
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Amphib. Reptile Sci. , 31 March 2025
Sec. Conservation
Volume 3 - 2025 | https://doi.org/10.3389/famrs.2025.1525965
This article is part of the Research Topic Reproductive Physiology, Reproductive Technologies, and Biobanking to Assist Amphibian and Reptile Conservation View all 4 articles
Amphibians are currently experiencing the highest extinction rate of any vertebrate class. Gamete cryopreservation and the biobanking of genetic resources are important conservation tools to safeguard the genetic diversity of imperiled species. While amphibian oocytes/embryos have proven difficult to cryopreserve, amphibian sperm cryopreservation has been achieved in a growing number of species, though with variable post-thaw recovery. Oxidative stress is a major cause of cell damage during cryopreservation and results in compromised post-thaw sperm quality. Supplementation of cryopreservation extenders with antioxidants has been shown to benefit the post-thaw recovery of sperm from a number of mammal and fish species, however research investigating potential benefits to amphibian sperm cryopreservation is lacking. The aim of the present study was to quantify the effect of antioxidant supplementation (2mM melatonin, 2mM ascorbic acid, 2mM uric acid, or control cryodiluent on sperm viability, motility, and velocity in the near-threatened red-crowned toadlet, Pseudophryne australis. A split-sample experimental design was adopted, whereby single-male sperm suspensions (n = 8) were evenly divided among four experimental treatments (control, melatonin, ascorbic acid, and uric acid). Sperm suspensions were cryopreserved, and post-thaw sperm quality metrics assessed (sperm viability [live/dead], percentage total sperm motility, percentage forward progressive motility [FPM], curvilinear velocity [VCL], and average path velocity [VAP]). Melatonin and uric acid treatments exhibited significantly higher sperm viability compared to the control treatment, with the ascorbic acid treatment exhibiting intermediate mean viability. Motility parameters were not significantly different among treatments, though motility and velocity metrics tended to be higher in the ascorbic acid treatment. Overall, this study provides the first evidence that antioxidant supplementation of cryopreservation extenders can improve post-thaw sperm quality in an amphibian, and paves the way for future research.
Biodiversity losses are occurring at an unprecedented rate due to factors such as rapid environmental change, increased stochasticity, emergent disease, and habitat loss. Amphibians are experiencing the highest extinction rates of any vertebrate class, with 41% of known amphibian species threatened with extinction, followed by 27% of mammals, 26% of freshwater fish, 21% of reptiles, and 12% of birds (IUCN, 2024). Conservation breeding programs (CBPs) are a safeguard for many imperilled species, contributing to species recovery by maintaining genetically representative captive colonies and providing individuals for wild release. As species decline, extant populations typically become increasingly fragmented and remnant in situ and ex-situ populations can suffer from restricted gene flow, loss of genetic diversity and heterozygosity, leading to increased extinction risk (Frankham, 2015). Consequently, it is imperative that the genetic diversity and adaptive potential of threatened species is sequestered by CBP managers to afford opportunities for populations to be augmented and genetic diversity reinvigorated to promote species recovery (Bell et al., 2019). The application of reproductive technologies (including hormone therapies, sperm cryopreservation, and assisted fertilisation), used in concert with the establishment of genome resource banks (GRBs; also known as biorepositories) affords valuable opportunities to extend the reproductive lifespan of individuals, and facilitate genetic rescue and assisted gene-flow (Byrne and Silla, 2022). Incorporating these technologies into conservation breeding programs for threatened species therefore has enormous potential to assist managers to reach genetic management goals and enhance conservation outcomes.
Sperm cryopreservation is a reproductive technology that offers a strategy to preserve viable amphibian genetic material ad infinitum. While amphibian sperm cryopreservation technologies have been available for many decades, the application of these technologies and establishment of GRBs for conservation efforts of threatened amphibians has occurred more recently (Silla and Kouba, 2022). The value of GRBs for species conservation relies on cryopreservation protocol refinement to ensure biobanked samples retain maximal functionality for subsequent inclusion in assisted fertilisation efforts and the production of viable offspring (Anastas et al., 2023). Successful assisted fertilisations for externally fertilising amphibians are associated with optimal sperm concentrations (Silla and Byrne, 2019), therefore ensuring that a high proportion of sperm survive the freeze–thaw process will ultimately enhance the generation of offspring from cryopreserved sperm. To date, sperm from 50 amphibian species have been successfully cryopreserved and a growing number of amphibian GRBs established globally (Calatayud and Della Togna, 2022). At present, however, standardised protocols for the routine cryopreservation of amphibian sperm are limited, and post-thaw recovery rates of sperm samples are highly variable. A number of factors are likely to contribute to the variation in post-thaw sperm qualities reported (Anastas et al., 2023), with each warranting further investigation, including freezing and thawing rates, dilution ratios and composition of the cryopreservation extender (also referred to as the cryodiluent, or cryoprotectant [CPA]) employed. One priority research area that may benefit amphibian sperm cryopreservation outcomes concerns investigating the use of antioxidant supplementation of cryopreservation extenders, to combat the deleterious effects of oxidative stress on post-thaw sperm function.
Oxidative stress, as a result of excess reactive oxygen species, is a major cause of decline in sperm quality and performance during and after cryopreservation (Aitken et al., 2014). Reactive oxygen species (ROS) are unstable oxygen-containing molecules generated as a by-product of cellular metabolic processes (Aitken et al., 2014; Gualtieri et al., 2021). They are required for crucial cell functions but can have harmful effects depending on their composition and concentration. Sperm cells are particularly vulnerable to oxidative stress due to sperm plasma membrane features that include polyunsaturated fatty acids (PUFAs) which are subject to lipid peroxidation when exposed to increased ROS levels (Amidi et al., 2016). Resultant peroxidation can lead to membrane leakage and DNA damage, and prevent oocyte penetration or fertilisation (Amidi et al., 2016). While ROS are required for cell signalling, sperm capacitation, and activation, the process of cryopreservation has been shown to result in a significant increase in ROS production in numerous species (Alevra et al., 2022; Félix et al., 2020; Medrano et al., 2017). The production of ROS during cryopreservation and thawing has been shown to result in lipid peroxidation, DNA damage, and decreased sperm function (Alevra et al., 2022; Kameni et al., 2021). Management of oxidative stress may therefore prove crucial to ensure optimal post-thaw sperm quality for fertilisation success and the production of viable cryo-offspring.
