- 1Centre for Ecological Sciences, Indian Institute of Science, Bengaluru, Karnataka, India
- 2Department of Ecology and Environmental Sciences, Pondicherry University, Kalapet, Puducherry, India
Urban areas comprise a matrix of natural and human-made microhabitats, with associated variation in microclimates. Since reptiles are dependent on environmental temperature for optimal functioning, their survival in cities depends on how well they can navigate microhabitat-level thermal heterogeneity. For the Mysore Day gecko (Cnemaspis mysoriensis) in the urban environment of Bengaluru, we determined if shifts in thermal physiology or behavioural thermoregulatory strategies were used to adapt to human-made microhabitats (e.g. walls) compared to natural microhabitats (tree trunks and roots). We collected active body temperatures and environmental temperatures in the field, and measured preferred temperature (Tset), thermal tolerance limits (CTmax and CTmin), and thermal performance curve (TPC) of locomotion in the lab. We found that human-made microhabitats had slightly higher and more variable environmental temperatures than the natural microhabitats. Thermal physiological variables (Tset, CTmax, CTmin, and TPC) of lizards caught from these distinct microhabitats did not vary, implying a conserved thermal physiology within the species. However, given the body temperatures of lizards in the wild, natural microhabitats seem to be of better thermal quality, providing a suitable temperature range that is closer to preferred temperatures for the species. Hence, in natural spaces, lizards can thermoregulate more accurately. We demonstrate that even small differences in thermal conditions at the microhabitat scale can influence accuracy of thermoregulation for lizards in the city. Our result emphasise the importance of retaining natural habitats in a cityscape for effective thermoregulation of small ectotherms, like C. mysoriensis.
Introduction
Urbanisation is a complex and dynamic process (Grimm et al., 2008) and one of the key drivers of change in ecosystems (Elmqvist et al., 2013). Global studies have shown that urban expansion has already caused 50% loss of local species richness and 38% loss in species abundance (Li et al., 2022). Along with changes in the physical and structural properties of the environment, which includes habitat fragmentation (Finand et al., 2024), urbanisation causes changes to local microclimatic conditions (Battles and Kolbe, 2019). The Urban Heat Island (UHI) effect, wherein cities tend to have higher surface and air temperatures than their rural surroundings, is one of the best documented examples of local climatic change due to urbanisation (Grimm et al., 2008; Chown and Duffy, 2015).
Reptiles are known to be sensitive to the environmental changes caused by urbanisation (Brum et al., 2023). As a consequence, reptiles in urban environments show altered physiology (Prosser et al., 2006; Thawley et al., 2019), behaviour (Batabyal et al., 2017; Pellitteri-Rosa et al., 2017; Mohanty et al., 2021) and morphology (Kolbe et al., 2016; Putman and Tippie, 2020; Balakrishna et al., 2021) compared to those in rural environments. Reliance of reptiles on environmental temperatures for thermoregulation makes them especially vulnerable to urban heating (Bodensteiner et al., 2021). For example, the patterns of persistence and dispersal of Anolis species in urban environments is strongly linked to the temperature experienced in the city (Battles and Kolbe, 2019). Warmer city temperatures are also known to increase the development rate of embryos of Anolis cristatellus, at the cost of reduced survival (Hall and Warner, 2018). For some species, the thermal impact of urbanisation can be negative to such a degree that it leads to local extinction (Baur and Baur, 1993; Duncan et al., 2011), but for others, there is evidence of genetic adaptations to the novel characteristics of urban landscapes (McKinney, 2008). For example, a recent study by Campbell-Staton et al. (2021) found that city dwelling Anolis criatatellus can endure higher environmental temperatures and display greater heat tolerances than their forest counterparts, because of changes in genes associated with heat tolerance.
The thermal environment of urbanised areas is different from natural environments not only at the scale of the entire landscape, which is typically captured as the UHI effect, but also at the microhabitat scale (Avilés-Rodríguez and Kolbe, 2019). In urban areas, natural microhabitats such as trees, grass, and rocks are interspersed with artificial microhabitats, such as walls and roads. Hence, an ectothermic species must also navigate thermal heterogeneity associated with microhabitat heterogeneity in order to thermoregulate in cities. Whether the increased temperatures and altered distribution of thermal microhabitats of urban areas affects the thermal ecology of reptiles is an open question (Battles and Kolbe, 2019). For species that are sensitive to habitat and thermal changes, their presence and survival in cities will be influenced by how well they can respond (French et al., 2018; Thaker et al., 2022).
Body temperature (Tb) is one of the most important physiological variable that affects performance in reptiles (Angilletta, 2009), and both behaviour and physiology are strongly sensitive to Tb (Huey and Stevenson, 1979). Reptiles need to actively maintain body temperatures that are optimal for maximal performance (Topt) while preventing overheating or excessive cooling. Before evolutionary adaptation can provide solutions to urban heating (Campbell-Staton et al., 2021; Vardi et al., 2023) and increased thermal heterogeneity (Angilletta et al., 2006; Angilletta, 2009), ectotherms already have an array of physiological and behavioural strategies to mitigate variation in environmental temperatures (Angilletta, 2009). Species can thermoregulate behaviourally by basking when conditions are favourable (Huey, 1974; Hertz, 1992) or physiologically by changing enzyme levels in response to heat (Seebacher and Franklin, 2005). The cost of thermoregulation is an important parameter that determines whether species can behaviourally thermoregulate to attain Tb close to their preferred temperatures (Tset), or perform sub-optimally at lower or higher Tb without acclimatization (Hertz et al., 1983). For those species that can acclimatise to new thermal environments, they could physiologically shift optimal performance temperature to lower or higher Tb to better match the environmental temperatures (Te). To understand which strategies are used by individuals for thermal adaptation, we need information on both the thermal quality of the habitat (de), which is how close Te is to Tset, as well as the accuracy of thermoregulation (db), which is how close Tb in the wild is to Tset. Together, this information allows us to determine the effectiveness of thermoregulation (E), which is the degree to which thermoregulatory activity compensates for the thermal shortcomings of the habitat (Hertz et al., 1993).
In this paper, we aim to understand the thermal adaptation of Cnemaspis mysoriensis, a small diurnal gecko found in the urban environments of the Bengaluru-Mysuru region of Southern India. In areas where it is found, the species is known to utilise trees, such as Ficus trunks and roots, as well as anthropogenically-created microhabitats, such as walls and cement gutters (Giri et al., 2009). Given their small size, low dispersal range, high site fidelity (Kabir et al., 2020) and communal breeding behaviours (Giri et al., 2009; pers. obs.), individuals found on these microhabitats are likely to have lived there for at least one or more generations. To live on novel microhabitats (e.g., walls) that are thermally distinct from natural microhabitats (e.g., trees), this species could use behavioural thermoregulation strategies or physiological acclimation, or both. While several studies have measured the effects of urbanisation on lizard species in tropical areas like India (Batabyal et al., 2017; Mohanty et al., 2021; Thaker et al., 2022), there is a lack of information on how lizards thermally adapt to cities. We aim to compare the thermal adaptation strategies of lizards who utilise natural microhabitats (trees) versus those who utilise human-made microhabitats (walls). Specifically, we measured the thermal tolerance range (Critical Thermal Maximum (CTmax) and the Critical Thermal Minimum (CTmin)), the optimum temperature of a performance parameter (Topt) and the thermal breadth of activity (B95) for lizards that live on these distinct microhabitats. We also determine how the microhabitat types utilised by the species have an impact on the thermoregulatory accuracy (db), thermal quality of the habitat (de) and Effectiveness of thermoregulation (E). Overall, these data will allow us to understand if trees or walls differ in their thermal suitability for the endemic gecko species, which has consequences for their persistence in the rapidly urbanising environments within their geographic distribution.
