
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Agron., 12 December 2023
Sec. Weed Management
Volume 5 - 2023 | https://doi.org/10.3389/fagro.2023.1235196
This article is part of the Research TopicWeed Management in Specialty CropsView all 5 articles
Weed management in container plant production is a serious problem and remains one of the most expensive and time-consuming aspects of the industry. Weeds cause severe reductions in crop growth due to the limited growing environment characteristic of container plant production. The container nursery industry relies heavily on a limited number of preemergence herbicide options. The use of herbicides as the primary means to manage weeds has resulted in some negative consequences such as high chemical costs, leaching, runoff, and concerns with recycling irrigation water. Additionally, nursery growers are shifting their focus toward different nonchemical weed management strategies because many ornamental plants are very sensitive to preemergence herbicides. One such method is using organic mulch to control weeds in container plant production. Mulching is the foundation of a nonchemical weed management protocol and acts as the first line of defense against weeds. Organic mulches used in container plant production include rice hulls, pine bark, wood chips, wood shavings, coconut coir, nut (peanut, pecan) shells, oyster shells, cacao bean hulls, pelletized newspaper, recycled newspaper, pine straw, and other materials; material selection often depends on the availability of the product. The objective of this manuscript is to provide a comprehensive review of existing research on the utilization of various mulch materials as a weed management tool in container plant production. Additionally, it aims to highlight any critical knowledge gaps and provide suggestions for possible future research.
Across pest categories, weeds are often considered the most problematic due to competitive interactions for light, nutrients, water and space that can severely reduce the yield and marketability of crops. Because of the confined growing environment within containers, weeds have the potential to decrease the growth of ornamental crops by over 60%, subsequently extending the production time (Berchielli-Robertson et al., 1990; Fretz, 1973). Furthermore, weeds can provide habitat for insects and vertebrate pests, serve as alternate hosts for pathogens, and facilitate disease development by altering the physical environment. i.e. weeds do not provide habitat for disease, which is the abnormal condition caused by the pathogen. Even when weed competition is not a primary concern, consumers expect containers to be weed free (Simpson et al., 2002).
Unlike agronomic crops, the container nursery industry has relatively few herbicide options available to use in or around ornamental plants (Fennimore and Doohan, 2008). Container plant production relies heavily on preemergence (PRE) herbicides and supplemental hand weeding to manage weeds. While the use of herbicides presents an easy and effective method for weed control, heavy reliance on them has led to several adverse outcomes, including high chemical costs, potential leaching, runoff, and concerns regarding recycling irrigation water (Poudyal and Cregg, 2019; Wilson et al., 1995). Consumer concerns over the impact of herbicides on human health and the environment are also on the rise. Additionally, the container nursery industry produces thousands of different taxa ranging from succulents, herbaceous annuals, perennials, ornamental grasses, to tropical plants, many of which are highly sensitive to herbicides. Additionally, it has been observed that relying solely on hand weeding for control is cost-prohibitive, with expenses reaching up to $10,000 per hectare per year (Case et al., 2005).
Increased labor costs, environmental worries regarding chemical weed control and the emphasis on sustainability in container-grown crops have motivated numerous growers to adopt non-chemical practices and explore alternative methods of weed control. Various non-chemical methods have been developed to manage weeds including mulching (Chalker-Scott, 2007; Marble et al., 2015), weed discs (Appleton and French, 2000), sub-irrigation (Wilen et al., 1999), substrate stratification (Khamare et al., 2022a), fertilizer placement (Saha et al., 2019; Khamare et al., 2020) and other methods. However, the most widely adopted non-chemical method in recent years is mulching. While mulching has been frequently employed in horticultural crop production and landscaping, recent research has shown their ability to manage weeds in container plant production (Altland and Krause, 2014; Marble et al., 2019; Saha et al., 2020; Poudel and Witcher, 2022). While sanitation and preventive measures such as scouting, using clean soil and sanitized containers, and sourcing weed-free liner sources represent the initial steps in every integrated weed management plan, mulching functions as the primary line of defense against weeds. Numerous reviews have been published in the past decade focusing on weed control practices within container plant production. However, there has been no review dedicated to summarizing research on the use of mulches as a weed management tool, specifically with an emphasis on container plant production. The primary objective of this manuscript is to provide a review of all the research concerning the utilization of various mulch materials as weed management tools in container plant production. The secondary objective is to identify and highlight key knowledge gaps while offering suggestions for potential future research directions.
The word “mulch” is derived from the German word ‘molsch,’ which means soft or decaying matter. This reflects the natural form of mulch in a forest. Functionally mulch is defined as any material placed or applied in a thick layer, coating, or protective covering onto the soil’s surface (Crutchfield et al., 1986). Mulching offers numerous benefits, including minimizing soil erosion (Chalker-Scott, 2007), improving soil moisture retention (Li et al., 2020), regulating soil temperature (Long et al., 2001; Cook et al., 2006; Kader et al., 2019), increasing soil organic matter (Tindall et al., 1991; Duiker and Lal, 1999), promoting plant establishment and growth (Foshee et al., 1996; Cregg and Schutzki, 2009; Maggard et al., 2012a), supporting root development (Patten et al., 1988), providing food and shelter for earthworms (Pelosi et al., 2009), and stimulating microbial activity in the soil (Doran, 1980).
While all these benefits of mulching are notable, one of its most crucial applications in container plant production is weed suppression. The precise mechanism through which different types of mulch control weeds is not yet fully understood and varies depending on the specific mulch material and weed species (Chalker-Scott, 2007). However, research has shown that the main factors contributing to weed suppression include light exclusion (Teasdale and Mohler, 2000), decreasing available air and water for germinating weed seeds (Richardson et al., 2008), leaching of allelochemicals (Saha et al., 2018) and creating a physical barrier (Chalker-Scott, 2007).
The importance of light in the germination of many species is well-researched. Although not universal for all plant species, light responsiveness during germination is particularly significant for small-seeded species (Pons and Fenner, 2000), which are often present in container plant production. Moreover, seed germination necessitates imbibition (Woodstock, 1988). Mulch contributes to enhanced soil moisture retention (Chalker-Scott, 2007), while simultaneously reducing water availability on the mulch surface. The mulch layer generates a top layer that can rapidly dry out, depriving weed seeds of the moisture necessary for germination. This dual action significantly suppresses weed growth. Altland et al., 2016 reported that greater control over flexuous bittercress (Cardamine flexuosa With.) was achieved by placing the seeds on the surface of the mulch, as opposed to positioning them beneath the mulch layer. In another study, pine bark and pine straw mulch retained less water than hardwood mulch and resulted in greater control of garden spurge (Euphorbia hirta L.) and large crabgrass (Digitaria sanguinalis (L.) Scop.) compared to hardwood mulch when the seeds were placed on top of the mulch (Saha et al., 2020). Consequently, the position of the seed relative to the mulch layer dictates whether light exclusion or reduced moisture availability acts as a mechanism of weed control (Ngouajio and Ernest, 2004; Altland et al., 2016; Saha et al., 2020).
Mulches also create a physical barrier by forming a layer near the soil surface that reduces the ability of weed seedlings to photosynthesize (Crutchfield et al., 1986; Chalker-Scott, 2007). The depth of mulch is a critical factor influencing the creation of this physical barrier. Numerous researchers have demonstrated that applying mulch at depths ranging from 2.5 cm to 7.5 cm provides effective and long-term weed control (Penny and Neal, 2003; Richardson et al., 2008; Cochran et al., 2009). The depth of mulch serves a dual purpose, it acts as a physical barrier, preventing weed seedlings from developing roots, and it also blocks light, hindering weed seed germination. However, the effect of the physical barrier is temporary and diminishes as the mulch material starts to degrade (Marble et al., 2015).
Various mulch materials can also suppress weeds through the process of allelopathy. Allelopathy is defined as the direct or indirect effect of plants on neighboring plants through the production of allelochemicals that interfere with their growth (IAS, 2018). Allelochemicals are the secondary metabolites or byproducts of the principal metabolic pathways in plants. These secondary metabolites are non-nutritional and be released through plant parts by leaching from leaves or litter on the ground, root exudation, volatilization from leaves, residue decomposition, and other processes in the natural and agricultural systems (Rice, 1984; Anaya et al., 1990). These allelochemicals can hinder the germination, growth, and establishment of nearby plants. Several studies have established that leachates released from the wood chip mulch of certain allelopathic species can inhibit weed seed germination and seedling growth (Rathinasabapathi et al., 2005). Duryea et al., 1999, compared the chemical, allelopathic, and decomposition characteristics of six mulches that included cypress (Taxodium distichum (L.) Rich.), eucalyptus (Eucalyptus grandis W.Hill), pine bark (Pinus elliottii Engelm.) pine needle, melaleuca (Melaleuca Quinquenervi (Cav.) S.T.Blake) and a utility trimming mulch (pruning from oaks (Quercus laurifolia Michx. and Quercus virginiana Mill) and cherry (Primus serotina Ehrh.), with a small amount of cedar and pine (Juniperus silicicola (Small) Bailey) and southern pines (Pinus spp.). The study reported that eucalyptus and utility trimming mulch had the highest decomposition rates and water extracts from all mulch materials inhibited germination of lettuce seeds. All mulch materials contained hydroxylated aromatic compounds that might have an allelopathic effect on lettuce seeds.
In terms of allelopathy, phenolic compounds encompass a range of substances, including simple aromatic phenols, hydroxy- and substituted benzoic acids and aldehydes, hydroxy- and substituted cinnamic acids, coumarins, tannins, and potentially a select few flavonoids (Zeng et al., 2008). These phenolic compounds have demonstrated to inhibit various plant root elongation, cell division, and reduce the growth and development of the plant. For example, coumarins have been found to significantly reduce the root elongation of lettuce (Li et al., 1993). Similarly, certain phenolic acids such as caffeic acid, coumaric acid, ferulic acid, cinnamic acid, and vanillic acid have been reported to inhibit the photosynthesis and chlorophyll content of soybean (Patterson, 1981). A comprehensive exploration of allelopathy and its practical applications can be found in a recent review (Khamare et al., 2022a).