Antioxidant supplementation of cryopreservation extenders has the potential to mitigate the damaging effects of oxidative stress by sequestering or converting harmful ROS. The beneficial effects of antioxidant supplementation on sperm cryopreservation outcomes have been investigated in a diversity of fish and mammal species (Amidi et al., 2016; Félix et al., 2020; Sandoval-Vargas et al., 2021; Hu et al., 2010; Varo-Ghiuru et al., 2015). Specifically in fish, the antioxidant compounds ascorbic acid and uric acid have been of particular interest due to their presence in the natural antioxidant defence systems of fish (Sandoval-Vargas et al., 2021; Lahnsteiner and Mansour, 2010; Ciereszko et al., 1999). Overall, ascorbic acid (vitamin C) has been the most widely applied antioxidant to the cryopreservation extenders of fish semen (Kolyada et al., 2023). The addition of ascorbic acid has been reported to result in increased post-thaw sperm motility and improved fertilisation rates in Russian sturgeon (Mirzoyan et al., 2006), and improved post-thaw motility in rainbow trout (Yavuz and Bozkurt, 2020). Uric acid supplementation has also been shown to improve post-thaw percent motility and motility duration in rainbow trout (Kutluyer et al., 2014). Melatonin is another potent antioxidant known to protect against oxidative stress and enhance post-thaw recovery of cryopreserved sperm from a number of mammalian species (Ofosu et al., 2021). The supplementation of fish cryopreservation extenders with melatonin has occurred more recently, with research in streaked prochilod, reporting an increase in post-thaw curvilinear and average path sperm velocities in response to the addition of melatonin (Motta et al., 2022). Additionally, supplementation of cryopreservation extenders with melatonin to Brazilian piracanjuba sperm increased sperm viability, percent motility, progressive motility, fertilisation, and hatching rates (Palhares et al., 2021).
Overall, the results from studies in several fish species highlight the potential for antioxidant supplementation of cryopreservation extenders to improve post-thaw sperm quality parameters. To date antioxidants including melatonin, ascorbic acid, and uric acid are yet to be applied to amphibian sperm cryopreservation extenders, and their potential to reduce sperm cryodamage is therefore currently unknown. Research investigating the refinement of sperm cryopreservation protocols for species in the Australian genus Pseudophryne are of particular importance because post-thaw sperm quality metrics reported for these species have been comparatively low (Anastas et al., 2023), and several of the 14 identified species in the genus are threatened. The aim of the present study was to investigate the effects of antioxidant supplementation on the post-thaw recovery of sperm from the red-crowned toadlet, Pseudophryne australis. Specifically, our study aimed to quantify, for the first time, the effect of supplementing cryopreservation extenders with melatonin, ascorbic acid, and uric acid on post-thaw sperm viability, motility, and velocity, in an amphibian.
The red-crowned toadlet, Pseudophryne australis is a small (males=19–25mm, females=23–28mm, snout–vent length) terrestrial frog from the family Myobatrachidae (Thumm and Mahony, 2002). Both males and females of the species are predominantly dark grey on their dorsal surface with a distinctive triangular red marking on the head (Figure 1). The species is restricted to fragmented populations associated with ephemeral pools among sandstone ridges throughout the Sydney Basin area of NSW, Australia (Anstis, 2017). The species is characterised by a terrestrial mode of reproduction, whereby a small clutch of eggs (10–51 eggs) are laid in shallow depressions underneath leaf litter, rocks, fallen logs, and woody debris (Thumm and Mahony, 2002). The species demonstrates continuous iteroparity, breeding opportunistically throughout the year following rainfall (Thumm and Mahony, 2002). The red-crowned toadlet is classified as Near Threatened by the International Union for the Conservation of Nature (IUCN), with related species, the Northern Corroboree frog, Pseudophryne pengilleyi, Southern Corroboree frog, Pseudophryne corroboree, and magnificent broodfrog, Pseudophryne covacevichae classified as Critically Endangered, Critically Endangered and Endangered, respectively (IUCN, 2024).

Figure 1. Adult male red-crowned toadlet, Pseudophryne australis. Images left-to-right show the; dorsal, lateral, anterior, and ventral views. Photographs courtesy of Aimee J. Silla.
On the 20th of February 2024, eight male red-crowned toadlets were collected from a large population associated with an ephemeral drainage pan, approximately 30m2 in area, located in the Heathcote National Park, NSW, Australia. Wild males were collected by hand between 20:30 and 22:30 hours, following triangulation of the males’ vocalisations, and gentle removal of the leaf litter to expose the calling male. The males collected for this study were located between a minimum distance of 1m and maximum distance of 6m apart. A small number of non-calling males were found in close proximity to calling males, however only calling males were collected for this study as calling behaviour is an indication that males are reproductively mature and in suitable breeding condition. Single-use powder free latex gloves were worn at all times during collection and subsequent handling of the frogs, and gloves were changed between each individual male to avoid potential spread of pathogens. Males were housed individually in ventilated plastic containers (9cm D x 5cm H) with three laboratory tissues (Kimwipes) wetted with 20ml of distilled water. Individual male containers were placed within a larger ventilated plastic container for transportation, within a 4WD vehicle, to a field station approximately 30 minutes from the collection site. Once at the field station, frogs were held in their individual containers, in a constant temperature room set to a 22°C/18°C day/night cycle. For biosecurity reasons, no other species were housed within the field station during the time of the study. Frogs were housed for approximately 4 days and monitored daily for signs of ill health or distress according to standard operating procedures approved by the University of Wollongong’s Animal Ethics Committee. Wetted tissues were replaced every two days. Males were similar in body size and their size consistent with previous records of mature adult males of this species; body mass ranged from 0.73 to 0.97 grams (mean ± SEM=0.83 ± 0.04g) and snout–vent length ranged from 21.5 to 24.1 mm (mean ± SEM=23.0 ± 0.27mm).
To confirm the disease status of individual males, skin swabs (sterile cotton-tipped; MWE Medical Wire MW100, UK) were taken from each frog using a standard swabbing protocol. Briefly, five strokes of the swab tip passed over each of the following skin surfaces; dorsal, ventral, lateral left, lateral right, armpit to left fingers, armpit to right fingers, inner thigh to left toes, and inner thigh to right toes (40 strokes in total). Skin swabs were sent to Cesar Australia (Cesar Pty Ltd, Victoria, Australia) for analysis whereby a Bd zoospore count was determined using real-time quantitative polymerase chain reaction (qPCR) with a Qiagen master mix (three replicate qPCRs per male). All three replicates for each individual male were negative for Batrachochytrium dendrobatidis (Bd) infection, and all males used in this study were therefore confirmed to be free from the amphibian chytrid fungus.