Methods
Study area
Bengaluru (12.9716°N, 77.5946°E), the capital of Karnataka, is one of the fastest growing cities in India (Sudhira et al., 2007). It is a densely populated region dominated by a large number of anthropogenic structures (Shetty et al., 2012). The city is situated 920m above sea level, and receives a mean annual rainfall of about 880mm and experiences ambient temperatures that range from 18°C to 38°C (Sudhira et al., 2007; Thaker et al., 2022). There has been substantial land use change in and around the city over the last 25 years, which includes a decrease in forest cover, as well as a reduction in water bodies, rocky outcrops, and open scrub habitat spaces (Shetty et al., 2012; Thaker et al., 2022). Within the city, much of the loss of green cover has been due to building development and the associated road network (Sudhira et al., 2007). However, even with the high rate of urbanisation, vegetation such as trees and scrub habitats remain in parks, private gardens, educational institutes, and around water bodies (Nagendra et al., 2012; Thaker et al., 2022). The complex mix of trees, scrub vegetation, natural rocky habitats, and anthropogenic structures harbours a great number of faunal species. Karthikeyan (1999) recorded approximately 38 species of reptiles, within a 40 km radius of Bengaluru city centre.
Capture and housing of individuals
To measure preferred body temperature (Tset), Critical thermal maximum (CTmax) and minimum (CTmin), and locomotor performance, we captured lizards from the wild and brought them to the lab at the Centre for Ecological Sciences, Indian Institute of Science, Bengaluru. Sampling was carried out from the beginning of winter (December) until early summer (April), during the active hours of the species from 1000 hrs to 1800 hrs. Lizards were collected from five different locations (approximately 6-9 km apart) in the urbanised areas of Bengaluru city. For the natural microhabitat type (hereafter referred to as “Tree”), individuals were sampled from tree trunks, tree crevices and prop roots of trees in vegetated areas of the city. For the human-made microhabitat types (hereafter referred to as “Wall”), individuals were collected from wall surfaces, wall crevices, on cement footpaths and from below cement bricks. The individuals (N=213; n=117 from Tree and 96 from Wall) were captured by hand and field body temperature (Tb) was immediately taken by carefully inserting a thermal probe (Amprobe Thermocouple Thermometer- K Type) inside the cloaca of the captured individual (Taylor et al., 2021). This was done within 10-20 seconds of capture to ensure that there is no transfer of heat from the researchers’ hands to the lizard (Taylor et al., 2021). We then recorded the sex, snout-to-vent length (SVL; measured with a centimetre ruler), microhabitat type, and date and time of capture. Sex of the individuals was determined on the basis of presence (in males) or absence (in females) of femoral pores (Giri et al., 2009; Kabir et al., 2019). Only adult individuals were collected (SVL >/= 2.4 cm), and we excluded females who were visibly gravid. Each individual was marked with a unique identity number on the ventral side of the body with a non-toxic marker (Johnson, 2005), and was then transferred to a well-ventilated plastic container for transportation to the laboratory (within 2-3 hours of capture).
In the laboratory, lizards were housed in individual well-ventilated plastic containers (22cm×14cm×10cm). Boxes were lined with clean tissue paper, and were placed in a dedicated lizard housing room at 26-28°C. Water was provided ad libitum and each lizard was given four to five Drosophila flies a day for food. All individuals were maintained under these conditions for 24 hours from the time of capture before starting the measurements described below. Preferred body temperature (Tset) and the thermal tolerance limits (CTmin and CTmax) were measured for one set of individuals (N=40). To minimise fatigue and stress, a different set of individuals (N=110) were used to generate the TPC. All individuals were released to their respective capture locations, within two days of capture.
Preferred body temperature
Preferred Body Temperature (Tset) measurements were obtained in a thermal gradient setup in the laboratory (N=40; n=19 for Tree; n=21 for Wall). The thermal gradient (55cm long ×22 cm wide ×18cm high) had an aluminium sheet base and acrylic walls, and ranged in temperature from 12-40°C, which was achieved by having a heating pad on one side and ice packs on the other. Lizards were placed individually - always at the centre of the thermal gradient at the start of the experiment and allowed 10 min to acclimate, after which the surface temperature of the head was taken with an infrared thermal gun (FLIR TG165- distance-to-spot ratio (D:S) of 24:1) every 5 min for one hour. Surface temperature was strongly correlated with cloacal temperature (see Supplementary Material 1). After one hour, the lizard was returned to its housing container at room temperature. Tset experiments were conducted throughout the day, from 0900 hrs to 2000 hrs to capture the temporal variation in activity of the geckos. For each individual, the median Tset was calculated from the 5 min intervals of head surface temperature. The range of Tset was the mean of the inter-quartile range of all the individuals for each of the microhabitat types. A linear model (LM) was used to predict Tset as a function of microhabitat type (Tree and Wall), sex (male and female) and snout-vent length (SVL).
Critical thermal minimum and critical thermal maximum
The critical thermal minimum (CTmin) and critical thermal maximum (CTmax) were measured for the same set of individuals as Tset (N=40; n=19 for Tree; n=21 for Wall). Lizards were placed individually in a tall metal container and tested one at a time. For CTmin, we gradually reduced the temperature of the container, starting at room temperature (~26°C) by increasing the number of ice packs around the container. When the lizard was visibly sluggish, we turned the lizard over and allowed it to right itself (Laspiur et al., 2021; Tatu et al., 2024). The cloacal temperature (measured with an Amprobe Thermocouple Thermometer- K Type) at which there was a loss of righting response was considered the CTmin. The individual was then transferred to its housing container at room temperature and allowed 3 hours to recover before CTmax was measured. For CTmax, the metal container was placed inside a water bath whose temperature was increased gradually from room temperature (~26°C). The lizard was monitored closely and the cloacal temperature at the onset of muscular spasms was considered the CTmax (Lutterschmidt and Hutchison, 1997). Linear models (LM) were used to predict the impacts of microhabitat type (Tree and Wall), sex (male and female) and snout-vent length (SVL) on CTmin and CTmax.
Locomotor performance
To generate a thermal performance curve (TPC), we measured sprint speed of lizards on a race track (N=110; n=55 for Tree; n=55 for Wall). The race track (84cm×10cm×20cm) had a wooden base with a sandpaper (grit) layer and acrylic walls. Before lizards were placed in the race track, we first exposed them to one of five different temperatures: 15°C, 20°C, 25°C, 30°C and 35°C (n = 11 lizards per temperature- for each substrate). When the body temperature of the lizard, which was monitored continuously by the thermal probe, reached the required temperature, the lizard was placed in the race track, acclimatised for 1 minute and then induced to run (by lightly touching the back with a soft brush). Each lizard was induced to run three times at the same body temperature, with at least 10‐min rest between successive runs. While resting, lizards were returned to the treatment apparatus to maintain their test body temperature. The timing of the TPC experiments varied from 1100 hrs to 2000 hrs.
A digital camera (GoPro Hero Black 8) was set up 20 cm above the race track to record the runs at 120 frames per second. All the videos were analysed using the Avidemux Version-2.8.1 Multi-platform Video Editor (similar to Tatu et al., 2024). The length of the race track was visually divided into six consecutive 10 cm intervals, and the fastest 10cm from all three runs for each individual was considered the maximal sprint speed (Vmax) for that individual. The corresponding temperature to the Vmax was determined and assigned as the optimal temperature of performance (Topt) for both the microhabitat types. Additionally, the performance breadth (B95), was determined to be the range of Tb at which performance is greater than or equal to 95% of Vmax (Cecchetto et al., 2020). The lower and upper end of the TPC was anchored at CTmin and CTmax respectively (derived from above), for which sprint speeds were assigned as 0 m/s (Cecchetto et al., 2020; Tatu et al., 2024). We analysed the sprint speed data by the Generalized Additive Model (GAM) (Cecchetto et al., 2020) in R using the ‘mgcv’ package (Wood and Wood, 2015) with body temperature as a fixed effect, and sex, SVL and microhabitat type as fixed parametric covariates. Sprint speed data contained two outliers, and we present the results and TPC graphs without the outlier in the main text and with the outlier in Supplementary Material 2. Results from the TPC was qualitatively unchanged by the outliers.
