- Weed Physiology Laboratory, Department of Crop, Soil, and Environmental Sciences, University of Arkansas, Fayetteville, AR, United States
The Echinochloa genus contains two of the world's top five worst weeds. The persistence and weediness of Echinochloa spp. are partly due to its seed longevity and variable seed dormancy. In the USA, specifically in Arkansas, multiple species infest the same field in many cases. Persistence could vary across species and dormancy affects infestation level. Studies were conducted to evaluate the seed production potential and dormancy of Echinochloa species in the State. Ninety-four accessions were characterized in a common garden in Fayetteville, Arkansas, USA. The species were Echinochloa colona (L.) Link, E. crus-galli (L.) P. Beauv, E. muricata P. Beauv and E. walteri (Pursh). Only one accession was identified as E. walteri and was excluded from data analysis. Seeds were after-ripened for 6 months at room temperature and germinated at 32°C day/23°C night with 12-h daylength. Germination was monitored for 14 d. The Echinochloa species in Arkansas were predominantly E. colona (78%). E. colona had the highest seed production and the lowest seed dormancy among species. Dormancy within each species varied greatly, especially for E. colona, with a germination capacity (GC) of 41–99%. Only 2.7% of 73 E. colona accessions were dormant. E. crus-galli had 56–79% GC; 33% of the accessions were dormant. E. muricata had 2–39% germination, with all accessions considered dormant. E. colona had the highest fecundity, with 72,973 seeds per plant. This was 2.3- and 2.6x higher than that of E. crus-galli and E. muricata, respectively. High seed production and high germination capacity must have contributed to the dominance of E. colona among other Echinochloa species. E. muricata is expected to persist longer in the soil seedbank compared to E. colona and E. crus-galli. Seed production, dormancy and longevity will affect interspecies population dynamics in response to management tactics.
Introduction
Echinochloa species are the most problematic weed in rice fields, with E. crus-galli and E. colona recorded among the ten most common weeds in vegetable farms, rice (Oryza sativa L.), and upland row crops in Arkansas including cotton (Gossypium hirsutum L.), sugarcane (Saccharum offcinarum L.), sorghum (Sorghum bicolor L.), peanut (Arachis hypogea L.) and cassava (Manihot esculenta Crantz) (Holm et al., 1991, Dowler, 1995; Ciocarlan, 2000; Norsworthy et al., 2013). These species are major global weeds in rice. It is important agronomically and economically to study the characteristics of these species under similar growing conditions since weedy Echinochloa can invade and dominate a crop field after only one season and cause significant yield losses (Ruiz-Santaella et al., 2006). Echinochloa species are prolific seed producers. E. crus-galli can produce up to 1,000,000 seeds plant−1 contingent upon environmental conditions. Seed size and weight vary across species and these traits are somewhat correlated with seed dormancy (Maun and Barrett, 1986). Studies on dormancy of Echinochloa species in the United States have been chiefly on E. crus-galli. Few studies have been conducted on the germination behavior of E. colona (Chun and Moody, 1987; Lin and Kuo, 1996; Kovach et al., 2010); one study was conducted on E. walteri; and none on E. muricata. Understanding the abundance and distribution of E. colona, E. muricata, and E. crus-galli, requires good comprehension of the dormancy behavior of these species (Bryson and Reddy, 2012). This knowledge informs long-term weed management strategies.
Echinochloa species have innate dormancy at seed maturity, as many plant species are, which is broken when the seed is separated from the mother plant. Notwithstanding, a proportion of the seeds will stay dormant for 3–7 months after maturity (Honek and Martinkova, 1996; ShengGan et al., 2007). In general, seed dormancy can be broken after prolonged after-ripening at room temperature (Finch-Savage and Leubner-Metzger, 2006). Different proportions of seeds of numerous weedy species do not germinate despite sufficient after-ripening period, showing some level of secondary dormancy. In the field, numerous non-dormant seeds are driven into secondary dormancy by unfavorable conditions. Seed dormancy in the field can be overcome by modulating soil temperature and light using synthetic or natural ground cover (Rahn, 1968; Benech-Arnold et al., 2000; Probert, 2000). Tillage can also encourage germination of weed seeds and allow for control measures of emerged seedlings prior to planting the crop.
Most research on seed dormancy focused on treatments or techniques to break dormancy. Little is known about interspecies variation in dormancy within the same genus (Finch-Savage and Leubner-Metzger, 2006). Furthermore, variation in seed germination behavior among ecotypes of the same species could be high and could impact the efficacy of weed management tactics. For example, the weedy relative of rice has a wide range of dormancy, with 84–100% germination capacity (GC) at 35°C and a much wider range at lower temperatures (Tseng et al., 2013). It is common to have more than one species in a field, which presents a problem in weed management because of variability in germination behavior (Grundy, 2003; Donohue, 2005; Finch-Savage and Leubner-Metzger, 2006).
