- 1Department of Pharmacy, College of Medicine and Health Sciences, Bahir Dar University, Bahir Dar, Ethiopia
- 2Department of Biomedical Science, College of Medicine and Health Sciences, Bahir Dar University, Bahir Dar, Ethiopia
Background: This study provides an overview of the clinical applications of drugs and their metabolites analysis in biological fluids and identifies commonly used analytical techniques for bioanalysis.
Methods: Original open-access articles published between 31 October 2005 and 31 October 2020 in Google Scholar, MEDLINE, PubMed, and Embase were reviewed, and pertinent findings of the individual studies were pooled and presented using tables. This review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA).
Results: Fifteen studies met the eligibility criteria and were included in the review. These studies show that qualitative identification and quantitative determination of drugs and their metabolites in biological fluids are important for therapeutic drug monitoring (TDM), pharmacokinetic and pharmacodynamic studies, drug abuse control, and forensic and toxicological analyses. Spectroscopic, electrochemical, and hyphenated and nonhyphenated chromatographic techniques are used to analyse drugs and their metabolites in biological fluids. However, hyphenated techniques are the preferred analytical methods because of their sensitivity, selectivity, accuracy, reproducibility, efficiency, and rapid analysis.
Conclusion/Recommendations: Bioanalysis is important for pharmacokinetic and pharmacodynamic studies, therapeutic drug monitoring, forensic and toxicological analyses, and drug abuse control using different bioanalytical techniques. However, hyphenated techniques are the most commonly used bioanalytical techniques. Bioanalysis of drugs and their metabolites needs to be improved to provide good medical and pharmaceutical care to patients, to confirm forensic and toxicological cases, and to control drug abuse.
1 Introduction
Bioanalysis is a subdiscipline of pharmaceutical analysis that focuses on the analysis of drug substances and their metabolites in biological fluids using bioanalytical techniques (Hansen et al., 2011). Drug analysis in biological fluids has been important for many years and has become the cornerstone of developing and formulating new chemical entities. However, most recently, patient care has included the identification and quantification of drugs and their metabolites in various biological systems (blood, plasma, serum, saliva, urine, skin, hair, and organ tissue (Wood, 1999; Thompson et al., 2002; Hansen et al., 2011).
The field of bioanalysis has matured significantly since early studies on drug metabolism using many simple and advanced techniques, and current bioanalysis is well-equipped to address modern challenges (Thompson et al., 2002). However, the analysis of drugs and their metabolites in biological fluids is complex for several reasons: drugs and their metabolites are found in complex biological matrices. The concentrations to be measured are often in the microgram to nanogram or picogram levels. These matrices normally contain large amounts of endogenous compounds, which can interfere with the chemical and physical analytical methods used to detect and determine materials of pharmacological interest. Consequently, unless an ultra-specific method of analysis is available for the substance of interest, physical separation of that substance from other interfering substances is usually necessary before quantitative determination can be achieved. To this end, most drug analyses involve different steps, such as sample collection, sample pre-treatment, separation from the matrix, detection, and analysis (Food and Drug Administration, 2001; Wells, 2003; Hansen et al., 2011).
Biological fluids serve as invaluable sources of information for various diagnostic, research, and therapeutic purposes in medicine and biology. Collecting these fluids with precision and care is crucial to ensure the integrity of the samples and the reliability of the subsequent analysis (Food and Drug Administration, 2001; Wells, 2003). Samples of biological fluids were collected from extracellular fluids, namely, blood (whole blood, serum, or plasma), cerebrospinal fluid (CSF), saliva, amniotic fluid, ocular fluid, pleural fluid (from the sac surrounding the lungs), urine, pericardial fluid (from the sac surrounding the heart), peritoneal fluid, also called ascetic fluid (from the abdomen), and synovial fluid (a fluid that is found in joint cavities). However, blood, plasma, urine, and serum are the fluids of choice (Wells, 2003; Food and Drug Administration, 2001; Thompson et al., 2002; Ritscher et al., 2019). A good bioanalysis starts with appropriate sample collection procedures and sample preparation, which are often the keys to successful analytical results. It has a direct impact on accuracy, precision, and quantification limits and is often the rate-determining step for many analytical methods. The sample preparation stage of the analysis is often the most critical and difficult part of the process, both in terms of the time involved and the difficulty of extracting the desired analyte from the matrix. In addition, each matrix has unique challenges (Hansen et al., 2011; Ritscher et al., 2019).
The method of sample preparation generally depends on the available analytical technique and the physical characteristics of the analytes under investigation. The purpose of sample preparation is to remove many endogenous interferences from the analyte and to ensure that the injection matrix is compatible with the chromatographic system (Van der Heijden et al., 2009; Hansen et al., 2011).
The choice of analytical methods and separation techniques for the analysis of drugs in biological fluids may depend on the type of sample matrix to be analysed, the physicochemical properties of the drug(s) and its metabolites, the chemical structures of the drug, the range of concentrations to be measured, the stability of the drug(s), the objective of the analysis (clinical, forensic), and the degree of experience of the analyst (Mark, 2003; Fríguls et al., 2010). Therefore, this study provides an overview of the clinical applications of drugs and their metabolites analysis in biological fluids and identifies the most effective analytical techniques for bioanalysis.
