- 1Department of Botany, Shoolini Institute of Life Sciences and Business Management, Solan, India
- 2University Institute of Biotechnology, Department of Biosciences, Chandigarh University, Mohali, India
- 3Department of Food Technology, School of Applied and Life Sciences, Uttaranchal University, Dehradun, Uttarakhand, India
- 4Department of Plant Sciences, Quaid-i-Azam University, Islamabad, Pakistan
- 5Institute of Life Sciences, Faculty of Food Science and Technology, University of Agriculture Sciences and Veterinary Medicine, Cluj-Napoca, Romania
- 6Food Engineering Department, Faculty of Food Science and Technology, University of Agriculture Sciences and Veterinary Medicine, Cluj-Napoca, Cluj-Napoca, Romania
Nowadays, it is generally accepted that medicinal plants play a crucial role in traditional healthcare operations, providing hints to new research fields and in biodiversity protection. However, there is a lack of information on the medicinal uses of plants in many of the interior Himalayan regions. In light of this, the current investigation was initiated in the tribally dominant western Himalayan hinterland. The current study examined five underutilized wild edible plants, namely, Allium rubellum, Berberis chitria, Capsella bursa-pastoris, Stellaria aquatica, and Rheum emodi, for their nutritional qualities, phytochemical analysis, antioxidant activity, and antibacterial activity, which are consumed as food by the Gaddi community of the Bharmour region of the Chamba District, Himachal Pradesh. In this study, the nutritional makeup of these plants was examined in terms of their carbohydrate, protein, sodium, potassium, crude fiber, and fat content. As compared to other investigated species, A. rubellum has the highest nutritional content: carbohydrate (6.93 mg/g), protein (10.18 mg/g), sodium (3.21 mg/g), potassium (16.32 mg/g), and fiber (6.46%). In addition, phenols, amino acids, tannins, terpenoids, carotenoids, and phytate were found to be the least significant phytochemicals in R. emodi, i.e., 4.81 mg/g, 0.594 mg/g, 2.204 mg/g, 1.482 mg/g, 156 µg/g, and 0.680%, respectively. The methanolic extract of these wild edible plants showed significant free radical scavenging activity by using ABTS and DPPH assays. Moreover, the antibacterial activity of the methanolic extract of studied plants based on the present study suggested that R. emodi exhibits a maximum zone of inhibition of 20.8 mm against Escherichia coli, whereas S. aquatica showed a maximum inhibition zone of 19.2 mm against S. aureus. The findings of this study validated that these wild edible plants are an alternate source of medicine and are an abundant source of various phytochemicals like protein, carbohydrates, vitamins, and minerals. These compositions offer dietary benefits, food security, health benefits, and therapeutic advantages. Hence, in the current study, it was analyzed that there is an urgent need for documentation, conservation, characterization, and evaluation of these underutilized plants for their therapeutic purpose and nutritional supplements.
1 Introduction
One of the major issues in developing countries is the problem of malnutrition. The causes of malnutrition are diverse, and a holistic and multidimensional approach is needed to address it (Obasohan et al., 2020). One way to meet the challenge of food security is by leveraging underutilized wild plants. According to FAO research, approximately 1 billion people in underdeveloped nations rely on edible uncultivated plants (Taylor et al., 2014).
One of the richest hotspots of biodiversity, the Indian Himalayan region is renowned for its diverse flora, fauna, and ecosystems. Migratory tribes used to live in the remote, high-altitude region of the Northwest Himalayas. Generally, wild food plants partially contribute to their meals and often have a higher amount of nutrients and bioactive compounds that cure several diseases (Vipin & Ashok, 2010; Hegazy et al., 2013; Kalita et al., 2014; Singh et al., 2021b). These reports indicate that plants may have a pool of novel drugs. It is a known fact that, since ancient times, poor people depend on wild edible plants for their food, which not only contribute essential nutrients but also meet the energy requirements of poor, especially tribal communities (Kumar et al., 2021; Singh et al., 2021). Thus, nowadays, there has been a lot of interest among the scientific community to evaluate and explore the potential of natural edibles used by tribes (Dangwal et al., 2014; Seal et al., 2017; Sunil & Sanjeev, 2018; Datta et al., 2019; Kumar et al., 2020; Singh et al., 2022).
Sometimes, human cells are not able to produce the required amount of antioxidants during the time synthetic antioxidants are provided to the human body, which sometimes cause a mutagenic effect and cancer because of its toxic nature (Bendiabdellah et al., 2012). Hence, an alternative safer natural source of antioxidant supplement is required. Positive response has been reported in the case of plant-based antioxidants because they play a vital role in protecting cells without any side effects (Prasad et al., 2010). Therefore, the demand for plant-based antioxidants as food additive is increasing day by day in pharmaceuticals and nutraceuticals.
There are many underutilized, less exploited plants that are used by tribal communities. Thus, the goal of the current study was to investigate five plants used by gaddis tribes for their nutritional value, antioxidant capacity, and antibacterial activity. The studied plants were Allium rubellum, Berberis chitria, Capsella bursa-pastoris, Stellaria aquatica, and Rheum emodi (Figure 1). The main aim of this study is to determine their potential as underutilized plants and the possibility of their inclusion in daily diets for the prevention of oxidative stress-related issues. In spite of the traditional use of these plants by tribes, their nutritional value was not studied. Both leaves and bulb of A. rubellum are used as condiments. The plant is also used for a range of common health problems including coughs and cold and skin rashes (Kunkel, 1984; Devi et al., 2014). B. chitria is eaten as a pickle. Berberin is the major alkaloid found in the stem and root of the plant and is known to cure a variety of ailments such as eyes and ear diseases, diabetes, fever, jaundice, stomach disorders, and skin diseases (Khan et al., 2016; Palai, 2022). C. bursa-pastoris has medicinal value and is used to treat edema caused by nephritis and hypertension (Kweon et al., 1996 and Song et al., 2007). About 23 species of Stellaria occur in India in which S. aquatica shoot and leaves are eaten, which are rich in iron (Sharma & Arora, 2012). R. emodi possesses antifungal, antioxidant, anticancer, antimicrobial, healing, and immune-enhancing action and is often known as “the wonder drug” because of its extensive medicinal uses (Bilal et al., 2015).
In addition, the main aim of the study is to determine their potential as underutilized plants and the possibility of their inclusion in daily diets for the prevention of the malnutrition problem and oxidative stress-related issues. Therefore, this study was to ascertain the medicinal and nutritional value of these plants used by tribal people. Moreover, phytochemical profiling was also performed to understand their antioxidant properties. The current study was not documented in the studied region earlier; hence, this study is useful for tribal people in the said region for them to better use these wild plants for nutrition and medicinal purposes.
2 Materials and methods
2.1 Sample collection and preparation
The important characteristics of the five underutilized plants used in the present study are given in Table 1. The plants were collected from the Bharmour region of the Chamba District, Himachal Pradesh. Bharmour stretches from 76° 31’ 35” to 76° 53’ 71” E longitude and from 32° 11’ 35” to 32° 41’ 54” N latitude. Aerial parts of A. rubellum, C. bursa-pastoris, and S. aquatica were collected in the first week of August 2019, whereas leaves of B. chitria and stems of R. emodi were collected in the third week of July 2019 for analysis. Plants were collected, preserved in blotting paper, rinsed with distilled water, and dried in a hot air oven at 40°C. The dried plants were crushed into a fine powder using a mortar and pestle, placed in a glass bottle, and refrigerated at 4°C for use in further phytochemical and nutritional analyses.

Figure 1 Wild edible plants used by Gaddis: (A) Allium rubellum, (B) Berberis chitria, (C) Capsella bursa-pastoris, (D) Stellaria aquatica, and (E) Rheum emodi.
2.2 Extract preparation
For each sample, 2 g of powdered plant parts was soaked in 20 ml of methanol and maintained for 48 h on an orbital shaker. The extract from the filtrate was utilized to test for antioxidant and antibacterial activities after it had been evaporated and dried at 40°C in a water bath.
