- 1School of Ecology and Environment Studies, Nalanda University, Bihar, India
- 2Biochemical Engineering Department, University College London, London, United Kingdom
Emerging contaminants includes diverse types of synthetic or natural chemical compounds which are not detected, monitored, or controlled in the environment regularly and are released from anthropogenic activities. Substantial quantities of emerging contaminants can be found in the wastewater, originating from agro-industrial and industrial outlets, containing oil and grease, heavy metals, and harmful chemicals. Different species of microalgae can be applied in biological remediation of such contaminants in wastewater. This research emphasizes the multifaceted roles of microalgae in wastewater treatment in context of pollutants, especially the removal of emerging contaminants. A comprehensive overview of different emerging contaminant removal processes was conveyed through an in-depth examination and depiction of the uptake mechanisms employed by microalgae in wastewater treatment in this review. The final section of this review focuses on the articulation of difficulties and prospects for the future of microalgae-based wastewater treatment technology. It is subsequently established how the microalgal technologies for emerging contaminant remediation can be helpful to achieve Sustainable Development Goals (SDGs). This review establishes the connection between phytoremediation technologies with Sustainable Development, and shows how successful implementation of such technologies can lead to the remediation of emerging contaminants and effective management of wastewater.
1 Introduction
Chemicals of emerging concern and are of anthropogenic origin, found in different water bodies because of commercial, agricultural, and domestic discharges with concentrations ranging from microgram to milligram per litre are generally referred to as emerging pollutants (EPs) (Wanda et al., 2017). These pollutants can be organic or inorganic in nature. Organic pollutants include pharmaceutical ingredients, personal care products, endocrine disruptors, hormones, etc.,; heavy metals are examples of inorganic pollutants. These contaminants ultimately cause ecosystem imbalances due to their exceptionally high biological and chemical oxygen demand (BOD and COD). Excess nutrients, such as nitrogen (N) and phosphorus (P), will lead to eutrophication of waterbodies, disrupting the health of water systems. COD, nitrogen, and phosphorus concentrations in various wastewaters were investigated (Amenorfenyo et al., 2019; Chai et al., 2021; Noorani et al., 2024). This phenomenon causes environmental concerns such as the generation of solid waste and byproducts, the emission of undesirable products into the atmosphere, the excessive growth of undesirable microbes that threaten aquatic life forms, and the deterioration of water quality, which leads to widespread health-related problems in areas close to the discharge range. The global issue of emerging pollutants (EPs) accumulated in wastewater has garnered attention. Recently, policymakers and researchers have shown interest in addressing emerging pollutants in wastewater due to their potential hazards to human health and the ecosystem. The treatment of emerging pollutants (EPs) is receiving significant attention due to the potential risks to human health and adverse effects on the environment. (Wanda et al., 2017).
The treatment of wastewater is conducted through primary, secondary, or tertiary levels, employing physical, biological, or chemical methods. Primary treatment targets the removal of easily settled materials, which could lead to operational challenges in subsequent treatment stages. On the contrary, secondary treatment involves physical or biological processes that break down the organic content in wastewater by utilizing dissolved organic matter and oxidizing essential nutrients into nitrate and orthophosphate. Consequently, secondary effluent contains elevated levels of inorganic nitrogen and phosphorus, contributing to eutrophication and posing severe threats to aquatic habitats and human health due to the release of unmanageable amount of organic compounds and heavy metals. (Gondi et al., 2022; Rathod, 2014; Shitu et al., 2024). However, tertiary treatment, which is a progressive treatment method that reduces nitrates, phosphates, and organic matter, is required to produce clean and harmless effluent that will be released into water bodies (Molazadeh et al., 2019). In tertiary treatment, denitrification involves the reduction of nitrate to nitrite, followed by the further reduction of nitrite to nitrogen gas, which is then released into the atmosphere. The inadequate performance of wastewater treatment facilities in certain countries can be attributed to design deficiencies in the water treatment process, a shortage of expertise, and insufficient financial resources. Consequently, the need for well-designed wastewater treatment methods and feasible economic approaches is becoming progressively crucial. (Farazaki and Gikas, 2019).
Conventional wastewater treatment systems focus primarily on eliminating solid suspension and reducing BOD through activated sludge. Consequently, the efficacy of traditional water treatment methods in removing micropollutants and inorganic nutrients remains suboptimal. In situations where water contains substantial quantities of additional components like heavy metals, xenobiotics, and nutrient loads, the biodegradation process, limited by the capabilities of traditional wastewater treatment methods to break down both organic and inorganic constituents, is likely to be ineffective. (Gondi et al., 2022; Wollmann et al., 2019). This phenomenon will result in a lethal environmental issue affecting the ecosystem, namely, oxygen depletion and a higher level of effluent toxicity to aquatic life (Umamaheswari and Shanthakumar, 2016). Furthermore, untreated nutrients in wastewater effluent will reduce the functionality of the disinfection stage, resulting in an increase in chlorine demand, which is harmful to the aquatic ecosystem and human health. As a result, there is a higher demand for treatment processes that can eradicate these nutrients prior to discharge (Chai et al., 2021).
According to the United Nations’ Report (United Nations, 2019), only 70% of domestic and industrial wastewater undergoes treatment in high-income countries. In contrast, the situation is significantly worse in middle- and low-income countries, where only 28%–38% and 8% of wastewater, respectively, is treated. This means, globally more than 80% of all wastewater is discharged into the environment without proper treatment. Traditional wastewater treatment plants (WWTPs), however, are only capable of removing approximately 50% of pharmaceutical contaminants typically present in wastewater (World Health Organization, 2024). These facilities are, in fact, a major source of pollutant discharge into natural water bodies, as illustrated by Zhang, et al. (2017).
For example, Castiglioni et al. (2018) reported that three WWTPs in Milan managed to treat only 13% of personal care products (PCPs) present in their influent. The occurrence of various classes of emerging contaminants (ECs) in wastewater has been studied globally, revealing significant variations in their concentrations depending on geographical location (Tran, et al., 2018). Ciprofloxacin, a commonly used antibiotic, has been detected at concentrations ranging from below detection limits to 6.4 μg L−1 in Asia, and from <LDL to 246.1 μg L−1 in North America. Similarly, ibuprofen concentrations in wastewater have ranged from 34.8 to 55,975 ng L−1 in Asia and from 2,500 to 45,000 ng L−1 in North America. Table 1 summarizes concentrations of widely used antibiotics in WWTP influent and effluent across Asia, Europe, and North America.
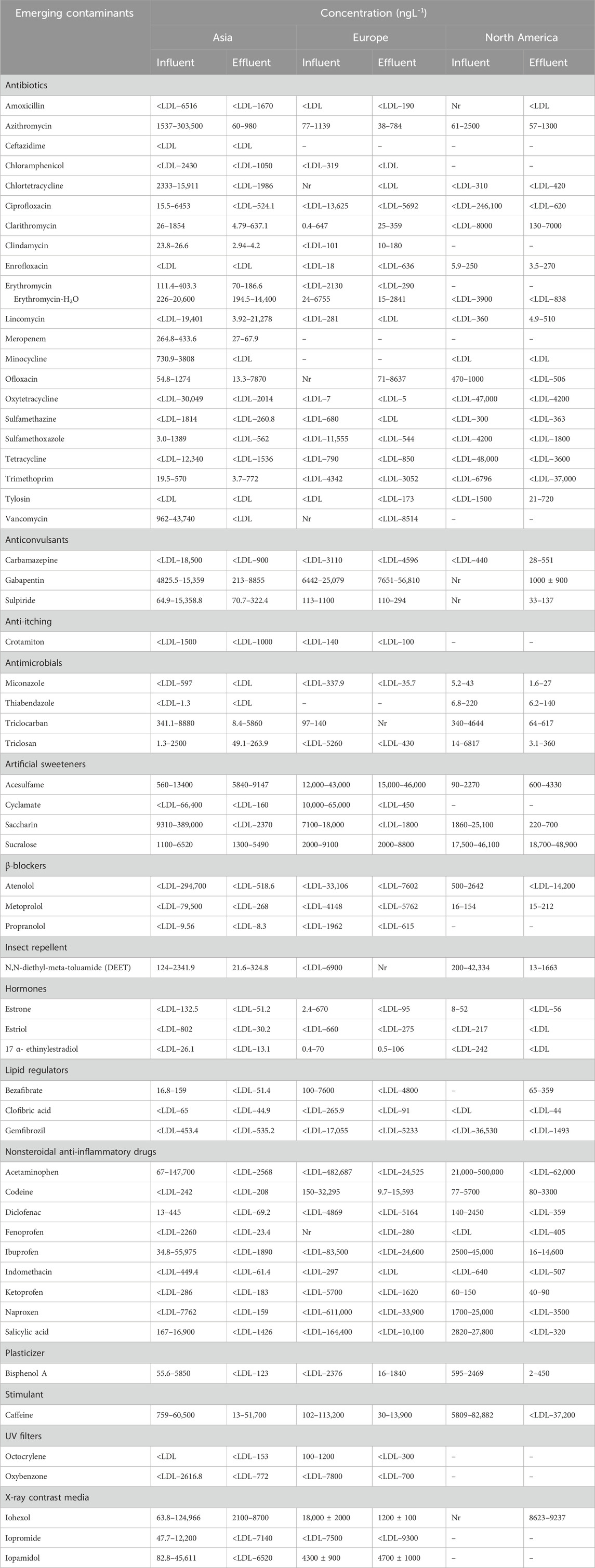
Table 1. Typical concentration ranges of various emerging contaminants (ECs) in raw influent and treated effluent from full-scale wastewater treatment plants (WWTPs). Adapted from (Tran, et al., 2018).
Pharmaceuticals in drinking water can pose significant risks to the environment and wildlife. For instance, natural and synthetic hormones like progesterone have been detected in wastewater (Houtman, et al., 2018). When animals ingest such compounds, their endocrine systems may be disrupted, potentially leading to fertility issues, delayed development of reproductive organs, and damage to the kidney or liver (Fang, et al., 2021). Observed that chronic or sub-chronic exposure to 17-β-estradiol in mosquitofish altered hormonal balances and vitellogenin protein levels. The growing production of pharmaceuticals further exacerbates the issue, increasing the concentrations of these ECs in domestic wastewater. For example, India’s pharmaceutical market is expanding at a rate of 10% annually, making it one of the fastest-growing globally (IQVIA, 2018). This raises concerns about the emergence of antibiotic resistance genes, which could undermine the efficacy of antibiotics. Additionally, microplastics have recently been classified as emerging contaminants.
Finally, it is important to emphasize that the “emerging” nature of these contaminants reflects the fact that many of their impacts remain insufficiently studied or understood, leaving significant gaps in our knowledge of their long-term effects.
Table 1 shows the concentration ranges of various emerging contaminants (ECs) in raw influent and treated effluent from full-scale wastewater treatment plants (WWTPs), adapted from Tran et al., 2018. Wastewater treatment technologies based on microalgae present an attractive solution due to their efficient fixation of inorganic compounds like carbon dioxide and heavy metals, and to deal with emerging contaminants (Inuwa et al., 2023). Microalgae exhibit a notable capacity for the uptake of inorganic nutrients, requiring nitrogen and phosphorus for protein synthesis and utilizing heavy metals as micronutrients for their development. Consequently, employing microalgae as agents for bioremediation in wastewater proves effective in extracting nitrogen and phosphorus from the wastewater, maintaining dissolved oxygen levels, and contributing to the reduction of pathogens and faecal bacteria present in the wastewater. The study’s findings indicate that the interaction between wastewater and microalgae resulted in a significant decrease in levels of several contaminants, especially the emerging contaminants in wastewater (Das et al., 2019; Rathod, 2014). Microalgae treatment is also a more efficient method of wastewater treatment because it can treat wastewater in a single step, as opposed to traditional wastewater treatment, which requires a number of steps to fix the carbon, nitrogen, and phosphorus ratios (C:N:P). It is also an environmentally sustainable option because it can convert carbon dioxide into chemical substances and fuel products without resulting in pollution, thereby aiding in the reduction of greenhouse gas emissions (Klinthong et al., 2015). Microalgal biomass harvested from wastewater treatment can be converted into valuable bio-based products such as health supplements, biohydrogen, bio-alcohols, and bio-hydrocarbons to offset its production costs (Koyande et al., 2019; Perez-Garcia and Bashan, 2015). Microalgae have found widespread application in wastewater treatment, with eukaryotic and prokaryotic blue-green algae being the most commonly employed species in experimental setups. The concentrations of non-renewable resource phosphorus and another essential nutrient, nitrogen, found in wastewater, are sufficient to support the cultivation of microalgae for cell growth substrates, biomass yields, and carbon neutrality. Consequently, there is a reduced reliance on freshwater and industrial nutrients typically needed for conventional biological purification, leading to a significant reduction in overall operational costs and the environmental impact of the treatment process. (Delrue et al., 2016).
This review aims to demonstrate the potential of microalgae in removal of emerging contaminants from wastewater through different treatment processes. Different sections discuss phycoremediation of wastewater in removal of several chemicals of emerging concern (ex. dyes, heavy metals, xenobiotic compounds, pesticides, pharmaceuticals, inorganic nutrients, and different microorganisms, especially the pathogens). Different mechanisms employed by microorganisms to remove the emerging contaminants (ex. biosorption, bioaccumulation, biodegradation, etc.) are explained. Also, the influences of several environmental factors (pH, temperature, light, etc.) on the actions of microalgae for wastewater treatment are discussed in this article. Finally, the advantages, limitations, and future perspectives of the microalgae-based treatment method are explained, and it is established that how these eco-friendly technologies can ensure sustainability.