The ideal mulch materials for container plant production should have several key characteristics. These include having minimal available nutrients, quick drying despite frequent irrigation, being easy to apply, demonstrating slow decomposition rates, being non-toxic to both humans and crops, and possessing aesthetic appeal for customers. In the United States, the most frequently utilized mulch materials in container plant production include pine bark (or other types of bark), rice hulls, and wood chips. (Figure 1). Common mulch materials used in container plant production consist of rice hulls, pine bark, wood chips, wood shavings, coconut coir, nut (peanut, pecan) shells, oyster shells, cacao bean hulls, pelletized newspaper, recycled wastepaper, pine straw, and other materials (Sibley et al., 2004; Ferguson et al., 2008; Richardson et al., 2008; Maggard et al., 2012b; Marble et al., 2015; Altland et al., 2016; Bartley et al., 2017; Marble et al., 2019; Somireddy, 2011; Wilen et al., 1999; Table 1). Although there exists a wide range of mulch materials, comprehensive research has been conducted on only a limited selection of these materials. Below, we present an overview of mulch materials that have been extensively studied and thoroughly investigated, with a primary focus on container plant production.

Figure 1 Examples of commonly used mulch material in container plant production consisting of (A) Crape myrtle (Lagerstroemia indica L.) mulched with pine bark, (B) Crape myrtle mulched with rice hulls, (C) Podocarpus (Podocarpus macrophyllum var. maki) mulched with shredded hardwood and (D) Podocarpus mulched with sawdust.
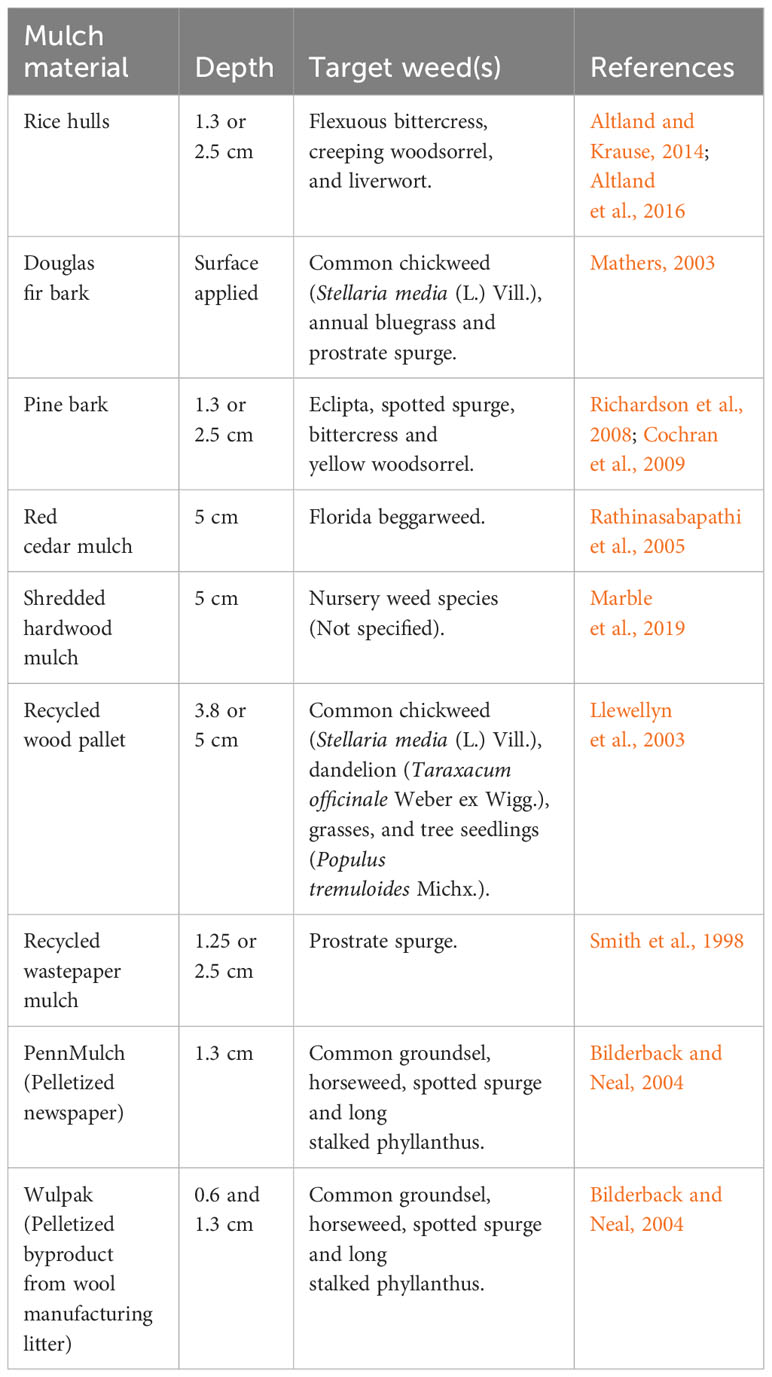
Table 1 Examples of effective mulch materials and required depths previously evaluated for use in container plant production.
Rice (Oryza sativa L.) hulls are a popular mulch material, mostly used in greenhouses and container plant production. It is a byproduct of the rice milling process and can be found at a reasonable price in large quantities from rice-producing states that include California, Louisiana, Mississippi, Arkansas, and Texas in the United States (Snyder and Slaton, 2001). Rice hulls are known to be hydrophobic; it has been suggested that their inability to retain water is a primary mechanism by which they suppress weeds (Altland et al., 2016). The germination and seedling growth of barnyardgrass (Echinochloa crus-galli (L.) P.Beauv.) in response to rice hull extracts from 91 rice cultivars was evaluated in a petri dish study (Ahn and Chung, 2000). The research indicated that there was significant variability observed among cultivars with ‘Jaganbyeo; exhibiting strong allelopathic characteristics. Seedling germination and dry weight were reduced 39% and 96%, respectively. This allelopathic ability of rice has been well-researched in both field and laboratory studies (Dilday et al., 1989; Olofsdotter et al., 1999). The most potent allelopathic compound present in rice is momilactone B (Kato-Noguchi and Ino, 2003). Interestingly, momilactone B was first isolated from rice hulls as a growth inhibitor to study the dormancy of rice seeds (Takahashi et al., 1976).
Altland and Krause, 2014 reported a nearly 100% control of flexuous bittercress and liverwort (Marchantia polymorpha L.) with either 1.3 or 2.5 cm depth of rice hulls. In a similar study, the emergence and growth of bittercress and creeping woodsorrel (Oxalis corniculate L.) was reduced with rice hulls mulch at a depth of 1.3 and 2.5 cm (Altland et al., 2016). However, in the same study better control of flexuous bittercress was observed when the seeds were placed on top of the mulch compared to the seeds placed under the mulch. In both of the above-mentioned studies, the authors observed that the key mechanism leading to weed suppression was attributed to the rice hull’s capacity to retain minimal water. Poudel and Witcher, 2022, reported that rice hull mulch at depths of 1.27 and 2.54 cm reduced the germination and shoot dry weight of creeping woodsorrel by more than 46% and 90% respectively.
Bark is a popular and regularly used material for mulch and as a part of the substrate in container plant production (Samtani et al., 2007). Bark is a byproduct of the timber industry, stripped from the logs after harvest. Subsequently, the bark undergoes a process of hammer milling, screening, and aging to achieve the desired particle size. The aging process reduces the likelihood of phytotoxicity caused by fresh bark. Douglas fir (Pseudotsuga menziesii (Mirbel) Franco) bark is commonly utilized in the Pacific Northwest, whereas Pine bark (Pinus spp.) is readily available in the southeast USA (Buamscha et al., 2007).
Pine bark nuggets come in a particle size range of 2.5 cm to 10 cm, often categorized as mini nuggets, standard-size nuggets, and jumbo nuggets. Pine bark and other tree derived mulch materials often have comparatively large particle sizes, and low fertility with hydrophobic properties resulting in a habitat that is not favorable for weed germination and growth (Richardson et al., 2008). Generally, larger particle sizes offer enhanced weed control by significantly reducing light penetration and facilitating faster drying, as opposed to smaller particle-sized pine bark (≤1.2 cm). On the other hand, fine-textured pine bark or other nutrient-dense materials like compost are not suitable for weed control and may even serve as a source of weed seeds, leading to increased weed germination (Chalker-Scott, 2007). Fine-textured pine bark has the capacity to retain larger amounts of water, as reducing particle size leads to smaller but more numerous pores (Handreck and Black, 2002). Pine bark mini nuggets mulch applied at a depth of 2.5 cm decreased the weed counts of eclipta (Eclipta alba (L.) L.) and spotted spurge (Euphorbia maculata L.) by 87% and 90%, respectively, compared to the non-mulched control (Cochran et al., 2009). In another study, pine bark mini nuggets applied at a depth of 7.6 cm in large containers (26 L) provided a season-long weed control of bittercress (Cardamine hirsuta L.) and yellow woodsorrel (Oxalis stricta L.) (Richardson et al., 2008). Pine bark mulch at a depth of ≥ 3.7 cm or weed seeds placed at a depth of ≥1.8 cm reduced the mulberry weed (Fatoua villosa (Thunberg) Nakai) emergence by 90% (Penny and Neal, 2003). A container study evaluated four organic mulches consisting of screened pine bark (0.6-0.9 cm), hardwood largely from oak (0.6-7.5 cm), cypress (0.6-7.5 cm), and decorative pine bark nuggets (7.5-10 cm) and concluded that decorative pine bark nuggets at a shallower depth (10 to 15 cm) provided the optimal control of purple nutsedge (Cyperus rotundus L.), crabgrass, johnsongrass (Sorghum halepense (L.) Pers.), and velvetleaf (Abutilon theophrasti Medic.) (Billeaud and Zajicek, 1989). Bartley et al., 2017, evaluated pine bark mini nuggets and mulch derived from readily available tree species including eastern red cedar (Juniperus virginiana L.), ground whole loblolly pine (Pinus taeda A.E.Murray), Chinese privet (Ligustrum sinense Lour.), and sweetgum (Liquidambar styraciflua L.) at different depths, with and without herbicide. At 30 days after treatment, mulch applied alone and with herbicide (dimethenamid-p) reduced weed fresh weight 82% to 100%; at 168 days after treatment, mulch depth, alone, was a significant factor in weed suppression as the herbicide treatment had broken down. The study also concluded that any of these tree-derived mulches applied at a depth of 5 cm can provide effective long-term weed control in container plant production.