On February 24th, 2024, frogs were euthanised according to methods approved by the University of Wollongong’s Animal Ethics Committee (see section 2.8 Ethics review statement below), and testis tissue immediately extracted, weighed, and macerated in 170μL of simplified amphibian ringer (SAR: 113mM NaCl, 1mM CaCl2, 2mM KCl, 3.6mM NaHCO3). Testes mass ranged from 0.0021 to 0.0035g (mean ± SEM=0.003 ± 0.0002g). Once sperm suspensions were prepared, samples were refrigerated for approximately 21 hours at 5°C. Sperm suspensions were homogenised and pre-freeze metrics for each of the eight samples assessed (Table 1). For each sperm suspension, sperm concentration was quantified by diluting a 2μL aliquot of sperm in 18μL of SAR (1:10 dilution), and homogenising and pipetting the sample into an Improved Neubauer Haemocytometer chamber (Bright Line, Optik Labor). The number of spermatozoa present in five quadrats was recorded and used to calculate total sperm concentration. Sperm viability was quantified by mixing a 2μL aliquot of sperm suspension with 5μL of a 1:50 dilution of SYBR-14, followed by 2μL of propidium iodide (Invitrogen, L-7011, Thermo Fisher Scientific, Melbourne, Australia). The sample was incubated in the dark for 5 minutes following the addition of each solution. Wet mounts were prepared, and the proportion of viable sperm was evaluated under a fluorescent microscope to determine proportion live/dead. To assess the pre-freeze sperm motility of each sperm suspension, a 2μL aliquot of sperm suspension was diluted in 10μL of chilled milli-Q water, the solution was homogenised and loaded onto a haemocytometer chamber. Following a 5-minute activation period, sperm motility was assessed using a computer-assisted-sperm-analysis system (CASA; CEROS II, Animal Breeder, software version: v1.11.5; Hamilton Thorne Inc., Beverly, MA, USA). The CASA system was set to the parameters detailed in section 2.5 below. Following pre-freeze assessment, each sperm suspension was divided into four 25μL aliquots, and 25μL of cryodiluent (CPA) corresponding to each experimental treatment was added gradually and cryopreserved.
A split-sample experimental design was used to test the effect of antioxidant supplementation to the cryopreservation extender on the post-thaw recovery of sperm. This approach involved homogenising and dividing sperm suspensions from each individual male across all experimental treatments (Figure 2). This process was then replicated for sperm suspensions from each of the eight males. Similar studies employing split-ejaculate designs to cryopreserve amphibian sperm have used variable, but typically low sample sizes (n=3–6; see Table 2, Anastas et al., 2023). The present study applied four experimental treatments: a standard cryopreservation extender (control CPA; constituents detailed below), CPA plus 2mM melatonin (melatonin), CPA plus 2mM ascorbic acid (ascorbic acid), and CPA plus 2mM uric acid (uric acid). Within males, the order that CPA treatments were applied to each of the four sperm subsamples was randomised using the randomisation function in excel. Split-sample designs are routinely adopted by studies quantifying sperm performance parameters in response to experimental treatments in order to account for individual male variation in sperm quality.
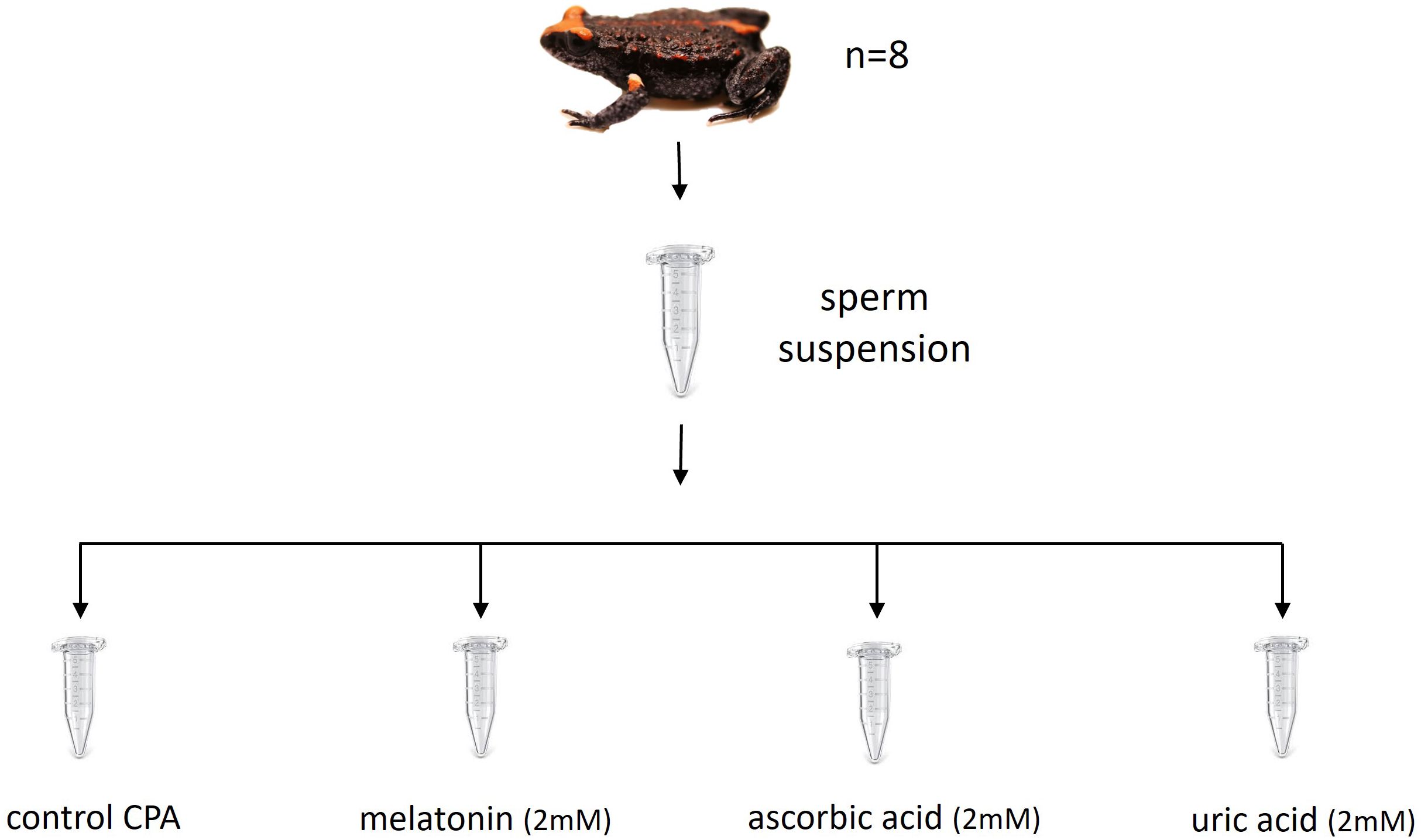
Figure 2. Split-sample experimental design. Each sperm suspension (n = 8) was evenly divided among four experimental treatments immediately prior to cryopreservation; control cryodiluent (CPA), CPA plus 2mM melatonin, CPA plus 2mM ascorbic acid, and CPA plus 2mM uric acid.
