- 1Institute of Crop Variety Resources, Xinjiang Academy of Agricultural Sciences, Urumqi, China
- 2Engineering Research Center of Plant Growth Regulator, Ministry of Education/State Key Laboratory of Plant Physiology and Biochemistry/College of Agronomy and Biotechnology, China Agricultural University, Beijing, China
- 3College of Resources and Environment, Xinjiang Agricultural University, Urumqi, China
- 4College of Grassland Science, Xinjiang Agricultural University, Urumqi, China
Water scarcity, over-fertilization, and improper crop management practices severely limit the sustainable cultivation of licorice (Glycyrrhiza uralensis Fisch) in the arid regions of Xinjiang. To elucidate the impacts of integrated water and fertilizer management on the growth characteristics and bioactive components (glycyrrhizic acid and liquiritin) of four-year-old licorice plants, a comprehensive four-year field experiment was conducted from 2019 to 2022.The experiment included four irrigation levels (W1: 2500 m³/ha, W2: 4000 m³/ha, W3: 5500 m³/ha, W4: 7000 m³/ha) and four fertilization rates (F1: 305 kg/ha, F2: 610 kg/ha, F3: 915 kg/ha, F4: 1220 kg/ha), following a completely randomized design. Results indicated that both irrigation and fertilization significantly influenced plant height, root length, root weight, root diameter, leaf area index, and root-to-shoot ratio. The optimal growth characteristics were observed under the W2F2 treatment. The contents of glycyrrhizic acid and liquiritin varied significantly among different water and fertilizer treatments, with the highest levels observed under the W2F2 treatment. Excessive irrigation (W4) and over-fertilization (F4) led to a decrease in these bioactive components. A comprehensive evaluation of the growth characteristics and bioactive components revealed that the ideal irrigation and fertilization parameters were 4000 m³/ha and 610 kg/ha, respectively. These parameters optimized plant development and bioactive component accumulation while ensuring efficient resource use. This study provides scientific evidence for optimizing irrigation and fertilization strategies to enhance licorice yield in arid regions, thereby supporting sustainable agricultural practices and improving economic benefits.
1 Introduction
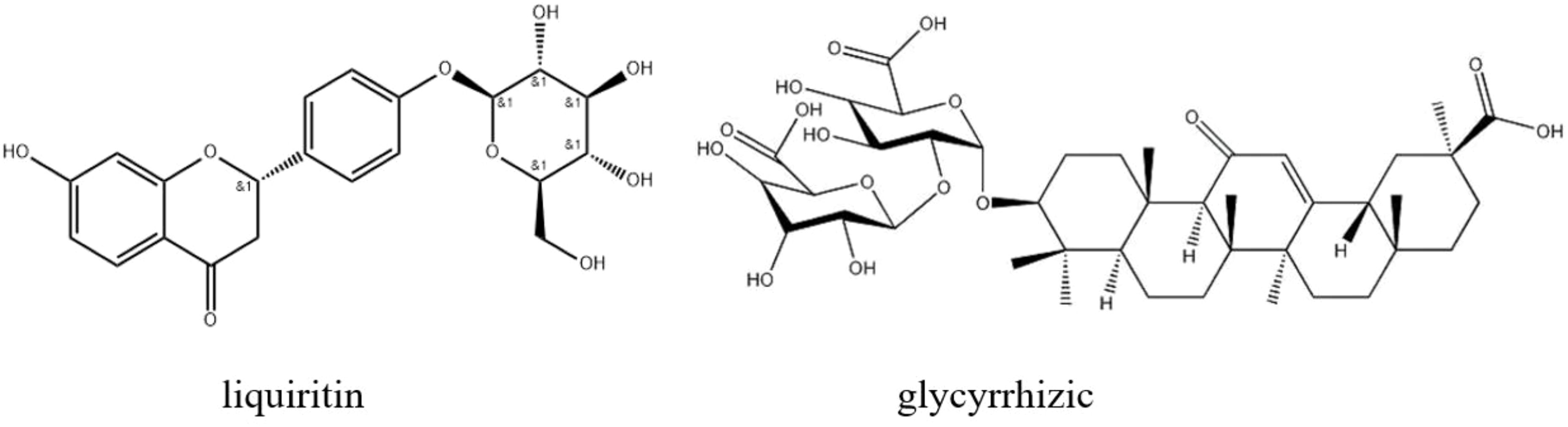
Licorice (Glycyrrhiza uralensis Fisch.), a member of the genus Glycyrrhiza, is frequently employed as a natural sweetening agent and in herbal medicine. Its dried roots and rhizomes are known as radix glycyrrhizae (RG). Moreover, it is a medicinal liquorice listed in the Chinese Pharmacopoeia (Chinese Pharmacopoeia Sheng, 2010). Licorice contains over 300 distinctive chemical compounds, many of which possess antibacterial, antiviral, antitumor, anti-inflammatory, antidiabetic, and hepatoprotective properties (Wang et al., 2015; Wang et al., 2013; Michel et al., 2013; Wu et al., 2010; Gou et al., 2020; Tyagi et al., 2018; Zhang et al., 2018). Glycyrrhiza root comprises secondary metabolites such as polysaccharides, triterpene saponins, and flavonoids, all of which exhibit notable medicinal properties (He et al., 2019; Assar et al., 2021). Glycyrrhizic acid, the principal component among triterpenoid saponins (Meng et al., 2022), is a highly valued and pharmacologically active compound renowned for its anti-inflammatory (Chen et al., 2017), antiviral, and immunoregulatory properties (Wu, 2013; Crance et al., 2003; Baba et al., 1987). Liquiritin, a vital constituent of flavonoids, boasts remarkable properties including antioxidant, antibacterial, and anti-inflammatory activities (Martins et al., 2015). However, research indicates that the levels of bioactive compounds in licorice are influenced by factors such as the cultivation area, plant maturity, environmental conditions, soil pH, temperature, weather, and the methods of harvesting and processing (Khaitov et al., 2021). For instance, licorice is abundant in triterpenoid saponins, particularly glycyrrhizic acid (GL), a principal component and active substance, with its concentration ranging from approximately 1.84% to 9.82%, contingent upon the origin and extraction methods (Liu et al., 2007; Chen et al., 2017; Wu et al., 2021). Another eminent group of bioactive compounds in licorice is flavonoids, comprising about 1.78% to 4.82% of the licorice content, depending on the sourcing and extraction techniques employed. This group includes various compounds such as isoliquiritigenin (ISL), isoliquiritin, and liquiritigenin (Figure 1) (Liu et al., 2007; Sheng, 2010).
Xinjiang is situated within the arid and semi-arid regions of China (Wang et al., 2018). However, the optimization of its growth conditions and the enhancement of its active components, particularly glycyrrhizic acid, remain challenging. Most current research focuses on the impact of individual factors such as soil type, irrigation, or fertilization independently. This approach overlooks the potential synergistic effects that combinations of these factors might have on the growth and active component synthesis in licorice plants. Such research gaps limit the effective application of optimized agricultural practices for licorice cultivation. To address these gaps, our study aims to investigate the synergistic effects of different irrigation and fertilization combinations on the growth and active component content of licorice. Our hypothesis posits that optimizing these combinations will significantly enhance both the growth rate and the glycyrrhizic acid concentration in the licorice plants.
Irrigation and nitrogen fertilizer applications are two pivotal, human-controlled factors of paramount importance (Rogers et al., 2022). Water-fertilizer coupling technology, an innovative agricultural approach, seamlessly integrates water and fertilizers, delivering them directly to the crop’s root zone via the irrigation system (Zhang et al., 2014; Wang et al., 2018). This scientific method excels in precise irrigation and fertilization, enhancing the efficient absorption and utilization of nutrients and water by crops. Furthermore, it plays a crucial role in regulating the interaction between fertilizer and water, fostering a symbiotic relationship where fertilizer regulates water and water enhances fertilizer effectiveness (Hao et al., 2022). The synergistic amalgamation of water and fertilizer possesses an impressive capacity to conserve water, enhance crop yield, and significantly elevate the quality of agricultural produce. Proper fertilizer management, meticulous irrigation practices, and the use of superior quality seeds have the remarkable potential to boost crop yield by an impressive margin of 35-40% (Shekhawat et al., 2012).
Researchers have increasingly focused on developing rational models to optimize the coupling of water and fertilizer, recognizing their importance in advancing agricultural practices. Currently, a growing body of research examines the effects of integrative water and fertilizer application on crop performance. For instance, scholars have explored the effects of synergistic water and fertilizer integration on the productivity and quality of wheat (Fan et al., 2022; Wang et al., 2021), cotton (Wang et al., 2018), sunflower (Wang et al., 2021), and sugarcane (Wu et al., 2022). Flood irrigation methods have resulted in suboptimal productivity and water efficiency in Glycyrrhiza cultivation. Moreover, in the quest for increased crop yields, the excessive application of fertilizers remains a common practice among farmers. Investigating the interplay between water and fertilizer within a drip irrigation framework is crucial for promoting sustainable agricultural development in arid regions like Xinjiang, China. This study aims to identify the best water and fertilizer management practices to enhance crop performance and conserve resources (Wang et al., 2018).
