- 1Department of Plant and Soil Sciences, Research and Education Center, University of Delaware, Georgetown, DE, United States
- 2Department of Plant Sciences and Landscape Architecture, Wye Research and Education Center, University of Maryland College Park, Queenstown, MD, United States
- 3Department of Entomology and Wildlife Ecology, University of Delaware, Newark, DE, United States
Weeds interfere with lima bean production by reducing crop yield, hindering harvest, and contributing contaminants to harvested beans, yet there are very few trials documenting the impact of weeds on lima bean. This research was designed to evaluate weeds on lima bean yield, quality, as well as Rhizoctonia solani and pod-feeding insects in order to assist in implementing a more integrated approach to pest management. Field studies at four sites evaluated the impact of common cocklebur (Xanthium strumarium L.), jimsonweed (Datura stramonium L.), and ivyleaf morningglory [Ipomoea hederacea (L.) Jacq.] at densities of 0, 7, 10, or 20 plants 10 m-1 row in the presence and absence of Rhizoctonia solani on lima bean (Phaseolus lunatus L.). The planting dates of late June to mid-July represented a typical planting period for the mid-Atlantic region of USA, while one site represented an early planting date in this region (28 May). Differences in response to weed competition for total lima bean yield, marketable yield, yield components, and R. solani discoloration on lima bean occurred at one or more sites. Weed competition from 7 plants 10 m-1 of row or higher, reduced number lima bean pods by as much as 40%. However, weed density had little impact on percentage of flat, plump, or dry pods. Marketable yield was reduced at two sites in response to 7 plants 10-1 row (19% yield loss) and higher weed densities resulted in 29 to 33% yield loss. The presence of lima bean resulted in 40 to 60% reduction of common cocklebur and jimsonweed biomass and burs or seeds compared to weeds grown without crop competition. Weed competition in lima bean was influenced by many factors including weed species and planting date. Weed management is important to not only preserve yield but limit weed seed return to the soil seedbank and maintain harvest efficiency.
Introduction
Green baby lima bean (Phaseolus lunatus L.) is an important crop in the Mid-Atlantic states of Delaware, Maryland, New Jersey, and Virginia (USDA, 2017), accounting for nearly 50% of the lima bean produced in the USA. They are harvested 75 to 86 days after planting before pods and seeds begin to dry down (Wyenandt and van Vuuren, 2024). The beans are shelled during harvesting and then processed for freezing or canning.
Weed management is a primary concern to lima bean growers for several reasons. Weeds compete with lima bean for water, nutrients, and light that can result in reduced yield; can interfere with harvest; and the weeds’ leaves, stems and seed capsules in the harvested commodity require additional cleaning measures. Important factors affecting the severity of weed competition in most crops include weed species, density, relative time of emergence, and the length of time they compete with the crop.
Sicklepod (Senna obtusifolia L.) is the only species for which weed thresholds have been determined in green lima bean (Glaze and Mullinix, 1984). An 18% lima bean yield loss was documented over a 3-yr period with 2.7 sicklepod plants m-2.
In snap bean (Phaseolus vulgaris L.), Neary and Majek (1990) documented up to 55% yield loss with common cocklebur (Xanthium strumarium L.) densities of 8 weeds m-2. Yield loss occurred with redroot pigweed (Amaranthus retroflexus L.) or large crabgrass (Digitaria sanguinalis [L.] Scop.) densities of >1 or >2 plants m-2, respectively in snap bean (Aguyoh and Masiunas, 2003a, Aguyoh and Masiunas, 2003b).
Since plant growth habits and length of the growing season influence weed competition, research from one species may not relate to a second species. Lima bean have a less upright growth habit than snap bean, and they require three to four additional weeks to reach the harvest stage. Dry bean (Phaseolus vulgaris L) also have a more upright growth habit than lima bean, but they are harvested at a different physiological stage than lima bean, when pods and seeds are dry. Urwin et al. (1996) reported differences among dry bean classes when evaluating weed emergence and late-season competition. Thus, there needs to be more research evaluating weed competition for lima bean.
Common cocklebur, ivyleaf morningglory [Ipomoea hederacea (L.) Jacq.], and jimsonweed (Datura stramonium L.) are three common weed species in Delaware. Common cocklebur and jimsonweed are upright growing plants (up to 1.5 m in height), but common cocklebur develops a wider canopy that intercepts more sunlight and is documented as causing more yield loss in soybean (Regnier and Stoller, 1989; Stoller et al., 1987). Ivyleaf morningglory has a vining growth habit with 2 to 2.5 m long stems (Mohler et al., 2021). In soybean, common cocklebur resulted in 57% yield loss over two years while entireleaf morningglory [Ipomoea hederacea (L.) Jacq.] resulted in 21% under the same conditions (Mosier and Oliver, 1995a). However, a better understanding of weed thresholds for these weed species in vegetable crops such as lima bean is needed for implementing an integrated approach to weed management.
Weed competition studies often do not consider weed seed production. In a limited number of studies on weed seed production, weed densities below economic thresholds have been shown to increase the level of the weed seedbank (Bauer and Mortensen, 1992; Bagavathiannan and Norsworthy, 2012). To implement an integrated approach to weed management, information is needed on seed production. Understanding seed production potential will allow for current year weed management decisions to account for future cropping seasons.
There are a limited number of weed control options for lima bean. Herbicide choices are limited due to crop safety as well as presence of herbicide-resistant biotypes (McNaughton et al., 2004; VanGessel et al, 2002). Lima bean have not responded well to some cultural practices to enhance weed control, such as narrower row spacing (Sankula et al., 2001; Korres et al., 2019). Thus, growers rely on a limited number of herbicides and row cultivation, which often leave surviving weeds in the crop row.
Weeds have been identified as hosts for pathogens detrimental to lima bean production such as downy mildew (Phytophthora phaseoli Thaxt.) (Dominiak, 2002) and Phytophthora capsici Leon (Abeysekara et al., 2019). As hosts for pathogens, weeds contribute to increased inoculum levels, allow carryover of pathogens in crop rotations, and provide a base for pathogenic variation (Hartman et al., 1986). In some cases, pathogens isolated from weeds showed increased pathogenicity when re-inoculated to soybean (Helbig and Carroll, 1984).
Environmental concerns related to pesticide use may force future pest management strategies to achieve maximum pest control with reduced pesticide input and to identify strategies to control more than one class of pest. This necessitates that weed management be integrated with other pest management strategies and cultural practices. Little information is available on how weeds influence disease severity (Black et al., 1996), especially in a crop such as lima bean. Additionally, few studies have examined these types of interactions under field conditions. A more complete understanding of weed/disease relationships is needed to implement a comprehensive integrated pest management program.
