- 1Ethiopian Institute of Agricultural Research, Ambo Agricultural Research Center, Ambo, Ethiopia
- 2International Center of Agricultural Research in the Dry Areas, Rabat, Morocco
- 3Ethiopian Institute of Agricultural Research, Addis Ababa, Ethiopia
- 4Department of Crop Productions, Faculty of Agriculture, University of Eswatini, Luyengo, Eswatini
Crenate broomrape, Orobanche crenata Forskal belongs to the family Orobanchaceae. It is a root holoparasitic weed devoid of chlorophyll and entirely dependent on host plants for its growth requirement. This parasite considerably infects plant species of the families Leguminosae, Apiaceae, and Asteraceae in highly infested drier and warmer areas of the world. It is well-known for its devastating effects on cool-season food legumes especially on faba bean (Vicia faba L.) and for threatening the livelihood of subsistence farmers. Yield losses of infected crops reach up to 100% depending on the level of infection by the parasitic weed. The long-term impact of the parasite is even more serious than its short-term effects as its numerous minute seeds are easily spread using different mechanisms and persist in the soil for up to 20 years. Besides, its management is difficult as no single method proved to be effective, economical, and complete in managing the weed. This review article gives an overview of information on the biological characteristics and harmful effects of crenate broomrape and summarizes scientifically proven management techniques for its effective management. Different approaches should be validated, demonstrated, and scaled for wider uses to manage crenate broomrape sustainably and boost the host crop productivity. Therefore, besides narrowing the knowledge gaps on the weed biology more strengthened efforts in searching for host plant resistance and/or tolerance based integrated management approaches by considering socio-economic and ecological conditions of faba bean growers are found paramount importance.
Introduction
Parasitic weeds infect economically important crops and seriously threaten the livelihoods of small-holder farmers. Upon infection, they exploit other flowering plants for water, nutrients, metabolites, and hormones with the help of one or more haustoria (Restuccia et al., 2009). Parasitic plants from the families Convolvulaceae, Loranthaceae, Viscaceae, and Orobanchaceae grow on their host plants (Joel, 2009; Heide-Jorgensen, 2011). Orobanchaceae are by far the largest family of parasitic plants with a large number of species but only a few are considered as important in leading to economic losses (Lambrada, 2008; Joel, 2009; Parker, 2009; Heide-Jorgensen, 2011).
Crenate broomrape (Orobanche crenata Forskal) is a root holoparasite belonging to the family Orobanchaceae, which infects various dicot plants (Parker and Riches, 1993; Bennett and Mathews, 2006; Joel, 2009; Heide-Jorgensen, 2011). It causes the most widespread damage on cool-season food and forage legumes in northern Africa, southern Europe, and western Asia countries (Joel, 2009; Restuccia et al., 2009), and also occurs sporadically on vegetables like carrot, eggplant, and tomato (Linke et al., 1993; Dahan and El-Mourid, 2004; Matusova and Bouwmeester, 2005; Rubiales et al., 2006; Lambrada, 2008).
Cool-season legumes [faba bean (Vicia faba L.), field pea (Pisum sativum L.), lentil (Lens culinaris Medik), grass pea (Lathyrus sativus L.), and chickpea (Cicer arietinum L.)] are cultivated for food, income generation, animal feed, and also as break crops to improve soil fertility and reduce pest infestation. Crenate broomrape causes high levels of damage on cool-season legume crops, in terms of both yield and quality, mainly because of its close underground physiological connection with host plants. It is especially damaging faba bean, peas and lentils, and has seriously reduced areas planted to these crops mainly around the Mediterranean region and Middle East countries (Parker, 2002, 2009).
Cool-season legume crops are grain legumes widely cultivated in Ethiopian farming systems. Faba bean is one of the major legume crops with various benefits for the farming community when included in cropping systems. It occupies the largest share of area under pulses and the production volume of pulse crops. It had occupied about 0.45 million hectares (Mha) of land with an annual production of about 0.85 metric tons (MT) and a productivity of 1.9 tha−1 (CSA, 2016). FAO (2019) reported that Ethiopia is cultivating faba bean on 0.519 Mha of land, producing on average 0.92 MT annually, and having a 21.03% share of the global production. This makes the country the second largest faba bean growing country in the world after China; the country that recorded 0.933 mil ha land, 1.64 mil ton production and 37.26% share of the world production.
Since its introduction in the northern part of Ethiopia in the 1980s, crenate broomrape parasitism on legume crops, especially on faba bean, has become increasingly important (Besufekad et al., 1999; Abebe et al., 2013). Faba bean cultivation is strongly hampered by the occurrence of the parasite, which threatens the livelihoods of many farmers (Besufekad et al., 1999; Rezene and Kedir, 2006; Abebe et al., 2013). According to these authors, yield losses of faba bean due to the weed interference can reach up to 100%, depending on the levels of infection in the crop, which vary with infestation levels of the growing fields, host-parasite interaction, and environmental conditions.
Different cultural practices have been tried to manage crenate broomrape in faba bean fields (Abebe et al., 2013; Mekonnen, 2016). So far, no effective management practices have been developed and adopted. Unrestricted and continuous spread and dense presence of the weed force farmers to scarify the cultivation of faba bean, the most valuable host crop, resulting in substantial reductions in both cultivated areas and production (Abebe et al., 2013). These have also limited the traditional practice of crop rotation, hindering the nutritional, economic, and ecological benefits from the diversified cropping systems. Although the parasitic weed is spreading and affecting the livelihoods of food legume growers, little emphasis is placed on developing knowledge and management practices.
The development of effective containment or management strategies depends on a better knowledge of the biology and physiology of crenate broomrape. Identity, distribution, host ranges, detailed knowledge of the specific mechanisms of parasitism, damage caused, and possible management options need to be studied for this obscure parasitic weed. As there is no standard integrated management “package” developed for the parasite that can be implemented, a relevant integrated management strategy needs to be developed and adjusted to meet the individual cropping system, local needs, and farmer preferences. To reduce the elevated infestation and impacts of the parasite, there is a strong need for integrated management practices based on host plant resistance and tolerance, taking into account the host-parasite association and the prevailing socio-economic and ecological conditions. The parasitic weed has, in general, considerable negative implications in farm productivity and in the country's economic development at large. Therefore, the aim of this article is to give an overview of the biology, economic impact, and management practices of crenate broomrape that can benefit researchers, development agents, and farming communities, mainly in Ethiopia.
Biological Characteristics of Crenate Broomrape
The Orobanchaceae family has more than 150 species, but only a few are considered economically important on agricultural crops (Lambrada, 2008; Joel, 2009; Parker, 2009; Heide-Jorgensen, 2011). The most severe species are O. crenata Forskal, Orobanche cumana Wallr, Orobanche cernua Loefl, Orobanche foetida Poiret, Orobanche minor Sm., Phelipanche ramosa (L.) Pomel, and Phelipanche aegyptiaca (Pers.) Pomel. The two genera Orobanche and Phelipanche were commonly named broomrape indicating the damage and impact made by these parasites on their host crops.
Crenate broomrape (O. crenata) naturally has unique morphological characteristics such as unbranched stem and dense inflorescence, which is usually white with purple veins (Lambrada, 2008). The plant has no green parts, with only leafless flowering stem up to 100 cm high, bearing alternate scales <2 cm long visible above the ground. The spike is loosely flowered, interrupted below, and continuous above. The base of the stem, below the ground, is normally swollen and tuberous. It is a short-lived plant growing on the roots of broadleaf host plants to obtain its growth requirement. Mathieu et al. (2012) reported that crenate broomrape has diploid chromosome numbers (2n = 38). The plant is normally outcrossing but is facultatively autogamous. The occurrence of allogamous O. crenata as a native species is unknown, like the exclusive in crop fields' appearance of autogamous Striga aciatica but unlike the allogamous S. hermontica (Roman et al., 2002).
The main host crops of the parasite are restricted to the families Leguminosae, Apiaceae, and Asteraceae but sporadically occurred on crops under the families Cucurbitaceae, Solanaceae, Lamiaceae, and Ranunculaceae (Parker, 2013). It has been reported by various authors and reviewers; Rezene and Kedir (2006), Joel et al. (2007), Abbes et al. (2008), and Restuccia et al. (2009) that crenate broomrape is parasitic on a wide range of legumes such as faba bean (V. faba L.), field peas (P. sativum L., P. sativum var. abyssinicum), lentil (L. culinaris Medik), chickpea (C. arietinum L.), vetch (Vicia sativa L.), clovers (Trifolium spp.), grass pea (L. sativus L.), and groundnut (Arachis hypogaea L.). Also, it infects carrot (Daucus carota L.), safflower (Carthamus tinctorious L.), geraniums (Geranium spp.), lettuce (Lactuca sativa L.), parsley [Petroselinum crispum (Mill.) Nym], celery (Apium graveolens Dulce), cumin (Cuminum cyminum L.), eggplant (Solanum melongena L.), tomato (Lycopersicon esculentum Mill.), verbenas (Verbena spp.), and many other cultivated vegetable, oil, spice, and ornamental plant species.
Normal development of the parasite starts with seed germination that comes in response to the reception of a chemical stimulus from host roots following a period of conditioning that is required for dormancy-germination transition (Zhou et al., 2004). According to Uematsu et al. (2007), germination preceding seeds requires an after-ripening period and next specific preconditioning, which has been linked with water imbibition and production of gibberellins (GAs) that seem to be the prerequisites for germination. A moist environment is required for several days together with suitable temperatures before the mature seed is responsive to germination stimulants. This preparatory period is known as conditioning or preconditioning.
Optimum temperatures for the conditioning and germination of crenate broomrape are in the region of 15–20°C (Restuccia et al., 2009), but prolonged exposure to these temperatures in the absence of a stimulant leads to secondary dormancy, while much higher temperatures are inhibitory (Timko et al., 1989; Joel et al., 1995; Kebreab and Murdoch, 1999). It only germinates in response to specific chemicals released by the host plant, host-derived strigolactones at the time of host detection (Das et al., 2015). The conditioned seeds remain responsive to germination stimulants for several months (Bouwmeester et al., 2007). Their ability to respond to germination stimuli fades gradually when the seeds dry and then they remain dormant until reconditioned. Natural strigolactones that are isolated and identified to date are strigol, alectrol, and orobanchol (Matusova and Bouwmeester, 2005; Yoneyama et al., 2009).
Crenate broomrape spends most of its life cycle underground where it undergoes processes of conditioning, the reception of host-encoded stimulus molecules, germination, chemotrophic radicle growth toward host root, haustorium differentiation from the radicle, haustorial penetration of the host, the formation of vascular connection with the host, acquisition of host nutrients, and the initiation and development of tubercles before the emergence of shoots and the diagnosis of infection establishing the host-parasite relationship (Dorr and Kollmann, 1995; Joel et al., 2007; Fernandez-Aparicio and Rubiales, 2011; Westwood, 2013).
Seed conditioning of crenate broomrape induces a decrease in abscisic acid (ABA) levels and GAs synthesis. Interestingly, experimentation carried out on broomrapes specialized in summer crops revealed their lower requirement for conditioning when compared with species specialized in winter annual crops highlighting the ecological adaptation of these parasites to the cropping system in which they become specialized (Plakhine et al., 2009). Crenate broomrape seedling must have contact with a host root immediately after the germination from such a minute seed size to ensure its survival. It is a complete root parasite with devastating effects on many legume crops (Fernandez-Aparicio et al., 2012).
