- 1School of Human Development and Health, Faculty of Medicine, University of Southampton, Southampton, United Kingdom
- 2School of Primary Care and Population Sciences, Faculty of Medicine, University of Southampton, Southampton, United Kingdom
- 3Centre for Trials Research, Cardiff University, Cardiff, United Kingdom
- 4Nuffield Department of Primary Care Health Sciences, University of Oxford, Oxford, United Kingdom
- 5NIHR Southampton Biomedical Research Centre, University Hospital Southampton NHS Foundation Trust and University of Southampton, Southampton, United Kingdom
Aging is associated with changes to the immune system, collectively termed immunosenescence and inflammageing. However, the relationships among age, frailty, and immune parameters in older people resident in care homes are not well described. We assessed immune and inflammatory parameters in 184 United Kingdom care home residents aged over 65 years and how they relate to age, frailty index, and length of care home residence. Linear regression was used to identify the independent contribution of age, frailty, and length of care home residence to the various immune parameters as dependent variables. Participants had a mean age (±SD) of 85.3 ± 7.5 years, had been residing in the care home for a mean (±SD) of 1.9 ± 2.2 years at the time of study commencement, and 40.7% were severely frail. Length of care home residence and frailty index were correlated but age and frailty index and age and length of care home residence were not significantly correlated. All components of the full blood count, apart from total lymphocytes, were within the reference range; 31% of participants had blood lymphocyte numbers below the lower value of the reference range. Among the components of the full blood count, platelet numbers were positively associated with frailty index. Amongst plasma inflammatory markers, C-reactive protein (CRP), interleukin-1 receptor antagonist (IL-1ra), soluble E-selectin and interferon gamma-induced protein 10 (IP-10) were positively associated with frailty. Plasma soluble vascular cell adhesion molecule 1 (sVCAM-1), IP-10 and tumor necrosis factor receptor II (TNFRII) were positively associated with age. Plasma monocyte chemoattractant protein 1 was positively associated with length of care home residence. Frailty was an independent predictor of platelet numbers, plasma CRP, IL-1ra, IP-10, and sE-selectin. Age was an independent predictor of activated monocytes and plasma IP-10, TNFRII and sVCAM-1. Length of care home residence was an independent predictor of plasma MCP-1. This study concludes that there are independent links between increased frailty and inflammation and between increased age and inflammation amongst older people resident in care homes in the United Kingdom. Since, inflammation is known to contribute to morbidity and mortality in older people, the causes and consequences of inflammation in this population should be further explored.
Introduction
The number and proportion of older people is increasing in many societies (GBD 2016 Causes of Death Collaborators, 2017; GBD 2016 DALYs and HALE Collaborators, 2017). Aging increases the risk of morbidity, bringing with it loss of independence, increased health and social care costs, and for many older people, the need to move to a care home. Aging is also associated with changes to the immune system, collectively termed immunosenescence (De Martinis et al., 2004; Agarwal and Busse, 2010; Pawelec et al., 2010; Castelo-Branco and Soveral, 2014; Bektas et al., 2017) and inflammageing (Franceschi, 2007; Calder et al., 2017; Ventura et al., 2017; Atienza et al., 2018). Immunosenescence involves changes in the numbers of different immune cells in the bloodstream and reductions in their function (De Martinis et al., 2004; Agarwal and Busse, 2010; Pawelec et al., 2010; Castelo-Branco and Soveral, 2014; Bektas et al., 2017). For example, there is reduced production and export of naïve T lymphocytes into the blood (and lymphoid tissues) during aging with a loss in T cell receptor diversity and an accumulation of memory T lymphocytes (Agarwal and Busse, 2010; Pawelec et al., 2010; Castelo-Branco and Soveral, 2014; Bektas et al., 2017). The overall result of these changes are lowered numbers of T lymphocytes in the blood and impaired T lymphocyte responsiveness (De Martinis et al., 2004; Agarwal and Busse, 2010; Pawelec et al., 2010; Castelo-Branco and Soveral, 2014; Bektas et al., 2017). Immunosenescence also affects B lymphocyte numbers and function and the function of antigen presenting cells and some components of innate immunity (De Martinis et al., 2004; Agarwal and Busse, 2010; Pawelec et al., 2010; Castelo-Branco and Soveral, 2014; Bektas et al., 2017). Inflammageing is seen as an increase in blood plasma or serum concentrations of the acute phase protein C-reactive protein (CRP) and of inflammatory cytokines like interleukin (IL)-6 (Franceschi, 2007; Calder et al., 2017; Ventura et al., 2017; Atienza et al., 2018). This may reflect sensitized pro-inflammatory signaling pathways in older people. Together these changes contribute to the increased prevalence and severity of infections (Yoshikawa, 2000; Pera et al., 2015), the poorer responses to vaccinations (Goodwin et al., 2006; Trzonkowski et al., 2009; Derhovanessian and Pawelec, 2012) and the increased likelihood to suffer illness and disability (Onder et al., 2012) that occur with aging. However, aging is heterogeneous and occurs at different rates in different individuals; different settings may influence the aging process, for example by providing different access to a good diet, physical activity, mental stimulation and social interactions. It is described that free-living older individuals have a significantly better quality of life when compared with older people in institutional care homes (Montoya and Mody, 2011; Olsen et al., 2016; Sampson et al., 2019). This may relate to the different experiences offered outside and inside care homes which may themselves contribute to the aging process.
Frailty is currently recognized as a ”geriatric syndrome” (Laksmi, 2014; Chen et al., 2016). Categorization of frailty has traditionally been according to physical mobility and strength (Dent et al., 2016), although there is also a cognitive component to frailty as recognized in some scales for evaluating the extent of frailty among older people (Morley et al., 2013). Frail older adults are at increased risk of adverse health outcomes, including falls, hospitalization, and mortality (Beck-Sague et al., 1994; Ahmed et al., 2007). It has been suggested that one of the important pathways of frailty development is the immune/inflammatory pathway (Wilson et al., 2017). Inflammation has also been linked to a wide range of chronic diseases of common prevalence within older populations (Franceschi, 2007; Bauer and Fuente, 2016). Age, frailty, and length of care home residence might be linked to adverse outcomes (Carneiro et al., 2017). In order to better understand the relationships of age, frailty and length of care home residence with immunosenescence and inflammageing, we measured a range of immune and inflammatory markers in 184 United Kingdom care home residents aged over 65 years and investigated the relevant associations. We assessed static measures in blood (full blood count, immune phenotypes, plasma immune mediator concentrations, plasma CRP) as well as blood immune cell responses after ex vivo challenge (phagocytosis, blood culture responses to immune stimulation) and included components of both innate and acquired immunity. Many of these markers have not been well explored in the context of aging or frailty or in older people in the care home setting.
Methods
Participants
This cross-sectional study is embedded within the “Probiotics to reduce infections in care home residents” (PRINCESS) trial which is a two-arm double-blind individually randomized controlled trial, involving three academic centers in the United Kingdom (Universities of Cardiff, Oxford and Southampton). The full protocol (Owen-Jones et al., 2019) and the main outcomes (Butler et al., 2020) of the PRINCESS trial have been published. The PRINCESS trial was approved by the Wales REC 3 (15/WA/0306) and is registered at www.controlled-trials.com as ISRCTN16392920. Care home residents were eligible for participation if they were aged 65 years or older and willing and able to give informed consent for participation; if they lacked capacity, a consultee could complete a consultee declaration for participation on their behalf. Exclusions were immunocompromize (ongoing immune-suppressants; long-term, high-dose, oral, intramuscular or intravenous steroids), lactose intolerance, taking ongoing regular probiotics, or temporary residence in the care home. Care homes were residential, nursing or mixed. Here we report frailty and immune parameters in a subset of participants whose data was available at study entry (n = 184, although not all immune parameters were available for all these participants). Data were not available for all participants in the main PRINCESS trial and in this sub-study because 1) participants did not consent to take part in the immune sub-study of PRINCESS; or 2) insufficient blood was collected to measure some or any of the immune parameters; or 3) the blood arrived at the University of Southampton, where immune measurements were made, outside of a time window pre-determined based upon an earlier study (Castro-Herrera et al., 2018).
