- 1Caesar Kleberg Wildlife Research Institute, Texas A&M University-Kingsville, Kingsville, TX, United States
- 2Arthur Temple School of Forestry and Agriculture, Stephen F. Austin State University, Nacogdoches, TX, United States
Texas tortoises (Gopherus berlandieri) were once considered common and abundant throughout southern Texas with densities as high as 16 tortoises per hectare. Today, density estimates are 0.25 tortoises per hectare, which constitutes about a 98% population decline. Because of their low numbers and elusive behavior, Texas tortoises can be difficult to find. We demonstrate the value of using a detector dog as a time saving method in locating Texas tortoises. We glued VHF radio transmitters onto 9 adult tortoises and released them in a 5-ha plowed and short-grass pasture that contained mesquite (Prosopsis glandulosa) mottes, habitat conducive for Texas tortoise habitat selection. We calculated the Detectability Index (DI) as the detection rate (# tortoises found/minute) × percent tortoises from the known population found within 60 minutes. We compared DIs via telemetry, detector dog, and “cold” (no equipment or knowledge) human searches. We used the time required to find all tortoises when a searcher had knowledge of locations as the baseline. Our baseline DI was 0.79, followed by telemetry (0.13) and detector dogs (0.11), while “cold” searches was 0.02. Telemetry, detector dog, and cold searches were 6-fold, 7-fold, and nearly 40-fold slower, respectively, than having knowledge of tortoise locations. However, the combination of using detector dogs with telemetry resulted in a 50% time savings than single methods. Telemetry was useful in locating a generalized area with a tortoise but a detector dog was 2X faster in visually locating the tortoise once the area was identified. Therefore, we recommend the use of detector dogs as a time-saving method when conducting research on Texas tortoises.
1 Introduction
The Texas tortoise (Gopherus berlandieri) is one of six species of tortoises that are native to North America (Rose and Judd, 1989). They are the smallest and most sexually dimorphic of the Gopherus spp. (Rose and Judd, 1982). Historically, the geographic distribution of Texas tortoises was located across the southern portion of Texas from an imaginary line from Del Rio to San Antonio to Victoria. Their historical range extends into Mexico southward from the Rio Grande through eastern Coahuila and Nuevo Leon into San Luis Potosi (Rose and Judd, 1982; 1989). However today, their abundance has declined and their distribution in their historical range within the United States has become sporadic. Past densities of Texas tortoises during the mid-1970s have ranged from 13 to 35 tortoises/ha (Judd and Rose, 1983), but more recent studies estimate densities at 0.26 tortoises/ha (Kazmaier et al., 2001b). As of 1977, Texas tortoises are listed as a threatened species in Texas.
Because of their threatened status and limited distribution, population surveys are often required for conservation and management purposes (Scott and Seigel, 1992). Population monitoring of tortoises have included transects (Luckenbach, 1982), systematic searches (Judd and Rose, 1983), and relative abundance indices such as burrow, track, and scat counts (Auffenberg and Franz, 1982). However, Witz et al. (1992) demonstrated that relative abundance indices can vary by season and region; thus, creating problems in relating the quantity of tortoise sign to an actual number of tortoises.
Dogs (Canis lupus familiaris), because of their keen sense of smell and ability to use olfaction in intra- and interspecies communication (Kokocinska-Kusiak et al., 2021), have been trained by humans as service animals for centuries (Woollett et al., 2013). Today, dogs are trained to detect the scent of specific substances. For example, dogs have been trained to: locate explosives (Gazit and Terkel, 2003); determine the presence of accelerants after arson events (Katz and Midkiff, 1998) or contaminants in an area for human safety (Arner et al., 1986); find criminal suspects (Schoon, 1997); search for and rescue missing people (Fenton, 1992); and to detect medical issues such as seizures prior to onset (Brown and Strong, 2001). Wildlife biologists have used detection dogs to locate invasive species such as brown tree snakes (Boiga irregularis) in cargo shipments from Guam (Engeman et al., 2002), to recognize bark beetle pheromone (Vosvrdova et al., 2023), and to find endangered species such as San Juaquin kit foxes (Vulpes macrotis mutica; Smith et al., 2006). Grimm-Seyfarth et al. (2020) reviewed 1220 publications that reviewed nearly 2500 cases where detection dogs were used in conservation and noted that detection dogs were used to locate 483 species across 16 Phylums and 4 Kingdoms. Cablk and Heaton (2006) reported that detector dogs were successful in finding desert tortoises (Gopherus agassizii) in the Mojave Desert.
