
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Aging Neurosci., 03 March 2025
Sec. Alzheimer's Disease and Related Dementias
Volume 17 - 2025 | https://doi.org/10.3389/fnagi.2025.1554834
Introduction: Inadequate primary care infrastructure and training in China and misconceptions about aging lead to high mis−/under-diagnoses and serious time delays for dementia patients, imposing significant burdens on family members and medical carers.
Main body: A flowchart integrating rural and urban areas of China dementia care pathway is proposed, especially spotting the obstacles of mis/under-diagnoses and time delays that can be alleviated by data-driven computational strategies. Artificial intelligence (AI) and machine learning models built on dementia data are succinctly reviewed in terms of the roadmap of dementia care from home, community to hospital settings. Challenges and corresponding recommendations to clinical transformation are then reported from the viewpoint of diverse dementia data integrity and accessibility, as well as models’ interpretability, reliability, and transparency.
Discussion: Dementia cohort study along with developing a center-crossed dementia data platform in China should be strongly encouraged, also data should be publicly accessible where appropriate. Only be doing so can the challenges be overcome and can AI-enabled dementia research be enhanced, leading to an optimized pathway of dementia care in China. Future policy-guided cooperation between researchers and multi-stakeholders are urgently called for dementia 4E (early-screening, early-assessment, early-diagnosis, and early-intervention).
Nowadays 16.99 million people in China are living with dementia [63–70% diagnosed with Alzheimer’s disease (AD)] (Wang et al., 2024). Without disease-modifying Alzheimer’s therapies, early diagnosis is critical to slowing disease progression and enhancing quality of life (Breijyeh and Karaman, 2020; Liss et al., 2021). In response, the National Health Commission of China, together with various medical organizations, has established a diagnostic process tailored to the Chinese population (Tian et al., 2019, 2021; National Center for Neurological Disorders et al., 2024) and is promoting the development of cognitive disorder treatment centers in township institutions (National Center for Neurological Disorders et al., 2024).
However, many patients delay seeking medical care due to misconceptions about aging, dementia-related stigma, financial and/or distant issues, and inadequate primary healthcare, especially in rural areas of China, resulting in long waiting time of 24 to 146 months and significant cognitive decline (Zhao et al., 2016; Mattke et al., 2023; Olwage, 2024). Additionally, China lacks dementia-related specialists and hospital memory clinics (Wu and Lam, 2016). Only 24.7% general practitioners (GPs) received relevant training and about 60% reported feeling confident in providing diagnostic advice on dementia, leading to a high misdiagnosis rate (Li et al., 2021). Therefore, this study integrates expert consensus and prevention guidelines (Tian et al., 2019, 2021; Zhang et al., 2020; Huang et al., 2024; National Center for Neurological Disorders et al., 2024) into a comprehensive China dementia care pathway (Figure 1), highlighting two critical barriers: mis/under-diagnoses and time delays (bolds in Figure 1).
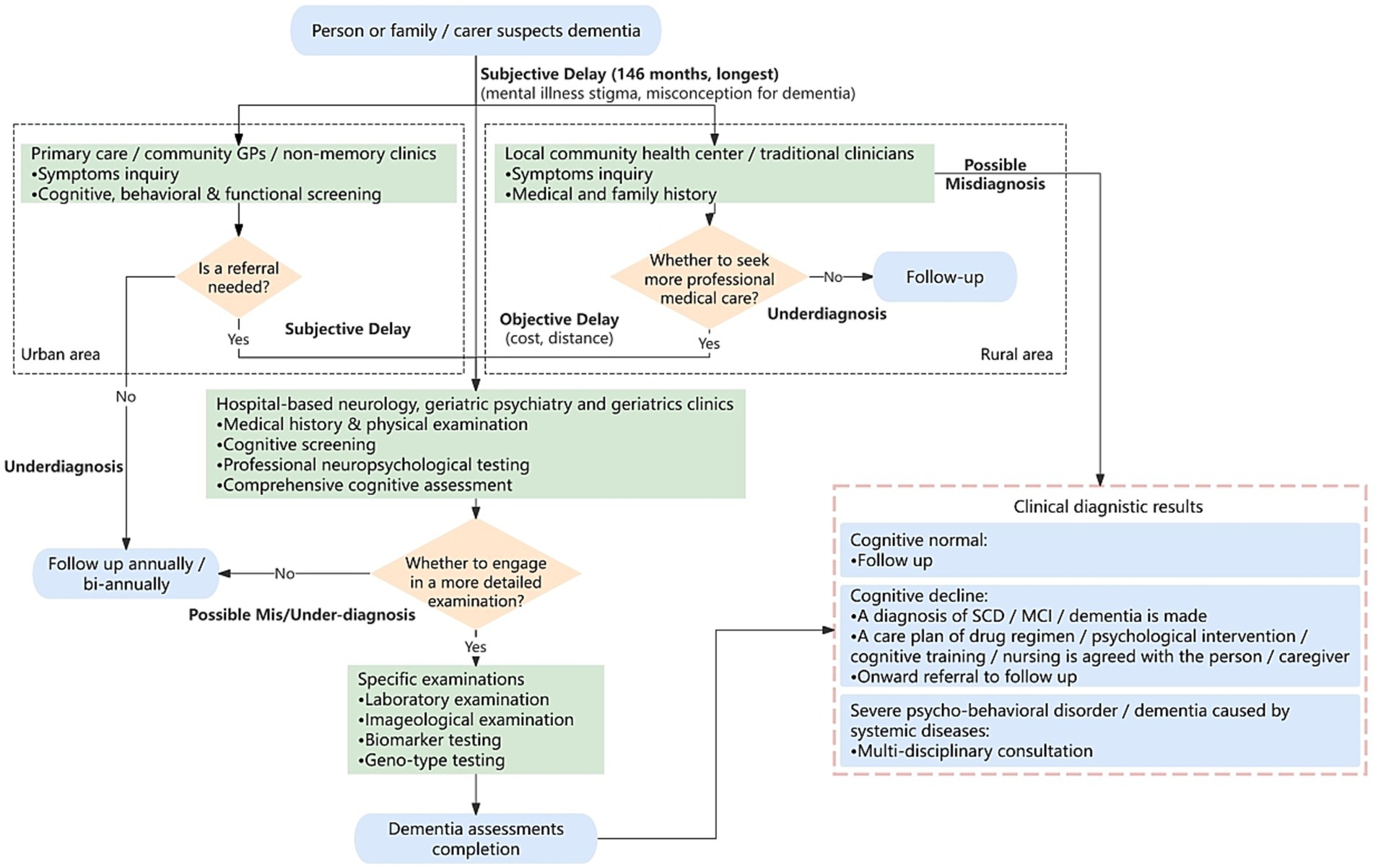
Figure 1. Flowchart of China dementia care pathway. Bolds: potential mis/under-diagnosis and time delay that could be alleviated by data-driven computational strategy. Flowchart proposed in the basis of studies in Tian et al. (2019, 2021), Zhang et al. (2020), Dementia and Cognitive Impairment Group of Chinese Society of Neurology, Cognitive Disorders Committee of Neurology Branch of Chinese Medical Doctor Association (2023), Huang et al. (2024), and National Center for Neurological Disorders et al. (2024). Blue rounded box: pathway starting/ending. Green box: dementia screening/assessing/examining. Orange diamond: if-then-else condition. GP, general practitioner; SCD, subjective cognitive decline; MCI, mild cognitive impairment.
