
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Agron. , 24 February 2025
Sec. Disease Management
Volume 7 - 2025 | https://doi.org/10.3389/fagro.2025.1526115
Arthropods threaten crop production by feeding on plants and, most importantly, by transmitting viruses. BYDV-PAV is the most prevalent virus species that causes barley yellow dwarf disease, one of the most economically important viral diseases affecting cereals worldwide. Maize plays a central role in BYDV-PAV epidemiology, serving as a “green bridge” for BYDV-PAV and its vector Rhopalosiphum padi in summer. Some studies have reported that the incidence of persistently transmitted viruses may be reduced in plants that are resistant to their insect vectors. In contrast, the choice test applied in our study revealed that R. padi is not repelled by the included BYDV-PAV-resistant maize inbreds. Significant differences in phloem architecture observed among the inbreds suggested that aphids feeding on BYDV-PAV-resistant maize may have difficulties reaching the phloem or establishing a stable feeding site. However, monitoring of aphid feeding behavior using the electrical penetration graph technique on maize inbreds that differed in their BYDV-PAV susceptibility revealed no correlation between R. padi feeding and BYDV-PAV resistance. Furthermore, we could not confirm the generation of reactive oxygen species (ROS), a typical reaction of plants during aphid infestation and infection of some viruses. In summary, we conclude that the BYDV-PAV resistance mechanisms in maize act directly on the virus and not on its vector, R. padi.
Arthropods are an economically relevant threat to crop production. They damage crops when they feed on them, leading to reduced plant productivity. Approximately 18% to 20% of annual crop production worldwide is destroyed by arthropods (Sharma et al., 2017). On a global scale, the most relevant insect pests affecting maize are Spodoptera frugiperda and Diabrotica virgifera. For wheat, aphids have the greatest potential to cause direct damage (Savary et al., 2019). Approximately 30% of all plant viruses described to date are transmitted by aphids, making them the most important virus vectors, followed by leafhoppers, whiteflies, and thrips (Brault et al., 2010; Hunter, 2008).
Virus-transmitting insects mostly feed on saps of the vascular bundle—either phloem or xylem —and have therefore developed specialized mouthparts, called stylets (Leybourne and Aradottir, 2022). To reach the nutrient source, aphids have to cross different tissues. Healthy, insect-resistant plants possess a variety of resistance factors located on the leaf surface or in leaf tissues such as the phloem to prevent insect feeding (Alvarez et al., 2006). Resistance to insect vectors may hinder virus acquisition from infected plants and/or transmission into healthy ones, which might negatively affect the spread of a virus within the field and can therefore be considered indirect virus resistance (Rodríguez-López et al., 2011).
At least 10 different phloem-limited viruses of the genus Luteovirus and Polarovirus, e.g., barley yellow dwarf virus (BYDV) and cereal yellow dwarf virus (CYDV), cause barley yellow dwarf (BYD) disease (Walls et al., 2019). Viruses associated with BYD disease are transmitted by at least 25 different aphid species (Halbert and Voegtlin, 1995). Symptoms of BYD disease are stunting and discoloration of leaves (Choudhury et al., 2017; Oswald and Houston, 1953; Walls et al., 2019). Furthermore, a negative effect on leaf and vascular bundle morphology has been observed in small grain cereals in association with decreased leaf width, vascular bundle area, sieve element area, and xylem vessel area (Choudhury et al., 2018; Esau, 1957; Paulmann et al., 2018). BYDV-PAV is the most prevalent BYD-causing virus in temperate regions and is predominantly transmitted by Rhopalosiphum padi (Aradottir and Crespo-Herrera, 2021). In addition, BYDV-PAV is the most economically important virus, causing yield losses of up to 80% in cereals such as barley and wheat (Choudhury et al., 2017; Nancarrow et al., 2021; Van den Eynde et al., 2020) and of approximately 15% to 25% in maize (Beuve et al., 1999; Loi et al., 1994).
Aphids acquire BYDV from infected winter cereals by ingesting virus particles together with phloem sap when feeding (Gildow and Gray, 1993; Ng and Perry, 2004). BYDV-carrying aphids preferentially move to uninfected plants and transmit the virus when saliva is injected during feeding (Gildow and Gray, 1993; Ingwell et al., 2012; Ng and Perry, 2004), but this was shown to depend upon the aphid clone (Kern et al., 2021). Maize plays a central role in the infection cycle of BYDV and functions as an intermediate host. Maize plants are infected by alate aphid vectors that migrate from ripening small grain cereals in early summer (Haack et al., 1999) and transfer BYDV when migrating to newly sown winter cereals in autumn (Haack et al., 1999; Henry and Dedryver, 1989). Maize inbreds were identified that vary with respect to their resistance and susceptibility to BYDV-PAV (Horn et al., 2013, Horn et al., 2015). The use of resistant genotypes in farmers’ fields could interrupt the transmission of BYDV. Horn et al. (2014) identified a candidate gene for BYDV-PAV resistance in maize that potentially confers resistance via H2O2 generation (Blanvillain et al., 2009). However, it is not known if the resistance mechanism targets the virus directly or indirectly by interfering with virus transmission via the vector R. padi as is the case for R. maidis in inbred maize line Mp708 (Pingault et al., 2021).
The electrical penetration graph (EPG) technique allows for the study of different feeding-associated stylet activities of aphids and other piercing-sucking insects in plant tissues (Jimenez et al., 2020; Leybourne and Aradottir, 2022). Differences in insect feeding activities between resistant and susceptible plants may indicate the location and mode of action of plant resistance factors (Alvarez et al., 2006; Leybourne and Aradottir, 2022). Features such as epidermal waxes, different types of leaf trichomes, fortified cell walls, and acyl-sucroses on the leaf surface serve as physical barriers that cause piercing-sucking insects to take longer to start probing (Alvarez et al., 2006; Leybourne and Aradottir, 2022; Rodríguez-López et al., 2011). The position of resistance factors within the leaf and the anatomy of the vascular tissues may also affect the accessibility of the phloem and, hence, insect acceptance of the plant (Leybourne et al., 2019). Restricted phloem accessibility is the most common and unspecific aphid resistance mechanism in plants (Leybourne and Aradottir, 2022). The latter can be associated with multiple factors such as secondary metabolites, specialized proteins, or the production of reactive oxygen species (ROS) (Will et al., 2013).
Plants are able to sense herbivores or pathogen attacks through specific receptors and activate molecular mechanisms that induce defense reactions (Jones and Dangl, 2006; Palukaitis and Yoon, 2020; Teixeira et al., 2019). The generation of ROS during pathogen and herbivore attacks is a central process linked to the plant defense response (Castro et al., 2021; Goggin and Fischer, 2021; Mittler et al., 2022). ROS are a group of highly reactive molecules derived from molecular oxygen (O2), including hydrogen peroxide (H2O2), superoxide (O2.−), singlet oxygen (1O2), the hydroxyl radical (HO.), and various forms of organic and inorganic peroxides (Mittler et al., 2022). ROS function in cells as signaling molecules and are essential for multiple biological processes. However, they are also toxic byproducts of aerobic metabolism. Therefore, balancing ROS production, scavenging, and transport is crucial to living organisms (Castro et al., 2021; Mittler et al., 2022). Biotic and abiotic stresses can disrupt this homeostasis, leading to a stress-specific accumulation of different ROS in different subcellular compartments (Mittler et al., 2022). In addition to their aforementioned function as local and systemic signaling molecules inducing further defense reactions, ROS are involved in direct defense as well (Castro et al., 2021; Mittler et al., 2022). Aphid-responsive H2O2 accumulation is widely conserved across plant families (Goggin and Fischer, 2021). For instance, saliva proteins have been identified that act as effectors and trigger ROS accumulation in host plants (Goggin and Fischer, 2021). Additionally, ROS accumulation is also involved in response mechanisms during plant virus infection (Hernández et al., 2016; Kappagantu et al., 2020; Király et al., 2021).
To determine if plant defense against R. padi that might cause BYDV resistance in the tested genotypes is present, this study investigates i) the possible presence of repellent factors affecting host choice, ii) the presence of resistance factors affecting feeding behavior, and iii) whether a general pathogen defense mechanism, the production of ROS, might be involved in BYDV-PAV resistance. Furthermore, iv) the influence of BYDV-PAV infection on the vascular system of susceptible, tolerant, and resistant maize inbreds is investigated and their influence on the feeding behavior of R. padi and a possible spread of viruses is discussed.
Five maize inbred lines—namely D408, FAP1360A, Ky226, P092, and W64A—were included in our study. These inbreds have been identified in the screenings of Horn et al. (2013; 2015) to differ with respect to their BYDV-PAV concentration measured with double antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) after artificial inoculation in field and greenhouse conditions. No or very few virus particles (extinction rate < 0.5) have been identified in D408, FAP1360A, and Ky226 which were therefore considered BYDV-PAV-resistant (Horn et al., 2015; Schmidt et al., 2024). A higher virus concentration (extinction rate > 0.75) was observed for P092 and W64A, where the former showed no BYDV-PAV-related symptoms but the latter did. Therefore, P092 was designated as BYDV-PAV-tolerant and W64A as BYDV-PAV-susceptible (Horn et al., 2015; Schmidt et al., 2024). These five inbreds were cultivated in a greenhouse (16h light, 20°C/8h darkness, 16°C) unless stated otherwise. These inbreds are the founders of connected segregating mapping populations that were used by Horn et al. (2015) to identify the QTLs for BYDV resistance in maize.
Apterous aphids carrying barley yellow dwarf virus PAV (BYDV-PAV) and virus-free apterous aphids of the species Rhopalosiphum padi clone R07 were reared under controlled environmental conditions on the BYDV-susceptible barley cv. ‘Haisa’ at room temperature under artificial daylight conditions with a light/dark period as described above. Viruliferous and virus-free aphid populations were kept at a physical distance from each other and checked regularly for the presence of BYDV-PAV using DAS-ELISA with in-house polyclonal antisera for BYDV-PAV from the Julius Kühn-Institute as described below.
The relative virus titer of BYDV-PAV-infected plants was determined by DAS-ELISA according to Clark and Adams (1977) using custom-made polyclonal antibodies (Julius Kühn-Institute, Quedlinburg/Germany). A total of 50mg of leaf material was collected from the sixth leaf of individual plants 6 weeks after infection and samples were processed as previously described (Horn et al., 2013). The extinction value was measured at 405 nm, 60 min after the addition of the enzyme substrate (p-nitrophenyl phosphate) using a microplate reader (Tecan Sunrise, Tecan, Männedorf, Switzerland). Leaf samples from healthy maize plants were used as negative controls. A positive infection was defined by Formula 2 as described by Lardeux et al. (2016).
Observation of aphid feeding behavior was conducted via electrical penetration graph (EPG) recordings (Tjallingii, 1978; Tjallingii and Esch, 1993). For preparation of the aphids, randomly selected adult apterous BYDV-PAV-carrying R. padi were starved for approximately 1 hour and were subsequently attached to a thin gold wire (2cm length, 18µm diameter) using water-based silver conductive glue (EPG-Systems, Wageningen, The Netherlands). The gold wire was connected via a copper wire to a pre-amplifier and the latter was connected to a Giga-8 EPG amplifier (EPG Systems). A total of 16 aphids were prepared in parallel and plants of the inbreds were distributed randomly to the eight positions of the EPG amplifier and two amplifiers were used in parallel. Aphids were placed on the lower side of the youngest mature leaf of the plants in the two-to-three-leaf stage and the aphid feeding behavior was monitored for 8 hours. For each test, new plants and aphids were used. For each maize inbred, the experiment was replicated 10 to 22 times.