2 Methodology
2.1 Search strategy
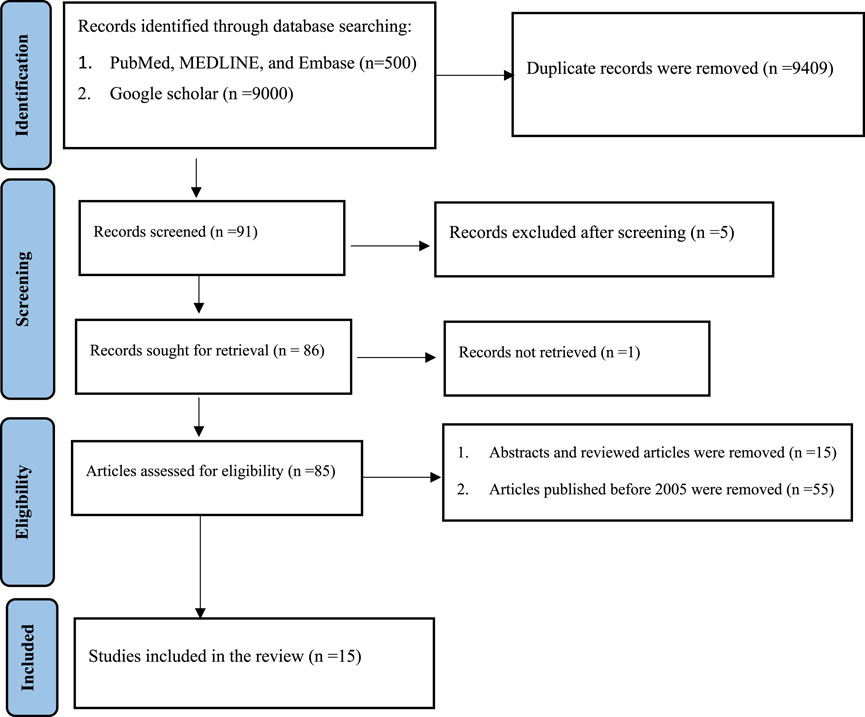
Different databases (PubMed, Medline, Embase, and Google Scholar) were used. Articles that were published between 31 October 2005 and 31 October 2020 were identified using keywords. The entire search was conducted using stepwise procedures from 15 October 2020, to 10 November 2020, based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 1) (Moher et al., 2009).
Keywords: (clinical application OR clinical importance OR clinical purpose) AND (analysis OR test) AND (drugs OR pharmaceutical products OR medicines OR medical products) AND (biological fluids OR biological matrices) AND (analytical techniques OR analytical methods).
2.2 Eligibility criteria
Original open-access articles in PDF format published within the past 15 years and studies clearly described their sample size, sampling drugs, the purpose of the study, biological fluids taken for bioanalysis, analytical techniques used for bioanalysis, and major findings were included in the review, whereas book reviews, abstracts, reviewed articles, and unrelated articles were excluded.
2.3 Data extraction
Important and relevant findings were extracted from the original articles using inclusion and exclusion criteria.
2.4 Data analysis
After extraction, the pertinent findings of the individual studies were pooled and presented in a table.
3 Results
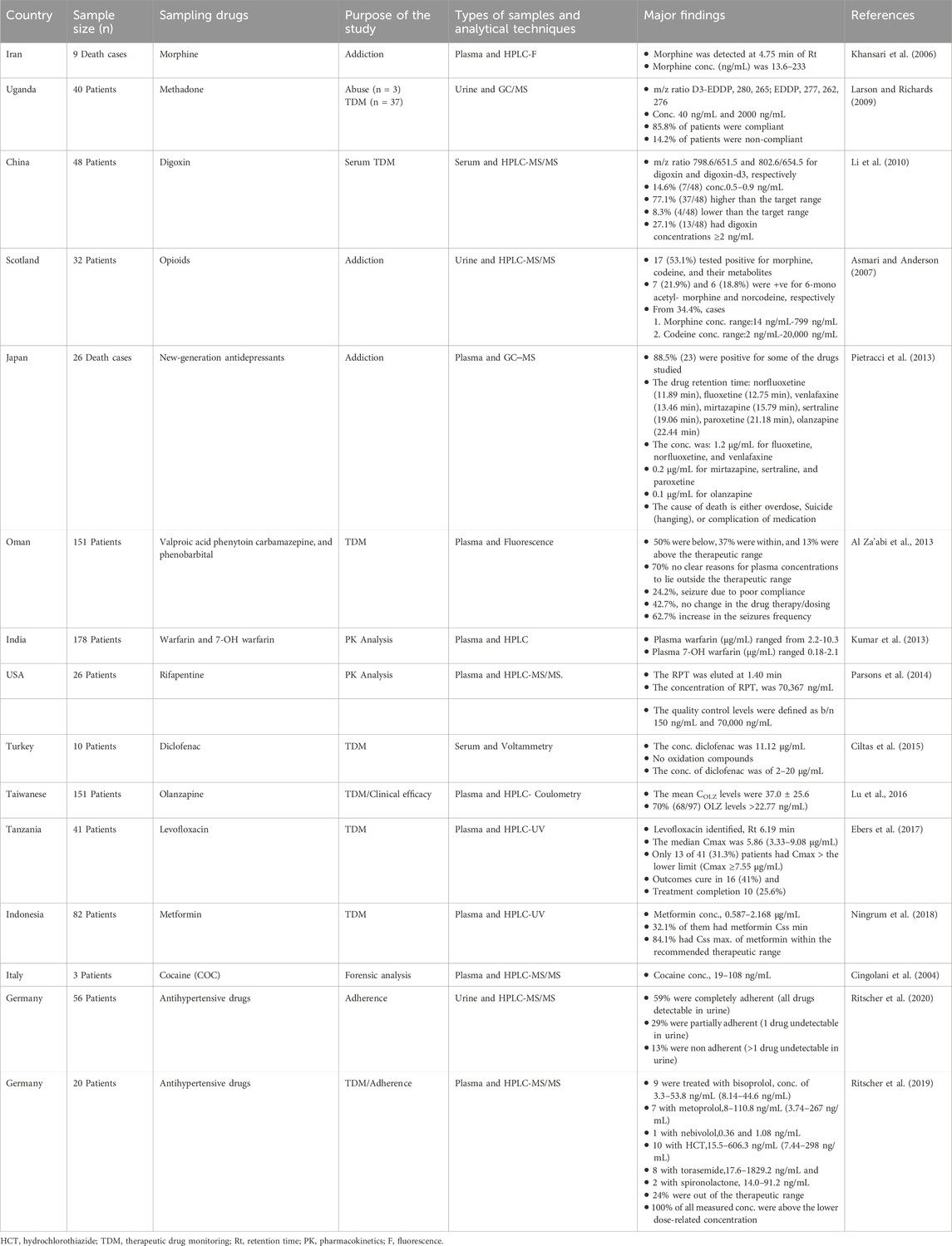
Fifteen studies related to the analysis of drugs in biological fluids and analytical techniques used for bioanalysis were searched; these studies focused on the importance of drug analysis in biological fluids, including therapeutic drug monitoring, pharmacokinetic and pharmacodynamic drug studies, drug abuse control, and forensic and toxicological analyses. In addition, studies focusing on the analytical techniques used in bioanalysis were included (Table 1).
4 Discussion
Bioanalysis represents a multifaceted field that is critical for unraveling the complexities of human health and disease. It is conducted in hospital laboratories, forensic toxicology laboratories, and doping control laboratories. Bioanalytical data obtained in these settings are highly important for therapeutic drug monitoring (TDM), pharmacokinetic and pharmacodynamic drug studies, drug abuse control, and forensic and toxicological analyses (Cingolani et al., 2004; Larson and Richards, 2009; Li et al., 2010; Kumar et al., 2013; Al Za’abi et al., 2013; Parsons et al., 2014; Ciltas et al., 2015; Lu M et al., 2016; Ebers et al., 2017; Ningrum et al., 2018; Ritscher et al., 2019; Ritscher et al., 2020).