Table 2. Hamilton Thorne CEROS II CASA settings utilised for red-crowned toadlet spermic urine sample assessment.
Sperm suspensions were split evenly between one of four cryopreservation extenders, corresponding to the four experimental treatments (Figure 2). The stock solution of control CPA consisted of 20% v/v dimethyl formamide (DMF) plus 20% v/v trehalose (TRE) diluted in simplified amphibian ringer (SAR). A concentrated stock (0.2M) of each antioxidant treatment was then prepared in SAR: melatonin (47mg/ml), ascorbic acid (35mg/ml) and uric acid (33mg/ml). The final CPA treatments were made by diluting each concentrated antioxidant stock with control CPA. The pH of the control CPA (pH 7.5 ~1002mOsm/kg; final concentration 20% DMF + 20% TRE) and each antioxidant extender was adjusted using 2M NaOH prior to sperm treatment; melatonin (pH 8.6; ~994mOsm/kg; final concentration 20% DMF + 20% TRE + 2mM melatonin), ascorbic acid (pH 7.7; ~1007mOsm/kg; final concentration 20% DMF + 20% TRE + 2mM ascorbic acid), and uric acid (pH 7.3; ~994mOsm/kg; final concentration 20% DMF + 20% TRE + 2mM uric acid).
Sperm suspensions held at 4°C were then diluted slowly at 1:1 v/v (25µL of sperm : 25µL of cryoprotectant) in pre-cooled (4°C) extender, either melatonin CPA, ascorbic acid CPA, uric acid CPA or control CPA over a period of 10 minutes with gentle homogenisation by flicking of the Eppendorf tube (Eppendorf Safe-Lock, Thermo Fisher Scientific). Treated samples were further incubated for 10 minutes at 4°C, during which time samples were loaded into 0.25mL semen straws (IMV technologies, l’Aigle, France; 50μL-treated sample per straw; counterweighted with 150µL of SAR; n = 1 straw per CPA treatment per male). The straws were then cryopreserved using a dry shipper protocol described previously (Hobbs et al., 2023). Briefly, two thermocouple probes were inserted into separate 0.25mL cryo-straws containing SAR and placed into the dry shipper to monitor cooling rates. Individual straws were placed in a 13mm cryogenic goblet attached to an aluminium storage cane and set in a pre-cooled (approx. 4°C) dry shipper canister. The canister was then quickly placed inside the charged dry shipper (model CX100, 4.1L, Worthington Industries, Ohio, USA), positioned flush with the neck of the dry shipper for 30 seconds, and then lowered to the bottom of the dry shipper and left for at least 10 minutes. Excess sperm sample from each male was cryopreserved in straws using control CPA following the same cryopreservation methods described above and accessioned into the Taronga CryoDiversity Bank. All experimental straws were transferred to a cryogenic liquid nitrogen dewar (35L; HC35 Taylor Wharton (Australia) P/L, Albury, Australia) for approximately 3 months (79 days) before thawing.
Frozen sperm samples were thawed and assessed on the 14th and 15th of May 2024 in a temperature-controlled room set to 22°C. The thawing order of cryostraws was randomised, whereby first male order was randomised, then, within each male the order that treatment straws were thawed was also randomised (note each of the four treatment straws were thawed for a male before moving to the next male). The cryopreserved straws were thawed individually by extracting each straw one at a time from the cryogenic goblet, holding the straw at room temperature for 2 seconds before plunging the straw into a water bath (DairyMac Superthaw™, Dairymac Limited, Hampshire, UK) containing SAR at 40°C for 5 seconds. The exterior of each straw was cleaned gently with a laboratory tissue (Kimwipe) to remove residual SAR before extracting the sperm suspension and transferring it to a sterilised Eppendorf tube by micropipette. Sperm suspensions were then homogenised by careful flicking of the Eppendorf tube, and a sub-sample was diluted 1:4 in milli-Q water (10µL of thawed treatment sample in 40µL of milli-Q water) to activate sperm by lowering osmolality. Post-thaw motility parameters were assessed using computer-assisted sperm analysis (CASA); a Zeiss microscope (Axiolab 5, Carl Zeiss Microscopy GmbH, Oberkochen, Germany) connected to a CASA CEROS II system (Animal Breeder, software version: v1.11.5; Hamilton Thorne Inc., Beverly, MA, USA). The CASA system was set to the parameters detailed in Table 2. Each sperm suspension (10µL) was pipetted into an Improved Neubauer Haemocytometer chamber (Bright Line, Optik Labor). Total time from thawing to motility assessment ranged from 3 to 5 minutes, with each sample exposed to a 2-minute settlement period after the sample was pipetted onto the slide before analysis. The fluid settlement period was utilised to ensure accurate sperm motility readings that were not altered by fluid dynamics. A thermocouple was used to quantify the temperature of the slide stage at the time of each assessment (temperature mean ± SEM= 21.52 ± 0.05°C). A 10x negative phase contrast objective (Zeiss 10× NH CEROS II 160 nm) was used to assess total sperm motility, progressive sperm motility, curvilinear velocity (VCL), straight-line velocity (VSL) and average path velocity (VAP) at 60 frames s-1 (x1.21 magnification). One researcher entered the male and treatment ID into the software package, while a second researcher mounted the slide and initiated CASA assessment blind to the treatment being assessed. Simultaneously, viability was assessed with fluorescence microscopy by a third researcher. Briefly, a 2µL aliquot of the thawed sperm suspension was stained with 5µL SYBR-14 and incubated for 5 minutes, before 2µL of propidium iodide (P.I) was added and incubated for a further 5 minutes. The suspension was then pipetted into a haemocytometer chamber (exact depth 0.1mm). The proportion of live/dead sperm was determined immediately for 100 sperm cells using a fluorescent microscope (Motic BA310 Epi-LED FL microscope with a 470nm long-pass filter; Motic Inc. Ltd., Causeway Bay, Hong Kong).
To test the effect of antioxidant treatment on post-thaw sperm quality parameters, linear mixed-effects models (LMMs) were constructed for each response variable. Testes mass and pre-freeze sperm concentration were initially added to the model, but subsequently removed as these co-variates were not significant. Within the final model, treatment was the fixed factor, the response variable was either viability (%), total motility (%), forward progressive motility (FPM; %), average path velocity (VAP), or curvilinear velocity (VCL), male ID was a random factor, and post-thaw sperm concentration and assessment time were added as co-variates. Prior to analysis, assumptions of normality were tested for each response variable using a Shapiro-Wilkes tests. Where significant model effects were detected (p ≤ 0.05), Tukey-Kramer HSD post hoc tests were performed to determine statistically significant differences between treatments. All statistical analyses were performed using JMP® 17.0 software package (SAS Institute Inc. North Carolina, USA). For all tests in this study, statistical significance was assumed at p ≤ 0.05.