Because Grimm-Seyfarth et al. (2020) reported that detector dogs performed better than other detection methods in 89% of 542 reported cases where comparisons were made, we hypothesized that the use of detector dogs would exceed the ability of humans with the aid of telemetry to locate tortoises and would greatly exceed the ability of humans to locate tortoises via “cold” searches. Therefore, we designed an experiment to assess the efficacy of a detector dog versus human searchers with and without the aid of telemetry within a 5-ha area of a known population of Texas tortoises.
2 Study area
We built a 5-ha enclosure within the South Texas Plains ecoregion approximately 10 km south of Kingsville (27° 28’ 21” N, 97 ° 52’ 58” W, 20 m elevation), Texas, during October–December 2021, as part of a companion study investigating the efficacy of translocation and soft release on Texas tortoises. To make the habitat more suitable for Texas tortoises (Kazmaier et al., 2001a), we cleared brush and overgrown vegetation via cutting with a chainsaw, followed by prescribed fire and mowing, in order to develop a grassland with several mottes of honey mesquite trees (Prosopis glandulosa) scattered throughout the enclosure (Figure 1). Mowing was conducted monthly thereafter to maintain a grassland habitat. Downed trees, areas of taller grasses around tree bases, and old animal burrows were maintained as refuge sites for tortoises. We erected a 90-cm tall silt fence, with the bottom 30-cm buried in the soil and the plastic side facing inside the enclosure so tortoises could not dig underneath or become entangled in the wire mesh of the fence. This designed was conducted because it allowed us to have a known number of Texas tortoises within a known area and it served as a soft release site for later translocated tortoises.

Figure 1 South Texas habitat before (A) and after (B) habitat modifications to make the study area more suitable for Texas tortoises (Gopherus berlandieri).
3 Methods
3.1 Data collection
Nine Texas tortoises were captured fortuitously from the surrounding area during March–May, 2022, measured for carapace length, width, height, and circumference, weighed, sexed, outfitted with a VHF telemetry transmitter (Model R2020, 142 MHz frequency; ATS, Isanti, MN 55040) that was glued to the back of the carapace with epoxy, and released within the 5-ha enclosure. Telemetry transmitters were placed on tortoises to assess distances from undetected tortoises to the search paths of human searchers and the detector dog. Tortoises were allowed at least 30 days as an acclimation period within the enclosure prior to the initiation of the experiment.