The life cycle of the parasite is strongly cued to that of its host (Figure 1). The life cycle of root parasitic angiosperms, i.e., orobanche, phelipanche, or striga is very similar to each other, comprising an independent and a proper parasitic phase (Joel, 2000). Upon host-induced germination, the seed embryo develops only a radicle independently with the functions of searching and contacting the host root. At the parasitic phase, the plant depends totally on its host for all nutrients and becomes an active sink comparable to an actively growing part of the host plant itself. Shortly after host penetration and connection, the parasite begins its heterotrophic growth at the expense of host resources. The haustorial structure allows intimate contact between the parasite and host, with consequent absorption of water and nutrients from the host plant (Hibberd et al., 1999), hormones (Suzuki et al., 1994), toxins (Rank et al., 2004), and almost everything able to travel through vascular connections, including genes (Mower et al., 2004).
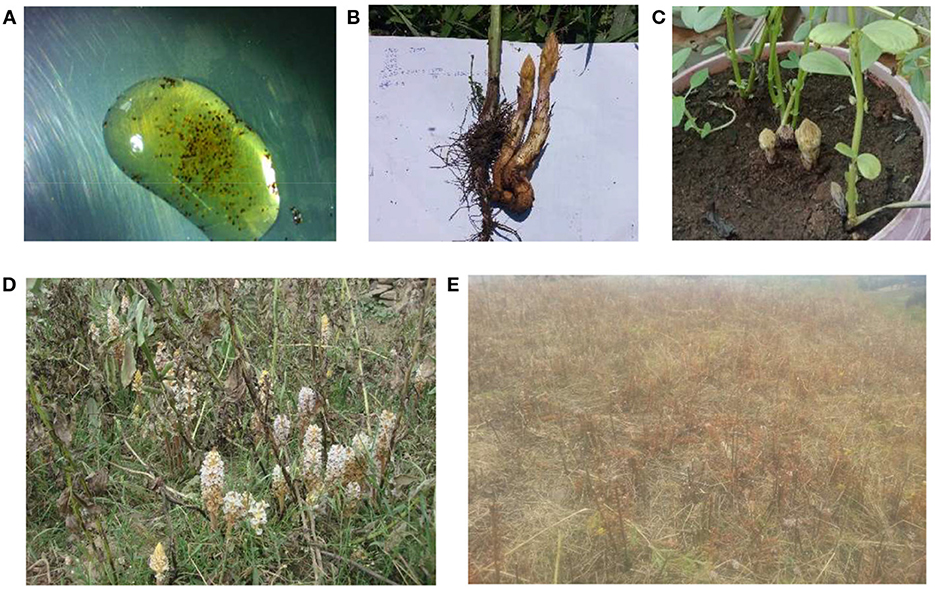
Figure 1. Life cycle of crenate broomrape: (A) seeds in water bubble, (B) underground tubercles, (C) emerging shoots, (D) flowering shoots, and (E) seeds shedding mature parasitic plant.
A few days after the host vascular connection, the part of the crenate broomrape seedling that remains outside the host root develops into a storage organ called a tubercle. As the tubercle matures, a crown of adventitious roots will emerge from it carrying a capacity of developing lateral haustorial connections. Underground shoots also develop from the tubercle, which eventually emerges through the soil surface leading to the development of reproductive organs. The particular characteristics of the parasite like underground development and attachment to host roots hamper the development of effective management strategies (Joel et al., 2007).
Most damages from crenate broomrape occurred before it emerged from the ground, and only 10–30% of the attached parasites emerged above the ground (Sauerborn, 1991). In crop plant fields, the parasite has now been found to be well-nourished, allowing its plant population to develop extremely well and sets a lot of seeds contained in capsules. The plant set about half a million-minute seeds per season, ranging in size from 0.2 to 2 mm, which are easily disseminated over long distances by irrigation and/or flood water, wind, animals, and human beings, and persist in the soil viable up to 20 years (Joel et al., 2007; Gevezova et al., 2012; Habimana et al., 2014). Thus, a single plant of the parasite is capable of heavily increasing soil seed bank and/or rapidly infesting new fields.
The important spreading agent is the uncontrolled grazing of animals (Panetta and Lawes, 2005). The seeds remain viable after passing through the alimentary system of animals, as result manure can be contaminated with its viable seeds. The seeds can also easily adhere to the hairs and feet of animals, and transfer to new fields upon the animals' free transference from infested to weed-free fields. Farm products, like seeds or hay, and farm tools can carry the weed seeds if contaminated in the infested field(s).
Crenate broomrape is a thermophilic plant that frequently requires dry conditions and light soils to be invasive. Because low atmospheric humidity ensures a high rate of transpiration, which enhances the movement of water and the solute from the host plant, it is speculated that the parasite occurrence is favored by such a condition (Parker and Riches, 1993). According to Mohamed et al. (2006), scenario analyses of climatic changes taking the form of increased temperatures and drought in many areas of the world could pose greater threats to the host crop production due to Orobanche spp. infestation. In addition, Parker (2014) indicated that where there is moisture stress and poor soils, there can be greater damage to the point of total crop failure due to Orobanche spp. It is likely that a negative effect of some agricultural pests can increase with a rapid climate change that results in favorable growth conditions for the parasite and environmental stress on host crops reducing their resistance and/or tolerance, particularly in less intensively managed production systems.
According to Fernandez-Aparicio et al. (2016a), many of Orobanche spp. traits such as the lack of photosynthesis, underground parasitism with the late appearance of shoots, prolific seed production, complex mechanisms of seed dispersal and longevity, and a physical and metabolic overlap with host crops or the lack of functional roots reduced the efficiency of conventional management programs. These provide the parasite species with great genetic adaptability to environmental changes, including host resistance, agronomical practices, and herbicide treatments (Joel et al., 2007). Due to this, available management efforts against broomrapes have not proven as effective, economical, and applicable as predicted (Goldwasser et al., 2008; Perez-de-Luque et al., 2010).
Impacts of Crenate Broomrape
The cultivation of cool-season food legumes is strongly hampered by the occurrence of crenate broomrape threatening the livelihoods of many farmers around the Mediterranean region. Upon infection, it causes severe and visible damage to host plants, including wilting, a reduction in stands, flower dropping, severe yield losses, and poor seed quality (Habimana et al., 2014; Fernandez-Aparicio et al., 2016a). Moreover, early and dense infection of the parasite can kill the host plant before physiological maturity. The earlier symptoms on the host plant can be observed even before the emergence of the weed shoots.
Crenate broomrape causes high levels of losses on host crop yield and seed quality. Yield losses reach up to 100% depending on the level of infection, which also depends on the extent of infestation of an area, host species resistance and/or tolerance, soil fertility, and weather conditions during crop growth (Lambrada, 2008; Habimana et al., 2014; Fernandez-Aparicio et al., 2016a). Heavy infestation of the parasite not only leads to a complete crop failure but also makes the field sick for many years. These situations pose a serious economic concern and necessitate effective management of the parasite. The species is aggressive in nature causing the most widespread damage to its host crops and tolerant to management measures (Dahan and El-Mourid, 2004). According to Fernandez-Aparicio et al. (2016a), the infection due to broomrape caused reductions in host biomass in both vegetative and reproductive organs, the latter being more affected. Infection at later stages affects host development, flowering, and fruiting by causing chlorosis or inhibiting growth. In many cases, due to parasitic infection, the number of host flowers was reduced, pollinated flowers shed, fruits drop before ripening, or fruits do not grow to their optimal size.
Crenate broomrape causes the most widespread damage, and it is considered a major constraint and also remained a constant threat to the cultivation of host crops in Ethiopia. It has increasingly become the actual threat in cultivating economically important cool-season legume crops in Amharara and the Tigray Regional States, as reported since the 1990s (Asefa and Endale, 1994; Adugna et al., 1998; Besufekad et al., 1999). Furthermore, Rezene and Kedir (2006) and Abebe et al. (2013) reported that it infects seriously the faba bean and also infects field peas, lentils, chickpea, and vetch. This parasite is found to be a serious problem in the country due to many major reasons. Initially, the host crop is widely grown, the local varieties are found to be susceptible to its infection in different locations, and both the parasite and its management methods are unknown by the growers of this area. Moreover, dry weather conditions, light soil, and poor soil fertility of the areas are found to be favorable for its invasion and also farmers of the areas are practicing less intensively managed production systems.
In the world, faba bean can be parasitized mainly by the three different species of broomrape, namely, O. crenata (crenate broomrape), O. foetida (foetida broomrape), and P. aegyptiaca formerly O. aegyptiaca (Egyptian broomrape) (Rubiales et al., 2006; Joel et al., 2007). The crenate broomrape that causes damage on faba bean is the commonest in countries adjacent to the Mediterranean. It extends sporadically eastwards as far as Pakistan and India, southwards into northern Africa, and northwards into southeastern Europe as indicated in Figure 2 (CABI, 2021).
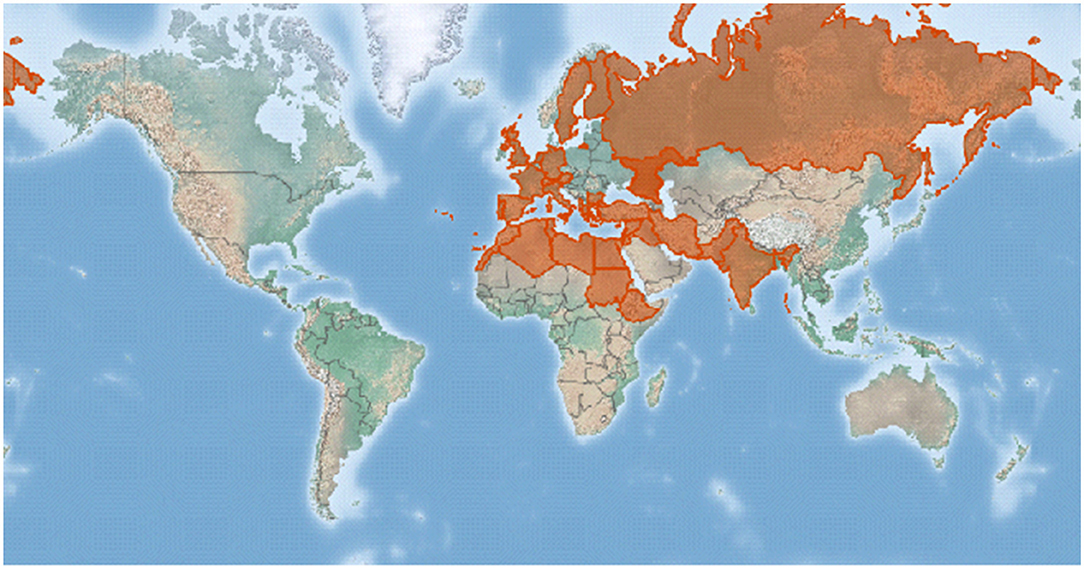
Figure 2. A map showing the crenate broomrape distribution represented by a red color in the world after CABI (2021).
It is speculated that crenate broomrape was introduced to the northern part of Ethiopia from abroad unknowingly with grains meant for food aid in the 1980s (Besufekad et al., 1999). Initially, it was a low-lying warm area problem but later on it has become a problem in high altitudes too (Abebe et al., 2013; Mekonnen, 2016). The spread of the parasite in Amhara and Tigray Regional States within a few years of its introduction demonstrates the limited efforts done in managing its spread and impacts. For instance, the community failed to implement the suggested joint program by agricultural researchers to eradicate, contain, or manage the species on the specific site where it is found essential (Rezene, 1998; Rezene and Kedir, 2006).
