
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Anal. Sci., 07 March 2025
Sec. Environmental Analysis
Volume 5 - 2025 | https://doi.org/10.3389/frans.2025.1527655
The persistence of brominated flame retardants (BFRs) in the environment and the associated toxicological risks have made the development of efficient and rapid detection methods increasingly urgent. Despite regulatory mitigation action in many countries, BFRs such as polybrominated diphenyl ethers (PBDEs) and tetrabromobisphenol A (TBBPA) continue to threaten ecosystems due to their resistance to degradation. BFRs persist in air, water, soil, and sediments, and bioaccumulate in the food chain, leading to prolonged exposure risks for both humans and wildlife. Additionally, in regions with less stringent regulations, products containing BFRs are still being manufactured, posing a challenge for customs agencies responsible for regulating imports. This scenario underscores the urgent need for rapid, sensitive, and cost-effective methods to monitor BFRs in commercial products and environmental matrices. Biosensors present a promising solution, offering rapid detection and screening of BFR contamination at trace levels. Their ability to provide accurate, real-time data makes them invaluable for environmental monitoring, product safety, and regulatory compliance. This review explores the recent advancements in biosensor technology for BFR detection, highlighting their potential for improving environmental and human health protection but also underlining the specific areas that require further research.
Brominated flame retardants (BFRs) have been widely incorporated into commercial products for several decades to reduce fire-related hazards, playing a critical role in fire prevention. These compounds are extensively used as additives in a wide range of materials, such as plastics, textiles, electronics, foams, rubbers, and building components, to ensure compliance with fire safety standards (Alaee et al., 2003; Hou et al., 2021; Lan et al., 2023). One of the prevalent groups of BFRs is represented by polybrominated diphenyl ethers (PBDEs), consisting of 209 organobromine compounds, which were used in three major commercial mixtures: Penta-BDE, Octa-BDE, and Deca-BDE. The high global demand for Deca-BDE, which in 1999 reached 54,800 tons (Alaee et al., 2003) and accounted for nearly 80% of the PBDE market in 2001 (BSEF, 2003), underscores its extensive historical usage. Despite regulatory bans implemented years later, the persistence of PBDE in the environment remains a critical concern due to its long-term resistance to degradation and bioaccumulation potential. As a consequence of their intensive past use, PBDEs have been detected in a variety of environmental matrices, including air, water, soil, sediments, and biota, as well as in human tissues such as blood, breast milk, and the brain (Li et al., 2024; Stadion et al., 2024; Struzina et al., 2024). Human exposure occurs primarily through inhalation, dermal absorption, and ingestion of contaminated food (Linares et al., 2015), which has been shown to disrupt endocrine functions (e.g., thyroid hormone regulation) and adversely affect neurodevelopment and behavior (Wu et al., 2020). Moreover, due to their ability to undergo long-range atmospheric transport, PBDEs have been detected in remote regions such as the Arctic and Antarctic (e.g., Cincinelli et al., 2016; Palaniswamy et al., 2024), with consequent harmful effects on the ecosystems. In response to their environmental and health risks, regulatory actions have been implemented worldwide. The European Union (EU) classified PBDEs as persistent organic pollutants (POPs) under the Stockholm Convention. As a result, the use of Penta-BDE and Octa-BDE was banned in 2004, followed by restrictions on Deca-BDE in 2017, due to concerns about its degradation into lower brominated congeners (Ganci et al., 2019; Palaniswamy et al., 2024). Similarly, PBDEs have been regulated in the U.S., Australia, and Canada, where restrictions on certain PBDE congeners began in 2008 (NICNAS, 2007; Chevrier et al., 2010; Kim et al., 2016; Oulhote et al., 2018). In 2016, further amendments banned the manufacture, use, sale, and import of PBDE-containing substances, with some exceptions (Government of Canada, 2020).
While the use of PBDEs has declined over the last two decades, tetrabromobisphenol A (TBBPA), one of the most widely used BFRs, is still widely available on the market. TBBPA differs from PBDEs in that it forms covalent bonds with polymers, which theoretically reduces its tendency to leach into the environment (Lyche et al., 2015). Nevertheless, environmental contamination from TBBPA can still occur, particularly through improper disposal of electronic waste. TBBPA has been detected in various environmental compartments, including surface water, sediment, and soil, and has also been found in human tissues (Huang et al., 2020; Hou et al., 2021). Concerns regarding its potential health effects continue to grow, as TBBPA has been linked to endocrine disruption and possible carcinogenicity (Jin et al., 2021). Although this compound has long been used as a flame retardant, its persistence and toxicity in the environment make it a subject of ongoing studies and regulations.
Despite all the regulatory efforts, recent studies still highlight the persistent widespread distribution of legacy BFRs in the environment and the correlated long-term risk (Gomes et al., 2024). Furthermore, even in countries where strict regulations exist, products containing BFRs (particularly electronics and textiles) are still being imported from regions with less stringent limitations, raising concerns about the need for customs and regulatory authorities to have effective screening tools to control imports.
In addition, because of the restrictions on older generation BFRs, alternative compounds, including new brominated flame retardants (NBFRs), have been developed to meet fire safety requirements while reducing environmental and health impacts (Zuiderveen et al., 2020). These NBFRs, such as hexabromocyclododecane (HBCD) and bis(2-ethylhexyl) tetrabromophthalate (TBPH), are increasingly being used as replacements for legacy BFRs. However, many of these new compounds share similar concerns regarding persistence, bioaccumulation, and potential toxicity.
Despite their widespread use, the detection of BFRs and NBFRs in environmental and biological matrices remains challenging. Traditionally, their quantification has relied on sophisticated analytical techniques such as gas chromatography (GC) and liquid chromatography (LC) coupled with mass spectrometry (MS) to detect trace-level concentrations. Although these methods are highly accurate and sensitive they present significant limitations, including high costs, the need for skilled personnel, and labor-intensive sample preparation. Additionally, their reliance on extensive sample pretreatment and complex instrumentation limits their applicability in contexts that require rapid, real-time detection and sustainable environmental monitoring. These challenges have underscored the need for simpler and faster alternatives. In this context, biosensors have emerged as promising tools, offering simplicity, low cost, rapid response times, portability and in-field applications, while maintaining sufficient sensitivity and specificity for BFRs detection. Addressing the limitations of traditional methods, biosensors represent a key advance in achieving efficient and sustainable monitoring of BFRs. They are typically composed of selective recognition element—such as an enzyme, antibody, nucleic acid, whole-cell, or molecularly imprinted polymer (MIP)—that specifically interacts with the target analyte and generates a detectable signal. This approach has shown great potential for the quantification of PBDEs and TBBPA, but research into biosensors for NBFR detection remains limited.
Given the increasing environmental and toxicological concerns associated with legacy and new-generation BFRs and the need for high-sensitive large-scale screenings, the development of efficient, rapid, and cost-effective detection systems is crucial for advancing regulatory enforcement and protecting public health. This review aims to provide a comprehensive overview of recent advancements in biosensor technologies for the detection of BFRs, focusing on PBDEs and TBBPA. It examines the progress in improving biosensor sensitivity, selectivity, and real sample applications while highlighting future developments and research gaps that need to be filled for biosensing legacy and new-generation BFRs.
For more than 20 years, it has been known that BFRs are released through human activity posing health risks to humans and the environment. This justifies the increasing number of papers dealing with their presence, fate, and toxicity in the environment over the past decade, as shown in Figure 1. The still strong interest in the research and analysis of BFRs in various environmental compartments, including their bioaccumulation in humans, despite restrictions to which they are subjected, highlights the need to implement fast and simple methods for their detection, such as biosensors.
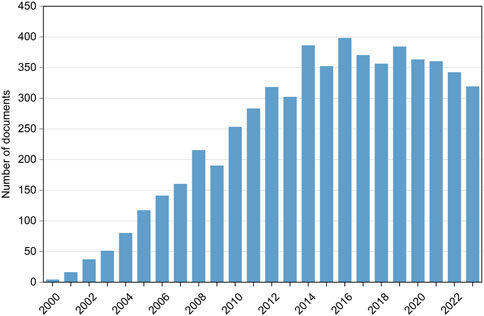
Figure 1. Number of documents per year studying BFRs and their presence in the environment from 2000 to 2023 (source: SCOPUS Database).
The following paragraphs outline the main characteristics and critical issues of typical pretreatments that environmental samples can undergo for BFR extraction and the commonly adopted analytical detection methods.
Sample preparation is often the most time-consuming and labor-intensive part of the analytical process, although essential for conventional chromatographic methods and, depending on the matrix, for biosensing techniques (Kelemen et al., 2019). For biosensor technologies, however, simpler and faster sample preparation methods are often required to preserve bioreceptor integrity and operational efficiency.
Detecting BFRs in environmental samples presents significant challenges due to their typically low concentrations and the complex nature of the matrices, which can interfere with instrumental analysis and biosensor performance. For this reason, pre-treatment steps, such as sample extraction and clean-up, are crucial to reduce matrix interferents, thereby enhancing sensitivity and selectivity. The low polarity of BFRs, such as PBDEs and TBBPA, requires the use of organic solvents for extraction. Commonly adopted extraction methods include Soxhlet extraction (SE) for solid samples and solid-phase extraction (SPE) for liquid samples, both of which provide high recovery rates (Dirtu et al., 2013). Regarding the extraction of liquid samples, liquid-liquid extraction (LLE) represents another established method, using solvents like ethyl acetate, hexane, isooctane, toluene, chloroform, and methylcyclohexane (Bredsdorff et al., 2023; Dimpe and Nomngongo, 2016). However, not all extraction and clean-up techniques are compatible with biosensing technologies, as many methods have been optimized primarily for chromatographic techniques. For instance, Soxhlet extraction (SE), liquid-liquid extraction (LLE), pressurized liquid extraction (PLE), and gel permeation chromatography (GPC) are widely used in GC-MS or LC-MS workflows, but they present several limitations when applied to biosensors (Vidal et al., 2023; Matamoros et al., 2012). These methods often require long extraction times, high temperatures, and large solvent volumes, which may degrade or inactivate biological recognition elements such as antibodies and enzymes. Additionally, derivatization steps commonly required in GC-MS and LC-MS workflows are incompatible with affinity-based biosensors, as they chemically modify the analytes, altering their recognition by the specific receptor. As a result, modern sample pre-treatment is shifting toward eco-friendly approaches characterized by lower solvent usage, automation, miniaturization, and simplicity (Berton et al., 2016), maintaining the integrity of the biorecognition elements. SPE, solid-phase microextraction (SPME), liquid-phase microextraction (LPME), ultrasound-assisted extraction (UAE), and QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) are among the most effective pre-treatment strategies for biosensors (Anastassiades et al., 2003; Liang et al., 2023).
For biosensor applications, SPE remains one of the most widely adopted methods, as it facilitates selective extraction while minimizing solvent usage and matrix effects (Vidal et al., 2023). Similarly, SPME and LPME provide miniaturized, solvent-free alternatives that can be easily integrated into sensor platforms (Liang et al., 2023) while offering high enrichment factors. These techniques simplify and accelerate sample preparation, although SPME faces limitations, including short fiber lifespan, fragility, high cost, and potential carry-over effects, particularly when used with complex matrices (Krylov et al., 2011; Kelemen et al., 2019).
