
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Aging , 25 June 2021
Sec. Aging and the Immune System
Volume 2 - 2021 | https://doi.org/10.3389/fragi.2021.718966
This article is part of the Research Topic Impact of Aging on Host Innate and Adaptive Immune Responses to Respiratory Viral Infections View all 4 articles
This article is a correction to:
Key Determinants of Cell-Mediated Immune Responses: A Randomized Trial of High Dose Vs. Standard Dose Split-Virus Influenza Vaccine in Older Adults
 Chris P. Verschoor1,2
Chris P. Verschoor1,2 Laura Haynes3
Laura Haynes3 Graham Pawelec1,4
Graham Pawelec1,4 Mark Loeb5
Mark Loeb5 Melissa K. Andrew6
Melissa K. Andrew6 George A. Kuchel3
George A. Kuchel3 Janet E. McElhaney1,2*
Janet E. McElhaney1,2*A Corrigendum on
Key Determinants of Cell-Mediated Immune Responses: A Randomized Trial of High Dose Vs. Standard Dose Split-Virus Influenza Vaccine in Older Adults
by Verschoor CP, Haynes L, Pawelec G, Loeb M, Andrew MK, Kuchel GA and McElhaney JE (2021) Front. Aging 2:649110. doi:10.3389/fragi.2021.649110
In the original article, there was a mistake in Figures 3 and 4 as published. A coding error with the statistical analysis led to erroneous estimates, mainly for associations with the frailty index. Although all estimates were altered slightly, the overall conclusion was not impacted greatly. The corrected Figures 3 and 4 appear below.
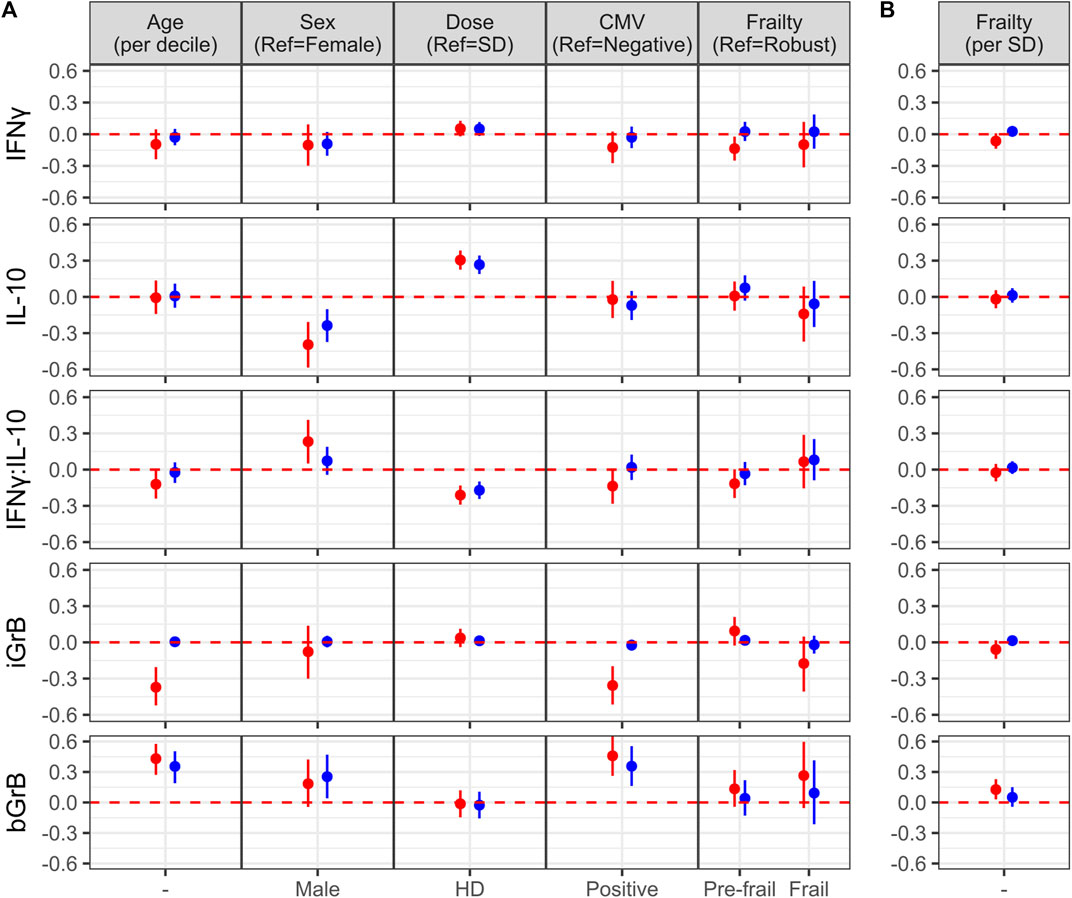
FIGURE 3. Factors associated with the of cell-mediated immune (CMI) response to vaccination. (A) The effect of different characteristics of older adult vaccinees are shown for each of the CMI measures in an unadjusted (red dots) and adjusted (blue dots) linear regression analysis, and includes frailty as a categorical variable. For (B), the association between each CMI measure and frailty as standardized continuous variable in both unadjusted and adjusted analyses is presented. The regression coefficient and 95% confidence interval is presented, which represents a 1-standard deviation (SD) change in each measure for a given change in a participant factor, [ie., relative to the reference (ref), or per SD change]. Points and error bars above the red, dashed line indicate a significant positive correlation with a CMI measure, whereas those below the red, dashed line indicate a significant inverse correlation. Note that due to the scale of the y-axis, certain confidence intervals are not visible beyond the point estimate.
