
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Aging , 25 September 2023
Sec. Interventions in Aging
Volume 4 - 2023 | https://doi.org/10.3389/fragi.2023.1211571
This article is part of the Research Topic Insights in Aging Interventions: 2022 View all 12 articles
Objectives: To investigate whether exposure history to two common loop diuretics, bumetanide and furosemide, affects the risk of developing Alzheimer’s disease (AD) after accounting for socioeconomic status and congestive heart failure.
Methods: Individuals exposed to bumetanide or furosemide were identified in the Stanford University electronic health record using the de-identified Observational Medical Outcomes Partnership platform. We matched the AD case cohort to a control cohort (1:20 case:control) on gender, race, ethnicity, and hypertension, and controlled for variables that could potentially be collinear with bumetanide exposure and/or AD diagnosis. Among individuals older than 65 years, 5,839 AD cases and 116,103 matched controls were included. A total of 1,759 patients (54 cases and 1,705 controls) were exposed to bumetanide.
Results: After adjusting for socioeconomic status and other confounders, the exposure of bumetanide and furosemide was significantly associated with reduced AD risk (respectively, bumetanide odds ratio [OR] = 0.23; 95% confidence interval [CI], 0.15–0.36; p = 4.0 × 10−11; furosemide OR = 0.42; 95% CI, 0.38–0.47; p < 2.0 × 10−16).
Discussion: Our study replicates in an independent sample that a history of bumetanide exposure is associated with reduced AD risk while also highlighting an association of the most common loop diuretic (furosemide) with reduced AD risk. These associations need to be additionally replicated, and the mechanism of action remains to be investigated.
Medical systems generate massive amounts of electronic health record (EHR) data, and researchers have analyzed these data to derive new insights and improve healthcare (Rajkomar et al., 2019; Shah et al., 2019). Stanford University has established a novel and secure data platform: Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM). Alzheimer’s disease (AD) is well suited for analysis with OMOP given its multifaceted complexity, prevalence, and the multitude of small sample size studies that claim benefit for certain interventions.
AD is a neurodegenerative disorder of uncertain cause and pathogenesis. In the United States, as many as one in nine people (10.7%) older than 65 years has AD (Rajan et al., 2021). Recently, repurposing bumetanide as an AD medication was proposed based on data that showed bumetanide “reversed” APOE genotype-dependent transcriptomic signatures in mouse and cell culture models (Taubes et al., 2021). This finding was investigated in two EHR-based cohorts demonstrating that in individuals older than 65 years, bumetanide exposure was associated with lower AD prevalence (Taubes et al., 2021). This finding warrants further validation as bumetanide is more expensive than the commonly prescribed loop diuretic (furosemide), and thus, potential socioeconomic status (SES) confounding such as insurance coverage needs to be investigated. Both furosemide and bumetanide are indicated, and often interchangeably used, for patients with hypertension, congestive heart failure (CHF), and kidney disease. In this study, using Stanford’s EHR data, we sought to replicate the bumetanide findings in an independent dataset accounting for SES, hypertension, and CHF, and additionally test the association of furosemide with AD risk.
Stanford’s de-identified OMOP instance hosts multi-factor and multi-modal data, including Stanford’s structured clinical data, clinical notes, meta-data on clinical notes, extracted concepts from clinical notes using natural language processing and other approaches, and radiological images. Participants or their caregivers provided written informed consent to store their data in OMOP. The Stanford University institutional review board granted the current study protocol an exemption because the analyses were carried out on de-identified data; therefore, additional informed consent was not required.
We have curated OMOP data for 656,683 patients older than 65 years at their last known visit. We focused on 5,872 patients with AD defined by ICD9 and ICD10 codes (ICD10: G30.1, G30.8, G30.9, and ICD9: 331.0, Table 1). We matched individual AD patients with up to 20 controls on age and exact match of sex, race, ethnicity, and hypertension (Table 2) using R package optmatch (Hansen and Olsen Klopfer, 2006). We excluded 937 matched controls with an age difference greater than 5 years and 373 matched controls that belonged to strata with fewer than 15 controls per case, resulting in 5,839 AD cases and 116,103 matched controls.