Bioanalysis is crucial for TDM in many hospital laboratories. Recently, TDM has become an essential tool for guiding rational clinical drug use and aiding the management of patients receiving drug therapy to alleviate or prevent disease. TDM combines pharmacokinetic and pharmacodynamic knowledge to optimize personalized drug therapy, which can improve treatment outcomes, reduce drug toxicity, and avoid the risk of developing drug resistance (Campbell and Bode, 1994; Al Za’abi et al., 2013; Ciltas et al., 2015).
Personalised medicine is a medical approach that optimises treatment efficacy and reduces adverse effects by designing the best treatment plan specifically for each patient (Kumar et al., 2013; Lu et al., 2016). The pharmacological response to a drug given in a selected dosage regimen depends on various factors, such as patient compliance, age, sex, body type, metabolic status, lifestyle, environmental factors, underlying diseases, drug interactions, bioavailability, pharmacokinetics, and the protein-binding ability of the drug(s) (Larson and Richards, 2009; Al Za’abi et al., 2013; Ebers et al., 2017; Ritscher et al., 2019).
Currently, TDM approaches depend on the pharmacokinetic/pharmacodynamic variability of patients, which causes variations in the dose–response relationship among different patients (Larson and Richards, 2009; Ritscher et al., 2020). It is important to know how quickly drugs are metabolised; that is, whether a patient’s daily doses of a drug are positively or negatively correlated with its metabolites, and the concentration of its metabolites also increases. Generally, pharmacokinetics (ADME) analysis determines how these four criteria influence performance and pharmacological activity, such as drug distribution in plasma and tissue (Humphrey, 1996; Kumar et al., 2013; Parsons et al., 2014; Ciltas et al., 2015; Lu et al., 2016).
It is well established that only unbound free drugs can bind to receptors to produce the desired effect. Serum drug concentrations and pharmacological responses are significantly correlated with dose-serum drug concentrations for some drugs. Therefore, monitoring serum concentrations of these drugs is beneficial for patient management, particularly when they have very narrow therapeutic ranges (Larson and Richards, 2009; Ritscher et al., 2019).