EPG waveforms were recorded with the software module EPG Stylet + d (EPG Systems), and data analysis was conducted using the software module EPG Stylet + a (EPG Systems). EPG waveforms were annotated in accordance with Tjallingii (1978) and Tjallingii and Esch (1993). EPG parameter analysis was conducted by using the Excel workbook NPAC-EPGv v1.0 (Garzo et al., 2024). Parameters in Table 1 were selected for analysis due to the focus on plant resistance against aphids and the potential of a negative effect on the transmission of BYDV, a persistently transmitted virus.
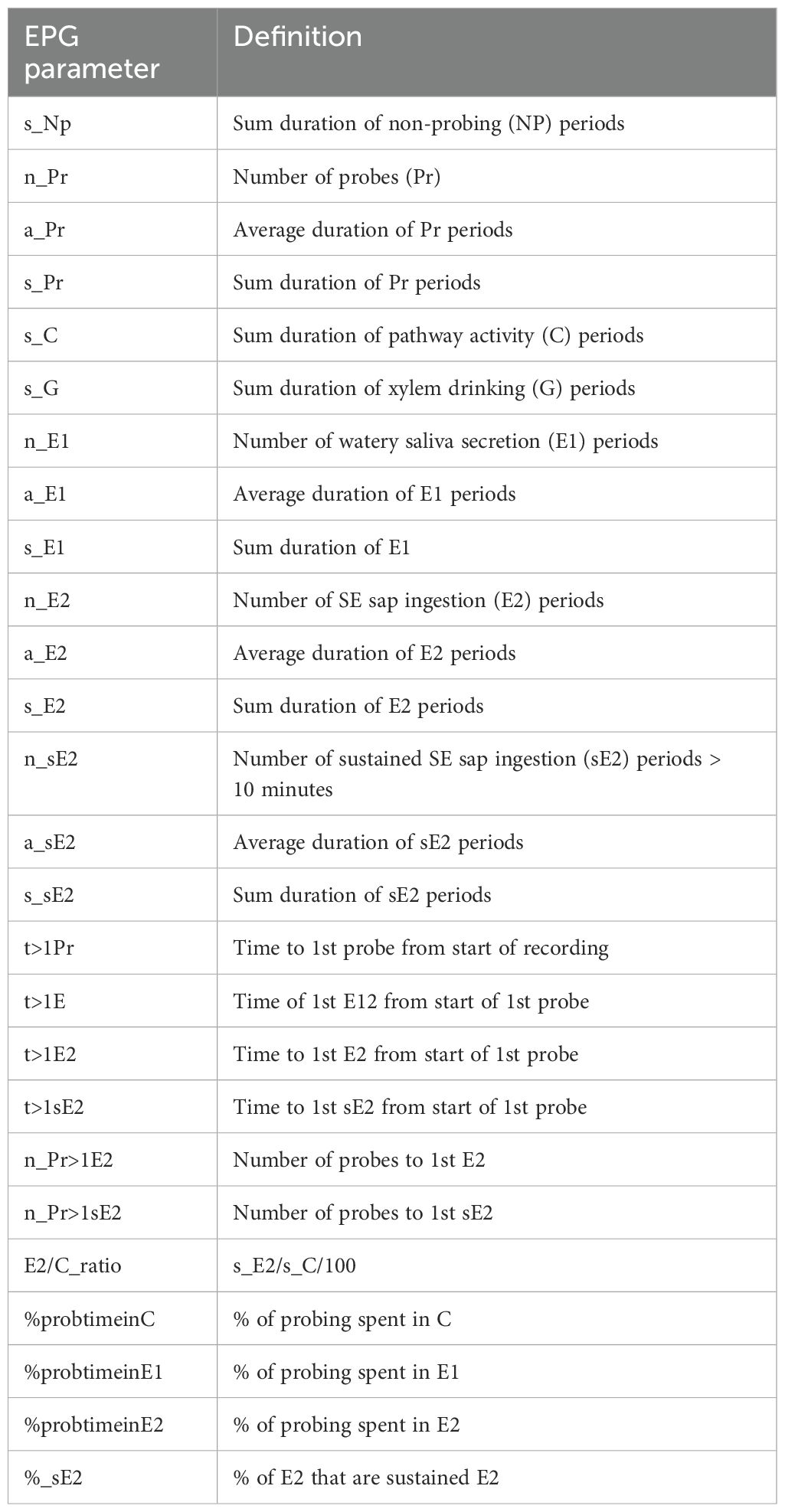
Table 1. Selected EPG parameters for analysis with a focus on plant resistance against aphids and relevance for transmission of BYDV.
To observe short-term [2h (Jiménez-Martínez et al., 2004)] and long-term host plant preference (24h) of R. padi, multi-choice tests were conducted. The front 5cm of leaves of maize inbreds D408, FAP 1360A, Ky226, P092, and W64A of plants in the two-or-three-leaf stage, still attached to an intact plant, were placed in a circular choice arena with an inner diameter of 11.5 cm as described by Hewer et al. (2010). Leaf tips were attached to the bottom of the choice arena with adhesive tape at equal distances from the middle of the arena in a randomized order. In total, 30 BYDV-PAV-carrying adult apterous R. padi were placed in an area of 5.3cm2 in the middle of the arena. The arena was covered with a plastic ring covered with Parafilm to prevent the aphids from escaping and, on the lower side, foam rubber was used to protect the leaves from injury (Supplementary Figure S1). The experimental setup was kept in the greenhouse under the same conditions used for plant rearing. Aphids on the upper and lower sides of each leaf were counted in intervals of 30 minutes for a period of 2 hours and after 24 hours (1,440 minutes). The experiment was replicated 17 times. Our study focuses on avoiding BYDV infection in order to interrupt the function of maize as a green bridge for BYDV. We therefore conducted our experiments exclusively with BYDV-carrying aphids, since we wanted to investigate which of the tested inbreds was less preferred by BYDV-carrying aphids.
This experiment examined the involvement of a general pathogen defense mechanism, the production of ROS, in the resistance mechanism against BYDV-PAV and R. padi. The experiment was based on three selected maize inbreds with different levels of susceptibility/resistance to BYDV: FAP1360A (BYDV-PAV-resistant), P092 (BYDV-PAV-tolerant), and W64A (BYDV-PAV-susceptible). Plants were cultivated in a climate chamber (16h light at 24°C and 8h dark at 22°C). When plants reached the two-leaf stage, they were treated with i) BYDV-PAV-carrying aphids, ii) virus-free aphids, or iii) left untreated as control. Samples were taken at the start of the experiment (0h) and at 6, 12, 24, 48, and 96 hours and 6 weeks (end of the experiment) after inoculation. Aphids were gently removed with a fine paintbrush immediately before the samples were harvested. The youngest fully developed leaves of eight plants per inbred and treatment were pooled and frozen immediately in liquid nitrogen. Leaves were homogenized and weighted under deep frozen conditions. The amount of ROS was determined using the method of Jambunathan (2010). Inoculation success was confirmed by DAS-ELISA.
Inbreds FAP1360A, P092, and W64A were grown in a climate chamber (16h light at 24°C, 8h darkness at 22°C). A total of 16 plants of each inbred were treated with BYDV-PAV-carrying R. padi at the two-leaf stage while 16 others were left untreated as a control. Cross-sections of the midrib in the middle of the leaf and at the widest point of the mature 8th leaf were examined microscopically and vascular bundle diameter, diameter of ten sieve elements, sieve element number, and diameter of the xylem vessels of different vein orders (major and minor veins) were measured. Additionally, leaf length, leaf width, and plant height were recorded. The experiment was conducted with three replicates. Inoculation success with BYDV was confirmed on a per-plant basis 6 weeks after infection by using DAS-ELISA.
The data collected at each timepoint in the choice test were analyzed using the following mixed linear model
where Yij is the phenotypic observation (number of aphids) for the ith inbred in the jth replicate, μ the general mean, gi the effect of the ith inbred, bj the effect of the jth replicate, and eij the residual. Inbred and replicate were considered as fixed effects. Because the replications did not differ significantly, the final model of the analysis was:
Residuals were checked graphically for normal distribution. As this was the case, ANOVA and Tukey’s HSD test were applied for further analyses.
Analyses of EPG data were conducted using the non-parametric Kruskal–Wallis test, as a normal distribution of the acquired data was not detected for most parameters. The Wilcoxon test was used as a post hoc test for pairwise comparisons. A correction for multiple testing was not applied, as the high degree of data scatter of behavioral data in general only leads to small significant differences in relation to the p-value. This leads to an increased risk of Type I errors but reduces the risk for Type II errors.
The data collected in the ROS experiment were analyzed using the following mixed linear model:
where Yijkl is the observed concentration of H2O2 equivalents, μ the general mean, gi the effect of the ith inbred, tj the effect of the jth treatment, hk the effect of the kth time of sampling, bl the effect of the lth block, and eijkl the residual. gi and tj were considered as fixed and all other effects as random.
Analyses of data collected from microscopic observation of leaves of BYDV-infected plants was conducted using the following mixed linear model
where Yijk is the phenotypic observation for the ith inbred and the jth treatment for the kth replicate, μ the general mean, gi the effect of the ith inbred, tj the effect of the jth treatment, bk the effect of the kth block, and eijk the residual. gi and tj were considered as fixed and all other effects as random.
Unless stated otherwise, all analyses were conducted using R version 3.6.3 (R Core Team, 2020).
In order to test aphid preferences for the respective maize inbred genotypes, plants were offered to BYDV-PAV-carrying R. padi in a multi-choice arena. The applied mixed linear model indicates that plant genotype and time interval significantly affect the number of aphids on the leaves. At all time intervals, a significant difference was detected for the genotypes tested (30’: df=4, SQ=6.518, F-value=2.743, p=0.034; 60’: df=4, SQ=14.588, F-value=3.647, p=0.009; 90’: df=4, SQ=18.659, F-value=4.48, p=0.003; 120’: df=4, SQ=15.459, F-value=4.38, p=0.003; 1440’: df=4, SQ=201.106, F-value=50.277, p<0.001) where the most aphids were detected on FAP1306A while the lowest number was detected for the genotypes Ky226 and P092. For all time points, Tukey’s HSD test (α = 0.05) showed significant differences between FAP1306A to one or multiple other genotypes regarding the preference of BYDV-carrying R. padi. This preference for FAP1360A became even more pronounced after 24h (Figure 1).