During July 2022, we conducted surveys each week for 4 weeks to determine tortoise detectability via telemetry, detector dog, and human searchers. All surveys began at the southeast corner of the enclosure, began at 0900 hr, and the order of searches (i.e., human searcher with telemetry, human searcher with no knowledge, and detector dog) were randomized each week. Using telemetry, the searcher, without knowledge of tortoise locations, recorded the time required to locate all nine tortoises and obtain a visual identification of each tortoise. The same person, now with knowledge of where each tortoise was located within the enclosure, would then record the time required to relocate all nine tortoises. This time was considered as the baseline time or the minimum time required to locate tortoises. Due to financial constraints (i.e., training costs, travel, per diem while on site; $10,000/dog [US-2021], a single dog was used for our study. Our dog was a 12-year-old, female Labrador retriever that had >10 years of experience locating wildlife, mainly white-tailed deer (Odocoileus virginianus). However, the dog was trained to locate Texas tortoises for 4 months prior to the initiation of this study using captive Texas tortoises. Texas tortoises are active from mid-March to November (Judd and Rose, 1983), during which time southern Texas experiences an average temperature of 33°C with 98 days of temperatures (2023) exceeding 38°C (Fulbright and Bryant, 2002, www.extremeweatherworld.com/cities/kingsville/yearly-days-of-100-degrees). The dog was from south-central Texas, and thus, acclimated to the Texas climate. The detector dog with handler would search the enclosure and record the time required for the dog to locate tortoises. The handler would walk approximately 40 meters behind the dog to avoid providing signals to the dog as to potential tortoise locations. The dog was allotted up to 60 minutes to locate the nine tortoises. Because we only had one dog that was trained to locate Texas tortoises available for our study, to reduce the likelihood of the dog learning locations of tortoises within our study area, we 1) only allowed the dog to survey our study area one time each week for 4 weeks, and 2) had the dog handler have the dog search surrounding areas for Texas tortoises for an equal amount of time at least twice before our next survey. A human searcher, who possessed prior tortoise search experience, was allotted up to 60 minutes to systematically search the enclosure for all nine tortoises. A different human searcher was used for each weekly survey to avoid past knowledge from previous searches to bias their search of the enclosure. The detector dog and human searcher without knowledge of tortoise location were equipped with a hand-held GNSS Trimble GPS unit (Model TDC650; Trimble Geospatial Technology, Westminster, Colorado 80021) that had an accuracy of ± 30 cm and obtained locations of their search path as they searched the 5-ha enclosure. Each search path was overlaid with the actual tortoise location via ArcMap 10.1 (Environmental Systems Research Institute 2013) and the closest distance from undetected tortoises to the search path of the detector dog and human searcher was recorded. The detector dog, dog handler, and human searchers were not present during the initial searches with telemetry and knowledge of tortoise location to avoid biasing their searches. In addition, prior to searches by the detection dog and human searchers, three people would randomly walk through the enclosure to make potential scent trails and vegetation paths to reduce the likelihood of creating search bias.
We calculated a Detectability Index (DI) as the detection rate (number of tortoises found/search-minute) × percent of tortoises from the known population found within 60 minutes. The DI was calculated rather than detection rate alone to adjust for differences in the number of tortoises found and varying minutes within surveys. For example, if 6 tortoises were found (i.e., 67% of the available tortoises) in 30 minutes, the detection rate would be 0.20 tortoises/minute but the DI would by 0.13. By contrast, if all 9 tortoises were found during the full allotted time (i.e., 60 minutes), then the detection rate and DI would be 0.15. We used the time it required to find all tortoises when the searcher had knowledge of locations as our baseline. Although such a baseline is artificial and other methods to locate tortoises would, in theory, require more time to locate tortoises, locating tortoises even with knowledge of their location does require some time and we used this time as our standard measurement to compare other methods. Therefore, methods that have a ratio approaching unity between their detectability index and the baseline detectability index would be comparable to having knowledge of tortoise location. Thus, we compared mean DIs via telemetry, detector dog, and cold searches to that of the baseline.
3.2 Analysis of data
We analyzed data as a completely randomized analysis of variance to compare mean Detectability Indices between treatments, inclusive of the baseline, full knowledge search (SAS Institute, Inc, 2012). Multiple comparisons were made with the Tukey’s studentized range (HSD) test when significant effects were found (Cochran and Cox, 1957). Homogeneity of variances among treatments was evaluated and verified with the Bartlett’s test (Steel and Torrie, 1980). Distributions of residual errors were tested and verified for normality via the Shapiro-Wilk Test (Shapiro and Wilk, 1965). Means are reported as ± 1 standard error.
