
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Agron. , 25 May 2023
Sec. Plant-Soil Interactions
Volume 5 - 2023 | https://doi.org/10.3389/fagro.2023.1179996
This article is part of the Research Topic Maximizing Nitrogen Fixation in Legumes as a Tool for Sustainable Agriculture Intensification: Volume II View all 7 articles
Inoculation of legumes is generally considered to increase yield and to lower the need of nitrogen (N) fertilization, especially in semiarid regions and on sandy soils. It has not been clear whether inoculation with Rhizobium sp. in cropping of faba beans (Vicia faba minor) under Swedish conditions would improve yield and protein content. In 2015–2016, three faba bean cultivars and two strains of Rhizobium were studied in field trials in Central Sweden, including analyses of N fixation capacities using 15N abundance. The study did not show any effects of inoculation of Rhizobium on yield or protein content of faba beans or subsequent spring wheat yields. Yields of faba beans varied between cultivars but were not connected to inoculation. 15N abundance was influenced by rhizobium. The study cannot support the opinion that, generally, inoculation is beneficial for improved outcome of faba bean cropping under Scandinavian field conditions. No residual effect of inoculation on subsequent spring wheat yield was found.
Vicia faba minor (L.), colloquially referred to as faba (or fava) bean, is an ancient legume crop cultivated worldwide for human consumption and livestock feed. Broad bean, horse bean, and field bean are commonly used names for the same species, often distinguished by the seed size (Cubero, 1974; Duc, 1997). The use of faba beans is in Northern Europe is, today, mainly connected to feed, whereas, in Southern Europe, Northern Africa, and the Middle East, it is still a major staple.
Seed yields of faba beans in Scandinavia vary between 3 and 4 metric tons (MT) per hectare (ha) (SJV, 2019; SCB, 2019), and protein content is typically around 30% of dry matter (Klingspor, 2017). The faba bean ability of biological nitrogen fixation (BNF) and its adaptation to various soils and cool climate conditions make it interesting to Scandinavian farmers as a break crop in normally grain-dominated crop rotations.
In Scandinavia, small seed varieties of faba bean (Vicia faba minor) have been favored for cultivation, mainly for feed purpose. Vicia faba minor has received much attention through development of new low-tannin varieties that are suitable as a protein fodder crop in pig and milk production. The cultivated area of faba beans increased steadily since the 1990s, and, today, the acreage in Sweden varies annually between 15,000 and 30,000 ha (SCB, 2019).
Inoculation of faba bean seeds with N-fixing bacteria has been shown to enhance BNF and to reduce the need for organic or mineral fertilization (Elsheikh and Elzidany, 1997) especially on sandy soils (Abdel-Ghaffar, 1988; Youseif et al., 2017). However, the inoculated strains must be competitive comparing with the native strains (Moawad and Beck, 1991), and the effect of various strains may differ with crop variety (Rodelas et al., 1999; Ntatsi et al., 2018). Furthermore, inoculation of faba beans may increase yields of non–N-fixing crops the following season, as, e.g., seen in crop rotations with wheat in Ethiopia (Habtemichial et al., 2007).
Scandinavian field trials in beans, peas, and lupines in the period 1910 to 1930 showed, in general, a positive reaction on yield and plant development by inoculation—why Swedish agricultural scientist recommended and produced inocula mixtures for farmers (Barthel and Rhodin, 1914; Barthel and Bjälfve, 1930; Bjälfve, 1935). More recent studies on inoculation in various soils and bean types (Rai, 1992; Raposeiras et al., 2006; Uaboi-Egbenni et al., 2010) have shown a general yield increase compared to non-inoculated plots.
It is currently not clear whether inoculation on productive soils in Scandinavia will affect yields and protein content in faba beans. The typically high clay content of Scandinavian soils gives them a better nutrient holding capacity than sandy soils, and inoculation may not be as effective as on lighter soils poorer in nutrients. The fact that cropping of faba beans in Sweden and Scandinavia has been carried out for centuries and thus maintained an active Rhizobium flora in the soil may also reduce the need for addition of new strains. Furthermore, it is unclear whether there is an interaction between faba bean cultivars and Rhizobium strains, i.e., if some bean varieties will be more affected than others when inoculated with Rhizobium.
The aim of this study was i) to investigate the effects of inoculation of faba bean seeds with Rhizobium sp. on yield and protein content of faba beans under Scandinavian conditions, ii) to investigate interactions between faba bean cultivars and Rhizobium strains, and iii) to investigate residual effects on yield of subsequent spring wheat crop.
In addition, differences in N fixation capacity between cultivars were investigated by analyzing 15N abundance.
In 2015 and 2016, field experiments were conducted in faba beans (Vicia faba minor). Three faba bean cultivars were studied in combination with inoculation with two strains of Rhizobium and one control with no inoculation.
The trials were located at Brunnby Farm close to Västerås, Central Sweden (N 59° 36.6001′ E 16° 39.0864′l). The field experiments were seeded and maintained by the regional Rural Economy and Agricultural Society in Västmanland, an organization specialized in field trials and agricultural advisory services (www.hushallningssallskapet.se).
The soil at the experimental site is a heavy clay soil with an organic matter content of 4%–5%. Additional soil properties are shown in Table 1. The soil parameters—pH, nitrate (NO3)–N, and ammonia (NH4)–N—were recorded by soil analyses of each treatment after harvest of winter wheat, with soil depth of 0–20 cm. Prior to our experiments, the field had been cropped with winter wheat in 2014 and grass ley in 2013. Weather data are shown in Figures 1, 2.
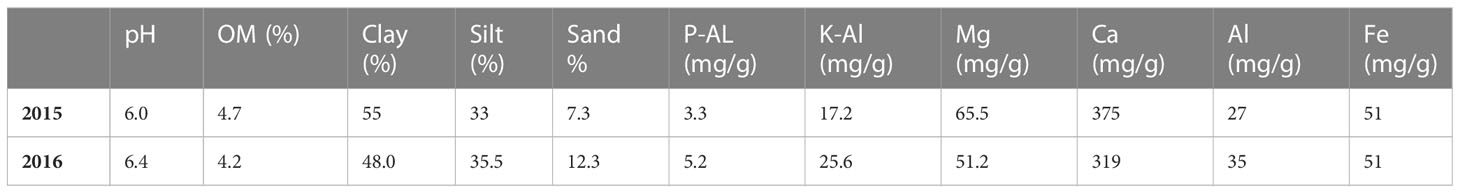
Table 1 Information on soil texture, organic matter content (OM), plant available P (P-Al), plant available K (K-Al), Mg, Ca, Al, and Fe in the topsoil at the experimental sites in 2015 and 2016.