Assessment of Frailty
Frailty index was determined according to the scale described elsewhere (Morley et al., 2013). The scale has nine categories defined as: 1 = Very fit for their age (active, energetic and motivated); 2 = Well (absent symptomatology of disease but less active); 3 = Managing well (medical problems under control but not regularly active); 4 = Vulnerable (symptoms that limit activities but not decedent on others); 5 = Mildly frail (impairment of daily activities); 6 = Moderately frail (progressive impairment and declined activities); 7 = Severely frail (completely dependent cognitively or physically but not terminally ill); 8 = Very severely frail (completely dependent and approaching the end of life); 9 = Terminally ill (life expectancy < 6 months).
Measurement of Immune Parameters
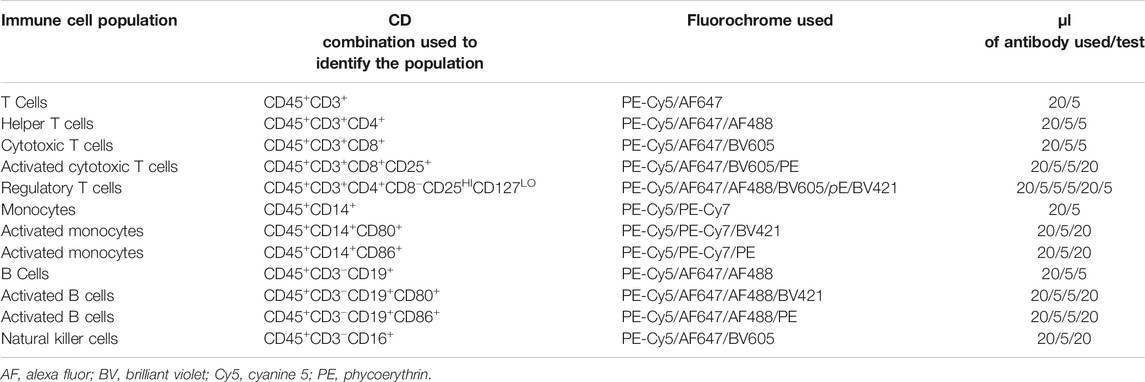
Blood was collected into EDTA or heparin at the care homes and was posted to the University of Southampton. Whole blood was used to determine full blood count, for immune phenotyping, for assessment of neutrophil and monocyte phagocytosis, and for cultures to determine production of immune mediators after stimulation. Plasma was prepared for measurement of CRP and immune mediator concentrations. Immune parameters were measured as described in detail previously (Castro-Herrera et al., 2018). Briefly, full blood count was determined in blood collected into EDTA using an automated UniCel Beckman Coulter Dxl 800 (Beckman Coulter, High Wycombe, United Kingdom). Full blood collected into heparin was used for immune phenotyping using flow cytometry following staining with fluorescently labelled antibodies to immune cell surface structures. Blood (500 µl) was placed in BD Trucount™ tubes (BD Pharmingen Oxford, United Kingdom). Antibodies were purchased from BD Pharmingen (Oxford, United Kingdom). Table 1 lists the details of the immune phenotyping. Staining was performed at room temperature for 20 min and protected from light. BD-FACS lysing solution (1 ml; BD Pharmingen Oxford, United Kingdom) was added and tubes were incubated for 20 min. Tubes were vortexed and placed at room temperature in a dark place. Tubes were analyzed on a BD FACS LSRF Fortessa TM X-20 Special order (BD Biosciences, San Jose, CA). Isotype controls were run at a medium flow rate. 10,000 events were collected for all samples in tubes containing Trucount beads. Beads were gated and 5,000 events were collected within the bead region. Data analyses were performed with BD FACSDiva 8.0.1 software. Instrument stability was checked daily using the cytometer setup and tracking to evaluate performance with Research Beads™ (BD Biosciences, Oxford, United Kingdom).
Phagocytic activity of blood neutrophils and monocytes toward E. coli was assessed in heparinsed whole blood (200 µl) using the commercially available Phagotest™ kit (Glycotope Biotechnology GmbH, Heidelberg Germany). Events (20,000) were collected using a BD FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). Both the percentage of cells (neutrophils and monocytes) involved in phagocytosis and their geometric mean fluorescence intensity (reflecting the number of ingested bacteria per cell) were analyzed.
For whole blood cultures, 500 μl heparinized whole blood was diluted 1:10 in Roswell Park Memorial Institute 1640 culture medium supplemented with penicillin (50 U/ml), streptomycin (50 μg/ml) and L-glutamine (2 mM) (Sigma Aldrich, Gillingham, United Kingdom). Diluted blood (990 μl) was added to the wells of a 24-well flat-bottomed cell culture plate. Then, 10 µl of either medium, lipopolysaccharide (LPS; from E. coli K12 strain), peptidoglycan (PGN; from Staphylococcus aureus) or phytohaemagglutinin (PHA; from Phaseolus vulgaris) was added to the wells to obtain final concentrations of 10 μg/ml LPS, 5 μg/ml PGN or 5 μg/ml PHA respectively. Cultures were incubated for 24 h at 37°C in an atmosphere of 95% air and 5% CO2. Supernatants were collected by centrifuging the plate at 2000 rpm for 5 min and were then stored at −80°C for analysis. Once all supernatants were ready to be analyzed, magnetic luminex assays (Bio-Techne, R&D Systems, Abingdon, United Kingdom) were used. Analytes were measured in negative controls and in the medium after stimulation with PGN or LPS and the assay limits of detection (pg/ml) were: tumor necrosis factor (TNF-α) (0.62), interleukin (IL)-1β (0.25), IL-6 (0.38), IL-10 (2.93), and IL-12p70 (2.39). Analytes measured following stimulation with PHA were TNF-α (limit of detection (pg/ml) (1.2) and interferon (IFN-γ) (0.4). Assays were performed according to manufacturer’s instructions. Microparticles were resuspended in buffer and read using a Bio-Rad-plex Luminex Analyzer.
Plasma was prepared from 1 ml of heparinized whole blood by centrifugation at 1,500 rpm for 5 min and stored at −20°C prior to analysis. CRP, immune mediators and soluble receptors were measured by magnetic luminex assays (Bio-Techne, R&D Systems, Abingdon, United Kingdom). Analytes measured and the assay limits of detection (pg/ml) were CRP (116), TNF-α (0.54), IL-6 (0.31), IL-10 (0.24), IL-17 (1.8), IL-12p70 (2.96), IL-1ra (18), TNF receptor II (TNFRII; 0.5), monocyte chemoattract protein (MCP-1; 9.9), soluble vascular cell adhesion molecule (sVCAM-1; 238), soluble E-selectin (sE-selectin; 18.8), soluble intercellular adhesion molecule (sICAM-1; 87.9), and interferon gamma-induced protein 10 (IP-10; 1.18). Assays were performed according to manufacturer’s instructions. Microparticles were resuspended in buffer and read using a Bio-Rad-plex Luminex Analyzer.
Statistical Analysis
As this is an exploratory study no power calculation was done. Normality of data was assessed by visual inspection of histogram distributions and by using the Shapiro Wilk and Kolmogorov-Smirnov tests. Data were not normally distributed. Thus, data are presented using median, interquartile range and percentiles. Comparisons of outcomes between sexes were made using the Mann-Whitney U test. Correlations amongst age (as a continuous variable), frailty index and length of care home residence (as a continuous variable) were investigated using Spearmans’s test. Associations between age, frailty index, length of care home residence and each immune parameter were investigated using linear regression. Multivariate analysis using linear regression models was used to examine the independent influence of age, frailty and length of care home residence on each immune parameter. All data were log10 transformed prior to conducting these analyses. Data collation and analysis were performed in SPSS version 22, Microsoft Excel and PRISM software. In all cases a value for p < 0.05 was taken to indicate statistical significance; no correction for multiple testing was made.
Results
Participants Characteristics
Table 2 shows the characteristics of the subset of participants studied here compared to those of the entire PRINCESS cohort; the characteristics are comparable. The age range of the included care home residents was 65–102 years. They had a mean age (±SD) of 85.3 (±7.5) yr and had been residing in the care home for a mean (±SD) of 1.89 (±2.16) yr at the time of study commencement (Table 2), although it is not known if they had previously resided in another care home. There were more women than men (63.4 vs 36.6%). Over one-third (40.7%) of included participants were severely frail (category 7) and 42% were moderately or mildly frail (categories 6 and 5) (Table 2). Age, frailty and duration of care home residence did not differ between women and men (data not shown).