Temperature and light are two major environmental factors that control seed dormancy. Light requirement for germination differs across species (Kovach et al., 2010), but E. colona, E. crus-galli, and E. walteri require light for germination (Maun and Barrett, 1986; Chauhan and Johnson, 2009; Kovach et al., 2010) and complete darkness induces secondary dormancy (Kovach et al., 2010). Thus, Echinochloa seeds buried deep in the soil profile where the seed can no longer perceive light will go into deep dormancy, helping build a persistent soil seedbank. Extreme soil temperature fluctuation is common in temperate regions, which also promotes secondary dormancy. Warm temperature in the summer breaks seed dormancy and allows seed emergence. The effectiveness of cultural practices, such as stale seedbed, for weed management requires knowledge of weed emergence patterns, which is a manifestation of the dormancy status and germination requirements of the seeds. Information on the inter-species variation in dormancy and fecundity would be useful for reducing soil seedbank and enhancing weed control (Vleeshouwers and Kropff, 2000; Fischer et al., 2009). Specifically, differences in emergence patterns, if known, will allow growers to adjust tillage operation and crop planting ahead of the anticipated early cohort of the weed. Growers would also know the critical weed-free period and conduct cultivation and herbicide applications at the proper time, in anticipation of further weed emergence. Knowledge of dormancy trait will inform growers or crop managers about roughly how many cropping seasons it would take to reduce the soil seedbank to a minimum. The objective of this study was to evaluate the fecundity and dormancy characteristics of Echinochloa species occurring in Arkansas, in the US Mid-south. The goal is to provide data that could improve the robustness of crop management decision models and better prediction models on how crop management practices affect the population dynamics of major weed species.
Materials and Methods
Seed Production Potential
Ninety-four (94) Echinochloa accessions were collected from rice and soybean fields in Arkansas, USA (Figure 1). Seedlings were raised in the greenhouse and transplanted at the 4-leaf stage in a common garden at 1.5 × 1.5 m spacing at the Milo Shult Agricultural Research and Extension Center, Fayetteville, AR. Transplanting occurred on July 5, 2012 and June 28, 2013. In 2012, the whole field was sprayed with a mixture of glyphosate (1.12 kg ae ha−1) and S-metolachlor (1.79 kg ai ha−1) 7 d before transplanting to control other weeds. The field was irrigated when needed and kept weed-free by hoeing other weeds. In 2013, a mixture of pendimethalin (1.0 kg ai ha−1) and S-metolachlor (1.0 kg ai ha−1) was sprayed 7 d after transplanting. Nitrogen fertilizer (60 kg N ha−1) was applied on the day of transplanting by manually incorporating the fertilizer around the base of each plant. Insecticide (imidacloprid, 0.22% granule) was applied at 0.20 kg ai ha−1 to control wireworms. Each accession had four biological replications. Mature panicles were harvested by hand and air-dried for 7 d. A subset of 10 panicles per plant were threshed and cleaned to remove sterile florets. The seeds from these 10 panicles were weighed and the weight of 500 seeds was recorded. The remaining panicles were also threshed and the weight of the seeds was recorded. The total number of seeds plant−1 was estimated based on the total seed weight of the representative panicles harvested, multiplied by the total panicles plant−1.
Figure 1. Counties from where Echinochloa samples were collected in Arkansas, USA. Rice-producing states in the US (https://www.nass.usda.gov/Charts_and_Maps/Crops_County/index.php#ar).
Pre-germination Temperature Treatments to Break Seed Dormancy of Echinochloa colona
Three accessions, identified morphologically as E. colona, were selected randomly out of 94 accessions to represent different seed sizes. Seeds were stored at room temperature (about 25°C) in the dark for 180 d. Batches of 50 seeds were counted and placed in Petri plates lined with filter paper. Seeds were incubated for 7 d in four conditions: (1) 50°C; (2) 4°C; (3) −20°C; and 4) ambient temperature (25 °C) with four replications per condition. At the end of incubation period, the Petri dishes were arranged, completely randomized, in a tray and were placed in a growth chamber at 32°C day/23°C night temperature with 12-h photoperiod. Seed germination was recorded at 7, 14, and 21 d after incubation. At each germination evaluation period, the germinated seeds were removed from the Petri dish after counting.
GC (%) was calculated using the formula:
Dormancy Evaluation of Echinochloa Species
Ten (10) representative panicles harvested from each field-grown plant were air-dried in the greenhouse (36°C) for 7 d, threshed and stored at room temperature (25–28°C) for 180 d in the dark. Ninety-two accessions comprised of 72 E. colona, 9 E. crus-galli, and 10 E. muricate were used in this study. The same method for seed germination was used as in Section Pre-germination Temperature Treatments to Break Seed Dormancy of Echinochloa colona, except that for this study, the seeds were stored only at room temperature prior to germination. The germination condition was similar to the ideal germination conditions for E. colona at 30°C day/20°C night temperature cycle (Chauhan and Johnson, 2009). Seed germination was evaluated at 7 and 14 d of incubation for accessions with high GC. The observation period was extended to 21 d for large-seeded accessions (generally E. muricata) with low GC. The GC was calculated using Equation (1).
Statistical Analysis
Data collected were analyzed using JMP for Windows software Version 11.0 (SAS, 2011). To determine the effect of temperature on seed dormancy, an analysis of variance (ANOVA) was conducted on the GC data. The GC of all accessions studied in 2012 and 2013 were pooled in the absence of year effect. Cluster analysis was done on the average GC of each accession to determine statistically supported grouping of accessions based on germination.