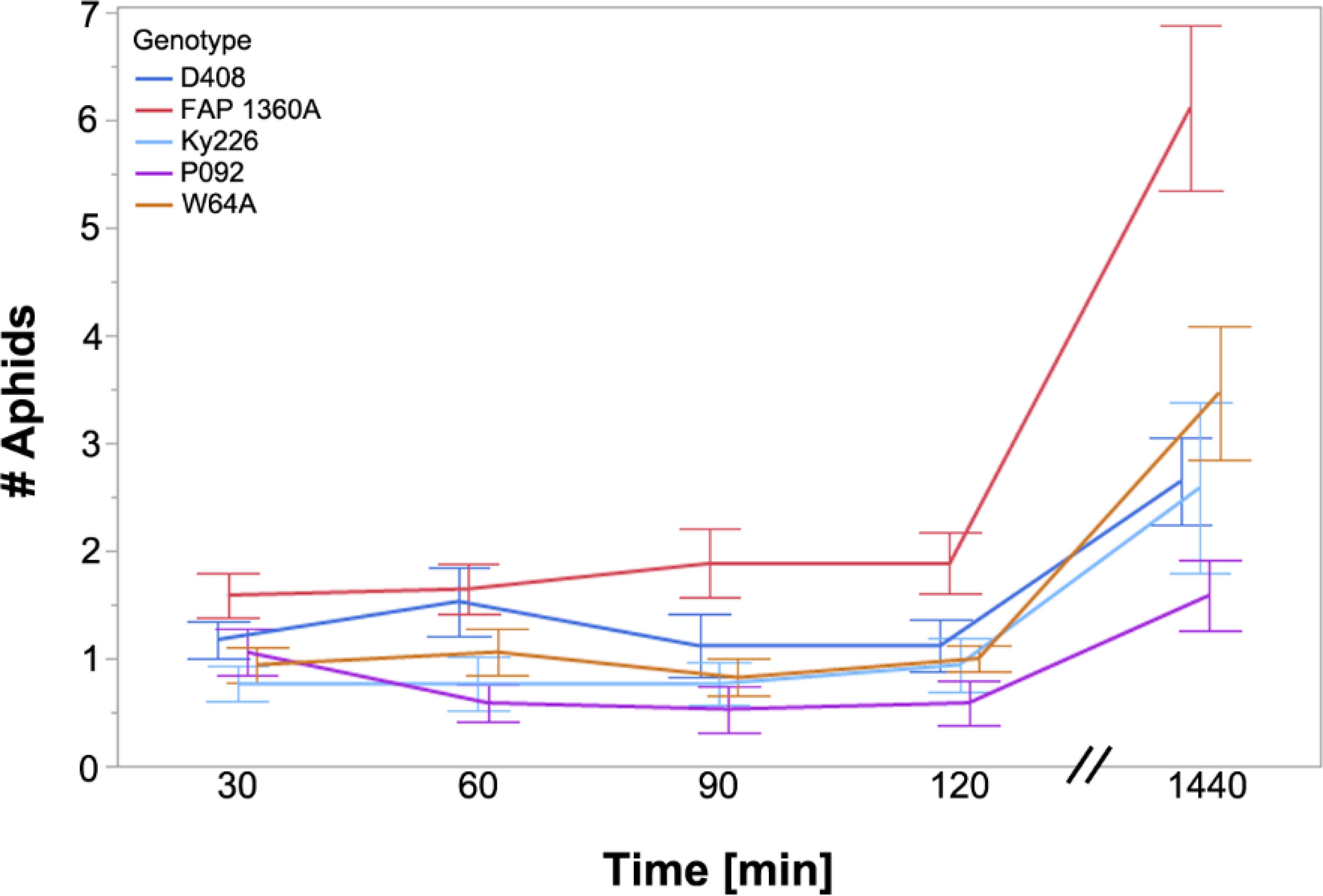
Figure 1. Number of aphids in a multi-choice experiment on maize inbred genotypes. BYDV carrying-R. padi were placed in a choice arena containing leaves of BYDV-PAV-free inbreds D408 (resistant), FAP1360A (resistant), Ky226 (resistant), P092 (tolerant), and W64A (susceptible) with differing susceptibility/resistance against BYDV (status given in brackets). Aphids were counted at different time points (note the break of the x-axis). Data points represent the mean (N=17) including the standard error of the mean.
We conducted an EPG analysis to test if the BYDV-PAV susceptibility of maize inbreds correlates with R. padi susceptibility based on the observation of feeding behavior on the respective inbreds. Regardless of the maize inbred, BYDV-PAV-carrying R. padi spent the majority of the 8-hour EPG experiment with probing activity, indicated by s_Pr with a mean in a range of 317 (FAP1360A) and 352 minutes (D408). This period represented 66% to 73% of the duration of the experiment and includes any kind of behavior that is associated with penetration of the leaf by the aphid stylet (waveform C, F, G, E1, and E2; Figure 2). In this context, no significant differences were observed for the EPG parameters s_Pr and s_Np between the genotypes tested (Tables 1, 2). For the average probing time (a_Pr), the Kruskal–Wallis test indicated a significant difference between the groups (Chi2 = 13.545, p = .0089). In detail, a significant difference was observed between FAP1360A and W64A (z = 3.503, p = .0005) and P092 (z = 2.889, p = .004), respectively with the lowest value for FAP1360A (Figure 2).

Figure 2. Non-sequential EPG parameters are shown as the sum of duration for the respective parameters and maize inbreds with differing susceptibility/resistance against BYDV (status given in brackets): D408 (resistant), FAP1360A (resistant), Ky226 (resistant), P092 (tolerant), and W64A (susceptible). The Wilcoxon test was used for pairwise comparison and different letters indicate significant differences between genotypes (p < 0.05). N = 10-22.
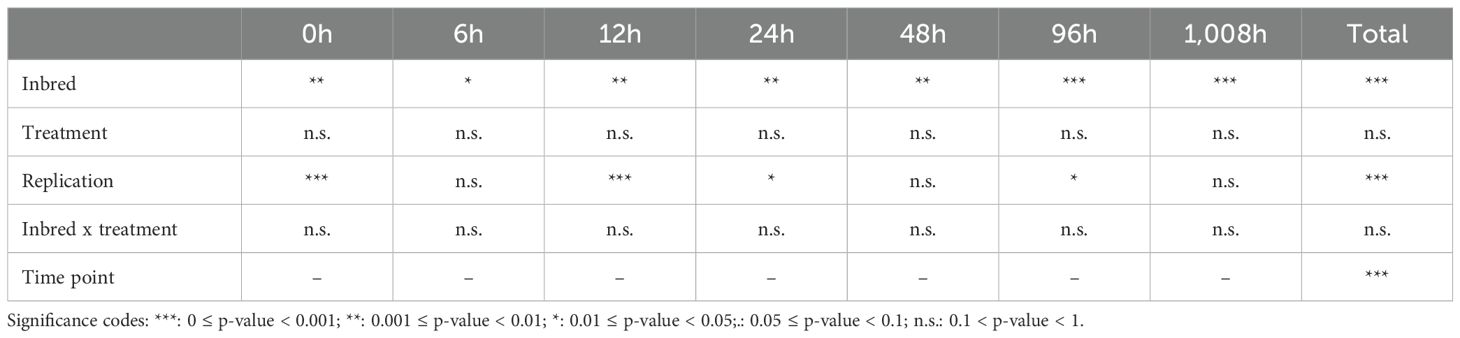
Table 2. Significance of the experimental factors that influence ROS level in Rhopalosiphum padi-infested, BYDV-PAV-infected, and control plants of maize inbreds FAP1360A, P092, and W64A.
Upon closer examination of the EPG parameters closely linked to the respective waveforms that are summarized by the term probing, significant differences were detected among inbreds for s_C (Chi2 = 9.974, p = .0409), a_E1 (Chi2 = 11.59, p = .0207), s_E1 (Chi2 = 17.878, p = .0013), and a_E2 (Chi2 = 13.976, p = .0074). In detail, aphids on the inbreds W64A and Ky226 spent less time on stylet propagation through the apoplast when compared with genotypes P092 [(W64A – P092 (z = 2.497, p = .0125); Ky226 – P092 (z = 2.053, p = .0401)] and D408 [(W64A – D408 (z = 2.497, p = .0125); Ky226 – D408 (z = -2.186, p = .0228)]. While the duration for s_C was reduced for W64A and Ky226 in a range of approximately 20% to 25%, the sum duration of ingestion of SE sap (s_E2) was increased for aphids on these genotypes by approximately 30% to 70% where these differences were not significant. The result of this observation was an E2/C ratio above one, indicating that aphids on the genotypes W64A and Ky226 spend more time ingesting SE sap than searching for SEs as food sources. The aphids on genotypes W64A and Ky226 showed a significant increase in the average duration of single ingestion events [(P092 - W64A (z = -2.488, p = .0129); P092 - Ky226 (z = -3.07, p = .0021) and D408 (Ky226 – D408 (z = 2.385, p = .0171)). Taking a step back to the beginning of SE penetration and the initial SE-associated behavior E1, the secretion of watery saliva relevant for suppression of plant defense, significant differences were detected among the inbreds for the parameters a_E1 [P092 - Ky226 (z = 3.191, p = .0014); Ky226 – D408 (z = -2.234, p = .0255); Ky226 – FAP1360A (z = -2.398, p = .0165)] with the lowest duration for aphids on the inbred Ky226. Aphids on Ky226 also showed the lowest duration for s_E1, up to 13.5-fold lower when compared with W64A, with a significant difference compared to all other genotypes [P092 (z = 4.127, p <.0001); FAP1360A (z = -2.714, p = .0066); W64A (z = -2.613, p = .009); D408 (z = -3.188, p = .0014)]. The time needed from the beginning of probing to the first phloem contact (t>1E), first ingestion (t>1E2), and first sustained ingestion (t>1sE2) did not differ between the groups whereat aphids on FAP1360A show for all parameters the longest duration.
Data were further analyzed at hourly intervals (Figure 3) to investigate temporal aspects of feeding behavior. Significant (α = 0.05) differences in EPG parameters between aphids feeding on D408, FAP1360A, Ky226, P092, and W64A were tested by using the Kruskal–Wallis test and in case of a significant difference, the Wilcoxon test was applied for pairwise comparison. As described for s_E1 for the entire recording time, the curve progression over time for s_E1 (Figure 3A) showed clear differences between aphids on the genotype Ky226 and the other genotypes tested with a mean duration below 1 minute for the respective hours. In contrast, for the genotypes D408, FAP1360A, and W64A, an increase over time was detected, although strong variations occurred between subsequent hours. Only for P092 did the value drop after a peak in hour 4. As the Kruskal–Wallis test did not indicate significant differences among inbreds for the respective hours, no pairwise comparisons were performed. For the other parameters tested [s_C, s_E2, and s_sE2 (Figure 3B)] aphids on the inbreds D408, FAP1360A, and P092 did not show any difference and the summed duration did not vary strongly over time. For the inbreds Ky226 and W64A, a continuous increase in s_E2 and s_sE2 was observed which was accompanied by a decrease in s_C. This trend stopped at hour 4 and was reversed from hour 5 onwards. A significant difference between inbreds was observed for s_C (Chi2 = 14.499, p = .0059), s_E2 (Chi2 = 14.532, p = .0058), and s_sE2 (Chi2 = 22.443, p = .0002) exclusively at hour 4: s_C: [Ky226 (Ky226 - FAP1360A (z = -2.442, p = .0146); Ky226 – D408 (z = -2.053, p = .04); Ky226 – P092 (z = 2.79, p = .0053)] and W64A [W64A - FAP1360A (z = -2.365, p = .018); W64A – D408 (z = -2.083, p = .0373); W64A – P092 (z = -2.549, p = .0108)]; s_E2: Ky226 [Ky226 - FAP1360A (z = 2.052, p = .0402); Ky226 – P092 (z = -1.996, p = .046)] and W64A [W64A - FAP1360A (z = 2.566, p = .0103); W64A – D408 (z = 2.658, p = .0079); W64A – P092 (z = 3.115, p = .0018)]; s_sE2: Ky226 [Ky226 - FAP1360A (z = 2.652, p = .008); Ky226 – D408 (z = 2.703, p = .0069); Ky226 – P092 (z = -3.195, p = .0014)] and W64A [W64A - FAP1360A (z = 2.653, p = .008); W64A – D408 (z = 2.893, p = .0038); W64A – P092 (z = 3.385, p = .0007)]. With the exception of FAP1360A, all aphids reached the sieve elements and showed secretion of watery saliva (E1) and ingestion (E2) during the observation period (Figure 4).