To prevent adverse reactions and drug toxicity, which can occur when the drug concentration in the blood exceeds the maximum therapeutic concentration, and when the dosage is customised to achieve maximum efficacy, it may also be more beneficial to adjust the dose based on the serum drug concentration rather than routine patient assessment for these medications, which have very narrow therapeutic ranges. Therefore, therapeutic drug monitoring is a cost-effective healthcare approach. However, more rapid, precise, and advanced analytical techniques are needed to successfully apply TDM, particularly at low sample concentrations (Cingolani et al., 2004; Larson and Richards, 2009; Li et al., 2010; Kumar et al., 2013; Al Za’abi et al., 2013; Lu et al., 2016; Ebers et al., 2017; Ningrum et al., 2018).
TDM is tested for concentration after a certain period of drug administration at corresponding time intervals, and drug administration is controlled according to the drug’s therapeutic effect at different treatment stages in combination with the drug treatment window (Kumar et al., 2013; Parsons et al., 2014; Lu et al., 2016; Ebers et al., 2017).
A patient’s venous blood sample (whole blood, plasma, serum, etc.) is collected and analysed to measure the concentrations of drugs and their metabolites in blood samples. First, the identification of the drug in the blood sample confirms whether the patient has taken the drug or not. This information is important because not all patients comply with the prescribed medication. Second, the exact concentration of the drugs and their metabolites measured in the blood samples confirmed that the amount of drug was appropriate. This refers to the individualisation of dosage by maintaining blood drug concentration within a target range to optimize efficacy and reduce the risk of adverse side effects by monitoring drug concentrations in the blood (Kumar et al., 2013; Parsons et al., 2014; Lu et al., 2016; Ritscher et al., 2020).
Bioanalysis is also very important for forensic and toxicological analyses in forensic and toxicology laboratories (Khansari et al., 2006; Asmari and Anderson, 2007; Pietracci et al., 2013). Drug analysis in forensic and toxicology laboratories is performed by taking biological fluids as samples following the death of any person to aid in legal investigations to determine the actual cause of death using different bioanalytical techniques. Several studies have used blood, urine, brain tissue, etc., to determine the presence of compounds, such as opioids, antidepressants, antipsychotic drugs, and cocaine, for forensic and toxicological analysis and have also been used to investigate the abuse of opioids, antidepressants, antipsychotic drugs, cocaine, and other toxic substances (Flanagan et al., 1995; Hoja et al., 1997; Cingolani et al., 2004; Khansari et al., 2006; Asmari and Anderson, 2007; De Castro et al., 2009; Pietracci et al., 2013).
The analyte is typically unknown in forensic toxicology. Therefore, samples were first screened for the presence of drugs or drugs of abuse. In the case of a positive sample, the presence of the drug or the drug of abuse is confirmed using a second bioanalytical method. Due to the serious legal consequences of forensic cases, particular emphasis is placed on the quality and reliability of bioanalytical results. The work always involves the application of at least two different analytical methods (screening and confirmation) based on different physical or chemical principles (Campbell and Bode, 1994; Cingolani et al., 2004; Li et al., 2010; Kumar et al., 2013; Al Za’abi et al., 2013; Ciltas et al., 2015; Ebers et al., 2017; Ningrum et al., 2018; Ritscher et al., 2019).
Bioanalysis is highly challenging because most target pharmaceutical substances are present in blood, urine, and saliva samples at very low concentrations. Typically, the concentration is low at the nanogram level; however, in some cases, target pharmaceuticals must be detected at the picogram level. This requires high operator skill and highly sensitive instrumentation (Cingolani et al., 2004; Li et al., 2010; Ningrum et al., 2018). In addition, target pharmaceuticals coexist with a broad range of endogenous compounds that are naturally present in biological samples, which can be thousands of different components, and many of them can be present at high concentrations. Therefore, before the final measurement using the sensitive instrument, target drugs must typically be isolated from the biological matrix for bioanalysis to be successful. Therefore, experience and skills in sample preparation and the selection of bioanalytical techniques are extremely important in bioanalysis (Kumar et al., 2013; Al Za’abi et al., 2013; Ciltas et al., 2015; Ebers et al., 2017; Ritscher et al., 2019).
Analytical technique is a set of techniques that allow us to qualitatively and quantitatively determine the analyte in a sample. Bioanalytical techniques are analytical techniques used to determine drugs or substances in biological matrices, such as urine, serum, and plasma. Drugs in biological fluids are identified and quantified using various analytical techniques, including spectroscopic, electrochemical, and hyphenated and nonhyphenated chromatography techniques (Li et al., 2010; Kumar et al., 2013; Ritscher et al., 2020).
Spectroscopic techniques that use the relationship between the amount of light absorbed or emitted and the concentration of the reference drug can be used to determine the concentration of the drug in biological fluids. Because of its high selectivity, low detection limits, and ability to detect drug concentrations ranging from ng/mL to pg/mL, the fluorescence technique is a spectroscopic method that has been widely used in biological fluid analysis. However, the use of fluorescence methods has become limited because only certain classes of compounds exhibit fluorescence. Furthermore, derivative spectrophotometry offers an alternative approach for enhancing the sensitivity and specificity of mixture analyses with spectral overlap (Sotomayor et al., 2008; Al Za’abi et al., 2013).