2 Materials and methods
2.1 General description of the experimental area.
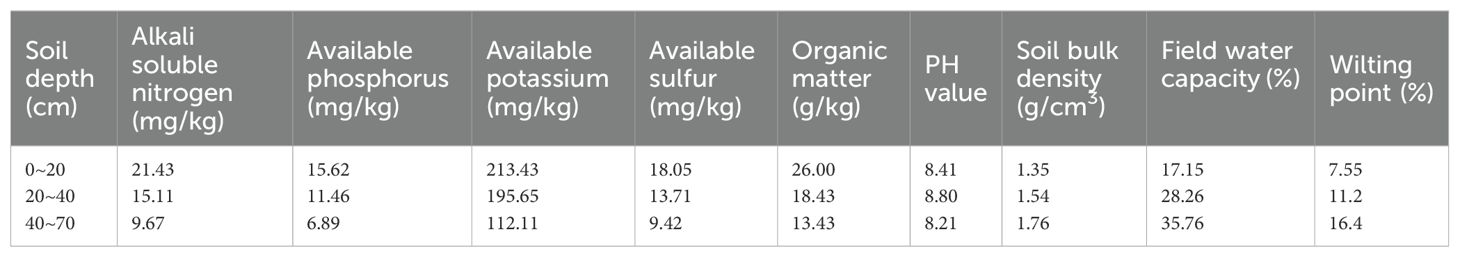
The four-year experiment was conducted at Xinjiang Agricultural University’s Sanping Internship Farm in Urumqi (87°28’08” N, 43°52’30” E, altitude 680 m) in the Xinjiang Uyghur Autonomous Region (Figure 2), northwest China. This area is characterized by a typical continental arid climate, with an average annual temperature of approximately 7.5-8.2°C. The average annual precipitation is 180-270 mm, predominantly concentrated in autumn and winter, while the average annual evaporation ranges from 1800-2500 mm. From 2019 to 2022, the region received a total of 2990 hours of sunshine. It benefits from a frost-free period of 170 days, with an average annual temperature of 8°C and a daily average temperature of 14°C. Extreme temperatures can drop to -31°C, with an average wind speed of 15.2 km/h. The groundwater is approximately 4 meters deep. In this region, irrigation is the primary agricultural practice. Table 1 details the primary physical characteristics and soil fertility of the tillage layer at a depth of 0-70 cm in the experimental area. The soil texture is characterized as clayey loam with a light composition. Notably, the soil contains a significant amount of stones, comprising 20-30% of the total soil volume, and the average pH is 8.56. The fertility characteristics at various soil depths were fundamentally identical. For all treatments, the lowest concentration range is 6.89 mg/kg and the highest concentration range is 26.00 mg/kg. The content of alkali-soluble nitrogen, available phosphorus, available potassium, available sulfur, and organic matter exhibited a declining trend with increasing soil depth. Moreover, the concentration of nitrate nitrogen in the 0-40 cm soil depth was higher than that in the 40-70 cm soil depth across all treatments. Additionally, the pH value, soil bulk density, field water capacity, and wilting point were 8.41, 1.35 g·cm−3, 17.15%, and 7.55%, respectively, in the upper 0.2 m soil layer (Table 1) (Abudurezike et al., 2024). The remaining soil properties are presented in Table 1.
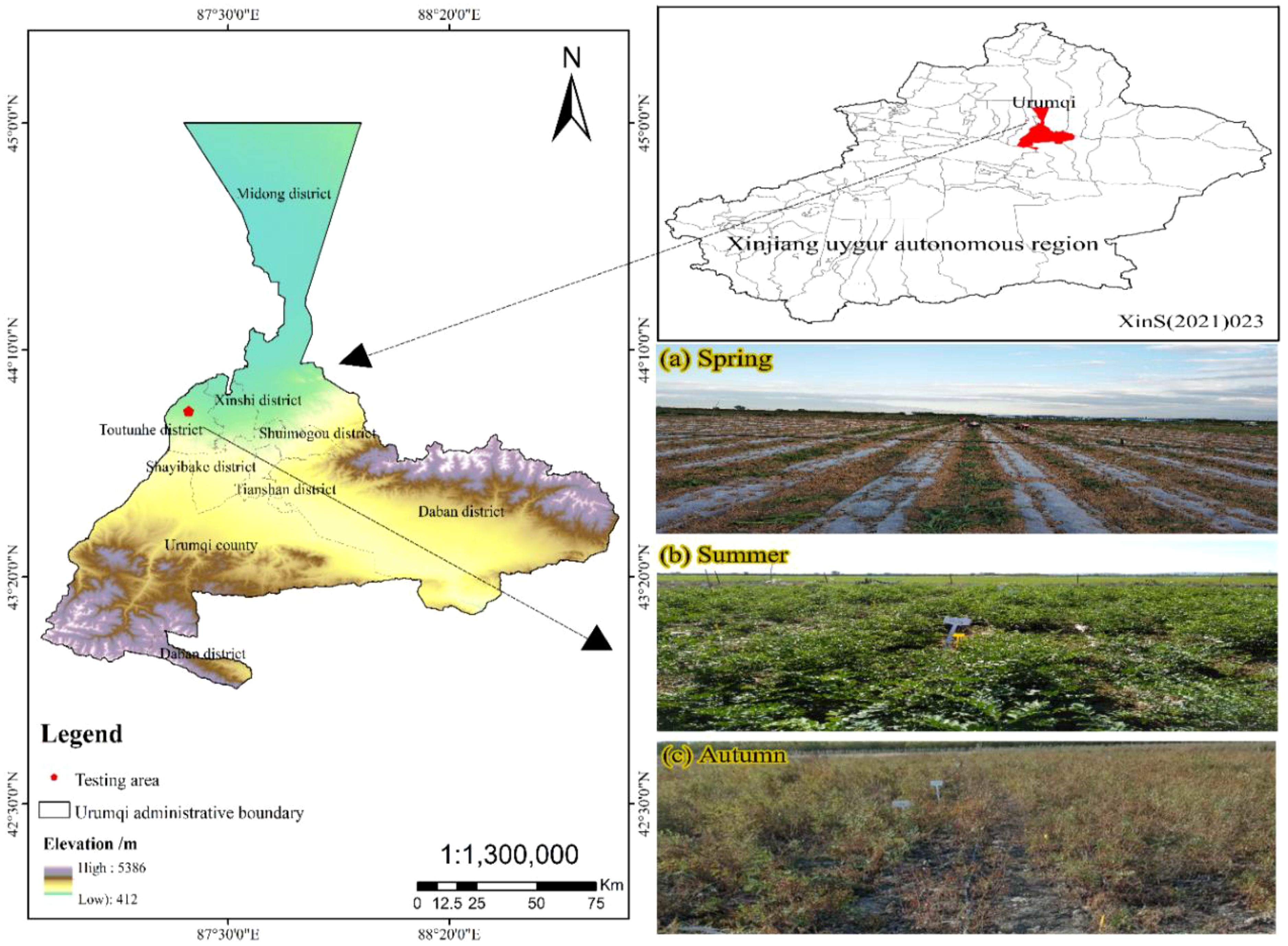
Figure 2. The location of the experimental site. (A) represents the experimental site in spring, (B) represents the experimental site in summer, and (C) represents the experimental site in autumn.
2.2 Experimental design
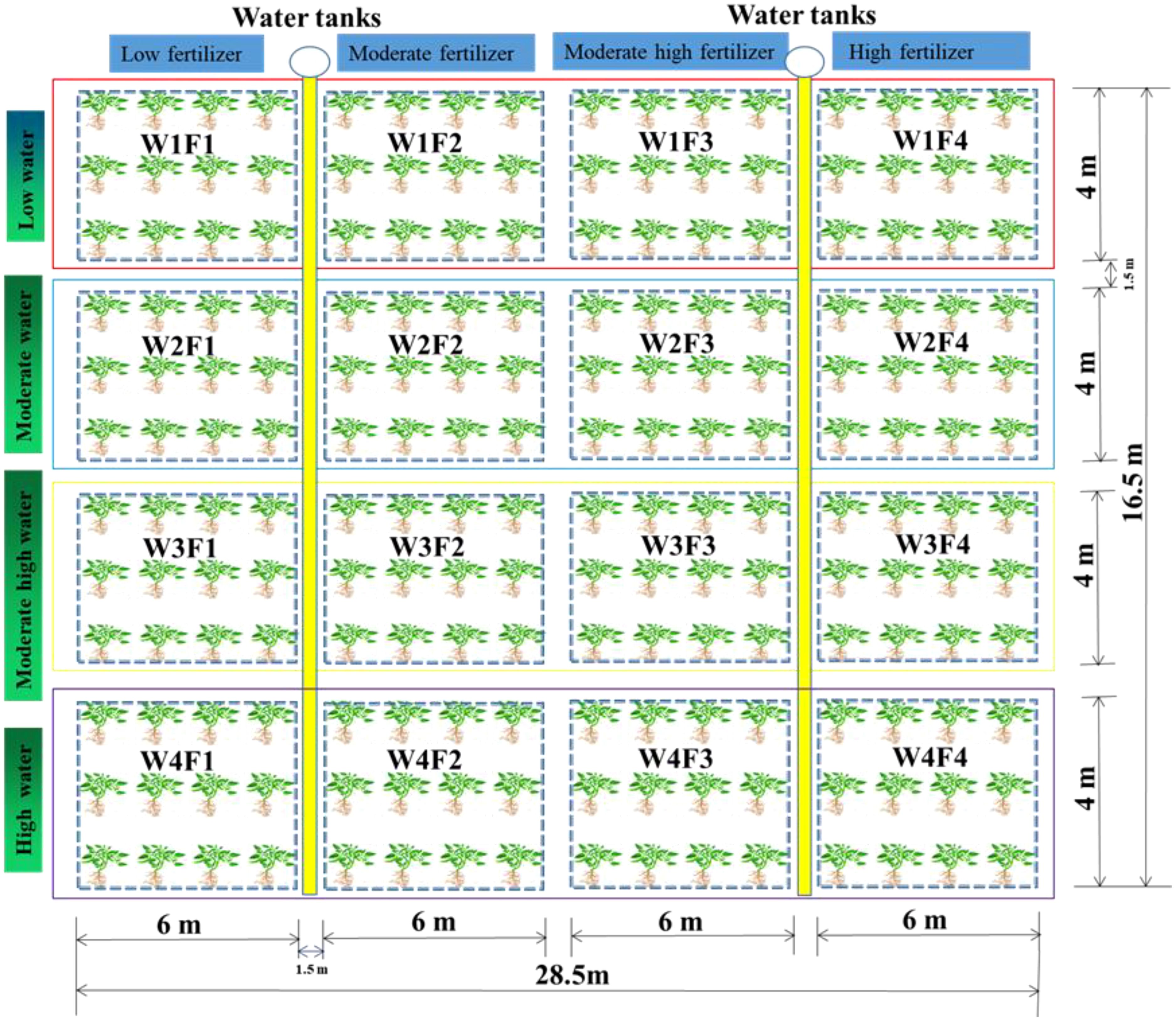
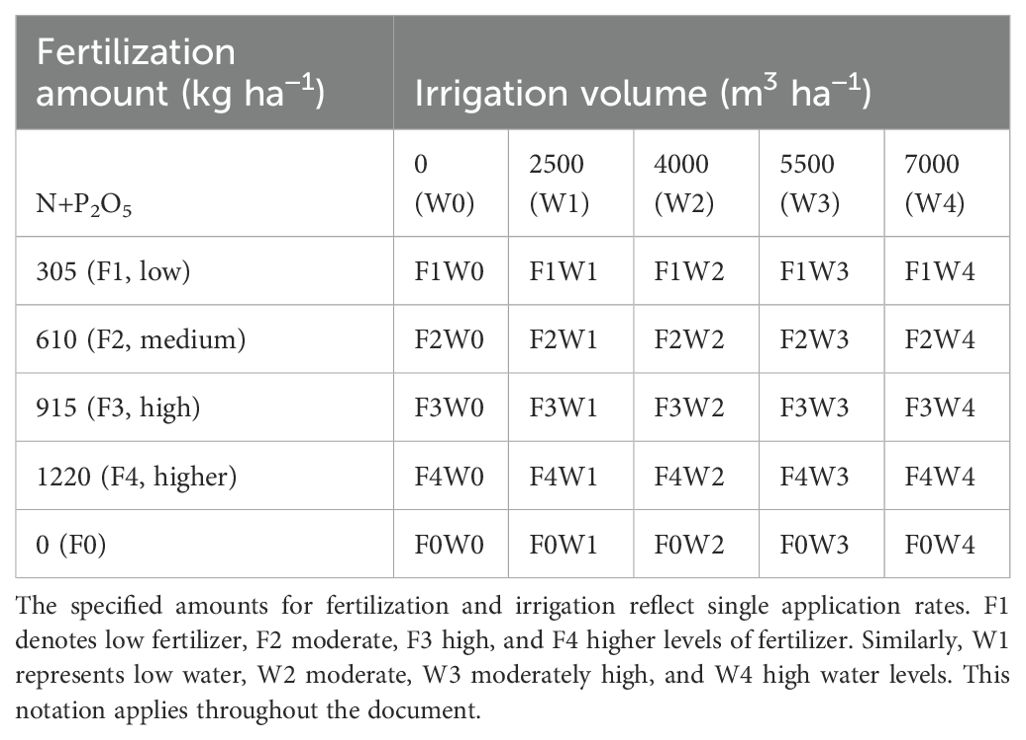
Liquorice seeds (Glycyrrhiza uralensis Fisch.) were sourced from Xinjiang Tianbo Grass Industry Corporation, China. The seeds boasted an impressive purity of 96%, with a weight of 18.68 g per 1,000 seeds. Moreover, the germination rate was a remarkable 87.6%, with a germination potential of 46.4%. The seeds were planted on May 20, 2019, at a soil depth of 2-3 cm at the experimental station of the Sanping Internship Farm, with seedlings cultivated for two years to establish a robust root system. The planting configuration featured 30 cm spacing between rows and 15 cm between plants, covering an area of 24 m² (6 m in length by 4 m in width). For perennial cultivation, the seed sowing rate was set at 15-16 kg/ha, with a planting density of 129,075 plants per hectare. Prior to sowing, the experimental area was ploughed to a depth of 30 cm to manually remove weeds. Additionally, 30 kg K2O per hectare, 54 kg P2O5 per hectare, and 94 kg N per hectare were applied as nutrients. To prevent groundwater infiltration, each test plot was demarcated with a 60-centimeter-wide film barrier. Additionally, a complex fertilizer containing essential elements nitrogen (N), phosphorus (P), and potassium (K) was selected for the study (N 46%, P2O5 53%, K2O 51%; Urumqi Furuike Biological Co., Ltd., Urumqi, China). Each plot was mulched with two sheets of plastic film, beneath which two drip irrigation lines were installed. The irrigation technique implemented was surface drip irrigation, with each plant row furnished with two drip pipes, equipped with pressure-compensated emitters to ensure uniform and controlled water distribution. The irrigation system used 16 mm external diameter drip tapes with a wall thickness of 0.3 mm and emitter spacings of 30 cm. These tapes, not covered by plastic film, delivered 1.2 L/m² at an operating pressure of 60 kPa. Emitters discharged at an average rate of 1.8 L/h, positioned 0.3 m apart. The system featured a water outlet stake, pressure gauge, water meter, ball valve, filter, venturi fertilizer injector, capillary tube, and drip head, all functioning at a pressure of 1.5 MPa. The components for the ZNHQ-P1 water and fertilizer precision dosing machine were supplied by Xinjiang Dayu Water Saving Co., Ltd., Urumqi, China. The study utilized a fully randomized two-factor experimental design featuring four irrigation levels: 2,500 m³/ha (W1), 4,000 m³/ha (W2), 5,500 m³/ha (W3), and 7,000 m³/ha (W4), consistent with the “three red lines” water resource management policy (Abudurezike et al., 2024; Wu et al., 2021).
The depths of the horizontal rhizomes, vertical rhizomes, and vertical root heads of Ural radix glycyrrhiza are primarily concentrated within the 30-80 cm soil layer (Ao et al., 2009). The water available for plants in the soil is known as soil available water, which lies between the field water capacity and the wilting point (Chang and Chang, 2021). In this paper, the maximum irrigation volume of the area within the experimental zone was calculated based on the available soil water content, test plot area, soil capacity, and irrigation depth, where the effective soil water content and the maximum irrigation volume were calculated using the following equations:
Where ASWC is the available soil moisture (%), FC is the field capacity (%), and PWP is the permanent wilting point (%).
Where Imax represents the maximum irrigation volume (kg), M denotes the area of the test zone (m2), B indicates the soil bulk density (kg m−3), and D represents the irrigation depth (the depth of root distribution is considered to be the irrigation depth, and D = 0.7 m).
Because the test area is subject to significant surface water loss, the amount of water available to the plants is often less than the maximum irrigation volume in the actual system (Zhang et al., 2014). In this study, the researchers simulated irrigation of the deepest parts of the licorice root system by controlling the amount of irrigation water with a water meter based on the maximum irrigation volume. Utilizing irrigation quotas was determined based on 40%, 60%, 80%, and 100% of the actual irrigation volume. The biological water requirement of Licorice was then calculated using the area quota method with the following formula:
Where W is the total biological water requirement of radix glycyrrhiza (m3), A is the area of radix glycyrrhiza cultivation (m2), and r is the ecologically appropriate water quota of radix glycyrrhiza (m3 m−2).
Based on the biological water requirements of radix glycyrrhiza, a water system standard was established with five levels: no irrigation (W0: 0 m3 ha−1, 0%), less irrigation (W1: 2500 m3 ha−1, 40%), medium irrigation (W2: 4000 m3 ha−1, 60%), high irrigation (W3: 5500 m3 ha−1, 80%), and higher irrigation (W4: 7000 m3 ha−1, 100%), in that order. In the seedling watering system, each level of the water system was irrigated 0, 4, 6, 8, and 10 times to meet the water requirements for optimal radix glycyrrhiza growth, while ensuring that the total irrigation remained stable. After the second year of regrowth stage, based on conventional drip irrigation management of perennial plant roots, it was assumed that the total yearly water system volume remained unchanged, and the number of water system times for each water system level was balanced at 0, 2, 4, 6, and 8 times. The equation below calculates the duration of the water system by dividing the drip irrigation tape laying, trickle head flow rate, and drip head dispersing (Zhang et al., 2003).
Where H is the dripping amount (L h-1), A is the drip irrigation area (ha), S is the drip tape distance (m), Se is the dribble head spacing (m), and G is the dribble head stream rate (L), in which the 1 ha dribble rate is 60 m3 h-1, the drip irrigation belt laying spacing is 30 cm, and the dribble head stream rate is 1.76 L h-1, i.e., 0.00176 m3 h-1, which is approximately 0.0018 m3 h-1.
Additionally, four fertilization rates were applied: 305 kg/ha (F1), 610 kg/ha (F2), 915 kg/ha (F3), and 1220 kg/ha (F4). Among the 17 treatment combinations (Figure 3), one served as a control with no fertilization or irrigation (W0F0). Fertilization was applied 30 minutes after the commencement of the operation and again 30 minutes before the conclusion of irrigation during the experiment (Table 2), which details the water-fertilizer coupling scheme for this study.
2.3 Sampling and measurements
2.3.1 Plant materials
Samples of licorice plants, including roots and rhizospheric soils, were collected in May, August, and October. Samples were taken from depths of 0-20 cm, 20-40 cm, and 40-60 cm. To enhance statistical significance, three healthy medicinal licorice plants were randomly selected from each location using the five-point sampling method. Root samples were carefully cut and trimmed using sterile scissors. Each plant’s roots were categorized into three segments: upper (0-20 cm), middle (20-40 cm), and lower (40-60 cm). To mitigate edge effects, three sample rows were randomly assigned within each plot. Representative licorice plant samples were carefully collected from each row for transport back to the laboratory.
2.3.2 Licorice plant bioactive components
The glycyrrhizal root samples were thoroughly dried until a consistent weight was achieved. The dried roots were then finely pulverized using a mortar and pestle, and sieved through a 60-mesh sieve. For comprehensive ingredient analysis, 0.2 g of sieved root powder was extracted using 71% chromatographic methanol in an ultrasonic bath (250 W, 40 kHz). The levels of liquiritin and glycyrrhizic acid in licorice powder mixtures were determined using high-performance liquid chromatography (HPLC), according to the Chinese Pharmacopeia guidelines (Sheng, 2010). Dried licorice plant samples were pulverized using a mortar and pestle and sieved through a 60-mesh sieve. Precisely 0.2 g of the powder was extracted in 70% ethanol (100 mL) using an ultrasonic cleaner (250 W, 40 kHz) at room temperature for 30 minutes. Liquiritin levels were measured using an Agilent-1260 HPLC system (USA) equipped with an Agilent Eclipse Plus C18 column (250 mm × 4.6 mm, 5 µm). Detection conditions were as follows: a Kromasil C18 column (4.6 × 200 mm, 5 µm) with a mobile phase composed of methanol (0.2 mol L^-1), ammonium acetate solution, and glacial acetic acid in a 67:32:1 ratio. The UV detection wavelength was set at 252 nm, with an injection volume of 20 µL and a flow rate of 0.001 L/min. The column temperature was maintained at room temperature, and the minimum theoretical plate number for the liquiritin and glycyrrhizic ammonium peaks was 4000 (Wang et al., 2013).