We scanned the data using the medication orders or medical history variable tables to identify those who had been exposed to bumetanide or, respectively, furosemide prior to AD diagnosis. We included any type of exposure to the drug, specifically oral or IV exposures, for any duration of time. We calculated the percent of AD cases and non-AD controls exposed to bumetanide and furosemide using a χ2 test. In addition, as post hoc sensitivity analyses, we calculated the odds ratio of AD diagnosis for those exposed to bumetanide (and separately furosemide) while adjusting for variables that could potentially be collinear with bumetanide exposure and/or AD diagnosis including diagnosis of CHF (defined by the ICD10 of I11, I13, and I50, Table 1), insurance type, and median income (defined by the patient’s recorded zip code, derived from publicly available data from the United States Census Bureau), and explored the relationship of AD with the other commonly used loop diuretic, furosemide. Statistical analyses were performed using R (version 3.6.3). The results are shown in Tables 3A,B.
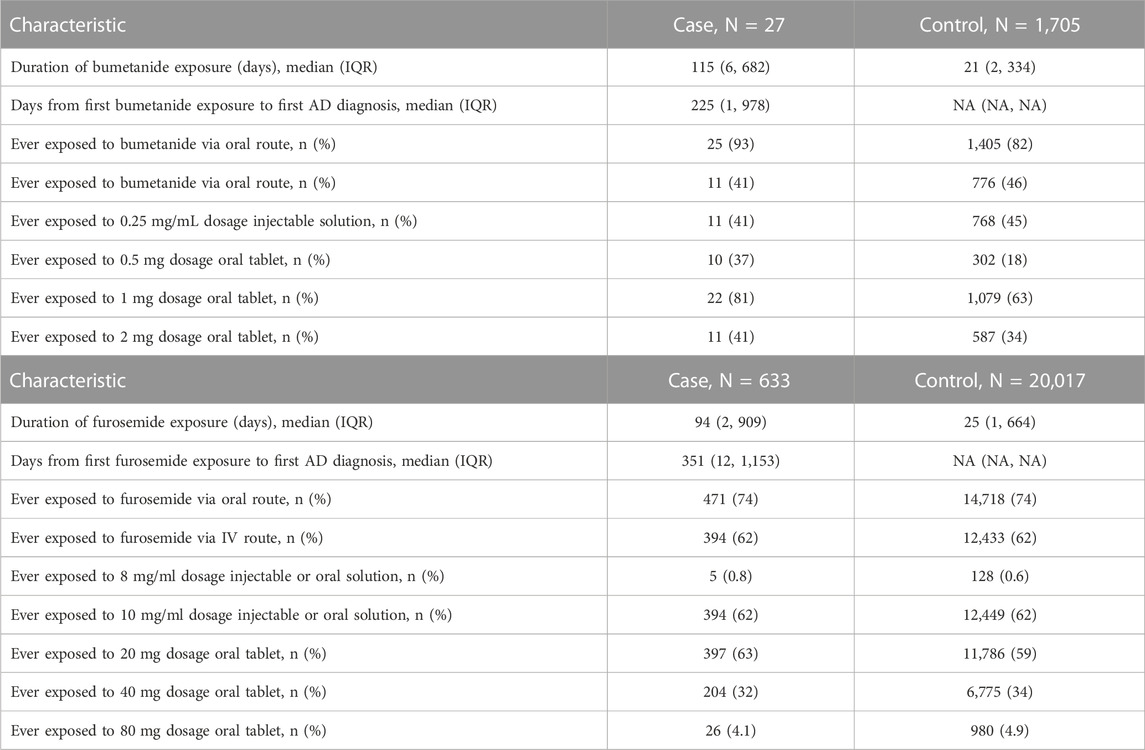
TABLE 3. (AB) Bumetanide and furosemide exposure details of exposed participants by diagnosis status (A). Bumetanide exposures for full matched cohort (B) and furosemide exposures for full matched cohort.
