- 1Department of Agricultural Production, School of Agricultural Sciences, College of Agricultural and Environmental Sciences, Makerere University, Kampala, Uganda
- 2Department of Crop Sciences, Tshwane University of Technology, Pretoria, South Africa
- 3Chemistry Department, Tshwane University of Technology, Pretoria, South Africa
Inhibition of N2 fixation in N-fertilized common bean (Phaseolus vulgaris L.) plants growing on the fields of farmers in the Eastern Cape of South Africa was measured using 15N natural abundance and tissue ureide analysis. The N-fertilized bean plants revealed greater soil N uptake, higher concentrations of nitrate in organs, low tissue ureide levels, and much lower percent relative ureide-N abundance when compared with unfertilized plants. In contrast, the unfertilized plants showed greater nodule fresh weight, higher N derived from fixation (e.g., 84.6, 90.4, and 97.1% at Lujecweni fields 2, 3, and 4, respectively), increased amount of N-fixed (e.g., 163.3, 161.3, and 140.3 kg ha−1 at Lujecweni fields 2, 3, and 4, respectively), greater ureide concentration in stems and petioles, higher % relative ureide-N abundance, and low soil N uptake. We also found that the percent N derived from fixation (%Ndfa) was very high for some bean plants receiving a double dose of N fertilizer [e.g., Lujecweni field 1 (51.8%) and Tikitiki field 1 (53.3%], and quite high for others receiving a single dose of N fertilizer [e.g., Tikitiki field 2 (50.1%), Mfabantu fields 1 and 2 (45.5 and 79.9%, respectively), and St. Luthberts field 1 (58.9%)]. Though not assessed in this study, it is likely that the rhizobia that effectively nodulated the N-fertilized bean plants and fixed considerable amounts of symbiotic N had constitutive and/or inducible nitrate reductase genes for reducing nitrate in nodules and bacteroids, hence their ability to form root nodules and derived high %Ndfa in bean with added N. While single- and double-dose N fertilizer applications increased plant growth and grain yield compared to unfertilized bean plants, the single-dose N fertilizer application produced much greater grain yield than the double dose. This indicates that farmers should stop using a double dose of N fertilizers on bean production, as it decreases yields and can potentially pollute the environment. This study has however shown that government supply of free N fertilizers to resource-poor farmers in South Africa increased bean yields for food/nutritional security.
Introduction
Legume N2 fixation is important for sustainable cropping systems in both tropical and subtropical environments, where the soils are naturally deficient in N (Graham and Vance, 2003). The legume-rhizobia symbiosis can provide symbiotic N for meeting the N demand of legumes and, in so doing, spare endogenous soil N for use by cereal crops, in addition to increasing soil N supply through decomposition of legume residues and improving soil structure and organic matter content (Maingi et al., 2000).
Several studies have assessed N nutrition in common bean (Farid and Navabi, 2015; Farid et al., 2016; Jiang et al., 2020), and symbiotic N2 fixation has generally been found to be lower in common bean when compared to other legumes (Farid et al., 2016). However, applying Rhizobium strain HB-429 to common bean crop increased plant growth, percent N derived from fixation (%Ndfa), the amount of N-fixed, and grain yield by 19, 17, 54, and 48%, respectively, over the uninoculated control in Ethiopia (Samago et al., 2018). These findings show that supplying rhizobial inoculants can replace chemical N fertilizer use and increase bean yields in Ethiopia. An additional benefit following grain harvest in legumes is the release of fixed-N by the legume residues into the soil, which is generally regarded as slow and can match plant uptake from the soil with symbiotic N supply by residues (Chikanai et al., 2018), thus reducing leaching, runoff, and environmental pollution, which are common with added N fertilizer.
In an attempt to increase grain yield and improve household food security, the South African government embarked on a program of providing free inputs, such as improved seeds and the fertilizers NPK, urea, and limestone ammonium nitrate (LAN), for use by resource-poor farmers instead of rhizobial inoculants. Although N fertilizers can be used to overcome the inherently low nutrient levels in African soils and increase crop yields, they are expensive and inaccessible to resource-poor farmers, hence the free N supply to poor farmers by the South African government. Although government supply of fertilizers has increased the production of maize and common bean, in particular, the two major staple foods in South Africa, no study has evaluated the effect of frequent N fertilizer use on plant growth and N2 fixation of common bean on the fields of farmers. However, the suppressive effects of N fertilizers on legume N2 fixation is known. For example, high concentrations of NH3 can inhibit nod-gene expression, nodule formation, and nitrogenase activity in legumes (Vieira et al., 1998; Li et al., 2009), thus reducing their symbiotic N yield. Nitrate is also known to reduce the oxygen diffusion barrier and inhibit nodule functioning via the formation of nitrosylleghemoglobin, which reduces oxygen supply to respiring bacteroids for ATP formation (Dakora and Atkins, 1989).
Several techniques are available for estimating legume N2 fixation in the field, which include the 15N natural abundance technique and the ureide assay. Although the 15N natural abundance technique is robust in quantifying N2 fixation in legumes (Unkovich et al., 1994; Mohale et al., 2013), it assumes plant uptake of only two sources of N (soil N and atmospheric N2), which therefore limits its application in situations where external N is added to the soil (Unkovch et al., 2008). Even if there are non-fixing, non-legume plant species growing on the field and sourcing N from the soil and added fertilizer, the 15N natural abundance technique can still be used to estimate N2 fixation in the legume species, such as the N-fertilized common bean plants on the fields of farmers. Additionally, tropical legumes belonging to the tribe Phaseoleae (e.g., common bean) export their fixed-N from nodules to shoots in the form of ureides (allantoin and allantoic acid) via the xylem stream (Herridge and People, 1990; Dakora et al., 1992). The concentration of ureides relative to other nitrogenous solutes (nitrate and amino acids) in the xylem sap, stems, or petioles of the tribe Phaseoleae is a reliable measure of N2 fixation (Herridge and People, 1990; Unkovich et al., 2008) that is not limited by external N inputs.
The application of N fertilizer to symbiotic legumes can decrease nodulation, N2 fixation, and even grain yield. We hypothesize that the program of providing free N fertilizers (NPK, urea, and LAN) by the South African government to resource-poor farmers could increase grain yield but decrease nodulation and N2 fixation in beans. This study aimed to assess the effect of N fertilization on plant growth, N2 fixation, and grain yield of common bean planted without rhizobial inoculation on the fields of farmers in the Eastern Cape Province of South Africa, using the 15N natural abundance technique and ureide assay.