2.3.3 Soil sampling
Soil specimens were collected biannually in May and October using a soil drill at depths of 0-20 cm, 20-40 cm, and 40-70 cm. The drill had a 5 cm diameter metal cylinder. After collection, the soil samples were air-dried at room temperature for two weeks. The dried soil was then finely pulverized and sifted through a 2-mm mesh for uniform particle size. The processed samples were then prepared for chemical analysis. Soil pH values were measured using pH meters after mixing soil with distilled water in a 1:5 ratio. The Tyurin and Lancaster (Jones, 2018) methods were used to extract organic matter and determine the available phosphate concentration in the soil.
2.3.4 Physicochemical analysis of the soil
For the physicochemical analysis of rhizospheric soil, samples were air-dried and sieved (2 mm mesh) following Bao (2008). Soil pH (1:5 soil: distilled water) was measured using a pH meter, and soil water content was determined by weighing. Organic matter content was measured by external heating with potassium dichromate and atomic absorption spectrometry. Soil nitrate-nitrogen levels were determined using the 0.01 M calcium chloride extraction method. Available phosphorus content was measured using the sodium bicarbonate extraction method (molybdenum-antimony colorimetry). Available potassium content was determined by the ammonium acetate extraction method using atomic absorption spectrometry.
2.4 Statistical analysis.
Data analyses were conducted using SPSS (Version 21.0, IBM Corp., Armonk, NY). Differences among treatments were evaluated using independent samples and the Kruskal-Wallis test (P ≤ 0.05). The tabulated data represent the means of three replicates.
3 Results
3.1 Effects of irrigation and fertilizer on the growth parameters of licorice
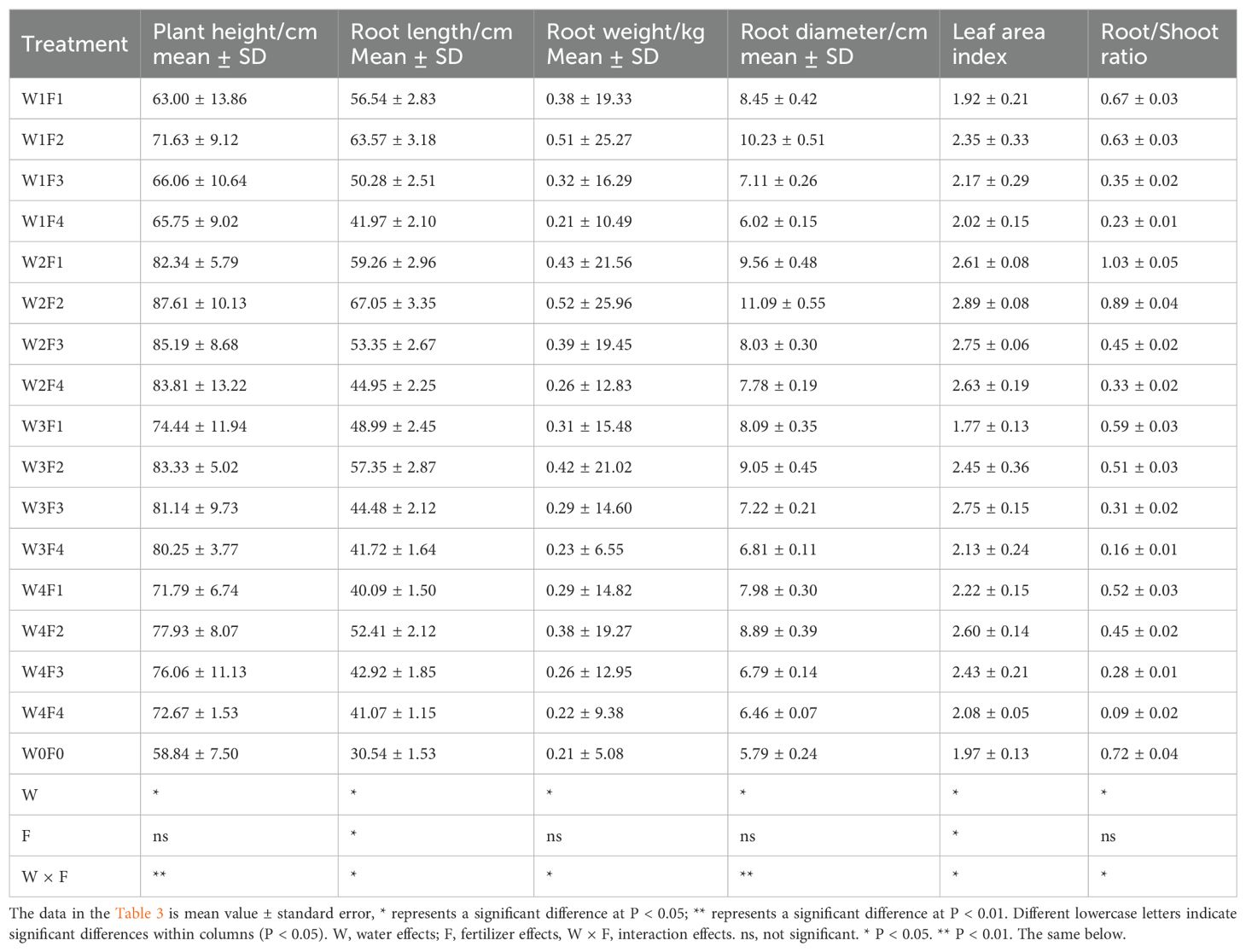
Licorice plants exhibit distinct characteristics, including a thick fibrous root, elongated taproot, and fewer branch roots and stems, which contribute to their recognized superior quality (Zhu et al., 2000). Significant variations were observed in various growth parameters, such as plant height, root length, root weight, root diameter, leaf area index, and root/shoot ratio (Table 3). Comparing the treatments to W0F0, all water-fertilizer combinations showed a significant increase in plant height and leaf area index (P< 0.05). Various treatment combinations significantly improved plant height and leaf area index compared to W0F0. This indicates that increasing water and fertilizer enhances overall plant vigor. The highest root lengths were observed in the W2F2 and W2F1 treatments, while the lowest were found in the W1F4 and W4F1 treatments. This suggests that moderate levels of both water and fertilizer promote optimal root elongation. Treatments W2F2, W2F1, and W1F2 displayed the highest root diameters, whereas the W1F4 treatment showed the lowest. This indicates that moderate fertilizer levels with moderate or reduced water irrigation enhance the root’s structural development. Among the various combinations, the W2 (4000 m³/ha) and F2 (610 kg/ha) treatment (W2F2) emerged as the most favorable. This combination demonstrates superior growth characteristics, such as the maximum plant height, root length, root weight, root diameter, and leaf area index. Moderate irrigation and appropriate fertilization significantly promote the growth and nutrient accumulation in licorice plants. Conversely, under the W4 (7000 m³/ha) and F4 (1220 kg/ha) combination, licorice growth characteristics declined, indicating that excessive irrigation and fertilization negatively impact licorice growth. Based on a comprehensive evaluation of growth characteristics, four-year-old licorice plants subjected to the W2F2 treatment (4000 m³/ha of water and 610 kg/ha of fertilizer) demonstrated a well-balanced and comprehensive growth pattern. This moderate treatment combination is the most beneficial, optimizing the overall growth and quality of licorice plants. Employing moderate levels of irrigation and fertilization enhances growth parameters collectively, promoting superior licorice development.
