- 1Laboklin GmbH & Co. KG, Bad Kissingen, Germany
- 2Department of Small Mammal, Reptile and Avian Diseases, University of Veterinary Medicine Hanover, Hanover, Germany
- 3Reptile Rescue Center Munich e.V., Munich, Germany
Species specific physiology, seasonal changes, sex, and husbandry factors all influence the blood chemistry of chelonians, including vitamin D3, calcium, phosphate and magnesium levels. Problems in the supply of many of these are commonly seen in captive reptiles. The goal of this study was to measure vitamin D3, calcium, phosphate and magnesium in plasma from captive, healthy, adult Hermann’s tortoises (Testudo hermanni) and pond sliders (Trachemys scripta). Samples were categorized and compared based on species, sex, season, and access to sunlight as a central husbandry element. Blood samples of 522 Hermann’s tortoises and 188 pond sliders, taken from March to September 2022, were included in the study. New reference intervals for vitamin D3, calcium, phosphate and magnesium were established, with specific reference intervals calculated for each species, sex, and season in those cases in which significant differences were found based on these factors. For the calculation of reference intervals for vitamin D3 the factors species, season and access to sunlight were considered. Vitamin D3 levels differed by access to sunlight in some seasons depending on the species and were generally higher in Hermann’s tortoises. Plasma vitamin D3 levels did not correlate with calcium, phosphorus or magnesium levels on a larger scale in either species. Calcium, phosphate, and magnesium were higher in females than in males of both species, and magnesium was higher in Hermann’s tortoises than in pond sliders. Our results can be helpful in a more specific interpretation of blood results and support previous findings that direct sunlight is an important factor in chelonian health. More studies are needed to better understand the role of other hormonal influence on the vitamin D3, calcium, phosphate and magnesium metabolism in chelonians.
1 Introduction
Vitamin D3 plays an important role in calcium (Ca) and phosphorus (PO4) metabolism in vertebrates. Unlike other vitamins, its active form is produced endogenously beginning with the activation of a precursor in the skin, 7-dehydrocholesterol, under the influence of UVB-light. In the blood stream, 7-dehydrocholesterol is bound to vitamin D binding protein and transported to the liver, where it is hydroxylated to 25(OH) vitamin D3 which is also the storage form of vitamin D3. After transportation to the kidneys, it is metabolized to 1,25(OH)2 vitamin D3, which is the active form (Lips, 2006). As a regulatory hormone, vitamin D3 increases the absorption of Ca and PO4 in the lower intestine. 1,25(OH)2 vitamin D3 was found in humans to alter proliferation and apoptosis in skeletal cells including hypertrophic chondrocytes (Holick and Garabedian, 2006). It interacts with other hormones including parathormone (PTH) and calcitonin, which are also involved in Ca and PO4 homeostasis (Lips, 2006). In reptiles, lack of sufficient UVB-radiation, resulting in low blood concentrations of vitamin D3, as well as dietary Ca deficits, can result in a cluster of clinical disorders summarized under the name metabolic bone disease (MBD) complex. Being among the most commonly diagnosed disorders in reptile clinical practice, MBD manifests in a variety of diseases such as hypocalcemia, osteomalacia, rachitis, and nutritional secondary hyperparathyroidism. Clinical signs arising from these conditions are seen in the form of carapace deformities, fractures, demineralized eggs, or even seizures (McArthur et al., 2004; Mader, 2006). UV-radiation and vitamin D3 are therefore centrally important for reptile health. Aside from bone metabolism, vitamin D3 has also been found in mammals to be involved in immune system modulation (Holick and Garabedian, 2006). In pond sliders, it also interacts with brain regions involved in the orchestration of season-specific processes such as reproduction and related behaviors (Bidmon and Stumpf, 1994; Holick and Garabedian, 2006).
Magnesium (Mg) plays a role in vitamin D3 synthesis. It is an important activator for over 300 enzymes including ones involved in the production of the active vitamin D3 metabolites (Uwitonze and Razzaque, 2018). Mg deficiency may lead to increased intracellular Ca levels resulting in a decrease in extracellular plasma Ca levels in mammals (Alam et al., 2016). Mg complexes with PTH receptors. Severe Mg deficiency can therefore inhibit PTH secretion leading to a decrease in Ca and PO4 levels in mammals (Favus et al., 2006). Similar studies on the role of magnesium in metabolism of reptiles are still lacking.
Hermann´s tortoises (Testudo hermanni) are a terrestrial species from the northern Mediterranean in Europe. Pond sliders (Trachemys scripta) are aquatic turtles native to the south-eastern USA. Both species are popular pets in many countries and are more frequently seen in European veterinary practice than most other chelonian species. Both species originate from subtropical climates characterized by marked seasonal temperature differences (Lovich and Gibbons, 2021). As ectotherms, chelonians from these climates evade frost by hibernating in the ground or under water. This period of decreased UV exposition impacts vitamin D3 synthesis, with lower amounts synthesized during hibernation (Acierno et al., 2006; Selleri and Di Girolamo, 2012). In central Europe, chelonians are often kept indoors or under glass or plastic to bridge the often too cold and wet early spring climate conditions right after hibernation. This impacts the availability of UV light as most glass and plastic will reduce the amount of UV light transmitted and many artificial light sources do not provide sufficient UVB-fractions in their spectrum (Burger et al., 2007; Sackey et al., 2015).
Physiological values of plasma vitamin D3 in chelonians have been the subject of a number of studies (Acierno et al., 2006; Eatwell, 2008; Wiedemann, 2010; Selleri and Di Girolamo, 2012; Watson et al., 2017; Garefino and Milton, 2022). Eatwell (2008) measured vitamin D3 in three species of European tortoises and found significantly higher levels in males than in females, but no seasonal influences. Some studies have documented the influence of UVB-irradiation on vitamin D3 plasma values in red eared sliders (Trachemys scripta elegans) (Acierno et al., 2006; Selleri and Di Girolamo, 2012) and Hermann´s tortoises (Selleri and Di Girolamo, 2012). Acierno et al. (2006) reported significant increases in vitamin D3 levels in those turtles with access to UV-light. Selleri and Di Girolamo (2012) evaluated vitamin D3 levels among three groups of tortoises that either had no access to UV-light, access to artificial UV-light, or access to natural sunlight. The group with access to natural sunlight had significantly higher vitamin D3 levels than tortoises in the other two groups. Selleri and Di Girolamo (2012) used an enzyme immunoassay for vitamin D3 measurements while Acierno et al. (2006) did not mention the detection method used. Watson et al. (2017) measured plasma vitamin D3 levels in two geographically different populations of box-turtles (Terrapene c. carolina) and found no significant differences between populations and no significant influence of age-class or sex on the values measured. They also found no significant correlation between vitamin D3 plasma levels and plasma Ca levels (Watson et al., 2017). Garefino and Milton (2022) measured significantly higher vitamin D3 levels and reported enhanced health and recovery from fibropapillomatosis in green sea turtles (Chelonia mydas) exposed to sunlight. None of the studies on pond sliders and Hermann’s tortoises established reference intervals (RI) for plasma vitamin D3 levels according to ASVCP guidelines (Friedrichs et al., 2012). Physiological blood values for Ca and PO4 have been evaluated for a number of chelonian species in previous studies (Lawrence, 1987; Christopher et al., 1999; Erler, 2003; Scope et al., 2013; Leineweber et al., 2019). Several of these studies have also documented differences in levels depending on season and sex of the animals (Erler, 2003; Scope et al., 2013; Leineweber et al., 2019).
The objective of this study was to measure the plasma levels of 25(OH)-vitamin D3 as well as Ca, PO4 and Mg in healthy adult pond sliders and Hermann’s tortoises under human care, under consideration of differences in the species, sexes, seasons, and access to sunlight, aiming to evaluate interactions between each of these factors and establish reference intervals for vitamin D3 in those cases in which the number of animals tested was sufficient. The central hypothesis was that vitamin D3 levels would be higher in animals with access to sunlight and would vary depending on species, sex and season. We also expected that Ca, PO4, and Mg levels would be interdependent and also depend on vitamin D3 levels, sex, and season.
2 Materials and methods
In the context of yearly routine health checks, blood samples from clinically healthy adult Hermann’s tortoises (319 males (M), 203 females (F)) and pond sliders (53 M, 135 F) were collected from March 2022 to September 2022. The use of the samples for this study was reviewed and approved by the ethics commission of the Faculty of Veterinary Medicine of the University of Leipzig (GZ: EK 21/2021). The body weight of the Hermann’s tortoises ranged from 110 g to 3500 g (x̄ = 1014 g) and that of the pond sliders from 400 g to 3500 g (x̄ = 1364 g). Blood samples were grouped according to the season in which they were collected. Samples collected from March to June were considered spring samples, July to August early summer and September late summer samples. As both of the two sampled species undergo hibernation, no samples were collected in fall or winter. The chelonians were kept by private keepers as well as public zoological institutions and reptile rescue centers. Animal collections sampled were distributed over 37 different locations all over Germany. Average temperatures and hours of sunlight per month in Germany in 2022 compared to overall averages in portions of the native habitats of each species are shown in Figures 1A, B. All of the Hermann’s tortoises sampled were kept in naturalistic environments providing them ad libitum access to various feeder plants. Additionally, they received wild herbs collected in the surroundings, hay and hay cobs, as well as small portions of leafy greens, vegetables and fruits. The pond sliders were fed a diet consisting of quality pellets, invertebrates, wild herbs and water plants but had a plethora of different food sources found in their pond enclosures. Some of the animals were kept in greenhouses paned with simple window glass, while others were kept outdoors with direct access to unfiltered sunlight. Access to natural sunlight was noted and considered as an independent variable. At the time of blood collection, all animals were considered healthy on the basis of a general health examination including evaluation of the nutritional status, total white blood cell count (WBC), and packed cell volume (PCV) (Barrows et al., 2004; Bielli et al., 2015). Animals sampled in spring had all begun eating following hibernation and were considered in good body condition compared to pre-hibernation weights when these were available.