In some cases, wood mulch, derived from various hardwood and softwood species, is used as a mulch material in container plant production. Shredded or chipped wood chips are organic materials that are cheap, renewable, convenient, and locally available. Unlike bark mulch, wood chips tend to decompose at a faster rate, often requiring reapplication on a seasonal basis (Duryea et al., 1999). Rathinasabapathi et al., 2005, evaluated the allelopathic potential of wood chips from red maple (Acer rubrum L.), swamp chestnut oak (Quercus michauxii Nutt.), red cedar (Juniperus silici- cola (Small) Bailey), neem (Azadirachta indica A. Juss.), and magnolia (Magnolia grandiflora L.). Water extracts from all of the wood chip treatments were able to inhibit the germination of lettuce seeds. The same study also reported that red cedar mulch inhibited the growth of Florida beggarweed (Desmodium tortuosum (Sw.) DC.) compared to the gravel-mulch and non-mulch containers and attributed it to its allelopathic potential. Marble et al., 2019 reported that shredded hardwood mulch derived from melaleuca trees provided similar weed control and reduction in weeding timing compared to pine bark, paper slurry with pine bark, and plastic mulch (Figure 1). Melaleuca is classified as a federal noxious weed in the United States and using the mulch derived from melaleuca trees can offset the control cost of the trees, remove the invasive plant, and reduce the transportation cost (Serbesoff-King, 2003).
Mulch derived from fresh and composted sugar gum eucalyptus (Eucalyptus cladocalyx F. Muell.), applied to a depth of 10 cm around California sycamore (Platanus racemose Nutt.), was effective in reducing the growth of annual weeds, similar to pine bark. Additionally, it improved soil moisture retention and increased the stem diameter of the tree (Downer and Faber, 2005). The allelopathic potential of eucalyptus species has been widely studied (Bajwa and Nazi, 2005; El-Khawas and Shehata, 2005). The major allelochemical present in the eucalyptus is 1,8-cineol which has been shown to decrease germination, root growth and inhibit mitosis (Baum et al., 1998; Romagni et al., 2000). In a greenhouse study, the leachates from fresh leaves of bluegum eucalyptus (Eucalyptus globulus Labill.) at a concentration of 20% (w/v) and 40% (w/v) reduced the resprouting of purple nutsedge by 57%-68% and bermudagrass (Cynodon dactylon (L.) Pers.) by 82%-89% (Babu and Kandasamy, 1997). Shredded hardwood bark derived from oak in a landscape study was able to suppress weeds almost as effectively as opaque synthetic mulches consisting of black polyethylene, woven polypropylene, and heavy-duty green plastic (Ashworth and Harrison, 1983).
The conventional mulch materials mentioned above are widely used, but several unconventional mulches have been evaluated in landscape and nursery settings, but not extensively in container production. There have been various unconventional materials tested as potential mulch that range from shredded paper, cardboard, corn cobs, spent hops, buckwheat hulls, fiberglass mat, sawdust, cocoa bean hulls, walnut hulls, coconut coir, crushed tires or shredded rubber, yard waste, gravel, and others. Pellet and Heleba, 1995 reported that chopped newspaper mulch applied in nursery crop rows at a depth of 10 cm and 15 cm was able to reduce weed germination for two years and also suppress weed growth. In a field experiment, chopped newspaper mulch at a depth of 7.6 cm was able to control weeds consistently compared to wheat straw, shredded newspaper, black plastic, and plastic landscape fabric (Monks et al., 1997). A study designed to compare shredded newspaper and wheat straw as crop mulch concluded that shredded newspaper was effective in suppressing most annual and some perennial weed species evaluated (Munn, 1992). Smith et al., 1998 evaluated recycle wastepaper crumble and pellets as a non-chemical alternative for weed control in container production (Table 1). Mulching with recycled paper pellets at a depth of 2.5 cm proved effective in reducing the germination of spotted spurge. The authors stated that the paper pellets were able to absorb more water causing the pellets to swell, forming a thick mat with a smooth surface that suppressed weed seed germination. In a landscape experiment, recycled paper crumble and recycled paper pellets provided effective weed control (90% and above) and, in some cases, in some cases, recycled paper crumble and pellets provided weed suppression similar to oxadiazon (Smith et al., 1997). Other products such as PennMulch (Penn State University, State College, PA, USA) which is comprised of pelletized newspaper with 1% nitrogen added, and Wulpak (Wilbro Inc., Norway, SC, USA) which consists of pelletized sweepings from the shearing floors of sheep operation with 5N-0.44P-2.64K added has shown good control of weeds (Wooten and Neal, 2000). Bilderback and Neal, 2004 reported that Wulpak was able to suppress common groundsel (Senecio vulgaris L.), horseweed (Conyza canadensis L.), spotted spurge, and long stalked phyllanthus (Phyllanthus tenellus Roxb.) at mulch depths of 0.6 and 1.3 cm, similar results were observed with PennMulch applied at a depth of 1.3 cm.
Unconventional inorganic materials such as recycled rubber chips, gravel, sand, and polyethylene fabrics have been used in several landscape studies (Martin et al., 1987; Calkins et al., 1996; Hanim et al., 2014; Winkel et al., 1996). However, there is a lack of research on the utilization of these materials as mulch in container production, possibly due to environmental and safety concerns. For instance, the use of recycled rubber mulch has been shown to leach high levels of heavy metals such as zinc, lead, and cadmium and organic chemicals such as benzothiazoles (Kumata et al., 2002; Kanematsu et al., 2009; Crampton et al., 2014; Mohajerani et al., 2022). Several studies have shown that rubber-modified materials have a higher potential of leaching when exposed to seasonal rainwater (Aoki, 2008; Bocca et al., 2009). Consequently, rubber mulches are not ideal for container systems due to daily irrigation requirements. Materials such as polyethylene fabrics or woven polypropylene have higher installation costs, require more time, and need to be replaced once the fabric degrades (Marble et al., 2015).
A modification of mulching is the use of weed discs that can be made from paper, jute, black polyethylene, fiberglass, wool, coco coir, various fabrics, biodegradable plastic, etc, (Appleton and Derr, 1990; Appleton and French, 2000; Chong, 2003). A container disk is circular with a diameter that matches the surface of the container to block light and act as a physical barrier. The disc has a single cut to allow the insertion of the disc around the stem of the container-grown crop (Figure 2). Optimal weed discs should possess certain characteristics, including ease of application, a snug fit atop the container, resistance to wind and water displacement, water permeability, durability, slow decomposition, cost-effectiveness, and availability in various sizes (Chong, 2003). Appleton and Derr, 1990 tested discs made from heavy brown wrapping paper, compressed peat moss paper, black polyethylene, fiberglass, eight geotextiles (white spunbonded, white spunbonded sprayed with yellow, red, blue, and black enamel paint, gray spunbonded, black woven type, and a white woven) and disc from bio-barrier geotextile with preemergence herbicide combination (Biobarrier, Reemay, Old Hickory, Tenn, USA). The study reported that the most consistent weed control was obtained with the bio-barrier geotextile with a preemergence herbicide combination (trifluralin). Most of the failure of the geotextile to control weeds was due to the lack of adequate fit on the surface of the container resulting in weeds growing on the edges or center cut of the disc. In another study, Tex-R Geodisc (Texel USA, Henderson, NC, USA), a nonwoven polypropylene disc coated with copper was able to keep the container weed free for 6 months (Appleton and French, 2000). Weed discs made from different materials such as PolyVulc made from crumb rubber disc (Tatum et al., 1999), Corrudisc made from pressed peat or cardboard (Chong and Purvis, 2000), coco-discs made from the byproduct of coconut fiber (Cocos nucifera L.) (Altland and Lanthier, 2007; Frangi et al., 2010) have provided acceptable weed control in container production. Llewellyn et al., 2003 evaluated the efficacy of recycled wood pallets, econo mulch (Econo), shredded pine mulch, and coco disc. The authors reported that the coco disc performed the best to reduce weed germination and growth, followed by shredded pine mulch. Researchers have also explored other unconventional forms of weed discs, such as the Mori weed bag (Mori Nurseries, Niagara on the Lake, Ontario, Canada), which comprises of polyethylene sleeves with punched holes, and Enviro LIDs (Enviro LID, Langley, British Columbia, Canada), consisting of plastic lids that fit over the container with watering holes (Mathers, 2003).

Figure 2 Crape myrtle covered with weed disc made up of recycled fiber cloth in container plant production.
In recent years, with the success of plastic mulch in field weed management programs, researchers are developing biodegradable mulching sprays suitable for container plant production. Biodegradable mulching films can be created using natural or synthetic polymers like starch, polylactic acid, or polyvinyl acid (Vox et al., 2013). On the other hand, biodegradable mulching sprays can be formulated using water-based solutions of natural polysaccharides such as sodium alginate, galactomannan, chitosan, and cellulose (Schettini et al., 2007; Immirzi et al., 2009; Massa et al., 2019). While this is a new area of research, there have been promising results.
A spray-on mulch slurry consisting of a byproduct of the newsprint recycling industry (Newstech Recycling Partners, Burnaby, BC, Canada) provided superior weed control in a commercial orchard in comparison with glyphosate treatment (Cline et al., 2011). The same study also reported that weed suppression was even better in the treatment with spray-on mulch was applied on top of compost, paper, and cloth barriers. An innovative biodegradable mulch was created by spraying water-based solution of sodium alginate in a field study that had the mulching effect for 6 months (Immirzi et al., 2009). The durability and life of the biodegradable mulching sprays highly depends on the growing environment. For example, Mater-Bi (Novamont, Shelton, CT, USA), a bioplastic made from starch, cellulose, and vegetable oil, exhibited a durability of 2 to 5 months when used in open-air cultivation. In a low tunnel cultivation environment, it maintained its effectiveness for up to 9 months (Morra et al., 2016).