Specifically, for those patients with subjective cognitive decline (SCD) or mild cognitive impairment (MCI), in urban areas, the initial consultation usually occurs at a community primary hospital (indicated in the left black-dotted box in Figure 1). While in rural areas, patients may visit a local health center (if there is) or consult traditional Chinese medical practitioners (Quail et al., 2020) (see the right black-dotted box). If suspicions persist, they may be referred to secondary or specialized hospitals. Whereas some patients skip primary care and seek comprehensive evaluations at higher-level hospitals directly, including medical history, physical exams, neuropsychological assessments, blood tests, computed tomography or magnetic resonance imaging (MRI) scans, positron emission tomography (PET) scans or cerebrospinal fluid (CSF) analysis. With the completion of all tests, the diagnosis and corresponding care plan will be made (bottom-right pink-dotted box).
In this pathway, both objective and subjective factors contribute to mis/under-diagnoses and time delays. Advancements in computing science, particularly data-driven methods, can help reduce subjective errors while improving diagnostic accuracy and efficiency (Muhammad et al., 2021; Rodrigues et al., 2023). Therefore, these approaches hold promise in addressing the existing challenges in China dementia care pathway (Devenney et al., 2024).
Digital neuropsychological testing can reduce errors from manual record-keeping and evaluator biases, improving diagnostic precision and streamlining the process, while also enabling better data storage and remote management (Dementia and Cognitive Impairment Group of Chinese Society of Neurology, Cognitive Disorders Committee of Neurology Branch of Chinese Medical Doctor Association, 2023). Some existing digital strategies are the computer assessment of MCI (CAMCI-Research, 2016), Cambridge neuropsychological test automated battery paired associates learning (CANTAB Assessments, 2012), and CogState digital cognitive assessment (CogState, 2013) etc., where CogState brief battery is a highly automated and standardized tool, lasting only 15 min and assessing multiple cognitive functions (Staffaroni et al., 2020). These tools support less experienced GPs in primary care, reducing misdiagnosis rates.
Each neuropsychological assessment focuses on specific areas. Previous research showed that some assessments performed poorly in certain cognitive domains, whereas multiple assessments together cover a broader range (Yin et al., 2015). However, comprehensive neuropsychological assessment is time-consuming, prompting computational studies into the most effective assessment combinations for diagnosing MCI and AD (Hemmy et al., 2020). For instance, Bucholc et al. (2019) used support vector machine (SVM) to assess dementia severity, achieving 83% accuracy by combining mini-mental state examination (MMSE), Montreal cognitive assessment (MoCA), functional activities questionnaire (FAQ), and AD assessment scale. Similarly, McCombe et al. (2022) employed random forest using nine selected sub-items from FAQ, MMSE, and the global deterioration scale, achieving an area under the receiver operating characteristic curve (AUC) of 0.865 for classifying cognitive normal (CN), MCI, and AD. These methods improve diagnostic accuracy even using fewer scales, demonstrating the potential of data-driven computational approaches to assist GPs in reducing misdiagnoses and shortening clinical management times.
Moreover, emerging data-driven techniques have shown great potential in the early detection, caregiver support and personalized management of AD, with the possibility of being widely adopted in home and community scenarios. For instance, wearable devices, as a non-invasive technology, collect real-time physical data from patients, e.g., retinal imaging (Wang et al., 2021; Gaire et al., 2024), language (Lindsay et al., 2021), hearing (Bucholc et al., 2021), and gait (Duan et al., 2023) etc. These devices, combined with artificial intelligence (AI) techniques, analyze patient’s physical condition, lighten care burdens, and aid doctors in making well-informed decisions remotely (Qi et al., 2022; Salehi et al., 2022; Vrahatis et al., 2023; Borna et al., 2024; Chen et al., 2024). A study used gait data collected via seven wearable devices served for 77 CN and 68 MCI subjects, with advanced machine learning (ML) models achieving classification accuracy of 0.73 in dual-task walking and 0.66 in normal walking (Jeon et al., 2023). In addition, other smart technologies can assist clinicians and patients through interactive devices and distributed systems, such as leveraging sensors in homes or care facilities to collect patient and environmental data and providing feedback to patients. For instance, Munteanu et al. (2022) designed an intelligent assistance system that uses AI to recognize human activities in videos. This system can detect when AD patients eat or drink and remind them with a voice message if they forget or overconsume. It also enables caregivers to remotely supervise and manage the patient’s nutrition plan. Integrating these technologies into routine dementia management can improve care efficiency and enhance quality of patients’ life (Gillani and Arslan, 2021). Therefore, data-driven computational approaches are efficient for early dementia detection and personalized care, optimizing the dementia care pathway.
Patients with referrals can further undergo comprehensive examinations at a hospital to determine the dementia type and corresponding treatment planning. Specifically, neuroimaging examinations can identify changes in the brain. Currently, structural MRI (sMRI) is the most important imaging detection method of prodromal AD (pAD), as it provides valuable imaging markers (Dementia and Cognitive Impairment Group of Chinese Society of Neurology, Cognitive Disorders Committee of Neurology Branch of Chinese Medical Doctor Association, 2023). A systematic review indicates that, for identifying MCI, the total hippocampal volume contributes a sensitivity of 0.73 and specificity of 0.71; medial temporal lobe atrophy provides a sensitivity of 0.64 and specificity of 0.65; and lateral ventricular volume presents a sensitivity of 0.57 and specificity of 0.60 (Lombardi et al., 2020). Nowadays, majority of imaging analyses utilize various deep learning (DL) methods to capture brain structural and pathological changes in sMRI images, eventually improving the accuracy of dementia diagnosis and prognosis (Yamanakkanavar et al., 2020; Frizzell et al., 2022; Borchert et al., 2023; Xu et al., 2023). Crucially, these decision-making support techniques significantly reduce errors caused by clinical fatigue or negligence in practice (Zhou et al., 2024).