A total of 1,732 patients (27 cases and 1,705 controls) were exposed to bumetanide during any of their visits (prior to their AD diagnosis for cases). For the full-matched cohort, among AD cases, 0.5% (27/5839) of patients were exposed to bumetanide prior to diagnosis compared to 1.5% (1705/116103) of controls exposed to bumetanide, suggesting patients exposed to bumetanide are less likely to develop AD. The unadjusted odds ratio (OR) for AD diagnosis among bumetanide exposed was 0.31 (95% CI, 0.21–0.46; p = 2.4 × 10−9).
To adjust SES variables, we included insurance information (if patients ever had Medicare/Medicaid/VA and if patients ever had private insurance) and median household income by the zip code. However, we did have some missingness in our data—insurance information was available for 94.0% of cases and 84.5% of controls. In addition, for the zip code data informing the median income component, we focused on patients from California, and due to de-identification reasons, data were only available for 76.8% of cases and 74.1% of controls. Because SES estimates were not easily imputable from our data, a sensitivity analysis was performed as a complete case analysis. In this sensitivity analysis, we restricted the cohort to patients with complete insurance and median income data (N = 77,688) and repeated the unadjusted analysis prior to fitting a multivariable model adjusted for CHF, insurance, and median income. In the complete case-restricted cohort, 21/4238 (0.50%) AD cases and 1,321/73,450 (1.8%) matched controls were exposed to bumetanide. The unadjusted OR remained similar to that in our primary analysis (OR = 0.27; 95% CI, 0.18–0.42; p = 3.7 × 10−9). After adjusting for CHF, insurance, and median income the estimated OR for AD diagnosis among bumetanide exposed was 0.23 (95% CI, 0.15–0.36; p = 4.0 × 10−11).
For the full-matched cohort, among AD cases, 10.8% (633/5839) of patients were exposed to furosemide prior to diagnosis compared to 17.2% (20023/116103) of controls exposed to furosemide, suggesting patients exposed to bumetanide are less likely to develop AD. The unadjusted OR for AD diagnosis among furosemide exposed was 0.58 (95% CI, 0.54–0.63; p < 2.0 × 10−16). In the same sensitivity analysis performed for bumetanide exposure, the unadjusted OR for the complete case-restricted cohort remained similar to that in our primary analysis (OR = 0.53; 95% CI, 0.48–0.58; p < 2.0 × 10−16); this protective effect was replicated after adjusting for CHF, insurance, and median income (OR = 0.42; 95% CI, 0.38–0.47; p < 2.0 × 10−16).
Most clinical trials in AD suffer from an inherent shortfall regarding primary prevention as they do not give insights on whether a compound reduces the incidence of AD as medications are tested after disease onset. Studying EHR using OMOP allows us to derive insight into possible primary prevention of AD (Datta et al., 2020).
In an independent dataset, our results replicate those of the original study that found a protective effect of bumetanide exposure on AD risk (Taubes et al., 2021). We further investigated whether this effect is generalizable to the more commonly used and less expensive medication in the same class and adjusted for potentially confounding variables such as SES and CHF. In our study, we calculated the odds ratio of AD diagnosis for those exposed to bumetanide (and separately furosemide) and found that the exposure of both bumetanide and furosemide was associated with reduced future AD diagnosis.