Materials and Methods
Soil Sampling and Analysis
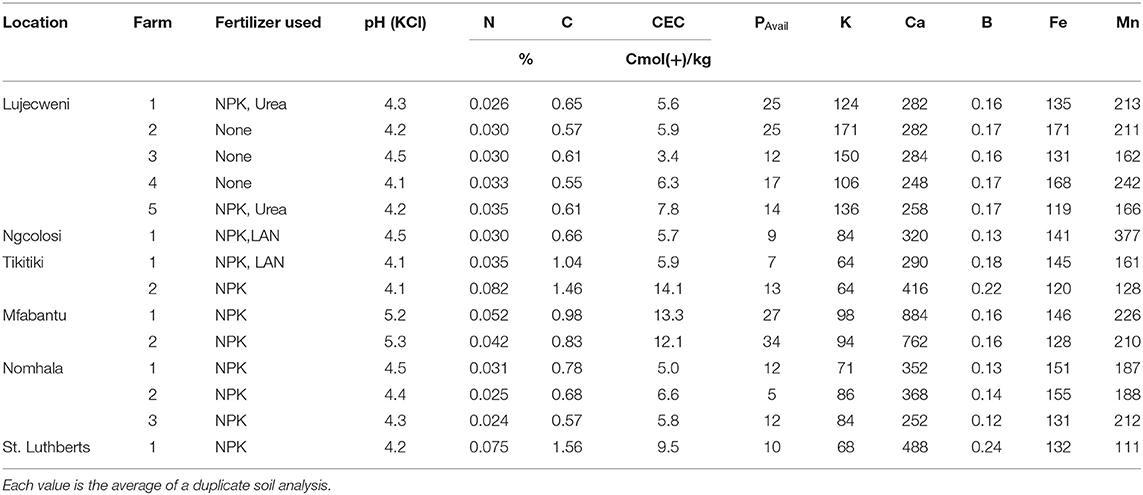
In each field, bulk soil was sampled (0–20 cm depth) from empty spaces in between plants using an augur. The soil samples from each farm were pooled, taken to the laboratory, air-dried, and sieved (2 mm). Subsamples were taken and analyzed for pH (KCl), %C (Walkley and Black, 1934), P (Bray and Kurtz, 1945), CEC, Ca, K, Mg, Fe, Mn, and B (ammonium acetate method), and total N using the Kjeldahl digestion.
Plant Sampling and Processing
In this study, plants were sampled from the fields of 14 farmers located in six villages [namely, Lujecweni (31.6103°S; 28.8619°E), Ngcolosi (31.4000°S; 28.7000°E), Tikitiki (31.4000°S; 28.7000°E), Mfabantu (31.5706°S; 29.0014°E), Nomhala (31.1523°S; 28.3714°E), and St. Luthberts] in the Eastern Cape Province of South Africa. The number of farmer fields sampled varied from village to village depending on the willingness of farmers to allow plants to be collected from their fields. A paired legume-reference plant sampling procedure was used, as described by Unkovich et al. (1994). Ten plants were randomly selected from each farm at the pod-filling stage, dug up, and separated into nodulated roots and shoots plus pods. The shoots of non-legume plant species growing among the common bean plants in the field of each farmer (and which received the same blanket N fertilizer treatment applied to the field before planting the beans) were also harvested as reference plants for the estimation of soil N uptake by legumes. All bean plant materials (i.e., shoots plus pods) and shoots only of reference plants were oven-dried separately at 60°C for 48 h, weighed, and milled to a fine powder (0.85 mm sieve) for 15N and 13C isotopic analysis.
Common Bean Stem and Petiole Collection, and Processing for Ureide and Nitrate Analysis
As the 10 bean plants were sampled from the field of each farmer for measuring N2 fixation using the 15N natural abundance method, the stems and petioles of each plant were removed, oven-dried separately at 60°C for 48 h, weighed, and milled to a fine powder (0.85 mm sieve) for ureide and nitrate analysis, after warm water extraction (Unkovich et al., 2008).
Ureide and Nitrate Assay
Ureides and nitrate in finely ground powder of stems and petioles were extracted using hot water, as described by Unkovch et al. (2008). The ureide concentration in plant extracts was analyzed using the Rimini–Schryver reaction as described by Young and Conway (1942), whereas nitrate was analyzed using the salicylic acid method (Cataldo et al., 1975), as outlined by Unkovich et al. (2008). The concentrations of nitrate and ureides in stems and petioles were expressed on per gram oven-dried organ basis, and total nitrate-N and ureide-N were estimated for each sample (Unkovich et al., 2008). The percent relative ureide abundance in stems and petioles was calculated as described by Herridge (1982a,b):
Measurement of N2 Fixation
15N/14N Isotopic Analysis
Finely ground plant material was weighed into Al tin capsules (1–2 mg/legume sample and 2–3 mg/reference plant) and analyzed for %Ndfa and 15N/14N ratio using a Carlo Erba NA1500 elemental analyzer (Fisons Instruments SpA, Strada, Rivoltana, Italy) coupled to a Finnigan MAT252 mass spectrometer via Conflo II open-split device. The 15N natural abundance was expressed as δ (delta) notation and is per mille deviation of the 15N natural abundance of the sample from atmospheric (atm) N2 (0.36637 atom %15N). The isotopic composition (δ15N) was measured as described by Unkovich et al. (2008):
where 15N/14Nsample is the abundance ratio of 15N and 14N in the common bean sample, and 15N/14Natm is the abundance ratio of 15N and 14N in the atmosphere.
Shoot N Content
The N content of common bean shoots was determined as the product of %Ndfa and shoot dry weight (Pausch et al., 1996; Peoples et al., 2009; Angus and Peoples, 2012).
Percent N Derived From Fixation
The percent N derived from the symbiotic fixation of atmospheric N2 was estimated using the equation (Shearer and Kohl, 1986; Unkovich et al., 2008):
where the δ15Nref is the 15N natural abundance of reference plants, δ15Nleg is the 15N natural abundance of legume, and the B-value is the 15N natural abundance of the test legume (common bean) wholly dependent on N2 fixation for its N nutrition. The B-value replaces the value of atmospheric N2 as it incorporates the isotopic fractionation associated with N2 fixation (Unkovich et al., 2008). In this study, the B-value used (−2.16%0) was obtained from Unkovich et al. (2008).
Amount of N-fixed
The amount of N-fixed was calculated as described by Maskey et al. (2001):
N-fixed per hectare was estimated as the product of N-fixed in shoots per plant and plant density per hectare. The plant density in each field was measured by counting the number of common bean plants in a 3-m2 quadrant, which was then used to estimate plant population per hectare.
Statistical Analysis
Statistical analyses were carried out using the STATISTICA package (StatSoft Inc., Tulsa, OK, USA). Data were subjected to a normal distribution test, followed by a one-way ANOVA. Where there were significant differences, treatment means were separated using the Duncan multiple range test at p ≤ 0.05.
Results
Soil Chemical Properties
All the sites used in this study were characterized by acidic soils with pH ranging from pH 4.1 at Tikitiki fields 1 and 2, and Lujecweni field 4 to pH 5.3 at Mfabantu field 2. Generally, the soils appeared to have low levels of N and intermediate to high levels of %C, Ca, Mg, P, Mn, B, and Fe. Nevertheless, plant-available P was lowest in the soil from Nomhala field 2, followed by Tikitiki field 1, Ngcolosi field 1, and St. Luthberts field 1 (Table 1).