2 Phycoremediation of wastewater
Microalgae, minute organisms consisting of eukaryotic cells, employ the photosynthetic process akin to that found in higher plants. The cellular structure of microalgae encompasses essential components such as cell walls, plasma membranes, cytoplasm, nuclei, and organelles. Notably, microalgae cells contain plastids housing chlorophyll, a pigment crucial for synthesizing food through photosynthesis. A distinguishing feature of microalgae is the absence of a vascular system for nutrient transport, a contrast to higher plants. This lack is compensated by the photoautotrophic nature of each microalgal cell, enabling direct absorption of nutrients without the need for an elaborate vascular network. This unique characteristic simplifies nutrient uptake processes in microalgae, setting them apart from higher plants in terms of nutrient transport strategies. (Emparan et al., 2019). Through their uptake mechanism, microalgae-based wastewater treatment is effective in removing inorganic compounds such as nitrate, phosphate, heavy metals, inorganic carbon, toxic substances (organic and inorganic), BOD, COD, and other impurities dissolved in wastewater (Borowitzka, 1998). Microalgae use photons as energy in their chloroplast cells and extract CO2 from exhaust gases generated by combustion or bacterial respiration, as well as nutrients from wastewater, to synthesise biomass while producing oxygen. As a result, the microalgae biomass is obtained and O2 is released into the atmosphere. The conversion of CO2 and water into organic compounds does not require any additional energy, thereby avoiding secondary pollution. The oxygen released by microalgae is sufficient to meet bacteria’s aerobic requirements for metabolising residual organic substances in treated wastewater (Chai et al., 2021).
2.1 Nutrient removal
The uptake and consumption of nitrates and phosphates by microalgae cells for growth can significantly reduce the nitrogen and phosphorus content of wastewater and improve the quality of the wastewater discharge. The ability of different microalgae strains to remove nitrogen and phosphate was investigated (Chlamydomonas sp., Chlorella sp., and Oocystis sp.) by assessing NO3−-N and PO43-- P loss. Algae require nitrogen and phosphorus to grow (Rasoul-Amini et al., 2014). Phosphorus is required for the synthesis of nucleic acids, phospholipids, and phosphate esters in the cells, whereas nitrogen binds to proteins in the algal cell, which account for 45%–60% of the dry weight. They use nitrogen and phosphorus-containing organic compounds derived from their carbon sources. The use of those compounds by algae results in the removal of nutrients from the wastewater, a process that can last from a few hours to a few days (Kaloudas et al., 2021).
2.1.1 Phosphorus removal
Inorganic phosphorus, which can be found naturally in lipids, nucleic acids, and proteins in wastewater, is important for microalgae energy metabolism and growth. Inorganic phosphates are transported across the plasma membrane of microalgae cells. Through phosphorylation, inorganic phosphorus in the forms of mono- and dihydrogen phosphate (HPO42- and H2PO4) is integrated into organic compounds such as adenosine diphosphate (ADP) in the case of algae. To produce its final product, ATP, the phosphorylation process requires energy. Energy can be obtained through the oxidation of respiratory substrates, the electron transport system of mitochondria found in eukaryotic microalgae, and light used in photosynthesis (Chai et al., 2021; Emparan et al., 2019).
2.1.2 Nitrogen removal
Organic nitrogen can enter wastewater from land where animal manure is stored or applied via sewage effluent. Organic nitrogen is a critical component of biological substances such as enzymes, peptides, proteins, chlorophylls, and energy transfer molecules like ADP and ATP. Organic nitrogen is derived from inorganic sources encompassing nitrite (NO2−), nitrate (NO3−), nitric acid (HNO3), ammonia (NH3), ammonium (NH4+), and nitrogen gas (N2). The presence of nitrogen in wastewater is usually in the form of NH4+, NO2− and NO3−. Assimilation can be used by eukaryotic microalgae to convert inorganic nitrogen into organic forms. transformation mechanism that takes place across the microalgae plasma membrane is the reduction of nitrate (NO3−) to nitrite (NO2−) and to ammonium (NH4+) subsequently, which is then integrated into amino acids (the organic form of nitrogen). The first step in nitrate assimilation involves nitrate reductase (NR), which is the reduced form of nicotinamide adenine dinucleotide (NADH), C21H27N7O14P2, which is found in microalgae and transfers two electrons in the reaction of converting nitrate to nitrite. Following that, ferrodoxin (Fd) from microalgae and nitrite reductase (NADPH, C21H29N7O17P3) produced from the photosynthesis reaction involving ADP, phosphate, and NADP transfer six electrons in the reaction of reducing NO2 to NH4+. All inorganic forms of nitrogen will be reduced to NH4+ as a result of this action within the intracellular fluid of microalgae. Finally, glutamic acids (Glu), C5H9NO4, neuroactive amino acids found in microalgae, and adenosine triphosphate (ATP) released from phosphorylation (the process of assimilation of phosphates into organic compounds) incorporate ammonium into amino acids (glutamine) within microalgae intracellular fluid (Chai et al., 2021; Emparan et al., 2019; Kaloudas et al., 2021).
In one of the recent studies, two microalgal species (Chlorella sorokiniana and Selenastrum sp.) were applied for fish processing wastewater (FPWW) after fat and oil removal, with an objective of facilitating the reuse of wastewater and recovery of several nutrients. The results showed that Chlorella sorokiniana cultivated in the unfiltered FPWW displayed the highest growth and nutrient removal (Khalatbari et al., 2024).
2.2 Xenobiotic compounds removal
Microalgae play an important role in the dispersion, chemical transformation, and bioaccumulation of many toxic xenobiotic compounds. Incomplete combustion of organic materials produces polycyclic aromatic hydrocarbons (PAHs). Because they are generally persistent and highly toxic, with carcinogenic and/or teratogenic properties, they are the subject of strict control and monitoring (Torres et al., 2008). Some microalgae species have a metabolic profile that allows them to survive in PAH-contaminated environments and even assimilate and degrade these contaminants under certain conditions. These organisms can bio-transform low-molecular-weight PAHs; naphthalene can be hydroxylated to form two-ringed PAHs, while phenanthrene (tricyclic PAH) can be bio-transformed to its hydroxylated intermediates (Olmos-Espejel et al., 2012; Lei et al., 2007) tested the possibility of removing fluoranthene (1.0 mg/L), pyrene (1.0 mg/L) and a mixture of fluoranthene (0.5 mg/L) and pyrene (0.5 mg/L) using four microalgae species (C. vulgaris, S. platydiscus, S. quadricauda, and Selenastrum capricornutum) incubated for 7 days. S. capricornutum performed best, with removal efficiencies ranging from 88% to 98% for various pollutant concentrations.
Monoaromatic hydrocarbons are also known to be mutagenic and carcinogenic. The production, transportation, and storage of oil and oil products are the primary sources of contamination by these compounds. They are highly soluble in water and are strongly absorbed by the soil. When macro- and micronutrients are available at ideal environmental conditions for microalgae growth, these pollutants serve as carbon sources (Semple et al., 1999; Paixão et al., 2007) performed controlled experiments in which they exposed T. chuii cultures to 14 different types of gasoline at varying concentrations (0%, 4.6%, 10.0%, 22.0%, 46.0%, and 100%) for 96 and 24 h. Growth inhibition and abnormalities occurred only at concentrations greater than 50% gasoline, demonstrating significant tolerance to such monoaromatic hydrocarbon mixtures. Chlorophenols are another type of highly toxic and persistent xenobiotic with carcinogenic properties. These compounds are widely used in the manufacture of pesticides and wood preservatives (Petroutsos et al., 2008). investigated the potential of 2,4-dichlorophenol (2,4-DCP) removal using the microalgae Tetraselmis marina and found that the microalga could metabolise more than 1 mmol L1 2,4-DCP in a 2 L photobioreactor after 6 days of exposure to the contaminant. Anabaena and Aulosira fertilissima demonstrated remarkable abilities to bioconcentrate and degrade DDT into its two main metabolites; dichlorodiphenyldichloroethylene (DDD) and dichlorodiphenyldichloroethane (DDE) (Lal et al., 1987).
2.3 Dyes removal
Because of their high surface area and binding affinity, microalgae have been used to remove colour and vinyl sulfone dye from textile wastewater. The cell wall of microalgae is involved in dye removal mechanisms such as biosorption, electrostatic attraction, complexation, and bioconversion (Andrade et al., 2018). Dye ions adhere and accumulate on the surface of algal biopolymers, then diffuse onto the biopolymer’s solid phase. Extracellular polymers with functional groups can aid in the biosorption of dye molecules onto polymer surfaces (Saha et al., 2023). Spirogyra biomass, a microalga species, has been demonstrated to be an effective biosorbent for reactive dye removal. Caulerpa lentillifera and Caulerpa scalpelliformis biomass can remove basic dyes via biosorption. Furthermore, C. vulgaris has been widely used as a biosorbent for the removal of reactive dyes like Remazol Black B (Aksu and Tezer, 2005). Microalgae disintegrate dyes into simpler compounds for bioconversion. Chlorella vulgaris can remove (63–69) % of the colour from mono-azo dye by converting it to aniline, as demonstrated by adding them to different concentrations of textile wastewater (Supranol Red 3BW) for a 10-day culture period. Furthermore, five microalgal strains, A. flos aquae, N. elepsosporum, N. linkia, A. variabilis, and C. vulgaris, were evaluated for their ability to remove red coloration from textile industrial effluent. The experiment revealed that all microalgae strains tested could remove the red dye from the treated textile wastewater effluent with varying reduction percentages. The dye was completely removed by N. elepsosporum, followed by C. vulgaris (96.16%), A. variabilis (88.71%), N. linkia (79.03%), and A. flos aquae (50.81%). The complexity of the textile wastewater, which contains entangled compositions of other chemicals such as heavy metals, does not appear to affect the efficacy of colour removal by microalgae (Ghazal et al., 2018; Kumar et al., 2014).
2.4 Heavy metals (HM) removal
Heavy metals are metals with atomic densities greater than 4000 kg/m3 (Vardhan et al., 2019). HMs pollution is a major issue worldwide due to their non-biodegradable properties, abundant sources, toxicity, and accumulative behaviour. Even at low concentrations, HMs are naturally toxic and cause serious diseases in humans and animals (Edelstein and Ben-Hur, 2018). HMs are classified as radionuclides (U, Ra, Am, and Th), precious metals (Au, Pd, Pt, and Ru), and toxic metals (Cu, Cr, As, Zn, Ni, Ag, Sn, Co, and Pb). HMs enter aquatic systems through industrial discharges and agricultural runoff, in addition to naturally occurring sources (Pavithra et al., 2020). Various treatment approaches are available, with varying degrees of success, to remove HMs from the aquatic environment. In any case, these traditional treatment methods generate secondary waste while incurring high operating and maintenance costs. As a result, developing effective, environmentally friendly, and economically viable treatments is critical. Metal bioaccumulation by microalgae may be a viable method of wastewater remediation (Vardhan et al., 2019). Most metal removal techniques use algae, both dry biomass and dead forms, with adsorption as the primary removal mechanism. Microalgae can accumulate toxic heavy metal ions from aqueous solutions at concentrations on the order of 15 mg/g biomass, demonstrating that the process is competitive with other treatment methods (Abdel-Raouf et al., 2012).
Several microalgae species, including C. vulgaris, Scenedesmus sp., Chlorococcum sp., Lyngbya spiralis, Tolypothirx tenuis, Stigonema sp., Phormidium molle, Aphanothece halophytica, and Chroococcus paris, can remove Hg (II), Cd (II), and Pb (II) (Tüzün et al., 2005). Metal absorption by microalgae occurs in two stages: first, the surface of algal cells interacts with metals via physical adsorption or ion exchange in a fast process. The following step, known as chemisorption, is slower and takes place intracellularly, and it is related to metabolic processes involving active binding groups. A cellular distribution analysis revealed that large amounts of metal ions bind to the cell wall, while an insoluble fraction accumulates intracellularly (Omar, 2002).
In a detoxification assay using C. vulgaris (Shen et al., 2013), investigated the mechanism of bioconversion of Cr (VI) into its less toxic form of Cr (III). Secondary alcohols were found to be primarily responsible for the reduction of Cr (VI) to Cr (III), with -NH2 and -COOH being the main functional groups associated with the biosorption and bio-fixation of these elements. Magro et al. report a chromium hexavalent (Cr VI) removal value of 60.92% when cultivating Spirulina platensis strain in a mixture of artificial medium and wastewater under controlled air, temperature, and lighting conditions.
(Chong et al., 2000) investigated the treatment of synthetic wastewater containing nickel (Ni) and zinc (Zn) using 11 microalga species with the same cell density. The S. quadricauda species was singled out for its ability to reduce an initial concentration of nickel (Ni) and zinc (Zn) of 30 mg/L to 0.9 mg/L. The removal of these metals reached 97% within the first 5 min, then dropped to 0.4 mg/L within the next 90 min. This efficiency can be attributed to the fact that it has a larger surface area than the other microalgae studied.
