- 1Caesar Kleberg Wildlife Research Institute, Texas A&M University – Kingsville, Kingsville, TX, United States
- 2Department of Chemistry and Biology, Texas A&M International University, Laredo, TX, United States
- 3Arthur Temple College of Forestry and Agriculture, Stephen F. Austin State University, Nacogdoches, TX, United States
Spot-tailed Earless Lizards (STEL; Plateau STEL, Holbrookia lacerata; and Tamaulipan STEL, Holbrookia subcaudalis) are lizard species that are in decline both in number and distribution. As a result, this species has been considered for federal threatened status and a 90-day finding by the United States Fish and Wildlife Service stated that federal protection may be warranted. To date, a decision for Endangered Species Act listing for the Plateau STEL has been denied, but a judgement for the Tamaulipan STEL has not yet been decided. The requests to list STEL as a threatened species initiated numerous studies to investigate the status of both species and to add to the knowledge base of STEL. Though many studies have been conducted, much of the resulting information exists in gray literature (e.g., governmental reports, project summaries, etc.) that are not readily available or peer-reviewed. Herein, we consolidate the research results from scientific literature, governmental reports, and petitions to assist future researchers of STEL. We describe the natural history of STEL, their current taxonomy, conservation status, and discuss potential causes for their decline. Our goal is to provide the needed background knowledge for future researchers and policy makers.
1 Introduction
Spot-tailed Earless Lizards (Plateau, Holbrookia lacerata (Cope, 1880); and Tamaulipan, Holbrookia subcaudalis (Cope, 1880); here after STEL) are small terrestrial lizards belonging to the family Phrynosomatidae that are found within semi-arid to humid grasslands (Hibbitts et al., 2021). Historically they were considered a species characteristic of the Great Plains of the United States, ranging from Comanche County, Oklahoma (Webb and Ortenberger, 1953), throughout central and southern Texas and into the Mexican states of Coahuila, Nuevo Leon, and Tamaulipas (Axtell, 1968; Conant and Collins, 1991; Texas Parks and Wildlife Department (TPWD), 2005, Hammerson et al., 2007). In recent years they have been elevated from subspecies status (H. l. lacerata and H. l. subcaudalis, respectively) to full species status (Hibbitts et al., 2019; Firneno et al., 2024). Geographically, these two species are split along the Balcones Escarpment, which is located in south-central Texas within the South Texas Plains ecoregion, with H. lacerata to the north and H. subcaudalis to the south (Figure 1; Hibbitts et al., 2019). Today, their population numbers and distributions have declined substantially within the United States, with a few disjunct populations occurring (Hibbitts and Ryberg, 2018; LaDuc et al., 2018).
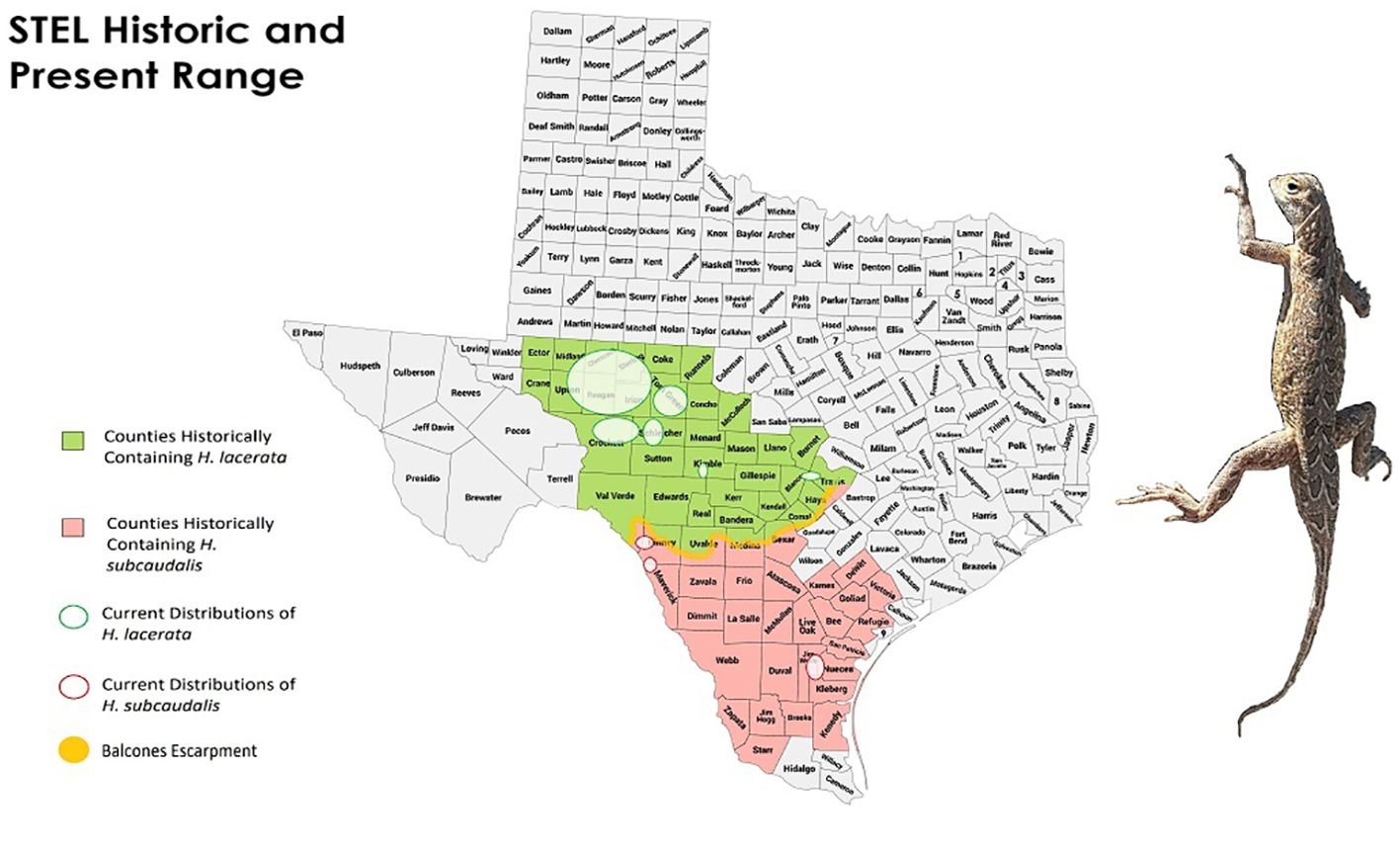
Figure 1. The historical and current distributions of Spot-tailed Earless Lizards (STEL; Plateau or Northern STEL (Holbrookia lacerata) highlighted in green and Tamaulipan or Southern STEL (H. subcaudalis) highlighted in pink. Currently, Plateau and Tamaulipan STEL occur within 6 and 3, respectively, areas that are too distant from each other for gene flow.
There is very little published information on the life history and basic ecology of STEL. An increased interest in their ecology was the result of The WildEarth Guardians (2010), a nonprofit environmental organization that advocates for wildlife and wild places, who petitioned for federal protection of STEL under the Endangered Species Act (hereafter ESA) in 2010 (Ingram, 2017). The United States Fish and Wildlife Service (USFWS) (2011) issued a 90-day finding stating that the petition presented substantial evidence that federal protection may be warranted, placing much emphasis on the distributional overlap between STEL and Red-imported Fire Ants (Solenopsis invicta; Department of the Interior United States Fish and Wildlife Service (USFWS), 2011). Critics of the USFWS findings argue that virtually nothing is known about STEL; hence, it is impossible to identify vulnerabilities for the species. Critics have suggested that federal listing of the Plateau STEL: would hamper crude oil production of the Eagle Ford Shale (Ingram, 2017). Unfortunately, without available data on the species’ abundances, distributions, and ecologies, such criticisms can continue (United States Fish and Wildlife Service (USFWS), 2016). Although the United States Fish and Wildlife Service (USFWS) (2024) recently decided that the Plateau STEL does not warrant listing as a federally endangered or threatened species, their decision on the status of the Tamaulipan STEL is still pending. With the decision for ESA listing awaiting, it has become more pertinent than ever to quantify and understand the factors contributing to the decline of STEL. Recent studies involving both species of STEL have begun to shed light on some of the factors affecting them; however, as more information concerning their life history is gained, researchers are left with more questions than answers.
As researchers of STEL, it became apparent that a dearth of information existed within the scientific literature for either species, which created difficulties to assess their conservation status. Therefore, our objectives were 1) to search the scientific literature, government reports, and communications of STEL to determine the natural history, ecology, taxonomy, and potential causes for the decline of these species, and 2) to report the known knowledge of STEL and develop a comprehensive reference section that will benefit future researchers of STEL. Such a document will offer a valuable starting point for future research of STEL.