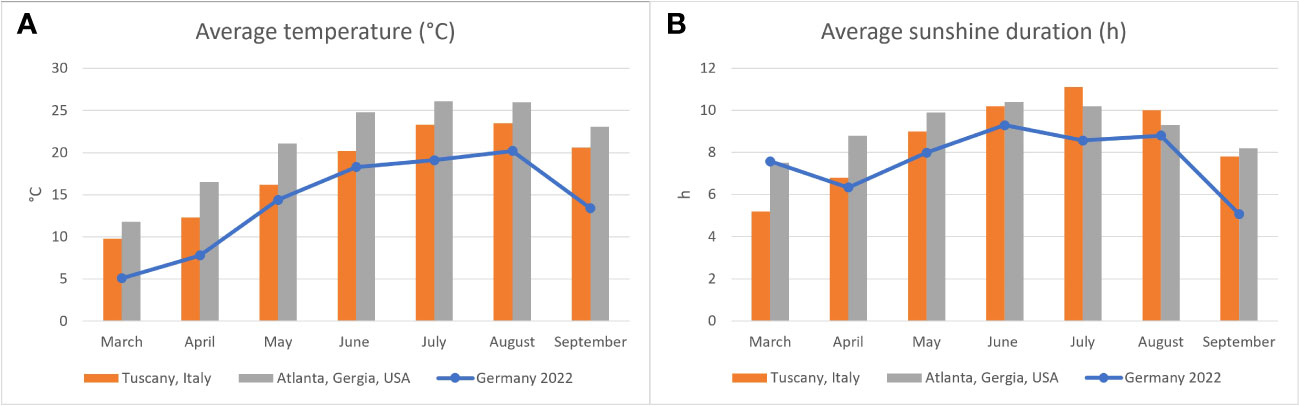
Figure 1 Average air temperature (A) and hours of sunlight (B) in Germany in 2022 (blue line) as reported by the Deutsche Wetterdienst (2022a) compared to areas in which the two species examined are found naturally: Italy for Hermann’s tortoises (Testudo hermanni) (Deutsche Wetterdienst, 2022b) and Georgia, USA for pond sliders (Trachemys scripta spp.) (Climate data, 2023).
Samples were collected from the dorsal coccygeal vein, in some cases from the subcarapacial plexus. No samples with visible lymph contamination or a PCV < 10% were included in the study. A total of 0.5 to 3.0 ml blood was collected depending on the animal’s body weight and never exceeded 0.7% of the total body weight. The blood was then transferred into lithium-heparinized tubes (4.5 ml tube, lithium heparin, Sarstedt, Nurnbrecht, Germany) and transported in an upright position overnight cool (4–8°C, 39.2–46.4°F) to the laboratory. For testing, priority was given to standard hematology and biochemistry analyses, including Ca, PO4, and Mg, followed by vitamin D3 measurement. Blood smears were made directly after sample collection and air-dried. PCV was determined using microhematocrit capillaries, which were centrifuged for 5 min at 12,000 g in a Haemofuge (Thermo Fisher Scientific Inc., Breda, The Netherlands). WBC was determined microscopically at 500x magnification in 10 fields, modified from the method described by (Sheldon et al., 2016). The hematologic results were used to further assess the health of the animals. Exclusion criteria were adopted according to Bielli et al. (2015). Specifically, WBC above 15.3 G/L was used as a cut off for both species. No samples were excluded based on these values. Samples were centrifuged at 4000 rpm for 3 min with a Thermo Scientific Megafuge ST Plus Series (Thermo Fisher Scientific Inc., Breda, The Netherlands) no later than 24 h after collection. The subsequent examinations requiring heparin plasma were done within 24 h after centrifugation, plasma samples were stored cool (4–8°C, 39.2–46.4°F) until examination. Ca, Mg, and PO4 were determined in the plasma using a cobas® 8000 module c701 analyzer series (Roche Diagnostics, Mannheim, Germany). Vitamin D3 in the plasma samples was first prepared using a Recipe ClinRep© HPLC kit for 25-OH-Vitamin D2/D3 (RECIPE Chemicals + Instruments, Munich, Germany) and then measured within 24 h after preparation using a Shimadzu Prominence High Performance Liquid Chromatography (HPLC) Modul System (Shimadzu Corporation, Kyoto, Japan). For vitamin D3, intra and inter assay variance tests were conducted prior to the study begin using plasma from a pooled tortoise sample, to control the validity of the method and effects of storage on our measurements using Westgard SD Calculator (Westgard QC, 2019). The coefficient of variation (CV) for the intraassay variance was 0.06, the CV for interassay variance was 0.162 with an acceptable maximal CV of 0.33 (FDA, 2021).
Study samples were grouped by species, sex, season, and access to sunlight for the statistical analyses, which were carried out using the SAS analysis software package (SAS; SAS Institute Inc., Cary, NC, USA) and SPSS analysis software (SPSS 28.0; IBM, Armonk, USA). A Shapiro-Wilk test was used for initial determination of normality for each analyte. An ANOVA mixed model was used to evaluate the influence of the factors species, sex, season, and access to sunlight on the blood vitamin D3, Ca, PO4, and Mg values, the cut off for significance for these models were set at a corrected P < 0.002. Specific correlations between vitamin D3, Ca, PO4 and Mg were tested by Spearman correlation coefficient, cut-off for significance was P < 0.002. In those cases, in which a sufficient number of samples were available, reference intervals (RIs) were determined according to the guidelines of the American Society of Veterinary Clinical Pathologists (ASVCP) (Friedrichs et al., 2012) using Reference Value Advisor v2.1 (Geffré et al., 2011). Outliers were determined using Tukey and Dixon-Reed test. Depending on group size and normality of distribution, RIs were either calculated using the parametric method or robust method. RIs for vitamin D3 were only calculated for those groups with access to natural sunlight, as keeping of these species under window glass was not considered optimal husbandry and therefore not considered useful for health evaluations in these species.
3 Results
3.1 25(OH) vitamin D3
Since blood sampling was carried out in the context of routine examinations, measurement of Ca, PO4, and Mg was prioritized before vitamin D3. Group sizes are detailed in Tables 1–4.
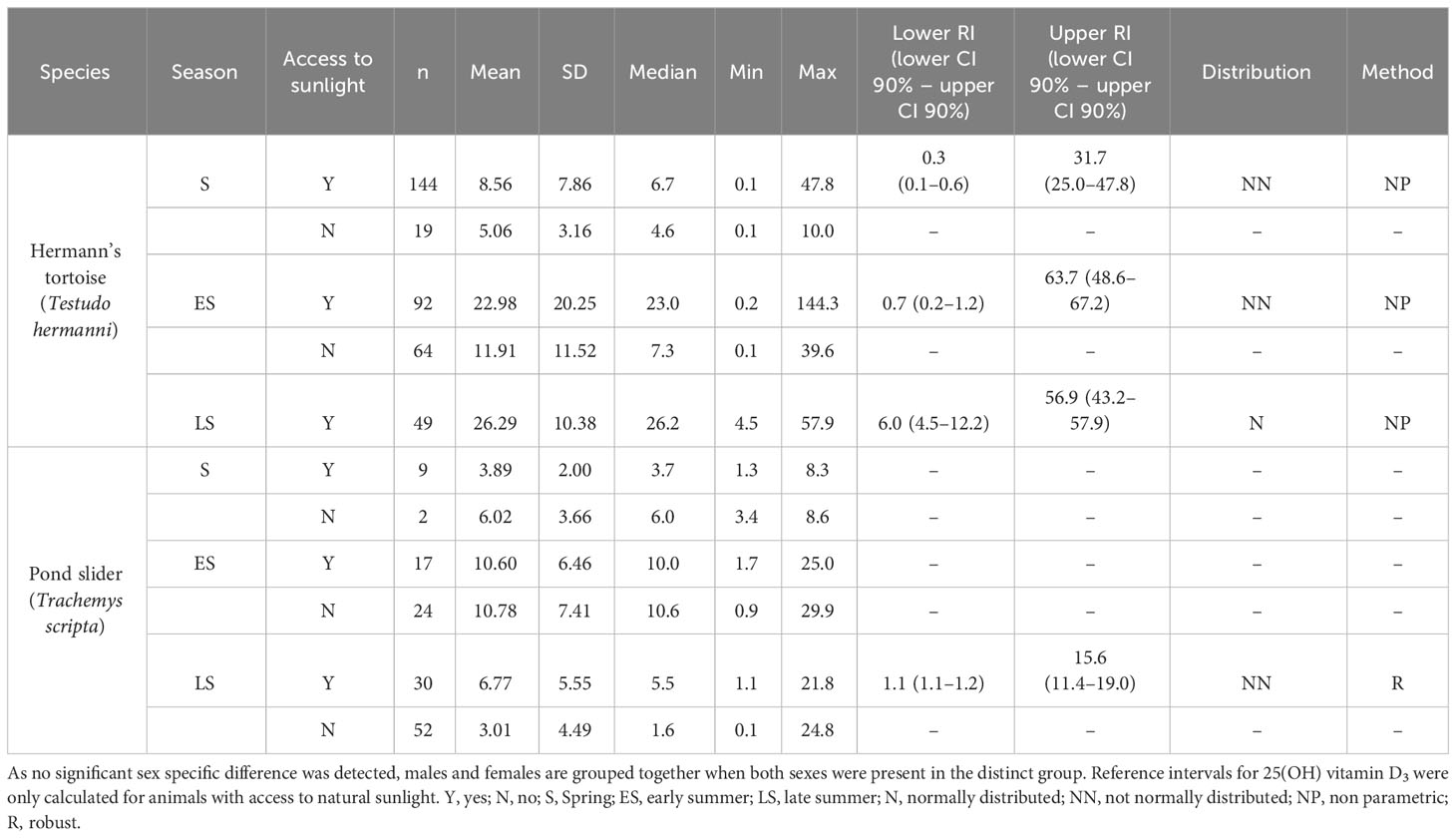
Table 1 Plasma 25-OH vitamin D3 levels (nmol/l) in Hermann’s tortoises (Testudo hermanni) and pond sliders (Trachemys scripta) divided by access to sunlight and the season.