2.3 Nutritional composition
2.3.1 Carbohydrate estimation
One hundred milligrams of the sample was weighed into a test tube, homogenized with 2 ml of 2.5 N HCl, heated in water for 2 h, and then allowed to cool to room temperature. Plant extract (0.2 ml) was mixed with 500 ml of distilled water and 4 ml of the anthrone reagent. The mixture was heated in a water bath for 5 min before chilling. The UV/visible spectrophotometer at 630 nm determined the green color of the mixture. The glucose calibration curve was used to calculate the sample’s carbohydrate content, with the results represented as 1 g of glucose equivalents per milligram of sample (Johnson et al., 2017). A protein estimate of 500 mg of the material was weighed and pulverized in a 2-ml buffer solution using a pestle and mortar. The supernatant from a centrifuge was used to determine the amount of protein. Three milliliters of copper sulfate reagent was added to 200 µl of supernatant, which was then thoroughly mixed and left to sit for 10 min. After that, 0.5 ml of the Folin–Ciocalteu reagent was added and carefully mixed. The combination in test tubes was kept at room temperature in a dark environment for approximately 30 min. The UV-visible spectrophotometer detected the reaction mixture’s blue color at 660 nm. The bovine albumin serum (BSA) calibration curve was used to estimate the sample’s protein content, and the results were expressed as 1 g of BSA equivalent/mg of sample (Winters & Minchin, 2005).
2.3.2 Sodium and potassium
The potassium and sodium content of plant samples were measured through acid digest by using a flame photometer. A 100-ml flask contained 500 mg of dried plant material. The flask was then pre-digested by being kept in an enclosed place for 6 to 8 h or overnight after receiving 10 ml of pure HNO3. After pre-digestion, 10 ml of concentrated HNO3 and 2–3 ml of HClO4 were added. This was kept on a hot plate for the first hour and then heated to 100°C and then 200°C in an acid-proof chamber with a fume exhaust system. By continuing to heat the acid at the same temperature, the acid content was reduced to roughly 2–3 ml. One hundred milliliters was manufactured following three to four washings in 10–15 ml of distilled water. Using a flame photometer, the solution’s Na+ and K+ concentrations were measured. By graphing the measurements against the Na and K readings, a standard curve was created (Chikhale & Chikhale, 2017).
2.3.3 Determination of crude fiber
The sample was digested by boiling it in 50 ml of a 1.25% H2SO4 solution for 30 min before being filtered under pressure. The residue was rinsed with hot water three times. Fifty milliliters of a 1.25% NaOH solution was used for the second time throughout the same procedure. After being chilled and dried at 100°C, the final residue was weighed (C1). After 3 h of 550°C heating in a muffle furnace, the crucible was cooled to ambient temperature and weighed once more (C2) (Aina et al., 2012).
The formula used to determine the crude fiber % was
2.3.4 Determination of crude fat
Twenty-five milliliters of diethyl ether was used to extract 1 g of the material, which was shaken for 24 h. After filtering, the extract was kept in a beaker that had already been weighed with the ether extract (W1). After being shaken for an additional 24 h and re-equilibrated with 25 ml of diethyl ether, the solution was maintained in the same beaker (W1). After the beaker’s ether was dried in a water bath at 40 to 60°C, it was reweighed (W2) (Unuofin et al., 2017).
2.4 Phytochemicals analysis
2.4.1 Determination of phenols
One hundred milligrams of the sample was homogenized in 3 ml of methanol and centrifuged at 10,000 rpm for 10 min. The Folin–Ciocalteu reagent was added to the supernatant, and the mixture was left to stand for 2 min. The mixture was then given 1 ml of a 35% Na2CO3 solution before being given 10 ml of distilled water as the final volume. The solution was left at room temperature for about 30 min while the OD against a blank was measured. The amount of phenol in an extract was represented as milligrams of gallic acid equivalents (GAE) per gram (Jing et al., 2015).
2.4.2 Determination of flavonoids
One hundred milligrams of the substance was weighed and centrifuged for 10 min at 10,000 rpm, 2 ml of methanol was added, and the supernatant was collected. Using distilled water, 200 ml of the plant extracts was diluted to 1.5 ml, and 75 ml of 5% NaNO2 was added. After waiting for 5 min, 150 ml of 10% AlCl3 was added to the solution. The reaction mixture was left at room temperature for 5 min. At 510 nm, absorbance was measured against a reagent blank after adding 0.5 ml of 1 M NaOH to it. Results were given in terms of milligrams of rutin equivalents (RE) per gram of sample (Wang et al., 2020).
2.4.3 Determination of ascorbic acid
Ascorbate was recovered by homogenizing 1 g of the material in 4% TCA and creating a final volume of 10 ml using 4% tricarboxylic acid. The supernatant was treated with a tiny amount of activated charcoal, violently mixed, and then left to stand for 10 min after being centrifuged for 10 min at 2,000 rpm. The remaining charcoal was removed by using centrifugation once more. Tricarboxylic acid (4%) was added to 1 ml of supernatant to make 2 ml. Osazones were created by mixing 500 µl of DNPH reagents with two drops of a thiourea solution at a concentration of 10% for 3 h at 35°C; 2.5 ml of 85% H2SO4 was used to dissolve the osazones. Following 30 min of incubation, the absorbance was measured at 540 nm, and the ascorbic acid concentrations were calculated using a standard graph and expressed as ascorbate mg/g (Roe & Kuether, 1943).
2.4.4 Determination of amino acid
Five hundred milligrams of the substance was weighed and then ground in a mortar and pestle with 5 ml of ethanol that contained 80% alcohol; 0.1 ml of the extract was centrifuged with 1 ml of the ninhydrin solution added. The volume was then increased to 2 ml with distilled water, and the tube was heated in a boiling bath for 15 min, followed by completely blending in 5 ml of propanol. After 15 min, the optical density (OD) of the purple color at 570 nm was measured using a spectrophotometer. The concentration of amino acid in the plant sample was determined and expressed as mg/g equivalent of leucine (Stein & Moobe, 1948).
2.4.5 Determination of tannin
A sample weighing 100 mg was homogenized with 3 ml of methanol and centrifuged for 10 min at 10,000 rpm before collecting the supernatant. After combining 1 ml of the extract with 0.5 ml of Folin’s phenol reagent, the mixture was allowed to sit for 10 min at room temperature before being mixed with 5 ml of 35% Na2CO3. The blue hue of the reaction mixture was picked up by the UV-visible spectrophotometer at 640 nm. Tannin content was assessed using a calibration curve for gallic acid, and the results were presented as mg/g of gallic acid equivalent (Moore & Stein, 1948).
2.4.6 Determination of terpenoids
One hundred milligrams of the sample was homogenized in 2 ml of methanol. After 10 min of centrifuging at 10,000 rpm, the supernatant was recovered. One hundred milliliters of supernatant received 3 ml of chloroform addition. A reddish-brown precipitate appeared after the mixture was allowed to remain at room temperature for 1.5 to 2 h in the dark. Without disturbing the precipitate, the supernatant of the reaction mixture was decanted. Three milliliters of 95% methanol was added and then the mixture was aggressively vortexed to ensure that all of the precipitate is thoroughly combined with the methanol; 95% methanol was used to calculate the 538-nm absorbance in relation to a blank. Linalool served as the benchmark for estimation. The terpenoid concentration was calculated using the linalool calibration curve, and the results were expressed as linalool equivalents (mg/g) (Ghorai et al., 2012).
2.4.7 Determination of tocopherol
The sample was gently dissolved in 0.1 N sulfuric acid with 100 mg of the sample, allowed to stand at room temperature the next day, and then filtered. Before centrifuging, 1.5 ml of xylene was added to 1.5 ml of tissue extract. After combining 1.0 ml of the 2,2-pyridyl reagent and 1.0 ml of the separated xylene layer, the OD was measured at 460 nm. Initially, 0.33 ml of FeCl3 was added to the blank and thoroughly mixed. The test and standard were read at 520 nm against the blank after 15 min. The tocopherol content was determined using the tocopherol calibration curve, and the results were given in tocopherol equivalents (mg/g) (Rosenberg, 1945).