2 Methods
We used keywords to search eight Search Engines (ResearchGate, Google Scholar, AGRIS, AGRICOLA, BioOne, CAB Abstracts, JSTOR, and Science Open) for peer-reviewed, scientific papers that included the terms: Ecology, Criteria for Endangered Species Act listing, Decline, Habitat, Holbrookia, Holbrookia lacerata, Holbrookia subcaudalis, Life history, Population, Species distribution, Spot-tailed Earless Lizard, and combinations of each term with either STEL scientific name. We used Boolean terms of ‘And’ and ‘Or’ for term combinations, and included literature dated from 1880 to April 2024. We collected published papers from our keyword search and added pertinent papers that were listed within the Literature Cited and Reference sections of those papers. Therefore, our final compilation included papers from scientific literature, books, book chapters, theses, dissertations, and government reports.
2.1 Definitions for each term used were
Holbrookia lacerata - a small terrestrial lizard in the family Phrynosomatidae, which is native to west-central Texas, north of the Balcones Escarpment and north-eastern Mexico. It is commonly known as the Plateau STEL or Northern STEL. Formerly known as Holbrookia maculata lacerata and Holbrookia lacerata lacerata.
Holbrookia subcaudalis- a small terrestrial lizard in the family Phrynosomatidae, which is native to the Rio Grande Valley and as far north as the Balcones Escarpment, and the adjacent states of Mexico. It is commonly known as the Tamaulipan STEL and Southern STEL. Formerly known as Holbrookia lacerata subcaudalis.
Holbrookia - the genus of lizards commonly known as “lesser earless lizards” within the family Phrynosomatidae. They are native to the south-western United States and northern Mexico. This genus contains six species characterized by their lack of an external ear opening.
Spot-tailed Earless Lizard - the common name for H. lacerata and H. subcaudalis collectively. Until recently, both species were recognized as subspecies: H. l. lacerata, and H. l. subcaudalis, respectively.
Life history - the pattern of reproduction and survival of a specific species and the traits that affect said patterns.
Ecology - the study of how organisms interact with one another and their environment.
Habitat - the biotic and abiotic factors that comprise the area in which an organism lives and interacts.
Population - a group of organisms of a particular species that can interact with and pass genes with one another.
Decline - loss in number; within ecology pertaining to a lowering number of individuals, genetic diversity, or available habitat.
Criteria for Endangered Species Act listing - “A species is added to the list when it is determined to be an endangered or threatened species because of any of the following factors: • the present or threatened destruction, modification, or curtailment of its habitat or range; • overutilization for commercial, recreational, scientific, or educational purposes; • disease or predation; • the inadequacy of existing regulatory mechanisms; • other natural or manmade factors affecting its survival.” (United States Fish and Wildlife Service (USFWS), 2011).
Species distribution - the spatial arrangement of populations within a particular taxon.
All papers that mentioned H. subcaudalis and H. lacerata, as well as papers referring to other species in the genus Holbrookia, were entirely read and catalogued to main topic of the paper. In each of the papers, main topics were STEL ecology, natural history, taxonomy, biology, physiology, behavior, geographic distribution, and proposed reasons for the decline of the species. Herein, we report the current knowledge of both species of STEL.
3 Results and discussion
3.1 Objective one: literature search
The literature referencing H. lacerata and H. subcaudalis was comparatively sparse (N = 181 and 48, respectively; Table 1). Even within these papers, the vast majority of them only listed the species and its distribution as a reptile in Texas (N = 139, 77% of H. lacerata citations and N = 39, 81% of H. subcaudalis citations; Table 1), and did not offer natural history or ecological information concerning either species. Thus, even though our search identified >200 papers concerning STEL, < 50 papers contained information other than their distribution. We identified 57 citations that contained ecological data concerning STEL, which are included within our reference section. Our search results from the reference sections of published papers indicated that approximately 40% of the literature concerning STEL information would be considered ‘gray’ literature (i.e., non-published government reports and communications); however, such reports contained the majority of ecological knowledge of both species known to date.
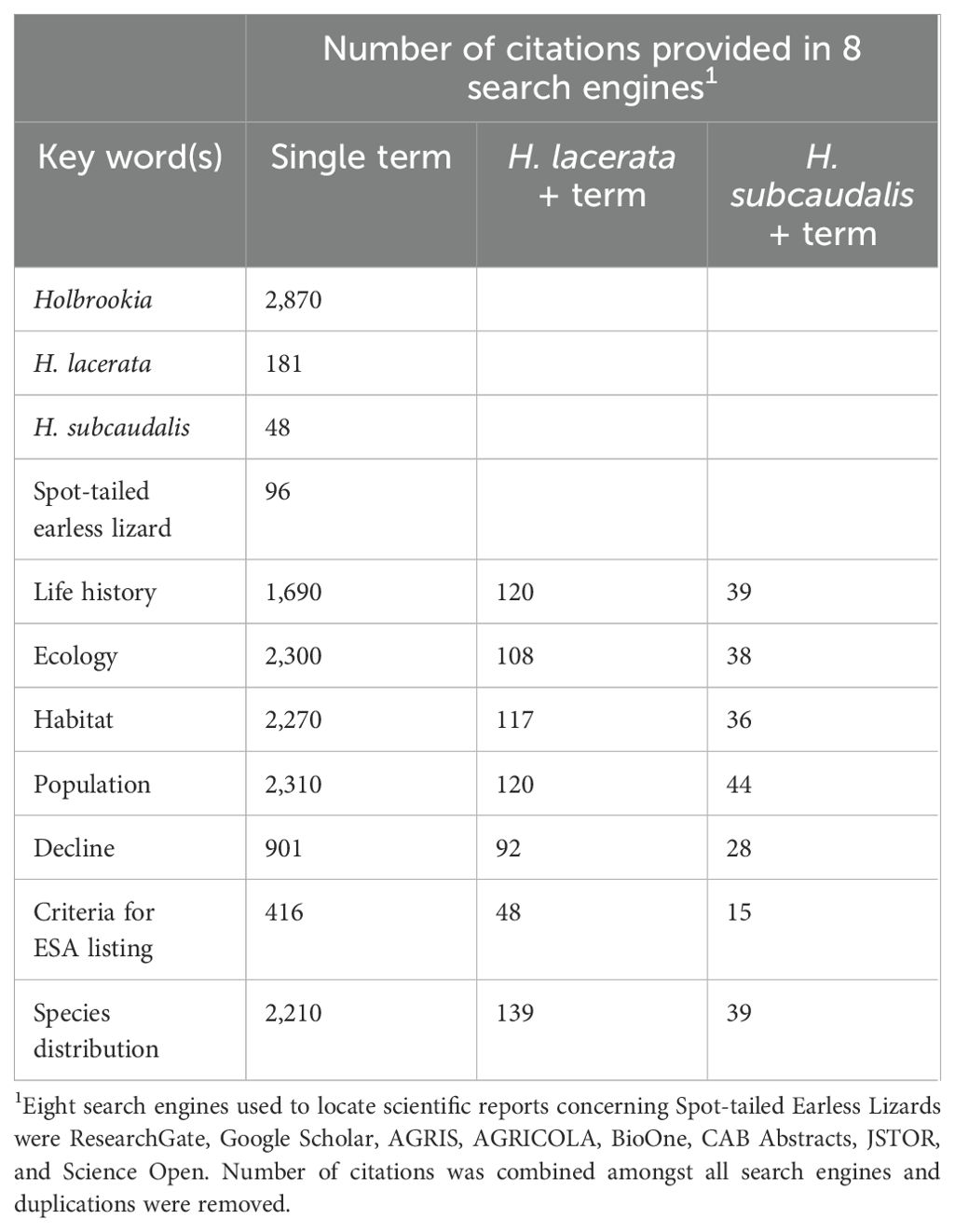
Table 1. Number of citations that occurred in a Google Scholar search by each single term and combination of terms with each scientific name for Spot-tailed Earless Lizards.
Conservation status of threatened and endangered species is imperative, but often, adequate knowledge of the species is lacking. Agencies must rely on unpublished reports to make assessments; however, gaining access to such reports can be difficult. Freedom of Information Act of 1967 allows public access to certain documents and data; however, a request form must be completed and the request must supply the exact document name and document number in order to be obtained. Thus, government reports and communications are often excluded from scientific literature due to inaccessibility. For this reason, we believe it is imperative to have a resource that consolidates the knowledge of STEL, from scientific and ‘gray’ literature, in order to advance the knowledge of these imperiled lizards.
3.2 Objective two: review of STEL knowledge
Both species of STEL have experienced sharp declines in population numbers and distribution and are considered as Near Threatened by the IUCN (Axtell, 1954, 1956, 1998, Duran and Axtell, 2010). Holbrookia lacerata was named a priority species in the Texas Wildlife Action Plan (Texas Parks and Wildlife Department, 2012) and The WildEarth Guardians (2010) petitioned for federal protection of STEL under the Endangered Species Act in 2010 (WildEarth Guardians, 2010). The United States Fish and Wildlife Service issued a 90-day finding stating that the petition presented substantial evidence that federal protection may be warranted. Since the time of the petition by The WildEarth Guardians (2010), the two subspecies of H. lacerata have been split into separate species (see section 3.4 Taxonomy; Hibbitts et al., 2019). On 6 February 2024, the United States Fish and Wildlife Service issued their findings that the Plateau STEL does not warrant listing as an endangered or threatened species because 13 populations have been identified, which constitute 91% of its historic range, and are currently in moderate to high condition (USFWS 2024). However, the United States Fish and Wildlife Service is investigating the status of Tamaulipan STEL separately and will make a determination as to the federal status of that species in the future.