High-performance liquid chromatography voltammetry and coulometry, are considered alternatives to spectroscopy and chromatography for determining drug concentrations in biological fluids (Gergov et al., 2009). These techniques are based on the continuous variation of the potential applied across the electrode-solution interface, and the resulting current is recorded. The current is a quantitative measurement related to the analyte concentration. The applied potential acts as the driving force for electrochemical reactions such as analyte reduction or oxidation. With the use of simple and affordable instrumentation, electrochemical methods offer high sensitivity and low detection limits. However, the application of these approaches to the analysis of drug concentrations in biological fluids is limited because of a lack of analysis of related substances (Gergov et al., 2009; Santos et al., 2009).
In recent years, chromatographic methods have become highly versatile for detecting and determining most drug concentrations in biological fluids. Hyphenated or nonhyphenated chromatographic techniques are preferred over other analytical techniques in terms of their sensitivity, selectivity, accuracy, reproducibility, small sample volumes, rapid analysis, and high separation efficiency (Gergov et al., 2009; Pietracci et al., 2013; Al Za’abi et al., 2013).
Gas chromatography (GC), but not high-performance liquid chromatography (HPLC), is limited to volatile and thermally stable compounds or molecules that undergo derivatization reactions to produce thermally stable products. HPLC is one of the most commonly used nonhyphenated methods; it is not dependent on the volatility of compounds and is very versatile for detecting and determining most drug concentration levels in biological fluids (Khansari et al., 2006; Asmari and Anderson, 2007; Larson and Richards, 2009; Santos et al., 2009; Pietracci et al., 2013; Kumar et al., 2013; Ciltas et al., 2015; Lu M et al., 2016; Ebers et al., 2017; Ningrum et al., 2018).
Extremely low concentrations of some drugs in biological fluids necessitate the combination of various chromatographic and spectroscopic techniques; such combinations are called hyphenated techniques. The hyphenation system provides the best drug identification and quantification through increasingly complex separations to optimize the sample response and discriminate against interferences using different detectors, accurate and rapid analysis, the best sample throughput, and the best degree of automation. Thus, it provides powerful methods for analyzing complex samples and provides more information than individual techniques. It is a multidisciplinary analysis method to avoid incorrect results, it saves time because two or more analyses can be run at the same time, sample preparation time is reduced, and it provides accurate analytical information without cross-contamination (Kallner, 1999; Valcárcel et al., 1999; Kantharaj et al., 2003; Pulido et al., 2003).
In hyphenation systems, chromatographic methods are largely used for separation, whereas interfaced methods are mostly used for quantification and detection. Spectrophotometric detectors, electrochemical detectors, and mass spectrometry are by far the most frequently employed in HPLC systems. HPLC coupled with UV/vis detectors, diode array detectors (DADs), and fluorescence detectors (Khansari et al., 2006; Ebers et al., 2017; Ningrum et al., 2018).
UV/vis detectors are most often used in HPLC-based quantitative analysis because they are easy to use, affordable, and capable of evaluating a wide range of compounds. However, its sensitivity and selectivity are low for some stronger substances. Although DADs are more complex and costly than UV/vis detectors, they allow the analyte’s UV absorption spectrum to be registered, thus increasing the identification power of HPLC. Additionally, DADs allow the evaluation of chromatographic peak purity, which is useful for determining the separation efficiency of HPLC systems (Khansari et al., 2006; Gergov et al., 2009; Ebers et al., 2017; Ningrum et al., 2018).
Luminescence and fluorescence detectors are the most common in HPLC, and they are used either for direct analyte detection or for detecting analyte derivative products. Fluorescence detectors are widely used in bioanalysis because of their better sensitivity than that of absorption-based detection. The use of fluorescence detectors in HPLC systems is an intriguing strategy to exploit their increased sensitivity. The importance of very sensitive detectors in HPLC systems is clearly demonstrated by the extremely small quantity of samples using this technique. Fluorescence detectors are therefore suitable for this purpose (Khansari et al., 2006).
Another type of detector that is widely used in HPLC for bioanalysis is the electrochemical detector. The separation capabilities of HPLC are combined with the attractive features of electrochemical techniques, including sensitivity, precision, and inexpensive instrumentation, for electrochemical detection. Electrochemical detection techniques can be helpful in HPLC because their sensitivity is higher than that of fluorimetric methods. Additionally, electrochemical detectors are more versatile because they do not require chemical derivation steps because electroactive substances are more common than fluorescent substances (Ciltas et al., 2015; Lu et al., 2016).
However, electrochemical detection fundamentally differs from spectrophotometric detection because it alters the sample by oxidation or reduction of the analyte. This characteristic causes the electrode surface to be passivated by redox reaction intermediates or products, which is a limitation of electrochemical detectors in HPLC. When determining organic species, like drugs, whose redox processes frequently involve numerous intermediates that can firmly adsorb onto the electrode surface, this restriction of electrochemical detection is particularly noticeable (Khansari et al., 2006; Gergov et al., 2009; Ciltas et al., 2015; Lu et al., 2016).