This research was designed to determine the impact of important weed species, exhibiting different growth habits, on lima bean yield, quality, as well as Rhizoctonia solani and pod-feeding insects in order to assist in implementation of a more complete integrated pest management program in lima bean.
Materials and methods
Field sites
Field studies were conducted in 1997, 1998, and 2000. In 1997, the study was conducted at University of Delaware’s Warrington Farm located near Harbeson, DE. Lima bean was planted at two sites in 1998 and at one site in 2000 all at University of Delaware’s Thurman Adams Research Farm located near Georgetown, Delaware. Soil at Warrington Farm was Downer loamy sand (coarse-loamy, siliceous, mesic, Typic Paleudult) and Fallsington sandy loam (fine-loamy, mixed, mesic, Typic Ochraquult) at the Adams Research Farm. Soil pH was 5.4, 5.5, and 5.7 with 1.2%, 1.2%, and 1.1% organic matter in 1997, 1998, and 2000, respectively. Sites in 1998 were adjacent to each other and thus, soil characteristics were the same. Lima bean cultivar ‘Maffei-15’ was used since it is susceptible to Rhizoctonia solani and commonly grown in Delaware at time of the study. Lima bean was planted on 27 June in 1997, 28 May and 13 July in 1998, and 10 July in 2000. The planting dates in 1998 represent an early and a typical date for the range of lima bean planting in the southern Delaware region. Plots were four rows, 76 cm apart (3 m wide) and 9 m long. Seeding depth was 2.5 to 3 cm. Total plant population was 175,000 plants ha-1 at all sites. In 1997, no nitrogen was applied, but in 1998 and 2000 nitrogen at 68 kgha-1 was fertigated in two applications, within 2 weeks after planting and at the mid-vegetative stage of lima bean. All sites were irrigated with overhead irrigation to prevent moisture stress.
In 1998, jimsonweed plots (all densities) were infested with Colorado potato beetles (Leptinotarsa decemilineata [Say] [Coleoptera: Chrysomelidae]) at both sites and carbaryl (carbaryl-1-nephthyl carbamate) was sprayed at 0.85 kg ai ha-1 to control them and limit jimsonweed damage. In 2000, all plots had a severe infestation of downy mildew (Phytophthora phaseoli). Copper hydroxide was sprayed for downy mildew management at 0.65 kg ai ha-1 for four consecutive weeks beginning at full-bloom stage. However, effectiveness was only marginal. Copper hydroxide was selected since it does not control R. solani.
Experimental design
Experiment was designed was a randomized complete block with treatments arranged as a three-factor factorial with four replications. Two factors included weed species and weed density in an additive design (Harper, 1983). The third factor was level of R. solani infestation (inoculated or non-inoculated). Weed species were common cocklebur, jimsonweed, or ivyleaf morningglory planted at 0, 7, 10, or 20 weeds 10 m-1 row (weed-free, 150, 100, or 50 cm apart, respectively). Weeds were chosen to represent different canopies, branching and growth characteristics. Seeding densities were based on previous additive design experiments conducted with other Phaseolus spp (Blackshaw, 1991; Chikoye et al., 1995; Neary and Majek, 1990). In addition, each weed species was grown at a density of 7 plants 10 m-1 row with no lima bean. These additional plots were used to determine the influence of lima bean plants on weed biomass accumulation. All plots were repeated with or without R. solani inoculation in 1997 and 1998 only. In 2000, only one plot per replication with common cocklebur at 20 plants m-1 row was inoculated with R. solani.
Each weed species was hand-seeded within 3 cm of the crop row immediately after planting lima bean, and each plot received a single weed species. The seeding of weeds within the crop row was designed to mimic plants that are not controlled with cultivation. The plots were kept weed-free except for the weed species of interest by placing a cup over the weeds and spraying 1.1 kg ai.ha-1 bentazon, [3-(1-methylethyl)-(1H)-2,1,3-benzothiadiazin-4(3H)-one 2,2-dioxide] plus 0.2 kg ai.ha-1 sethoxydim, {2-[1-(ethoxyimino)butyl]-5-[2-ethylthio)propyl]-3-hydroxy-2-cyclohexen-1-one} plus 0.25% v/v non-ionic surfactant at second trifoliolate stage of lima bean. Bentazon was used for broadleaf weed control and sethoxydim for grass control. Thereafter, plots were hoed and handweeded at 1-to-2-week intervals.
Pod rot inoculum
Inoculum was produced from an R. solani hyphal tip isolate from infected lima bean pods collected in Delaware. Pathogenicity was confirmed on lima bean; however, the anastomosis group was not identified (Ginn et al., 2023). R. solani was increased by growing on quarter strength potato dextrose agar. Four mm diameter plugs were cut and mixed with twice-autoclaved barley seed. The barley was incubated for 2 weeks at 25 C until the fungal mycelia covered the entire grain surface. The barley was then dried, ground, and mixed with corn meal to disperse the inoculum onto the soil surface. Inoculum consisted of hyphal pieces and colonized barley seed pieces. Infested barley-cornmeal mixture was broadcast by hand in the inoculated plots at mid-vegetative stage in 1997 and at mid-flowering in 1998 and 2000.
Jimsonweed and morningglory species are considered hosts of Rhizoctonia solani, the causal agent of pod rot although this factor did not impact weed biomass or bur/seed parameters (Black et al., 1996). Although there is little information on how susceptible these species are to R. solani. Common cocklebur is not a host of R. solani.
Data collection
The height and width of lima bean canopy were measured 65 days after planting (DAP) to assess the impact of weeds on canopy development. Throughout the season, all plots were surveyed for pest insects, most notably corn earworm (Chloridea obsoleta L.) and lygus bugs [Lygus lineolaris (Palisot de Beauvois)].
At crop harvest, lima bean pods from ten randomly selected plants of the center two rows were separated into flat or plump pods based on seed development. In addition, number of seeds per pod (1, 2, or 3), dried pods, pod rot incidence, and insect damaged pods were determined for 5 plants. Pods per plant and number of seeds per pod served as indicators of yield components while number of succulent and dry pods indicated if weed presence or R. solani affected crop maturity.