Cluster Analysis
The accessions were grouped using the cubic clustering criterion in SAS-JMP (12.1) (Figure 2). The number of statistically distinct clusters was the point where the cubic clustering criterion reached a maximum, beyond which the curve declines with each additional number of clusters.

Figure 2. Cubic clustering criterion. The point where the cubic clustering criterion reached a maximum, beyond which the curve declines with each additional number of clusters.
Results
Seed Production Potential of Echinochloa Species in a Common Garden
E. colona produced the highest amount of seed (539 seeds panicle−1) and E. muricata produced the lowest (259 seeds panicle−1) (Table 1). Accounting for the total number of panicles, the estimated total seed production per plant was significantly different among species. E. colona produced the highest estimated number of seeds (72,973 seeds plant−1), E. crus-galli had 31,911 seeds plant−1, and E. muricata produced an estimated 27,589 seeds plant−1. The lone E. walteri accession produced 25,392 seeds plant−1, which was within the range of E. muricata seed production.
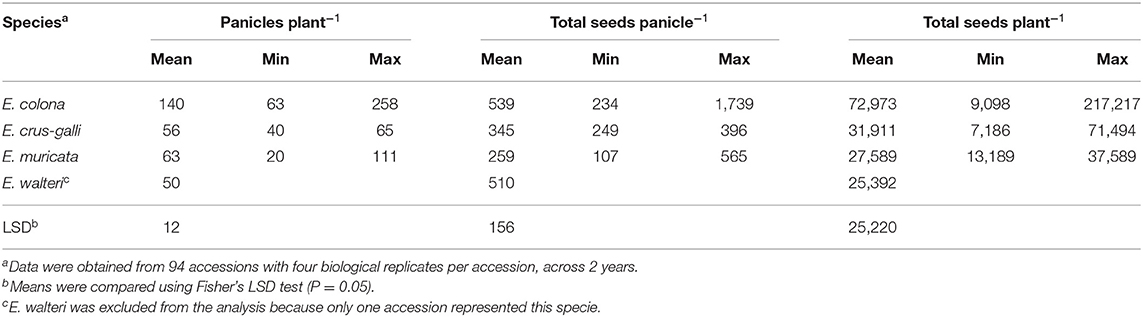
Table 1. Seed production of Echinochloa species in a common garden, averaged across accessions and years, Milo Shult Agricultural Research and Extension Center, University of Arkansas, Fayetteville, AR, USA.
Dormancy-Breaking Temperature Treatment for E. colona
In this study, seed storage at different pre-germination temperatures, from freezing (−20°C) to hot (50°C), did not have a big effect on GC (Table 2). All pre-germination incubation temperature treatments resulted in 65–77% germination at 32/23°C day/night temperature with 12-h photoperiod. Seven days of incubation at 25°C (room temperature) resulted in the highest GC, which was significantly higher only than the GC at 4°C. Incubation at 4°C for 7 d resulted in 65% germination.
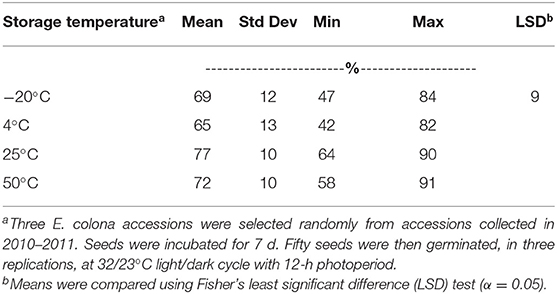
Table 2. Germination capacity of Echinochloa colona from Arkansas, averaged across accessions, after storage at various temperatures for 7 d, Altheimer Laboratory, Milo Shult Agricultural Research and Extension Center, University of Arkansas, Fayetteville, USA.
Species Differences in Seed Dormancy
Among the three species tested, E. colona had the highest average GC (77%) with a range of 41–99% (Table 3). Of the 74 E. colona accessions tested, 73% had a maximum GC of 80–100% and 47% of accessions had an average GC of at least 80% (Supplementary Table 1). Therefore, this specie is not highly dormant. The temperature used was within the optimum germination temperature for E. colona and E. crus-galli (Chauhan and Johnson, 2009). E. crus-galli had the second highest average GC (53%) with a range of 11–79% across accessions. This was at least twice as high as the GC reported previously for E. crus-galli (25.7 ± 24.9%) and inferior to var. oryzicola after 270 d of after-ripening (92.9 ± 7.3%), germinated at 30°C with 12 h photoperiod (Barrett and Wilson, 1983). While it is not possible to make direct comparisons to other studies on different species of Echinochloa, it is useful to list other germination reports to provide an overview of the germination behavior of Echinochloa in a wide range of environments. Brod (1968) reported 79–86% germination of E. crus-galli after 5 mo of storage at room temperature (Brod, 1968). All previous studies determined that 30°C is the optimum temperature for germination of E. crus-galli (Kasahara and Kinoshita, 1952; Arai and Miyahara, 1963,Brod, 1968). In the present study, E. muricata had the lowest GC (18%) with a range of 2–73% across accessions (Table 3).