δ15N of Reference Plants
At least five non-legume plant species were collected from the field of each farmer for 15N/14N isotopic analysis. Across the fields of farmers, the δ15N of all reference plants ranged from +0.02 to +9.37%0, whereas the combined δ15N of the reference plant species sampled from the different fields ranged from +3.03 to +6.87%0 (Table 2).
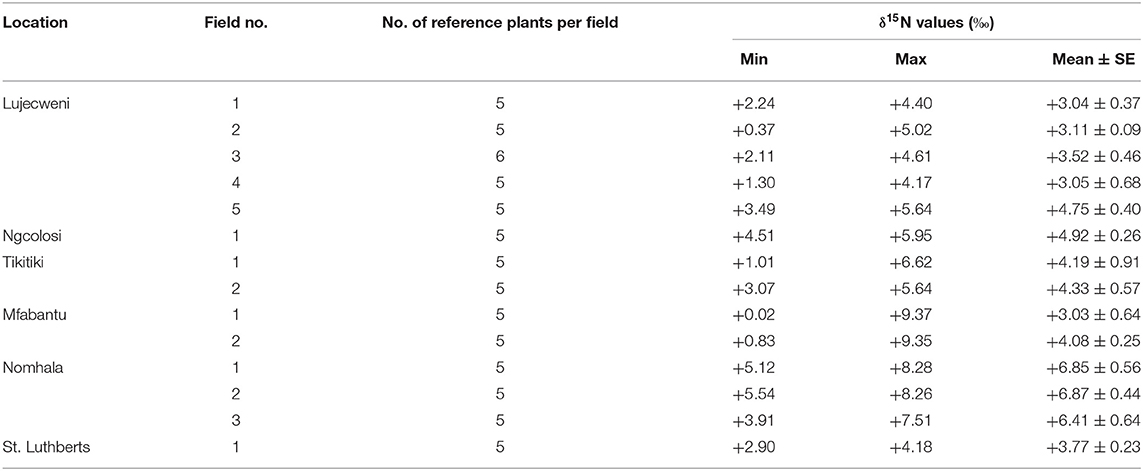
Table 2. Shoot δ15N values of reference plants sampled from the fields of farmers in the Eastern Cape and Limpopo Provinces for estimating percent N derived from fixation (%Ndfa) and soil N uptake by common bean.
Plant Growth
Plant growth (measured as shoot dry matter) of common bean variety PAN128 was significantly (p ≤ 0.05) greater at Nomhala fields 1 (156.92 g plant−1) and 2 (134.44 g plant−1), followed by those grown at Mfabantu fields 2 (107.86 g plant−1) and 1 (106.96 g plant−1) (Figure 1C). On the other hand, the common bean plants from Nomhala field 2 ranked least in nodule number and nodule fresh weight, despite recording high plant growth (Figures 1A–C). However, the plants sampled from Mfabantu field 2 ranked highest in nodule number, nodule fresh weight, and plant growth. Plant growth was lowest at Tikitiki field 1 (25.72 g plant−1), Lujecweni fields 1 (51.32 g plant−1) and 3 (53.30 g plant−1), Ngcolosi field 1 (54.76 g plant−1), and Lujecweni field 4 (57.54 g plant−1). The least nodulation and plant growth were recorded in the common beans that were sampled from Lujecweni field 1 and Ngcolosi field 1 (Figures 1A–C).
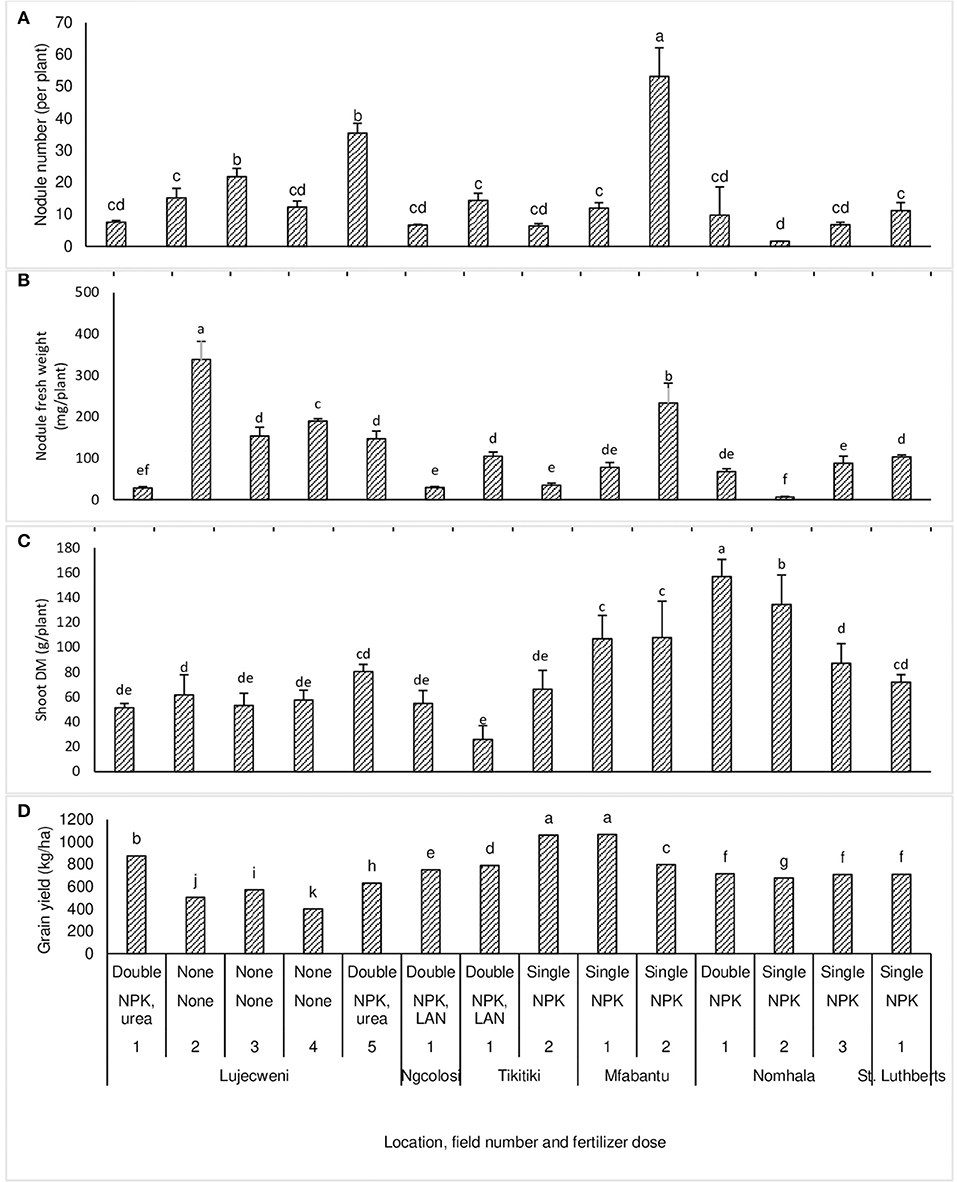
Figure 1. Nodulation (A,B), plant biomass (C), and grain yield (D) of common bean variety PAN128 sampled from the fields of 14 farmers in Eastern Cape Province, in the 2014 cropping season. NPK (2:3:2), limestone ammonium nitrate (LAN, 28% N), and urea were each used at rates of 100 kg ha−1. For each location on the x-axis, the numbers 1, 2, 3, … represent different fields within that location. Bars with dissimilar letters are significantly different at p ≤ 0.05.