Unlike MRI, a PET scan is more expensive and invasive, but it reveals molecular metabolic activity and brain function, making it a valuable tool for diagnosing and assessing neurological disorders like dementia (Borchert et al., 2023; Ricci et al., 2020; Bao et al., 2021). It turns out that conventional ML and DL has been effectively utilized for PET image analytics to detect lesion size, morphology, and changes over time and further to improve the accuracy of early-stage dementia diagnosis and prognosis (Tang et al., 2024). For instance, SVM achieved >85% accuracy in detecting AD-specific hypometabolic patterns with fluorodeoxyglucose (FDG)-PET and outperforming sMRI (Ferreira et al., 2017; De Carli et al., 2019). It also performed promising in distinguishing AD vs. CN (>86% accuracy) and MCI vs. CN (>78.8% accuracy) as well as predicting MCI-to-AD conversions within 12–60 months (72–80% accuracy), all based on FDG-PET images (Pan et al., 2019; Li et al., 2019; Shen et al., 2019; Teng et al., 2020). Similarly, applying SVM to amyloid-PET data achieved >85% accuracy for predicting MCI-to-AD conversions and diagnosing AD (Nozadi and Kadoury, 2018; Yang et al., 2020). In terms of DL, convolutional neural networks have presented a range of accuracy of 75–100% on FDG-and amyloid-PET images, depending on the variables of data size, imaging modality, and image preprocessing (Ding et al., 2019; Huang et al., 2019; Son et al., 2020). These findings highlight the potential of ML to enhance PET imaging analytics for AD diagnosis and prognosis (Borchert et al., 2023) in hospital setting. In addition, leveraging the combination of PET and MRI images enables more comprehensive assessments. More studies regarding DL on PET/MRI focus on image segmentation and reconstruction, pathological features visualization, thereby facilitating dementia detection and prediction (Dyrba et al., 2021; Zhao et al., 2023; Zhou et al., 2024).
Another rich source of biomarkers for AD comes from CSF because it reflects changes in the central nervous system caused by neuronal metabolic disturbances (McGrowder et al., 2021; Bouwman et al., 2022; Ardanaz et al., 2022). CSF Aβ protein concentration has shown strong diagnostic performance, with sensitivity and specificity of 0.86 and 0.80 for distinguishing AD from CN, and 0.79 and 0.61 for differentiating AD from non-AD dementias. Other CSF biomarkers, such as T-tau and P-tau181, have also demonstrated high diagnostic efficacy (Tian et al., 2019). Recent studies have pointed out that the clinically diagnosed AD group may include patients with other types of dementia, while the control group often contains individuals with other neurological disorders, complicating the definition of cut-off values between the groups (Bellomo et al., 2021). To address this, Bellomo et al. used unsupervised ML methods to calculate unbiased cut-off values based on CSF biomarker distribution, reducing inter-laboratory variability and improving biochemical phenotyping. For instance, a study utilizing proximity extension-based assays to analyze CSF from dementia patients identified dopamine decarboxylase (DDC) as the most significantly dysregulated protein. DDC effectively classified Lewy body dementia (LBD) vs. CN with an AUC of 0.91, and LBD vs. AD with an AUC of 0.81. The study further established a biomarker panel comprising seven CSF proteins via the constructed classification model, achieving an improved AUC performance of 0.93 for differentiating LBD vs. AD (Del Campo et al., 2023). Subsequently, to determine the optimal combination of CSF biomarkers for predicting disease progression in AD and other neurodegeneration, a study analyzed data from 1,983 participants across three cohorts. Statistical analysis revealed that P-tau/Aβ42 is sufficient for predicting progression in AD with AUC performance greater than 0.87 (Salvadó et al., 2023). As such, data-driven computational strategies have made significant contributions in precise patient stratification, the discovery of novel biomarkers, and the identification of effective marker combinations that enhance clinical diagnosis, thereby further reducing dementia misdiagnosis rates.
Researchers are also exploring peripheral biomarkers, such as blood tests, which offer a less invasive and more accessible method for detecting pAD, with high sensitivity and specificity (Dementia and Cognitive Impairment Group of Chinese Society of Neurology, Cognitive Disorders Committee of Neurology Branch of Chinese Medical Doctor Association, 2023). The measurement of plasma Aβ protein concentrations demonstrates a combined sensitivity and specificity of 0.88 and 0.90, respectively, in distinguishing AD and MCI from CN. Similarly, plasma tau concentrations show a combined sensitivity and specificity of 0.96 and 0.93, respectively, for the same differentiation. These findings highlight plasma biomarkers as a promising option for AD diagnosis (Tian et al., 2021). A recent study using a Markov model to predict the dementia care burden in China from 2024 to 2043 indicated that using blood tests could significantly shorten dementia care pathway, typically in reducing patient waiting time for diagnosis (Mattke et al., 2023). Importantly, AI techniques can be utilized in analyzing high-dimensional and complex blood data (Lee and Lee, 2020; Palmqvist et al., 2024). For instance, an exciting research based on ML had identified a small set of blood transcripts capable of effectively distinguishing CN from those with neurodegenerative diseases, including AD (Huseby et al., 2022).
In terms of genetic biomarkers, it has already been reported that apolipoprotein E (ApoE) ε4 allele is associated with higher AD risk than the more common ApoE ε3 allele (Neu et al., 2017; Narasimhan et al., 2024), highlighting the importance of genetic testing in dementia assessment (Koriath et al., 2021). However, challenges remain in translating human genetic findings [such as genome-wide association studies (GWAS)] into the pathobiology and therapeutic discoveries for AD. To address this, a DL framework was proposed to identify disease-associated genes (Xu et al., 2022). This framework identified 156 AD-related genes, enriched in druggable molecules, and discovered four drugs linked to reduced AD incidence. These breakthroughs emphasize the potential of DL in both understanding AD pathobiology and in identifying genetic markers for early disease prediction and prevention. Given AD’s polygenic nature, polygenic risk scores (PRS) assess genetic susceptibility. Zhou et al. (2023) used DL to model genetic data more comprehensively, showing clear advantages over traditional PRS and least absolute shrinkage and selection operator models in identifying genetic risk and uncovering biological mechanisms. In addition, the integration of computational models into genetic evaluation enables the early identification of individuals at higher risk for AD, allowing for targeted prevention strategies and more timely interventions. In summary, computational models excel at processing large-scale datasets from genomics and proteomics, not only enabling the discovery of novel biomarkers but also predicting their associations with disease onset and progression. By detecting complex data patterns, these models may uncover insights overlooked by human experts, thereby enhancing screening efficiency for dementia patients and high-risk populations and consequently speeding dementia care pathway (Javaid et al., 2022).
In clinical settings, single-modal data typically provides only a partial view of the disease and cannot comprehensively reflect its full scope (Elazab et al., 2024). Multi-modal data and corresponding techniques can capture various aspects of the disease and biomarkers related to AD pathology, leading to more accurate and personalized diagnostic results (Muhammad et al., 2021). Recently, a considerable AI-model built on multimodal data over diverse cohorts has been proposed to differentiate dementia etiologies (Xue et al., 2024), achieving a microaveraged AUC of 0.94 for classifying CN, MCI, and dementia. In a random subset of 100 cases, the AUC of neurologist assessments increased by 26.25% with AI assistance compared to assessments made by neurologists alone, underscoring the significant enhancement that AI provides in supporting expert evaluations for dementia diagnosis. In addition, Rahim et al. (2024) used multimodal data to predict patient outcomes 3 years later with a DL model, achieving 88% generalization accuracy and an AUC of 0.88. This kind of multimodal data study of AD progression can provide information support for clinicians to intervene in treatment. Hospitals in China are actively engaging in multi-sectional cohort studies to collect, consolidate and integrate multimodal data.