Environmental temperature
Size, shape and color contribute very little to body temperature changes in small lizards (Vitt and Sartorius, 1999), and therefore, to measure the environmental temperatures in different microhabitats, Thermocron i-buttons DS1921G (n = 8) without a biophysical model were used. Four thermocrons were placed on the Wall microhabitats, including in crevices and open areas. While the other four thermocrons were placed in the Tree microhabitats, including crevices and the trunk of trees. These thermocrons were set to record temperature at 10 min intervals from January to April.
Thermoregulatory indices
The effectiveness of thermoregulation (E) was calculated by comparing the extent to which a lizard experiences its Tb within its Tset range (thermoregulatory accuracy; db) with the extent to which its habitat allows its Tb to be with Tset (thermal quality of the habitat; de; Hertz et al., 1993). The db was calculated by using the Tset calculated for that sex (male or female) in the lab and field active body temperature (Tb) of lizards from the two microhabitats (Wall or Tree). If Tb was greater than the upper limit of Tset, the upper limit of Tset from Tb was subtracted. If Tb was less than the lower limit of Tset, the Tb from the lower limit of Tset was subtracted. If the Tb fell within the Tset range, db was assigned as 0. Similarly, to calculate de, the Tset calculated for that sex (male or female) and environmental temperature (Te) for each microhabitat was used. If Te of the microhabitat was greater than the upper limit of Tset, the upper limit of Tset was subtracted from Te. If Te was less than the lower limit of Tset, the Te was subtracted from the lower limit of Tset. E was calculated as 1- mean db/mean de (Hertz et al., 1993).
All thermal experiments for Cnemaspis mysoriensis were carried out under animal ethics clearance (CAF/Ethics/882/2022) granted by the Institutional Animal Ethics Committee of the Indian Institute of Science.
Results
Preferred body temperature
Tset estimates ranged from 24.4°C to 27.7°C (mean = 26.26 ± 0.57°C S.E.; N = 40) across all individuals from both the microhabitat types. Microhabitat type was not a significant predictor of Tset (t =-0.32, p =0.75), and Tset was also not significantly influenced by sex (t =-1.65, p =0.11) and SVL (t = 1.29, p = 0.21) of the lizard (Table 1, Figure 1).
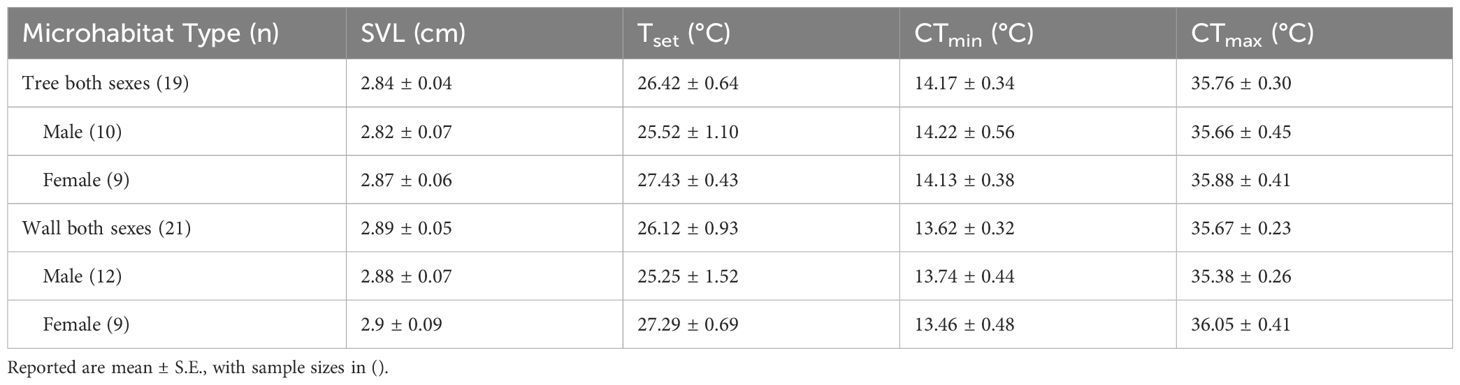
Table 1. Preferred temperature (Tset), Critical thermal minima (CTmin), Critical thermal maxima (CTmax), and snout-to-vent length (SVL) of males and females of Cnemaspis mysoriensis from Tree and Wall microhabitats.
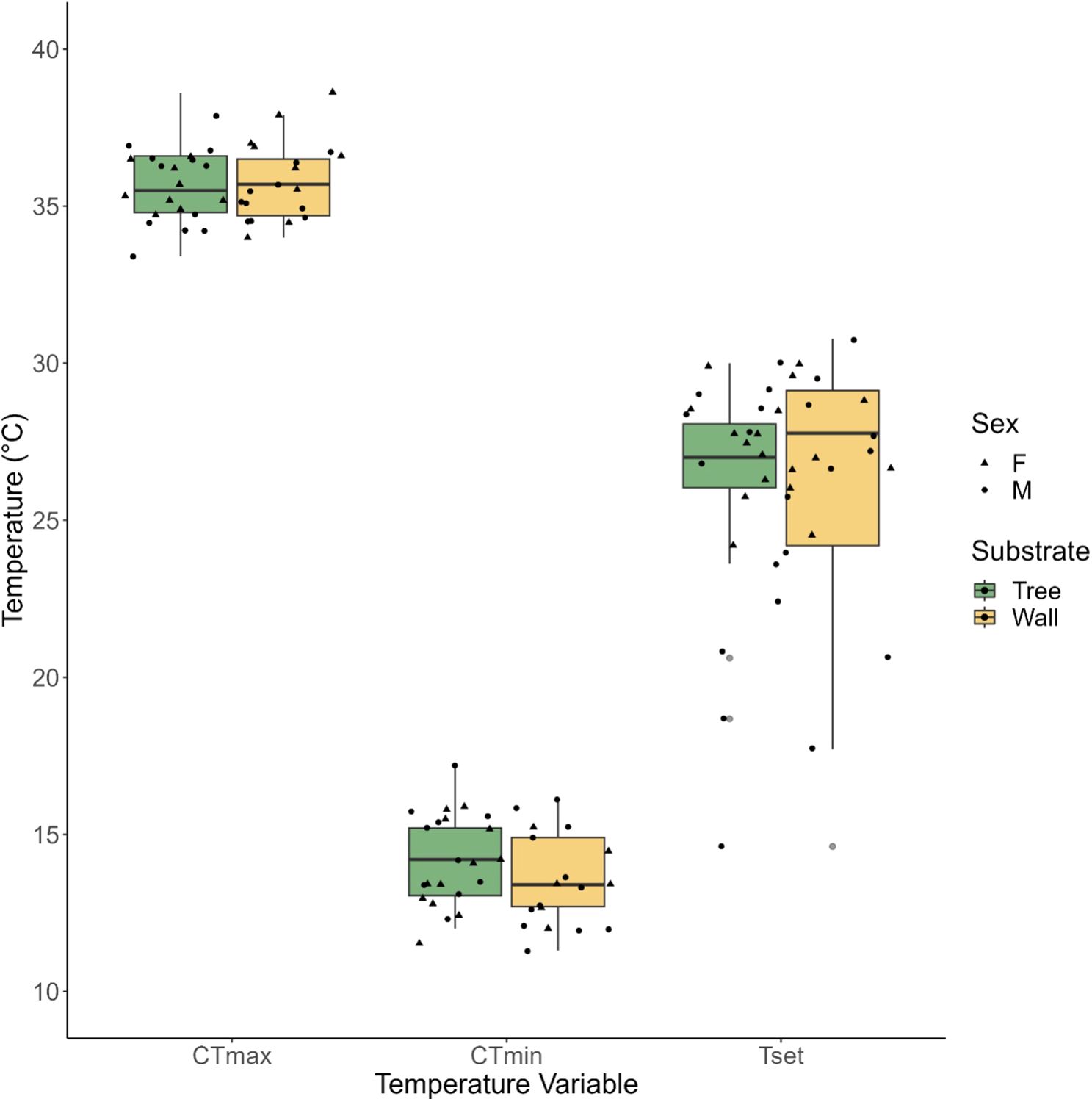
Figure 1. The Critical thermal maximum (CTmax, °C), Critical thermal minimum (CTmin, °C) and Thermal preference (Tset, °C) of C. mysoriensis from the Tree (green) and Wall (yellow) microhabitat types. Box plots show medians, 25% and 75% quartiles, and extreme values (grey points). Values for individual lizards are shown as circles (males) and triangles (females).