In general, the common bean plants grown without fertilizer application (e.g., Lujecweni fields 2, 3, and 4) recorded much higher nodulation but lower shoot DM when compared to their counterparts supplied with N fertilizers by farmers in the Eastern Cape (Figures 1A–C).
Grain Yield
There were marked (p ≤ 0.05) differences in the grain yield of the common bean plants from the fields of various farmers (Figure 1D). PAN128, the test bean variety, recorded the highest grain yield at Mfabantu field 1 (1,065 kg ha−1) and Tikitiki field 2 (1,060 kg ha−1), followed by Lujecweni field 1 (875 kg ha−1), Mfabantu field 2 (797 kg ha−1), and Tikitiki 1 (787 kg ha−1). The fields that showed the highest yields (Mfabantu field 1 and Tikitiki field 2) each received a single dose of mineral N fertilizer, whereas intermediate yields were recorded at Lujecweni field 5 (630 kg ha−1), Nomhala field 1 (715 kg ha−1), St. Luthberts field 1 (710 kg ha−1), and Ngcolosi field 1 (750 kg ha−1). Most of the fields producing intermediate yields received a double dose of mineral N. In contrast, the lowest grain yield was recorded at Lujecweni fields 4 (400 kg ha−1), 2 (503 kg ha−1), and 3 (570 kg ha−1), which were all unfertilized (Figure 1D).
Nodulation
Nodulation (measured as nodule number and nodule fresh weight per plant) varied significantly (p ≤ 0.05) among common bean plants collected from the fields of 14 farmers in the Eastern Cape Province (Figures 1A,B). The bean plants grown at Nomhala field 2 (1.6 nodules per plant) had the least number of nodules, followed by the plants grown at Tikitiki field 2 (6.4 nodules per plant), Ngcolosi field 1 (6.7 nodules per plant), and then Lujecweni field 1 (7.6 nodules per plant). The plants from Mfabantu field 2 recorded the highest nodulation (53.2 nodules per plant), followed by Lujecweni field 5 (35.4 nodules per plant), Lujecweni field 3 (21.8 nodules per plant), Lujecweni field 2 (15.2 nodules per plant), Lujecweni field 4 (12.3 nodules per plant), Mfabantu field 1 (11.9 nodules per plant), and then St. Luthberts field 1 (11.2 nodules per plant). The fresh weight of nodules was markedly greater at Lujecweni field 2 (338.41 mg plant−1), Mfabantu field 2 (233.56 mg plant−1), Lujecweni field 4 (189.43 mg plant−1), Lujecweni field 3 (153.86 mg plant−1), Lujecweni field 5 (147.23 mg plant−1), Tikitiki field 1 (104.88 mg plant−1), and St. Luthberts field 1 (103.06 mg plant−1). In contrast, the common bean plants from Nomhala field 2, Lujecweni field 1, Ngcolosi field 1, and Tikitiki field 2 recorded much lower nodule fresh weights of 6.5, 28.1, 29.11, and 34.83 mg plant−1, respectively (Figures 1A,B). There was a strong correlation between nodule number and nodule fresh weight (r = 0.82***) of the common bean plants sampled from the unfertilized fields. In contrast, there was no significant correlation between nodule number and nodule fresh weight of plants sampled from the N-fertilized fields.
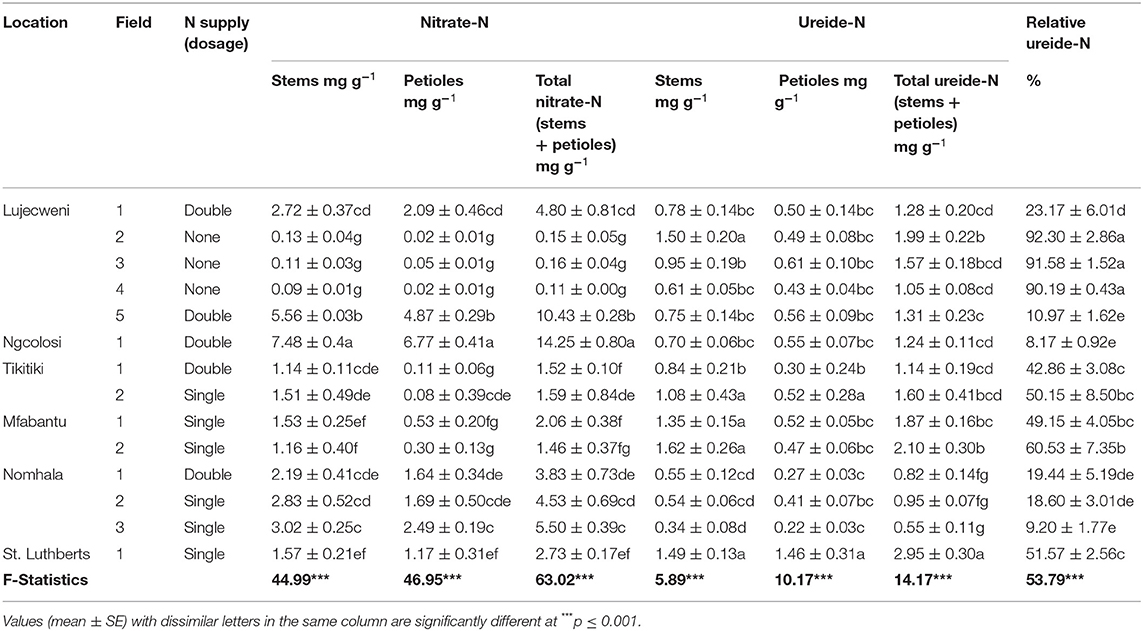
Ureides, Nitrate, and Percent Relative Ureide-N Abundance in Plant Organs
Warm water extracts of ground common bean stems and petioles were analyzed for ureides and nitrate. The sum of the two nitrogenous solutes from both organs was used to estimate nitrate-N, ureide-N, and percent relative ureide-N abundance (Table 3). The common bean plants grown without N fertilization at Lujecweni fields 2, 3, and 4 in the Eastern Cape exhibited greater nodulation but low shoot biomass and also recorded the lowest nitrate concentration in their stems and petioles (Table 3). Total nitrate-N of plants from Lujecweni field 4 was 0.11 mg g−1, whereas those of Lujecweni fields 2 and 3 were 0.15 and 0.16 mg g−1, respectively. The common bean plants that exhibited intermediate levels of nitrate in their stems and petioles, and intermediate total nitrate in the two organs were sampled from Nomhala fields 1 (3.83 mg g−1) and 2 (4.53 mg g−1) and also from Lujecweni field 1 (4.80 mg g−1). As noted before, plants sampled from Nomhala fields 1 and 2 showed better growth, despite low nodulation in field 2. It is interesting to note that, although plants from Lujecweni field 5 showed intermediate shoot growth (80.50 g plant−1), those from Ngcolosi field1 had much lower shoot DM (54.76 g plant−1), and they both recorded much higher nitrate in stems and petioles (Table 3).
