
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Agron. , 16 November 2022
Sec. Plant-Soil Interactions
Volume 4 - 2022 | https://doi.org/10.3389/fagro.2022.1028195
 Simon Thierry Okiobe1
Simon Thierry Okiobe1 Peter Meidl1
Peter Meidl1 Timon Koths1
Timon Koths1 Dustin Olschewsky1
Dustin Olschewsky1 Matthias C. Rillig1,2
Matthias C. Rillig1,2 Daniel R. Lammel1,2*
Daniel R. Lammel1,2*Arbuscular mycorrhizal fungi (AMF) form symbioses with many agricultural crops and can improve plant biomass and health. The performance of the AM symbiosis is context dependent, for example, usually the inoculation of the AMF Rhizophagus irregularis benefits plant biomass, but benefits can be suppressed by high soil fertility levels. Nevertheless, the importance of many other agricultural management practices on AMF, such as fungicides application, is poorly understood. Also, pesticide regulations usually neglect a comprehensive safety testing of fungicides on AMF and lawmakers require empirical support to improve such laws. The objective of this study was to evaluate the effects of spraying fungicides on tomato plants and the subsequent root colonization of plants grown in natural soil containing AMF and inoculated with R. irregularis. We detected that the inoculation of R. irregularis increased the total root colonization of the control plants that did not receive fungicides and that spraying the plants with the fungicides Signum ® and Topas ® reduced total root colonization. The effect on specific AM fungal structures was variable according to the product. Signum ® reduced the occurrence of arbuscules, while Topas ® reduced the occurrence of AM hyphae in the colonized roots. Cuprozin ® did not reduce total root colonization but reduced the occurrence of AM vesicles. Sampling time was also relevant. Effects were detected at 90 days, but not at 35 days. Our results show that fungicides safety should be evaluated for their effects on root colonization of crops in non-sterilized soils and at adequate sampling time.
Arbuscular mycorrhizal fungi (AMF) are plant symbionts that are represented across global biomes, forming symbiotic associations with more than 80% of all terrestrial plants, including many agricultural crops (Brundrett and Tedersoo, 2018). AMF can provide multiple benefits to the host plant, such as increased nutrient uptake, drought resistance, and resistance to a plethora of pathogens (Jacott et al., 2017; Begum et al., 2019; Rillig et al., 2019; Kuila and Ghosh, 2022). AMF payouts to plants are context dependent, for example, there are fewer benefits of the symbiosis on plant biomass in higher fertility soil (Hoeksema et al., 2010). However, AMF-related benefits extend beyond plant biomass, and include positively affecting plant stability, e.g. improved resistance to stress, and to environmental quality, e.g. by reducing leaching and promoting soil aggregation (Jacott et al., 2017; Begum et al., 2019; Rillig et al., 2019; Kuila and Ghosh, 2022).
Because of the benefits of this symbiosis, farmers have considered boosting AM fungal abundance in soils by using specific management practices, such as using crop rotations or inoculation of the AMF Rhizophagus irregularis or with a mixture of AM fungal species (Rillig et al., 2019; Kuila and Ghosh, 2022). Inoculating AMF could not only increase AM community abundance but also modulate rhizosphere-associated microbiota with potential consequences on soil and plant-microbe interactions and plant production (Ardestani et al., 2019). However, the natural roles carried out in the key AM-plant symbioses in agro-ecosystems have been hampered by a broad range of farming practices including plant protection measures using pesticides with far-reaching ecological consequences (Hernández-Dorrego and Mestre-Parés, 2010; Rillig et al., 2019; Baibakova et al., 2019; Gaspar et al., 2002; Kuila and Ghosh, 2022). For example, usually pesticide regulations and laws require fungicides to not be harmful for R. irregularis spore germination, but there are generally no requirements for the evaluation of root colonization of adult plants (EFSA Panel on Plant Protection Products and their Residues (PPR) et al., 2017; European Comission, 2021).