3.3 Species description
3.3.1 Plateau STEL
Plateau STEL are a small (albeit medium compared to other species in the genus Holbrookia) lizard species measuring 50 – 55mm SVL and averaging 7 – 8g body mass (Hibbitts and Hibbitts, 2015). Overall, the lizard is cryptically colored and primarily tan-to-caramel with grey undertones (Hibbitts et al., 2019). Extending dorsally from the neck to the base of the tail, Plateau STEL have two rows of darker brown chevron-shaped blotches (Axtell, 1968). The center two rows of blotches run paravertebral and extend to the tip of the tail (Axtell, 1968). The outer two rows run dorsolateral, ceasing at the base of the tail (Axtell, 1968). These blotches are in noticeable contrast to the lizards’ primary colors; however, aid in camouflaging STEL against dirt and rocks. Beyond the large blotches, Plateau STEL also have darker pigmented scales scattered across their back providing a speckled effect (Axtell, 1968). The ventral side of the lizard is generally a white to off-white color with some specimens having striations of black pigment in the gular region (Axtell, 1968). The limbs of Plateau STEL follow the same pattern as the body, having a tan-to-caramel dorsum with blotches and a white venter (Axtell, 1968). Just under the dorsolateral fold, most specimens have a variable number of the namesake black spots on the ventral side of the tail (Axtell, 1968). The ventral tail spots are variable in number but are a dark black color starting just behind the cloaca and fading to grey as they proceed down the tail (Axtell, 1968). In 37% of males, the tail length exceeds the SVL, whereas, in females and juveniles of either sex, the tail is < SVL (Axtell, 1968). Generally, Plateau STEL are smaller, have shorter hind limbs, less spots on their legs, less spots on their sides, and have more connected dorsal blotches than Tamaulipan STEL (Figure 2A; Axtell, 1956).

Figure 2. Dorsal and ventral views of (A) Plateau (Holbrookia lacerata) and (B) Tamaulipan (H. subcaudalis) Spot-tailed Earless Lizards (STEL). Plateau STEL are smaller in size, tan-colored, and have fewer caudal spots than the larger, gray-colored, more caudal-spotted Tamaulipan STEL. (Photographs by Brian Loflin).
3.3.2 Tamaulipan STEL
Tamaulipan STEL are slightly larger than Plateau STEL, averaging 62 – 67mm SVL and a body mass of 10 – 12g (Hibbitts and Hibbitts, 2015; LaDuc et al., 2018; Hibbitts et al., 2019). Overall, the species is a slate gray color. Tamaulipan STEL exhibit darker chevron-shaped blotches, though the contrast to the rest of the body is not as extreme as that of Plateau STEL (Axtell, 1956). Dorsal blotch shape is fused in Plateau STEL and unfused in Tamaulipan STEL (LaDuc et al., 2018). The center two rows of blotches run paravertebral and extend to the tip of the tail (Axtell, 1968). The outer two rows run dorsolateral, ceasing at the base of the tail (Axtell, 1968). In addition to the large blotches, there are also darker pigmented scales scattered across the dorsum giving a speckled effect (Axtell, 1968). The ventral side of these species is generally a white to off-white color with some specimens having striations of black pigment in the gular region (Axtell, 1968). The dorsal and ventral pattern and ventral tail spots of Tamaulipan STEL are similar to that of Plateau STEL (Axtell, 1956). In 80% of males the length of the tail is greater than the SVL, whereas in females and juveniles it is not. Generally, Tamaulipan STEL have longer rear limbs, more spots on their legs, more femoral pores (approximately four more femoral pores than Plateau STEL), more lateral spots, and fewer connected dorsal blotches than Plateau STEL (Figure 2B; Axtell, 1956; LaDuc et al., 2018).
3.3.3 Lack of external ears
These species lack an external auditory canal; however, they do possess the internal ear structure that is observed in other lizards (Earle, 1961). This lack of an external auditory canal is thought to have evolved as a result of the burying behavior characteristic to the group of lizards known as “Sand Lizards” (Genera Uma, Callisaurus, Cophisaurus, Holbrookia; Earle, 1961). In addition to the lack of an external auditory canal, other subtle traits that aid in burying are elongation of the skull and reduction of the mandible, creating a shovel-like head shape (Earle, 1961). Similar to their head shape, the lack of an external ear reduces drag in the substrate (Earle, 1961).
3.3.4 Breeding colors
At the end of brumation, breeding season begins immediately (i.e., March – August, with peaks in April and July) likely as a result of ovulation, during which female Plateau STEL and Tamaulipan STEL develop vibrant yellow and green colors that are concentrated on the gular region of the lizards and fade posteriorly (Duran, 2017). This color lasts from the initial emergence from brumation in March through August. The colors are most vibrant laterally on the neck and shoulders and fade down the dorsolateral fold to the base of the tail (Duran, 2017). In Plateau STEL this is typically accompanied by a bright orange color on the gular region (Figure 3), which does not extend as far along the body as the yellow-green colors (Duran, 2017). One study found that 87% of female Plateau STEL and 19% of male Plateau STEL exhibited orange coloration in the gular region (LaDuc et al., 2018). In some cases, the orange coloration can appear red (E. D. Rangel, pers. observ.). This orange coloration is completely absent in Tamaulipan STEL (Axtell, 1956). In Tamaulipan STEL; however, males occasionally will develop a slight yellow-green hue along their dorsolateral fold, but not as extensively as females (Axtell, 1956).

Figure 3. Reproductive colors of the (A) female Plateau (Holbrookia lacerata) Spot-tailed Earless Lizard (STEL) displaying the bright yellow-green body and orange gular colorations. (B) Female Tamaulipan (H. subcaudalis) STEL also display the yellow-green body coloration, but it is more subdued (faded) compared to the Plateau STEL. Tamaulipan STEL do not display the orange-red gular color during reproductive periods. (Photographs by Brian Loflin).
3.4 Taxonomy
The taxonomic classification of both species of STEL are Domain Eukaryota, Kingdom Animalia, Phylum Chordata, Subphylum Vertebrata, Class Reptilia, Order Squamata, Suborder Iguania, Family Phrynosomatidae, Genus Holbrookia, and Species H. lacerata (Plateau STEL) and H. subcaudalis (Tamaulipan STEL). The genetic distinction and accompanying taxonomic classifications of the genus Holbrookia have been debated since first described by Cope (1880). There was some early confusion as a result of Cope (1880) including two distinct species (H. lacerata and H. maculata) under the single specific name Holbrookia lacerata. This confusion was exacerbated further when Stejneger (1890) reduced the then newly described species, Holbrookia lacerata, to subspecies status under Holbrookia maculata (Axtell, 1956). The first comprehensive review of the genus Holbrookia was put forth by Schmidt and Dickerson (1922), where even then they acknowledged difficulty in distinguishing between Plateau STEL and other Holbrookia species, including Tamaulipan STEL (Axtell, 1956). Duran (2017) noted that some early confusion may have arisen from four Plateau STEL specimens that were sent from Comanche County, Texas (i.e., the most northern locality) in 1870, to the Academy of Natural Sciences of Philadelphia (ANSP), which were actually Lesser Earless Lizards (H. maculata). However, those specimens remain as Plateau STEL in the ANSP collection today.
Schmidt and Dickerson (1922) documented distinct features of Plateau STEL, but in reference to the previous publications of Cope (1880) and Stejneger (1890), deemed them variable. Schmidt and Dickerson (1922) did, however, refute the subspecies status and referred to Plateau STEL as a full species. Axtell (1956) acknowledged the geographic separation of the presence of subcaudal spots amongst Holbrookia lizards. While Axtell (1956) was originally examining specimens from the Edwards Plateau region of Texas, he was able to acquire specimens, which he recognized as Plateau STEL from 10 counties in southern Texas and three localities from the adjacent state of Coahuila, Mexico. Axtell (1956) proposed these southern examples be given the subspecies designation of Holbrookia lacerata subcaudalis. This designation, persisted until Hibbitts et al. (2019), who used molecular genetic evidence, species morphology, and environmental niche of both subspecies, as a means to recommend full species status for Plateau STEL and Tamaulipan STEL. Originally conveyed in a report by LaDuc et al. (2018) and later published by Roelke et al. (2018), mitochondrial NADH dehydrogenase subunit 2 gene (ND2) was selected as it was informative in reconstructing diversification patterns within the sand lizard clade of Phrynosomatidae, and Roelke et al. (2018) found that Plateau STEL (i.e., including H. l. lacerata and H. l. subcaudalis) encompassed three strongly supported, reciprocally monophyletic lineages (northern, southeastern, and southwestern groups). They found that the evidence across data sources and methods of analyses was sufficient to raise both subspecies to full species status, designating the northern lineage as Plateau STEL and the two southern lineages as Tamaulipan STEL (Hibbitts et al., 2019). With this taxonomic change and the petition for federal protection, the USFWS decided to evaluate Plateau STEL and Tamaulipan STEL separately as it pertains to ESA listing. Most recently, Firneno et al. (2024) supports the recognition of Plateau and Tamaulipan STEL as two separate species, based on strong phylogenetic and genomic divergence and species delimitation that exceed those of other recognized species within the Holbrookia genera.