2.4.8 Determination of carotenoids
The method used by Zakaria et al. (1979) to estimate the carotenoid content was also followed in the present study. Five grams of the sample should be saponified for approximately 30 min at 37°C in a shaking water bath after the alcoholic KOH has been extracted. Saponified extract was added to a different funnel containing 10–15 ml of petroleum ether to dissolve the carotenoid pigments. The bottom aqueous layer was moved to a different separating funnel while the petroleum ether extract containing the carotenoid pigments was transferred to an amber-colored bottle. Petroleum ether was used to repeatedly extract the aqueous phase in a similar manner until it was colorless. A small amount of sodium sulfate was added to the petroleum ether layer to eliminate turbidity after the aqueous layer was removed. The absorbance at 450 nm was measured using a spectrophotometer and petroleum ether as a control (Zakaria et al., 1979).
2.4.9 Determination of alkaloid
Omoruyi et al. (2012) method was used to determine the amount of alkaloids. Twenty-five milliliters of 10% acetic acid in ethanol was used to dissolve 1 g of it. For 2 h, it was covered and left to stand. The mixture was filtered, and the filtrate was heated while being reduced to one-fourth of its initial volume. Until precipitation, drops of concentrated NH4OH were added to the extract. The mixture continued to resolve after being filtered and washed with mild NH4OH. The accumulated precipitate was dried and weighed (Omoruyi et al., 2012).
The following formula was used to determine alkaloid content:
2.4.10 Determination of phytate
A flask containing 50 ml of 2% HCl and 1 g of the weighted sample was incubated for 2 h before being filtered. Twenty-five milliliters of the solution and 5 ml of 0.3% ammonium thiocyanate were added to a 250-ml flask to serve as an indicator; 53.5 ml of distilled water was added to give the mixture some acidity. It was titrated using a typical FeCl2 solution (0.00195 g of Fe/ml) until a brownish yellow color emerged (Damilola et al., 2013). Phytate content was calculated as:
2.5 Antioxidant activity
2.5.1 The 2,2-azinobis (3-ethylbenzthiazoline)-6-sulfonic acid assay
The antioxidant activity [2,2-azinobis (3-ethylbenzthiazoline)-6-sulfonic acid (ABTS)] of wild food plants was measured. Solutions for potassium persulfate and ABTS (7 mM) were made in 100 ml of methanol (2.45 mM). In order to create free radicals, these two solutions were completely combined and left in the dark all night. The OD at 745 nm was set to 0.76 and the stock solution was diluted to roughly 3 ml (control solution). A solution of 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) was dissolved in 2 ml of the test sample and maintained at 25°C for 15 min. The optical density (OD) of the combination was calculated using a spectrophotometer at a wavelength of 745 nm. OD was determined after preparing various ascorbic acid dilutions using the same process (positive control) (Re et al., 1999).
2.5.2 2,2-Diphenyl-1-picrylhydrazyl assay
The antioxidant activity [2,2-diphenyl-1-picrylhydrazyl (DPPH)] of wild food plants was evaluated. Twenty milligrams of DPPH was dissolved in 100 ml of methanol (stock solution) to create DPPH solution. The OD at 515 nm (control solution) of 3 ml of this solution was fixed at 0.75. For the creation of stock solutions, 5 mg of each extract was dissolved in 5 ml of methanol. A solution of 2 ml of DPPH was combined with about 2 ml of each dilution, and the mixture was then maintained in the dark for 15 min. In all of the experiments used for the comparative study, ascorbic acid served as a standard antioxidant component (Barros et al., 2007). Using the equation, the percentage of DPPH that extracts inhibited was determined.
2.6 Antibacterial activity
2.6.1 Collection of test organisms
The bacterial strains used in this experiment were Staphylococcus aureus (MTCC 96) and Escherichia coli (MTCC 82). These strains go through 14–16 h of incubation at 37°C while being cultured in nutrient broth.
2.6.2 Antibacterial activity of plants extract
To assess each plant extract’s antibacterial properties, the disc diffusion method was performed (Razmavar et al., 2014). Upon reaching a final concentration of 50 L/disc, the plant extract leftovers (100 mg) were dissolved in 1 ml of 10% DMSO before being applied to sterile filter paper discs (8 mm in diameter). Filter paper discs were loaded with 10 µl of streptomycin as a positive control. After being kept at 4°C in the refrigerator for 2 h to allow plant extracts to diffuse, the plates were incubated at 35°C for 24 h. By using a measuring scale, the occurrence of inhibitory zones was determined, and the extract’s antibacterial activity was demonstrated.
2.7 Statistical analysis
GraphPad Prism® 5.2 was used for the statistical analysis. The means of two species were compared using the least significant difference (LSD) at the 5% level. Three replicates’ worth of data (mean ± SD) were analyzed using a two-way ANOVA and then a Bonferroni multiple comparison post-test.
3 Results
Methanol extracts from A. rubellum, B. Chitria, C. bursa–pastoris, S. aquatica, and R. emodi retain a percentage yield of 24.00%, 24.82%, 15.33%, 22.60%, and 17.33%, respectively. Variation in percentage yield of plants was observed, which might be due to the composition of different phytochemicals in plants.
3.1 Nutritional composition
The nutritional composition of the five wild edible plants studied is presented in Table 2. Among all the species studied, A. rubellum was highly rich in carbohydrates, proteins, sodium, potassium, and crude fiber compared to the other four species. The minimum amount of these nutrients was found in R. emodi. The carbohydrates were found to be highest in A. rubellum (6.936 ± 0.175 mg/g) and lowest in R. emodi (4.681 ± 0.177 mg/g); protein content was found to be highest in A. rubellum (10.183 ± 0.153 mg/g) and lowest in R. emodi (2.320 ± 0.235 mg/g); sodium was found to be highest in A. rubellum (3.212 ± 0.359 mg/g) and lowest in R. emodi (0.553 ± 0.150 mg/g); potassium was found to be highest in A. rubellum (16.325 ± 1.139 mg/g) and lowest in R. emodi (5.776 ± 0.108 mg/g); crude fiber was found to be highest in A. rubellum (6.466%) and lowest in R. emodi (2.566%); crude fat content was found to be highest in B. chitria and lowest in C. bursa-pastoris. B. chitria was 2.14 times higher as compared to C. bursa-pastoris. Wild species of plants are well known for their nutritional value. The selection of plant species for systematic classification, plant improvement initiatives, and nutraceutical relevance depends on the amount and quality of crude protein content discovered in plant samples (Nisar et al., 2018). In earlier studies, it was shown that leaf samples have significant levels of crude fiber, which support digestion; proteins, which serve as the building blocks of cells; and lipids, which offer energy and help with the assimilation of fat-soluble vitamins (Aberoumand, 2011).