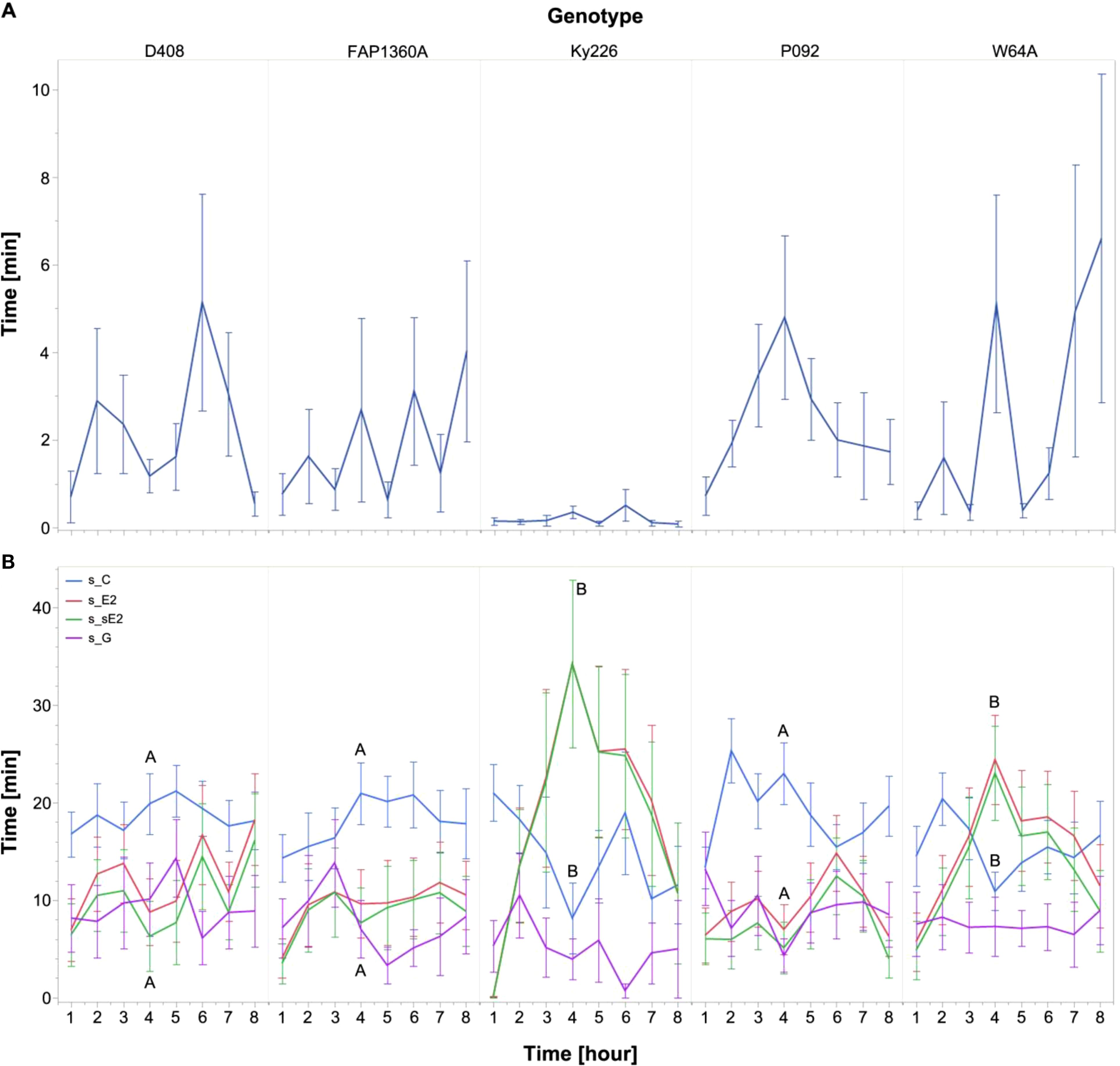
Figure 3. Non-sequential EPG parameters are shown as the sum of the duration for the respective parameters sE1 (A) as well as sC, sE2, ssE2, and sG (B) and maize inbreds for the respective hours. The Wilcoxon-Test was used for pairwise comparisons of inbreds for each hour, and different letters indicate significant differences between inbreds (p < 0.05). For clarity, significant differences of sE2 and s_sE2 between inbreds are presented together as no huge difference was observed for these parameters. N = 10-22.
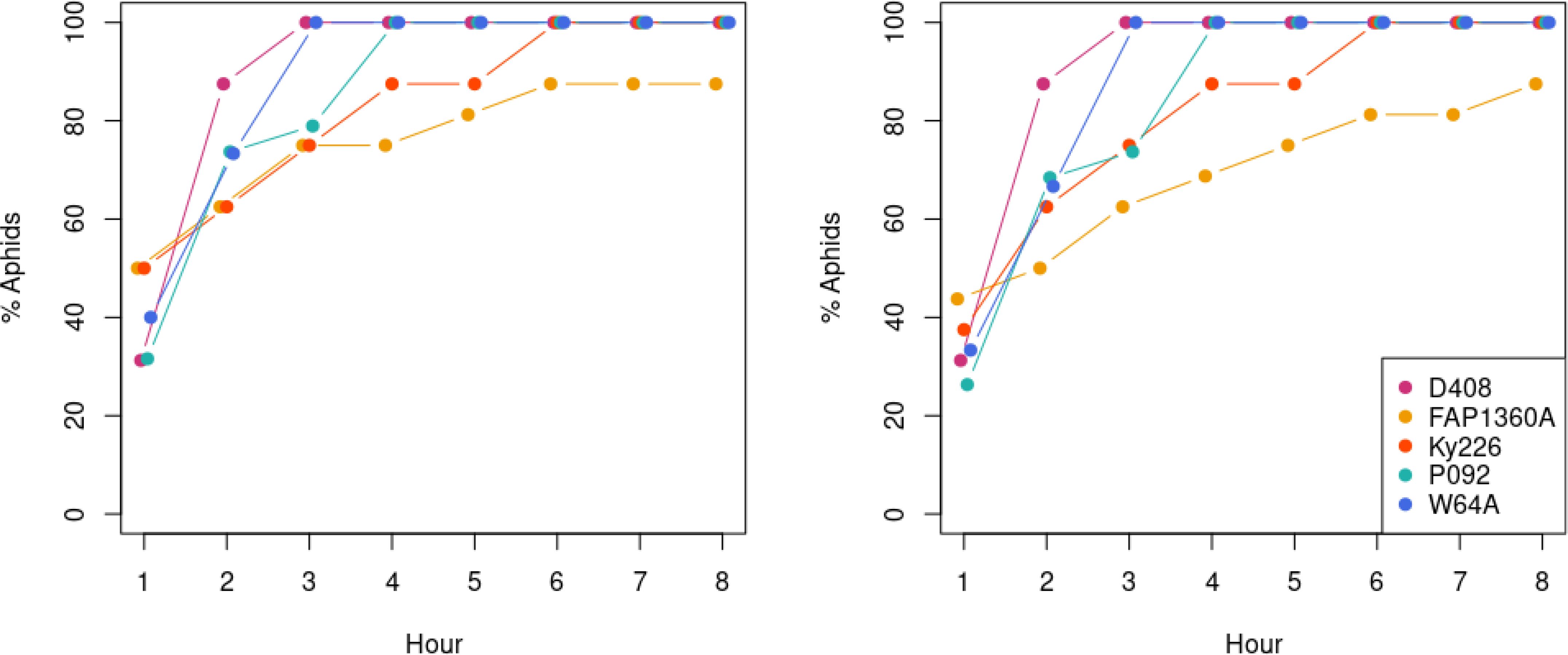
Figure 4. Percentage of BYDV-carrying Rhopalosiphum padi that reached phloem salivation E1 (left) and phloem sap ingestion E2 (right) on BYDV-resistant (D408, FAP1360A, and Ky226), BYDV-tolerant (P092), and BYDV-susceptible (W64A) maize inbred lines during the observation period of the EPG experiment.
Concentrations of ROS varied between 38µmol/g-1 fresh weight (FW) and 898µmol/g-1FW across all inbred*treatment combinations (Figure 5). For all inbreds, the initial ROS concentration at t0 was high, dropped within the first 6 hours of incubation, and subsequently increased. The maize inbred, the time point of sampling, and the block had a significant influence on the measured ROS concentration (Table 2). However, the treatment (BYDV-PAV infection, aphid infestation, or control) did not significantly affect ROS concentrations—neither across the whole time of the experiment nor at single time points (Figure 5). Pairwise comparisons revealed that all three maize inbreds were significantly (α = 0.05) different from each other (Table 2) with FAP1360A having the lowest ROS concentrations (average of 219µmol/g-1FW across all time points and treatments) and P092 having the highest ROS concentrations (average of 462µmol g-1FW). ROS concentrations of W64A were one and a half times higher than that of FAP1360A (average of 338µmol g-1FW).