Additionally, some studies on AD care recommendations use additional care assessments, such as those related to daily living care problems, behavioral and psychological symptoms, and safety risks. For example, a study using a knowledge graph to develop a care recommendation system achieved 98.92% accuracy, providing decision support for personalized AD patient care and improving the care process (Sun et al., 2024).
The complexity of dementia pathology challenges the acquirement of large-scale, clean data across multi-centers, thus hindering the translation of computational strategies to clinical settings (Afzal et al., 2019; Bron et al., 2022). Consequently, most studies rely on openly-sourced datasets like Alzheimer’s Disease Neuroimaging Initiative (Mueller et al., 2005), Australian Imaging Biomarkers & Lifestyle (Ellis et al., 2009), National Alzheimer’s Coordinating Center (Beekly et al., 2007), and UK BioBank (Ollier et al., 2005). Although ClinicalTrials.gov reports Chinese dementia cohort recruitment, patient data are still inaccessible.
Implementing data-driven strategies in China requires government-supported collaboration between medical and research institutions to build a multi-centered big-data platform integrating massive clinical records (National Center for Neurological Disorders et al., 2024). Poor data interoperability often brings redundant testing after referrals. Therefore, standardized data structuring and quality evaluation processes are essential to ensure data integrity and usability (Fan and Fu, 2023), enabling computational models to address clinical challenges like mis/under-diagnoses, drug efficacy, and health economics, ultimately providing more accurate and timely diagnoses for dementia patients (Xian et al., 2023; Gao et al., 2021).
Many computational models emphasize improving accuracy to support clinicians in making diagnostic decision, but the “black-box” issue (lack of interpretability) hinders trust from patients and doctors (Bron et al., 2022; Mirzaei and Adeli, 2022; Wang et al., 2023; Chen and Cheng, 2024). Other challenges include terminology inconsistencies, preprocessing methods variety, unclear evaluation criteria, and model optimization complexity (Bron et al., 2022). As a result, varying implementation details across studies hinder practical validation.
Recommendations include traditional ML algorithms (e.g., Bayesian networking, distance-based modeling), offering inherent interpretability but require extensive manual feature engineering (Ding et al., 2018; Yang et al., 2024). In contrast, post-hoc interpretable methods like class activation mapping (CAM) and Grad-CAM enhance transparency for complex models such as neural networks (Zhou et al., 2016; Selvaraju et al., 2017). Moreover, occlusion methods improve trust in AI by identifying key features through output image comparison (Kwak et al., 2022a,b). Crucially, before large-scale deployment of data-driven models, clinical requirements elicitation, policy guidance, and extensive validation are the foundation to ensure clinical relevance, reliability, and transparency.
Clear evidence exists for using data-driven computational strategy to speed up clinical administration time and reduce mis/under-diagnosis rate, i.e., optimize China dementia care pathway. Therefore, dementia cohort study along with developing a center-crossed dementia platform in China should be strongly encouraged. Data should also be publicly accessible where appropriate. Only be doing so can the challenges be overcome and can AI-enabled dementia research be enhanced, thereby optimizing China dementia care pathway. Clinical transformation urgently requests substantive cooperation of multi-stakeholders, including computational researchers, medical professionals, healthcare specialists, policymakers, and industrial developers etc., across regions.
PL: Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. XLL: Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. XFL: Data curation, Validation, Writing – original draft, Writing – review & editing. MC: Data curation, Formal analysis, Investigation, Visualization, Writing – review & editing. CL: Data curation, Validation, Visualization, Writing – review & editing. HY: Project administration, Supervision, Writing – review & editing. YW: Supervision, Writing – review & editing. XD: Conceptualization, Formal analysis, Investigation, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This project was supported by the Research Development Projects of Fujian Normal University, China (DH-1736 and DH-1711).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Afzal, S., Maqsood, M., Nazir, F., Khan, U., Aadil, F., Awan, K. M., et al. (2019). A data augmentation-based framework to handle class imbalance problem for Alzheimer's stage detection. IEEE Access. 7, 115528–115539. doi: 10.1109/ACCESS.2019.2932786
Ardanaz, C. G., Ramírez, M. J., and Solas, M. (2022). Brain metabolic alterations in Alzheimer’s disease. Int. J. Mol. Sci. 23:3785. doi: 10.3390/ijms23073785
Bao, W., Xie, F., Zuo, C., Guan, Y., and Huang, Y. H. (2021). PET neuroimaging of Alzheimer's disease: radiotracers and their utility in clinical research. Front. Aging Neurosci. 13:624330. doi: 10.3389/fnagi.2021.624330
Beekly, D. L., Ramos, E. M., Lee, W. W., Deitrich, W. D., Jacka, M. E., Wu, J., et al. (2007). The National Alzheimer's coordinating center (NACC) database: the uniform data set. Alzheimer Dis. Assoc. Disord. 21, 249–258. doi: 10.1097/WAD.0b013e318142774e
Bellomo, G., Indaco, A., Chiasserini, D., Maderna, E., Paolini Paoletti, F., Gaetani, L., et al. (2021). Machine learning driven profiling of cerebrospinal fluid core biomarkers in Alzheimer’s disease and other neurological disorders. Front. Neurosci. 15:647783. doi: 10.3389/fnins.2021.647783
Borchert, R. J., Azevedo, T., Badhwar, A. P., Bernal, J., Betts, M., Bruffaerts, R., et al. (2023). Artificial intelligence for diagnostic and prognostic neuroimaging in dementia: a systematic review. Alzheimers Dement. 19, 5885–5904. doi: 10.1002/alz.13412
Borna, S., Maniaci, M. J., Haider, C. R., Gomez-Cabello, C. A., Pressman, S. M., Haider, S. A., et al. (2024). Artificial intelligence support for informal patient caregivers: a systematic review. Bioengineering 11:483. doi: 10.3390/bioengineering11050483
Bouwman, F. H., Frisoni, G. B., Johnson, S. C., Chen, X., Engelborghs, S., Ikeuchi, T., et al. (2022). Clinical application of CSF biomarkers for Alzheimer's disease: from rationale to ratios. Alzheimer Dement. 14:e12314. doi: 10.1002/dad2.12314
Breijyeh, Z., and Karaman, R. (2020). Comprehensive review on Alzheimer’s disease: causes and treatment. Molecules 25:5789. doi: 10.3390/molecules25245789
Bron, E. E., Klein, S., Reinke, A., Papma, J. M., Maier-Hein, L., Alexander, D. C., et al. (2022). Ten years of image analysis and machine learning competitions in dementia. Neuroimage 253:119083. doi: 10.1016/j.neuroimage.2022.119083
Bucholc, M., Ding, X., Wang, H., Glass, D. H., Wang, H., Prasad, G., et al. (2019). A practical computerized decision support system for predicting the severity of Alzheimer's disease of an individual. Expert Syst. Appl. 130, 157–171. doi: 10.1016/j.eswa.2019.04.022
Bucholc, M., McClean, P. L., Bauermeister, S., Todd, S., Ding, X., Ye, Q., et al. (2021). Association of the use of hearing aids with the conversion from mild cognitive impairment to dementia and progression of dementia: a longitudinal retrospective study. Alzheimer Dement. 7:e12122. doi: 10.1002/trc2.12122
CAMCI-Research. (2016). Computer assessment of memory and cognitive impairment (CAMCI). Available online at: https://pstnet.com/products/camci-research/ (Accessed October 3, 2024).