Arsenic (As) is a toxic element that can be found in nearly all terrestrial environments. The organic forms are less toxic than the inorganic forms [arsenite, As (III) and arsenate, As (V)]. Arsenic exposure through drinking water is linked to a variety of skin diseases, respiratory, neurological, cardiovascular, gastrointestinal, and urinary disorders, as well as an increased risk of high blood pressure and diabetes (Mestrot et al., 2013; Zhang et al., 2013) demonstrated that the marine microalga Ostreococcus tauri can convert inorganic arsenic into a less toxic organic form that can be easily incorporated into biogeochemical cycles and can promote As volatilization via the biomethylation metabolic mechanism.
2.5 Pathogens removal
Pathogen removal mechanisms of microalgae in wastewater include nutrient competition, pH and dissolved oxygen level elevation, pathogen adhesion and sedimentation, and algal toxins (Dar et al., 2019). The CO2 assimilation in photosynthesis causes the pH value to rise during microalgae cultivation. Nitrogen absorption by microalgae raises the pH of the medium because every nitrate ion converted to ammonia produces one OH− ion. This phenomenon will result in pathogen eradication. Because of the limited transfer of carbon dioxide from the atmosphere and the process of microbial oxygenation, microalgae will also raise pH levels, potentially causing pathogens to die off. Fluctuations in pH are also known to have an adverse effect on E. coli survival, resulting in a remarkable elimination of faecal coliforms such as Escherichia coli, Enterococci, and Clostridium perfringens in waterbodies (Ansa et al., 2011). Oxygenation caused by bacterial respiration in treatment ponds that contributes to algal growth has been linked to faecal bacteria annihilation due to the presence of toxic oxygen formations. Microalgae photosynthesis activity is also sufficient to raise oxygen concentrations in waterbodies to levels that are harmful to faecal bacteria. Oxygen concentrations greater than 0.5 mg/L have been linked to faecal bacteria removal (Ansa et al., 2011). The adhesion of faecal bacteria to microalgae in wastewater is critical because it ensures that bacterial cells are in close proximity when microalgae elevate pH and dissolved oxygen. Pathogens will first attach to the solid matter that will sink as sediment and deposit on the surface of microalgal cells for adhesion to occur. Following that, the polysaccharides that are available for expression by bacterial cells will form positively charged amino groups. The positively charged polymers will then neutralise the negatively charged microalgal surface, resulting in the formation of a bridge between the particles and bacterial cell adhesion to microalgae (Dar et al., 2019). Furthermore, a toxin called microcystin-LR produced by an algal strain called Synechocystis sp. and toxins of long-chain fatty acids produced by C. vulgaris under high pH conditions have been found to be toxic to pathogens and faecal bacteria (Mohamed, 2008). In another experiment, Salmonella enterica was found to be eliminated by a microalgae species, Scenedesmus sp. (Mezzari et al., 2017). The effect of elevated microalgae pH and dissolved oxygen (DO) levels on the removal and inactivation of E. coli, Enterococci, and C. perfringens was studied (Liu et al., 2020).
2.6 Pesticides removal
Through biosorption and biodegradation, microalgae can assimilate a wide range of organic pollutants, including pesticides, as an energy source for their growth in wastewater. Biosorption includes mechanisms of absorption, adsorption, surface complexation, ion exchange, and precipitation that occur in both living and dead cell walls. Biodegradation occurs when microalgae produce enzymes that break down the bonds in pesticide molecules. Chlorella vulgaris was exposed to four common fungicides, propamocarb, mandipropamid, cyprodinil, and metalaxyl, in two experiments: short-term involving biosorption (60 min) and long-term involving biodegradation (4 days) in a study conducted by (Chai et al., 2021). Another set of short and long-term experiments was carried out to determine the percentage of pesticides removed by microalgae. Chlorella vulgaris was used in the experiment, and the pesticides used were molinate, simazine, isoproturon, atrazine, propanil, carbofuran, dimethoate, pendimethalin, metoalcholar, and pyproxin. The results showed that microalgae removed more pesticides in the long-term experiment than in the short-term experiment (Hussein et al., 2016).
Removal of pathogens, pesticides, dyes and heavy metals by microalgae with associated mechanisms are shown in Figure 1.

Figure 1. Removal mechanisms of pathogens, pesticides, dyes and heavy metals by microalgae. Reprinted with permission from Elsevier (Chai et al., 2021), licence number 5705400764493.
2.7 Pharmaceuticals removal
Pharmaceutical compounds (PCs) are a broad class of chemical substances that are widely used around the world. PC residues pollute aquatic environments from agricultural operations, hospital effluents, industrial pollution, and household trash. The removal of emerging pharmaceuticals in conventional wastewater treatment plants ranges from 10% to 100%, depending on the method and the emerging pollutant (Kruglova et al., 2016). Pharmaceutical compounds have measured concentrations ranging from 0.008 to 55.78 g/L, while pesticides have measured concentrations ranging from 0.01 to 8845 g/L. Some of the negative effects on aquatic organisms include behavioural changes, the accumulation of pharmaceutical and cosmetic products, reproductive damage, and the inhibition of cell proliferation (Mascolo et al., 2010). Microalgae extracellular enzymes convert certain antibiotics into less toxic or even non-toxic intermediates. Enzymes in cells then bioaccumulate and degrade these intermediates. Furthermore, the degree of biodegradability is determined by the complexity of the structures (Naghdi et al., 2018). Both ketoprofen and ibuprofen, which are both nonsteroidal anti-inflammatory drugs, are eliminated from wastewater in different ways. Ibuprofen was eliminated more efficiently than ketoprofen using vascular plants and microalgae-mediated treatment. Chlorella sorokiniana completely destroyed paracetamol and ibuprofen (Bai and Acharya, 2017). Adsorption, accumulation, and eventually biodegradation of various substances is facilitated by the EPS matrix. Because of the presence of carboxyl, hydroxyl, and phosphoryl functional groups, microalgae are predominantly negatively charged, which is advantageous for capturing positively charged PC molecules (Hom-Diaz et al., 2015). Many recent studies have demonstrated the rapid uptake of PCs onto the cell wall using EPS. In general, the studies suggest that PCs are eliminated through a variety of mechanisms, with bio-adsorption being the most common. Bio-adsorption contributes differently depending on the microalgae used and the micropollutant absorbed (Bai and Acharya, 2017). Study has demonstrated that Scenedesmus dimorphus can biodegrade about 85% of 17 a-estradiol within 7 days (Zhang et al., 2014).
2.8 Carbon dioxide fixation
Industrial effluents contain about 10%–15% CO2, making them an excellent source of CO2 for microalgae cultivation and a potentially more efficient route for bio-fixation (Ji et al., 2013). Microalgae have received a lot of attention among biological CO2 fixation technologies because they can convert CO2 (and complementary nutrients) into biomass at much higher rates than conventional crops (Farhadian et al., 2008). Furthermore, they are a sustainable ecological alternative to CO2 mitigation in the atmosphere. Microalgae contain approximately 50% carbon in their composition, and to produce 1 tonne of microalgae requires approximately 1.83 tonnes of CO2. Direct use of combustion gases from chimneys in microalgae cultures reduces pre-treatment costs but requires tolerance to extreme conditions such as high CO2 concentrations and the presence of inhibitory compounds such as NOx and SOx (Kumar et al., 2010; Cheng et al., 2006) tested different concentrations of CO2 as the input gas when controlling CO2 levels in a culture of C. vulgaris in membrane photobioreactors with a closed environment and achieved the best results for CO2 removal at a concentration of 1.0% (Chiu et al., 2011). Investigated the growth and potential for on-site bioremediation of Chlorella sp. MTF-7, a thermotolerant and CO2-tolerant strain. This microalga was grown in a tubular photobioreactor with an artificial directly aerated medium and an intermittent flow of steel industry combustion gas. In this cultivation, average CO2, SO2, and NO removal efficiencies of 60, 70, and 50%, respectively, were obtained.
2.9 Biological oxygen demand (BOD) reduction
High levels of biochemical oxygen demand can deplete the oxygen in the water, causing the fish to suffocate and creating conditions for anaerobiosis in the water. When it comes to wastewater treatment, removing biochemical oxygen demand is a primary goal. Because microalgae produce oxygen through photosynthesis, they can reduce biological oxygen demand in wastewater. The removal of phenolic compounds reduces the waterbody’s biochemical oxygen demand (Kaloudas et al., 2021). Microalgae such as Chlorella pyrenoidosa, Chlorella kessleri, and Spirulina sp. have been shown in experimental procedures to have the ability to remove phenolic compounds from water and can be prominent species for phenol removal (Zhang C. et al., 2020). Although the removal of phenolic compounds by algae in wastewater treatment may be difficult because microalgae can only biodegrade phenol under limited carbon source conditions in which they use phenol as an alternative carbon source, wastewaters are typically rich in carbon sources for the algae to utilise, reducing the potential use of phenol compounds as an alternative energy source (Kaloudas et al., 2021).
3 Microalgal mechanisms for the removal of emerging pollutants
Pollutant removal via a microalgal-based treatment system involves a variety of reaction mechanisms. The bioremediation mechanisms of the microalgae-based treatment method for removing emerging pollutants are depicted in Figure 1. The mechanisms underlying microalgal-based EP bioremediation are described as follows, (a) During bio uptake, pollutants pass through the algal cell wall and attach to intracellular proteins in living cells and (b) in the non-living cell during bioaccumulation (c) during biosorption, pollutants are adsorbed into the algal cell wall or Extra Polymeric substances (EPS) (d) During biodegradation, complex pollutants are broken down into simpler, less toxic compounds (e) During photodegradation, they undergo exposure to direct or UV light (Gondi et al., 2022). Table 2 shows the quantitative comparison between the steps of phyco-remediation.
3.1 Biosorption
Biosorption refers to the adherence of pollutants to the surface of microorganisms. Algae has the potential to be used as a bio-sorbent. On the algal cell surface, different functional groups such as polysaccharides, lipids, and proteins serve as adsorption sites (El-Naggar et al., 2019). There are two types of biosorption; physisorption (physical biosorption) and chemisorption (chemical biosorption). Solute molecules interact with the binding sites on the sorbent surface during physical adsorption, whereas chemical bonds are formed during chemisorption. The advantages of this method include lower costs, simple processes, no sludge production, and the ability to remediate large amounts of wastewater with low pollutant concentrations (Lo et al., 2014; Olawale, 2021). Microalgae, which can produce Exopolysaccharides (EPSs), are used as surface-active agents for eliminating pollutants (primarily heavy metals). Algal biosorption is influenced by a variety of factors, including cell wall composition, which is essential for electrostatic attraction and chelation or complexation. Algal bioremediation is facilitated by functional groups on the algal cell surface, especially the carboxyl group. The interaction of positively charged pollutants and negatively charged microalgae cellular walls is involved in the biosorption mechanism. The rate of biosorption is influenced by dissolved oxygen, pollutants concentration, initial pH level, and hydraulic retention time (Gusain et al., 2020; Ma et al., 2020).
3.2 Bioaccumulation and bio-uptake
Bioaccumulation is defined as the accumulation of contaminants from the external environment within the cytoplasm of a cell. Spirogyra, for example, can efficiently absorb and collect contaminants inside their cells and use them to grow. Nannochloris sp. absorbed only 11% of sulfamethoxazole, 11% of trimethoprim, 27% of triclosan, and 13% of carbamazepine (Rezania et al., 2016). Pollutant bio-adsorption and bioaccumulation mechanisms are completely distinct. The estimation of bioaccumulation and bio-adsorption of pollutants is difficult because both processes occur in most cases in parallel. The first stage of the bioaccumulation process is bio-adsorption. Molecules must be adsorbed within the microalgal cell in order to bioaccumulate there. Pharmaceuticals (triclosan and sulfamethoxazole) have been found to bioaccumulate in microalgae, resulting in an excess of reactive oxygen species and, eventually, cell death (Bai and Acharya, 2017).
In bioaccumulation and bio-uptake, contaminants can enter algal cells through the cell wall and bind to intracellular proteins. The primary distinction among bioaccumulation and bio-uptake is that bio-uptake of contaminants can only occur in living microalgae cells. The transfer of hazardous substances or pollutants from the surrounding environment into the cells is known as biological or bio-uptake. Microalgae can absorb nutrients and can be mass cultivated (Mulla et al., 2019).