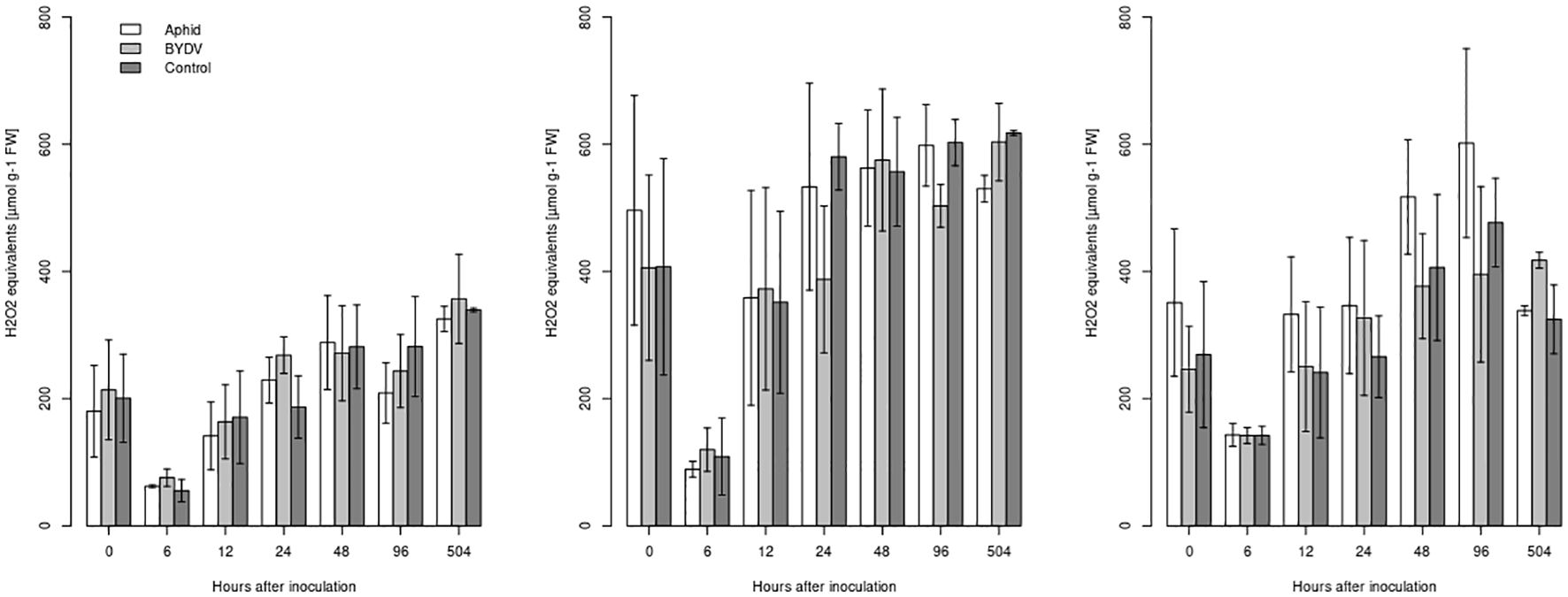
Figure 5. Reactive oxygen species level in Rhopalosiphum padi-infested, BYDV-PAV-infected, and control plants of maize inbreds FAP1360A (left), P092 (middle), and W64A(right). Error bars represent the standard error of the mean.
ROS concentrations measured in blocks A and B were significantly (α = 0.05) lower than in blocks C and D. This was true for all time points. ROS concentrations measured at time points 0h and 12h were both significantly (α = 0.05) lower than concentrations measured at 48h and 96h, respectively.
The diameter of the vascular bundles varied across all inbred*treatment combinations between 140 and 310µm (Figure 6). The size of the sieve elements was between 8 and 17µm and the xylem vessel sizes ranged between 42 and 81µm.
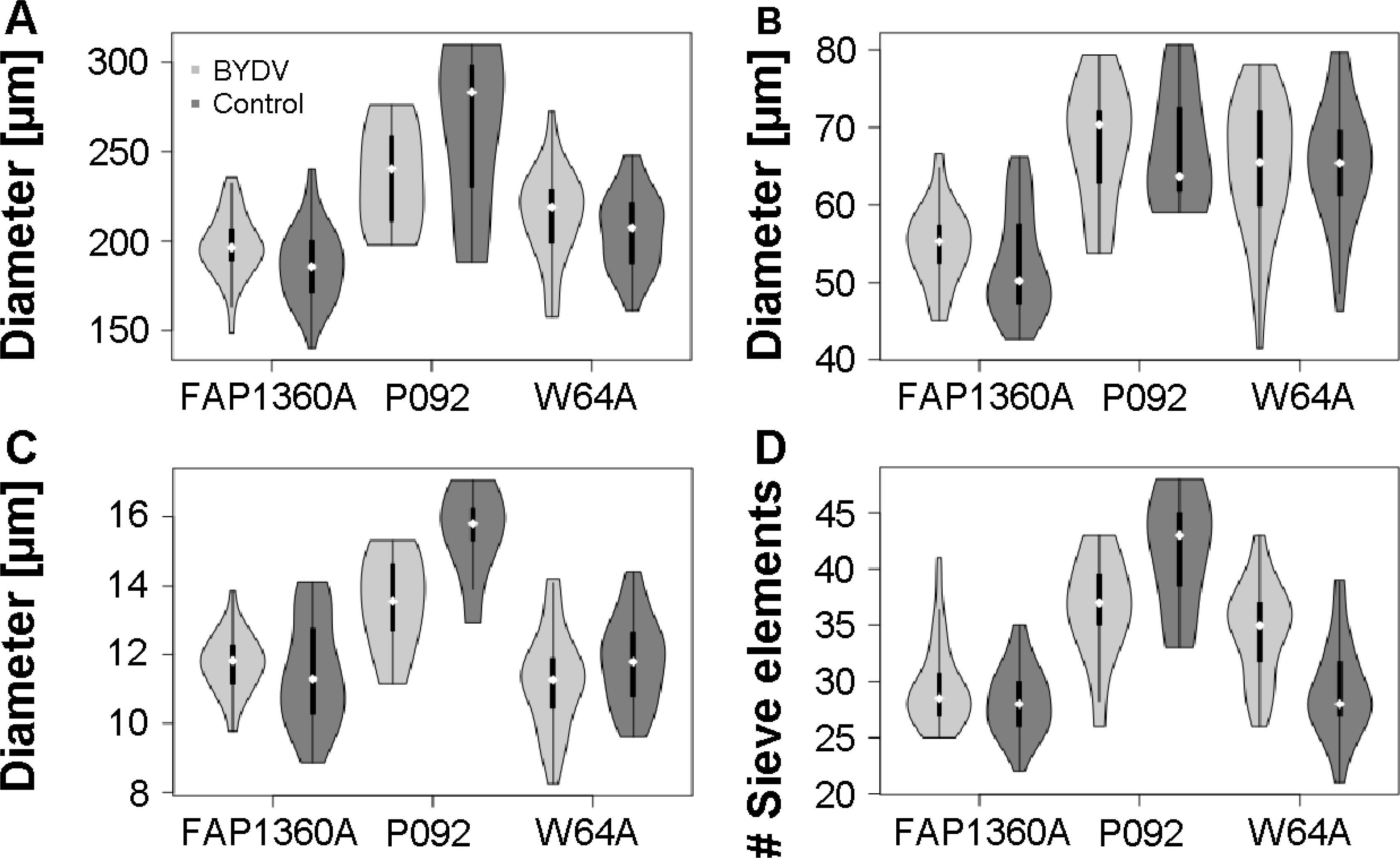
Figure 6. Morphological comparison of major vein vascular bundle parameters of BYDV-PAV-infected and uninfected control maize inbreds. (A) vascular bundle diameter, (B) xylem diameter, (C) sieve element diameter, (D) number of sieve elements. Note the suppressed zero at the y-axis scale. The white diamond shape represents the median.
The maize inbred significantly influenced the diameter of the vascular bundle, sieve elements, and xylem vessels and the number of sieve elements in both major and minor veins (Table 3; Supplementary Figure S2). These parameters were highest in P092 and lowest in FAP1360A. However, the xylem diameter of the minor veins was highest in W64A (Supplementary Figure S2).
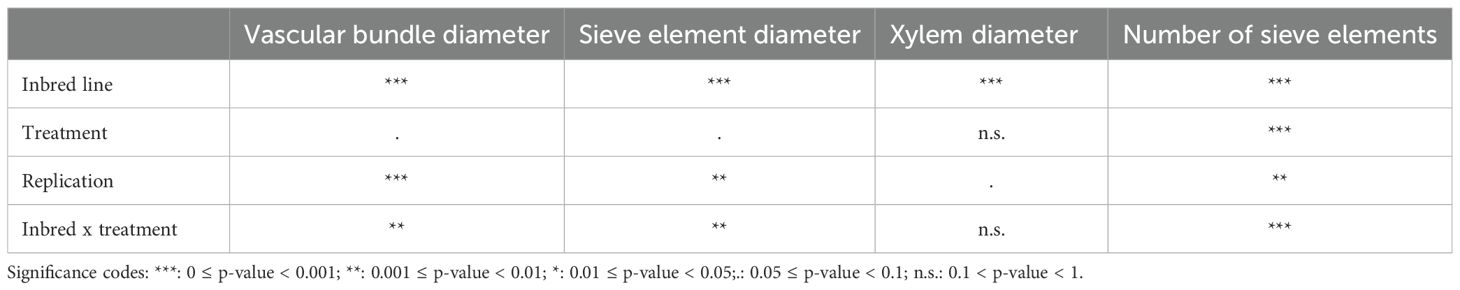
Table 3. Significance of the experimental factors influencing morphological traits of the vascular bundle of the major vein of BYDV-PAV-infected and control plants of maize inbreds FAP1360A, P092, and W64A from an analysis of variance.
BYDV-PAV infection significantly influenced the number of sieve elements and diameter of the vascular bundle and sieve elements in both major and minor veins but not the xylem diameter (Table 3). We observed a significant inbred*treatment interaction effect for vascular bundle diameter, sieve element diameter, and number of sieve elements of the major and number of sieve elements of the minor veins (Table 3 and Supplementary Table S1). However, changes evoked by BYDV-PAV infection were usually of small scale. Parameters measured in BYDV-PAV-infected plants differed from control plants by less than ±6% except for the diameter of the vascular bundle and sieve elements of the major vein of P092 that were strongly reduced in BYDV-PAV-infected plants in comparison to control plants (-10,5% and -13,5%, respectively), and the number of sieve elements in the major veins (-12.0%) of P092. Conversely, the number of sieve elements in the major vein (+18.7%) and minor veins (+16,6%) of inbred W64A was increased in BYDV-PAV-infected plants compared to control plants. Additionally, BYDV-PAV infection strongly reduced the plant height of inbred P092 (-18.5%) and the leaf width of inbred W64A (-9.1%), compared to control plants.
World-wide inbred lines of maize show broad genetic variation (Buckler et al., 2006) and can be a source of new traits that can be exploited in breeding. In this context, BYDV tolerance and resistance (Horn et al., 2013) are of potential interest for maize breeding as BYDV infection causes direct effects such as chlorotic spots and discoloration (Eweida et al., 1983) and yield losses (Beuve et al., 1999). In addition, maize also acts as a green bridge for BYDV and its vector (Rashidi et al., 2020), meaning that resistance would reduce the risk of spreading BYDV infection and, thus, protect other crops.