4 Results
The nine (5M:4F) tortoises captured were adult-sized individuals with average weight, carapace length, width, height, and circumference of 1.0 ± 0.02 kg, 162 ± 1.4 mm, 138 ± 1.6 mm, 84 ± 0.8 mm, and 516 ± 4.1 mm, respectively.
With knowledge of tortoise location, the searcher was able to find all 9 tortoises within 11.5 ± 1.3 minutes, on average; whereas, no other method was able to consistently locate all 9 tortoises within the 60-minute allotted time (Table 1). The baseline detectability index was 0.79 ± 0.04 (Table 1). With the use of telemetry, the searcher would locate the tortoise signal and be within 10 m of the tortoise location within 51 ± 4.7 seconds (~12% of search time), but would require, on average, an additional 6.4 ± 1.3 minutes/tortoise to locate and visually see the tortoise (~88% of search time). During the 4 searches with the use of telemetry, the searcher had 2, 0, 1, and 0 undetected tortoises, and was between 3 and 12 m from the undetected tortoises when the 60-minute time limit expired. The detector dog required 37.2 ± 2.8 minutes to locate 6 tortoises (i.e., ~6.2 minutes/tortoise), on average. Although anecdotal, the detector dog appeared to spend about 57% of its search time (i.e., ~3.5 minutes/tortoise) in a random search sweep, and about 43% of its search time (~2.7 minutes) with its nose within the ground litter and its tail in a pointed position. The detector dog had 2, 5, 2, and 4 undetected tortoises during its four sequential searches and was 43–72 m away from its undetected tortoises. Whereas, human searchers, on average, only found 3.5 ± 0.3 tortoises within the 60-minute allotted time (Table 1) and had 22 undetected tortoises during the 4 searches, of which the searcher ranged from 1 to 12 m away from the undetected tortoises. Detectability Indices ranged from 0.02 to 0.13 and were different (F3,10 = 177.4, P < 0.0001) between search methods (Table 1). Time required to locate tortoises was quickest with knowledge of their location, followed by telemetry and detector dogs, and then by human searchers. Telemetry, detector dog, and cold searches were 6-fold, 7-fold, and nearly 40-fold slower, respectively, than searches with knowledge of tortoise locations.
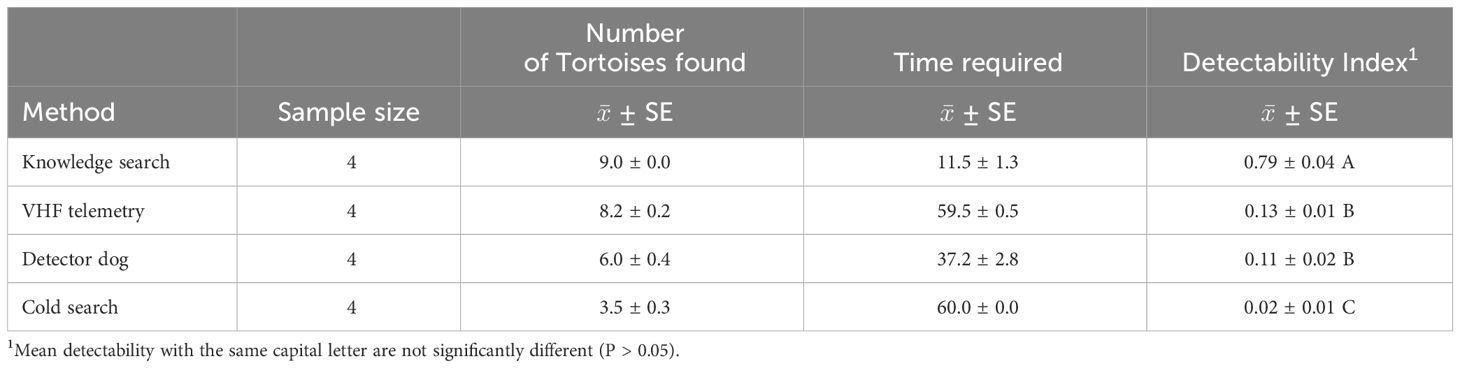
Table 1 Detectability indices to locate 9 adult Texas tortoises via telemetry, detector dog, and human searches within a 5-ha enclosure in southern Texas during July 2022.