Crenate broomrape has already spread in South Tigray, South Gondar, and the South and North Wollo Zones (Belay, 2015; Mekonnen, 2016; Brhane, 2017). It has been a cause of great concern among farmers who grow legumes extensively. As a result, many farmers have been forced to abandon the growth of cool-season legume crops, like faba bean, and weed-free neighboring areas are also potentially under the abovementioned threat. Abundance and invasiveness of the parasite are more serious in the eastern Amhara and southeastern Tigray National Regional States than in other areas (Figure 3). The infested districts in the two regions include Kutaber, Dessie-Zuria, Tenta, Mekidela, Meket, Wadla, Tach-Gaint, Farta, Fogera, Ofla, Enda-Mehoni, Raya-Alamata, Emba-Alage, and Raya-Azebo (Abebe et al., 2013; Mekonnen, 2016; Brhane, 2017).
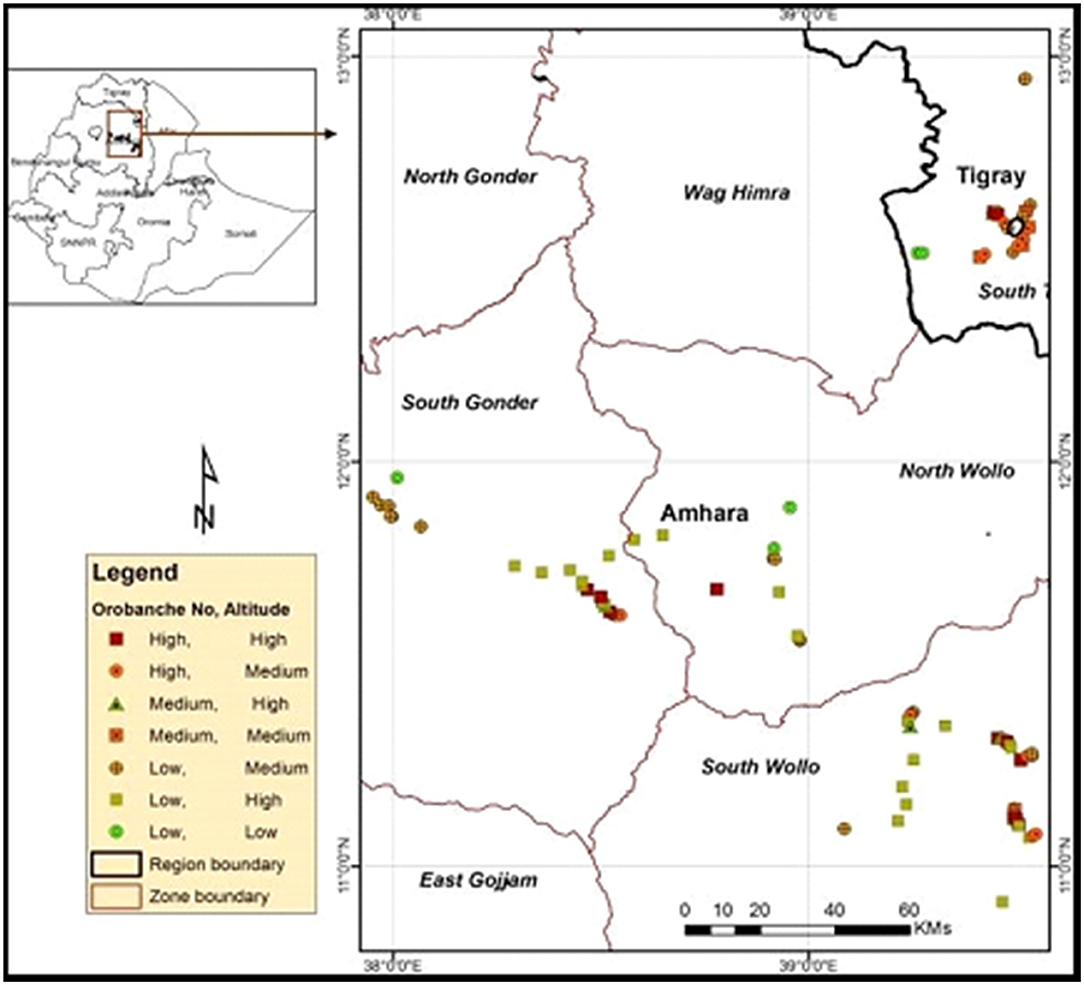
Figure 3. Crenate broomrape distribution in the Amhara and Tigray regions, northern Ethiopia after Belay (2015).
Frequent cultivation of host crops, moisture deficit, poor soil fertility, and the lack of awareness of the biological nature of crenate broomrape seem to be the main constraints aggravating the impacts of the parasite in Ethiopia. These limitations, coupled with ever increasing population pressure fastening ecological degradation and climate change, are exacerbating parasite infestation and impacts year after year. Animal grazing on infested fields has widely spread the parasite and enriched the soil seed bank. In the highly infested areas, as a result of the complete devastation caused by crenate broomrape, growers at large avoided growing host crops, resulting in substantial reductions in both cultivated areas and crop production. Besides causing yield loss and reduction in the cultivated area of host crops, the parasite also reduces the crop quality (Lal et al., 2017). These situations can considerably affect the genetic resources of food legumes in the country too. Moreover, the unrestricted continuous spread and dense occurrence of the parasite have also limited traditional crop rotation practices, hindering the economic, nutritional, and ecological benefits of diversified cropping systems.
Abebe et al. (2013) stated that crenate broomrape is putting all food legumes at jeopardy and indirectly productivity of cereals regular diminution due to limitation on rotational crops that resulted cereal based mono-cropping system. Thus, the authors suggested the development of a future strategy for checking the dispersal of the weed by the creation of necessary attitudinal changes and legislative measures to tackle the problem. Currently, large areas of northern Ethiopia are endangered, which causes huge amounts of yield losses from infected legume crops and also the remaining parts are at risk of a new infestation. According to Belay (2015), more than 0.05 Mha of faba bean production area in the country is at risk of collapse due to the rapid expansion of parasite infestation since its introduction.
It can be realized that although the problem of devastating weed infestation is widely increasing in the area, research and management efforts made in the country are too small. Animals' free grazing in infested fields and the movement of contaminated farm products, such as seeds and straw, and farm tools are the commonest agents that assist the dispersal of parasite seeds among neighboring areas. In addition, one should learn from others' experience that a serious infection by crenate broomrape in specific host crops today might extend to other related crops in the future unless possible efforts are undertaken in restricting the infestation. In general, the harmful effects of the parasite have considerable negative implications on farm productivity and the country's economic development at large.
Management of Crenate Broomrape
In Ethiopia, farmers are not even aware of the biological nature of crenate broomrape and use their own usual management options while managing the weed (Asefa, 2008). As a result, they end up with ineffective parasite management. Farmers in different localities gave the parasite different names such as Akenchira, Daymerch, Kitign, Gelmit, Metselema, and Yejibras indicating the harmfulness and difficulty in managing the nature of the parasitic plant. Several weed management options against the weed have been reported in different parts of the world. The available management options are reviewed below so that they can be used in consideration of farming conditions to minimize their dispersal and impacts.
Preventive Measures
If implemented consistently in an organized manner, preventive measures are valuable techniques to avoid the entry, dispersal, and dense occurrence of crenate broomrape. It needs to have a preventive program targeting the difficulty of managing the parasitic weed at the national level. Besides strict preventive measures, the creation of adequate know-how among farming communities helps to avoid the introduction and dispersal in parasite-free areas. To prevent further expansion to the central and southern parts of the country, restricting the local movement of infested farm inputs and creating knowhow about the potential impacts of the weed at the farmer level are crucial.
Species of Orobanche are listed as a prohibited pest in virtually all countries with developed plant quarantine systems. Due to increased global travel and trades, the number of entering or emerging serious weeds and their impacts may increase unless quarantine actions at ports and containment in already infested areas are strengthened and implemented. Preventive measures are unknown and are not adopted by most farmers, which contributes to further dissemination of the parasite. Very large weed-free areas are at risk of invasion if care is not taken to limit the spread of already introduced species and prevent the introduction of new species by raising awareness among agricultural experts and farmers.
Quarantine Measures
Appropriate quarantine measures are useful in preventing the spread of weed seeds in farming systems. Strict local quarantine measures at various levels—regional, zonal, district, or community jurisdiction help in preventing the introduction of crenate broomrape into parasite-free areas. Prevention of seed distribution and containment of infested areas are practices that are considered necessary in weed management strategies. The important task for winning against such a parasite is to avoid fighting it (Habimana et al., 2014); therefore, local quarantine is an essential element in the prevention program for weed-free faba bean growing areas.
The alarming cross-border and local spread of Orobanche is largely due to the effective way in which the parasite spreads to a large extent through human-mediated dispersal (Goldwasser and Rodenburg, 2013). Strong local quarantine legislation and community awareness or ownership of the problem are crucial to successfully avoid or curb the spread of the parasite in high-risk areas. Eradication programs can be considered in the early stages of weed emergence in new areas, and containment is essential to prevent the spread of the infestation (Habimana et al., 2014).
Phytosanitary Measures
The strength of the parasitic weed lies in its ability to form a seed bank in the soil. Phytosanitary measures aim to reduce this seed bank while minimizing the production of new seeds and their dispersal to new sites (Panetta and Lawes, 2005). Phytosanitary measures could be effective and one of the most economical methods to reduce parasite infestation in agricultural fields.
It is crucial to prevent the movement of parasite seeds from infested to non-infested agricultural fields by contaminated seed lots, straw, farm tools, or machinery is crucial (Panetta and Lawes, 2005). Weeding before seed setting, stubble burning, and the use of uncontaminated seeds ensure clean fields during the season if the soil was not previously infested. It is also important to know that agricultural products of various crops can carry the parasite seed if harvested from an infested field, so it is necessary to avoid the use of such seeds and hay from infected plants and infested fields. Farm tools need to be always cleaned after being used in an infested field to avoid the transfer of the weed seeds to non-infested fields. The use of fermented or thoroughly composted manure is essential, as the fermentation process kills parasite seeds. In addition, free grazing of animals in weed-infested areas should be avoided as their hairs and legs can carry and transfer seeds. It also needs to divert wind and water of erosion or irrigation water moving from field to field carrying the parasite seed.
Thus, phytosanitary measures could be effective and one of the most economical methods to reduce crenate broomrape infestation in agricultural fields. It is believed that the weed seeds were introduced into northern Ethiopia with grains meant for food aid in the 1980s (Besufekad et al., 1999). It then spread from the place of introduction to the other areas, mainly through seed exchange among farmers and also due to the fact that agricultural development agents and non-governmental organizations moved farm inputs throughout the country without any restrictions. Therefore, proper phytosanitary measures are necessary both to reduce the weed seed inoculums at the site of infestation and to prevent their spread into new areas.
Cultural Practices
Several cultural practices have been described for the management of parasitic weeds in food legumes. In faba bean fields, cultural practices against crenate broomrape include crop rotation, adjustment of sowing dates, intercropping, fertilizer application, and hand weeding.
Crop Rotation
Crop rotation is an integral part of the crop production system. A well-planned cropping sequence can considerably reduce pests, improve organic matter levels and soil structure, and consequently increase farm productivity. To combat crenate broomrape infection in the host crop, Mekonnen (2016) indicated that farmers should be advised to practice crop rotation over long periods. Crop rotation involving non-host crops and fallows is a key option in reducing the impacts of the weed. Crops such as alfalfa (Medicago sativa), wheat (Triticum spp.), and cultivated oat (Avena sativa) used in crop rotation to reduce soil seed bank of the weed (Abbes et al., 2008; Perez-de-Luque et al., 2010). However, crop rotation is not very effective as it takes several years to reduce the parasite's seed bank. Proper management can be achieved after a nine-course rotation to prevent the seed bank from increasing (Grenz et al., 2005). In Ethiopia, in areas heavily infested by crenate broomrape, farmers cannot grow cool-season food legumes and instead they grow common beans (Phaseolus vulgaris L.) and fenugreek (Trigonella foenum-graecum L.) crops.
