- 1Department of Evolution, Ecology, & Organismal Biology, University of California, Riverside, Riverside, CA, United States
- 2U.S. Geological Survey, Western Ecological Research Center, Reno, NV, United States
Populations of the threatened desert tortoise (Gopherus agassizii) continue to decline throughout the geographic range, in part because of degraded and fragmented habitats in the Mojave and western Sonoran deserts. The species is herbivorous and highly selective in choice of plant species. To increase options for recovery, we analyzed behaviors, patterns of movements while foraging, and parts of plants consumed during a superbloom. We characterized foraging routes and the habitat strata and microhabitats where tortoises traveled to eat preferred wildflower species. Tortoises walked one foraging route per day in early spring, often switched to two routes per day in middle and late spring with rise of midday temperatures. They chose habitat strata (primarily hills and ephemeral stream channels) and three of seven microhabitats for foraging on preferred food plants. Preferred microhabitats were intershrub open space and small (1–2 m wide) ephemeral stream channels. They rarely took bites of forbs growing under and in the dripline of shrubs or nonnative forbs and grasses. Tortoises typically did not select specific plant parts to eat but important exceptions occurred. For example, they usually ignored the inflorescences of the annual Eremothera boothii and, when eating the non-native annual Erodium cicutarium, tended to focus on fruits. All such information aids recovery efforts to restore declining tortoise populations.
1 Introduction
Agassiz’s desert tortoise (Gopherus agassizii, herein tortoise) is a federally threatened species under the Endangered Species Act (U.S. Fish and Wildlife Service [USFWS], 1990) and is a globally critically endangered species (Berry et al., 2021). Despite recovery efforts, most populations are no longer viable (U.S. Fish and Wildlife Service [USFWS], 2015; Berry and Murphy, 2019). Allison and McLuckie (2018) noted that the desert tortoise was on the path to extinction under current conditions. The status is primarily the result of habitat degradation, fragmentation, and loss; disease and hyper-predation are also contributing to declines (Berry and Murphy, 2019). The sources of habitat degradation and loss include urban and agricultural development, roads and utility corridors, mining, livestock grazing, military activities, authorized and unauthorized off-road vehicle use, and many other anthropogenic activities. Disturbances to soil and native plant communities contributed to invasion and establishment of non-native forbs and combustible grasses, which created a new fire cycle in the Mojave Desert (Brooks and Matchett, 2006; Klinger et al., 2021). Recovery of tortoise populations and restoration of deteriorated land in the Mojave and western Sonoran deserts where desert tortoises live present major challenges (Abella et al., 2023). Tortoises are long-lived and slow to reproduce; mortality rates are high for young individuals (Woodbury and Hardy, 1948; Hardy, 1976; Turner et al., 1987; Medica et al., 2012). Additionally, they are herbivorous, highly selective in choice of plant foods, and rely on rains for free water to drink (Burge and Bradley, 1976; Jennings and Fontenot, 1993; Henen et al., 1998; Jennings and Berry, 2015). The amounts and timing of precipitation during fall and winter months drive germination, growth, and flowering of ephemeral plants during the subsequent spring in the western Mojave Desert (Rowlands et al., 1982; Jennings, 2001). In years of low or no rainfall, little or no food is available, whereas in years of high precipitation, a superbloom may result with abundant wildflowers (Jennings, 2001; Brooks and Berry, 2006; Jennings and Berry, 2015).
After the federal listing of the tortoise as threatened in 1990 (U.S. Fish and Wildlife Service [USFWS], 1990), several observational and experimental research projects were undertaken to determine the plants tortoises consumed and avoided, because of threats to habitat from such disturbances as livestock grazing (Webb and Stielstra, 1979; U.S. Fish and Wildlife Service [USFWS], 1990; U.S. Fish and Wildlife Service [USFWS], 1994). Research on the digestibility and nutritional characteristics of potential plants available to wild desert tortoises provided answers to why tortoises selected and preferred certain species in altered habitats (e.g., Brooks and Berry, 2006). Barboza (1995), in experimental studies of feeding different diets to tortoises, reported loss of body mass from the grass diet, a finding later supported by feeding trials with both native and non-native annuals (forbs and grasses) for juvenile tortoises (Hazard et al., 2009; Hazard et al., 2010; Drake et al., 2016). Further, young tortoises gained weight and grew when eating forbs but did not thrive on either native or non-native grasses (Hazard et al., 2009; Hazard et al., 2010). Jennings (2002) and Jennings and Berry (2015) detailed the preferences of wild tortoises for selected species of forbs and herbaceous perennial plants using observations of bite counts by species during a wet year. Oftedal et al., (1994, Oftedal, 2002; Oftedal et al., 2002) explained the selectivity for a few species of forbs by content of potassium, ubiquitous in desert plants and difficult to metabolize without free water. Wild tortoises needed to select ephemeral plants high in water and nitrogen but low in potassium to maintain a healthy diet, grow, and reproduce (Oftedal et al., 1994). Oftedal (2002) developed the Potassium Excretion Potential (PEP) index of a plant: a positive PEP index occurs when a plant contains more nitrogen and water than is needed to excrete the ingested potassium, whereas a negative PEP signified that a plant contained insufficient protein and water for a tortoise to dispose of ingested potassium (Oftedal, 2002). Observational studies indicated that tortoises were highly selective in choices of annual and herbaceous plant species (Jennings, 2002; Jennings and Berry, 2015) and and favored species low in potassium (Oftedal, 2002).
Our objectives were to build off previous work on tortoise foraging by characterizing preferences as plant resource availability and phenology changed throughout the spring in the western Mojave Desert (Jennings and Berry, 2015). Here we address foraging behaviors and choices of plant parts consumed by the tortoises during a year with a superbloom and when confronted with a suite of choices of plant foods. We asked three questions about foraging in spring during a superbloom: 1) do tortoises walk among habitat strata in a random manner to forage, 2) do distances walked within each habitat stratum change as phenological stages of plants change, and 3) do tortoises randomly select microhabitats to forage? In addition, for each plant species, we estimated the percentage of the plants consumed and relative proportions eaten of different plant parts (stems, leaves, flowers/buds, and fruits). We evaluated occurrences and proportions of years of high and low precipitation before and after the study. Our findings will contribute to the knowledge of tortoise movements and foraging behaviors, important elements in critical recovery efforts to build viable populations (U.S. Fish and Wildlife Service [USFWS], 2011).
2 Study area
The study was conducted on a ~15 km2 site within the Desert Tortoise Research Natural Area (DTRNA; 35°14'34", −117°51'45''), at elevations of 800–915 m, in the western Mojave Desert, California, USA. The ~101 km2 area received designation in 1980 by U.S. Congress and was protected in 1978–1979 with a fence to exclude recreational vehicle use and sheep grazing (Berry et al., 2014). The vegetation alliance of perennial shrubs was diverse with 11 species, including creosote bush (Larrea tridentata), white bursage (Ambrosia dumosa), Nevada ephedra (Ephedra nevadensis), goldenhead (Acamptopappus sphaerocephalus), Mojave indigo bush (Psorothamnus arborescens), Mojave California buckwheat (Eriogonum fasciculatum), Anderson boxthorn (Lycium andersonii), winterfat (Krascheninnikovia lanata), hop-sage (Grayia spinosa), and Mojave aster (Xylorhiza tortifolia).
3 Materials and methods
3.1 Data collection
3.1.1 Precipitation
The study area is in the western region of the Mojave Desert, where most rainfall occurs during fall and winter and can produce, depending on amounts of rain, annual plants in late winter and spring (Rowlands et al., 1982; Rowlands, 1995). We used high-resolution gridded data on climate that were geographically and elevation-weighted from PRISM to summarize monthly estimates of precipitation from 1961 to the present. PRISM is a gridded data product (Daly et al., 2008) using elevation, latitude, and longitude of the study area (2) at 4-km resolution from the Prism Climate Group (https://www.prism.oregonstate.edu, accessed 5 April and 10 October 2023). We recognize that several variables affect climate in the American Southwest—precipitation, temperature, and soil moisture are probably the most important (Ault et al., 2016; Cook et al., 2021; Dannenberg et al., 2022). Because amounts of winter and summer precipitation are prime drivers of almost every aspect in the lives of tortoises, we used precipitation here instead of other variables (e.g., Henen, 1997; Henen et al., 1998; Christopher et al., 1999; Duda et al, 1999; Henen, 2002; Berry et al., 2023).