3.3 Biodegradation
Enzymes catalyse the metabolic breakdown or degradation of contaminants during biodegradation. In general, microalgae degrade complex parent compounds into simpler ones. Several enzymatic processes, such as hydroxylation, glycosylation dehydrogenation, hydrogenation, and hydrolysis, may be involved (Varjani, 2017). Pollutant biodegradation by algal-bioremediation occurs in three stages, with several enzymes involved in the catalysis. The first phase of cytochrome P450 detoxification involves hydrolysis, oxidation, or reduction activities. During this phase, the addition of the hydroxyl group transforms the lipophilic molecules to hydrophilic molecules. In the second phase, compounds with electrophilic groups develop a conjugate bond with glutathione in the second phase that shields the cell from oxidative damage. A variety of enzymes (such as carboxylase, dehydrogenase, laccases, and decarboxylase) are used in the third phase. The biodegradability of compounds is determined by the complexity of their structures (Xiong et al., 2016). Compounds with a linear and unsaturated structure, as well as electron-donating groups, biodegrade faster than complex compounds with a cyclic structure, as demonstrated by Navicula sp., C. pyrenoidosa, and S. obliquus, which showed 95% biodegradation. Other examples include the biodegradation of levofloxacin by C. vulgaris. Due to the presence of few extracellular enzymes, EPS is hygroscopic, forming a matrix around the cells that aids in adsorption, accumulation, and biodegradation (Xiong et al., 2016; Posadas et al., 2014) proposed that contaminants biodegradability is affected by the C:N:P ratio of wastewater, and that the ideal C:N:P for optimum biodegradability is 100:18:2. Biodegradation, as opposed to bioaccumulation or biosorption, can reduce contaminants toxicity in algae cells, and algal biomass could be used to produce biofuels and value-added bioproducts. Biodegradation can occur in two ways; co-metabolism and metabolic biodegradation. The co-metabolism biodegradation process includes the breakdown of contaminants catalysed by enzymes, with contaminants serving as a carbon source for algae throughout metabolic biodegradation (Norvill et al., 2016).
Biosorption, biodegradation and detoxification of heavy metals by microalgae is shown in Figure 2 with possible mechanisms.
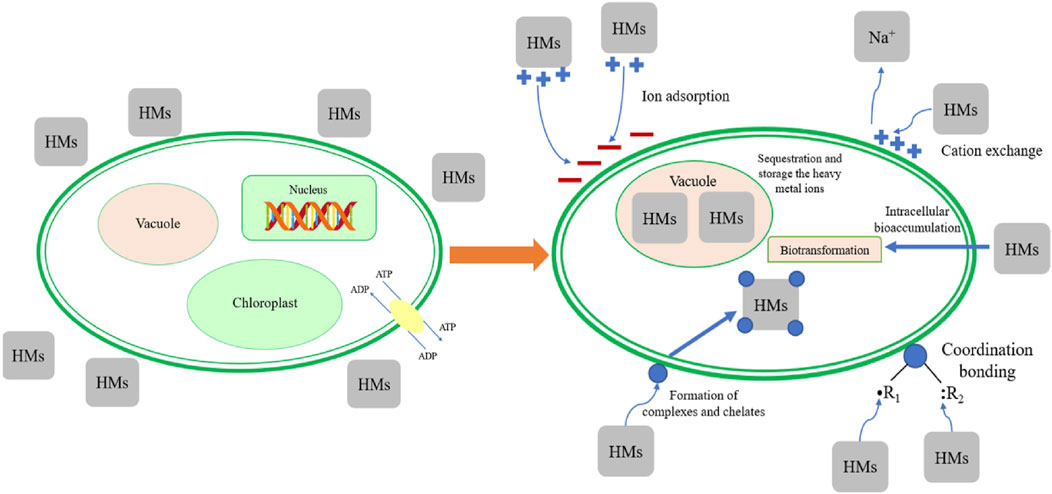
Figure 2. Biosorption, biodegradation and detoxification of heavy metals by microalgae. Reprinted with permission from Elsevier (Yan et al., 2022), licence number 5705480505642.
3.4 Photodegradation
Even if a pollutant cannot be bioremediated using the techniques described above, microalgae can still play an essential part in effective bioremediation via photodegradation. There are two types of photodegradation: photooxidative degradation and photolysis (Abo et al., 2016). When a pollutant interacts with oxidants like hydroxyl radicals formed as a result of photooxidation, photooxidative degradation occurs. Photolysis occurs when a pollutant absorbs light, causing a change in the pollutant’s conformation and, as a result, its degradation. The photodegradation process is controlled by the physicochemical characteristics of the pollutant and wastewater, as well as the intensity and wavelength of light. The introduction of Dissolved Organic Molecules (DOM) is another method for increasing the rate of photodegradation. DOM is a class of compounds that include fulvic acids, humic acids, and hemicellulose and play an active part in enhancing photodegradation through mechanisms such as hydroxyl radical production or redox cycling. Pollutants that absorb less light become photosensitized and transformed as a result of interactions with EOMs (Extracellular Organic Matter) and DOMs (Gondi et al., 2022). There are two types of photo-degradation; direct and indirect photo-degradation. Algal systems use algal scrubbers and turbulent mixing in both photobioreactors to increase light exposure. This process, however, is highly selective, as not all pollutants can be photodegraded (Reymann et al., 2020).
3.5 Hydrolysis
In conventional treatment processes, hydrolysis can be used to deactivate some emerging pollutants. This process is primarily determined by the pollutant structure. The disadvantage of the method is the fact that numerous emerging pollutants, particularly pharmaceuticals, can withstand hydrolysis. Hydrolysis, for example, can only be used to break down a few pharmaceuticals, such as lactam antibiotics. This process is resistant to pharmaceuticals that include sulphonamide and fluoroquinolone (Banu et al., 2020). Chemical structure, pollutant concentration, temperature, and pH are all factors that influence hydrolysis. Although information on antibiotic hydrolysis in micro-algal bioremediation is limited, the chemical reactions involved are pollutant-specific. The rate of hydrolysis in an algal pond can increase as the temperature rises (Zhang S. et al., 2020). Furthermore, because the pH of algal ponds can vary greatly, new contaminants that hydrolyse at high pH levels can breakdown significantly during the day. Emerging pollutants that were hydrolysed at neutral pH, on the other hand, may still degrade at night while the pH values return to neutral bandwidth (Gondi et al., 2022).
4 Factors affecting wastewater treatment by microalgae
The efficiency of microalgal-bioremediation processes is affected by various physicochemical factors. These factors include pH levels, redox potential, temperature, the duration and intensity of light exposure, hydraulic retention time, and the size of the adsorbent. These elements collectively play a crucial role in shaping the rate and effectiveness of microalgae-mediated bioremediation mechanisms. The pH of the environment, the redox state, and the prevailing temperature can significantly impact the metabolic activity and growth of microalgae, influencing their capacity to remediate contaminants. The duration and intensity of light exposure are essential considerations, as they directly affect photosynthesis, a key process in microalgal remediation. Hydraulic retention time, representing the duration of water within the system, and the size of the adsorbent particles also contribute to the overall efficiency of microalgal-bioremediation processes by influencing contact time and surface area availability for remediation processes.
4.1 pH
pH is a critical parameter that can influence the mechanism of microalgal bioremediation. pH also influences the ionisation states of various functional groups on the adsorbent’s surface (Ummalyma et al., 2016). Any change in the optimal pH of any biological process may slow down the rate of the reaction. Because at lower pH, the algal surface becomes positively charged, reducing molecule adsorption (Chen et al., 2016). When the pH rises above the isoelectric point, the algal surface becomes negatively charged, causing absorption to increase. A pH greater than 9 has a negative impact on algal growth because the capacity for carbon dioxide absorption is significantly reduced and RuBisCO activity cannot be maintained (Sutherland et al., 2015).
4.2 Temperature
Industrial wastewater effluents, when discharged, often carry elevated temperatures, posing a risk of thermal pollution in aquatic ecosystems. Microalgae, despite having strain-specific optimal temperatures, exhibit a versatile ability to thrive across a broad temperature spectrum. This adaptability allows them to withstand and potentially mitigate the thermal impacts associated with the immediate disposal of heated industrial wastewater into aquatic habitats. In essence, while microalgae have temperature preferences, their capacity to flourish within a wide range makes them valuable in scenarios where temperature fluctuations in wastewater discharges are a concern. The optimal temperature range for commonly cultivated microalgal strains is 15°C–35°C. Once the optimal temperature is reached, biomass productivity decreases dramatically with increasing temperature (Chen et al., 2020). Temperature fluctuations are a major source of concern in microalgae cultivation facilities. Microalgal species such as Spirulina plantesis thrive at 35C, while Scenedesmus sp. thrives at varying temperatures. As a result, microalgae that can grow in wastewater at high temperatures (30°C–40°C) are critical microorganisms for algal-bioremediation of emerging pollutants (Cheah et al., 2015).
4.3 Light intensity
Algae, being phototrophic organisms, harness light energy to synthesize the essential chemical compounds necessary for their growth. The photosynthetic process, facilitated by the presence of inorganic carbon, adequate light, and suitable temperatures, enables algae to absorb nutrients vital for their development. The growth of algae is intricately linked to the availability and intensity of light, a factor that plays a pivotal role in shaping the nutrient utilization efficiency within the waterbody. The interplay of these factors underscores the complex relationship between light, photosynthesis, and nutrient dynamics, influencing the overall ecological balance of the aquatic environment. (Whitton et al., 2015). Microalgae photosynthetic systems are more productive in the blue and red regions of the spectrum, 400 and 600–700 nm, respectively, resulting in better utilisation of nitrogen and phosphorus from wastewater, with red light stimulating algal growth. Although the use of artificial light increases the cost of wastewater treatment, the technology of the light-emitting diode, which provides a longer lightbulb lifespan in combination with lower electricity consumption, makes the use of artificial light sources to enhance the photosynthetic activity of the algae and thus enhancing nutrient uptake from the waterbody more prominent (Ibrahim et al., 2014). The best light source for algae cultivation is thought to be developed light-emitting diode technology with narrowband wavelengths. A study using Spirulina platensis found that red light increased the growth rate of the microalgae by 38%. Green light was used to achieve maximum productivity with C. vulgaris (Wang et al., 2007).
4.4 Hydraulic retention time
The efficiency of microalgae in taking up pollutants can be influenced by the hydraulic retention time. A shorter hydraulic retention time may lead to incomplete or partial removal of pollutants in certain instances. The interplay between microalgal biomass and hydraulic retention time is a critical factor influencing pollutant removal. There is an observable trend where, up to a certain threshold, the removal rate increases with higher concentrations of microalgae and longer hydraulic retention times. However, once the hydraulic retention time surpasses a certain limit, or if it is prolonged excessively, the removal efficiencies may experience a notable decrease. This highlights the importance of optimizing hydraulic retention time in conjunction with microalgal biomass for effective pollutant removal in wastewater treatment processes. (De-Bashan and Bashan, 2010).
4.5 Dose of adsorbent and particle size
The greater the permeability of the pollutant particle, the greater the absorption into the cell wall. This interaction is also affected by the pollutant’s toxicity. It is due to the availability of more surface area, which increases the availability of binding sites. Nano compounds are more readily absorbed. Surface area, electrostatic capacity, and the functional groups involved in the interaction are all factors that influence algal bioremediation via adsorption (Hlongwane et al., 2019). The absorption was found to increase as the hydraulic retention time between the pollutants and the adsorbent increased (Sarkar and Dey, 2021).
5 Transformation and fate of contaminants inside the microalgal cells
Despite the benefits of the bioaccumulation process, many studies fail to consider the fate of emerging contaminants within the algal cell. There is still much debate about how to safely dispose of hazardous algal biomass after bioaccumulation (Gojkovic et al., 2019). The conversion and breakdown of complex compounds into simpler molecules is known as biodegradation or biotransformation. Compound breakdown can take place both intracellularly and extracellularly. Biodegradation has the capability to minimise the toxic effects of contaminants within algal cells and in bulk medium when compared to bio-adsorption or bioaccumulation processes. Microalgae biomass can be converted into additional value-added products. Biodegradation can take place through two main mechanisms: metabolic degradation and co-metabolism. Contaminants provides carbon to microalga throughout metabolic degradation. The degradation of contaminants in the co-metabolism process is mediated by enzymes that catalyse the substrates in the bulk medium (Tiwari et al., 2017).
Considerable research efforts have been dedicated to exploring the effectiveness of microalgal biodegradation in removing pharmaceuticals and personal care products from wastewater. This ongoing research aims to understand and optimize the capabilities of microalgae in breaking down and eliminating these contaminants, offering a potential environmentally friendly solution for wastewater treatment. The investigation focuses on the mechanisms and efficiency of microalgal biodegradation, with the goal of developing sustainable and effective methods to address the presence of pharmaceuticals and personal care products in wastewater. (Nguyen et al., 2021). Microalgal biodegradation was found to be the most effective method of removing estrogenic hormones. It was discovered that enzymes oversee biodegradation, and enzyme activation can be measured by the concentration of EC in the bulk medium. Thus, the EC threshold concentration is critical for triggering enzyme activity and microalgal biodegradation (Xiong et al., 2018). Microalgae biotransformation of these constant and robust ECs is complex. There is still substantial disagreement about the role of enzymes in the biodegradation process (Sutherland and Ralph, 2019). Further investigation into the function of enzymes and their degradation procedure in wastewater medium is required.
It is also worth noting that not all the contaminants are readily biodegradable, and some can be toxic to microalgal cells (Villar-Navarro et al., 2018). Some non-biodegradable pharmaceutical contaminants (e.g., carbamazepine) were found to be resistant to photolysis in high-rate algal ponds. It is proposed that microalgal strains can be pre-acclimated to target EC concentrations that are not toxic. This is a critical first step towards effective toxic substance remediation. Studies have shown that when microalgae are exposed to contaminants, their metabolisms and cellular processes improve. Microalgae tolerance to ECs appeared to increase in response to chronic exposure to target EC. This is because enzymatic pathways are activated to combat the toxic effects of ECs (Norvill et al., 2016; Chen et al., 2015) reported that when C. pyrenoidosa was pre-exposed to the antibiotic cefradine, its removal efficiency increased.