3.5 Distribution
Originally, Plateau STEL were considered a Great Plains prairie species whose range extended northward to Comanche County, Oklahoma (Webb and Ortenberger, 1953), across central and southern Texas and into the Mexican states of Coahuila, Nuevo Leon, and Tamaulipas (Axtell, 1968; Conant and Collins, 1991; Dixon, 2000; Texas Parks and Wildlife Department (TPWD), 2005; Hammerson et al., 2007). The two species of STEL are now thought to be geographically split along the Balcones Escarpment, with Plateau STEL to the north and Tamaulipan STEL to the south (Hibbitts et al., 2019). The distribution of Plateau STEL extends north to the Colorado River, east to the edge of the Balcones Escarpment and west to the Pecos River and southern edge of the Llano Estacado (Texas Parks and Wildlife Department (TPWD), 2013, LaDuc et al., 2018). The distribution of Tamaulipan STEL includes populations south of the Balcones Escarpment in Texas, west to the Sierra Madre Oriental mountains in Coahuila, Nuevo Leon, and Tamaulipas, Mexico (LaDuc et al., 2018). In combination, STEL historically ranged in Texas from Del Rio to Corpus Christi and from the Rio Grande Valley and the adjacent states in Mexico to north of Odessa, Texas (Axtell, 1968). STEL formerly occurred in the Gulf Coast Prairies ecoregion, but they are believed to have been extirpated in the southeastern portion of their former range as well as from urbanized counties of Bexar, Comal, Hayes, and Travis counties, Texas (Axtell, 1968; Duran and Axtell, 2010; LaDuc et al., 2018). Sometime after 1970, STEL populations experienced a sharp decline (Axtell, 1998; Duran and Axtell, 2010; Duran et al., 2011; LaDuc et al., 2018). When comparing the historic ranges reported by Axtell (1954, 1956) with contemporary estimates, the range of Plateau STEL has shrunk by 39% (Hibbitts et al., 2021). In addition, Tamaulipan STEL were reported in 21 Texas counties, then known to occur in San Patricio, Nueces, Live Oak, McMullen, Kleberg, Jim Wells, Zapata, and Val Verde counties, Texas, as recently as 2005 (Texas Parks and Wildlife Department (TPWD), 2005). Presently, there are only three known populations of Tamaulipan STEL remaining: one in the Agua Dulce and Bishop area (Nueces County, Texas), another approximately 20 km west in Jim Wells County, Texas, and what is thought to be the last population in the Del Rio and Brackettville (Val Verde and Kinney counties, respectively) areas (LaDuc et al., 2018; Rangel, 2023). Rangel (2023) found one juvenile Tamaulipan STEL in McMullen County during a 2022 survey; however, the property was within a future strip mine zone and will likely no longer be viable habitat in the near future. Therefore, Tamaulipan STEL appear to have undergone a substantial reduction in distribution, with nearly 400 km between populations with no geographically intermediate populations (iNaturalist.org, 2024; LaDuc et al., 2018). Plateau STEL, while still severely reduced and isolated, has more known metapopulations in West-Central Texas. Plateau STEL are currently thought to persist in Edwards, Real, Crocket, Schleicher, Sutton, Kimble, Mason, McCulloch, Tom Green, Reagan, Irion, Glasscock, Sterling, Coke, and Runnels counties (LaDuc et al., 2018). Kahl et al. (2022) added nine sightings of Plateau STEL in Concho County, Texas, during 2021. Duran (2017) noted that the one record of Plateau STEL from Mason County (Duran et al., 2012) could not be verified in subsequent surveys following the initial observation. LaDuc et al. (2018) reported an average relative abundance of 0.28 STEL/hour during 623 hours of driving surveys. However, LaDuc et al. (2018) noted that flooded fields during their 2015 surveys may have forced STEL onto roads, which could have caused increased STEL numbers during their driving surveys. In addition, LaDuc et al. (2018), on average, encountered twice as many Plateau STEL during driving surveys (i.e., 0.49/hr) than Tamaulipan STEL (i.e., 0.24/hr) during 2015 – 2017. Kahl et al. (2022), only considering surveys where STEL were found, calculated a relative abundance of 1 STEL per 20.8 minutes; however, they did not report a standard error with their estimate.
Recapture rates of marked STEL have been low regardless of year or survey method (LaDuc et al., 2018). Axtell (1958, 1998) reported boom and bust cycles in STEL populations. A cyclic trend in populations was also observed by Duran (2017) who noted that he found numerous STEL in 2015 where populations had not been found for decades, but he was unable to locate a single individual in the same places during the following two years.
3.6 Natural history
Little is known regarding the natural history of Plateau and Tamaulipan STEL. Both species are small, cryptic, and seldom encountered, which has resulted in limited scientific literature (Hibbitts et al., 2019). Both Plateau and Tamaulipan STEL are diurnal and their activity is primarily limited to the warmest time of day, typically between 1100 – 1600 hrs (LaDuc et al., 2018). This is different than many Phrynosomid lizards, which display bimodal activities during the summer with peak activity after sunrise and before sunset (Henke and Montemayor, 1998). Henke et al. (2023) noted that STEL emerge from underground burrows during daytime hours subsequent to dispersal of morning cloud cover, increase in ambient temperature, and during peak ultraviolet light (UV) and light intensity levels. Thus, STEL rarely are found aboveground before 1100 hr, which is atypical of many ecotherms (Rangel, 2023). However, due to STEL behavior of frequently burying and emerging during the diurnal period, Henke et al. (2023) noted that approximately only 42% of the potential population of STEL were aboveground at any point in time.
STEL historically occupied grasslands but showed a preference for the comparatively open areas within their environment (LaDuc et al., 2018). Today, the majority of observations occur in areas of regular disturbance, such as row crop agricultural fields, the regularly mowed airfield of Laughlin Airforce Base (LAB), and private lands under prescribed fire regimens (Hibbitts et al., 2021; Rangel, 2023; Henke et al., 2023). It also has been theorized that they exhibit a boom-and-bust cycle, and thus can vary in presence in a particular area from year to year (Axtell, 1958, 1998, Duran, 2017; Hibbitts et al., 2021).
LaDuc et al. (2018) found that STEL may actively forage at dawn and dusk because stomach contents of STEL contained legs of Sun Spiders (Order: Solifugae) and Carpenter Ants (Camponotus sp.), both of which are crepuscular or nocturnal in their activity. Tamaulipan STEL are believed to cease activity when temperatures drop below 28°C; however, Kahl et al. (2022) believed that the temperature threshold for STEL was lower than previously thought, but did not state a lower threshold level.
Activity period for STEL varies and may be species and location dependent. LaDuc et al. (2018) found STEL active between April – September with gravid females detected in early April – late July and juveniles active from mid-June – September. Only surveying Tamaulipan STEL, Rangel (2023) found STEL active from March – December, but adult STEL appeared to enter brumation earlier (September – October) than juveniles, which remained active, albeit in low population numbers as late as the end of December. Hatchling Tamaulipan STEL were observed by late May, had two population peaks (i.e., one in June and another in August), and most likely obtained juvenile size by October (Rangel, 2023).
Plateau and Tamaulipan STEL have been reported as having home ranges as large as 2.1 ha and 7.1 ha, respectively, which is large in comparison to similarly sized insectivorous lizards (Hibbitts et al., 2021). However, Henke and Eversole (2021) using a crossover statistical design, noted STEL maintained much smaller home ranges when undisturbed, but fled when approached and did not return to the original site; thus, giving the appearance of larger home ranges.