The center two rows (6 m long) of each plot were harvested for final yield. Entire plants were pulled, pods were mechanically threshed, and seed weight was recorded. A 600 gm sub-sample from the final yield was passed through a sieve of 95 mm diameter holes to determine the marketable yield. Seeds that were retained on the sieve were considered marketable. Weight of 200-seeds retained on the sieve was determined. Finally, a random sample of 200-seeds was collected and examined for pest insect damage and staining from R. solani (Dillard, 1987). Weight and damage of these 200 seeds served as indicators of lima bean quality.
Three weeds per plot for common cocklebur and jimsonweed were randomly selected at harvest for weed seed production and biomass accumulation. The vining growth habit of ivyleaf morningglory prevented us from identifying separate plants. Weed plants were cut at ground level and placed in individual paper bags. Plants were oven-dried to constant weight and weed dry weights recorded. Common cocklebur burs were removed and total weight of 200 burs was recorded. Jimsonweed seed capsules were opened and seed was collected and cleaned. Total seed weight and weight per 200 weed seeds were determined for individual jimsonweed plants. Additionally, the number of viable jimsonweed seeds out of the 200-seed sample was counted in 1998 and 2000. Seeds that resisted the gentle pressure of forceps were considered to be viable.
Statistical analysis
All data were subjected to analysis of variance using PROC Mixed version 9.4 (SAS Institute Inc., Cary, NC). Year and replications within year were treated as random effects while weed species, weed density and presence of R. solani inoculum were fixed effects. Fisher’s protected LSD was used for mean separation at P = 0.05. In 1997, jimsonweed and ivyleaf morningglory did not emerge, thus, only common cocklebur was used for evaluations.
Results
Weed parameters
Lima bean competition had marked impact on weed biomass production (Table 1). Comparing seven weeds 10-1 m of row without the lima bean crop with the same density in the presence of lima bean, common cocklebur biomass reduction ranged from 48 to 65% as a result of interspecific competition. Similarly, lima bean competition reduced jimsonweed biomass, ranging from 38 to 74% in two out of the three site-years; with no differences detected for the May-planted lima bean in 1998.
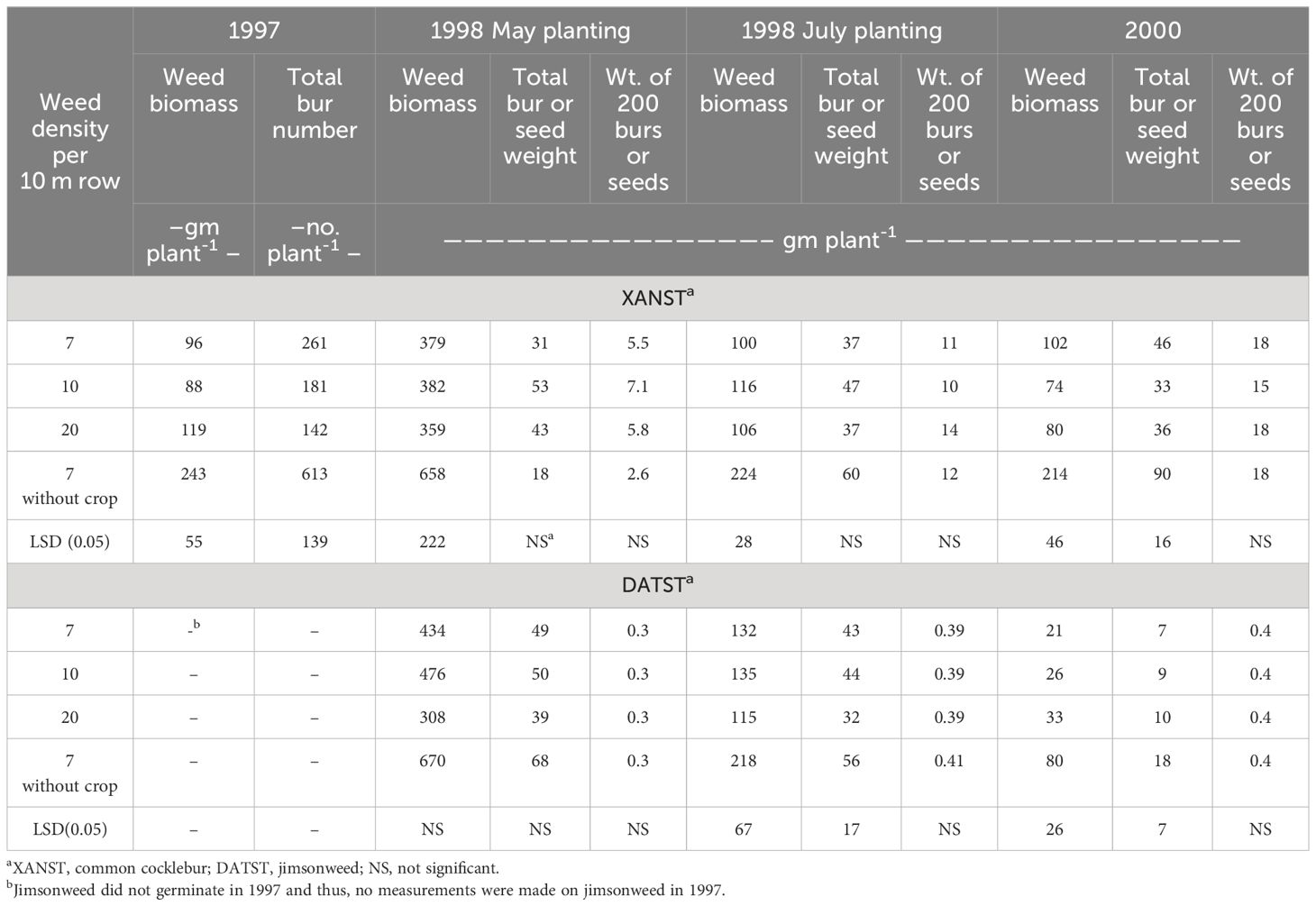
Table 1 Biomass and seed production per plant of common cocklebur (XANST) and jimsonweed (DATST) in 1997, 1998, and 2000 as influenced by density and presence of the lima bean crop.
Competition from the lima bean crop also reduced bur or seed production at four out of the seven weeds by site observations (Table 1). Burs or seeds were reduced by as much as 77% in 1997. However, the crop competition did not reduce the size of individual seeds as noted by weight of 200 burs or seeds.
Weed biomass was at least 2X greater with the May-planted lima bean in 1998 than the other three sites (Table 1). Conversely, 200-bur/seed weight and viable seed production increased with the typical planting date of July. There was an increase of at least 40 and 30% in 200-bur/seed weight for common cocklebur and jimsonweed, respectively. Viability of jimsonweed seeds also increased by at least 29% with the typical planting in 1998 (data not presented).