Fungicides can be uptaken by roots and AMF from the soil or when fungicides are systemically transported through the above-ground plant parts to the roots and can then affect AM fungal structures including spores, hyphae, arbuscules and vesicles (Hage-Ahmed et al., 2019). The effects of fungicide on AM fungal spore production have been shown to vary considerably across studies and fungicides (Hage-Ahmed et al., 2019). Schreiner and Bethlenfalvay (1997) performed an in vitro study and found that the fungicides Captan, pentachloronitrobenzene (PCNB) and benomyl inhibited the germination of three species of arbuscular mycorrhizal fungi (Glomus etunicatum, G. mosseae and Gigaspora rosea) at 20 mg kg-1 (Schreiner and Bethlenfalvay, 1997; Hage-Ahmed et al., 2019). Moreover, the effects of fungicides on AM root colonization have been found to be usually neutral or detrimental. For example, Benomyl (1 µg g-1s oil), fenpropimorph (125 µg g-1 soil) and propiconazole (0.21 µg g-1 soil), Teldor (1.5 g L-1) were reported detrimental to root colonization, while Topas was neutral (0.4 ml l-1) ( (Kjøller and Rosendahl, 2000; Hernández-Dorrego and Mestre-Parés, 2010; Baibakova et al., 2019; Hage-Ahmed et al., 2019). These results show that the effects of fungicides on AMF are not fully understood and require to be continuously assessed with different fungicide types and contexts.
Recently it was highlighted that the implementation of AMF plant protection product assessment is limited by critical knowledge and research gaps to ensure a better risk‐assessment (Sweeney et al., 2022). Although some studies have addressed the impacts of some fungicides on AMF, many of these have been performed in vitro or using soilless media and usually such studies only partially reflect the performance of fungicides in commercial greenhouse settings or field trials (Hage-Ahmed et al., 2019). Furthermore, most of the experimental settings trials, including greenhouse studies, have been conducted using sterilized soil substrates to eliminate indigenous soil microbial communities, and thereby limit the establishment of a root-colonizing AM fungal community that interacts with the local soil microbial community as occurs in field conditions (Hernández-Dorrego and Mestre-Parés, 2010; Hage-Ahmed et al., 2019). Due to this, it is of utmost importance to investigate the effects of both novel and established fungicides that are present in the market for application as in commercial greenhouse and or field settings (Baibakova et al., 2019; de Novais et al., 2019; European Comission, 2022; Kuila and Ghosh, 2022).
The objective of this study was to test for potential putative effects of four widely used fungicides on the association of arbuscular mycorrhiza and tomato plants cultivated in non-sterilized soils. We hypothesized that systemic fungicides sprayed on the plant leaves can be detrimental to the root colonization of AMF, and that effects depend on the fungicide.
We conducted a greenhouse experiment to test for the putative effects of four widely used fungicides: Cuprozin ® progress (383 g.l-1 copper hydroxide, Certis, Germany), Signum ® (267.0 g.kg-1 boscalid, 67.0 g.kg-1 F 500® - pyraclostrobin, Basf, Germany), Teldor ® (500g.kg-1 fenhexamide, Bayer, Germany), and Topas ® (10 g.l-1 penconazole, Syngenta, Germany) on arbuscular mycorrhiza and tomato plant symbiosis including the effects of fungicides on AMF root-colonization structures and tomato plant biomass. We used a non-sterilized meadow soil that closely relates to the natural symbiotic community present in agricultural settings. We mimicked farmer practices of growing tomatoes in a greenhouse and also when inoculating the plants with the model AMF species Rhizophagus irregularis (R.i.), a common inoculant found on the market. We performed an experiment with a completely randomized design with the following treatments inoculated with R.i.: 1) control without fungicide, 2) Cuprozin, 3) Signum, 4) Teldor, and 5) Topas. Each treatment was applied to eight pots, for a total of 40 pots. In addition to these treatments, we tested an additional control with plants non-inoculated with R. irregularis and without receiving fungicide to check the efficiency of the R.i. inoculation (Supplementary Figure 1).