Critical thermal minimum
Average CTmin was 13.89 ± 0.23°C S.E. (N=40) for all individuals from both the microhabitat types. Microhabitat type (t=-1.01, p=0.32) and sex (t=0.21, p=0.83) did not significantly affect CTmin, however CTmin decreased with an increase in SVL (t= -2.57, p=0.01) (Table 1; Figure 1).
Critical thermal maximum
Average CTmax was 35.71 ± 0.18°C S.E. (N=40) across all individuals from both the microhabitat types. Microhabitat type (t=-0.29, p=0.77), sex (t=-1.14, p=0.26) and SVL (t=0.95, p= 0.35) did not significantly influence CTmax (Table 1; Figure 1).
Optimum temperature, performance breadth and maximum sprint speed from the thermal performance curve
Maximum sprint speed (Vmax) for this species was 0.70 ± 0.06 m/s S.E. (N=190) at an optimum temperature (Topt) of 26.46°C. Microhabitat type (t=-0.99, p=0.33) and sex (t=0.59, p=0.56) did not significantly influence sprint speed (Table 2; Figure 2; Supplementary Figure S2 for plot with outliers). However, sprint speed increased significantly with SVL (t=3.32, p=0.001). The performance breadth (B95) for both the microhabitat types ranged from 23.71°C to 28.95°C.
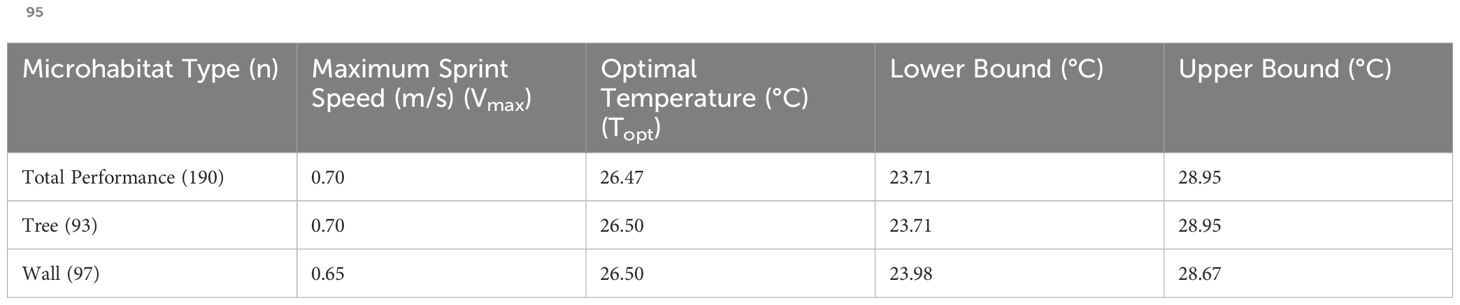
Table 2. Maximum Sprint Speed (Vmax in m/s), Optimal Temperature (Topt in °C) and Lower and Upper bounds for the 95% performance breadth (B95 in °C), for lizards from both the Tree and Wall microhabitat types, and for the species regardless of microhabitat.
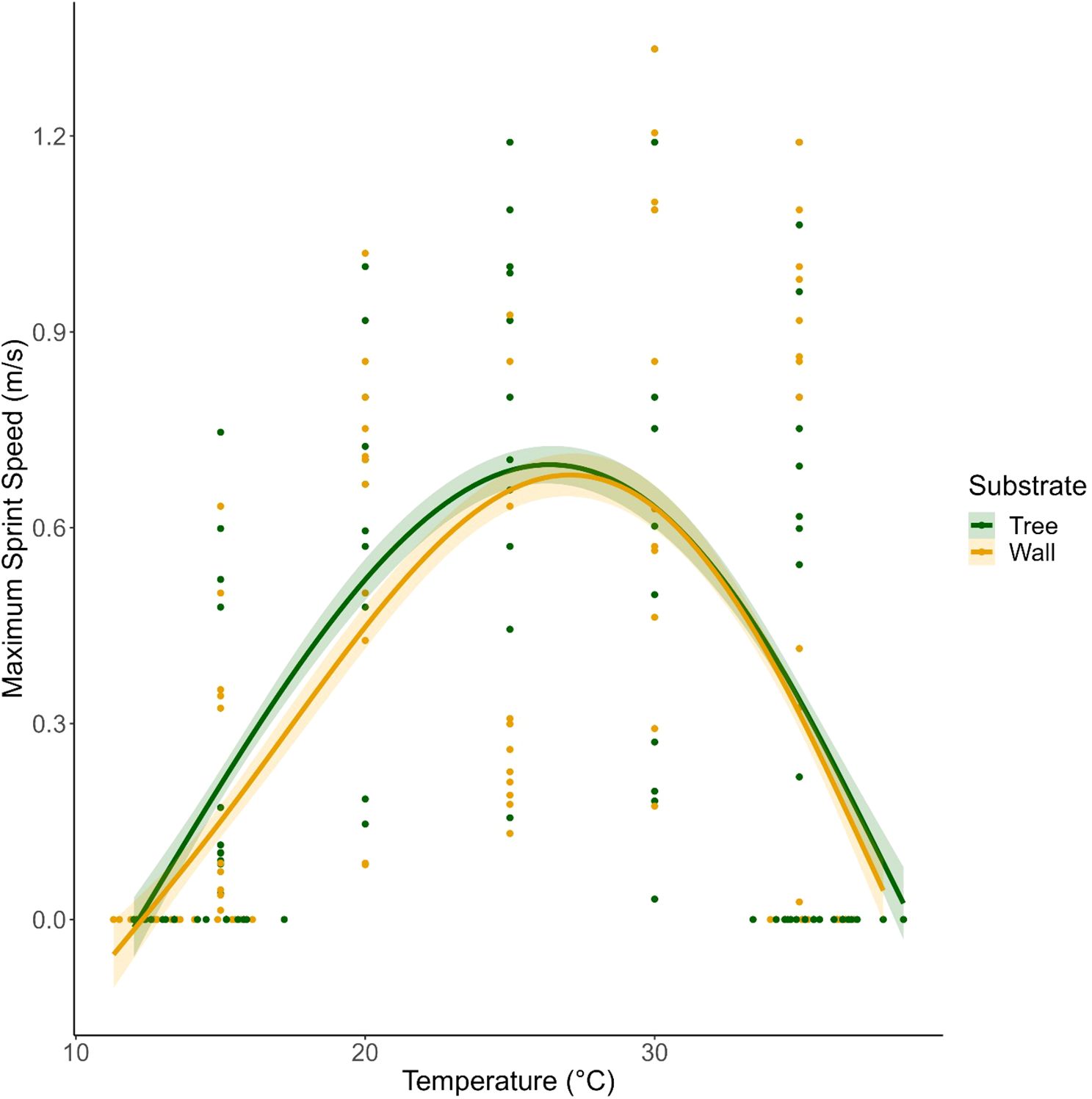
Figure 2. Maximum Sprint Speed (m/s) in Cnemaspis mysoriensis from the Tree (green) and Wall (yellow) microhabitats. Shown are the global smoothing lines from the Generalized Additive Model for each microhabitat type across the range of body temperatures. Note that Vmax for lizards from Wall microhabitats is 0.65 m/s at Topt of 26.50°C, while that from Tree microhabitat is 0.70 m/s at Topt of 26.50°C.