Because telemetry was quick in locating areas with a tortoise, but detector dogs were faster finding the tortoise that were hidden by vegetation (Figure 2), we combined the two methods and conducted a post hoc test. Because this test lacked independence from the other methods, it was not included within the original analysis of variance comparison. The searcher using telemetry and a detector dog were able to find and visually observe all 9 tortoises within 33 minutes, which yielded a DI of 0.27. The combination of telemetry and detector dog yielded results that were 3-fold slower than having knowledge of tortoise location, but twice as fast as either using telemetry or a detector dog as separate methods.

Figure 2 Texas tortoises (Gopherus berlandieri) underneath vegetation were not always observed by human searchers (A) and required more time for human searchers to visually locate with the aid of telemetry (B), while detector dogs were twice as fast to locate telemetered tortoises human searchers with telemetry receivers (C).
5 Discussion
The reasons to capture and study wild animals is more diverse today than ever before (i.e., for management and regulation purposes, for wildlife damage and disease issues, and for protection of threatened and endangered species). Hence, locating specimens is a critical initial component. However, even after animals are initially captured and marked, such as with telemetry, it is essential to occasionally relocate those individuals to verify their actual location and health status. For example, when estimating survivability through telemetered animals, acquiring a signal does not always equate to a live animal, nor does a triggered mortality sensor on a transmitter equate to death if the transmitter is placed on a sedentary species, such as a Texas tortoise during brumation. In addition, a transmitter that dislodges from an animal could be lying on the ground, but if the GPS accuracy is ± 5 m, it could give the appearance of an animal moving, albeit small movements. Hence, physically locating the animal often is required.
Our hypothesis was only partially correct in that detector dogs performed much better than “cold” (i.e., no knowledge of actual tortoise locations) human searches, even though the searchers used in our study had previous experience conducting surveys for Texas tortoises. Detector dogs did not surpass the overall time required of humans locating telemetered tortoises. This could be a function of: 1) how far a dog is from the tortoise, or 2) if the dog can recognize the odor as coming from a tortoise, and follow the odor to its source. Detection distances for detector dogs of Mojave desert tortoises ranged from 0.5 m to 62.8 m (Cablk et al., 2008). However, we acknowledge that we adapted the searches to fit ideal conditions for human searchers, and not necessarily for ideal search conditions for the dog.
Because our enclosure was ~224 m/side, which represented 3.5X the largest detection distance of the dogs in Cablk et al. (2008), it is reasonable to assume if our detector dog displayed similar results as those in Cablk et al. (2008), then our detector dog would require additional time to randomly search and cover the entire 5-ha enclosure to locate every tortoise. Our study demonstrated that our detector dog typically missed locating a tortoise when the tortoise was toward the extreme detection distance described by Cablk et al. (2008). Because Texas tortoises use animal burrows or shallow pallets under thick vegetation as resting sites (Rose and Judd, 1975, 1982), we assume that such sites would reduce odor transmission; and thus, dogs must approach closer to tortoises before such detection occurs. Approach distances could potentially be reduced if the dog handler and dog would search the area in a methodical, serpentine style, rather than a random approach.
Detector dogs were superior to human searchers in visually locating tortoises under brush and heavy vegetative cover. If Texas tortoises were not actively moving in the open, mowed grassland, they were found in thick vegetation similar to that observed by (Kazmaier et al., 2001a). A human searcher using telemetry could quickly acquire the signal of a telemetered tortoise and get within 10 m of it, but required up to 15 minutes to visually find the tortoise within the vegetative cover. Detector dogs were twice as fast to locate tortoises in thick vegetation. Similar results were reported by Nussear et al. (2008). By contrast, our human searchers without knowledge of tortoise location often walked past a tortoise that was obscured by vegetative cover. Our human searchers attempted a logical, serpentine search path where each search swath was about 10 m apart in order to cover the entire 5-ha area. However, the majority of tortoises went undetected due to their secretive and cryptic nature, even though the searcher often was quite close to a tortoise.