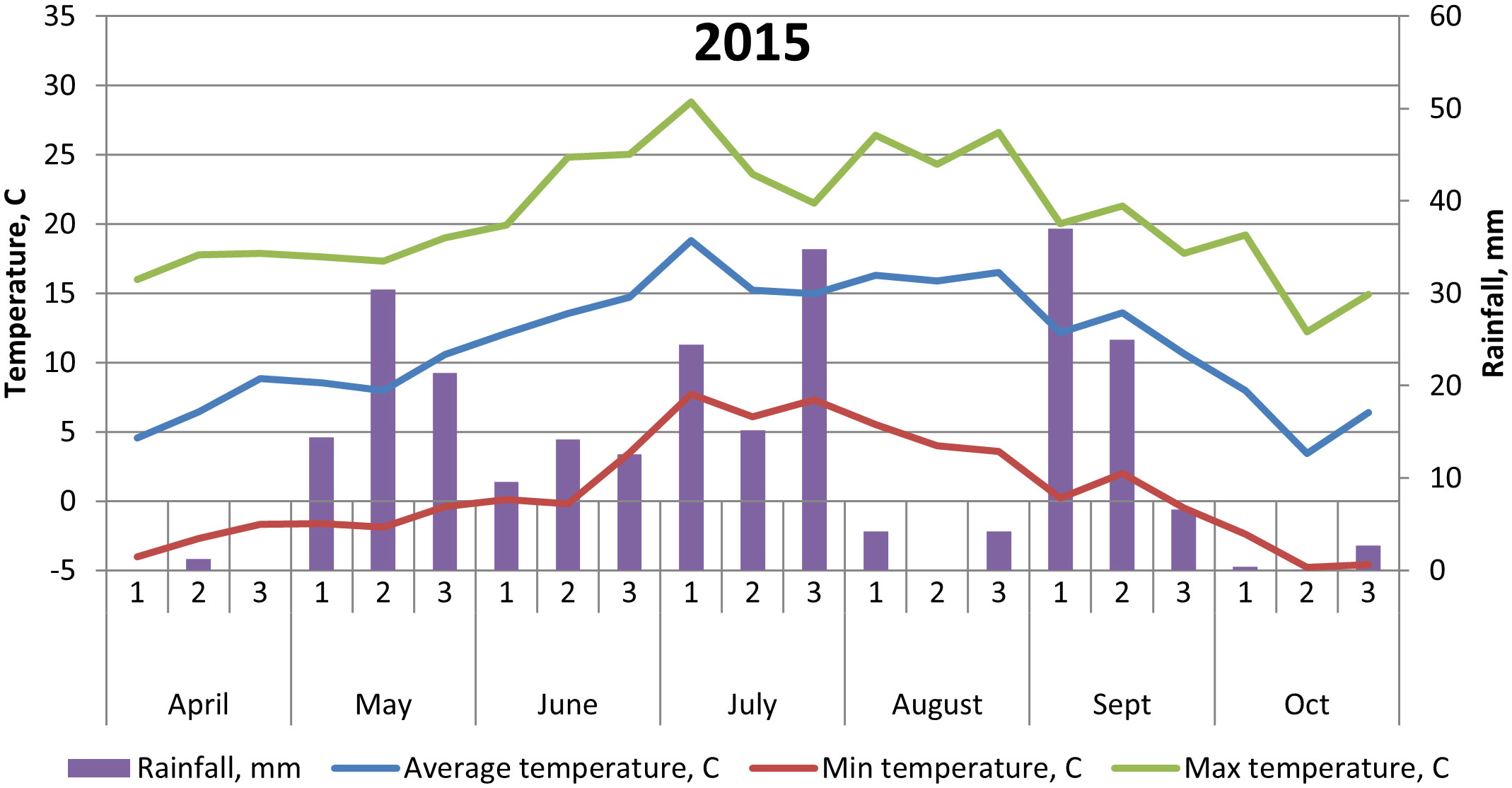
Figure 1 Temperature (°C) and precipitation (mm) at Brunnby test site, Sweden, April to October 2015.
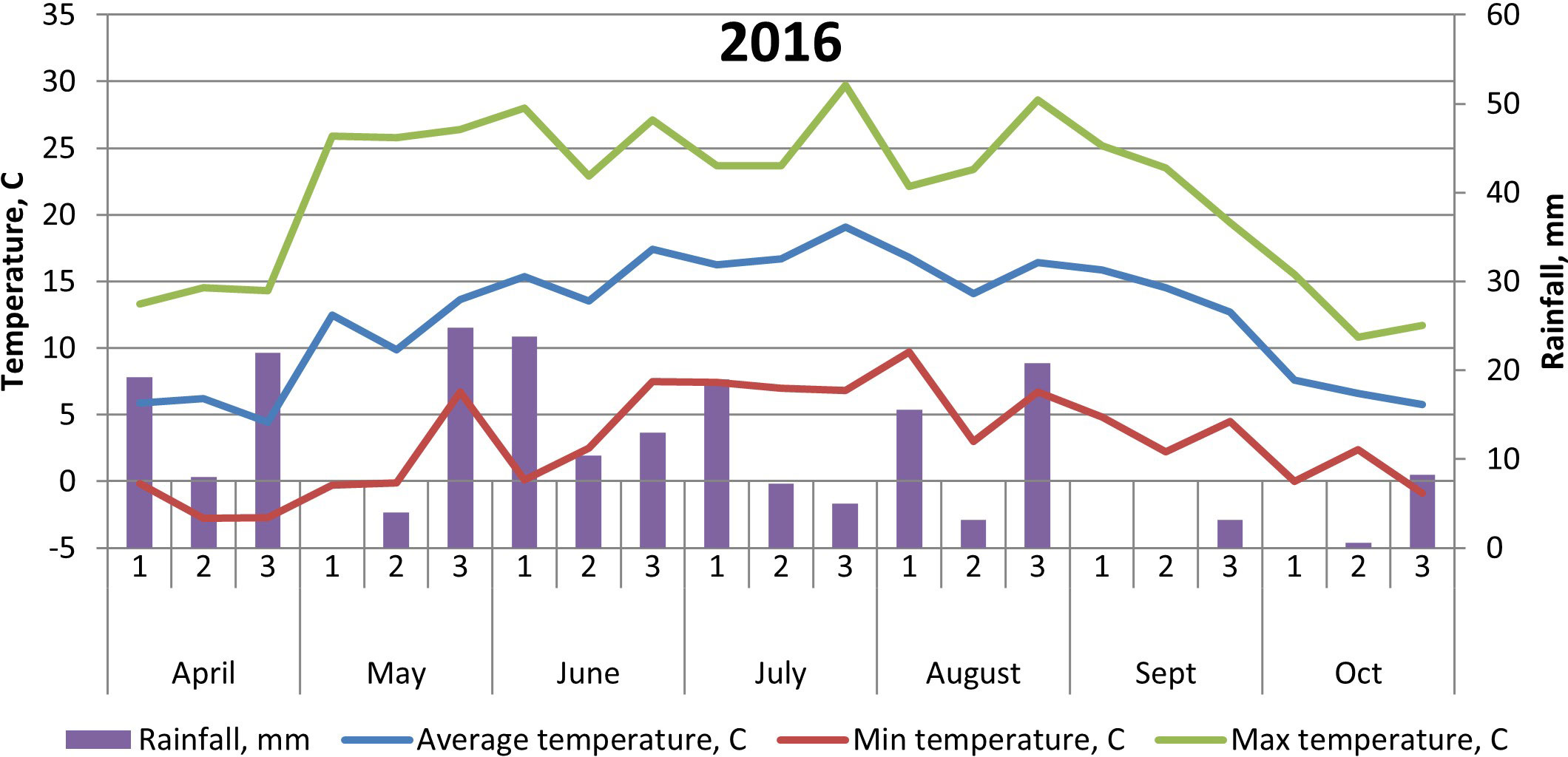
Figure 2 Temperature (°C) and precipitation (mm) at Brunnby test site, Sweden, April to October 2016.
The trials had a randomized complete block design with four replicates of each treatment (Figure 3). A block thus contained three plots with bean cultivars without inoculation, three plots with bean cultivars inoculated with Swedish standard inoculation, and three plots with new N-fixing strain from Latvia. Each plot had a gross size of 42 m−2. The plots of the field trial were recorded by Global Positioning System (GPS) plotting to use the same plots in the following year for the spring wheat trial.
