- 1Bioversity International, Kampala, Uganda
- 2Independent Consultant, Kampala, Uganda
- 3Bioversity International, Addis Ababa, Ethiopia
The adoption of tool sterilization using either 3.5% sodium hypochlorite (household bleach) or fire, a core element of the cultural control packages for Xanthomonas wilt (XW) of banana has been poorly adopted hampering XW control in East and Central Africa. Household bleach is costly and not accessible to the rural poor while repeated heating weakens metal blades of garden tools (machetes, knives, and hoes). Identification of economically viable tool sterilization options is thus crucial for XW management. We explored a range of practices including tool insertion for varying time periods into cold and hot ash, fire and boiling water; tool exposure over varying time periods to the sun while under black or transparent plastic sheets; and washing tools with cold water and laundry soap or detergent. Cleaning with household bleach served as a negative control while uncleaned tools as positive control. Like for household bleach, no Xanthomonas vasicola pv. musacearum (Xvm) the causal agent of XW was recovered from tools washed with cold water and different laundry soaps or detergents. Culturing Xvm in varying detergent and soap concentrations (0.00125–0.035 g/mL), only resulted in growth at lower concentrations of 0.00125 and 0.0025 g/mL. The cleaning effect of soap could thus be due to both an anti-bacterial effect and dislodgment of bacteria from tools. Laundry soaps/detergents are cheaper than household bleach and used for various purposes within and across households, including the resource poor and rural households, hence a cheaper and convenient tool sterilization alternative. Tool insertion into boiling water was effective from the 40th second and thus a viable alternative. Heating tools in fire required up to a minute to clear all bacteria. The currently recommended 20–40 s heating could thus be inadequate. Repeated heating for 1 min may also damage tools. Other practices (washing with cold water only, use of solar radiation, repeatedly and forcefully inserting tools into the soil, tool insertion into hot and cold ash for up to 5 min) only reduced Xvm populations on tools, thus not independently recommended. We recommend expanding the tool sterilization options to include washing with soap/ detergents and tool insertion into boiling water for at least 1 min.
Introduction
Xanthomonas wilt (XW) of banana is an important disease of banana in the east and central African region (Kalyebara et al., 2006; Biruma et al., 2007; Tripathi et al., 2009; Blomme et al., 2014, 2017a; Ocimati et al., 2019). Yield losses due to XW can reach 100% in case of poor or delayed management. A 35% drop in sales and doubling of bunch prices due to XW have been reported in Tanzania and Rwanda (Nkuba et al., 2015). It might also not be a coincidence that the period 2001–2014 has also seen a 50% and a 39% decline in banana production and area with banana, respectively, in Uganda (FAO, 2020).
The disease is caused by the Gram-negative bacteria Xanthomonas vasicola pv. musacearum (previously Xanthomonas campestris pv. musacearum). Cultural practices have been predominantly used for XW management mainly through interventions that prevent pathogen introduction and or eliminate/ reduce disease inoculum. The core cultural control practices promoted include the rouging of entire diseased mats and/ or singly cutting at soil level of diseased plants, sterilization of farm tools by flaming/heating with fire or cleaning with 3.5% sodium hypochlorite (NaOCl/ household bleach), timely removal of the floral male buds with a forked stick and the use of clean planting materials (Karamura et al., 2008; Blomme et al., 2014, 2019; PROMUSA, 2020). With respect to tool sterilization with fire, the recommendation advised to heat the metal tool until when too hot to touch, and 20–40 s over a strong flame was perceived as sufficient to achieve this (PROMUSA, 2020). The control practices are promoted as complete packages comprising of at least three individual technologies. The main packages include the (i) complete diseased mat uprooting (CDMU) which comprises uprooting entire mats, farm tool sterilization and male bud removal; and (ii) single-diseased stem removal (SDSR) which consists of the removal of single diseased stems, farm tool sterilization and early male bud removal.
Full adoption of these practices has been reported to have positive results. For example, the SDSR control package has been reported to rapidly recover fields even when the initial incidence on farm was as high as 80% (Blomme et al., 2017b, 2019). Full adoption of the SDSR technology package in Uganda was reported to improve annual banana production per acre by US$187 compared to no adoption and by US$ 139 when two practices out of the full SDSR XW control package were adopted (Kikulwe et al., 2019). The adoption of one, two control practices and the full package translated to a 36, 193, and 216% improvement in revenue obtained (USD/acre/annum) from banana on farm. Despite the success of control packages e.g., the SDSR package in controlled experiments, adoption level, application intensity and correctness, and success on farms has been variable. Several factors including the timing of application, wrong application and dis-adoption of some elements of the control packages have been observed (Blomme et al., 2019; Ntamwira et al., 2019).
Tool sterilization (using either household bleach or fire), which is a core part of the XW control packages, has been poorly adopted, reducing the effectiveness the packages. For example, tool sterilization adoption rates of <30% and about 55% have been, respectively, reported in eastern DR Congo (Blomme et al., 2014) and Uganda (Kikulwe et al., 2019). The reach/availability of household bleach in the rural areas is poor in addition to being costly. In a more recent study, farmers reported that repeated heating of metal garden tools with fire between plants or after cutting all diseased plants, a practice previously thought to be more feasible among the resource poor households, to weaken the metal blades of their tools (Blomme et al., 2019). The reported damage to metal tools could be attributed to heating the tools for a much longer time than the recommended 20–30 s. Although the cost of a machete is only about 3 USD, this cost is often perceived as too high to subsistence small-scale rural farmers. More still, lighting a small fire in the field during the rainy season is also not feasible as dry mulch, twigs or leaves collected from within the fields are normally used by farmers. Fostering the adoption of the entire XW control package is crucial for maintaining or improving banana production levels. Adoption of technologies in agriculture has remained a challenge and is directly related to the efficiency of any agricultural technology (Bozeman, 2000). The adoption of new technologies has also been reported to vary among farm households due to differences in their socio-economic characteristics (Asfaw and Admassie, 2004; Somda and Kamuanga, 2005). There is a need to investigate additional or alternative options for tool sterilization other than the use of fire or chemical disinfectants that are out of reach of most farm households.
Farmers who dis-adopted heating of farm tools on fire reported that tool sterilization should preferably be carried out by pushing the metal blade in hot ash, and not directly in a flame, to protect the metal tool against heat damage (Blomme et al., 2019). However, hot ash most likely has a lower temperature compared to flames and this could potentially fail to eliminate all bacteria on the blade and have a negative effect on disease control. This practice needs to be evaluated under controlled situations, e.g., in the laboratory and screenhouse. Another potential option could be the use of boiling water to sterilize the metal tools, after all diseased plants have been cut. Washing a machete with water and soap, and subsequently drying the machete in the sun, could potentially eliminate the bacteria on metal blades or reduce the number of bacteria to levels that do not cause infection when subsequently used on a healthy plant. Sterilizing garden tools through solar radiation could have potential as the central African region lies on the equator with regular sunshine and would need to be investigated. The effect of solar radiation especially during days of intense sunshine could also be hastened by wrapping the tools in black or transparent polythene sheets. Other potential options to evaluate include cleaning the farm tool blade by repeatedly pushing it in loosened soil, scrubbing with organic materials/plant debris and biorationals. These options nevertheless require thorough investigation. The exploration of less burdensome tool sterilization options and increase in tool sterilization options could potentially lead to the full adoption of XW control packages and ultimately improve XW management. This study therefore explored different options for tool sterilization with the hope of identifying more farmer-friendly options for scaling.
Materials and Methods
This study was conducted between January 2019 and November 2020 through four rounds of experiments at the National Agricultural Research Laboratories (NARL) in Kawanda, central Uganda. The study aimed at identifying cost-effective alternative methods to household bleach and heating tools with fire for sterilizing metal surfaces of tools (e.g., machetes, knives and hoes used by banana farmers for field operations, and during XW control operations).
Exploration of Alternative Tool Sterilization Options
Xanthomonas vasicola pv. musacearum (Xvm) inoculum preparation: For each round of experimentation, a pseudostem cut from a single banana plant showing recent (i.e., less than a week old) XW characteristic symptoms was sourced from the same banana field (located at NARL) for obtaining Xvm inoculum. The transversally cut banana pseudostems were placed on a work bench in the laboratory and allowed to ooze. The fresh ooze containing Xvm was then scooped from the cut pseudostem surface into a petri dish for the subsequent procedures. The bacterial ooze used in this study were all confirmed, with the help of a polymerase chain reaction (PCR), to contain Xvm using Xvm-specific AvP1 primers that amplify genes encoding the Avirulence protein KFA14425.1 of the bacteria as described in Nakato et al. (2018).
Application of Xvm on Farm Tools
Bacterial ooze was scooped and applied gently using a paint brush on the surface of knives previously sterilized with 70% (v/v) ethanol and a flame. Caution was taken to pick and apply a uniform amount of the bacterial ooze on the surfaces of the knives. The amount of Xvm bacteria applied on each blade of the knife was approximately 3.5 × 106 CFU (colony forming units)/mL.