UAE, which uses ultrasound radiation in a water bath or other devices to enhance extraction efficiency (Lestido-Cardama et al., 2022), is attractive due to the low solvent volumes and short extraction times but shows limitations in terms of selectivity and enrichment capacity (Kelemen et al., 2019). Additionally, the potential degradation of analytes during sonication poses a risk when applying UAE to organic compounds (Ridgway et al., 2007). Conversely, MAE employs microwave energy to efficiently extract the analytes while reducing environmental impact, allowing the simultaneous pre-treatment of multiple samples, and improving productivity (Bartolomé et al., 2005). QuEChERS combines both extraction and clean-up steps, streamlining sample preparation. Originally developed for pesticide analysis in food (Anastassiades et al., 2003), this technique has later been adapted for environmental contaminants such as BFRs (Tavoloni et al., 2020). UAE and QuEChERS, which enable rapid extraction with minimal solvent use, have demonstrated good compatibility with biosensors, making them attractive options for environmental sample pre-treatment (Tavoloni et al., 2020; Lestido-Cardama et al., 2022).
For biosensors, the primary concern in sample preparation is minimizing matrix effects while preserving the activity of biorecognition elements. Unlike traditional chromatographic methods, which often rely on extensive purification steps, biosensor-compatible extraction techniques should avoid harsh solvents and chemical modifications that could degrade biological receptors (Kelemen et al., 2019). Therefore, the use of milder sample preparation strategies that maintain analyte integrity and enhance detection sensitivity is crucial for successful biosensor applications.
While pre-treatment and sample preparation are essential for the accurate detection of BFRs, biosensor technologies require the adaptation of methods that avoid harsh solvents, chemical modifications, and excessive purification steps that could compromise sensor performance. The continued development of biosensor-compatible pre-treatment strategies will play a key role in expanding the applicability of these techniques for environmental monitoring.
Over the past decades, various analytical methods have been developed for the quantification of PBDEs, TBBPA, and related compounds in different environmental samples. Given their low vapor pressure and polarity, gas chromatography (GC) has emerged as the conventional analytical technique for their analysis in a variety of matrices, even if, several challenges, such as thermal degradation and isomeric interconversion, necessitate careful optimization of the injection technique and column type (Śmiełowska and Zabiegała, 2020). Common injection techniques include split/splitless, on-column, and programmable temperature vaporization (PTV) injections. Although split/splitless injection is widely used due to its ability to handle complex samples (typically injecting 1–3 µL) it poses the risk of thermal degradation for higher molecular weight compounds, such as BDE-209. To minimize this risk, on-column injection can be used, but an extensive sample clean-up is required to prevent co-extracted compounds and macromolecular residues from reaching the column, which could result in peak tailing, increased noise, retention shifts, and reduced column lifespan. More recently, PTV inlets have gained popularity as they minimize the degradation of high-molecular-weight thermolabile compounds and allow for larger injection volumes (up to 125 μL). Another critical step in GC method development is column selection. Short (10–15 m) nonpolar DB columns with thin stationary phases (0.1 μm) are typically preferred to minimize thermal degradation and isomerization of higher molecular weight PBDEs.
For BFR detection and quantification, mass spectrometry (MS), both high resolution (HR) and low resolution (LR), has been widely applied. LR-MS instruments can be operated in electron ionization (EI), negative chemical ionization (NCI), or atmospheric pressure ionization (API) modes. While EI offers better selectivity and structural confirmation of target PBDEs, it may cause excessive fragmentation. NCI, on the other hand, is more suitable for detecting higher brominated PBDEs, as it primarily monitors bromide ions (m/z 79 and 81), but this reduces selectivity and limits the use of 13C-labeled compounds as internal standards, except for BDE-209. API techniques, which provide softer ionization and thus reduce in-source fragmentation, have seen recent advancements with increasing applications (Ayala-Cabrera et al., 2021). Despite these developments, the most reliable spectrometric method for both qualitative and quantitative determination of BFRs in complex environmental samples remains high-resolution gas chromatography coupled with electron capture ionization mass spectrometry (GC-HRMS), whose use is often limited by the high cost of instrumentation, complicated sample pre-treatment procedures, long data processing times and the need for skilled and trained operators.
The selection of an analytical method for BFR detection requires a balance between cost, reproducibility, resolution, sensitivity, and analysis time, especially when large sample sets are involved. While environmental occurrence and health implications associated with these compounds are well documented, the development of biosensors targeting BFRs has not been widely explored in recent years (Huang et al., 2023; Wang et al., 2023). The limited number of recent biosensor studies on BFRs, in contrast to broader advancements in biosensor technology for other pollutants, highlights a lack in current research that deserves further investigation. To address this gap, the main results for PBDE and TBBPA biosensing are summarized and classified according to the type of transducer in Tables 1, 2, respectively, detailing the analyte, bioreceptor type, detection system, limit of detection (LOD), linear range, and applications in real samples, providing a comprehensive overview of the current state of research. The decision to address PBDEs and TBBPA separately was driven by their distinct environmental behaviors, toxicological profiles, and analytical challenges, as PBDEs encompass a diverse class of compounds with varying bromination levels, while TBBPA differs in its covalent bonding and contamination pathways, necessitating tailored biosensor strategies for each.
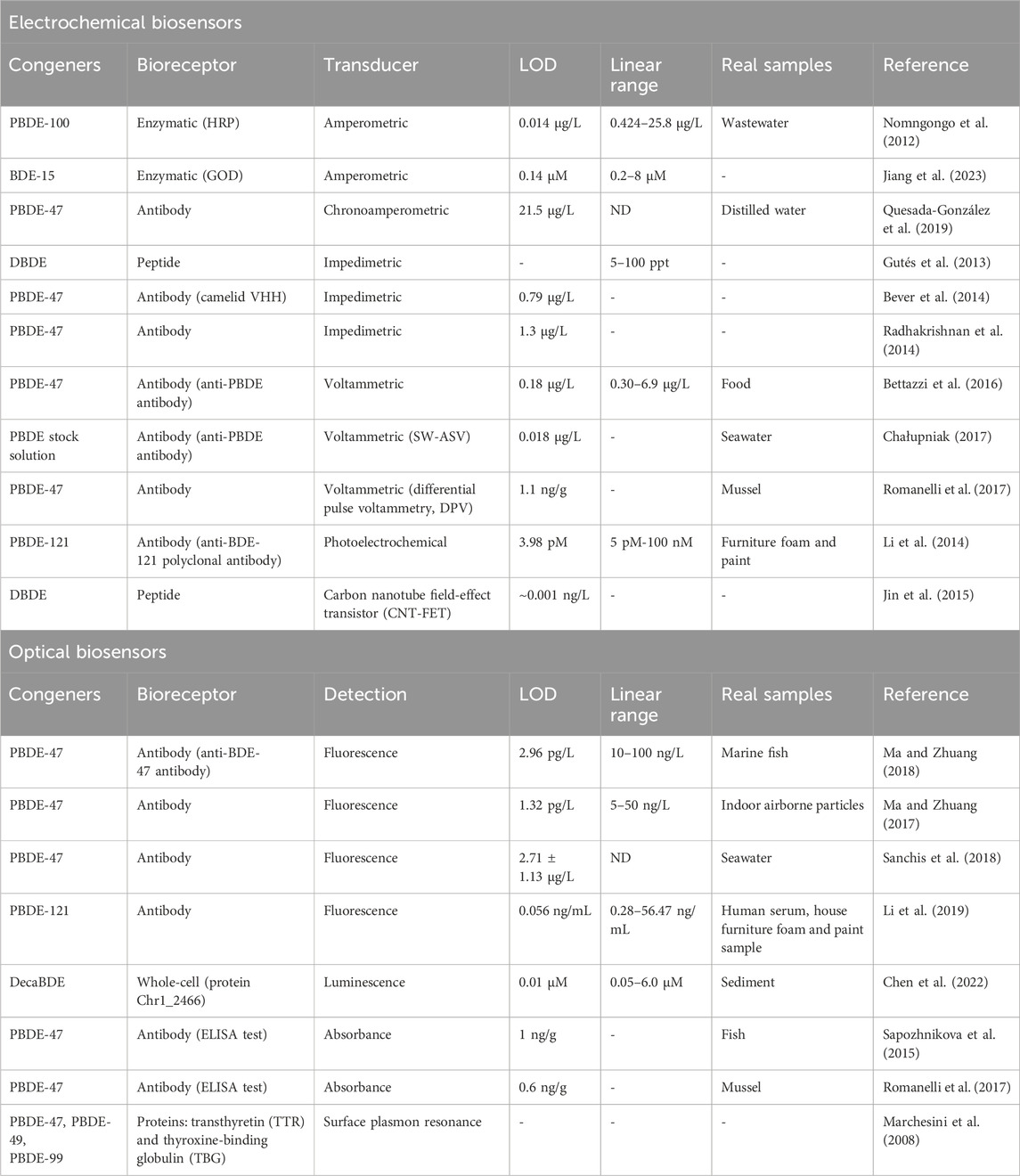
Table 1. Summary of various biosensors for PBDE determination, classified by transducer type. The respectively studied congeners, bioreceptor, detection limit, linear range and real samples on which they were tested are reported.