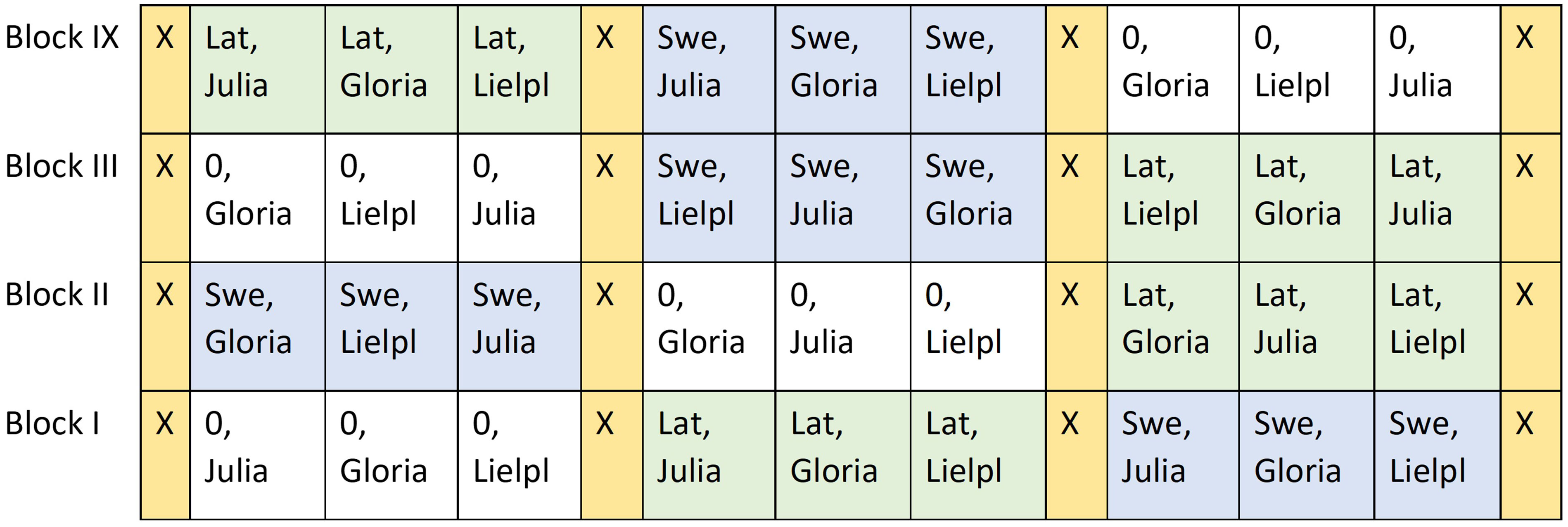
Figure 3 Design of the faba bean field trials in 2015 and 2016 with three inoculation treatments: no inoculation (0), Latvian strain (Lat), and Swedish strain (Swe) in three cultivars of faba beans: Julia, Gloria, and Lielplatones (Lielpl). X = boarder strips sown with untreated faba beans.
To investigate the possible residual effects from inoculation of the faba bean seeds, spring wheat (cv. ‘Diskett’) was seeded on the experimental site in the consecutive year. The wheat crop was harvested, and the yield was registered plot-wise using the same plots previously sown with faba beans.
Three commercial faba bean cultivars available on the Nordic-Baltic market were selected: ‘Gloria’, a white flowering bean commonly used for feed purpose; ‘Julia’, a colored flowering bean commonly used for feed purpose; and ‘Lielplatones’, a white flowering bean originating from Latvia. The ‘Julia’ is considered to have higher tannin content and higher yield than ‘Gloria’. ‘Lielplatones’ has a low Thousand Kernel Weight (TKW) and is thus suitable for seeding with grain seeders. Earlier field cultivar trials in Sweden with ‘Lielplatones’ have shown a potential for high yield and protein content compared with standard faba bean cultivars used in Sweden.
We used two strains for the inoculations: a commercial standard strain from Inocula Scandinavia AB, Hovmantorp, Sweden; and an experimental strain from Latvia (RV50501) supplied by Dr. Ina Alsina at the Latvian Agricultural University. The Swedish product constituted of a fine-milled peaty powder and the Latvian of a liquid.
The inoculum (5 g of powder or 5 cl of liquid to 500 g of seeds according to instructions by the provider) was applied on the seeds immediately prior to seeding. Beans intended for seeding were placed in a plastic container, and inoculum was added. The beans and inoculum were mixed thoroughly with a plastic stick. We used separate plastic containers and sticks for each mixture of cultivar and inoculum to reduce cross-contamination. Furthermore, to reduce risk of plots being affected by cross-contamination, the seeding machine coulters, hoses, and other parts touched by the inoculated seeds were washed with 95% ethanol between the two different strains. Inoculated plots were also separated by a plot (21 m−2) not used for sampling and a non-seeded strip of 1 m in the beginning of each plot. Plots without inoculation were seeded first.
In 2015, beans were seeded on 5 May using a standard plot seeder type “Öyjord”. Prior to seeding, the field was harrowed twice using standard methods for the region. The plots were neither fertilized nor treated with herbicides or insecticides. In 2016, beans were seeded in another field nearby on 18 May. As in 2015, the plots were harrowed twice, and neither fertilized nor treated with herbicides or insecticides.
The experiment was harvested at maturity using a field trial combine harvester. For each plot, the yield was registered from a net area of 19.6 m−2. The yield was weighed and dried for later analyses.
In spring 2016, the faba bean trial area of 2015 was seeded with spring wheat cv. ‘Diskett’ (300 kg of seeds ha−1, row distance of 12.5 cm). On 12 September 2016, the number of wheat heads in the former bean plots was recorded on an area of 0.5 m−2 in each plot. Harvest was carried out on 21 September in an area of 19.6 m−2 in each former bean plot. In 2017, a similar operation was carried out in the trial area of 2016. A number of heads were recorded on 5 September and yield of spring wheat cv. ‘Diskett’ was recorded on 28 September 2017. All samples were dried and weighed for further analyses.
To investigate whether N fixation increased by the use of inoculum, a 15N abundance analysis was carried out. This technique quantifies the amount of N derived from N fixation in air versus taken up from the soil. Atmospheric N2 consists of two stable isotopes, where 14N constitutes ∼99.6337% and 15N constitutes ∼0.3663%. The 15N abundance analysis is based on the discrimination against 15N uptake over the lighter 14N isotope from atmospheric N2 in N fixing plants, leading to lower abundance of 15N compared to in non–N2-fixing plants (He et al., 2009). The ratio between 14N and 15N in the air is constant but varies in other biological systems, for example, in soil where microbial fractionation has an impact (Dijkstra et al., 2006). Delta15N, expressed (δ15N) in parts per thousand ‰, represents the deviation from atmospheric 15N. It is typically a positive value when measured in non–N2-fixing plants because 15N is more abundant in these plants (Paul et al., 2012).
By comparing δ15N of inoculated plants and controls, it was possible to determine the effect of inoculation on N fixation.
Samples of the leaves and the stem of faba beans were taken for analyses of 15N abundance in 2015 and 2016, respectively. Ten plants from each plot were collected in July (before pod setting) each year, and the above-ground parts were oven-dried at 106°C for 24 h. The plant material was then ground into <1-mm particle size, and 4 g of biomass of each sample was placed in tin capsules. The analyses were performed by UC Davis Stable Isotope Facility, Davis, California, USA, using a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20-20 isotope ratio mass spectrometer (Sercon Ltd., Cheshire, UK).
The statistical analyses of all trials were carried out by the authors using the Statgraphics Centurion XVI software. Multifactor ANOVA with Tukey’s Honest Significant Difference (HSD) was used as standard analysis. All observations and registrations were used in the statistical analyses. Results are presented as mean values ± SE. Statistical differences are presented as p-values using the 95% level as standard.
In the 2015 trial ‘Lielplatones’ yielded between 5.8 and 6.0 MT ha−1 marketable yield, which was significantly higher (p = 0.0002) compared to ‘Julia’ (4.8 to 5.1 MT ha−1) and ‘Gloria’ (5.4 to 5.6 MT ha−1). There were no statistical differences in yield (p = 0.8916) between inoculated and non-treated seeds, regardless of cultivar (Table 2).