The efficacy of crop rotation for crenate broomrape management can be increased if trap and/or catch crops are included in the rotation (Habimana et al., 2014). Particularly, commercially valuable trap crops promote parasite seed germination but do not support parasitism. Flax (Linum usitatissimum L.), coriander (Coriandrum sativum L.), basil (Ocymum basilicum L.), fenugreek (T. foenum-graecum L.), and Egyptian clover (Trifolium alexandrinum L.) were recommended as successful trap crops on the parasite (Restuccia et al., 2009; Rubiales and Fernandez-Aparicio, 2012; Habimana et al., 2014). While catch crops promote seed germination and support parasitism, they are destroyed prior to parasitic flowering to reduce the crenate broomrape seed bank in the soil. Catch crops like the susceptible faba bean variety are usually used for the green pod, forage, or green manure before the emergence or flowering of the parasite to reduce the seed stock by more than 30% in a season (Nadal et al., 2005).
Adjustment of Sowing Dates
Planting dates could be manipulated to minimize crenate broomrape infection, as environmental factors affect the germination and development of the parasite (Manschadi et al., 2001). It is reported that shifting faba bean sowing from October to November and from December to January reduced the numbers and dry weight of attached and emerged broomrapes, both O. crenata and O. foetida (Grenz et al., 2005). In late-sown crops, two factors are known to reduce parasite damage: decreasing seed germination due to suboptimal soil temperatures and obstructing seedling development during underground stages (Habimana et al., 2014).
Early planted faba bean was severely infected by crenate broomrape than the delayed sowing one in the Tach-Gaint district of the South Gondar zone (Kemal and Olivera, 2016). The authors indicated that delayed sowing resulted in a reduction in seed size and yield compared to early planting. Indeed, faba bean has a long growth period and requires early sowing while delaying the sowing date implies shortening the grain filling, which is detrimental to yield if extending for more than 2 weeks from the optimum period (Grenz et al., 2005). According to Manschadi et al. (2001), only in moderately infested plots, shifting of the planting time of faba bean result in a significant decrease in parasite dry weight and an increase in crop seed yield.
Intercropping
Intercropping is a method that facilitates simultaneous crop production and ecological conservation. Tomato intercropping with maize and snap bean has been used in the regions of Africa as a low-cost technology for broomrape management (Oswald et al., 2002). Maize and snap bean as trap crops showed better performance in stimulating germination of the Orobanche soil seed bank that is raised by more than 70%. These two crops also complemented each other in intercropping, and the soil seed bank of O. ramosa and O. cernua was depleted by 72.5% per season. Also, the yield of tomato increased significantly due to the reduction of Orobanche soil seed bank in the 3rd season due to the trap crop, maize (Abebe et al., 2005).
Zemrag and Bajja (2001) indicated that fenugreek and coriander intercropping with faba bean increased the crop yield by 18 and 15%, respectively. These trap crops decreased the number of attached parasites per host plant and disturbed their development. It is reported that intercropping cereals, fenugreek, or berseem clover with faba bean or field pea reduced crenate broomrape infection due to allelopathic interactions (Fernandez-Aparicio et al., 2010). Fenugreek root exudates produce trigoxazonane, which is believed to inhibit parasite seed germination (Evidente et al., 2007). Field experiments showed that intercropping faba bean or pea with oat reduced parasite infection due to the release of allelochemicals by the oat roots (Fernandez-Aparicio et al., 2013).
Fertilizer Application
Dense infection of crenate broomrape on faba bean tends to be associated with less fertile soils (Trabelsi et al., 2017). In contrast, a high level of composted manure or nitrogen (N) fertilizer showed a suppressive effect on the parasite (FAO, 2008). Nutrient management can promote both resistance and tolerance to broomrape parasitism in crops at pre-attachment and also after establishment stages (Parker, 2009). Moreover, in addition to the toxic effects on seeds and seedlings of the parasite, fertilization can protect crops from parasitism by means of downregulating crop synthesis and exudation of strigolactones, the most potent germination-inducing factors for root parasites (Fernandez-Aparicio et al., 2016b). Soil fertility management can be categorized into two groups, considering its reducing effect on the parasite; management using organic and inorganic fertilizers.
Organic Fertilizer
Manure application can aid a sustainable farming strategy by improving soil fertility and reducing parasite infection of host crops (FAO, 2008). In addition, manure fertilization augments the killing effect of solarization on crenate broomrape seeds (Haidar and Sidahmed, 2000). An increase in soil organic matter encourages the development of soil microflora and fauna that accelerates decomposition and facilitates predation of target weed seed (Ayongwa et al., 2011). Moreover, in soils with adequate organic matter content, many legumes, including faba bean, can have an adequate symbiotic relationship with beneficial microbes. There are conflicting ideas among farmers concerning the impact of soil fertility improvement using manure on crenate broomrape infested fields (SARC, 2015). In some cases, a good increase in crop yield was recorded, but in others, the crop was damaged after manure application. Nevertheless, manure application seems to be a useful asset if implemented after composting and proper rate adjustment.
Inorganic Fertilizer
Phosphorus (P) and N are described in downregulating strigolactones exudation in some crop species (Yoneyama et al., 2012). In the Tach-Gaint district of the South Gondar zone, ammonium N fertilization at 75 kg N ha−1 reduced crenate broomrape infection on faba bean with a substantial increase in the grain yield of the crop (Kemal and Olivera, 2016). Trabelsi et al. (2017) found that both N and P deficiencies enhanced the exudation of strigolactones on faba bean genotypes. With an increase in the application of nitrogen fertilizers, the extent of parasite infection in host crops decreases (Fernandez-Aparicio et al., 2016b). The authors pointed out that in some cases, a good increase in crop yield was recorded but in others, the crop was damaged and that implies the need to maintain a correct balance between N and P fertilizers.
Hand Weeding
Manual weeding is useful to avoid the spread of seeds and further accumulation of the soil seed bank at the commencement of crenate broomrape infestation in the field. Hand pulling of flowering shoots is an effective management method if labor is available and the weed population is found sparsely (Etagegnehu, 2005; Asefa, 2008). In addition, combined with other methods, it can serve for efficient removal of the weed even in heavily infested areas (FAO, 2008).
Some disadvantages of hand weeding include: (1) damage from the parasite normally occurs prior to its appearance, (2) while handing removal at early vegetative growth stage, uprooting of crop plants together with the parasite take place and new flashes occur within a few days following the parasite shoot uprooting, and (3) it becomes unpractical with the escalation of the dense weed infestation. The pulled-out shoots also need to be immediately collected, pilled, and burned. Hand pulling as a management practice does not show any yield increase in the current crop but plays an important role in reducing the parasitic soil seed bank in the long run (FAO, 2008).
Mechanical Control
The host location is a critical part of the life cycle of most damaging obligate parasitic weeds that depend on the limited reserves available in their seeds to quickly locate suitable hosts. Host location disruption in respect to the parasite thus seems a promising practice for its management strategies. Deep plowing to 50–60 cm is suggested to bring the seeds of broomrapes to a depth with less oxygen availability and therefore a reduction in their germination capacity (Van Delft et al., 2000). On the other hand, Lopez-Bellido et al. (2009) indicated that minimum tillage reduced the number of viable seeds incorporated into the soil and then their capacity to reach the crop root. The same authors also reported that zero tillage can considerably reduce O. crenata infection in faba bean.
Physical Control
Physical control methods are very useful in managing broomrapes but are tedious, time-consuming, and costly. Physical control methods that can be used for crenate broomrape management include flooding, soil solarization, microwave or electromagnetic radiation, and others.
Flooding
Flooding for a period of at least 6 weeks caused the decay of crenate broomrape seeds in the soil (Linke, 1999). This practice proved to be useful in irrigated areas or if food legumes are planted after waterlogged soils have dried. Another exemplary finding for good suppression of O. cernua was achieved by maintaining continuous flooding for about 2 months prior to tomato draining and planting at Upper Awash Agro-Industry (Ahmed and Mohammed, 1992).
Soil Solarization
Solarization, warm mulching, or solar pasteurization of moist soil, usually for periods lasting 45–60 days during the warmest season, have proven to be the most effective in reducing crenate broomrape in open crop fields (Haidar and Sidahmed, 2000; Mauromicale et al., 2001). Ashrafi et al. (2009) observed that effective solarization was accomplished by the application of clear polythene sheets on moist soil for 63 days in the hot season. This treatment killed about 95% of the buried viable seeds and induced secondary dormancy in the remaining percentage. Similarly, Habimana et al. (2014) indicated that temperatures of 48–57°C killed broomrape seeds that were in the imbibition state; hence, the soil must be wet at the time of treatment. It is not applicable in rain-fed systems for economic reasons, but under irrigation systems, soil solarization with polythene sheets could be a much more interesting measure to reduce weed infestation (Restuccia et al., 2009). Relatively, the high cost of this method can be partly compensated by managing difficult-to-control (obnoxious) pests, including other problematic weed seeds, nematodes, and fungal and bacterial pathogens besides an improvement in the nutrient status of soil and also its eco-compatibility. This approach has attracted the interest of many warm-climate countries because of its effectiveness, simplicity, and safety for humans, plants, and the environment.
Growing Resistant and/or Tolerant Varieties
Plant species that are not parasitized by a parasitic plant under natural conditions are considered non-hosts, resulting in the avoidance of parasitism, whereas plants capable of supporting parasite growth to physiological maturity are considered host plants, and within host species level of resistance and tolerance to parasitism may vary (Westwood et al., 2010). So far, it is well-agreed among breeders that no complete resistance has been found in legume crops against crenate broomrape. Most of the breeding for crenate broomrape resistance is mainly focused on faba bean (Roman et al., 2002; Maalouf et al., 2011).
The most widely used selection index to select partially resistant cultivars is the total number of emerged shoots per host plant. Food legume resistance to broomrapes is a multifaceted response, and several defense mechanisms have been detected, but the main mechanisms are cell wall reinforcement (Perez-de-Luque et al., 2005, 2006a, 2007), the production of toxic compounds (Lozano et al., 2007), and sealing of vascular tissues (Perez-de-Luque et al., 2006b). Resistance mechanisms identified in faba bean against the weed were due to a low induction of seed germination, and the inhibition of haustorium penetration and tubercle development (Perez-de-Luque et al., 2005; Abbes et al., 2010; Fernandez-Aparicio et al., 2012). Low production of strigolactones was reported as a good trait in faba bean and field pea breeding programs (Pavan et al., 2016). Breeding for crenate broomrape resistance is in its infancy and only one partially resistant faba bean cultivar called Hashenge (ILB 4358) was released and used by some farmers in northern parts of the country (Abebe et al., 2015).
Genetic diversity of O. crenata is reported in some countries. Using the results of 11 SSRs, 2 populations of O. crenata were identified irrespective of their geographic collections (Belay et al., 2020). Similar diversity was reported from Spain, Israel, and Morocco (Roman et al., 2002; Ennami et al., 2017). The existence of genetic diversity may favor the increase of the aggressive population that can attack partially resistant food legume cultivars.
Biological Control
A biological control refers to the use of natural antagonist(s) to exert pressure on the host population to reduce it to levels below the economic threshold. Unlike herbicides, bioagents have the advantage of being specific to the weed type and do not, directly and indirectly, contribute to environmental pollution (Sauerborn et al., 2007). Living organisms found to be effective in affecting parasitic weeds are insects, fungi, and bacteria (Amsellem et al., 2001; Boari and Vurro, 2004).