Active body temperature and environmental temperature
Mean active body temperature (Tb) of lizards caught from the Tree microhabitat was 24.8 ± 1.63°C S.D., while those from the Wall microhabitat was 25.1 ± 2.08°C S.D. Tb was not significantly influenced by microhabitat (t=1.30, p=0.20), sex (t=0.47, p=0.64), hour of the day (t=0.50, p=0.62), month of the year (t=1.38,p=0.17), or the interactive effect of hour of the day and month of the year (t=-1.60, p=1.11) (Figure 3A).
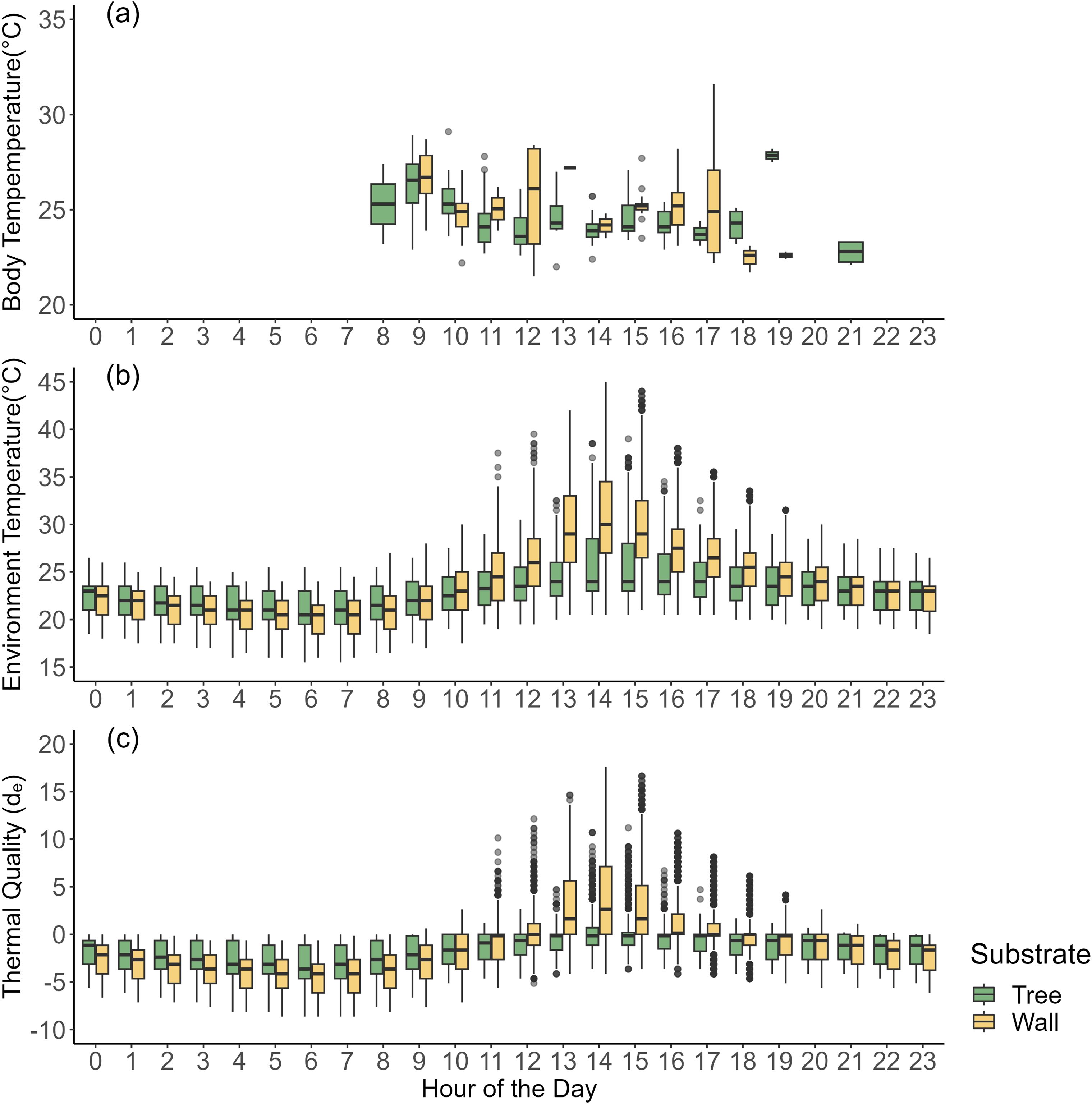
Figure 3. (A) Body temperature (Tb) and (B) Environment temperature (Te) for Cnemaspis mysoriensis throughout the day in the Tree and Wall microhabitats. (C) Thermal Quality (de) of the Tree and Wall microhabitats for C. mysoriensis throughout the day. Values approaching zero indicate high thermal quality. Positive or negative values indicate that environment temperatures (Te) are higher or lower than preferred temperature (Tset) for the species, therefore indicating poor thermal quality. Note the higher variability in de for Wall microhabitat than Tree microhabitat.
Environmental temperature (Te) on Wall microhabitats (23.8 ± 4.3°C S.D.) was significantly higher than that on the Tree microhabitats (23.0 ± 2.7°C S.D.) (t=28.39, p<0.001). The range of Te on Wall microhabitats (16-45°C) was also wider than those on Tree microhabitats (15.5-39°C). Te was significantly influenced by month of the year (t=61.62, p<0.001), hour of the day (t=42.25, p<0.001), and the interaction between month of the year and hour of the day (t=-4.15, p<0.001) (Figure 3B).
Thermoregulatory indices
The accuracy of thermoregulation (db) was 0.44 ± 0.28 S.E. for Wall microhabitats while db was 0.16 ± 0.08 S.E. for Tree microhabitats. The thermal quality (de) of Wall microhabitats was 2.40 ± 0.01 S.E., while for Tree microhabitats, de was 1.87 ± 0.01 S.E. Based on these values, effectiveness of thermoregulation (E) was valued at 0.81 for lizards on Wall microhabitats and 0.91 for lizards on Tree microhabitats (Figure 3C).
Discussion
With urbanisation resulting in the creation of novel environments and structures, species in anthropogenic areas not only experience altered microhabitats, but also microclimates (Thawley et al., 2019). For reptiles, changes in the thermal environment of urban areas can have consequences for their thermal ecology. In urban Bengaluru, we found that the substrate temperatures of microhabitats made from cement (e.g. walls, bricks, footpaths) were slightly higher and more variable than substrates on trees (e.g. trunks, tree crevices, prop roots). Despite the differences in environmental temperature (Te), the preferred temperature (Tset), critical limits of tolerance (CTmin and CTmax), and optimal temperature (Topt) for locomotor performance did not differ for C. mysoriensis found on these distinct microhabitats. Differences in Te between Wall and Tree microhabitats, however, did influence the thermal quality of the environment and therefore, how accurately lizards can thermoregulate to achieve Tset. The thermal environment of the city, and strategies like higher values of Effectiveness of thermoregulation, can have consequences for the persistence of species in urbanised areas (Ackley et al., 2015; Campbell-Staton et al., 2020; Tucker et al., 2023).