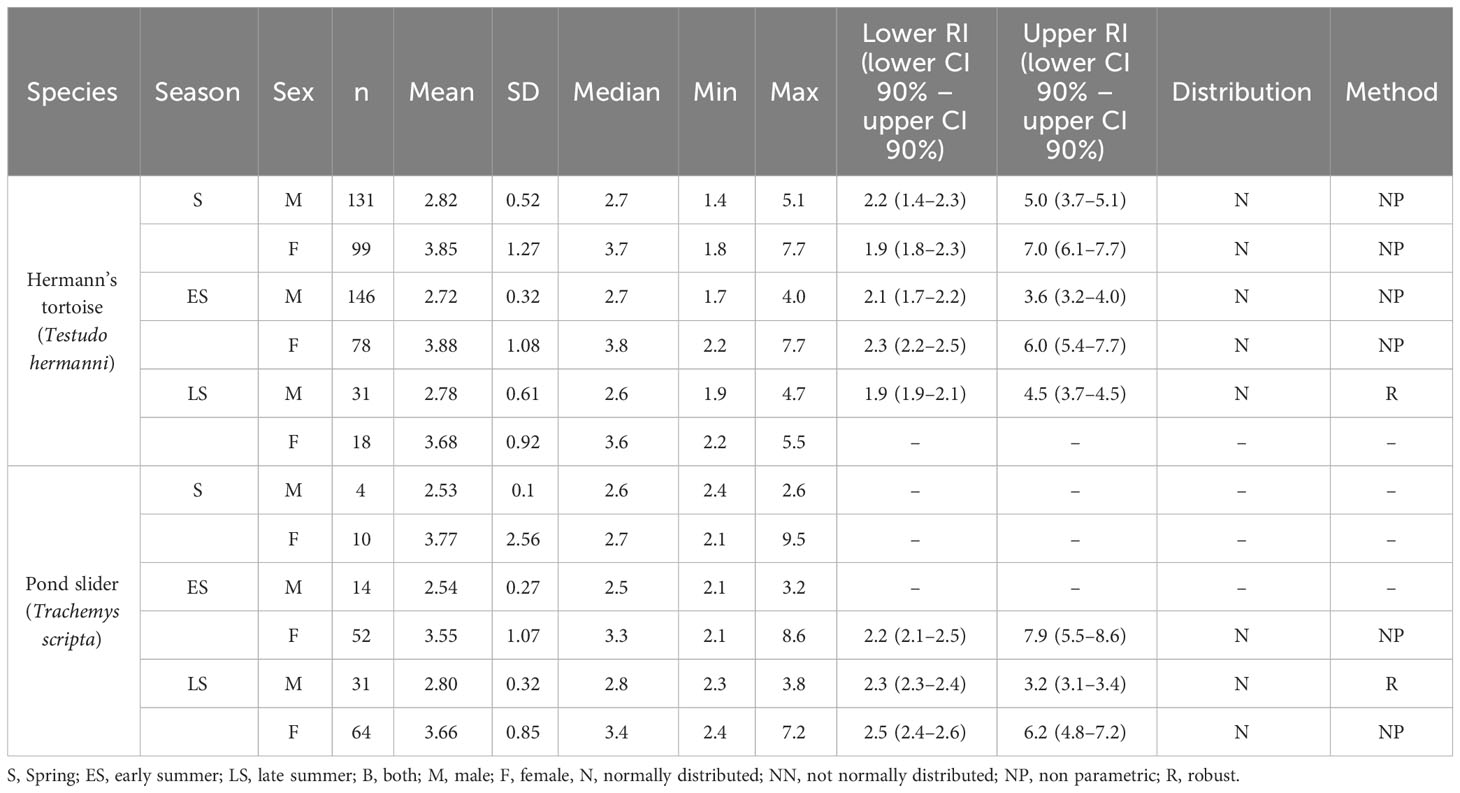
Table 2 Plasma Ca levels and reference intervals (mmol/l) in Hermann’s tortoises (Testudo hermanni) and pond sliders (Trachemys scripta) divided by season and sex.
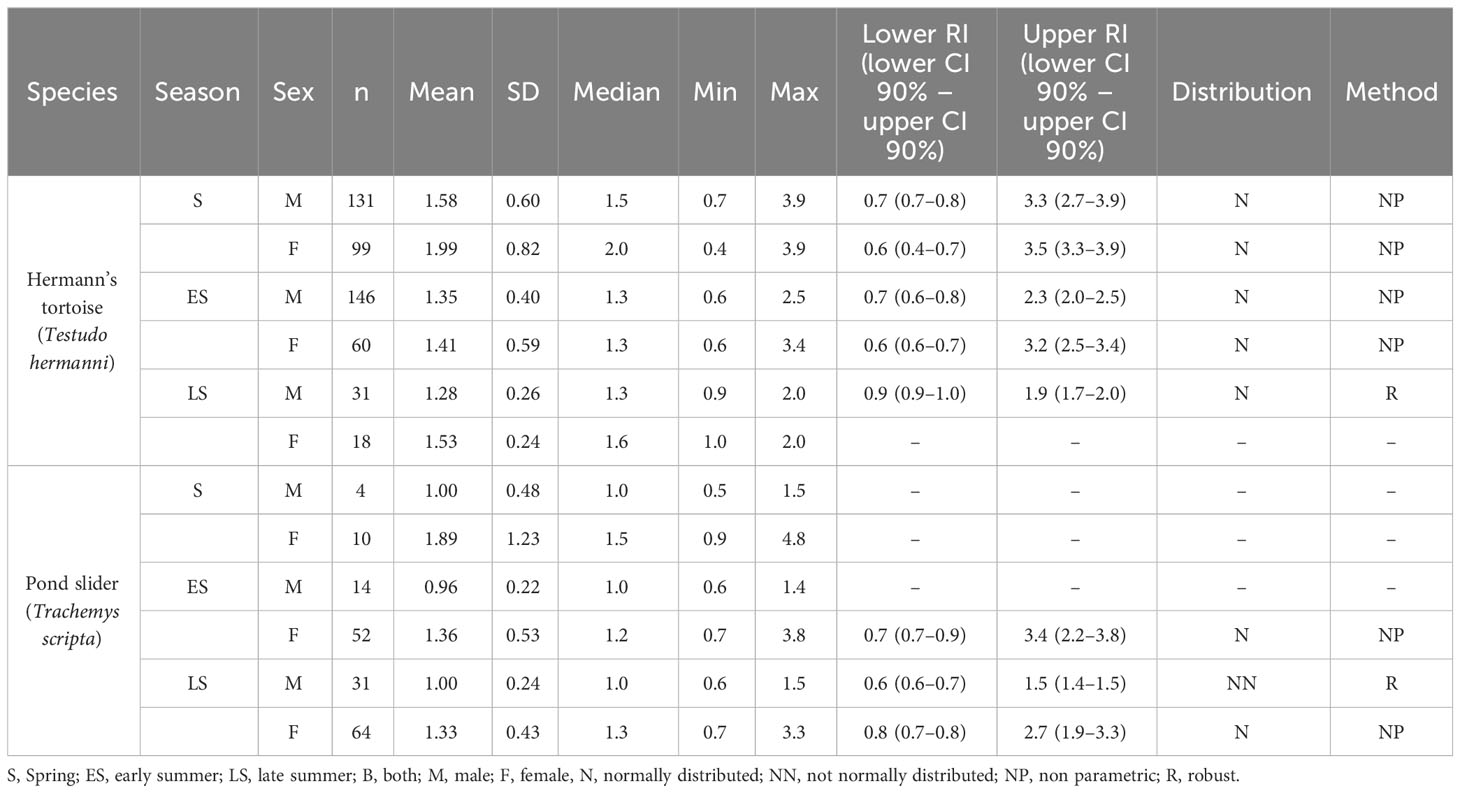
Table 3 Plasma PO4 levels and reference intervals (mmol/l) in Hermann’s tortoises (Testudo hermanni) and pond sliders (Trachemys scripta) divided by season and sex.