CANTAB Assessments. (2012). Cambridge neuropsychological test automated battery. Available online at: https://cambridgecognition.com/digital-cognitive-assessments/ (Accessed October 3, 2024).
Chen, Z., and Cheng, G. (2024). Algorithm black box in medical artificial intelligence and its core ethical issues. Med. Philos. 45, 6–10. doi: 10.12014/j.issn.1002-0772.2024.12.02
Chen, X., Xie, H., Tao, X., Wang, F. L., Leng, M., and Lei, B. (2024). Artificial intelligence and multimodal data fusion for smart healthcare: topic modeling and bibliometrics. Artif. Intell. Rev. 57:91. doi: 10.1007/s10462-024-10712-7
CogState (2013). CogState digital cognitive assessment. Available online at: https://www.cogstate.com/digitalcognitive-assessment/ (Accessed October 3, 2024).
De Carli, F., Nobili, F., Pagani, M., Bauckneht, M., Massa, F., Grazzini, M., et al. (2019). Accuracy and generalization capability of an automatic method for the detection of typical brain hypometabolism in prodromal Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 46, 334–347. doi: 10.1007/s00259-018-4197-7
Del Campo, M., Vermunt, L., Peeters, C. F. W., Sieben, A., Hok-A-Hin, Y. S., Lleó, A., et al. (2023). CSF proteome profiling reveals biomarkers to discriminate dementia with Lewy bodies from Alzheimer's disease. Nat. Commun. 14:5635. doi: 10.1038/s41467-023-41122-y
Dementia and Cognitive Impairment Group of Chinese Society of Neurology, Cognitive Disorders Committee of Neurology Branch of Chinese Medical Doctor Association (2023). Chinese expert consensus on brief screening of prodromal Alzheimer's disease (2023). Chin. J. Neuromed. 22, 433–444. doi: 10.3760/cma.j.cn115354-20230330-00191
Devenney, E. M., Anh, N., Nguyen, Q., Tse, N. Y., Kiernan, M. C., and Tan, R. H. (2024). A scoping review of the unique landscape and challenges associated with dementia in the Western Pacific region. Lancet Reg. Health Western Pacific. 50:101192. doi: 10.1016/j.lanwpc.2024.101192
Ding, X., Bucholc, M., Wang, H., Glass, G. H., Wang, H., Clarke, D. H., et al. (2018). A hybrid computational approach for efficient Alzheimer's disease classification based on heterogeneous data. Sci. Rep. 8:9774. doi: 10.1038/s41598-018-27997-8
Ding, Y., Sohn, J. H., Kawczynski, M. G., Trivedi, H., Harnish, R., Jenkins, N. W., et al. (2019). A deep learning model to predict a diagnosis of Alzheimer disease by using 18F-FDG PET of the brain. Radiology 290, 456–464. doi: 10.1148/radiol.2018180958
Duan, Q., Zhang, Y., Zhuang, W., Li, W., He, J., Wang, Z., et al. (2023). Gait domains may be used as an auxiliary diagnostic index for Alzheimer's disease. Brain Sci. 13:1599. doi: 10.3390/brainsci13111599
Dyrba, M., Hanzig, M., Altenstein, S., Bader, S., Ballarini, T., Brosseron, F., et al. (2021). Improving 3D convolutional neural network comprehensibility via interactive visualization of relevance maps: evaluation in Alzheimer's disease. Alzheimers Res. Ther. 13, 1–18. doi: 10.1186/s13195-021-00924-2
Elazab, A., Wang, C., Abdelaziz, M., Zhang, J., Gu, J., Gorriz, J. M., et al. (2024). Alzheimer's disease diagnosis from single and multimodal data using machine and deep learning models: achievements and future directions. Expert Syst. Appl. 255:124780. doi: 10.1016/j.eswa.2024.124780
Ellis, K. A., Bush, A. I., Darby, D., De Fazio, D., Foster, J., Hudson, P., et al. (2009). The Australian imaging, biomarkers and lifestyle (AIBL) study of aging: methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer's disease. Int. Psychogeriatr. 21, 672–687. doi: 10.1017/S1041610209009405
Fan, M., and Fu, H. (2023). Research on the mechanism and path of digital transformation promoting the development of hospital information management. Chin. Hosp. Manag. 43, 60–63.
Ferreira, L. K., Rondina, J. M., Kubo, R., Ono, C. R., Leite, C. C., Smid, J., et al. (2017). Support vector machinebased classification of neuroimages in Alzheimer’s disease: direct comparison of FDG-PET, rCBF-SPECT and MRI data acquired from the same individuals. Braz. J. Psychiatry 40, 181–191. doi: 10.1590/1516-4446-2016-2083
Frizzell, T. O., Glashutter, M., Liu, C. C., Zeng, A., Pan, D., Hajra, S. G., et al. (2022). Artificial intelligence in brain MRI analysis of Alzheimer’s disease over the past 12 years: a systematic review. Ageing Res. Rev. 77:101614. doi: 10.1016/j.arr.2022.101614
Gaire, B. P., Koronyo, Y., Fuchs, D. T., Shi, H., Rentsendorj, A., Danziger, R., et al. (2024). Alzheimer's disease pathophysiology in the retina. Prog. Retin. Eye Res. 101:101273. doi: 10.1016/j.preteyeres.2024.101273
Gao, J., Zhai, Y., and Li, M. (2021). Research status of health and medical big data processing in precision medicine field. Chin. Hosp. Manag. 41, 8–13.