3.2 Effects of irrigation and fertilizer on bioactive components of licorice
3.2.1 Impact of various irrigation and fertilizer combinations on the content of liquiritin and glycyrrhizic acid in different parts of the licorice
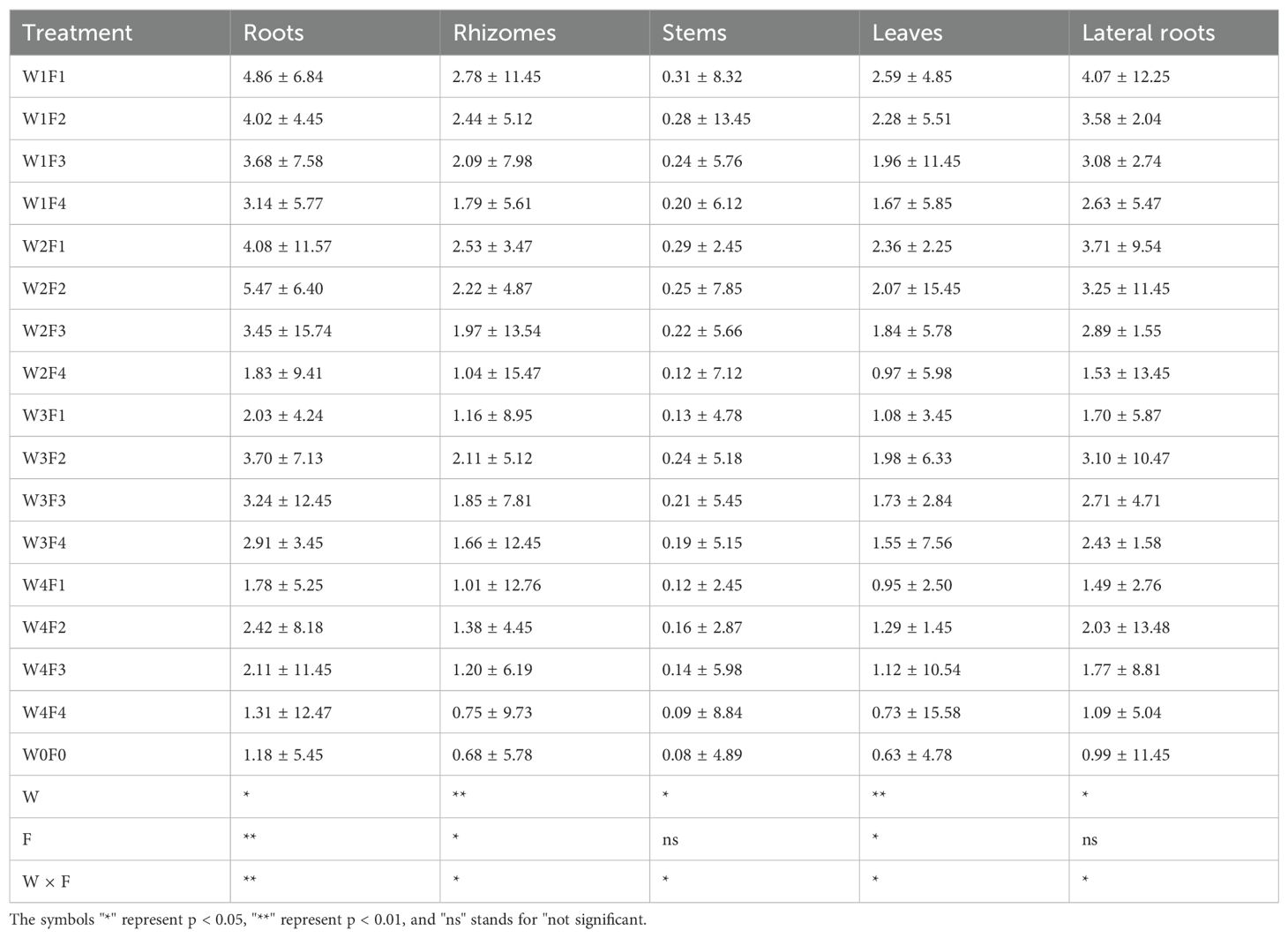
Different water and fertilizer treatments significantly affect the glycyrrhizic acid content in roots. The W2F2 treatment results in the highest glycyrrhizic acid content in roots, at 5.47 ± 6.40 mg/g, while the W4F4 treatment shows the lowest content, at 1.31 ± 12.47 mg/g. This indicates that an appropriate combination of water and fertilizer can increase the glycyrrhizic acid content in roots. The glycyrrhizic acid content in rhizomes also varies significantly between treatments. The highest content is observed under the W2F2 treatment, at 2.22 ± 4.87 mg/g, while the lowest is under the W0F0 treatment, at 0.68 ± 5.78 mg/g. Proper water and fertilizer management can significantly enhance the glycyrrhizic acid content in rhizomes. Although the variation in glycyrrhizic acid content in stems between treatments is smaller, it still shows some changes. The W1F3 treatment yields the highest content in stems, at 0.24 ± 5.76 mg/g, while the W0F0 treatment has the lowest, at 0.08 ± 4.89 mg/g. This suggests that a certain amount of fertilizer treatment can help increase glycyrrhizic acid content in stems. There is a significant difference in the glycyrrhizic acid content in leaves across treatments. The W1F1 treatment has the highest glycyrrhizic acid content in leaves, at 2.59 ± 4.85 mg/g, whereas the W4F3 treatment shows the lowest, at 1.29 ± 4.54 mg/g. An appropriate combination of water and fertilizer can enhance the glycyrrhizic acid content in leaves. The glycyrrhizic acid content in lateral roots also shows significant differences among treatments. The highest content is under the W2F4 treatment, at 5.13 ± 13.45 mg/g, while the lowest is under the W0F0 treatment, at 0.99 ± 11.45 mg/g. This indicates that proper water and fertilizer treatment can significantly increase the glycyrrhizic acid content in lateral roots. According to the statistical data in Table 4, the independent effects of water (W) and fertilizer (F) and their interaction (W×F) significantly affect the glycyrrhizic acid content in different parts of licorice (P<0.05 or P<0.01). This shows that different combinations of water and fertilizer significantly influence the accumulation of glycyrrhizic acid in various parts.
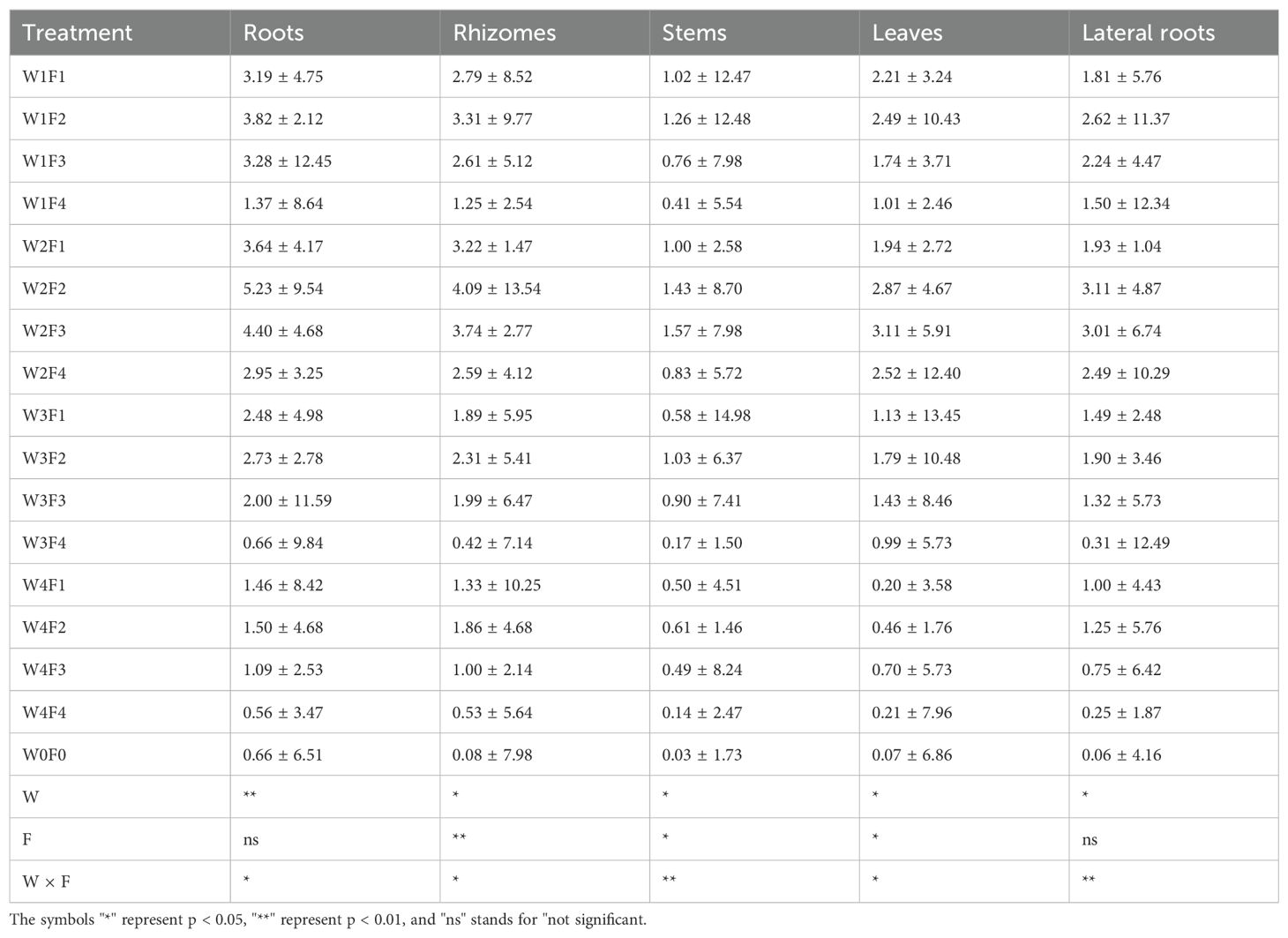
Table 5 shows the variations in liquiritin content in different parts of licorice (roots, rhizomes, stems, leaves, and lateral roots) under different water and fertilizer treatments. Different water and fertilizer treatments significantly affect the liquiritin content in roots. The W2F2 treatment results in the highest liquiritin content in roots, at 5.23 ± 9.54 mg/g, while the W0F0 treatment shows the lowest content, at 0.66 ± 5.61 mg/g. This indicates that an appropriate combination of water and fertilizer can increase the liquiritin content in roots. The liquiritin content in rhizomes also varies significantly between treatments. The highest content is observed under the W2F2 treatment, at 4.09 ± 13.54 mg/g, while the lowest is under the W0F0 treatment, at 0.88 ± 7.98 mg/g. Proper water and fertilizer management can significantly enhance the liquiritin content in rhizomes. Although the variation in liquiritin content in stems between treatments is smaller, it still shows some changes. The W2F3 treatment yields the highest content in stems, at 1.57 ± 9.98 mg/g, while the W0F0 treatment has the lowest, at 0.03 ± 1.73 mg/g. This suggests that a certain amount of fertilizer treatment can help increase liquiritin content in stems. There is a significant difference in the liquiritin content in leaves across treatments. The W2F3 treatment has the highest liquiritin content in leaves, at 3.11 ± 5.91 mg/g, whereas the W0F0 treatment shows the lowest, at 0.07 ± 6.86 mg/g. An appropriate combination of water and fertilizer can enhance the liquiritin content in leaves. The liquiritin content in lateral roots also shows significant differences among treatments. The highest content is under the W2F3 treatment, at 3.01 ± 6.74 mg/g, while the lowest is under the W0F0 treatment, at 0.06 ± 4.16 mg/g. This indicates that proper water and fertilizer treatment can significantly increase the liquiritin content in lateral roots. According to the statistical data in Table 5, the independent effects of water (W) and fertilizer (F) and their interaction (W×F) significantly affect the liquiritin content in different parts of licorice (P<0.05 or P<0.01). This shows that different combinations of water and fertilizer significantly influence the accumulation of liquiritin in various parts.
