
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Aging Neurosci., 20 February 2025
Sec. Neurocognitive Aging and Behavior
Volume 17 - 2025 | https://doi.org/10.3389/fnagi.2025.1550319
This article is part of the Research TopicArtificial Intelligence-based Diagnosis and Neuromodulation in Neurological and Psychiatric DiseasesView all 8 articles
Lung diseases induce changes in brain structure and function, leading to a range of cognitive, emotional, and motor deficits. The concept of the lung-brain axis has been proposed through neuroanatomy, endocrine, and immune pathway, while a considerable number of studies also explored the existence of the lung-brain axis from a neuroimaging perspective. This survey summarizes studies exploring the relationship between lung disease and brain structure and function from neuroimaging perspective, particular in magnetic resonance imaging (MRI). We have collated existing lung diseases studies and categorized them into four types: chronic obstructive pulmonary disease (COPD), coronavirus disease 2019 (COVID-19), lung cancer and other lung diseases. The observed structural and functional changes in the brain and cognitive dysfunction induced by lung diseases are discussed. We also present distinct pattern of brain changes in various lung diseases. Neuroimaging changes in COPD are concentrated in the frontal lobes, including gray matter atrophy, white matter damage, and reduced perfusion. Patients with COVID-19 exhibit extensive microhemorrhages and neuroinflammation, brain regions functionally connected to the primary olfactory cortex show greater changes. For lung cancer patients, brain changes are mainly attributed to the neurotoxicity of radiotherapy and chemotherapy, with damage concentrated in subcortical structures, patients with cancer pain demonstrate hyperconnectivity in motor and visual networks. The survey also discusses the pathological mechanisms revealed in neuroimaging studies and clinical significance of current studies. Finally, we analyzed current limitations, mainly in terms of small sample size, non-standardized criteria, reliance on correlation analyses, lack of longitudinal studies, and absence of reliable biomarkers. We suggest future research directions should include leveraging artificial intelligence for biomarker development, conducting longitudinal and multicenter studies, and investigating the systemic effects of lung disease on the brain and neuromodulation strategies.
The central nervous system (CNS) and respiratory system are intricately interconnected through the autonomic nervous system. This connection facilitates reflexive responses like coughing and sneezing in asthma and chronic obstructive lung disease (COPD) (De Virgiliis and Di Giovanni, 2020). The respiratory system also impact the CNS via CO2-sensitive brainstem nuclei to regulate cortisol level (Abelson et al., 2010). Neuroendocrine lung tumors influence central neuroendocrine function by releasing endocrines like adrenocorticotrophic (Gandhi and Johnson, 2006). Immune responses in the lungs, including autoreactive T cell proliferation, can lead to autoimmune effects, and lung microbiome may regulate the susceptibility of CNS to autoimmune diseases (Li et al., 2023; Hosang et al., 2022). Evidences from neuroanatomy, endocrine, immune pathways support that lung pathology can impact brain structure and function. Exploring the brain changes in lung diseases may reveal new avenues for lung disease monitoring and targeted treatment strategies. While many studies have explored the impact of lung diseases on the brain using neuroimaging techniques. This survey integrates these studies to explore the multifaceted effects of lung diseases on brain structure and function, analyze related mechanisms, and summarize the clinical significance and future research directions.
Advanced neuroimaging techniques can visualize the impact of lung diseases on brain structure and function. These techniques include structural magnetic resonance imaging (MRI), diffusion tensor imaging (DTI), resting-state functional MRI (rs-fMRI), perfusion MRI and positron emission tomography (PET). The non-invasive, high resolution and multimodal capabilities of neuroimaging technologies render them powerful tools for investigating lung-brain interactions. Each modality offers unique advantages and limitations. Structural MRI can capture anatomical details. It is widely used to assesses gray matter (GM) atrophy, cortical morphology changes and white matter (WM) changes in COPD, coronavirus disease 2019 (COVID-19), lung cancer, and other lung diseases (Esser et al., 2016; Chen et al., 2016; Qin et al., 2020; van der Knaap et al., 2024; Parsons et al., 2021; Douaud et al., 2022; Simó et al., 2016a; Simó et al., 2016b; Liu et al., 2022c; Hopkins et al., 2006; Roy et al., 2021; Mohammadi-Nejad et al., 2022; Vandiver et al., 2022). However, it cannot detect WM microstructural changes. DTI detects WM microstructural damage by measuring fractional anisotropy (FA) and other diffusion metrics in COPD and lung cancer patients (Lee S. et al., 2019; Simó et al., 2016b; Simó et al., 2016a; Liu et al., 2022c). Rs-fMRI reveals altered functional connectivity (FC) in the default mode network (DMN) in asthma (Zhang et al., 2017; Wang et al., 2023; Zhu et al., 2023; Wu et al., 2023), and alterations in brain network topology and abnormal FC in pain perception circuits (Chammah et al., 2021; Hu et al., 2022; Wei et al., 2024; Zhou X. et al., 2022). Rs-fMRI can assess brain activity, but depend on data preprocessing and subject conditions. Perfusion MRI offers insights into cerebrovascular dynamics, implicating vascular pathology in long-term neurological sequelae of post-COVID-19 patients (Ajčević et al., 2023; Mohammadi and Ghaderi, 2024). PET imaging elucidates metabolic and neurotransmitter alterations in lung diseases (Fontana et al., 2020; Golan et al., 2009). But PET has lower spatial resolution compared to MRI and needs radioactive tracers. These neuroimaging techniques complement each other and are often combined to provide a more comprehensive understanding of lung-brain axis.