The fly Phytomyza orobanchia (Diptera: Agromyzidae) is reported to attack Orobanche species. This insect prevents seed production through the development of larvae inside the seed capsules of its target hosts and, thus, contributes to reducing its reproductive capacity and spread (Klein and Kroschel, 2002). Biological control on Orobanche spp. was tried in Ethiopia a long year back by Abuelgasim (1996) but since then no progressive work has been done. Because of their short lifetime aerially and enormous seed production, and vast damage on the host by unemerged plants, Orobanche cannot be regarded as ideal organisms for biological control by insects.
Moreover, numerous microorganisms potentially useful for the biocontrol of Orobanche spp. have been isolated and reported, but none of them have been subjected to continuous widespread use (Boari and Vurro, 2004). Preliminary results demonstrated a reduction in the infection of crenate broomrape by the application of Myrothecium verrucaria to the soil of faba bean field (El-Kassas et al., 2005). Bacterial and fungal species like Pseudomonas aeruginosa, Pseudomonas fluorescens, Bacillus atrophaeus, Bacillus subtilis, arbuscular mycorrhizal fungi (AMF), and Fusarium spp. are reported as biological control agents affecting the growth of broomrape radicles (Shabana et al., 2003; Hershenhorn et al., 2006; Fernandez-Aparicio et al., 2016b; Brhane, 2017). It has been suggested that soil-dwelling organisms can provide effective control by attacking seeds and early stages of the developing parasite; they are less sensitive to environmental conditions and expected to survive in soil by producing resting structures at population levels sufficient to provide residual control of the parasite seeds compared with aerial bioagents (Sauerborn et al., 2007). In peas, inoculation with Rhizobium leguminosarum decreased crenate broomrape seed germination by inducing systemic resistance (Mabrouk et al., 2007; Fernandez-Aparicio et al., 2009).
Thus, future investigation toward the selection of bioagents against crenate broomrape shall focus on soil-dwelling microorganisms that might confer both mechanical and chemical barriers confronting the invading parasite. Biochemicals like strigolactone can be used to stimulate the parasite seed germination in the absence of host plants and play roles in reducing the soil seed bank. Moreover, research has shown other functions of strigolactone in the rhizosphere promoting hyphal branching in AMF and rhizobial nodulation in legume crops (Soto et al., 2010) that indirectly contribute to reducing the parasite infection on host crops. Interesting molecules that hamper ability of broomrapes radicle to reach host have been discovered from different microbial and plant origins too (Cimmino et al., 2014; Fernandez-Aparicio et al., 2014).
Chemical Control
The chemical control method to manage parasitic weeds includes soil fumigation, crop seed dressing with selective herbicides, and preplanting, preemergence, or postemergence application of systemic herbicides like imidazolinones, glyphosate, and sulphonel urea chlorsulfuron (Eizenberg et al., 2004, 2006; Restuccia et al., 2009). Soil fumigation with methyl bromide was effective, especially in light soils for suicidal germination of parasitic weeds (Ahmed et al., 1990; Beyenesh and Etagegnehu, 1992), but its use has now been banned due to its residual effect on the environment. Systemic herbicides used for broomrape management include glyphosate [Isopropyl amine salt of N-(phosphonomethyl)glycin] and herbicides belonging to imidazolinones or sulfonylureas (Colquhoun et al., 2006; Eizenberg et al., 2006).
The herbicide Imazethapyr is applied as a seed dressing and spray and showed better parasitic weed management in faba bean (Bayaa et al., 2000). Incorporation of sulfosulfuron and rimsulfuron directly into the soil provides successful suppression of preattached stages of broomrape (Eizenberg et al., 2012). It was also reported that excellent management of crenate broomrape was achieved in the faba bean field with a single glyphosate application at 60 g ha−1 at the growth stage of the weed when the shoot bud is already visible or shoot and vestigial roots are well-developed (Restuccia et al., 2009). On the other hand, glyphosate can be used on few crops like faba bean without a visible negative effect on crop plants if applied at low dosages, but the necessary number of applications for effective management depends on environmental conditions (Mekonnen, 2016). Conversely, its selectivity to the host crop is marginal, it can subdue the weed only for a short duration after application, necessitating repeated applications with all environmental and economic consequences, resistance can evolve rapidly because of prolific seed production of the parasite (Slavov et al., 2005), and thus, its use is not widely adopted by farmers.
Chemical control of broomrape has been extensively explored since the 1970s. On the other hand, this form of management is complicated by several factors: (i) it is only effective as a prophylactic treatment, as in most cases its infestation level is not well-known; (ii) the parasite is directly connected to the host; (iii) if the herbicide is to be applied to the parasite through the conductive tissues of its host, the host must be selective to the herbicide without reducing its phytotoxicity on to the parasite; and (iv) the parasite can often continuously germinate throughout the season, developing new infections (Perez-de-Luque et al., 2010). Nonetheless, the value of herbicide-resistant crop varieties has already been demonstrated and near-perfect selectivity is achieved with different herbicides in correspondingly modified commercial crops. With target-site resistance, herbicides can be translocated unmetabolized to the underground parasite part via haustorium inflicting its suppressive action on the parasite (Gressel, 2009). This approach can become an important weapon in managing crenate broomrape.
Integrated Management of Crenate Broomrape
Integrated management of crenate broomrape means combining different preventive and curative measures into a given farming system to manage the infestation and to overcome impacts of the parasite satisfactorily. Many reviews are published on the integrated management of parasitic weeds in food legumes (Abang Mathew et al., 2007; Habimana et al., 2014; El-Rokiek et al., 2015; Fernandez-Aparicio et al., 2016b; Mekonnen, 2016).
Khalil et al. (2004), Babalola et al. (2007), and Stoddard et al. (2010) suggested that a combination of genetic resistance, hygiene, or inoculating legume crops with beneficial microbes with monitoring of crops for threshold levels of infestation allow the most economic and effective management of Orobanche, and then maximize economic yields. Intercropping with fenugreek or fertilizing with NH4NO3 along with two times application of imazapic herbicide (2-[4,5-dihydro-4-methyl-4-(1-methylethyl)-5-oxo-1Himidazol-2-yl]-5-methyl-3-pyridinecarboxylic acid) was effective in managing crenate broomrape in faba bean (Ghalwash et al., 2012).
Mohammed et al. (2012) reported that faba bean inoculated with Rhizobium strain TAL 1399 alone or in combination with Bacillus megatherium var phosphaticum (BMP) or Azospirillum brasiliense (Ab) plus chicken manure at 35 g pot−1 displayed no emergence of orobanche above the ground. Furthermore, the TAL 1399 plus chicken manure at 35 g pot−1 treated crop was significantly higher in the root, shoot, and total dry weight than the control and other treatments. Abu-Shall and Ragheb (2014) reported that intercropping trap crops like radish (Raphanus sativus), Egyptian clover, celery, and fenugreek with faba bean plants in integration with the early release of Phytomyza orobancia achieved successful suppression of crenate broomrape. Habimana et al. (2014) concluded that successful weed management in faba bean can only be achieved by the integration of a range of options. Fernandez-Aparicio et al. (2016b) also have suggested that successful root parasite suppression should target its underground earlier life stages, prior to attachment or as soon as attached to the host, because of its highest vulnerability at those stages and the avoidance of yield loss in the current crop. Combinations of T. harzanium, BMP, Rhizobium isolate TAL 1399, and 95.2 kg ha−1 rate NP fertilizers significantly (p ≤ 0.05) increased the number of pods per plant, seeds per pod, 100 seed weight, and crop yield (kg ha−1), as compared to the check (Yahia et al., 2019).
A partially resistant faba bean variety Hashenge planted with a bio-inoculant (R. leguminosarum and/or Trichoderma harzianum), two times of glyphosate application (0.3 L ha−1) and one hand weeding at the flowering stage of faba bean increases the productivity of the crop (SARC, 2015; Mekonnen, 2016; Brhane, 2017). Alongside an adequate knowledge of crenate broomrape biology, an integrated and sustainable management strategy composed of several possible measures acting at early life stages of the parasite is found to be crucial in successfully keeping away or minimizing its problem in particular agroecology.
Conclusion
The scopes of inflicting damages to faba bean due to crenate broomrape are many and diverse, but the degree of attention and work done to solve the problems caused by the parasite is still very low. The biological nature of the parasite is the main problematic attribute limiting the development of successful management measures that can be accepted and used by subsistence farmers. The most severe effect of the parasite is the elimination of important host crops from cultivation in suitable cropping areas. The high potential of faba bean cultivation demands urgent and apt protection measures to solve the imminent problem due to this emerging issue. There is a dire need for strong attention to work on the parasite management research and technology transfer having professionals for technological innovation and active extension staff to transfer available management technology. Strict in-country preventive regulations should be implanted to delay further spread to other food legume growing areas. Crops like common bean and fenugreek should be widely included in the cropping system to reduce the soil seed bank of the parasite in crop fields. In conclusion, no single technique provides complete suppression of crenate broomrape parasitism; thus, an integrated approach to combine several available preventive and curative techniques might be more effective. Then, major efforts need to be directed to genetic improvement of host crops and management measures targeting the earlier life stages of the parasite, which seem to be the most appropriate and sustainable practices for subsistence farmers. Therefore, the legislative policies of the government bodies and the commitment of the community at all levels are very important to make the history of the current serious problem of the parasite.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to the management and the coworkers of the Ethiopian Institute of Agricultural Research (EIAR) and Haramaya University (HrU) who have been involved directly and/or indirectly in encouraging and supporting our efforts throughout the period of development of this manuscript.
References
Abang Mathew, M., Bassam, B., Barakat, A.-I., and Amor, Y. (2007). A participatory farming system approach for sustainable broomrape (Orobanche spp.) management in the Near East and North Africa. Crop Protect. 26, 1723–1732. doi: 10.1016/j.cropro.2007.03.005
Abbes, Z., Kharrat, M., and Chaibi, W. (2008). Seed germination and tubercle development of Orobanche foetida and Orobanche crenata in presence of different plant species. Tunisian J. Plant Protect. 3, 101–109.
Abbes, Z., Kharrat, M., Pouvreau, J. B., Delavault, P., Chaibi, W., and Simier, P. (2010). The dynamics of faba bean (Vicia faba L.) parasitism by Orobanche foetida. Phytopathol. Mediterran. 49, 239–248. Available online at: https://www.jstor.org/stable/26458597
Abebe, G., Sahile, G., and Al-Tawaha, A. M. (2005). Evaluation of potential trap crops on Orobanche soil seed bank and tomato yield in the Central Rift Valley of Ethiopia. World J. Agric. Sci. 1, 148–151.
Abebe, T., Beyene, H., and Nega, Y. (2013) Distribution economic importance of broomrape (Orobanche crenata) in food legumes production of South Tigray, Ethiopia. ESci J. Product. 2, 101–106. Available online at: http://www.escijournals.net/EJCP
Abebe, T., Nega, Y., Mehari, M., Mesele, A., Workineh, A., and Beyene, H. (2015). Genotype by environmental interaction of some faba bean genotypes under diverse broomrape environments of Tigray, Ethiopia. J. Plant Breed. Crop Sci. 7, 79–86. doi: 10.5897/JPBCS2014.0493
Abuelgasim, E. M. (1996). An investigation on the possibility of Biological control of Orobanche spp. in Ethiopia (M.Sc. Thesis). Addis Ababa University, Addis Ababa, Ethiopia.