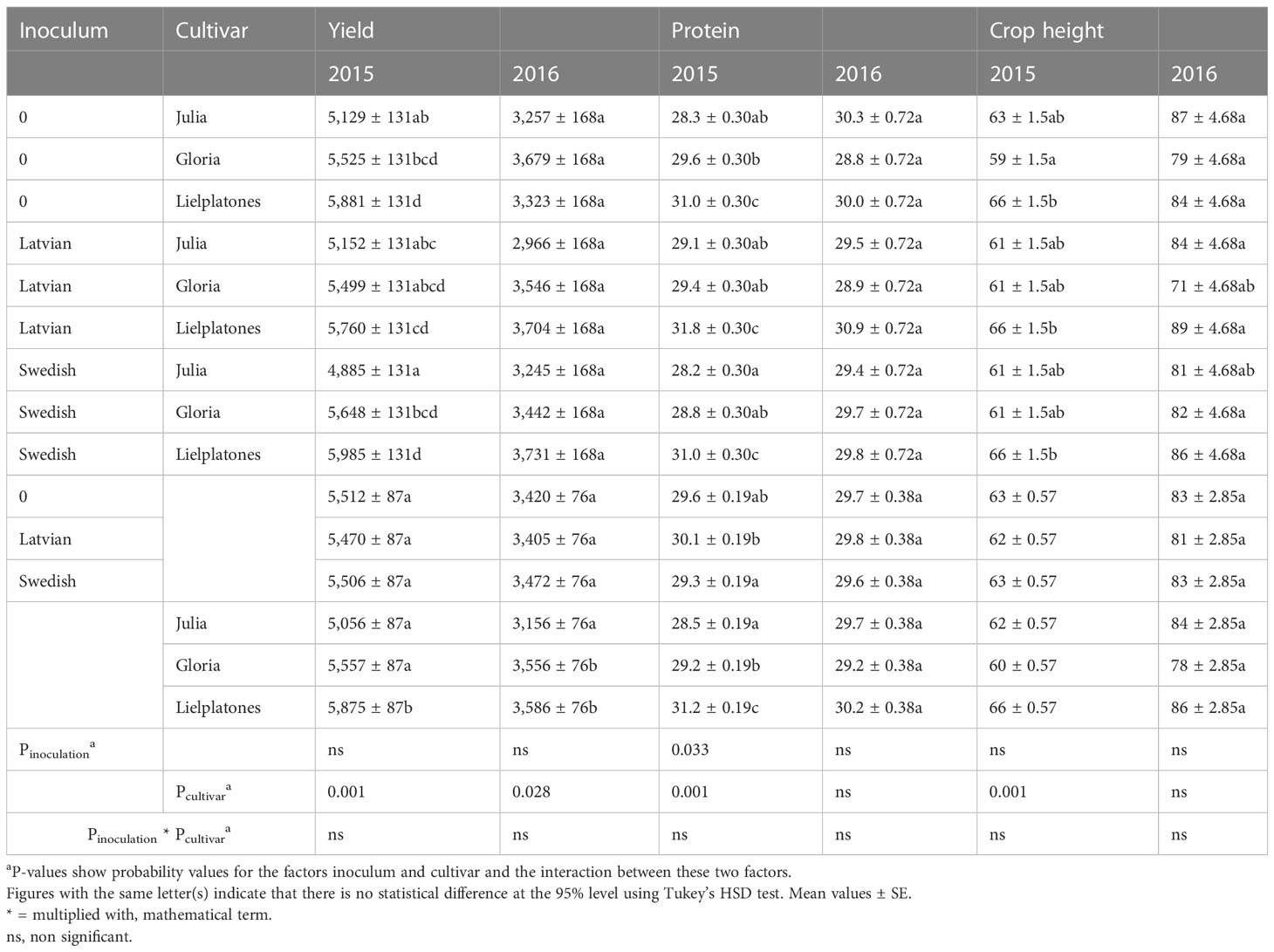
Table 2 Yields (kg ha−1), protein content (% of DM) and crop stand height (cm) of faba beans in the 2015 and 2016 trials.
Protein content varied between cultivars: 28.2%–29.1% of DM for ‘Julia’; 28.8%–29.6% for ‘Gloria’, and 31.0%–31.8% for ‘Lielplatones’, the latter having a significantly higher protein content than the other cultivars (p = 0.0014), regardless of inoculation strains including non-treated (Table 2).
There were generally small differences in crop stand heights (heights varied between 59 and 66 cm). The only significant variation (p = 0.0077) was recorded between ‘Lielplatones’ regardless of treatment and untreated ‘Gloria’ (Table 2).
The overall observation of marketable yields was like those of plant height and protein content: small non-statistical differences between ‘Julia’ and ‘Gloria’ with ‘Lielplatones’ generally significantly higher in yield than ‘Julia’ (Table 2). Furthermore, the analyses did not show any statistical interactions between cultivar and inoculum (treated or non-treated).
Compared to the 2015 trial, the yields were overall lower in 2016. ‘Lielplatones’ yielded 3.3 to 3.7 MT ha−1, ‘Julia’ (2.9 to 3.2 MT ha−1), and ‘Gloria’ (3.4 to 3.6 MT ha−1). There were no statistical differences in yield (p = 0.051) between inoculated and non-treated seeds, regardless of cultivar (Table 3).
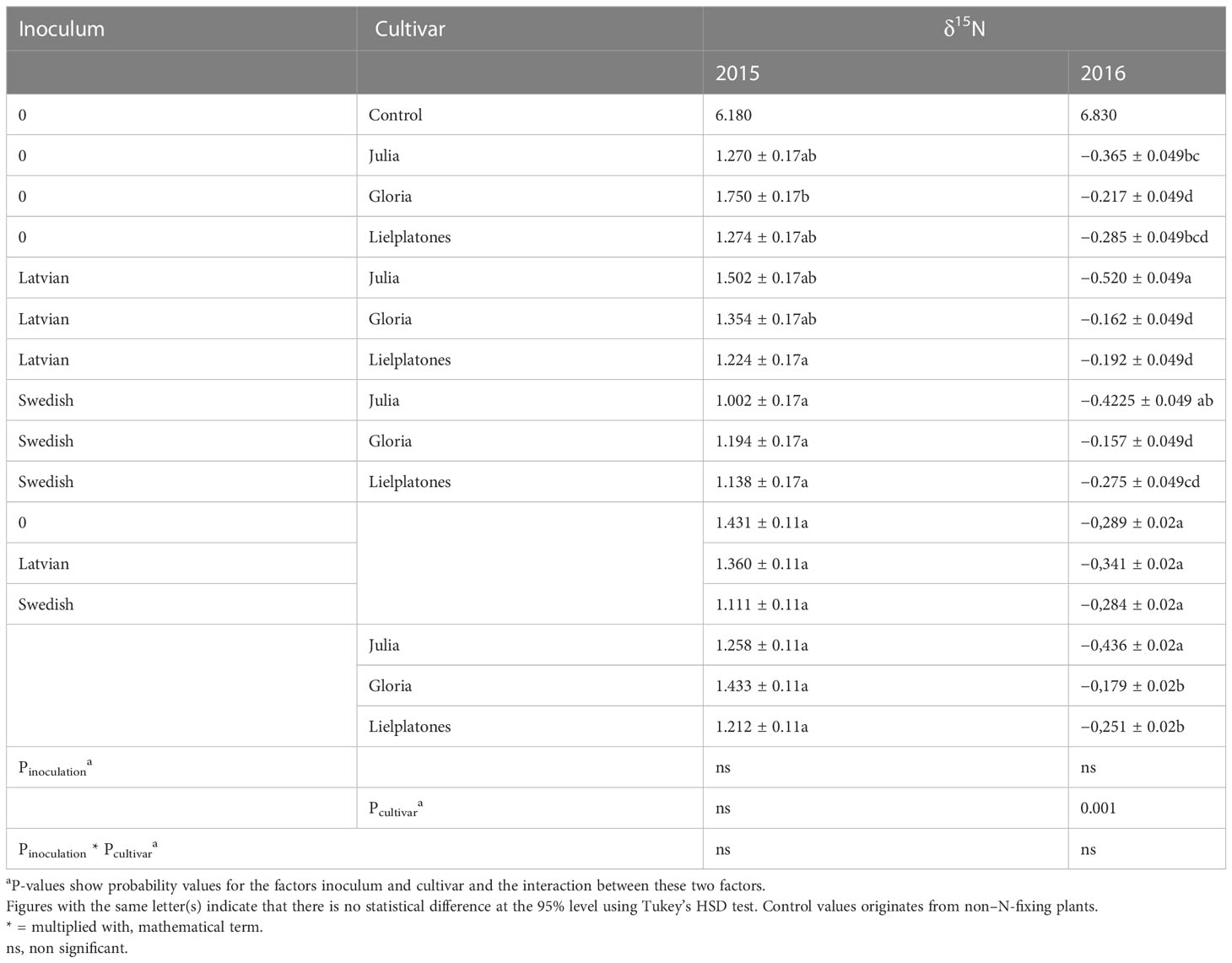
Table 3 Nitrogen fixation (δ15N) in different cultivars in presence or no presence of inoculum from either Latvia or Sweden in the field trials of 2015 and 2016.