In summary, appropriate combinations of water and fertilizer significantly impact the liquiritin and glycyrrhizic acid contents in various parts of licorice. Through proper irrigation and fertilization management, the liquiritin and glycyrrhizic acid contents in roots, rhizomes, stems, leaves, and lateral roots can be effectively increased. These findings provide a scientific basis and practical guidance for the efficient production and quality improvement of licorice. This analysis reveals that different water and fertilizer treatments significantly influence the bioactive components of licorice, and reasonable management measures will help enhance the medicinal value and economic benefits of licorice.
3.2.2 Impact of various irrigation and fertilizer combinations on the liquiritin and glycyrrhizic acid contents of the licorice reproductive organs
The combination of various irrigation and fertilizer treatments significantly impacted the concentrations of liquiritin and glycyrrhizic acid in the reproductive organs of licorice. As shown in Figures 4 and 5, the concentrations of liquiritin and glycyrrhizic acid in the reproductive organs of licorice-including buds, flowers, pods, and seeds-exhibited significant variations (P< 0.05) across different combinations of water and fertilizer treatments. Notably, the licorice seed consistently displayed significantly higher levels of liquiritin and glycyrrhizic acid compared to the bud, flower, and pod, regardless of the treatment. Moreover, when compared to the W0F0 treatment, the accumulation of liquiritin and glycyrrhizic acid in licorice plants was significantly enhanced (p< 0.05) in the bud, flower, pod, and seed (Figures 4A–D). The concentrations of liquiritin and glycyrrhizic acid in the reproductive organs of licorice in each treatment surpassed those of the W0F0 treatment. This observation suggests that the licorice plants exhibited improved fertilizer absorption and utilization across all water and fertilizer treatments compared to the W0F0 treatment. Particularly, the reproductive organs of licorice subjected to moderate fertilizer and water treatments (W2F2 and W3F3) displayed significantly higher levels of liquiritin and glycyrrhizic acid compared to the other treatments. This observation suggests that the optimal combination for achieving high concentrations of liquiritin and glycyrrhizic acid in licorice, while minimizing environmental impact, is moderate fertilizer and moderate water (W2F2 and W3F3). Following this, the next best combinations are low fertilizer and low water (W1F1) and moderate fertilizer and moderate water (W2F2).

Figure 4. Impact of various irrigation and fertilizer combinations on the content of liquiritin in licorice reproductive organs. (A) variation of the content of liquiritin in licorice bud; (B) variation of the content of liquiritin in licorice flower; (C) variation of the content of liquiritin in licorice pod; (D) variation of the content of liquiritin in licorice seed. Different letters above the bars indicate a significant difference at P< 0.05 according to an LSD test. Applies similarly below.
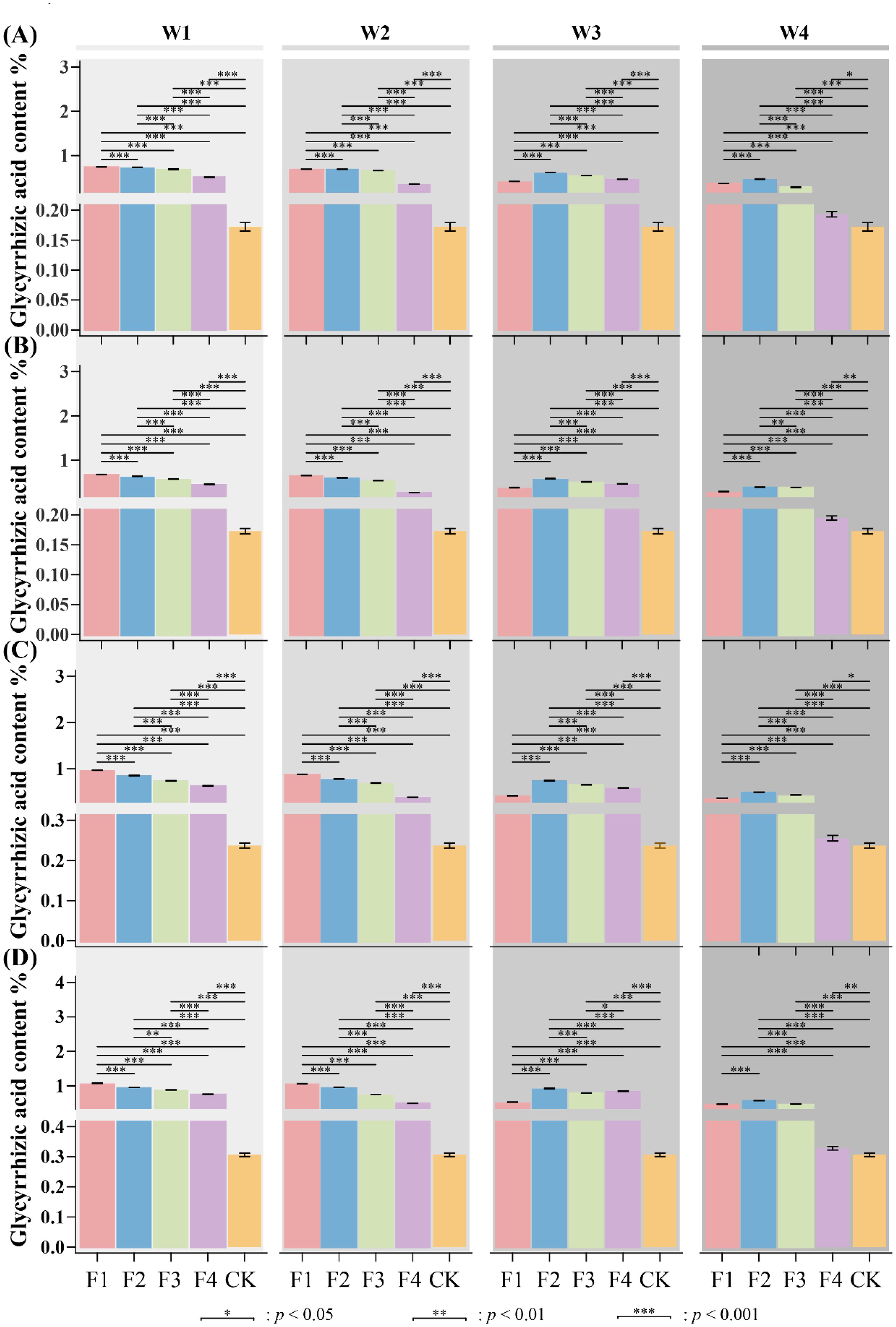
Figure 5. Impact of various irrigation and fertilizer combinations on the content of liquiritin in licorice reproductive organs the content of glycyrrhizic acid in licorice reproductive organs. (A) variation of the content of liquiritin in licorice bud; (B) variation of the content of liquiritin in licorice flower; (C) variation of the content of liquiritin in licorice pod; (D) variation of the content of liquiritin in licorice seed. Different letters above the bars indicate a significant difference at P< 0.05 according to an LSD test.
3.2.3 Analysis of mixed effects between different irrigation and fertilizer coupling combinations and material content in licorice roots
From Figure 6, it is evident that both irrigation amount and fertilization amount have significant impacts on the contents of liquiritin and glycyrrhizic acid. Specifically: Effect of Irrigation Amount: Contribution rate to liquiritin content: 53.47%. Contribution rate to glycyrrhizic acid content: 53.91%. This indicates that irrigation amount is one of the major factors affecting the contents of liquiritin and glycyrrhizic acid. Effect of Fertilization Amount: Contribution rate to liquiritin content: 46.53%. Contribution rate to glycyrrhizic acid content: 46.09%. This indicates that fertilization amount is also an important factor affecting the contents of liquiritin and glycyrrhizic acid. Interaction between Irrigation Amount and Fertilization Amount: Contribution rate to liquiritin content: 8.64%. Contribution rate to glycyrrhizic acid content: 7.84%. Although the contribution rate of the interaction is relatively low, it is still significant (p< 0.001), indicating a synergistic effect between irrigation amount and fertilization amount on the contents of liquiritin and glycyrrhizic acid. The data in Figure 6 indicates that both irrigation amount and fertilization amount have significant impacts on the contents of liquiritin and glycyrrhizic acid, and there is a significant interaction between the two factors. The impact of irrigation amount on these two compounds is slightly greater than that of fertilization amount, but the impact of fertilization amount is also considerable. To optimize licorice production and enhance its medicinal component content, it is necessary to consider the combination of irrigation and fertilization strategies comprehensively.