Microalgae may indirectly improve the biodegradation process through symbiotic interaction with bacteria. The synthesis of reactive oxygen species during photosynthesis was thought to be aided by photosynthetically mediated pH changes and high oxygen production. Because algal biomass is less toxic after biodegradation, it can be used for a variety of purposes, including biofuel. However, there is a chance that accumulation and sorption will leave some ECs after bioremediation (Agüera et al., 2020).
6 Advantages of microalgal-bioremediation systems for removal of emerging pollutants
Flocculation, chemical precipitation, activated charcoal, reverse osmosis, ultraviolet disinfection (UV disinfection), ultrafiltration, electro-coagulation, and ion exchange are the most common wastewater treatment methods. These approaches are not cost-effective because they necessitate a significant amount of energy and labour (Sankaran et al., 2020). Microalgal-bioremediation systems have been shown to be an effective method of wastewater treatment. Microalgae are abundant in most environments, do not generate toxic substances, grow quickly, and have a large surface area.
• Cultivating microalgae recovers essential nutrients from wastewater and prevents eutrophication of freshwater, whereas in conventional treatments, nitrogen is removed as atmospheric nitrogen, carbon is oxidised to carbon dioxide, while phosphorus is precipitated (Nagarajan et al., 2020).
• Microalgae can withstand a variety of emerging pollutants. Scenedesmus, Chlamydomonas, and Chlorella Sp. were discovered to be among the most reported microalgal strains in proof-of-concept studies and are extensively studied (Wang et al., 2016).
• Microalgae play a crucial role in mitigating global warming by capturing atmospheric carbon dioxide through photosynthesis. Utilizing this natural process, microalgae absorb carbon dioxide from the atmosphere and convert it into organic compounds, thereby serving as an effective carbon sink. This carbon sequestration by microalgae helps reduce the concentration of greenhouse gases in the atmosphere, ultimately contributing to the mitigation of climate change and alleviating the severity of global warming. (Kannah et al., 2021).
• Cultivating microalgae on barren land offers a valuable opportunity to alleviate pressure on traditional agricultural land. By utilizing barren or non-arable areas, microalgae cultivation provides an alternative and sustainable approach to food and biomass production. This method not only optimizes land use but also mitigates the competition for fertile soil resources with traditional crops. Additionally, microalgae cultivation on barren land has the potential to contribute to ecosystem restoration and enhance resource efficiency in areas where conventional agriculture may be impractical or unsustainable. (Farooq et al., 2015).
• Value-added products are bioproducts that have been modified or improved as a result of a process. Possibility of producing a variety of value-added bioproducts for various industries and applications (fish feed, lipids, biofuel, sugars, bio pigments, enzymes, biofertilizers, algal plastics, and biomaterials). Furthermore, determining the protein and oil content is critical for determining what types of bioproducts can be produced (Banu et al., 2020).
• Microalgae grow at a rate that is 10–50 times faster than that of other terrestrial plants. Numerous studies have shown that cultivating microalgae is effective. Several studies have reported successful cultivation of several microalgae species for wastewater treatment, including Chlorella, Chlamydomonas, Botryococcus, Scenedesmus, Arthrospira, and Phormidium (Pittman et al., 2011).
7 Limitations/challenges associated with microalgae-based wastewater treatment
Traditional wastewater treatment plants (WWTPs) primarily target organic matter and nutrients but are ineffective at fully removing emerging contaminants (ECs) such as pharmaceuticals, hormones, antibiotic resistance genes (ARGs), and microplastics, with removal rates for some contaminants as low as 50% (World Health Organization, 2024; Zhang et al., 2017). Advanced methods like ozonation, reverse osmosis, and advanced oxidation processes (AOPs) offer higher efficiency but are energy-intensive, costly, and often produce harmful byproducts, such as bromates. Emerging biological approaches, including microalgae-based treatments, show promise but face challenges such as scalability, large space requirements, and the need for controlled environmental conditions. These limitations are further compounded in low- and middle-income countries, where only a small fraction of wastewater is treated due to financial and operational barriers. Additionally, there are significant gaps in understanding the long-term environmental and health impacts of ECs, as well as variability in contaminant concentrations across regions, which complicates the standardization of treatment technologies. Residual sludge management and the lack of real-world validation for many pilot-scale innovations also remain critical challenges.
The limitations of microalgae-based wastewater treatment are as follows:
• While microalgae-based wastewater treatment is geared towards efficient nitrogen and phosphorus removal, not all emerging pollutants and heavy metals can be effectively eliminated. Before integrating microalgae with wastewater treatment, the inhibition factors from the environment and wastewater itself must be considered because they have a large impact on the growth and treatment efficiency of microalgae (Chai et al., 2021).
• Significant amounts of solids suspensions and high turbidity in industrial wastewater may affect light radiation through the wastewater, thereby affecting photosynthesis and interfering with microalgae growth. As a result, an additional wastewater method with high solids removal efficiency, such as sedimentation, adsorption, coagulation, and so on, can be used to ensure high photosynthesis efficiency (Amenorfenyo et al., 2019).
• Even though the cultivation of microalgae in wastewater is simple and effective, it is not an enticing alternative wastewater treatment method in terms of cost. According to (Umamaheswari and Shanthakumar, 2016) high downstream processing costs, a small scale of production, and only selected microalgae species and cultivation modes can yield high quality biomass that can be converted into useful bioproducts all contribute to this treatment method’s less profitable property. Furthermore, enclosed photobioreactors that require an artificial light source and chemical agents for sterilisation raise the overall cost of production.
• It is critical to choose the right species of microalgae for wastewater treatment. The selected microalgae species should be able to cope with variations in environmental factors due to the different physical and chemical composition of wastewater from various sources. Furthermore, the species should be able to share metabolites in order to accommodate stress and survive any attack by unwanted species as well as nutrient limitations. Microalgae species that are facultative for utilising organic carbons as sole substrates and cut off any light source for cultivation are also limited for heterotrophic and mixotrophic microalgae (Amenorfenyo et al., 2019).
• Higher altitudes make it inconceivable to cultivate microalgae, which has a direct impact on algal biomass concentration. Selection and cultivation of extremophile varieties could be an option (Gondi et al., 2022). However, the effectiveness of such strains’ microalgal bioremediation must also be considered.
Different processes involving wastewater treatment by microalgae with the advantages and disadvantages are summarized in Figure 3.
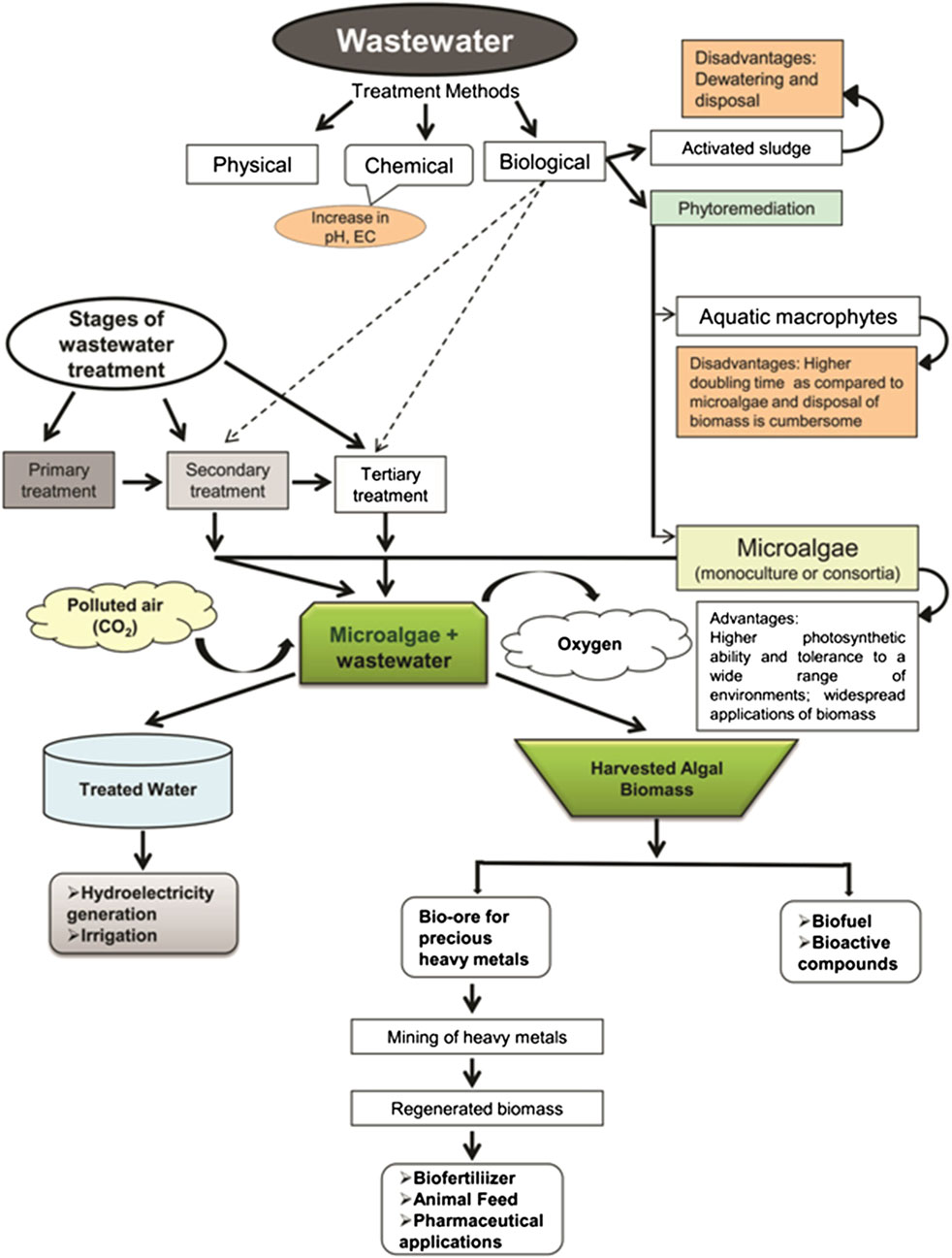
Figure 3. Different processes involving wastewater treatment by microalgae with their advantages and disadvantages. Reprinted with permission from Springer-Nature (Renuka et al., 2015), licence number 5705400031262.
8 Applications of microalgae to achieve sustainable development goals (SGDs)
Goal 6 in UN Sustainable Development Goals (SDGs) aims to ensure availability and sustainable management of water and sanitation for all. Especially, target 6.a. under Goal 6 stated “By 2030, expand international cooperation and capacity-building support to developing countries in water-and sanitation-related activities and programmes, including water harvesting, desalination, water efficiency, wastewater treatment, recycling and reuse technologies” (United Nations, 2022). Target 6.3. stated “By 2030, improve water quality by reducing pollution, eliminating dumping and minimizing release of hazardous chemicals and materials, halving the proportion of untreated wastewater and substantially increasing recycling and safe reuse globally.” wastewater treatment using microalgae can be one of the sustainable solutions in this regard. Advanced application of different microalgal strains in wastewater remediation can play a direct role in advancing Sustainable Development Goal 6, which aims to ensure the availability and sustainable management of clean water. The removal of emerging contaminants from wastewater can thereby mitigate the potential health risks associated with the exposure of emerging contaminants and can foster overall wellbeing.
The approaches and findings discussed in this research can be applied to real-world wastewater treatment by integrating innovative and sustainable technologies into existing systems. Upgrading conventional wastewater treatment plants (WWTPs) with advanced processes like advanced oxidation processes (AOPs), membrane bioreactors (MBRs), or activated carbon adsorption can significantly enhance the removal of emerging contaminants (ECs), such as pharmaceuticals, hormones, and microplastics. This hybrid approach closes the efficiency gap in addressing these pollutants. Additionally, nature-based solutions, such as microalgae and constructed wetlands, offer sustainable, cost-effective methods for EC removal while reducing energy consumption and generating valuable byproducts like biofuels and fertilizers, promoting a circular economy (Dutta et al., 2023).
Real-time monitoring systems can further enhance treatment effectiveness by detecting and targeting high-priority contaminants. Scaling up pilot technologies, such as microalgae-based treatments, through collaboration between academia, industry, and government can validate their practicality in diverse environments. In low- and middle-income regions, decentralized and affordable solutions like algal ponds or modular bioreactors can address infrastructure limitations, offering scalable and cost-effective alternatives.
Policy support and public awareness are crucial to the success of these innovations. Stricter regulations on EC discharges and campaigns promoting responsible pharmaceutical disposal can reduce contaminant loads in wastewater systems. Additionally, incorporating technologies that target antibiotic residues and resistance genes can mitigate global health risks associated with antibiotic resistance. Resource recovery technologies that reclaim nutrients or water for reuse can further enhance the sustainability and economic viability of treatment systems, particularly in water-scarce areas. These strategies collectively provide practical, scalable, and eco-friendly solutions for improving wastewater treatment while safeguarding public health and ecosystems.