3.1.2 Desert tortoises
Field work occurred between 26 March and 23 June 1992. One person (WBJ) recorded all daily movements and foraging behaviors for 16 adult tortoises with an average (±SE) carapace length (mm) at the midline for eight males of 230 ±14.42 mm (range 179–280 mm) and eight females, 222 ±4.14 mm (range 210–239 mm). Twelve of the study animals were part of another study and carried radio transmitters (Model 2B-2 AVM Instrument Co., Livermore, California, powered by lithium C-cells, Tadiran Co., Israel) with 48-cm copper antennae affixed to the shells (Christopher et al., 1999). Using a receiver and a 2-element antenna (Telonics, Mesa, Arizona), selected individuals were located each day (see details below). Data were also collected opportunistically on movements and foraging for four incidentally encountered adults without radio transmitters.
Throughout the field season, a focal animal was selected to observe and record foraging and movements for each field day. When possible, observations of foraging were recorded for tortoises over the entire course of the daily or bi-daily foraging route, i.e., a complete foraging route = from emergence from a cover site (pallet or burrow) to retreat to a cover site after foraging ended. Typically, tortoises emerged from cover sites during morning hours. If the selected focal animal did not emerge, an alternate was selected, usually an individual inside or outside a cover site. The footpath (m) distances walked by each tortoise during foraging routes were also measured for each of three habitat strata (% of occurrence within the study area): 1) broad and sandy alluvial fans (48%); 2) low, rocky hills (42%); and 3) dry, sandy, ephemeral stream channels (10%) (Jennings and Berry, 2015). Tortoises used two patterns of movement on foraging routes: 1) round trips, with returns to the same cover site occupied earlier in the day and generally remaining within the same habitat stratum to forage; and 2) non-round trips in which a tortoise left a cover site to forage, traveled among >1 habitat strata and ended foraging at a different cover site. On several occasions, observations were recorded for multiple tortoises during the same day, e.g., observations of foraging activities of one individual in the morning and another during the afternoon—thus recording data for the foraging route of each tortoise. Observations were also recorded simultaneously for two to three individuals foraging <10 m apart when all were feeding on the same plant species. Focal individuals were followed at distances of 4–8 m, frequently using binoculars, which minimized disturbance to the tortoise.
While watching a tortoise, data were recorded onto a microcassette recorder (Sony M560V, China): start and end times of observations; times for each foraging bout (1 bout = all bites taken from a single food item); species of plant eaten; number of bites taken (Jennings and Berry, 2015); the estimated percentage of the plant eaten (above-ground biomass only); parts of the plant consumed (leaves, stems, flowers, fruits); condition of the plant (succulent or dry); and the microhabitat where the plant grew. Note that our estimates of the percentage of plant eaten are based on simple visual estimates of the portion of a plant eaten (i.e., visual comparisons of a plant before and after a tortoise fed from it). These estimates provide data on whether tortoises ate a plant in its entirety, ate part of it, or just took a few bites, etc. The microhabitats were: 1) beneath creosote bush; 2) at or under the dripline of a creosote bush; 3) intershrub space between shrubs; 4) outer edge of species of non-creosote bushes; 5) margin of small (1–2 m wide) ephemeral stream channels, 6) center of small stream channels; and 7) sandy areas within large ephemeral stream channels (>2 m wide). The recognition of two distinct microhabitat categories for the small stream channels was warranted because tortoises showed a strong tendency to walk along the edges of small stream channels where some preferred plants grew (see Jennings and Fontenot, 1993). Plant nomenclature was from the Jepson Flora Project (2023). Data for plants available for forage are in Jennings and Berry (2015).
3.2 Analyses of data
3.2.1 Precipitation
We evaluated precipitation data monthly for winter rain (October 1 – March 31) and for the hydrologic year (October 1 – September 30). We identified three blocks of years for analysis of annual and winter rainfall averages: the 30 years prior to the study (1961–1990) as a benchmark; the eight years, 1991–1998, bracketing when the study occurred and before the start of the megadrought; and from the start of the megadrought in 1999 through the potential end point in 2022 (Ault et al., 2016; Williams et al., 2020; Cook et al., 2022). According to Ault et al. (2016: abstract), “Megadroughts are comparable in severity to the worst droughts of the 20th century but are of much longer duration.” We identified numbers of years with: 1) unusually high winter precipitation, when superblooms occurred, which we defined as winter rainfall exceeding the long-term averages by ≥70%; 2) above average, average, and slightly below average precipitation; and 3) dry years or droughts for the tortoise, when winter precipitation fell well below long-term averages.
3.2.2 Phenological periods
To account for seasonal variation in availability of food plants and plant parts, we analyzed foraging behaviors and movement patterns within each of three successive phenological periods in spring, defining them as: early spring, characterized by early-spring species of blooming plants in March and April; middle spring with the middle-spring bloomers in May; and late spring, the late-spring bloomers in the final phase of the spring wildflower season in June (Jennings and Berry, 2015).
3.2.3 Selection of habitat strata by tortoises
Observations from the pilot study of tortoise foraging behavior conducted the previous year at the same site suggested that the tortoises walk among different habitat strata during their daily or bi-daily foraging walks or travels (Jennings and Fontenot, 1993). We therefore tested the null hypothesis that tortoises randomly walked among habitat strata while they were foraging. To perform this test, we compared the distances (m) walked by tortoises within each of three strata to the randomly expected values (derived using the relative coverages of each stratum) using a χ2 test. Data for each phenological period were tested separately. We addressed the question whether distances walked by tortoises within each habitat stratum varied across phenological periods using a Kruskal-Wallis test; we tested each habitat stratum with phenological period as the independent variable. We addressed the question whether lengths of foraging routes differed among phenological periods by conducting a Kruskal-Wallis test with phenological period as the independent variable. Tests yielding significant P-values were followed with post hoc pairwise comparisons using Wilcoxon rank sum tests to determine which group(s) were statistically different from other.
3.2.4 Selection of microhabitats by tortoises
We addressed the question whether tortoises randomly selected microhabitats for foraging by comparing the expected frequencies of plants consumed (assumed to be equal among the seven microhabitats) vs. observed frequencies using a χ2 test. We analyzed each phenological period separately. All statistical tests were conducted using the R statistical package (version 4.3.0, R Development Core Team, 2023; https://cran.r-project.org/ accessed June 15, 2023). For χ2 and Kruskal-Wallis tests, we designated α = 0.05 as the level of significance. In all post hoc Wilcoxon rank sum tests, we adjusted α using a Bonferroni correction.
3.2.5 Patterns of plant consumption
We estimated the relative percentage of each anatomical part consumed (i.e., flowers, leaves, stems, and fruits) by plant species using the equation:
Each species was analyzed separately for each phenological period. We did not test these data statistically because of inter-individual variation in the phenological states of the plants.
4 Results
4.1 Precipitation
During the study in the spring of 1992, the fall–winter rainfall was 240.5 mm, 96.2% above the long-term average of 122.59 mm for winter, calculated for the previous 30 years. The result was a superbloom of wildflowers. In the 30 previous years, three superblooms occurred, an average of one per decade (Table 1). In comparison, in the 1990s, eight years prior to the beginning of the megadrought, five high rainfall years and superblooms occurred. These eight years were unusually wet compared to the years prior to the study. During the ensuing megadrought (1999–2023 and possibly ongoing), five of 22 winters of high rainfall produced superblooms.
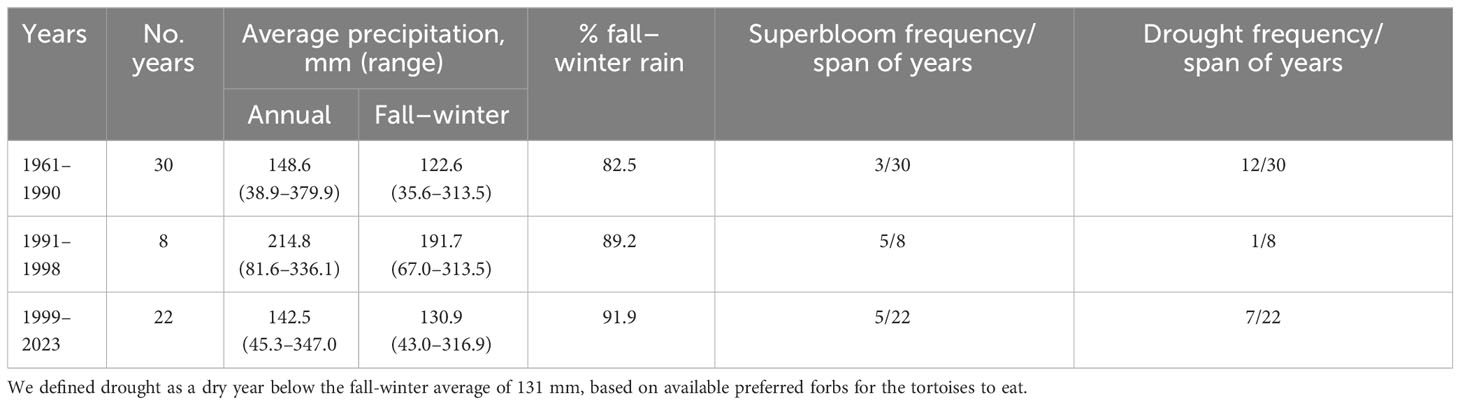
Table 1 Frequency of years with high fall–winter precipitation and associated superblooms at the Desert Tortoise Research Natural Area, western Mojave Desert, California, USA, in the 30 years before and after the study, including the 1999–2023 megadrought.