Both medications have similar indications and mechanisms of action: potential protective molecular modulation of neuronal transmembrane chloride gradients by blocking NKCC1 in the central nervous system (Kharod et al., 2019), which is the mechanism that led to proposed investigations to treat autism (Lemonnier et al., 2012), schizophrenia (Rahmanzadeh et al., 2017), and epilepsy (Eftekhari et al., 2013; Rahmanzadeh et al., 2017). Both bumetanide and furosemide have been shown to penetrate the blood–brain barrier, albeit at low concentrations (Javaheri et al., 1994; Töllner et al., 2015). The brain bumetanide concentrations following systemic administration are below those required for effective NKCC1 inhibition (Johanson et al., 1992; Holtkamp et al., 2003; Römermann et al., 2017; Brandt et al., 2018). However, other potential explanations for the protective effects including unique effects on the APOE genotype-dependent transcriptomic signature (Taubes et al., 2021), potent diuretic effects, and off-target metabolic, cardiorespiratory, and hormonal alterations that may be indirectly linked to reducing the risk of AD are also a possibility (Brater, 1991; Puskarjov et al., 2014). Our OMOP EHR dataset analysis demonstrated a potential inverse association between past bumetanide and furosemide exposures (Table 4) and AD onset. These associations remained significant even after correcting for SES and CHF, indicating that the results are not driven by differences in SES or severity of cardiac disease.
These results should be treated cautiously as they are based on retrospective data. Bumetanide and furosemide are potent loop diuretics that if given excessively, can lead to a profound diuresis with water and electrolyte depletion, which is particularly problematic in the elderly population. In addition, insurance and income were modeled through proxies available in OMOP and may not fully account for differences in SES. Last, additional functional studies are warranted to investigate the biological mechanism through which bumetanide and furosemide exposures are associated with reduced AD risk. The current findings do not support the use of bumetanide for the prevention or treatment of AD. There is a need for prospective, randomized, double-blinded, and placebo-controlled clinical trials to confirm the findings in patients without comorbidities and determine the lowest effective dose that may reduce the risk of AD without causing intolerable side effects.
The data analyzed in this study are subject to the following licenses/restrictions: Electronic Health Record from Stanford University. Requests to access these datasets should be directed to emlodWFpQHN0YW5mb3JkLmVkdQ==.
The requirement of ethical approval was waived by the Stanford University institutional review board for the studies involving humans because the analyses were carried out on de-identified data; therefore, additional informed consent was not required. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board also waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the analyses were carried out on de-identified data; therefore, additional informed consent was not required.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This research was supported by NIH/NIA awards AG066206 (ZH) and AG066515 (ZH, HY, and MG).
This research used data or services provided by STARR, “STAnford medicine Research data Repository,” a clinical data warehouse containing live epic data from Stanford Health Care (SHC), the Stanford Children’s Hospital (SCH), the University Healthcare Alliance (UHA), and Packard Children’s Health Alliance (PCHA) clinics, and other auxiliary data from Hospital applications such as radiology PACS. The STARR platform was developed and operated by the Stanford Medicine Research IT team and was made possible by the Stanford School of Medicine Research Office.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Brandt, C., Seja, P., Töllner, K., Römermann, K., Hampel, P., Kalesse, M., et al. (2018). Bumepamine, a brain-permeant benzylamine derivative of bumetanide, does not inhibit NKCC1 but is more potent to enhance phenobarbital’s anti-seizure efficacy. Neuropharmacology 143, 186–204. doi:10.1016/j.neuropharm.2018.09.025
Brater, D. C. (1991). Clinical pharmacology of loop diuretics. Drugs 41, 14–22. doi:10.2165/00003495-199100413-00004
Datta, S., Posada, J., Olson, G., Li, W., O’Reilly, C., Balraj, D., et al. (2020). A new paradigm for accelerating clinical data science at Stanford Medicine.