Studies of urban lizards around the world have yielded some interesting insights on how the thermal ecology of species can change. Some studies have found that species physiologically respond to increased temperatures in urban areas by increasing their preferred temperatures (Tset) and critical limits (Putman and Tippie, 2020). Others find directional selection for individuals with inherently higher Tset in warmer areas (Gilbert and Miles, 2017). In C. mysoriensis, CTmax and CTmin values were within the range published for geckos (Diele-Viegas et al., 2018; Romero-Báez et al., 2020; Luzete et al., 2022), and we found that Tset, CTmin and CTmax were not significantly different between the sexes or between lizards that use different microhabitats. We did, however, find that CTmax was more conserved than CTmin in lizards across microhabitats and sexes, which supports the general expectation that CTmax evolves at a slower rate than CTmin (Sunday et al., 2011; Muñoz et al., 2014; Bodensteiner et al., 2021). The conservatism of thermal physiology in C. mysoriensis could be due to gene flow, as movement and breeding of individuals between both the microhabitat types is possible. Equally likely is the fact that C. mysoriensis can behaviourally thermoregulate, and thus the Bogert effect, wherein thermoregulatory behaviour buffers individuals against variation in the thermal environment, can lead to slower physiological adaptation (Bodensteiner et al., 2021).
An important component of thermal adaptation strategies of a species is how temperature influences performance (Hertz et al., 1993; Angilletta et al., 2006). Maximal sprint speed is one of the most common parameters measured in a TPC, as sprint speed has direct consequences for survival in encounters with predators or even competitors (Huey and Bennett, 1987). Apart from the expected positive relationship between sprint speed and body size, we found no measurable differences in maximal sprint speed (Vmax), the thermal performance breadth (B95), and the optimal temperature for performance (Topt) of C. mysoriensis from different microhabitats. Such conserved thermally-sensitive performance parameters might be due to similar predation pressures and other ecological factors, such as competition, in the different microhabitat types that favour high performance at similar temperatures (Cecchetto et al., 2020). Evolutionary conservatism of thermally-sensitive performance traits within populations of a species can also be attributed to the high costs associated with synthesising new enzymes, changing membrane structures, and redistributing resources to enable the genetic changes in the physiology of a species (Angilletta et al., 2006). In the evolutionary time-scale of this species, changes to the thermal environment due to urbanisation might still be too recent for genetic adaptations to evolve. Conserved TPC for this species, however, does not rule out the possibility of different maximal sprint speeds on wall surfaces and tree surfaces because of the inherent differences in rugosity (Pillai et al., 2020). Thus, urbanisation-induced microhabitat change could influence performance and fitness for this species, and other species, even without altering the TPC.
Several recent studies have found that, at the landscape scale, Bengaluru city does not experience an urban heat island effect (Ambinakudige, 2011; Thaker et al., 2022). The relatively cool temperatures of this city compared to its immediate surroundings can be attributed to its high-altitude location (~900m above sea level), numerous waterbodies, and dense vegetation (Siddiqui et al., 2021). At the microhabitat scale experienced by C. mysoriensis, however, we found that Wall surfaces were slightly hotter and reached higher temperatures than Tree surfaces during the day. These differences were expected given that Tree microhabitats are more shaded than Walls in the city. Tree microhabitats also have better thermal quality (de values were closer to zero) and have lower variation throughout the day than Wall microhabitats, enabling lizards on trees to have higher thermoregulatory accuracy than those on walls (db values were lower). Greater accuracy of thermoregulation implies less energy expenditure to attain Tb for activity (Huey and Slatkin, 1976; Hertz et al., 1993; Angilletta et al., 2002). Thus, for C. mysoriensis, trees provide the most suitable microclimatic conditions in urban environments.
Overall, our results indicate that C. mysoriensis is behaviourally thermoregulating to adapt to the thermal heterogeneity associated with anthropogenic microhabitat types in urban Bengaluru. Indeed, active body temperatures did not vary significantly across the two microhabitat types, but the effort needed for thermoregulation was higher on Walls than on Trees. Hence, for C. mysoriensis, the Tree microhabitats are thermally more suitable than Wall ones. We do acknowledge that the differences in the effectiveness of thermoregulation (E) between lizards on trees and walls is small, but for a small-bodied diurnal lizard, even minor differences could influence survival, especially if walls continue to increase in temperature with climate warming, and if lizards on walls need to use crevices or shaded areas more often than those on trees. Trees, especially those with buttresses and crevices, are already less abundant than walls in Bengaluru, and this may be why lizards are currently using walls. With increasing urbanisation, trees may become an even more limited resource. Our study shows the importance of retaining natural spaces in a city, with its importance even at the microhabitat level for species abundantly found in the cityscape. With the ongoing environmental warming (IPCC, 2014), behavioural buffering (via thermoregulation) and physiological plasticity are key mechanisms that can help individuals compensate for the increase in environmental temperature (Domínguez–Guerrero et al., 2019). Most reptiles are already experiencing elevated temperatures in their habitats (Sunday et al., 2014), and genetic-based responses of thermal traits are unlikely, limiting the potential of an adaptive rescue of thermal biology (Giacometti et al., 2024). Hence, for species with conserved physiology, the degree to which behavioural thermoregulation will help the species to persist is an open question. On another note, not much ecological information exists about C. mysoriensis (but see Kabir et al., 2019, 2020; Joshi and Thaker, 2020). Our study is the first to generate basal thermal information on a gecko species in India. Such data are crucial, especially in the current climate warming scenario. With the continual increased pace of urbanisation and increasing population in Bengaluru (United Nations, 2016), our study provides new evidence for why the maintenance of natural habitats is essential.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Institute Animal Ethics Committee of the Indian Institute of Science. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
VA: Formal Analysis, Investigation, Visualization, Writing – original draft. AT: Conceptualization, Formal Analysis, Investigation, Visualization, Writing – review & editing. MT: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding for this research was supported by the Indian Institute of Science to MT.