Plants are able to sense herbivore or pathogen attack through specific receptors and activate molecular mechanisms that induce specific defense reactions (Castro et al., 2021; Couto and Zipfel, 2016; Erb and Reymond, 2019; Jiang et al., 2019; Radchenko et al., 2022), leading to antibiosis and antixenosis (Peterson et al., 2017). In addition to toxic secondary metabolites, plants have evolved a range of mechanisms to combat herbivores and pathogens (for review see Erb and Reymond, 2019; Radchenko et al., 2022). Volatile organic compounds (VOCs) and visual cues of host plants may attract or repel aphids (Jiménez-Martínez et al., 2004), the latter of which can be considered a mechanism of antixenosis and, thus, a first line of defense against herbivores. We observed that BYDV-PAV-carrying R. padi significantly preferred the BYDV-PAV-resistant maize inbred line FAP1360A compared to other BYDV-PAV-resistant inbreds (D408 and Ky226), the BYDV-PAV-tolerant inbred P092, and the BYDV-PAV-susceptible line W64A (Figure 1). This can be interpreted as an attraction to FAP1360A or may be caused by a repellent effect of the other inbred lines tested. During the observation period, the difference between FAP1360A and the other tested inbreds intensified and was strongest after 24h, indicating that presumably short-term (volatile) and long-term interactions (feeding associated factors) are responsible for attraction and repellence in the respective inbreds. However, the presence of repellent or attracting VOCs was not tested in this study. For wheat, Medina-Ortega et al. (2009) identified the VOC Z-3-hexenyl acetate as a relevant compound for host plant preference, which was confirmed in maize by Schröder et al. (2015) and that was also detected as a VOC for barley (Kern et al., 2021). Schröder et al. (2015) detected repellent VOCs that were emitted by only two of three tested maize genotypes and whose presence/absence was obviously relevant in the context of host plant selection. Thus, the VOC pattern can differ between respective genotypes/inbreds and a comparable situation could have probably been observed for the inbred lines in our test, leading to the observed preference of the line FAP1360A by R. padi.
After host-plant recognition and colonization of a plant, the complex feeding behavior of aphids becomes apparent. In a meta-study comprising 76 individual studies on host-plant resistance against aphids, Leybourne and Aradottir (2022) found that phloem access is restricted in aphid-resistant plants. Aphids probing on resistant plants take a longer time to reach the phloem, as indicated by the longer time taken until E1. This is common in all plant families studied and defense responses are effective against aphids with broad and narrow host ranges, respectively (Leybourne and Aradottir, 2022). These factors might be activated only through aphid feeding, which has been demonstrated for plant reaction to virus-free and viruliferous aphids (Ahmad et al., 2011; Givovich and Niemeyer, 1991; Leybourne et al., 2019; Louis et al., 2015; Meihls et al., 2013). Differences in R. padi feeding behavior were reported for R. padi resistant and -susceptible maize cultivars (Sytykiewicz et al., 2019). In comparison to aphids feeding on a susceptible cultivar, R. padi feeding on the resistant cultivar spend a longer time with non-penetration (Np), exhibited a prolonged duration of secretion of watery saliva into sieve elements (E1), and reduced sieve element sap ingestion (E2). These behavior changes are characteristic of poor-host interaction as described by Escudero-Martinez et al. (2021).
Observing the detailed feeding behavior by EPG analysis revealed that BYDV-carrying R. padi feeding on the five virus-free maize inbreds D408, FAP1360A, Ky226, P092, and W64A did not differ with regard to the sum of the probing period (Figure 2), indicating that initial access to host plants does not differ between the inbred lines. With regard to average probing time, FAP1360A showed the lowest average probing time together with the highest number of probing events indicating that probing is interrupted more often, probably due to mesophyll-located plant defense responses. The total duration aphids spent in the pathway phase (C) revealed that R. padi spent more time searching for the food source on the BYDV-PAV-resistant lines FAP1360A and D408 compared to Ky226 and the BYDV-PAV-susceptible or tolerant inbreds. On the BYDV-resistant inbred Ky226, aphids spent the least time in pathway phase C, indicating that aphids find the sieve elements as a nutrition source faster than on other inbred lines. Combined with the observation that aphids on Ky226 show the lowest period of all lines tested on average and summed duration of the secretion of watery saliva into sieve elements (a_E1, s_E1), the data indicate that resistance factors in the mesophyll are not strongly expressed and are nearly absent in sieve elements for Ky226. The secretion of watery saliva serves to suppress plant defense responses, whereby detoxifying enzymes and effectors have been described for various aphid species (van Bel and Will, 2016). Furthermore, the secretion of watery saliva is important for the transmission of persistent viruses such as BYDV, as virus particles are injected into sieve elements along with the aphid’s saliva while acquisition occurs during ingestion of sieve element sap (Prado and Tjallingii, 1994). As the duration of a_E1 and s_E1 did not differ significantly between R. padi feeding on the other maize inbreds, none of these lines appear to show an increased defense against R. padi including the potential risk of acquiring an increased amount of virus particles during aphid-plant interaction.
Interestingly, BYDV-infected R. padi were strongly attracted to the BYDV-resistant inbred FAP1360A when aphids were allowed to choose freely between leaves of the tested inbred lines (Figure 1). This was accompanied by a reduced percentage of aphids on FAP1360 showing sieve element penetration indicated by E1 and 12.5% of aphids failed to reach the ingestion phase (E2) until the end of the observation period of the EPG experiment, indicating antixenosis. With a mean duration from the start of probing to the first phloem contact (t>1E) longer than for any other inbred line and a mean duration of s_E2 and s_sE2 in the midfield of all tested inbred lines, the maize inbred FAP1360A is only moderately susceptible to R. padi in contrast to the other tested inbreds. The choice and EPG data indicate the presence of a potential resistance mechanism located in the mesophyll that might be overwhelmed during later periods of aphid-plant interaction indicated by the high preference during later periods of the choice tests. In contrast, data for the BYDV-resistant inbred line Ky226 indicate that no resistance mechanisms were present, e.g., in the mesophyll and the sieve elements. The presence of sustained plant resistance against aphids could have explained the BYDV resistance of the lines Ky226, D408, and FAP1360A but it appears obvious that the observed BYDV resistance for which we expect a comparable molecular mechanism between the inbred lines (Schmidt et al., 2024), is independent of the degree of susceptibility to the vector R. padi.
This observation is in contrast to other cereals, where a correlation of the resistance against a virus and its vector insect has been suggested. In wild barley relatives, for instance, initial data suggested that YDV and aphid resistance can both occur in parallel in one genotype. Furthermore, R. padi feeding on H. bulbosum clone A17 with a qualitative resistance to BYDV and CYDV, which is caused by the Ryd4Hb gene (Scholz et al., 2009), showed reduced numbers and duration of E1 and E2 phases compared to aphids feeding on a BYDV-susceptible H. bulbosum clone (Schliephake et al., 2013). The authors suggested that reduced phloem feeding might result in resistance to BYDV (Schliephake et al., 2013). However, the results of Pidon et al. (2024) illustrated that reduced phloem feeding is not the resistance mechanism underlying Ryd4Hb. On wild barley, R. padi feeding is characterized by a reduced duration of sustained E2 in comparison to a BYDV-susceptible barley variety (Leybourne et al., 2019). However, this change of behavior was linked to reduced nutritional quality of the phloem sap (Leybourne et al., 2019).
An R. padi-resistant maize cultivar was reported to show increased H2O2 (ROS) levels in response to aphid infestation when compared with a susceptible cultivar (Sytykiewicz, 2015). ROS act as local and systemic signaling molecules inducing defense responses (Castro et al., 2021; Couto and Zipfel, 2016) and ROS accumulation is described as being a conserved response to aphid infestation in a diverse range of plant species including monocots (Goggin and Fischer, 2021). With regard to vector-transmitted virus infection of cucumber mosaic virus (CMV), a non-persistently transmitted virus, an increased ROS level was reported to lead to changes of aphid behavior with an increased duration to reach phloem ingestion, reduced sieve element sap ingestion, and an increased percentage of aphids that change the host plant whereat a change of aphid behavior was associated with the acquisition of a higher copy number of the virus per aphid and a probable enhancement of transmission (Guo et al., 2019). Regarding the transmission of persistently transmitted viruses such as BYDV by aphids, some similarities might be suggested. This is because previous studies showed that the ROS level of a susceptible variety increases in response to infection (Paulmann et al., 2018; Rong et al., 2018; Wang et al., 2018), while resistant barley inbreds carrying the resistance gene Ryd2 did not show an altered ROS level (Paulmann et al., 2018). Similarly, ROS levels were significantly increased in BYDV-GAV-infected susceptible wheat compared to uninfected plants or infected plants carrying the Bdv2 resistance gene three, four, and five weeks after inoculation (Paulmann et al., 2018; Rong et al., 2018; Wang et al., 2018). These observations can be related to CMV-induced accumulation of H2O2 as reported by (Guo et al., 2022).
Our data did not indicate any effect on ROS level with regard to an aphid infestation—neither in BYDV-PAV-resistant nor BYDV-PAV-susceptible or tolerant maize inbreds (Figure 5). At first glance, this appears to contradict the previous results of Sytykiewicz (2015), who additionally proposed a connection between the level of ROS accumulation and the degree of resistance to aphids. However, in our study, we observed only small differences in aphid attractivity and resistance among the tested maize inbreds compared to the study of Sytykiewicz (2015) but found strong differences in BYDV resistance/tolerance. Therefore, it is not surprising that the inbred line-specific differences observed in our study indicated that the BYDV-resistant inbred line FAP1360A showed the lowest ROS level, which is in accordance with resistant genotypes from studies on other cereals (Guo et al., 2019; Paulmann et al., 2018). It should be noted that a BYDV-associated ROS increase likely has no negative effect on aphid behavior, but rather leads to easier access as indicated by increased ingestion (Kern et al., 2021).
The fact that we did not detect statistical differences between the “control” and “aphid-infested” treatments in our study (Figure 5) suggests that unknown stressors might have affected the plants of both treatments during the experiment, possibly covering treatment-specific responses. Regarding the spatiotemporal dynamics of ROS production in response to aphid and especially virus infection, current knowledge is limited, making it difficult to compare data from different stressors and different plant species. A comparable biphasic increase in ROS, which was shown by our data, was previously described for Arabidopsis thaliana in response to aphid infestation (Jaouannet et al., 2015; Prince et al., 2014). For maize, no such course has been observed as demonstrated by Jiang and Zhang (2002) studying ROS level in the context of water stress. However, the magnitude of ROS induction varies depending upon the aphid species, the aphid biotype, the plant cultivar, plant age, location of the aphid infestation on the plant, and aspects of the experimental design such as infestation levels and timing of measurement (Goggin and Fischer, 2021).
In addition to plant defense responses on multiple levels, the functional integrity of the vascular bundle and the sieve elements must also be considered. This is because Esau (1957) described that BYDV infection leads to the accumulation of “wound gum” inside sieve elements, which potentially disturbs mass flow by reducing the conductivity inside sieve elements (Mullendore et al., 2010), ultimately leading to necrosis of the affected cells. Paulmann et al. (2018) demonstrated that, in contrast to detrimental effects on the anatomy and physiology of vascular bundles and sieve elements of a susceptible barley genotype, no effect was observed for a barley genotype with a quantitative BYDV resistance as a consequence of BYDV infection. For the BYDV-resistant maize inbred line FAP1360A, we observed no effects of BYDV infection on major and minor veins either, suggesting that physiological processes are not affected by virus infection. However, it appears that in maize, BYDV-induced effects on the anatomy of vascular bundles and sieve elements of the susceptible inbred maize line W64A and the tolerant line P092 were rather weak compared to the previous reports in barley. In addition, the trend varied for the susceptible inbred line in some points, e.g., with regard to the diameter of the vascular bundle of major veins that increased slightly in response to BYDV infection. This is most likely caused by the observed increase in the number of sieve elements. However, the question as to whether virus infection directly regulates cell division and differentiation inside the leaf tissue or if this was an indirect effect of the plant compensating, e.g., smaller diameters of individuals cells, remains unanswered.