3.7 Reproduction
There is a dearth of information about the reproductive habits of STEL. When the lizards emerge in spring, females display breeding colors that include vibrant yellows and greens predominantly in the gular region but also along the dorsolateral fold (Duran, 2017). In Plateau STEL this includes bright red-orange on the gular region (Duran, 2017). This red-orange color is not present in Tamaulipan STEL (Axtell, 1956). It is unclear whether these vibrant colors in female STEL are a signal to attract males, or simply a byproduct of the hormones associated with reproduction. These colors have been found to play a role in sex recognition in the closely related Keeled Earless Lizard (Holbrookia propinqua; Cooper, 1984). Male Plateau and Tamaulipan STEL typically do not exhibit much color change; however, there have been documented cases of some males becoming “somewhat yellowish” (Axtell, 1956). Both STEL species are known to double clutch, lay their eggs underground, and eggs hatch after 4-5 weeks of incubation (Fitch, 1970). During the initial sightings after emergence from brumation, most females are already gravid suggesting some method of sperm storage from the previous summer (Axtell, 1956). Axtell (1956) noted that in females observed early in the season, most had two complements of eggs: one group of large ova and a second set of smaller ones. The first clutch is laid in May and June and the second clutch in July and August (Axtell, 1956). Axtell (1956) also asserted that the clutch size varied with age, in that yearling females will have 4 – 6 eggs in the first clutch and 5 – 7 in the second; whereas, an older female will have 7 – 12 eggs in both clutches (Axtell, 1956). The clutches observed by Axtell (1954, 1956) hatched roughly five weeks after laying. The number of eggs produced by these lizards is remarkable with consideration to their stature. On average, the eggs laid in the second clutch are usually larger than those of the first, with average lengths of 14.6 mm and 12.9 mm, respectively (Axtell, 1956). Hatchlings averaged 20.5 ± 0.5 mm SVL (Axtell, 1956). Both eggs and offspring are slightly larger when clutch size is smaller (Axtell, 1956). Both species are considered to have boom-bust population cycles, with booms thought to occur more frequently during wet years (Axtell, 1954; Duran, 2017).
3.8 Growth
Spot-tailed Earless Lizards are a remarkably fast-growing species, reaching adult size in their first year of life. Hatchlings emerge at 20 – 21 mm SVL (Axtell, 1956). Both Plateau and Tamaulipan STEL grow rapidly, reaching average adult minimum lengths of 59 mm and 61 mm SVL, respectively, within their hatchling season (Hibbitts and Hibbitts, 2015). Females will breed soon after reaching the minimum size for reproduction (Pianka and Parker, 1972). Captive female STEL raised at the Fort Worth Zoo have laid their first eggs as early as six months old, but this may have been a result of artificially eliminating brumation (D. Barber, pers. comm. to Duran, 2017).
3.9 Longevity and survival
There is a dearth of information concerning the survivability and longevity of STEL. However, because they are Phrynosomatids, researchers speculate that STEL display similar survivorship patterns and longevity as that of Texas horned lizards (Phrynosoma cornutum). A population viability analysis for STEL using the software VORTEX found that changes in the mortality rates of hatchlings, followed by age at first reproduction, percentage of female breeding, female sex ratios at birth, and clutch size had the strongest effects on population growth rates and extinction risk (Ayala, 2023). Severe drought was noted to affect STEL reproduction, which can cause population crashes due to low rates of juvenile recruitment (Ayala, 2023). Simulations of anthropogenic impacts showed that small increases (e.g., ~2%) can exacerbate extinction risk even for stable STEL populations (Ayala, 2023).
3.10 Mortality
There is no published literature regarding mortality rates of STEL; however, there have been a few reports of individual mortality events. Hit-by-vehicle is commonly offered as a potential mortality factor; however, road surveys are the common survey method (Hibbitts et al., 2021); thus, death by vehicle is considered logical. Vehicular death is a major factor when assessing the effect of urbanization, agriculture, and oil and gas development on STEL, as all of these factors either create roads through habitat, or increase traffic on existing roads (Wolaver et al., 2018). Literature documenting cases of depredation on either species is extremely limited. To our knowledge, there are only four documented depredation events on Plateau and Tamaulipan STEL: two by snakes (i.e., Chihuahuan Nightsnake (Hypsiglena jani) that ate a Plateau STEL (Walkup et al., 2018), and a Checkered Garter Snake (Thamnophis marcianus) that consumed a Tamaulipan STEL (Rangel et al., 2023a); one by a Rio Grande Ground Squirrel (Ictidomys parvidens) (Neuharth et al., 2018a), and an experimental population held in an open, outdoor enclosure lost nearly 80% of their STEL population (i.e., a mix of 40 Plateau and Tamaulipan STEL) to Loggerhead Shrikes (Lanius ludovicianus) during a two-week period (Rangel et al., 2023b).
Diseases and parasites have been considered as a potential threat to STEL populations (WildEarth Guardians, 2010). However, to date, to our knowledge no studies have investigated diseases and parasites of STEL.
3.11 Habitat
Both species of STEL are considered grassland species, and are commonly found in the grassy areas of south and central Texas, which is characterized by Oak-Juniper (Quercus fusiformis and Juniperus ashei) woodlands, ploughed fields, and Mesquite (Prosopis glandulosa) brushlands (Kahl et al., 2022). Even though STEL have a propensity to be linked to grasslands, STEL seem to prefer the comparatively more open areas within ecosystems (Hibbitts et al., 2021). LaDuc et al. (2018) noted they found STEL in relatively undisturbed, but grazed grasslands with minimal woody encroachment and in heavily disturbed agricultural fields. The lizards avoid obstructions such as waterways, buildings, and pavements (WildEarth Guardians, 2010). STEL appear to be an early successional species that prefer areas of bare ground created by disturbance, the presence of small forbs and grasses, areas of low woody encroachment, and areas that lack canopy cover or trees (Axtell, 1954, 1958, Duran, 2017; Hibbitts and Ryberg, 2018; LaDuc et al., 2018). Rangel (2023) also found STEL in similar locations as LaDuc et al. (2018), but noted the relative abundance of STEL was greater along the periphery of crop fields. Rangel (2023) also found a population of Tamaulipan STEL along the runways of Laughlin Airforce Base near Del Rio, Texas, and noted the grassland was highly disturbed because it was mowed several times per week. Preferred soils are considered loam, clayey loam, loamy clay, and clay in nearly flat areas (<3% slope; LaDuc et al., 2018). A disparity exists within the scientific literature concerning STEL preference of soil compaction (Hibbitts et al., 2021; Rangel et al., 2022c); however, STEL have been documented to use a variety of compactness ranging from the ploughed, loose soil in agricultural fields (Rangel et al., 2022c), to concrete of the runways at Laughlin Airforce Base (Neuharth et al., 2018b; Hibbitts et al., 2021). Additionally, STEL habitat consisted of a moderate variety of soil composition, ranging from the clay to clay-loam soils of the Edwards Plateau to the sandy-clay soils of the Gulf Coast (Axtell, 1968). However, they have not been documented to occur on pure sandy soils (Hibbitts et al., 2019). Amongst these habitats, STEL can be found basking in full sun at the peak UV index of the day on rocks, clumps of dirt, or small corn stalks (LaDuc et al., 2018). In congruence with being terrestrial, these basking spots are never more than 0.3 m off the ground, except in the Tamaulipan STEL population on Laughlin Airforce Base, where male Tamaulipan STEL have been documented perching as high as 3 m off the ground on fence posts (Owen et al., 2023). Beyond the newly documented perching behavior of a single population, STEL have been found on, or close to the ground, and utilize vegetation, rocks, detritus, rodent holes, or shimmying into loose soil as cover (Owen et al., 2023; Duran and Yandell, 2014).
3.12 Diet
Most of what is known regarding STEL dietary habits has come from the exploration of museum specimen stomachs performed by LaDuc et al. (2018). In one of the early studies of STEL diet, Axtell (1954) found that it primarily consisted of “hopping and flying insects” and in captivity STEL accepted moths. Axtell (1954) also noted that movement was requisite for consumption to occur and that STEL were visual predators. Rangel (2023) confirmed the observation reported by Axtell (1954), noting that captive STEL would not consume dead crickets. Rangel et al. (unpubl. data) glued dead crickets to a thin microfilament wire and slowly pulled the cricket across the floor of an aquarium, upon which STEL responded by attacking and consuming the cricket; thus, noting the importance of prey movement for STEL. The diet of STEL was confirmed by LaDuc et al. (2018) via dissection of 129 museum specimens, in which they described STEL as an opportunistic generalist after finding a variety of arthropods in the stomach contents. Grasshoppers accounted for the majority of stomach contents (42.0%) by volume across sex and age categories (i.e., ~65% of volume for male STEL), followed by beetles (12.4%), ants and wasps (12.0%), termites (10.1%), caterpillars and spiders (9.3% each), true bugs (4.4%), and centipedes (0.5%; LaDuc et al., 2018). Females contained a higher proportion of beetles than did males or juveniles (LaDuc et al., 2018). Juveniles had the most even diet composition compared to adult males and females, where the major diet item by volume was grasshoppers, while other taxa were represented in relatively equal proportion (LaDuc et al., 2018). Juveniles also consumed the largest number of individual termites, though by volume they did not exceed that of grasshoppers (LaDuc et al., 2018). Juvenile STEL also ate a large number of Springtails (Order: Collembolan) and Harvestmen (Order: Opilone), both very small and most likely overlooked by adult STEL as a sufficient food source (LaDuc et al. (2018). Also, LaDuc et al. (2018) noted that juvenile STEL did not consume caterpillars like what was found for adult STEL. LaDuc et al. (2018) noted that juveniles were not as adept at climbing on vegetation or at foraging within vegetation as an explanation for the lack of consumption of caterpillars by juvenile STEL. LaDuc et al. (2018) reported that there seemed to be little difference between Plateau STEL diet and that of Tamaulipan STEL, but acknowledged that Tamaulipan STEL dietary habits were less clear due to low sample size.