Abu-Shall, A. M. H., and Ragheb, E. I. M. (2014). Management of Orobanche crenata using trap crops and Phytomaiza orobanchia Kalt. in broad bean (Vicia faba) fields in Egypt. Egypt. J. Biol. Pest Control 24, 217–223. Available online at: https://web.s.ebscohost.com/abstract?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=11101768&AN=97137206&h=jIdTiVZqeOQtUv8MKb2Sk1P4PMVBp6BUWjEDVXNdqDOqdHIn3VErNPuif7VqCVDfKVDjH8vq55cwbsw2%2fe4oTg%3d%3d&crl=c&resultNs=AdminWebAuth&resultLocal=ErrCrlNotAuth&crlhashurl=login.aspx%3fdirect%3dtrue%26profile%3dehost%26scope%3dsite%26authtype%3dcrawler%26jrnl%3d11101768%26AN%3d97137206
Adugna, W., Amare, G., Kemal, A., and Berhanu, B. (1998). Crop losses assessment of 1997 in Gojam and Gondar of Amhara National Regional State. A survey report submitted to HARC, Holetta, IAR, Addis Ababa, Ethiopia.
Ahmed, M. S., and Mohammed, G. (1992). “Orobanche control research in Nura Era Horticulture Enterprise. pp. 56-60,” in Problems and Control of Parasitic Weeds in Ethiopia. Proceedings of the Second Ethiopian Weed Science Workshop, 29-30 September, 1988, EWSC, eds F. Rezene and C. Parker (Addis Ababa: Ethiopian Weed Science Society (EWSS)).
Ahmed, M. S., Mohammed, G., and Parker, C. (1990). “Control of Orobanche cernua by soil fumigation at Nura Era Horticulture Enterprise,” in Proceedings of the Six Annual Meeting of EWSC, edsC. Parker and F. Rezene (Addis Ababa: EWSC), 67–74.
Amsellem, Z., Barghouthi, S., Cohen, B., Goldwasser, Y., Gressel, J., Hornok, L., et al. (2001). Recent advances in the biocontrol of Orobanche (broomrape) species. Biocontro, 46, 211–228. doi: 10.1023/A:1011496114707
Asefa, A. (2008). “Integrated orobanche management in food legumes (faba bean): experience of farmers' field school (FFS) in Dessie-Zuria District, Ethiopia,” in Progress on Farmer Training in Parasitic Weed Management, ed R. Labrada (Rome: FAO).
Asefa, A., and Endale, B. (1994). Orobanche crenata: A potential threat of food legumes in Ethiopia. Ethiopian Weed Sci. Soc. Newslett. 2, 1.
Ashrafi, Z. Y., Hassan, M. A., Mashhadi, H. R., and Sadeghi, S. (2009). Applied soil solarization for control of Egyptian broomrape (Orobanche aegyptiaca) on the cucumber (Cucumis sativus) in two growing seasons. J. Agricult. Technol. 5, 201–212.
Ayongwa, G. C., Stomph, T. J., Beder, P., Leffelaar, P. A., and Kuyper, T. W. (2011). Organic matter and seed survival of Striga hermonthica – mechanisms for seed depletion in the soil. Crop Protect. 30, 1594–1600. doi: 10.1016/j.cropro.2011.08.012
Babalola, O. O., Sanni, A. I., Odhiambo, G. D., and Torto, B. (2007). Plant growth-promoting rhizobacteria do not pose any deleterious effect on cowpea and detectable amounts of ethylene are produced. World J. Microbiol. Biotechnol. 23, 747–752. doi: 10.1007/s11274-006-9290-6
Bayaa, B., El-Hossein, N., and Erskine, W. (2000). Attractive but deadly. In lentils parasitic weeds O. crenata control: Cultural control; sowing date, integrated control and herbicides; imazapic, imazaquin and imazethapyr. ICARDA Caravan 12, 16. Available online at: https://www.cabdirect.org/cabdirect/abstract/20003015385
Belay, G. (2015). Genetic diversity analysis of Ethiopian Orobanche crenata population using microsatellite markers (MSc Thesis). School of Graduate Studies, Institute of Biotechnology, Addis Ababa University, Ethiopia, 86.
Belay, G., Tesfaye, K., Hamwieh, A., Ahmed, S., Dejene, T., Lustosa de Oliveira, J. S., et al. (2020). Genetic diversity of Orobanche crenata populations in ethiopia using microsatellite markers. Int. J. Genomics. 2020, 1–8. doi: 10.1155/2020/3202037
Bennett, J. R., and Mathews, S. (2006). Phylogeny of the parasitic plant family Orobanchaceae inferred from phytochrome A. Am. J. Bot. 93, 1039–1051. doi: 10.3732/ajb.93.7.1039
Besufekad, T., Legesse, A., and Rezene, F. (1999). “Orobanche problem in south Wollo,” in Proceedings of the Ethiopian Weed Sience Workshop, Arem, Vol 5, eds F. Reda and D. G. Tanner (Addis Ababa: Ethiopian Weed Science Society (EWSS)), 1–10.
Beyenesh, Z., and Etagegnehu, G. (1992). “Review of weed research activities in Ethiopia,” in Horticulture Research and Development in Ethiopia. Proceedings of the Second National Horticulture Workshop of Ethiopia, eds E. Herath and L. Desalegne (Addis Ababa: IAR), 243–253.
Boari, A., and Vurro, M. (2004). Evaluation of Fusarium spp. and other fungi as biological control agents of broomrape (Orobanche ramosa). Biol. Control 30, 212–219. doi: 10.1016/j.biocontrol.2003.12.003
Bouwmeester, H. J., Roux, C., Lopez-Raez, J. A., and Becard, G. (2007). Rhizosphere communication of plants, parasitic plants and AM fungi. Tends Plant Sci. 12, 224–230. doi: 10.1016/j.tplants.2007.03.009
Brhane, T. (2017). Evaluation of the synergistic effect of host plant resistance, rhizobial inoculant and Trichoderma on orobanche infestation of faba beans (Vicia faba L.) in southern Tigray, Ethiopia (MSc Thesis). Hawassa University, Ethiopia.
CABI (Center for Agriculture Bioscience International). (2021). Invasive Species Compendium: Orobanche crenata (crenate broomrape). Avaialble online at: https://www.cabi.org/isc/datasheet/37744 (accessed May 10, 2022).
Cimmino, A., Fernandez-Aparicio, M., Andolfi, A., Basso, S., Rubiales, D., and Evidente, A. (2014). Effect of fungal and plant metabolites on broomrapes (Orobanche and Phelipanche spp.) seed germination and radicle growth. J. Agric. Food Chem. 62, 10485–10492. doi: 10.1021/jf504609w
Colquhoun, J. B., Eizenberg, H., and Mallory-Smith, C. A. (2006). Herbicide placement site affects small broomrape (Orobanche minor) control in red clover. Weed Technol. 20, 356–360. doi: 10.1614/WT-04-327R2.1
CSA (Central Statistical Agency of Ethiopian). (2016). Central Statistical Agency of Ethiopian report on key findings of agricultural sample surveys during 2015/16. Addis Ababa: Country summary, 25.
Dahan, R., and El-Mourid, M. (2004). “Integrated management of orobanche in food legumes in the Near East and North Africa,” in: Proceedings of the Expert Consultation on IPM for Orobanche in Food Legume Systems in the Near East and North Africa, 7-9 April 2003, Rabat, Morocco, eds R. Dahan and M. El-Mourid (Aleppo: ICARDA/INRA/FAO. ICARDA), 120.
Das, M., Fernandez-Aparicio, M., Yang, Z. Z., Huang, K., Wickett, N. J., and Alford, S. (2015). Parasitic plants Striga and Phelipanche dependent upon exogenous strigolactones for germination have retained genes for strigolactone biosynthesis. Am. J. Plant Sci. 6, 1151–1166. doi: 10.4236/ajps.2015.68120
Dorr, I., and Kollmann, R. (1995). Symplasmic sieve element continuity between Orobanche and its host. Botanica Acta 108, 47–55.
Eizenberg, H., Aly, R., and Cohen, Y. (2012). Technologies for smart chemical control of broomrape (Orobanche spp. and Phelipanche spp.). Weed Sci. 60, 316–323. doi: 10.1614/WS-D-11-00120.1
Eizenberg, H., Colquhoun, J. B., and Mallory-Smith, C. A. (2006). Imazamox application timing for small broomrape (Orobanche minor) control in red clover (Trifolium pratense). Weed Sci. 54, 923–927. doi: 10.1614/WS-05-151R.1
Eizenberg, H., Goldwasser, Y., Golan, S., Plakhine, D., and Hershenhorn, J. (2004). Egyptian broomrape (Orobanche aegyptiaca Pers.) control in tomato with sulfonylurea herbicides–greenhouse studies. Weed Technol. 18, 490–496. doi: 10.1614/WT-03-023R3
El-Kassas, R., Karam El-Din, Z., Beale, M. H., Ward, J. L., and Strange, R. N. (2005). Bioassay led isolation of Myrothecium verrucaria as germination inhibitors of Orobanche crenata. Weed Res. 45, 212–219. doi: 10.1111/j.1365-3180.2005.00448.x
El-Rokiek, G., Kowthar, I. M., El-Metwally, M., Nadia, K., and El-Din Samia Amin, S. (2015) Controlling Orobanche crenata in faba bean using the herbicides glyphosate imazapic with some additives. Int. J. ChemTech Res. 8, 18–26. Available online at: http://sphinxsai.com/2015/ch_vol8_no10/1/(18-26)V8N10CT.pdf
Ennami, M., Fatima, Z. B., Joseph, M. M., Fatima, G., Lamiae, G., Loubna, B., et al. (2017). Genetic diversity of Moroccan Orobanche crenata populations revealed by sequence-related amplified polymorphism markers. J. Agric. Sci. 9, 164–175. doi: 10.5539/jas.v9n4p164
Etagegnehu, G. (2005). Integrated control of branched broomrape (Orobanche ramosa L.) in tomato (Lycopersicon esculentum Mill.) in Central Rift Valley of Ethiopia (PhD Dissertation). Graduate School of Kasetsart University, Bangkok.
Evidente, A., Fernandez-Aparicio, M., Andolfi, A., Rubiales, D., and Motta, A. (2007). Trigoxazonane mono substituted trioxazonane by Trigonella foenum-graecum root exudate, inhibiting agent of Orobanche crenata seed germination. Phytochemistry 68, 2487–2492. doi: 10.1016/j.phytochem.2007.05.016
FAO (Food and Agriculture Organization). (2008). Progress on Farmer Training in Parasitic Weed Management. Rome: FAO, 156.
FAO. (2019). The Global Economy of Pulses. FAO statistical database. Rome, Italy, 190. Avaialble online at: https://www.fao.org/publications/card/en/c/a65cd75d-1c49-5696-a325-d8c160dbe633/ (accessed May 10, 2022).