Our experimental data suggest that BYDV resistance in the tested maize inbreds is not mediated through vector resistance (antixenosis) although both occur for some of the tested inbred lines as a combined trait. With regard to the anatomical data, we confirm previously observed data from barley showing that vascular anatomy of a resistant inbred line/genotype is not negatively affected by virus infection in cases of quantitative resistance, which is relevant for the transport of photoassimilates and, thus, for biomass and grain yield. In this context, interesting trait combinations for the inbred line FAP1360A were observed that are suitable for future breeding combining BYDV resistance with vector host plant preference and reduced feeding of R. padi.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
MS: Formal analysis, Investigation, Writing – original draft, Writing – review & editing. TW: Formal analysis, Investigation, Writing – original draft, Writing – review & editing. BR: Formal analysis, Investigation, Writing – review & editing. AH: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. BS: Conceptualization, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – project number 403095468. The funder did not influence the study design, the collection, analysis and interpretation of data, the writing of the manuscript, or the decision to submit the manuscript for publication.
We would like to thank Sascha Feldt for conducting the microscopy of vascular bundles. Furthermore, we would like to thank Stephanie Krey, Florian Esser, Vesna Lamesic, Konstantin Shek, and Katja Dlouhy for their technical support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2025.1526115/full#supplementary-material
Ahmad S., Veyrat N., Gordon-Weeks R., Zhang Y., Martin J., Smart L., et al. (2011). Benzoxazinoid metabolites regulate innate immunity against aphids and fungi in maize. Plant Physiol. 157, 317–327. doi: 10.1104/pp.111.180224
Alvarez A. E., Tjallingii W. F., Garzo E., Vleeshouwers V., Dicke M., Vosman B. (2006). Location of resistance factors in the leaves of potato and wild tuber-bearing Solanum species to the aphid Myzus persicae. Entomologia Experimentalis Applicata 121, 145–157. doi: 10.1111/j.1570-8703.2006.00464.x
Aradottir G. I., Crespo-Herrera L. (2021). Host plant resistance in wheat to barley yellow dwarf viruses and their aphid vectors: a review. Curr. Opin. Insect Sci. 45, 59–68. doi: 10.1016/j.cois.2021.01.002
Beuve M., Naïbo B., Foulgocq L., Lapierre H. (1999). Irrigated hybrid maize crop yield losses due to barley yellow dwarf virus-PAV luteovirus. Crop Sci. 39, 1830–1834. doi: 10.2135/cropsci1999.3961830x
Blanvillain R., Kim J. H., Wu S., Lima A., Ow D. W. (2009). OXIDATIVE STRESS 3 is a chromatin-associated factor involved in tolerance to heavy metals and oxidative stress. Plant J. 57, 654–665. doi: 10.1111/j.1365-313X.2008.03717.x
Brault V., Uzest M., Monsion B., Jacquot E., Blanc S. (2010). Aphids as transport devices for plant viruses. Comptes Rendus. Biologies 333, 524–538. doi: 10.1016/j.crvi.2010.04.001
Buckler E. S., Gaut B. S., McMullen M. D. (2006). Molecular and functional diversity of maize. Curr. Opin. Plant Biol. 9, 172–176. doi: 10.1016/j.pbi.2006.01.013
Castro B., Citterico M., Kimura S., Stevens D. M., Wrzaczek M., Coaker G. (2021). Stress-induced reactive oxygen species compartmentalization, perception and signalling. Nat. Plants 7, 403–412. doi: 10.1038/s41477-021-00887-0
Choudhury S., Hu H., Larkin P., Meinke H., Shabala S., Ahmed I., et al. (2018). Agronomical, biochemical and histological response of resistant and susceptible wheat and barley under BYDV stress. PeerJ 6, e4833. doi: 10.7717/peerj.4833
Choudhury S., Hu H., Meinke H., Shabala S., Westmore G., Larkin P., et al. (2017). Barley yellow dwarf viruses: infection mechanisms and breeding strategies. Euphytica 213. doi: 10.1007/s10681-017-1955-8
Clark M. F., Adams A. N. (1977). Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 34, 475–483. doi: 10.1099/0022-1317-34-3-475
Couto D., Zipfel C. (2016). Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 16, 537–552. doi: 10.1038/nri.2016.77
Erb M., Reymond P. (2019). Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 70, 527–557. doi: 10.1146/annurev-arplant-050718-095910
Esau K. (1957). Anatomic effects of barley yellow dwarf virus and maleic hydrazide on certain gramineae. Hilgardia 17, 15–69. doi: 10.3733/hilg.v27n01p015
Escudero-Martinez C., Leybourne D. J., Bos J. I. B. (2021). Plant resistance in different cell layers affects aphid probing and feeding behaviour during non-host and poor-host interactions. Bull. Entomological Res. 111, 31–38. doi: 10.1017/S0007485320000231
Eweida M., Tomenius K., Oxelfelt P. (1983). Reactions in maize infected with Swedish isolates of barley yellow dwarf (BYDV). J. Phytopathol. 108, 251–261. Available at: https://api.semanticscholar.org/CorpusID:84654032.
Garzo E. A.-O., Álvarez A. A.-O., Moreno A., Walker G. P., Tjallingii W. F., Fereres A. A.-O. (2024). Novel program for automatic calculation of EPG variables. J. Insect Sci. 24, 28. doi: 10.1093/jisesa/ieae063
Gildow F. E., Gray S. M. (1993). The aphid salivary gland basal lamina as a selective barrier associated with vector-specific transmission of barley yellow dwarf luteoviruses. Phytothology 83, 1293–1302. doi: 10.1094/PHYTO-83-1293
Givovich A., Niemeyer H. M. (1991). Hydroxamic acids affecting barley yellow dwarf virus transmission by the aphid Rhopalosiphum padi. Entomologia Experimentalis Applicata 59, 79–85. doi: 10.1111/j.1570-7458.1991.tb01489.x
Goggin F. L., Fischer H. D. (2021). Reactive oxygen species in plant interactions with aphids. Front. Plant Sci. 12, 811105. doi: 10.3389/fpls.2021.811105
Guo H., Bi X., Wang Z., Jiang D., Cai M., An M., et al. (2022). Reactive oxygen species-related genes participate in resistance to cucumber green mottle mosaic virus infection regulated by boron in Nicotiana benthamiana and watermelon. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1027404
Guo H., Gu L., Liu F., Chen F., Ge F., Sun Y. (2019). Aphid-borne viral spread is enhanced by virus-induced accumulation of plant reactive oxygen species. Plant Physiol. 179, 143–155. doi: 10.1104/pp.18.00437
Haack L., Courbon R., Riault G., Tanguy S., Le Vilain D., Henry M., et al. (1999). A plant and field study of BYDV-PAV and -MAV distribution on maize in France. J. Plant Dis. Prot. 106 (3), 297–303.
Halbert S., Voegtlin D. (1995). “Biology and taxonomy of vectors of barley yellow dwarf viruses,” in Barley yellow dwarf—40 years of progress. Eds. Arcy C. J. D., Burnett P. A. (St. Paul, MN, USA: APS Press), p. 217–258.
Henry M., Dedryver C. A. (1989). Fluctuations in cereal aphid populations on maize (Zea mays) in western France in relation to the epidemiology of barley yellow dwarf virus (BYDV). J. Appl. Entomology 107, 401–410. doi: 10.1111/j.1439-0418.1989.tb00275.x
Hernández J. A., Gullner G., Clemente-Moreno M. J., Künstler A., Juhász C., Díaz-Vivancos P., et al. (2016). Oxidative stress and antioxidative responses in plant–virus interactions. Physiol. Mol. Plant Pathol. 94, 134–148. doi: 10.1016/j.pmpp.2015.09.001
Hewer A., Will T., van Bel A. J. (2010). Plant cues for aphid navigation in vascular tissues. J. Exp. Biol. 213, 4030–4042. doi: 10.1242/jeb.046326
Horn F., Habekuß A., Stich B. (2013). Natural variation for BYDV resistance in maize. Maydica 58, 173–181.
Horn F., Habekuss A., Stich B. (2014). Genes involved in barley yellow dwarf virus resistance of maize. Theor. Appl. Genet. 127, 2575–2584. doi: 10.1007/s00122-014-2400-1
Horn F., Habekuss A., Stich B. (2015). Linkage mapping of barley yellow dwarf virus resistance in connected populations of maize. BMC Plant Biol. 15, 29. doi: 10.1186/s12870-015-0420-x
Hunter W. B. (2008). “Plant viruses and insects,” in Encyclopedia of entomology. Ed. Capinera J. L. (Dordrecht: Springer Netherlands), 2938–2945. doi: 10.1007/978-1-4020-6359-6_2991
Ingwell L. L., Eigenbrode S. D., Bosque-Pérez N. A. (2012). Plant viruses alter insect behavior to enhance their spread. Sci. Rep. 2, 578. doi: 10.1038/srep00578
Jambunathan N. (2010). Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. Methods Mol. Biol. 639, 292–298. doi: 10.1007/978-1-60761-702-0_18
Jaouannet M., Morris J. A., Hedley P. E., Bos J. I. B. (2015). Characterization of Arabidopsis transcriptional responses to different aphid species reveals genes that contribute to host susceptibility and non-host resistance. PLoS Pathog. 11, e1004918. doi: 10.1371/journal.ppat.1004918
Jiang M., Zhang J. (2002). Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 53, 2401–2410. doi: 10.1093/jxb/erf090
Jiang Y., Zhang C.-X., Chen R., He S. Y. (2019). Challenging battles of plants with phloem-feeding insects and prokaryotic pathogens. Proc. Natl. Acad. Sci. 116, 23390–23397. doi: 10.1073/pnas.1915396116
Jimenez J., Arias-Martin M., Moreno A., Garzo E., Fereres A. (2020). Barley yellow dwarf virus can be inoculated during brief intracellular punctures in phloem cells before the sieve element continuous salivation phase. Phytopathology 110, 85–93. doi: 10.1094/PHYTO-07-19-0260-FI
Jiménez-Martínez E. S., Bosque-Pérez N. A., Berger P. H., Zemetra R. S., Ding H., Eigenbrode S. D. (2004). Volatile cues influence the response of Rhopalosiphum padi (Homoptera: Aphididae) to barley yellow dwarf virus–infected transgenic and untransformed wheat. Environ. Entomology 33, 1207–1216. doi: 10.1603/0046-225X-33.5.1207
Jones J. D., Dangl J. L. (2006). The plant immune system. Nature 444, 323–329. doi: 10.1038/nature05286
Kappagantu M., Collum T. D., Dardick C., Culver J. N. (2020). Viral hacks of the plant vasculature: The role of phloem alterations in systemic virus infection. Annu. Rev. Virol. 7 (1), 351–370. doi: 10.1146/annurev-virology-010320-072410
Kern M., Meiners T., Schliephake E., Habekuss A., Ordon F., Will T. (2021). Infection of susceptible/tolerant barley genotypes with barley yellow dwarf virus alters the host plant preference of Rhopalosiphum padi clones depending upon their ability to transmit BYDV. J. Pest Sci. 95, 215–229. doi: 10.1007/s10340-021-01367-2
Király L., Albert R., Zsemberi O., Schwarczinger I., Hafez Y. M., Künstler A. (2021). Reactive oxygen species contribute to symptomless, extreme resistance to potato virus X in tobacco. Phytopathology 111, 1870–1884. doi: 10.1094/PHYTO-12-20-0540-R
Lardeux F., Torrico G., Aliaga C. (2016). Calculation of the ELISA's cut-off based on the change-point analysis method for detection of Trypanosoma cruzi infection in Bolivian dogs in the absence of controls. Mem. Inst. Oswaldo Cruz 111, 501–504. doi: 10.1590/0074-02760160119
Leybourne D. J., Aradottir G. I. (2022). Common resistance mechanisms are deployed by plants against sap-feeding herbivorous insects: insights from a meta-analysis and systematic review. Sci. Rep. 12, 17836. doi: 10.1038/s41598-022-20741-3
Leybourne D. J., Valentine T. A., Robertson J. A. H., Pérez-Fernández E., Main A. M., Karley A. J., et al. (2019). Defence gene expression and phloem quality contribute to mesophyll and phloem resistance to aphids in wild barley. J. Exp. Bot. 70, 4011–4026. doi: 10.1093/jxb/erz163
Loi N., Osler R., Pertot I., Snidaro M., Lorenzoni C., Reffati E. (1994). The influence of barley yellow dwarf luteovirus and maize dwarf mosaic potyvirus on maize. Barley Yellow Dwarf Newsl 5, 23–24.