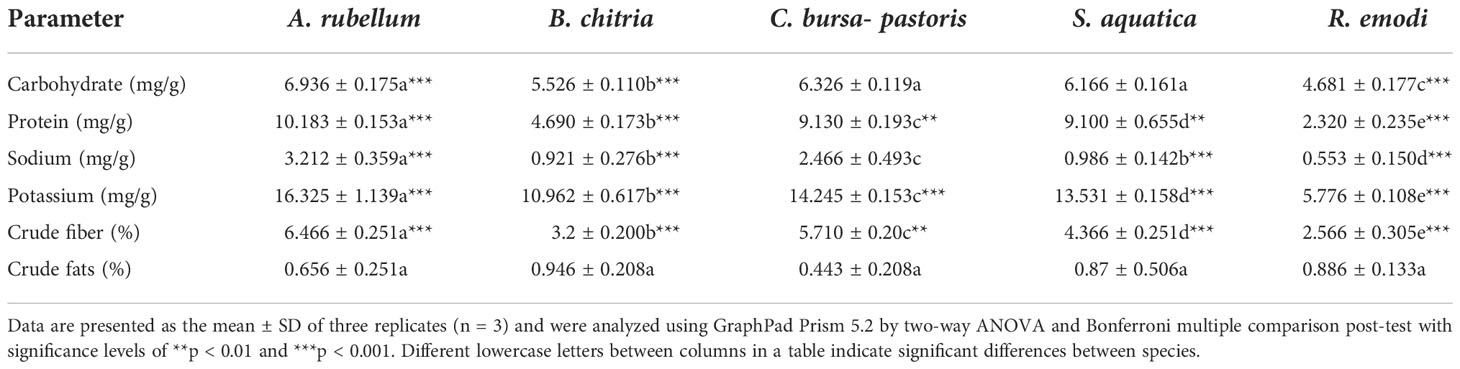
Table 2 Allium rubellum, Berberis chitria, Capsella bursa-pastoris, Stellaria aquatica, and Rheum emodi nutritional content.
3.2 Phytochemicals analysis
Phytochemicals analysis of the five wild edible plants studied is shown in Table 3. In general, substantial variability was observed for phytochemicals tested in the five species. The highest phenol content was observed in B. chitria and the lowest was observed in R. emodi. Phenol and flavonoid content were higher in B. chitria (14.219 ± 0.11 mg/g and 4.991 ± 0.221 mg/g, respectively). The lowest phenol and flavonoid content was found in R. emodi, which is approximately 4.815 ± 0.599 mg/g and 1.571 ± 0.359mg/g, respectively. Ascorbic acid content was found the highest in R. emodi (4.157 ± 0.424 mg/g) and the lowest in C. bursa-pastoris (0.389 ± 0.185 mg/g). Amino acid content was found to be 5.85 times higher in A. rubellum (3.475 ± 0.180 mg/g) in comparison with R. emodi (0.594 ± 0.123 mg/g). Tannin content was found to be the highest in C. bursa-pastoris (3.594 ± 0.229 mg/g) and the lowest in A. rubellum (1.991 ± 0.260 mg/g). It was 81% higher in C. bursa-pastoris than in A. rubellum. The order of presence of tannin was C. bursa-pastoris >B. chitria > R. emodi > S. aquatica > A. rubellum. The highest terpenoid content was found in B. chitria (6.291 ± 0.202 mg/g) and the lowest was found in R. emodi (1.482 ± 0.178 mg/g). Terpenoid content ranged between 1.482 ± 0.178 mg/g and 6.291 ± 0.202 mg/g in the five studied species. Carotenoids content was observed to be 61% higher in A. rubellum (251.603 ± 0.781 µg/g) than in R. emodi (156.21 ± 0.196 µg/g). Tocopherol content was found to be the highest in S. aquatica (14.603 ± 0.39µg/g) and was 6.12 times higher in B. chitria (2.387 ± 0.419 µg/g). The highest alkaloids content was found in R. emodi (1.271%) and the lowest was found in A. rubellum (0.366%) and was 3.47 times higher. The alkaloids, in general, were low in quantity in tested species and it varied between 0.36% and 1.27%. However, phytate content was the highest in A. rubellum (1.491%) and the lowest in R. emodi (0.680%). However, interestingly, the content of crude fats among the five species showed no significant variation (Tables 2, 3). It was observed that there was a significant difference among all the five species for protein, potassium, crude fiber, phenols, ascorbic acid, amino acid, terpenoids, carotenoids, and tocopherol contents. Elements accumulate differently in different plant species depending on factors like the kind of soil, plant species, fertilization technique, and environmental conditions (Bengtsson et al., 2003). On the basis of overall studies, we conclude that these plants are a good source of mineral composition.
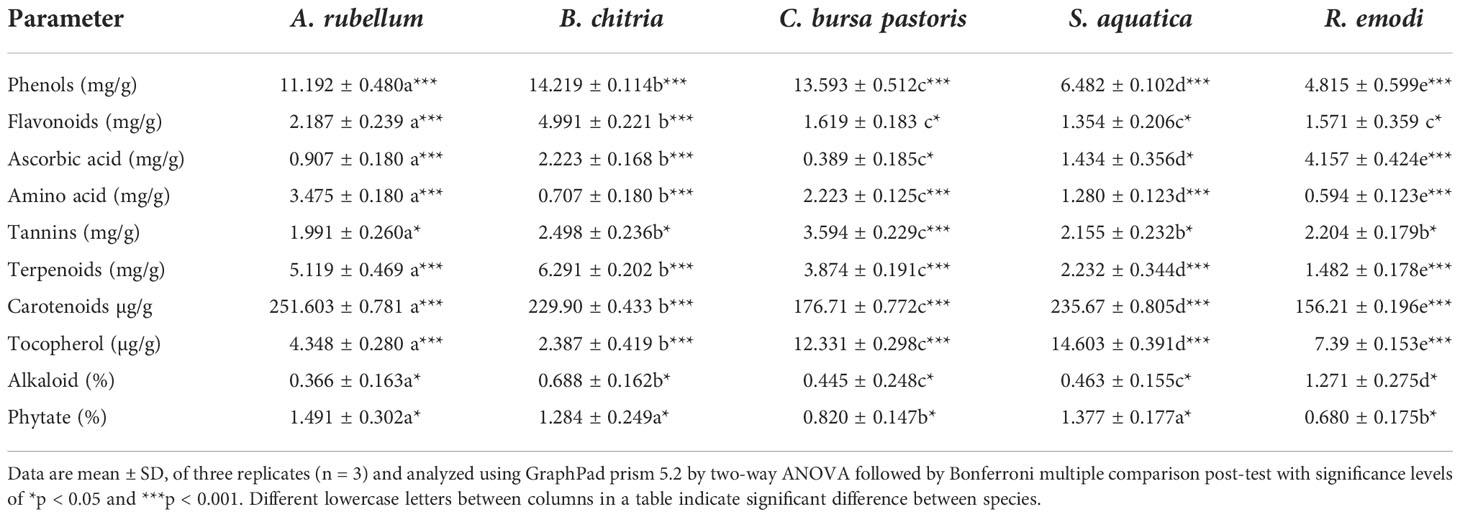
Table 3 Phytochemical compositionof Allium rubellum, Berberis chitria, Capsella bursa-pastoris, Stellaria aquatica, and Rheum emodi.
3.3 Antioxidant activity
Radical scavenging activity in the five species studied, using DPPH radical and ABTS radical, is shown in Table 4. The highest antioxidant activity was observed in R. emodi because it has the lowest IC50 value (1.512 µg/ml), and S. aquatica showed the lowest antioxidant activity because it has a high IC50 value evaluated by the ABTS assay. Again, through the DPPH assay, the highest antioxidant activity was found in R emodi because it has the lowest IC50 value (2.816 µg/ml). However, the IC50 of the positive control (ascorbic acid) was 0.6 µg/ml and 0.8 µg/ml with ABTS and DPPH, respectively. Our results indicate significant antioxidant in all the five extracts, whose IC50 ranged from 1.5 µg/ml to 6.5 µg/ml in the ABTS assay and 2.8 µg/ml to 9.6 µg/ml in the DPPH assay.
3.4 Antibacterial activity
The antibacterial activity of the methanolic extract of the five wild edible plants is presented in Table 5. Maximum zone of inhibition was found in R. emodi (20.133 ± 0.152 mm) and the lowest was found in A. rubellum (13.33 ± 0.493 mm) against E. coli. Zone of inhibition was observed the highest in S. aquatica (19.2 ± 0.305 mm) and the lowest in C. pastoris (14.5 ± 0.173 mm) against S. aureus. Results of antibacterial activity of the five plant extracts suggested that S. aureus was the resistant strain to plant extracts compared to E. coli. The main contributors to the antimicrobial mode of action of plant extracts are phenolic chemicals, which cause microbial membrane breakdown, cytoplasmic leakage, and cytoplasmic component coagulation; adapt anti-quorum sensing activity; and interfere with microbial cellular metabolism (Chandra et al., 2017). Here, studies revealed that each of the five test plant extracts included powerful substances that aided in the antimicrobial activity. The activity of the bioactive chemicals that give plant extracts their medicinal value, their methods of action, and other potential advantages of employing extracts should be thoroughly studied.