Assessment of Tool Sterilization Options
After allowing the bacterial ooze to set on the knives for about 30 min, three knives, each acting as a replicate were then subjected to nine different tool sterilization options, including a positive and negative control as described below.
i) Inserting tools with shear force into loosened soil or ground: knives with Xvm ooze were repeatedly inserted forcefully into the ground/ soil with shear force. It was presumed that the shear force of insertion would result in a cleaning effect on the knives.
ii) Inserting tools into hot ash: Xvm ooze laden knives were completely inserted in hot ash freshly obtained from a charcoal stove for time periods of 10, 20, 40, 60, 120, 180, and 300 s. This was to mimic a common practice of inserting knives into hot ash by farmers. It was presumed that that the hot ash would desiccate and kill the bacteria on the blades of the knives.
iii) Inserting tools in cold dry ash: Blades of knives covered with Xvm ooze were inserted, fully covering all blades in to cold dry ash for 10, 20, 40, 60, 120, 180, 300 s; 1, 2, and 6 h. It was postulated that the ash could dehydrate the bacteria, thus reducing its population on farm tools.
iv) Heating farm tools with fire: Blades of the knives covered with Xvm ooze were completely inserted into a red hot fire made out of charcoal (in a charcoal stove) for various time durations of 10, 20, 40, 60, 180, and 300 s. Heating or flaming farm tools is a common practice currently being promoted for sterilizing farm tools used on XW infected plants or fields.
v) Inserting farm tools into boiling water: Tools with Xvm were fully immersed into boiling water for time durations of 10, 20, 40, 60, 180, and 300 s. Hot water was postulated to denature the structural and physiological properties of the bacteria.
vi) Washing farm tools with common laundry soap and cold water. The common practice of washing household utensils/dishes within the East African region was used for this treatment. To attain this, the tools were submerged into 0.005 g/mL of laundry soap-water solution (prepared by dissolving 5 g of laundry soap in 1 L of sterile water) for 20 s followed by a thorough scrubbing with cotton wool soaked in the same solution for about 10 s. This amount of soap was able to form adequate amount of foam as observed while cleaning dishes within households. Thereafter the tools were rinsed twice with autoclaved sterile water and allowed to drain dry for about 5–10 min before the subsequent step of recovering bacteria from the surface of the knives. The laundry soap used in the study contained no antibiotics and was obtained from a local market in Kampala, Uganda.
vii) Solarisation of farm tools while wrapped into a black polythene sheet: The tools were wrapped into a black polythene sheet and thereafter subjected to solar radiation for time durations of 1, 2, 3, and 6 h.
viii) Solarisation of farm tools while wrapped into a transparent polythene sheet: The tools with bacterial ooze were wrapped into a transparent polythene sheet and thereafter subjected to solar radiation for time durations of 1, 2, 3, and 6 h.
ix) Leave Xvm-laden farm tools in a dry place for varying time durations: Tools with Xvm ooze were placed in a dry place for 24 h, 3 days, and 7 days before the recovery and enumeration of the bacteria.
x) Positive control: Xvm ooze laden tools assessed for Xvm presence and population without application of any treatment.
xi) Negative control: Farm tools were cleaned with 3.5% sodium hypochlorite (NaOCl) solution that has been proven to eliminate the bacteria from farm tools.
Recovery and Enumeration of Xvm
To recover bacteria from the knives subjected to the above treatments, the surface of each knife was washed with 10 mL of double distilled water under a laminar flow hood. A clean sterile paint brush was used to repeatedly swab the surface of the knives followed by repeated rinsing to increase the chance of dislodging all bacteria from the surface of the knives. Each suspension was thoroughly mixed through repeated pipetting and serially diluted to 10−2. From two dilutions (100, and 10−2), 10 μL of the suspension was plated in two replicates (resulting in a total of four replicates) on Petri plates containing a non-selective media of yeast peptone glucose agar (YPGA, containing per liter of distilled water: 5 g of yeast, 5 g of peptone, 10 g of glucose, and 15 g of agar; Schaad et al., 2001). The plates were then incubated for a period of 72 h at 28°C, the presence/absence of Xvm noted, and where present the number of Xvm colonies counted. Xvm CFU per mL of the original suspension was then computed for each replicate Petri plate as below.
Assessment of a Range of Laundry Soaps and Detergents
Due to the observed effectiveness of the laundry soap in the above trial, eight brands of laundry/washing soaps (without antibiotics) and six brands of powder detergents were assessed for their effectiveness in eliminating Xvm from farm tools. All the laundry soaps and detergents were procured from the local market in Kampala, Uganda and are all readily available brands. The laundry soaps included a locally made crude soap [a mixture of different soap salts, water, liberated glycerol (glycerine) and surplus fat or alkali (Chukwulozie et al., 2014)]. All the laundry soaps and detergents were coded, and a 0.005 g/mL laundry soap-water or detergent-water solution prepared from each laundry soap or detergent as described above. The other steps of the experiment i.e., inoculum preparation, application of Xvm ooze on tools and tool sterilization by washing with soap and cold water were undertaken as for the laundry soap in the section above. The laundry soap used in the section above and household bleach served as negative controls while washing tools with cold water only acted as the positive control. The recovery and enumeration of Xvm on tools were conducted as described in the above section. This experiment was repeated four times with three knives used per soap and detergent type in each repeat experiment.
Survival of Xvm in a Soap or a Detergent Solution
This experiment sought to determine if the effect of using laundry soap was either through its washing effect only or in combination with killing of the pathogen.
Inoculum Preparation
Yellow Xvm characteristic ooze from a single banana plant showing recent (i.e., less than a week old) XW characteristic symptoms from the same field described above was harvested into sterile falcon tubes, and thoroughly vortexed for about 3 min to weaken the gum structure of Xanthan (a polysaccharide that protects Xvm) and to separate bacterial cells. Subsequently, the Xvm suspension was diluted to an OD600 of 0.5 with sterile water for the subsequent steps.
Preparation of Soap and Detergent Solutions
Laundry soap and detergent solutions of varying concentrations (i.e., 0.0025 g/mL, 0.005, 0.01, 0.02, 0.05, 0.06, and 0.07 g/mL) were prepared by dissolving their appropriate weights into 50 mL of sterile cold water. To fasten the dissolution, the soap/detergents were regularly vortexed until full dissolution.
Experimental Setup and Renumeration of Xvm
5 mL each of the different concentrations of laundry soap-water or detergent-water solutions were then mixed with an equal volume (i.e., 5 mL) of the Xvm-water suspension in separate 15 mL falcon tubes. This resulted in halving the soap or detergent concentrations (g/mL) to 0.00125, 0.0025, 0.005, 0.01, 0.025, 0.03, and 0.035, respectively, each soap/detergent concentration acting as a treatment. Each treatment mixture was thereafter vortexed for about 3 min to break up any clogged Xvm colonies and to increase the chance for the soap/detergent molecules to interact with individual bacterium. Each treatment was then incubated at 28°C for 1 h. For the control treatment, 5 mL of the above Xvm suspension was diluted to 10 mL by addition of more sterile water, vortexed for 3 min and incubated for an hour as above. 1 mL of each treatment (soap or detergent—Xvm—water mixture) was pipetted after an hour, serially diluted to 10−2. 10 μL of the 100 and 10−2 dilutions were then plated on Petri plates containing YPGA and incubated at 28°C as described in the sections above. The experiment was repeated three times, with each repeat acting as a replicate.
Data Analysis
All data on Xvm colony counts were log10 transformed to reduce the variability within the data set. Linear mixed model analysis of the relationship between Xvm incidence (i.e., percentage of tools with Xvm) or the natural log of mean Xvm colony counts on tools and different tool sterilization options was performed using the lmer function of the lme4 package (Winter, 2013; Bates et al., 2015) and R statistical software (R Core Team, 2018). A linear mixed model was used to overcome potential errors due to presence of random effects arising from four repeat experiments performed using different pseudostem tissues and separate days. A preliminary analysis of variance using the GenStat v. 12 statistical software (VSN International Ltd., 2009) showed significant (p < 0.05) differences between the four repeat experiments. The random effect (i.e., repeat experiments or replications) was entered as a nested random effect with intercepts. The Xvm incidence (%) or natural log of colony counts served as the fixed effects. The above model(s) [here after full model(s)] and their null models (were fitted to the same data using a maximum likelihood criterion and compared using the Akaike's Information Criterion (AIC) (Sakamoto et al., 1986), the deviance of the parameter estimates and p-values. The models were considered suitable when the model fit criterions for the full models were smaller than those of the null models and the P values less than 0.05. The models were then fit with the restricted maximum likelihood criterion (REML) that is default for lmer (Bates, 2010) to obtain the random and fixed effects. The lmerTest package gave the lmer4 package an extended output using the Satterthwaite's (Kenward-Roger's) approximations for the t test and corresponding p-values (Kuznetsova et al., 2017). P-values and treatment effects were then used for comparison of fixed effects. The R statistical software and statistical packages ggplot2 (Wickham, 2016), ggpubr (Kassambara, 2018) and patchwork (Pedersen, 2017) were used for data visualization (i.e., drawing box plots).