Louis J., Basu S., Varsani S., Castano-Duque L., Jiang V., Williams W. P., et al. (2015). Ethylene contributes to maize insect resistance1-mediated maize defense against the phloem sap-sucking corn leaf aphid. Plant Physiol. 169, 313–324. doi: 10.1104/pp.15.00958
Medina-Ortega K. J., Bosque-Pérez N. A., Ngumbi E., Jiménez-Martínez E. S., Eigenbrode S. D. (2009). Rhopalosiphum padi (Hemiptera: Aphididae) responses to volatile cues from Barley yellow dwarf virus-infected wheat. Environ Entomol. 38 (3), 836–845. doi: 10.1603/022.038.0337
Meihls L. N., Handrick V., Glauser G., Barbier H., Kaur H., Haribal M. M., et al. (2013). Natural variation in maize aphid resistance is associated with 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one glucoside methyltransferase activity. Plant Cell 25, 2341–2355. doi: 10.1105/tpc.113.112409
Mittler R., Zandalinas S. I., Fichman Y., Van Breusegem F. (2022). Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 23, 663–679. doi: 10.1038/s41580-022-00499-2
Mullendore D. L., Windt C. W., Van As H., Knoblauch M. (2010). Sieve tube geometry in relation to phloem flow. Plant Cell 22, 579–593. doi: 10.1105/tpc.109.070094
Nancarrow N., Aftab M., Hollaway G., Rodoni B., Trębicki P. (2021). Yield losses caused by barley yellow dwarf virus-PAV infection in wheat and barley: A three-year field study in South-Eastern Australia. Microorganisms 9, 645. doi: 10.3390/microorganisms9030645
Ng J. C. K., Perry K. L. (2004). Transmission of plant viruses by aphid vectors. Mol. Plant Pathol. 5, 505–511. doi: 10.1111/j.1364-3703.2004.00240.x
Oswald J. W., Houston B. (1953). The yellow-dwarf virus disease of cereal crops. Phytopathology 43, 128–136.
Palukaitis P., Yoon J. Y. (2020). R gene mediated defense against viruses. Curr. Opin. Virol. 42, 65. doi: 10.1016/j.coviro.2020.07.007
Paulmann M. K., Kunert G., Zimmermann M. R., Theis N., Ludwig A., Meichsner D., et al. (2018). Barley yellow dwarf virus infection leads to higher chemical defense signals and lower electrophysiological reactions in susceptible compared to tolerant barley genotypes. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00145
Peterson R. K. D., Varella A. C., Higley L. G. (2017). Tolerance: the forgotten child of plant resistance. PeerJ 5, e3934. doi: 10.7717/peerj.3934
Pidon H., Ruge-Wehling B., Will T., Habekuß A., Wendler N., Oldach K., et al. (2024). High-resolution mapping of Ryd4(Hb), a major resistance gene to Barley yellow dwarf virus from Hordeum bulbosum. Theor. Appl. Genet. 137, 60. doi: 10.1007/s00122-024-04542-y
Pingault L., Varsani S., Palmer N., Ray S., Williams W. P., Luthe D. S., et al. (2021). Transcriptomic and volatile signatures associated with maize defense against corn leaf aphid. BMC Plant Biol. 21, 138. doi: 10.1186/s12870-021-02910-0
Prado E., Tjallingii W. F. (1994). Aphid activities during sieve element punctures. Entomologia Experimentalis Applicata 72, 157–165. doi: 10.1111/j.1570-7458.1994.tb01813.x
Prince D. C., Drurey C., Zipfel C., Hogenhout S. A. (2014). The leucine-rich repeat receptor-like kinase BRASSINOSTEROID INSENSITIVE1-ASSOCIATED KINASE1 and the Cytochrome P450 PHYTOALEXIN DEFICIENT3 contribute to innate immunity to aphids in Arabidopsis. Plant Physiol. 164, 2207–2219. doi: 10.1104/pp.114.235598
Radchenko E. E., Abdullaev R. A., Anisimova I. N. (2022). Genetic resources of cereal crops for aphid resistance. Plants (Basel) 11, 1490. doi: 10.3390/plants11111490
Rashidi M., Cruzado R. K., Hutchinson P. J. S., Bosque-Pérez N. A., Marshall J. M., Rashed A. (2020). Grassy weeds and corn as potential sources of barley yellow dwarf virus spread into winter wheat. Plant Dis. 105, 444–449. doi: 10.1094/PDIS-05-20-1004-RE
R Core Team (2020). R: A language and environment for statistical computing. Available online at: https://www.R-project.org (Accessed November 18, 2020).
Rodríguez-López M. J., Garzo E., Bonani J. P., Fereres A., Fernández-Muñoz R., Moriones E. (2011). Whitefly resistance traits derived from the wild tomato Solanum pimpinellifolium affect the preference and feeding behavior of Bemisia tabaci and reduce the spread of tomato yellow leaf curl virus. Phytopathology 101, 1191–1201. doi: 10.1094/PHYTO-01-11-0028
Rong W., Wang X., Wang X., Massart S., Zhang Z. (2018). Molecular and ultrastructural mechanisms underlying yellow dwarf symptom formation in wheat after infection of barley yellow dwarf virus. Int. J. Mol. Sci. 19, 1187. doi: 10.3390/ijms19041187
Savary S., Willocquet L., Pethybridge S. J., Esker P., McRoberts N., Nelson A. (2019). The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 3, 430–439. doi: 10.1038/s41559-018-0793-y
Schliephake E., Habekuss A., Scholz M., Ordon F. (2013). Barley yellow dwarf virus transmission and feeding behaviour of Rhopalosiphum padi on Hordeum bulbosum clones. Entomologia Experimentalis Applicata 146, 347–356. doi: 10.1111/eea.12033
Schmidt M., Guerreiro R., Baig N., Habekuß A., Will T., Ruckwied B., et al. (2024). Fine mapping a QTL for BYDV-PAV resistance in maize. Theor. Appl. Genet. 137, 163. doi: 10.1007/s00122-024-04668-z
Scholz M., Ruge-Wehling B., Habekuss A., Schrader O., Pendinen G., Fischer K., et al. (2009). Ryd4 (Hb): a novel resistance gene introgressed from Hordeum bulbosum into barley and conferring complete and dominant resistance to the barley yellow dwarf virus. Theor. Appl. Genet. 119, 837–849. doi: 10.1007/s00122-009-1093-3
Schröder M. L., Glinwood R., Webster B., Ignell R., Krüger K. (2015). Olfactory responses of Rhopalosiphum padi to three maize, potato, and wheat cultivars and the selection of prospective crop border plants. Entomologia Experimentalis Applicata 157, 241–253. doi: 10.1111/eea.12359
Sharma S., Kooner R., Arora R. (2017). “Insect pests and crop losses,” in Breeding insect resistant crops for sustainable agriculture. Eds. Arora R., Sandhu S. (Singapore: Springer), 45–66. doi: 10.1007/978-981-10-6056-4_2
Sytykiewicz H. (2015). Transcriptional responses of catalase genes in maize seedlings exposed to cereal aphids' herbivory. Biochem. Systematics Ecol. 60, 131–142. doi: 10.1016/j.bse.2015.04.015
Sytykiewicz H., Łukasik I., Goławska S., Chrzanowski G. (2019). Aphid-triggered changes in oxidative damage markers of nucleic acids, proteins, and lipids in maize (Zea mays L.) seedlings. Int. J. Mol. Sci. 20, 3742. doi: 10.3390/ijms20153742
Teixeira R. M., Ferreira M. A., Raimundo G. A. S., Loriato V. A. P., Reis P. A. B., Fontes E. P. B. (2019). Virus perception at the cell surface: revisiting the roles of receptor-like kinases as viral pattern recognition receptors. Mol. Plant Pathol. 20, 1196–1202. doi: 10.1111/mpp.12816
Tjallingii W. F. (1978). Electronic recording of penetration behaviour by aphids. Entomologia Experimentalis Applicata 24, 721–730. doi: 10.1111/j.1570-7458.1978.tb02836.x
Tjallingii W. F., Esch T. H. (1993). Fine structure of aphid stylet routes in plant tissues in correlation with EPG signals. Physiol. Entomology 18, 317–328. doi: 10.1111/j.1365-3032.1993.tb00604.x
van Bel A. J. E., Will T. (2016). Functional evaluation of proteins in watery and gel saliva of aphids. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01840
Van den Eynde R., Van Leeuwen T., Haesaert G. (2020). Identifying drivers of spatio-temporal dynamics in barley yellow dwarf virus epidemiology as a critical factor in disease control. Pest Manag Sci. 76, 2548–2556. doi: 10.1002/ps.5851
Walls J., Rajotte E., Rosa C. (2019). The past, present, and future of barley yellow dwarf management. Agriculture 9, 23. doi: 10.3390/agriculture9010023
Wang X., Rong W., Liu Y., Wang X., Zhang Z. (2018). Investigation of the mechanism of adult-stage resistance to barley yellow dwarf virus associated with a wheat–Thinopyrum intermedium translocation. Crop J. 6, 394–405. doi: 10.1016/j.cj.2018.02.002
Keywords: maize, BYDV, resistance, aphids, Rhopalosiphum padi
Citation: Schmidt M, Will T, Ruckwied B, Habekuß A and Stich B (2025) Characterization of the resistance mechanism against BYDV-PAV in maize. Front. Agron. 7:1526115. doi: 10.3389/fagro.2025.1526115
Received: 11 November 2024; Accepted: 28 January 2025;
Published: 24 February 2025.
Edited by:
Hakim Manghwar, Lushan Botanical Garden (CAS), ChinaReviewed by:
Adnan Akhter, University of the Punjab, PakistanCopyright © 2025 Schmidt, Will, Ruckwied, Habekuß and Stich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin Stich, YmVuamFtaW4uc3RpY2hAanVsaXVzLWt1ZWhuLmRl
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.