Film forming, natural materials can also play a crucial role in addressing the challenges associated with spray mulches. Chitosan is the second most abundant polysaccharide present in nature and has a film-forming capacity. Giaccone et al., 2018, replaced alginate with chitosan to use as a mulching spray in a container study. The study concluded that chitosan-based spray mulch performed better than the herbicide; oxadiazon at 2% (Ronstar® Bayer Crop Science, Monheim, Germany), but the mulch started to degrade after three months of its application. A bio-based liquid mulch material comprising corn, potato, wheat, and cellulose (Advanced Micro Polymers Inc, Milton, Ontario, Canada) was tested for its efficacy on weed suppression in a container production setting (Shen and Zheng, 2017). While the liquid mulch successfully reduced both weed quantity and fresh weight, a significant issue arose as the liquid mulch dried and shrunk within a few days following its application. This contraction led to a space forming between the container’s edge and the mulch, which subsequently allowed weeds to sprout within this gap. In a container study, recycled wastepaper mulch slurry mixed with tackifier or soil glue (Granite Seed, North Lehi, UT, USA) reduced weeding time compared to the non-treated control and reduced the weed weight for the first four months compared to the control (Marble et al., 2019). A similar problem mentioned above by Shen and Zheng, 2017 was also reported in this study, the recycled paper slurry in the container began to shrink at 3 months, and with higher rainfall, it started to break down at a faster rate (Figure 3). In a study conducted by Massa et al., 2019, the efficacy of weed control was evaluated for a hydro-compacting organic mulch made from organic fibers sourced from Hibiscus cannabinus L., combined with a polyvinyl alcohol-based adhesive. This mulch type was compared against alternative organic mulches and herbicide treatments. The findings indicated that the hydro-compacting mulch exhibited a similar reduction in weed growth when compared to the effects of the herbicide treatment.
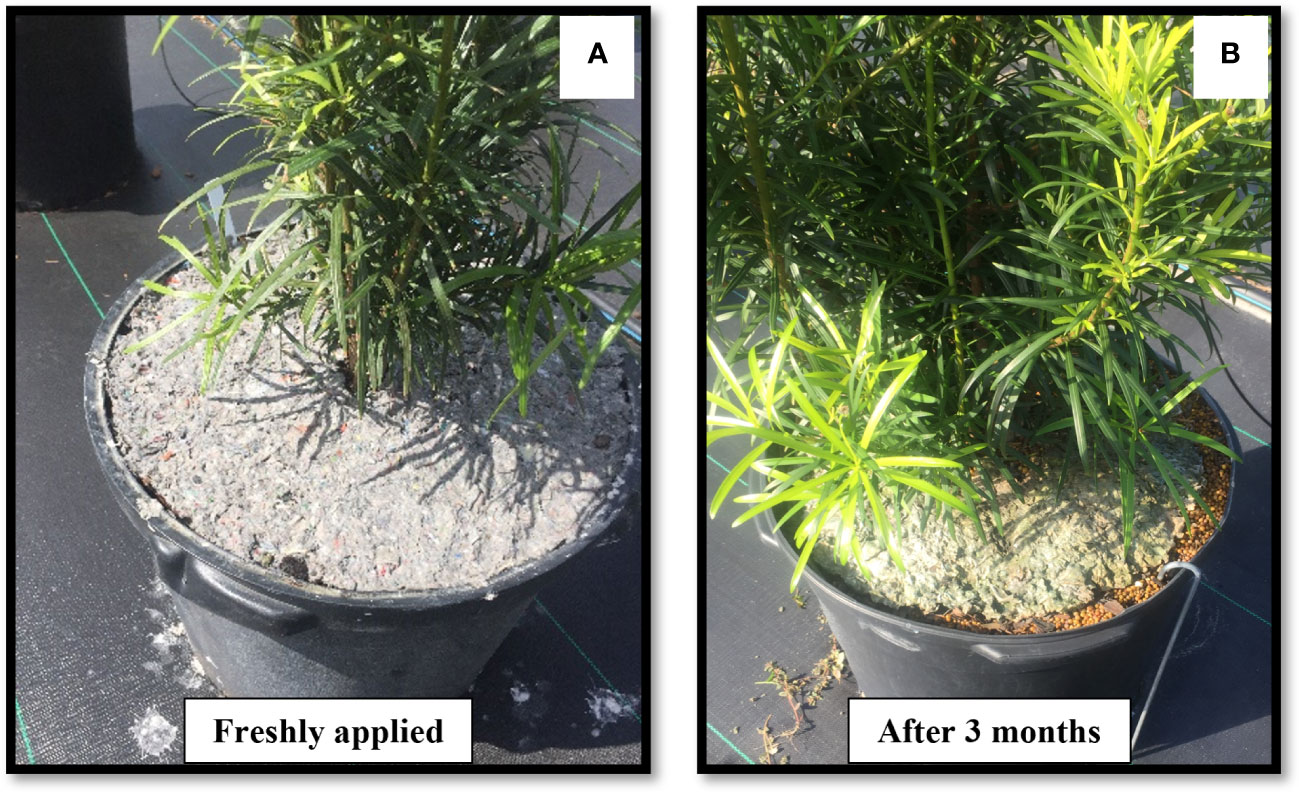
Figure 3 An example of (A) recycled wastepaper slurry mulch applied fresh and (B) after 3 months in a container grown plant.
This efficiency of herbicides in container production can be compromised by factors such improper application rates, incorrect application timing, improper selection of herbicides, and non-target losses due to container spacing (Gilliam et al., 1992; Altland et al., 2003). In container plant production, managing weeds effectively often involves employing preemergence herbicides and applying organic mulches, both of which are commonly utilized methods. In addition to providing a physical barrier for weed suppression, mulches used in combination with herbicides can reduce the amount of chemical applied, reduce herbicide leaching, and act as a slow-release carrier for the herbicides. Researchers have often observed herbicides applied in combination with mulch improve weed control. Case and Mathers, 2006 in a container study, compared herbicide-treated mulches with untreated mulches, over-the-top sprays of herbicides, and a combination spray of two herbicides. Four out of five herbicide-treated mulches in this study provided acceptable weed control at 115 days after treatment. Milled pine bark incorporated with chlorpropham, chlorpropham plus p-chlorophenyl-N-methylcarbamate (PPG-124), S-ethyl dipropylthiocarbamate (EPTC), N, N-dimethyl-2,2-diphenylacetamide (diphenamid) and dichlobenil at two different rates provided commercially acceptable control of broadleaf weed species in containers (Fretz, 1973). In another study, incorporating dichlobenil into several organic mulches resulted in equal or better weed control than dichlobenil or mulch alone (Lanphear, 1968). Mathers, 2003 reported that herbicide-treated bark provided a 1.8-fold increase in efficacy and 2.8-fold increase in the duration of efficacy compared to bark alone, whereas compared to herbicide alone, it provided a 1.5-fold increase in efficacy and 2.2-fold decrease in phytotoxicity. The study also compared the herbicide-treated bark to PennMulch (Penn State University, State College, PA, USA), wulpack (Wilbro Inc, Norway, SC, USA), Mori weed bag (Mori Nurseries, Niagara on the Lake, Ontario, Canada), and Enviro LIDs (Enviro LID, Langley, British Columbia, Canada) and stated that herbicide-treated bark provided the most promising results. Other research demonstrated that rice hulls, landscape leaf waste pellets, and pine bark act as carriers for diuron and oryzalin and provide long-term weed control of up to 120 days (Samtani et al., 2007). Research has shown that herbicide-treated mulches in nursery containers can be effective for 130 days (Mathers, 2003) and 310 days (Case, 2003), whereas in field applications, they can be effective for 1 year or longer (Mathers and Case, 2006). While herbicide-treated mulches are effective, there are several difficulties associated with them. Over-mulching of herbicide-treated mulches can cause higher or excessive application of herbicide, furthermore, they will be difficult to use in ornamental plants sensitive to herbicides (Derr, 1994). The nursery operation will also have to store herbicide-treated mulches like herbicides and users will not be able to use them without any protective equipment and proper licensing.
Research has also focused on innovative ways of applying mulch in containers to reduce weed growth and improve crop growth. One such area of research is in the form of substrate stratification and strategic fertilizer placement (Fields et al., 2021; Khamare et al., 2022b). Substrate stratification involves different textures of the same substrate or different substrates applied in layers in a container. To utilize substrate stratification as a weed control method, larger particles are applied as the top layer containing no fertilizer, and a fine textured, highly moisture-retentive substrate with fertilizer as the bottom layer. This method combines the benefits of mulching and strategic fertilizer placement into one method. The upper layer of the substrate lacks nutrients and sufficient moisture for weed seeds to establish, while the lower layer contains nutrients and greater water retention capacity beneficial for the intended crop. Substrate stratification has shown to reduce the growth of bittercress by 80% to 97%, spotted spurge by 14% to 55%, and liverwort 97% to 100% (Khamare et al., 2022b; Khamare et al., 2022c). Additionally, the stratified substrates had no impact on the growth or quality of two commonly produced nursery crops.
Mulching provides a range of advantages in container plant production; nonetheless, it is important to acknowledge that there are also several drawbacks. A significant characteristic of numerous organic mulches is their rapid degradation, leading to the need for frequent reapplication for longer-term nursery crops (i.e. trees, large shrubs, etc.). This results in higher material costs and increased demand for labor during the mulching process. Another potential drawback associated with placing mulch against the trunks of container-grown plants is the heightened risk of pest pressure (e.g. pathogens) if irrigation is not managed properly and the potential formation of girdling roots which has been noted in landscape evaluations. In a field study, the plots mulched with pine bark had a dense population of Diptera dominated by Asyndetus spp (Gill et al., 2011). Additionally, applying mulch at a depth of 10 cm or more can adversely affect plant growth due to reduced soil aeration and slower soil warming (Greenly and Rakow, 1995). However, in the context of container plant production, mulch is typically applied at a depth of 2.5 to 7.5 cm. It is important to note that concerns about potential damage from rodents, termites, and other insects associated with mulch have primarily been focused on landscape-grown plants and not within the context of commercial nursery production settings. Another disadvantage linked to mulch materials is the potential loss of material resulting from container blow-over or the susceptibility of lighter materials like rice hulls or sawdust to be carried away by strong winds or heavy rain.