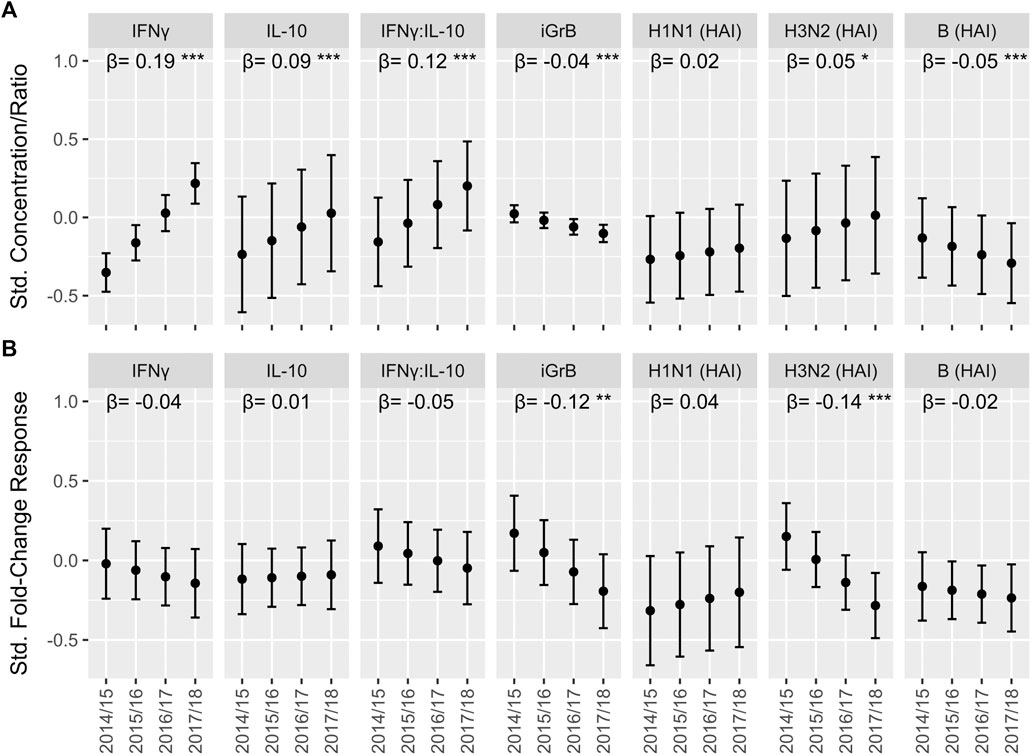
FIGURE 4. The trend in cell-mediated immune (CMI) responses to live A/H3N2 virus and hemagglutination inhibition (HAI) antibody titers over the course of the study in older adults. For the absolute CMI/HAI measures (A) and fold-change in HAI/CMI measures from pre-to 4 weeks post-vaccination (B), the estimated marginal mean and 95% confidence interval of the standardized value (mean 0, standard deviation 1) in each year, which was derived from a linear mixed model accounting for repeated measures within participant, is presented on the y-axis. The slope (β) and significance (asterisks) of the regression coefficient for year in these models is presented along the top of each plot. ***p < 0.001; **p < 0.01; *p < 0.05.
Due to the coding error in the statistical analysis, the description of the findings presented in the Abstract, Results, and Discussion could also be misleading.
A change was made in the Abstract, sub-section “Results,” Line 6. The corrected line now reads: “In a regression analysis, female sex and HD-SVV were associated with higher IL-10 levels, while SD-SVV was associated with lower iGrB levels.”