The soil was collected from a meadow at the experimental field of Freie Universität Berlin (Rillig et al., 2010). The soil is an Albic Luvisol and had the following characteristics, texture: sand, 74%, silt, 18% and clay, 8%, pH 7, 6.9 mg 100 g-1 of P, and 5 mg 100 g-1of K, 4.5 mg 100 g-1 Mg, 0.32 mg kg-1 B, 4.6 mg kg-1 Cu, 64 mg kg-1 Mn, 23 mg kg-1 Zn, <0,1 mg 100 g-1 NH4-N and 0.3 mg 100 g-1 NO3-N (Rillig et al., 2010). The soil was air dried, sieved (10 mm) and homogenized and then filled into 2 l pots. Seeds of the tomato variety Harzfeuer F1 were germinated in sterilized sand for two weeks and then two seedlings transferred to each pot. Each pot received 1 ml of the AM inoculant BioMyc™ Vital which contains 200.000 infective propagules including spores and hyphae (BioMyc™ Environment GmbH, Germany). After one week all the pots were thinned to one plant per pot. Plants were fertilized weekly with 100 ml of a modified Hoagland Solution nutrient solution minus-phosphorus (0.5 g l-1MgSO4, 0.7 g l-1 KCl, 0.7 g l-1 CaCl2, 0.3 g l-1 NH4NO3, 30 mg l-1 FeSO4, 3 mg l-1 H3BO3, 2 mg l-1 MnCl2, 1 mg l-1 ZnCl2, 40 mg l-1 CuCl2, 30 mg l-1 H2MoO4) and at 40 and 80 days fertilized with 100 ml of full solution containing phosphorus (Sarruge, 1975). After 18 days of planting, the fungicidal treatments were sprayed separately onto each tomato plant according to doses recommended by the companies. The fungicides were applied one time at the rates Cuprozin (0.2 ml m-2), Signum (0.075 g m-2), Teldor (0.1g m-2) and Topas (0.025 ml m-2), respectively. For this, pots were covered with a plastic bag with a hole at the top to avoid fungicide drifting across the treatments. After the application of the fungicides the plastic bags were all removed. Similar to commercial production settings, the fungicides sprayed here on tomato plants could also run-off into the soil. Our visual evaluation indicated that most of the product stayed on the plants. Plants were grown under greenhouse conditions with temperatures ranging from 25 ± 3°C day-time to 19 ± 3°C night-time, with lights adjusted for a 16h photoperiod, and relative humidity of 60-80%. Plants were watered with non-sterile tap water every two days with an automated drip irrigation system connected to each individual pot. The experiment was harvested at two sampling times, first at an early stage at 35 days (one week after fungicide treatment), five pots (five replicates), and another part at a later stage at 90 days, three pots (three replicates). The plants were also visually evaluated for diseases, and they looked all heathy with no visible damage to their leaves and stems. The plants were then harvested and divided into shoots and roots (both harvests) and also in fruits for sampling at 90 days. Plant biomass was dried at 60° C until stabilizing at a constant mass and then weighed.
To determine AM fungal root colonization, 10 subsamples of the roots were collected prior to drying for each pot and placed in 10% KOH at 80°C for 30 min, washed with water three times and acidified in 1 M HCl at room temperature and stained for 15 min in 0.05% Trypan Blue at 80°C (Phillips and Hayman, 1970; Gerdemann, 1975). AM fungal root colonization was quantified at 200 X magnification using the magnified root intersections method (McGonigle et al., 1990). AM fungal structures were then recorded separately, and arbuscules, hyphae, vesicles, and the total percentage of root length colonized by AM fungal structures in tomato roots were calculated.