3.13 Movements
Research regarding STEL movements is limited. Hibbitts et al. (2021) reported a telemetry study performed on Tamaulipan STEL at Laughlin Airforce Base and Plateau STEL on a private ranch in Crockett County, Texas. They found that Tamaulipan STEL movements were predominantly along the runways and roads on Laughlin Airforce Base (Hibbitts et al., 2021). Movements of Plateau STEL also were concentrated along road edges and oil well pads (Hibbitts et al., 2021). Amongst the STEL that met their criteria for estimating home range, they found two areas of concentrated use in six of nine Plateau STEL, and two of 11 Tamaulipan STEL (Hibbitts et al., 2021). No significant differences were found pertaining to movement or home range between male and female Plateau and Tamaulipan STEL (Hibbitts et al., 2021). Overall, Tamaulipan STEL had significantly larger home ranges and movements than Plateau STEL (Hibbitts et al., 2021). They also found that amongst the Plateau STEL home ranges, there was limited home range overlap (Hibbitts et al., 2021). They found a few instances of female-male and female-female overlap, but no male-male overlap. For Tamaulipan STEL, overlap was much greater (Hibbitts et al., 2021). On average, any given Tamaulipan STEL overlapped with 4 – 6 other STEL (Hibbitts et al., 2021). Further in contrast to Plateau STEL, they found examples of female-female, female-male, and male-male overlap in Tamaulipan STEL (Hibbitts et al., 2021). Hibbitts et al. (2021) believed that such overlap indicated the species is not territorial. It was also mentioned that they had observed mate-guarding behavior in Tamaulipan STEL, and suggested it was a non-territorial species because of this behavior. Using the Minimum Convex Polygon (MCP) method, Hibbitts et al. (2021) found Plateau and Tamaulipan STEL to have 2.1 ha and 7.1 ha home ranges, respectively. These are particularly large home ranges when compared to other similarly sized lizard species. This could be a result of the correlation with the roads, runways, and well pads, as these features open up the habitat at a landscape scale and could extend the species’ movement and home range (Hibbitts et al., 2021). Although a small sample size, LaDuc et al. (2018) estimated home range using the MCP method for STEL to be 0.35 ± 0.1 ha, much smaller than estimated by Hibbitts et al. (2021). Henke et al. (2023) hypothesized that STEL most likely have home ranges of <50 m2, but flee when approached by researchers and do not return to their site of origin; thus, multiple disturbances by researchers during telemetry studies create what appears to be a larger estimates of home range.
3.14 Survey methodology
Detection of rare and cryptic species is notoriously difficult. Pertaining specifically to herpetofauna, vehicular surveys (a.k.a. “road cruising”, “driving surveys”) are a predominant method of search due to reptiles’ ectothermic physiology drawing them to roads, which absorb and retain the heat of the sun better than dirt or plant matter (Hibbitts et al., 2021). Hibbitts et al. (2021) claimed that road cruising was 32 times more efficient than walking surveys for Plateau STEL and twice as efficient as walking surveys for Tamaulipan STEL. They suggested this was likely due to STEL’s cryptic nature and aptitude to flee. While driving, surveyors can generally spot the lizards from a further distance; whereas, while walking, STEL likely see surveyors before they are detected and accounted for. However, it is worthy to note that individual surveyors differ in their ability to locate objects (Henke, 1998). LaDuc et al. (2018) noted that walking surveys were less successful than driving surveys for STEL. However, there is disagreement as to the speed at which to conduct the driving surveys. Hibbitts and Ryberg (2018) state a slow speed of 24 kph can be conducted, while Rangel (2023) state that speed should be <15 kph; otherwise, some STEL will not be detected. In addition, Rangel et al. (2022a) noted that by driving slowly (i.e., < 10 kph), surveyors can hear movement in the vegetation and ground litter that often resulted in STEL detection, which was not possible at increased speeds. Rangel et al. (2022a) found that both STEL species will allow a vehicle to approach at a closer distance before fleeing than if approached by a human on foot. Rangel et al. (2022a) also noted that upon fleeing, STEL will run further when startled by a human on foot than they will when startled by a vehicle. STEL also are wary and exhibit efficient predator evasion via burying behavior (Neuharth et al., 2018b; Rangel et al., 2022b). There is also a discrepancy in tolerance between the two species in that Plateau STEL are more tolerant to approach than Tamaulipan STEL and upon fleeing, Tamaulipan STEL will run >2X as far as Plateau STEL (Rangel et al., 2022a). This greater tolerance of approach by vehicles than by humans on foot, in combination with the lizards being easier to see on a road’s more uniform surface makes road cruising a superior method of detection for both species of STEL (Rangel et al., 2022a).
Rangel (2023) evaluated nine standard reptile search techniques (i.e., pitfall traps, funnel traps, rock mound searches, cover board surveys, remote camera surveys, use of detector dogs, environmental DNA presence, visual systematic searches, and road cruising surveys) at five known STEL densities (5, 10, 20, 30, and 40 STEL/ha) and only visual systematic surveys and road cruising surveys were successful methods. A density threshold was not apparent nor was the number of STEL observed useful in predicting the density of STEL (Rangel, 2023). Henke et al. (2023) determined that high relative humidity, high UV light intensity, and humic substrate, all common to southern Texas, degrade eDNA too quickly for eDNA to be a practical survey method. Similar results were noted for detector dogs, which were unable to locate buried STEL (Henke et al. (2023).
Hibbitts and Ryberg (2018) reported a discrepancy between years as to the best months to survey STEL. During 2015 and 2016 surveys, Hibbitts and Ryberg (2018) stated April through June yielded the most successful surveys; however, during 2017, the hotter and drier months of July and August provided the best STEL results.
3.15 Proposed factors for population number and distributional decline
Axtell (1968, 1998) and Duran and Axtell (2010) noted a sharp population decline of Plateau STEL after 1970. Brush encroachment, invasive grasses, use of pesticides and herbicides in agricultural practices, urbanization and fragmentation by road construction, and predation have been proposed as the most likely potential causes for the observed decline (LaDuc et al., 2018; Kahl et al., 2022). Land use changes from native land to croplands, grazing land, land development, and invasion by red-imported fire ants were considered the most serious threats to STEL survival (WildEarth Guardians, 2010; United States Fish and Wildlife Service (USFWS), 2011). Although development of oil and gas pads was the cause of 48% of the land-use change in western and central Texas, such change only amounted to 0.5% of the land mass (LaDuc et al., 2018). However, LaDuc et al. (2018) did state that future drilling and associated oil pad construction could cause reductions in important STEL habitat such as grasslands and row crops. Urbanization is typically listed as a concern for reptile populations (French et al., 2018); however, considering the current distributions of STEL, only the Plateau STEL surrounding San Angelo, Texas, would most likely be threatened by urbanization (LaDuc et al., 2018). The potential of agricultural practices as a driver of STEL decline warrants investigation but is likely a minor contribution to the decline of STEL populations. Currently, the vast majority of STEL sightings occur in agricultural fields (Kahl et al., 2022). The practices of concern are the regular ploughing of fields, and the spraying of herbicides and pesticides. Because STEL spend the majority of time on or in the soil, the potential for them to be killed by ploughs or agricultural equipment is likely high. The various chemical and fertilizer applications utilized by farmers appear to be a larger potential threat. It is assumed that the lizards come into contact with the pesticides and herbicides; however, it is unknown to what degree, if any, mortality occurs. Greater potential risks to STEL include the elimination of the prey base and the possibility of non-lethal carry-over effects such as reduced or loss of fertility.
Considering that STEL are thought to historically inhabit native grasslands, the ever-increasing percentage of invasive grasses could potentially be a significant problem (Hibbitts et al., 2019). It has been observed that even among native grasslands, STEL prefer comparatively more open areas (Hibbitts et al., 2019). As invasive grasses occupy areas and form dense monocultures, they could potentially be eliminating STEL by inhibiting their ability to navigate the landscape and find proper microhabitats. The shifting of grass species composition, particularly in the case of monocultures, also affects the types of insect fauna present on the landscape (Bezemer et al., 2014). It could be possible that the changing grass composition could be extirpating a key component(s) of STEL diet.