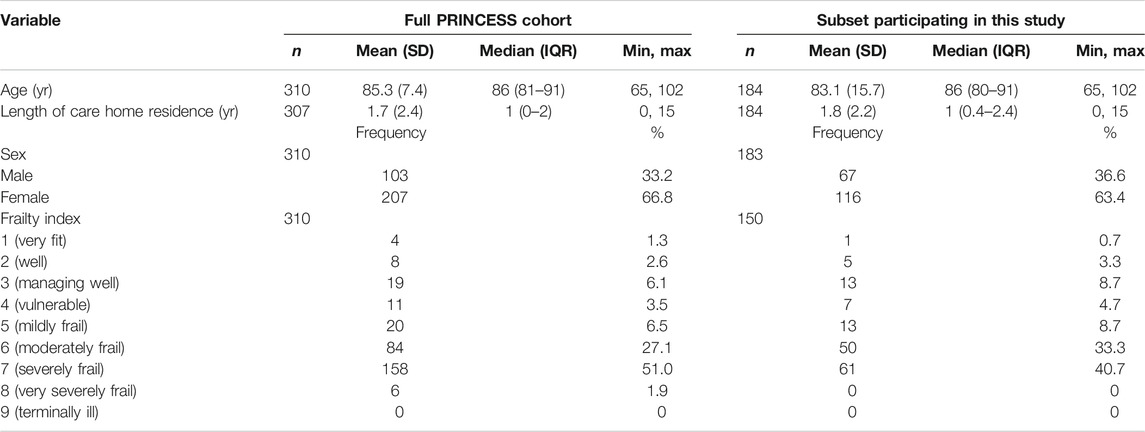
TABLE 2. Characteristics of participants in this study and those of the full PRINCESS cohort at commencement of study and enrollment.
Association Amongst Age, Frailty and Length of Care Home Residence
There was a significant positive correlation between length of care home residence and frailty index (Spearman’s correlation coefficient = 0.185; p = 0.023) as shown in Figure 1A. Age and frailty index and age and length of care home residence were not significantly correlated (Figures 1B,C).
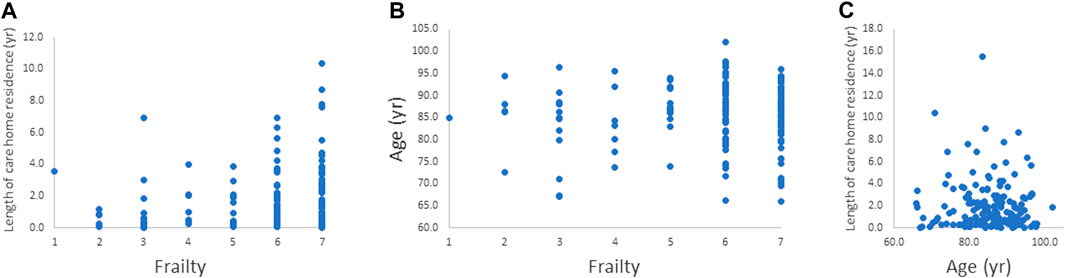
FIGURE 1. Relationships between. (A) frailty index and length of care home residence, (B) frailty index and age, and (C) age and length of care home residence. The relationship between frailty index and length of care home residence was significant (p = 0.023).
Full Blood Count and Immune Parameters
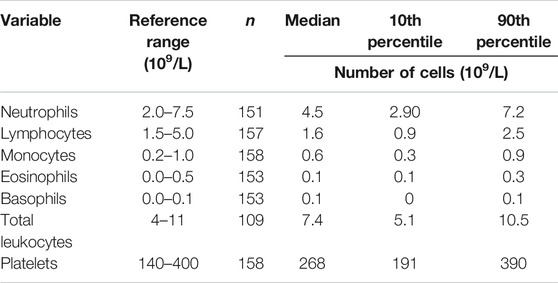
Data for the components of the full blood count were mainly within the reference range, apart from lymphocyte numbers (Table 3). Many participants had low blood lymphocyte numbers, with 31% (n = 49) having numbers below the lower value of the reference range. The percentage of women and men with lymphocyte numbers below the lower value of the reference range did not differ. Age, frailty, and length of care home residence were not different between those with blood lymphocyte numbers below or within the reference range. A small proportion of participants (n = 12; 7.6%) had platelet numbers above the upper value of the reference range. Platelet numbers were higher in women than men (median (10th and 90th centile) 293 (211, 389) vs 251 (168, 386) 109/L; p = 0.039). Data for immune phenotypes, neutrophil and monocyte phagocytosis, plasma CRP and immune mediator concentrations, and concentrations of immune mediators in stimulated whole blood cultures are shown in Tables 4–7, respectively. There are no reference values for these immune outcomes, but Table 4 lists a selection of previously reported vales for immune phenotypes in older individuals (Seidler et al., 2010; Tavares et al., 2014; Qin et al., 2016). Participants in the current study had lower numbers of T lymphocytes and natural killer cells and a lower ratio of CD4+ to CD8+ T lymphocytes than reported in these other studies of older adults. Ten percent of participants had a ratio of CD4+ to CD8+ T lymphocytes less than 1 (Table 4). The only immune outcome that differed between sexes was plasma IL-10 concentration, which was higher in men than women (median (10th and 90th centile) 0.66 (0.25, 3.59) vs 0.56 (0.12, 1.64) pg/ml; p = 0.039).
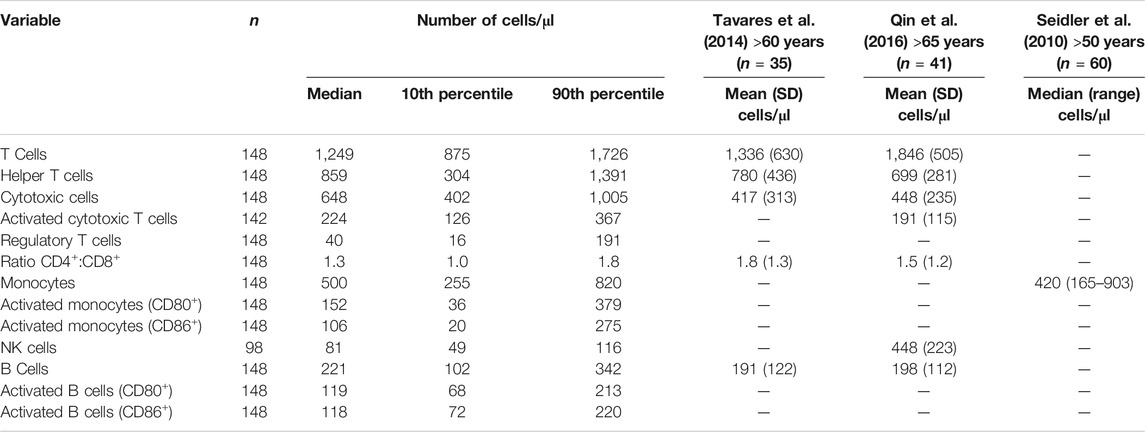
TABLE 4. Blood immunophenotypes in older people resident in care homes along with a comparison of values from the literature.
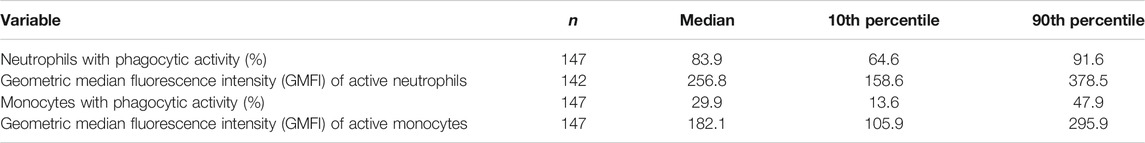
TABLE 5. Phagocytosis of E. coli by blood neutrophils and monocytes from older people resident in care homes.
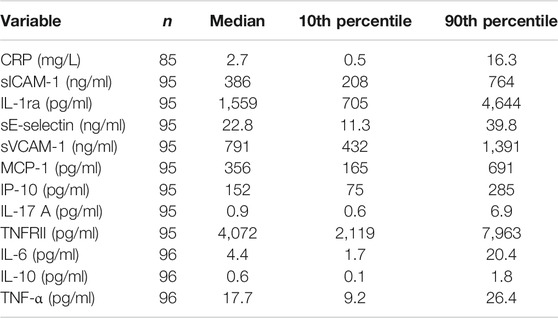
TABLE 6. Concentrations of CRP and immune mediators in plasma from older people resident in care homes.