Study animals were collected under NSW Department of Planning and Environment’s Scientific Licence SL102771. Although wild release was requested, the scientific licence was approved on the condition that animals could not be released and must be euthanised. The study animals used in the present study were not euthanised specifically for the present study, but the study employed testicular tissues that were made available post-mortem, affording the opportunity to a) develop sperm cryopreservation protocols and b) biobank excess sperm, for a species that no biobanked sperm previously existed. Animals were euthanised via pithing (brain destruction), and testicular tissue extracted for the present study according to the protocols approved by the University of Wollongong’s Animal Ethics Committee, protocol number AEPR231.
Overall, mean post-thaw sperm viability ranged from 37 to 53% among antioxidant treatments (Table 3). Antioxidant treatment had a significant effect on post-thaw sperm viability (LMM, p<0.05; Table 3), with the sperm viability of samples frozen in CPA supplemented with melatonin or uric acid significantly higher than the control CPA treatment (Tukey-Kramer HSD posthoc tests p<0.05; Table 3). The sperm viability of samples frozen in CPA supplemented with ascorbic acid were of intermediate viability and not significantly different from the other treatments (Tukey-Kramer HSD posthoc tests p>0.05; Table 3).
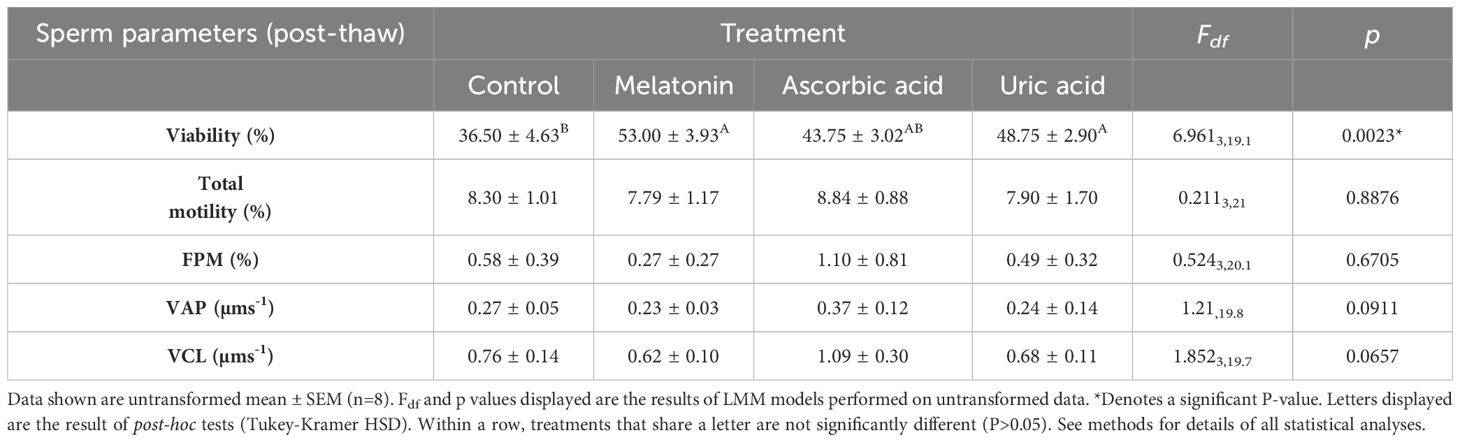
Table 3. Effect of antioxidant treatment on post-thaw sperm parameters in the red-crowned toadlet, P.australis.
Overall mean percent sperm motility and percent forward progressive motility were low across treatments (<10% and <1.5%, respectively), with samples frozen in the cryopreservation extender supplemented with ascorbic acid tending to exhibit the highest motilities (Table 3). Treatment means were statistically similar across antioxidant treatments (LMM, p>0.05; Table 3). Similarly, mean sperm velocities (VAP and VCL) were higher in samples frozen in CPA supplemented with ascorbic acid, however, differences among antioxidant treatments were not statistically significant (LMMs, p<0.05; Table 3).
In the face of severe loss of amphibian biodiversity, integrated conservation management strategies, that employ reproductive technologies and biobanking, are needed to assist the genetic management of threatened species (Silla and Byrne, 2019; Silla and Kouba, 2022). As amphibian conservation breeding programs increasingly adopt cryopreservation technologies to facilitate the long-term storage of sperm suspensions, protocol refinement is required to maximise post-thaw sperm recovery. Investing in protocol refinement is critical to ensuring maximum post-thaw sperm quality, which will ultimately enhance the successful generation of offspring from cryopreserved sperm, as successful amphibian assisted fertilisations are associated with functional sperm concentrations. Protecting sperm from cryodamage is also important to ensure high quality frozen repositories for future application as science and technology progress beyond what is presently achievable (Silla and Kouba, 2022). To date, no studies have assessed the effect of supplementing cryopreservation extenders with the antioxidants, melatonin, ascorbic acid, or uric acid, on post-thaw sperm quality in an amphibian. The aim of this study was to quantify the effect of supplementing the cryopreservation extender with various antioxidants (melatonin, ascorbic acid, or uric acid) on post-thaw sperm viability, motility, and velocity in the red-crowned toadlet (Pseudophryne australis). Results from the present study demonstrate that antioxidant supplementation had a significant effect on sperm viability, with melatonin and uric acid improving post-thaw sperm viability by 12–17% above controls. Motility parameters (total motility, FPM, VAP and VCL) tended to be higher in samples frozen in CPA supplemented with ascorbic acid, however, differences were not statistically significant. These findings provide the first evidence that antioxidant supplementation of cryopreservation extenders can improve post-thaw sperm quality in an amphibian.