4.2 Tortoises
Tortoise foraging and movement data were obtained for 52 observation days: 24 in early spring, 20 in middle spring, and eight in late spring. The average (± SE) number of foraging bouts was 244.1 ±170.8 (range 2–1,415) for the eight male tortoises and 133.3 ±65.5 (range 2–568) for the eight females. We obtained data for 40 foraging routes among the three phenological periods.
4.3 Foraging among habitat strata and movements
During early spring, 16% of foraging routes were in alluvial fans, 68% were in hills, and 16% were in ephemeral stream channels. These values differed from the random expected percentages of 48%, 42%, and 10%, respectively (χ2 = 2054.8, df = 2, P = 0.001). The patterns of movements for foraging routes differed during the three phenological periods. In early spring, tortoises tended to embark on foraging routes from a cover site in one stratum and continued through >1 strata—usually hills, where they fed on solitary Mirabilis laevis, to small ephemeral stream channels, where they ate small groups of Astragalus layneae—before terminating at a second cover site. In middle spring, tortoises again selected foraging routes, because 88% were in hills, and 12% were in ephemeral stream channels (χ2 = 2199.9, df = 2, P = 0.001). They departed from and returned to the same cover sites, usually within the hill stratum, where they foraged on Acmispon brachycarpus or clusters of Prenanthella exigua. Eleven of the 12 recorded foraging routes only occurred in the hill stratum, whereas one was in a small ephemeral stream channel. In late spring, tortoises took roundtrips from the same cover site to forage on patches of Chamaesyce albomarginata in the ephemeral stream channel stratum (37% more than expected), while they walked in alluvial fans (41%) and hills (22%) less than expected (χ2 = 841.7, df = 2, P = 0.001). Although the tortoises frequently walked along stream channels while they were foraging, there was no standing surface water in these channels—or anywhere else on the study site—during the entire spring activity season.
4.4 Within seasonal variations in movements
The average length of foraging routes was 268 ± 45.7 m (n = 18) in early spring, 185 ± 39.9 m (n = 12), and 161 ± 37.8 m (n = 6) in middle and late spring periods, respectively. The lengths of foraging routes did not vary during the three periods (Kruskal-Wallis test, P = 0.424).
Distances of foraging routes differed by habitat strata and phenological period. Within the alluvial fan stratum, distances varied among spring periods (Kruskal-Wallis test, P = 0.035). During middle spring, tortoises completely avoided alluvial fan areas, whereas they foraged in this stratum during the early and late spring periods (post hoc Wilcoxon rank sum tests, P = 0.025, P = 0.012, respectively). The distances walked in the alluvial fan stratum between early and late spring did not differ (post hoc Wilcoxon rank sum tests, P = 0.710). In contrast, tortoises showed no difference in the distances of foraging routes within hill and stream channel strata over the entire spring season (Kruskal-Wallis tests, P = 0.078 and P = 0.070, respectively).
4.5 Selective use of microhabitats
Foraging in microhabitats varied depending on the phenological period. During early spring, tortoises fed on plants growing in the intershrub space microhabitat and mostly ignored plants in other microhabitats (χ2 = 1720.5, df = 6, P = 0.001). Nearly 60% of the plants eaten were in the intershrub space, including some preferred species, i.e., Mirabilis laevis and Astragalus didymocarpus. Other preferred forage species, particularly A. layneae and Eremothera boothii, were in small and large sandy ephemeral stream channel microhabitats, respectively. These microhabitats provided open spaces for foraging. In middle spring, nearly 90% of plants consumed were in the intershrub space microhabitat (χ2 = 6363, df = 6, P = 0.001). Comparable to the early spring period, tortoises fed extensively on the herbaceous perennial A. layneae along the margins of small stream channel microhabitats. They rarely foraged in dripline and canopy microhabitats. During the late spring period, tortoises continued to feed on plants primarily located in the intershrub space (~60%) and small stream channel (~37%) microhabitats (χ2 = 1041.8, df = 6, P = 0.001). The most frequently eaten species was the herbaceous perennial Chamaesyce albomarginata, which appeared restricted to intershrub space and small stream channel microhabitats.
4.6 Plant parts consumed by tortoises
Tortoises clipped off bite-sized pieces of plants before swallowing them. The tortoises only fed on above-ground plant parts and never pulled plants from the ground or ate roots. In early spring, tortoises, on average, consumed 46% of aboveground biomass from each plant encountered (Table 2). On average, tortoises ate ~60% (range, 59–63%) of flowers, leaves, and stems on each plant across all forage species (Table 2). In contrast, they only consumed fruits on ~9% of each plant across all forage species (Table 2). Though tortoises appeared to generally eat food plants indiscriminately, close inspection of the data suggested that they were selective about the plant parts they consumed for some forage plants. For example, tortoises ate fruits from: 36 of the 40 (90%) Erodium cicutarium plants, 9 of 11 Pectocarya spp. individuals, and both individuals of Tropidocarpum gracile (Table 2). We repeatedly observed an apparent avoidance of flowers, leaves, and stems of E. cicutarium, a non-native species (Table 2). When tortoises encountered Eremothera boothii, the fourth favored forage species by bite counts, they bit off the entire inflorescence, which dropped to the ground, before eating other parts of the plant (Table 2). This behavior was not observed for other forage species. It is important to add that none of the preferred food plants were so large that they were inaccessible to the tortoises (e.g., fruits).
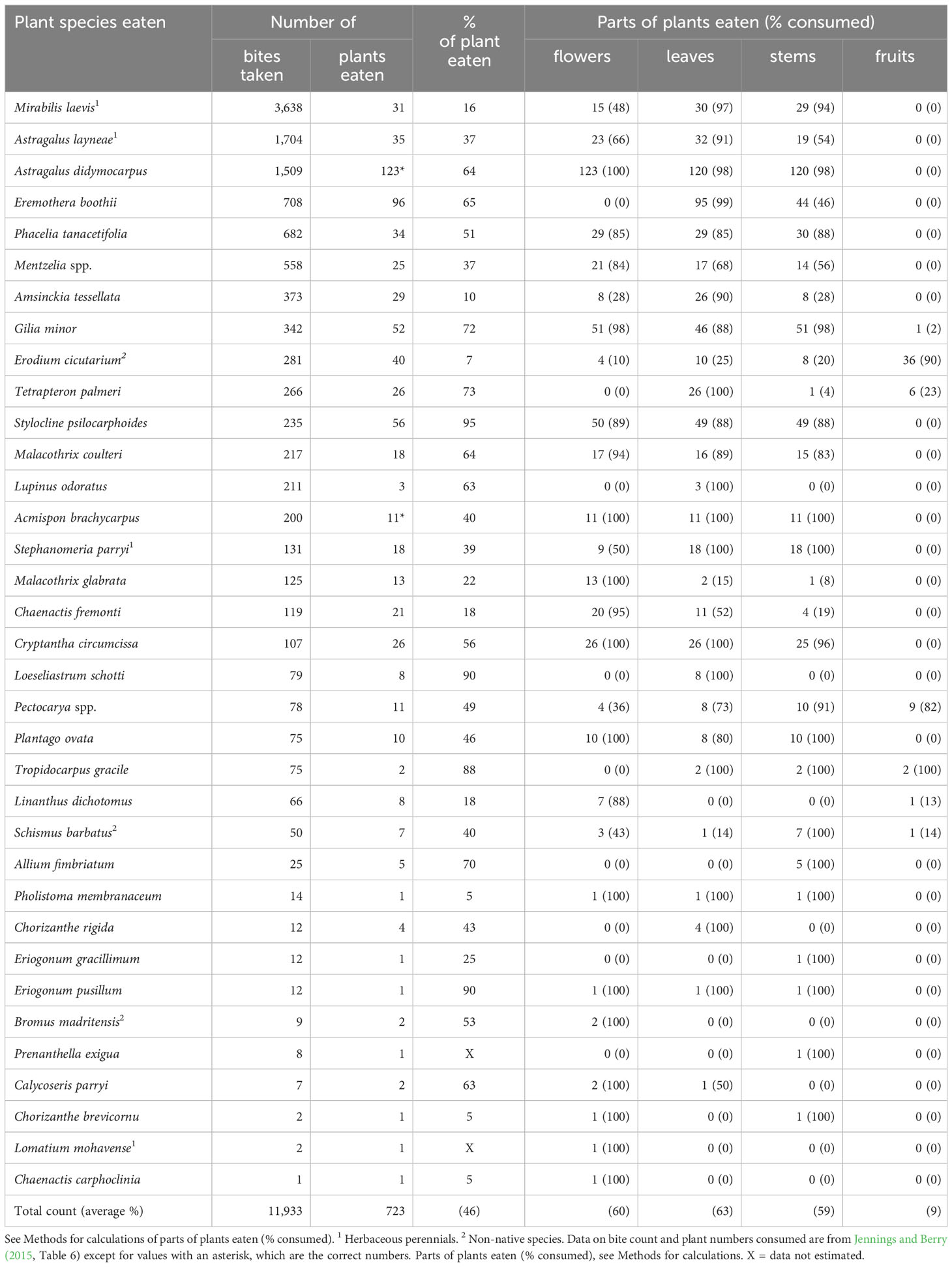
Table 2 Characteristics of winter annual and herbaceous perennial species of plants and plant parts consumed by adult desert tortoises (Gopherus agassizii) during early spring (the first phenological period: March and April) at the Desert Tortoise Research Natural Area, western Mojave Desert, California, USA, in 1992.