Fernandez-Aparicio, M., Cimmino, A., Evidente, A., and Rubiales, D. (2013). Inhibition of Orobanche crenata seed germination and radicle growth by allelochemicals identified in cereals. J. Agric. Food Chem. 61, 9797–9803. doi: 10.1021/jf403738p
Fernandez-Aparicio, M., Emeran, A. A., and Rubiales, D. (2010). Inter-cropping with berseem clover (Trifolium alexandrinum) reduces infection by Orobanche crenata in legumes. Crop Protect. 29, 867–871. doi: 10.1016/j.cropro.2010.03.004
Fernandez-Aparicio, M., Flores, F., and Rubiales, D. (2016a). The effect of Orobanche crenata infection severity in faba bean, field pea and grass pea productivity. Front. Plant Sci. 7, 1409. doi: 10.3389/fpls.2016.01409
Fernandez-Aparicio, M., Moral, A., Kharrat, M., and Rubiales, D. (2012). Resistance against broomrapes (Orobanche and Phelipanche spp.) in faba bean (Vicia faba) based in low induction of broomrape seed germination. Euphytica 186, 897–905. doi: 10.1007/s10681-012-0686-0
Fernandez-Aparicio, M., Reboud, X., and Gibot-Leclerc, S. (2016b). Broomrape weeds. Underground mechanisms of parasitism and associated strategies for their control: A review. Front. Plant Sci. 7, 135. doi: 10.3389/fpls.2016.00135
Fernandez-Aparicio, M., and Rubiales, D. (2011). Differential response of pea (Pisum sativum) to Orobanche crenata, Orobanche foetida and Phelipanche aegyptiaca. Crop Protect. 31, 27–30. doi: 10.1016/j.cropro.2011.08.021
Fernandez-Aparicio, M., Soto, M. J., Rubiales, D., Ocampo, J. A., and Garcia-Garrido, J. M. (2009). The potential of Rhizobium mutants for biological control of Orobanche crenata. Haustorium 54, 3–4.
Fernandez-Aparicio, M., Takaya, K., Xiaonan, X., Diego, R., and Koichi, Y. (2014) Low strigolactone root exudation: A novel mechanism of broomrape (Orobanche Phelipanche spp.) resistance available for faba bean breeding. J. Agric. Food Chem. 62, 7063–7071. doi: 10.1021/jf5027235
Gevezova, M., Dekalska, T., Stoyanov, K., Hristeva, T., Kostov, K., Batchvarova, R., et al. (2012). Recent advances in Broomrapes research. J. Biosci. Biotechnol. 1, 91–105. Available online at: https://www.semanticscholar.org/paper/Recent-advances-in-Broomrapes-research-Gevezova-Dekalska/6f813232cc7d4a92ce458564663f55af9c64541b
Ghalwash, A. M., Gharib, H. S., and Khaffagy, A. E. (2012). Integrated broomrape (Orobanche crenata Forsk.) control in faba bean (Vicia faba L.) with nitrogen fertilizer, intercropping and herbicides. Egypt. J. Agron. 34, 301–319. doi: 10.21608/agro.2012.106
Goldwasser, Y., and Rodenburg, J. (2013). “Integrated agronomic management of parasitic weeds seed banks,” in Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies, eds D. M. Joel, J. Gressel, and L. J. Musselman (Berlin, Heidelberg: Springer Verlag), 393–413. doi: 10.1007/978-3-642-38146-1_22
Goldwasser, Y., Yoneyama, K., Xie, X., and Yoneyama, K. (2008). Production of strigolactones by Arabidopsis thaliana responsible for Orobanche aegyptiaca seed germination. Plant Growth Regul.55, 21–28. doi: 10.1007/s10725-008-9253-z
Grenz, J. H., Manschadi, A. M., Uygurc, F. N., and Sauerborn, J. (2005). Effects of environment and sowing date on the competition between faba bean (Vicia faba L.) and the parasitic weed Orobanche crenata. Field Crops Res. 93, 300–313. doi: 10.1016/j.fcr.2004.11.001
Gressel, J. (2009). Crops with target-site herbicide resistance for Orobanche and Striga control. Pest Manag. Sci. 65, 560–565. doi: 10.1002/ps.1738
Habimana, S. A., Nduwumuremyi, J. D., and Chinama, R. (2014). Management of Orobanche in field crops- A review. J. Soil Sci. Plant Nutr. 14, 43–62. doi: 10.4067/S0718-95162014005000004
Haidar, M. A., and Sidahmed, M. M. (2000). Soil solarization and chicken manure for the control of Orobanche crenata and other weeds in Lebanon. Crop Protect. 19, 169–173. doi: 10.1016/S0261-2194(99)00083-6
Heide-Jorgensen, H. S. (2011). “Parasitic plants,” in Encyclopedia of Biological Invasions, eds D. Simberloff and M. Rejmanek (Berkeley, CA; Los Angeles, CA: University of California Press), 504–510. doi: 10.1525/9780520948433-113
Hershenhorn, J., Dor, E., Alperin, B., Lati, R., Eizenberg, H., Lande, T., et al. (2006). “Integrated broomrape control - resistant lines, chemical and biological control and sanitation - can we combine them together?” in Workshop Parasitic Plant Management in Sustainable Agriculture Final Meeting of COST849, 23–24. November 2006 (Oeiras-Lisbon: ITQB), 1–8.
Hibberd, J. M., Quick, W. P., Press, M. C., Scholes, J. D., and Jeschke, W. D. (1999). Solute fluxes from tobacco to the parasitic angiosperm Orobanche cernua and the influence of infection on host carbon and nitrogen relations. Plant Cell Environ. 22, 937–947. doi: 10.1046/j.1365-3040.1999.00462.x
Joel, D. M. (2000). The long-term approach to parasitic weeds control: Manipulation of specific developmental mechanisms of the parasite. Crop Protect. 19, 753–758. doi: 10.1016/S0261-2194(00)00100-9
Joel, D. M. (2009). The new nomenclature of Orobanche and Phelipanche. Eur. Weed Res. Soc. Weed Res. 49, 6–7. doi: 10.1111/j.1365-3180.2009.00748.x
Joel, D. M., Hershenhorn, J., Eizenberg, H., Aly, R., Ejeta, G., Rich, P. J., et al. (2007). Biology and management of weedy root parasites. Hortic. Rev. 33, 267–349. doi: 10.1002/9780470168011.ch4
Joel, D. M., Steffens, J. C., and Matthews, D. E. (1995). Germination of Weedy Root Parasites. New York, NY: Marcel Dekker Inc., 567–598. doi: 10.1201/9780203740071-22
Kebreab, E., and Murdoch, A. J. (1999). Modeling the effects of water stress and temperature on germination rate of Orobanche aegyptiaca seeds. J. Exp. Bot. 50, 655–664. doi: 10.1093/jxb/50.334.655
Kemal, S., and Olivera, J. R. (2016). Narrowing the yield gap of food legumes through integrated management of parasitic weeds in the highlands of Ethiopia, ICARDAEMBRAPA Report for the period 20 Nov 2013-19 May 2016.
Khalil, S., Kharrat, M., Malhotra, R., Saxena, M., and Eriskne, W. (2004). “Breeding faba bean for orobanche resistance,” in: Integrated Management of Orobanche in Food Legumes in the Near East and North Africa. Proceedings of the Expert Consultation on IPM for Orobanche in Food Legume Systems in the Near East and North Africa, 7-9 April 2003, Rabat Morocco, eds R. Dahan and M. El-Mourid (Aleppo: ICARDA/INRA/FAO. ICARDA), 1–18.
Klein, O., and Kroschel, J. (2002). Biological control of Orobanche spp. With Phytomyza orobanchia, a review. BioControl 47, 245–277. doi: 10.1023/A:1014862302818
Lal, H., Devendra, S., and Jat, B. L. (2017). Orobanche infestation in Indian Brassica juncea L. in Ajmer districts of Rajasthan and its management. Asian J. Environ. Sci. 12, 1–22. doi: 10.15740/HAS/AJES/12.1/1-22
Linke, K. H. (1999). “Status quo of Orobanche management: preventive, cultural and physical control,” in Joint Action to Control Orobanche in the WANA-Region: Experiences from Morocco. Proceedings of the Regional Workshop (Rabat: CTZ GmbH), 107–133.
Linke, K. H., Abd El-Moneim, A. M., and Saxena, M. C. (1993). Variation in resistance of some forage legumes species to Orobanche crenata Forsk. Field Crops Res. 32, 277–285. doi: 10.1016/0378-4290(93)90037-N
Lopez-Bellido, R. J., Benitez-Vega, J., and Lopez-Bellido, L. (2009). No-tillage improves broomrape control with glyphosate in faba-bean. Agron. J. 101, 1394–1399. doi: 10.2134/agronj2009.0014
Lozano, M. D., Moreno, M. T., Rubiales, D., and Perez-De-Luque, A. (2007). Medicago truncatula as a model for non-host resistance in legume-parasitic plants interactions. Plant Physiol. 145, 437–449. doi: 10.1104/pp.107.097089
Maalouf, F., Shaaban, K., Seid, A., Akinnola, N. A., Mohammed, K., Khaled El, S., Samir, H., and Rajinder, S. M. (2011). Yield stability of faba bean lines under diverse broomrape prone production environments. Field Crops Res. 124, 288–294. doi: 10.1016/j.fcr.2011.06.005
Mabrouk, Y., Zourgui, L., Sifi, B., Delavault, P., Simier, P., and Belhadj, O. (2007). Some compatible Rhizobium leguminosarum strains in peas decrease infections when parasitised by Orobanche crenata. Weed Res. 47, 44–53. doi: 10.1111/j.1365-3180.2007.00548.x
Manschadi, A. M., Sauerborn, J., and Stutzel, H. (2001). Quantitative aspect of Orobanche crenata infestation in faba beans as affected by abiotic factors and parasite soil seed bank. Weed Res. 41, 311–324. doi: 10.1046/j.1365-3180.2001.00240.x
Mathieu, P., Andre, J., Gerald, M., Schneeweiss, J., Macas Petr, N., Heidrun, G., et al. (2012). Next-generation sequencing reveals the impact of repetitive DNA across phylogenetically closely related genomes of Orobanchaceae. Mol. Biol. Evol. 29, 3601–3611. doi: 10.1093/molbev/mss168
Matusova, R., and Bouwmeester, H. J. (2005). “The biosynthetic origin of strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp,” in Joint Working Groups and MC Meeting of Cost Action 849, Broomrape Biology, Control and Management 15-17 September 2005 (London: Reading University), 44.
Mauromicale, G., Restuccia, G., and Marchese, A. (2001). Soil solarization, a non- chemical technique for controlling Orobanche crenata and improving yield of faba bean. Agronomie 21, 757–765. doi: 10.1051/agro:2001167
Mekonnen, M. (2016). Integrated weed (Orobanche crenata) management on faba bean. Am. J. Agric. Res. 1, 0029–0034. doi: 10.28933/misganaw-ajar-2016
Mohamed, K. I., Papes, M., Williams, R., Benz, B. W., and Reterson, A. T. (2006). Global invasive potential of ten parasitic witch weeds and related Orobanchaceae AMBIO. J. Hum. Environ. 35, 281–288. doi: 10.1579/05-R-051R.1
Mohammed, M. H., Awad, G. O., Samia, O. Y., Ashraf, M. S., Ahmed, M. E. R., Ibrahim, S. M., et al. (2012). Effects of bacterial strains and chicken manure on Orobanche crenata infesting faba bean. Agric. J. 7, 122–127. doi: 10.3923/aj.2012.122.127
Mower, J. P., Stefanovic, S., Young, G. J., and Palmer, J. D. (2004). Gene transfer from parasitic to host plants. Nature 432, 165–166. doi: 10.1038/432165b
Nadal, S., Moreno, M. T., Cubero, J. I., and Rubiales, D. (2005). Determinate faba bean young pod response to glyphosate and crenate broomrape (Orobanche crenata). J. Sust. Agric. 25, 19–27. doi: 10.1300/J064v25n04_04
Oswald, A., Ransom, J. K., Kroschel, J., and Sauerborn, J. (2002). Intercropping controls Striga in maize based farming systems. Crop Protect. 21, 367–374. doi: 10.1016/S0261-2194(01)00104-1
Panetta, F. D., and Lawes, R. (2005). Evaluation of weed eradication programs: the delimitation of extent. Divers. Distribut. 11, 435–442. doi: 10.1111/j.1366-9516.2005.00179.x
Parker, C. (2002). “Parasitic higher plants,” in CAB International, Plant Pathologists' Pocketbook, eds J. M. Waller, J. M. Lanne, and S. J. Waller (Biristol: Royal York Crescent), 187–188.