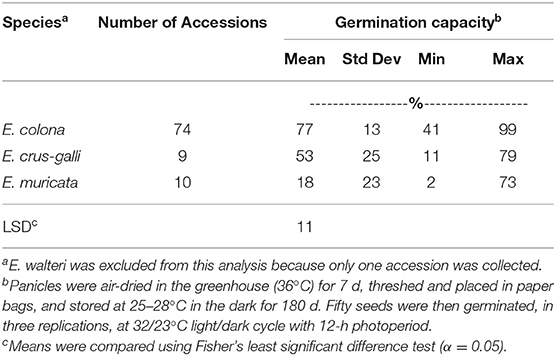
Table 3. Germination capacity of Echinochloa species from Arkansas, USA, grown in a common garden at the Milo Shult Agricultural Research and Extension Center, Fayetteville, AR, averaged by species across 2 years (2012 and 2013).
Echinochloa accessions separated into three clusters based on GC (Figure 3). Cluster 1 was the largest group with 65 accessions (i. 61 E. colona; ii. 3 E. crus-galli; iii. 1 E. muricata). The average GC of each accession in this cluster ranged from 66 to 99 % (Table 4). Cluster 1 was the least dormant group of accessions. The majority (84%) of the E. colona accessions, 33% of E. crus-galli, and 10% of E. muricata accessions were in this group. The second cluster had 18 accessions (12 E. colona, 4 E. crus-galli and 2 E. muricata) with an average GC of 52%. A marginally larger category (44%) of E. crus-galli accessions grouped in this intermediate cluster relative to Cluster 1. The third cluster had nine accessions, mainly E. muricata, with only two E. crus-galli accessions. This cluster consisted of the most dormant accessions, with an average GC of 9%. Most of the E. muricata (70%) were among the most dormant accessions.
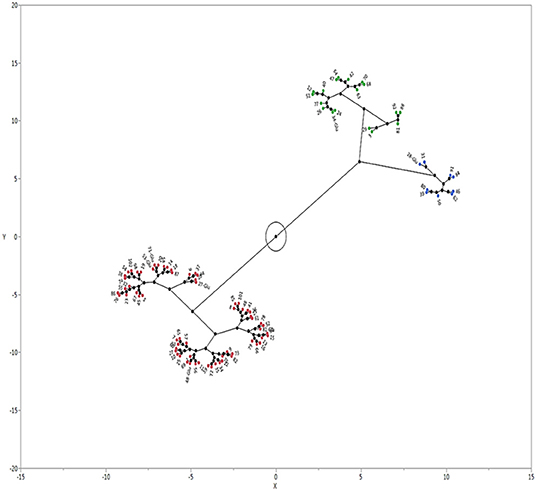
Figure 3. Castellation plot showing three clusters of Echinochloa species from Arkansas, USA based on germination capacity. The accessions were grown in a common garden in Fayetteville, Arkansas. Seeds were after-ripened for 180 d at 25°C and germinated in a growth chamber at 32°C day/23°C night temperature with 12-h photoperiod. Cluster 1 = E. colona (red); Cluster 2 = E. crus-galli (green); cluster 3 = E. muricata (blue).
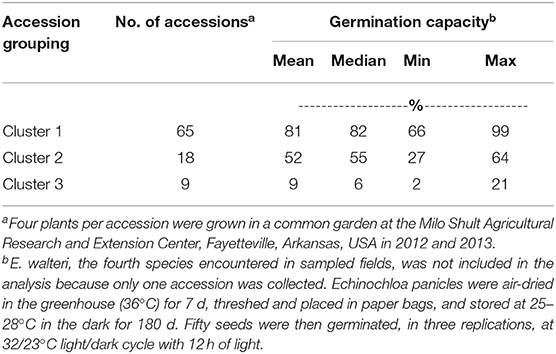
Table 4. Germination capacity of Echinochloa species E. colona, E. crus-galli, and E. muricata from Arkansas, USA, averaged across accessions in a cluster.
Intra-Species Variation in Seed Dormancy
Cluster Analysis of E. colona Germination Capacity
As indicated in the previous section, there was a large variation in the GC of E. colona, while the vast majority of accessions were in the low dormancy group. When analyzed with other species, the E. colona accessions were divided into four clusters (Figure 4). Cluster 1 (blue) had 46 accessions, with GC ranging from 74 to 92% and an average of 83% (Table 5). Cluster 2 (red) consisted of 20 accessions with a GC range of 51–71% and an average of 63%. Cluster 3 (brown) consisted of four non-dormant accessions with the highest average GC of 97%. This focused study showed that the majority (63%) of E. colona accessions had low dormancy level, with a few non-dormant accessions. This supports the overall classification of E. colona as the least dormant species in the previous section. Cluster 4 (green) consisted of the two most dormant E. colona accessions with a GC of 42%. Compared to other species, the most dormant E. colona were less dormant than most E. muricata accessions and the most dormant E. crus-galli accessions (see Section Species Differences in Seed Dormancy).