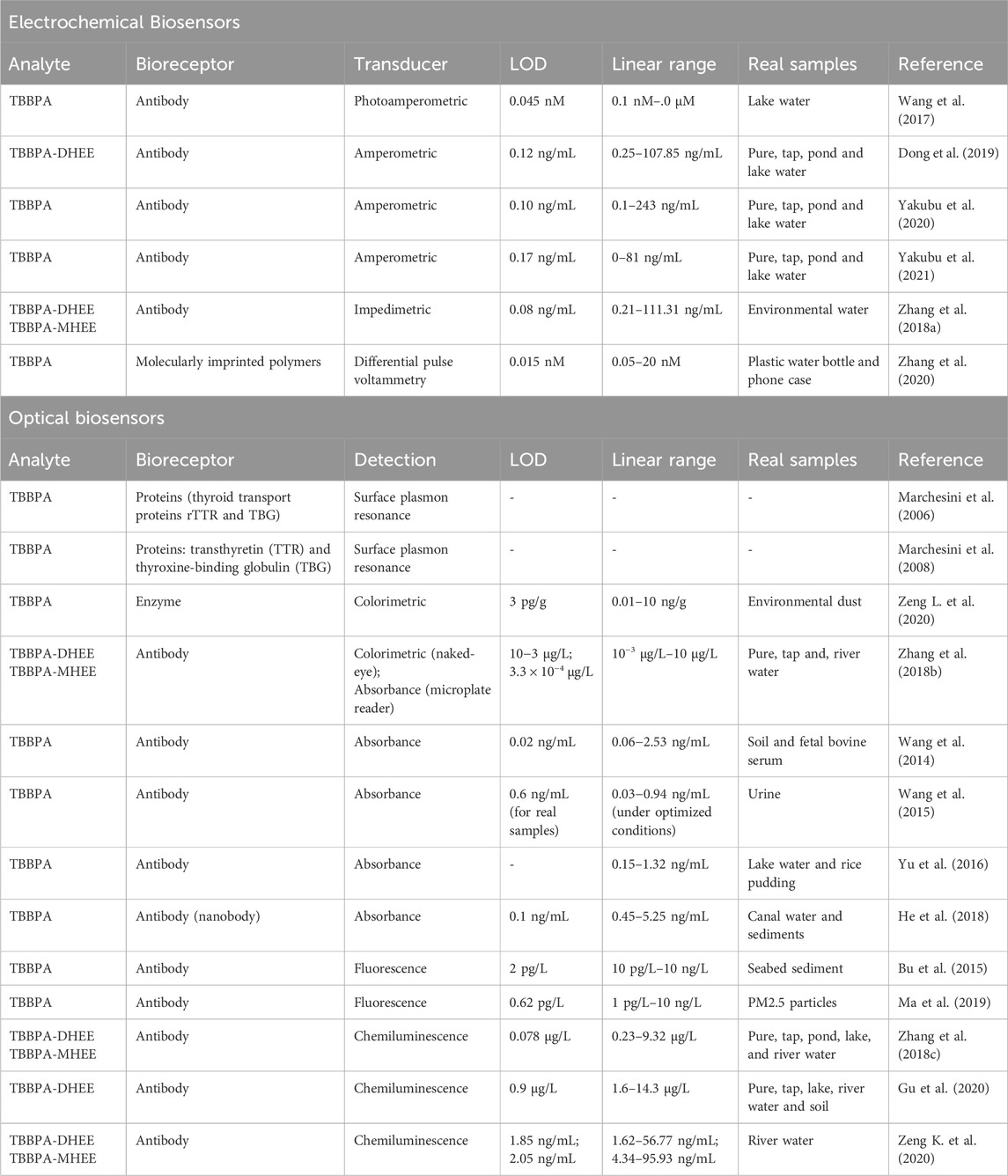
Table 2. Summary of various biosensors for TBBPA and related compound detection, categorized by transducer type. The respectively studied congeners, bioreceptor, detection limit, linear range and real samples on which they were tested are reported.
In this section, the most significant approaches reported in the literature for the electrochemical biosensing of PBDEs are discussed and subdivided based on the different detection techniques.
To the best of our knowledge, the first study reporting the quantification of PBDEs using biosensors in environmental matrices was published by Nomngongo et al. (2012). This study developed an amperometric biosensor for determining BDE-100, along with other classes of persistent organic pollutants such as polybrominated biphenyls (PBBs) and polychlorinated biphenyls (PCBs) in landfill leachates. The biosensor utilized horseradish peroxidase (HRP) enzyme, which was immobilized via electrostatic attachment onto a polyaniline-modified platinum electrode. For PBDE-100, the biosensor achieved a LOD of 0.014 μg/L and displayed a linear range between 0.424 and 25.8 μg/L. Inhibition studies on the HRP biosensor’s activity toward H₂O₂ reduction in the presence and absence of target analytes indicated a competitive inhibition mechanism for PBDEs, unlike for PCBs and PBBs. The biosensor was tested on real landfill leachate samples, where BDE-100 was not detected, but PCB congeners PCB-28 and PCB-101 were found at concentrations of 0.28 ± 0.03 μg/L and 0.31 ± 0.02 μg/L, respectively. To validate the sensor’s accuracy, GC-MS was used as a cross-check method, and the results from both techniques were in close agreement (Nomngongo et al., 2012).
More recently, Jiang et al. (2023) developed a highly sensitive amperometric biosensor based on the blocking effect on glucose oxidase (GOD). The biosensor employs a GOD∼/AuNPs#rGO-CHIT/GC electrode, where gold nanoparticles (AuNPs) and reduced graphene oxide (rGO) were used to modify the electrode. This device measures the inhibition effect of PBDEs like BDE-15 on glucose oxidase activity by monitoring the reduction in current, making it suitable for the quantification of low-brominated diphenyl ethers. The device displayed a linear range for BDE-15 detection between 0.2 and 8 μM, with a LOD of 0.14 μM.
Furthermore, for the detection of BFR in environmental matrices, biosensors have been developed that use chronoamperometry, an electroanalytical technique that measures the current flow through an electrode over time. For instance, Quesada-González et al. (2019) introduced a biosensor for PBDE quantification based on screen-printed carbon electrodes (SPCEs), enabling electrochemical monitoring of the water oxidation reaction (WOR) catalyzed by iridium oxide (IV) nanoparticles (IrO₂ NPs) (Figure 2). Using chronoamperometric measurements, the biosensor achieved a LOD of 21.5 ppb for BDE-47 in distilled water. The authors demonstrated that this biosensor is a promising tool for the rapid and cost-effective detection of PBDEs, as it eliminates the need for enzymes and additional reagents. The catalytic reaction occurs in an aqueous buffer, simplifying the setup. Additionally, IrO₂ NPs are stable over time and resilient to temperature variations, making them ideal for long-term use. Future developments could include miniaturization of the device and potential integration with mobile phones, further enhancing its portability and ease of use. Impedance biosensors have garnered significant attention in academic research for environmental monitoring, as they offer simplicity and portability. However, their commercial application remains limited due to several challenges, including difficulties in detecting small analytes, stability issues with bioreceptor immobilization, the complexity of impedance detection, and susceptibility to non-specific adsorption (Radhakrishnan et al., 2014). Gutés et al. (2013) developed the first impedimetric biosensor for decabromodiphenyl ether (DBDE). The biosensor employed monolayer graphene modified with AuNPs and a synthetic peptide designed specifically for DBDE detection. The device showed good linearity within the range of 5–100 ppt, demonstrating strong selectivity for brominated species by not responding to structurally similar non-brominated molecules. However, no tests on real environmental samples were reported. Bever et al. (2014) developed an impedance-based biosensor for the selective detection of BDE-47, using an antibody as the bioreceptor, specifically a variable domain of heavy chain antibodies (VHH) obtained from an alpaca immunized with a BDE-47 surrogate attached to a carrier protein. The mRNA encoding the VHH was isolated, transcribed into cDNA, and cloned into a phagemid vector to construct a phage display library. VHHs that specifically recognized BDE-47 were selected through panning. The VHH antibody was then immobilized on a gold electrode, enabling impedimetric biosensing with a LOD of 0.79 μg/L for BDE-47. Cross-reactivity studies confirmed the biosensor’s high selectivity for BDE-47 and its hydroxylated metabolites. Radhakrishnan et al. (2014) developed another impedance-based immunosensor for the selective detection of endocrine-disrupting chemicals (EDCs), including BDE-47. This device achieved a LOD of 1.3 ng/mL for BDE-47, slightly higher than the one reported by Bever et al. (2014). Additional studies indicated that increasing concentrations of potential interferents, such as norfluoxetine, did not affect the selectivity of the BDE-47 antibody-coated electrode, demonstrating the antibodies’ specificity and lack of cross-reactivity with non-target compounds.
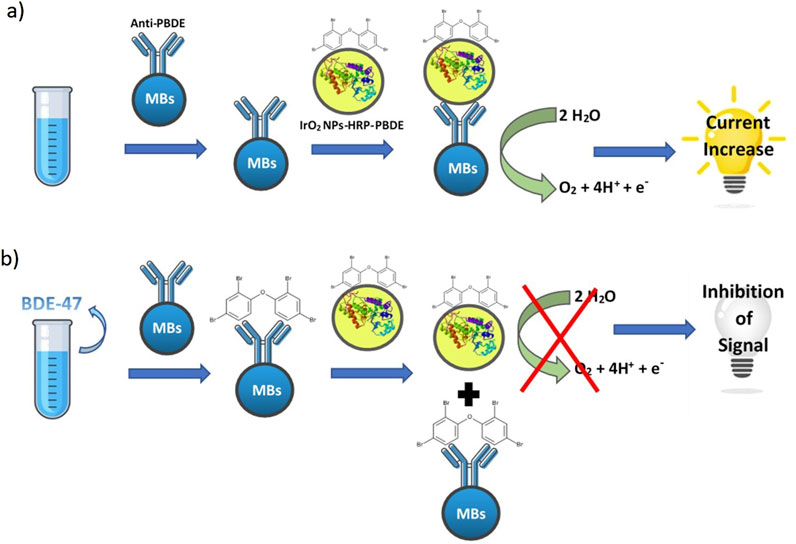
Figure 2. Schematic representation of the biosensor developed by Quesada-González et al. (2019). (A) in absence of BDE-47 and (B) in presence of BDE-47.
Another possible electrochemical detection technique for biosensing of environmental samples is represented by voltammetry. Bettazzi et al. (2016) developed a voltammetric biosensor for the detection of BDE-47 in food samples that consisted of a competitive electrochemical enzyme immunoassay on magnetic particles, using an alkaline phosphatase-labeled BDE-47 congener as a tracer. Anti-PBDE antibodies were immobilized on magnetic particles, which were then captured on a disposable carbon sensor array. Quantification was achieved via differential pulse voltammetry (DPV), yielding a LOD of 0.18 ng/mL and a limit of quantification (LOQ) of 0.30 ng/mL, with a linear range between 0.30 and 6.9 ng/mL. Tests on food sample extracts showed a strong correlation with results obtained by traditional GC-MS, demonstrating the device’s potential for rapid screening. Additionally, the use of screen-printed sensors mitigated issues like electrode poisoning and fouling, while also reducing the cost of analysis (Bettazzi et al., 2016).
A different voltammetric biosensor, based on a miniaturized microfluidic system, was developed by Chałupniak (2017). The device consists of a polydimethylsiloxane (PDMS) microfluidic chip for the immunoreaction step, a PDMS chip with an integrated screen-printed electrode (SPCE) for detection, and a reduced graphene oxide (rGO) PDMS chip for physical adsorption and removal of PBDE residues. The system employs a competitive immunoassay between PBDE and horseradish peroxidase-modified PBDE (HRP-PBDE), with quantification achieved by monitoring the enzymatic oxidation of o-aminophenol (o-AP) using anodic stripping voltammetry (SW-ASV). This biosensor demonstrated high sensitivity, with a LOD of 0.018 ppb, and proved suitable for analyzing complex matrices like seawater. Additionally, it required lower reagent volumes and shorter analysis times compared to previous systems (Chałupniak, 2017).
Romanelli et al. (2017) employed a voltammetric immunoassay to determine BDE-47 in mussel samples, which had been extracted and purified using the QuEChERS technique. The method used a competitive biosensing approach with an alkaline phosphatase (AP)-labeled congener as a tracer. Anti-PBDE antibody-modified magnetic particles were captured on a carbon-based screen-printed electrode, and the reaction was monitored via DPV, achieving a detection limit of 1.1 ng/g. The biosensor was tested on real mussel samples and certified reference material, with parallel GC-MS analysis for validation. Although the immunoassay showed lower sensitivity than GC-MS, it proved to be a useful tool for the rapid detection of highly contaminated seafood samples, which pose a significant potential threat to human health. However, the method’s limitations included the need for calibration to account for matrix effects and the potential cross-reactivity of various PBDE congeners (Romanelli et al., 2017).