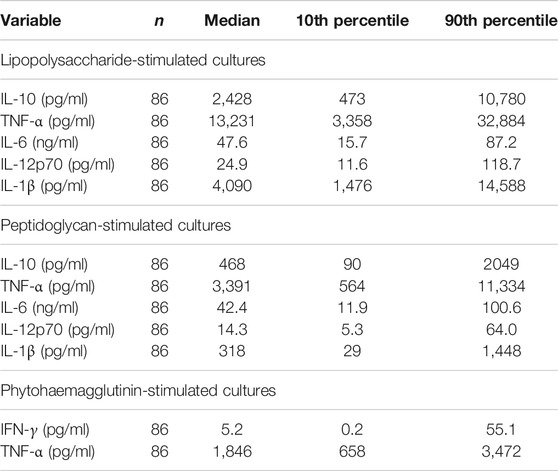
TABLE 7. Immune mediator concentrations in stimulated cultures of whole blood from older people resident in care homes.
Relationship Between Immune Markers and Age, Frailty and Length of Care Home Residence
Univariate Analysis
Associations of each immune marker with age, frailty and length of care home residence were investigated. In most cases there was no statistically significant association (Supplementary Tables S1–S7). Exceptions were:
Platelet numbers were positively associated with frailty index (p = 0.003).
Plasma CRP, IL-1ra, sE-selectin, and IP-10 were positively associated with frailty index (p = 0.014, 0.023, 0.015, and 0.016, respectively) (Figure 2).
PGN-stimulated IL-10 production was inversely associated with frailty index (p = 0.031).
Plasma sVCAM-1, IP-10 and TNFRII were positively associated with age (p = 0.023, 0.002, and 0.002, respectively) (Figure 3).
Plasma MCP-1 was positively associated with length of care home residence (p = 0.012).
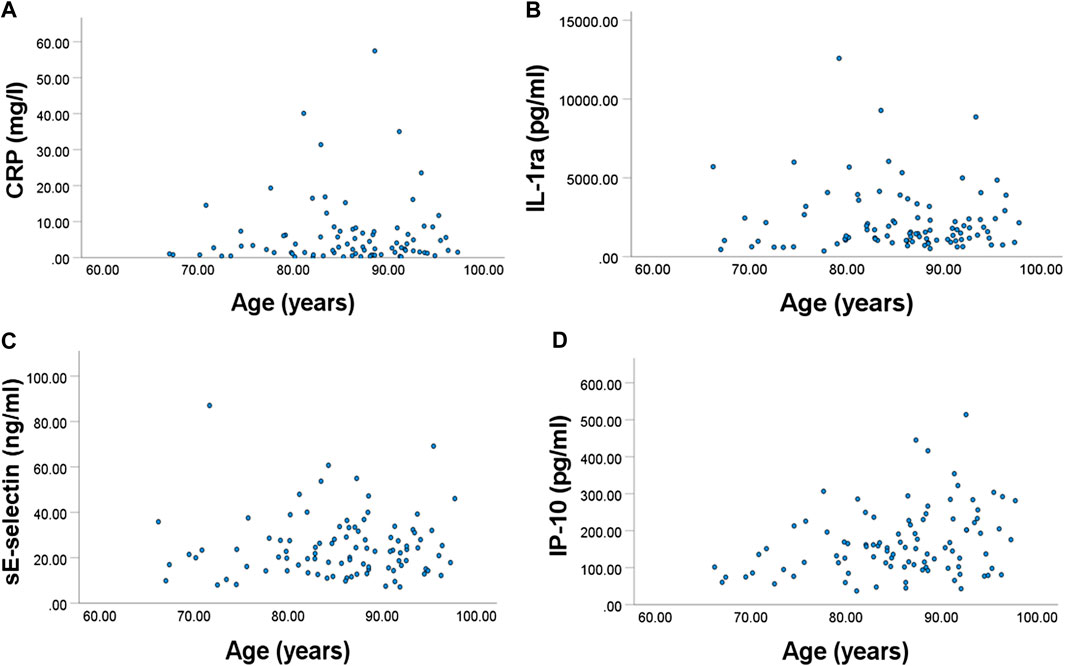
FIGURE 2. Relationships between frailty index and plasma concentration of (A) CRP, (B) IL-1ra, (C) sE-selectin, and (D) IP-10. All were significant.
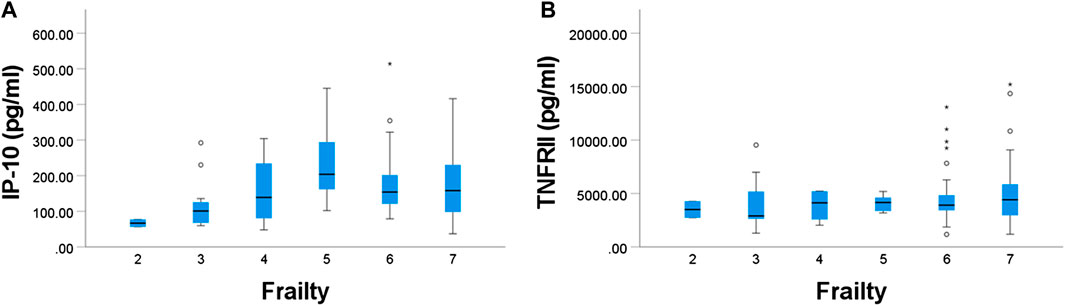
FIGURE 3. Relationships between age and plasma concentration of (A) IP-10 and (B) TNF-RII. Both were significant.
Multivariate Analysis
A linear regression model was used to identify the independent contribution of age, frailty and length of care home residence to the various immune parameters as dependent variables (Supplementary Tables S1–S7). Among the parameters included as part of the full blood count, frailty was a significant predictive factor for platelet numbers (adjusted coefficient 0.23 (95% CI: 0.08, 0.37), p = 0.002; Supplementary Table S1). Among the immune phenotypes, age was a significant predictive factor for activated monocytes as determined by CD86 expression (adjusted coefficient 2.78 (95% CI: 0.87, 4.70), p = 0.005; Supplementary Table S2). Apart from these, none of the covariates was found to contribute significantly to the individual components of the full blood count (Supplementary Table S1) or the immune cell phenotypes (Supplementary Table S2). There were also no predictive associations between the covariates and neutrophil or monocyte phagocytosis (Supplementary Table S3). For immune mediators after stimulation of whole blood cultures, the only predictive association was between frailty and PGN-stimulated IL-10 (adjusted coefficient −0.79 (95% CI: 1.54, −0.04), p = 0.038, Supplementary Table S5). Frailty index, age and length of care home residence each independently predicted some plasma immune mediators (Supplementary Table S4). Age was a significant predictor of plasma IP-10 (adjusted coefficient 1.77 (95% CI: 0.61, 2.93), p = 0.003), TNFRII (adjusted coefficient 1.76 (95% CI: 0.60, 2.92), p = 0.003) and sVCAM-1 (adjusted coefficient 1.19 (95% CI: 0.13, 2.26), p = 0.029). Frailty index was an independent predictor of CRP (adjusted coefficient 1.18 (95% CI: 0.34, 2.01), p = 0.006), IL-1ra (adjusted coefficient 0.43 (95% CI: 0.00, 0.87), p = 0.050), sE-selectin (adjusted coefficient 0.35 (95% CI: 0.05, 0.66), p = 0.024) and IP-10 (adjusted coefficient 0.32 (95% CI: 0.32, 0.64), p = 0.042). Lastly, length of care home residence was an independent predictor of MCP-1 (adjusted coefficient 0.10 (95% CI: 0.01, 0.19), p = 0.026).
Discussion
Few studies have described immune parameters in older people resident in care homes. Here we describe a selection of immune and inflammatory markers in blood and ex vivo immune cell functions in a sample of 184 older people resident in care homes aged 65–102 years and their association with frailty, age and length of care home residence. Almost a third of the participants had low total lymphocyte numbers. Moreover, participants had lower numbers of T lymphocytes and natural killer cells and a lower ratio of CD4+ to CD8+ T lymphocytes than reported in other studies of older adults (Tavares et al., 2014; Qin et al., 2016). These findings are consistent with the hallmarks of immunosenescence (Boraschi et al., 2013; Pera et al., 2015; Weyand and Goronzy, 2016) and would indicate an increased risk of infections and poor vaccination responses (Crétel et al., 2010; Bektas et al., 2017). Lymphocyte numbers were not associated with age or frailty. This contrasts with the report of Collerton et al. (2012) who found an inverse association of lymphocyte numbers with frailty, assessed using two different models, in 845 85 years olds in the United Kingdom. Furthermore, Bernabeu-Wittel et al. (2019) identified that low lymphocyte numbers were associated with frailty in hospitalized older people with poly-pathologies; they also identified that frailty was a risk factor for mortality at 12 months. In another study, there was an inverse association between frailty score and lymphocyte count in institutionalized older people, but lymphocyte count did not predict hospitalisations or mortality, although frailty did predict mortality (Fernandez-Garrido et al., 2018). Recently, low lymphocyte counts were shown to associate with frailty in patients with cardiovascular disease (Bodolea et al., 2020).