Neither weed density (7, 10, or 20 plants 10 m-1 row) nor R. solani impacted common cocklebur or jimsonweed biomass production, total bur/seed production, or 200-bur/seed weight at any site (Table 1). Viable seed production of jimsonweed also was not affected by weed densities, presence or absence of the lima bean crop, or pod rot in all three years (data not shown).
Lima bean yield
Total lima bean yield was not impacted by pod rot (data not presented). No differences were noted in total lima bean yield between different weed densities in 1997 or 2000 (Table 2). Poor lima bean yield in 2000 was influenced by downy mildew infestation during the reproductive stage. Total lima bean yield loss was recorded with both planting dates in 1998, with losses of 26 and 33% recorded for May and July planting, respectively. Yield loss was greater with 10 or 20 weeds 10 m-1, compared to 7 weeds 10 m-1, but no differences were detected between the two higher weed densities.
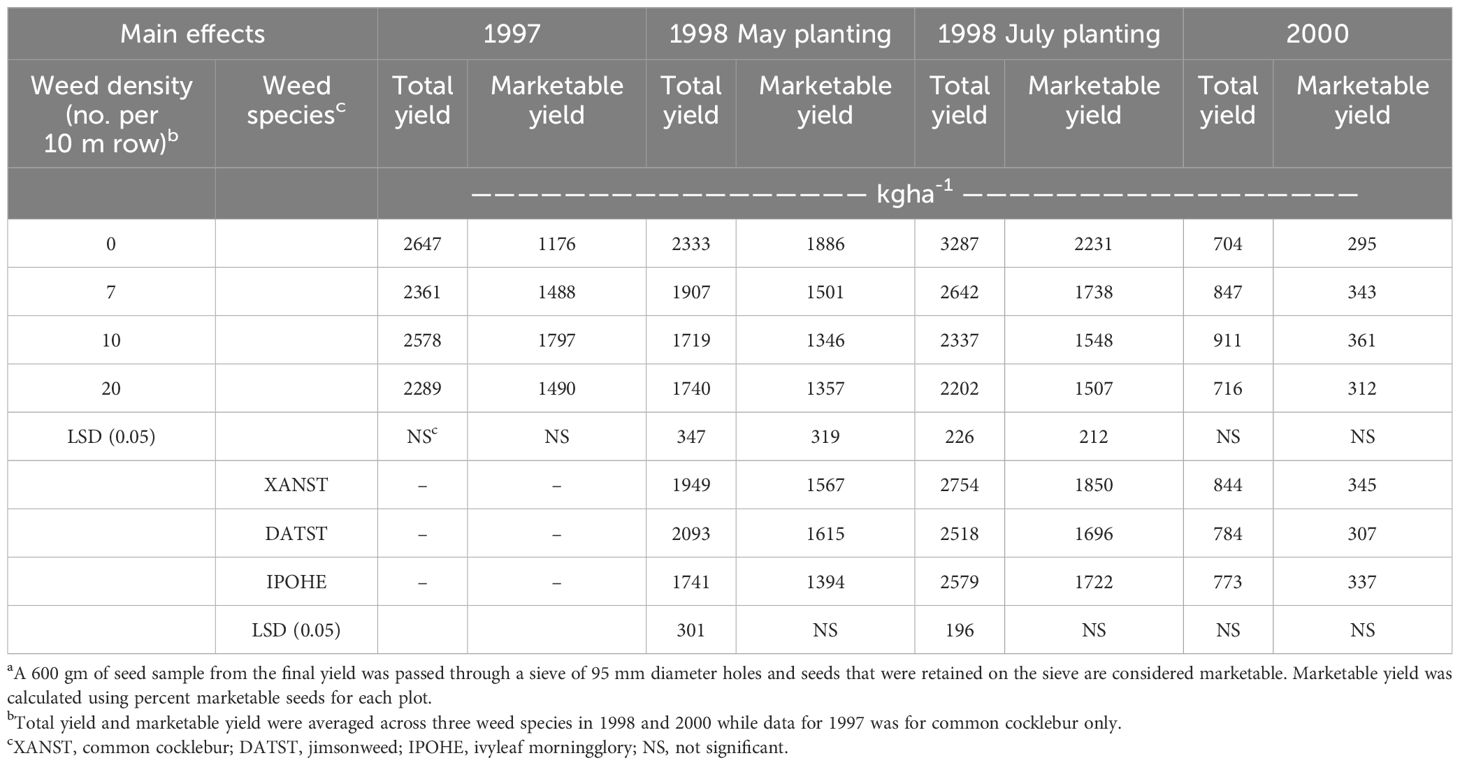
Table 2 Total yield and marketable yielda of lima bean as influenced by density of weed species in 1997, 1998, and 2000.
When yield loss was detected, ivyleaf morningglory reduced total yield more than either common cocklebur or jimsonweed with the May-planting in 1998. Ivyleaf morningglory or jimsonweed reduced yield more than common cocklebur for July-planted lima bean in 1998. Yield loss from ivyleaf morningglory averaged 25 and 22% across all densities in May- and July-plantings, respectively, in 1998. Weed species was not a significant factor in 2000.
Weed densities reduced marketable yield for both planting dates in 1998, but not in 1997 or 2000 (Table 2). Marketable lima bean yield was reduced by 26% when weeds were present for the May planting date in 1998 with no differences observed among the three weed densities tested. For the July-planting date in 1998, yield was highest with the weed-free check with 3287 kg ha-1. Weed densities of 7 and 10 weeds 10 m-1 of row reduced marketable yield similarly, averaging 26% yield loss. Densities of 20 weeds 10 m-1 row resulted in the greatest yield reduction with 33% yield loss. The factors of weed species and pod rot did not have an influence on marketable yield of lima bean.
In 1998, weed dry weights were greater with the May planting date by 3X compared to July planting, while lima bean yields were 30% lower at the May planting date compared to the typical planting date. However, yield reduction due to weed competition was 26 to 33% for both planting dates. Furthermore, common cocklebur biomass in 1997 and 2000 was similar to July planted site in 1998 (214 to 243 gm plant-1), yet yield loss only occurred in 1998. Thus, yield loss cannot be explained by weed biomass data alone.
Lima bean attributes
Lima bean height at harvest was not influenced by weed density, weed species, or R. solani (data not presented). Lima bean width was less in the presence of weeds in the July planting in 1998 and 2000, by 7 and 13%, respectively. There was no difference for weed densities.