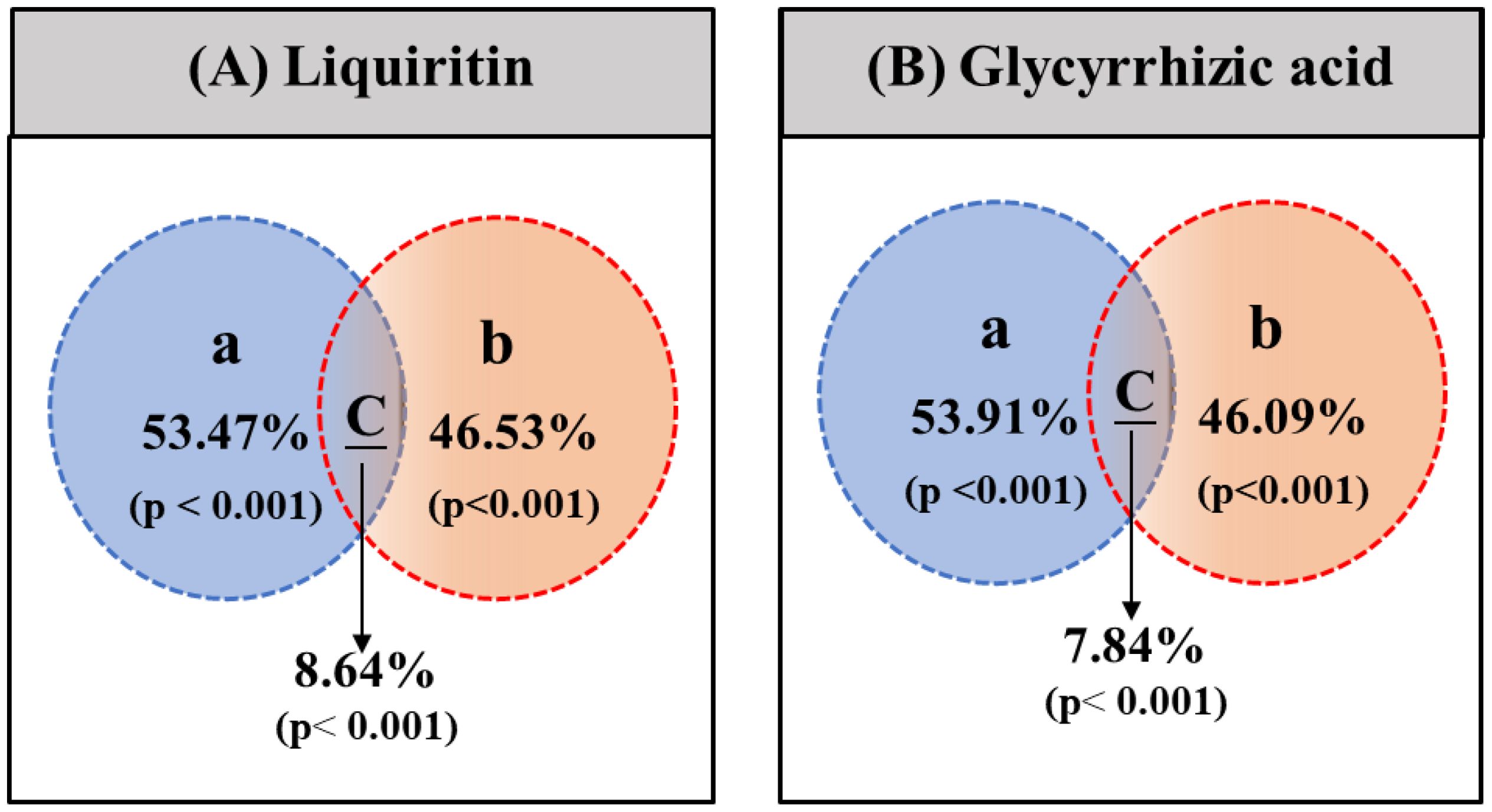
Figure 6. The results of main effect analysis of mixed variance analysis of irrigation amount and fertilization amount. (A) represents liquiritin content, and (B) represents glycyrrhizic acid content.
4 Discussion
4.1 Characteristics of licorice growth
The growth characteristics of licorice are influenced significantly by environmental conditions, particularly soil moisture and nutrient availability, which play pivotal roles in plant development. As observed in studies on other crops such as potatoes (Zhang et al., 2023), the growth and quality of licorice are similarly dependent on the optimization of irrigation and fertilization practices. Irrigation plays a critical role in mitigating drought stress, which is particularly crucial during critical growth phases of licorice such as root development and biomass accumulation. Adequate water supply not only alleviates the stress on licorice roots but also provides a conducive environment for root expansion and nutrient uptake. This, in turn, enhances the photosynthetic efficiency of the plant, promoting the accumulation and transformation of photosynthetic products into essential bioactive compounds such as glycyrrhizic acid and liquiritin. The study results indicate that moderate irrigation levels (e.g., W2: 4000 m³/ha) optimize water availability without causing waterlogging, thereby supporting optimal growth and development. This is akin to findings in potato cultivation, where excessive irrigation diluted key nutritional components in the tubers. In licorice, similar trends were observed where excessive water supply could potentially dilute the concentration of bioactive compounds in the roots, underscoring the importance of maintaining balanced irrigation practices. Similar findings have been reported for maize by Suat et al. (2022) and Khuynh et al. (2022). Hou et al. (2010) and Botir et al (Khaitov et al., 2021). have also documented similar impacts of water stress on licorice plants. Fertilization significantly influences the growth characteristics of licorice by improving soil fertility, which in turn enhances root growth and the plant’s overall nutrient uptake efficiency. Optimal fertilization (e.g., F2: 610 kg/ha) promotes the synthesis of bioactive compounds by providing essential nutrients that act as precursors for biosynthetic pathways involved in their production. Moreover, similar to observations in potato studies, excessive fertilization leads to nutrient leaching and can have a toxic effect on plant roots, thereby reducing the quality of the licorice roots. The study highlighted that moderate levels of fertilization maximize the content and quality of bioactive compounds in licorice, aligning with the findings that excessive nutrient supply can adversely affect plant health and product quality. The interaction between irrigation and fertilization levels plays a crucial role in determining the overall growth and quality of licorice. The study’s findings suggest that a synergistic approach, where both water and fertilizer are optimally managed, leads to the best growth outcomes and highest concentration of bioactive compounds. This is consistent with the principles observed in other agricultural practices where the balance of water and nutrients is essential for maximizing crop yield and quality. In conclusion, the growth characteristics of licorice in arid regions can be significantly enhanced through careful management of irrigation and fertilization. Adopting strategies similar to those used in optimizing potato quality, such as adjusting water and nutrient levels to suit specific growth stages and environmental conditions, can lead to improved licorice root quality and higher concentrations of valuable medicinal compounds. Future research should focus on refining these practices to suit local conditions and exploring the underlying biological mechanisms that govern the response of licorice to these environmental variables. This study solely evaluated the fertilizer and water requirements of licorice in the Xinjiang production area. Other licorice varieties, such as Glycyrrhiza inflata and Glycyrrhiza glabra, exhibit different ecological characteristics. Therefore, future research should focus on determining the optimal water and fertilization requirements for these varieties in Xinjiang.
4.2 Licorice bioactive components
As people’s quality of life improves, agricultural production is increasingly aimed at achieving not only high yields but also high quality. Bioactive components such as glycyrrhizin and glycyrrhetinic acid in licorice are important indicators of its quality. Our study has found that the supply of water and fertilizer significantly impacts the accumulation of these bioactive components in licorice. Firstly, appropriate irrigation can optimize the growth environment for licorice, alleviate root drought stress, and provide a comfortable environment that enhances the plant’s photosynthetic characteristics. This in turn promotes the accumulation and transformation of photosynthetic products, thereby affecting the quality of licorice. Our results indicate that with an increase in irrigation, the content of glycyrrhizin and glycyrrhetinic acid initially increases and then decreases. This phenomenon may be due to the fact that a reasonable amount of water meets the normal hydration needs of licorice, promotes photosynthetic enzyme activity and chlorophyll synthesis, enhances the metabolism of reactive oxygen species in plants, and positively impacts the quality of licorice. However, excessive irrigation leads to a rapid increase in soil water content, accelerating the transfer of phloem juice to the roots and diluting the concentration of bioactive components. Additionally, the impact of fertilization on the quality of licorice is also significant. Moderate application of nitrogen fertilizer is an important way to improve the quality of licorice. The study shows that the highest contents of glycyrrhizin and glycyrrhetinic acid are achieved at a moderate fertilization level, while excessive fertilization reduces the quality of licorice. When the amount of fertilizer is reduced from a high to a moderate level, the soil fertility improves, which promotes root growth and effectively delays the maturation time of licorice. This also prolongs the photosynthesis duration of the plant, significantly increasing the content of bioactive components. Moderate fertilization also promotes the transformation of plant materials and the synthesis of organic macromolecules in storage organs, improving the quality of licorice. However, when the fertilization rate is too high, the nutrient content in the soil significantly exceeds the plant’s uptake capacity, resulting in a high residual nutrient content in the root zone and a high soil ion concentration per unit volume, which has a toxic effect on licorice roots and reduces the quality of licorice. Based on these findings, it is advisable for licorice growers in Xinjiang to adopt a cultivation strategy that optimizes the concentration of active substances in licorice roots. This can be accomplished through a strategy that either employs a combination of lower fertilizer use and effective water management, or through a regimen that integrates minimal fertilizer use with moderate watering levels.
5 Conclusion
The more effective utilization of water and fertilizer resources, along with the development of rational management schemes, are among the urgent challenges faced by China. Optimizing irrigation schemes, quotas, fertilization amounts, and the water-fertilizer coupling effect will promote the production of licorice roots. The experimental results enhance the scientific foundation for implementing the strictest water management system in Xinjiang’s arid regions. The primary goal of this study was to assess the specific water and fertilizer needs for cultivating licorice in Xinjiang. A detailed analysis of various water and fertilizer application techniques was conducted to understand their impact on the growth characteristics and bioactive compounds in four-year-old licorice plants, and proposed a multi-objective optimization scheme for drip irrigation in the cultivation of licorice in Xinjiang’s arid regions. The study found a positive correlation between fertilizer use and the concentration of active constituents in different parts of the licorice plant. Initially, increased irrigation levels led to higher active component content, but this trend reversed at higher fertilizer application rates. Liquiritin and glycyrrhizic acid primarily accumulated in the licorice roots, with higher concentrations compared to other plant parts like the rhizome, stem, leaves, and lateral roots. Appropriate water and fertilizer application (305-610 kg/hm² for fertilizer and 2500-4000 m³/hm² for irrigation) enhanced the accumulation of these compounds in both vegetative and reproductive organs of the licorice. This improved the plant’s ability to absorb and utilize fertilizer, increasing the content of active components. The method strengthened the growth characteristics of licorice, leading to higher active component concentrations across various plant segments. The most effective water-fertilizer coupling mode was found to be moderate application levels, which resulted in the highest concentration of active constituents. This outcome is primarily due to the synergistic effect of moderate water and fertilizer levels, enhancing photosynthesis and leading to increased dry matter accumulation, thereby improving overall plant growth.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
AA: Writing – original draft, Writing – review & editing. XL: Data curation, Writing – original draft. AS: Funding acquisition, Writing – review & editing, Project administration. GA: Funding acquisition, Writing – review & editing, Investigation, Methodology, Resources.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Natural Science Foundation of China (41867044,41361069), the Key Research and Development of Xinjiang Uygur Autonomous region (2022A03004-1), agricultural science and technology cooperation project of Zhejiang Department of Agriculture and Rural Affairs(2024SNJF035), the “Tianshan Talent” Agricultural Backbone Personnel Project in Xinjiang Uygur Autonomous Region (2023SNGGNT058), Xinjiang Uyghur Autonomous Region Science and Technology Commissioner Rural Science and Technology Entrepreneurship Action Project (2024KY017).