Other associations identified in the current study indicate links between greater frailty and increased inflammation and between increasing age and increased inflammation. The association between frailty and inflammation is consistent with the proposal that frailty is an inflammatory condition (Bruunsgaard and Pedersen, 2003; Angulo et al., 2016), while the associations between age and inflammatory markers or responses are consistent with the concept of inflammageing (Breitbart and Stollar, 2000; Ferrucci and Fabbri, 2018).
A proportion of participants (7.6%) had a platelet count above the upper limit of the reference range. The exact threshold at which platelet numbers become a marker of chronic inflammation has not been clearly defined, but high platelet numbers are related to inflammatory conditions, cancer and infection as well as endothelial dysfunction (Jones, 2016; Haynes et al., 2017) and atherosclerotic plaque formation (Stolla et al., 2016). Moreover, platelet numbers increased across categories of frailty, findings also confirmed through modelling, where frailty emerged as a significant independent predictor of platelet numbers. Recently, Bodolea et al. (2020) found that platelet numbers associate with frailty in patients with cardiovascular disease. Fuentes et al. (2017) report that platelet oxidative stress is a novel marker of cardiovascular risk in frail older people and Starr and Deary (2011) observed increased platelet numbers over a time-frame of 8 years in individuals initially aged over 79 years. The current study did not reveal a significant association of platelet numbers with age. Nevertheless, increased platelet numbers could be a marker of mortality risk through increased frailty. Platelets trigger leukocyte adhesion which favors their aggregation. The mechanism seems to be linked to platelet-induced production of adhesion molecules (Garraud et al., 2012; Jenne et al., 2013).
CD80 and CD86 were used as markers of activated blood monocytes. The linear regression model showed that age was a significant independent predictor of CD86+ monocytes over frailty and length of care home residence. Busse et al. demonstrated that monocytes expressing CD86 were increased in elderly individuals (Busse et al., 2015) and concluded this to be a consequence of immunosenescence/inflammageing, as this trait appeared in both a cohort of elderly individuals with dementia and in healthy age-matched controls (Busse et al., 2015).
Phagocytic function has been reported to decline with age leading to a failure to remove foreign antigenic particles and autologous senescent cells (Goronzy and Weyand, 2012; Li, 2013). In the current study, phagocytic function of neutrophils and monocytes was not significantly associated with age, frailty or length of care home residence. These findings do not confirm what has been shown by others where phagocytic function, especially of neutrophils, declined with age (Butcher et al., 2000; Butcher et al., 2001). However, this may be because the current study only investigated older participants. A previous comparison of neutrophil phagocytosis among three age groups (21–36, 38–56, and 62–83 years) found a significant age-dependent reduction in the number of phagocytosed E. coli (Wenisch et al., 2000). Thus, that study investigated a much wider age range than in the current study. It is possible that beyond 65 years of age, the alteration in phagocytic activity of neutrophils and monocytes becomes less dramatic than the change between young and older or middle-aged and older individuals.
Previous studies have associated markers of inflammation with different chronic and age-related conditions (e.g., cardiovascular disease and dementia (Assar et al., 2016; Calabrese et al., 2018). Others have reported that age and frailty are factors associated with inflammatory biomarkers (Bruunsgaard and Pedersen, 2003; Angulo et al., 2016; Branas et al., 2018). Indeed, researchers have reported that there is a characteristic “cytokinome” (Costantini et al., 2010) in older people with physical frailty and sarcopenia (Marzetti et al., 2019), suggesting IP-10 to be a marker of frailty and sarcopenia. The current study identified that IP-10 was associated with frailty. In the current study frailty was also an independent predictor of CRP, IL-1ra and sE-selectin. Previous studies have shown that aging is associated with increased concentrations of sICAM-1 and sVCAM-1 (Calder et al., 2017; Calabrese et al., 2018). The current study found that sVCAM-1 concentration had an association with age, as did IP-10 and TNFRII. These findings support the idea that inflammatory pathways are upregulated in aging and in age-related diseases (Cardoso et al., 2018).
Beyer et al. suggest that inflammation is related to muscle wasting, facilitating progression of frailty: in a population of 33 geriatric individuals, those with higher MCP-1 showed a significantly lower grip strength and lower lean body mass (Beyer et al., 2012). Animal research has suggested that MCP-1 is a potential biomarker of biological aging (Yousefzadeh et al., 2018). However, one study reported lower plasma MCP-1 in frail compared with non-frail older care home residents (Seto et al., 2015), while in the current study frailty was not a predictor of MCP-1 concentration.
Other inflammatory markers where frailty appeared as a significant contributory factor over age and length of stay at care home—identified through the regression model—were IL-1ra and the soluble adhesion molecule sE-selectin. IL-1ra opposes the action of pro-inflammatory IL-1 and may be released in an effort to mitigate inflammation. Nevertheless, IL-1ra has been linked as an independent risk factor of morbidity and mortality in the older people resident in care homes (Bruunsgaard and Pedersen, 2003). Upregulation of the expression of adhesion molecules with frailty has been reported (Licastro et al., 2005; Jenny, 2012).
Inflammageing, either low grade or chronic, is commonly linked to morbidity and mortality (Ahmad et al., 2009; Ahmadi-Abhari et al., 2014). Our findings support an association of inflammation with frailty in older people resident in care homes. Inflammageing is a predictor of frailty in elderly (Zhang et al., 2017). Edvardsson et al. have demonstrated that inflammatory markers are related to reduced survival in a follow-up study for one year with frail older people resident in care homes (Edvardsson et al., 2019).
Experiments to assess cellular responses ex vivo were performed through whole blood cultures. These experiments allowed assessment of inflammatory and immune mediator production via stimulation of toll-like receptor (TLR)2 and TLR4 with PGN and LPS, respectively, as well as T cell stimulation with PHA. The activation of TLR2 and TLR4 leads to increased production of multiple cytokines (Schwandner et al., 1999; Skinner et al., 2005). Findings herein presented showed that IL-10, TNF-α and IL-1β were potently induced by LPS in comparison to PGN. LPS induced median production values 5-fold higher for IL-10, 3.9-fold higher for TNF-α and almost 12-fold higher for IL-1β when compared with PGN. Furthermore, a superior production of IL-12p70 was induced by LPS when compared with PGN, but the difference was less (two-fold). Lastly, IL-6 was similarly induced by both PGN and LPS. PHA stimulates T cells. The production of TNF-α following PHA stimulation was lower than with LPS and PGN. The potent effects exerted by LPS agree with what has been shown by others (Andersson et al., 1992). The association of health and TLR responsiveness, particularly TLR4, in older people resident in care homes has not been widely explored. McFarlin et al. have suggested that TLR4 appears to have a role in regulating the linkage between cytokine production (IL-1β and TNF-α) and physically active lifestyle regardless of age. In that study, a group of older (60–80 years) and young (18–30 years) adults were categorized as “active” or “inactive.” There was significantly higher production of IL-1β and TNF-α in the inactive group in both young and older people (McFarlin et al., 2006). McFarlin et al. (2006) also reported lower expression of TLR4 in the active group. Similar observations were reported in a group of older women exposed to regular training (McFarlin et al., 2004). Current findings certainly suggest an active TLR4 pathway in the older people resident in care homes according to the cytokine production detected in the cultures following LPS stimulation. A predisposition to active responses of innate immune cells via TLR4, and perhaps other pattern recognition receptors, may be one reason for higher circulating concentrations of inflammatory cytokines in older people, one of the hallmarks of inflammageing.
IL-10 induced by PGN was significantly inversely associated with frailty. IL-10 is an anti-inflammatory cytokine that counterbalances pro-inflammatory responses (Iyer and Cheng, 2012). The older people resident in care homes appeared to show an imbalance in IL-10 and TNF-α.