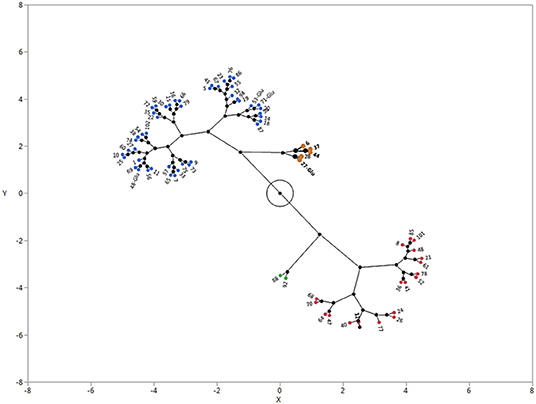
Figure 4. Castellation plot showing four clusters of E. colona from Arkansas, USA based on germination capacity. The accessions were grown in a common garden in Fayetteville, Arkansas. Seeds were after-ripened for 180 d at 25°C and germinated in a growth chamber at 32°C day/23°C night temperature with 12-h photoperiod. Cluster 1 = blue; Cluster 2 = red; cluster 3 = brown, Cluster 4 = green.

Table 5. Germination capacity of Echinochloa colona from Arkansas, USA, averaged across accessions in a cluster.
Cluster Analysis of E. crus-galli Germination Capacity
The nine E. crus-galli accessions were split into two groups (Figure 5). Two-thirds of these grouped in cluster 1, with GC ranging from 56 to 79% and an average of 68% (Table 6). One-third of the accessions were more dormant, showing an average GC of 22%, with the maximum GC of only 34%. Overall, the highest GC of E. crus-galli was 79% and the lowest was 11% when germinated at 30/20°C with 12-h photoperiod.

Figure 5. Castellation plot showing two clusters of E. crus-galli from Arkansas, USA based on germination capacity. The accessions were grown in a common garden in Fayetteville, Arkansas. Seeds were after-ripened for 180 d at 25°C and germinated in a growth chamber at 32°C day/23°C night temperature with 12-h photoperiod. Cluster 1 = green; Cluster 2 = red.

Table 6. Germination capacity of Echinochloa crus-galli in Arkansas, USA, averaged across accessions in a cluster.
Cluster Analysis of Germination Capacity of E. muricata
The E. muricata accessions separated into two clusters on the basis of germination (Figure 6). Cluster 1 had seven accessions, with a GC between 2 and 15% and an average of 6% (Table 7). The three accessions in cluster 2 had lower dormancy, with an average GC of 46%, and the highest germination being 73%. This one accession in Cluster 2 had the highest GC among E. muricata accessions. The bulk of E. muricata accessions were highly dormant. Given that this specie also has the largest seeds, E. muricata will most likely persist for the longest time in the soil compared to E. colona and E. crus-galli.

Figure 6. Castellation plot showing two clusters of E. muricata from Arkansas, USA based on germination capacity. The accessions were grown in a common garden in Fayetteville, Arkansas. Seeds were after-ripened for 180 d at 25°C and germinated in a growth chamber at 32°C day/23°C night temperature with 12-h photoperiod. Cluster 1 = Red; Cluster 2 = green.

Table 7. Germination capacity of Echinochloa muricata from Arkansas, USA, averaged across accessions in a cluster.
Discussion
Seed Production Potential
Assessment of the relative contribution of seed production to weed population dynamics, persistence, or weediness of species entails growing such plants in the same environment at the same time without competition. For example, weed-weed and weed-crop associations, the duration of interference, and season of the year alter weed seed production tremendously (Bagavathiannan et al., 2012). The seed production potential of E. crus-galli in the common garden (without competition) ranged from about 7,000 to about 72,000 per plant across 2 years. The main factors affecting this large variation are high intraspecies diversity in plant type and climate-related variability across years. The year effect cannot be overstated. E. crus-galli growing with cotton full-season, can produce 35,500 seeds plant−1 in year 1 and about half that in year 2 (Bagavathiannan et al., 2012). The crop effect may not be as large as that of year or climatic effect. In the same study, E. crus-galli growing with rice full season produced about 39,000 seeds plant−1 in year 1, very similar to that with cotton. The same principle applies to seed production of other species. Seed production data from other regions or locations generated at different times can only be examined in conjunction with information on associated plant and environmental factors. A large dataset across space and time would allow one to detect species-specific behavioral patterns, if any, and would be highly informative in formulating location-specific and broadscale management strategies.