Protein content varied slightly between cultivars: on average, 29.7% of DM for ‘Julia’, 29.2% for ‘Gloria’, and 30.3% for ‘Lielplatones’. There were no statistical differences neither between cultivars (p = 0.17) nor treatments (p = 0.6182).
There were differences in crop stand heights between treatments or cultivars. Plant heights varied between 71 and 87 cm, but none of these variations were statistically separated on the 95% level (p = 0.3037).
The δ15N values of analyzed faba bean samples ranged from 1.00 to 1.75 in the 2015 trials and from −0.52 to −0.16 in 2016. The δ15N in controls (non-legume plants) were 6.18 and 6.83, respectively (Table 3). These results clearly show that the bean plants have fixed N from air. The effect is more pronounced in 2016. However, as there are few statistical differences between inoculated and non-inoculated plots, the results do not show that inoculation, in general, improved the uptake of N from the air.
In 2015, there was no statistical difference between treatments (p = 0.182), but a highly significant difference between blocks (p = 0.01). In 2016, the δ15N values varied significantly between treatments (p = 0.0002). In 2016, the varieties ‘Gloria’ and ‘Lielplatones’ fixed N to a higher degree compared to Julia’ (p = 0.001) independent of inoculation strategy (inoculation vs. no inoculation and Swedish vs. Latvian).
Statistical analyses over inoculation treatments showed no significant differences between the two inoculum products (p = 0.75) and no statistical difference between the products and non-inoculated plots (p = 0.98).
To evaluate the effects of the faba beans and the two inoculums on the subsequent crop, we analyzed yields, number of heads, and protein content of spring wheat cropped in the previous plots of the bean trials. In the 2016 trial, wheat yields varied between 6.0 and 6.4 MT ha−1 and the number of heads varied between 527 and 586 (Table 4). There were no statistical differences in number of head (p = 0.2321), but marketable yield varied between cultivars (p = 0.036) and between inocula (p = 0.001). We registered a significant interaction between cultivar and inocula (p = 0.003).
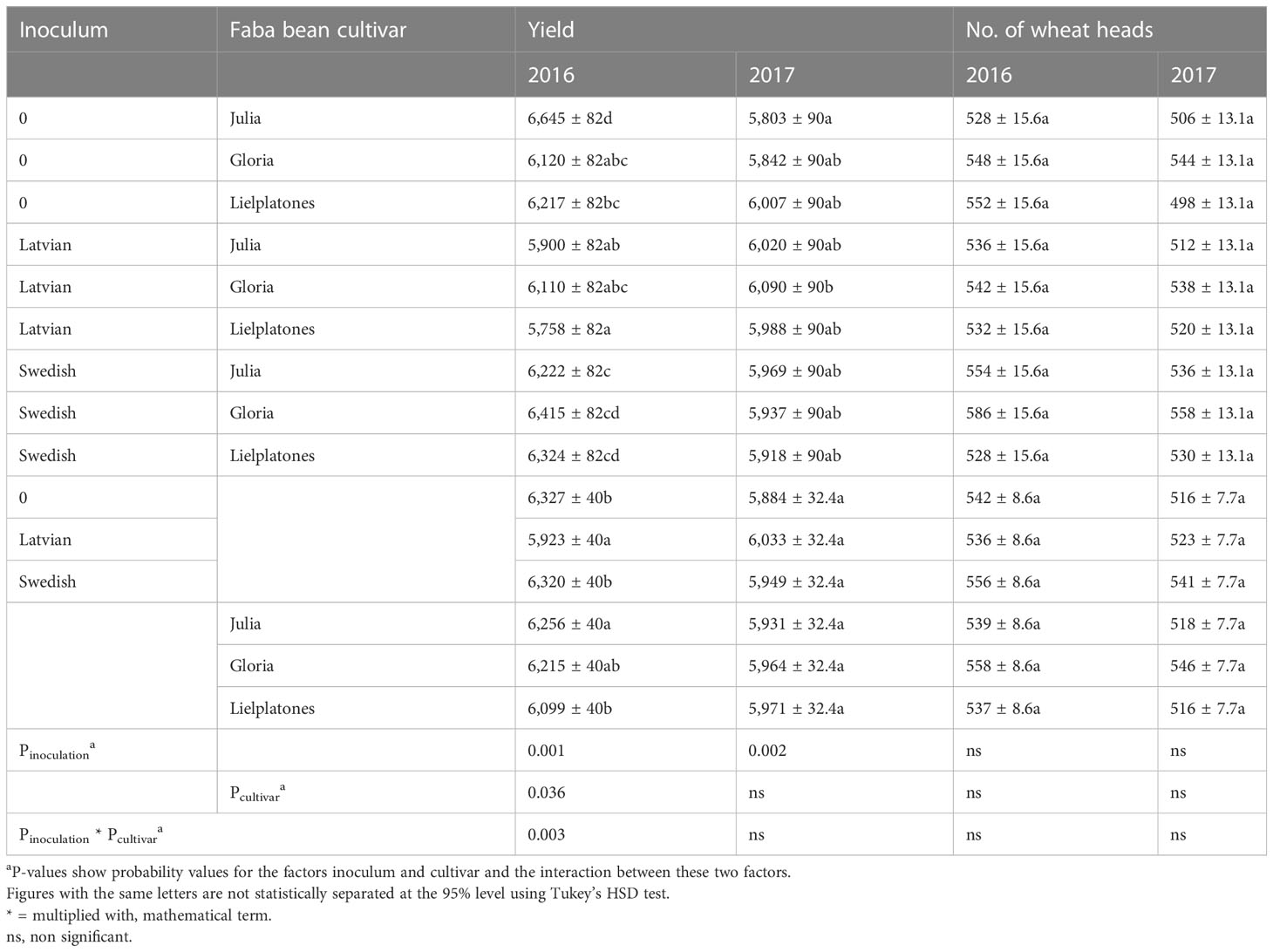
Table 4 Yields (kg ha−1) and number of heads (m−2) of spring wheat in 2016 and 2017 using the same plots as used for growing faba beans in 2015 and 2016, respectively.
The results of the 2017 trial were similar to those of 2016 (Table 4). There were no significant differences in wheat yield, number of head, or protein content. The number of heads m−2 varied between 498 and 558 (p = 0.3745), yields varied between 5.8 MT and 6.2 MT ha−1 (p = 0.3933), and protein content varied between 11.2% and 11.6% of dry matter (DM) (p = 0.4343). Top soil concentrations of mineral N after harvest of winter wheat are presented in Figures 4, 5.