The effects of fungicide application were accessed by analysis of variance (ANOVA) and Tukey post-hoc tests. All response variables were tested by the Shapiro-Wilk and Levene’s Homogeneity tests to check the assumptions of normality and homoscedasticity of variance. Root colonization data was arcsine root square transformed prior analysis. Data was reported as box-plots with the Tukey post-hoc letters classifications in the main paper and details are reported in the Supplementary Material. We also calculated and plotted the effect sizes of each treatment. All statistical analyses were performed in R version 3.0.2 (R Core Team, 2013).
The experiment was evaluated at two sampling times, at 35 and 90 days. At 35 days, the plants presented low total root colonization (1-4%) and there was no evidence of differences between treatments regarding root colonization (ANOVA P>0.05, Supplementary Figure 2). For sampling time 35 days, plant biomass was slightly higher for the treatment Teldor (ANOVA P<0.05, Supplementary Figure 2). But for sampling time 90 days, the treatments had no effects (ANOVA P>0.05) on total plant biomass, including shoot, root or fruit weight (Figure 1A). At 90 days, we found statistically significant effects of the fungicides on total root colonization (Figure 1B, F=4.83; P=0.02), arbuscular (Figure 2A, F=4.01; P=0.03), hyphal (Figure 2B, F=3.44; P=0.05) and vesicular colonization (Figure 2C, F=3.92; P=0.03) (details of the ANOVA models statistics in Supplementary Information). The Tukey post-hoc (P<0.05) test showed, that compared to the control, the treatment Signum and Topas reduced total colonization by AMF, but the control was not statistically different to Cuprozin and Teldor (Figure 1B). Furthermore, in comparison to control treatment, Signum reduced arbuscule occurrence (Figure 2A), while Topas reduced hyphal colonization (Figure 2B) and Cuprozin reduced vesicle occurrences (Figure 2C). All the other treatments were not statistically different to the control (Figures 2A–C). In addition, at 90 days we also compared the experiment inoculated control to an additional non-inoculated control and founded that the root colonization of the inoculated-control (68%) was statistically significantly higher than the non-inoculated control (43%) (P<0.05, Supplementary Figure 1).
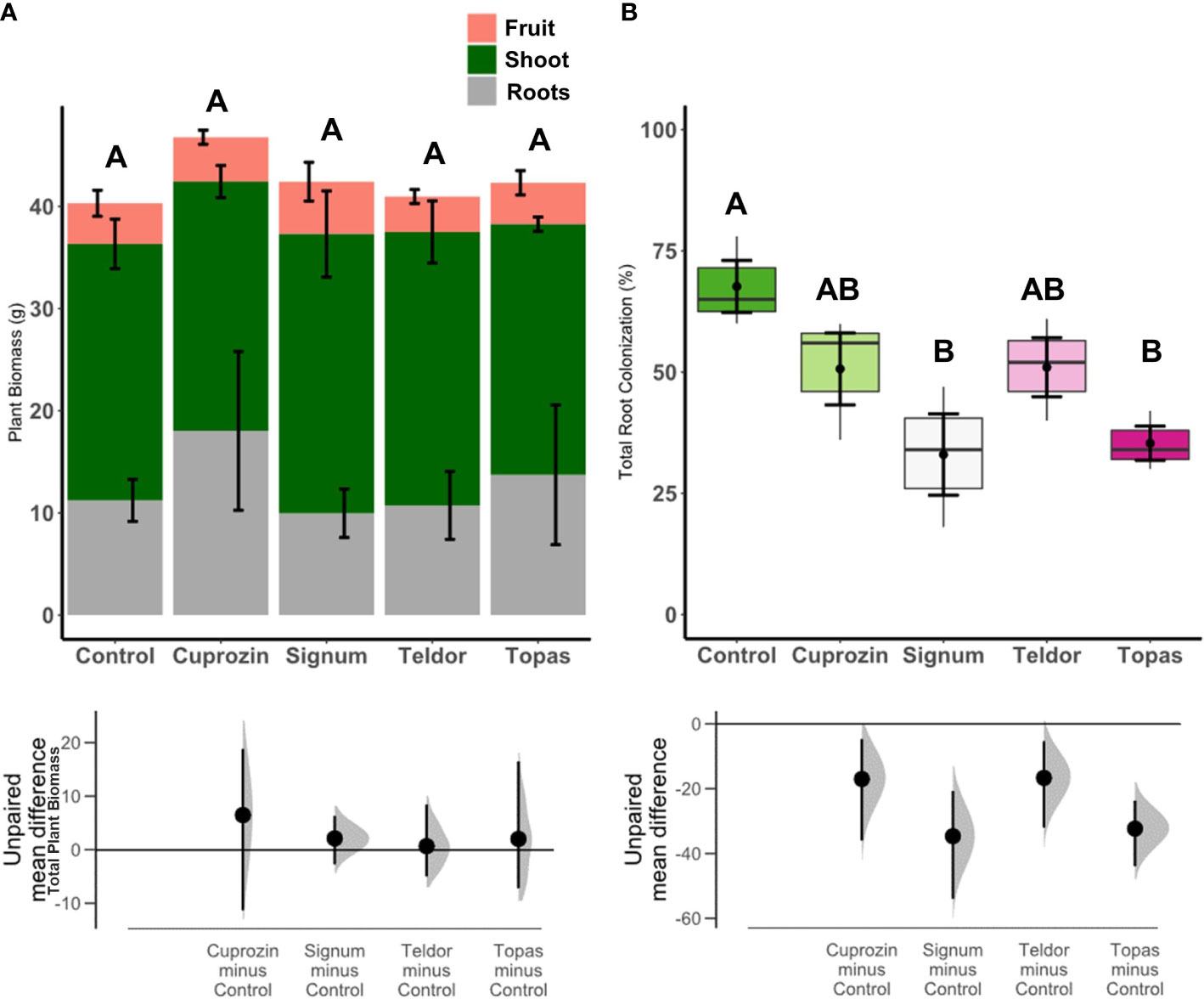