High-performance liquid chromatography-mass spectrometry-mass spectrometry (HPLC-MS/MS) is the most effective and efficient technique (Katagi et al., 2001; Rivier, 2003). HPLC systems through a chromatographic column allow separating compounds in relation to polarity and affinity to the column, while a mass spectrometer generates multiple ions from the sample and separates these ions according to their specific mass-to-charge ratio, recording the relative abundance of each ion type (Hubert et al., 2002; Matuszewski et al., 2003; Asmari and Anderson, 2007; Siddiqui et al., 2009; Parsons et al., 2014).
HPLC-MS/MS is now the gold standard for TDM and offers many advantages in its application in TDM: sensitivity, precision, accuracy, speed of analysis, simple preparation, and determination of multiple analytes in one short run (Cingolani et al., 2004; Li et al., 2010).
Gas chromatography coupled with mass spectrometry (GC‒MS) (Bressolle et al., 1996; Mark, 2003; Larson and Richards, 2009; Pietracci et al., 2013), high performance liquid chromatography coupled with mass spectrometry (HPLC-MS) and ultraviolet (HPLC-UV) (Ebers et al., 2017; Ningrum et al., 2018), high performance liquid chromatography with atomic emission spectrometry (HPLC-AES) (Khansari et al., 2006), and high performance liquid chromatography coupled with electrochemical detection (HPLC-ECD) were also used for the identification and quantification of drugs and their metabolites in biological fluids (Katagi et al., 2001; Link et al., 2007; Lu et al., 2016).
5 Potential future indications
Bioanalysis of drugs and their metabolites need to be improved to provide good medical and pharmaceutical care to patients, to confirm forensic and toxicological cases, and to control drug abuse. In addition, newly developed or advanced bioanalytical techniques are required to obtain more reliable, accurate, and precise results by overcoming the challenges associated with bioanalysis.
6 Conclusion
Identification and quantification of drugs and their metabolites in various biological fluids (whole blood, plasma, serum, saliva, urine, etc.) using different bioanalytical techniques are used for therapeutic drug monitoring (TDM), pharmacokinetic and pharmacodynamic drug studies, drug abuse control, and forensic and toxicological analyses. Spectroscopic, electrochemical, and hyphenated or nonhyphenated chromatography techniques are used for bioanalysis. However, hyphenated techniques such as GC–MS, GC–MS/MS, HPLC-MS, HPLC-ECD, HPLC-MS/MS, and HPLC-AES are the most commonly used bioanalytical techniques for bioanalysis.
Author contributions
BM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing–original draft, Writing–review and editing. MY: Supervision, Validation, Writing–original draft, Writing–review and editing. KA: Conceptualization, Data curation, Formal Analysis, Methodology, Supervision, Validation, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al Za’abi, M., Al Asmi, A., Ahmed, R., Al-Masrouri, S., Al-Lawati, R., Nandhagopal, R., et al. (2013). Therapeutic antiepileptic drug monitoring pattern in a tertiary care hospital in Oman. Afr. J. Pharm. Pharmacol. 7 (2), 58–62. doi:10.5897/AJPP12.1203
Asmari, A. I., and Anderson, R. A. (2007). Method for quantification of opioids and their metabolites in autopsy blood by liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 31 (7), 394–408. doi:10.1093/jat/31.7.394
Bressolle, F., Bromet-Petit, M., and Audran, M. (1996). Validation of liquid chromatographic and gas chromatographic methods Applications to pharmacokinetics. J. Chromatogr. B Biomed. Sci. Appl. 686 (1), 3–10. doi:10.1016/s0378-4347(96)00088-6
Campbell, B., and Bode, G. (1994). Guest editor's note: toxicokinetics—the way forward. Drug Inf. J. 28 (1), 141–142. doi:10.1177/009286159402800115
Ciltas, U., Yilmaz, B., Kaban, S., Akcay, B. K., and Nazik, G. (2015). Square wave voltammetric determination of diclofenac in pharmaceutical preparations and human serum. Iran. J. Pharm. Res. IJPR 14 (3), 715–722.
Cingolani, M., Cippitelli, M., Froldi, R., Gambaro, V., and Tassoni, G. (2004). Detection and quantitation analysis of cocaine and metabolites in fixed liver tissue and formalin solutions. J. Anal. Toxicol. 28 (1), 16–19. doi:10.1093/jat/28.1.16
De Castro, A., Concheiro, M., Shakleya, D. M., and Huestis, M. A. (2009). Development and validation of a liquid chromatography mass spectrometry assay for the simultaneous quantification of methadone, cocaine, opiates and metabolites in human umbilical cord. J. Chromatogr. B 877 (27), 3065–3071. doi:10.1016/j.jchromb.2009.07.028
Ebers, A., Stroup, S., Mpagama, S., Kisonga, R., Lekule, I., Liu, J., et al. (2017). Determination of plasma concentrations of levofloxacin by high performance liquid chromatography for use at a multidrug-resistant tuberculosis hospital in Tanzania. PLoS One 12 (1), e0170663. doi:10.1371/journal.pone.0170663
Flanagan, R. J., Braithwaite, R. A., Brown, S. S., Widdop, B., and de Wolff, F. A. (1995). Basic analytical toxicology-world health organization.
Food and Drug Administration (2001). Guidance for industry: bioanalytical method validation. Available at: http://www.fda.gov/cder/Guidance/4252fnl.pdf.