Our findings may be compared with those of Collerton et al. (2012) who measured a range of immune and inflammatory parameters in 845 85 years olds in the United Kingdom and related these to frailty assessed with two different models. As mentioned earlier, that study reported an inverse association between frailty and lymphocyte numbers which was not observed in the current study. This may represent differences in the characteristics of the participants included in the two studies (all were resident in care homes in the current study whereas this was not the case in Collerton et al. (2012)); age range was 66–102 years in the current study but all participants were aged 85 years in Collerton et al. (2012) or the smaller sample size of the current study. Collerton et al. (2012) also reported positive associations of frailty with total leukocyte and neutrophil counts, which we did not observe. Collerton et al. (2012) reported a positive association between frailty and CRP concentrations, as observed in the current study. They also identified a lack of association of frailty with monocyte, basophil, and eosinophil counts, ratio of CD4+ to CD8+ lymphocytes, and LPS-stimulated TNF-α and IL-6 production; our observations are consistent with this. Collerton et al. (2012) did not report platelet numbers or plasma concentrations of inflammatory mediators, which were associated with frailty in the current study.
The current study has several strengths. There were few restrictions on participant inclusion. A broad range of immune and inflammatory outcomes were measured, representing several different components of the immune system; these included static measures in blood (full blood count, immune phenotypes, plasma mediators, CRP) as well cell responses after challenge (phagocytosis, blood culture responses to LPS, PGN, and PHA) and components of innate (phagocytosis, blood culture responses to LPS and PGN) and acquired immunity (blood culture responses to PHA). Finally, linear regression modelling was used to identify independent effects of age, frailty, and time of care home residence on the outcomes reported. However, the study also has some limitations. Firstly, not all immune outcomes were available for all 184 participants; this is mainly because some blood samples did not arrive at the laboratory within a predetermined time to assure the viability of the immune assay (Castro-Herrera et al., 2018). Secondly, the samples were from participants in a randomized controlled trial (Owen-Jones et al., 2019; Butler et al., 2020) and this required exclusion of some of the care home residents; thus the findings are not generalisable to all care home residents. Thirdly, we did not collect data on co-morbidities (other than frailty index) or medication use, which might be relevant to immune and inflammatory biomarkers. Finally, since the study was exploratory no power calculation was done, and so non-significant findings must be interpreted with caution, and significant findings interpreted cautiously since we did not correct for the multiple statistical comparisons performed.
Data Availability Statement
The datasets presented will be linked with clinical data produced as part of the same project. Requests to access the datasets should be directed to David Gillespie R2lsbGVzcGllRDFAY2FyZGlmZi5hYy51aw==.
Ethics Statement
The studies involving human participants were reviewed and approved by Wales REC 3 (15/WA/0306). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MLo, MLa, RL, KH, DG, FH, PL, CB, and PC conceptualized and designed the PRINCESS trial; EO-J and RL provided support for the PRINCESS trial; CB oversaw the conduct of the PRINCESS trial; VC-H conducted all laboratory research supported by HF under the supervision of EM and PC; VC-H, KH, and DG conducted the statistical analysis; VC-H and PC drafted the manuscript; all authors commented on the manuscript and agreed the final version.
Funding
This research was supported by the Efficacy and Mechanism Evaluation Program which is funded by the Medical Research Council (MRC) and National Institute for Health Research (NIHR), with contributions from CSO in Scotland, HCRW in Wales and the HSC R& D, Public Health Agency in Northern Ireland (Efficacy and Mechanism Evaluation (EME), grant number 13/95/10). VMC-H is supported by Colciencias, Colombia. FDRH acknowledges part-funding from the NIHR School for Primary Care Research, the NIHR Collaboration for Leadership in Health Research and Care (CLARHC) Oxford, the NIHR Oxford Biomedical Research Center, and the NIHR Oxford Medtech and In-Vitro Diagnostics Co-operative. PCC is supported by the NIHR Southampton Biomedical Research Center. The views expressed in this publication are those of the authors and not necessarily those of the MRC, the National Health Service, the NIHR or the Department of Health.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Center for Trials Research, Cardiff University and the University of Oxford Primary Care and Vaccines Clinical Trials Collaborative for providing support in the conduct of the trial, and the staff of participating care homes.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fragi.2021.599084/full#supplementary-material.
References
Agarwal, S., and Busse, P. J. (2010). Innate and adaptive immunosenescence. Ann. Allergy Asthma Immunol. 104, 183–190. doi:10.1016/j.anai.2009.11.009
Ahmad, A., Banerjee, S., Wang, Z., Kong, D., Majumdar, A., and Sarkar, F. (2009). Aging and inflammation: etiological culprits of cancer. Curr. Aging Sci. 2, 174–186. doi:10.2174/1874609810902030174
Ahmadi-Abhari, S., Kaptoge, S., Luben, R. N., Wareham, N. J., and Khaw, K.-T. (2014). Longitudinal association of C-reactive protein and lung function over 13 years: the EPIC-Norfolk study. Am. J. Epidemiol. 179, 48–56. doi:10.1093/aje/kwt208
Ahmed, N., Mandel, R., and Fain, M. J. (2007). Frailty: an emerging geriatric syndrome. Am. J. Med. 120, 748–753. doi:10.1016/j.amjmed.2006.10.018
Andersson, J., Björk, L., Dinarello, C. A., Towbin, H., and Andersson, U. (1992). Lipopolysaccharide induces human interleukin-1 receptor antagonist and interleukin-1 production in the same cell. Eur. J. Immunol. 22, 2617–2623. doi:10.1002/eji.1830221022
Angulo, J., El Assar, M., and Rodríguez-Mañas, L. (2016). Frailty and sarcopenia as the basis for the phenotypic manifestation of chronic diseases in older adults. Mol. Aspects Med. 50, 1–32. doi:10.1016/j.mam.2016.06.001
Assar, M. E., Angulo, J., and Rodríguez-Mañas, L. (2016). Diabetes and ageing-induced vascular inflammation. J. Physiol. 594, 2125–2146. doi:10.1113/JP270841
Atienza, M., Ziontz, J., and Cantero, J. L. (2018). Low-grade inflammation in the relationship between sleep disruption, dysfunctional adiposity, and cognitive decline in aging. Sleep Med. Rev. 42, 171–183. doi:10.1016/j.smrv.2018.08.002
Bauer, M. E., and Fuente, M. D. l. (2016). The role of oxidative and inflammatory stress and persistent viral infections in immunosenescence. Mech. Ageing Development 158, 27–37. doi:10.1016/j.mad.2016.01.001
Beck-Sague, C., Villarino, E., Giuliano, D., Welbel, S., Latts, L., Manangan, L. M., et al. (1994). Infectious diseases and death among nursing home residents: results of surveillance in 13 nursing homes. Infect. Control. Hosp. Epidemiol. 15, 494–496. doi:10.1086/646957
Bektas, A., Schurman, S. H., Sen, R., and Ferrucci, L. (2017). Human T cell immunosenescence and inflammation in aging. J. Leukoc. Biol. 102, 977–988. doi:10.1189/jlb.3RI0716-335R
Bernabeu-Wittel, M., González-Molina, Á., Fernández-Ojeda, R., Díez-Manglano, J., Salgado, F., Soto-Martín, M., et al. (2019). Impact of sarcopenia and frailty in a multicenter cohort of polypathological patients. J. Clin. Med. 8, 535. doi:10.3390/jcm8040535
Beyer, I., Njemini, R., Bautmans, I., Demanet, C., Bergmann, P., Mets, T., et al. (2020). Inflammation-related muscle weakness and fatigue in geriatric patients. Exp. Gerontol. 47, 52–59. doi:10.1016/j.exger.2011.10.005
Bodolea, C., Hiriscau, E. I., Buzdugan, E.-C., Grosu, A. I., Stoicescu, L., Vesa, S., et al. (2020). The association between peripheral blood cells and the frailty syndrome in patients with cardiovascular diseases. EMIDDT 20, 1419–1433. doi:10.2174/1871530320666200813135905
Boraschi, D., Aguado, M. T., Dutel, C., Goronzy, J., Louis, J., et al. (2013). The gracefully aging immune system. Sci. Transl. Med. 5, 185ps8. doi:10.1126/scitranslmed.3005624
Brañas, F., Azcoaga, A., García Ontiveros, M., and Antela, A. (2018). Cronicidad, envejecimiento y multimorbilidad. Enferm. Infecc. Microbiol. Clin. 36 (Suppl. 1), 15–18. doi:10.1016/S0213-005X(18)30241-6
Breitbart, E., and Stollar, B. D. (2000). Aging and the human immune system. Isr. Med. Assoc. J. 2, 703–707.