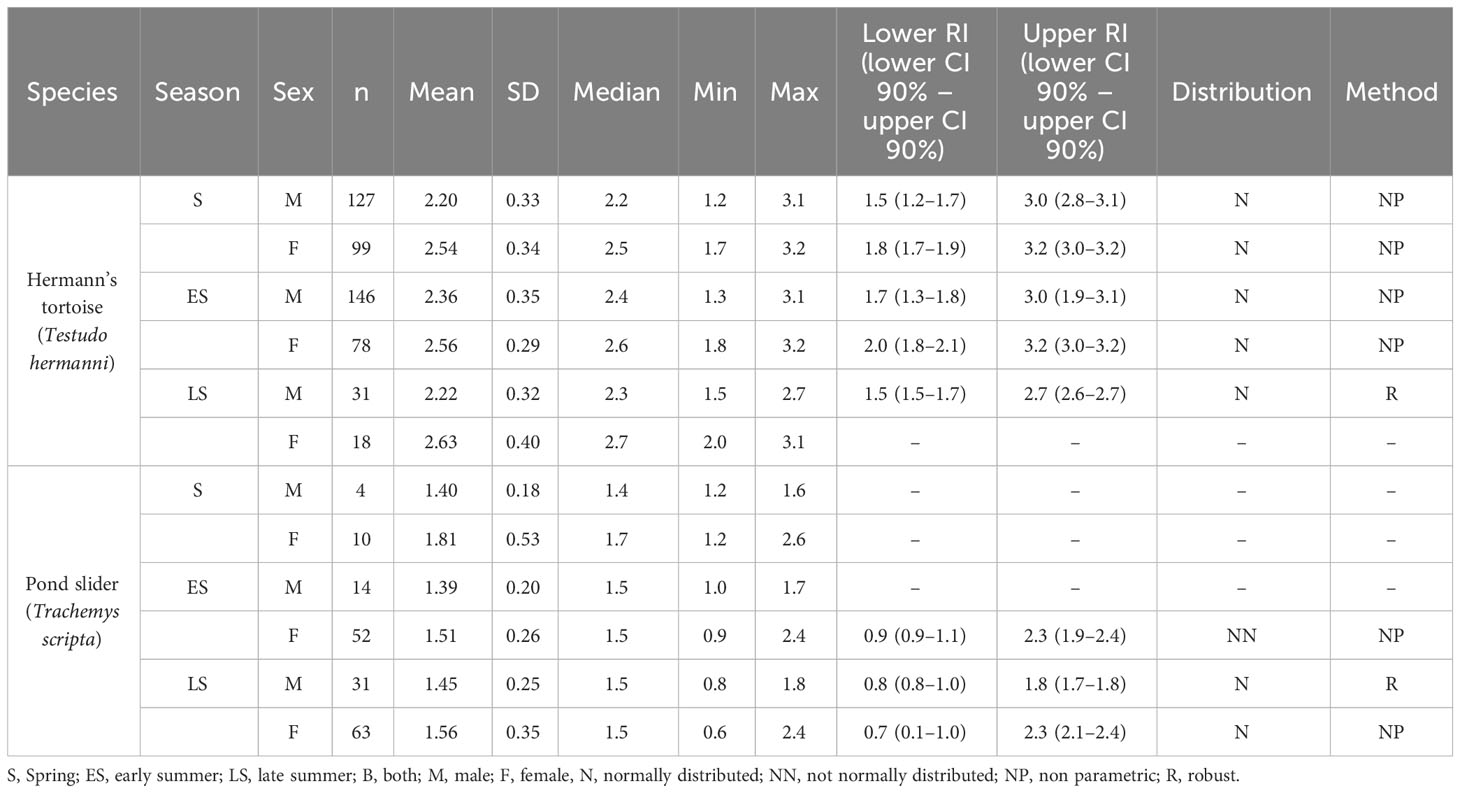
Table 4 Plasma Mg levels and reference intervals (mmol/l) in Hermann’s tortoises (Testudo hermanni) and pond sliders (Trachemys scripta) divided by season and sex.
The variance analysis showed that some factors and the interaction of these factors had a significant effect on the measured vitamin D3 values, specifically species (df = 1; F = 24.887; P < 0.001) and the combination of season x access to sunlight (df = 2; F = 7.205; P < 0.001). Hermann’s tortoises had significantly higher vitamin D3 values than pond sliders. In both species, vitamin D3 values were higher in animals with access to sunlight in early and late summer, but not in spring (Figure 2).
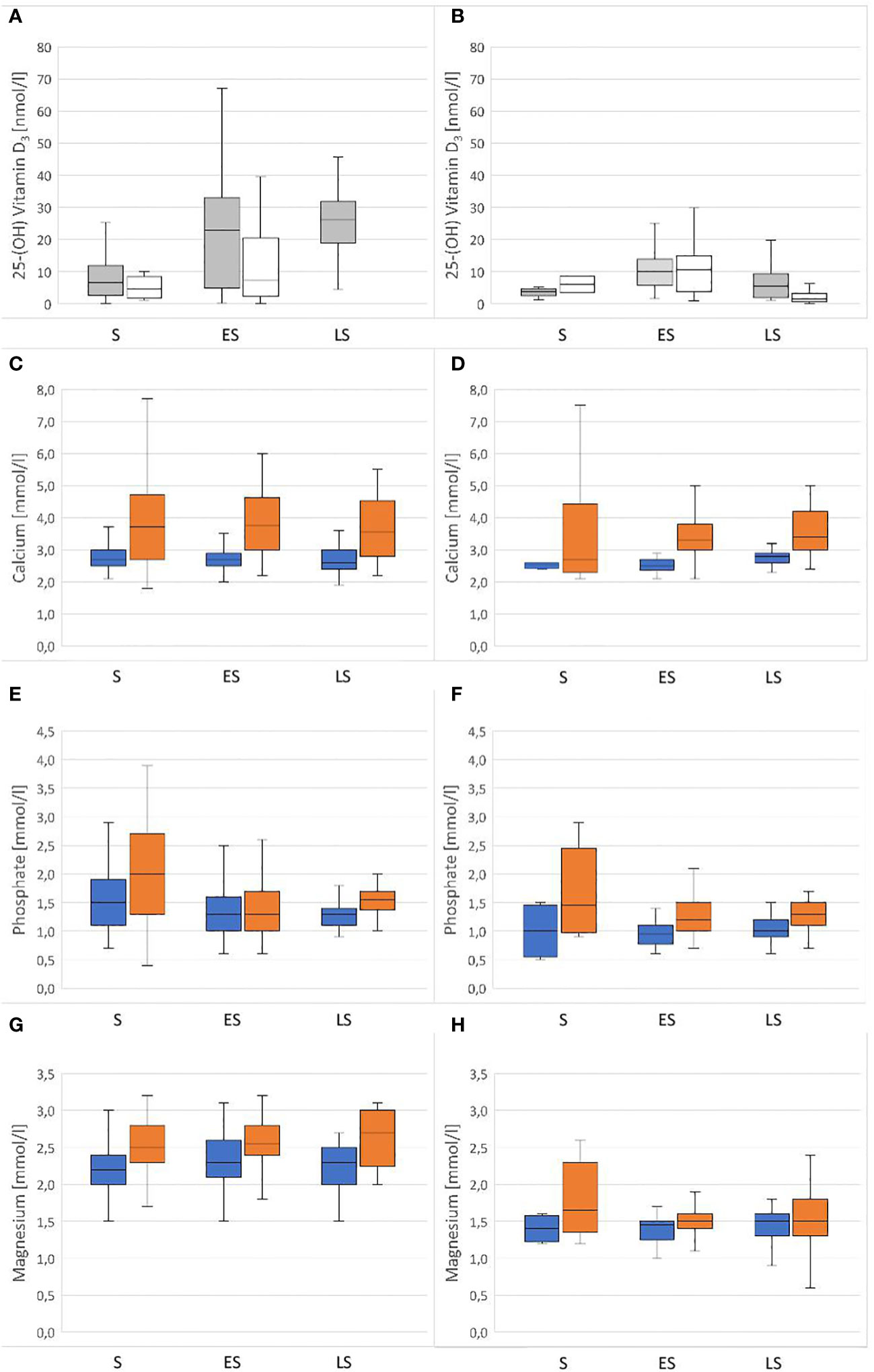
Figure 2 Plasma concentrations of vitamin D3, Ca, PO4 and Mg in Hermann’s tortoises (Testudo hermanni – A, C, E, G) and pond sliders (Trachemys scripta – B, D, F, H) in spring (S), early summer (ES) and late summer (LS). For Vitamin D3 the animals are divided by their access to sunlight (Grey = with access to sunlight; white = without access to sunlight). For Ca, PO4 and Mg the animals are divided by sex (blue = males orange = females).
Sex (df = 1; F = 0.170; P = 0.681), season (df = 2; F = 0.479; P = 0.620), access to sunlight (df = 1; F = 2.284; P < 0.131), species x sex (df = 1; F = 0.249; P = 0.618), species x season (df = 2; F = 3.674; P = 0.026), species x access to sunlight (df = 1; F = 1.159; P = 0.282), sex x season (df = 2; F = 0.163; P = 0.849), sex x access to sunlight (df = 1; F = 0.246; P = 0.620), species x sex x season (df = 2; F = 0.103; P = 0.902), species x sex x access to sunlight (df = 1; F = 1.375; P = 0.242), species x season x access to sunlight (df = 1; F = 2.971; P = 0.085), and sex x season x access to sunlight (df = 2; F = 1.165; P = 0.313) all had no significant effect on vitamin D3 values.
3.2 Calcium
The variance analysis showed that sex (df = 1; F = 76.898; P = < 0.001) had a significant effect on the measured Ca values, with significantly higher values found in females than in males (Figure 2). Other factors did not significantly influence Ca values: species (df = 1; F = 1.334; P = 0.248), season (df = 2; F = 0.254; P = 0.776), access to sunlight (df = 1; F = 0.041; P = 0.839), species x sex (df = 1; F = 0.020; P = 0.888), species x season (df = 2; F = 0.031; P = 0.969), species x access to sunlight (df = 1; F = 0.003; P = 0.958), sex x season (df = 2; F = 0.619; P = 0.539), sex x access to sunlight (df = 1; F = 0.302; P = 0.583), season x access to sunlight (df = 2; F = 2.640; P = 0.072), species x sex x season (df = 2; F = 0.134; P = 0.874), species x sex x access to sunlight (df = 1; F = 0.008; P = 0.929), species x season x access to sunlight (df = 1; F = 0.016; P = 0.898), sex x season x access to sunlight (df = 2; F = 4.350; P = 0.013).
3.3 Phosphate
The variance analysis showed that species (df = 1; F = 13.127; P < 0.001) and sex (df = 1; F = 17.285; P < 0.001) each had a significant effect on the measured PO4 values, with higher values found in females than males of both species and higher values found in Hermann’s tortoises than in pond sliders (Figure 2). No significant effects were found for other factors and combinations of factors: season (df = 2; F = 0.448; P = 0.639), access to sunlight (df = 1; F = 2.652; P = 0.104), species x sex (df = 1; F = 0.417; P = 0.519), species x season (df = 2; F = 1.008; P = 0.365), species x access to sunlight (df = 1; F = 3.736; P = 0.054), sex x season (df = 2; F = 0.139; P = 0.871), sex x access to sunlight (df = 1; F = 0.511; P = 0.475), season x access to sunlight (df = 2; F = 4.274; P = 0.014), species x sex x season (df = 2; F = 0.049; P = 0.952), species x sex x access to sunlight (df = 1; F = 0.011; P = 0.918), species x season x access to sunlight (df = 1; F = 0.053; P = 0.818), sex x season x access to sunlight (df = 2; F = 0.132; P = 0.876).