Historically, the vast majority of STEL habitat was grassland, interspersed with patches of woody cover, which would seasonally burn, creating a patchwork mosaic across the landscape. A significant portion of these grasslands has now been lost to brush encroachment. Between 1937 and 1999 alone, untreated areas have seen a 5-fold increase in woody cover (Asner et al., 2003). Brush, once established, is difficult and expensive to manage and it requires multiple management techniques to reduce and control (Asner et al., 2003). Brush encroachment often results in drastic changes to the natural disturbance regime. Interestingly, the areas where STEL have persisted experience regular disturbance (Hibbitts et al., 2021). Whether this is constantly ploughed agricultural fields or the weekly mowed airstrips on Laughlin Airforce Base, STEL appear to only be consistently found in areas of regular disturbance.
LaDuc et al. (2018) developed several model scenarios, which included climate change, to predict the effect on STEL. Within their climate change model. they predicted a substantial decline in the range of Plateau STEL during the remainder of the 21st century, a loss of all available habitat for the southwestern population of Tamaulipan STEL, and a range expansion for the southeastern population of Tamaulipan STEL. However, the United States Fish and Wildlife Service (USFWS) (2024) did not conclude that this model provided enough evidence to warrant a ‘threatened’ status “within the foreseeable future,” as expressed by the Endangered Species Act (United States Fish and Wildlife Service (USFWS), 2019).
4 Conclusions
After reviewing the available literature, a singular cause for the shrinking geographic distribution of Plateau and Tamaulipan STEL is not evident. Among the factors proposed by previous studies, brush encroachment, invasive grasses, and the resulting ecosystem change seem more substantial than others. However, it is likely that the decline stems from multiple factors, and other processes working in tandem.
Our goal is for future researchers to benefit from this consolidation of information concerning Plateau and Tamaulipan STEL and apply it to current and future STEL conservation.
Author contributions
ER: Writing – original draft. SH: Writing – review & editing, Writing – original draft, Supervision, Project administration, Funding acquisition, Conceptualization. RA: Writing – original draft, Resources. CE: Writing – review & editing, Funding acquisition, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by Texas Comptroller of Public Accounts.
Acknowledgments
We thank Christin Moeller and Duston Duffie for their contributions to earlier drafts of this review, and the Texas Comptroller of Public Accounts for financial support of the project. This is contribution number 24-109 of the Caesar Kleberg Wildlife Research Institute.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Asner G. P., Archer S., Hughes R. F., Ansley R. J., Wessman. C. A. (2003). Net changes in regional woody vegetation cover and carbon storage in Texas drylands 1937–1999. Global Change Biol. 9, 316–335. doi: 10.1046/j.1365-2486.2003.00594.x
Axtell R. W. (1954). The systematic relationships of certain lizards in two species groups of the genus Holbrookia. University of Texas, Austin, Texas, USA, 57 pp. Master’s Thesis.
Axtell R. W. (1956). A solution to the long neglected Holbrookia lacerata problem, and the description of two new subspecies of Holbrookia. Chicago Acad. Sci. 10, 163–181.
Axtell R. W. (1958). A monographic revision of the Iguanid genus Holbrookia. University of Texas, Austin, Texas, USA, 222 pp. PhD dissertation.
Axtell R. W. (1968). Holbrookia lacerata. Catalogue of American amphibians and reptiles, (CAAR), Vol. 56. 1–2.
Axtell R. W. (1998). Holbrookia lacerata cope. Interpretative atlas Texas lizards. (Self-published) 20, 1–12.
Ayala R. A. (2023). A population viability analysis of the Plateau (Holbrookia lacerata) and Tamaulipan (Hobrookia subcaudalis) spot-tailed earless lizards. Texas A&M International University, Laredo, Texas, USA, 111 pp. M. S. Thesis.
Bezemer T. M., Harvey J. A., Cronin. J. T. (2014). Response of native insect communities to invasive plants. Annu. Rev. Entomology 59, 119–141. doi: 10.1146/annurev-ento-011613-162104
Conant R., Collins J. T. (1991). A field guide to reptiles and amphibians: Eastern and central North America. 3rd ed. (Boston, Massachusetts, USA: Houghton Mifflin Company), 616 pp.
Cooper J. R. W. E. (1984). Female secondary sexual coloration and sex recognition in the keeled earless lizard, Holbrookia propinqua. Anim. Behav. 32, 1142–1150. doi: 10.1016/S0003-3472(84)80230-4
Cope E. D. (1880). No.17 – On the zoological position of Texas Vol. 20 (Washington DC: Washington Government Printing Office. Bulletin of The United States National Museum), 1–51.
Dixon J. R. (2000). Amphibians and reptiles of Texas. Second edition (Texas, USA: Texas A&M University Press, College Station), 460 pp.
Duran C. M. (2017). “A survey of a subset of historically known localities of the spot-tailed earless lizard (Holbrookia lacerata),” in Final Report to Texas Parks and Wildlife Department and The U. S. Fish and Wildlife Service Cooperative Endangered Species Fund Grant TX E-165-R-1, vol. 458178–31. (Austin, Texas: Texas Parks and Wildlife), 1–41.
Duran C. M., Anderson W. M., Perry G. (2012). Holbrookia lacerata lacerata (Northern Spot-tailed earless lizard). USA: Texas: Mason Co. Geographic distribution. Herpetological Rev. 43, 305–306.
Duran C. M., Axtell R. W. (2010). “A rangewide inventory and habitat model for the spot-tailed earless lizard (Holbrookis lacerata),” in Report to Texas Parks and Wildlife Department(Austin, Texas, USA: Texas Parks and Wildlife), 37 pp.
Duran C. M., Axtell R. W., Gilbert S., Valdez J., Elliot. L. (2011). Response to a request for information from the Department of Interior U. S. Fish and Wildlife Service. 50 CFR Part 17 [Docket No. FWS-R2-ES-2011-0017; MO 92210-0-0008B2]. Endangered and Threatened Wildlife and Plants: 90-Day Finding on a Petition to List the Spot-tailed Earless Lizard as Threatened or Endangered: The Status of and a Predictive Model for Holbrookia lacerata (the Spot-tailed earless lizard). (Washington, DC: United States Fish and Wildlife Service), 3 pp.
Duran C. M., Yandell D. L. (2014). Holbrookia lacerata subcaudalis (Southern spot-tailed earless lizard). Refugia and commensalism. Herpetological Rev. 45, 500–501.
Earle A. M. (1961). The middle ear of Holbrookia and Callisaurus. Copeia 1961, 405–410. doi: 10.2307/1439581
Firneno T. J. Jr., Roelke C. E., Leache A. D., Rains N. D., Hibbitts T. J., Ryberg W. A., et al. (2024). Genome-wide data reinforces the evolutionary relationships of previously problematic earless lizards (Phrynosomatidae: Holbrookia). Bull. Soc. Systematic Biologists 3, 1–16. doi: 10.18061/bssb.v312.9459
Fitch H. S. (1970). Reproductive cycles of lizards and snakes Vol. 52 (Lawrence, KS: University of Kansas Museum Natural History Miscellaneous Papers), 1–247.
French S. S., Webb A. C., Hudson S. B., Virgin. E. E. (2018). Town and country reptiles: A review of reptilian responses to urbanization. Integr. Comp. Biol. 58, 948–966. doi: 10.1093/icb/icy052
Hammerson G. A., Lavin P., Mendoza-Quijano F. (2007).Holbrookia lacerata. In: IUCN 2009. IUCN Red List of Threatened Species. Version 2009.1. Available online at: www.iucnredlist.org (Accessed 15 November 2023).
Henke S. E. (1998). The effect of multiple search images and item abundance on the efficiency of human searchers. J. Herpetology 32, 112–115. doi: 10.2307/1565489
Henke S. E., Eversole C. B. (2021). Identifying best practices for spot-tailed lizards: Interim report to Texas Comptroller of Public Accounts; Contract 21-7255BG (Austin, Texas, USA: Texas Comptroller of Public Accounts), 36 pp.
Henke S. E., Eversole C. B., Wester D. B., Rangel E. D., Ayala. R. A. (2023). “Identifying best practices to survey for spot-tailed earless lizards,” in Final report to Texas Comptroller of Public Accounts(Austin, Texas, USA: Texas Comptroller of Public Accounts), 237 pp.
Henke S. E., Montemayor M. (1998). Diel and monthly variation in capture success of Phrynosoma cornutum via road cruising in southern Texas. Herpetological Rev. 29, 148–150.
Hibbitts T. D., Hibbitts T. J. (2015). Texas lizards: A field guide (Austin, Texas, USA: University of Texas Press), 333 pp.
