
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging , 14 September 2023
Sec. Aging and the Immune System
Volume 4 - 2023 | https://doi.org/10.3389/fragi.2023.1260053
 Miodrag Vrbic1*
Miodrag Vrbic1* Ana Milinkovic2
Ana Milinkovic2Background: The immune-inflammatory response is the basis of the pathophysiology of SARS-Cov-2 infection. In severe cases of COVID-19 uncontrolled systemic inflammatory response causes multiorgan dysfunction (MODS), as the most common immediate cause of death. Unfavorable outcome of the COVID-19 most often occurs in elderly patients. The aim of the study was to establish parameters with prognostic significance in severe cases of COVID-19 according to life years, laboratory markers of sepsis and MODS, as well as the number of peripheral CD4+ and CD8+T lymphocytes in 20 consecutively selected critically ill patients.
Results: Eleven subjects were male, 9 female, mean age 73.45 ± 11.59, among which the oldest patient was 94 and the youngest 43 years. All the patients met the sepsis and MODS criteria. Increased age and low CD4+ and CD8+T cell counts were identified as independent predictors of death. Only the two youngest patients (43 and 50 years old) survived 28 days, and they are the only ones with a CD4 lymphocyte count above 500 cells/mm3.
Conclusion: Senescence of the immune system is mostly characterized by reduced regenerative capacity of adaptive immunity with diminished ability to respond to new antigens and a manifested proinflammatory phenotype. Additional reduction of protective capacity by further deterioration of T cell quantity and quality due to sepsis itself and mutual interaction of senescent T cells and vascular endothelial cells in the induction of cytokine storm represent two complementary vicious cycles in the development of sepsis-related multiorgan dysfunction.
Today, much is known about the clinical course and mortality of SARS-CoV-2 infection. Furthermore, current findings emphasize that the immune-inflammatory response plays a key role in its pathophysiology. Considering the wide distribution of angiotensin-converting enzyme 2 (ACE2), the functional receptor through which SARS-CoV-2 enters host cells, COVID-19 is a systemic disease. The first step in understanding of its pathogenesis is the abundant expression of ACE2 on respiratory tract epithelial cells and vascular endothelial cells (Pons et al., 2020). They represent an important component of innate immunity, causing a proinflammatory reaction, particularly violent in the presence of SARS-CoV-2 viremia, that is not detectable in mild and asymptomatic cases of COVID-19 (Richter et al., 2021). In severe cases of COVID-19, systemic inflammatory hyperactivation in the form of a cytokine storm causes sepsis-related MODS as the most common immediate cause of death (De Roquetaillade et al., 2021; Elezkurtaj et al., 2021).
Adverse disease outcomes are far more common in older patients (Santesmasses et al., 2020). The aging process itself is accompanied and guided by the aging of the immune system, primarily senescence of adoptive immunity, which is characterized by a reduction of its regenerative capacity, with a reduced protective capacity and a manifested pro-inflammatory phenotype. (Anna et al., 2019; Cunha et al., 2020). Dealing with these circumstances could contribute to a better understanding of the pathophysiological role of adaptive immunity, which is still not fully explained, in the development of severe forms of COVID-19.
In accordance with the given assumptions, the aim of the study was to establish the years of life, presence of sepsis and MODS, values of additional factors with confirmed prognostic significance and number of peripheral CD4+ and CD8+T lymphocytes in critically ill patients with COVID-19.
The study was conducted at the Clinic for Infectious Disease of University Clinical Center Nis, Serbia, from January to March 2022. Twenty patients were consecutively selected at the beginning of invasive mechanical ventilation, during hospitalization due to COVID-19.
Presence of mechanical ventilation, frequent need for sedation and use of vasoactive agents made impossible for us to use the clinical criteria (such as SOFA or qSOFA scoring) in the assessment of the presence and severity of sepsis and MODS. Therefore, various laboratory markers of sepsis and MODS were determined, including: interleukin 6 (IL-6), procalcitonin (PCT), C-reactive protein (CRP), white blood cell count (WBC), lymphocyte %, platelets count (PLT), glycemia, creatinine, lactate, international normalized ratio (INR), and PaO2/FiO2. Additionally, the values of lactate dehydrogenase (LDH), ferritin and D-dimer, with a confirmed prognostic significance for COVID-19, were determined. The numbers of peripheral CD4+ and CD8+T lymphocytes were determined by flow cytometry (BD FACSCount™ Reagent Kit).