3.4 Magnesium
The variance analysis showed that species (df = 1; F = 270.491; P < 0.001) and sex (df = 1; F = 39.712; P < 0.001) each had a significant effect on the measured Mg values. Females of both species had higher Mg levels than males and Hermann’s tortoises had higher levels than pond sliders (Figure 2). Season (df = 2; F = 0.119; P = 0.888), access to sunlight (df = 1; F = 2.057; P = 0.152), species x sex (df = 1; F = 0.885; P = 0.347), species x season (df = 2; F = 4.348; P = 0.013), species x access to sunlight (df = 1; F = 0.127; P = 0.722), sex x season (df = 2; F = 1.665; P = 0.190), sex x access to sunlight (df = 1; F = 0.025; P = 0.874), season x access to sunlight (df = 2; F = 0.981; P = 0.375), species x sex x season (df = 2; F = 1.202; P = 0.301), species x sex x access to sunlight (df = 1; F = 1.084; P = 0.298), species x season x access to sunlight (df = 1; F = 3.022; P = 0.083) and sex x season x access to sunlight (df = 2; F = 4.558: P = 0.011) all had no significant effect on vitamin D3 values.
3.5 Correlations
Correlations between the measured analytes are shown in Tables 5, 6. A positive correlation between vitamin D3 and Ca levels was only seen in female pond sliders without access to sunlight in early summer. Correlations between vitamin D3 and PO4 and between vitamin D3 and Mg occurred only occasionally and showed no distinct trend. When there was a correlation between Ca and Mg, this was always positive, and was seen more often in Hermann’s tortoises than in pond sliders. While Mg showed a strong positive correlation with Ca in both male and female Hermann’s tortoises this correlation was only seen in female pond sliders. In both species, PO4 generally correlated significantly positively with Ca, but no correlation was found for males.
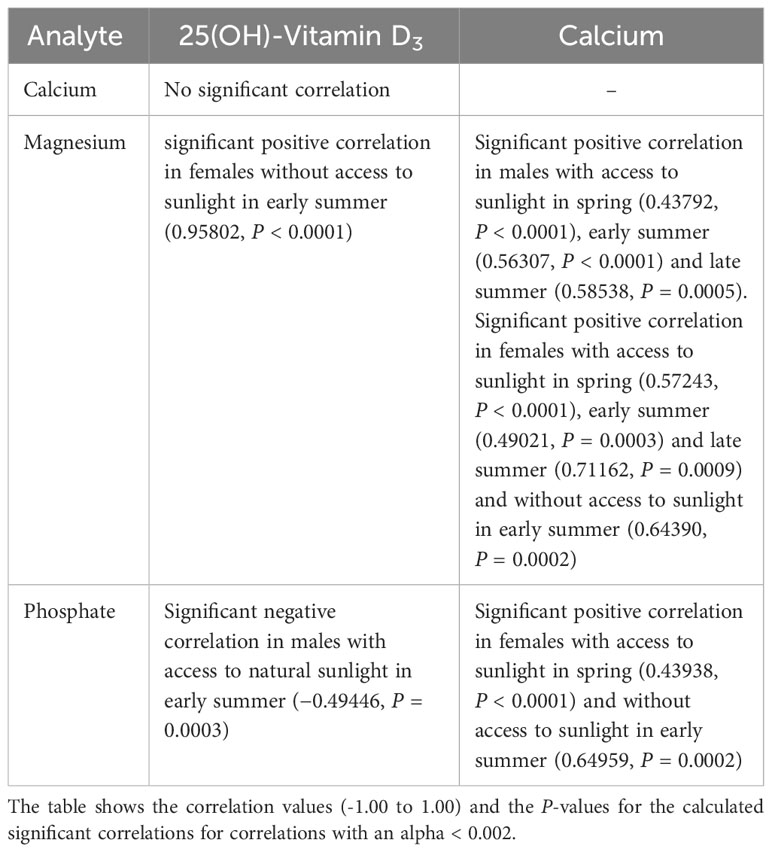
Table 5 Significant correlations between plasma vitamin D3, Ca, PO4 and Mg levels in Hermann’s tortoises (Testudo hermanni).
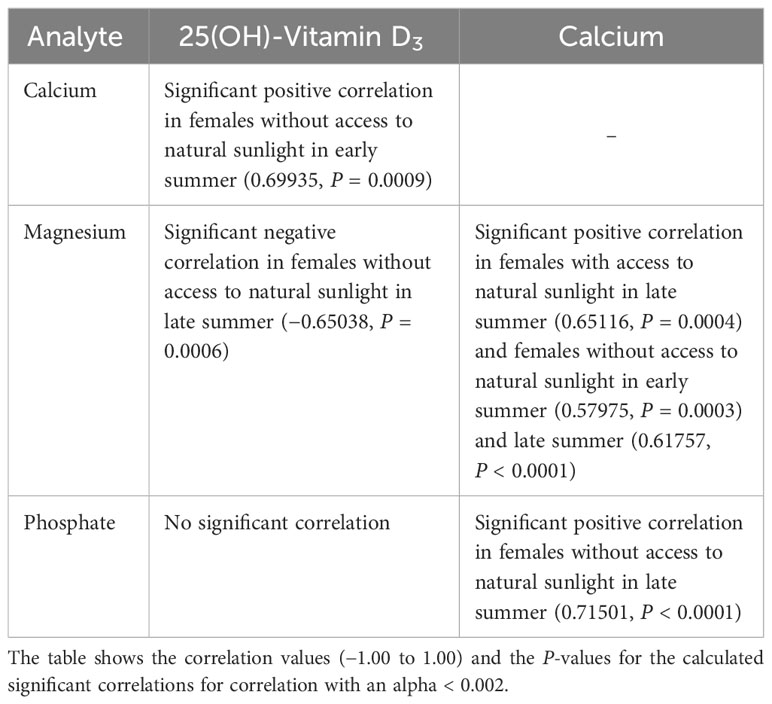
Table 6 Significant correlations between plasma vitamin D3, Ca, PO4 and Mg levels in pond sliders (Trachemys scripta).
4 Discussion
The relevance of a sufficient supply of vitamin D3 for reptiles has been well documented (Dickinson and Fa, 1997; Ferguson et al., 2003; Karsten et al., 2009). Access to natural sunlight or high-quality artificial lighting is generally agreed to be a critical factor for the health of many reptiles, in large part due to its importance in vitamin D3 synthesis. Increased vitamin D3 levels have been documented in various reptile species exposed to adequate UV light (Holick et al., 1995; Laing and Fraser, 1999; Carman et al., 2000; Ferguson et al., 2002; Acierno et al., 2008; Oonincx et al., 2010; Bos et al., 2018). Previous studies on a variety of chelonian species have demonstrated significantly higher vitamin D3 concentrations in animals exposed to a UVB source. Absence of UV-light was accompanied by declining plasma vitamin D3 levels in green sea turtles (Chelonia mydas) (Purgley et al., 2009). In Hermann’s tortoises and sea turtles, comparisons of groups with different UV sources revealed the highest vitamin D3 values for those animals exposed to natural, unfiltered sunlight (Selleri and Di Girolamo, 2012; Scott et al., 2019; Garefino and Milton, 2022). Access to natural sunlight was also clearly a factor in plasma vitamin D3 levels in both of the species included in our study, but only beginning in early summer, not in spring shortly after the animals had emerged from hibernation. In both species, plasma vitamin D3 values appear to decrease during the hibernation period with no access to UVB, and then slowly increase after emergence. One possible explanation for the higher vitamin D3 values measured in Hermann’s tortoises compared to pond sliders in our study could be differences in basking behavior between the two species. As an aquatic species, pond sliders are expected to experience less UV exposure time and intensity (Hoskins et al., 2022). However, both species are known to exhibit basking behavior, and pond sliders leave the water to bask directly in sunlight (Hill and Vodopich, 2013). Vitamin D3 levels reported in plasma of Hermann’s tortoises previously have varied. One study found vitamin D values similar to our results (Eatwell, 2008), while another study reported far higher levels (Selleri and Di Girolamo, 2012). For pond sliders, the values reported by Acierno et al. (2006) were higher than those calculated in our study, even in turtles that received no supplemental UV-radiation. Other studies on Emydidae also report higher values (Watson et al., 2017; Hoskins et al., 2022). For green sea turtles (Chelonia mydas) higher levels are reported as well (Purgley et al., 2009; Stringer et al., 2010; Scott et al., 2019). All of these previous studies used a radioimmunoassay to measure vitamin D levels (Acierno et al., 2006; Eatwell, 2008; Purgley et al., 2009; Stringer et al., 2010; Watson et al., 2017; Scott et al., 2019; Garefino and Milton, 2022; Hoskins et al., 2022). This method detects a range of vitamin D metabolites including 24,25-(OH)2-D3 and 25-OH-D2, increasing the reported total vitamin D level measured (Wiedemann, 2010; Selleri and Di Girolamo, 2012), while the method used in the present study specifically measures 25(OH)D3. Also, the geographical location in which the studies were conducted could contribute to differences in plasma vitamin D levels. Chelonians kept in regions closer to the equator have a prolonged activity period, more hours of sun and more intense UV-radiation due to solar altitude than those further from the equator. The animals included in this study were all kept in Germany, in which the amount of UV radiation encountered by individual animals was likely somewhat lower than animals studied e.g., in Italy (Selleri and Di Girolamo, 2012). Direct comparison of average temperatures and hours of sunlight in Germany compared to the natural habitats of each of the species included in our study shows lower average temperatures in Germany (Figure 1A), while the average sunshine duration is similar in spring, but longer in the natural ranges of both species in summer (Figure 1B). These differences could influence the overall health of captive Hermann’s tortoises and pond sliders in central Europe and could influence various blood chemistry levels, including vitamin D3. However, a study in box turtles showed no differences in vitamin D levels based on location for populations in Illinois and Tennessee in the USA (Watson et al., 2017).