Figure 1 Tomato plant biomass and root colonization analyzed after 90 days of growth including: total biomass (A) and total root colonization (B). The upper part of the figures are represented by stacked bar plots with the biomass partitioning (in fruits, shoot and roots) for the plant biomass (A), and box-plots containing the average values and standard errors for the root colonization (B). Treatments with the same letter for each variable are not statistically significant by the Tukey post-hoc test (p < 0.05, details of ANOVA model are in Supplementary Material). There was no statistically significant difference for the biomass partitioning between treatments, so statistics are only reported for the total plant biomass. The bottom part of the figure are the effect sizes of the treatments in relation to the control.

Figure 2 Root colonization of tomato plants by AMF structures after 90 days: arbuscules (A), intraradical hyphae (B) and vesicles (C). The upper part of the figures are box-plots containing the average values and standard errors. Treatments with the same letter for each variable are not statistically significant by the Tukey post-hoc test (p < 0.05, details of ANOVA model are in Supplementary Material). The bottom part of the figure are the effect sizes of the treatments in relation to the control.
This study evaluated the effect of four widely used fungicides on the root colonization of tomato plants by arbuscular mycorrhizal fungi. We detected low root colonization at sampling time 35 days, which is common, since sometimes the symbiosis needs some weeks to become fully established or perhaps due to a low density of viable AM fungal propagules. At the second sampling time, 90 days, we detected significantly higher rates of root colonization in the control, with the fungicides Signum and Topas reducing the total root colonization via AMF. These products are systemic products, which means that they are translocated through the plants to their roots. The Topas (triazole) fungicide mechanism is interfering with the biosynthesis of sterols and Signum (boscalid and pyraclostrobin) interferes with mitochondrial respiration (Baibakova et al., 2019). Furthermore, the fungicide Cuprozin (copper hydroxide) reduced vesicle occurrence; this is a fungicide based on copper that can disrupt fungal proteins and mycelium growth (Baibakova et al., 2019). Since these fungicides have effects on fungi generally, they likely also affected AMF by similar mechanisms (Baibakova et al., 2019; Hage-Ahmed et al., 2019).