Among photoelectrochemical immunosensors (PECs), Li et al. (2014) developed a rapid detection method for PBDEs, using BDE-121 as a model compound. The immunosensor features a core-shell ZnS/CdTe/Mn-CdS/ZnS sensitized microporous ZnO nanosheet (NS) photoelectrode, onto which anti-BDE-121 polyclonal antibodies were immobilized. The immunoreaction leads to changes in the photocurrent signal, enabling highly specific quantification of BDE-121, with a detection limit of 3.98 pM and a linear range between 5 pM and 100 nM. Analysis of BDE-121 in paint samples produced results comparable to those obtained through gas chromatography-mass spectrometry, demonstrating the immunosensor’s applicability (Li et al., 2014).
Lastly, the selective and sensitive biosensor proposed by Jin et al. (2015) for detecting decabromodiphenyl ether (DBDE) is reported. This biosensor utilizes DBDE peptide receptors integrated with carbon nanotube field-effect transistors (CNT-FETs). The peptides were selected through a high-throughput screening process of phage library display and possess a unique consensus binding pocket featuring two Trp-His/Asn-Trp repeats, which bind to DBDE in a multivalent manner. While FET-based biosensors are not traditionally classified as electrochemical biosensors due to their reliance on field-effect principles rather than direct electron transfer, they share similarities in using electrical signals as outputs. The estimated LOD for DBDE is approximately 0.001 ng/L, significantly lower than the maximum allowable concentration (MAC) environmental quality standards (EQS) for water outlined in Directive 2013/39/EC, and comparable to the sensitivity of the most advanced GC methods (Jin et al., 2015).
The following section presents various electrochemical biosensors designed for the quantitative determination of TBBPA, along with its derivative tetrabromobisphenol A mono (hydroxyethyl) ether (TBBPA-MHEE) and by-product tetrabromobisphenol A bis(2-hydroxyethyl) ether (TBBPA-DHEE).
Wang et al. (2017) employed a photoamperometric technique to detect TBBPA in lake water. The device used dodecahedral gold nanocrystals (AuNCs) self-assembled on molybdenum disulfide (MoS₂) nanosheets to create an AuNCs/MoS₂ nanocomposite, which was drop-coated onto a glassy carbon electrode. Transthyretin (TTR) was immobilized on the electrode to enable specific recognition of TBBPA. Under optimal conditions, the photocurrent change ratio was linearly related to the logarithm of the TBBPA concentration, with a LOD of 0.045 nM and a linear range of 0.1 nM–1.0 μM. The immunosensor was successfully applied to contaminated water samples from South Lake in Wuhan, China (Wang et al., 2017).
In 2019, Dong et al. developed a competitive immunosensor for TBBPA-DHEE analysis using an electrochemical amperometric strategy. This method recorded the reduction of hydrogen peroxide (H₂O₂), catalyzed by synthesized catalase-functionalized AuNPs loaded with self-assembled polymer nanospheres. The biosensor achieved a LOD of 0.12 ng/mL, seven times lower than conventional ELISA tests, with good accuracy, low sample consumption (6 mL), and low cost. It was applied to detect TBBPA-DHEE in real water samples (pure water, tap water, pond water, and lake water) (Dong et al., 2019).
Additionally, Yakubu et al. (2020) developed another competitive amperometric immunosensor, using bimetallic gold-palladium nanoparticles synthesized from amine-functionalized nanoflower-like manganese oxide (NH₂-fMnO₂) for rapid TBBPA quantification in water. TBBPA antigens were adsorbed onto MWCNT-modified glassy carbon electrodes, while AuPd-NH₂-fMnO₂ acted as the signal amplifier, catalyzing H₂O₂. The device demonstrated excellent sensitivity (LOD = 0.10 ng/mL) and a wide linear range (0.1–243 ng/mL). It also showed great potential for detecting trace contaminants in pure, tap, pond, and lake water (Yakubu et al., 2020). One year later, Yakubu et al. (2021) designed an indirect competitive immunosensor for TBBPA detection, utilizing in situ reduced Pd nanospheres on hybrid MnO₂ nanosheets (MnO₂/Pd) as labels for the secondary antibody via Pd-N binding. This enhanced the catalytic activity and signal response. Additionally, multi-walled carbon nanotubes loaded with a blue gold-toluidine compound (MWCNTs/Au-TB) improved electron transfer and sensitivity. The immunosensor exhibited good stability, reproducibility, and sensitivity, with a LOD of 0.17 ng/mL (approximately five times lower than conventional ELISA) and a linear range of 0–81 ng/mL. It has also been successfully tested on environmental water samples (pure water, tap water, pond water, and lake water) and shows promise for emerging contaminant monitoring (Yakubu et al., 2021).
A competitive immunosensor using electrochemical impedance spectroscopy (EIS) for the quantification of TBBPA derivatives, including TBBPA-DHEE and TBBPA-MHEE, in environmental water samples, was developed by Zhang et al. (2018a). Signal amplification was achieved through the biocatalytic precipitation of 4-chloro-1-naphthol (CN) on the electrode surface using horseradish peroxidase (HRP) and enhanced enzyme loading with HRP-loaded silica nanoparticles carrying poly (SiO₂@PAA) brushes as labels. The method showed a linear range of 0.21–111.31 ng/mL and a LOD of 0.08 ng/mL for both targets (Zhang et al., 2018a).
Zhang et al. (2020) developed an electrochemical sensor based on DPV for the detection of TBBPA in plastic products. The sensor is based on a nanocomposite of reduced graphene oxide (rGO) and silver nanodendrites (AgNDs) combined with MIPs for selective detection. The proposed device exhibited excellent sensitivity with a LOD of 0.015 nM and a linear range from 0.05 to 20 nM. Real samples, including plastic water bottles and mobile phone cases, were tested and the results were consistent with those obtained by LC-MS.
Electrochemical biosensors offer high sensitivity, portability, low cost, and rapid response times, making them ideal for in situ and real-time environmental monitoring. Recent advancements in nanomaterials, such as graphene and metal nanoparticles, have further enhanced their performance, improving sensitivity, selectivity, and stability. These features make electrochemical biosensors valuable tools for detecting pollutants like BFRs, providing cost-effective and reliable solutions for environmental monitoring and health risk assessment.
Optical biosensors are compact analytical devices that integrate a biorecognition element (e.g., enzymes, antibodies, antigens, receptors, nucleic acids) with a transducer system, emitting an optical signal (such as light absorption, fluorescence, luminescence, reflectance, Raman scattering, or refractive index) proportional to the concentration of the analyte. These biosensors offer several advantages over traditional analytical methods, including rapid response, high sensitivity, robustness, real-time monitoring, small size, cost-effectiveness, and the ability to conduct high-frequency measurements of biological and chemical substances without requiring sample concentration or pre-treatment. Despite their potential, significant opportunities remain for further research and development, particularly in the field of environmental pollution control and early detection of health associated risks (Long et al., 2013).
In order to estimate the general population’s exposure to PBDEs through diet, it is crucial to develop highly sensitive and high-throughput techniques for detecting BDE-47 in food. Optical biosensors are particularly appealing in food safety as they can detect contaminants in complex matrices with minimal sample treatment. In this context, a few studies have utilized fluorescence-based measurements for the quantitative determination of target molecules. The first device was proposed by Ma & Zhuang in 2017 for determining BDE-47 in indoor air particles. Using the DNA-GNP-pAb-BDE-47 compound, they optimized the sensitivity of the immunoassay, achieving a LOD of 1.32 pg/L and a linear range of 5–50 pg/L. The analysis of real environmental samples confirmed that the biosensor results aligned well with GC-MS data, highlighting the biosensor’s reliability for highly sensitive quantification of analytes (Ma and Zhuang, 2017). A similar approach was proposed by the same authors a year later (Ma and Zhuang, 2018), in which they developed a highly sensitive real-time immuno-polymerase chain reaction (iPCR) assay using the biotin-streptavidin (BA-IPCR) system to monitor BDE-47 in various marine fish species commonly consumed in China. The method involves a competitive reaction between BDE-47 in the sample and coating antigens, immobilized in the PCR tube, to bind with biotinylated pAb-BDE-47. The higher the BDE-47 concentration in the sample, the less bio-pAb-BDE-47 binds to the coating antigens. As a result, fewer template DNAs are present on the solid phase, releasing less target DNA, which increases the number of amplification cycles required to reach the fluorescence threshold during PCR analysis. Thus, BDE-47 concentrations can be quantified by the number of cycles needed. A summary of the procedure is provided in Figure 3. This method achieved LOD of 2.96 pg/L, with a linear range between 10 pg/L and 100 ng/L for BDE-47. The BA-IPCR results were consistent with those obtained through conventional GC-MS, demonstrating the accuracy and sensitivity of the immunoassay for BDE-47 trace-level quantification in fish samples (Ma and Zhuang, 2018).
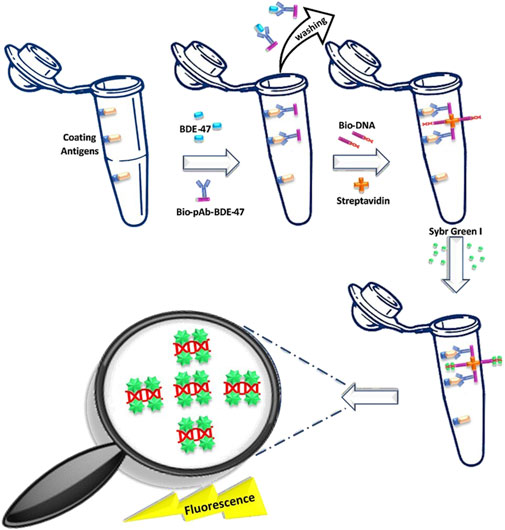
Figure 3. Schematic representation of the BA-IPCR assay developed by Ma and Zhuang (2018).
Additionally, Sanchis et al. (2018) reported the development of a fluorescent multiplexed microarray platform for the monitoring of a wide variety of pollutants in seawater samples. Using a cocktail of six monoclonal and polyclonal antibodies, the platform targeted analytes such as triazine biocides, sulfonamides, chloramphenicol, PBDEs (specifically BDE-47), 17β-estradiol, and algae toxins. The device demonstrated simultaneous detection of these contaminants directly in seawater, achieving a LOD of 2.71 ± 1.13 μg/L for BDE-47, with an analysis time of 1 h and 30 min, without sample pre-treatment. Although the LOD did not meet the Environmental Quality Standards (EQS) of the Water Framework Directive (2000/60/EC), sensibility could be improved with sample pre-treatment and pre-concentration techniques.
Li et al. (2019) developed another optical biosensor for the selective detection of BDE-121 using a competitive fluoroimmunoassay (FIA). The assay employed two different fluorescent labels: carbon quantum dots (CQD) and fluorescein isothiocyanate (FITC). Both bioconjugates (CQDs-BSA-BDE-121 and FITC-BSA-BDE-121) demonstrated good sensitivity and selectivity, but the CQD-labeled method showed superior performance, likely due to CQDs’ biocompatibility and photostability, with a LOD of 0.056 ng/mL and a linear range of 0.28–56.47 ng/mL.