Gillani, N., and Arslan, T. (2021). Intelligent sensing technologies for the diagnosis, monitoring and therapy of Alzheimer’s disease: a systematic review. Sensors 21:4249. doi: 10.3390/s21124249
Hemmy, L. S., Linskens, E. J., Silverman, P. C., Miller, M. A., Talley, K. M., Taylor, B. C., et al. (2020). Brief cognitive tests for distinguishing clinical Alzheimer-type dementia from mild cognitive impairment or normal cognition in older adults with suspected cognitive impairment. Ann. Intern. Med. 172, 678–687. doi: 10.7326/M19-3889
Huang, L., Li, Q., Lu, Y., Pan, F., Cui, L., Wang, Y., et al. (2024). Consensus on rapid screening for prodromal Alzheimer's disease in China. Gen. Psychiatry 37:e101310. doi: 10.1136/gpsych-2023-101310
Huang, Y., Xu, J., Zhou, Y., Tong, T., and Zhuang, X. (2019). Diagnosis of Alzheimer’s disease via multimodality 3D convolutional neural network. Front. Neurosci. 13:509. doi: 10.3389/fnins.2019.00509
Huseby, C. J., Delvaux, E., Brokaw, D. L., and Coleman, P. D. (2022). Blood transcript biomarkers selected by machine learning algorithm classify neurodegenerative diseases including Alzheimer’s disease. Biomol. Ther. 12:1592. doi: 10.3390/biom12111592
Javaid, M., Haleem, A., Singh, R. P., Suman, R., and Rab, S. (2022). Significance of machine learning in healthcare: features, pillars and applications. Int. J. Intell. Netw. 3, 58–73. doi: 10.1016/j.ijin.2022.05.002
Jeon, Y., Kang, J., Kim, B. C., Lee, K. H., Song, J. I., and Gwak, J. (2023). Early Alzheimer’s disease diagnosis using wearable sensors and multilevel gait assessment: a machine learning ensemble approach. IEEE Sensors J. 23, 10041–10053. doi: 10.1109/JSEN.2023.3259034
Koriath, C. A., Kenny, J., Ryan, N. S., Rohrer, J. D., Schott, J. M., Houlden, H., et al. (2021). Genetic testing in dementia – utility and clinical strategies. Nat. Rev. Neurol. 17, 23–36. doi: 10.1038/s41582-020-00416-1
Kwak, K., Niethammer, M., Giovanello, K. S., Styner, M., and Dayan, E. (2022a). Differential role for hippocampal subfields in Alzheimer's disease progression revealed with deep learning. Cereb. Cortex 32, 467–478. doi: 10.1093/cercor/bhab223
Kwak, K., Stanford, W., and Dayan, E. (2022b). Identifying the regional substrates predictive of Alzheimer's disease progression through a convolutional neural network model and occlusion. Hum. Brain Mapp. 43, 5509–5519. doi: 10.1002/hbm.26026
Lee, T., and Lee, H. (2020). Prediction of Alzheimer's disease using blood gene expression data. Sci. Rep. 10:3485. doi: 10.1038/s41598-020-60595-1
Li, Y., Jiang, J., Lu, J., Jiang, J., Zhang, H., and Zuo, C. (2019). Radiomics: a novel feature extraction method for brain neuron degeneration disease using 18F-FDG PET imaging and its implementation for Alzheimer’s disease and mild cognitive impairment. Ther. Adv. Neurol. Disord. 12:1756286419838682. doi: 10.1177/1756286419838682
Li, J., Wang, M., Shao, S., Xu, X., and Du, J. (2021). Review of dementia knowledge, abilities and attitudes of general practitioners. Med. Educ. Manag. 7, 217–222. doi: 10.3969/j.issn.2096045X.2021.02.023
Lindsay, H., Tröger, J., and König, A. (2021). Language impairment in Alzheimer's disease—robust and explainable evidence for AD-related deterioration of spontaneous speech through multilingual machine learning. Front. Aging Neurosci. 13:642033. doi: 10.3389/fnagi.2021.642033
Liss, J. L., Seleri Assunção, S., Cummings, J., Atri, A., Geldmacher, D. S., Candela, S. F., et al. (2021). Practical recommendations for timely, accurate diagnosis of symptomatic Alzheimer’s disease (MCI and dementia) in primary care: a review and synthesis. J. Intern. Med. 290, 310–334. doi: 10.1111/joim.13244
Lombardi, G., Crescioli, G., Cavedo, E., Lucenteforte, E., Casazza, G., Bellatorre, A. G., et al. (2020). Structural magnetic resonance imaging for the early diagnosis of dementia due to Alzheimer's disease in people with mild cognitive impairment. Cochrane Database Syst. Rev. 3:CD009628. doi: 10.1002/14651858.CD009628.pub2
Mattke, S., Loh, W. K., Yuen, K. H., and Yoong, J. (2023). Preparedness of China's health care system to provide access to a disease-modifying Alzheimer's treatment. Alzheimers Dement. 19, 5596–5604. doi: 10.1002/alz.13348
McCombe, N., Ding, X., Prasad, G., Gillespie, P., Finn, D. P., Todd, S., et al. (2022). Alzheimer's disease assessments optimized for diagnostic accuracy and administration time. IEEE J. Transl. Eng. Health Med. 10, 1–9. doi: 10.1109/JTEHM.2022.3164806
McGrowder, D. A., Miller, F., Vaz, K., Nwokocha, C., Wilson-Clarke, C., Anderson-Cross, M., et al. (2021). Cerebrospinal fluid biomarkers of Alzheimer’s disease: current evidence and future perspectives. Brain Sci. 11:215. doi: 10.3390/brainsci11020215
Mirzaei, G., and Adeli, H. (2022). Machine learning techniques for diagnosis of Alzheimer disease, mild cognitive disorder, and other types of dementia. Biomed. Signal Proces. Control. 72:103293. doi: 10.1016/j.bspc.2021.103293
Mueller, S. G., Weiner, M. W., Thal, L. J., Petersen, R. C., Jack, C., Jagust, W., et al. (2005). The Alzheimer's disease neuroimaging initiative. Neuroimaging Clin. N. Am. 15, 869–877. doi: 10.1016/j.nic.2005.09.008
Muhammad, G., Alshehri, F., Karray, F., El Saddik, A., Alsulaiman, M., and Falk, T. H. (2021). A comprehensive survey on multimodal medical signals fusion for smart healthcare systems. Inform. Fusion 76, 355–375. doi: 10.1016/j.inffus.2021.06.007
Munteanu, D., Bejan, C., Munteanu, N., Zamfir, C., Vasić, M., Petrea, S. M., et al. (2022). Deep-learning-based system for assisting people with Alzheimer’s disease. Electronics 11:3229. doi: 10.3390/electronics11193229
Narasimhan, S., Holtzman, D. M., Apostolova, L. G., Cruchaga, C., Masters, C. L., Hardy, J., et al. (2024). Apolipoprotein E in Alzheimer’s disease trajectories and the next-generation clinical care pathway. Nat. Neurosci. 27, 1236–1252. doi: 10.1038/s41593-024-01669-5
National Center for Neurological Disorders, Xuanwu Hospital, Capital Medical University; National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention; National Health Commission Capacity Building and Continuing Education Center; China Population and Development Research Center; Project Group of "Blue Paper on Alzheimer's Disease in China" (2024). Blue paper on Alzheimer's disease in China (simplified version). Chin. Med. J. 104, 1–27. doi: 10.3760/cma.j.cn12137-20240416-00883