Typically, pesticide risk assessments do not include testing the effects of fungicides on root colonization by AMF in non-sterilized soils or field conditions (European Comission, 2021). However, fungicide-based legislation should likely include testing for non-targets organisms, such as AMF (EFSA Panel on Plant Protection Products and their Residues (PPR) et al., 2017). Suggestions have been made to test the effect of pesticides on AM fungal spore germination in vitro (Mallmann et al., 2018). However, testing only spores may miss the opportunity for a more realistic evaluation of the effects of fungicides on AMF. In realistic conditions, fungicides may affect AMF directly by suppressing their metabolism or indirectly through systemic responses in the host plant (Baibakova et al., 2019; Hage-Ahmed et al., 2019). AM structures, such as arbuscules, hyphae, and vesicles, can be exposed directly to fungicides via root or hyphal uptake from the soil or when fungicides are transported systemically from above-ground plant parts to the roots while altering the metabolism of AMF and the host plant (Hage-Ahmed et al., 2019). A number of fungicides have been shown to have an effect on AMF symbiosis with plants in different contexts, mainly in sterilized soils (Kjøller and Rosendahl, 2000; Hernández-Dorrego and Mestre-Parés, 2010; Hage-Ahmed et al., 2019). For example, the fungicide Benomyl was shown to inhibit both internal and external AM fungal hyphae at the low application level (1 µg g-1soil) while fenpropimorph had a moderate effect on AMF at high application level (125 µg g-1soil) and propiconazole decreased the external AM fungal hyphae but not the internal AM fungal hyphae at the low concentration level (0.21 µg g-1soil) (Kjøller and Rosendahl, 2000). Other fungicides had different results in different experimental sets, for example, the application of Teldor and Topas at 1.5 g l-1 and 0.4 ml l-1 respectively, showed neutral or negative effects on AM fungal root colonization depending on the plant and substrate type (Hernández-Dorrego and Mestre-Parés, 2010; de Novais et al., 2019). Other products are understudied, for example, we have not found previous reports for the effect of Signum on AMF, tested here.
Fungicide-induced alteration in AM fungal structures could potentially uncouple the multifunctional properties and benefits that AM symbiosis provides for a more sustainable agriculture. For example, a decrease in the percentage of vesicles could mean that the fungi allocate less to lipid structure, which could influence future AM fungal generations in soil. Moreover, a decrease in the percentage of vesicles by Cuprozin could result in a negative effect on stress protection; vesicles are capable to accumulate and immobilize high amounts of Cu ions thus alleviating heavy metal stress and avoiding Cu translocation to aerial parts. A decrease in arbuscules could reduce symbiotic activity and thus reduce nutrient transport to the host plant (Jacott et al., 2017; Begum et al., 2019; Rillig et al., 2019; Kuila and Ghosh, 2022).
Overall, we did not observe statistically significant effects of the fungicides or AMF inoculation on plant biomass. The benefits of AMF to plants are context dependent, and in our experimental conditions mimicking commercial greenhouse production, the plants were supplied with adequate levels of nutrients and were not challenged by stress or diseases. In such optimal conditions, neither the AM inoculation, nor the fungicides applications provided any clear benefits to the plants, and this could be different from dynamic field conditions (Rillig et al., 2019). Experiments testing the consequences of pesticides on AMF could be expanded in the future for testing a variety of contexts, such as testing at low and high soil fertility and in the presence of fungal diseases. In that context, effects of the treatments on plant biomass are more likely, since AMF often benefits crops in terms of production stability and reducing stressors effects (Rillig et al., 2019).