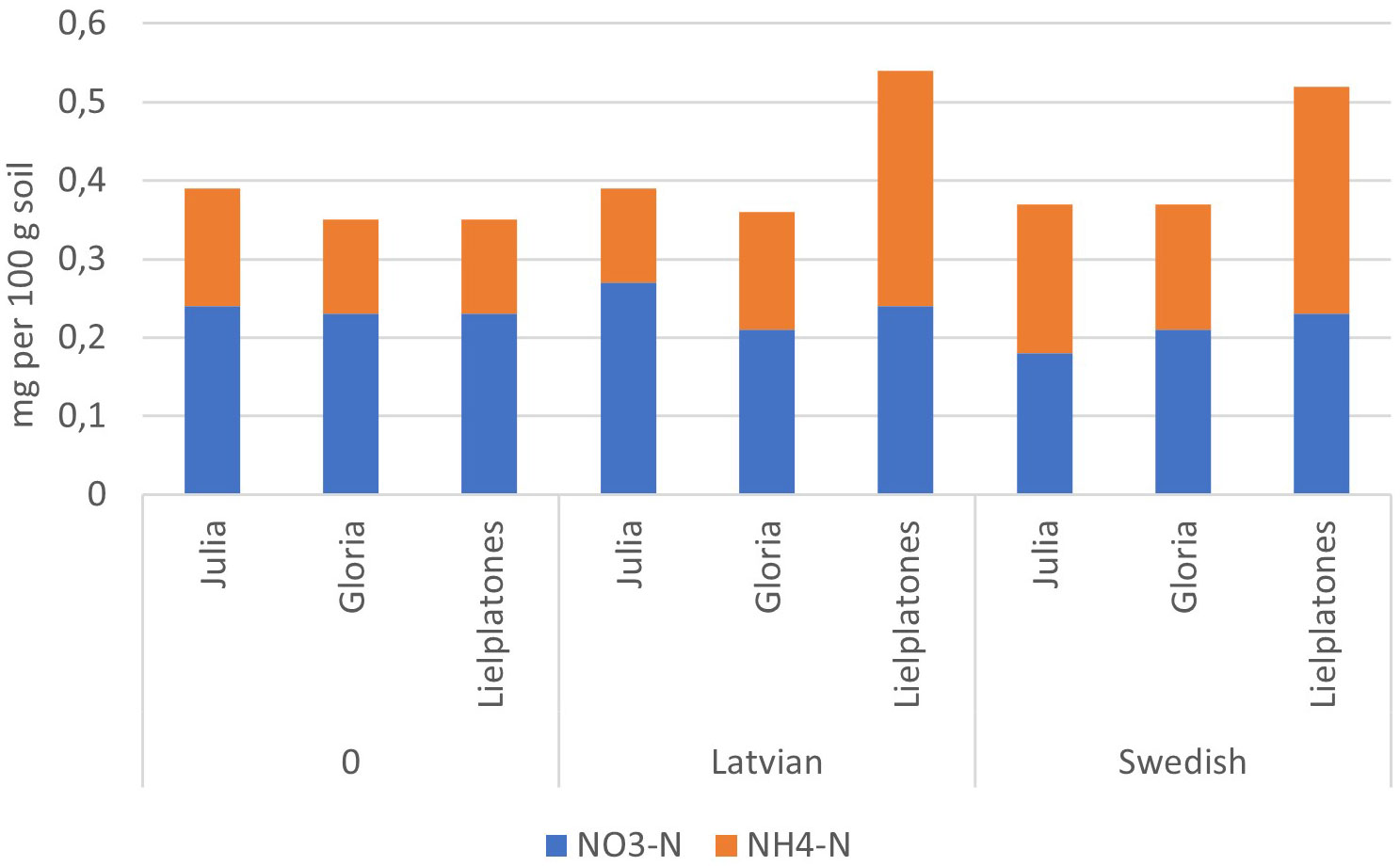
Figure 4 Soil mineral–N (g 100 g jord−1) in the top soil (depth of 0–20 cm) after harvest of winter wheat (2016) following on faba bean (2015).
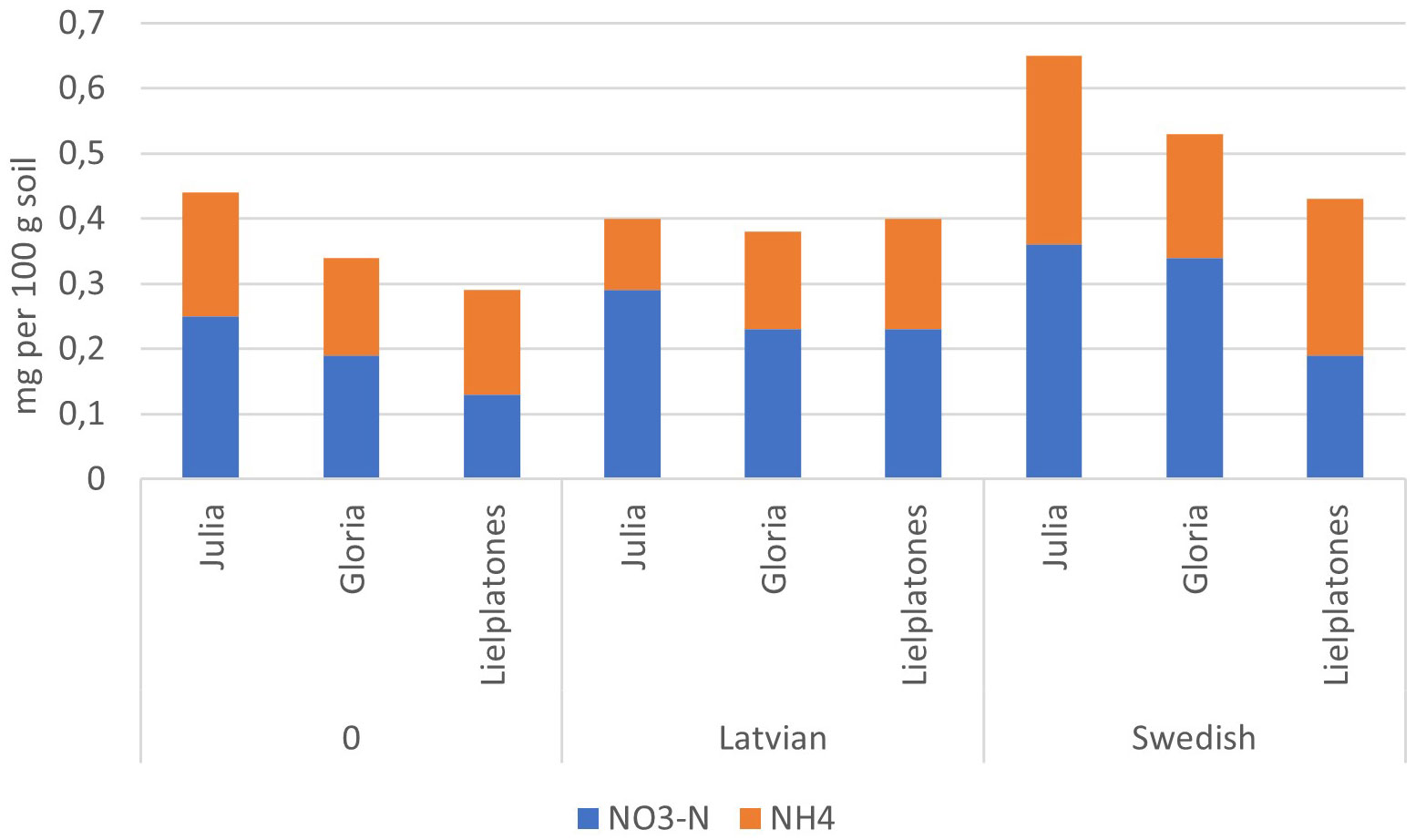
Figure 5 Soil mineral–N (g 100 g jord−1) in the top soil (depth of 0–20 cm) after harvest of winter wheat (2017) following on faba bean (2016).