Ureide analysis of tissue extracts revealed significant (p ≤ 0.05) differences in the concentration of ureide-N per organ (stem and petiole) of the bean plants (Table 3). Common bean grown at St. Luthberts showed significantly higher concentration of ureides in stems (1.49 mg g−1), petioles (1.46 mg g−1), and total ureide-N in the two organs (2.95 mg g−1) (Table 3). The next higher ureide concentrations were recorded in the tissues of plants grown at Mfabantu field 2 (2.10 mg g−1) and Lujecweni field 2 (1.99 mg g−1). Intermediate concentrations of total ureide-N were recorded for the stems and petioles of common bean plants sampled from Mfabantu field 1 (1.87 mg g−1), Tikitiki field 2 (1.60 mg g−1), and Lujecweni field 3 (1.57 mg g−1). In contrast, plants from Nomhala fields 1, 2, and 3 showed the lowest concentrations of ureide-N in organs (0.55 mg g−1 in field 3, 0.82 mg g−1 in field 1, and 0.95 mg g−1 in field 2; see Table 3).
The percent relative ureide-N abundance varied markedly (p ≤ 0.05) between and among plants sampled from the fields of different farmers (Table 3). Common bean plants grown without N fertilizer application at Lujecweni fields 2, 3, and 4 recorded much higher relative ureide-N abundance, in the order of 92.3, 91.6, and 90.2%, respectively. Mfabantu fields 1 (49.15%) and 2 (60.53%), St. Luthberts field 1 (51.57%), and Tikitiki field 2 (50.15%), which received a single dose of N fertilizer at the planting stage, produced plants with intermediate relative ureide-N abundance (Table 3). In contrast, the plants grown at Ngcolosi fields 1 (8.17%), Nomhala field 3 (9.20%), and Lujecweni field 5 (10.97%), which were supplied with N fertilizer both at planting and flowering, revealed the lowest relative ureide-N abundance (Table 3).
Shoot N, δ15N, %Ndfa, and Amount of N-fixed
All the bean fields surveyed in the Eastern Cape received mineral N at the planting stage and/or the pod-filling stage, except for Lujecweni fields 2, 3, and 4. That notwithstanding, the 15N natural abundance technique was applied to estimate N2 fixation in the N-fertilized bean plants using reference plants sampled from among the bean plants in each field. This was based on the understanding that, like the bean plants, the reference plants also took up the added mineral N.
Shoot N concentration in bean plants ranged from 1.61 to 2.77%, and N content from 0.61 to 3.59 g plant−1 (Table 4). Shoot δ15N values were much lower in the unfertilized bean plants at Lujecweni fields 2, 3, and 4, and much higher in plants from Nomhala fields 1, 2, and 3 (Table 4). As a result, the %Ndfa was higher at Lujecweni fields 2, 3, and 4 (85, 90, and 97%, respectively) and much lower at Nomhala fields 1 and 2 (12 and 4%, respectively). Symbiotic N contribution by common bean plants ranged from 0.14 to 2.27 g plant−1 or 16 to 163 kg ha−1 (Table 4). Soil N uptake also varied from 0.05 to 3.14 g plant−1 or 6 to 337 kg ha−1 (Table 4).
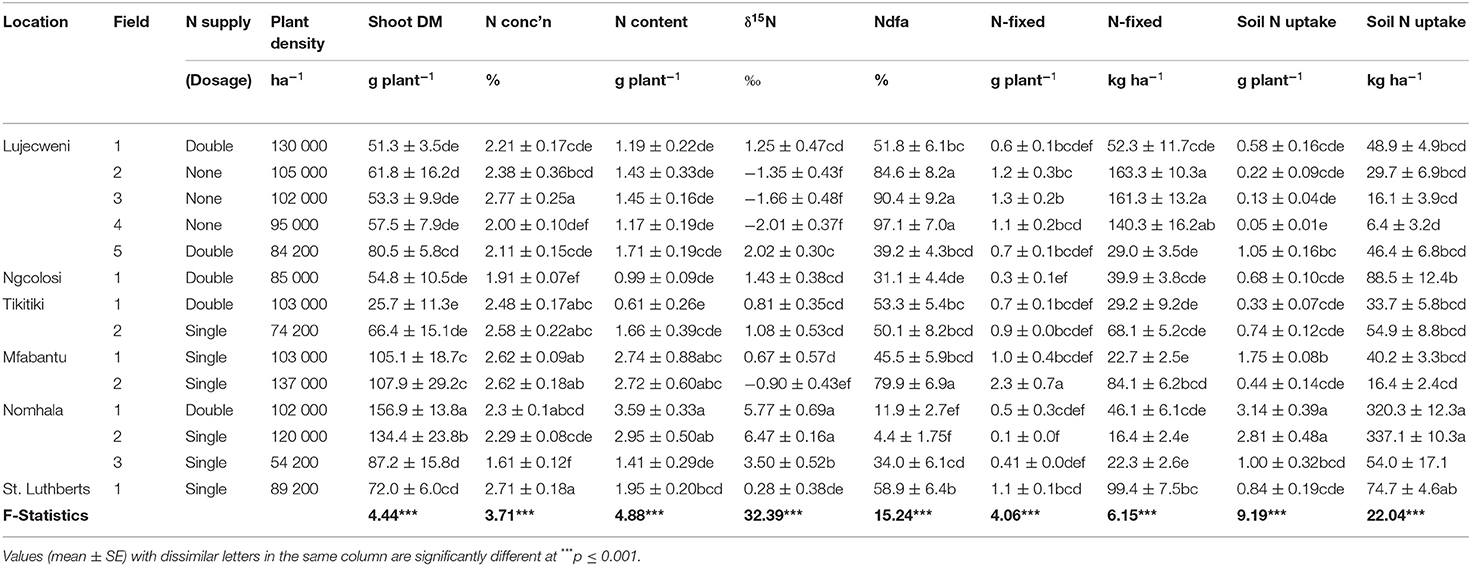
Table 4. Growth and symbiotic performance of common bean variety PAN128 plants sampled from the fields of farmers.
Correlation Analysis
Correlation analysis revealed a significantly positive relationship between % relative ureide-N abundance and nodule fresh weight (r = 0.50***) and also between % relative ureide-N abundance and %Ndfa (r = 0.75***) for common bean plants sampled from the N-fertilized fields. In contrast, the % relative ureide-N abundance was negatively correlated with shoot DM (r = −0.31*), whereas relative ureide-N abundance and %Ndfa from 15N natural abundance were both negatively correlated with tissue nitrate concentration (r = −0.78*** and r = −0.32***, respectively) for N-fertilized bean plants. With the unfertilized common bean plants, the correlation analysis showed a significantly positive relationship between nodule number and nodule fresh weight (r = 0.82***) and between %Ndfa measured from 15N natural abundance and % relative ureide-N abundance (r = 0.96***). But %Ndfa was negatively correlated with tissue nitrate. Furthermore, % relative ureide-N was positively correlated with nodule number (r = 0.56*) and nodule fresh weight (r = 0.82***). In contrast, there was a significantly negative correlation between relative ureide-N abundance and shoot DM (r = −0.61**), and tissue NO concentration (r = −0.84***).