Dormancy-Breaking Treatments for E. colona
Conducting successful research on weedy species requires the capability to obtain sufficient germination to grow enough plants for an indoor test, or establish a large enough population for a field test. This need is most acute in evaluating germination requirements, germination behavior, seed longevity, plant growth traits, or response to treatments. Sufficient after-ripening time is a primary requirement for good germination of Echinochloa (Chauhan and Johnson, 2009) as it is for the majority of plant species. Research in the Philippines revealed that optimum GC occurred after 60 d of after-ripening. Extending the after-ripening duration to 90 d did not increase the germination further (Chauhan and Johnson, 2009). Given sufficient after-ripening period, and sufficient moisture in the soil, temperature becomes the primary determinant for germination. The temperature to which the seeds are exposed prior to germination, and the duration of such exposure, largely determine whether the seed will germinate or stay dormant. Hence, we tested various seed storage temperatures to break dormancy. We learned that storage at room temperature is the best condition for E. colona. Research by Chauhan and Johnson (2009) also showed the same with incubation at room temperature (25°C) resulting in the highest germination (76%) of E. colona, which is almost identical to our findings about E. colona from Arkansas. Exposure to a wide range of pregermination temperatures has a small effect on E. colona GC, as long as the seeds are germinated under optimum conditions at some point. Extended exposure to extreme temperatures could drive the seeds into deep dormancy, while fluctuating temperatures could help break dormancy (Benech-Arnold et al., 1990; Martínez-Ghersa et al., 1997) as this weakens the hard seed coat. Storing dry seed at a constant temperature promotes secondary dormancy (Martínez-Ghersa et al., 1997), but soil temperature fluctuates diurnally and seasonally in the field. When briefly exposed to extremely high temperature, i.e., 120°C for 5 min, more than 70% of the seeds germinated; germination decreased at temperatures higher than this and the seeds were killed at 180°C (Chauhan and Johnson, 2009). That means, at least for E. colona, brief exposure to high temperatures will not reduce germination. Conditions that break dormancy can vary across species. Thus, it would be beneficial to determine such condition for each specie that contributes to the weed community composition.
Dormancy Across and Within Species
The four Echinochloa species tested vary widely in GC. E. colona was the least dormant of the species tested. About 16% of E. colona accessions had an intermediate level of dormancy (Table 4). The separation of E. colona accessions into four clusters indicates a significant dormancy level grouping. The range of GC within E. colona cluster 1 was also especially large, showing differences among accessions within the cluster. Such intra-cluster variation may not be statistically significant, but in terms of weed management to reduce the soil seed bank, a 33% difference in germination means a substantial difference in population size and the amount of possible new seed deposits in cases where the population is not managed effectively. At the species level, this large variation in seed dormancy entails long-term weed management goals.
E. crus-galli looks generally like E. colona, but with higher dormancy. E. crus-galli normally has 5–50% non-dormant seed that can germinate soon after maturation; the rest will stay dormant and remain in the soil seedbank for a long time (Honek and Martinkova, 1996). Only 39% of freshly deposited E. crus-galli seeds germinate in the field at one time; the rest germinate at various times thereafter (Kon et al., 2007; Chauhan and Johnson, 2009). The extended germination period presents a challenge to farmers in achieving effective season-long weed control. The E. crus-galli accessions from Arkansas separated into three germination categories, with almost equal proportions in Clusters 1 and 2, showing the greatest intra-species variability in dormancy among the species tested. In that sense, E. crus-galli may be the most unpredictable in terms of population response to management tactics. Barrett and Wilson (1983) reported similar findings. They tested E. crus-galli var. crus-galli accessions that have been post-ripened for 9 mo and germinated at 30°C with 12-h photoperiod for 14 d. Eleven accessions had GCs between 0 and 25%, four had 26–50%, two had 51–75%, and one had >75% (Barrett and Wilson, 1983).
E. muricata was the most dormant among the Arkansas species tested and the most heavily biased toward the opposite end of the spectrum compared to E. colona. Here it is tempting to argue that seed size is a contributing factor to seed dormancy (Barrett and Wilson, 1983). However, this hypothesis needs to be tested empirically and is best illustrated with different seed sizes of the same species. It may be comforting to a rice farmer to know that E. muricata is rarely found in the rice field; rather, it thrives in ditches and field edges (N. Roma-Burgos, observation during sample collection). It is expected to persist the longest, based on its large seed size and deep dormancy.
Of the accessions collected, only one was identified as E. walteri based on morphological traits (Hussain, 2016). Our data on this specie is, therefore, not conclusive. Nevertheless, the GC of E. walteri (49%) was consistent with what Kovach et al. (2010) and Buhler and Hoffman (1999) reported under similar germination conditions as the current experiment. They also reported that E. walteri requires alternating light and dark germination conditions, and does not germinate in total darkness. Light is therefore a crucial trigger for E. walteri germination. This means that E. walteri seed, when buried deep in the soil profile where the seed can no longer sense light, will go into deep dormancy. Light requirement, however, is species-specific; both E. colona and E. crus-galli germinate in darkness, but light increases germination (Kovach et al., 2010). In earlier studies, Buhler and Hoffman (1999) reported high dormancy of Echinochloa species and interspecies variability in GC.
Significance of Findings
Weedy Echinochloa species, specifically E. colona and E. crus-galli are among the world's worst weeds and are the primary weeds in rice production. These two species are the most common in Arkansas (U.S. Mid-south). Little is known about what drives species dominance, among which could be seed production and dormancy. The fact that E. colona produces the highest seed number (up to about 217,000 plant−1) and has the lowest dormancy implies that it would be predominant in any growing season, as was captured in the collection of samples. The difference in seed dormancy between and within species of Echinochloa is high, which means that there will be consistently high level of infestation and a persistent soil seed bank to maintain species dominance. This data set partly explains the relative species abundance in the US Mid-south. Exposure of E. colona seeds to freezing (−20°C) or high (50°C) temperature does not reduce its germination capacity. This implies that extreme winters or superhot summers are not going to reduce infestation levels. In temperate regions, we could not rely on winter kill to reduce weed population size. E. muricata is deeply dormant and thrives more in ditches and field edges rather than in the crop field, but has been observed to invade rice fields in a few cases. Growers need to be vigilant in stopping any encroachment because this specie will have a very large, persistent seedbank that can plague the crop indefinitely.