Our study shows that some widely used fungicides suppress AM fungal root colonization and AM structures in tomato roots. This data suggests that legislation for the registration of fungicides needs to be improved and should include analysis of root colonization by AMF in adult crops. Our data reveals that even products that are sprayed on the plants, and apparently with no direct physical contact to the AMF, can suppress root colonization and AM structures in roots. Thus, current agrochemical legislation should be reviewed and expanded to assess the risks for this integral symbiosis. Lastly, farmers should be aware that their choice of using fungicides can potentially impact the AM symbiosis and could potentially affect crop production stability, e.g. if the plants are challenged by stressors where the AM symbiosis could be beneficial. Such contexts should thus be further studied experimentally in realistic conditions including commercial greenhouse and agricultural field settings.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
SO, TK, DO, MR, and DL designed the study. TK, DO, and SO conducted the experiment. SO and PM analyzed the data. SO and DL wrote the manuscript with contribution from all authors. All authors contributed to the article and approved the submitted version.
DL acknowledges a postdoctoral fellowship from the Alexander von Humboldt Foundation (Germany) in cooperation with the Brazilian “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES) agency. We acknowledge support by the Open Access Publication Initiative of Freie Universität Berlin.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2022.1028195/full#supplementary-material
Ardestani M. M., Jilkova V., Bonkowski M., Frouz J. (2019). The effect of arbuscular mycorrhizal fungi rhizophagus intraradices and soil microbial community on a model plant community in a post-mining soil. Plant Ecol. 220, 789–800. doi: 10.1007/s11258-019-00953-w
Baibakova E. V., Nefedjeva E. E., Suska-Malawska M., Wilk M ., Sevriukova G. A., Zheltobriukhov V. F., et al. (2019). Modern fungicides: Mechanisms of action, fungal resistance and phytotoxic effects. Annu. Res. Rev. Biol. 32, 1–16. doi: 10.9734/arrb/2019/v32i330083
Begum N., Qin C., Ahanger M. A., Raza S., Khan M. I., Ashraf M., et al. (2019). Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01068
Brundrett M. C., Tedersoo L. (2018). Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 220, 1108–1115. doi: 10.1111/nph.14976
de Novais C. B., Giovannetti M., de Faria S. M., Sbrana C. (2019). Two herbicides, two fungicides and spore-associated bacteria affect Funneliformis mosseae extraradical mycelium structural traits and viability. Mycorrhiza 29, 341–349. doi: 10.1007/s00572-019-00901-6
EFSA Panel on Plant Protection Products and their Residues (PPR), Ockleford C., Adriaanse P., Berny P., Brock T., Duquesne S., et al. (2017). Scientific opinion addressing the state of the science on risk assessment of plant protection products for in-soil organisms. EFSA J. 15, e04690. doi: 10.2903/j.efsa.2017.4690
European Comission. (2021). Approval of active substances. Available at: https://ec.europa.eu/food/plants/pesticides/approval-active-substances_en (Accessed 5 May 2022).
European Comission. (2022). The use of plant protection products in the European union. Available at: https://ec.europa.eu/eurostat/web/products-statistical-books/-/KS-76-06-669 (Accessed 18 September 2022).
Gaspar M., Cabello M., Cazau M., Pollero R. (2002). Effect of phenanthrene and Rhodotorula glutinis on arbuscular mycorrhizal fungus colonization of maize roots. Mycorrhiza 12, 55–59. doi: 10.1007/s00572-001-0147-4
Gerdemann J. W. (1975). “Vesicular-arbuscular mycorrhizae,” in The development and function of roots. Eds. Torrey J. G., Clarkson D. T. (London: Academic Press), 575–591.