Presence of weeds reduced number of total lima bean pods in 1997 and 1998 by 20 to 39%. No difference in lima bean pod number was observed in 2000 when pod number was low (Table 3). Total number of pods was highest for the weed-free plots. The presence of weeds reduced pod number, but differences between weed densities were only detected in 1997. Total number of pods were 26, 34, and 22 for 7, 10 and 20 weeds 10 m-1 of row, respectively.
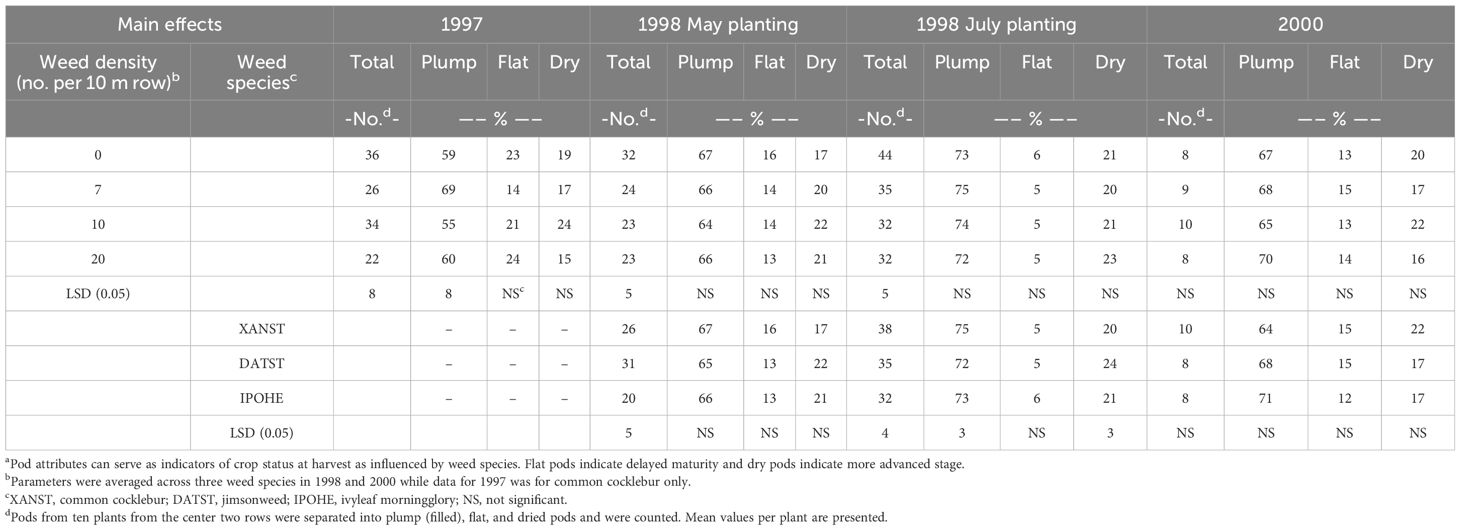
Table 3 Lima bean pod attributes in 1997, 1998, and 2000 as influenced by weed species and weed densitya.
Differences in total number of pods was detected among the weed species with both plantings in 1998. Total number of pods was lowest when ivyleaf morningglory was present, with 20 pods per plant, compare to jimsonweed and common cocklebur with 31 and 26 pods per plant, respectively at the May planting date in 1998. With the July planting date, ivyleaf morningglory resulted in the fewest pods while common cocklebur had higher pod number with 38.
Weed species, weed densities, or pod rot had a limited effect on the percent plump, flat, or dry pods. Jimsonweed had 3% fewer plump pods and 4% more dry pods compared to common cocklebur for July-planted lima bean in 1998 only.
Yield components measured as number of 1-seeded, 2-seeded, or 3-seeded pods per five plants were not influenced by weed density in 1997 or 2000, however differences were observed in 1998 (Table 4). Number of 1- and 2-seeded pods produced by May-planted lima bean was highest in the weed-free plots compared to the presence of weeds. There were no differences among the weed densities. In the absence of weeds, there were 5 and 9.5 pods per plant for 1- and 2-seed pods, while 1- and 2-seed pods averaged 2.7 and 7.3 in the presence of weeds. Similar trends were observed for the July planting, with more 1-, 2-, and 3-seed pods in the absence of weeds, and 34, 23, and 31% reduction, respectively, in the presence of weeds, regardless of weed density.
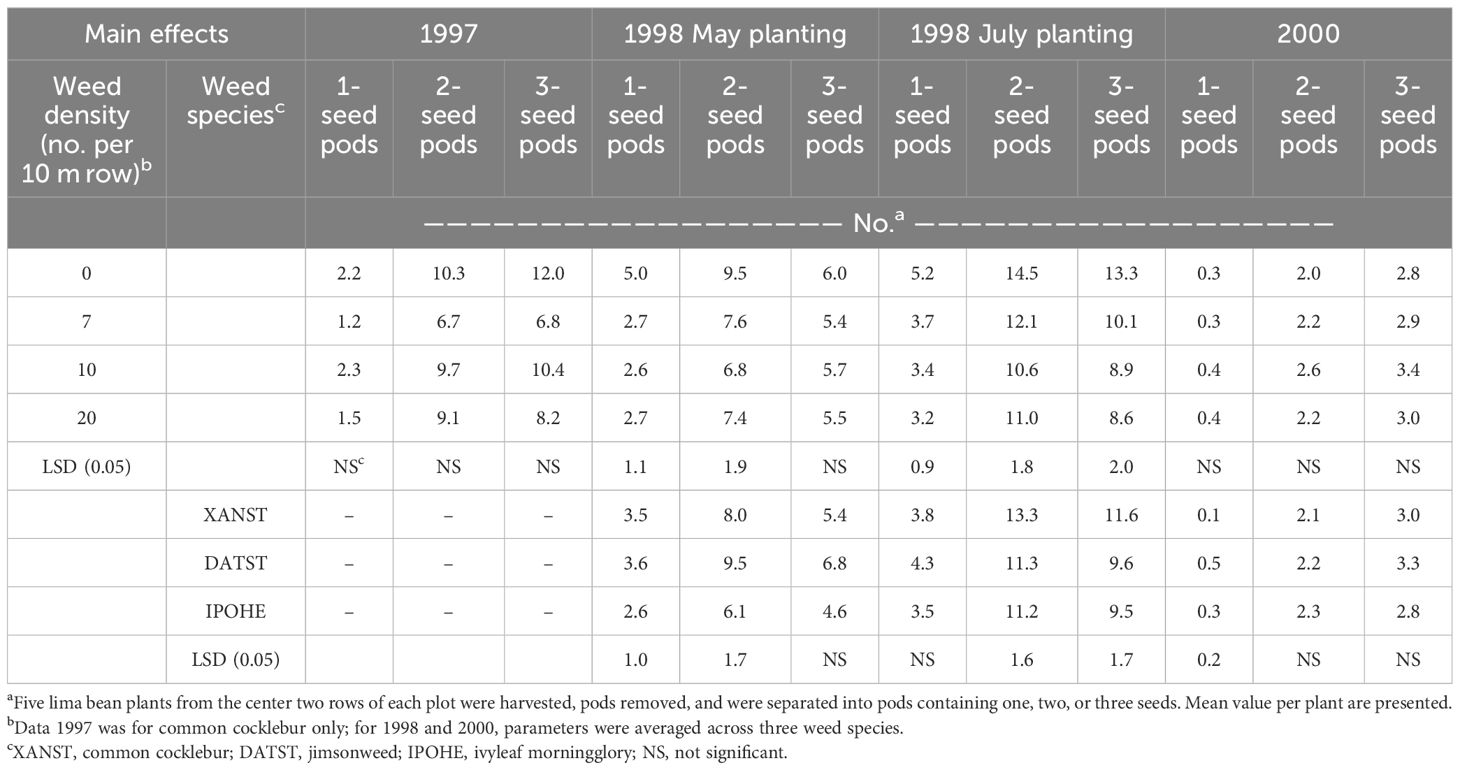
Table 4 Yield components of lima bean as influenced by weed species and weed densitya.