Throughout middle spring, tortoises maintained a pattern of consuming nearly half (41%) of each forage plant (Table 3); that is, they almost always consumed the leaves, stems, and fruits of Acmispon brachycarpus, a species that largely finished flowering but remained green and succulent (Table 3). Similarly, whenever tortoises fed on the annual Prenanthella exigua, the second most-preferred plant species by bite counts, they generally only ate flowers and stems, the only available parts (Table 3). Some foraging behaviors observed in early spring were repeated during middle spring (e.g., the apparent preference for the fruits of E. cicutarium and avoidance of flowers of E. boothii; Table 3). Overall, tortoises appeared to focus more on eating the vegetative parts of plants; on average they consumed leaves and stems 73% and 76% of the time, respectively (Table 3). The frequency of eating flowers (43%) was reduced, and consumption of fruits (32%) increased (Table 3).
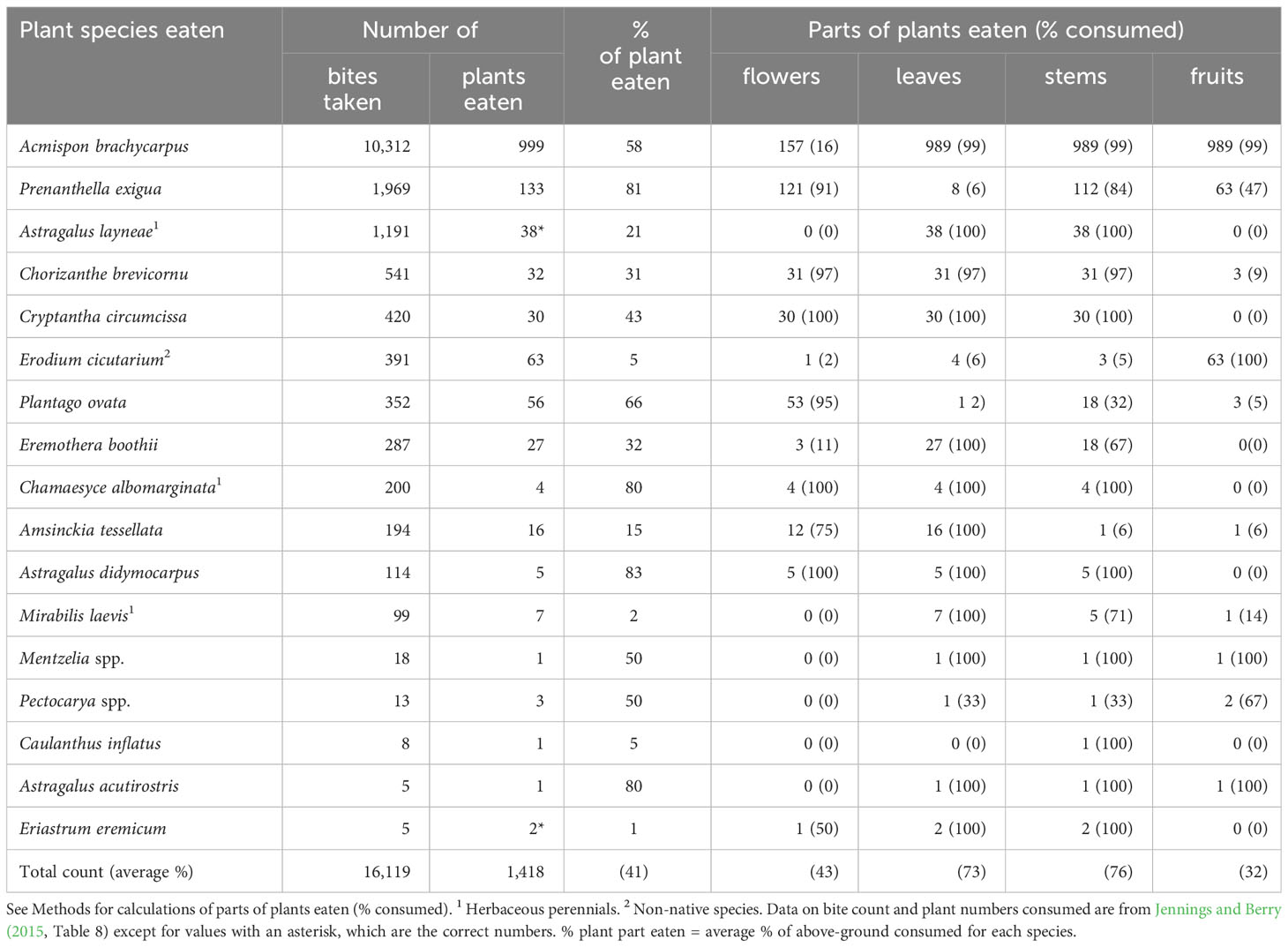
Table 3 Characteristics of winter annual and herbaceous perennial species of plants and plant parts consumed by adult desert tortoises (Gopherus agassizii) during middle spring (the second phenological period: May) at the Desert Tortoise Research Natural Area, western Mojave Desert, California, USA, in 1992.
In late spring, tortoise foraging tactics differed from the other time periods. On average, they consumed a smaller percentage of each forage plant (31%) compared to earlier periods. For the preferred forage species, Chamaesyce albomarginata, tortoises tended to eat ~30% of each plant (Table 4). They ate a larger fraction of the green and succulent E. cicutarium (20%) compared with early and middle spring periods (7% and 5%, respectively). The leaves, stems, and fruits of succulent E. cicutarium were consumed in a largely equal manner compared to early spring (Table 4). The tortoises also were less selective about parts to eat on succulent E. boothii; they ate flowers more frequently than leaves and stems (Table 4). Another change in foraging habits was the substantial inclusion of dried annual plants—annuals that had flowered earlier in the year but had flowered and dried a year earlier (Table 4). The tortoises appeared to eat many dried plants comparable to when the plants were succulent (Table 4).