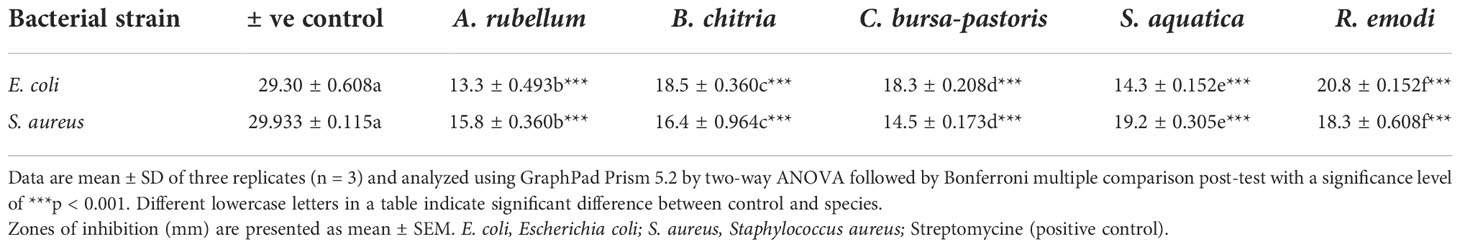
Table 5 Antibacterial activity of Allium rubellum, Berberis chitria, Capsella bursa-pastoris, Stellaria aquatica, and Rheum emodi.
4 Discussion
A major portion of the Gaddis’ tribe food while migrating is made up of plant resources, which is rich in carbohydrates, proteins, fats, and fiber. The current study examined five neglected wild food plants’ nutritional, phytochemical, antioxidant, and antibacterial properties, i.e., A. rubellum, B. chitria, C. bursa-pastoris, S. aquatica, and R. emodi consumed by the Gaddis tribe. These wild plants are the cheapest source of food and have medicinal properties. Polar solvent methanol was used as a solvent for the extraction of plants metabolites because it is reported that polar solvents are more effective in extracting bioactive metabolites (Al-Mansoub et al., 2014). It was observed that there was a large variability in content of parameters studied except for fats in different species. The nutrient analysis was within the range of prior research, according to an overall examination of the literature. The significant variation from the other stated value may be due to a number of factors, including the season and level of plant maturity used for the analysis. It was seen that the nutritional value of A. rubellum was better than the other species. The antioxidant and antibacterial activity of R. emodi was higher than that of the other studied species.
Carbohydrates are known to be a major and indispensable source of energy in metabolic processes (Ogungbenle & Omosola, 2015). A. rubellum has the highest carbohydrate content compared to other species, indicating that it is a good energy supplier. In one of the studies, 130 g of carbohydrates has been recommended for human consumption (Datta et al., 2019), while in the present study, it varied between 4.6 and 6.9 mg/g in the five species. It implies that in order to meet the daily human requirement, more than 200 g of those dried plants need to be consumed. Similarly, proteins constitute an essential and pivotal component for living organisms. The rich protein content in A. rubellum, C. bursa-pastoris, and S. aquatica potentially offers essential dietary supplements. Both carbohydrate and protein content were higher in the studied species compared to the wild plants consumed as vegetables (Hassan & Umar, 2006). Excess fat is also not good for the health as it is a cause of aging, cancer, and cardiovascular diseases (Blessing et al., 2011). In our studies, all the species had low crude fat, indicating that these species are effective in preventing certain diseases. The amount of crude fat was similar to that found in Drymeria cordata and Blumea lanceolaria (0.54%) wild edible plants consumed by the Bodos tribes of Assam (Brahma et al., 2014). The presence of high fiber in food is considered good for digestion and reduces illnesses associated with metabolic conditions (Ikewuchi & Ikewuchi, 2008). A. rubellum and C. bursa-pastoris had high amounts of crude fibers compared to other studied species, indicating that these plants help to reduce disease better than the other species studied. However, if fiber content is high, it might interfere with how well some minerals are absorbed like iron (López and Martos, 2004).
Protein, carbohydrate, and fat are the three important components of food that provide energy. It is a known fact that 1 g of carbohydrate and protein yields 4 kcal of energy while an equal amount of fat yields 9 kcal of energy (Mandario et al., 2019). The energy content of the studied species can be determined by multiplying values obtained for carbohydrate, protein, and fat by 4, 4, and 9, i.e., 144, and adding up these values. It is evident that S. aquatica had a maximum energy amounting to 7,029.5 kcal/g, followed by A. rubellum (6,671.9 kcal/g) and the least was in R. emodi (1,385.5 kcal/g). The energy content in B. chitria and C. bursa-pastoris was nearly half that of S. aquatica.
For the physical and mental growth of humans, sodium and potassium play an important role besides being essential components of teeth, bone, blood, and muscle (Zoroddu et al., 2019). In contrast to potassium, which is required due to its diuretic properties, sodium aids in the transfer of metabolites. It has been reported that 0.03 to 1.2 mg/g of sodium can be found in cultivated vegetables and fruits (Walid et al., 2012). However, in the present study, it ranged between 0.55 and 3.21 mg/g, the highest amount being in A. rubellum. Similarly, the potassium content was high in our studied plants. Sodium-to-potassium ratio is considered to be important in controlling hypertension and arteriosclerosis. In literature, it has been reported that Na enhances blood pressure while K decreases blood pressure (Saupi et al., 2009). In our studies, this ratio was 5.0 for A. rubellum, 5.9 for C. bursa-pastoris, 11.4 for R. emodi, 12.1 for B. chitria, and 13.7 for S. aquatica. In contrast, high ratios were observed in Amaranthus virdis (32.4), Achyranths aspera (67.0), and Phyllanthus emblica (45.0) in one study (Sundriyal & Sundriyal, 2001). However, they also reported a low ratio in tomato (11.3) and papaya (11.5).
Phenols and flavonoids are secondary metabolites and have scavenging capabilities (Williams et al., 2004). In contrast to the 3.3 mg/g and 2.5 mg/g of phenols found in Euphorbia thymiafolia and Pouzolzia hirta, two edible plants from the Western Himalayas (Prasad & Chandra, 2018), in our studies, phenol concentration ranges from 4.8 to 14.2 mg/g.