Results from the present study show that supplementation with melatonin or uric acid significantly improve post-thaw sperm viability in the red-crowned toadlet. Increases in post-thaw sperm viability of sperm samples that have been cryopreserved in extenders supplemented with antioxidants have also been reported in other taxa (Li et al., 2018; Lee and Lee, 2023; Succu et al., 2011; Karimfar et al., 2015), with increased viability attributed to protection of the spermatozoan acrosome or prevention of lipid peroxidation and support of membrane integrity (Li et al., 2018; Lee and Lee, 2023). For example, a study of sturgeon reported significantly higher sperm membrane integrity than controls when sturgeon spermatozoa were treated with ascorbic acid prior to freezing (Li et al., 2018). A study of pig semen reported increased post-thaw viability with melatonin supplementation, in the absence of a significant effect on sperm motility, consistent with the results of the present study (Lee and Lee, 2023). The beneficial effects of antioxidant supplementation on post-thaw sperm viability as reported herein may be the results of protective effects to the sperm acrosome, a membranous organelle located at the tip of the sperm head that plays a role in sperm penetration into the egg and egg activation during fertilisation (Alavi et al., 2012). Alternatively, antioxidant supplementation may have reduced lipid peroxidation. Lipid peroxidation is a primary manifestation of oxidative damage (due to altered membrane structure and enzyme inactivation), which can lead to reduced sperm viability. Sperm membranes contain a high concentration of polyunsaturated fatty acids (PUFAs), which are essential to sperm function (Sandoval-Vargas et al., 2021). However, an abundance of PUFAs has also been related to increased risk of lipid peroxidation due to reactivity of methylene groups present in the carbon chains (Sandoval-Vargas et al., 2021). Antioxidants, such as melatonin may counteract intracellular ROS, reducing lipid peroxidation while also supporting the action of other primary antioxidants (Varo-Ghiuru et al., 2015). While the mechanism by which melatonin and uric acid improved post-thaw sperm viability in the present study remains unknown, the positive effects reported highlight a need for further research, which would benefit from quantifying ROS and oxidative stress simultaneously with sperm parameters to elucidate the biochemical processes causing changes in post-thaw sperm recovery.
Previous aquaculture research has suggested that antioxidant effectiveness at enhancing post-thaw sperm quality in fish may be both concentration-dependent and species-specific. Specifically, ascorbic acid has been previously applied to the semen suspensions of several fish species at a range of concentrations, with varying results on post-thaw sperm parameters and fertilisation rates (Cabrita et al., 2011; Figueroa et al., 2018; Li et al., 2018; Mirzoyan et al., 2006; Chelewani et al., 2024). A low dose of 1mM ascorbic acid applied to the cryopreservation extender resulted in reduced DNA fragmentation in gilthead seabream, while the same dose applied to European seabass semen led to an increase in DNA fragmentation (Cabrita et al., 2011). A comparatively high dose of 10mM ascorbic acid, resulted in increased post-thaw sperm motility and improved fertilisation rates in Russian sturgeon (Mirzoyan et al., 2006), and improved post-thaw motility in rainbow trout (Yavuz and Bozkurt, 2020). In contrast, the same dose of 10mM ascorbic acid applied to masu salmon semen resulted in reduced sperm motility, viability, and fertilisation rates (Chelewani et al., 2024). These studies highlight that optimal antioxidant dose-curves need to be established on a species-specific basis. Suboptimal concentrations may be insufficient to inhibit ROS-induced reduction of sperm quality, while doses surpassing the optimal dose may switch to prooxidant activity and be detrimental. The present study applied a single dose of 2mM melatonin, 2mM ascorbic acid, or 2mM uric acid to sperm suspensions of the red-crowned toadlet. This is the first study to apply these antioxidants to the cryopreservation extender of any amphibian species, and our results are therefore a critically important first step toward the application and refinement of antioxidant supplementation for amphibians. Future amphibian cryopreservation studies would now benefit from establishing dose-response relationships for antioxidant supplementation across a range of species.
In addition to dose-dependent effects of antioxidant supplementation to cryopreservation extenders, previous research also suggests that antioxidant action may be enhanced through co-supplementation. For example a study in Atlantic salmon, applied α-tocopherol alone at two doses (0.1mM and 0.5mM), ascorbic acid alone at two doses (1mM and 10mM), or in combination at two doses (α-tocopherol/ascorbic acid; 0.1mM/1.0mM and 0.5mM/10mM) (Figueroa et al., 2018). The study reported enhanced antioxidant defences, higher post-thaw sperm motility, and higher fertilisation with the combined supplementation of α-tocopherol with ascorbic acid at a dose of 0.1mM/1.0mM, compared with the supplementation of either α-tocopherol or ascorbic acid alone, which showed no significant improvement in these parameters (Figueroa et al., 2018). Antioxidant defence systems typically comprise a number of antioxidant compounds acting simultaneously and/or synergistically. Research investigating the antioxidant systems in semen of different teleost fish species, revealed the presence of several antioxidants, including ascorbic acid, carnitine, glutathione, methionine, tocopherol, and uric acid (Lahnsteiner and Mansour, 2010). At present, characterisation of the natural antioxidants present in anuran seminal fluid has not occurred for any species. Biochemical assays to determine amphibian endogenous antioxidant compounds, as performed for teleost (Lahnsteiner and Mansour, 2010), would not only direct the specific antioxidant compounds to target for future research refining amphibian cryopreservation protocols, but may elucidate antioxidant combinations that may beneficial.
Overall, mean post-thaw sperm motility ranged from 7.8 to 8.8%, and relative motility (relative motility=post-thaw motility/pre-freeze motility) from 9.6 to 12.9% across antioxidant treatments in the present study. To date, sperm suspensions from only a small number of individuals from four species of the Australian ground frog family, the Myobatrachidae, have been cryopreserved (Anastas et al., 2023). Sperm from the common eastern froglet and Bibron’s toadlet, exhibited less than 20% relative motility (15 ± 14% n=4, 20 ± 2% n=2, respectively) (Browne et al., 2002), while post-thaw motility of the Northern corroboree frog was 8%, and relative motility 18.2% (n=1; Hobbs and O’Brien, unpublished data). Motilities reported in the present study for the red-crowned toadlet are consistent with the low motilities reported for other species in the Myobatrachidae family. By contrast, studies cryopreserving sperm from the Australian tree frog family, the Hylidae, typically report much higher post-thaw relative motilities, for example Litoria latopalmata, L. nasuta, L. phyllochroa, and L. peronii, report relative motilities between 70% and 90% (Anastas et al., 2023). Lower post-thaw motilities reported for myobatrachid species, suggests that the sperm of these species are either relatively less resilient to current cryopreservation protocols compared with species from other anuran families, or that comparatively lower sperm concentrations found in these species may be affecting their freeze–thaw capacity and post-thaw sperm parameters. As such, additional research investigating alternative cryopreservation extenders (including novel antioxidant-supplemented cryoprotectants), as well as investigations of the effects of sperm concentration, and methods for concentrating sperm suspensions, may be important for species within this family.
Cryopreservation technologies have enormous potential to assist the long-term genetic management of threatened species and safeguard valuable genetic resources in perpetuity (Silla and Kouba, 2022). Amphibian conservation breeding programs are increasingly adopting sperm cryopreservation techniques as a way of biobanking amphibian genetic resources. At present, post-thaw sperm recovery is highly variable, and many thousand threatened amphibian species are yet to have sperm biobanked. The production of ROS and subsequent oxidative stress generated through the process of cryopreservation and thawing of cryopreserved sperm, is believed to be a major contributor of cryodamage. While several studies have investigated the beneficial effects of supplementing cryopreservation extenders with antioxidants on post-thaw sperm parameters and fertilisation rates in a diversity of fish species, research on amphibians is lacking. Herein, we report the results of the first study to apply melatonin, ascorbic acid, and uric acid to the cryopreservation extenders of amphibian sperm. Overall, the addition of melatonin significantly increased post-thaw sperm viability, while ascorbic acid increased all sperm motility and velocity parameters, though differences were not significant. This research highlights the potential for antioxidants to improve amphibian cryopreservation outcomes and paves the way for further investigation of the effects of antioxidant supplementation in other amphibian species.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The animal study was approved by the University of Wollongong’s Animal Ethics Committee. The study was conducted in accordance with the local legislation and institutional requirements.