Data Availability Statement
The dataset presented in this article can be made available upon request to the corresponding author Nilda Roma-Burgos via email: bmJ1cmdvc0B1YXJrLmVkdQ==.
Author Contributions
NR-B conceptualized and designed the experiment, supervised the implementation of the experiment, wrote the manuscript with HT, revised the manuscript, and secured research funding. HT conducted the experiment, collected and analyzed data, prepared figures and tables, and wrote the first draft of the manuscript with guidance from NR-B. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by Bayer Crop Science, the Arkansas Soybean and Promotion Board, and the University of Arkansas Hatch Project #02606. Funding for the graduate scholarship of HT was provided by the Government of Malaysia and Public Service Department of Malaysia in 2012–2014.
Conflict of Interest
The authors declare that this study received initial funding from Bayer Crop Science for the collection of samples and screening for baseline response to glufosinate. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgments
The authors thank the former members of the Weed Physiology Laboratory for their assistance in various research activities indoors and in the field: Dr. Reiofeli A. Salas-Perez, Dr. Te Ming Tseng, Dr. Leopoldo Estorninos Jr., Dr. Vijay Singh, Dr. Mariccor Batoy, Shilpa Singh, Dr. Muhammad Ather Nadeem, Dr. Fernando Martini, Dr. Fernando Ramirez, Dr. Ana Carolina Roso, Dr. Caroline Bevilacqua, George Macmillan Botha, and Dr. Seth B. Abugho. Dr. Andy Mauromoustakos (Statistician) provided guidance in data analysis. Dr. Johnnie L. Gentry (Taxonomist) provided guidance in species identification.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2021.623425/full#supplementary-material
References
Arai, M., and Miyahara, M. (1963). Physiological and ecological studies on Barnyard Grass (Echinochloa crus-galli Beauv. var. oryzicola Ohwi): V. On the germination of the seed. Jap. J. Crop Sci. 31, 362–366. doi: 10.1626/jcs.31.362
Bagavathiannan, M. V., Norsworthy, J. K., Smith, K. L., and Neve, P. (2012). Seed production of barnyardgrass (Echinochloa crus-galli) in response to time of emergence in cotton and rice. J. Agric. Sci. 150, 717–714. doi: 10.1017/S0021859611000876
Barrett, S. C. H., and Wilson, B. F. (1983). Colonizing ability in the E. crus-galli complex (barnyardgrass). II. Seed biology. Can. J. Bot. 61, 556–562. doi: 10.1139/b83-063
Benech-Arnold, R. L., Ghersa, C. M., Sanchez, R. A., and Insausti, P. (1990). Temperature effects on dormancy release and germination rate in Sorghum halepense (L.) Pers. seeds: a quantitative analysis. Weed Res. 30, 81–89. doi: 10.1111/j.1365-3180.1990.tb01690.x
Benech-Arnold, R. L., Sanchez, R. A., Forcella, F., Kruk, B. C., and Ghersa, C. M. (2000). Environmental control of dormancy in weed seedbanks in soil. Field Crops Res. 67, 105–122. doi: 10.1016/S0378-4290(00)00087-3
Brod, G. (1968). Unter Suchengen zur Biologie und Okologie der Huhner-hirse E. crus-galli (L.) Beauv. Weed Res. 8, 115–127. doi: 10.1111/j.1365-3180.1968.tb01409.x
Bryson, C. T., and Reddy, K. N. (2012). “Diversity of Echinochloa in the US Mid-South,” in Abstract retrieved from Proc. 2012 Weed Science Society of America Annual Meeting (Waikoloa, HI).
Buhler, D. D., and Hoffman, M. L. (1999). Echinochloa, in Andersen's Guide to Practical Methods of Propagating Weeds and Other Plants. Lawrence, KS: Weed Science Society of America.
Chauhan, B. S., and Johnson, D. E. (2009). Seed germination ecology of junglerice (Echinochloa colona): a major weed of rice. Weed Sci. 57, 235–240. doi: 10.1614/WS-08-141.1
Chun, J. C., and Moody, K. (1987). “Ecotypic variation in Echinochloa colona,” in Abstract extracted from Proc. 11th Asian Pacific Weed Science Society Conference (Taipei: Asian Pacific Weed Science Society), 3–27.
Ciocarlan, V. (2000). “The genus Echinochloa Beauv,” in Flora ilustratǎ a României: Pteridophyta et Spermatophyta (Bucureşti: Ceres), 1141.
Donohue, K. (2005). Seeds and seasons: interpreting germination timing in the field. Seed Sci. Res. 15, 175–187. doi: 10.1079/SSR2005208
Dowler, C. C. (1995). “Weed Survey-Southern States,” in Proc. 1995 (Memphis, TN: Southern Weed Science Society), 290–325.