Fríguls, B., Joya, X., García-Algar, O., Pallás, C. R., Vall, O., and Pichini, S. (2010). A comprehensive review of assay methods to determine drugs in breast milk and the safety of breastfeeding when taking drugs. Anal. Bioanal. Chem. 397, 1157–1179. doi:10.1007/s00216-010-3681-0
Gergov, M., Nokua, P., Vuori, E., and Ojanperä, I. (2009). Simultaneous screening and quantification of 25 opioid drugs in post-mortem blood and urine by liquid chromatography–tandem mass spectrometry. Forensic Sci. Int. 186 (1-3), 36–43. doi:10.1016/j.forsciint.2009.01.013
Hansen, S., Hansen, S. H., Pedersen-Bjergaard, S., and Rasmussen, K. (2011). Introduction to pharmaceutical chemical analysis. John Wiley and Sons.
Hoja, H., Marquet, P., Verneuil, B., Lotfi, H., Pénicaut, B., and Lachâtre, G. (1997). Applications of liquid chromatography-mass spectrometry in analytical toxicology: a review. J. Anal. Toxicol. 21 (2), 116–126. doi:10.1093/jat/21.2.116
Hubert, P., Chiap, P., Crommena, J., Boulanger, B., Chapuzet, E. N., Laurentie, M., et al. (2002). The SFSTP guide on the validation of chromatographic methods for drug bioanalysis: from the Washington Conference to the laboratory. Anal. Chim. Acta 391, 135–148. doi:10.1016/s0003-2670(99)00106-3
Humphrey, M. J. (1996). Application of metabolism and pharmacokinetic studies to the drug discovery process. Drug metab. Rev. 28 (3), 473–489. doi:10.3109/03602539608994012
Kallner, A. (1999). Quality specifications based on the uncertainty of measurement. Scand. J. Clin. laboratory investigation 59 (7), 513–516. doi:10.1080/00365519950185256
Kantharaj, E., Tuytelaars, A., Proost, P. E., Ongel, Z., Van Assouw, H. P., and Gilissen, R. A. (2003). Simultaneous measurement of drug metabolic stability and identification of metabolites using ion-trap mass spectrometry. Rapid Commun. mass Spectrom. 17 (23), 2661–2668. doi:10.1002/rcm.1228
Katagi, M., Nishikawa, M., Tatsuno, M., Miki, A., and Tsuchihashi, H. (2001). Column-switching high-performance liquid chromatography–electrospray ionization mass spectrometry for identification of heroin metabolites in human urine. J. Chromatogr. B Biomed. Sci. Appl. 751 (1), 177–185. doi:10.1016/s0378-4347(00)00469-2
Khansari, M., Zendehdel, R., Pirali-Hamedani, M., and Amini, M. (2006). Determination of morphine in the plasma of addicts in using Zeolite Y extraction following high-performance liquid chromatography. Clin. Chim. acta 364 (1-2), 235–238. doi:10.1016/j.cccn.2005.07.002
Kumar, D. K., Shewade, D. G., Parasuraman, S., Rajan, S., Balachander, J., Chandran, B. S., et al. (2013). Estimation of plasma levels of warfarin and 7-hydroxy warfarin by high performance liquid chromatography in patients receiving warfarin therapy. J. Young Pharm. 5 (1), 13–17. doi:10.1016/j.jyp.2013.02.001
Larson, M. E., and Richards, T. M. (2009). Quantification of a methadone metabolite (EDDP) in urine: assessment of compliance. Clin. Med. and Res. 7 (4), 134–141. doi:10.3121/cmr.2009.859
Li, S., Liu, G., Jia, J., Miao, Y., Gu, S., Miao, P., et al. (2010). Therapeutic monitoring of serum digoxin for patients with heart failure using a rapid LC-MS/MS method. Clin. Biochem. 43 (3), 307–313. doi:10.1016/j.clinbiochem.2009.09.025
Link, B., Haschke, M., Wenk, M., and Krähenbühl, S. (2007). Determination of midazolam and its hydroxy metabolites in human plasma and oral fluid by liquid chromatography/electrospray ionization ion trap tandem mass spectrometry. Rapid Commun. Mass Spectrom. An Int. J. Devoted Rapid Dissem. Up-to-the-Minute Res. Mass Spectrom. 21 (9), 1531–1540. doi:10.1002/rcm.2987
Lu, M. L., Wu, Y. X., Chen, C. H., Kuo, P. T., Chen, Y. H., Lin, C. H., et al. (2016). Application of plasma levels of olanzapine and N-desmethyl-olanzapine to monitor clinical efficacy in patients with schizophrenia. PloS one 11 (2), e0148539. doi:10.1371/journal.pone.0148539
Mark, H. (2003). Application of an improved procedure for testing the linearity of analytical methods to pharmaceutical analysis. J. Pharm. Biomed. analysis 33 (1), 7–20. doi:10.1016/s0731-7085(03)00346-7
Matuszewski, B. K., Constanzer, M. L., and Chavez-Eng, C. M. (2003). Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC− MS/MS. Anal. Chem. 75 (13), 3019–3030. doi:10.1021/ac020361s
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Prisma Group*, T. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 151 (4), 264–W64. doi:10.7326/0003-4819-151-4-200908180-00135