Brüünsgaard, H., and Pedersen, B. K. (2003). Age-related inflammatory cytokines and disease. Immunol. Allergy Clin. North Am. 23, 15–39. doi:10.1016/s0889-8561(02)00056-5
Busse, S., Steiner, J., Alter, J., Dobrowolny, H., Mawrin, C., Bogerts, B., et al. (2015). Expression of HLA-DR, CD80, and CD86 in healthy aging and Alzheimer’s disease. J. Alzheimers Dis. 47, 177–184. doi:10.3233/Jad-150217
Butcher, S., Chahel, H., and Lord, J. M. (2000). Ageing and the neutrophil: no appetite for killing? Immunology 100, 411–416. doi:10.1046/j.1365-2567.2000.00079.x
Butcher, S. K., Chahal, H., Nayak, L., Sinclair, A., Henriquez, N. V., Sapey, E., et al. (2001). Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J. Leukoc. Biol. 70, 881–886. doi:10.1189/jlb.70.6.881
Butler, C. C., Lau, M., Gillespie, D., Owen-Jones, E., Lown, M., Wootton, M., et al. (2020). Effect of probiotic use on antibiotic administration among care home residents:a randomized clinical trial. JAMA 324, 47–56. doi:10.1001/jama.2020.8556
Calabrese, V., Santoro, A., Monti, D., Crupi, R., Di Paola, R., Latteri, S., et al. (2018). Aging and Parkinson’s Disease: inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free Radic. Biol. Med. 115, 80–91. doi:10.1016/j.freeradbiomed.2017.10.379
Calder, P. C., Bosco, N., Bourdet-Sicard, R., Capuron, L., Delzenne, N., Doré, J., et al. (2017). Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev. 40, 95–119. doi:10.1016/j.arr.2017.09.001
Cardoso, A. L., Fernandes, A., Aguilar-Pimentel, J. A., de Angelis, M. H., Guedes, J. R., Brito, M. A., et al. (2018). Towards frailty biomarkers: candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res. Rev. 47, 214–277. doi:10.1016/j.arr.2018.07.004
Carneiro, J. A., Cardoso, R. R., Durães, M. S., Guedes, M. C. A., Santos, F. L., Costa, F. M. D., et al. (2017). Frailty in the elderly: prevalence and associated factors. Rev. Bras. Enferm. 70, 747–752. doi:10.1590/0034-7167-2016-0633
Castelo-Branco, C., and Soveral, I. (2014). The immune system and aging: a review. Gynecol. Endocrinol. 30, 16–22. doi:10.3109/09513590.2013.852531
Castro-Herrera, V., Lown, M., Lewith, G., Miles, E. A., and Calder, P. C. (2018). Influence of delayed sample processing on blood immune cell phenotypes, immune cell responses and serum anti-influenza vaccine antibody titres. J. Immunol. Methods 458, 8–14. doi:10.1016/j.jim.2018.03.012
Chen, L. K., Hwang, A. C., Liu, L. K., Lee, W. J., and Peng, L. N. (2016). Frailty Is a geriatric syndrome characterized by multiple impairments: a comprehensive approach is needed. J. Frailty Aging 5, 208–213. doi:10.14283/jfa.2016.109
Collerton, J., Martin-Ruiz, C., Davies, K., Hilkens, C. M., Isaacs, J., Kolenda, C., et al. (2012). Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mech. Ageing Dev. 133, 456–466. doi:10.1016/j.mad.2012.05.005
Costantini, S., Castello, G., and Colonna, G. (2010). Human cytokinome: a new challenge for systems biology. Bioinformation 5, 166–167. doi:10.6026/97320630005166
Crétel, E., Veen, I., Pierres, A., Bongrand, P., and Gavazzi, G. (2010). Immunosénescence et infections, mythe ou réalité? Med. Mal. Infect. 40, 307–318. doi:10.1016/j.medmal.2009.12.008
De Martinis, M., Modesti, M., and Ginaldi, L. (2004). Phenotypic and functional changes of circulating monocytes and polymorphonuclear leucocytes from elderly persons. Immunol. Cell Biol. 82, 415–420. doi:10.1111/j.0818-9641.2004.01242.x
Dent, E., Kowal, P., and Hoogendijk, E. O. (2016). Frailty measurement in research and clinical practice: a review. Eur. J. Intern. Med. 31, 3–10. doi:10.1016/j.ejim.2016.03.007
Derhovanessian, E., and Pawelec, G. (2012). Vaccination in the elderly. Microb. Biotechnol. 5, 226–232. doi:10.1111/j.1751-7915.2011.00283.x
Edvardsson, M., Sund-Levander, M., Milberg, A., Ernerudh, J., and Grodzinsky, E. (2019). Elevated levels of CRP and IL-8 are related to reduce survival time: 1-year follow-up measurements of different analytes in frail elderly nursing home residents. Scand. J. Clin. Lab. Invest. 79, 288–292. doi:10.1080/00365513.2019.1609695
Fernandez-Garrido, J., Ruiz-Ros, V., Navarro-Martínez, R., Buigues, C., Martínez-Martínez, M., et al. (2018). Frailty and leucocyte count are predictors of all-cause mortality and hospitalization length in non-demented institutionalized older women. Exp. Gerontol. 103, 80–86. doi:10.1016/j.exger.2018.01.007
Ferrucci, L., and Fabbri, E. (2018). Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15, 505–522. doi:10.1038/s41569-018-0064-2
Franceschi, C. (2007). Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr. Rev. 65, S173–S176. doi:10.1301/nr.2007.dec.s173-s176
Fuentes, F., Palomo, I., and Fuentes, E. (2017). Platelet oxidative stress as a novel target of cardiovascular risk in frail older people. Vasc. Pharmacol. 93, 14–19. doi:10.1016/j.vph.2017.07.003
Garraud, O., Hamzeh-Cognasse, H., and Cognasse, F. (2012). Platelets and cytokines: how and why? Transfus. Clin. Biol. 19, 104–108. doi:10.1016/j.tracli.2012.02.004
GBD 2016 Causes of Death Collaborators (2017). Global, regional, and national age-sex specific mortality for 264 causes of death, 1980 -2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1151–1210. doi:10.1016/S0140-6736(17)32152-9
GBD 2016 DALYs and HALE Collaborators (2017). Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet 390, 1260–1344. doi:10.1016/S0140-6736(17)32130-X
Goodwin, K., Viboud, C., and Simonsen, L. (2006). Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 24, 1159–1169. doi:10.1016/j.vaccine.2005.08.105
Goronzy, J. J., and Weyand, C. M. (2012). Immune aging and autoimmunity. Cell. Mol. Life Sci. 69, 1615–1623. doi:10.1007/s00018-012-0970-0
Haynes, A., Linden, M. D., Robey, E., Naylor, L. H., Cox, K. L., Lautenschlager, N. T., et al. (2017). Relationship between monocyte-platelet aggregation and endothelial function in middle-aged and elderly adults. Physiol. Rep. 5, e13189. doi:10.14814/phy2.13189
Iyer, S. S., and Cheng, G. (2012). Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 32, 23–63. doi:10.1615/CritRevImmunol.v32.i1.30
Jenne, C. N., Urrutia, R., and Kubes, P. (2013). Platelets: bridging hemostasis, inflammation, and immunity. Int. J. Lab. Hematol. 35, 254–261. doi:10.1111/ijlh.12084
Jones, C. I. (2016). Platelet function and ageing. Mamm. Genome 27, 358–366. doi:10.1007/s00335-016-9629-8
Laksmi, P. W. (2014). Frailty syndrome: an emerging geriatric syndrome calling for its potential intervention. Acta Med. Indones 46, 173–174.