Results
All the models not only performed better than corresponding null models (i.e., lower AIC scores for models compared to the nulls) but were also significantly different (p < 0.001) from their null models (Table 1). These models were thus subsequently used to determine the fixed effects, Xvm incidence and Xvm colony counts of the different tool sterilization options.
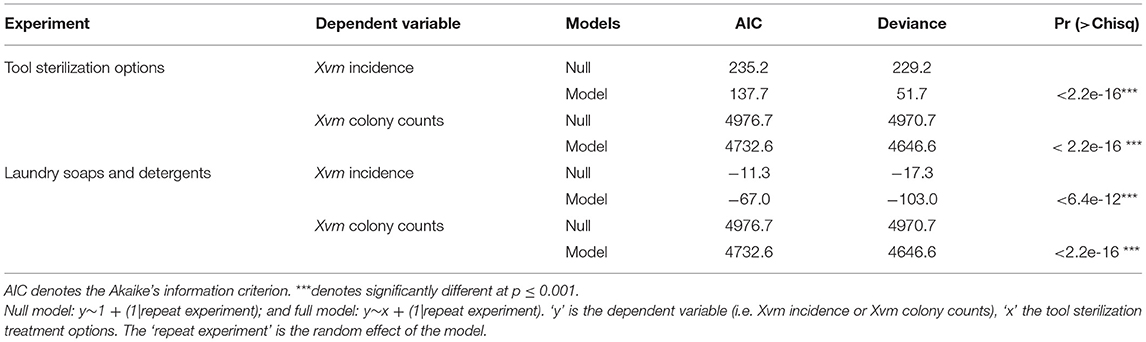
Table 1. Model fit criterions comparing the null and full models for different fixed effects assessing the efficacy of different tool sterilization options on elimination of Xanthomonas vasicola pv. musacearum (Xvm) from farm tools.
Xanthomonas vasicola pv. musacearum Incidence and Colony Forming Units (CFU) on Farm Tools Cleaned Using Different Options and Stored Over Different Time Durations
Significant differences (p < 0.001) in the fixed effects i.e. incidence of tools with Xvm (Table 2) and mean Xvm CFU on tools (Table 3) were visible between the tool sterilization options explored in this study. Similar estimates for the fixed effects (i.e., Xvm incidence on tools and CFU recovered from tools) were attained for washing tools with cold water and laundry soap; cleaning with 3.5% sodium hypochlorite (household bleach); tool insertion into boiling water for at least 40 s, 60 s, 3 min and 5 min; and tool insertion into fire for about 60 s, 3 min and 5 min (Tables 2, 3; Figures 1A,B). For these treatments, no Xvm colonies were recovered from the blade of the knives (Figures 1A,B), suggesting they are effective for tool sterilization. Heating the farm tools on fire, one of the currently recommended practices for tool sterilization however only eliminated all the bacteria on farm tools after at least 60 s of heating (Figures 1A,B). Other sterilization options explored did not eliminate all the bacteria (Table 2, Figures 1A,B), with no significant (p > 0.05) differences in the incidence of tools with Xvm visible between the negative control (household bleach) and tool insertion into hot ash for at least 3–5 min, and repeated tool insertion into soil with shear force (Figure 1A, Table 2). In hot ash, Xvm incidence on tools declined with increasing time, suggesting a longer time of tool exposure to hot ash can potentially eliminate all the bacteria. Xvm was recovered from all the farm tools stored in a dry place for up to 7 days. Immersion of tools into boiling water for 10–20 s, using transparent polythene sheets, using shear force to insert tools repeatedly into the soil and only washing with clean water resulted in moderate estimates in the mean number of Xvm CFU per mL (between 1.3 and 4.9) (Table 3, Figure 1B). In contrast, most of the hot and cold ash treatments, storing the tools for 1–7 days had large estimates of mean Xvm CFU per mL and did not differ from the untreated control (Table 3, Figure 1B). These results suggest that, though not effective practices such as solarization, hot and cold ash treatment, forceful repeated insertion into the soil and washing with plain cold water were still able to reduce the bacterial load on the tools.
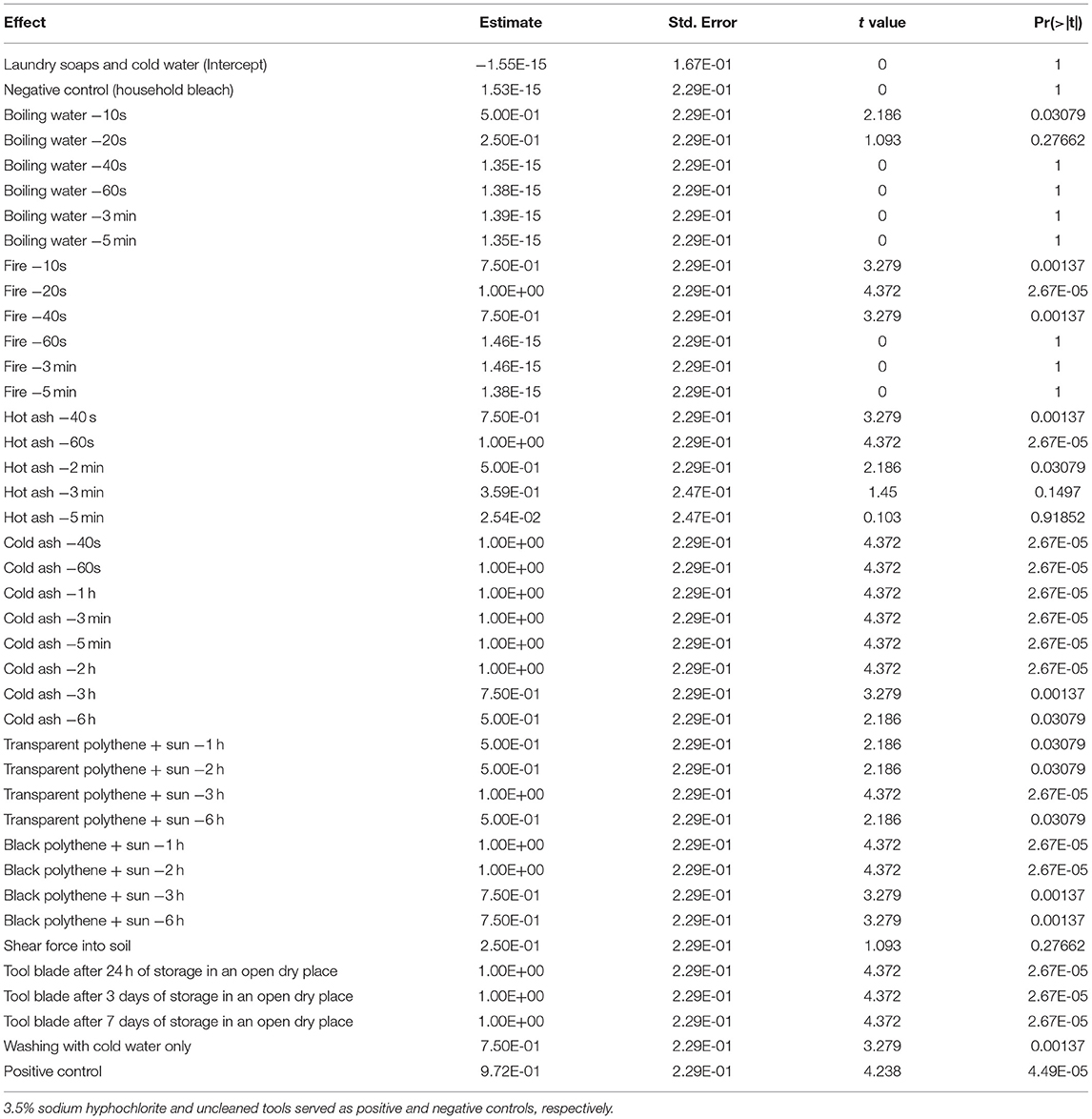
Table 2. Fixed effects of different methods of tool sterilization on the percentage (%) of tool blades with Xanthomonas vasicola pv. musacearum.