Hibbitts T. J., Ryberg W. A. (2018). “Survey results for the spot-tailed earless lizard (Holbrookia lacerata) in Texas, (2015 – 2017). Pages 18 – 39,” in Final Report: Collaborative research on the natural history of the enigmatic spot-tailed earless lizard (Holbrookia lacerata) in Texas. Texas Data Repository, vol. 1 . Eds. LaDuc T. J., Wolaver B. D., Pierre J. P., Duran C. M., Labay B. J., Ryberg W. A., Hibbitts T. J., Roelke C. E., Fujita M. K., Wright I. M., Surya G., Shank C. J., Holloway P., Andrews J. R., Ikonnikova S. A., McDaid G. (Austin, TX: Texas Comptroller of Public Accounts), 1–259. doi: 10.18738/T8/C1C7X7
Hibbitts T. J., Ryberg W. A., Harvey J. A., Voelker G., Lawing A. M., Adams C. S., et al. (2019). Phylogenetic structure of Holbrookia lacerata (Cope 1880) (Squamata: Phrynosomatidae): One species or two? Zootaxa 4619, 139–154. doi: 10.11646/zootaxa.4619.1
Hibbitts T. J., Walkup D. K., LaDuc T. J., Wolaver B. D., Pierre J. P., Duran M., et al. (2021). Natural history of the spot-tailed earless lizards (Holbrookia lacerata and H. subcaudalis). J. Natural History 55, 495–514. doi: 10.1080/00222933.2021.1907469
iNaturalist.org (2024). Web application. Available online at: http://www.inaturalist.org/taxa/36352-Holbrookia-lacerata (Accessed 18 January 2024).
Ingram M. (2017). The Endangered Species Act in Texas: A survey and history (Austin, Texas, USA: Texas Policy Foundation), 16 pp.
Kahl S. S., Portillo-Quintero C., Cox R., McIntyre N., Perry. G. (2022). “Landscape assessment of west and south Texas grasslands to inform conservation of spot-tailed earless lizards in Texas,” in Final report to the Texas Comptroller Office of Public Accounts(Austin, Texas, USA: Texas Comptroller of Public Accounts), 50 pp.
LaDuc T. J., Wolaver B. D., Pierre J. P., Duran C. M., Labay B. J., Ryberg W. A., et al. (2018). Collaborative research on the natural history of the enigmatic spot-tailed earless lizard (Holbrookia lacerata) in Texas. Texas Data Repository 1, 1–259. doi: 10.18738/T8/C1C7X7
Neuharth D. B., Frizzell S., Ryberg W. A., Hibbitts T. J. (2018a). Holbrookia lacerata (Spot-tailed earless lizard). Predation. Herpetological Rev. 49, 537.
Neuharth D. B., Walkup D. K., Frizzell S. L., Kachel J. Z., Adams C. S., Johnson T. E., et al. (2018b). Holbrookia lacerata (Spot-tailed earless lizard). Burying behavior. Herpetological Rev. 49, 536–537.
Owen J. D., Johnson D., Rangel E. D., Henke S. E., Eversole. C. B. (2023). Holbrookia subcaudalis (Tamaulipan spot-tailed earless lizard). Perching behavior. Herpetological Rev. 54, 128–129. doi: 10.17161/ch.vi1.11960
Pianka E. R., Parker W. S. (1972). Ecology of the iguanid lizard Callisaurus draconoides. Copeia 1972, 493–508. doi: 10.2307/1442922
Rangel E. D. (2023). Identifying best practices to survey for spot-tailed earless lizards. Texas A&M University-Kingsville, Kingsville, Texas, USA, 150 pp. M. S. Thesis.
Rangel E. D., Henke S. E., Eversole C. B., Ayala R. (2023a). Holbrookia subcaudalis predation. Herpetological Rev. 54, 297–298.
Rangel E. D., Henke S. E., Moeller C., Willard L., Eversole C. B. (2022b). Holbrookia lacerata (Plateau spot-tailed earless lizard) and Holbrookia subcaudalis (Tamaulipan spot-tailed earless lizard). Burying behavior. Herpetological Rev. 53, 497–498.
Rangel E. D., Moeller C., Henke S. E., Eversole C. B., Ayala R. (2022c). Holbrookia lacerata (Plateau spot-tailed earless lizard) and Holbrookia subcaudalis (Tamaulipan spot-tailed earless lizard). Habitat use. Herpetological Rev. 53, 498.
Rangel E. D., Moeller C., Willard L., Henke S. E., Eversole C. B., Ayala R. (2022a). Approach tolerance and escape distances of plateau (Holbrookia lacerata) and Tamaulipan (Holbrookia subcaudalis) spot-tailed earless lizards. Herpetology Notes 15, 267–270.
Rangel E. D., Reyes J., Henke S. E., Eversole C. B., Ayala R. (2023b). Holbrookia lacerata and Holbrookia subcaudalis Avian predation. Herpetological Rev. 54, 296–297.
Roelke C. E., Maldonado J. A., Pope B. W., Firneno T.J. Jr, LaDuc T. J., Hibbitts T. J., et al. (2018). Mitochondrial genetic variation within and between Holbrookia lacerata lacerata and Holbrookia lacerata subcaudalis, the spot-tailed earless lizards of Texas. J. Natural History 2018, 1–11. doi: 10.1080/00222933.2018.1436726
Schmidt K. P., Dickerson M. C. (1922). A review of the North American genus of lizards Holbrookia. Bull. Am. Museum Natural History 46, 709–725.
Stejneger L. H. (1890). Annotated list of reptiles and batrachians collected by Dr. C. Hart Merriam and Vernon Bailey on the San Francisco Mountain Plateau and the desert of the Little Colorado, Arizona, with descriptions of a new species. North Am. Fauna 3, 103–118. doi: 10.3996/nafa.3.0006
Texas Parks and Wildlife Department (2012). Texas conservation action plan 2012 – 2016: Statewide/multi-region handbook. Ed. Connally W. (Austin, Texas, USA: Texas Parks and Wildlife), 78 pp.
Texas Parks and Wildlife Department (TPWD) (2005). Rare, threatened, and endangered species of Texas: Spot-tailed earless lizard (Holbrookia lacerata). Potential known presence map. Available at: https://tpwd.texas.gov/gis/rtest/.
Texas Parks and Wildlife Department (TPWD) (2013). “The NDD Report, Data highlights: Spot-tailed earless lizard (Holbrookia lacerata),” in The Texas Natural Diversity Database, Wildlife Diversity program(Austin, Texas, USA: Texas Parks and Wildlife). Available at: https://tpwd.texas.gov/huntwild/wild/wildlife_diversity/txndd/.
United States Fish and Wildlife Service (USFWS) (2011). Endangered and threatened wildlife and plants; 90-day finding on a petition to list the spot-tailed earless lizard as endangered or threatened. Federal Register 76, 30082–30087.
United States Fish and Wildlife Service (USFWS) (2016). National listing workplan: 7-year workplan. Available online at: https://www.fws.gov/endangered/esa-library/pdf/ (Accessed January 18, 2024).
United States Fish and Wildlife Service (USFWS) (2019). Endangered and threatened wildlife and plants; regulations for listing species and designating critical habitat. Federal Register 84, 45020–45053.
United States Fish and Wildlife Service (USFWS) (2024). Endangered and threatened wildlife and plants; Two species not warranted for listing as endangered or threatened species. Docket FWS-R2-ES-2023-0260. Federal Register 89, 8137–8149.
Walkup D. K., Adams C. S., Ryberg W. A., Hibbitts. T. J. (2018). Holbrookia lacerata (Spot-tailed earless lizard). Predation. Herpetological Rev. 49, 742–743.
Webb R. G., Ortenberger A. I. (1953). Reptiles of the wichita mountains wildlife refuge, comanche county, Oklahoma. Proc. Oklahoma Acad. Sci. 1953, 87–92.
WildEarth Guardians (2010). Petition to list the spot-tailed earless lizard (Holbrookia lacerata) under the U. S. Endangered Species Act. Petition submitted to the U. S. Secretary of Interior acting through the U. S. Fish and Wildlife Service, (2010) (Washington, D.C: United States Fish and Wildlife Services), 22 pp.
Wolaver B. D., Pierre J. P., Labay B. J., LaDuc T. J., Duran C. M., Ryberg W. A., et al. (2018). An approach for evaluating changes in land-use from energy sprawl and other anthropogenic activities with implications for biotic resource management. Environ. Earth Sci. 77, 1–14. doi: 10.1007/s12665-018-7323-8
Keywords: decline, distribution, Endangered Species Act, habitat, natural history, taxonomy, threatened
Citation: Rangel ED, Henke SE, Ayala RA and Eversole CB (2024) A comprehensive review of the ecology and conservation status of spot-tailed earless lizards (Holbrookia lacerata and Holbrookia subcaudalis). Front. Amphib. Reptile Sci. 2:1412860. doi: 10.3389/famrs.2024.1412860
Received: 05 April 2024; Accepted: 27 September 2024;
Published: 14 October 2024.
Edited by:
Robert Nathan Fisher, United States Geological Survey (USGS), United StatesReviewed by:
John C. Murphy, Field Museum of Natural History, United StatesThilina Dilan Surasinghe, Bridgewater State University, United States
Copyright © 2024 Rangel, Henke, Ayala and Eversole. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Scott E. Henke, c2NvdHQuaGVua2VAdGFtdWsuZWR1
 E. Drake Rangel1
E. Drake Rangel1 Scott E. Henke
Scott E. Henke Cord B. Eversole
Cord B. Eversole