Neu, S. C., Pa, J., Kukull, W., Beekly, D., Kuzma, A., Gangadharan, P., et al. (2017). Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. JAMA Neurol. 74, 1178–1189. doi: 10.1001/jamaneurol.2017.2188
Nozadi, S. H., and Kadoury, S. (2018). Classification of Alzheimer’s and MCI patients from semantically parceled PET images: a comparison between AV45 and FDG-PET. Int. J. Biomed. Imaging 2018:1247430. doi: 10.1155/2018/1247430
Ollier, W., Sprosen, T., and Peakman, T. (2005). UK biobank: from concept to reality. Pharmacogenomics 6, 639–646. doi: 10.2217/14622416.6.6.639
Olwage, L. A. (2024). Dementia-related stigma is still pervasive. Lancet 404, 572–628. doi: 10.1016/S14744422(24)00404-6
Palmqvist, S., Tideman, P., Mattsson-Carlgren, N., Schindler, S. E., Smith, R., Ossenkoppele, R., et al. (2024). Blood biomarkers to detect Alzheimer disease in primary care and secondary care. J. Am. Med. Assoc. 332, 1245–1257. doi: 10.1001/jama.2024.13855
Pan, X., Adel, M., Fossati, C., Gaidon, T., Wojak, J., and Guedj, E. (2019). Multiscale spatial gradient features for 18F-FDG PET image-guided diagnosis of Alzheimer’s disease. Comput. Methods Prog. Biomed. 180:105027. doi: 10.1016/j.cmpb.2019.105027
Qi, J., Wu, C., Yang, L., Ni, C., and Liu, Y. (2022). Artificial intelligence (AI) for home support interventions in dementia: a scoping review protocol. BMJ Open 12:e062604. doi: 10.1136/bmjopen-2022-062604
Quail, Z., Wei, A., Zhang, V. F., and Carter, M. M. (2020). Barriers to dementia diagnosis and care in China. BMJ Case Reports. 13:e232115. doi: 10.1136/bcr-2019-232115
Rahim, N., El-Sappagh, S., Rizk, H., El-serafy, O. A., and Abuhmed, T. (2024). Information fusion-based Bayesian optimized heterogeneous deep ensemble model based on longitudinal neuroimaging data. Appl. Soft Comput. 162:111749. doi: 10.1016/j.asoc.2024.111749
Ricci, M., Cimini, A., Chiaravalloti, A., Filippi, L., and Schillaci, O. (2020). Positron emission tomography (PET) and neuroimaging in the personalized approach to neurodegenerative causes of dementia. Int. J. Mol. Sci. 21:7481. doi: 10.3390/ijms21207481
Rodrigues, P. M., Madeiro, J. P., and Marques, J. A. L. (2023). Enhancing health and public health through machine learning: decision support for smarter choices. Bioengineering 10:792. doi: 10.3390/bioengineering10070792
Salehi, W., Gupta, G., Bhatia, S., Koundal, D., Mashat, A., and Belay, A. (2022). IoT-based wearable devices for patients suffering from Alzheimer disease. Contrast Media Mol. Imaging 2022:3224939. doi: 10.1155/2022/3224939
Salvadó, G., Larsson, V., Cody, K. A., Cullen, N. C., Jonaitis, E. M., Stomrud, E., et al. (2023). Optimal combinations of CSF biomarkers for predicting cognitive decline and clinical conversion in cognitively unimpaired participants and mild cognitive impairment patients: a multi-cohort study. Alzheimers Dement. 19, 2943–2955. doi: 10.1002/alz.12907
Selvaraju, R. R., Cogswell, M., Das, A., Vedantam, R., Parikh, D., and Batra, D. (2017). Grad-CAM: Visual explanations from deep networks via gradient-based localization. Proceedings of the IEEE International Conference on Computer Vision, Venice, Italy. 618–626. doi: 10.1109/ICCV.2017.74
Shen, T., Jiang, J., Lu, J., Wang, M., Zuo, C., Yu, Z., et al. (2019). Predicting Alzheimer disease from mild cognitive impairment with a deep belief network based on 18F-FDG-PET images. Mol. Imaging 18:1536012119877285. doi: 10.1177/1536012119877285
Son, H. J., Oh, J. S., Oh, M., Kim, S. J., Lee, J. H., Roh, J. H., et al. (2020). The clinical feasibility of deep learning-based classification of amyloid PET images in visually equivocal cases. Eur. J. Nucl. Med. Mol. Imaging 47, 332–341. doi: 10.1007/s00259-019-04595-y
Staffaroni, A. M., Tsoy, E., Taylor, J., Boxer, A. L., and Possin, K. L. (2020). Digital cognitive assessments for dementia: digital assessments may enhance the efficiency of evaluations in neurology and other clinics. Pract. Neurol., 24–45. Available at: https://pubmed.ncbi.nlm.nih.gov/33927583/
Sun, Y., Leng, M., Lu, W., Li, B., Lv, F., Zhang, W., et al. (2024). A knowledge graph-based recommender system for dementia care: design and evaluation study. Int. J. Med. Inform. 191:105554. doi: 10.1016/j.ijmedinf.2024.105554
Tang, C., Ruan, W., Lan, X., and Sun, X. (2024). Advancements in the application of PET-based radiomics in the assessment of Alzheimer's disease. Chin. J. Med. Imaging 32, 504–509. doi: 10.3969/j.issn.1005-5185.2024.05.017
Teng, L., Li, Y., Zhao, Y., Hu, T., Zhang, Z., Yao, Z., et al. (2020). Predicting MCI progression with FDG-PET and cognitive scores: a longitudinal study. BMC Neurol. 20, 1–10. doi: 10.1186/s12883-020-01728-x
Tian, J. Z., Xie, H. G., Qin, B., Fan, D. S., Shi, J., Xiao, W. Z., et al. (2019). The diagnostic framework for screening Alzheimer's disease in the Chinese population. Chin. J. Internal Med. 58, 91–101. doi: 10.3760/cma.j.issn.0578-1426.2019.02.005
Tian, J. Z., Xie, H. G., Wang, L. N., Wang, Y. H., Wang, H. L., Shi, J., et al. (2021). Chinese guideline for the diagnosis and treatment of Alzheimer's disease dementia (2020). Chin. J. Geriatr. 40, 269–283. doi: 10.3760/cma.j.issn.0254-9026.2021.03.001
Vrahatis, A. G., Skolariki, K., Krokidis, M. G., Lazaros, K., Exarchos, T. P., and Vlamos, P. (2023). Revolutionizing the early detection of Alzheimer's disease through non-invasive biomarkers: the role of artificial intelligence and deep learning. Sensors 23:4184. doi: 10.3390/s23094184
Wang, J., Ke, M., Dong, Z., Wang, L., and Li, L. (2023). Application of neuroimaging-based deep learning model interpretability methods in Alzheimer's disease recognition. Chin. J. Biomed. Eng. 42, 475–485. doi: 10.3969/j.issn.0258-8021.2023.04.010
Wang, G., Qi, J., Liu, X., Ren, R., Lin, S., Hu, Y., et al. (2024). China Alzheimer report 2024. J. Diagnost. Conc. Pract. 23:219. doi: 10.16150/j.1671-2870.2024.03.001
Wang, X., Zhao, Q., Tao, R., Lu, H., Xiao, Z., Zheng, L., et al. (2021). Decreased retinal vascular density in Alzheimer's disease (AD) and mild cognitive impairment (MCI): an optical coherence tomography angiography (OCTA) study. Front. Aging Neurosci. 12:572484. doi: 10.3389/fnagi.2020.572484
Wu, D., and Lam, T. P. (2016). Underuse of primary care in China: the scale, causes, and solutions. J. Am. Board Fam. Med. 29, 240–247. doi: 10.3122/jabfm.2016.02.150159
Xian, C., Zhu, X., and Wang, G. (2023). Construction and application of big medical data platform for mental disorders. Chin. Hosp. Manag. 43, 64–66.