Chen et al. (2022) developed a novel biosensor to detect BDE-209 using a whole-cell luminescent biosensor based on genetically engineered Sphingobium xenophagum (strain C1). The detection method relied on luminescence, where luciferase activity generated the optical signal, allowing the interaction between PBDEs and the bioreceptor to be measured. The bioreceptor, the extracellular Cache domain-containing sensor protein Chr1_2466, specifically recognizes PBDEs, primarily BDE-209, leading to a LOD of 0.01 µM (∼9.59 μg/L), with a linear range extending from 0.05 to 6.0 µM. The biosensor was tested on sediment samples from Southern China, showing a good correlation with results obtained through HPLC and underscoring the potential of whole-cell biosensors for in situ and real-time environmental monitoring.
Absorbance-based biosensors have also been designed for PBDE detection. Sapozhnikova et al. (2015) developed a biosensor using absorbance measurements at 450 nm for the semi-quantitative determination of BDE-47 in biotic samples. The biosensor was tested on fish tissues, previously extracted and purified with QuEChERS technique. The device achieved a LOD of 1 ng/g for BDE-47, and its performance was validated with LPGC-MS/MS, showing consistent results with the immunoassay. Similarly, Romanelli et al. (2017) designed an immunosensor for BDE-47 quantification in mussel samples using a colorimetric screening method. The study proposes an ELISA kit with absorbance measurements at 450 nm on a microtiter plate reader, achieving a LOD of 0.6 ng/g, lower than the 1.1 ng/g obtained with an electrochemical detection. Results from real mussel samples and certified reference materials aligned with GC data, validating the sensor’s accuracy.
Surface plasmon resonance (SPR) biosensors are particularly promising for environmental applications due to their ability to monitor molecular interactions at the transducer, by detecting the changes in the refractive index at the metal surface. Marchesini et al. (2008) developed an SPR-based biosensor to quantify thyroid-disrupting chemicals, including PBDEs. The authors designed a SPR optical detection method to screen chemicals affecting the transport of thyroxine (T4) by thyroid-binding globulin (TBG) and transthyretin (TTR), both key transport proteins of thyroid hormones. The detection system allowed for real-time, label-free quantification of binding interactions between the chemicals and transport proteins. While the study did not report specific LODs, the biosensor showed strong binding to hydroxylated PBDE metabolites (especially BDE-47, BDE-49, and BDE-99), demonstrating its potential as a broad screening method for environmental contaminants.
One of the earliest examples of optical biosensors for the selective detection of TBBPA reported in the literature utilizes the SPR technique. In 2006, Marchesini et al. developed SPR-based biosensor assays for screening chemicals with thyroid-disrupting activity. Two thyroid transport proteins (TPs), thyroxine-binding globulin (TBG) and recombinant transthyretin (rTTR), were used in an inhibition assay format on a Biacore 3000 system with CM5 biosensor chips coated with L-thyroxine (T4), the main hormone of the thyroid system. Among the various analytes tested was TBBPA. This study was one of the first to drive the development of increasingly sensitive, rapid, and cost-effective biosensing techniques for selectively detecting and screening environmental contaminants (Marchesini et al., 2006). The same authors (Marchesini et al., 2008) designed another SPR sensor assay, already described in Section 3.1.2, based on thyroid hormone transport proteins (TBG and TRR) in combination with a T4-coated biosensor chip for the monitoring of several thyroid-disrupting chemicals, including PBDEs and TTBPA. TBBPA showed strong binding to TTR with a relative potency (RP) of 1 but was inactive for TBG, indicating selective interaction. The author did not specifically state the LOD for TBBPA alone, but the assay was sensitive enough to differentiate chemicals with varying affinities for the transport proteins.
In recent years, one of the most successful approaches for biosensing environmental contaminants in real samples, due to its simplicity, cost-effectiveness, and speed of analysis, is represented by colorimetric methods, which do not require expensive instrumentation or highly trained operators. Zhang et al. developed a colorimetric strategy to create a plasmonic enzyme-linked immunosorbent assay (pELISA) using a polyclonal antibody for the simultaneous ultra-sensitive naked-eye detection of two TBBPA-related compounds, TBBPA-MHEE and TBBPA-DHEE. The method leveraged.
AuNPs, which were synthesized in the presence of glucose oxidase (GOx). The reaction between GOx and glucose generated H₂O₂, facilitating the growth of AuNPs. To enhance the enzyme load, a large number of GOx molecules were immobilized on silica nanoparticles carrying poly brushes (SiO₂@PAA), and this complex was conjugated to the secondary antibody, resulting in signal amplification. Under optimized conditions, the colorimetric method allowed naked-eye detection with a LOD of 10⁻³ μg/L for TBBPA-DHEE. Sensitivity was further improved using a microplate reader, achieving a LOD of 3.3 × 10⁻⁴ μg/L. The biosensor’s analytical performance was successfully tested on real water samples, including pure, tap, and river water, demonstrating its potential for effective environmental monitoring (Zhang et al., 2018b). Two years later, Zeng L. et al. (2020) proposed a colorimetric detection method for TBBPA based on enzyme-mimicking activity and the molecular recognition capabilities of MIPs, composed of a copper-based metallorganic framework on a paper support. The biorecognition of TBBPA by the MIP weakens the enzymatic mimicking properties of HKUST-1 under the MIP layer, reducing the number of imprinted cavities. The adsorbed TBBPA is degraded in the presence of H₂O₂, and the combined effect of H₂O₂ and HKUST-1 reduces the intensity of the staining caused by the catalytic oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB). Since the grey intensity of the stain is proportional to the logarithmic concentration of TBBPA, ranging from 0.01 to 10 ng/g, this amplification strategy enables ultrasensitive and highly selective colorimetric detection. The reported LOD is 3 pg/g, and the method’s selectivity remains unaffected by the sample matrix complexity and possible interferents. This approach has also been successfully applied to the analysis of TBBPA in environmental dust samples, demonstrating satisfactory recoveries (Zeng L. et al., 2020).
Moreover, one of the most widely used methods for the quantitative determination of TBBPA involves absorbance measurements. In 2014, Wang et al. proposed an absorbance biosensing technique for TBBPA detection, using camelid antibodies, which naturally lack light chains. Despite their rarity in applications for small molecules, these antibodies show great potential as efficient tools for monitoring environmental contaminants. In this study, an alpaca was immunized with a TBBPA hapten conjugated to thyroglobulin, and a highly selective variable domain of heavy-chain antibody (VHH) T3−15 was isolated from a phage-displayed VHH library using heterologous coating antigens. Under optimized conditions, the assay demonstrated linearity in the range of 0.06–2.53 ng/mL, with a LOD of 0.02 ng/mL for TBBPA. The method was successfully applied to soil and fetal bovine serum samples, with results aligning with those obtained using LC-MS/MS (Wang et al., 2014). The following year, the same research group developed a one-step immunoassay for TBBPA, using a single-domain camelid alkaline phosphatase-antibody fusion protein. This required the expression of a highly selective anti-TBBPA heavy-chain (VHH) T3-15 antibody fused to alkaline phosphatase (AP) from E. coli, combining TBBPA binding capability with enzymatic activity. The device, with a linear range of 0.03–0.94 ng/mL under optimized conditions, was also applied to urine samples, showing excellent correlation with a high-performance HPLC-MS/MS method (Wang et al., 2015). In 2016, Yu et al. proposed an immunosensor coupled with absorbance measurements for TBBPA detection in lake water and food samples. Using an indirect competitive enzyme immunoassay, a monoclonal antibody (mAb) against TBBPA was produced, establishing a correlation between absorbance and analyte concentration within a linear range of 0.15–1.32 ng/mL. This method demonstrated high sensitivity and specificity in both water and rice pudding samples (Yu et al., 2016). A similar approach was adopted by He et al. (2018) for the quantitative determination of TBBPA in environmental water and sediment samples. In this study, the variable domain of the camelid-derived heavy-chain antibody (nanobody, Nb) was employed. An anti-TBBPA nanobody genetically integrated with a C-terminal cysteine residue was immobilized on bacterial magnetic particles (BMPs) enclosed within a protein membrane, using a heterobifunctional N-succinimidyl-3-(2-pyridyldithiol) propionate reagent to form a solid BMP-Nb complex. A rapid and sensitive enzyme immunoassay (ELISA) based on the combination of BMP-Nb and T5 horseradish peroxidase was developed for the analysis of TBBPA. This assay achieved quantitative recoveries of analyte from canal water (114%–124%) and sediment (109%–113%) samples, with a LOD of 0.1 ng/mL and a linear range of 0.45–5.25 ng/mL (He et al., 2018).
Several optical biosensing techniques, beyond absorbance, exploit the emission of electromagnetic radiation resulting from the interaction between a bioreceptor and analyte for its quantitative determination. For instance, Bu et al. (2015) employed fluorescence analysis to detect TBBPA in seabed sediment samples. This study introduced an ultrasensitive real-time competitive indirect immuno-PCR (rt-iPCR). The TBBPA coating antigen, adsorbed on the surface of an Eppendorf tube, competed with the analyte for binding to biotinylated polyclonal antibodies (pAbs). Streptavidin acted as a bridge between the biotinylated pAbs and biotinylated standard DNA. During the real-time PCR reaction, SYBR Green I, a fluorescent molecule, intercalated into double-stranded DNA at each amplification cycle, enabling quantitative analysis through fluorescence. The assay exhibited a linear range of 10 pg/L to 10 ng/L, with a LOD of 2 pg/L, and demonstrated high selectivity with low cross-reactivity to TBBPA analogs. Marine sediment samples were analyzed in parallel by rt-iPCR and LC, yielding consistent results (Bu et al., 2015). A few years later, Ma et al. (2019) developed an ultrasensitive real-time immuno-polymerase chain reaction (IPCR) assay for TBBPA detection, also utilizing fluorescence measurements. This immunochemical assay required the preparation of TBBPA antigens and a polyclonal anti-TBBPA antibody (pAb-TBBPA), along with functionalized nanoprobes (FNPs-IPCR) for signal amplification. AuNPs modified with thiol-linked DNA and pAb-TBBPA were prepared as antibody-DNA conjugates, leading to strong signal intensification. SYBR Green I intercalated with DNA during the real-time PCR system, enabling the fluorescence-based determination of TBBPA. The assay demonstrated linearity ranging from 1 pg/L to 10 ng/L, with a LOD of 0.62 pg/L. The method was tested on PM2.5 particulate samples, producing results consistent with those of HPLC, suggesting that the FNPs-IPCR technique is suitable for trace TBBPA screening in environmental samples (Ma et al., 2019).