Although the UVB-intensity was not measured in this study, direct access to sunlight vs. access to sunlight filtered through window glass was the main difference in husbandry conditions between the groups. There were several cases in which groups of animals were kept either with or without direct access to sunlight by the same owner. In these cases, the animals in the different groups all received the same diet. Window glass is known to absorb UVB-radiation nearly completely (Sackey et al., 2015). Therefore, it is likely that the animals without access to sunlight had lower plasma vitamin D3 levels as a result of lower amounts of UV-radiation received due to exposition to glass-filtered light.
The finding that sex did not appear to strongly influence plasma vitamin D3 levels, especially in spring following hibernation, matches with findings in previous studies in European tortoises with access to natural sunlight. Eatwell (2008) found that vitamin D plasma levels did not differ significantly between males and females in spring, but that this changed later in the year. That study reported higher levels in males in early and late summer, while we found higher levels in female Hermann’s tortoise in late summer, although the difference to males was not significant. This might be explained by species-specific differences as Eatwell (2008) used samples from a variety of Mediterranean tortoise species for their study. Another possible explanation is the smaller sample size examined by Eatwell (2008) compared to our study.
When examining the relationship between plasma vitamin D3, Ca, and PO4 levels, we saw only little correlation between the various analytes. Although vitamin D3 is a central factor in Ca homeostasis, a number of other factors, such as PTH and estrogens, help regulate plasma Ca and PO4 levels and could have masked any specific effect of vitamin D3 in the animals in the present study. Since PTH and estrogen were not measured in the current study, exact interactions between these hormones and Ca metabolism in the chelonians tested cannot be determined. Further research on the mechanisms controlling vitamin D3 and Ca interactions in chelonians is needed.
Our study showed significant sex-dependent differences in Ca and PO4 levels in both species, with females having significantly higher levels of both. Peaks in Ca levels in female reptiles are induced by estrogen (Callard et al., 1978). Estrogen induces vitellogenin production in oviparous vertebrates which appears in the blood as calcium binding protein complex, increasing blood Ca concentration by mobilization from the bones (Urist and Scheijde, 1961; Munday et al., 1968). The correlation of high Ca with seasonal yolk production and egg development has been described by various authors in multiple chelonian species (Clark, 1967; Callard et al., 1978; Lawrence, 1987; Christopher et al., 1999; Lagarde et al., 2003). Male painted turtles (Chrysemys picta) had hypercalcemia after estrogen treatment underlining the hormone’s importance in sex-specific Ca differences (Clark, 1967). Previous studies have also documented a strong influence of sex on plasma Ca concentrations in Hermann’s tortoises (Holz, 2007; Leineweber et al., 2019), while other studies have reported an increase of plasma Ca in males during the year (Erler, 2003; Scope et al., 2013). Studies looking at Ca and PO4 levels in other Testudo spp. have found similar sex dependent dynamics of these analytes and have reported no species-specific differences for these analytes between European tortoise species (Testudo spp.) (Mathes et al., 2006; Holz, 2007), while no sex-specific differences for Ca and PO4 were reported for Russian tortoises (Testudo horsfieldi) (Knotková et al., 2002). Sex-dependent differences in Ca and PO4 levels have also been reported for other members of the Testudinidae across the globe (Ghebremeskel et al., 1991; Raphael et al., 1994; Christopher et al., 1999; Gülen and Gül, 2018). The majority of these studies report overlapping Ca and PO4 ranges for various tortoise species and genera. They also repeatedly show the occurrence of significantly higher levels in females. These findings indicate a high degree of consistency regarding the regulatory mechanisms for these agents throughout the family Testudinidae.
Similar sex-dependent effects on Ca and PO4 were also found in the pond sliders in this study. Other studies on emydid turtles have reported mixed results when examining sex and season-specific changes in these values in various species. Research done on Caspian river turtles (Mauremys caspica) showed higher values for Ca and PO4 in females than in males, but did not examine seasonal changes (Hidalgo-Vila et al., 2007). Other studies conducted on Emydidae in early summer showed no differences in Ca and PO4 levels between the sexes in European pond turtles (Emys orbicularis) but higher Ca levels in female Caspian river turtles and higher PO4 levels in male Balkan pond turtles (Mauremys rivulata) (Metin et al., 2006; Metin et al., 2008). In common box turtles (Terrapene c. carolina), gravid females had higher Ca levels than males and nongravid females in spring. The plasma Ca of those females dropped significantly after oviposition while the plasma Ca levels of males and nongravid females increased (Kimble and Williams, 2012). In the same study, no effects of sex or season on plasma PO4 levels were detected.
The close association of Ca and Mg metabolism is well known in humans and severe hypomagnesemia can lead to hypocalcemia due to impaired PTH release and decreased heteroionic Ca/Mg exchange at the skeletal surface (al-Ghamdi et al., 1994). In laying hens, a normocalcemic diet deficient in Mg led to hypocalcemia and hypomagnesemia. Only supplementation of both electrolytes led to normalization (Cox and Sell, 1967). In Chinese softshell turtles (Pelodiscus sinensis), animals fed with a Mg richer diet developed a stronger carapace (Chen et al., 2014). In mammals, blood Mg levels are kept within very tight ranges, and the majority of the body’s Mg is found intracellularly and is therefore undetectable in plasma samples (Uwitonze and Razzaque, 2018). There is little information available on plasma Mg levels in chelonians. We saw significantly higher Mg levels in female tortoises than in males but both sexes had a similar width in their respective ranges. Studies conducted on other testudinids have not found sex-related differences (Hamooda et al., 2014; Gülen and Gül, 2018; Berg et al., 2021). In one case, this could be due to the young age of the animals; Berg et al. (2021) examined juvenile animals, while all of the animals in our study were adult. Development of sex-related physiologic differences may not have occurred in the juvenile tortoises (Berg et al., 2021). Ranges reported in Greek tortoises (Testudo graeca) and red-footed tortoises (Chelonoides carbonaria) were similar to ours (Hamooda et al., 2014; Berg et al., 2021). One study reported much lower ranges in Greek tortoises with a significant drop from March and April to the reproductive period in June and July in both sexes, which our results do not reflect (Gülen and Gül, 2018). Our results showed that Hermann’s tortoises have significantly higher Mg levels than pond sliders. Higher Mg levels in the herbivorous Hermann’s tortoises compared to the omnivorous pond sliders might be a result of dietary differences between the two species. Similar to our results, significant species differences have been reported for Mg in sea turtles. The more carnivorous Kemp´s ridley sea turtles had higher Mg levels than the more herbivorous green sea turtles, even when fed the same diet (Anderson et al., 2011).
Because the aim of the current study was to evaluate the influences of a wide variety of physiological and husbandry factors on plasma vitamin D3, Ca, PO, and Mg levels, calculations were based on relatively small groups of animals in some cases. This affected the power of the calculations. In spring, for example, plasma samples were only available from two male pond sliders housed without access to natural sunlight (Tables 1–4). This prohibited direct comparisons between some groups as well as the establishment of RIs for specific groups. In order to allow for larger groups, animals of both sexes were grouped together if no significant differences were found between the sexes. Because access to unfiltered sunlight is considered a key component of good husbandry in both of the species included in this study, we avoided grouping animals with and without access to sunlight together for calculations and comparisons of vitamin D3 levels. For Ca, PO and Mg, on the other hand, we did group animals with and without access to sunlight together when no significant differences were seen. Since the animals came from different locations, details in husbandry and nutrition may vary between the individual animals and groups, which could have influenced the results. Animals were categorized by their access to sunlight, but no UV-measurements were taken. A number of studies provide UV-ranges for different light sources or geographical factors (Acierno et al., 2006; Eatwell, 2008; Purgley et al., 2009; Selleri and Di Girolamo, 2012; Garefino and Milton, 2022; Hoskins et al., 2022). UV levels received by our specimens with access to sunlight are likely to have been similar to those measured by Eatwell (2008) based on the latitudes at which the two studies were conducted. An estimation of UV radiation passing through to those specimens kept in the green houses can only be vague. Many factors, including age and cleanliness of the glass as well as the height of the roof and therefor distance between the glass and the basking animal, influence the amount of UV radiation the animals will have received. The UV levels both species are exposed to in their natural habitats (Selleri and Di Girolamo, 2012; Garefino and Milton, 2022) is likely to be higher than in central Europe. Differences in the times between sampling and analysis and prolonged transportation before analysis might also have influenced the results. All samples were cooled constantly and stored upright in order to minimize any effects of storage and transportation on blood analytes. The interassay variance test conducted prior to the examinations indicate a robust stability of vitamin D3.
Environmental temperature has been well documented to strongly influence the metabolism of ectothermic reptiles, as does time of year, especially for animals from temperate climates. The classification of the samples according to seasons was carried out in order to roughly depict this seasonal cycle. However, annual variation in weather conditions and geographical influences on individual animals were not considered in this categorization.
Our work provides a comparison of vitamin D3 values of two ecologically different species while also examining the influences of sex, season and access to natural sunlight. Sufficient group sizes allowed us to establish RIs for different groups of Hermann’s tortoises and pond sliders according to the ASVCP guidelines (Friedrichs et al., 2012). The results of this study document the positive influence of direct, unfiltered sunlight in connection with the season on vitamin D3 levels in Hermann’s tortoises and pond sliders in captivity in central Europe, as well as demonstrating that sex has only a minor influence on these values. The calculated RIs and a better understanding of dynamics between UV, and plasma vitamin D3, Ca, PO4, and Mg levels can help to improve chelonian husbandry as well as serving as a basis for clinical health evaluations in these species. Further studies are needed to better understand interdependencies with other hormones regulating the Ca and PO metabolism in chelonians, such as PTH, calcitonin and estrogen as well as the role of diet on vitamin D3, Ca, PO, and Mg levels in the blood.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal studies were approved by Ethics Commission of the Faculty of Veterinary Medicine of the University of Leipzig, Germany. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was not obtained from the owners for the participation of their animals in this study because the study used remaining blood from animals for which standard health checks were performed. The majority of the tests (except vitamin D) were performed at the request of the owners or veterinarians in the course of these health checks.
