials the prevalence of hypertension ranged between 30 and 40%, the incidence of diabetes ranged between 7 and 14% and the incidence of coronary artery disease was not presented at all [1, 7]. Consequently, 70.5% of our patients were ASA III to IV, which was associated with higher mortality. Based on these facts it would have been obvious that our 23.7% mortality rate was driven by cardiovascular complications. Against our expectation that mortality cardiovascular related, 80% of our patients died of cancer-related events.
We also evaluated the association between preoperative and postoperative maximum NT-proBNP, Troponin T, Copeptin, vWF, sORP and cORP concentrations and two-year mortality. A previous observational study evaluated the association between NT-proBNP, Troponin T and Copeptin measured at hospital admission and two-year mortality in cancer patients [20]. Approximately 75% of these patients had planned surgery for treatment [20]. They showed that baseline elevated NT-proBNP, Troponin T and Copeptin levels was significantly associated with two-year mortality [20]. All our patients underwent surgery for cancer treatment. Interestingly, preoperative NT-proBNP and Troponin concentrations were not significantly associated with long-term mortality. Preoperative Copeptin, vWF, sORP, and cORP were significantly associated with long-term mortality in the univariable analyses. In fact, our association between preoperative Copeptin and two-year mortality was highly significant in our study group. Copeptin is a relatively novel marker for pathophysiological and psychological stress. Thus, it seems reasonable that preoperative Copeptin are more likely to be elevated in patients with chronic stress [21, 22]. It is evident, that stress is a potential trigger for the development of several diseases including cardiovascular diseases as well as the development and progression of cancer [23, 24]. Interestingly, it has been shown previously that Copeptin concentrations in ICU patients were significantly associated with 30-day mortality [25]. Therefore, it seems likely that stress represented by elevated Copeptin concentrations is also associated with increased long-term mortality after major noncardiac surgery. Nevertheless, this still must be verified in future large outcome trials.
In contrast, vWF activity is a more specific marker to predict cardiovascular events. In the non-surgical setting for example it has been shown that elevated vWF activity is strong predictor for development of atherosclerosis, as well as coronary heart disease and stroke in cardiac-risk patients [26–30]. Due to our small number of cardiovascular events we did not evaluate the association between elevated vWF and postoperative cardiovascular complications. However, we observed that preoperative and postoperative vWF activity was significantly associated with long-term mortality. This could be explained by the fact that vWF activity is significantly increased in patients with cancer and cancer progression and metastasis is associated with increases in vWF activity [30]. To completely understand the association between vWF and long-term mortality, more detailed research is needed, to fully understand the complex patho-mechanisms.
Our study has some limitations, which must be considered. First, this is a follow-up analysis of 260 patients from a study, originally planned for another outcome. Thus, our study is probably underpowered to detect an effect of supplemental oxygen on mortality. Nevertheless, based on most recent and largest studies it seems very unlikely that perioperative hyperoxia increases long-term mortality [6, 7]. Furthermore, due to our inclusion criteria, we were able to evaluate the effect of hyperoxia in a homogenous patient population of mostly elderly patients with cardiovascular comorbidities. Thus, our results may be extrapolated for these specific patients.
Secondly, our time point of follow-up was 2 years after surgery, which is similar to the follow-up analysis by Meyhoff et al. [5] but earlier than other previous follow-up analyses [6, 7]. However, Jiang et al. showed that a later time point of follow-up did not lead to a significant effect of supplemental oxygen on long-term mortality in their patient population [7]. Nevertheless, it cannot be excluded that in elderly and sicker patients, supplemental oxygen might significantly affect long-term mortality after 2 years.
Third, our follow-up data was obtained from a national registry. Therefore, for example data regarding rehospitalizations, reoperations, severe cardiovascular events or further cancer-related treatments were not available and can therefore not be presented. Furthermore, the actual cause of death in our study population was not available by using the data of the national registry. Therefore, we were not able to evaluate the association between our biomarkers and the specific causes of death in this study.
In summary, we did not observe a significant difference in two-year all-cause mortality between the 80 and 30% oxygen groups, which is consistent with most follow-up analyses. We evaluated that even in mostly elderly patients with cardiovascular risk factors the administration of supplemental oxygen seems not to have a significant effect on mortality. Based on previous studies and our results, there is evidence that supplemental oxygen during surgery exerts no long-term harmful effects, irrespective of the preoperative prevalence of comorbidities.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Medical University of Vienna. The patients/participants provided their written informed consent to participate in this study.