MH: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. PB: Formal Analysis, Supervision, Writing – review & editing. JO’B: Data curation, Methodology, Supervision, Writing – review & editing. RH: Data curation, Methodology, Writing – review & editing. AS: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Additional procedural contributions: MH: Animal collection. PB: Animal collection. JO’B: Sperm thawing and motility analyses. RH: Preparation of CPAs, Cryopreservation of sperm samples. AS: Animal monitoring, Animal euthanasia, Dissection & sperm suspension preparation, Sperm motility analyses, Sperm viability assessment.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Australian Research Council, Discovery Early Career Researcher Award (DE210100812) awarded to AS, with ancillary funding by the University of Wollongong and Taronga Conservation Society Australia.
The authors acknowledge the Dharawal people as the Traditional Custodians of the lands on which the study animals were collected and research conducted, in addition to the Cammeraigal people as the Traditional Custodians of the lands on which the sperm samples were biobanked and are held to safeguard the biodiversity of our study species for future generations. We thank the contributions of staff from NSW National Parks and Wildlife Service for their dedication to managing the biodiversity and habitats within the National Park where individuals were collected for this study. This project was made possible by facilities provided by the University of Wollongong and Taronga Institute of Science and Learning.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aitken R. J., Smith T. B., Jobling M. S., Baker M. A., De Iuliis G. N. (2014). Oxidative stress and male reproductive health. Asian J. androl. 16, 31–38. doi: 10.4103/1008-682X.122203
Alavi S. M. H., Hatef A., Pšenička M., Kašpar V., Boryshpolets S., Dzyuba B., et al. (2012). Sperm biology and control of reproduction in sturgeon:(II) sperm morphology, acrosome reaction, motility and cryopreservation. Rev. Fish Biol. Fish. 22, 861–886. doi: 10.1007/s11160-012-9270-x
Alevra A. I., Exadactylos A., Mente E., Papadopoulos S. (2022). The protective role of melatonin in sperm cryopreservation of farm animals and human: lessons for male fish cryopreservation. Animals 12, 791. doi: 10.3390/ani12060791
Amidi F., Pazhohan A., Shabani Nashtaei M., Khodarahmian M., Nekoonam S. (2016). The role of antioxidants in sperm freezing: a review. Cell Tissue bank. 17, 745–756. doi: 10.1007/s10561-016-9566-5
Anastas Z. M., Byrne P. G., O’brien J. K., Hobbs R. J., Upton R., Silla A. J. (2023). The increasing role of short-term sperm storage and cryopreservation for conserving threatened amphibian species. Animals 13, p.2094. doi: 10.3390/ani13132094
Anstis M. (2017). Tadpoles and Frogs of Australia. 2nd ed. (Sydney, Australia: New Holland Publishers).
Bell D. A., Robinson Z. L., Funk W. C., Fitzpatrick S. W., Allendorf F. W., Tallmon D. A., et al. (2019). The exciting potential and remaining uncertainties of genetic rescue. Trends Ecol. Evol. 34, 1070–1079. doi: 10.1016/j.tree.2019.06.006
Browne R., Clulow J., Mahony M. (2002). The short-term storage and cryopreservation of spermatozoa from hylid and myobatrachid frogs. CryoLetters 23, 129–136.
Byrne P. G., Silla A. J. (2022). “Genetic management of threatened amphibians: Using artificial fertilisation technologies to facilitate genetic rescue and assisted gene flow,” in Reproductive technologies and biobanking for the conservation of amphibians. Eds. Silla A. J., Kouba A. & Heatwole H. (Melbourne, Australia: Csiro Publishing).
Cabrita E., Ma S., Diogo P., Martínez-Páramo S., Sarasquete C., Dinis M. (2011). The influence of certain aminoacids and vitamins on post-thaw fish sperm motility, viability and DNA fragmentation. Anim. Reprod. Sci. 125, 189–195. doi: 10.1016/j.anireprosci.2011.03.003
Calatayud N., Della Togna G. (2022). “Appendix: principal institutions recently participating in the development and application of reproductive technologies and biobanking for amphibians,” in Reproductive Technologies and Biobanking for the Conservation of Amphibians. Eds. Silla A. J., Kouba A. J., Heatwole H. (CSIRO Publishing, Melbourne, Australia).
Chelewani A. P., Takahashi E., Nishimura T., Fujimoto T. (2024). Optimizing the post-thaw quality of cryopreserved masu salmon (Oncorhynchus masou) sperm: Evaluating the effects of antioxidant-supplemented extender. Aquaculture 593, 741332. doi: 10.1016/j.aquaculture.2024.741332
Ciereszko A., Dabrowski K., Kucharczyk D., Dobosz S., Goryczko K., Glogowski J. (1999). The presence of uric acid, an antioxidantive substance, in fish seminal plasma. Fish Physiol. Biochem. 21, 313–315. doi: 10.1023/A:1007886121663
Félix F., Oliveira C. C., Cabrita E. (2020). Antioxidants in fish sperm and the potential role of melatonin. Antioxidants 10, 36. doi: 10.3390/antiox10010036
Figueroa E., Farias J., Lee-Estevez M., Valdebenito I., Risopatrón J., Magnotti C., et al. (2018). Sperm cryopreservation with supplementation of α-tocopherol and ascorbic acid in freezing media increase sperm function and fertility rate in Atlantic salmon (Salmo salar). Aquaculture 493, 1–8. doi: 10.1016/j.aquaculture.2018.04.046
Frankham R. (2015). Genetic rescue of small inbred populations: Meta-analysis reveals large and consistent benefits of gene flow. Mol. Ecol. 24, 2610–2618. doi: 10.1111/mec.2015.24.issue-11
Gualtieri R., Kalthur G., Barbato V., Di Nardo M., Adiga S. K., Talevi R. (2021). Mitochondrial dysfunction and oxidative stress caused by cryopreservation in reproductive cells. Antioxidants 10, 337. doi: 10.3390/antiox10030337
Hobbs R. J., Upton R., Calatayud N. E., Silla A. J., Daly J., Mcfadden M. S., et al. (2023). Cryopreservation cooling rate impacts post-thaw sperm motility and survival in litoria booroolongensis. Animals 13, 3014. doi: 10.3390/ani13193014
Hu J.-H., Tian W.-Q., Zhao X.-L., Zan L.-S., Wang H., Li Q.-W., et al. (2010). The cryoprotective effects of ascorbic acid supplementation on bovine semen quality. Anim. Reprod. Sci. 121, 72–77. doi: 10.1016/j.anireprosci.2010.04.180
IUCN (2024). The IUCN Red List of Threatened Species. Version 2024-1. Available online at: https://www.iucnredlist.org (Accessed August 22, 2024).