Eftekhari, S., Mehvari Habibabadi, J., Najafi Ziarani, M., Hashemi Fesharaki, S. S., Gharakhani, M., Mostafavi, H., et al. (2013). Bumetanide reduces seizure frequency in patients with temporal lobe epilepsy. Epilepsia 54, e9–e12. doi:10.1111/j.1528-1167.2012.03654.x
Hansen, B. B., and Olsen Klopfer, S. (2006). Optimal full matching and related designs via network flows. J. Comput. Graph. Statistics 15, 609–627. doi:10.1198/106186006X137047
Holtkamp, M., Matzen, J., Buchheim, K., Walker, M. C., and Meierkord, H. (2003). Furosemide terminates limbic status epilepticus in freely moving rats. Epilepsia 44, 1141–1144. doi:10.1046/j.1528-1157.2003.14003.x
Javaheri, S., Corbett, W., Adams, J. M., Davis, P. J., and Gartside, P. S. (1994). Acute respiratory acidosis: large-dose furosemide and cerebrospinal fluid ions. J. Appl. Physiology 76, 2651–2655. doi:10.1152/jappl.1994.76.6.2651
Johanson, C. E., Murphy, V. A., and Dyas, M. (1992). Ethacrynic acid and furosemide alter Cl, K, and Na distribution between blood, choroid plexus, CSF, and brain. Neurochem. Res. 17, 1079–1085. doi:10.1007/BF00967284
Kharod, S. C., Kang, S. K., and Kadam, S. D. (2019). Off-label use of bumetanide for brain disorders: an overview. Front. Neurosci. 13, 310. doi:10.3389/fnins.2019.00310
Lemonnier, E., Degrez, C., Phelep, M., Tyzio, R., Josse, F., Grandgeorge, M., et al. (2012). A randomised controlled trial of bumetanide in the treatment of autism in children. Transl. Psychiatry 2, e202–e208. doi:10.1038/tp.2012.124
Puskarjov, M., Kahle, K. T., Ruusuvuori, E., and Kaila, K. (2014). Pharmacotherapeutic targeting of cation-chloride cotransporters in neonatal seizures. Epilepsia 55, 806–818. doi:10.1111/epi.12620
Rahmanzadeh, R., Eftekhari, S., Shahbazi, A., Khodaei Ardakani, M. R., Rahmanzade, R., Mehrabi, S., et al. (2017). Effect of bumetanide, a selective NKCC1 inhibitor, on hallucinations of schizophrenic patients; a double-blind randomized clinical trial. Schizophrenia Res. 184, 145–146. doi:10.1016/j.schres.2016.12.002
Rajan, K. B., Weuve, J., Barnes, L. L., McAninch, E. A., Wilson, R. S., and Evans, D. A. (2021). Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimer’s Dementia 17, 1966–1975. doi:10.1002/alz.12362
Rajkomar, A., Dean, J., and Kohane, I. (2019). Machine learning in medicine. N. Engl. J. Med. 380, 1347–1358. doi:10.1056/NEJMra1814259
Römermann, K., Fedrowitz, M., Hampel, P., Kaczmarek, E., Töllner, K., Erker, T., et al. (2017). Multiple blood-brain barrier transport mechanisms limit bumetanide accumulation, and therapeutic potential, in the mammalian brain. Neuropharmacology 117, 182–194. doi:10.1016/j.neuropharm.2017.02.006
Shah, N. H., Milstein, A., and Bagley PhD, S. C. (2019). Making machine learning models clinically useful. JAMA 322, 1351–1352. doi:10.1001/jama.2019.10306
Taubes, A., Nova, P., Zalocusky, K. A., Kosti, I., Bicak, M., Zilberter, M. Y., et al. (2021). APOE4 -related Alzheimer ’ s disease, 1.
Keywords: Alzheimer’s disease, bumetanide, electronic health record informatics, furosemide, quantitative pharmacology
Citation: Graber-Naidich A, Lee J, Younes K, Greicius MD, Le Guen Y and He Z (2023) Loop diuretics association with Alzheimer’s disease risk. Front. Aging 4:1211571. doi: 10.3389/fragi.2023.1211571
Received: 24 April 2023; Accepted: 07 September 2023;
Published: 25 September 2023.
Edited by:
Yadong Huang, Gladstone Institutes, United StatesReviewed by:
Misha Zilberter, Gladstone Institutes, United StatesCopyright © 2023 Graber-Naidich, Lee, Younes, Greicius, Le Guen and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yann Le Guen, eWxlZ3VlbkBzdGFuZm9yZC5lZHU=; Zihuai He, emlodWFpQHN0YW5mb3JkLmVkdQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.