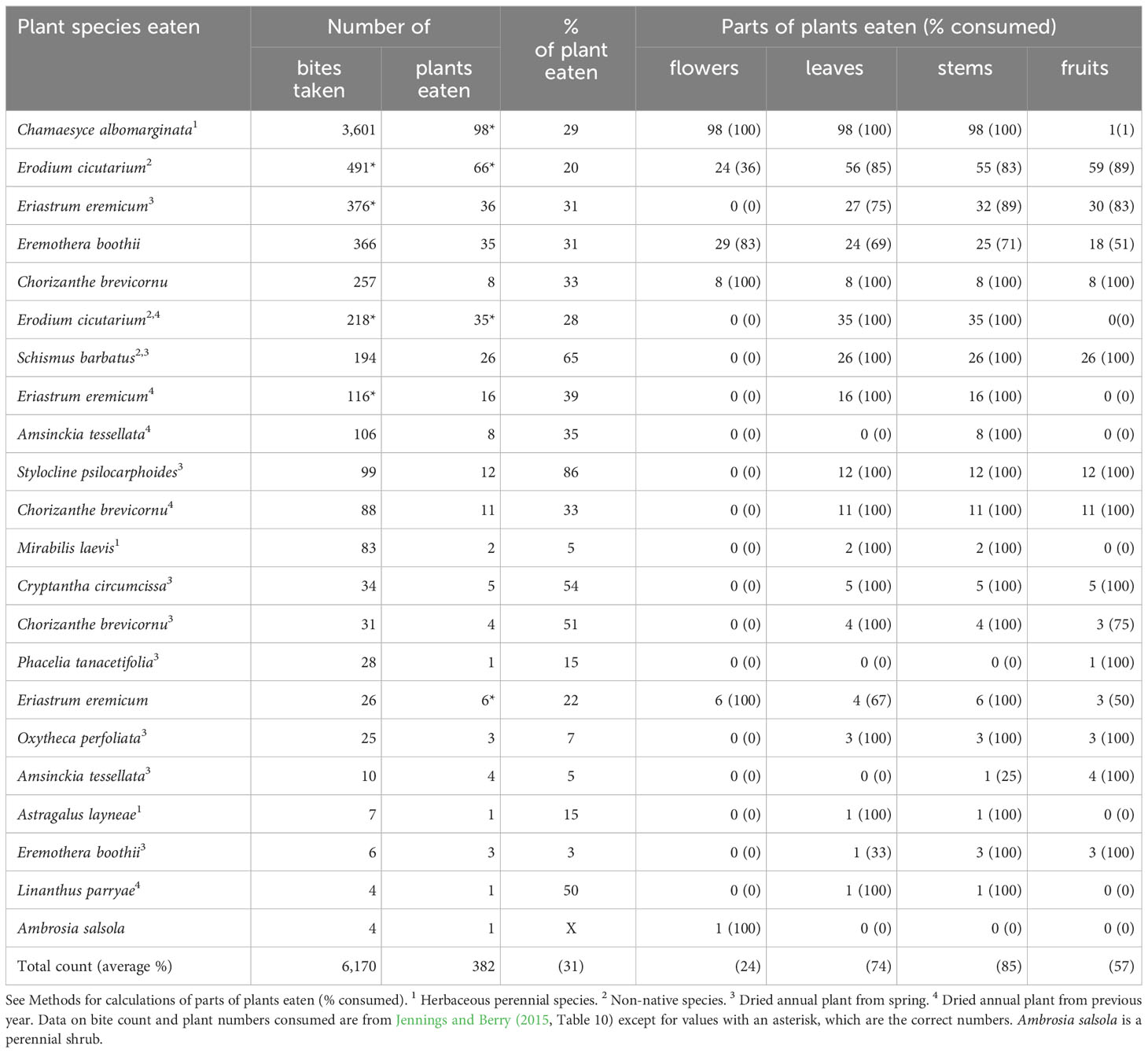
Table 4 Characteristics of winter annual and herbaceous perennial species of plants and plant parts consumed by adult desert tortoises (Gopherus agassizii) during late spring (the third phenological period: June) at the Desert Tortoise Research Natural Area, western Mojave Desert, California, USA, in 1992.
5 Discussion
5.1 Frequency of superblooms in a warming climate
The proportions of high winter rains that produce superblooms varied in the last 60 years in the vicinity of the study site from approximately one every 10 years between 1961 and 1990, to a cluster of five in eight years between 1991 and 1998. Since 1999, the return frequency of superblooms averaged less than five years during the megadrought. The increase in extreme precipitation events was predicted to occur with increases in global temperatures as part of anthropogenic climate warming (Williams et al., 2020; Cook et al., 2022; Williams et al., 2022). The superblooms during the megadrought years and beyond may not carry the same high productivity of annual blooms or length of the spring blooming season, because of decreases in soil moisture and increasing heat waves (Williams et al., 2020; Cook et al., 2021; Cook et al., 2022; Dannenberg et al., 2022; Thompson et al., 2022; Williams et al., 2022). Annual and herbaceous perennial plants may dry up earlier, limiting the foraging time for tortoises.
Superblooms and years of average and above rainfall are times for tortoises of all sizes to consume succulent green plants, develop fat bodies, fill bladders with dilute fluid, and grow (Henen et al., 1998; Berry et al., 2023). This is especially important for hatchling, juvenile, and immature tortoises, e.g., Berry et al. (2023). Although our findings pertain to a single site during a superbloom, we note that the preferred forage plants observed during a superbloom at the DTRNA were also present and consumed by tortoises at a site in the Soda Mountains in the central Mojave Desert in other years where tortoises fed on Acmispon brachycarpus and Prenanthella exigua in a year of above average rainfall (Berry et al., 2006; KHB, personal observations). The same forage plant species also occurred during years of average and above average rainfall, but not at the high levels of abundance (data not shown). Depending on the timing of winter precipitation, not all species bloom in such years, even during superblooms. Notably, in a multi-year study of juvenile, immature, and young adult tortoises at a site south of the DTRNA, immature and young adult tortoises grew during a dry year (winter rain, 54.01 mm, water year 71.07 mm) (Table 1 in Berry et al., 2023).
Dry or drought years associated with decadal changes in precipitation between 1961 and 2023 at the study site and elsewhere deserve consideration too, because tortoises cannot live for long periods without free water and food (Henen et al., 1998). Increased mortality from dehydration and starvation occurred when one dry year followed another in wild populations, with small, young tortoises especially vulnerable (Peterson, 1996; Berry et al., 2002; Longshore et al., 2003). From 1961 to 1990, droughts occurred in 12 out of 30 years, whereas during 1991–1998, only one drought occurred in eight years (Table 1). From the start of the megadrought through winter rains in 2022–2023, dry years occurred in seven out of 22 years, an increase of 25% from the 1961 to 1990 period. Except during 1991–1998, two years of drought occasionally occurred sequentially.
Summer and winter rains are important to tortoises for drinking free water throughout the Mojave Desert (Henen et al., 1998). Prior to this study, from 1961–1990, summer rains averaged 18% of annual rainfall, whereas during the megadrought, summer rains had declined to 8% of annual rain (Table 1). The western Mojave Desert rarely receives the extreme rainfall events typical of monsoons experienced in the eastern and northeastern Mojave Desert (e.g., Bhattacharya et al., 2023), although greater portions of annual rainfall occur in summer in these regions of the Mojave Desert than in the western Mojave Desert (Rowlands, 1995).
5.2 Regional and local differences in annual and herbaceous perennial plant species
Species of available annual and herbaceous perennial native and non-native plants may differ depending on desert region, precipitation patterns, surficial geology, topography, elevation, and history of anthropogenic uses (e.g., Burge and Bradley, 1976; Avery and Neibergs, 1997; Drake et al., 2015). For example, Burge and Bradley (1976) described bite counts and frequency of use of plant species eaten by tortoises at a site where homes were under construction in the northeastern Mojave Desert of Nevada. Nineteen species of annuals, 13 species of shrubs, herbaceous perennials, and cacti occurred onsite; of these, tortoises ate parts of three species of herbaceous perennials, four species of cacti, and 10 species of forbs and grasses during spring and summer. Of the 100 bites observed, 73% were on Sphaeralcea ambigua, Plantago insularis, and the native annual grass, Festuca octoflora. Drake et al. (2015) also reported use of S. ambigua by tortoises in a burned area in Nevada. At a site in Ivanpah Valley, California, also in the northeastern Mojave Desert, observers reported dietary overlap between tortoises and cattle (Avery and Neibergs, 1997). The site had been continually grazed between the 1870s and mid-1990s. Seven major forage species and 12 minor species were noted as part of the tortoise diet, but no bite counts were reported (Avery and Neibergs, 1997). Of the 19 species, two were common non-natives. In the studies by Burge and Bradley (1976) and Avery and Neibergs (1997), some species eaten by the tortoises were on the plant lists described in Tables 2–4, some were new, and some were summer annuals.
5.3 Composition and productivity of plants during superblooms and other years
During the superbloom of 1992, annual and herbaceous perennial plants began to grow in the weeks following the arrival of substantial late winter precipitation. Overall species richness and biomass of these ephemeral plants peaked in April before the plants gradually dried out and became senescent during middle to late spring periods (Jennings, 2001; SI-1). Native annuals formed most of the annual biomass, especially throughout the early spring period (SI-1). Non-native grasses in the genera Bromus spp. and Schismus spp. and forbs can be successful competitors of native annual plants in the Mojave Desert (Brooks, 2000). The effects of competition can vary by year and level of precipitation. Because the non-native grasses and forb (Erodium cicutarium) are so successful, they have negative effects on biomass of native forbs eaten by tortoises (Jennings and Berry, 2015). Results differ by year (Jennings and Berry, 2015).