Similarly, in one of the studies, flavonoid content as reported in Amaranthus retroflexus, Chaerophyllum byzantinum, and Ornithogalum umbellatum (Prasad & Chandra, 2018) are in agreement with our studied species. In our study, 50% inhibition of ABTS and DPPH radicals (IC50) by the methanolic extract in different species ranged from 1.5 to 6.5 µg/ml and 2.8 to 9.6 µg/ml, indicating potential antioxidant activity in these plants. The IC50 value of the DPPH assay was found to be higher than the ABTS assay, which showed that all these species have more antioxidant activities. Additionally higher phenolic content obtained than flavonoids showed more antioxidant activity as phenols have a higher redox potential and can absorb and neutralize free radicals (Jimoh et al., 2011). Flavonoids are also referred to as a natural constituent of plants and show antioxidant activity through a scavenging or chelating process (Pourmorad et al., 2006). The presence of ascorbic acid (0.38 to 4.15 mg/g) and tocopherol (2.3 to 14.6 µg/g) in wild plants is considered to be sufficient to play a role in deactivating and absorbing free radicals (Islary et al., 2016). Tannins have a reputation for treating cancer and ulcerated tissues. Among the five species studied, it varied from 1.9 to 3.5 mg/g, which are in range to the species (Hegazy et al., 2013). In one study, it has been reported that 13.2 mg/g of terpenoid in Pueraria tuberose was consumed by the tribes of Maharashtra, while in our studied species, it was found to be quite low (1.4 to 6.2 mg/g) (Ojewumi et al., 2021); this might be due to climatic and seasonal variations. Terpenoids are known for their flavor due to their aromatic quality, and in the studied species, low amounts of terpenoids indicate that these species lack aroma and will have no potential effect on human behavior. Similarly, consumption of high alkaloid-containing food is not good for the health as high alkaloid causes damage to muscles, blood vessels, and other soft tissues (Gemede & Ratta, 2014). It was found to be low in all the five species, which justifies the use of these plants as food by Gaddis. Alkaloids and phytate are both regarded as being antinutritional (Samtiya et al., 2020). The amount of phytate in the studied five species ranged between 0.68% and 1.49%. It has been found that the amount of phytate in wild edible plants Portulaca oleracea and Solanum indicum is 0.82% and 0.69%, respectively (Aberoumand & Deokule, 2009). In one of the studies, it has been found that 1%–16% of phytate taken reduces the bioavailability of minerals with time (Oke, 1969). However, by boiling, soaking, and heating, alkaloid and phytate concentrations in food can be reduced (Ekop & Eddy, 2005). The antibacterial activity of the methanolic extract of the five studied species revealed that the extract inhibited E. coli growth comparatively more than S. aureus. The results are in line with that reported in wild edible plants Datura stramonium, Ricinus communis, and Calotropis gigantean (Jain et al., 2010). The range of inhibition was also almost the same as that reported here. In one study, it has also been found that the ethanolic extract of Catunargam spinosa was effective against E. coli and Klebsiella pneumoniae (Anand et al., 2017).
Previous research indicates that major chemical constituents like number of anthraquinone (such as emodin) occur in R. emodi. Reports suggest that anthraquinone derivatives show proof of antimicrobial, antifungal, anti-Parkinson’s, immuno-enhancing, antiproliferative, antiviral, and antioxidant activities (Zargar et al., 2011). It is reported that 3-hydroxy-methyl furan is a major chemical constituent present in S. aquatica, which is effective against fistula and piles (Li, 1973; Kameoka et al., 1978). Furthermore, ADMET analysis revealed that both compounds exhibited drug-like behavior. As a result, it is possible that these could be used as a natural antioxidant and as a safe compound for the treatment of a variety of diseases. More research, such as the identification and extraction of reliable specific antioxidant and antimicrobial compounds, is needed and suggested for its broad-spectrum application, in order to validate the specific activity.
5 Conclusion
With the advancement of modern techniques, it has become possible to screen the nutritional composition, phytochemical, natural antioxidant, and antimicrobial potential of wild edible plants to feed the next generation. In addition, natural antioxidants have the capacity to improve food quality and stability and to act as nutraceuticals to terminate free radical chain reaction in biological systems, and thus may provide additional health benefits to consumers. The current investigation showed that the five edible plants are rich in proteins, carbohydrates, fibers, fats, phenols, flavonoids, etc., which help maintain normal body functions and provide protection against oxidative stress. Thus, it should be emphasized how crucial it is to preserve traditional ethnomedical knowledge before this rich resource is lost. Lastly, the present study records wild species with therapeutic potentials, which should be explored further for pharmacological studies due to their immense potential.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AT, SS, KD and NS designed the article, performed the work and wrote the first draft of the article. BA, AH, DV, and RM corrected the article and finally all authors read and approved the final article.
Funding
The publication was supported by funds from the National Research Development Projects to Finance excellence (PFE) - 14/2022-2024 granted by the Romanian Ministry of Research and Innovation.
Acknowledgments
The Shoolini University of Biotechnology and Management Sciences in Solan (H.P.), India, provided the research facilities, which the authors gratefully acknowledge.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aberoumand A. (2011). Protein, fat, calories, minerals, phytic acid and phenolic in some plant foods based diet. J. Food Process. Technol. 2 (3), 14. doi: 10.4172/2157-7110.1000114
Aberoumand A., Deokule S. S. (2009). Studies on nutritional values of some wild edible plants from Iran and India. Pak. J. Nutr. 8 (1), 26–31. doi: 10.4172/2157-7110.1000114
Aina V. O., Sambo B., Zakari A., Haruna M. S. H., Umar H., Akinboboye R. M., et al. (2012). Determination of nutritional and anti-nutrient content of Vitisvinifera (Grapes) grown in bomo (Area c) zaria, Nigeria. Adv. J. Food Sci. Tech 4 (6), 445–448.
Al-Mansoub M. A., Asmawi M. Z., Murugaiyah V. (2014). Effect of extraction solvents and plant parts used on the antihyperlipidemic and antioxidant effects of garcinia atroviridis: a comparative study. J. Sci. Food Agric. 94 (8), 1552–1558. doi: 10.1002/jsfa.6456
Anand S. P., Deborah S., Velmurugan G. (2017). Antimicrobial activity, nutritional profile and phytochemical screening of wild edible fruit of Catunaregam spinosa (Thunb.) triveng. Phar. Innova 6 (10), 106–109. doi: 10.1155/2021/3257732
Barros L., Ferreira M. J., Queiros B., Ferreira I., Baptista P. (2007). Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 103 (2), 413–419. doi: 10.1016/j.foodchem.2006.07.038
Bendiabdellah A., El Amine Dib M., Djabou N., Allali H., Tabti B., Muselli A., et al. (2012). Biological activities and volatile constituents of daucus muricatus l. from Algeria. Chem. Cent. J. 6, 48–58. doi: 10.1186/1752-153X-6-48
Bengtsson H., Oborn I., Jonsson S., Nilsson I., Andersson A. (2003). Field balances of some mineral nutrients and trace elements in organic and conventional dairy farming–a case study at Öjebyn, Sweden. Eur. J. Agron. 20 (1-2), 101–116. doi: 10.1016/S1161-0301(03)00079-0
Bilal S., Bhat S. A., Hussain I., Parrah J. D., Ahmad S. P., Mir M. R. (2015). The use of a rabbit model to evaluate the influence of age on excision wound healing. DNA 11, 13.
Blessing A. C., Ifeanyi U. M., Chijioke O. B. (2011). Nutritional evaluation of some Nigerian pumpkins (Cucurbitaspp.). Frui. Veg.and Cer. Sci. Biotech. 5 (2), 64–71.
Brahma J., Singh B., Rethy P., Gajurel P. (2014). Nutritional analysis of some selected wild edible species consumed by the bodos tribes of kokrajhar district, BTC, Assam. Asian J. Pharm. Clin. Res. 7 (3), 34–37.
Chandra H., Bishnoi P., Yadav A., Patni B., Mishra A. P., Nautiyal A. R. (2017). Antimicrobial resistance and the alternative resources with special emphasis on plant-based antimicrobials–a review. Plants 6 (2), 16. doi: 10.3390/plants6020016
Chikhale H. U., Chikhale P. U. (2017). Flame photometric estimation of sodium and potassium ion present in water sample of darna and godavari river. Int. J. Sci. Eng. Res. 8 (1), 131–136.
Damilola O., Joseph O. B., Olufemi A., Amoo I. (2013). Chemical composition of red and white cocoyam (Colocosia esculenta) leaves. Int. J. Sci. Res. 11, 121–125.
Dangwal L. R., Singh T., Singh A. (2014). Exploration of wild edible plants used by gujjar and bakerwal tribes of district rajouri (J&K), India. J. Appl. Nat. Sci. 6 (1), 164–169. doi: 10.31018/jans.v6i1.394
Datta S., Sinha B. K., Bhattacharjee S., Seal T. (2019). Nutritional composition, mineral content, antioxidant activity and quantitative estimation of water-soluble vitamins and phenolics by RP-HPLC in some lesser-used wild edible plants. Heliyo 5 (3), e01431. doi: 10.1016/j.heliyon.2019.e01431
Devi A., Rakshit K., Sarania B. (2014). Ethnobotanical notes on Allium species of arunachal pradesh, India. Indian Journal of Traditional Knowledge 13 (3), 606–612.