Discussion
The South African government policy of providing free N fertilizers to resource-poor farmers has had a positive impact on food security, especially among rural households. Both maize and common bean yields rose with the supply of free fertilizer inputs to emerging small-scale farmers (Figure 1D). In this study, the low endogenous soil N concentrations (0.030–0.033%) found in the unfertilized fields of the Eastern Cape do justify the program of supplying free fertilizer by the government to resource-poor farmers. However, %Ndfa of 84.6, 90.4, and 97.1%, and symbiotic N contribution of 163.3, 161.3, and 140.3 kg ha−1 by unfertilized common bean plants at Lujecweni fields 2, 3, and 4, respectively, when compared to their N-fertilized counterparts, suggest that the use of chemical N fertilizers in common bean production in South Africa is not sustainable due to (i) the high symbiotic performance of unfertilized bean plants relative to their fertilized counterparts, (ii) the high cost of N fertilizers, and (iii) the negative impact of N fertilizers on the environment with frequent use. Exploiting symbiotic N for increased dry bean production in South Africa is therefore a better and sustainable alternative to the use of chemical N fertilizers for increased food and nutritional security.
Additionally, in this study, N-fertilization had a directly negative effect on the symbiotic process of common bean plants. For example, with the unfertilized bean plants, there was a significantly positive correlation between nodule number and nodule fresh weight (r = 0.82***) and between %Ndfa measured from 15N natural abundance and % relative ureide-N abundance (r = 0.96***). Furthermore, the % relative ureide-N was positively correlated with nodule number (r = 0.56*) and nodule fresh weight (r = 0.82***), a finding consistent with an uninhibited functional symbiosis. The significantly positive relationship between % relative ureide-N abundance and nodule fresh weight (r = 0.50***) and between % relative ureide-N abundance and %Ndfa (r = 0.75***) in N-fertilized common bean plants suggests that the N-fertilized common bean plants were still able to fix N2 for their growth as they could achieve up to 60% relative ureide-N abundance with a single N-dose application. Tsai et al. (1993) and Tahir et al. (2009) reported that the use of some starter N at planting was beneficial in achieving a synergistic effect on N2 fixation in common bean by stimulating nodule formation, nitrogenase activity, and plant growth, especially in infertile soils. However, in this study, the % relative ureide-N abundance was negatively correlated with shoot DM (r = −0.31*), an indication of inadequate symbiotic N supply to shoots. Several studies have shown that plant growth and grain yield of legumes in Africa are directly correlated with symbiotic functioning, and that grain legumes with high levels of N2 fixation generally elicit greater biomass accumulation, leading to higher grain yield, and vice versa (Belane and Dakora, 2009, 2010; Peoples et al., 2009; Mohale et al., 2013).
The low relative ureide-N (9.2%) observed at Nomhala field 3 that received a double N-dose application (Table 3) shows that, in this study, farmer application of N fertilizers suppressed N2 fixation in common bean (Vargas et al., 2000; Salvagiotti et al., 2008; Li et al., 2009; Reinprecht et al., 2020); as a result, those bean plants had to complement symbiotic N supply with greater soil N uptake in order to meet their N demand (Van Vessel and Hartley, 2000; Adu-gyamfi et al., 2007). This argument is reinforced by the high nitrate concentration found in stems and petioles of the common bean plants receiving a single and a double dose of N-fertilizer. In fact, Unkovich et al. (2008) have shown that the N solutes in the xylem stream and aqueous stem extracts of members of the tribe Phaseoleae can change from one dominated by ureides in N2-dependent plants to a system dominated by nitrate and amino acids in plants utilizing soil N. This could therefore also explain the strong inverse relationship found between relative ureide-N abundance and tissue NO concentrations of the bean plants in this study. Given that the ureide technique provides an instantaneous measure of legume N2 fixation at the time of sampling (Alves et al., 2000; Unkovich et al., 2008), the higher ureide-N concentration in stems than petioles could suggest that the stem extract contained xylem ureides that were in transit from root nodules to the leaves, the major sink for fixed-N assimilation.
In this study, although N fertilization markedly suppressed nodulation and nodule symbiotic functioning in bean plants (Tables 3, 4), its application as a single dose generally increased grain yield over either double-dose application or zero-N fertilization (Figure 1D). The fact that double-dose application increased shoot growth at the expense of grain yield is consistent with a recent report by Boddey et al. (2017), who observed higher vegetative growth and lower grain yields in cowpea receiving N fertilizer. The findings of this study therefore suggest that, if N must be applied to bean plants, a single dose supply could help to increase yields while reducing government expenditure to resource-poor farmers. However, the fact that symbiotic dependence of bean plants was markedly decreased by a single dose of N fertilizer applied suggests the need to explore the possibility of achieving greater symbiotic N nutrition and increased grain yield at lower starter N doses in low N soils.
A comparison of the data obtained for symbiotic performance from measuring N2 fixation using the 15N natural abundance technique and ureide assay revealed similar trends for plants sampled from the fields of the same farmer, an indication of their precision in measuring N2 fixation in bean plants on the fields of farmers. For example, %Ndfa by unfertilized bean plants at Lujecweni fields 2, 3, and 4 were 84.6, 90.4, and 97.1%, respectively, using the 15N natural abundance method, values comparable to the 92.3, 91.6, and 90.2% relative ureide abundance estimated using the ureide technique. Similarly, symbiotic N contribution by unfertilized common bean plants at Lujecweni fields 2, 3, and 4 using the 15N method was 163.3, 161.3, and 140.3 kg ha−1 respectively, with values similar in magnitude to the total ureides in stems and petioles of 2.0, 1.6, and 1.1 mg g−1 (Tables 3, 4). These trends indicate that the two techniques were similar in their precision and robustness in estimating N2 fixation in the bean plants on the fields of farmers.