Kameni S. L., Meutchieye F., Ngoula F. (2021). Liquid storage of ram semen: associated damages and improvement. Open J. Anim. Sci. 11, 473–500. doi: 10.4236/ojas.2021.113033
Karimfar M., Niazvand F., Haghani K., Ghafourian S., Shirazi R., Bakhtiyari S. (2015). The protective effects of melatonin against cryopreservation-induced oxidative stress in human sperm. Int. J. immunopathol. Pharmacol. 28, 69–76. doi: 10.1177/0394632015572080
Kolyada M. N., Osipova V. P., Berberova N. T. (2023). Use of cryoprotectors and antioxidants in sturgeon semen cryopreservation. Cryobiology 111, 30–39. doi: 10.1016/j.cryobiol.2023.02.003
Kutluyer F., Kayim M., Öğretmen F., Büyükleblebici S., Tuncer P. B. (2014). Cryopreservation of rainbow trout Oncorhynchus mykiss spermatozoa: Effects of extender supplemented with different antioxidants on sperm motility, velocity and fertility. Cryobiology 69, 462–466. doi: 10.1016/j.cryobiol.2014.10.005
Lahnsteiner F., Mansour N. (2010). A comparative study on antioxidant systems in semen of species of the Percidae, Salmonidae, Cyprinidae, and Lotidae for improving semen storage techniques. Aquaculture 307, 130–140. doi: 10.1016/j.aquaculture.2010.07.011
Lee S.-H., Lee S. (2023). Effects of melatonin and silymarin on reactive oxygen species, nitric oxide production, and sperm viability and motility during sperm freezing in pigs. Animals 13, 1705. doi: 10.3390/ani13101705
Li P., Xi M., Du H., Qiao X., Liu Z., Wei Q. (2018). Antioxidant supplementation, effect on post-thaw spermatozoan function in three sturgeon species. Reprod. Domest. Anim. 53, 287–295. doi: 10.1111/rda.2018.53.issue-2
Medrano A., Contreras C. F. B., Herrera F. M., Alcantar-Rodriguez A. M. (2017). Melatonin as an antioxidant preserving sperm from domestic animals. Asian Pacific J. Reprod. 6, 241–246. doi: 10.4103/2305-0500.217317
Mirzoyan A., Nebesikhina N., Voynova N., Chistyakov V. (2006). Preliminary results on ascorbic acid and lysine suppression of clastogenic effect of deep-frozen sperm of the Russian sturgeon (Acipenser gueldenstaedti). Int. J. Refrigeration 29, 374–378. doi: 10.1016/j.ijrefrig.2005.07.008
Motta N. C., Egger R. C., Monteiro K. S., Vogel De Oliveira A., Solis Murgas L. D. (2022). Effects of melatonin supplementation on the quality of cryopreserved sperm in the neotropical fish Prochilodus lineatus. Theriogenology 179, 14–21. doi: 10.1016/j.theriogenology.2021.11.012
Ofosu J., Qazi I. H., Fang Y., Zhou G. (2021). Use of melatonin in sperm cryopreservation of farm animals: A brief review. Anim. Reprod. Sci. 233, 106850. doi: 10.1016/j.anireprosci.2021.106850
Palhares P. C., Assis I. D. L., MaChado G. J., De Freitas R. M. P., De Freitas M. B. D., Paula D., et al. (2021). Sperm characteristics, peroxidation lipid and antioxidant enzyme activity changes in milt of Brycon orbignyanus cryopreserved with melatonin in different freezing curves. Theriogenology 176, 18–25. doi: 10.1016/j.theriogenology.2021.09.013
Sandoval-Vargas L., Silva Jimenez M., Risopatron Gonzalez J., Villalobos E. F., Cabrita E., Valdebenito Isler I. (2021). Oxidative stress and use of antioxidants in fish semen cryopreservation. Rev. Aquacult. 13, 365–387. doi: 10.1111/raq.12479
Silla A. J., Byrne P. G. (2019). The role of reproductive technologies in amphibian conservation breeding programs. Annu. Rev. Anim. Biosci. 7, 499–519. doi: 10.1146/annurev-animal-020518-115056
Silla A. J., Kouba A. J. (2022). “Integrating Reproductive technologies into the conservation toolbox for the recovery of amphibian species,” in Reproductive Technologies and Biobanking for the Conservation of Amphibians. Eds. Silla A. J., Kouba A. J., Heatwole H. (CSIRO Publishing, Melbourne, Australia).
Succu S., Berlinguer F., Pasciu V., Satta V., Leoni G. G., Naitana S. (2011). Melatonin protects ram spermatozoa from cryopreservation injuries in a dose-dependent manner. J. Pineal Res. 50, 310–318. doi: 10.1111/j.1600-079X.2010.00843.x
Thumm K., Mahony M. (2002). Evidence for continuous iteroparity in a temperate-zone frog, the red-crowned toadlet, Pseudophryne australis (Anura: Myobatrachidae). Aust. J. zool. 50, 151–167. doi: 10.1071/ZO01038
Varo-Ghiuru F., Miclea I., Hettig A., Ladoşi I., Miclea V., Egerszegi I., et al. (2015). Lutein, trolox, ascorbic acid and combination of trolox with ascorbic acid can improve boar semen quality during cryopreservation. CryoLetters 36, 1–7.
Keywords: amphibian, conservation, sperm, reproductive technologies, biobanking, cryopreservation, antioxidant, melatonin
Citation: Howard MS, Byrne PG, O’Brien JK, Hobbs RJ and Silla AJ (2025) Antioxidant supplementation of cryopreservation extenders improves post-thaw sperm viability in the red-crowned toadlet, Pseudophryne australis. Front. Amphib. Reptile Sci. 3:1525965. doi: 10.3389/famrs.2025.1525965
Received: 11 November 2024; Accepted: 07 March 2025;
Published: 31 March 2025.
Edited by:
José Luis Ros-Santaella, Czech University of Life Sciences Prague, CzechiaReviewed by:
Lola Brookes, National Centre for the Replacement Refinement and Reduction of Animals in Research, United KingdomCopyright © 2025 Howard, Byrne, O’Brien, Hobbs and Silla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aimee J. Silla, YXNpbGxhQHVvdy5lZHUuYXU=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.