Ningrum, V. D., Ikawati, Z., Sadewa, A. H., and Ikhsan, M. R. (2018). Patient-factors associated with metformin steady-state levels in type 2 diabetes mellitus with therapeutic dosage. J. Clin. and Transl. Endocrinol. 12, 42–47. doi:10.1016/j.jcte.2018.05.001
Parsons, T. L., Marzinke, M. A., Hoang, T., Bliven-Sizemore, E., Weiner, M., Mac Kenzie, W. R., et al. (2014). Quantification of rifapentine, a potent antituberculosis drug, from dried blood spot samples using liquid chromatographic-tandem mass spectrometric analysis. Antimicrob. agents Chemother. 58 (11), 6747–6757. doi:10.1128/AAC.03607-14
Pietracci, E., Bermejo, A. M., Álvarez, I., Cabarcos, P., Balduini, W., and Tabernero, M. J. (2013). Simultaneous determination of new-generation antidepressants in plasma by gas chromatography–mass spectrometry. Forensic Toxicol. 31, 124–132. doi:10.1007/s11419-012-0152-7
Pulido, A., Ruisánchez, I., Boqué, R., and Rius, F. X. (2003). Uncertainty of results in routine qualitative analysis. TrAC Trends Anal. Chem. 22 (9), 647–654. doi:10.1016/S0165-9936(03)01104-X
Ritscher, S., Hoyer, M., Georges, C., Wunder, C., Wallemacq, P., Persu, A., et al. (2020). Benefit of serum drug monitoring complementing urine analysis to assess adherence to antihypertensive drugs in first-line therapy. PLoS One 15 (8), e0237383. doi:10.1371/journal.pone.0237383
Ritscher, S., Hoyer, M., Wunder, C., Obermüller, N., and Toennes, S. W. (2019). Evaluation of the dose-related concentration approach in therapeutic drug monitoring of diuretics and β-blockers - drug classes with low adherence in antihypertensive therapy. Sci. Rep. 9 (1), 15652. doi:10.1038/s41598-019-52164-y
Rivier, L. (2003). Criteria for the identification of compounds by liquid chromatography–mass spectrometry and liquid chromatography–multiple mass spectrometry in forensic toxicology and doping analysis. Anal. Chim. acta 492 (1-2), 69–82. doi:10.1016/s0003-2670(03)00889-4
Santos, A. L., Takeuchi, R. M., and Stradiotto, N. R. (2009). Electrochemical, spectrophotometric and liquid-chromatographic approaches for analysis of tropical disease drugs. Curr. Pharm. Anal. 5 (1), 69–88. doi:10.2174/157341209787314927
Siddiqui, M. R., Tariq, A., Ahmad, A., Chaudhary, M., Shrivastava, S. M., and Singh, R. K. (2009). Application of DDQ and p-chloranilic acid for the spectrophotometric estimation of milrinone in pharmaceutical formulations. Asian J. Sci. Res. 2 (3), 135–145. doi:10.3923/ajsr.2009.135.145
Sotomayor, M. D., Dias, I. L. T., Lanza, M. R., Moreira, A. B., and Kubota, L. T. (2008). Aplicação e avanços da espectroscopia de luminescência em análises farmacêuticas. Quím. Nova 31, 1755–1774. doi:10.1590/s0100-40422008000700031
Thompson, M., Ellison, S. L., and Wood, R. (2002). Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl. Chem. 74 (5), 835–855. doi:10.1351/pac200274050835
Valcárcel, M., Cárdenas, S., and Gallego, M. (1999). Sample screening systems in analytical chemistry. TrAC Trends Anal. Chem. 18 (11), 685–694. doi:10.1016/s0165-9936(99)00167-3
Van der Heijden, J., De Beer, Y., Hoogtanders, K., Christiaans, M., De Jong, G. J., Neef, C., et al. (2009). Therapeutic drug monitoring of everolimus using the dried blood spot method in combination with liquid chromatography–mass spectrometry. J. Pharm. Biomed. analysis 50 (4), 664–670. doi:10.1016/j.jpba.2008.11.021
Wells, D. A. (2003). “High throughput bioanalytical sample preparation-methods and automation strategies,” in Progress in pharmaceutical and biomedical analysis.
Keywords: drug, medicine, bioanalysis, biological fluids, analytical techniques
Citation: Mekonnen BA, Yizengaw MG and Adugna KF (2024) The clinical applications of drugs and their metabolites analysis in biological fluids and commonly used analytical techniques for bioanalysis: review. Front. Anal. Sci. 4:1490093. doi: 10.3389/frans.2024.1490093
Received: 02 September 2024; Accepted: 11 November 2024;
Published: 26 November 2024.
Edited by:
Jayant Gowda, BLDEA’s Commerce BHS Arts and TGP Science College, IndiaReviewed by:
J. G. Manjunatha, Mangalore University Constituent College, IndiaJyothi C. Abbar, BMS Institute of Technology, India
Copyright © 2024 Mekonnen, Yizengaw and Adugna. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biset Asrade Mekonnen, YmlzZXQyMDA2bWVAZ21haWwuY29t
 Biset Asrade Mekonnen
Biset Asrade Mekonnen Muluabay Getie Yizengaw2
Muluabay Getie Yizengaw2