Hage-Ahmed K., Rosner K., Steinkellner S. (2019). Arbuscular mycorrhizal fungi and their response to pesticides. Pest Manag Sci. 75, 583–590. doi: 10.1002/ps.5220
Hernández-Dorrego A., Mestre-Parés J. (2010). Evaluation of some fungicides on mycorrhizal symbiosis between two Glomus species from commercial inocula and Allium porrum l. seedlings. Spanish J. Agric. Res. 8, 43–50. doi: 10.5424/sjar/201008S1-1222
Hoeksema J. D., Chaudhary V. B., Gehring C. A., Johnson N. C., Karst J., Koide R. T., et al. (2010). A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 13, 394–407. doi: 10.1111/j.1461-0248.2009.01430.x
Jacott C. N., Murray J. D., Ridout C. J. (2017). Trade-offs in arbuscular mycorrhizal symbiosis: Disease resistance, growth responses and perspectives for crop breeding. Agronomy 7, 75. doi: 10.3390/agronomy7040075
Kjøller R., Rosendahl S. (2000). Effects of fungicides on arbuscular mycorrhizal fungi: differential responses in alkaline phosphatase activity of external and internal hyphae. Biol. Fertil Soils 31, 361–365. doi: 10.1007/s003749900180
Kuila D., Ghosh S. (2022). Aspects, problems and utilization of arbuscular mycorrhizal (AM) application as bio-fertilizer in sustainable agriculture. Curr. Res. Microbial Sci. 3, 100107. doi: 10.1016/j.crmicr.2022.100107
Mallmann G. C., Sousa J. P., Sundh I., Pieper S., Arena M., da Cruz S. P., et al. (2018). Placing arbuscular mycorrhizal fungi on the risk assessment test battery of plant protection products (PPPs). Ecotoxicology 27, 809–818. doi: 10.1007/s10646-018-1946-0
McGonigle T. P., Miller M. H., Evans D. G., Evans D. G, Fairchild G. L., Swan J. A. (1990). A new method which gives an objective measure of colonization of roots by vesicular–arbuscular mycorrhizal fungi. New Phytol. 115, 495–501. doi: 10.1111/j.1469-8137.1990.tb00476.x
Phillips J. M., Hayman D. S. (1970). Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycological Soc. 55, 158–IN18. doi: 10.1016/S0007-1536(70)80110-3
R Core Team. (2013). R: A language and environment for statistical computing. (Vienna: R Foundation for Statistical Computing). http://www.Rproject.org/.
Rillig M. C., Aguilar-Trigueros C. A., Camenzind T., Cavagnaro T. R., Degrune F., Hohmann P., et al. (2019). Why farmers should manage the arbuscular mycorrhizal symbiosis. New Phytol. 222, 1171–1175. doi: 10.1111/nph.15602
Rillig M. C., Mardatin N. F., Leifheit E. F., Antunes P. M. (2010). Mycelium of arbuscular mycorrhizal fungi increases soil water repellency and is sufficient to maintain water-stable soil aggregates. Soil Biol. Biochem. 42, 1189–1191. doi: 10.1016/j.soilbio.2010.03.027
Schreiner R. P., Bethlenfalvay G. J. (1997). Mycorrhiza, biocides and biocontrol 3. the effects of three different fungicides on developmental stages of three arbuscular mycorrhizal fungi. Biol. Fertil Soils 24, 18–26. doi: 10.1007/BF01420215
Keywords: arbuscular mycorrhizal fungi, root colonization, pesticides, fungicides, tomato
Citation: Okiobe ST, Meidl P, Koths T, Olschewsky D, Rillig MC and Lammel DR (2022) Root colonization by arbuscular mycorrhizal fungi is reduced in tomato plants sprayed with fungicides. Front. Agron. 4:1028195. doi: 10.3389/fagro.2022.1028195
Received: 25 August 2022; Accepted: 25 October 2022;
Published: 16 November 2022.
Edited by:
Ashwani Kumar, Dr. Harisingh Gour Central University, IndiaReviewed by:
Debasis Mitra, National Rice Research Institute (ICAR), IndiaCopyright © 2022 Okiobe, Meidl, Koths, Olschewsky, Rillig and Lammel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel R. Lammel, bGFtbWVsQHplZGF0LmZ1LWJlcmxpbi5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.