Xu, X., Lin, L., Sun, S., and Wu, S. (2023). A review of the application of three-dimensional convolutional neural networks for the diagnosis of Alzheimer’s disease using neuroimaging. Rev. Neurosci. 34, 649–670. doi: 10.1515/revneuro-2022-0122
Xu, J., Mao, C., Hou, Y., Luo, Y., Binder, J. L., Zhou, Y., et al. (2022). Interpretable deep learning translation of GWAS and multi-omics findings to identify pathobiology and drug repurposing in Alzheimer’s disease. Cell Rep. 41:111717. doi: 10.1016/j.celrep.2022.111717
Xue, C., Kowshik, S. S., Lteif, D., Puducheri, S., Jasodanand, V. H., Zhou, O. T., et al. (2024). AI-based differential diagnosis of dementia etiologies on multimodal data. Nat. Med., 30, 1–13. doi: 10.1038/s41591024-03118-z
Yamanakkanavar, N., Choi, J. Y., and Lee, B. (2020). MRI segmentation and classification of human brain using deep learning for diagnosis of Alzheimer’s disease: a survey. Sensors 20:3243. doi: 10.3390/s20113243
Yang, B. H., Chen, J. C., Chou, W. H., Huang, W. S., Fuh, J. L., Liu, R. S., et al. (2020). Classification of Alzheimer’s disease from 18F-FDG and 11C-PiB PET imaging biomarkers using support vector machine. J. Med. Biol. Eng. 40, 545–554. doi: 10.1007/s40846-020-00548-1
Yang, H., Mao, J., Ye, Q., Bucholc, M., Liu, S., Gao, W., et al. (2024). Distance-based novelty detection model for identifying individuals at risk of developing Alzheimer’s disease. Front. Aging Neurosci. 16:1285905. doi: 10.3389/fnagi.2024.1285905
Yin, Z., Zhao, Y., Lu, X., and Duan, H. (2015). A hybrid intelligent diagnosis approach for quick screening of Alzheimer’s disease based on multiple neuropsychological rating scales. Comput. Math. Methods Med. 2015:258761, 1–13. doi: 10.1155/2015/258761
Zhang, H. N., Wang, M. R., Chen, X. L., Xu, X. J. Y., Li, J., Wang, H. L., et al. (2020). The content construction of dementia management in community based on Delphi method. Chin. Gen. Pract. 23:2072. doi: 10.12114/j.issn.1007-9572.2020.00.031
Zhao, Y., Guo, Q., Zhang, Y., Zheng, J., Yang, Y., Du, X., et al. (2023). Application of deep learning for prediction of Alzheimer's disease in PET/MR imaging. Bioengineering 10:1120. doi: 10.3390/bioengineering10101120
Zhao, M., Lv, X., Tuerxun, M., He, J., Luo, B., Chen, W., et al. (2016). Delayed help seeking behavior in dementia care: preliminary findings from the clinical pathway for Alzheimer's disease in China (CPAD) study. Int. Psychogeriatr. 28, 211–219. doi: 10.1017/S1041610215000940
Zhou, X., Chen, Y., Ip, F. C. F., Jiang, Y., Cao, H., Lv, G., et al. (2023). Deep learning-based polygenic risk analysis for Alzheimer’s disease prediction. Commun. Med. 3:49. doi: 10.1038/s43856-023-00269-x
Zhou, B., Khosla, A., Lapedriza, A., Oliva, A., and Torralba, A. (2016). “Learning deep features for discriminative localization” in Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA. 2921–2929.
Zhou, Y., Zhou, Q., Yin, C., Wu, R., Gai, Y., Su, Y., et al. (2024). Research progress in PET/MR for diagnosis of Alzheimer's disease within ATN framework. Chin. J. Magn. Reson. Imaging 15, 159–165. doi: 10.12015/issn.1674-8034.2024.06.025
AD - Alzheimer’s disease
AI - Artificial intelligence
ApoE - Apolipoprotein E
AUC - Area under the receiver operating characteristic curve
CAM - Class activation mapping
CN - Cognitive normal
CSF - Cerebrospinal fluid
DDC - Dopamine decarboxylase
DL - Deep learning
FAQ - Functional activities questionnaire
FDG-PET - Fluorodeoxyglucose positron emission tomography
GP - General practitioner
LBD - Lewy body dementia
MCI - Mild cognitive impairment
ML - Machine learning
MMSE - Mini-mental state examination
MoCA - Montreal cognitive assessment
MRI - Magnetic resonance imaging
NCND - National center for neurological disorders
pAD - Prodromal AD
PRS - Polygenic risk scores
SCD - Subjective cognitive decline
sMRI - Structural magnetic resonance imaging
SVM - Support vector machine
Keywords: dementia, Alzheimer’s disease, China dementia care pathway, computational strategy, machine learning, optimization, interpretability
Citation: Lu P, Lin X, Liu X, Chen M, Li C, Yang H, Wang Y and Ding X (2025) A mini review of transforming dementia care in China with data-driven insights: overcoming diagnostic and time-delayed barriers. Front. Aging Neurosci. 17:1554834. doi: 10.3389/fnagi.2025.1554834
Received: 03 January 2025; Accepted: 17 February 2025;
Published: 03 March 2025.
Edited by:
Li Su, The University of Sheffield, United KingdomReviewed by:
Weijie Huang, Beijing Normal University, ChinaCopyright © 2025 Lu, Lin, Liu, Chen, Li, Yang, Wang and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuhua Wang, eXVod2FuZ0Bmam51LmVkdS5jbg==; Xuemei Ding, eC5kaW5nQHVsc3Rlci5hYy51aw==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.