It was unlikely that the detector dog learned tortoise locations with subsequent searches because 1) we conducted the study during early morning and during July when tortoise daily movements are at their peak (Auffenberg and Weaver, 1969; Rose and Judd, 2014); thus, tortoises were not found at same locations during subsequent searches, and 2) the number of undetected tortoises by the dog fluctuated between searches while the search time by the dog was fairly consistent between searches. A potential shortcoming to the use of a single detector dog was that our dog became less motivated as time passed; hence, the reason why the average search time for the detector dog was 37 minutes, even though not all tortoises were located. This possibly was a function of 1) the dog’s age (i.e. 12-year-old Labrador retriever), and 2) southern Texas heat during the summer (temperature = 35°C, range = 24–43°C; relative humidity = 74%, range = 60–98%; https://weather-and-climate.com/kingsville-texas-us-july-averages). After locating several tortoises, the dog would lie down in the shade to rest, at which point the dog would receive a drink of water. We stopped recording the time at this point because the dog was no longer actively searching for tortoises. However, our calculated DI compensated for differing search times and number of tortoises found; therefore, comparisons between all treatments are valid. To reduce this potential stopping behavior, we conducted all surveys during the early morning prior to the peak of summer temperatures during the afternoons (Fulbright and Bryant, 2002). However, perhaps beginning dog searches at sunrise would be best, and allow the dog frequent breaks with water to reduce panting due to excessive heat, which would allow the sense of smell to work ideally, and ultimately, could improve the DI of the detection dog.
At the initiation of many wildlife studies, the study species often needs to be acquired for demographic information, marking, transmitter attachment, etc. Researchers seeking species that are rare, cryptic, or secretive may find it beneficial to render aid from detector dogs. Detector dogs have been used in a myriad of studies to locate >400 animal, 42 plant, 26 fungi, and 6 bacteria species (Grimm-Seyfarth et al., 2020). We found that the use of detector dogs reduced the amount of time searching for our desired species.
6 Conclusion
We recommend the use of detector dogs as a time-saving method when conducting surveys for and research on Texas tortoises. Detector dogs found Texas tortoises 5.5X faster than human searchers when originally locating tortoises, and 2X faster than humans when tortoises were telemetered.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Texas A&M University Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SH: Conceptualization, Formal analysis, Funding acquisition, Methodology, Supervision, Writing – original draft. CM: Data curation, Project administration, Writing – review & editing. SP: Investigation, Writing – review & editing. WR: Investigation, Writing – review & editing. SR-H: Supervision, Writing – review & editing. CE: Conceptualization, Formal analysis, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding for the project was provided by the Rob and Bessie Welder Wildlife Foundation and Next Decade. The Caesar Kleberg Wildlife Research Institute provided funding for publication costs.
Acknowledgments
We thank E. D. Rangel, L. Willard, P. Richardson, and J. Reyes for assistance with tortoise surveys. We thank the Welder Wildlife Foundation and Next Decade for financial support and Rio Grande LNG as a source for Texas tortoises. This is contribution number WC 740 of the Welder Wildlife Foundation and contribution number 24-105 of the Caesar Kleberg Wildlife Research Institute. This study was approved by the Texas A&M University-Kingsville Animal Care and Use Committee (Protocol Henke_S_2021-03-08/1463).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Arner L. D., Johnson G. R., Skovronek. H. S. (1986). Delineating toxic areas by canine olfaction. J. Hazardous Materials 13, 375–381. doi: 10.1016/0304-3894(86)85009-9
Auffenberg W. A., Franz R. (1982). “The status and distribution of the gopher tortoise (Gopherus polyphemus),” in North american tortoise: conservation and ecology. Ed. Bury R. B. (US Fish and Wildlife Service, Washington, DC), Pages 95–126. Report 12.