Estimates of native and non-native annual biomass varied between wet years, between wet and dry years, and between protected and unprotected sites, following the variations in patterns of precipitation and levels of disturbance (SI-1). During dry years (1989, 1994, 1999, 2012), non-native annual species dominated the annual plant biomass (91–100%) at unprotected sites in the western, southern, and central Mojave Desert but were lower in protected habitats (SI-1). In contrast, non-native annual plant biomass tended to be lower during wet years (1993, 1995, 1997); estimates were 56–69% on protected sites and 30–86% on unprotected sites (SI-1).
Nonetheless, because production of richness and biomass of winter-annual species depends on the amounts of fall-winter precipitation, the predicted swings between extreme dry and wet years as the climate warms are likely to present tortoises with varying nutritional challenges (Rowlands et al., 1982; Rowlands, 1995; Brooks and Berry, 2006; Williams et al., 2020; Cook et al., 2021; Cook et al., 2022). During years with high, above average precipitation, a high diversity of native annual species is likely to bloom and thus be available as potential forage for tortoises (SI-1). In contrast, in extremely dry years—such as occurred in 1989 and 2012—most annuals did not germinate, leaving tortoises with little or no available food—or only with poor quality non-native annual grasses (Hazard et al., 2009; Hazard et al., 2010; SI-1).
Climate warming may alter the flowering phenologies of preferred tortoise forage species comparable to observations for other plant species (e.g., Anderson et al., 2012). Changes in availability of food plants may have severe consequences for tortoises in the Mojave Desert and elsewhere because tortoises track the flowering phenologies of preferred species during a superbloom and probably in other years with differing precipitation levels (Jennings, 2002; Jennings and Berry, 2015).
Another determinant of annual plant production is the level of anthropogenic disturbance to the land (e.g., livestock grazing and off-highway vehicle use). Inside protected areas such as the DTRNA, the biomass of native annuals tended to be higher than non-protected sites in both dry and wet years (Brooks, 2000; Brooks and Berry, 2006; Berry et al., 2020). Thus, production of native annual plants is likely to be limited on disturbed lands during drought years (e.g., Brooks and Berry, 2006).
5.4 Other factors potentially affecting forage preferences
The presence of contaminants and toxicants in some food plants may affect nutritional qualities and taste. Chaffee and Berry (2006) analyzed samples of soils and ephemeral stream bed sediments, and plants known or thought to be eaten by tortoises in six desert regions for potential contaminants or toxicity. Elevated levels of some elements were identified locally in some plant species, and sources were associated with contamination from mines, mine wastes, roads, railroads, and other disturbances (Chaffee and Berry, 2006). For example, elevated concentrations of arsenic, potentially toxic to tortoises, were identified in regions (and plants) associated with two mining districts (Chaffee and Berry, 2006). Elevated levels of arsenic were evident in an ill, necropsied tortoise from a mining district (Homer et al., 1998; Homer et al., 2001; Selzer and Berry, 2005). Dust from human disturbances settling on plants may contribute to uptake of such toxicants, or by contact with or breathing contaminated soils (Chaffee and Berry, 2006).
5.5 Forage routes and preferred microhabitats
The foraging routes taken by adult tortoises offer valuable information for ongoing and future recovery actions, e.g., projects for restoration of degraded and burned habitats and microhabitats that could benefit from protection from human disturbance. Tortoises more frequently used habitat strata in hills and ephemeral stream channels than on alluvial fans; microhabitats selected for foraging were the intershrub spaces and ephemeral stream channels. These microhabitats were open spaces in the environment and where most bites were taken. Importantly, the ephemeral stream channels were small, not the often wide, axial valley stream channels or washes with large shrubs and trees, but the third, fourth, or higher-level ephemeral stream channels upslope and draining into secondary channels to the axial valley ephemeral stream channel.
Vegetation in general and microhabitats favored by tortoises are vulnerable to disturbance from anthropogenic uses: livestock grazing, vehicle use, whether by recreationists or other users, and other sources. Livestock grazing occurred in desert tortoise habitats from the 1860s and continues in some critical habitat units (e.g., U.S. Bureau of Land Management, 1980; Muhn and Stuart, 1988; U.S. Fish and Wildlife Service [USFWS], 1994; U.S. Bureau of Land Management, 2019). Livestock grazing contributed to alteration of composition of perennial and annual plants and invasion of non-native species in some designated critical habitat units for desert tortoises (e.g., Webb and Stielstra, 1979; Fleischner, 1994; Brooks and Berry, 2006; Brooks et al., 2006; Berry and Murphy, 2019). Livestock walk in ephemeral stream channels and intershrub spaces to feed, feed on annual plants and within shrubs, and seek shade within shrubs or under trees. Recreational vehicle use increased in intensity in the 1970s, with continuing expansion (e.g., U.S. Bureau of Land Management, 2019). This use remains a major factor in deterioration and fragmentation of tortoise habitats within the geographic range (e.g., U.S. Bureau of Land Management, 1973; U.S. Bureau of Land Management, 1980; Webb and Wilshire, 1983; U.S. Fish and Wildlife Service [USFWS], 1990; Brooks and Lair, 2009; U.S. Bureau of Land Management, 2019). Both authorized and unauthorized travel continues to occur in ephemeral stream channels and cross-country in intershrub spaces, and results in damaging shrubs (Berry et al., 2014; Berry et al., 2020).
6 Conclusions
In the American West where tortoises occur, climate warming and droughts are expected to bring greater aridity, prolonged and severe dry spells (Overpeck and Udall, 2020), more extreme heat waves (Dannenberg et al., 2022), and reductions in soil moisture (Cook et al., 2021; Cook et al., 2022). The frequency of superblooms and production of annual herbaceous perennial plants described here are likely to change as the century progresses. Superblooms were previously important to desert tortoises because they provided an abundance of succulent, green annual forbs, and herbaceous perennial plants, some of which are low in potassium and edible to tortoises. Years with superblooms were important in providing tortoises with sufficient resources to recover from droughts and other years of low rainfall, to develop fat stores, and to grow. New data on parts of plants utilized by tortoises provide a greater understanding of how tortoises forage. Similarly, the movements and travels focused on foraging added clarity to the value of different parts of the habitat and specific microhabitats essential for feeding. Maintenance and protection of these microhabitats from anthropogenic disturbances can contribute to recovery efforts for the species.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because the study was observational, and thus did not require handling of desert tortoises. This study did receive reviews prior to its commencement from J. Campbell at the University of Texas, Arlington and the U.S. Bureau of Land Management.
Author contributions
WBJ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. KHB: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The U.S. Bureau of Land Management (Contract No. B950-C2-0014) and U.S. Geological Survey (Order No. 140G0322P0158) provided funds to WBJ. Both agencies supported KHB in writing and reviewing.
Acknowledgments
We thank J. Gooch for assistance in the field. We appreciate reviews from D. Elam, J. Perkins, A. M. Ray, R. I. Bancila, and S. Henke, which increased the quality of the manuscript. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) KHB declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/famrs.2023.1283255/full#supplementary-material
References
Abella S. R., Berry K. H., Ferrazzano S. (2023). Techniques for restoring damaged Mojave and western Sonoran habitats, including those for threatened desert tortoises and Joshua trees. Desert Plants 38, 4–52.
Allison L. J., McLuckie A. M. (2018). Population trends in desert tortoises (Gopherus agassizii). Herpetol. Conserv. Biol. 13, 433–452.
Anderson J. T., Inouye D. W., McKinney A. M., Colautti R. I., Mitchell-Olds T. (2012). Phenotypic plasticity and adaptive evolution contribute to advancing flowering phenology in response to climate change. Proc. Royal Soc. B 279.1743, 3843–3852. doi: 10.1098/rspb.2012.1051
Ault T. R., Mankin J. S., Cook B. I., Smerdon J. E. (2016). Relative impacts of mitigation, temperature, and precipitation on 21st century megadrought risk in the American Southwest. Sci. Adv. 2, 1–8. doi: 10.1126/sciadv.1600873
Avery H. W., Neibergs A. (1997). “Effects of cattle grazing on the desert tortoise.” Van Abbema J., In Proceedings: Conservation, Restoration, and Management of Tortoises and Turtles—An International Conference, (New York: New York Turtle and Tortoise Society and WCS Turtle Recovery Program), 13–19.