Author contributions
GG: Formal Analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. CL: Conceptualization, Formal Analysis, Methodology, Supervision, Writing – review & editing. MP: Writing – review & editing, Formal Analysis. SÖ: Conceptualization, Writing – review & editing, Resources. RM: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was partially funded by the DGHT AG ARK Ingo and Waltraud Pauler Fund.
Conflict of interest
Three of the authors GG, CL and RM are employed by a private lab Laboklin that offers diagnostic services for veterinarians. This employment did not influence study design, interpretation, or publication preparation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors REM and MP declared that they were editorial board members of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/famrs.2023.1268801/full#supplementary-material
References
Acierno M. J., Mitchell M. A., Roundtree M. K., Zachariah T. T. (2006). Effects of ultraviolet radiation on 25-hydroxyvitamin D3 synthesis in red-eared slider turtles (Trachemys scripta elegans). Am. J. Vet. Res. 67, 2046–2049. doi: 10.2460/ajvr.67.12.2046
Acierno M. J., Mitchell M. A., Zachariah T. T., Roundtree M. K., Kirchgessner M. S., Sanchez-Migallon Guzman D. (2008). Effects of ultraviolet radiation on plasma 25-hydroxyvitamin D3 concentrations in corn snakes (Elaphe guttata). Am. J. Vet. Res. 69, 294–297. doi: 10.2460/ajvr.69.2.294
Alam M. K., Begum N., Begum S. (2016). Relationship of serum ionized calcium and magnesium concentration with parasympathetic nerve Function in type 2 diabetes mellitus. J. Bangladesh Soc. Physiol. 11, 70–73. doi: 10.3329/jbsp.v11i2.30654
al-Ghamdi S. M., Cameron E. C., Sutton R. A. (1994). Magnesium deficiency: pathophysiologic and clinical overview. Am. J. Kidney Dis. 24, 737–752. doi: 10.1016/S0272-6386(12)80667-6
Anderson E. T., Minter L. J., Clarke E. O., Mroch R. M., Beasley J. F., Harms C. A. (2011). The effects of feeding on hematological and plasma biochemical profiles in green (Chelonia mydas) and Kemp’s Ridley (Lepidochelys kempii) Sea Turtles. Vet. Med. Internat 2011, 890829. doi: 10.4061/2011/890829
Barrows M., McArthur S., Wilkinson R. (2004). “Chapter 6 diagnosis,” in Medicine and Surgery of Tortoises and Turtles. Eds. McArthur S., Wilkinson R., Meyer J. (Oxford: Blackwell), 109–138.
Berg K. J., Schexnayder M., Grasperge B. J., Diaz-Figueroa O., Mitchell M. A., Nevarez J. G. (2021). Single time point reference intervals for complete blood counts and select biochemistries in juvenile red-footed tortoises (Chelonoidis carbonaria). J. Herpetol Med. Surg. 31, 124–131. doi: 10.5818/JHMS-S-20-00006
Bidmon H. J., Stumpf W. E. (1994). Distribution of target cells for 1,25-dihydroxyvitamin D3 in the brain of the yellow bellied turtle Trachemys scripta. Brain Res. 640, 277–285. doi: 10.1016/0006-8993(94)91883-x
Bielli M., Nardini G., Di Girolamo N., Savarino P. (2015). Hematological values for adult eastern Hermann’s tortoise (Testudo hermanni boettgeri) in semi-natural conditions. J. Vet. Diag Invest. 27, 68–73. doi: 10.1177/1040638714561251
Bos J. H., Klip F. C., Oonincx D. G. A. B. (2018). Artificial ultraviolet B radiation raises plasma 25-hydroxivitamin D3 concentrations in Burmese pythons (Python bivittatus). J. Zoo Wildl Med. 49, 810–812. doi: 10.1638/2017-0243.1
Burger R. M., Gehrmann W. H., Ferguson G. W. (2007). Evaluation of UVB reduction by materials commonly used in reptile husbandry. Zoo Biol. 26, 417–423. doi: 10.1002/zoo.20148
Callard I. P., Lance V., Salhanick A. R., Barad D. (1978). The annual ovarian cycle of Chrysemys picta: correlated changes in plasma steroids and parameters of vitellogenesis. Gen. Comp. Endocrinol. 35, 245–257. doi: 10.1016/0016-6480(78)90069-2
Carman E. N., Ferguson G. W., Gehrmann W. H., Chen T. C., Holick M. F. (2000). Photobiosynthetic opportunity and ability for UV-B generated vitamin D synthesis in free-living house geckos (Hemidactylus turcicus ) and Texas spiny lizards (Sceloporus olivaceous ). Copeia 2000, 245–250. doi: 10.1643/0045-8511(2000)2000[0245:POAAFU]2.0.CO;2
Chen C.-Y., Chen S.-M., Huang C.-H. (2014). Dietary magnesium requirement of soft-shelled turtles, Pelodiscus sinensis, fed diets containing exogenous phytate. Aquaculture 432, 80–84. doi: 10.1016/j.aquaculture.2014.05.001
Christopher M. M., Berry K. H., Wallis I. R., Nagy K. A., Henen B. T., Peterson C. C. (1999). Reference intervals and physiologic alterations in hematologic and biochemical values of free-ranging desert tortoises in the Mojave Desert. J. Wildl Dis. 35, 212–238. doi: 10.7589/0090-3558-35.2.212
Clark N. B. (1967). Influence of estrogens upon serum calcium, phosphate and proteins concentrations of fresh-water turtles. Comp. Biochem. Physiol. 20, 823–834. doi: 10.1016/0010-406X(67)90056-4
Climate data. (2023). Klima atlanta (Vereinigte staaten von amerika). In: Klima Atlanta: Temperatur, Klimatabelle & Klimadiagramm für Atlanta + Wetter. Available at: climate-data.org. (Accessed September 4, 2023).
Cox A. C., Sell J. L. (1967). Magnesium deficiency in the laying hen. Poult Sci. 46, 675–680. doi: 10.3382/ps.0460675
Deutsche Wetterdienst (2022a). “Klimastatusbericht deutschland jahr 2022,” in Klimastatusbericht Deutschland Jahr 2022. (Offenbach, Germany: dwd.de).
Deutsche Wetterdienst (2022b). “Klimatafel von grosseto, toskana / italien,” in ak_162060_kt.pdf. (Offenbach, Germany: dwd.de).
Dickinson H., Fa J. (1997). Ultraviolet light and heat source selection in captive spiny-tailed iguanas (Oplurus cuvieri). Zoo Biol. 16, 391–401. doi: 10.1002/(SICI)1098-2361(1997)16:53.3.CO;2-G
Eatwell K. (2008). Plasma concentrations of 25-hydroxycholecalciferol in 22 captive tortoises (Testudo species). Vet. Rec 162, 342–345. doi: 10.1136/vr.162.11.342
Erler M. (2003). Saisonale Veränderungen hämatologischer und blutbiochemischer Werte bei europäischen Landschildkröten (Testudo graeca, Testudo hermanni, Testudo marginata) (Munich: Ludwig-Maximilian University, Faculty of Veterinary Medicine, Institute for Zoology, Fish Diseases and Fishery Biology). Dissertation.
Favus M. J., Bushinsky D. A., Lemann J. (2006). “Chapter 13. Regulation of calcium, magnesium, and phosphate metabolism,” in Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism: An official publication of the American Society for Bone and Mineral Research. Ed. Favus M. J. (Washington: American Society for Bone and Mineral Research), 76–83.
FDA (2021). Q2(R1) Validation of Analytical Procedures: Text and Methodology: Guidance for Industry (Silver Spring, MD, United States: FDA).
Ferguson G. W., Gehrmann W. H., Chen T. C., Dierenfeld E. S., Holick M. F. (2002). Effects of artificial ultraviolet light exposure on reproductive success of the female panther chameleon (Furcifer pardalis) in captivity. Zoo Biol. 21, 525–537. doi: 10.1002/zoo.10054
Ferguson G. W., Gehrmann W. H., Karsten K. B., Hammack S. H., McRae M., Chen T. C., et al. (2003). Do panther chameleons bask to regulate endogenous vitamin D3 production? Physiol. Biochem. Zool 76, 52–59. doi: 10.1086/374276
Friedrichs K. R., Harr K. E., Freeman K. P., Szladovits B., Walton R. M., Barnhart K. F., et al. (2012). ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet. Clin. Pathol. 41, 441–453. doi: 10.1111/vcp.12006
Garefino V. E., Milton S. L. (2022). Influence of sunlight on vitamin D and health status in green (Chelonia mydas) sea turtles with fibropapillomatosis. Anim. (Basel) 12. doi: 10.3390/ani12040488
Geffré A., Concordet D., Braun J. P., Trumel C. (2011). Reference Value Advisor: a new freeware set of macroinstructions to calculate reference intervals with Microsoft Excel. Vet. Clin. Pathol. 40, 107–112. doi: 10.1111/j.1939-165X.2011.00287.x
Ghebremeskel K., Williams G., Spratt D., Samour H. J. (1991). Plasma biochemistry of free-living giant tortoises (Geochelone gigantea) on Curieuse Island (Republic of Seychelles). Comp. Biochem. Physiol. Part A: Physiol. 99, 65–67. doi: 10.1016/0300-9629(91)90236-6
Gülen E., Gül C. (2018). Determining of plasma biochemical parameters according to different reproductive periods in the population of Testudo graeca (Çanakkale, Turkey). Appl. Ecol. Env. Res. 16, 3305–3313. doi: 10.15666/AEER/1603_33053313
Hamooda A. E. F., El-Mansoury A., Mehdi A. (2014). Some blood indexes of the tortoise Testudo graeca Linn. 1758 from benghazi province, Libya. Scientif Res. J. 36–40.