Parker, C. (2009). Observations on the current status of Orobanche and Striga problems worldwide. Pest Manag. Sci. 65, 453–459. doi: 10.1002/ps.1713
Parker, C. (2013). “The parasitic weeds of the Orobanchaceae,” in Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies, eds D. M Joel, J. Joel, and L. J. Musselman (Berlin Heidelberg: Springer-Verlag), 313–344. doi: 10.1007/978-3-642-38146-1_18
Parker, C. (2014). Orobanche crenata in UK-an update. Haustorium 65, 5–6. Available online at: http://ww2.odu.edu/~lmusselm/haustorium/pdf/HAUST65.pdf
Parker, C., and Riches, C. R. (1993). Parasitic Weeds of the World: Biology and Control. Wallingford; Oxon: CAB International, 332.
Pavan, S., Adalgisa, S., Angelo, R. M., Nicoletta, B., Valentina, B., Francesca, R., et al. (2016). Characterization of Low-Strigolactone Germplasm in Pea (Pisum sativum L.) Resistant to Crenate Broomrape (Orobanche crenata Forsk.). Mol Plant Microbe Interact 9, 743–749. doi: 10.1094/MPMI-07-16-0134-R
Perez-de-Luque, A., Eizenberg, H., Grenz, J. H., Sillero, J. C., Avila, C., Sauerborn, J., and Rubiales, D. (2010). Broomrape management in faba bean. Field Crops Res. 115, 319–328. doi: 10.1016/j.fcr.2009.02.013
Perez-de-Luque, A., Gonzalez-Verdejo, C. I., Lozano, M. D., Dita, M. A., Cubero, J. I., Gonzalez-Melendi, P., et al. (2006a). Protein cross-linking, peroxidase and B-1.3-endoglucanase involved in resistance of pea against Orobanche crenata. J. Exp. Bot. 57, 1461–1469. doi: 10.1093/jxb/erj127
Perez-de-Luque, A., Lozano, M. D., Cubero, J. I., Gonzalez-Melendi, P., Risue, M. C., and Rubiales, D. (2006b). Mucilage production during the incompatible interaction between Orobanche crenata and Vicia sativa. J. Exp. Bot. 57, 931–942. doi: 10.1093/jxb/erj078
Perez-de-Luque, A., Lozano, M. D., Moreno, M. T., Testillano, P. S., and Rubiales, D. (2007). Resistance to broomrape (Orobanche crenata) in faba bean (Vicia faba): cell wall changes associated with pre-haustorial defensive mechanisms. Ann. Appl. Biol. 151, 89–98. doi: 10.1111/j.1744-7348.2007.00164.x
Perez-de-Luque, A., Rubiales, D., Cubero, J. I., Press, M. C., Scholes, J., and Yoneyama, K. (2005). Interaction between Orobanche crenata and its host legumes: unsuccessful haustorial penetration and necrosis of the developing parasite. Ann. Bot. 95, 935–942. doi: 10.1093/aob/mci105
Plakhine, D., Ziadna, H., and Joel, D. M. (2009). Is seed conditioning essential for Orobanche germination? Pest Manag. Sci. 65, 492–496. doi: 10.1002/ps.1716
Rank, C., Rasmussen, L. S., Jensen, S. R., Pierce, S., Press, M. C., and Scholes, J. D. (2004). Cytotoxic constituents of Alectra and Striga species. Weed Res. 44, 265–270. doi: 10.1111/j.1365-3180.2004.00398.x
Restuccia, A., Marchese, M., Mauromicale, G., and Restuccia, G. (2009). Biological characteristics and control of Orobanche crenata Forsk., A review. Ital. J. Agron. Rev. Agron. 1, 53–68. doi: 10.4081/ija.2009.1.53
Rezene, F. (1998). Review of Broomrapes (Orobanche spp.) in Ethiopia. NVRSRP Newsletter, Addis Ababa, 20–22.
Rezene, F., and Kedir, N. (2006). “Weed research in high land food legumes of Ethiopia,” in Food and Forage Legumes of Ethiopia: Progress and Prospects. Proceedings of the Workshop on Food and Forage Legumes, 22-26 September 2003, Addis Ababa, Ethiopia, eds A. Kemal, K. Gemechu, A. Seid, R. Malhotra, S. Beniwal, K. Makkouk, and M. H. Halila (Aleppo: ICARDA), 278–87.
Roman, B., Satovic, Z., Rubiales, D., Torres Ana, M., Cubero, J. I., Katzir, N., et al. (2002). Variation among and within populations of the parasitic weed Orobanche crenata from two sides of the Mediterranean revealed by Inter Simple Sequence Repeat Markers. Phytopathology 92, 1262–1266. doi: 10.1094/PHYTO.2002.92.12.1262
Rubiales, D., and Fernandez-Aparicio, M. (2012). Innovations in parasitic weeds management in legume crops: a review. Agron. Sust. Dev. 32, 433–449. doi: 10.1007/s13593-011-0045-x
Rubiales, D., Perez-de-Luque, A., Fernandez-Aparico, M., Sillero, J. C., Roman, B., Kharrat, M., et al. (2006). Screening techniques and sources of resistance against parasitic weeds in grain legumes. Euphytica 147, 187–199. doi: 10.1007/s10681-006-7399-1
SARC (Sirinka Agricultural Research Center). (2015). Sirinka Agricultural Research Center Progress Report for the period 2014/15.
Sauerborn, J. (1991). Parasitic flowering plants in agricultural ecosystems of West Asia. Flora Veget. Mundi 9, 83–91.
Sauerborn, J., Muller-Stover, D., and Hershenhorn, J. (2007). The role of biological control in managing parasitic weeds. Crop Protect. 26, 246–254. doi: 10.1016/j.cropro.2005.12.012
Shabana, Y. M., Muller-Stover, D., and Sauerborn, J. (2003). Granular pesta formulation of Fusarium oxysporum f. sp. orthoceras for biological control of sunflower broomrape: efficacy and shelf-life. Biol. Control 26, 189–201. doi: 10.1016/S1049-9644(02)00130-5
Slavov, S., Valkov, V., Batchvarova, R., Atanassova, S., Alexandrova, M., and Atanassov, A. (2005). Chlorsulfuron resistant transgenic tobacco as a tool for broomrape control. Transgenic Res. 14, 273–278. doi: 10.1007/s11248-004-8081-9
Soto, M. J., Fernandez-Aparicio, M., Castellanos-Morales, V., Garcia-Garrido, J. M., Ocampo, J. A., Delgado, M. J., et al. (2010). First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa). Soil Biol. Biochem. 42, 383–385. doi: 10.1016/j.soilbio.2009.11.007
Stoddard, F. L., Nicholas, A. H., Rubiales, D., Thomas, J., and Villegas-Fernandez, A. M. (2010). Integrated pest management in faba bean. Field Crops Res. 115, 308–318. doi: 10.1016/j.fcr.2009.07.002
Suzuki, Y., Murofushi, N., Zhang, Y. H., and Takeuchi, Y. (1994). Endogenous gibberellins in clover broomrape, a parasitic plant, and its host, clover: Dependency of the parasite on the host for gibberellin production. J. Plant Growth Regul. 13, 63–67. doi: 10.1007/BF00210948
Timko, M. P., Florea, C. S., and Riopel, J. L. (1989). “Control of germination and early development in parasitic angiosperms,” in Recent Advances in the Development and Germination of Seeds (Springer US), 225–40. doi: 10.1007/978-1-4613-0617-7_17
Trabelsi, I., Yoneyama, K., Abbes, Z., Amri, M., Xie, X., Kisugi, T., et al. (2017). Characterization of strigolactones produced by Orobanche foetida and Orobanche crenata resistant faba bean (Vicia faba L.) genotypes and effects of phosphorous, nitrogen, and potassium deficiencies on strigolactone production. South Afr. J. Bot. 108, 15–22. doi: 10.1016/j.sajb.2016.09.009
Uematsu, K., Nakajima, M., Yamaguchi, I., Yoneyama, K., and Fukui, Y. (2007). Role of cAMP in gibberellin promotion of seed germination in Orobanche minor (Smith). J. Plant Growth Regul. 26, 245–254. doi: 10.1007/s00344-007-9012-9
Van Delft, G. J., Graves, J. D., Fitter, A. H., and Van Ast, A. (2000). Striga seed avoidance by deep planting and no-tillage in sorghum and maize. Int. J. Pest Manag. 46, 251–256. doi: 10.1080/09670870050206019
Westwood, J. H. (2013). “The physiology of the established parasite-host association,” in: Parasitic Orobanchaceae, eds D. M. Joel, J. Gressel, and L. J. Musselman (Berlin, Springer), 87–114. doi: 10.1007/978-3-642-38146-1_6
Westwood, J. H., Yoder, J. I., Timko, M. P., and de Pamphilis, C. W. (2010) The evolution of parasitism in plants-A review. Trends Plant Sci. 15, 227–235. doi: 10.1016/j.tplants.2010.01.004
Yahia, M. Y. A., Hassan, M. M., Elamien, M. A. M., Abdalla, N. K., Rugheim, A. M. E., Abusin, R. M. A., et al. (2019). Counteracting the Effect of Orobanche crenata Infestation on Faba Bean (Vicia faba L.) by Soil Microorganisms and Chemical Fertilizers. Int. J. Agric. Environ. Res.5, 469–484.
Yoneyama, K., Xie, X., Kim, H. I., Kisugi, T., Nomura, T., and Sekimoto, H. (2012). How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 235, 1197–1207. doi: 10.1007/s00425-011-1568-8
Yoneyama, K., Xie, X., and Takeuchi, Y. (2009). Strigolactones: structures and biological activities. Pest Manag. Sci. 65, 467–470. doi: 10.1002/ps.1726
Zemrag, A., and Bajja, M. (2001). “Characterization of Orobanche spp. in Morocco and the effect of some trap crops on Orobanche crenata Forsk in faba bean (Vicia faba L.),” in Proceedings of the Seventh International Parasitic Weed Symposium. Phytoprotection, Vol. 88, eds A. Fer, P. Thalouarn, D. M. Joel, L. J. Musselman, C. Parker, and J. A. C. Verkleij (Nantes: Phytoprotection), 83–92. doi: 10.7202/018953ar
Keywords: broomrape, holoparasite, infection, infestation, integrated management
Citation: Negewo T, Ahmed S, Tessema T and Tana T (2022) Biological Characteristics, Impacts, and Management of Crenate Broomrape (Orobanche crenata) in Faba Bean (Vicia faba): A Review. Front. Agron. 4:708187. doi: 10.3389/fagro.2022.708187
Received: 11 May 2021; Accepted: 22 February 2022;
Published: 25 March 2022.
Edited by:
Daizy Rani Batish, Panjab University, IndiaReviewed by:
Simerjeet Kaur, Punjab Agricultural University, IndiaJibin Baby, University of Missouri, United States
Copyright © 2022 Negewo, Ahmed, Tessema and Tana. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takele Negewo, dGFrZWxlbmVnZXdvMjdAZ21haWwuY29t
 Takele Negewo
Takele Negewo Seid Ahmed
Seid Ahmed Taye Tessema3
Taye Tessema3