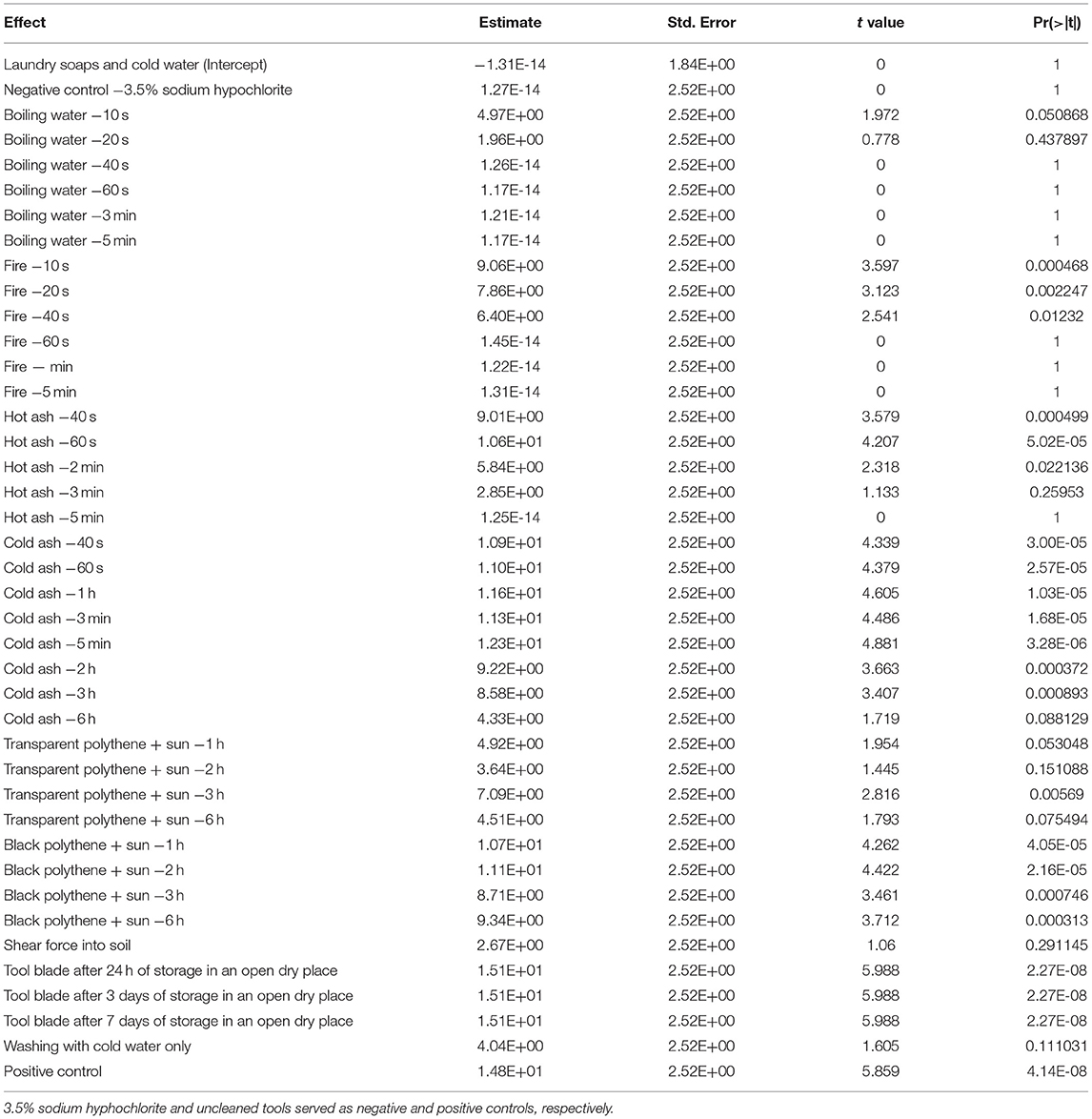
Table 3. Fixed effects of different methods of tool sterilization on the colony forming units of Xanthomonas vasicola pv. musacearum (Xvm) per mL of a suspension obtained from the surface of Xvm-contaminated farm tools.
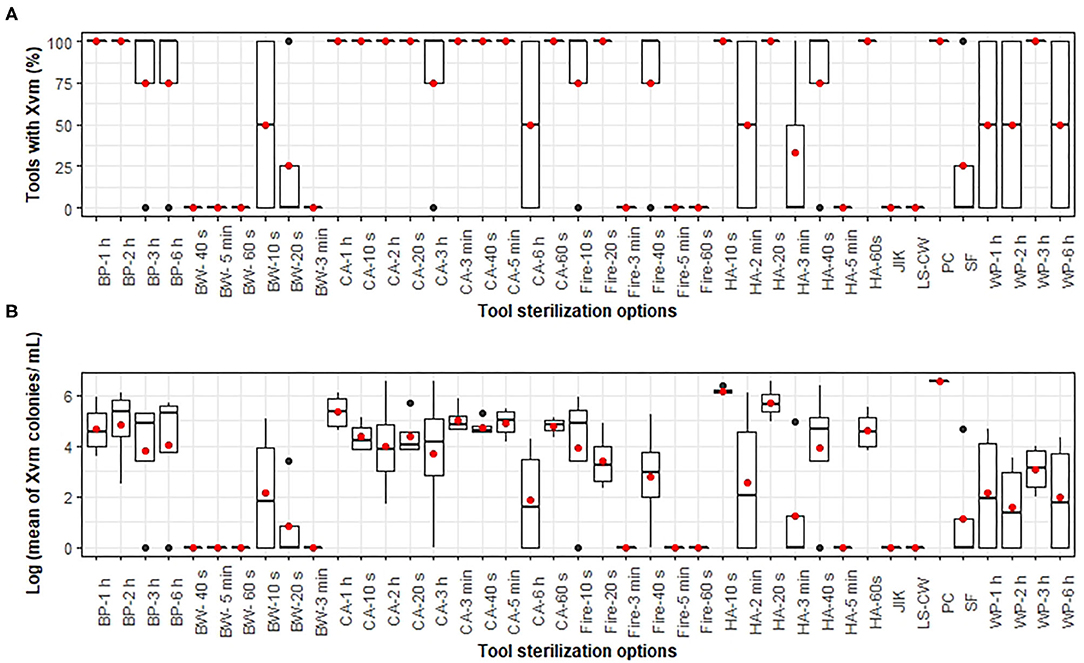
Figure 1. The (A) percentage of tools with Xanthomonas vasicola pv. musacearum (Xvm) and (B) log10 of the mean number of Xvm colonies per mL recovered from contaminated farm tools following cleaning with different methods or storage. 3.5% sodium hypochlorite (Household bleach) served as the negative control or best cleaning method whereas uncleaned freshly contaminated tool blades served as the positive control (PC). BP, BW, CA, HA, LS-CW, SF, and WP, respectively denote black polythene sheet, boiling water, cold ash, hot ash, laundry soap, and cold water, repeatedly inserting tool with shear force into the soil, and white polythene sheet. The horizontal “black lines” and “red points” within the boxes are, respectively, the median and mean values of the percentage of tools with Xvm or Xvm colony counts. The lower and upper boundaries of the boxes are, respectively, the 25th and 75th percentile; the bars/whiskers below, above the box are the 10th and 90th percentile and points beyond the 25th and 75th percentiles are outliers.
Soap and Detergent Options
Different laundry soaps and detergents were examined for their efficacy in eliminating Xvm from farm tools. All the seven laundry soaps (including one crude soap) and the six detergents used with cold water to wash contaminated tools eliminated all the bacteria on the tools and were thus as effective as household bleach and the control laundry soap used in the study above in eliminating Xvm (Table 4). In contrast, a significantly higher number of Xvm CFU (i.e., 1,239 CFU/mL; p < 0.01) and treatment effect (0.75; p < 0.001; Table 4) was observed in the positive control treatment in which tools were washed with cold water only. This suggests that the available laundry soaps and detergents in the [Ugandan] market can potentially eliminate the bacteria from farm tools.
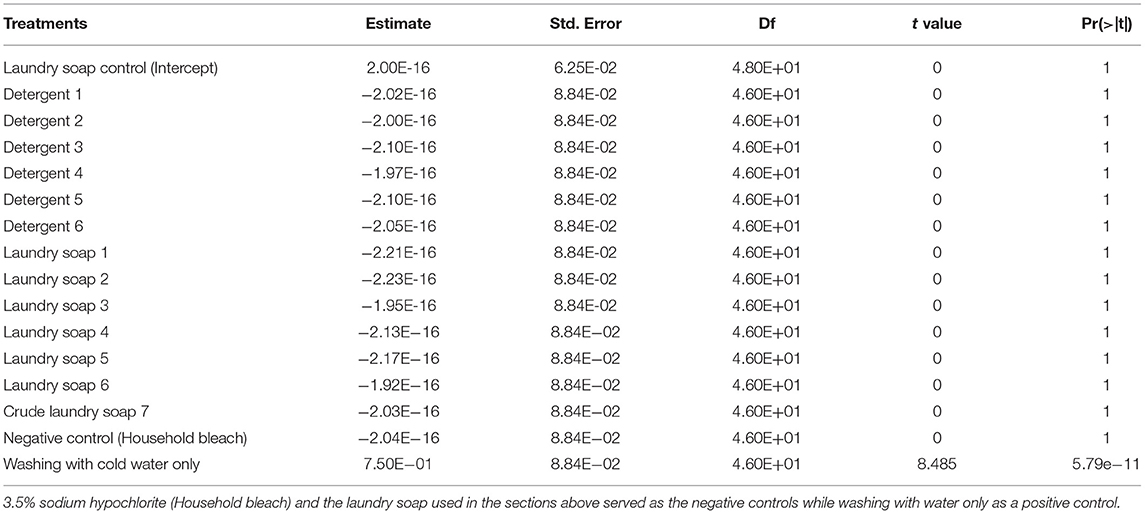
Table 4. Fixed effects of different laundry soaps and detergents obtained from within Kampala, Uganda on the incidence of farm tools with Xanthomonas vasicola pv. musacearum.