Ekop A. S., Eddy N. O. (2005). Comparative studies of the level of toxicants in the seed of Indian almond (Terminalia catappa) and African walnut (Coula edulis), chem. Class J. 2, 74–76.
Gemede H. F., Ratta N. (2014). Antinutritional factors in plant foods: Potential health benefits and adverse effects. Inter. J. Nutr. Food Sci. 3 (4), 284–289. doi: 10.11648/j.ijnfs.20140304.18
Ghorai N., Chakraborty S., Gucchait S., Saha S. K., Biswas S. (2012). Estimation of total terpenoids concentration in plant tissues using a monoterpene, linalool as standard reagent. Protocol Exchange, 5 doi: 10.1038/protex.2012.055
Hassan L. G., Umar K. J. (2006). Nutritional value of balsam apple (Momordicabalsamina l.) leaves. Pak. J. Nutr. 5 (6), 522–529. doi: 10.3923/pjn.2006.522.529
Hegazy A. K., Al-Rowaily S. L., Faisal M., Alatar A. A., El-Bana M. I., Assaeed A. M. (2013). Nutritive value and antioxidant activity of some edible wild fruits in the middle East. J. Med. Plant Res. 7 (15), 938–946.
Ikewuchi C. C., Ikewuchi J. C. (2008). Chemical profile of Pleurotus tuberregium (Fr) sing’s sclerotia. Pacific. J. Sci. Techn 10, 295–299.
Islary A., Sarmah J., Basumatary S. (2016). Proximate composition, mineral content, phytochemical analysis and in vitro antioxidant activities of a wild edible fruit (Grewiasapida roxb. ex DC.) found in Assam of north-East India. Ameri. J. Phy Bioche. Pharmaco 5 (1), 1–11. doi: 10.5455/jib.20160422015354
Jain P., Bansal D., Bhasin P. A., Anjali A. (2010). Antimicrobial activity and phytochemical screening of five wild plants against Escherichia coli, Bacillus subtilis and Staphylococcus aureus. J. Pharm. Res. 3 (6), 1260–1262.
Jimoh F. O., Adedapo A. A., Afolayan A. J. (2011). Comparison of the nutritive value, antioxidant and antibacterial activities of Sonchus asper and Sonchus oleraceus. Rec. Natu. Prod 5 (1), 29–42.
Jing L., Ma H., Fan P., Gao R., Jia Z. (2015). Antioxidant potential, total phenolic and total flavonoid contents of Rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in PC12 cells. BMC Comple. Alter. Med. 15 (1), 1–12. doi: 10.1186/s12906-015-0820-3
Johnson A. M., Kim H., Ralph J., Mansfield S. D. (2017). Natural acetylation impacts carbohydrate recovery during deconstruction of Populus trichocarpa wood. Biotech. Biofu 10 (1), 1–12. doi: 10.1186/s13068-017-0734-z
Kalita P., Tag H., Sarma H. N., Das A. K. (2014). Evaluation of nutritional potential of five unexplored wild edible food plants from Eastern Himalayan biodiversity hotspot region (India). Inter. J. Nutr. Food Eng. 8 (3), 215–218.
Kameoka H., Wang C. P., Yamaguchi T. (1978). The constituents of the essential oil from Stellaria aquatica scop. Nipp. Nog. Kag. Kaishi 52 (8), 335. doi: 10.1271/nogeikagaku1924.52.8_335
Khan I., Najeebullah S., Ali M., Shinwari Z. K. (2016). Phytopharmacological and ethnomedicinal uses of the genus berberis (Berberidaceae): A review. Trop. J. Pharm. Res. 15 (9), 2047–2057. doi: 10.4314/tjpr.v15i9.33
Kumar A., Kumar R., Singh N., Mansoori A. (2020). “Regulatory framework and policy decisions for genome-edited crops,” in CRISPR/Cas genome editing (Cham: Springer), 193–201. doi: 10.18006/2021.9(1).87.99
Kumar A., Singh N., Mansoori A., Jiwani G., Solanke A. U., Kumar R. (2021). Evaluation of antioxidant and antimicrobial potential of the spesia lampas root extracts. Journal of Experimental Biology and Agricultural Sciences 9 (1), 87–99.
Kunkel G. (1984). “Plants for human consumption,” in Koeltz scientific books(San Francisco, CA: Georgetown Press).
Kweon M. H., Kwak J. H., Ra K. S., Sung H. C., Yang H. C. (1996). Structural characterization of a flavonoid compound scavenging superoxide anion radical isolated from Capsella bursa-pastoris. BMB Repo 29 (5), 423–428.
Li S. C. (1973). Chinese Medicinal herbs (San Francisco, USA: Georgetown Press). translated by Smith, FP, Stuart, GA.
López M. A. A., Martos F. C. (2004). Iron availability: An updated review. Inter. J. Food Sci. Nutr. 55 (8), 597–606. doi: 10.1080/09637480500085820
Mandario M. A. E., Alava V. R., Anasco N. C. (2019). Evaluation of the bioremediation potential of mud polychaete Marphysasp. in aquaculture pond sediments. Envir. Sci. Pollu. Res. 26 (29), 29810–29821. doi: 10.1007/s11356-019-06092-z
Moore S., Stein W. H. (1948). Photometric nin-hydrin method for use in the chromatography of amino acids. J.of Biol. Chem. 176, 367–388. doi: 10.1016/S0021-9258(18)51034-6
Nisar T., Wang Z. C., Yang X., Tian Y., Iqbal M., Guo Y. (2018). Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. macromolecules 106, 670–680. doi: 10.1016/j.ijbiomac.2017.08.068
Obasohan P. E., Walters S. J., Jacques R., Khatab K. (2020). Risk factors associated with malnutrition among children under-five years in sub-Saharan African countries: a scoping review. Inter. J. Envir. Res. Pub. Health 17 (23), 8782. doi: 10.3390/ijerph17238782
Ogungbenle H. N., Omosola S. M. (2015). The comparative assessment of nutritive values of dry Nigerian okra (Abelmoschus esculentus) fruit and oil. Inter. J. Food Sci. Nutr. Eng. 5 (1), 8–14. doi: 10.5923/j.food.20150501.02
Ojewumi A. W., Bamkefa B. A., Kuku F. B. (2021). Survey and chemo-physiological assessment of biochemical preservatives and physicochemical properties of plants used for preservation of fruits. J. Nige. J. Pure Appl. Sci. 34 (2), 4058–4071. doi: 10.48198/ NJPAS/21.xxx
Oke O. L. (1969). Chemical studies on the more commonly used vegetables in Nigeria. Afr. Sci. Ass 11, 42–48. doi: 10.13140/RG.2.1.3697.9689
Omoruyi B. E., Bradley G., Afolayan A. J. (2012). Antioxidant and phytochemical properties of Carpobrotus edulis (L.) bolus leaf used for the management of common infections in HIV/AIDS patients in Eastern cape province. BMC Compl. Alter. Med. 12 (1), 1–9. doi: 10.1186/1472-6882-12-215
Palai S. (2022). “Berberine,” in Nutraceuticals and health care (San Diego, CA, USA: Elsevier Academic Press), 359–368.
Pourmorad F., Hosseinimehr S. J., Shahabimajd N. (2006). Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afri. J. Biotech. 5 (11), 1142–45.
Prasad K., Chandra D. (2018). Amino acid and antioxidant composition of some wild edible medicinal plants of uttarakhand Himalayas. J. Chem. Pharmac. Res. 10, 40–48.