Auffenberg W. A., Weaver W. G. (1969). Gopherus berlandieri in southeastern Texas. Bull. Florida State Museum 13, 141–203. doi: 10.58782/flmnh
Brown S. W., Strong V. (2001). Use of seizure-alert dogs. Seizure 10, 39–41. doi: 10.1053/seiz.2000.0481
Cablk M. E., Heaton J. S. (2006). Accuracy and reliability of dogs in surveying for desert tortoises in the western Mojave Desert. Ecol. Appl. 4, 446–460. doi: 10.1890/1051-0761
Cablk M. E., Sagebiel J. C., Heaton J. S., Valentin C. (2008). Olfaction-based detection distance: A quantitative analysis of how far away dogs recognize tortoise odor and follow it to source. Sensors 8, 2208–2222. doi: 10.3390/s8042208
Cochran W. G., Cox G. M. (1957). Experimental design. 2nd ed. (New York, New York, USA: John Wiley and Sons).
Engeman R. M., Vice D. S., York D., Gruver K. S. (2002). Sustained elevation of the effectiveness of detector dogs for locating brown tree snakes in cargo outbound from Guam. Int. Biodeterioration Biodegradation 49, 101–106. doi: 10.1016/S0964-8305(01)00109-3
Fenton V. (1992). Use of dogs in search, rescue, and recovery. J. Wilderness Med. 3, 292–300. doi: 10.1580/0953-9859-3.3.292
Fulbright T. E., Bryant F. C. (2002). The last great habitat (Kingsville, Texas, USA: Special Publication 1 of the Caesar Kleberg Wildlife Research Institute, Texas A&M University-Kingsville).
Gazit I., Terkel J. (2003). Explosives detection by sniffer dogs following strenuous physical activity. Appl. Anim. Behav. Sci. 81, 149–161. doi: 10.1016/S0168-1591(02)00274-5
Grimm-Seyfarth A., Harms W., Berger A. (2020). Detection dogs in nature conservation: A database on their world-wide deployment with a review on breeds used and their performance compared to other methods. Methods Ecol. Evol. 12, 568–579. doi: 1111/2041-210X.13560
Judd F. W., Rose F. L. (1983). Population structure, density, and movements of the Texas tortoise. Southwestern Nat. 28, 398–387. doi: 10.2307/3670817
Katz S. R., Midkiff C. R. (1998). Unconfirmed canine accelerant detection: a reliable issue in court. J. Forensic Sci. 43, 329–333. doi: 10.1520/JFS16142J
Kazmaier R. T., Hellgren E. C., Ruthven D. (2001a). Habitat selection by the Texas tortoise in a managed thornscrub ecosystem. J. Wildlife Manage. 65, 653–660. doi: 10.2307/3803016
Kazmaier R. T., Hellgren E. C., Synatzske D. R., Rutledge J. C. (2001b). Mark-recapture analysis of population parameters in a Texas tortoise (Gopherus berlandieri) population in southern Texas. J. Herpetology 35, 410–417. doi: 10.2307/1565959
Kokocinska-Kusiak A., Woszczylo M., Zybala M., Maciocha J., Barlowka K., Dzieciol M. (2021). Canine olfaction: Physiology, behavior, and possibilities for practical application. Animals 11, 2463. doi: 10.3390/ani11082463
Luckenbach R. A. (1982). “Ecology and management of the Desert Tortoise (Gopherus agassizzi),” in North american tortoise: conservation and ecology. Ed. Bury R. B. (US Fish and Wildlife Service, Washington, DC), Pages 1–37. Report 12.