The utilization of mulch materials composed of bark, sawdust, and wood chips from different allelopathic species can also lead to phytotoxicity in the desired crop. The chemical composition of the mulch material can result in nutritional deficiencies, salinity, or metabolic alterations (Ortega et al., 1996). The phytotoxicity of these materials varies depending on the species (Allison, 1965) and the presence of propagating mixture around the root system can reduce or eliminate this effect. The harmful impact of mulch materials has primarily been noticed in the case of young seedlings, plant cuttings, and bare root plants (Allison, 1965; Gruda et al., 2009). Research has shown that mulch materials composed of large portions of wood can result in a high rate of N immobilization (Pickering and Shepherd, 2000). This immobilization usually occurs due to the incorporation of high carbon, low nutrient materials where the nitrogen is extracted by microorganisms during the decomposition process. Nevertheless, the nitrogen rates commonly used in container plant production are sufficient to counteract this nitrogen immobilization (Buamscha et al., 2008). Methods such as aging the bark, composting wood chips, parboiling rice hulls, washing and incorporation of fertilizer eliminate these negative effects (Estaún et al., 1985; Ortega et al., 1996). Additionally, container nursery growers use plant liners with well-established root systems, and only very rarely would seeds be planted directly into a nursery container. These liners typically contain a certain amount of fertilizer and propagation mix within the root ball. This practice further minimizes any potential toxic impact of mulch materials on the intended crop. Research involving different types of bark and wood chips as components of substrates in numerous greenhouse and nursery experiments have indicated that crops can be grown with comparable quality to those grown in peat moss (Boyer et al., 2008; Fain et al., 2008; Jackson and Wright, 2009; Boyer et al., 2009; Khamare et al., 2022b; Khamare et al., 2022c).
In general, research indicates that organic mulches are a valuable addition to weed management programs. A remarkable reduction of 92% in weed growth was observed in containers that utilized mulch compared to those without mulch (Wilen et al., 1999). Research shows that incorporating organic mulch would likely provide benefits in every weed management program for container plant production (Billeaud and Zajicek, 1989; Chalker-Scott, 2007; Cochran et al., 2009; Altland et al., 2016; Bartley et al., 2017). The choice of mulch materials will significantly depend on their availability and cost based upon the region or area, and mulch that is available which can be obtained regularly and be of consistent quality. Several mulch materials, including pine bark, pine tree chips, wood chips, rice hulls, and newspaper, have been shown to have no negative impact on numerous ornamental plants (Lohr and Pearson-Mims, 2001; Richardson et al., 2008; Altland and Krause, 2014; Khamare et al., 2022c; Wilen et al., 1999). Depth of mulch can impact weed control success; research indicates that applying mulch at a depth of 2.5 to 5 cm to achieve efficient weed control while minimizing costs. For example, Bartley et al., 2017 found that applying pine bark nuggets at a 5 cm depth led to a 99.5% control of spotted spurge and eclipta. Furthermore, employing coarse mulches with larger particle sizes that are placed at a depth adequate to cover the container media surface would achieve optimal weed control. These larger particles block more light and facilitate quicker drying compared to smaller particles (Keddy and Constabel, 1986). Additionally, choosing a reputable and certified source of mulch is advisable to reduce the risk of weed contamination. Based upon available research, it is recommended to avoid the use of inorganic mulches like gravel, stones, rocks, sand, and rubber in container plant production. Rubber mulches have been linked to the leaching of heavy metals such as selenium, lead, and cadmium (Kanematsu et al., 2009). Additionally, rocks, gravel, and stones would make the containers heavy and impractical or cost-prohibitive for transport.
The mulch materials available for container plant production are limited. This limitation may be the result of material availability, aesthetic appeal to consumers, effects of crop growth and weed suppressive ability. Consequently, there is a need to investigate new mulch materials for nursery containers. Several potential mulch species can be evaluated for their use in container plant production. Black walnut (Juglans nigra L.) is one of the well-researched allelopathic species. The allelopathic compound present in black walnut is called juglone (Davis, 1928). There have been numerous studies demonstrating its allelopathic effect on vegetables, field crops, ornamental species, and various weed species (Topal et al., 2007; Shrestha, 2009; Strugstad and Despotovski, 2012). Many allelopathic invasive species are problematic to the local environment. Utilizing invasive species, such as the melaleuca mulch used in Florida, as mulch can effectively reduce the control cost of trees and contribute to decreasing the population of invasive species. Moreover, repurposing these invasive species into mulch not only controls costs but also provides an opportunity to generate value from the removal process. Tree of heaven (Ailanthus altissima (Mill.) Swingle) contains an allelochemical called ailanthone that has shown both pre- and postemergence activity (Heisey, 1996). In a container study, Heisey, 1990 reported oven dried root bark of the tree of heaven reduced the germination and growth of garden cress (Lepidium sativum L.). Comprehensive research has been performed on the effect of crop residues on controlling weeds in agricultural production. Crop residues from wheat (Triticum aestivum L.), rice, sorghum (Sorghum bicolor (L.) Moench), alfalfa (Medicago sativa L.), sunflower (Helianthus sp.), and corn (Zea mays L.) have demonstrated to control weed growth through allelopathy (Singh et al., 2003). The incorporation of sorghum stems, roots, and leaves in soil has been shown to decrease weed growth by 25-50% (Cheema et al., 2012). Khaliq et al., 2011 reported that the crop residue of brassica, sunflower, and sorghum reduced the growth of horse purslane (Trianthema portulacastrum L.) better than the sole application of the crop residues. Kenaf (Hibiscus cannabinus) has been shown to suppress weed growth similar to that of black polyethylene plastic mulch (Russo et al., 1997a). In another study, Russo et al., 1997b further demonstrated that extracts from kenaf plant material were able to suppress the germination of redroot pigweed (Amaranthus retroflexus L.), annual ryegrass (Lolium multiflorum Lam.), tomato, and cucumber. Many of the studies mentioned above are carried out in field conditions, and their findings can be applied to container plant production (Singh et al., 2003; Khaliq et al., 2011; Cheema et al., 2012).
The research for container plant production needs to focus on finding new mulch materials, innovative ways of mulch application, and combining mulching with other weed control methods. The agriculture byproducts used in field production can be further processed to use as mulch in nursery containers. The food processing industry produces large and rising amounts of waste each year (Virtanen et al., 2017). Similar to rice hulls, which have garnered significant use on a broad commercial scale, other food processing byproducts may hold potential to be mulch materials and would be inexpensive and widely available, at least in different regions.
Novel techniques for weed control using mulching could also be devised by observing approaches employed in studies related to organic agriculture. As an example, the concept of living mulch could be applied to container plant production. Living mulch comprised of plant species that could cover the container surface and not become overly competitive with the container-grown crop may hold potential. As an example, an annual grass species such as annual ryegrass could be seeded in large containers, provide quick cover, and then be killed by postemergence application of a graminicide, providing a short-term mulch for weed management. Another area which deserves further investigation is the economic aspects of utilizing different mulch types in container production. To date, no study has specifically addressed the cost or return on investment of utilizing different types of mulch in lieu of preemergence herbicides. Knowing how many preemergence herbicide applications can be eliminated with the use of different mulch materials applied at various depths is needed in order for more broad adoption by the industry.
The environmental concerns associated with herbicides, the cost of handweeding, and the availability of mulch material can be addressed by focusing research on the above-mentioned methods. These materials and innovative methods have great potential to improve weed management, reduce the use of herbicides, and overall improve the sustainability of the container plant production. Collaboration between researchers, nursery operators, local government, and manufacturers will be fundamental in creating these effective weed management methods.
YK conducted the literature searching, reviewed and wrote the manuscript with assistance of SM. All authors contributed to the article and approved the submitted version.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahn J. K., Chung I. M. (2000). Allelopathic potential of rice hulls on germination and seedling growth of barnyardgrass. Agron. J. 92 (6), 1162–1167. doi: 10.2134/agronj2000.9261162x
Allison F. E. (1965). Decomposition of wood and bark sawdusts in soil, nitrogen requirements and effects on plants (No. 1332) (US Department of Agriculture: Agricultural Research Service).
Altland J. E., Boldt J. K., Krause C. C. (2016). Rice hull mulch affects germination of bittercress and creeping woodsorrel in container plant culture. Am. J. Plant Sci. 7 (16), 2359–2375. doi: 10.4236/ajps.2016.716207
Altland J. E., Gilliam C. H., Wehtje G. (2003). Weed control in field nurseries. HortTechnology 13 (1), 9–14. doi: 10.21273/HORTTECH.13.1.0009
Altland J., Krause C. (2014). Parboiled rice hull mulch in containers reduces liverwort and flexuous bittercress growth. J. Environ. Horticulture 32 (2), 59–63. doi: 10.24266/0738-2898.32.2.59
Altland J., Lanthier M. (2007). Influence of container mulches on irrigation and nutrient management. J. Environ. Horticulture 25 (4), 234–238. doi: 10.24266/0738-2898-25.4.234
Anaya A., Calera M., Mata R., Pereda-Miranda R. (1990). Allelopathic potential of compounds isolated from Ipomoea tricolor cav. (Convolvulaceae). J. Of Chem. Ecol. 16 (7), 2145–2152. doi: 10.1007/bf01026926
Aoki T. (2008). Leaching of heavy metals from infills on artificial turf by using acid solution. Football Sci. 5, 51–53. doi: 10.57547/jssfenfs.5.1_51
Appleton B. L., Derr J. F. (1990). Use of geotextile disks for container weed control. HortScience 25 (6), 666–668. doi: 10.21273/HORTSCI.25.6.666
Appleton B. L., French S. C. (2000). Weed suppression for container-grown willow oak using copper-treated fabric disks. HortTechnology 10 (1), 204–206. doi: 10.21273/HORTTECH.10.1.204
Ashworth S., Harrison H. (1983). Evaluation of mulches for use in the home garden. HortScience 18 (2), 180–182. doi: 10.21273/HORTSCI.18.2.180
Babu R. C., Kandasamy O. S. (1997). Allelopathic effect of Eucalyptus globulus Labill. on Cyperus rotundus L. and Cynodon dactylon L. Pers. J. Agron. Crop Sci. 179 (2), 123–126. doi: 10.1111/j.1439-037X.1997.tb00507.x
Bajwa R., Nazi I. (2005). Allelopathic effects of Eucalyptus citriodora on growth, nodulation and AM colonization of Vigna radiata (L) Wilczek. Allelopathy J. 15 (2), 237–246.