Inoculation of legumes is generally carried out to improve the N2 fixation of the legume plants and thereby reduce the need for application of mineral or organic fertilizers (Amanuel et al., 2000; Dubova et al., 2015). Growing legumes can in some regions enhance yields of a following crop (Cuthforth et al., 2007; Habtemichial et al., 2007). A range of studies (e.g., Abdel-Ghaffar, 1988; Raposeiras et al., 2006; Uaboi-Egbenni et al., 2010; Youseif et al., 2017; Liu et al., 2019; and Guinet et al., 2020) have shown that inoculation of legumes such as faba beans, common beans, chickpeas, lentils, and field peas will result in, e.g., increased yields of these crops, increased N-content in soil, and increased yields of the succeeding crop. However, its known that nodulation and biomass production depend on the compatibility between faba bean genotype and rhizobium strain and its interaction with soil bio-physical conditions (Allito et al., 2021). Many studies, including those mentioned above, have focused on Rhizobia to enhance yields and to reduce the need of mineral fertilization in arid or semiarid regions (Allito et al., 2020), saline-sodic soils (Rai, 1992), acid soils (van Zwieten et al., 2015), or on regions previously not commonly cropped with faba beans. There are few recent studies on the effect of inoculation of common beans or faba beans under North European conditions. Early works by Barthel and Bjälfve (1930) and Bjälfve (1935) are still used as a basis for inoculation of legumes in Scandinavia. The results of our trials did, however, not support these early North European studies where inoculation of faba bean seeds improved the bean yield and accumulated N into the system as well as increase the yield of a subsequent crop. Furthermore, our 15N results show that native rhizobia present in the soil of non-inoculated plots were as effective as the inoculated strains in sustaining BNF, a finding also discussed by Irisarri et al. (2019).
The 15N abundance analyses of our trials showed that the ability to fix atmospheric N is not dependent on cultivar or presence of inoculum. Legumes, including Vicia faba, are known N-fixing crops, whereas the chosen controls in the trials are not able to fix atmospheric N. Thus, δ15N was significantly higher in the controls as expected. These results indicate that use of inoculum under Swedish cropping conditions does not increase N fixation and implies no effect on yields or subsequent crops.
In contrast to our results, Denton et al. (2017) noticed an increase in faba bean yield from 0.48 to 1.94 MT ha−1 using various inoculation products. However, this increase was noticed in eastern Australia where seeding normally takes place in dry soils prior to seasonal rains and legume cropping is an exception in the crop rotation. Sweden has a long tradition of cropping peas and faba beans, which likely has developed and maintained the BNF in the soils to a higher extent than in many other areas such as in Australia and parts of the Middle East. In line with our results, Zhang et al. (2010) reported a lack of yield increase from Rhizobium inoculation of bean, and they attributed this to a relatively high level of native rhizobia in the soil that annulled the effect of rhizobium inoculation.
Moreover, the N content in Swedish clay soils (a dominating soil type in Sweden and the soil type in the field trials) is generally high compared to in sandy soils in the Middle East or Northern Africa—a consequence of both soil type and climatic conditions. With more N in the soil, a somewhat lower effect of inoculation on the N-fixation and on yield could be expected, as the plant need for fixed N will be less pronounced. Moreover, Liu et al. (2019) reported that even a higher amount of BNF does not always correlate to a higher yield of seeds.
The cultivar ‘Julia’ generally yielded less than both ‘Gloria’ and ‘Lielplatones’, which was somewhat unexpected. Official statistics from cultivar testing in Sweden 2016 (Klingspor, 2017) shows that the average yield (MT ha−1) is 4.8 for ‘Julia’ and 4.6 for ‘Gloria’. White flowering cultivars are considered to yield less, in general, compared to multi-colored cultivars. The overall lower yields in our trials in 2016 are most likely connected to differences in weather conditions upon flowering (Figures 1, 2) with 26.2-mm precipitation in July 2016 compared to 39.6 mm in July 2015. Although faba beans have a deep tap root, plants are favored by cooler and wetter seasons due to a high soil water requirement. A drier season will also affect nutrient uptake negatively. As the trials were not fertilized, only nutrients already present in the soil or fixed from the air were available for the crop stand. Studies by Ntatsi et al. (2018) carried out in Greece on faba beans for fresh seed production investigated a wide range of factors influencing crop yield. Basically, their findings showed that protein content, pod length, and total yield were influenced by bean variety, whereas cropping system (conventional/organic farming) had a minor effect. Finnish studies (Lizarazo et al., 2015) found significant interactions between year and faba bean variety on protein content of the beans, probably connected with genotypic differences in N metabolism. In our study, there were no interactions between year and faba bean varieties on yields or protein content. This might be an effect of our choice of cultivars, soil conditions at the test site, and weather conditions in 2015–2016.
The two tested inocula did not affect yields, number of heads, nor protein content in the kernels of the subsequent spring wheat crop in 2017, but we did register the effects of faba bean cultivar as well as inocula on wheat yield in 2016. Although statistically significant, the result is inconclusive; the Latvian strain seemed to lower overall yields of wheat, whereas the Swedish inocula did not affect yields compared to untreated plots. Although the BNF of the faba bean crops most likely supplied the wheat with N, there seemed to be no increase in N supply by inoculation. These results support the lack of inoculation effect on BNF measured in the faba beans by the use of 15N. Soil type and season had probably a higher effect on wheat yield than choice of bean cultivar or inoculum product used in a previous crop and year.
Sweden has long history of faba bean cropping that most likely has resulted in natural and native Rhizobium strains in the soil. For Swedish conditions, there are few, if any, reasons to inoculate with rhizobia at cropping of faba beans in conventional agriculture. However, in the case that faba beans are seeded on sandy soils with low levels of plant available N and previously not known to be cropped with any legumes, an addition of commercially available Rhizobium can be beneficial.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
FF: Conceptualization, Methodology, Validation, Writing- Original draft preparation, Writing - Review and Editing. JÖ: Validation, Investigation, Writing- Original draft preparation, Writing - Review and Editing. ÅM: Validation, Investigation, Writing- Original draft preparation, Writing - Review and Editing. All authors contributed to the article and approved the submitted version.
The project was financed by European Union’s Seventh Framework Programme for Research, Technological Development and Demonstration under grant agreement no 613781.
The authors wish to thank the staff at Hushållningssällskapet Västmanland for their help in maintenance of the field trials.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdel-Ghaffar A. S. (1988). “Effect of edaphic factors on biological nitrogen fixation in vicia faba under Egyptian field conditions,” in Nitrogen fixation by legumes in Mediterranean agriculture, Eds. Beck D. P., Materon L. A. (Developm. Plant Soil Sci.) (The Netherlands: Springer Dordrecht).
Allito B. B., Ewusi-Mensah N., Logah V. (2020). Legume-rhizobium strain specificity enhances nutrition and nitrogen fixation in faba bean (Vicia faba l.). Agronomy 10, 826. doi: 10.3390/agronomy10060826www.mdpi.com/journal/agronomy
Allito B. B., Ewusi-Mensah N., Logah V., Hunegnaw D. K. (2021). Legume-rhizobium specificity effect on nodulation, biomass production and partitioning of faba bean (Vicia faba l.). Sci. Rep. 11, 3678. doi: 10.1038/s41598-021-83235-8
Amanuel G., Kühne R. F., Tanner D. G., Vlek P. L. G. (2000). Biological nitrogen fixation in faba bean (Vicia faba l.) in the Ethiopian highlands as affected by p fertilization and inoculation. Biol. Fertil Soils 32, 353–359. doi: 10.1007/s003740000258
Barthel C., Bjälfve G. (1930). Baljväxtodling med bakteriekulturer. erfarenheter och praktiska försök. Bakteriologiska avdelningen, Report 52. (Stockholm: Centralanstalten för försöksväsendet på jordbruksområdet).