A change was also made in the Results, sub-section “Effects of Age, Sex, Cytomegalovirus Status, Frailty and Vaccine Dose in Older Adults,” Paragraphs 1–3. The corrected text appears below:
Effects of Age, Sex, Cytomegalovirus Status, Frailty and Vaccine Dose in Older Adults
To determine the effect of age, sex, vaccine dose, CMV serostatus and frailty on standardized post-vaccination CMI measures (IFNγ, IL-10, iGrB), we performed linear regression on univariate and fully-adjusted multivariable models, accounting for the random effect of visit, site, year and participant; the latter was adjusted for pre-vaccination CMI and all aforementioned factors. In fully-adjusted models, IFNγ levels were not significantly affected by age, sex, CMV, dose or frailty (Figure 3). In contrast, IL-10 levels were significantly lower in males vs. females (std. β [95% CI] = −0.24 [−0.37, −0.10]) and significantly higher in HD vs. SD vaccine recipients (0.27 [0.19, 0.34]) (Figure 3A). The IFNγ:IL-10 ratio was significantly lower in HD vs. SD recipients (std. β [95% CI] = −0.17 [−0.24, −0.10]) (Figure 3A). In contrast to measures of the cytokine response to influenza challenge, none of the factors studied affected iGrB levels post-vaccination (Figure 3). Interestingly, bGrB activity in resting T cells significantly increased with age (std. β [95% CI] = 0.35 [0.19, 0.50]), male sex (0.25 [0.04, 0.47]), and CMV seropositivity (0.36 [0.16, 0.55]) (Figure 3A), while frailty as a continuous variable was not significantly associated (Figure 3B).
We found that the absolute levels of IFNγ (Std. β [95% CI] = 0.19 [0.15, 0.23]), IL-10 (0.09 [0.05, 0.13]) and the IFNγ:IL-10 ratio (0.12 [0.08, 0.16]) increased significantly with each study year, while iGrB levels exhibited a smaller but statistically significant decline (−0.042 [−0.059, −0.025]) (Figure 4A). In contrast, absolute hemagglutination inhibition (HAI) antibody titers to A/H3N2 (0.049 [0.005, 0.091]) strains increased significantly while HAI titers to influenza B declined significantly over the 4 years (−0.054 [−0.084, −0.023]) (Figure 4A).
Finally, a change was made to the Discussion, Paragraph 5, Line 1, which now reads: “In univariate regression analysis, we found that increasing age and CMV seropositivity are significant correlates of the decreased iGrB response to ex vivo influenza challenge.”
Paragraph 5, Line 19 now reads: “Our previous studies suggest that the addition of a toll-like receptor (TLR4) agonist to SVV can overcome the suppressive effects of IL-10 and improve the GrB response to influenza challenge (Behzad et al., 2012).”
A change was also made in Paragraph 6, Line 1, which now reads: “In light of the current controversy over the effects of repeated annual influenza vaccination potentially leading to a loss of vaccine-mediated protection against influenza, we analyzed the effect of vaccination in each study year according to the absolute levels of antibody, cytokines (IFNγ, IL-10, and IFNγ:IL-10) and iGrB at 4-, 10-, and 20-weeks post-vaccination and the pre- to 4-weeks post-vaccination fold-change response in older adults. We found a significant decline over the 4 study years only in the post-vaccination A/H3N2 iGrB levels and influenza B antibody titers.”
The authors apologize for these errors and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Keywords: cell-mediated immune responses, high-dose vs. standard-dose split-virus influenza vaccine, older adults, granzyme B, interferon-gamma, interleukin-10, cytomegalovirus, frailty index
Citation: Verschoor CP, Haynes L, Pawelec G, Loeb M, Andrew MK, Kuchel GA and McElhaney JE (2021) Corrigendum: Key Determinants of Cell-Mediated Immune Responses: A Randomized Trial of High Dose Vs. Standard Dose Split-Virus Influenza Vaccine in Older Adults. Front. Aging 2:718966. doi: 10.3389/fragi.2021.718966
Received: 03 June 2021; Accepted: 08 June 2021;
Published: 25 June 2021.
Edited and reviewed by:
Moisés Evandro Bauer, Pontifical Catholic University of Rio Grande do Sul, BrazilCopyright © 2021 Verschoor, Haynes, Pawelec, Loeb, Andrew, Kuchel and McElhaney. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Janet E. McElhaney, am1jZWxoYW5leUBoc25yaS5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.