In this study, %Ndfa and ureide levels in stems/petioles were also comparatively similar in trend when the two techniques were used on N-fertilized bean plants from Lujecweni field 1, Mfabantu fields 1 and 2, St. Luthberts field 1, and, to some extent, Tikitiki fields 1 and 2 (Tables 3, 4), and therefore this further confirmed the robustness of the two methods used. However, we also found that the values of %Ndfa at Lujecweni field 1 (51.8%) and Tikitiki field 1 (53.3%) were very high for plants receiving a double dose of N fertilizer and quite high for Tikitiki field 2 (50.1%), Mfabantu fields 1 and 2 (45.5 and 79.9%), and St. Luthberts field 1 (58.9%), which received a single dose of N fertilizer. Some legume symbioses are tolerant of nitrate (Dakora 1998). The only possible explanation for this observation would be that the rhizobia, and therefore bacteroids in root nodules of these N-fertilized bean plants, had a constitutive and/or inducible nitrate reductase activity. Rhizobia with high expression of constitutive nitrate reductase or inducible nitrate reductase genes can form root nodules in the presence of nitrate, but nitrate reductase-minus strains cannot (Serrano and Chamber, 1990). Similarly, bacteroids with a high expression of constitutive nitrate reductase and inducible nitrate reductase genes can fix N2 in the presence of nitrate, but nitrate reductase-minus strains cannot (Serrano and Chamber, 1990). Therefore, the observed differences in N2 fixation of bean plants supplied with high doses of mineral N on the fields of farmers could be attributed to the presence of native soil rhizobia with the ability to reduce mineral N and convert N2 into NH3 in root nodules, following differentiation into bacteroids.
Conclusion
In conclusion, this study has shown that government supply of free N fertilizers to resource-poor farmers in South Africa increased bean yields for food/nutritional security. Although single- and double-dose N fertilizer application increased plant growth and grain yield compared to unfertilized bean plants, single-dose N fertilizer application produced a much greater grain yield than double dose. This suggests that farmers should stop using a double dose of N fertilizers on bean production, as it decreases yields and can potentially pollute the environment. However, relative to the N-fertilized bean plants, their unfertilized counterparts produced greater nodule fresh weight, higher N derived from fixation, increased amount of symbiotic N, higher percent relative ureide-N abundance, and greater ureide concentration in stems and petioles. Furthermore, contrary to the hypothesis of reduced N2 fixation with N supply, %Ndfa was very high for some bean plants receiving a double dose of N fertilizer [e.g., Lujecweni field 1 (51.8%) and Tikitiki field 1 (53.3%)] and high for those receiving a single dose of N fertilizer [e.g., Tikitiki field 2 (50.1%), Mfabantu fields 1 and 2 (45.5 and 79.9%), and St. Luthberts field 1 (58.9%)]. Although this was not assessed in our study, it is likely that the rhizobia that effectively nodulated the N-fertilized bean plants and fixed considerable amounts of symbiotic N had constitutive and/or inducible nitrate reductase genes for reducing nitrate in nodules and bacteroids, hence their ability to form root nodules and derived high percent N from fixation in bean with added N.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
SH and SM collected the samples. SH analyzed data and drafted the manuscript. FD conceived the idea, edited and approved the final version of the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was supported with grants from the Bill and Melinda Gates Foundation Project on Capacity Building in Legume Sciences in Africa, the South African Department of Science and Technology, the Tshwane University of Technology, the National Research Foundation in Pretoria, and the South African Research Chair in Agrochemurgy and Plant Symbioses. SH is grateful for a competitive masters fellowship from the Bill and Melinda Gates Foundation Project on Capacity Building in Legume Sciences in Africa.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adu-gyamfi, J. J., Myaka, F. A., Sakala, W. D., Odgaard, R., Vesterager, J. M., and Høgh-Jensen, H. (2007). Biological nitrogen fixation and nitrogen and phosphorus budgets in farmer-managed intercrops of maize-pigeon pea in semi-arid Southern and Eastern Africa. Plant Soil. 295, 127–136. doi: 10.1007/s11104-007-9270-0
Alves, B., Resende, A., Urquiaga, S., and Boddey, R. (2000). Biological nitrogen fixation by two tropical forage legumes assessed from the relative ureide abundance of stem solutes: 15N calibration of the technique in sand culture. Nutr. Cycling Agroecosyst. doi: 10.1023/A:1009865816182
Angus, J. F., and Peoples, M. B. (2012). Nitrogen from Australian dryland pastures. Crop Pasture Sci. doi: 10.1071/CP12161
Belane, A. K., and Dakora, F. D. (2009). Measurement of N2 fixation in 30 cowpea (Vigna unguiculata L. Walp.) genotypes under field conditions in Ghana, using the 15N natural abundance technique. Symbiosis 48, 47–56. doi: 10.1007/BF03179984
Belane, A. K., and Dakora, F. D. (2010). Symbiotic N2 fixation in 30 field-grown cowpea (Vigna unguiculata L. Walp.) genotypes in the Upper West Region of Ghana measured using 15N natural abundance. Biol. Fertil. Soils 46, 191–198. doi: 10.1007/s00374-009-0415-6
Boddey, R. M., Fosu, M., Atakora, W. K., Miranda, C. H., Boddey, L. H., Guimaraes, A. P., et al. (2017). Cowpea (Vigna unguiculata) crops in Africa can respond to inoculation with rhizobium. Exp. Agric. 53, 578–587. doi: 10.1017/S0014479716000594
Bray, R. H., and Kurtz, L. T. (1945). Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 59, 39–46.
Cataldo, D., Maroon, M., Schrader, L., and Youngs, V. (1975). Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. doi: 10.1080/00103627509366547
Chikanai, V., Chikowo, R., and Vanlauwe, B. (2018). Response of common bean (Phaseolus vulgaris L.) to nitrogen, phosphorus and rhizobia inoculation across variable soils in Zimbabwe. Agric. Ecosyst. Environ. doi: 10.1016/j.agee.2018.08.010
Dakora, F. D., and Atkins, C. A. (1989). Diffusion of oxygen in relation to structure and function in legume root nodules. Funct. Plant Biol. 16, 131–140. doi: 10.1071/PP9890131
Dakora, F. D., Atkins, C. A., and Pate, J. S. (1992). Effect of NO3 on N2 fixation and nitrogenous solutes of xylem in two nodulated West African geocarpic legumes, Kersting's bean (Macrotyloma geocarpum L.) and Bambara groundnut (Vigna subterranea L.). Plant Soil. 140, 255–262. doi: 10.1007/BF00010602
Farid, M., Earl, H. J., and Navabi, A. (2016). Yield stability of dry bean genotypes across nitrogen-fixation-dependent and fertilizer-dependent management systems. Crop Sci. 56, 173–182. doi: 10.2135/cropsci2015.06.0343