Barboza P. S. (1995). Nutrient balances and maintenance requirements for nitrogen and energy in desert tortoises (Xerobates agassizii) consuming forages. Comp. Biochem. Physiol. 112A, 537–545. doi: 10.1016/0300-9629(95)02023-3
Berry K. H., Allison L. J., McLuckie A. M., Vaughn M., Murphy R. W. (2021) Gopherus agassizii, Mojave Desert tortoise (The International Union for Conservation of Nature Red List of Threatened Species™ (IUCN 2021). Available at: https://www.iucnredlist.org/species/97246272/3150871 (Accessed 14, 2023).
Berry K. H., Bailey T. Y., Anderson K. M. (2006). Attributes of desert tortoise populations at the National Training Center, Central Mojave Desert, California, USA. J. Arid Environ. 67 Suppl, 165–191. doi: 10.1016/j.jaridenv.2006.09.026
Berry K. H., Lyren L. M., Yee J. L., Bailey T. Y. (2014). Protection benefits desert tortoise (Gopherus agassizii) abundance: the influence of three management strategies on a threatened species. Herpetological Monogr. 28, 66–92. doi: 10.1655/Herpmonographs-D-14-00002
Berry K. H., Mack J. S., Anderson K. M. (2023). Variations in climate drive behavior and survival of small desert tortoises. Front. Ecol. Evol. doi: 10.3389/fevo.2023.1164050
Berry K. H., Murphy R. W. (2019). Gopherus agassizii (Cooper 1861) – mojave desert tortoise, agassiz’s desert tortoise. Chelonian Res. Monogr. 5, 1–45. doi: 10.3854/crm.5.109.agassizii.v1.2019
Berry K. H., Spangenberg E. K., Homer B. L., Jacobson E. R. (2002). Deaths of desert tortoises following periods of drought and research manipulations. Chelonian Conserv. Bio. 4, 436–448.
Berry K. H., Yee J. L., Shields T. A., Stockton L. (2020). The catastrophic decline of tortoises at a fenced Natural Area. Wildl. Monogr. 205, 1–53. doi: 10.1002/wmon.1052
Bhattacharya T., Feng R., Maupin C. R., Coats S., Brennan P. R., Carter E. (2023). California margin temperatures modulate extreme summer precipitation in the desert Southwest. Environ. Res. Lett. 18, 104048. doi: 10.1088/1748-9326/acfd43
Brooks M. L. (2000). Competition between alien annual grasses and native annual plants in the Mojave Desert. Am. Midl. Nat. 144, 92–108. doi: 10.1674/0003-0031(2000)144[0092:CBAAGA]2.0.CO;2
Brooks M. L., Berry K. H. (2006). Dominance and environmental correlates of alien annual plants in the Mojave Desert, USA. J. Arid Environ. 67 (Supplement), 100–124. doi: 10.1016/j.aridenv.2006.09.021
Brooks M. L., Lair B. M. (2009). “Ecological effects of vehicular routes in a desert ecosystem,” in The Mojave Desert. Eds. Webb R. H., Festermaker L. F., Heaton J. S., Hughson D. L., McDonald E. V., Miller D. M. (Reno, NV: University of Nevada Press), 168–195.
Brooks M. L., Matchett J. R. (2006). Spatial and temporal patterns of wildfires in the Mojave Desert 1980–2004. J. Arid Environ. Supplement), 67, 148–164. doi: 10.1016/j.jaridenv.2006.09.027
Brooks M. L., Matchett J. R., Berry K. H. (2006). Effects of livestock watering sites on alien and native plants in the Mojave Desert, USA. J. Arid Environ. Supplement) 67, 125–147. doi: 10.1016/j.aridenv.2006.09.027
Burge B. L., Bradley W. G. (1976). “Population density, structure and feeding habits of the desert tortoise, Gopherus agassizi, in a low desert study area in southern Nevada,”. In: Proc. 1st Ann. Desert Tortoise Council Symp (Las Vegas, Nevada, USA: The Desert Tortoise Council). Available at: https://deserttortoise.org (Accessed 2, 2023).
Chaffee M. A., Berry K. H. (2006). Abundance and distribution of selected elements in soils, stream sediments, and selected forage plants from desert tortoise habitat in the Mojave and Colorado deserts, USA. J. Arid Environ. Supplement) 67, 35–87. doi: 10.1016/j.jaridenv.2006.09.018
Christopher M. M., Berry K. H., Wallis I. R., Nagy K. A., Henen B. T., Peterson C. C. (1999). Reference intervals and physiologic alterations in hematologic and biochemical values of free ranging desert tortoises in the Mojave Desert. J. Wildl. Dis. 35, 212–238. doi: 10.7589/0090-3558-35.2.212
Cook B. I., Mankin J. S., Williams A. P., Marvel K. D., Smerdon J. E., Liu H. (2021). Uncertainties, limits, and benefits of climate change mitigation for soil moisture drought in southwestern North America. Earth’s Future 9, e2021ER002013. doi: 10.1029/2021EF002014
Cook B. I., Smerdon J. E., Cook E. R., Williams A. P., Anchukaitis K. J., Mankin J. S., et al. (2022). Megadroughts in the common era and the anthropocene. Nat. Rev. Earth Environ. 3, 741–757. doi: 10.1038/s43017-022-00329-1
Daly C., Halbleib M., Smith J. I., Gibson W. P., Doggett M. K., Taylor G. H., et al. (2008). Physiographically sensitive mapping of climatological temperature and precipitation across the conterminous United States. Int. J. Climatol. 28, 2031–2064. doi: 10.1002/joc.1688
Dannenberg M. P., Yan D., Barnes M. L., Smith W. K., Johnston M. R., Scott R. L., et al. (2022). Exceptional heat and atmospheric dryness amplified losses of primary production during the 2020 U.S. Southwest hot drought. Global Change Biol. 22, 4794–4806. doi: 10.111/gcb.16214
Drake K. K., Bowen L., Nussear K. E., Esque T. C., Berger A. J., Custer N. A., et al. (2016). Negative impacts of invasive plants on conservation of sensitive desert wildlife. Ecosphere 7, e01531. doi: 10.1002/ecs2.1531
Drake K. K., Esque T. C., Nussear K. E., DeFalco L. A., Scoles-Sciulla S. J., Modlin A. T., et al. (2015). Desert tortoise use of burned habitat in the eastern Mojave Desert. J. Wildlife Manage. 79, 618–629. doi: 10.1002/ecs2.1531
Duda J. J., Krzysik A. J., Freilich J. E. (1999). Effects of drought on desert tortoise movement and activity. J. Wildl. Manage. 63, 1181–1192. doi: 10.2307/3802836
Fleischner T. L. (1994). Ecological costs of livestock grazing in western North America. Conserv. Biol. 8, 629–644. doi: 10.1046/j.1523-1739.1994.08030629.x
Hardy R. (1976). The Utah population—a look in the 1970’s. In: Proc. 1st Ann. Desert Tortoise Council Symp (Nevada, USA: The Desert Tortoise Council). Available at: https://www.deserttortoise.org (Accessed 10, 2023).
Hazard L. C., Shemanski D. R., Nagy K. A. (2009). Nutritional quality of natural foods of juvenile desert tortoises (Gopherus agassizii): energy, nitrogen, and fiber digestibility. J. Herpetol. 43, 38–48. doi: 10.1670/07-160R1.1
Hazard L. C., Shemanski D. R., Nagy K. A. (2010). Nutritional quality of natural foods of juvenile and adult desert tortoises (Gopherus agassizii): calcium, phosphorus, and magnesium digestibility. J. Herpetol. 44, 135–147. doi: 10.1670/08-123.1
Henen B. T. (1997). Seasonal and annual energy budgets of female desert tortoises (Gopherus agassizii). Ecology 78, 283–296. doi: 10.1890/0012-9658(1997)078[0283:SAAEBO]2.0.CO;2
Henen B. T., Peterson C. C., Wallis I. R., Berry K. H., Nagy K. A. (1998). Effects of climatic variation on field metabolism and water relations of desert tortoises. Oecologia 117, 365–373. doi: 10.1007/s004420050669
Henen B. T. (2002). Energy and water balance, diet, and reproduction of female desert tortoises (Gopherus agassizii). Chelonian Conserv. Bio. 4, 319–328.