The Survival of Xvm in Soap and Detergent Solutions
Both the detergent and soap solution were observed to kill Xvm compared to the positive control (water-Xvm suspension, PC) that had a significantly higher (p < 0.05) Xvm colony count after an hour's incubation (Figure 2). For the same concentration (g/mL), the detergent solution was observed to have a higher impact on the bacteria compared to the soap solution (Figure 2). For the detergent, Xvm characteristic colonies were recovered from the 0.00125 g/mL detergent solution while no colonies were recovered from the detergent solution with a concentration varying between 0.0025 and 0.035 g/mL. In contrast, Xvm like colonies grew in the 0.00125 and 0.0025 g/mL of laundry soap-water-Xvm mixture, with no growth observed in the soap concentrations varying between 0.05 and 0.035 g/mL (Figure 2).
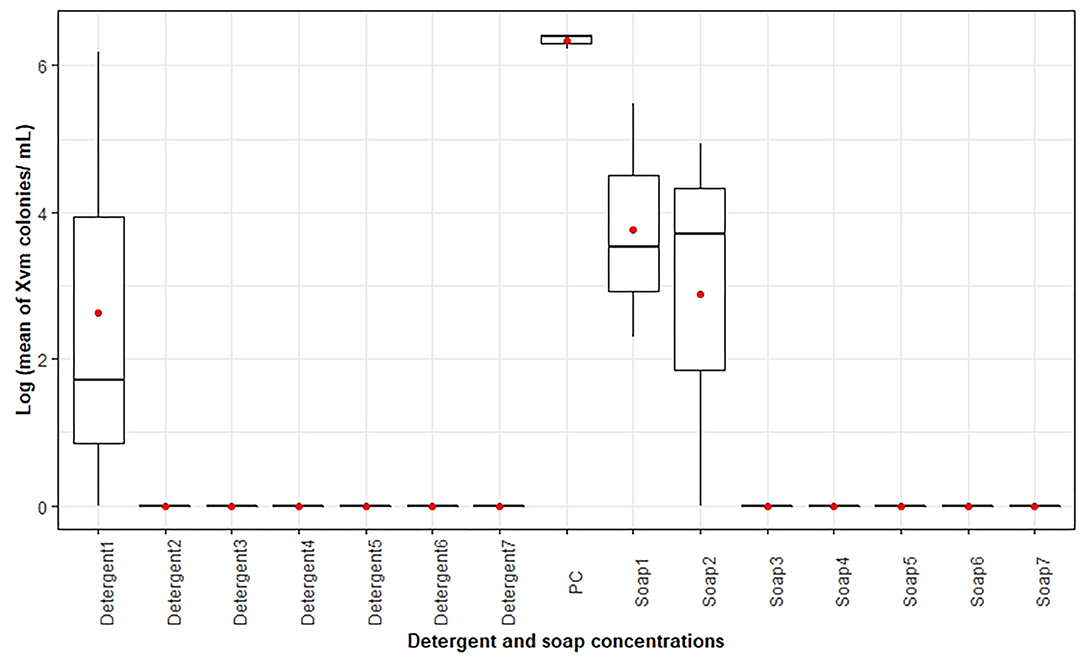
Figure 2. Log10 of the mean Xanthomonas vasicola pv. musacearum (Xvm) colony counts per mL recovered according to detergent and laundry soap concentration. One to seven (on the horizontal axis), respectively, stand for detergent and soap concentrations of 0.00125, 0.0025, 0.005, 0.01, 0.025, 0.03, and 0.035 g/mL. PC denotes the positive control treatment in which Xvm was only suspended in sterile water. The horizontal black lines within the boxes are the median values while the “red circle” is the mean value of the Xvm colony counts. The lower and upper boundaries of the boxes are respectively, the 25th and 75th percentile; the bars/whiskers below and above the box are the 10th and 90th percentile.
Discussion
Sanitation through removing diseased and asymptomatic infected plant tissue, as well as decontaminating tools, equipment and washing hands is one of many tactics for an effective disease management strategy (Salamanca, 2015). Decontamination of farm tools with fire and 3.5% sodium hypochlorite (household bleach) that are critical components of the field sanitation measures for the management of the XW disease in banana have been poorly adopted. This study explored a range of simple, cost effective and less cumbersome potential practices that could be promoted for the sterilization of farm tools as a measure for XW control on farm. Household bleach served as a control in this study and consistently no bacteria were recovered from tools cleaned with it. Household bleach rapidly dissociates in water to form the highly reactive hypochlorous acid which can alter the three-dimensional structure of a protein, thus killing bacteria (Ledford, 2008; Winter et al., 2008). In contrast, fire only eliminated all Xvm from the tools after at least 1 minute of exposure to fire. Dry heat from fire kills microbes through dehydration, altering membranes, denaturing proteins, and incineration (Jay et al., 2005; Talaro, 2008). However, the temperature and duration of exposure are important determinants of success (Talaro, 2008; Vu Thanh et al., 2012). For example, Xanthomonas translucens pv. pistaciae was observed to survive in wood exposed to 40–55°C for at least 60 min but killed when exposed to 60°C for at least 15 min (Vu Thanh et al., 2012). The results of this study suggest that the current advise to heat farm tools above fire for about 20–40 s (PROMUSA, 2020) may be leading to partial survival of Xvm and a continued disease spread through tools. The time of exposure to heat and effectiveness of sterilizing with fire could also potentially be influenced by the strength of the heat or flame. More still, the advice to heat the metal blade till too hot to touch or between 20 and 40 s, may be ambiguous for farmers potentially resulting in under or over-exposure to heat, explaining farmer reports of tool damage and linked dis-adoption of the practice. Repeated 1-min exposures of farm tools to fire can potentially damage farm tools as reported by farmers.
In this study, thorough washing of farm tools with cold water and different locally available laundry soaps that had no antibiotics and detergents, just like household bleach eliminated all the bacteria on farm tools. Soaps are the alkali salts of fatty acids and are obtained through alkaline hydrolysis of fats and oils by strong alkali/bases of sodium or potassium hydroxide (Learning Teaching Scotland, 2011). Soaps are thus composed of a hydrophobic (water hating), oil soluble (non-polar) tail and a charged carboxylate group head that is hydrophilic (attracted to water) (Talaro, 2008; Learning Teaching Scotland, 2011). To remove stains/grease off objects, the hydrophobic, non-polar, tails burrow into the greasy, non-polar molecule while the polar hydrophilic ends get attached to the polar water molecules. The polar-to-polar interactions are stronger and cause mechanical lift of the grease molecule (Learning Teaching Scotland, 2011; Talaro, 2008). In combination with water, soaps are therefore surfactants, reducing the surface tension of water and the interfacial tension between oil/grease/dirt and water. This surfactant action coupled with agitation help to pull off grease/ dirt off surfaces. This soap mechanism also potentially applied to the elimination of Xvm from the farm tools by dislodging the sticky sap of banana and bacterial ooze off farm tools. Using soap to wash hands has been reported to be more effective than using water only because the surfactants in soap lift microbes and soil off the skin, a process that further improves with thorough scrubbing of hands with soap (Luby et al., 2005, 2011; Burton et al., 2011). Burton et al. (2011) reported a 92–100% reduction in the number of different types of bacteria on hands following hand washing with plain soap and water. Similar action is anticipated from the detergents explored in the study. Detergents in addition to a surfactant (accounting for about 35% of detergents' cleaning performance) contain bleach (~27.5%), polymer (15%), builder (15%), and enzyme. Bleach enhances the appearance and effect of whiteness, polymers bind and remove certain types of dirt, builders provide the formulations (e.g., liquids, gels and tablets) while enzymes remove biological stains such as blood, coffee and wine (Learning Teaching Scotland, 2011).