Author contributions
AT: data curation, investigation, methodology, validation, writing–original draft, and writing–review and editing. EF: conceptualization, formal analysis, investigation, project administration, supervision, visualization, validation, and writing–review and editing. BK: conceptualization, formal analysis, project administration, supervision, and writing–review and editing. MFa, NA, KH, TC, and DE: data curation and methodology. MFr: data curation, formal analysis, methodology, software, and validation. AG: data curation, formal analysis, methodology, validation, and writing–review and editing. CR: investigation, methodology, project administration, resources, supervision, visualization, and writing–review and editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanes.2023.1108921/full#supplementary-material
References
1. Meyhoff CS, Wetterslev J, Jorgensen LN, Henneberg SW, Høgdall C, Lundvall L, et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery. JAMA. (2009) 302:1543–50. doi: 10.1001/jama.2009.1452
2. Kurz A, Kopyeva T, Suliman I, Podolyak A, You J, Lewis B, et al. Supplemental oxygen and surgical-site infections: an alternating intervention controlled trial. Br J Anaesth. (2018) 120:117–26. doi: 10.1016/j.bja.2017.11.003
3. Reiterer C, Kabon B, Taschner A, Falkner von Sonnenburg M, Graf A, Adamowitsch N, et al. Perioperative supplemental oxygen and NT-proBNP concentrations after major abdominal surgery–A prospective randomized clinical trial. J Clin Anesth. (2021) 73:110379. doi: 10.1016/j.jclinane.2021.110379
4. Cohen B, Ruetzler K, Kurz A, Leung S, Rivas E, Ezell J, et al. Intra-operative high inspired oxygen fraction does not increase the risk of postoperative respiratory complications: alternating intervention clinical trial. Eur J Anaesthesiol. (2019) 36:320–6. doi: 10.1097/EJA.0000000000000980
5. Meyhoff CS, Jorgensen LN, Wetterslev J, Christensen KB, Rasmussen LS. Increased long-term mortality after a high perioperative inspiratory oxygen fraction during abdominal surgery: follow-up of a randomized clinical trial. Anesth Analg. (2012) 115:849–54. doi: 10.1213/ANE.0b013e3182652a51
6. Podolyak A, Sessler DI, Reiterer C, Fleischmann E, Akça O, Mascha EJ, et al. Perioperative supplemental oxygen does not worsen long-term mortality of colorectal surgery patients. Anesth Analg. (2016) 122:1907–11. doi: 10.1213/ANE.0000000000001316
7. Jiang Q, Kurz A, Zhang X, Liu L, Yang D, Sessler DI. Supplemental intraoperative oxygen and long-term mortality: subanalysis of a multiple crossover cluster trial. Anesthesiology. (2021) 134:709–21. doi: 10.1097/ALN.0000000000003694
8. Meyhoff CS, Jorgensen LN, Wetterslev J, Siersma VD, Rasmussen LS. Risk of new or recurrent cancer after a high perioperative inspiratory oxygen fraction during abdominal surgery. Br J Anaesth. (2014) 113 (Suppl 1):i74–81. doi: 10.1093/bja/aeu110
9. Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med. (2015) 373:2258–69. doi: 10.1056/NEJMra1502824
10. Mouren S, Souktani R, Beaussier M, Abdenour L, Arthaud M, Duvelleroy M, et al. Mechanisms of coronary vasoconstriction induced by high arterial oxygen tension. Am J Physiol. (1997) 272:H67–75. doi: 10.1152/ajpheart.1997.272.1.H67
11. Welsh DG, Jackson WF, Segal SS. Oxygen induces electromechanical coupling in arteriolar smooth muscle cells: a role for L-type Ca 2+ channels. Am J Physiol. (1998) 274:H2018–24. doi: 10.1152/ajpheart.1998.274.6.H2018
12. Hafner C, Wu J, Tiboldi A, Hess M, Mitulovic G, Kaun C, et al. Hyperoxia induces inflammation and cytotoxicity in human adult cardiac myocytes. Shock. (2017) 47:436–44. doi: 10.1097/SHK.0000000000000740
13. Reiterer C, Kabon B, von Sonnenburg MF, Starlinger P, Taschner A, Zotti O, et al. The effect of supplemental oxygen on perioperative brain natriuretic peptide concentration in cardiac risk patients–A protocol for a prosprective randomized clinical trial. Trials. (2020) 21:1–9. doi: 10.1186/s13063-020-04336-9
14. Reiterer C, Fleischmann E, Taschner A, Adamowitsch N, von Sonnenburg MF, Graf A, et al. Perioperative supplemental oxygen and oxidative stress in patients undergoing moderate- to high-risk major abdominal surgery–A subanalysis of randomized clinical trial. J Clin Anesth. (2022) 77:110614. doi: 10.1016/j.jclinane.2021.110614
15. Taschner A, Kabon B, von Sonnenburg MF, Graf A, Adamowitsch N, Fraunschiel M, et al. Perioperative supplemental oxygen and plasma catecholamine concentrations after major abdominal surgery–secondary analysis of a randomized clinical trial. J Clin Med. (2022) 11:1767. doi: 10.3390/jcm11071767
16. Taschner A, Kabon B, Graf A, Adamowitsch N, von Sonnenburg MF, Fraunschiel M, et al. Perioperative supplemental oxygen and postoperative copeptin concentrations in cardiac-risk patients undergoing major abdominal surgery–a secondary analysis of a randomized clinical trial. J Clin Med. (2022) 11:2085. doi: 10.3390/jcm11082085
17. Hopf HW, Holm J. Hyperoxia and infection. Best Pract Res Clin Anaesthesiol. (2008) 22:553–69. doi: 10.1016/j.bpa.2008.06.001
18. Hofmann R, James SK, Jernberg T, Lindahl B, Erlinge D, Witt N, et al. Oxygen therapy in suspected acute myocardial infarction. N Engl J Med. (2017) 377:1240–9. doi: 10.1056/NEJMoa1706222
19. Pavo N, Raderer M, Hülsmann M, Neuhold S, Adlbrecht C, Strunk G, et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart. (2015) 101:1874–80. doi: 10.1136/heartjnl-2015-307848
20. Katan M, Morgenthaler N, Widmer I, Puder JJ, König C, Müller B, et al. Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level. Neuro Endocrinol Lett. (2008) 29:341–6.