Homer B. L., Berry K. H., Brown M. B., Ellis G., Jacobson E. R. (1998). Pathology of diseases in wild desert tortoises from California. J. Wildlife Dis. 35, 508–523. doi: 10.7589/0090-3558-34.3.508
Homer B. L., Li C., Berry K. H., Denslow N. D., Jacobson E. R., Sawyer R. H., et al. (2001). Soluble scute proteins of healthy and ill desert tortoises (Gopherus agassizii). Am. J. Veterinary Res. 62, 104–110. doi: 10.2460/ajvr.2001.62.104
Jennings W. B. (2001). Comparative flowering phenology of plants in the western Mojave Desert. Madroño 48, 162–171.
Jennings W. B. (2002). Diet selection by the desert tortoise in relation to the flowering phenology of ephemeral plants. Chelonian Conserv. Biol. 4, 353–335.
Jennings W. B., Berry K. H. (2015). Desert tortoises (Gopherus agassizii) are selective herbivores that track the flowering phenology of their preferred food plants. PloS One 10, e0116716. doi: 10.371/journal.pone.0116716
Jennings W. B., Fontenot C. L. (1993). Observations on the feeding behavior of desert tortoises (Gopherus agassizii) at the Desert Tortoise Research Natural Area, Kern County, California. In: Proc. Desert Tortoise Counc. Symp (Nevada, USA: The Desert Tortoise Council). Available at: https://www.deserttortoise.org (Accessed 20, 2023).
Jepson Flora Project (2023) Jepson eFlora. Available at: https://ucjeps.berkeley.edu/eflora/ (Accessed 6, 2023).
Klinger R., Underwood E. C., McKinley R., Brooks M. L. (2021). Contrasting geographic patterns of ignition probability and burn severity in the Mojave Desert. Front. Ecol. Evol. 9. doi: 10.3389/fevo.2021.593167
Longshore K. M., Jaeger J. R., Sappington J. M. (2003). Desert tortoise (Gopherus agassizii) survival at two eastern Mojave Desert sites: death by short-term drought? J. Herpetology 37, 169–177. doi: 10.1670/0022-1511(2003)037[0169:DTGASA]2.0.CO;2
Medica P. A., Nussear K. E., Esque T. C., Saethre M. B. (2012). Long-term growth of desert tortoises (Gopherus agassizii) in a southern Nevada population. J. Herpetol. 46, 213–220. doi: 10.1670/11-327
Muhn J., Stuart H. R. (1988). Opportunity and Challenge, The Story of BLM (Washington, D.C: U.S. Dept. of the Interior, Bureau of Land Management, U.S. Government Printing Office).
Oftedal O. T. (2002). “Nutritional ecology of the desert tortoise in the Mohave and Sonoran deserts,” in The Sonoran Desert Tortoise: Natural History, Biology, and Conservation. Ed. Van Devender T. R. (Tucson, AZ: University of Arizona Press and the Arizona-Sonora Desert Museum), 194–241.
Oftedal O. T., Allen M. E., Chung A. L., Reed R. C., Ullrey D. E. (1994). Nutrition, urates, and desert survival: Potassium and the desert tortoise (Gopherus agassizii). Proc. Am. Assoc. Zoo Veterinarians Ann. Meeting 1994 308–313.
Oftedal O. T., Hillard S., Morafka D. J. (2002). Selective spring foraging by juvenile desert tortoises (Gopherus agassizii) in the Mojave Desert: evidence of an adaptive nutritional strategy. Chelonian Conserv. Biol. 4, 341–352.
Overpeck J. T., Udall B. (2020). Climate change and the aridification of North America. Proc. Natl. Acad. Sci. U.S.A. 117, 11856–11858. doi: 10.1073/pnas.2006323117
Peterson C. C. (1996). Anhomeostasis: seasonal water and solute relations in two populations of the desert tortoise (Gopherus agassizii) during chronic drought. Physiol. Zool 69, 1324–1358. doi: 10.1086/physzool.69.6.30164263
R Development Core Team (2023) R: A Language and Environment for Statistical Computing. Available at: https://cran.r-project.org/ (Accessed 15, 2023).
Rowlands P. (1995). “Regional bioclimatology of the California Desert,” in The California Desert: An Introduction to Natural Resources and Man’s Impact. Eds. Latting J., Rowlands P. G. (Riverside, CA: June Latting Books), 95–134.
Rowlands P., Johnson H., Ritter E., Endo A. (1982). “The Mojave Desert,” in Reference Handbook on the Deserts of North America. Ed. Bender G. (Westport, CT: Greenwood Press), 103–162.
Selzer M. D., Berry K. H. (2005). Laser ablation ICP-MS profiling and semiquantitative determination of trace element concentrations in desert tortoise shells: documenting the uptake of elemental toxicants. Sci. Total Environ. 339, 253–265. doi: 10.1016/j.scitotenv.2004.07.027
Thompson V., Kennedy-Asser A. T., Vosper E., Lo Y. T. E., Huntingford C., Andrews O., et al. (2022). The 2021 western North America heat wave among the most extreme events ever recorded globally. Sci. Adv. 8, eabm6860. doi: 10.1126/sciadv.abm6860
Turner F. B., Berry K. H., Randall D. C., White G. C. (1987). Population ecology of the desert tortoise at Goffs, California 1983–1986 (Rosemead, California, USA: Report to Southern California Edison).
U.S. Bureau of Land Management (1973). Interim Critical management Plan for Recreational Vehicle Use in the California Desert (U.S. Dept. of the Interior, Bureau of Land Management, California Desert Program, USA).
U.S. Bureau of Land Management (1980). The California Desert Conservation Area Pla (California, USA: U.S. Department of the Interior, Bureau of Land Management, Desert District).
U.S. Bureau of Land Management (2019). West Mojave route network project. Final supplemental environmental impact statement for the California Desert District. BLM/CA/DOI-BLM-CA-2018-008-EIS (Moreno Valley, California, USA: USBLM).
U.S. Fish and Wildlife Service [USFWS] (1990). Endangered and threatened wildlife and plants; determination of threatened status for the Mojave population of the desert tortoise. Fed. Regist. 55, 12178–12191.
U.S. Fish and Wildlife Service [USFWS] (1994). Desert tortoise (Mojave population) recovery plan (Portland, Oregon. USA: USFWS).
U.S. Fish and Wildlife Service [USFWS] (2011). Revised Recovery Plan for the Mojave Population of the desert tortoise (Gopherus agassizii) (Reno, Nevada, USA: USFWS).
U.S. Fish and Wildlife Service [USFWS] (2015). Range-wide Monitoring of the Mojave Desert Tortoise (Gopherus agassizii): 2013–2014. Annual Report (Reno, NV: U.S. Department of the Interior, Fish and Wildlife Service).
Webb R. H., Stielstra S. S. (1979). Sheep grazing effects on Mojave Desert vegetation and soils. Environ. Manage. 3, 517–529. doi: 10.1007/BF01866321
Webb R. H., Wilshire H. G. (1983). Environmental Effects of Off-Road Vehicles (New York, New York: Springer Verlag).
Williams A. P., Cook B. I., Smerdon J. E. (2022). Rapid intensification of the merging southwestern North American megadrought in 2021. Nat. Clim. Change 12, 232–234. doi: 10.1038/s41558-022-01290-z
Williams A. P., Cook E. R., Smerdon J. E., Cook B. L., Abatzoglou J. T., Bolles K., et al. (2020). Large contribution from anthropogenic warming to an emerging North American megadrought. Science 368, 314–318. doi: 10.1126/science.aaz9600
Keywords: foraging, Gopherus agassizii, microhabitats, movements, plant parts
Citation: Jennings WB and Berry KH (2023) Selection of microhabitats, plants, and plant parts eaten by a threatened tortoise: observations during a superbloom. Front. Amphib. Reptile Sci. 1:1283255. doi: 10.3389/famrs.2023.1283255
Received: 25 August 2023; Accepted: 16 November 2023;
Published: 30 November 2023.
Edited by:
Scott Henke, Texas A&M University Kingsville, United StatesReviewed by:
Andrew M. Ray, National Park Service, United States Department of the Interior, Washington D.C., United StatesRaluca Ioana Bancila, Romanian Academy, Romania
Copyright © 2023 Jennings and Berry. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: W. Bryan Jennings, YnJ5YW4uamVubmluZ3NAdWNyLmVkdQ==; Kristin H. Berry, a3Jpc3Rpbl9iZXJyeUB1c2dzLmdvdg==
†These authors have contributed equally to this work and share first authorship
 W. Bryan Jennings
W. Bryan Jennings Kristin H. Berry
Kristin H. Berry