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abudurezike A., Liu X., Aikebaier G., Shawuer A., Tian X. (2024). Effect of different irrigation and fertilizer coupling on the liquiritin contents of the licorice in Xinjiang arid area. Ecol. Indic. 158, 111451–111451. doi: 10.1016/j.ecolind.2023.111451
Ao M., Miura R., Tominaga T. (2009). Root and rhizome systems of perennial grasses grown in Inner Mongolian grassland, China. Grassland Sci. 55, 187–192. doi: 10.1111/j.1744-697x.2009.00158.x
Assar D. H., Elhabashi N., Mokhbatly A.-A. A., Ragab A. E., Elbialy Z. I., Rizk S. A., et al. (2021). Wound healing potential of licorice extract in rat model: Antioxidants, histopathological, immunohistochemical and gene expression evidences. Biomedicine Pharmacotherapy 143, 112151. doi: 10.1016/j.biopha.2021.112151
Baba M., Shigeta S. (1987). Antiviral activity of glycyrrhizin against varicella-zoster virus in vitro. Antiviral Res 7 (2), 99–107. doi: 10.1016/0166-3542(87)90025-8
Chang X. S., Chang G. Q. (2021). Advances in research and prospect on soil moisture in aridand semi-arid areas. J. Desert Res. 41, 156–163. doi: 10.7522/j.issn.1000-694X.2020.00067
Chen M., Yang G., Sheng Y., Li P., Qiu H., Zhou X., et al. (2017). Glomus mosseae Inoculation Improves the Root System Architecture, Photosynthetic Efficiency and Flavonoids Accumulation of Liquorice under Nutrient Stress. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00931
Crance J. M., Scaramozzino N., Jouan A., Garin D. (2003). Interferon, ribavirin, 6-azauridine and glycyrrhizin: antiviral compounds active against pathogenic flaviviruses. Antiviral Res. 58, 73–79. doi: 10.1016/s0166-3542(02)00185-7
Fan Y., Ma C., li P., Cao H., Cao Y. (2022). Water–fertilizer coupling effect on the growth traits of winter wheat under conditions of light and small sprinklers. SHS Web Conferences 140, 1028. doi: 10.1051/shsconf/202214001028
Gou S., He M., Li B., Zhu N., Ni J. (2020). Hepatoprotective effect of total flavonoids from Glycyrrhiza uralensis Fisch in liver injury mice. Natural Product Res. 35, 6083–6087. doi: 10.1080/14786419.2020.1824223
Hao K., Fei L., Liu L., Jie F., Peng Y., Liu X., et al. (2022). Comprehensive evaluation on the yield, quality, and water-nitrogen use efficiency of mountain apple under surge-root irrigation in the loess plateau based on the improved TOPSIS method. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.853546
He C., Wang W., Hou J. (2019). Plant growth and soil microbial impacts of enhancing licorice with inoculating dark septate endophytes under drought stress. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.02277
Hou J. L., Li W. D., Zheng Q. Y., WANG W. Q., Xiao B., Xing D. (2010). Effect of low light intensity growth and accumulation of secondary metabolites in roots of Glycyrrhiza uralensis Fisch. Biochem. Syst. Ecol. 38, 160–168. doi: 10.1016/j.bse.2009.12.026
Khaitov B., Urmonova M., Karimov A., Sulaymonov B., Allanov K., Israilov I., et al. (2021). Licorice (Glycyrrhiza glabra)-Growth and Phytochemical Compound Secretion in Degraded Lands under Drought Stress. Sustainability-Base 13, 2923. doi: 10.3390/su13052923
Khuynh T. B., Toshiya N., Yoshida H., Toda Y., Omori Y., Tsuda M., et al. (2022). Effects of irrigation on root growth and development of soybean: A 3-year sandy field experiment. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1047563
Liu J., Wu L., Wei S., Xiao X., Su C., Jiang P., et al. (2007). Effects of arbuscular mycorrhizal fungi on the growth, nutrient uptake and glycyrrhizin production of licorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul. 52, 29–39. doi: 10.1007/s10725-007-9174-2
Martins N., Barros L., Dueñas M., Santos-Buelga C., Ferreira I. C. F. R. (2015). Characterization of phenolic compounds and antioxidant properties of Glycyrrhiza glabra L. rhizomes and roots. RSC Advances. 5 (34), 26991–26997. doi: 10.1039/c5ra03963k
Meng X., Zhang X., Su X., Liu X., Ren K., Ning C., et al. (2022). Daphnes Cortex and its licorice-processed products suppress inflammation via the TLR4/NF-κB/NLRP3 signaling pathway and regulation of the metabolic profile in the treatment of rheumatoid arthritis. J. Ethnopharmacology. 283, 114657. doi: 10.1016/j.jep.2021.114657
Michel H. E., Tadros M. G., Abdel-Naim A. B., Khalifa A. E. (2013). Prepulse inhibition (PPI) disrupting effects of Glycyrrhiza glabra extract in mice: A possible role of monoamines. Neurosci. Letters. 544, 110–114. doi: 10.1016/j.neulet.2013.03.055
Rogers C. W., Dari B., Neibling H., Walling J. G. (2022). Barley yield and malt characteristics as affected by nitrogen and final irrigation timing. Agron. J. 114, 1461–1474. doi: 10.1002/agj2.21036
Shekhawat K., Rathore S. S., Premi O. P., Kandpal B. K., Chauhan J. S. (2012). Advances in agronomic management of Indian mustard (Brassica juncea (L.) czernj. Cosson): an overview. Int. J. Agronomy., 408284. doi: 10.1155/2012/408284
Sheng W. (2010). Pharmacopeia of people’s republic of China. Beijing: China. China Med. Sci. Press, 1.
Suat I., Mohammed A. T., Kukal M. S. (2022). Maize response to coupled irrigation and nitrogen fertilization under center pivot, subsurface drip and surface (furrow) irrigation: Growth, development and productivity. Agricultural Water Management. 263, 107457–107457. doi: 10.1016/j.agwat.2022.107457
Tyagi P., Sharma S. K., Kumar P. (2018). Evaluation of antihyperlipidemic activity of ethanolic root extract of Glycyrrhiza glabra Linn. J Drug Deliv Ther. 8, 120–124. doi: 10.22270/jddt.v8i6-s.2098
Wang L., Yang R., Yuan B., Liu Y., Liu C. (2015). The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B. 5, 310–315. doi: 10.1016/j.apsb.2015.05.005
Wu Q. (2013). Research progress on the immunomodulatory effects of glycyrrhizin. Int. J. Immunol. 36, 276–280. doi: 10.3760/cma.j.issn.1673-4394.2013.04.008
Wu W. X., Fu W. X., Alatalo J. M., Ma Z. X., Bai Y. (2022). Effects of coupling water and fertilizer on agronomic traits, sugar content and yield of sugarcane in guangxi, China. Agron. J. 12, 321. doi: 10.3390/agronomy12020321
Wu T. Y., Khor T. O., Saw C. L. L., Loh S. C., Chen A. I., Lim S. S., et al. (2010). Anti-inflammatory/Anti-oxidative Stress Activities and Differential Regulation of Nrf2-Mediated Genes by Non-Polar Fractions of Tea Chrysanthemum zawadskii and Licorice Glycyrrhiza uralensis. AAPS J. 13, 1–13. doi: 10.1208/s12248-010-9239-4
Wu B., Zeng H., Zhu W., Yan N., Ma Z. (2021). Enhancing China’s Three Red Lines strategy with water consumption limitations. Sci. Bull. 66, 2057–2060. doi: 10.1016/j.scib.2021.06.012
Zhang F., Chen M., Fu J., Zhang X., Li Y., Shao Y., et al (2023). Coupling effects of irrigation amount and fertilization rate on yield, quality, water and fertilizer use efficiency of different potato varieties in Northwest China. Agricultural Water Management 287, 108446–108446. doi: 10.1016/j.agwat.2023.108446
Zhang L., Dong Z. C., Zhao B. (2003). Method for estimating ecological water requirement of natural vegetation in arid area. Adv. Water Science. 14, 745–748.
Zhang Y., Yang Y., Gong H., Zhu H. (2018). A systematic review of the comparison of three medicinal licorices, based on differences of the types and contents about their bioactive components. J. Chem. Biol. Pharm. Chem. 1, 1–6.
Zhang Y., Zhang F. C., Yuan Y. X., Qiang S. C., Fang D. P. (2014). The effect of irrigation and fertilization on growth and quality of tomato under fertigation in greenhouse. Agric. Res. Arid Areas. 32, 206–212. doi: 10.7606/j.issn.1000-7601.2014.02.033
Keywords: Water-Fertilizer Interaction, licorice, medicinal plant chemistry, growth characteristics, sustainable agriculture, Xinjiang
Citation: Abudurezike A, Liu X, Shawuer A and Aikebaier G (2024) Coupling effects of irrigation amount and fertilization rate on growth and bioactive components of four-year-old licorice (Glycyrrhiza uralensis Fisch) in arid regions of Xinjiang. Front. Agron. 6:1462005. doi: 10.3389/fagro.2024.1462005
Received: 09 July 2024; Accepted: 09 September 2024;
Published: 08 October 2024.
Edited by:
Haruyuki Fujimaki, Tottori University, JapanReviewed by:
Muhammad Saqlain Zaheer, Khwaja Fareed University of Engineering and Information Technology (KFUEIT), PakistanFathia Elmokh, Institut des Régions Arides, Tunisia
Copyright © 2024 Abudurezike, Liu, Shawuer and Aikebaier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ayixiamu Shawuer, QXlpeGlhbXVuZEAxNjMuY29t; Gulimila Aikebaier, ZGFndWxpbWlsYUAxNjMuY29t
†These authors share first authorship
 Abudukeyoumu Abudurezike
Abudukeyoumu Abudurezike Xinghong Liu3,4†
Xinghong Liu3,4†