Jimsonweed and common cocklebur plots for May planting in 1998 had similar number of 1- and 2-seeded pods, averaging 3.6 and 8.7 pods, respectively, while ivyleaf morningglory had the fewest number of 1- and 2-seeded pods with 2.6 and 6.1. Number of 3-seeded pods was similar for all treatments, averaging 5.6 pods per plant.
For the July-planting, common cocklebur plots had highest number of 2- and 3-seeded pods (13.3 and 11.6 pods per plant, respectively) with jimsonweed and ivyleaf morningglory having similar number of 2- and 3-seeded pods (11.2 and 9.5 pods per plant respectively. At all planting dates, 4-seeded pods were present, but it was a very low number (<1 per plant) (data not presented).
Other pests
In 1998, pod rot infection and insect damage on the pods of July-planted lima bean was affected by R. solani inoculation and weed density (data not presented). Although differences did exist, they were slight. Pod rot infection on the pods was highest in the uninoculated weed-free plots and lowest in the inoculated plots with 10 plants per 10 m-1 row, 16 and 14 infected pods, respectively. Insect damage on pods was greater on pod rot infected weed-free plots (16 damaged pods per 10 plants) than plots with 7 and 10 weeds 10-1 m row that were inoculated with pod rot and inoculated plots with 0 and 20 weeds 10-1, averaging 8 damaged pods per 10 plants. Environmental factors may have contributed to the inconsistent or erratic results.
Rhizoctonia staining on seeds (referred to as “brown bean”) was influenced by weed density and R. solani inoculation of July-planted lima bean in 1998 (data not presented). Overall, pod rot severity was low; only 5 out of 200 seeds were infested with R. solani in the inoculated plots. The uninoculated plots had lower number of seeds infested with the R. solani (2 out of 200 seeds). Pod rot infection on the seeds was greater when weeds competed with lima bean, however, no differences were observed between the three densities or species, averaging 4.6 out of 200 seeds (data not presented). Black et al. (1996) reported no differences for R. solani infestation of common cocklebur and entireleaf morningglory but they did not examine the potential R. solani infestation on a host crop plant.
Downy mildew infestation on the pods was evaluated in 2000 only. No treatments differences were noted.
Discussion
Season-long weed competition in this study impacted yield and yield components and this is in concurrence with previous research with Phaseolus species. Aguyoh and Masiunas (2003b) noted pod number, length, and diameter were dependent on redroot pigweed density over a range of 0 to 8 plants m-1 of row.
Weed density had limited and inconsistent effects on crop height and biomass in this study (data not presented). In contrast to our results, Aguyoh and Masiunas (2003a); Aguyoh and Masiunas (2003b) noted reduction in snap bean biomass when higher weed densities were included. Weed densities used in this study were based on previous additive design experiments using other Phaseolus spp (Blackshaw, 1991; Chikoye et al., 1995; Neary and Majek, 1990). However, the results of this study indicate that a wider range of weed densities are needed to detect density differences. Thus, weed densities less than 7 plants 10 m-1 and greater than 20 plants 10 m-1 row are needed to evaluate the impact of weed competition on lima bean.
Similar to our results, research with snap bean indicated broadleaf weeds (common cocklebur or redroot pigweed) emerging with snap bean and exceeding a density of 0.5 to 1 weed m-2 can reduce snap bean yield and therefore warrant control measures (Neary and Majek, 1990; Aguyoh and Masiunas, 2003b). Lima bean typically require 2 to 3 weeks longer to reach harvest stage than snap bean, but it appears that the competitive relationship between these two Phaseolus species and weeds are similar.
Percent plump, flat, and dry pods are an indicator if weed presence or density influenced crop maturity. Weed density and weed species did not have a consistent impact on lima bean maturity (Table 3). Flat pods are an indication of delayed maturity while more dry pods would represent more physiologically mature plants. Weed density or species influenced crop maturity at only one site based on flat and dry pods.
This study was not designed to evaluate the influence of planting dates on lima bean-weed competition. However, two planting dates were used in 1998 to compensate for no jimsonweed emergence the previous year, and this allowed for planting date comparisons. Lima bean yield losses in 1998 were 18, 29, or 25% for May-planted lima bean and 17, 26, or 31% for July planted lima bean when weeds were present at densities of 7, 10, or 20 plants 10 m-1, respectively. Percent yield reduction as a result of weed competition was similar regardless of planting date although individual weed growth was much greater with the May planted lima bean. In similar research conducted with soybean, common cocklebur biomass production was lower with a July planting date compared to April or May planting dates (Rushing and Oliver, 1998). However, they also reported percent yield loss was similar for the respective common cocklebur densities across the three planting dates.
Harvesting is a practical consideration that is often not included in yield loss models (Glaze and Mullinix, 1984). Machine-harvest of the May-planted lima bean in the present study would have been difficult, or even impossible as a result of the weed size (H. Seamons, personal communication). On the other hand, July-planted lima bean could have been harvested with machinery due to less vigorous and smaller sized weeds.