Bartley P. C., Wehtje G. R., Murphy A. M., Foshee W. G., Gilliam C. H. (2017). Mulch type and depth influences control of three major weed species in nursery container production. HortTechnology 27 (4), 465–471. doi: 10.21273/HORTTECH03511-16
Baum S. F., Karanastasis L., Rost T. L. (1998). Morphogenetic effect of the herbicide Cinch on Arabidopsis thaliana root development. J. Plant Growth Regul. 17, 107–114. doi: 10.1007/PL00007015
Berchielli-Robertson D. L., Gilliam C. H., Fare D. C. (1990). Competitive effects of weeds on the growth of container-grown plants. HortScience. 25 (1), 77–79. doi: 10.21273/HORTSCI.25.1.77
Bilderback T. E., Neal J. C. (2004). “Wulpak used as a mulch or an amendment for nursery potting substrates,” in International Symposium on Growing Media and Hydroponics, Vol. 644. 139–143. doi: 10.17660/ActaHortic.2004.644.16
Billeaud L. A., Zajicek J. M. (1989). Influence of mulches on weed control, soil pH, soil nitrogen content, and growth of Ligustrum japonicum. J. Environ. Horticulture 7 (4), 155–157. doi: 10.24266/0738-2898-7.4.155
Bocca B., Forte G., Petrucci F., Costantini S., Izzo P. (2009). Metals contained and leached from rubber granulates used in synthetic turf areas. Sci. total Environ. 407 (7), 2183–2190. doi: 10.1016/j.scitotenv.2008.12.026
Boyer C. R., Fain G. B., Gilliam C. H., Gallagher T. V., Torbert H. A., Sibley J. L. (2008). Clean chip residual: A substrate component for growing annuals. HortTechnology 18 (3), 423–432. doi: 10.21273/HORTTECH.18.3.423
Boyer C. R., Gilliam C. H., Fain G. B., Gallagher T. V., Allen Torbert H., Sibley J. L. (2009). Production of woody nursery crops in clean chip residual substrate. J. Environ. Horticulture 27 (1), 56–62. doi: 10.24266/0738-2898-27.1.56
Buamscha M. G., Altland J. E., Sullivan D. M., Horneck D. A. (2007). Micronutrient availability in fresh and aged Douglas fir bark. HortScience 42 (1), 152–156. doi: 10.21273/HORTSCI.42.1.152
Buamscha M. G., Altland J. E., Sullivan D. M., Horneck D. A., McQueen J. P. (2008). Nitrogen availability in fresh and aged douglas fir bark. HortTechnology 18 (4), 619–623. doi: 10.21273/HORTTECH.18.4.619
Calkins J. B., Swanson B. T., Newman D. L. (1996). Weed control strategies for field grown herbaceous perennials. J. Environ. Horticulture 14 (4), 221–227. doi: 10.24266/0738-2898-14.4.221
Case L. T. (2003). Herbicide-treated mulches for ornamental weed control (Doctoral dissertation (Columbus, Ohio, USA: The Ohio State University). Available at: http://rave.ohiolink.edu/etdc/view?acc_num=osu1399623802.
Case L. T., Mathers H. M. (2006). Herbicide-treated mulches for weed control in nursery container crops. J. Environ. Horticulture 24 (2), 84–90. doi: 10.24266/0738-2898-24.2.84
Case L. T., Mathers H. M., Senesac A. F. (2005). A review of weed control practices in container nurseries. HortTechnology 15 (3), 535–545. doi: 10.21273/HORTTECH.15.3.0535
Chalker-Scott L. (2007). Impact of mulches on landscape plants and the environment—A review. J. Environ. Horticulture 25 (4), 239–249. doi: 10.24266/0738-2898-25.4.239
Cheema Z. A., Farooq M., Wahid A. (2012). Allelopathy: current trends and future applications (New York, USA: Springer Science & Business Media).
Chong C. (2003). Experiences with weed discs and other nonchemical alternatives for container weed control. HortTechnology 13 (1), 23–27. doi: 10.21273/HORTTECH.13.1.0023
Chong C., Purvis P. (2000). Searching for nonchemical alternatives to container weed control. Landscape Trades 22 (1), 48.
Cline J., Neilsen G., Hogue E., Kuchta S., Neilsen D. (2011). Spray-on-mulch technology for intensively grown irrigated apple orchards: Influence on tree establishment, early yields, and soil physical properties. HortTechnology 21 (4), 398–411. doi: 10.21273/HORTTECH.21.4.398
Cochran D. R., Gilliam C. H., Eakes D. J., Wehtje G. R., Knight P. R., Olive J. (2009). Mulch depth affects weed germination. J. Environ. Horticulture 27 (2), 85–90. doi: 10.24266/0738-2898-27.2.85
Cook H. F., Valdes G. S., Lee H. C. (2006). Mulch effects on rainfall interception, soil physical characteristics and temperature under Zea mays L. Soil tillage Res. 91 (1-2), 227–235. doi: 10.1016/j.still.2005.12.007
Crampton M., Ryan A., Eckert C., Baker K. H., Herson D. S. (2014). Effects of leachate from crumb rubber and zinc in green roofs on the survival, growth, and resistance characteristics of Salmonella enterica subsp. enterica serovar Typhimurium. Appl. Environ. Microbiol. 80 (9), 2804–2810. doi: 10.1128/AEM.03565-13
Cregg B. M., Schutzki R. (2009). Weed control and organic mulches affect physiology and growth of landscape shrubs. HortScience 44 (5), 1419–1424. doi: 10.21273/HORTSCI.44.5.1419
Crutchfield D. A., Wicks G. A., Burnside O. C. (1986). Effect of winter wheat (Titicum aestivum) straw mulch level on weed control. Weed Sci. 34, 110–114. doi: 10.1017/S0043174500026564
Davis E. F. (1928). The toxic principle of Juglans nigra as identified with synthetic juglone and its toxic effects on tomato and alfalfa plants. Amer. J. Bot. 15, 620.
Derr J. F. (1994). Innovative herbicide application methods and their potential for use in the nursery and landscape industries. HortTechnology 4 (4), 345–350. doi: 10.21273/HORTTECH.4.4.345
Dilday R. H., Nastasi P., Smith R. J. Jr. (1989). Allelopathic observations in rice (Oryza sativa L.) to ducksalad (Heteranthera limosa). J. Arkansas Acad. Sci. 43 (1), 21–22. Available at: https://scholarworks.uark.edu/jaas/vol43/iss1/7.
Doran J. W. (1980). Microbial changes associated with residue management with reduced tillage. Soil Sci. Soc. America J. 44 (3), 518–524. doi: 10.2136/sssaj1980.03615995004400030016x
Downer A. J., Faber D. (2005). Effect of Eucalyptus cladocalyx mulch on establishment of California sycamore (Platanus racemosa). Journal of Applied Horticulture 7 (2), 90–94. Available at: https://www.horticultureresearch.net/jah/2005_7_2_90_94.PDF
Duiker S. W., Lal R. (1999). Crop residue and tillage effects on carbon sequestration in a Luvisol in central Ohio. Soil Tillage Res. 52 (1-2), 73–81. doi: 10.1016/S0167-1987(99)00059-8
Duryea M. L., English R. J., Hermansen L. A. (1999). A comparison of landscape mulches: chemical, allelopathic, and decomposition properties. J. Arboriculture 25, 88–97. doi: 10.48044/jauf.1999.014
El-Khawas S. A., Shehata M. M. (2005). The allelopathic potentialities of Acacia nilotica and Eucalyptus rostrata on monocot (Zea mays L.) and dicot (Phaseolus vulgaris L.) plants. Biotechnology 4 (1), 23–34.
Estaún V., Calvet C., Pagés M., Grases J. M. (1985). Chemical determination of fatty acids, organic acids and phenols, during olive marc composting process. Composts as Hortic. Substrates 172, 263–270. doi: 10.17660/ActaHortic.1985.172.29
Fain G. B., Gilliam C. H., Sibley J. L., Boyer C. R., Witcher A. L. (2008). Wholetree substrate and fertilizer rate in production of greenhouse-grown petunia (Petunia× hybrida Vilm.) and marigold (Tagetes patula L.). HortScience 43 (3), 700–705. doi: 10.21273/HORTSCI.43.3.700
Fennimore S. A., Doohan D. J. (2008). The challenges of specialty crop weed control, future directions. Weed Technology 22 (2), 364–372. doi: 10.1614/WT-07-102.1
Ferguson J., Rathinasabapathi B., Warren C. (2008). Southern redcedar and southern magnolia wood chip mulches for weed suppression in containerized woody ornamentals. HortTechnology 18 (2), 266–270. doi: 10.21273/HORTTECH.18.2.266
Fields J. S., Owen Jr J. S., Altland J. E. (2021). Substrate stratification: Layering unique substrates within a container increases resource efficiency without impacting growth of shrub rose. Agronomy 11 (8), 1454. doi: 10.3390/agronomy11081454
Foshee W. G., Goff W. D., Tilt K. M., Williams J. D., Bannon J. S., Witt J. B. (1996). Organic mulches increase growth of young pecan trees. HortScience 31 (5), 811–812. doi: 10.21273/HORTSCI.31.5.811
Frangi P., Piatti R., Amoroso G., Fini A. (2010). “Non-chemical alternatives for weed control in containerized plants,” in I International Symposium on Woody Ornamentals of the Temperate Zone, Vol. 885. 119–122. doi: 10.17660/ActaHortic.2010.885.15
Fretz T. A. (1973). Herbicide-impregnated mulches for weed control in container nursery stock. Scientia Hortic. 1 (2), 165–170. doi: 10.1016/0304-4238(73)90027-7
Giaccone M., Cirillo C., Scognamiglio P., Teobaldelli M., Mataffo A., Stinca A., et al. (2018). Biodegradable mulching spray for weed control in the cultivation of containerized ornamental shrubs. Chem. Biol. Technol. Agric. 5 (1), 1–8. doi: 10.1186/s40538-018-0134-z
Gill H. K., McSorley R., Branham M. (2011). Effect of organic mulches on soil surface insects and other arthropods. Florida Entomologist 94 (2), 226–232. doi: 10.1653/024.094.0215
Gilliam C. H., Fare D. C., Beasley A. (1992). Nontarget herbicide losses from application of granular Ronstar to container nurseries. J. Environ. Horticulture 10 (3), 175–176. doi: 10.24266/0738-2898-10.3.175
Greenly K. M., Rakow D. A. (1995). The effect of wood mulch type and depth on weed and tree growth and certain soil parameters. J. Arboriculture 21, 225–225. doi: 10.48044/jauf.1995.036
Gruda N., Rau B. J., Wright R. D. (2009). Laboratory bioassay and greenhouse evaluation of a pine tree substrate used as a container substrate. Eur. J. Hortic. Sci. 74 (2), 73.