Barthel C., Rhodin S. (1914). Försök med kulturer av baljväxtbakterier för blå lupin och blå lucern. Bakteriologiska avdelningen, Report 10. (Stockholm: Centralanstalten för försöksväsendet på jordbruksområdet).
Bjälfve G. (1935). Baljväxtbakteriernas rotknölar hos olika sorters; baljväxternas kvävehalt samt deras kvävehushållning i åkerjorden. Bakteriologiska avdelningen, Report 65. Stockholm.
Cubero J. I. (1974). On the evolution of vicia faba l. Theor. Appl. Genet. 45, 47–51. doi: 10.1007/BF00283475
Cuthforth H. W., McGinn S. M., McPhee K. E., Miller P. R. (2007). Adaption of pulse crops to the changing climate of the northern great plains. Agron. J. 99, 1684–1699. doi: 10.2134/agronj2006.0310s
Denton M. D., Philips L. A., Peoples M. B., Pearce D. J., Swan A. D., Mele P. M., et al. (2017). Legume inoculant application methods: effects n nodulation patterns, nitrogen fixation, crop growth and yield in narrow-leaf lupin and faba bean. Plant Soil 419, 25–39. doi: 10.1007/s11104-017-3317-7
Dijkstra P., Ishizu A., Doucett R., Hart S. C., Schwartz E., Menyailo O. V., et al. (2006). 13C and 15N natural abundance of the soil microbial biomass. Soil Biol. Biochem. 38, 3257–3266. doi: 10.1016/j.soilbio.2006.04.005
Dubova L., Senberga A., Alsina I. (2015). The effect of double inoculation on the broad beans (Vicia faba l.) yield quality. Res. Rural Dev. 1, 34–39.
Duc G. (1997). Faba bean (Vicia faba l.). Field Crops Res. 53, 99–109. doi: 10.1016/S0378-4290(97)00025-7
Elsheikh E. A., Elzidany A. A. (1997). Effects of rhizobium inoculation, organic and chemical fertilizers on yield and physical properties of faba bean seeds. Plant Foods Hum. Nutr. 51, 137–144. doi: 10.1023/A:1007937614660
Guinet M., Nicolardot B., Voisin A. S. (2020). Nitrogen benefits of ten legume pre-crops for wheat assessed by field measurements and modelling. Europ. J. Agron. 120, 126–151. doi: 10.1016/j.eja.2020.126151
Habtemichial K. H., Singh B. R., Aune J. B. (2007). Wheat response to N2 fixed by faba bean (Vicia faba l.) as affected by sulfur fertilization and rhizobial inoculation in semi-arid northern Ethiopia. J. Plant Nutr. Soil Sci. 170, 412–418. doi: 10.1002/jpln.200625006
He X., Xu M., Qiu G. Y., Zhou J. (2009). Use of 15N stable isotope to quantify nitrogen transfer between mycorrhizal plants. J. Plant Ecol. 2, 107–118. doi: 10.1093/jpe/rtp015
Irisarri P., Cardozo G., Tartaglia C., Reyno R., Gutierrez P., Lattanzi F. A., et al. (2019). Selection of competitive and efficient rhizobia strains for white clover. Front. Microb. 10. doi: 10.3389/fagro.2021.796717
Klingspor. (2017). Johan Klingspor, Scandinavian seed AB, Lidköping, Sweden. personal communication 2017.
Liu L., Knight J. D., Lemke R. L., Farrell R. F. (2019). A side-by-side comparison of biological nitrogen fixation and yield of four legume crops. Plant Soil 442, 169–182. doi: 10.1007/s11104-019-04167-x
Lizarazo C. I., Lampi A.-M., Liu J., Sontag-Strohm T., Piironen V., Stoddard F. L. (2015). Nutritive quality and protein production from grain legumes in a boreal climate. J. Sci. Food Agric. 95, 2053–2064. doi: 10.1002/jsfa.6920
Moawad H., Beck D. P. (1991). Some characteristics of rhizobium leguminosarum isolates from uninoculated field-grown lentil. Soil Biol. Biochem. 23, 933–937. doi: 10.1016/0038-0717(91)90173-H
Ntatsi G., Karkanis A., Yfantopoupos D., Olle M., Travlos I., Thanopoulos R., et al. (2018). Impact of variety and farming practices on growth, yield, weed flora and symbiotic nitrogen fixation in faba bean cultivated for fresh seed production. Acta Agric. Scand. Section B – Soil Plant Sci. 68, 619–630. doi: 10.1080/09064710.2018.1452286
Paul E., Melillo J., Knowles R., Blackburn H. (2012). Nitrogen isotope techniques (London, UK: Academic Press Inc.).
Rai R. (1992). Effect of nitrogen levels and rhizobium strains on symbiotic N2 fixation and grain yield of phaseolus vulgaris l. genotypes in normal and saline-sodic soils. Biol. Fertil Soils 14, 293–299. doi: 10.1007/BF00395466
Raposeiras R., Marriel I. E., Scotti Muzzi M. R., Paiva E., Pereira Filho I. A., Costa Carvalhais L. C., et al. (2006). Rhizobium strains competitiveness on bean nodulation in cerrado soils. Pesq. Agropec. Bras. 41 (3). doi: 10.3389/fendo.2013.00006
Rodelas B., Gonzalez-Lopez J., Pozo C., Salmeron V., Martinez-Toledo M. V. (1999). Response of faba bean (Vicia faba l.) to combined inoculation with azotobacter and rhizobium leguminosarum bv. viceae. Appl. Soil Ecol. 12 (1), 51–59.
SJV, (Swedish board of agriculture). (2019). Jordbruksstatistik sammanställning 2019, kap. 11. Available at: https://www2.jordbruksverket.se/download/18.2532524316cca0df48ab2548/1566885388130/JS_2019v2.pdf.
SCB. (2019). Statistics Sweden. Available at: www.scb.se/en/.
Uaboi-Egbenni P. O., Okolie P. N., Okafor C. N., Akinyemi O., Bisi-Johnson M. A., Teniola O. D. (2010). Effects of soil types and mixtures on nodulation of some beans and groundnuts varieties. Afr. J. Food Agriculture Nutr. Dev. 10 (3), 2272–2290. doi: 10.4314/ajfand.v10i3.54083
van Zwieten L., Rose T., Herridge D., Kimber S., Rust J., Cowie A., et al. (2015). Enhanced biological N2 fixation and yield of faba bean (Vicia faba l.) in an acid soil following biochar addition: dissection of casual mechanisms. Plant Soil 375, 7–20. doi: 10.1007/s11104-015-2427-3
Youseif S. H., Abd El-Megeed F., Saleh S. (2017). Improvement of faba bean yield using rhizobium/agrobacterium inoculant in low-fertility sandy soil. Agronomy 7, 2–12. doi: 10.3390/agronomy7010002
Keywords: legume cropping, horse bean, soil improvement, nitrogen, wheat
Citation: Fogelberg F, Östlund J and Myrbeck Å (2023) Effect of cultivar and inoculant on yields of faba beans (Vicia faba minor) and subsequent spring wheat (Triticum aestivum) under Scandinavian cropping conditions. Front. Agron. 5:1179996. doi: 10.3389/fagro.2023.1179996
Received: 05 March 2023; Accepted: 28 April 2023;
Published: 25 May 2023.
Edited by:
Fernando Alfredo Lattanzi, National Institute for Agricultural Research (INIA), UruguayReviewed by:
Cristina Abbate, University of Catania, ItalyCopyright © 2023 Fogelberg, Östlund and Myrbeck. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fredrik Fogelberg, RnJlZHJpay5Gb2dlbGJlcmdAcmkuc2U=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.