Prasad K. N., Chew L. Y., Khoo H. E., Kong K. W., Azlan A., Ismail A. (2010). Antioxidant capacities of peel, pulp, and seed fractions of canariumodontophyllum miq fruit. J. Biomed. Biotech. 2010, 871379. doi: 10.1155/2010/871379
Razmavar S., Abdulla M. A., Ismail S. B., Hassandarvish P. (2014). Antibacterial activity of leaf extracts of baeckea frutescens against methicillin-resistant Staphylococcusaureus. Bio. Med. Res. Inter 2014, 5. doi: 10.1155/2014/521287
Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radi. Bio. Med. 26 (9–10), 1231–1237. doi: 10.1016/S0891-5849(98)00315-3
Roe J. H., Kuether C. A. (1943). The determination of ascorbic acid in whole blood and urine through the 2, 4-dinitrophenylhydrazine derivative of dehydroascorbic acid. J. Bio. Chem. 147, 399–407. doi: 10.1016/S0021-9258(18)72395-8
Samtiya M., Aluko R. E., Dhewa T. (2020). Plant food anti-nutritional factors and their reduction strategies: an overview. Food Production Process. Nutr. 2 (1), 1–14. doi: 10.1186/s43014-020-0020-5
Saupi N., Zakaria M. H., Bujang J. S. (2009). Analytic chemical composition and mineral content of yellow velvetleaf (Limnocharis flava l. buchenau)’s edible parts. J. App. Sci. 9 (16), 2969–2974. doi: 10.3923/jas.2009.2969.2974
Seal T., Pillai B., Chaudhuri K. (2017). Evaluation of nutritional potential of five unexplored wild edible plants consumed by the tribal people of arunachal pradesh state in India. J. Food Nutr. Res. 5 (1), 1–5. doi: 10.12691/jfnr-5-1-1
Sharma A., Arora D. (2012). Phytochemical and pharmacological potential of genus stellaria: a review. J. Pharm. Res. 5 (7), 3591–3596.
Singh N., Mansoori A., Dey D., Kumar R., Kumar A. (2021a). “Potential of metabolomics in plant abiotic stress management,” in Omics technologies for sustainable agriculture and global food security (Springer, Singapore), 193–214.
Singh N., Mansoori A., Jiwani G., Solanke A. U., Thakur T. K., Kumar R., et al. (2021b). Antioxidant and antimicrobial study of schefflera vinosa leaves crude extracts against rice pathogens. Arab. J. Chem. 14 (7), 103243. doi: 10.1016/j.arabjc.2021.103243
Singh N., Pandey R., Chandraker S. K., Pandey S., Malik S., Patel D. (2022). “Use of wild edible plants can meet the needs of future generation,” in Agro-biodiversity and agri-ecosystem management (Springer, Singapore), 341–366.
Song N., Xu W., Guan H., Liu X., Wang Y., Nie X. (2007). Several flavonoids from Capsella bursa-pastoris (L.) medic. Asian Journal of Traditional Med 2, 6, 218–222. Available at: http://asianjtm.syphu.edu.cn/EN/Y2007/V2/I6/218
Stein W. H., Moobe S. (1948). Chromatography of amino acids on starch columns. separation of phenylalanine, leucine, isoleucine, methionine, tyrosine, and valine. J. Biol. Chem. 176, 337–365. doi: 10.1016/S0021-9258(18)51033-4
Sundriyal M., Sundriyal R. C. (2001). Wild edible plants of the sikkim himalaya: Nutritive values of selected species. Econ. Bot. 55 (3), 377–390. doi: 10.1007/BF02866561
Sunil P., Sanjeev K. (2018). Diversity and use of wild edible plants by migratory shepherds in the himachal pradesh of the western Himalayas, India. J. Med. Plants Res. 12 (30), 601–610. doi: 10.5897/JMPR2018.6682
Taylor M., Kambuou R., Lyons G. H., Hunter D., Morgan E. H., Quartermain A., et al. (2014). “Realizing the potential of indigenous vegetables through improved germplasm information and seed systems,” in XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014) (USA, Department of Agriculture, Acta horticulturae), Vol. 1102. 29–42.
Unuofin J. O., Otunola G. A., Afolayan A. J. (2017). Nutritional evaluation of Kedrostisafricana (L.) cogn: An edible wild plant of south Africa. Asian Paci. J. Trop. BioMed. 7 (5), 443–449. doi: 10.1016/j.apjtb.2017.01.016
Vipin P., Ashok A. (2010). Traditional uses of ethnomedicinal plants of lower foothills of himachal pradesh-I. Indian J. Trad. Knowl. 9 (3), 519–521.
Walid E., Hedia H., Nizar T., Yassine Y., Nizar N., Ali F. (2012). Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J. Med. Plants Res. 6 (32), 4724–4730. doi: 10.5897/JMPR11.995
Wang G., Chen S., Gu G., Qiu J., Chen Y., Jia Z., et al. (2020). Comparison of the content of flavonoids, total phenols, carotenoids and antioxidant activity in guang Citri reticulatae pericarpium during the aging time. Pharmacog. Magaz 16 (69), 375. doi: 10.4103/pm.pm_295_19
Williams R. J., Spencer J. P. E., Rice-Evans C. (2004). Flavonoids: antioxidants or signalling molecules? Free Radi. Bio. Med. 36 (7), 838–849. doi: 10.1016/j.freeradbiomed.2004.01.001
Winters A. L., Minchin F. R. (2005). Modification of the lowry assay to measure proteins and phenols in covalently bound complexes. Analy. Bioch 346 (1), 43–48. doi: 10.1016/j.ab.2005.07.041
Zakaria M., Simpson K., Brown P. R., Krstulovic A. (1979). Use of reversed-phase high-performance liquid chromatographic analysis for the determination of provitamin a carotenes in tomatoes. J. Chromato. A 176 (1), 109–117. doi: 10.1016/S0021-9673(00)92091-0
Zargar B. A., Masoodi M. H., Ahmed B., Ganie S. A. (2011). Phytoconstituents and therapeutic uses of Rheum emodi wall. ex meissn. Food Chem. 128 (3), 585–589. doi: 10.1016/j.foodchem.2011.03.083
Keywords: wild fruits, nutrition, phytochemicals, antioxidant, antibacterial, food
Citation: Thakur A, Singh S, Dulta K, Singh N, Ali B, Hafeez A, Vodnar DC and Marc RA (2022) Nutritional evaluation, phytochemical makeup, antibacterial and antioxidant properties of wild plants utilized as food by the Gaddis-a tribal tribe in the Western Himalayas. Front. Agron. 4:1010309. doi: 10.3389/fagro.2022.1010309
Received: 02 August 2022; Accepted: 25 October 2022;
Published: 19 December 2022.
Edited by:
Nikhil Malhotra, National Bureau of Plant Genetic Resources (ICAR), IndiaReviewed by:
Prashant Vikram, Shriram Bioseed Genetics, IndiaAbdel Rahman Mohmmad Said Al -Tawaha, Al-Hussein Bin Talal University, Jordan
Somayeh Ghahari, Sari University of Agricultural Sciences and Natural Resources, Iran
Copyright © 2022 Thakur, Singh, Dulta, Singh, Ali, Hafeez, Vodnar and Marc. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arti Thakur, YXJ0aXRoYWt1cjc1OEBnbWFpbC5jb20=; Romina Alina Marc, cm9taW5hLnZsYWljQHVzYW12Y2x1ai5ybw==; Somvir Singh, c2FtcmFqcHV0MDAwMkBnbWFpbC5jb20=; Nitesh Singh, bml0ZXNoaWdudHVAZ21haWwuY29t
†ORCID: Arti Thakur, orcid.org/0000-0001-5925-2227
Somvir Singh, orcid.org/0000-0002-0571-864X
Kanika Dulta, orcid.org/0000-0002-3661-5205
Nitesh Singh, orcid.org/0000-0002-7779-174X
 Arti Thakur1*†
Arti Thakur1*† Somvir Singh
Somvir Singh Nitesh Singh
Nitesh Singh Baber Ali
Baber Ali Aqsa Hafeez
Aqsa Hafeez Romina Alina Marc
Romina Alina Marc