Large-scale algae production for wastewater treatment typically involves systems like open ponds, raceway ponds, or photobioreactors. Open or raceway ponds are cost-effective and simple to operate, while photobioreactors offer better control over environmental conditions (Qin et al., 2019), enhancing algae growth efficiency but with higher initial costs. The time required to achieve large-scale algal biomass depends on factors like species, nutrient availability, light, temperature, and system design (Pruvost et al., 2016). Sustainable approaches in developing large-scale remediation facilities can be effective in wastewater bio-treatment. In terms of cost-effectiveness, algae-based treatment can be competitive, especially when considering the co-benefits. Harvested algae can be converted into valuable byproducts like biofuels, bioplastics, animal feed, or fertilizers, generating additional revenue. The initial investment may be higher than conventional systems, but operational costs are often offset by reduced chemical usage, energy savings, and the potential for resource recovery, making algae systems increasingly viable in the long term (Dutta et al., 2023) the outline of different processes involved in microalgae-based wastewater system to achieve sustainable development goals is shown in Figure 4.
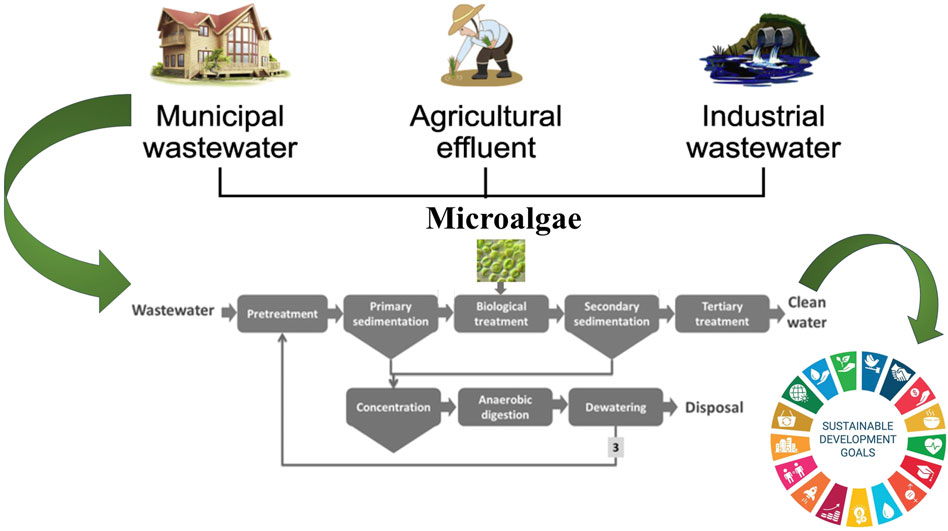
Figure 4. Different processes of microalgae-based wastewater system to achieve sustainable development goals.
9 Recommendation and future perspectives
Several investigations have been conducted to pave the way for industrial scale microalgae application by determining the associated processes and technologies. However,
• The transition from pilot to industrial scale activities frequently exposes microalgae to unfavourable conditions, resulting in significantly lower bioproduct yields. As a result, more studies and research are needed to ensure the monetary and environmental feasibility of integrating robust microalgae cells and bioprocess engineering methods. Further genetic or metabolic engineering research should be carried out to ensure the cultivation of genome-modified microalgal cells with improved characteristics related to unfavourable environment adaptation and higher performance for removing pollutants and bioproduct yields.
• Sorting microalgal biomass from treated wastewater after bioremediation is a significant challenge, particularly in suspended cultivation. To address this, securing microalgae cultivation to a media/supporter may be used, ensuring the separation process more accessible and decreasing hydraulic retention time.
• Only a few studies have been completed, so cost-effectiveness studies of microalgae-based wastewater treatment and assessments to traditional methods must be investigated. The lack of basic design and operation guidelines for microalgae-based wastewater treatment, on the other hand, encourages researchers to investigate harder to provide basic guidance and recommendations for enhancing the resilience and flexibility of microalgal strains to deal with different types of wastewaters.
• More research on incorporating microalgae into the biological treatment process, as well as a thorough understanding of the relationships between microalgae and existing bacteria in wastewater, is required.
• Before introducing microalgae, it is recommended to regulate the influents with adequate input and/or pretreat. Furthermore, the wide variety of nutrients and their strength in wastewater necessitate several pretreatments in order to produce an ideal balance of nutrients for the microalgae. In such cases, researchers should use optimised mixtures of multiple wastewater sources as a single, well-balanced nutrient media for microalgae.
• Assessing wastewater from industries and microalgae cultivation modes may differ depending on geographical zone due to variations in sunlight availability and temperature. As a result, colder zones should concentrate on photobioreactor-based microalgae cultivation with the goal of producing high-value-added products from microalgae grown on wastewater.
• The microalgae-based wastewater treatment processes and overall method monitoring (e.g., pH, temperature, microalgae cell conditions, BOD, and DO) are complicated operations that necessitate the development of innovative technologies such as online monitoring and remote control.
10 Conclusion
The bioremediation capabilities of microalgae in wastewater have been demonstrated and confirmed by recent and available literature studies. Microalgae are promising possibility for carbon capture technology because they have been shown to be effective at removing heavy metals and nutrients from various types of wastewaters. Microalgae have an accurately high potential to remove newly emerging contaminants. However, each species of microalgae has distinctive traits and the capacity to eliminate different kinds of contaminants. Because different types of microalgae have different inherent abilities, particularly nutrient uptake, tolerance for harsh or extreme environmental conditions, and competitive potential relative to native organisms, the response and development of different types of microalgae in wastewater also varies. The ultimate objective of wastewater treatment is to reduce the biochemical oxygen demand as well as organic, inorganic, and synthetic elements like high levels of ammonium, bicarbonate, phosphate, potassium, sulphur, heavy metals, dyes, pesticides, pharmaceuticals, and a wide range of pathogenic bacteria. Phyco-remediation, or the bioremediation of wastewater using algal species, can remove biological and chemical compounds using microalgae. High concentrations of nutrients found in domestic, industrial, and agricultural wastewaters encourage the growth of microalgae, reducing or even eliminating the need for supplemental feeding. After the consumption of heavy metals or toxic substances, algae do not cause secondary pollution. Even dead algal biomass can eliminate heavy metals from wastewaters through the biosorption process, though this method is less efficient than using live algae cells. Utilising algae to treat wastewater is a key component of new technologies. Molecular techniques are also used to develop novel algal strains with improved phyco-remediation capabilities. In order to increase the viability and compatibility of microalgae cultivated at full scale, more research is needed to examine the industrial scale of microalgae and the improvement of bioproduct quality. For the future advancement of microalgal technology, numerous experiments evaluating the removal effectiveness of a wide range of heavy metal ions by various microalgal strains either individually or in combination should be carried out. To increase the opportunities for the application of microalgal treatment in wastewater plants, more research on the integration of current treatment systems and microalgal treatment needs to be conducted and reported.
Author contributions
PK: Writing–original draft. ND: Writing–original draft, Writing–review and editing. SB: Conceptualization, Supervision, Validation, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Raouf, N., Al-Homaidan, A., and Ibraheem, I. (2012). Microalgae and wastewater treatment. Saudi J. Biol. Sci. 19, 257–275. doi:10.1016/j.sjbs.2012.04.005
Abo, R., Kummer, N.-A., and Merkel, B. J. (2016). Optimized photodegradation of Bisphenol A in water using ZnO, TiO 2 and SnO 2 photocatalysts under UV radiation as a decontamination procedure. Drink. Water Eng. Sci. 9, 27–35. doi:10.5194/dwes-9-27-2016
Agüera, A., Plaza-Bolaños, P., and Fernández, F. A. (2020) “Removal of contaminants of emerging concern by microalgae-based wastewater treatments and related analytical techniques,” in Current developments in biotechnology and bioengineering. Elsevier, 503–525.
Aksu, Z., and Tezer, S. (2005). Biosorption of reactive dyes on the green alga Chlorella vulgaris. Process Biochem. 40, 1347–1361. doi:10.1016/j.procbio.2004.06.007
Amenorfenyo, D. K., Huang, X., Zhang, Y., Zeng, Q., Zhang, N., Ren, J., et al. (2019). Microalgae brewery wastewater treatment: potentials, benefits and the challenges. Int. J. Environ. Res. public health 16, 1910. doi:10.3390/ijerph16111910
Andrade, C., Andrade, L., Dias, M., Nascimento, C., and Mendes, M. (2018). Microalgae for bioremediation of textile wastewater. An overview. MOJ Food Process Technol. 6, 432–433. doi:10.15406/mojfpt.2018.06.00200
Ansa, E., Lubberding, H. J., Ampofo, J., and Gijzen, H. (2011). The role of algae in the removal of Escherichia coli in a tropical eutrophic lake. Ecol. Eng. 37, 317–324. doi:10.1016/j.ecoleng.2010.11.023
Bai, X., and Acharya, K. (2017). Algae-mediated removal of selected pharmaceutical and personal care products (PPCPs) from Lake Mead water. Sci. Total Environ. 581, 734–740. doi:10.1016/j.scitotenv.2016.12.192
Banu, J. R., Kannah, R. Y., Kavitha, S., Ashikvivek, A., Bhosale, R. R., and Kumar, G. (2020). Cost effective biomethanation via surfactant coupled ultrasonic liquefaction of mixed microalgal biomass harvested from open raceway pond. Bioresour. Technol. 304, 123021. doi:10.1016/j.biortech.2020.123021
Borowitzka, M. A. (1998). Limits to growth. Wastewater Treat. algae, 203–226. doi:10.1007/978-3-662-10863-5_12
Castiglioni, S., Davoli, E., Riva, F., Palmiotto, M., Camporini, P., Manenti, A., et al. (2018). Mass balance of emerging contaminants in the water cycle of a highly urbanized and industrialized area of Italy. Water Res. 131, 287–298. doi:10.1016/j.watres.2017.12.047
Chai, W. S., Tan, W. G., Munawaroh, H. S. H., Gupta, V. K., Ho, S.-H., and Show, P. L. (2021). Multifaceted roles of microalgae in the application of wastewater biotreatment: a review. Environ. Pollut. 269, 116236. doi:10.1016/j.envpol.2020.116236
Cheah, W. Y., Show, P. L., Chang, J.-S., Ling, T. C., and Juan, J. C. (2015). Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour. Technol. 184, 190–201. doi:10.1016/j.biortech.2014.11.026
Cheirsilp, B., Maneechote, W., Srinuanpan, S., and Angelidaki, I. (2023). Microalgae as Tools for bio-circular-green economy: zero-waste approaches for sustainable production and biorefineries of microalgal biomass. Bioresour. Technol. 387, 129620. doi:10.1016/j.biortech.2023.129620
Chen, J., Zheng, F., and Guo, R. (2015). Algal feedback and removal efficiency in a sequencing batch reactor algae process (SBAR) to treat the antibiotic cefradine. PLoS One 10, e0133273. doi:10.1371/journal.pone.0133273
Chen, R., Liu, Y., and Liao, W. (2016). Using an environmentally friendly process combining electrocoagulation and algal cultivation to treat high-strength wastewater. Algal Res. 16, 330–337. doi:10.1016/j.algal.2016.03.032
Chen, Z., Shao, S., He, Y., Luo, Q., Zheng, M., Zheng, M., et al. (2020). Nutrients removal from piggery wastewater coupled to lipid production by a newly isolated self-flocculating microalga Desmodesmus sp. PW1. Bioresour. Technol. 302, 122806. doi:10.1016/j.biortech.2020.122806
Cheng, L., Zhang, L., Chen, H., and Gao, C. (2006). Carbon dioxide removal from air by microalgae cultured in a membrane-photobioreactor. Sep. Purif. Technol. 50, 324–329. doi:10.1016/j.seppur.2005.12.006
Chiu, S.-Y., Kao, C.-Y., Huang, T.-T., Lin, C.-J., Ong, S.-C., Chen, C.-D., et al. (2011). Microalgal biomass production and on-site bioremediation of carbon dioxide, nitrogen oxide and sulfur dioxide from flue gas using Chlorella sp. cultures. Bioresour. Technol. 102, 9135–9142. doi:10.1016/j.biortech.2011.06.091
Chong, A., Wong, Y., and Tam, N. (2000). Performance of different microalgal species in removing nickel and zinc from industrial wastewater. Chemosphere 41, 251–257. doi:10.1016/s0045-6535(99)00418-x
Dar, R. A., Sharma, N., Kaur, K., and Phutela, U. G. (2019). Feasibility of microalgal technologies in pathogen removal from wastewater. Appl. Microalgae Wastewater Treat. Domest. Industrial Wastewater Treat. 1, 237–268. doi:10.1007/978-3-030-13913-1_12
Das, A., Adhikari, S., and Kundu, P. (2019). Bioremediation of wastewater using microalgae. Environ. Biotechnol. soil wastewater Implic. Ecosyst., 55–60. doi:10.1007/978-981-13-6846-2_8
De-Bashan, L. E., and Bashan, Y. (2010). Immobilized microalgae for removing pollutants: review of practical aspects. Bioresour. Technol. 101, 1611–1627. doi:10.1016/j.biortech.2009.09.043
Delrue, F., Álvarez-Díaz, P. D., Fon-Sing, S., Fleury, G., and Sassi, J.-F. (2016). The environmental biorefinery: using microalgae to remediate wastewater, a win-win paradigm. Energies 9, 132. doi:10.3390/en9030132
Dutta, N., Kundu, P., Lee, J. T. E., and Bhattacharya, S. (2023). Implementation and optimization of algal biomass in value-added products recovery: a step towards algae-based green economy. Hydrobiolog 2, 326–346. doi:10.3390/hydrobiology2020021
Edelstein, M., and Ben-Hur, M. (2018). Heavy metals and metalloids: sources, risks and strategies to reduce their accumulation in horticultural crops. Sci. Hortic. 234, 431–444. doi:10.1016/j.scienta.2017.12.039
El-Naggar, N. E.-A., Hamouda, R. A., Rabei, N. H., Mousa, I. E., and Abdel-Hamid, M. S. (2019). Phytoremediation of lithium ions from aqueous solutions using free and immobilized freshwater green alga Oocystis solitaria: mathematical modeling for bioprocess optimization. Environ. Sci. Pollut. Res. 26, 19335–19351. doi:10.1007/s11356-019-05214-x
Emparan, Q., Harun, R., and Danquah, M. (2019). Role of phycoremediation for nutrient removal from wastewaters: a review. Appl. Ecol. and Environ. Res. 17, 889–915. doi:10.15666/aeer/1701_889915
Fang, G. Z., Huang, G. Y., Ying, G. G., Qiu, S. Q., Shi, W. J., Xie, L., et al. (2021). Endocrine disrupting effects of binary mixtures of 17β-estradiol and testosterone in adult female western mosquitofish (Gambusia affinis). Ecotoxicol. Environ. Saf. 208, 111566. doi:10.1016/j.ecoenv.2020.111566
Farazaki, M., and Gikas, P. (2019). Nitrification-denitrification of municipal wastewater without recirculation, using encapsulated microorganisms. J. Environ. Manag. 242, 258–265. doi:10.1016/j.jenvman.2019.04.054
Farhadian, M., Vachelard, C., Duchez, D., and Larroche, C. (2008). In situ bioremediation of monoaromatic pollutants in groundwater: a review. Bioresour. Technol. 99, 5296–5308. doi:10.1016/j.biortech.2007.10.025
Farooq, W., Suh, W. I., Park, M. S., and Yang, J.-W. (2015). Water use and its recycling in microalgae cultivation for biofuel application. Bioresour. Technol. 184, 73–81. doi:10.1016/j.biortech.2014.10.140
Ghazal, F., Mahdy, E., El-Fattah, M., El-Sadany, A., Elg, Y., and Doha, N. (2018). The use of microalgae in bioremediation of the textile wastewater effluent. Nat. Sci. 16, 98–104.