In addition to the described optical techniques, various optical biosensors specific for TBBPA-related compounds exploit the emission or inhibition of electromagnetic radiation during the biorecognition process, particularly through chemiluminescence. For example, in 2018, Zhang et al. developed a novel chemiluminescence immunoassay based on luminol-modified gold nanoclusters (AuNCs@Peps@luminol) for the simultaneous detection of TBBPA-DHEE and TBBPA-MHEE. In this system, alkaline phosphatase (ALP) was labeled on the secondary antibody (Ab2) for signal amplification. When ALP-Ab2 is captured by the primary antigen-antibody complex (Ab1), disodium phenyl phosphate (PPNa) generates a large amount of phenol under ALP catalysis, which greatly inhibits the chemiluminescence intensity of AuNCs@Peps@luminol. Under optimized conditions, the immunosensor demonstrated a linear range of 0.23–9.32 mg/L and a LOD of 0.078 mg/L for TBBPA-DHEE. The method was successfully applied to the analysis of real environmental water samples, including tap, pond, lake, and river water, underscoring its potential as a tool for the systematic investigation of trace concentrations of TBBPA-related compounds in environmental samples (Zhang et al., 2018c).
These chemiluminescence-based approaches complement other optical biosensing methods, such as fluorescence-based detection like those developed by Bu et al. (2015) and Ma et al. (2019), where real-time competitive indirect immuno-PCR (rt-iPCR) and fluorescence-based immuno-PCR were used for the ultrasensitive detection of TBBPA in seabed sediment and PM2.5 particulate samples, respectively. Together, these optical biosensing techniques provide a range of highly sensitive tools for the detection and analysis of TBBPA and its derivatives in complex environmental matrices. A chemiluminescence immunoassay specific for TBBPA-DHEE was also proposed 2 years later by (Gu et al., 2020). This method utilized an indirectly competitive approach, where the synthesized PS@hemin@Co2⁺ was labeled by a secondary antibody (Ab2) instead of common natural enzymes, providing excellent catalysis for the decomposition of illumination-induced H₂O₂ to generate the chemiluminescence signal. A schematic illustration of the chemiluminescent biosensing steps for the determination of TBBPA-DHEE is shown in Figure 4. Under optimized conditions, this optical immunosensor provides a LOD, 5 times lower than that using a conventional ELISA with the same antibody (LOD, 0.9 μg/L), good linearity (1.6–14.3 μg/L), and satisfactory accuracy. The proposed technique was applied for the analysis of water and soil samples collected in Jiangsu and Zhejiang Province, China (Gu et al., 2020).

Figure 4. High-throughput chemiluminescence immunoassay based on Co2+/hemin synergistic catalysis for sensitive detection tetrabromobisphenol A bis(2-hydroxyethyl) ether in environmental samples (Gu et al., 2020).
In the same year, Zeng K. et al. followed a similar strategy and developed a chemiluminescent immunoassay for the simultaneous detection of TBBPA-DHEE and TBBPA-MHEE in aquatic environments. This multiplexed competitive chemiluminescent immunoassay (CLIIA) enabled the quantification of both analytes using two specific monoclonal antibodies. Sensitivity was enhanced, achieving LOD of 1.85 ng/mL for TBBPA-MHEE and 2.05 ng/mL for TBBPA-DHEE, by utilizing AuNPs as a solid support to load labeled horseradish peroxidase, forming multi-enzyme particles. This biosensing technique was applied for the investigation of contaminants in the inner rivers of Zhenjiang, Jiangsu Province, demonstrating its effectiveness as a screening tool (Zeng K. et al., 2020).
The typical features of optical biosensors, including speed, simplicity, automation, and miniaturization potential, along with their robustness and capability for long-term and high-frequency online detection, make them valuable tools for sensitive and selective quantification of various targets. Recent advancements in functionalized nanomaterials further enhance their effectiveness. Consequently, optical biosensors can offer productive solutions for environmental pollution control and early warning systems (Long et al., 2013).
Despite advances in biosensor technologies, major gaps and challenges need to be addressed to fully exploit these techniques for comprehensive environmental monitoring of legacy and new BFRs. A major goal is to improve the sensitivity and selectivity of biosensors to detect trace levels of BFRs in complex environmental matrices. Advances in materials science, such as the incorporation of nanomaterials (e.g., graphene, metal nanoparticles, and carbon nanotubes), have shown significant potential in enhancing the sensitivity of transducers. Selectivity improvements are being pursued through the development of advanced bioreceptors, including recombinant antibodies, MIPs, and peptide-based receptors. These innovations aim to address the detection challenges posed by structurally similar BFR congeners and matrix interferences. However, it is important to note that the lack of selectivity in some biosensors may also offer advantages, particularly in allowing the simultaneous detection of structurally related BFR congeners. Multiplex detection strategies could improve the applicability of biosensors for environmental screening, compensating for this limitation.
Based on the studies reviewed, fluorescence-based biosensors coupled with antibody receptors showed the lowest LOD for both PBDEs and TBBPA in different environmental matrices. For instance, Ma and Zhuang (2017) and Ma and Zhuang (2018) achieved LODs of 1.32 pg/L and 2.96 pg/L for BDE-47, which are comparable to traditional chromatographic methods. Similarly, Bu et al. (2015) and Ma et al. (2019) reported LODs ranging from 0.62 to 2 pg/L for TBBPA. Fluorescence biosensors offer several advantages over traditional laboratory-based methods, such as ultrahigh sensitivity, portability, potential for real-time analysis, and miniaturization. However, their specificity may limit their ability to detect multiple congeners simultaneously, whereas chromatographic techniques excel in multi-target detection. Despite this limitation, fluorescence-based biosensors appear to be very promising for environmental applications, especially for rapid and field-deployable monitoring of specific BFRs.
Miniaturization is another critical trend, enabling the possibility of real-time and field monitoring. Portable biosensor devices offer the ability to rapidly assess environmental contamination without the need for additional laboratory analysis. Their use in remote or resource-limited settings can allow regulatory agencies and scientists to monitor contamination hotspots effectively and in real time. However, long-term stability and bioreceptors’ functionality, such as enzymes and antibodies remain critical issues. Complex environmental matrices can compromise biosensor performance. Advanced sample preparation techniques and robust biosensor designs are necessary to mitigate these effects. Additionally, expanding detection capabilities to include emerging NBFRs and their metabolites is an urgent research priority. Concerning NBFRs, biosensor development must prioritize the creation of platforms capable of detecting both NBFRs and their degradation products to address regulatory and public health needs, as multiplex detection capabilities are becoming increasingly important.
Furthermore, unlike conventional analytical techniques, biosensors offer unique advantages in toxicity analysis and the detection of biologically relevant interactions. By integrating bioactive recognition elements, biosensors can provide insight into the bioavailability and potential toxic effects of BFRs in environmental and biological matrices. This capability makes biosensors not only valuable for contamination assessment but also for evaluating exposure risks and environmental impact.
Lastly, the scale-up of biosensor production while maintaining cost-effectiveness, reproducibility, and reliability may represent an obstacle to widespread adoption. Rigorous field validation of biosensors in real large-scale conditions is essential to ensure their practicality and reliability, while the establishment of standardized validation protocols will enhance biosensor acceptance and comparability with traditional methods.
The physicochemical and toxicological properties of BFRs such as PBDEs and TBBPA, along with their widespread presence in various environmental matrices, underscore the critical need for accurate, rapid, and cost-effective detection in complex samples. Recent advances in biosensor technologies for the detection of BFRs show great potential in meeting this need. Although biosensors may not fully replace established methods such as GC-MS, they can serve as valuable tools for rapid and accurate screening before the costly instrumentation analysis, particularly in the field of food quality and safety control or identification of critical contamination zones, thereby helping to reduce the risk of exposed individuals. However, the advancement of biosensor technologies for environmental monitoring of BFRs requires addressing the challenges of stability, real-time usability in the field, multiple detection, and scalable manufacturing while ensuring rigorous validation and cost-effectiveness.
Despite the promising studies on the biosensing of legacy BFRs, there is a notable lack of research focused on NBFRs. This gap underscores the urgent need to focus attention and resources on the detection and analysis of these emerging compounds, as they present unique challenges and potential risks that warrant further investigation.
CS: Conceptualization, Data curation, Writing–original draft. LS: Data curation, Visualization, Writing–original draft. TM: Supervision, Visualization, Writing–review and editing. AC: Project administration, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. CS gratefully acknowledges MUR and EU-FSE for financial support of the PhD fellowship PON Research and Innovation 2014–2020 (D.M 1061/2021) XXXVII Cycle in Chemical Sciences: “Green deal and Zero Pollution strategy: innovative solutions for emerging contaminants removal”.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alaee, M., Arias, P., Sjödin, A., and Bergman, Å. (2003). An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ. Int. 29 (6), 683–689. doi:10.1016/S0160-4120(03)00121-1
Anastassiades, M., Lehotay, S. J., Stajnbaher, D., and Schenck, F. J. (2003). Multiresidue method for determination of 90 pesticides in fresh fruits and vegetables using solid-phase extraction and gas chromatography-mass spectrometry. J. Chromatogr. A 1015 (1–2), 185–198. doi:10.1016/s0021-9673(03)01211-1
Ayala-Cabrera, J. F., Santos, F. J., and Moyano, E. (2021). Recent advances in analytical methodologies based on mass spectrometry for the environmental analysis of halogenated organic contaminants. Trends Environ. Anal. Chem. 30, e00122. doi:10.1016/j.teac.2021.e00122
Bartolomé, L., Cortazar, E., Raposo, J. C., Usobiaga, A., Zuloaga, O., Etxebarria, N., et al. (2005). Simultaneous microwave-assisted extraction of polycyclic aromatic hydrocarbons, polychlorinated biphenyls, phthalate esters and nonylphenols in sediments. J. Chromatogr. A 1068 (2), 229–236. doi:10.1016/J.CHROMA.2005.02.003
Berton, P., Lana, N. B., Ríos, J. M., García-Reyes, J. F., and Altamirano, J. C. (2016). State of the art of environmentally friendly sample preparation approaches for determination of PBDEs and metabolites in environmental and biological samples: a critical review. Anal. Chim. Acta 905, 24–41. doi:10.1016/j.aca.2015.11.009
Bettazzi, F., Martellini, T., Shelver, W. L., Cincinelli, A., Lanciotti, E., and Palchetti, I. (2016). Development of an electrochemical immunoassay for the detection of polybrominated diphenyl ethers (PBDEs). Electroanalysis 28 (8), 1817–1823. doi:10.1002/elan.201600127
Bever, C. R. S., Majkova, Z., Radhakrishnan, R., Suni, I., McCoy, M., Wang, Y., et al. (2014). Development and utilization of camelid VHH antibodies from alpaca for 2,2′,4,4′-tetrabrominated diphenyl ether detection. Anal. Chem. 86 (15), 7875–7882. doi:10.1021/ac501807j
Bredsdorff, L., Olesen, P. T., Beltoft, V. M., Sharma, A. K., Hansen, M., Nørby, K., et al. (2023). Identifying and collecting relevant literature related to the toxicity of polybrominated diphenyl ethers (PBDEs), tetrabromobisphenol A (TBBPA) and brominated phenols. EFSA Support. Publ. 20 (4), 8014E. doi:10.2903/SP.EFSA.2023.EN-8014
Bromine Science and Environmental Forum (BSEF) (2003). Major brominated flame retardants volume estimates: total market demand by region in 2001. Belgium: BSEF.