Analyzes were performed on blood samples taken on the same day, during the patients' stay in the intensive care unit. The results were presented as arithmetic means and standard deviations, with assessment of their association with the probability of death by univariate and multivariate Cox regression analysis and with Kaplan-Meier curve of 28 days survival.
All the patients met the sepsis and MODS criteria. Also, increased values of LDH, ferritin and D-dimer, as well as depletion of CD4 + T and CD8 + T lymphocytes were recorded (Table 1).
By univariate regression analysis, increased age and low CD4+T and CD8+T cells counts were identified as independent predictors of death. In the multivariate model of Cox regression analysis, containing the 3 given variables, only age retained statistical significance as the factor associated with mortality, probably as a cumulative expression of changes in the number of CD4+T and CD8+T lymphocyte cells (Table 2).
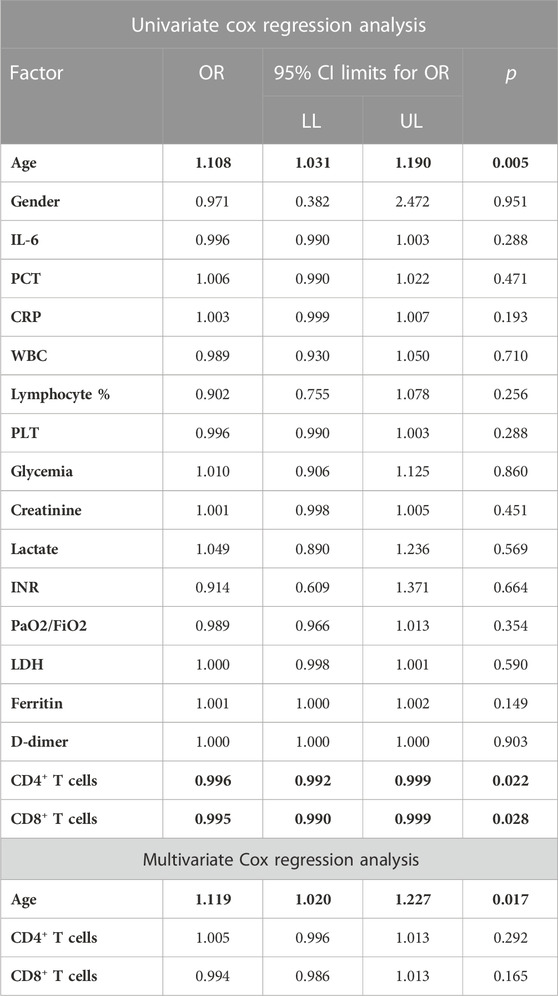
TABLE 2. Association with the probability of death by univariate and multivariate Cox regression analysis.
Only the two youngest patients (43 and 50 years old) survived 28 days, and these were the only ones with a CD4+T lymphocyte count above 500 cells/mm3 (Figure 1).
Before the COVID-19 pandemic, in a cohort of patients with acute hypoxemic respiratory failure death was rarely due to refractory pulmonary dysfunction. In SARS-CoV-2 infection, pulmonary dysfunction is present in the vast majority of cases at the time of death, as the most conspicuous sign of MODS (Ketcham et al., 2021).