Gojkovic, Z., Lindberg, R. H., Tysklind, M., and Funk, C. (2019). Northern green algae have the capacity to remove active pharmaceutical ingredients. Ecotoxicol. Environ. Saf. 170, 644–656. doi:10.1016/j.ecoenv.2018.12.032
Gondi, R., Kavitha, S., Kannah, R. Y., Karthikeyan, O. P., Kumar, G., Tyagi, V. K., et al. (2022). Algal-based system for removal of emerging pollutants from wastewater: a review. Bioresour. Technol. 344, 126245. doi:10.1016/j.biortech.2021.126245
Gusain, R., Kumar, N., and Ray, S. S. (2020). Recent advances in carbon nanomaterial-based adsorbents for water purification. Coord. Chem. Rev. 405, 213111. doi:10.1016/j.ccr.2019.213111
Hlongwane, G. N., Sekoai, P. T., Meyyappan, M., and Moothi, K. (2019). Simultaneous removal of pollutants from water using nanoparticles: a shift from single pollutant control to multiple pollutant control. Sci. Total Environ. 656, 808–833. doi:10.1016/j.scitotenv.2018.11.257
Hom-Diaz, A., Llorca, M., Rodríguez-Mozaz, S., Vicent, T., Barceló, D., and Blánquez, P. (2015). Microalgae cultivation on wastewater digestate: β-estradiol and 17α-ethynylestradiol degradation and transformation products identification. J. Environ. Manag. 155, 106–113. doi:10.1016/j.jenvman.2015.03.003
Houtman, C. J., Ten Broek, R., and Brouwer, A. (2018). Steroid hormonal bioactivities, culprit natural and synthetic hormones and other emerging contaminants in waste water measured using bioassays and UPLC-tQ-MS. Sci. Total Environ. 630, 1492–1501. doi:10.1016/j.scitotenv.2018.02.273
Hussein, M., Abdullah, A., Eladal, E., and El-Din, N. B. (2016). Phytoremediation of some pesticides by microchlorophyte alga, Chlorella Sp. J. Fertil. Pestic. 7, 2. doi:10.4172/2471-2728.1000173
Ibrahim, M. A., MacAdam, J., Autin, O., and Jefferson, B. (2014). Evaluating the impact of LED bulb development on the economic viability of ultraviolet technology for disinfection. Environ. Technol. 35, 400–406. doi:10.1080/09593330.2013.829858
Inuwa, A. B., Pervez, ·.A., Nazir, ·.R., and Huang, B. (2023). Microalgae-based wastewater treatment system: current state, antibiotic resistant bacteria and antibiotic resistance genes reduction potentials. Int. J. Environ. Sci. Technol. 20, 14053–14072. doi:10.1007/s13762-023-05069-3
IQVIA (2018). Winning the Indian pharmaceutical market. Available at: https://www.iqvia.com/locations/india/library/white-papers/winning-in-the-indian-pharmaceutical-market (accessed on November 24, 2024).
Ji, M.-K., Abou-Shanab, R. A., Kim, S.-H., Salama, E.-S., Lee, S.-H., Kabra, A. N., et al. (2013). Cultivation of microalgae species in tertiary municipal wastewater supplemented with CO2 for nutrient removal and biomass production. Ecol. Eng. 58, 142–148. doi:10.1016/j.ecoleng.2013.06.020
Kaloudas, D., Pavlova, N., and Penchovsky, R. (2021). Phytoremediation of wastewater by microalgae: a review. Environ. Chem. Lett. 19, 2905–2920. doi:10.1007/s10311-021-01203-0
Kannah, R. Y., Kavitha, S., Karthikeyan, O. P., Rene, E. R., Kumar, G., and Banu, J. R. (2021). A review on anaerobic digestion of energy and cost effective microalgae pretreatment for biogas production. Bioresour. Technol. 332, 125055. doi:10.1016/j.biortech.2021.125055
Khalatbari, S., Sotaniemi, V., Suokas, M., Taipale, S., and Leiviskä, T. (2024). Microalgae technology for polishing chemically-treated fish processing waste water. Groundw. Sustain. Dev. 24, 101074. doi:10.1016/j.gsd.2023.101074
Klinthong, W., Yang, Y.-H., Huang, C.-H., and Tan, C.-S. (2015). A review: microalgae and their applications in CO2 capture and renewable energy. Aerosol Air Qual. Res. 15, 712–742. doi:10.4209/aaqr.2014.11.0299
Koyande, A. K., Chew, K. W., Rambabu, K., Tao, Y., Chu, D.-T., and Show, P.-L. (2019). Microalgae: a potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 8, 16–24. doi:10.1016/j.fshw.2019.03.001
Kruglova, A., Kråkström, M., Riska, M., Mikola, A., Rantanen, P., Vahala, R., et al. (2016). Comparative study of emerging micropollutants removal by aerobic activated sludge of large laboratory-scale membrane bioreactors and sequencing batch reactors under low-temperature conditions. Bioresour. Technol. 214, 81–88. doi:10.1016/j.biortech.2016.04.037
Kumar, A., Ergas, S., Yuan, X., Sahu, A., Zhang, Q., Dewulf, J., et al. (2010). Enhanced CO2 fixation and biofuel production via microalgae: recent developments and future directions. Trends Biotechnol. 28, 371–380. doi:10.1016/j.tibtech.2010.04.004
Kumar, S. D., Santhanam, P., Nandakumar, R., Anath, S., Prasath, B. B., Devi, A. S., et al. (2014). Preliminary study on the dye removal efficacy of immobilized marine and freshwater microalgal beads from textile wastewater. Afr. J. Biotechnol. 13, 2288–2294. doi:10.5897/ajb2013.13242
Lal, S., Lal, R., and Saxena, D. (1987). Bioconcentration and metabolism of DDT, fenitrothion and chlorpyrifos by the blue-green algae Anabaena sp. and Aulosira fertilissima. Environ. Pollut. 46, 187–196. doi:10.1016/0269-7491(87)90076-5
Lei, A.-P., Hu, Z.-L., Wong, Y.-S., and Tam, N. F.-Y. (2007). Removal of fluoranthene and pyrene by different microalgal species. Bioresour. Technol. 98, 273–280. doi:10.1016/j.biortech.2006.01.012
Liu, L., Hall, G., and Champagne, P. (2020). The role of algae in the removal and inactivation of pathogenic indicator organisms in wastewater stabilization pond systems. Algal Res. 46, 101777. doi:10.1016/j.algal.2019.101777
Lo, Y.-C., Cheng, C.-L., Han, Y.-L., Chen, B.-Y., and Chang, J.-S. (2014). Recovery of high-value metals from geothermal sites by biosorption and bioaccumulation. Bioresour. Technol. 160, 182–190. doi:10.1016/j.biortech.2014.02.008
Ma, Y., Shen, Y., and Liu, Y. (2020). State of the art of straw treatment technology: challenges and solutions forward. Bioresour. Technol. 313, 123656. doi:10.1016/j.biortech.2020.123656
Mascolo, G., Balest, L., Cassano, D., Laera, G., Lopez, A., Pollice, A., et al. (2010). Biodegradability of pharmaceutical industrial wastewater and formation of recalcitrant organic compounds during aerobic biological treatment. Bioresour. Technol. 101, 2585–2591. doi:10.1016/j.biortech.2009.10.057
Mestrot, A., Xie, W.-Y., Xue, X., and Zhu, Y.-G. (2013). Arsenic volatilization in model anaerobic biogas digesters. Appl. Geochem. 33, 294–297. doi:10.1016/j.apgeochem.2013.02.023
Mezzari, M., Prandini, J., Kich, J. D., and Silva, M. (2017). Elimination of antibiotic multi-resistant Salmonella typhimurium from swine wastewater by microalgae-induced antibacterial mechanisms. J. Bioremediat. Biodegr. 8, 379.
Mohamed, Z. A. (2008). Polysaccharides as a protective response against microcystin-induced oxidative stress in Chlorella vulgaris and Scenedesmus quadricauda and their possible significance in the aquatic ecosystem. Ecotoxicology 17, 504–516. doi:10.1007/s10646-008-0204-2
Molazadeh, M., Ahmadzadeh, H., Pourianfar, H. R., Lyon, S., and Rampelotto, P. H. (2019). The use of microalgae for coupling wastewater treatment with CO2 biofixation. Front. Bioeng. Biotechnol. 7, 42. doi:10.3389/fbioe.2019.00042
Mulla, S. I., Bharagava, R. N., Belhaj, D., Ameen, F., Saratale, G. D., Gupta, S. K., et al. (2019). A review of micropollutant removal by microalgae. Appl. Microalgae Wastewater Treat. Domest. Industrial Wastewater Treat. 1, 41–55. doi:10.1007/978-3-030-13913-1_3
Nagarajan, D., Lee, D.-J., Chen, C.-Y., and Chang, J.-S. (2020). Resource recovery from wastewaters using microalgae-based approaches: a circular bioeconomy perspective. Bioresour. Technol. 302, 122817. doi:10.1016/j.biortech.2020.122817
Naghdi, M., Taheran, M., Brar, S. K., Kermanshahi-Pour, A., Verma, M., and Surampalli, R. Y. (2018). Removal of pharmaceutical compounds in water and wastewater using fungal oxidoreductase enzymes. Environ. Pollut. 234, 190–213. doi:10.1016/j.envpol.2017.11.060
Nguyen, H. T., Yoon, Y., Ngo, H. H., and Jang, A. (2021). The application of microalgae in removing organic micropollutants in wastewater. Crit. Rev. Environ. Sci. Technol. 51, 1187–1220. doi:10.1080/10643389.2020.1753633
Noorani, KRPM, Flora, G., Surendarnath, S., Mary Stephy, G., Amesho, K. T. T., Chinglenthoiba, C., et al. (2024). Recent advances in remediation strategies for mitigating the impacts of emerging pollutants in water and ensuring environmental sustainability. J. Environ. Manage 351, 119674. doi:10.1016/j.jenvman.2023.119674
Norvill, Z. N., Shilton, A., and Guieysse, B. (2016). Emerging contaminant degradation and removal in algal wastewater treatment ponds: identifying the research gaps. J. Hazard. Mater. 313, 291–309. doi:10.1016/j.jhazmat.2016.03.085
Olawale, S. A. (2021). Biosorption of heavy metals from aqueous solutions: an insight and review. Arch. Ind. Eng. 3, 1–31.