Farid, M., and Navabi, A. (2015). N2 fixation ability of different dry bean genotypes. Can. J. Plant Sci. 95, 1243–1257. doi: 10.4141/cjps-2015-084
Graham, P. H., and Vance, C. P. (2003). Legumes: importance and constraints to greater use. Plant Physiol. doi: 10.1104/pp.017004
Herridge, D. F. (1982a). Relative abundance of ureides and nitrate in plant tissues of soybean as a quantitative assay of nitrogen fixation. Plant Physiol. doi: 10.1104/pp.70.1.1
Herridge, D. F. (1982b). Use of the ureide technique to describe the nitrogen economy of field-grown Soybeans. Plant Physiol. doi: 10.1104/pp.70.1.7
Herridge, D. F., and People, M. B. (1990). Ureide assay for measuring nitrogen fixation by nodulated soybean calibrated by 15N methods. Plant Physiol. doi: 10.1104/pp.93.2.495
Jiang, Y., MacLean, D. E., Perry, G. E., Marsolais, F., Hill, B., and Pauls, K. P. (2020). Evaluation of beneficial and inhibitory effects of nitrate on nodulation and nitrogen fixation in common bean (Phaseolus vulgaris). Legume Sci. 2:e45. doi: 10.1002/leg3.45
Li, Y., Yu, C., Cheng, X., Li, C., Sun, J., Zhang, F., et al. (2009). Intercropping alleviates the inhibitory effect of N fertilisation on nodulation and symbiotic N2 fixation of faba bean. Plant and Soil. doi: 10.1007/s11104-009-9938-8
Maingi, J. M., Shisanya, C. A., Nkanata, M. G., and Hornetz, B. (2000). Nitrogen fixation by common bean (Phaseolus vulgaris L.) in pure and mixed stands in semi-arid south-east Kenya. Eur. J. Agron. doi: 10.1016/S1161-0301(00)00080-0
Maskey, S. L., Bhattarai, S., Peoples, M. B., and Herridge, D. F. (2001). On-farm measurements of nitrogen fixation by winter and summer legumes in the Hill and Terai regions of Nepal. Field Crops Res. doi: 10.1016/S0378-4290(01)00140-X
Mohale, K. C., Belane, A. K., and Dakora, F. D. (2013). Symbiotic N nutrition, C assimilation, and plant water use efficiency in Bambara groundnut (Vigna subterranea L. Verdc) grown in farmers' fields in South Africa, measured using 15N and 13C natural abundance. Bio. Fertil. Soils doi: 10.1007/s00374-013-0841-3
Pausch, R. C., Charles, L., Mulchi, C. L., Lee, E. H., and Meisinger, J. J. (1996). “Use of 13C and 15N isotopes to investigate O3effects on C and N metabolism in soybeans,” in Part II. Nitrogen uptake, fixation, and partitioning. Agriculture Ecosystem and Environment. Available online at: doi: 10.1016/S0167-8809(96)01062-6
Peoples, M. B., Brockwell, J., Herridge, D. F., Rochester, I. J., Alves, B. J. R., Urquiaga, S., et al. (2009). The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis. doi: 10.1007/BF03179980
Reinprecht, L., Vidholdová, Z., and Iždinský, J. (2020). Bacterial and mold resistance of selected tropical wood species. BioResources 15, 5198–5209.
Salvagiotti, F., Cassman, K. G., Specht, J. E., Walters, D. T., Weiss, A., and Dobermann, A. (2008). Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crops Res. doi: 10.1016/j.fcr.2008.03.001
Samago, T. Y., Endalkachew, W. A., and Dakora, F. D. (2018). Grain yield of common bean (Phaseolus vulgaris L.) varieties is markedly increased by rhizobial inoculation and phosphorus application in Ethiopia. Symbiosis 75, 245–255. doi: 10.1007/s13199-017-0529-9
Serrano, A., and Chamber, M. (1990). Nitrate reduction in Bradyrhizobium sp. (Lupinus) strains and its effects on their symbiosis with Lupinus luteus. J. Plant Physiol. 136, 240–246. doi: 10.1016/S0176-1617(11)81673-1
Shearer, G., and Kohl, D. H. (1986). N2 fixation in field settings: Estimation based on natural 15N abundance. Aus. J. Plant Physiol. doi: 10.1071/PP9860699
Tahir, M. M., Abbasi, M. K., Rahim, N., Khaliq, A., and Kazmi, M. H. (2009). Effect of Rhizobium inoculation and NP fertilisation on growth, yield and nodulation of soybean (Glycine max L.) in the sub-humid hilly region of Rawalakot Azad Jammu and Kashmir, Pakistan. Afr. J. Biotech. 8, 6191–6200. doi: 10.5897/AJB09.1039
Tsai, S. M., Bonetti, R., Agbala, S. M., and Rossetto, R. (1993). “Minimizing the effect of mineral nitrogen on biological nitrogen fixation in common bean by increasing nutrient levels,” in Enhancement of Biological Nitrogen Fixation of Common Bean in Latin America. Developments in Plant and Soil Sciences,eds F. A. Bliss, G. Hardarson (Dordrecht: Springer). Available online at: doi: 10.1007/978-94-011-2100-2_14
Unkovch, M. J., Herridge, D. F., Peoples, M. B., Cadisch, G., Boddey, R. M., and Giller, K. E. (2008). Measuring Plant-Associated Nitrogen Fixation in Agricultural Systems. Canberra: ACIAR.
Unkovich, M., Herridge, D., Peoples, M., Cadisch, G., Boddey, B., Giller, K., et al. (2008). Measuring Plant-Associated Nitrogen Fixation in Agricultural Systems. Canberra, ACT: Australian Centre for International Agricultural Research.
Unkovich, M. J., Pate, J. S., Sanford, P., and Amstrong, E. L. (1994). Potential precision of the δ15N natural abundance method in field estimation of nitrogen fixation by crop and pasture legumes in South-west Australia. Aust. J. Agric. Res. doi: 10.1071/AR9940119
Van Vessel, C., and Hartley, C. (2000). Agricultural management of grain legumes: has it led to an increase in nitrogen fixation? Field Crops Res. doi: 10.1016/S0378-4290(99)00085-4
Vargas, M. A., Mendes, I. C., and Hungria, M. (2000). Response of field-grown bean (Phaseolus vulgaris L.) to Rhizobium inoculation and nitrogen fertilization in two Cerrados soils. Biol. Fertil. Soils 32, 228–233. doi: 10.1007/s003740000240
Vieira, R. F., Cardoso, E. J. B. N., Vieira, C., and Cassini, S. J. A. (1998). Foliar application of molybdenum in common beans: Nitrogenase and reductase activities in a soil of high fertility. J. Plant Nutri. doi: 10.1080/01904169809365391
Walkley, A., and Black, I. A. (1934). An examination of the degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38. doi: 10.1097/00010694-193401000-00003
Keywords: symbiosis, soil N uptake, N-fixed, %Ndfa, ureide, percent relative ureide abundance
Citation: Habinshuti SJ, Maseko ST and Dakora FD (2021) Inhibition of N2 Fixation by N Fertilization of Common Bean (Phaseolus vulgaris L.) Plants Grown on Fields of Farmers in the Eastern Cape of South Africa, Measured Using 15N Natural Abundance and Tissue Ureide Analysis. Front. Agron. 3:692933. doi: 10.3389/fagro.2021.692933
Received: 09 April 2021; Accepted: 21 June 2021;
Published: 08 September 2021.
Edited by:
Jose Palacios, Polytechnic University of Madrid, SpainReviewed by:
Mahaveer P. Sharma, ICAR Indian Institute of Soybean Research, IndiaGlaciela Kaschuk, Federal University of Paraná, Brazil
Copyright © 2021 Habinshuti, Maseko and Dakora. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Felix D. Dakora, RGFrb3JhRkRAdHV0LmFjLnph
 Simon J. Habinshuti
Simon J. Habinshuti Sipho T. Maseko2
Sipho T. Maseko2 Felix D. Dakora
Felix D. Dakora