Acknowledgments
We thank Harish Prakash, Mihir Joshi, Subhashmita Patro and Amanda Ben for their help in running experiments in the lab. We also thank Dr. Kartik Shanker for his support during early stages of the project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/famrs.2024.1522805/full#supplementary-material
References
Ackley J. W., Angilletta M. J., DeNardo D., Sullivan B., Wu J. (2015). Urban heat island mitigation strategies and lizard thermal ecology: landscaping can quadruple potential activity time in an arid city. Urban Ecosyst. 18, 1447–1459. doi: 10.1007/s11252-015-0460-x
Ambinakudige S. (2011). Remote sensing of land cover’s effect on surface temperatures: a case study of the urban heat island in Bangalore, India. Appl. GIS 7, 1–12. doi: 10.4225/03/57E1D1618522A
Angilletta M. J. (2009). Thermal adaptation: A theoretical and empirical synthesis. Oxford Univ. Press. doi: 10.1093/acprof:oso/9780198570875.001.1
Angilletta M. J., Bennett A. F., Guderley H., Navas C. A., Seebacher F., Wilson R. S. (2006). Coadaptation: A unifying principle in evolutionary thermal biology. Physiol. Biochem. Zool. 79, 282–294. doi: 10.1086/499990
Angilletta M. J., Niewiarowski P. H., Navas C. A. (2002). The evolution of thermal physiology in ectotherms. J. Therm. Biol. 27, 249–268. doi: 10.1016/S0306-4565(01)00094-8
Avilés-Rodríguez K. J., Kolbe J. J. (2019). Escape in the city: urbanization alters the escape behavior of Anolis lizards. Urban Ecosyst. 22, 733–742. doi: 10.1007/s11252-019-00845-x
Balakrishna S., Amdekar M. S., Thaker M. (2021). Morphological divergence, tail loss, and predation risk in urban lizards. Urban Ecosyst. 24, 1391–1398. doi: 10.1007/s11252-021-01122-6
Batabyal A., Balakrishna S., Thaker M. (2017). A multivariate approach to understanding shifts in escape strategies of urban lizards. Behav. Ecol. Sociobiol. 71, 83. doi: 10.1007/s00265-017-2307-3
Battles A. C., Kolbe J. J. (2019). Miami heat: Urban heat islands influence the thermal suitability of habitats for ectotherms. Glob. Change Biol. 25, 562–576. doi: 10.1111/gcb.14509
Baur B., Baur A. (1993). Climatic warming due to thermal radiation from an urban area as possible cause for the local extinction of a land snail. J. Appl. Ecol. 30, 333. doi: 10.2307/2404635
Bodensteiner B. L., Agudelo-Cantero G. A., Arietta A. Z. A., Gunderson A. R., Muñoz M. M., Refsnider J. M., et al. (2021). Thermal adaptation revisited: How conserved are thermal traits of reptiles and amphibians? J. Exp. Zool. Part Ecol. Integr. Physiol. 335, 173–194. doi: 10.1002/jez.2414
Brum P. H. R., Gonçalves S. R. A., Strüssmann C., Teixido A. L. (2023). A global assessment of research on urban ecology of reptiles: patterns, gaps and future directions. Anim. Conserv. 26, 1–13. doi: 10.1111/acv.12799
Campbell-Staton S. C., Velotta J. P., Winchell K. M. (2021). Selection on adaptive and maladaptive gene expression plasticity during thermal adaptation to urban heat islands. Nat. Commun. 12, 6195. doi: 10.1038/s41467-021-26334-4
Campbell-Staton S. C., Winchell K. M., Rochette N. C., Fredette J., Maayan I., Schweizer R. M., et al. (2020). Parallel selection on thermal physiology facilitates repeated adaptation of city lizards to urban heat islands. Nat. Ecol. Evol. 4, 652–658. doi: 10.1038/s41559-020-1131-8
Cecchetto N. R., Medina S. M., Ibargüengoytía N. R. (2020). Running performance with emphasis on low temperatures in a Patagonian lizard, Liolaemus lineomaculatus. Sci. Rep. 10, 14732. doi: 10.1038/s41598-020-71617-3
Chown S. L., Duffy G. A. (2015). Thermal physiology and urbanization: perspectives on exit, entry and transformation rules. Funct. Ecol. 29, 902–912. doi: 10.1111/1365-2435.12478
Diele-Viegas L. M., Vitt L. J., Sinervo B., Colli G. R., Werneck F. P., Miles D. B., et al. (2018). Thermal physiology of Amazonian lizards (Reptilia: Squamata). PLOS ONE. 13, e0192834. doi: 10.1371/journal.pone.0192834
Domínguez–Guerrero S. F., Muñoz M. M., Pasten–Téllez D. D. J., Arenas–Moreno D. M., Rodríguez–Miranda L. A., Manríquez–Morán N. L., et al. (2019). Interactions between thermoregulatory behavior and physiological acclimatization in a wild lizard population. J. Therm. Biol. 79, 135–143. doi: 10.1016/j.jtherbio.2018.12.001
Duncan R. P., Clemants S. E., Corlett R. T., Hahs A. K., McCarthy M. A., McDonnell M. J., et al. (2011). Plant traits and extinction in urban areas: a meta-analysis of 11 cities: Plant traits and extinction in cities. Glob. Ecol. Biogeogr. 20, 509–519. doi: 10.1111/j.1466-8238.2010.00633.x
Elmqvist T., Fragkias M., Goodness J., Güneralp B., Marcotullio P. J., McDonald R. I., et al. (2013). Urbanization, biodiversity and ecosystem services: challenges and opportunities (Springer Netherlands: Dordrecht). doi: 10.1007/978-94-007-7088-1
Finand B., Loeuille N., Bocquet C., Fédérici P., Ledamoisel J., Monnin T. (2024). Habitat fragmentation through urbanization selects for low dispersal in an ant species. Oikos 2024, e10325. doi: 10.1111/oik.10325
French S. S., Webb A. C., Hudson S. B., Virgin E. E. (2018). Town and country reptiles: A review of reptilian responses to urbanization. Integr. Comp. Biol. 58 (5), 948–966. doi: 10.1093/icb/icy052
Giacometti D., Palaoro A. V., Leal L. C., De Barros F. C. (2024). How seasonality influences the thermal biology of lizards with different thermoregulatory strategies: a meta-analysis. Biol. Rev. 99, 409–429. doi: 10.1111/brv.13028
Gilbert A. L., Miles D. B. (2017). Natural selection on thermal preference, critical thermal maxima and locomotor performance. Proc. R. Soc B Biol. Sci. 284, 20170536. doi: 10.1098/rspb.2017.0536
Giri V. B., Agarwal I., Bauer A. M. (2009). Designation of a neotype for Cnemaspis mysoriensis (Jerdon 1853) (Sauria: Gekkonidae), with a redescription and notes on its distribution and habitat. Russ. J. Herpetol. 16, 256–264.
Grimm N. B., Faeth S. H., Golubiewski N. E., Redman C. L., Wu J., Bai X., et al. (2008). Global change and the ecology of cities. Science 319, 756–760. doi: 10.1126/science.1150195
Hall J. M., Warner D. A. (2018). Thermal spikes from the urban heat island increase mortality and alter physiology of lizard embryos. J. Exp. Biol. 221, jeb181552. doi: 10.1242/jeb.181552
Hertz P. E. (1992). Temperature regulation in puerto rican anolis lizards: A field test using null hypotheses. Ecology 73, 1405–1417. doi: 10.2307/1940686
Hertz P. E., Huey R. B., Nevo E. (1983). Homage to santa anita: thermal sensitivity of sprint speed in agamid lizards. Evolution 37, 1075–1084. doi: 10.2307/2408420
Hertz P. E., Huey R. B., Stevenson R. D. (1993). Evaluating temperature regulation by field-active ectotherms: the fallacy of the inappropriate question. Am. Nat. 142, 796–818. doi: 10.1086/285573
Huey R. B. (1974). Behavioral thermoregulation in lizards: importance of associated costs. Science 184, 1001–1003. doi: 10.1126/science.184.4140.1001
Huey R. B., Bennett A. F. (1987). Phylogenetic studies of coadaptation: preferred temperatures versus optimal performance temperatures of lizards. Evolution 41, 1098–1115. doi: 10.1111/j.1558-5646.1987.tb05879.x
Huey R. B., Slatkin M. (1976). Cost and benefits of lizard thermoregulation. Q. Rev. Biol. 51, 363–384. doi: 10.1086/409470
Huey R. B., Stevenson R. D. (1979). Integrating thermal physiology and ecology of ectotherms: A discussion of approaches. Am. Zool. 19, 357–366. doi: 10.1093/icb/19.1.357
IPCC (2014). Climate change 2014: synthesis report (Geneva, Switzerland: Intergovernmental Panel on Climate Change). Available at: https://www.ipcc.ch/report/ar5/syr/ (Accessed October 7, 2024).
Joshi M., Thaker M. (2020). The role of male chemical secretion components in sex recognition, mate assessment and mate choice in the diurnal gecko Cnesmaspis mysoriensis (Pune: Indian Institute of Science education and Research). Available at: http://dr.iiserpune.ac.in:8080/xmlui/bitstream/handle/123456789/4856/Mihir%20Joshi_MS%20Thesis_Final.pdf?sequence=1&isAllowed=n (Accessed November 17, 2022).
Kabir M. S., Radhika V., Thaker M. (2019). Mismatch in receiver responses to multimodal signals in a diurnal gecko. Anim. Behav. 147, 115–123. doi: 10.1016/j.anbehav.2018.11.011
Kabir M. S., Venkatesan R., Thaker M. (2020). Multiple sensory modalities in diurnal geckos is associated with the signalling environment and evolutionary constraints. Integr. Org. Biol. 2 (1), obaa027. doi: 10.1093/iob/obaa027
Karthikeyan S. (1999). The fauna of Bangalore-The vertebrates and butterflies of Bangalore. A checklist. Available online at: https://www.wildwanderer.com/publications/ (Accessed October 7, 2024).