Olmos-Espejel, J. J., de Llasera, M. P. G., and Velasco-Cruz, M. (2012). Extraction and analysis of polycyclic aromatic hydrocarbons and benzo [a] pyrene metabolites in microalgae cultures by off-line/on-line methodology based on matrix solid-phase dispersion, solid-phase extraction and high-performance liquid chromatography. J. Chromatogr. A 1262, 138–147. doi:10.1016/j.chroma.2012.09.015
Omar, H. H. (2002). Bioremoval of zinc ions by Scenedesmus obliquus and Scenedesmus quadricauda and its effect on growth and metabolism. Int. Biodeterior. and Biodegrad. 50, 95–100. doi:10.1016/s0964-8305(02)00048-3
Paixão, J. F., Nascimento, I. A., Pereira, S. A., Leite, M., Carvalho, G. C., Silveira, J., et al. (2007). Estimating the gasoline components and formulations toxicity to microalgae (Tetraselmis chuii) and oyster (Crassostrea rhizophorae) embryos: an approach to minimize environmental pollution risk. Environ. Res. 103, 365–374. doi:10.1016/j.envres.2006.06.015
Pavithra, K. G., Kumar, P. S., Jaikumar, V., Vardhan, K. H., and SundarRajan, P. (2020). Microalgae for biofuel production and removal of heavy metals: a review. Environ. Chem. Lett. 18, 1905–1923. doi:10.1007/s10311-020-01046-1
Perez-Garcia, O., and Bashan, Y. (2015). Microalgal heterotrophic and mixotrophic culturing for bio-refining: from metabolic routes to techno-economics. Algal Biorefineries Volume 2 Prod. Refin. Des., 61–131. doi:10.1007/978-3-319-20200-6_3
Petroutsos, D., Katapodis, P., Samiotaki, M., Panayotou, G., and Kekos, D. (2008). Detoxification of 2, 4-dichlorophenol by the marine microalga Tetraselmis marina. Phytochemistry 69, 707–714. doi:10.1016/j.phytochem.2007.09.002
Pittman, J. K., Dean, A. P., and Osundeko, O. (2011). The potential of sustainable algal biofuel production using wastewater resources. Bioresour. Technol. 102, 17–25. doi:10.1016/j.biortech.2010.06.035
Posadas, E., Bochon, S., Coca, M., García-González, M., García-Encina, P., and Muñoz, R. (2014). Microalgae-based agro-industrial wastewater treatment: a preliminary screening of biodegradability. J. Appl. Phycol. 26, 2335–2345. doi:10.1007/s10811-014-0263-0
Pruvost, J., Cornet, J. F., and Pilon, L. (2016). “Large-scale production of algal biomass: photobioreactors,” in Algae biotechnology. Editors F. Bux,, and Y. Chisti (Cham: Green Energy and Technology. Springer).
Qin, L., Alam, M. A., and Wang, Z. (2019). “Open pond culture systems and photobioreactors for microalgal biofuel production,” in Microalgae biotechnology for development of biofuel and wastewater treatment. Editors M. Alam,, and Z. Wang (Singapore: Springer).
Rasoul-Amini, S., Montazeri-Najafabady, N., Shaker, S., Safari, A., Kazemi, A., Mousavi, P., et al. (2014). Removal of nitrogen and phosphorus from wastewater using microalgae free cells in bath culture system. Biocatal. Agric. Biotechnol. 3, 126–131. doi:10.1016/j.bcab.2013.09.003
Rathod, H. (2014) “Algae based wastewater treatment,” in A seminar report of master of technology in civil engineering. Uttarakhand, India: Roorkee.
Renuka, N., Sood, A., Prasanna, R., and Ahluwalia, A. S. (2015). Phycoremediation of wastewaters: a synergistic approach using microalgae for bioremediation and biomass generation. Int. J. Environ. Sci. Technol. 12, 1443–1460. doi:10.1007/s13762-014-0700-2
Reymann, T., Kerner, M., and Kümmerer, K. (2020). Assessment of the biotic and abiotic elimination processes of five micropollutants during cultivation of the green microalgae Acutodesmus obliquus. Bioresour. Technol. Rep. 11, 100512. doi:10.1016/j.biteb.2020.100512
Rezania, S., Taib, S. M., Din, M. F. M., Dahalan, F. A., and Kamyab, H. (2016). Comprehensive review on phytotechnology: heavy metals removal by diverse aquatic plants species from wastewater. J. Hazard. Mater. 318, 587–599. doi:10.1016/j.jhazmat.2016.07.053
Saha, I., Datta, S., and Biswas, D. (2023). Mechanistic approach on comparative biosorption of dyes by extracellular polymer of Ochrobactrum pseudintermedium C1 utilizing waste mineral lubricating oil. Bioremedia. Journal. 28 (3), 266–285. doi:10.1080/10889868.2023.2269220
Sankaran, R., Cruz, R. A. P., Pakalapati, H., Show, P. L., Ling, T. C., Chen, W.-H., et al. (2020). Recent advances in the pretreatment of microalgal and lignocellulosic biomass: a comprehensive review. Bioresour. Technol. 298, 122476. doi:10.1016/j.biortech.2019.122476
Sarkar, P., and Dey, A. (2021). Phycoremediation–an emerging technique for dye abatement: an overview. Process Saf. Environ. Prot. 147, 214–225. doi:10.1016/j.psep.2020.09.031
Semple, K. T., Cain, R. B., and Schmidt, S. (1999). Biodegradation of aromatic compounds by microalgae. FEMS Microbiol. Lett. 170, 291–300. doi:10.1016/s0378-1097(98)00544-8
Shen, Q.-H., Zhi, T.-T., Cheng, L.-H., Xu, X.-H., and Chen, H.-L. (2013). Hexavalent chromium detoxification by nonliving Chlorella vulgaris cultivated under tuned conditions. Chem. Eng. J. 228, 993–1002. doi:10.1016/j.cej.2013.05.074
Shitu, A., Tadda, M. A., Zhao, J., Danhassan, U. A., Ye, Z., Liu, D., et al. (2024). Review of recent advances in utilising aquaculture wastewater for algae cultivation and microalgae-based bioproduct recovery. Environ. Geochem Health 46 (12), 485. doi:10.1007/s10653-024-02286-8
Sutherland, D. L., Howard-Williams, C., Turnbull, M. H., Broady, P. A., and Craggs, R. J. (2015). Enhancing microalgal photosynthesis and productivity in wastewater treatment high rate algal ponds for biofuel production. Bioresour. Technol. 184, 222–229. doi:10.1016/j.biortech.2014.10.074
Sutherland, D. L., and Ralph, P. J. (2019). Microalgal bioremediation of emerging contaminants-Opportunities and challenges. Water Res. 164, 114921. doi:10.1016/j.watres.2019.114921
Tiwari, B., Sellamuthu, B., Ouarda, Y., Drogui, P., Tyagi, R. D., and Buelna, G. (2017). Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 224, 1–12. doi:10.1016/j.biortech.2016.11.042
Torres, M. A., Barros, M. P., Campos, S. C., Pinto, E., Rajamani, S., Sayre, R. T., et al. (2008). Biochemical biomarkers in algae and marine pollution: a review. Ecotoxicol. Environ. Saf. 71, 1–15. doi:10.1016/j.ecoenv.2008.05.009
Tran, N. H., Reinhard, M., and Gin, K. Y. (2018). Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 133, 182–207. doi:10.1016/j.watres.2017.12.029
Tüzün, İ., Bayramoğlu, G., Yalçın, E., Başaran, G., Celik, G., and Arıca, M. Y. (2005). Equilibrium and kinetic studies on biosorption of Hg (II), Cd (II) and Pb (II) ions onto microalgae Chlamydomonas reinhardtii. J. Environ. Manag. 77, 85–92. doi:10.1016/j.jenvman.2005.01.028
Umamaheswari, J., and Shanthakumar, S. (2016). Efficacy of microalgae for industrial wastewater treatment: a review on operating conditions, treatment efficiency and biomass productivity. Rev. Environ. Sci. bio/technology 15, 265–284. doi:10.1007/s11157-016-9397-7
Ummalyma, S. B., Mathew, A. K., Pandey, A., and Sukumaran, R. K. (2016). Harvesting of microalgal biomass: efficient method for flocculation through pH modulation. Bioresour. Technol. 213, 216–221. doi:10.1016/j.biortech.2016.03.114
United Nations (2019). The united Nations world water development report 2023: partnerships and cooperation for water. Paris: UNESCO.
United Nations (2022). Sustainable development goals. Available at: https://sdgs.un.org/goals August 17, 2022).
Vardhan, K. H., Kumar, P. S., and Panda, R. C. (2019). A review on heavy metal pollution, toxicity and remedial measures: current trends and future perspectives. J. Mol. Liq. 290, 111197. doi:10.1016/j.molliq.2019.111197
Varjani, S. J. (2017). Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 223, 277–286. doi:10.1016/j.biortech.2016.10.037
Villar-Navarro, E., Baena-Nogueras, R. M., Paniw, M., Perales, J. A., and Lara-Martín, P. A. (2018). Removal of pharmaceuticals in urban wastewater: high rate algae pond (HRAP) based technologies as an alternative to activated sludge based processes. Water Res. 139, 19–29. doi:10.1016/j.watres.2018.03.072
Wanda, E. M., Nyoni, H., Mamba, B. B., and Msagati, T. A. (2017). Occurrence of emerging micropollutants in water systems in gauteng, mpumalanga, and North west provinces, South Africa. Int. J. Environ. Res. public health 14, 79. doi:10.3390/ijerph14010079
Wang, C.-Y., Fu, C.-C., and Liu, Y.-C. (2007). Effects of using light-emitting diodes on the cultivation of Spirulina platensis. Biochem. Eng. J. 37, 21–25. doi:10.1016/j.bej.2007.03.004
Wang, Y., Ho, S.-H., Cheng, C.-L., Guo, W.-Q., Nagarajan, D., Ren, N.-Q., et al. (2016). Perspectives on the feasibility of using microalgae for industrial wastewater treatment. Bioresour. Technol. 222, 485–497. doi:10.1016/j.biortech.2016.09.106
Whitton, R., Ometto, F., Pidou, M., Jarvis, P., Villa, R., and Jefferson, B. (2015). Microalgae for municipal wastewater nutrient remediation: mechanisms, reactors and outlook for tertiary treatment. Environ. Technol. Rev. 4, 133–148. doi:10.1080/21622515.2015.1105308
Wollmann, F., Dietze, S., Ackermann, J. U., Bley, T., Walther, T., Steingroewer, J., et al. (2019). Microalgae wastewater treatment: biological and technological approaches. Eng. Life Sci. 19, 860–871. doi:10.1002/elsc.201900071
World Health Organization (2024). Water, sanitation and hygiene (WASH). Available at: https://www.who.int/health-topics/water-sanitation-and-hygiene-wash#tab=tab_1 (accessed on December 6, 2024).
Xiong, J.-Q., Kurade, M. B., Abou-Shanab, R. A., Ji, M.-K., Choi, J., Kim, J. O., et al. (2016). Biodegradation of carbamazepine using freshwater microalgae Chlamydomonas mexicana and Scenedesmus obliquus and the determination of its metabolic fate. Bioresour. Technol. 205, 183–190. doi:10.1016/j.biortech.2016.01.038
Xiong, J.-Q., Kurade, M. B., and Jeon, B.-H. (2018). Can microalgae remove pharmaceutical contaminants from water? Trends Biotechnol. 36, 30–44. doi:10.1016/j.tibtech.2017.09.003
Zhang, C., Wang, X., Ma, Z., Luan, Z., Wang, Y., Wang, Z., et al. (2020a). Removal of phenolic substances from wastewater by algae. A review. Environ. Chem. Lett. 18, 377–392. doi:10.1007/s10311-019-00953-2
Zhang, S., Xiao, J., Wang, G., and Chen, G. (2020b). Enzymatic hydrolysis of lignin by ligninolytic enzymes and analysis of the hydrolyzed lignin products. Bioresour. Technol. 304, 122975. doi:10.1016/j.biortech.2020.122975
Zhang, S.-Y., Sun, G.-X., Yin, X.-X., Rensing, C., and Zhu, Y.-G. (2013). Biomethylation and volatilization of arsenic by the marine microalgae Ostreococcus tauri. Chemosphere 93, 47–53. doi:10.1016/j.chemosphere.2013.04.063
Zhang, X., Zhao, H., Du, J., Qu, Y., Shen, C., Tan, F., et al. (2017). Occurrence, removal, and risk assessment of antibiotics in 12 wastewater treatment plants from Dalian, China. Environ. Sci. Pollut. Res. Int. 24 (19), 16478–16487. doi:10.1007/s11356-017-9296-7
Zhang, Y., Habteselassie, M. Y., Resurreccion, E. P., Mantripragada, V., Peng, S., Bauer, S., et al. (2014). Evaluating removal of steroid estrogens by a model alga as a possible sustainability benefit of hypothetical integrated algae cultivation and wastewater treatment systems. ACS Sustain. Chem. and Eng. 2, 2544–2553. doi:10.1021/sc5004538
Keywords: microalgae, emerging contaminants, phycoremediation, wastewater treatment, biodegradation, sustainable development
Citation: Kundu P, Dutta N and Bhattacharya S (2024) Application of microalgae in wastewater treatment with special reference to emerging contaminants: a step towards sustainability. Front. Anal. Sci. 4:1513153. doi: 10.3389/frans.2024.1513153
Received: 17 October 2024; Accepted: 09 December 2024;
Published: 23 December 2024.
Edited by:
Marianne Fillet, University of Liège, BelgiumReviewed by:
Abdul Ghaffar Memon, NED University of Engineering and Technology, PakistanHarnish H. Soni, Maharaja Sayajirao University of Baroda, India
Copyright © 2024 Kundu, Dutta and Bhattacharya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sayan Bhattacharya, sayan.evs@gmail.com, sbhattacharya@nalandauniv.edu.in
 Pritha Kundu1
Pritha Kundu1