Bu, D., Zhuang, H., Yang, G., and Ping, X. (2015). A real-time immuno-PCR assay for the detection of tetrabromobisphenol A. Anal. Methods 7 (1), 99–106. doi:10.1039/c4ay01343c
Chałupniak, A. (2017). Development of novel electrochemical and optical Lab-on-a-chip platforms for contaminants and biomarkers sensing.
Chen, X., Yao, H., Song, D., Sun, G., and Xu, M. (2022). Extracellular chemoreceptor of deca-brominated diphenyl ether and its engineering in the hydrophobic chassis cell for organics biosensing. Chem. Eng. J. 433, 133266. doi:10.1016/J.CEJ.2021.133266
Chevrier, J., Harley, K. G., Bradman, A., Gharbi, M., Sjodin, A., and Eskenazi, B. (2010). Polybrominated diphenyl ether (PBDE) flame retardants and thyroid hormone during pregnancy. Environ. Health Perspect. 118 (10), 1444–1449. doi:10.1289/ehp.1001905
Cincinelli, A., Martellini, T., Pozo, K., Kukučka, P., Audy, O., and Corsolini, S. (2016). Trematomus bernacchii as an indicator of POP temporal trend in the Antarctic seawaters. Environ. Pollut. 217, 19–25. doi:10.1016/j.envpol.2015.12.057
Dimpe, K. M., and Nomngongo, P. N. (2016). Current sample preparation methodologies for analysis of emerging pollutants in different environmental matrices. TrAC - Trends Anal. Chem. 82, 199–207. doi:10.1016/j.trac.2016.05.023
Dirtu, A. C., Covaci, A., Dirtu, A. C., and Abdallah, M. (2013). Advances in the sample preparation of brominated flame retardants and other brominated compounds. TrAC - Trends Anal. Chem. 43, 189–203. doi:10.1016/j.trac.2012.10.004
Dong, S., Wang, S., Gyimah, E., Zhu, N., Wang, K., Wu, X., et al. (2019). A novel electrochemical immunosensor based on catalase functionalized AuNPs-loaded self-assembled polymer nanospheres for ultrasensitive detection of tetrabromobisphenol A bis(2-hydroxyethyl) ether. Anal. Chim. Acta 1048, 50–57. doi:10.1016/j.aca.2018.10.018
Ganci, A. P., Vane, C. H., Abdallah, M. A. E., Moehring, T., and Harrad, S. (2019). Legacy PBDEs and NBFRs in sediments of the tidal River Thames using liquid chromatography coupled to a high resolution accurate mass Orbitrap mass spectrometer. Sci. Total Environ. 658, 1355–1366. doi:10.1016/j.scitotenv.2018.12.268
Gomes, J., Begum, M., and Kumarathasan, P. (2024). Polybrominated diphenyl ether (PBDE) exposure and adverse maternal and infant health outcomes: systematic review. Chemosphere 347, 140367. doi:10.1016/j.chemosphere.2023.140367
Government of Canada (2020). Polybrominated diphenyl ethers (PBDEs). Available at: https://www.canada.ca/en/health-canada/services/chemical-substances/other-chemical-substances-interest/polybrominated-diphenyl-ethers-risk-assessment.html.
Gu, L., Zou, Y., Li, Y., Zeng, K., Zhu, N., Zhu, F., et al. (2020). High-throughput chemiluminescence immunoassay based on Co2+/hemin synergistic catalysis for sensitive detection tetrabromobisphenol A bis(2-hydroxyethyl) ether in the environments. Sci. Total Environ. 714, 136880. doi:10.1016/j.scitotenv.2020.136880
Gutés, A., Lee, B. Y., Carraro, C., Mickelson, W., Lee, S. W., and Mabouduan, R. (2013). Impedimetric graphene-based biosensors for the detection of polybrominated diphenyl ethers. Nanoscale 5 (13), 6048–6052. doi:10.1039/c3nr01268a
He, J., Tian, J., Xu, J., Wang, K., Li, J., Gee, S. J., et al. (2018). Strong and oriented conjugation of nanobodies onto magnetosomes for the development of a rapid immunomagnetic assay for the environmental detection of tetrabromobisphenol-A. Anal. Bioanal. Chem. 410 (25), 6633–6642. doi:10.1007/s00216-018-1270-9
Hou, R., Lin, L., Li, H., Liu, S., Xu, X., Xu, Y., et al. (2021). Occurrence, bioaccumulation, fate, and risk assessment of novel brominated flame retardants (NBFRs) in aquatic environments — a critical review. Water Res. 198, 117168. doi:10.1016/j.watres.2021.117168
Huang, C. W., Lin, C., Nguyen, M. K., Hussain, A., Bui, X. T., and Ngo, H. H. (2023). A review of biosensor for environmental monitoring: principle, application, and corresponding achievement of sustainable development goals. Bioengineered 14 (1), 58–80. doi:10.1080/21655979.2022.2095089
Huang, M., Li, J., Xiao, Z., and Shi, Z. (2020). Tetrabromobisphenol A and hexabromocyclododecane isomers in breast milk from the general population in Beijing, China: contamination levels, temporal trends, nursing infant’s daily intake, and risk assessment. Chemosphere 244, 125524. doi:10.1016/j.chemosphere.2019.125524
Jiang, X., Bai, X., Liu, X., Wang, X., and Shiu, K. K. (2023). Detecting low-brominated diphenyl ethers by highly sensitive biosensors based on the blocking effect on glucose oxidase. Sensors and Diagnostics 2 (1), 176–187. doi:10.1039/D2SD00161F
Jin, H. E., Zueger, C., Chung, W. J., Wong, W., Lee, B. Y., and Lee, S. W. (2015). Selective and sensitive sensing of flame retardant chemicals through phage display discovered recognition peptide. Nano Lett. 15 (11), 7697–7703. doi:10.1021/acs.nanolett.5b03678
Jin, X., Su, H., Xu, L., Wang, Y., Su, R., Zhang, Z., et al. (2021). Different co-culture models reveal the pivotal role of TBBPA-promoted M2 macrophage polarization in the deterioration of endometrial cancer. J. Hazard. Mater. 413 (February), 125337. doi:10.1016/j.jhazmat.2021.125337
Kelemen, H., Hancu, G., and Papp, L. A. (2019). Analytical methodologies for the determination of ticagrelor. Biomed. Chromatogr. 33 (7), e4528. doi:10.1002/bmc.4528
Kim, S. K., Kim, K. S., and Sang, H. H. (2016). Overview on relative importance of house dust ingestion in human exposure to polybrominated diphenyl ethers (PBDEs): international comparison and Korea as a case. Sci. Total Environ. 571, 82–91. doi:10.1016/j.scitotenv.2016.07.068
Krylov, V. A., Krylov, A. V., Mosyagin, P. V., and Matkivskaya, Y. O. (2011). Liquid-phase microextraction preconcentration of impurities. J. Anal. Chem. 66 (4), 331–350. doi:10.1134/S1061934811040101
Lan, Y., Liu, Y., Cai, Y., Du, Q., Zhu, H., Tu, H., et al. (2023). Eight novel brominated flame retardants in indoor and outdoor dust samples from the E-waste recycling industrial park: implications for human exposure. Environ. Res. 238, 117172. doi:10.1016/J.ENVRES.2023.117172
Lestido-Cardama, A., Paseiro-Cerrato, R., Ackerman, L. K., Sendón, R., and de Quirós, A. R. B. (2022). Determination of BFRs in food contact articles: an analytical approach using DART-HRMS, XFR and HPLC-MS/MS. Food Packag. Shelf Life 33, 100883. doi:10.1016/J.FPSL.2022.100883
Li, M., Gong, X., Tan, Q., Xie, Y., Tong, Y., Ma, J., et al. (2024). A review of occurrence, bioaccumulation, and fate of novel brominated flame retardants in aquatic environments: a comparison with legacy brominated flame retardants. Sci. Total Environ. 939, 173224. doi:10.1016/J.SCITOTENV.2024.173224
Li, W., Sheng, P., Cai, J., Feng, H., and Cai, Q. (2014). Highly sensitive and selective photoelectrochemical biosensor platform for polybrominated diphenyl ether detection using the quantum dots sensitized three-dimensional, macroporous ZnO nanosheet photoelectrode. Biosens. Bioelectron. 61, 209–214. doi:10.1016/j.bios.2014.04.058
Li, W., Yao, L., Geng, H., Sheng, P., and Cai, Q. (2019). Use of carbon quantum dots and fluorescein isothiocyanate in developing an improved competitive fluoroimmunoassay for detecting polybrominated diphenyl ether. J. Iran. Chem. Soc. 16 (8), 1641–1650. doi:10.1007/s13738-019-01639-w
Liang, Y., Hu, W., Jia, C., Wang, Y., Dong, C., Cai, Y., et al. (2023). Rapid screening of polybrominated diphenyl ethers in water by solid-phase microextraction coupled with ultrahigh-resolution mass spectrometry. Anal. Bioanal. Chem. 415 (8), 1437–1444. doi:10.1007/S00216-023-04531-Y
Linares, V., Bellés, M., and Domingo, J. L. (2015). Human exposure to PBDE and critical evaluation of health hazards. Archives Toxicol. 89 (3), 335–356. doi:10.1007/S00204-015-1457-1
Long, F., Zhu, A., and Shi, H. (2013). Recent advances in optical biosensors for environmental monitoring and early warning. Sensors Switz. 13 (10), 13928–13948. doi:10.3390/s131013928
Lyche, J. L., Rosseland, C., Berge, G., and Polder, A. (2015). Human health risk associated with brominated flame-retardants (BFRs). Environ. Int. 74, 170–180. doi:10.1016/j.envint.2014.09.006
Ma, Z., and Zhuang, H. (2017). A highly sensitive and rapid biological probe enhanced real-time immuno-polymerase chain reaction for detecting 2,2′,4,4′-tetrabromodiphenyl ether in indoor airborne particles. Int. J. Environ. Anal. Chem. 97 (14–15), 1405–1419. doi:10.1080/03067319.2018.1424332
Ma, Z., and Zhuang, H. (2018). Biotin–streptavidin system-based real-time immuno-polymerase chain reaction for sensitive detection of 2,2′,4,4′-tetrabromodiphenyl ether in marine fish. Food Agric. Immunol. 29 (1), 1012–1027. doi:10.1080/09540105.2018.1489374
Ma, Z., Zhuang, H. S., and Yang, G. X. (2019). An ultrasensitive real-time immuno-polymerase chain reaction assay for detection of tetrabromobisphenol A in PM 2.5 particles using functionalized nanoprobes. Chin. J. Anal. Chem. 47 (4), e19045–e19052. doi:10.1016/S1872-2040(19)61151-5
Marchesini, G. R., Meimaridou, A., Haasnoot, W., Meulenberg, E., Albertus, F., Mizuguchi, M., et al. (2008). Biosensor discovery of thyroxine transport disrupting chemicals. Toxicol. Appl. Pharmacol. 232 (1), 150–160. doi:10.1016/j.taap.2008.06.014