Kolbe J. J., Battles A. C., Avilés-Rodríguez K. J. (2016). City slickers: poor performance does not deter Anolis lizards from using artificial substrates in human-modified habitats. Funct. Ecol. 30, 1418–1429. doi: 10.1111/1365-2435.12607
Laspiur A., Santos J. C., Medina S. M., Pizarro J. E., Sanabria E. A., Sinervo B., et al. (2021). Vulnerability to climate change of a microendemic lizard species from the central Andes. Sci. Rep. 11, 11653. doi: 10.1038/s41598-021-91058-w
Li G., Fang C., Li Y., Wang Z., Sun S., He S., et al. (2022). Global impacts of future urban expansion on terrestrial vertebrate diversity. Nat. Commun. 13, 1628. doi: 10.1038/s41467-022-29324-2
Lutterschmidt W. I., Hutchison V. H. (1997). The critical thermal maximum: data to support the onset of spasms as the definitive end point. Can. J. Zool. 75, 1553–1560. doi: 10.1139/z97-782
Luzete J., Pinho L. O., Oliveira I., Klaczko J. (2022). Thermal physiology of the invasive tropical house gecko, hemidactylus mabouia (Squamata, gekkonidae). Herpetol. Conserv. Biol. 17, 511–520.
McKinney M. L. (2008). Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 11, 161–176. doi: 10.1007/s11252-007-0045-4
Mohanty N. P., Joshi M., Thaker M. (2021). Urban lizards use sleep sites that mirror the structural, thermal, and light properties of natural sites. Behav. Ecol. Sociobiol. 75, 166. doi: 10.1007/s00265-021-03101-5
Muñoz M. M., Stimola M. A., Algar A. C., Conover A., Rodriguez A. J., Landestoy M. A., et al. (2014). Evolutionary stasis and lability in thermal physiology in a group of tropical lizards. Proc. R. Soc B Biol. Sci. 281, 20132433. doi: 10.1098/rspb.2013.2433
Nagendra H., Nagendran S., Paul S., Pareeth S. (2012). Graying, greening and fragmentation in the rapidly expanding Indian city of Bangalore. Landsc. Urban Plan. 105, 400–406. doi: 10.1016/j.landurbplan.2012.01.014
Pellitteri-Rosa D., Bellati A., Cocca W., Gazzola A., Martín J., Fasola M. (2017). Urbanization affects refuge use and habituation to predators in a polymorphic lizard. Anim. Behav. 123, 359–367. doi: 10.1016/j.anbehav.2016.11.016
Pillai R., Nordberg E., Riedel J., Schwarzkopf L. (2020). Geckos cling best to, and prefer to use, rough surfaces. Front. Zool. 17, 32. doi: 10.1186/s12983-020-00374-w
Prosser C., Hudson S., Thompson M. B. (2006). Effects of urbanization on behavior, performance, and morphology of the garden skink, lampropholis guichenoti. J. Herpetol. 40, 151–159. doi: 10.1670/38-05A.1
Putman B. J., Tippie Z. A. (2020). Big city living: A global meta-analysis reveals positive impact of urbanization on body size in lizards. Front. Ecol. Evol. 8. doi: 10.3389/fevo.2020.580745
Romero-Báez Ó., Santos-Bibiano R., Domínguez-Godoy M. A., Miles D. B., Muñoz-nolasco F. J. (2020). Thermal ecophysiology of a native and an invasive gecko species in a tropical dry forest of Mexico. J. Therm. Biol. 90, 102607. doi: 10.1016/j.jtherbio.2020.102607
Seebacher F., Franklin C. E. (2005). Physiological mechanisms of thermoregulation in reptiles: a review. J. Comp. Physiol. B 175, 533–541. doi: 10.1007/s00360-005-0007-1
Shetty P. J., Gowda S., Gururaja K. V., Sudhira H. S. (2012). Effect of landscape metrics on varied spatial extents of Bangalore, India. Asian J. Geoinform. 12, 1–11.
Siddiqui A., Kushwaha G., Raoof A., Verma P. A., Kant Y. (2021). Bangalore: Urban heating or urban cooling? Egypt. J. Remote Sens. Space Sci. 24, 265–272. doi: 10.1016/j.ejrs.2020.06.002
Sudhira H. S., Ramachandra T. V., Subrahmanya M. H. B. (2007). Bangalore. Cities 24, 379–390. doi: 10.1016/j.cities.2007.04.003
Sunday J. M., Bates A. E., Dulvy N. K. (2011). Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc B Biol. Sci. 278, 1823–1830. doi: 10.1098/rspb.2010.1295
Sunday J. M., Bates A. E., Kearney M. R., Colwell R. K., Dulvy N. K., Longino J. T., et al. (2014). Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl. Acad. Sci. 111, 5610–5615. doi: 10.1073/pnas.1316145111
Tatu A., Dutta S., Thaker M. (2024). Hotter deserts and the impending challenges for the spiny-tailed Lizard in India. Biol. Open 13, bio060150. doi: 10.1242/bio.060150
Taylor E. N., Diele-Viegas L. M., Gangloff E. J., Hall J. M., Halpern B., Massey M. D., et al. (2021). The thermal ecology and physiology of reptiles and amphibians: A user’s guide. J. Exp. Zool. Part Ecol. Integr. Physiol. 335, 13–44. doi: 10.1002/jez.2396
Thaker M., Amdekar M. S., Mohanty N. P., Nageshkumar A. K., Prakash H., Seshadri K. S. (2022). An expanding cityscape and its multi-scale effects on lizard distribution. Front. Conserv. Sci. 3. doi: 10.3389/fcosc.2022.839836
Thawley C. J., Moniz H. A., Merritt A. J., Battles A. C., Michaelides S. N., Kolbe J. J. (2019). Urbanization affects body size and parasitism but not thermal preferences in Anolis lizards. J. Urban Ecol. 5. doi: 10.1093/jue/juy031
Tucker M. R., Biffi D., Williams D. A. (2023). Thermal refugia and persistence of Texas horned lizards (Phrynosoma cornutum) in small towns. Ecol. Evol. 13, e10245. doi: 10.1002/ece3.10245
Vardi R., Dubiner S., Ben Bezalel R., Meiri S., Levin E. (2023). Do urban habitats induce physiological changes in Mediterranean lizards? J. Zool. 321, 75–82. doi: 10.1111/jzo.13089
Vitt L. J., Sartorius S. S. (1999). HOBOs, Tidbits and lizard models: the utility of electronic devices in field studies of ectotherm thermoregulation. Funct. Ecol. 13, 670–674. doi: 10.1046/j.1365-2435.1999.00357.x
Keywords: heterogeneity, cnemaspis, reptile, CTmax, CTmin, sprint, environment temperature
Citation: Apte V, Tatu A and Thaker M (2025) Microhabitat level thermal physiology and thermoregulation of a diurnal gecko in an urban landscape. Front. Amphib. Reptile Sci. 2:1522805. doi: 10.3389/famrs.2024.1522805
Received: 05 November 2024; Accepted: 20 December 2024;
Published: 10 January 2025.
Edited by:
Duminda S. B. Dissanayake, University of Canberra, AustraliaReviewed by:
Theja Abayarathna, Rajarata University of Sri Lanka, Sri LankaJonathan Webb, University of Technology Sydney, Australia
Copyright © 2025 Apte, Tatu and Thaker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vaishnavi Apte, dmFpc2hhcHRlOTlAZ21haWwuY29t; Maria Thaker, bXRoYWtlckBpaXNjLmFjLmlu
†Present address: Vaishnavi Apte, CEROS, Nature Conservation Foundation, Mysuru, Karnataka, India
Avichal Tatu, School of Biosciences, University of Melbourne, Parkville, VIC, Australia
 Vaishnavi Apte
Vaishnavi Apte Avichal Tatu
Avichal Tatu Maria Thaker
Maria Thaker