The development of severe forms and mortality from COVID 19 are associated with age-related changes in the immune response (Bartle et al., 2021). The pathogenetic substrate of MODS development is the cytokine storm primarily triggered by vascular endothelial damage (Otifi and Adiga, 2022). Further, as a conditional part of the acquired immunity, endothelial cells represent the interface between the innate and adaptive immune responses (Schleimer et al., 2007). They are important for the recruitment and activation of T-cells, which with aging exhibit increased production of proinflammatory cytokines (Fukushima et al., 2018; Degauque et al., 2021). At the same time, the senescent T cells may contribute to the immunopathological mechanism of additional endothelial damage, in the vicious circle of inflammatory hyperactivation (Covre et al., 2020; Zhang et al., 2021). Clinically, the significant role of adaptive immunity is indicated by the time required for its activation, 2 weeks on average, considering that, in addition to the incubation period, serious forms of the disease usually develop 10 days after the appearance of the first symptoms (Blair et al., 2021).
Another vicious circle present is a deterioration of the quantity and quality of T cells, that generally characterizes sepsis, which results in an additional reduction of the protective capacity of adaptive immunity, already weakened by immunosenescence (Cabrera-Perez et al., 2014; Danahy et al., 2016). From there, the presence of SARS-CoV-2 viremia and its duration were directly correlated with the degree of inflammation and mortality (Hagman et al., 2022), while depletion rate of peripheral CD8 + T cells reflected the severity of the disease, and reduced CD4 +T cell count was independently associated with increased in-hospital mortality in patients with COVID-19 (Wen et al., 2021).
The development of severe forms and mortality from COVID-19, as well as sepsis in general, is associated with an overwhelming life-threatening immune response associated with aging.
Driven primarily by changes of adaptive immunity, this process is mostly genetically programmed, which greatly reduces the possibility of therapeutic interventions.
From there, the most promising are therapeutic interventions which reduce the possibility of excessive immune activation, such as the administration of virustatic agents and neutralizing monoclonal antibodies early in the course of infection.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the ethical committee of the University Clinical Center of Niš approved the study under number 4711/2. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
MV: Conceptualization, Data curation, Investigation, Methodology, Writing–original draft. AM: Conceptualization, Supervision, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Anna, A., Farzaneh, F., Candore, G., Caruso, C., Davinelli, S., Gambino, C. M., et al. (2019). Immunosenescence and its hallmarks: how to oppose aging strategically? A review of potential options for therapeutic intervention. Front. Immunol. 10, 2247. doi:10.3389/fimmu.2019.02247
Bartleson, J. M., Radenkovic, D., Covarrubias, A. J., Furman, D., Winer, D. A., and Verdin, E. (2021). SARS-CoV-2, COVID-19 and the ageing immune system. Nat. Aging 1 (9), 769–782. doi:10.1038/s43587-021-00114-7
Blair, P. W., Brown, D. M., Jang, M., Antar, A. A. R., Keruly, J. C., Bachu, V. S., et al. (2021). The clinical course of COVID-19 in the outpatient setting: A prospective cohort study. Open Forum Infect. Dis. 8 (2), ofab007. doi:10.1093/ofid/ofab007
Cabrera-Perez, J., Condotta, S. A., Badovinac, V. P., and Griffith, T. S. (2014). Impact of sepsis on CD4 T cell immunity. J. Leukoc. Biol. 96 (5), 767–777. doi:10.1189/jlb.5MR0114-067R
Covre, L. P., De Maeyer, R. P. H., Gomes, D. C. O., and Arne, N. A. (2020). The role of senescent T cells in immunopathology. Aging Cell 19 (12), e13272. doi:10.1111/acel.13272