Li, W. (2013). Phagocyte dysfunction, tissue aging and degeneration. Ageing Res. Rev. 12, 1005–1012. doi:10.1016/j.arr.2013.05.006
Licastro, F., Candore, G., Lio, D., Porcellini, E., Colonna-Romano, G., Franceschi, C., et al. (2005). Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immun. Ageing 2, 8. doi:10.1186/1742-4933-2-8
Marzetti, E., Picca, A., Marini, F., Biancolillo, A., Coelho-Junior, H. J., Gervasoni, J., et al. (2019). Inflammatory signatures in older persons with physical frailty and sarcopenia: the frailty “cytokinome” at its core. Exp. Gerontol. 122, 129–138. doi:10.1016/j.exger.2019.04.019
McFarlin, B. K., Flynn, M. G., Campbell, W. W., Craig, B. A., Robinson, J. P., Stewart, L. K., et al. (2006). Physical activity status, but not age, influences inflammatory cytokine production and Toll-like receptor 4. Med. Sci. Sports Exerc. 38, S308. doi:10.1249/00005768-200605001-02204
McFarlin, B. K., Flynn, M. G., Campbell, W. W., Stewart, L. K., and Timmerman, K. L. (2004). TLR4 is lower in resistance-trained older women and related to inflammatory cytokines. Med. Sci. Sports Exerc. 36, 1876–1883. doi:10.1249/01.Mss.0000145465.71269.10
Montoya, A., and Mody, L. (2011). Common infections in nursing homes: a review of current issues and challenges. Aging Health 7, 889–899. doi:10.2217/AHE.11.80
Morley, J. E., Vellas, B., Abellan van Kan, G., Anker, S. D., Bauer, J. M., Bernabei, R., et al. (2013). Frailty consensus: a call to action. J. Am. Med. Dir. Assoc. 14, 392–397. doi:10.1016/j.jamda.2013.03.022
Olsen, C., Pedersen, I., Bergland, A., Enders-Slegers, M.-J., Jøranson, N., Calogiuri, G., et al. (2016). Differences in quality of life in home-dwelling persons and nursing home residents with dementia-a cross-sectional study. BMC Geriatr. 16, 137. doi:10.1186/s12877-016-0312-4
Onder, G., Carpenter, I., Finne-Soveri, H., Gindin, J., Frijters, D., Henrard, J. C., et al. (2012). Assessment of nursing home residents in europe: the services and health for elderly in long TERm care (SHELTER) study. BMC Health Serv. Res. 12, 5. doi:10.1186/1472-6963-12-5
Owen-Jones, E., Lowe, R., Lown, M., Gillespie, D., Addison, K., Bayer, T., et al. (2019). Protocol for a double-blind placebo-controlled trial to evaluate the efficacy of probiotics in reducing antibiotics for infection in care home residents: the Probiotics to Reduce Infections iN CarE home reSidentS (PRINCESS) trial. BMJ Open 9, e027513. doi:10.1136/bmjopen-2018-027513
Pawelec, G., Larbi, A., and Derhovanessian, E. (2010). Senescence of the human immune system. J. Comp. Pathol. 142 (Suppl. 1), S39–S44. doi:10.1016/j.jcpa.2009.09.005
Pera, A., Campos, C., López, N., Hassouneh, F., Alonso, C., Tarazona, R., et al. (2015). Immunosenescence: implications for response to infection and vaccination in older people. Maturitas 82, 50–55. doi:10.1016/j.maturitas.2015.05.004
Qin, L., Jing, X., Qiu, Z., Cao, W., Jiao, Y., Routy, J.-P., et al. (2016). Aging of immune system: immune signature from peripheral blood lymphocyte subsets in 1068 healthy adults. Aging 8, 848–859. doi:10.18632/aging.100894
Sampson, E. L., Feast, A., Blighe, A., Froggatt, K., Hunter, R., Marston, L., et al. (2019). Evidence-based intervention to reduce avoidable hospital admissions in care home residents (the Better Health in Residents in Care Homes (BHiRCH) study): protocol for a pilot cluster randomised trial. BMJ Open 9, e026510. doi:10.1136/bmjopen-2018-026510
Schwandner, R., Dziarski, R., Wesche, H., Rothe, M., and Kirschning, C. J. (1999). Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 274, 17406–17409. doi:10.1074/jbc.274.25.17406
Seidler, S., Zimmermann, H. W., Bartneck, M., Trautwein, C., and Tacke, F. (2010). Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol. 11, 30. doi:10.1186/1471-2172-11-30
Seto, E., Setiati, S., Laksmi, P. W., and Tamin, T. Z. (2015). Diagnostic test of a scoring system for frailty syndrome in the elderly according to cardiovascular health study, study of osteoporotic fracture and comprehensive geriatric assessment based frailty index compared with frailty index 40 items. Acta Med. Indones 47, 183–187.
Skinner, N. A., MacIsaac, C. M., Hamilton, J. A., and Visvanathan, K. (2005). Regulation of Toll-like receptor (TLR)2 and TLR4 on CD14dimCD16+ monocytes in response to sepsis-related antigens. Clin. Exp. Immunol. 141, 270–278. doi:10.1111/j.1365-2249.2005.02839.x
Starr, J. M., and Deary, I. J. (2011). Sex differences in blood cell counts in the Lothian Birth Cohort 1921 between 79 and 87 years. Maturitas 69, 373–376. doi:10.1016/j.maturitas.2011.05.007
Stolla, M., Grozovsky, R., Lee-Sundlov, M. M., Falet, H., and Hoffmeister, K. M. (2016). Effects of platelet circulatory age on platelet function. Blood 128, 413. doi:10.1182/blood.v128.22.413.413
Tavares, S. M. Q. M. C., Junior, W. d. L. B., and Lopes e Silva, M. R. (2014). Normal lymphocyte immunophenotype in an elderly population. Rev. Bras. Hematol. Hemoter. 36, 180–183. doi:10.1016/j.bjhh.2014.03.021
Trzonkowski, P., Myśliwska, J., Pawelec, G., and Myśliwski, A. (2009). From bench to bedside and back: the SENIEUR Protocol and the efficacy of influenza vaccination in the elderly. Biogerontology 10, 83–94. doi:10.1007/s10522-008-9155-5
Ventura, M. T., Casciaro, M., Gangemi, S., and Buquicchio, R. (2017). Immunosenescence in aging: between immune cells depletion and cytokines up-regulation. Clin. Mol. Allergy 15, 15–21. doi:10.1186/s12948-017-0077-0
Wenisch, C., Patruta, S., Daxböck, F., Krause, R., and Hörl, W. (2000). Effect of age on human neutrophil function. J. Leukoc. Biol. 67, 40–45. doi:10.1002/jlb.67.1.40
Weyand, C. M., and Goronzy, J. J. (2016). Aging of the immune system. Mechanisms and therapeutic targets. Ann. Am. Thorac. Soc. 13 (Suppl. 5), S422–S428. doi:10.1513/AnnalsATS.201602-095AW
Wilson, D., Jackson, T., Sapey, E., and Lord, J. M. (2017). Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res. Rev. 36, 1–10. doi:10.1016/j.arr.2017.01.006
Yoshikawa, T. T. (2000). Epidemiology and unique aspects of aging and infectious diseases. Clin. Infect. Dis. 30, 931–933. doi:10.1086/313792
Yousefzadeh, M. J., Schafer, M. J., Noren Hooten, N., Atkinson, E. J., Evans, M. K., Baker, D. J., et al. (2018). Circulating levels of monocyte chemoattractant protein-1 as a potential measure of biological age in mice and frailty in humans. Aging Cell 17, e12706. doi:10.1111/acel.12706
Keywords: care home residents, aging, frailty, immunity, inflammation, immunosenescence, inflammageing
Citation: Castro-Herrera VM, Lown M, Fisk HL, Owen-Jones E, Lau M, Lowe R, Hood K, Gillespie D, Hobbs FDR, Little P, Butler CC, Miles EA and Calder PC (2021) Relationships Between Age, Frailty, Length of Care Home Residence and Biomarkers of Immunity and Inflammation in Older Care Home Residents in the United Kingdom. Front. Aging 2:599084. doi: 10.3389/fragi.2021.599084
Received: 12 November 2020; Accepted: 03 February 2021;
Published: 17 March 2021.
Edited by:
Moisés Evandro Bauer, Pontifical Catholic University of Rio Grande do Sul, BrazilReviewed by:
Omar Cauli, University of Valencia, SpainFernanda Calvo-Fortes, Federal University of Minas Gerais, Brazil
Copyright © 2021 Castro-Herrera, Lown, Fisk, Owen-Jones, Lau, Lowe, Hood, Gillespie, Hobbs, Little, Butler, Miles and Calder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vivian M. Castro-Herrera, di5jYXN0cm9oMDlAZ21haWwuY29t
 Vivian M. Castro-Herrera
Vivian M. Castro-Herrera Mark Lown2
Mark Lown2 Philip C. Calder
Philip C. Calder