Nussear K. E., Esque T. C., Heaton J. S., Clark M. E., Drake K. K., Valentin C., et al. (2008). Are wildlife detector dogs or people better at finding desert tortoises (Gopherus agassizii). Herpetological Conserv. Biol. 3, 103–115.
Rose F. L., Judd F. W. (1975). Activity and home range size of the Texas tortoise, Gopherus berlandieri, in South Texas. Herpetologica 31, 448–456.
Rose F. L., Judd F. W. (1982). “Texas tortoise,” in North american tortoise: conservation and ecology. Ed. Bury R. B. (US Fish and Wildlife Service, Washington, DC), Pages 57–70. Report 12.
Rose F. L., Judd F. W. (1989). “Texas tortoise of southern Texas,” in The conservation biology of tortoises. Eds. Swingland I. R., Klemens M. W. (World Conservation Union, Dice, England, UK), Pages 8–9.
Rose F. L., Judd F. W. (2014). The texas tortoise: A natural history (Norman, Oklahoma, USA: University of Oklahoma Press).
SAS Institute, Inc (2012). SAS/STAT software, version 9.3 (Cary, North Carolina, USA: SAS Institute, Inc.).
Schoon G. A. A. (1997). Scent detection by dogs (Canis familiaris): a new experimental design. Behaviour 134, 531–550. doi: 10.1163/156853997X00511
Scott N. J. Jr., Seigel R. A. (1992). “The management of amphibian and reptile populations: Species priorities and methodological and theoretical constraints,” in Wildlife 2001: populations. Eds. McCullough D. R., Barrett R. H. (Elsvier Applied Science, London, UK), Pages 343–368.
Shapiro S. S., Wilk M. B. (1965). An analysis of variance test for normality. Biometrika 52, 591–611. doi: 10.1093/biomet/52.3-4.591
Smith D. A., Ralls K., Cypher B. L., Clark. H. O. Jr., Kelly P. A., Williams D. F., et al. (2006). Relative abundance of endangered San Juaquin kit foxes (Vulpes macrotis mutica) based on scat detection dog surveys. Southwestern Nat. 51, 210–219. doi: 10.1894/0038-4909(2006)51[210:RAOESJ]2.0.CO;2
Steel R. G. D., Torrie J. H. (1980). Principles and procedures of statistics. 2nd ed. (New York, New York, USA: McGraw-Hill).
Vosvrdova N., Johansson A., Turcani M., Jakus R., Tyser D., Schlyter f., et al. (2023). Dogs trained to recognize a bark beetle pheromone locate recently attacked spruces better than human experts. For. Ecol. Manage. 528, 120626. doi: 10.1016/j.foreco.2022.120626
Witz B. W., Wilson D. S., Palmer M. D. (1992). Estimating population size and hatchling mortality of Gopherus polyphemus. Florida Sci. 55, 14–19. doi: 10.2307/2426159
Keywords: detectability index, detector dog, Gopherus berlandieri, search, survey, Texas tortoise
Citation: Moeller C, Perales S, Rodriguez W, Henke SE, Rideout-Hanzak S and Eversole CB (2024) Playing “hide and seek” with Texas tortoises: value of a detector dog. Front. Amphib. Reptile Sci. 2:1382591. doi: 10.3389/famrs.2024.1382591
Received: 05 February 2024; Accepted: 11 April 2024;
Published: 24 April 2024.
Edited by:
Anssi Laurila, Uppsala University, SwedenReviewed by:
Simon Kärvemo, Swedish University of Agricultural Sciences, SwedenJelena Mausbach, Artenspürhunde Schweiz, Switzerland
Copyright © 2024 Moeller, Perales, Rodriguez, Henke, Rideout-Hanzak and Eversole. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Scott E. Henke, c2NvdHQuaGVua2VAdGFtdWsuZWR1
 Christin Moeller1
Christin Moeller1 Scott E. Henke
Scott E. Henke Cord B. Eversole
Cord B. Eversole