July planting of lima bean resulted in improved yield. Similar results were reported by others (Glaze and Mullinix, 1984; Wootten, 1994). The cooler night temperatures associated with July planted lima bean in southern Delaware are more favorable for flower and pod development than high temperature and high relative humidity at nights during the reproductive phases associated with early planting (Fisher and Weaver, 1974; Wootten, 1994). As a result, lima bean are typically planted in late June through mid-July.
Monks and Oliver (1988) noted that biomass of common cocklebur and tall morningglory [Ipomea purpurea (L.) Roth] was reduced greater than 90% in response to soybean competition. Weed biomass reduction due to lima bean competition was less in this study compared to the reductions from soybean noted by Monks and Oliver (1988), suggesting that lima bean is less competitive with weeds than soybean.
Weed seed production was greater for weeds growing without a crop in four out of seven situations (Table 1). Weed seed viability and 200-seed weight were not different between treatments, regardless of planting date. Although the early planted lima bean allowed for larger weeds, these weeds did not produce more weed seed. Mosier and Oliver (1995b) reported entireleaf morningglory seed production was reduced by over 70% when grown in combination with soybean, compared to no soybean competition. This was one of the few studies examining the impact of crop competition on weed growth and weed seed production. This limits the ability to compare the real impact crop and crop management has on weed growth and fecundity.
As noted, planting date can impact the outcome of weed and crop competition, but so can length of growing season. Snap bean have a shorter growing season than lima bean by three to four weeks. Soybean have a longer growing season and most trials collecting weed biomass or reproductive output at soybean harvest, occurs twelve weeks after planting or longer. Weed production can increase rapidly in the late summer and so understanding the interactions of planting date, length of growing season and when weed fecundity is recorded are all important variables (Hill et al., 2016).
Research in snap bean has noted interactions between insect infestations and weeds (Aguyoh et al., 2004). We did not observe this in our study. However, few trials investigate these types of interactions and future research should note the interaction of multiple pests.
Weed management decision depends on understanding the relationship between weed species, density, and lima bean yield loss. Decisions on planting date of lima bean should consider the crop yield loss associated with early planting, intensity of weed management program to control larger, and more robust weeds when lima bean are planted early.
Practical implications
Lima bean have few herbicide options and growers rely on cultivation to supplement weed control, thus growers are often faced with the decision of whether or not to control weed escapes. Common cocklebur, jimsonweed, and ivyleaf morningglory are common weed species in the Mid-Atlantic region of the USA and have different growth habits, yet yield loss was not consistent for these three species and as a result they often had the same impact on yield at similar densities. Weed density of 7 plants 10 m-1 of row resulted in similar yield loss to higher densities and need to be controlled. Additional research is needed to establish a more refined weed threshold. Planting date is an important factor that is not often considered for weed management, with an earlier planting date resulting in larger weeds. Percent yield loss was similar across a range of planting dates, yet when lima bean was planted in May it resulted in larger weeds that would have hindered machine harvest. Lima bean producers need to implement effective weed management to prevent yield loss and prevent the production of weed seeds that can impact future cropping seasons.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SS: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. KE: Methodology, Writing – review & editing. JW: Methodology, Writing – review & editing. MV: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by a grant from United States Department of Agriculture’s Northeastern Regional Integrated Pest Management Program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abeysekara N. S., Hickman H., Westhafer S., Johnson G. C., Evans T. A., Gregory N. F., et al. (2019). Characterization of Phytophthora capsici isolates from lima bean grown in Delaware, United States of America. Phytopathol. Mediterr. 58, 535–546. doi: 10.13128/Phyto-10823
Aguyoh J. N., Masiunas J. B. (2003a). Interference of large crabgrass (Digitaria sanguinalis) with snap beans. Weed Sci. 51, 202–207. doi: 10.1614/0043-1745(2003)051[0202:IORPAR]2.0.CO;2
Aguyoh J. N., Masiunas J. B. (2003b). Interference of redroot pigweed (Amaranthus retroflexus) with snap beans. Weed Sci. 51, 202–207. doi: 10.1614/0043-1745(2003)051[0202:IORPAR]2.0.CO;2
Aguyoh J. N., Masiunas J. B., Eastman C. (2004). Interaction of insects and weeds in a snap beans agroecosystem. HortSci. 39, 287–290. doi: 10.21273/HORTSCI.39.2.287
Bagavathiannan M. V., Norsworthy J. K. (2012). Late-season seed production in arable weed communities: management implications. Weed Sci. 60, 325–334. doi: 10.1614/WS-D-11-00222.1
Bauer T. A., Mortensen D. A. (1992). A comparison of economic and optimum thresholds for two annual weeds in soybean. Weed Tech. 6, 228–235. doi: 10.1017/S0890037X00034606
Black B. D., Griffin J. L., Russin J. S., Snow J. P. (1996). Weed hosts for Rhizoctonia solani, causal agent for Rhizoctonia foliar blight of soybean (Glycine max). Weed Technol. 10, 865–869. doi: 10.1017/S0890037X00040938
Blackshaw R. E. (1991). Hairy nightshade (Solanum sarrachoides) interference in dry beans (Phaseolus vulgaris). Weed Sci. 39, 48–53. doi: 10.1017/S0043174500057854
Chikoye D., Weise S. F., Swanton C. J. (1995). Influence of common ragweed (Ambrosia artemisiifolia) time of emergence and density on white bean (Phaseolus vulgaris). Weed Sci. 43, 375–380. doi: 10.1017/S0043174500081352
Dillard H. R. (1987). Characterization of isolates of Rhizoctonia solani from lima beans grown in New York State (USA). Phytopathol. 77, 748–751. doi: 10.1094/Phyto-77-748
Dominiak J. D. (2002). Downy mildew of lima bean: alternate hosts, time of infection and control. University of Delaware, Newark.
Fisher V. J., Weaver C. K. (1974). Flowering, pod set, and pod retention of lima bean in response to night temperature, humidity, and soil moisture. J. Amer. Soc Hortic. Sci. 99, 448–450. doi: 10.21273/JASHS.99.5.448
Ginn A. N., Evans T. A., Ernest E. G., Koehler A. M. (2023). First report of Rhizoctonia solani AG 4 causing brown bean of lima bean in Delaware. Plant Dis. 107, 214. doi: 10.1094/PDIS-01-22-0118-PDN
Glaze N. C., Mullinix J. B. G. (1984). Competitive effects of sicklepod on lima beans. Weed Sci. 32, 1–3. doi: 10.1017/S0043174500058392
Harper J. L. (1983). “Mixtures of species. I. Spaces and proportion,” in Population biology of plants (Academic Press, New York, NY), 237–267.