Incubating Xvm in laundry soap and detergent solutions for an hour resulted in a significant decline in Xvm colony counts whereas at higher concentrations between 0.005 and 0.035 mg/mL no Xvm growth on YPGA occurred. This suggests that the cleaning effect of the soaps and detergents could be due to both Xvm being dislodged off tools and killed or demobilized. Xvm are Gram-negative bacteria and the outer membranes of Gram-negative bacteria are made of Lipopolysaccharides (LPSs) comprising of three important structural components, the O-specific polysaccharide (or O-antigen), the core region and the lipid A (the endotoxic active moiety) (Zähringer et al., 1999; Silipo et al., 2004). The lipid A layer of Xvm could have got attracted and attached to the non-polar, hydrophobic end of the soap or detergent molecules in water. With the non-polar ends of the soap molecules buried into the lipid, non-polar layers of the bacteria and the polar ends attached to the water molecules, the soap possibly perforates and corrodes the protective covering of the bacteria. This can potentially lead to loss of virulence, leaking of the internal viscera of the bacteria, desiccation and ultimately death. Baker et al. (1941) reported a range of cationic detergents to inhibit the metabolism of Gram-negative and Gram-positive microorganisms to the same degree (i.e. 74–100% inhibition), with some detergents being extremely potent and irreversibly inhibiting the metabolism of some bacterial species. In contrast, Kim and Rhee (2016) observed Gram-negative bacteria to be more susceptible to soaps than Gram-positive bacteria. Xvm are thus potentially highly susceptible to common laundry soaps and detergents.
The action of the synthetic detergents on bacterial metabolism has been reported to be influenced by several factors including the charge on ions containing the hydrophobic group; molecule's hydrophilic-hydrophobic balance; pH; chemical structure of the detergent and the characteristics of the microorganisms (Baker et al., 1941). For example, cationic detergents were more effective than the anionic ones while maximum efficiency in detergents with straight chain alkyl compounds occurred when the chains contained 12–16 carbon atoms (Baker et al., 1941). The cationic detergents were more active in the alkaline pH range while the anionic ones in the acid range (Eggerth, 1926; Baker et al., 1941). In the current study, all the laundry soaps and detergents evaluated were found to eliminate bacteria. Thus, soaps and detergents could be promoted across XW affected landscapes for the management of the disease. Soap has a wide reach and is very accessible even in the most rural and hard to reach communities. Secondly, laundry soaps serve multiple purposes including cleaning utensils, clothing and the human body in the study region, thus making it easily accessible by even the resource poor households. Apart from being accessible it is cheap and thus cost effective. However, at the time of promoting the practice, caution must be taken to understand the pH of the water sources in different communities and its effect on potential efficacy of the soaps in the elimination of the bacteria from farm tools.
Inserting farm tools in boiling water for at least 40 s also effectively removed all the bacteria off farm tools and could thus be promoted. Wet heat from boiling water has been reported to kill or inactivate bacteria, viruses, protozoa, and other pathogens through damaging structural components and denaturing proteins (Talaro, 2008; Center for Disease Control, 2009; World Health Organization, 2015; New York State Department of Health, 2018). Wet heat has been reported to be more effective in killing micro-organisms than dry heat (Jay et al., 2005; Talaro, 2008). Wet heat is reported to be effective at lower temperatures and over shorter exposure time, whereas dry heat requires moderate to high temperatures (Talaro, 2008). Jay et al. (2005) also reports heat resistance to decrease with increasing humidity, moisture, or water activity. The use of boiling water could especially be suitable for women farmers who often couple banana management work with household food preparation tasks.
Practices such as tool solarization or exposure to the sun (uncovered/in open sun or while covered with black polythene sheets), forceful and repeated insertion of tools into the soil, tool insertion in to hot or cold ash, and washing contaminated tools with plain water significantly reduced the bacterial population on farm tools compared to the un treated control. Solarization more often with a transparent polythene cover has been widely used as an environmentally friendly method to control pathogens and pests such as bacteria, fungi, insects, nematodes, mites, weeds, and weed seeds in the soil (DeVay et al., 1991; Stapleton et al., 2000). The effect of the sun on Xvm populations can be attributed to the effect of the high temperature due to the accumulated radiant sun energy captured by the polythene sheets (DeVay et al., 1991; Stapleton, 2000). Tool exposure to the sun was however profoundly affected by the changes in weather conditions e.g., cloud cover, rainy weather. The reduction in bacteria due to the repeated and forceful insertion into the soil can be attributed to the corrosive and cleaning effect of the practice.
Reduction in Xvm population on farm tools after insertion into ash could be due the high pH of ash and a possible dehydrating effect from the ash. Structural changes, more vacuolated cytoplasm and discontinuities in the membrane cells that is a sign of cell lysis has been reported in the bacterium cells after alkali stress (Luvielmo et al., 2016). Alkali stress has also been observed to lower the viscosity and weaken the gum structure of Xanthan, a polysaccharide that protects Xanthomonas campestris (Luvielmo et al., 2016). In soils, wood ash has been reported to increase pH and pore water electrical conductivity, strongly impacting soil bacterial population and community composition (Augusto et al., 2008; Kim et al., 2016; Merghache et al., 2018). Bang-Andreasen et al. (2017) observed a strong decline in bacterial richness and diversity in soil with increasing application of wood ash. Antibacterial activity of ash has also been reported, though with a higher sensitivity reported in Gram-positive bacteria than the Gram-negative bacteria (Merghache et al., 2018). Though not explored in this study, like soap, lye (mixture of ash with clean water allowed to rest for at least 15 days) is also reported to be used as a cleansing and disinfecting agent (Howard et al., 2002) and has been reported to be as effective as soap in removing bacteria (Anuradha et al., 1999; Laskar et al., 2005; Baker et al., 2014).
In the case of hot ash, death of the bacteria could have been hastened by heat. The number of bacteria significantly decline in the 5-min hot ash treatment suggesting a prolonged exposure could eliminate all bacteria. However, it is not clear how a prolonged and repeated exposure of the tools to hot ash would impact the blades of the tools. Washing with plain water also dislodged as significant number of bacteria. Nevertheless, the ability of tools to still transmit the bacteria after cleaning with these less-effective practices (cold and hot ash, water only, insertion into soil) remains high though not investigated in this study. These practices could however be potentially used in combination with some of the effective measures such as tool insertion into hot water, heating of the tool with fire and washing with soap.
Storing the tools in an open dry place over a period of up to 7 days did not affect the population of Xvm bacteria on the tools. Maina and Muthoni (2008) had a similar observation but over a shorter period of 72 h. Weekly monitoring and cutting of diseased banana stems/plants has been recommended for a more effective control of XW (Blomme et al., 2017b). The finding on storing tools in open air suggests that transmission will still be feasible if the farm tools are not sterilized over this week-long period, though the transmission efficiency was not determined in this study. Maina and Muthoni (2008), reported that the pathogenicity of the bacteria was not affected after the tools were stored for 72 h in a dry place. The survival of Xvm on farm tools for a long period of time can be attributed to the presence of the protective bacterial exopolysaccharide (EPS), Xanthan. Xanthan is produced by the bacterium species Xanthomonas campestris (Sutherland, 1993). It is a slimy and gummy in texture, and yellow bacterial exudate consisting of ring-shaped sugar molecules put together into a very stable configuration (Sutherland, 1993; Yoquinto, 2013). Xanthan offers several biological functions to the bacterium including protection from heat or temperature changes, ultraviolet rays, dryness, chemical products, destructive enzymes and adhesion on to inert surfaces (Jenkins and Starr, 1982; Sutherland, 1993; López et al., 1999; Yoquinto, 2013). Brominated arylpolyene (xanthomonadin) pigments produced by Xanthomonas campestris have been shown to protect bacteria from photo-biological destruction (Jenkins and Starr, 1982).
Conclusion
Washing metallic farm tools (machetes, knives, and hoes) contaminated with Xvm with cold water and laundry soaps or detergents was as effective as 3.5% sodium hypochlorite in the elimination of the bacteria. We thus recommend thorough scrubbing of farm tools with soap or detergents followed by rinsing with water as an additional measure for tool sterilization. This study revealed that heating tools, a currently widely promoted practice, eliminated all the bacteria after 1 min of exposure. Thus, the promotion of fire as a tool sterilization measure must incorporate this time dimension, though lengthening time will, as reported by farmers, continue to result in damaging of the metal tool blades. Despite the negative impact of fire, resource-endowed farmers could continue to use the practice on their farms. Tool immersion into boiling water was also found to be an effective alternative measure, as it eliminated all bacteria after 40 s of exposure. We recommend the inclusion of thorough washing of tools with soap and cold water and the immersion of tools into boiling water for at least 1 min into the current pool (i.e., use of sodium hypochlorite and heating tools on fire) of tool sterilization measures. The new expanded list of sterilization options will offer farmers a wider set of options to select from for managing the disease. These practices are potentially also useful for management of XW in the enset (Ensete ventricosum) systems. Other practices such as tool insertion into cold and hot ash for up to 5 min, washing with plain water, forceful and repeated insertion into the soil did not eliminate all bacteria, and are thus not recommended. Studies to explore the effect of long storage time in cold and hot ash on bacterial survival and tool damage are recommended.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
WO and GB conceived and developed the research concept, contributed to data and result interpretation, writing, and editing the manuscript. WO conducted laboratory experiments and analyzed the data. AFT contributed to laboratory experimentation and manuscript editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by funds from the Belgium Development Cooperation to CIALCA and the CGIAR Fund Donors (http://www.cgiar.org/about-us/our-funders/) through the CGIAR Research Program on Roots, Tubers and Bananas.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful for the support of CIALCA and the CGIAR Research Program on Roots, Tubers and Bananas. We also acknowledge Ms. Florence Nakamanya, Ms. Dorcus Nassazi, and Ms. Leniru Salome for their assistance with laboratory work.