Hidalgo-Vila J., Díaz-Paniagua C., Pérez-Santigosa N., Laza A., Camacho I., Recio F. (2007). Hematologic and biochemical reference intervals of free-living Mediterranean pond turtles (Mauremys leprosa). J. Wild Dis. 43, 798–801. doi: 10.7589/0090-3558-43.4.798
Hill S. K., Vodopich D. S. (2013). Habitat use and basking behavior of a freshwater turtle community along an urban gradient. Chelon Conserv. Biol. 12, 275–282. doi: 10.2744/CCB-0961.1
Holick M. F., Garabedian M. (2006). “Chapter 17. Vitamin D: photobiology, metabolism, mechanism of action, and clinical application,” in Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism: An official publication of the American Society for Bone and Mineral Research. Ed. Favus M. J. (Washington: American Society for Bone and Mineral Research), 106–114.
Holick M. F., Tian X. Q., Allen M. (1995). Evolutionary importance for the membrane enhancement of the production of vitamin D3 in the skin of poikilothermic animals. Proc. Natl. Acad. Sci. U.S.A. 92, 3124–3126. doi: 10.1073/pnas.92.8.3124
Holz A. (2007). Bestimmung hämatologischer und biochemischer Parameter bei der gesunden Europäischen Landschildkröte—Testudo hermanni, Testudo graeca, Testudo marginata, Testudo horsfieldii (Hannover: University of Veterinary Medicine Hannover, Clinic for Small Animals). Dissertation.
Hoskins A., Thompson D., Mitchell M. A. (2022). Effects of artificial ultraviolet B radiation on plasma 25-hydroxyvitamin D3 concentrations in juvenile Blanding’s turtles (Emydoidea blandingii). J. Herpetol Med. Surg. 32, 225–229. doi: 10.5818/JHMS-D-21-00039
Karsten K. B., Ferguson G. W., Chen T. C., Holick M. F. (2009). Panther chameleons, Furcifer pardalis, behaviorally regulate optimal exposure to UV depending on dietary vitamin D3 status. Physiol. Biochem. Zool 82, 218–225. doi: 10.1086/597525
Kimble S. J. A., Williams R. N. (2012). Temporal variance in hematologic and plasma biochemical reference intervals for free-ranging eastern box turtles (Terrapene carolina carolina). J. Wildl Dis. 48, 799–802. doi: 10.7589/0090-3558-48.3.799
Knotková Z., Doubek J., Knotek Z., Hájková P. (2002). Blood cell morphology and plasma biochemistry in Russian tortoises (Agrionemys horsfieldii). Acta Vet. Brno 71, 191–198. doi: 10.2754/avb200271020191
Lagarde F., Bonnet X., Henen B., Nagy K., Corbin J., Lacroix A., et al. (2003). Plasma steroid and nutrient levels during the active season in wild Testudo horsfieldi. Gen. Comp. Endocrinol. 134, 139–146. doi: 10.1016/s0016-6480(03)00245-4
Laing C. J., Fraser D. R. (1999). The vitamin D system in iguanian lizards. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 123, 373–379. doi: 10.1016/S0305-0491(99)00081-4
Lawrence K. (1987). Seasonal variation in blood biochemistry of long term captive Mediterranean tortoises (Testudo graeca and T. hermanni). Res. Vet. Sci. 43, 379–383. doi: 10.1016/S0034-5288(18)30808-7
Leineweber C., Stöhr A. C., Öfner S., Mathes K., Marschang R. E. (2019). Changes in plasma chemistry parameters in Hermann’s tortoises (Testudo hermanni) influenced by season and sex. J. Herpetol Med. Surg. 29, 113. doi: 10.5818/18-07-159.1
Lips P. (2006). Vitamin D physiology. Prog. Biophys. Mol. Biol. 92, 4–8. doi: 10.1016/j.pbiomolbio.2006.02.016
Lovich J. E., Gibbons W. (2021). Turtles Of The World: A Guide to Every Family (Princeton, Oxford: Princeton University Press).
Mader D. R. (2006). “Chapter 61 metabolic bone disease,” in Reptile Medicine and Surgery. Ed. Mader D. R. (St. Louis, Mo: Saunders Elsevier), 841–851.
Mathes K. A., Holz A., Fehr M. (2006). Blutreferenzwerte in Deutschland gehaltener europäischer Landschildkröten (Testudo spp.). Tierarztl Prax Ausg K 34, 268–274. doi: 10.1055/s-0037-1622540
McArthur S., Meyer J., Innis C. (2004). “Chapter 3 anatomy and physiology,” in Medicine and Surgery of Tortoises and Turtles. Eds. McArthur S., Wilkinson R., Meyer J. (Oxford: Blackwell), 35–72.
Metin K., Koca Y. B., Kiral F. K., Koca S., Türkozan O. (2008). Blood cell morphology and plasma biochemistry of captive Mauremys caspica (Gmelin 1774) and mauremys rivulata (Valenciennes 1833). Acta Vet. Brno 77, 163–174. doi: 10.2754/avb200877020163
Metin K., Türkozan O., Kargin F., Basumoglu Y. K., Taskavak E., Koca S. (2006). Blood cell morphology and plasma biochemistry of the captive European pond turtle Emys orbicularis. Acta Vet. Brno 75, 49–55. doi: 10.2754/avb200675010049
Munday K. A., Ansari A. Q., Oldroyd D., Akhtar M. (1968). Oestrogen-induced calcium-binding protein in Xenopus laevis. Biochim. Biophys. Acta 166, 748–751. doi: 10.1016/0005-2787(68)90393-6
Oonincx D. G. A. B., Stevens Y., van den Borne J. J. G. C., van Leeuwen J. P. T. M., Hendriks W. H. (2010). Effects of vitamin D3 supplementation and UVb exposure on the growth and plasma concentration of vitamin D3 metabolites in juvenile bearded dragons (Pogona vitticeps). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 156, 122–128. doi: 10.1016/j.cbpb.2010.02.008
Purgley H., Jewell J., Deacon J. E., Winokur R. M., Tripoli V. M. (2009). Vitamin D 3 in captive green sea turtles (Chelonia mydas). Chelon Conserv. Biol. 8, 161–167. doi: 10.2744/CCB-0765.1
Raphael B. L., Klemens M. W., Moehlman P., Dierenfeld E., Karesh W. B. (1994). Blood values in free-ranging pancake tortoises (Malacochersus tornieri). J. Zoo Wildl Med. 25, 63–67.
Sackey S. S., Vowotor M. K., Owusu A., Mensah-Amoah P., Tatchie E. T., Sefa-Ntiri B., et al. (2015). Spectroscopic study of UV transparency of some materials. EP 4, 1–17. doi: 10.5539/ep.v4n4p1
Scope A., Schwendenwein I., Schauberger G. (2013). Characterization and quantification of the influence of season and gender on plasma chemistries of Hermann’s tortoises (Testudo hermanni, Gmelin 1789). Res. Vet. Sci. 95, 59–68. doi: 10.1016/j.rvsc.2013.02.017
Scott G. N., Nollens H. H., Schmitt T. L. (2019). Evaluation of plasma 25-hydroxyvitamin D, ionized calcium, and parathyroid hormone in green sea turtles (Chelonia mydas) exposed to different intensities of ultraviolet B radiation. J. Zoo Wildl Med. 50, 421–426. doi: 10.1638/2017-0115
Selleri P., Di Girolamo N. (2012). Plasma 25-hydroxyvitamin D(3) concentrations in Hermann’s tortoises (Testudo hermanni) exposed to natural sunlight and two artificial ultraviolet radiation sources. Am. J. Vet. Res. 73, 1781–1786. doi: 10.2460/ajvr.73.11.1781
Sheldon J. D., Stacy N. I., Blake S., Cabrera F., Deem S. L. (2016). Comparison of total leukocyte quantification methods in free-living Galapagos tortoises (Chelonoidis spp.). J. Zoo Wildl Med. 47, 196–205. doi: 10.1638/2015-0159.1
Stringer E. M., Harms C. A., Beasley J. F., Anderson E. T. (2010). Comparison of ionized calcium, parathyroid hormone, and 25-hydroxyvitamin D in rehabilitating and healthy wild green sea turtles (Chelonia mydas). J. Herpetol Med. Surg. 20, 122. doi: 10.5818/1529-9651-20.4.122
Urist M. R., Scheijde A. O. (1961). The partition of calcium and protein in the blood of oviparous vertebrates during estrus. J. Gen. Physiol. 44, 743–756. doi: 10.1085/jgp.44.4.743
Uwitonze A. M., Razzaque M. S. (2018). Role of magnesium in vitamin D activation and function. J. Am. Osteopath Assoc. 118, 181–189. doi: 10.7556/jaoa.2018.037
Watson M. K., Byrd J., Phillips C. A., Allender M. C. (2017). Characterizing the 25-hydroxyvitamin D status of two populations of free-ranging eastern box turtles (Terrapene carolina carolina). J. Zoo Wildl Med. 48, 742–747. doi: 10.1638/2016-0236.1
Westgard QC (2019). Westgard QC Method Validation Tools: SD Calculator. Available at: https://www.westgard.com/mvtools.htm (Accessed November 11, 2022).
Keywords: vitamin D3, tortoise, turtle, reference interval, season, sunlight, calcium, magnesium
Citation: Geisler G, Leineweber C, Pees M, Öfner S and Marschang RE (2023) The effects of sex, season, and natural sunlight on plasma vitamin D3 levels in two chelonian species (Testudo hermanni, Trachemys scripta) and their interaction with calcium, phosphate, and magnesium as associated plasma compounds. Front. Amphib. Reptile Sci. 1:1268801. doi: 10.3389/famrs.2023.1268801
Received: 28 July 2023; Accepted: 27 September 2023;
Published: 23 October 2023.
Edited by:
Joana Sabino Pinto, University of Groningen, NetherlandsReviewed by:
Sid Knotek, Avian and Exotic Animal Clinic, CzechiaScott Henke, Texas A&M University Kingsville, United States
Copyright © 2023 Geisler, Leineweber, Pees, Öfner and Marschang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel E. Marschang, bWFyc2NoYW5nQGxhYm9rbGluLmNvbQ==
 Gregor Geisler1
Gregor Geisler1 Michael Pees
Michael Pees Sabine Öfner
Sabine Öfner Rachel E. Marschang
Rachel E. Marschang