Cunha, L. L., Felix Perazzio, S., Azzi, J., Cravedi, P., and Riella, L. (2020). Remodeling of the immune response with aging: immunosenescence and its potential impact on COVID-19 immune response. Front. Immunol. 11, 1748. doi:10.3389/fimmu.2020.01748
Danahy, D. B., Strother, R. K., Badovinac, V. P., and Griffith, T. S. (2016). Clinical and experimental sepsis impairs CD8 T-Cell-Mediated immunity. Crit. Rev. Immunol. 36 (1), 57–74. doi:10.1615/CritRevImmunol.2016017098
De Roquetaillade, C., Bredin, S., Lascarrou, J., Soumagne, T., Cojocaru, M., Chousterman, B. G., et al. (2021). Timing and causes of death in severe COVID-19 patients. Crit. Care 25 (1), 224. doi:10.1186/s13054-021-03639-w
Degauque, N., Haziot, A., Brouard, S., and Mooney, N. (2021). Endothelial cell, myeloid, and adaptive immune responses in SARS-CoV-2 infection. FASEB J. 35 (5), e21577. doi:10.1096/fj.202100024R
Elezkurtaj, S., Greuel, S., Jana, I., Michaelis, E. G., Bischoff, P., Kunze, C. A., et al. (2021). Causes of death and comorbidities in hospitalized patients with COVID-19. Sci. Rep. 11 (1), 4263. doi:10.1038/s41598-021-82862-5
Fukushima, Y., Minato, N., and Hattori, M. (2018). The impact of senescence-associated T cells on immunosenescence and age-related disorders. Inflamm. Regen. 38, 24. doi:10.1186/s41232-018-0082-9
Hagman, K., Hedenstierna, M., Rudling, J., Gille-Johnson, P., Hammas, B., Grabbe, M., et al. (2022). Duration of SARS-CoV-2 viremia and its correlation to mortality and inflammatory parameters in patients hospitalized for COVID-19: A cohort study. Diagn Microbiol. Infect. Dis. 102 (3), 115595. doi:10.1016/j.diagmicrobio.2021.115595
Ketcham, S. W., Bolig, T. C., Molling, D. J., Sjoding, M. W., Flanders, S. A., and Prescott, H. C. (2021). Causes and circumstances of death among patients hospitalized with COVID-19: A retrospective cohort study. Ann. Am. Thorac. Soc. 18 (6), 1076–1079. doi:10.1513/AnnalsATS.202011-1381RL
Otifi, H. M., and Adiga, B. K. (2022). Endothelial dysfunction in Covid-19 infection. Am. J. Med. Sci. 363 (4), 281–287. doi:10.1016/j.amjms.2021.12.010
Pons, S., Arnaud, M., Loiselle, M., Eden, A., Azoulay, E., and Zafrani, L. (2020). Immune consequences of endothelial cells' activation and dysfunction during sepsis. Crit. Care Clin. 36 (2), 401–413. doi:10.1016/j.ccc.2019.12.001
Richter, E., Al Arashi, D., Schulte, B., Bode, C., Marx, B., Aldabbagh, S., et al. (2021). Detectable SARS-CoV-2 RNAemia in critically ill patients, but not in mild and asymptomatic infections. Transfus. Med. Hemother 48 (3), 154–160. doi:10.1159/000515841
Santesmasses, D., Pedro Castro, J., Zenin, A. A., Shindyapina, A. V., Gerashchenko, M. V., Zhang, B., et al. (2020). COVID-19 is an emergent disease of aging. Aging Cell 19 (10), e13230. doi:10.1111/acel.13230
Schleimer, R. P., Kato, A., Kern, R., Douglas, K., and Pedro, C. (2007). Epithelium: at the interface of innate and adaptive immune responses. J. Allergy Clin. Immunol. 120 (6), 1279–1284. doi:10.1016/j.jaci.2007.08.046
Wen, X., Jiang, D., Gao, L., Zhou, J. Z., Xiao, J., Cheng, X. C., et al. (2021). Clinical characteristics and predictive value of lower CD4+T cell level in patients with moderate and severe COVID-19: A multicenter retrospective study. BMC Infect. Dis. 21 (1), 57. doi:10.1186/s12879-020-05741-w
Keywords: COVID-19, MODS, senescence of adoptive immunity, sepsis, pathogenesis
Citation: Vrbic M and Milinkovic A (2023) Two vicious circles associated with the aging of the immune system in the development of severe forms of COVID-19. Front. Aging 4:1260053. doi: 10.3389/fragi.2023.1260053
Received: 21 July 2023; Accepted: 04 September 2023;
Published: 14 September 2023.
Edited by:
Calogero Caruso, University of Palermo, ItalyReviewed by:
Atefe Ghamar Talepoor, Shiraz University of Medical Sciences, IranCopyright © 2023 Vrbic and Milinkovic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miodrag Vrbic, bXZyYmljQG10cy5ycw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.