Handreck K. A., Black N. D. (2002). Growing media for ornamental plants and turf (Sydney, Australia: University of New South Wales Press).
Hanim A., Nazera A. A. B., Kahar S., Hamdan M. N. (2014). Effects of different inorganic mulches on seed germination, weed biomass and plant growth. J. Trop. Agric. Fd. Sci. 42 (1), 29–36. Available at: http://jtafs.mardi.gov.my/jtafs/42-1/Seed%20germination.pdf.
Heisey R. M. (1990). Evidence for allelopathy by tree-of-heaven (Ailanthus altissima). J. Chem. Ecol. 16, 2039–2055. doi: 10.1007/BF01020515
Heisey R. M. (1996). Identification of an allelopathic compound from Ailanthus altissima (Simaroubaceae) and characterization of its herbicidal activity. Am. J. Bot. 83 (2), 192–200. doi: 10.1002/j.1537-2197.1996.tb12697.x
IAS (2018) International allelopathy society. Available at: http://allelopathy-society.osupytheas.fr/about/.
Immirzi B., Santagata G., Vox G., Schettini E. (2009). Preparation, characterisation and field-testing of a biodegradable sodium alginate-based spray mulch. Biosyst. Eng. 102 (4), 461–472. doi: 10.1016/j.biosystemseng.2008.12.008
Jackson B. E., Wright R. D. (2009). Pine tree substrate: an alternative and renewable substrate for horticultural crop production. Acta Hort 819, 265–272. doi: 10.17660/ActaHortic.2009.819.30
Kader M. A., Singha A., Begum M. A., Jewel A., Khan F. H., Khan N. I. (2019). Mulching as water-saving technique in dryland agriculture. Bull. Natl. Res. Centre 43 (1), 1–6. doi: 10.1186/s42269-019-0186-7
Kanematsu M., Hayashi A., Denison M. S., Young T. M. (2009). Characterization and potential environmental risks of leachate from shredded rubber mulches. Chemosphere 76 (7), 952–958. doi: 10.1016/j.chemosphere.2009.04.026
Kato-Noguchi H., Ino T. (2003). Rice seedlings release momilactone B into the environment. Phytochemistry 63 (5), 551–554. doi: 10.1016/S0031-9422(03)00194-8
Keddy P. A., Constabel P. (1986). Germination of ten shoreline plants in relation to seed size, soil particle size and water level: an experimental study. J. Ecol. 74, 133–141. doi: 10.2307/2260354
Khaliq A., Matloob A., Farooq M., Mushtaq M. N., Khan M. B. (2011). Effect of crop residues applied isolated or in combination on the germination and seedling growth of horse purslane (Trianthema portulacastrum). Planta Daninha 29, 121–128. doi: 10.1590/S0100-83582011000100014
Khamare Y., Chen J., Marble S. C. (2022a). Allelopathy and its application as a weed management tool: A review. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1034649
Khamare Y., Marble S. C., Altland J. E., Pearson B. J., Chen J., Devkota P. (2022b). Effect of substrate stratification on growth of common nursery weed species and container-grown ornamental species. HortTechnology 32 (1), 74–83. doi: 10.21273/HORTTECH04965-21
Khamare Y., Marble S. C., Altland J. E., Pearson B. J., Chen J., Devkota P. (2022c). Effect of substrate stratification without fine pine bark particles on growth of common nursery weed species and container-grown ornamental species. HortTechnology 32 (6), 491–498. doi: 10.21273/HORTTECH05113-22
Khamare Y., Marble S., Chandler A. (2020). Fertilizer placement effects on eclipta (Eclipta prostrata) growth and competition with container-grown ornamentals. Weed Sci. 68 (5), 496–502. doi: 10.1017/wsc.2020.44
Kumata H., Yamada J., Masuda K., Takada H., Sato Y., Sakurai T., et al. (2002). Benzothiazolamines as tire-derived molecular markers: sorptive behavior in street runoff and application to source apportioning. Environ. Sci. Technol. 36 (4), 702–708. doi: 10.1021/es0155229
Lanphear F. (1968). Incorporation of dichlobenil in mulches. Weed Sci. 16 (2), 230–231. doi: 10.1017/S004317450004697X
Li H. H., Inoue M., Nishimura H., Mizutani J., Tsuzuki E. (1993). Interactions of trans-cinnamic acid, its related phenolic allelochemicals, and abscisic acid in seedling growth and seed germination of lettuce. J. Chem. Ecol. 19, 1775–1787. doi: 10.1007/BF00982307
Li R., Li Q., Zhang J., Liu Z., Pan L., Huang K., et al. (2020). Effects of organic mulch on soil moisture and nutrients in karst area of southwest China. Polish J. Environ. Stud. 29 (6), 4161–4174. doi: 10.15244/pjoes/119477
Llewellyn J., Osborne K., Steer-George C., West J. (2003). Commercially available organic mulches as a weed barrier for container production. Comp. Proc. Intl. Plant Prop. Soc. 53, 590–593. Available at: https://ena.ipps.org/uploads/docs/53_152.pdf.
Lohr V. I., Pearson-Mims C. H. (2001). Mulching reduces water use of containerized plants. HortTechnology 11 (2), 277–278. doi: 10.21273/HORTTECH.11.2.277
Long C. E., Thorne B. L., Breisch N. L., Douglass L. W. (2001). Effect of organic and inorganic landscape mulches on subterranean termite (Isoptera: Rhinotermitidae) foraging activity. Environ. entomology 30 (5), 832–836. doi: 10.1603/0046-225X-30.5.832
Maggard A. O., Will R. E., Hennessey T. C., Cole J. C. (2012a). Tree-based mulches and their leachate suppress weed seed emergence. J. Environ. Horticulture 30 (3), 146–149. doi: 10.24266/0738-2898.30.3.146
Maggard A. O., Will R. E., Hennessey T. C., McKinley C. R., Cole J. C. (2012b). Tree-based mulches influence soil properties and plant growth. HortTechnology 22 (3), 353–361. doi: 10.21273/HORTTECH.22.3.353
Marble S. C., Koeser A. K., Hasing G. (2015). A review of weed control practices in landscape planting beds: Part I–nonchemical weed control methods. HortScience 50 (6), 851–856. doi: 10.21273/HORTSCI.50.6.851
Marble S. C., Steed S. T., Saha D., Khamare Y. (2019). On-farm evaluations of wood-derived, waste paper, and plastic mulch materials for weed control in Florida container nurseries. HortTechnology 29 (6), 866–873. doi: 10.21273/HORTTECH04437-19
Martin C. A., Ponder H. G., Gilliam C. H. (1987). Ability of polypropylene fabric to inhibit the growth of six weed species. Research Report Series, Alabama Agricultural Experiment Station, Auburn University 5, 25–26.
Massa D., Benvenuti S., Cacini S., Lazzereschi S., Burchi G. (2019). Effect of hydro-compacting organic mulch on weed control and crop performance in the cultivation of three container-grown ornamental shrubs: Old solutions meet new insights. Scientia Hortic. 252, 260–267. doi: 10.1016/j.scienta.2019.03.053
Mathers H. M. (2003). Novel methods of weed control in containers. HortTechnology 13 (1), 28–34. doi: 10.21273/HORTTECH.13.1.0028
Mathers H. M., Case L. T. (2006). Field evaluation of various herbicide and mulch combinations for ornamental weed control. Ornamental Plants 125, 997E–998. doi: 10.21273/HORTSCI.40.4.997E
Mohajerani A., Kurmus H., Conti D., Cash L., Semcesen A., Abdurahman M., et al. (2022). Environmental impacts and leachate analysis of waste rubber incorporated in construction and road materials: A review. Sci. Total Environ. 835, 155269. doi: 10.1016/j.scitotenv.2022.155269
Monks C. D., Monks D. W., Basden T., Selders A., Poland S., Rayburn E. (1997). Soil temperature, soil moisture, weed control, and tomato (Lycopersicon esculentum) response to mulching. Weed Technol. 11 (3), 561–566. doi: 10.1017/S0890037X00045425
Morra L., Bilotto M., Cerrato D., Coppola R., Leone V., Mignoli E., et al. (2016). The Mater-Bi® biodegradable film for strawberry (Fragaria x ananassa Duch.) mulching: effects on fruit yield and quality. Ital. J. Agron. 11 (3), 203–206. doi: 10.4081/ija.2016.731
Munn D. A. (1992). Comparisons of shredded newspaper and wheat straw as crop mulches. HortTechnology 2 (3), 361–366. doi: 10.21273/HORTTECH.2.3.361
Ngouajio M., Ernest J. (2004). Light transmission through colored polyethylene mulches affects weed populations. HortScience 39 (6), 1302–1304. doi: 10.21273/HORTSCI.39.6.1302
Olofsdotter M., Navarez D., Rebulanan M., Streibig J. C. (1999). Weed-suppressing rice cultivars: does allelopathy play a role? Weed Res. (Print) 39 (6), 441–454. doi: 10.1046/j.1365-3180.1999.00159.x
Ortega M. C., Moreno M. T., Ordovás J., Aguado M. T. (1996). Behaviour of different horticultural species in phytotoxicity bioassays of bark substrates. Scientia Hortic. 66 (1-2), 125–132. doi: 10.1016/0304-4238(96)00900-4
Patten K. D., Neuendorff E. W., Peters S. C. (1988). Root distribution of ‘Climax’rabbiteye blueberry as affected by mulch and irrigation geometry. J. Am. Soc. Hortic. Sci. 113 (5), 657–661. doi: 10.21273/JASHS.113.5.657
Patterson D. T. (1981). Effects of allelopathic chemicals on growth and physiological responses of soybean (Glycine max). Weed Sci. 29 (1), 53–59. doi: 10.1017/S0043174500025820
Pellett N. E., Heleba D. A. (1995). Chopped newspaper for weed control in nursery crops. J. Environ. Horticulture 13 (2), 77–81. doi: 10.24266/0738-2898-13.2.77
Pelosi C., Bertrand M., Roger-Estrade J. (2009). Earthworm community in conventional, organic and direct seeding with living mulch cropping systems. Agron. Sustain. Dev. 29, 287–295. doi: 10.1051/agro/2008069
Penny G. M., Neal J. C. (2003). Light, temperature, seed burial, and mulch effects on mulberry weed (Fatoua villosa) seed germination. Weed Technol. 17 (2), 213–218. doi: 10.1614/0890-037X(2003)017[0213:LTSBAM]2.0.CO;2
Pickering J. S., Shepherd A. (2000). Evaluation of organic landscape mulches: composition and nutrient release characteristics. Arboricultural J. 24 (2-3), 175–187. doi: 10.1080/03071375.2000.9747271
Pons T. L., Fenner M. (2000). “Seed responses to light,” in Seeds: the ecology of regeneration in plant communities, vol. 2. (New York, USA: CABI publishing), 237–260.