Hartman G. L., Manandhar J. B., Sinclair J. B. (1986). Incidence of Colletotrichum spp. on soybeans and weeds in Illinois and pathogenicity of Colletotrichum truncatum. Plant Dis. 70, 80–782. doi: 10.1094/PD-70-780
Helbig J. B., Carroll R. B. (1984). Dicotyledonous weeds as a source of Fusarium oxysporum pathogenic on soybean. Plant Dis. 68, 694–696. doi: 10.1094/PD-69-694
Hill E. C., Renner K. A., VanGessel M. J., Bellinder R. R., Scott B. A. (2016). Late-season weed management to stop viable weed seed production. Weed Sci. 64, 112–118. doi: 10.1614/WS-D-15-00096.1
Korres N. E., Hausman N., Moody J. L., Kitis Y. E., illiams II, M.M. (2019). Integrated weed management strategies with cereal rye mulch in processing vegetable legumes. Agron. J. 112, 4264–4275. doi: 10.1002/agj2.20349
McNaughton K. E., Sikkema P. H., Robinson D. E. (2004). Herbicide tolerance of lima bean (Phaseolus lunatus) in Ontario. Weed Technol. 18, 106–110. doi: 10.1614/WT-03-038R1
Mohler C. L., Teasdale J. R., DiTommaso A. (2021) Manage weeds on your farm, a guide to ecological strategies (Sustainable Agriculture Research and Education). Available online at: https://www.sare.org/resources/manage-weeds-on-your-farm/ (Accessed March 10, 2024).
Monks D. W., Oliver R. L. (1988). Interactions between soybean (Glycine max) cultivars and selected weeds. Weed Sci. 36, 770–774. doi: 10.1017/S0043174500075809
Mosier D. G., Oliver L. R. (1995a). Common cocklebur (Xanthium strumarium) and entireleaf morningglory (Ipomoea hederacea var. integriuscula) interference on soybeans (Glycine max). Weed Sci. 43, 239–246. doi: 10.1017/S0043174500081133
Mosier D. G., Oliver L. R. (1995b). Soybean (Glycine max) interference on common cocklebur (Xanthium strumarium) and entireleaf morningglory (Ipomoea hederacea var. integriuscula). Weed Sci. 43, 402–409. doi: 10.1017/S004317450008139X
Neary P. E., Majek B. A. (1990). Common cocklebur (Xanthium strumarium) interference in snap beans (Phaseolus vulgaris). Weed Technol. 4, 743–748. doi: 10.1017/S0890037X00026324
Regnier E. E., Stoller E. W. (1989). The effects of soybean (Glycine max) interference on the canopy architecture of common cocklebur (Xanthium strumarium), jimsonweed (Datura stramonium), and velvetleaf (Abutilon theophrasti). Weed Sci. 37, 187–195. doi: 10.1017/S0043174500071769
Rushing G. S., Oliver L. R. (1998). Influence of planting date on common cocklebur (Xanthium strumarium) interference in early-maturing soybean (Glycine max). Weed Sci. 46, 99–104. doi: 10.1017/S0043174500090238
Sankula S., VanGessel M. J., Kee J. W. E., Beste C. E., Everts K. L. (2001). Narrow row spacing does not affect lima bean yield or management of weeds and other pests. HortSci. 36, 884–888. doi: 10.21273/HORTSCI.36.5.884
Stoller E. W., Harrison S. K., Wax L. M., Regnier E. E., Nafziger E. D. (1987). Weed interference in soybeans (Glycine max). Rev. Weed Sci. 3, 155–181.
Urwin C. P., Wilson R. G., Mortensen D. A. (1996). Late season weed suppression from dry bean (Phaseolus vulgaris) cultivars. Weed Technol. 10, 699–704. doi: 10.1017/S0890037X00040677
USDA (2017) Delaware agricultural statistics 2017 and 2012 (Washington, DC: U.S. Department of Agriculture). Available online at: https://www.nass.usda.gov/Publications/AgCensus/2017/Full_Report/Volume_1,_Chapter_1_State_Level/Delaware/st10_1_0036_0036.pdf (Accessed August 7, 2023).
VanGessel M. J., Monks D. W., Johnson Q. R. (2002). Herbicides for potential use in lima bean (Phaseolus lunatus) production. Weed Technol. 14, 279–286. doi: 10.1614/0890-037X(2000)014[0279:HFPUIL]2.0.CO;2
Wootten T. L. (1994). The effects of heat stress on retention and abscission of lima bean reproductive structures. Newark, DE: University of Delaware. 197.
Wyenandt C. A., van Vuuren M. M. I. (2024) 2024/2025 Mid-Atlantic commercial vegetable production recommendations. Available online at: www.udel.edu/content/dam/udelImages/canr/pdfs/extension/sustainable-agriculture/commericial-veg-recommendations/2024-entirerecommendations.pdf (Accessed February 14, 2024).
Keywords: lima bean, Phaseolus lunatus, common cocklebur, Xanthium strumarium, jimsonweed, Datura stramonium, ivyleaf morningglory, Ipomoea hederacea, weed competition, weed seed production
Citation: Sankula S, Everts KL, Whalen JM and VanGessel MJ (2024) Influence of weed species and density on lima bean yield and other pests. Front. Agron. 6:1364232. doi: 10.3389/fagro.2024.1364232
Received: 01 January 2024; Accepted: 06 June 2024;
Published: 27 June 2024.
Edited by:
Ricardo Alcántara-de la Cruz, Universidade Federal de Viçosa, BrazilReviewed by:
Ahmet Uludag, Çanakkale Onsekiz Mart University, TürkiyeMuhammad Saqlain Zaheer, Khwaja Fareed University of Engineering and Information Technology (KFUEIT), Pakistan
Copyright © 2024 Sankula, Everts, Whalen and VanGessel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark J. VanGessel, bWp2QHVkZWwuZWR1
†Present address: Sujatha Sankula, Environmental Protection Agency, Office of Pesticide Programs, Washington DC, United States
‡First authorship
§These authors have contributed equally to this work
 Sujatha Sankula1†‡
Sujatha Sankula1†‡ Kathryne L. Everts
Kathryne L. Everts Mark J. VanGessel
Mark J. VanGessel