References
Anuradha, P., Devi, P. Y., and Prakash, M. S. (1999). Effect of handwashing agents on bacterial contamination. Indian J. Pediatr. 66, 7–10. doi: 10.1007/BF02752341
Asfaw, A., and Admassie, A. (2004). The role of education on the adoption of chemical fertiliser under different socioeconomic environments in Ethiopia. Agr. Econ. 30, 215–228. doi: 10.1111/j.1574-0862.2004.tb00190.x
Augusto, L., Bakker, M. R., and Meredieu, C. (2008). Wood ash applications to temperate forest ecosystems—potential benefits and drawbacks. Plant Soil 306, 181–198. doi: 10.1007/s11104-008-9570-z
Baker, K. K., Farzana, F. D., Ferdous, F., Ahmed, S., Das, S. K., Faruque, A. S. G., et al. (2014). Association between moderate-to-severe diarrhea in young children in the global enteric multicenter study (GEMS) and types of handwashing materials used by caretakers in Mirzapur, Bangladesh. Am. J. Trop. Med. Hyg. 91, 181–189. doi: 10.4269/ajtmh.13-0509
Baker, Z., Harrison, R. W., and Miller, B. F. (1941). Action of synthetic detergents on the metabolism of bacteria. J. Exp. Med. 73:249. doi: 10.1084/jem.73.2.249
Bang-Andreasen, T., Nielsen, J. T., Voriskova, J., Heise, J., Rønn, R., Kjøller, R., et al. (2017). Wood ash induced pH changes strongly affect soil bacterial numbers and community composition. Front. Microbiol. 8:1400. doi: 10.3389/fmicb.2017.01400
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Biruma, M., Pillay, M., Tripathi, L., Blomme, G., Abele, S., Mwangi, M., et al. (2007). Banana Xanthomonas wilt: a review of the disease, management strategies and future research directions. Afr. J. Biotechnol. 6, 953–962. doi: 10.4314/AJB.V6I8.56989
Blomme, G., Dita, M., Jacobsen, K. S., Pérez Vicente, L., Molina, A., Ocimati, W., et al. (2017a). Bacterial diseases of bananas and enset: current state of knowledge and integrated approaches toward sustainable management. Front. Plant Sci. 8:1290. doi: 10.3389/fpls.2017.01290
Blomme, G., Jacobsen, K., Ocimati, W., Beed, F., Ntamwira, J., Sivirihauma, C., et al. (2014). Fine-tuning banana Xanthomonas wilt control options over the past decade in East and Central Africa. Eur. J. Plant Pathol. 139, 271–287. doi: 10.1007/s10658-014-0402-0
Blomme, G., Ocimati, W., Sivirihauma, C., Lusenge, V., Bumba, M., Kamira, M., et al. (2017b). A control package revolving around the removal of single diseased banana stems is effective for the restoration of Xanthomonas wilt infected fields. Eur. J. Plant Pathol. 149, 385–400. doi: 10.1007/s10658-017-1189-6
Blomme, G., Ocimati, W., Sivirihauma, C., Lusenge, V., Bumba, M., and Ntamwira, J. (2019). Controlling Xanthomonas wilt of banana: Influence of collective application, frequency of application, and social factors on the effectiveness of the Single Diseased Stem Removal technique in eastern Democratic Republic of Congo. Crop Prot. 118, 79–88. doi: 10.1016/j.cropro.2018.12.015
Bozeman, B. (2000). Technology transfer and public policy: a review of research and theory. Res. Policy, 29, 627–655. doi: 10.1016/S0048-7333(99)00093-1
Burton, M., Cobb, E., Donachie, P., Judah, G., Curtis, V., and Schmidt, W. P. (2011). The effect of handwashing with water or soap on bacterial contamination of hands. Int. J. Environ. Res. Public Health 8, 97–104. doi: 10.3390/ijerph8010097
Center for Disease Control Prevention. (2009). A Guide to Drinking Water Treatment and Sanitation for Backcountry and Travel Use. Available online at: http://www.cdc.gov/healthywater/drinking/travel/backcountry_water_treatment.html (accessed April 2020).
Chukwulozie, P. O., Chukwuemeka, D. E., Chinwe, O. I., and Jude, E. S. (2014). Optimization of a soap production mix using response surface modelling: A case of Niger bar soap manufacturing industry Onitsha, Anambra State, Nigeria. Int. J. Sci. Technol. Res. 3, 346–352. Available online at: http://www.ijstr.org/final-print/sep2014/Optimization-Of-A-Soap-Production-Mix-Using-Response-Surface-Modeling-A-Case-Of-Niger-Bar-Soap-Manufacturing-Industry-Onitsha-Anambra-State-Nigeria.pdf (accessed March 26, 2021).
DeVay, J. E., Stapleton, J. J., and Elmore, C. L. (1991). Soil Solarization. Plant Production and Protection Paper 109. Rome: FAO/UN. p. 396.
Eggerth, A. H. (1926). The effect of the pH on the germicidal action of soaps. J. Gen. Physiol, 10, 147–160. doi: 10.1085/jgp.10.1.147
FAO (2020). Crops. Available online at: http://www.fao.org/faostat/en/#data/QC (accessed November 15, 2020).
Howard, G., Bogh, C., Goldstein, G., Morgan, J., Pruess, A., Shaw, et al. (2002). Healthy villages: a Guide for Communities and Community Health Workers. Chapter 8: Personal, Domestic and Community Hygiene. WHO. Available online at: http://www.who.int/water_sanitation_health/hygiene/settings/hvchap8.pdf (accessed March 1, 2021).
Jay, J. M., Loessner, M. J., and Golden, D. A. (2005). Modern Food Microbiology, 7th Edn. New York, NY: Springer Scientific and Business Media, Inc.
Jenkins, C. L., and Starr, M. P. (1982). The brominated arylpolyene (xanthomonadin) pigments of Xanthomonas juglandis protect against photobiological damage. Curr. Microbiol. 7, 323–326. doi: 10.1007/BF01566872
Kalyebara, M. R., Ragama, P. E., Kikulwe, E., Bagamba, F., Nankinga, K. C., and Tushemereirwe, W. K. (2006). Economic importance of the banana bacterial wilt in Uganda. Afri. Crop Sci. J. 14, 93–103. doi: 10.4314/acsj.v14i2.27915
Karamura, E. B., Turyagyenda, F. L., Tinzara, W., Blomme, G., Ssekiwoko, F., Eden-Green, S., and Markham, R. (2008). “Xanthomonas wilt of bananas in East and Central Africa,” in Diagnostic and Management Guide. Bioversity International, Uganda. INIBAP ISBN, 978–982.
Kassambara, A. (2018). ggpubr:' ggplot2' Based Publication Ready Plots. R Package Version 0.2.999. Available online at: http://www.sthda.com/english/rpkgs/ggpubr (accessed February 28, 2020).
Kikulwe, E. M., Kyanjo, J. L., Kato, E., Ssali, R. T., and Erima, R. (2019). Management of banana Xanthomonas wilt: evidence from impact of adoption of cultural control practices in Uganda. Sustainable 11:2610. doi: 10.3390/su11092610
Kim, J. M., Roh, A. S., Choi, S. C., Kim, E. J., Choi, M. T., Ahn, B. K., et al. (2016). Soil pH and electrical conductivity are key edaphic factors shaping bacterial communities of greenhouse soils in Korea. J. Microbiol. 54, 838–845. doi: 10.1007/s12275-016-6526-5
Kim, S. A., and Rhee, M. S. (2016). Microbicidal effects of plain soap vs triclocarban-based antibacterial soap. J. Hosp. Infect. 94, 276–280. doi: 10.1016/j.jhin.2016.07.010
Kuznetsova, A., Brockho, P. B., and Christensen, R. H. B. (2017). lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26. doi: 10.18637/jss.v082.i13
Laskar, M. S., Mahbub, M. H., and Harada, N. (2005). Effect of handwashing on child health. Lancet 366:893. doi: 10.1016/S0140-6736(05)67314-X
Learning Teaching Scotland (2011). Chemistry: Soaps and Emulsions. Available online at: http://www.new.chemistry-teaching-resources.com/Resources/Higher/LTS/SoapsAndEmulsions%20(H)_tcm4-664888.pdf (accessed April 13, 2020).