Poudel I., Witcher A. L. (2022). Effect of mulch type and depth on rooting of stem cuttings and weed control in containers. HortTechnology 32 (2), 140–146. doi: 10.21273/HORTTECH04937-21
Poudyal S., Cregg B. M. (2019). Irrigating nursery crops with recycled run-off: a review of the potential impact of pesticides on plant growth and physiology. HortTechnology 29 (6), 716–729. doi: 10.21273/HORTTECH04302-19
Rathinasabapathi B., Ferguson J., Gal M. (2005). Evaluation of allelopathic potential of wood chips for weed suppression in horticultural production systems. HortScience 40 (3), 711–713. doi: 10.21273/HORTSCI.40.3.711
Richardson B., Gilliam C. H., Fain G., Wehtje G. (2008). Nursery container weed control with pinebark mininuggets. J. Environ. Horticulture 26 (3), 144–148. doi: 10.24266/0738-2898-26.3.144
Romagni J. G., Allen S. N., Dayan F. E. (2000). Allelopathic effects of volatile cineoles on two weedy plant species. J. Chem. Ecol. 26, 303–313. doi: 10.1023/A:1005414216848
Russo V. M., Cartwright B., Webber C. L. III (1997a). Mulching effects on erosion of soil beds and on yield of autumn and spring planted vegetables. Biol. Agric. Horticulture 14 (2), 85–93. doi: 10.1080/01448765.1997.9754799
Russo V. M., Webber C. L. III, Myers D. L. (1997b). Kenaf extract affects germination and post-germination development of weed, grass and vegetable seeds. Ind. Crops Products 6 (1), 59–69. doi: 10.1016/S0926-6690(96)00206-3
Saha D., Marble S. C., Pearson B. J. (2018). Allelopathic effects of common landscape and nursery mulch materials on weed control. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00733
Saha D., Marble S. C., Pearson B., Pérez H., MacDonald G., Odero D. (2020). Emergence of garden spurge (Euphorbia hirta) and large crabgrass (Digitaria sanguinalis) in response to different physical properties and depths of common mulch materials. Weed Technol. 34 (2), 172–179. doi: 10.1017/wet.2019.88
Saha D., Marble S., Torres N., Chandler A. (2019). Fertilizer placement affects growth and reproduction of three common weed species in pine bark–based soilless nursery substrates. Weed Sci. 67 (6), 682–688. doi: 10.1017/wsc.2019.49
Samtani J. B., Kling G. J., Mathers H. M., Case L. (2007). Rice hulls, leaf-waste pellets, and pine bark as herbicide carriers for container-grown woody ornamentals. HortTechnology 17 (3), 289–295. doi: 10.21273/HORTTECH.17.3.289
Schettini E., Vox G., De Lucia B. (2007). Effects of the radiometric properties of innovative biodegradable mulching materials on snapdragon cultivation. Scientia Hortic. 112 (4), 456–461. doi: 10.1016/j.scienta.2007.01.013
Serbesoff-King K. (2003). Melaleuca in Florida: a literature review on the taxonomy, distribution, biology, ecology, economic importance and control measures. J. Aquat. Plant Manage. 41 (1), 98–112.
Shen K., Zheng Y. (2017). Efficacy of bio-based liquid mulch on weed suppression and water conservation in container nursery production1. J. Environ. Horticulture 35 (4), 161–167. doi: 10.24266/0738-2898-35.4.161
Shrestha A. (2009). Potential of a black walnut (Juglans nigra) extract product (NatureCur®) as a pre-and post-emergence bioherbicide. J. Sustain. Agric. 33 (8), 810–822. doi: 10.1080/10440040903303397
Sibley J. L., Cole D. M., Lu W. (2004). “Waste is a terrible thing to mind,” in Combined proceedings-international plant propagators society, vol. 54, 596. (South Carolina, USA: International Plant Propagators Society); 1998.
Simpson C. V., Gilliam C. H., Altland J. E., Wehtje G. R., Sibley J. L. (2002). Postemergence oxalis control in container grown crops Southern Nursery Assoc. Res. Conf. 47, 376–379.
Singh H. P., Batish D. R., Kohli R. K. (2003). Allelopathic interactions and allelochemicals: new possibilities for sustainable weed management. Crit. Rev. Plant Sci. 22 (3-4), 239–311. doi: 10.1080/713610858
Smith D. R., Gilliam C. H., Edwards J. H., Eakes D. J., Williams J. D. (1997). Recycled waste paper as a landscape mulch. J. Environ. Horticulture 15 (4), 191–196. doi: 10.24266/0738-2898-15.4.191
Smith D. R., Gilliam C. H., Edwards J. H., Olive J. W., Eakes D. J., Williams J. D. (1998). Recycled waste paper as a non-chemical alternative for weed control in container production. J. Environ. Horticulture 16 (2), 69–75. doi: 10.24266/0738-2898-16.2.69
Snyder C. S., Slaton N. A. (2001). Rice production in the United States: An overview. Better Crops 85 (3), 3–7. Available at: http://www.ipni.net/publication/bettercrops.nsf/0/42436536BD1BBB9A852579800081F86C/$FILE/Better%20Crops%202001-3%20p03.pdf.
Somireddy U. R. (2011) Effect of herbicide-organic mulch combinations on weed control and herbicide persistence (Order no. 3497714). Available from proQuest dissertations & Theses A&I; proQuest dissertations & Theses global; sciTech premium collection, (925813442). Available at: https://login.lp.hscl.ufl.edu/login?url=https://www.proquest.com/dissertations-theses/effect-herbicide-organic-mulch-combinations-on/docview/925813442/se-2.
Strugstad M., Despotovski S. (2012). A summary of extraction, synthesis, properties, and potential uses of juglone: A literature review. J. Ecosyst. Manage. 13 (3), 1–16. doi: 10.22230/jem.2012v13n3a119
Takahashi N., Kato T., Tsunagawa M., Sasaki N., Kitahara Y. (1976). Mechanisms of dormancy in rice seeds: II. New growth inhibitors, momilactone-A and-B isolated from the hulls of rice seeds. Japanese J. Breed. 26 (2), 91–98. doi: 10.1270/jsbbs1951.26.91
Tatum D., Johnson K., Winter N. (1999). The use of crumb rubber for weed control in ornamental containers. Proc. South. Nur. Assoc. Res. Conf 44, 388.
Teasdale J. R., Mohler C. L. (2000). The quantitative relationship between weed emergence and the physical properties of mulches. Weed Sci. 48 (3), 385–392. doi: 10.1614/0043-1745(2000)048[0385:TQRBWE]2.0.CO;2
Tindall J. A., Beverly R. B., Radcliffe D. E. (1991). Mulch effect on soil properties and tomato growth using micro-irrigation. Agron. J. 83 (6), 1028–1034. doi: 10.2134/agronj1991.00021962008300060019x
Topal S., Kocacaliskan I., Arslan O., Tel A. Z. (2007). Herbicidal effects of juglone as an allelochemical. Phyton. 46 (2), 259–269.
Virtanen S., Chowreddy R. R., Irmak S., Honkapää K., Isom L. (2017). Food industry co-streams: potential raw materials for biodegradable mulch film applications. J. Polymers Environ. 25, 1110–1130. doi: 10.1007/s10924-016-0888-y
Vox G., Santagata G., Malinconico M., Immirzi B., Mugnozza G. S., Schettini E. (2013). Biodegradable films and spray coatings as eco-friendly alternative to petro-chemical derived mulching films. J. Agric. Eng. 44 (s2). doi: 10.4081/jae.2013.286
Wilen C. A., Schuch U. K., Elmore C. L. (1999). Mulches and subirrigation control weeds in container production. J. Environ. Horticulture 17 (4), 174–180. doi: 10.24266/0738-2898-17.4.174
Wilson P. C., Whitwell T., Riley M. B. (1995). Effects of ground cover and formulation on herbicides in runoff water from miniature nursery sites. Weed Science 43 (4), 671–677. doi: 10.1017/S0043174500081819
Winkel V. K., Medrano J. C., Stanley C., Walo M. D. (1996). “Effects of gravel mulch on emergence of galleta grass seedlings,” in Wild Land Shrub and Arid Land Restoration Symposium: Proceedings (Las Vegas, NV, USA: Wildland shrub and arid land restoration symposium).
Woodstock L. W. (1988). Seed imbibition: a critical period for successful germination. J. Seed Technol. 12, 1–15. Available at: https://www.jstor.org/stable/23432691.
Wooten R. E., Neal J. C. (2000). “Evaluations of PennMulch, Wulpak and Geodisk for weed control in containers,” in Proceedings of the northeastern weed science society (New York, USA: Northeastern Weed Science Society).
Keywords: container nursery, container plant production, nonchemical weed control, ornamental plants, mulch, weed management
Citation: Khamare Y and Marble SC (2023) Mulching as a weed management tool in container plant production - review. Front. Agron. 5:1235196. doi: 10.3389/fagro.2023.1235196
Received: 05 June 2023; Accepted: 29 November 2023;
Published: 12 December 2023.
Edited by:
Muhammad Ikram, Pir Mehr Ali Shah Arid Agriculture University, PakistanReviewed by:
Hafeez Ur Rahim, University of Ferrara, ItalyCopyright © 2023 Khamare and Marble. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuvraj Khamare, eWtoYW1hcmVAdWZsLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.