21. Siegenthaler J, Walti C, Urwyler SA, Schuetz P, Christ-Crain M. Copeptin concentrations during psychological stress: the PsyCo study. Eur J Endocrinol. (2014) 171:737–42. doi: 10.1530/EJE-14-0405
22. Dai S, Mo Y, Wang Y, Xiang B, Liao Q, Zhou M, et al. Chronic stress promotes cancer development. Front Oncol. (2020) 10:1492. doi: 10.3389/fonc.2020.01492
23. Kivimäki M, Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol. (2018) 15:215–29. doi: 10.1038/nrcardio.2017.189
24. Krychtiuk KA, Honeder MC, Lenz M, Maurer G, Wojta J, Heinz G, et al. Copeptin predicts mortality in critically ill patients. PLoS ONE. (2017) 12:e0170436. doi: 10.1371/journal.pone.0170436
25. Kovacevic KD, Mayer FJ, Jilma B, Buchtele N, Obermayer G, Binder CJ, et al. Von Willebrand factor antigen levels predict major adverse cardiovascular events in patients with carotid stenosis of the ICARAS study. Atherosclerosis. (2019) 290:31–6. doi: 10.1016/j.atherosclerosis.2019.09.003
26. Fan M, Wang X, Peng X, Feng S, Zhao J, Liao L, et al. Prognostic value of plasma von Willebrand factor levels in major adverse cardiovascular events : a systematic review and meta-analysis. BMC Cardiovasc Disord. (2020) 20:1–9. doi: 10.1186/s12872-020-01375-7
27. Whincup PH, Danesh J, Walker M, Lennon L, Thomson A, Appleby P, et al. Von Willebrand factor and coronary heart disease: prospective study and meta-analysis. Eur Heart J. (2002) 23:1764–70. doi: 10.1053/euhj.2001.3237
28. Páramo JA, Beloqui O, Colina I, Diez J, Orbe J. Independent association of von Willebrand factor with surrogate markers of atherosclerosis in middle-aged asymptomatic subjects. J Thromb Haemost. (2005) 3:662–4. doi: 10.1111/j.1538-7836.2005.01305.x
29. Bongers TN, de Maat MPM, van Goor MLPJ, Bhagwanbali V, van Vliet HHDM, García EBG, et al. High von Willebrand factor levels increase the risk of first ischemic stroke: influence of ADAMTS13, inflammation, and genetic variability. Stroke. (2006) 37:2672–7. doi: 10.1161/01.STR.0000244767.39962.f7
Keywords: supplemental oxygen, postoperative mortality, cardiac risk patients, perioperative hyperoxia, major abdominal surgery
Citation: Taschner A, Fleischmann E, Kabon B, Falkner von Sonnenburg M, Adamowitsch N, Horvath K, Christian T, Emler D, Fraunschiel M, Graf A and Reiterer C (2023) Supplemental oxygen did not significantly affect two-year mortality in patients at-risk for cardiovascular complications undergoing moderate- to high-risk abdominal surgery–A follow-up analysis of a prospective randomized clinical trial. Front. Anesthesiol. 2:1108921. doi: 10.3389/fanes.2023.1108921
Received: 26 November 2022; Accepted: 16 January 2023;
Published: 01 February 2023.
Edited by:
Ke Peng, The First Affiliated Hospital of Soochow University, ChinaReviewed by:
Christian Bohringer, UC Davis Medical Center, United StatesMichele Carron, University of Padua, Italy
Yusuke Mazda, Saitama Medical University, Japan
Copyright © 2023 Taschner, Fleischmann, Kabon, Falkner von Sonnenburg, Adamowitsch, Horvath, Christian, Emler, Fraunschiel, Graf and Reiterer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Edith Fleischmann,  ZWRpdGguZmxlaXNjaG1hbm5AbWVkdW5pd2llbi5hYy5hdA==
ZWRpdGguZmxlaXNjaG1hbm5AbWVkdW5pd2llbi5hYy5hdA==
 Alexander Taschner
Alexander Taschner Edith Fleischmann1,2*
Edith Fleischmann1,2* Christian Reiterer
Christian Reiterer