López, N. I., Haedo, A. S., and Méndez, B. S. (1999). Evaluation of Xanthomonas campestris survival in a soil microcosm system. Intern. Microbiol. 2, 111–114.
Luby, S. P., Agboatwalla, M., Feikin, D. R., Painter, J., Billhimer, W., Altaf, A., et al. (2005). Effect of handwashing on child health: a randomised controlled trial. Lancet 366, 225–233. doi: 10.1016/S0140-6736(05)66912-7
Luby, S. P., Halder, A. K., Huda, T., Unicomb, L., and Johnston, R. B. (2011). The effect of handwashing at recommended times with water alone and with soap on child diarrhoea in rural Bangladesh: an observational study. PLoS Med. 8:1001052. doi: 10.1371/journal.pmed.1001052
Luvielmo, M. D. M., Borges, C. D., Toyama, D. D. O., Vendruscolo, C. T., and Scamparini, A. R. P. (2016). Structure of xanthan gum and cell ultrastructure at different times of alkali stress. Braz. J. Microbiol. 47, 102–109. doi: 10.1016/j.bjm.2015.11.006
Maina, M., and Muthoni, S. (2008). Survival of Xanthomonas vasicola pv. musacearum on metallic tools. J. Appl. Biosci. 3, 92–94. Available online at: http://www.m.elewa.org/JABS/2008/3/4.pdf (accessed March 26, 2021).
Merghache, D., Boucherit-Otmani, Z., El Haci, I. A., Chikhi, I., and Boucherit, K. (2018). Inhibitory Effect of Quercus ilex wood ash on the growth of pathogenic microorganisms. Phytothérapie 16, S269–S272. doi: 10.3166/phyto-2018-0071
Nakato, G. V., Wicker, E., Coutinho, T. A., Mahuku, G., and Studholme, D. J. (2018). A highly specific tool for identification of Xanthomonas vasicola pv. musacearum based on five Xvm-specific coding sequences. Heliyon 4:e01080. doi: 10.1016/j.heliyon.2018.e01080
New York State Department of Health (2018). Boil Water Response—Information for the Public Health Professional. Available online at: https://www.health.ny.gov/environmental/water/drinking/boilwater/response_information_public_health_professional.htm (accessed April 14, 2020).
Nkuba, J., Tinzaara, W., Night, G., Niko, N., Jogo, W., Ndyetabula, I., et al. (2015). Adverse impact of Banana Xanthomonas wilt on farmers livelihoods in Eastern and Central Africa. Afri. J. Plant Sci. 9, 279–86. doi: 10.5897/AJPS2015.1292
Ntamwira, J., Blomme, G., Bahati, L., and Ocimati, W. (2019). Effect of timing of diseased plant cutting, altitude and banana cultivar on efficacy of singly removing Xanthomonas wilt infected banana plants. Eur. J. Plant Pathol. 1–13. doi: 10.1007/s10658-019-01671-9
Ocimati, W., Bouwmeester, H., Groot, J. C. J., Tittonell, P., Brown, D., and Blomme, G. (2019). The risk posed by Xanthomonas wilt disease of banana: mapping of disease hotspots, fronts and vulnerable landscapes. PLoS ONE 14:e0213691. doi: 10.1371/journal.pone.0213691
Pedersen, T. L. (2017). Patchwork: The Composer of Ggplots. R Package Version 0.0.1. Available online at: https://github.com/thomasp85/patchwork (accessed February 28, 2020).
PROMUSA (2020). Xanthomonas Wilt of Banana. Musapedia, the Banana Knowledge Compendium. Available online at: http://www.promusa.org/Xanthomonas+wilt (accessed April 19, 2020).
R Core Team (2018). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at: https://www.R-project.org/ (accessed February 28, 2020).
Sakamoto, Y., Ishiguro, M., and Kitagawa, G. (1986). Akaike Information Criterion Statistics. D. Reidel: Dordrecht, p. 81.
Salamanca, L. R. (2015). Sanitation Is Critical to Prevent Plant Diseases Part 2: Field Sanitation, Michigan State University. Available online at: https://www.canr.msu.edu/news/sanitation_is_critical_to_prevent_plant_diseases_part_2_field_sanitation (accessed April 18, 2020).
Schaad, N. W., Jones, J. B., and Chun, W. (2001). Laboratory Guide for Identification of Plant Pathogenic Bacteria, 3rd Edn. St. Paul, MN: APS Press.
Silipo, A., Molinaro, A., Lanzetta, R., Parrilli, M., Lindner, B., and Holst, O. (2004). The structures of the lipid a moieties from the lipopolysaccharides of two phytopathogenic bacteria, Xanthomonas campestris pv. pruni and Xanthomonas fragariae. Eur. J. Org. Chem. 2004, 1336–1343. doi: 10.1002/ejoc.200300721
Somda, J., and Kamuanga, M. (2005). Characteristics and economic viability of milk production in the smallholder farming systems in The Gambia. Agric. Syst. 85, 42–58 doi: 10.1016/j.agsy.2004.07.011
Stapleton, J., Elmore, C., and DeVay, J. (2000). Solarization and biofumigation help disinfest soil. Calif. Agric. 54, 42–45. doi: 10.3733/ca.v054n06p42
Stapleton, J. J. (2000). Soil solarization in various agricultural production systems. Crop Prot. 19, 837–841. doi: 10.1016/S0261-2194(00)00111-3
Sutherland, I. W. (1993). “Xanthan,” in Xanthomonas, ed M. E. Bushell, (Dordrecht: Springer), 363–388. doi: 10.1007/978-94-011-1526-1_8
Talaro, P. K. (2008). Foundation in Microbiology, 7th Edn. New York, NY: McGraw-Hill Publishing Company. p. 928.
Tripathi, L., Mwangi, M., Abele, S., Aritua, V., Tushemereirwe, W. K., and Bandyopadhyay, R. (2009). Xanthomonas wilt: a threat to banana production in East and Central Africa. Plant Dis. 93, 440–451. doi: 10.1094/PDIS-93-5-0440
VSN International Ltd. (2009). GenStat 12th Edition. www.vsni.co.uk.
Vu Thanh, T. A., Sosnowski, M. R., Giblot-Ducray, D., Taylor, C., and Scott, E. S. (2012). Effect of burning and high temperature on survival of Xanthomonas translucens pv. pistaciae in infected pistachio branches and twigs. Plant Pathol. 61, 1082–1092. doi: 10.1111/j.1365-3059.2012.02596.x
Wickham, H. (2016). ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer-Verlag. doi: 10.1007/978-3-319-24277-4_9
Winter, B. (2013). Linear models and linear mixed effects models in R with linguistic applications. arXiv. arXiv preprint arxiv:1308.5499. Available online at http://arxiv.org/pdf/1308.5499.pdf (accessed February 28, 2020).
Winter, J., Ilbert, M., Graf, P. C. F., Özcelik, D., and Jakob, U. (2008). Bleach activates a redox-regulated chaperone by oxidative protein unfolding. Cell 135, 691–701. doi: 10.1016/j.cell.2008.09.024
World Health Organization (2015). Boil Water. Technical brief. Available online at: https://www.who.int/water_sanitation_health/dwq/Boiling_water_01_15.pdf (accessed April 18, 2020).
Yoquinto, L. (2013). The Truth About Xanthan Gum. Live Science, Future. New York. Available online at: https://www.livescience.com/36009-truth-xanthan-gum.html (accessed April 22, 2020).
Keywords: bacteria, detergent, hot water, soap, sodium hypochlorite, tool sterilization
Citation: Ocimati W, Tazuba AF and Blomme G (2021) Farmer Friendly Options for Sterilizing Farm Tools for the Control of Xanthomonas Wilt Disease of Banana. Front. Agron. 3:655824. doi: 10.3389/fagro.2021.655824
Received: 19 January 2021; Accepted: 15 March 2021;
Published: 16 April 2021.
Edited by:
Francesco Spinelli, University of Bologna, ItalyReviewed by:
Jaindra Nath Tripathi, International Institute of Tropical Agriculture (IITA), KenyaSophie Trouvelot, Université de Bourgogne, France
Copyright © 2021 Ocimati, Tazuba and Blomme. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Walter Ocimati, dy5vY2ltYXRpQGNnaWFyLm9yZw==
 Walter Ocimati
Walter Ocimati Anthony Fredrick Tazuba
Anthony Fredrick Tazuba Guy Blomme
Guy Blomme