Marchesini, G. R., Meulenberg, E., Haasnoot, W., Mizuguchi, M., and Irth, H. (2006). Biosensor recognition of thyroid-disrupting chemicals using transport proteins. Anal. Chem. 78 (4), 1107–1114. doi:10.1021/ac051399i
Matamoros, V., Calderón-Preciado, D., Domínguez, C., and Bayona, J. M. (2012). Analytical procedures for the determination of emerging organic contaminants in plant material: a review. Anal. Chim. Acta 722, 8–20. doi:10.1016/j.aca.2012.02.004
Nomngongo, P. N., Catherine Ngila, J., Msagati, T. A. M., Gumbi, B. P., and Iwuoha, E. I. (2012). Determination of selected persistent organic pollutants in wastewater from landfill leachates, using an amperometric biosensor. Phys. Chem. Earth 50–52, 252–261. doi:10.1016/j.pce.2012.08.001
Oulhote, Y., Tremblay, E., ´Arbuckle, T. E., Fraser, W. D., Lemelin, J.-P., S´eguin, J. R., et al. (2018). Prenatal exposure to polybrominated diphenyl ethers and predisposition to frustration at 7 months: results from the MIREC study. Environ. Int. 119, 79–88. doi:10.1016/j.envint.2018.06.010
Palaniswamy, S., Nevala, L., Pesonen, P., Rautio, A., Järvelin, M. R., Abass, K., et al. (2024). Environmental contaminants in Arctic human populations: trends over 30 years. Environ. Int. 189, 108777. doi:10.1016/J.ENVINT.2024.108777
Quesada-González, D., Baiocco, A., Martos, A. A., de la Escosura-Muñiz, A., Palleschi, G., and Merkoçi, A. (2019). Iridium oxide (IV) nanoparticle-based electrocatalytic detection of PBDE. Biosens. Bioelectron. 127, 150–154. doi:10.1016/j.bios.2018.11.050
Radhakrishnan, R., Suni, I. I., Bever, C. S., and Hammock, B. D. (2014). Impedance biosensors: applications to sustainability and remaining technical challenges. ACS Sustain. Chem. Eng. 2 (7), 1649–1655. doi:10.1021/sc500106y
Ridgway, K., Lalljie, S. P. D., and Smith, R. M. (2007). Sample preparation techniques for the determination of trace residues and contaminants in foods. J. Chromatogr. A 1153 (1–2), 36–53. doi:10.1016/j.chroma.2007.01.134
Romanelli, S., Bettazzi, F., Martellini, T., Shelver, W. L., Cincinelli, A., Galarini, R., et al. (2017). Evaluation of a QuEChERS-like extraction approach for the determination of PBDEs in mussels by immuno-assay-based screening methods. Talanta 170, 540–545. doi:10.1016/j.talanta.2017.04.027
Sanchis, A., Salvador, J. P., Campbell, K., Elliott, C. T., Shelver, W. L., Li, Q. X., et al. (2018). Fluorescent microarray for multiplexed quantification of environmental contaminants in seawater samples. Talanta 184, 499–506. doi:10.1016/j.talanta.2018.03.036
Sapozhnikova, Y., Simons, T., and Lehotay, S. J. (2015). Evaluation of a fast and simple sample preparation method for polybrominated diphenyl ether (PBDE) flame retardants and dichlorodiphenyltrichloroethane (DDT) pesticides in fish for analysis by ELISA compared with GC-MS/MS. J. Agric. Food Chem. 63 (18), 4429–4434. doi:10.1021/jf505651g
Śmiełowska, M., and Zabiegała, B. (2020). Current trends in analytical strategies for determination of polybrominated diphenyl ethers (PBDEs) in samples with different matrix compositions – Part 1.: screening of new developments in sample preparation. TrAC - Trends Anal. Chem. 132, 115255. doi:10.1016/j.trac.2018.09.019
Stadion, M., Blume, K., Hackethal, C., Lüth, A., Schumacher, D. M., Lindtner, O., et al. (2024). Germany’s first Total Diet Study - occurrence of non-dioxin-like polychlorinated biphenyls and polybrominated diphenyl ethers in foods. Food Chem. X 22, 101274. doi:10.1016/J.FOCHX.2024.101274
Struzina, L., Pineda, M., and Yargeau, V. (2024). Occurrence and removal of legacy plasticizers and flame retardants through a drinking water treatment plant. Sci. Total Environ. 912, 169333. doi:10.1016/J.SCITOTENV.2023.169333
Tavoloni, T., Stramenga, A., Stecconi, T., Siracusa, M., Bacchiocchi, S., and Piersanti, A. (2020). Single sample preparation for brominated flame retardants in fish and shellfish with dual detection: GC-MS/MS (PBDEs) and LC-MS/MS (HBCDs). Anal. Bioanal. Chem. 412 (2), 397–411. doi:10.1007/s00216-019-02250-x
Vidal, L. G., De Oliveira-Ferreira, N., Torres, J. P. M., Azevedo, A. F., Meirelles, A. C. O., Flach, L., et al. (2023). Brominated flame retardants and natural organobrominated compounds in a vulnerable delphinid species along the Brazilian coast. Sci. Total Environ. 905, 167704. doi:10.1016/J.SCITOTENV.2023.167704
Wang, J., Bever, C. R. S., Majkova, Z., Dechant, J. E., Yang, J., Gee, S. J., et al. (2014). Heterologous antigen selection of camelid heavy chain single domain antibodies against tetrabromobisphenol A. Anal. Chem. 86 (16), 8296–8302. doi:10.1021/ac5017437
Wang, J., Majkova, Z., Bever, C. R. S., Yang, J., Gee, S. J., Li, J., et al. (2015). One-step immunoassay for tetrabromobisphenol a using a camelid single domain antibody-alkaline phosphatase fusion protein. Anal. Chem. 87 (9), 4741–4748. doi:10.1021/ac504735p
Wang, L., Wang, H., Tizaoui, C., Yang, Y., Ali, J., and Zhang, W. (2023). Endocrine disrupting chemicals in water and recent advances on their detection using electrochemical biosensors. Sensors Diagnostics 2 (1), 46–77. doi:10.1039/D2SD00156J
Wang, Y., Chen, F., Ye, X., Wu, T., Wu, K., and Li, C. (2017). Photoelectrochemical immunosensing of tetrabromobisphenol A based on the enhanced effect of dodecahedral gold nanocrystals/MoS2 nanosheets. Sensors Actuators, B Chem. 245, 205–212. doi:10.1016/j.snb.2017.01.140
Wu, Z., He, C., Han, W., Song, J., Li, H., Zhang, Y., et al. (2020). Exposure pathways, levels and toxicity of polybrominated diphenyl ethers in humans: a review. Environ. Res. 187 (March), 109531. doi:10.1016/j.envres.2020.109531
Yakubu, S., Jia, B., Guo, Y., Zou, Y., Song, N., Xiao, J., et al. (2021). Indirect competitive-structured electrochemical immunosensor for tetrabromobisphenol A sensing using CTAB-MnO2 nanosheet hybrid as a label for signal amplification. Anal. Bioanal. Chem. 413, 4217–4226. doi:10.1007/s00216-021-03368-7
Yakubu, S., Xiao, J., Gu, J., Cheng, J., Wang, J., Li, X., et al. (2020). A competitive electrochemical immunosensor based on bimetallic nanoparticle decorated nanoflower-like MnO2 for enhanced peroxidase-like activity and sensitive detection of Tetrabromobisphenol A. Sensors Actuators, B Chem. 325 (August), 128909. doi:10.1016/j.snb.2020.128909
Yu, L., Liu, L., Song, S., Kuang, H., and Xu, C. (2016). Development of an immunochromatographic test strip and ic-ELISA for tetrabromobisphenol: a detection in lake water and rice pudding samples. Food Agric. Immunol. 27 (4), 460–470. doi:10.1080/09540105.2015.1126234
Zeng, K., Zhang, X., Gyimah, E., Bu, Y., Meng, H., and Zhang, Z. (2020). Chemiluminescence imaging immunoassay for simultaneous determination of TBBPA-DHEE and TBBPA-MHEE in aquatic environments. Anal. Bioanal. Chem. 412 (15), 3673–3681. doi:10.1007/s00216-020-02604-w
Zeng, L., Cui, H., Chao, J., Huang, K., Wang, X., Zhou, Y., et al. (2020). Colorimetric determination of tetrabromobisphenol A based on enzyme-mimicking activity and molecular recognition of metal-organic framework-based molecularly imprinted polymers. Microchim. Acta 187 (2), 142. doi:10.1007/s00604-020-4119-9
Zhang, K., Kwabena, A. S., Wang, N., Lu, Y., Cao, Y., Luan, Y., et al. (2020). Electrochemical assays for the detection of TBBPA in plastic products based on rGO/AgNDs nanocomposites and molecularly imprinted polymers. J. Electroanal. Chem. 862, 114022. doi:10.1016/J.JELECHEM.2020.114022
Zhang, Z., Dong, S., Ge, D., Zhu, N., Wang, K., Zhu, G., et al. (2018a). An ultrasensitive competitive immunosensor using silica nanoparticles as an enzyme carrier for simultaneous impedimetric detection of tetrabromobisphenol A bis(2-hydroxyethyl) ether and tetrabromobisphenol A mono(hydroxyethyl) ether. Biosens. Bioelectron. 105 (January), 77–80. doi:10.1016/j.bios.2018.01.029
Zhang, Z., Zhu, N., Dong, S., Huang, M., Yang, L., Wu, X., et al. (2018b). Plasmonic ELISA based on nanospherical brush-induced signal amplification for the ultrasensitive naked-eye simultaneous detection of the typical tetrabromobisphenol A derivative and byproduct. J. Agric. Food Chem. 66 (11), 2996–3002. doi:10.1021/acs.jafc.7b02803
Zhang, Z., Zhu, N., Zou, Y., Zhao, Z., Wu, X., Liang, G., et al. (2018c). A novel and sensitive chemiluminescence immunoassay based on AuNCs@pepsin@luminol for simultaneous detection of tetrabromobisphenol A bis(2-hydroxyethyl) ether and tetrabromobisphenol A mono(hydroxyethyl) ether. Anal. Chim. Acta 1035, 168–174. doi:10.1016/J.ACA.2018.06.039
Keywords: brominated flame retardants (BFRs), polybrominated diphenyl ethers (PBDEs), tetrabromobisphenol A (TBBPA), biosensing, real-time monitoring
Citation: Sarti C, Sforzi L, Martellini T and Cincinelli A (2025) Status and trends of biosensor technologies for environmental monitoring of brominated flame retardants. Front. Anal. Sci. 5:1527655. doi: 10.3389/frans.2025.1527655
Received: 13 November 2024; Accepted: 12 February 2025;
Published: 07 March 2025.
Edited by:
Marcos Tascon, National University of General San Martín, ArgentinaReviewed by:
Ignacio Boron, National Scientific and Technical Research Council (CONICET), ArgentinaCopyright © 2025 Sarti, Sforzi, Martellini and Cincinelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandra Cincinelli, YWxlc3NhbmRyYS5jaW5jaW5lbGxpQHVuaWZpLml0; Chiara Sarti, Y2hpYXJhLnNhcnRpQHVuaWZpLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.