Finch-Savage, W. E., and Leubner-Metzger, G. (2006). Seed dormancy and the control of germination. New Phytol. 171, 501–523. doi: 10.1111/j.1469-8137.2006.01787.x
Fischer, A. J., Linquist, B., Moechnig, M., Mutters, R., Hill, J. E., Greer, C., et al. (2009). “Alternative rice stand establishment systems to manage herbicide- resistant weeds,” in Abstract extracted from Proc. Weed Science Society of America Annual Meeting (Orlando, FL).
Grundy, A. C. (2003). Predicting weed emergence: a review of approaches and future challenges. Weed Res. 43, 1–11. doi: 10.1046/j.1365-3180.2003.00317.x
Holm, L. G., Plucknett, D. L., Pancho, J. V., and Herberger, J. P. (1991). The World's Worst Weeds. Malabar, FL: Krieger.
Honek, A., and Martinkova, Z. (1996). Geographic variation in seed dormancy among populations of E. crus-galli. Oecologia 108, 419–423. doi: 10.1007/BF00333716
Hussain, T. (2016). Characterization of Echinochloa spp. in Arkansas (Master's thesis). Fayetteville, AR: University of Arkansas.
Kasahara, Y., and Kinoshita, O. (1952). Studies on the control of barnyardgrass in the paddy field. Proc. Crop Sci. Soc. Jpn. 21, 319–320. doi: 10.1626/jcs.21.319
Kon, K. F., Follas, G. B., and James, D. E. (2007). Seed dormancy and germination phenology of grass weeds and implications for their control in cereals. NZ Plant Prot. 60, 174–182. doi: 10.30843/nzpp.2007.60.4597
Kovach, D. A., Widrlechner, M. P., and Brenner, D. M. (2010). Variation in seed dormancy in Echinochloa and the development of a standard protocol for germination testing. Seed Sci. Technol. 38, 559–571. doi: 10.15258/sst.2010.38.3.04
Lin, R. J., and Kuo, W. H. J. (1996). Seasonal changes in the germinability of buried seeds of Echinochloa colonum (L.) Link. and Alopecurus aequalis Sobol. var. amurensis. Mem. Coll. Agric. 36, 233–244.
Martínez-Ghersa, M. A., Satorre, E. H., and Ghersa, C. M. (1997). Effect of soil water content and temperature on dormancy breaking and germination of three weeds. Weed Sci. 45, 791–797. doi: 10.1017/S0043174500088986
Maun, M. A., and Barrett, S. C. H. (1986). The biology of Canadian weeds. 77. E. crus-galli (L.) Beauv. Can. J. Plant Sci. 66, 739–759. doi: 10.4141/cjps86-093
Norsworthy, J. K., Bond, J., and Scott, R. C. (2013). Weed management practices and needs in Arkansas and Mississippi Rice. Weed Technol. 27, 623–630. doi: 10.1614/WT-D-12-00172.1
Probert, R. J. (2000). “The role of temperature in the regulation of seed dormancy and germination,” in Seeds: The Ecology of Regeneration in Plant Communities, ed. M. Fenner (Wallingford: CAB International), 261–292.
Rahn, E. M. (1968). Life history studies as related to weed control in the Northeast. 5. Barnyardgrass. Agric. Exp. Stat. Univ. Delaware Bull. 368, 1–46.
Ruiz-Santaella, J. P., De Prado, R., Wagner, J., Fischer, A. J., and Gerhards, R. (2006). Resistance mechanisms to cyhalofop-butyl in a biotype of Echinochloa phyllopogon (Stapf) Koss. from California. J. Plant Dis. Prot. 20, 95–100.
ShengGan, W., Qiang, W., XuePing, Z., ChangXing, W., LiPing, C., and JinLiang, S. (2007). Dormancy and dormancy breaking in barnyardgrass E. crus-galli. Acta Agric. Univ. Zhejiangensis 19, 225–228.
Tseng, T. M., Burgos, N. R., Shivrain, V. K., Alcober, E. A., and Mauromoustakos, A. (2013). Inter- and intra-population variation in dormancy of Oryza sativa (weedy red rice) and allelic variation in dormancy-linked loci. Weed Res. 53, 440–451. doi: 10.1111/wre.12044
Keywords: dormancy-breaking treatment, Echinochloa species, E. crus-galli, E. walteri, E. colona, E. muricata, seedbank, seed dormancy
Citation: Tahir H and Roma-Burgos N (2021) Fecundity and Seed Dormancy Variation Within and Among Echinochloa Species. Front. Agron. 3:623425. doi: 10.3389/fagro.2021.623425
Received: 30 October 2020; Accepted: 15 January 2021;
Published: 15 February 2021.
Edited by:
Lauren M. Lazaro, Louisiana State University Agricultural Center, United StatesCopyright © 2021 Tahir and Roma-Burgos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nilda Roma-Burgos, bmJ1cmdvc0B1YXJrLmVkdQ==
†Present address: Hussain Tahir, Plant Biosecurity Division, Department of Agriculture, Kuala Lumpur, Malaysia
 Hussain Tahir
Hussain Tahir Nilda Roma-Burgos
Nilda Roma-Burgos