This survey summarizes neuroimaging findings across various lung diseases. It focuses on brain structural and functional changes caused by these conditions. It aims to clarify the neuroimaging mechanisms underlying lung-brain interactions using multimodal imaging. The survey assesses the clinical relevance of these findings. These insights lay a foundation for advancing the diagnosis and treatment of multisystem diseases.
Figure 1A highlights four major lung diseases studied in this field. Among these, COPD is the most extensively investigated condition, followed by lung cancer, COVID-19, and other less-explored conditions such as asthma and pulmonary artery hypertension. Figure 1B summarizes the neuroimaging modalities employed in current studies, categorizing them into structural and functional imaging techniques. Figure 2 builds on the categorization of lung diseases in Figure 1A, outlining the neuroimaging methods and associated data analysis approaches applied in studies of these conditions. Table 1 summarizes studies associating lung function indices with neuroimaging, and lists the main findings.

Table 1. List of research that found associations between lung function indicators and brain changes.
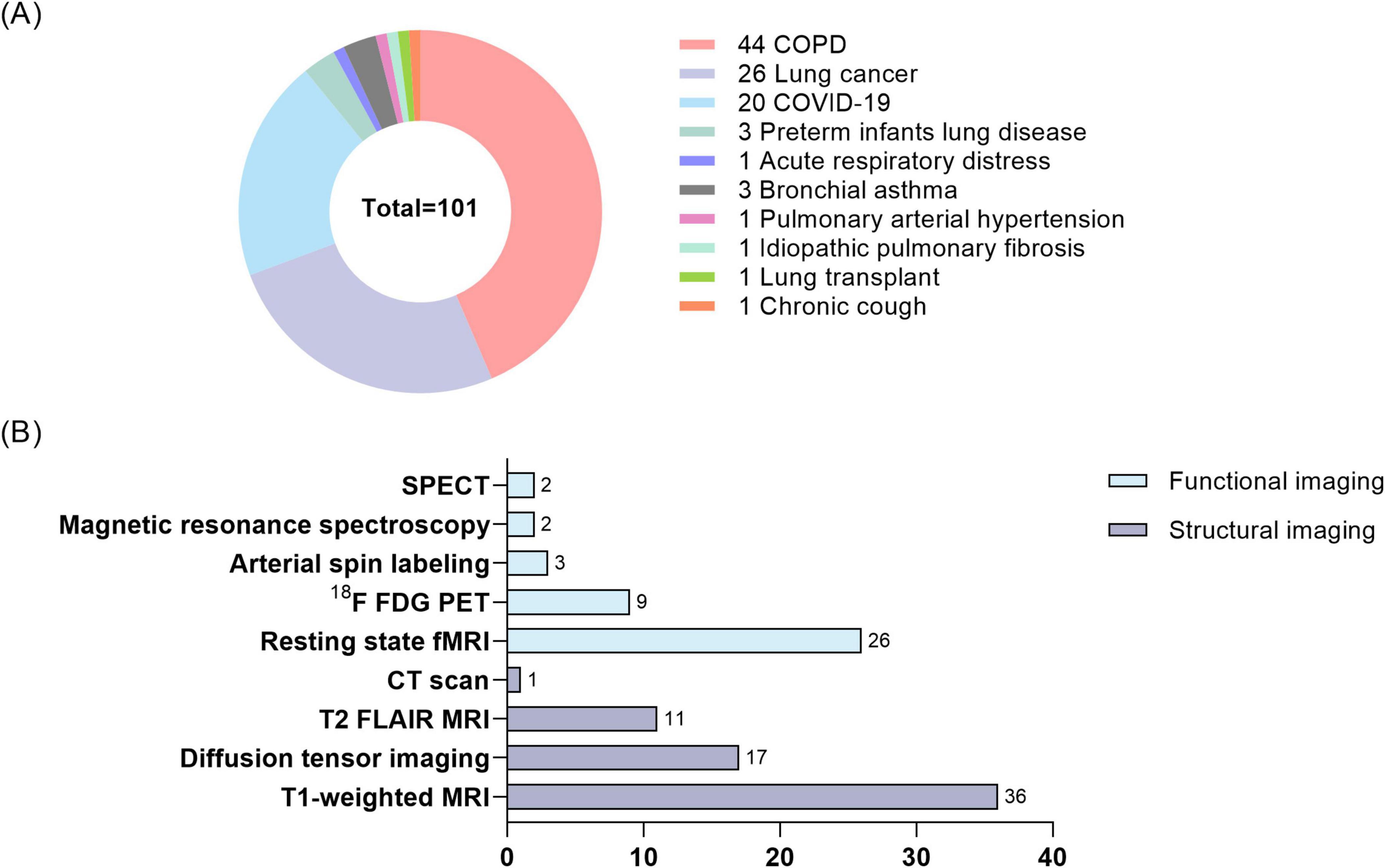
Figure 1. Distribution of lung disease studies and number of neuroimaging techniques. (A) Percentage of lung diseases studied in lung-brain interaction studies. (B) Number of neuroimaging modalities employed in lung-brain interaction studies. COPD, chronic obstructive lung disease; COVID-19, coronavirus disease 2019; SPECT, single-photon emission computed tomography. 18F-FDG-PET, 18F-FDG positron emission tomography.
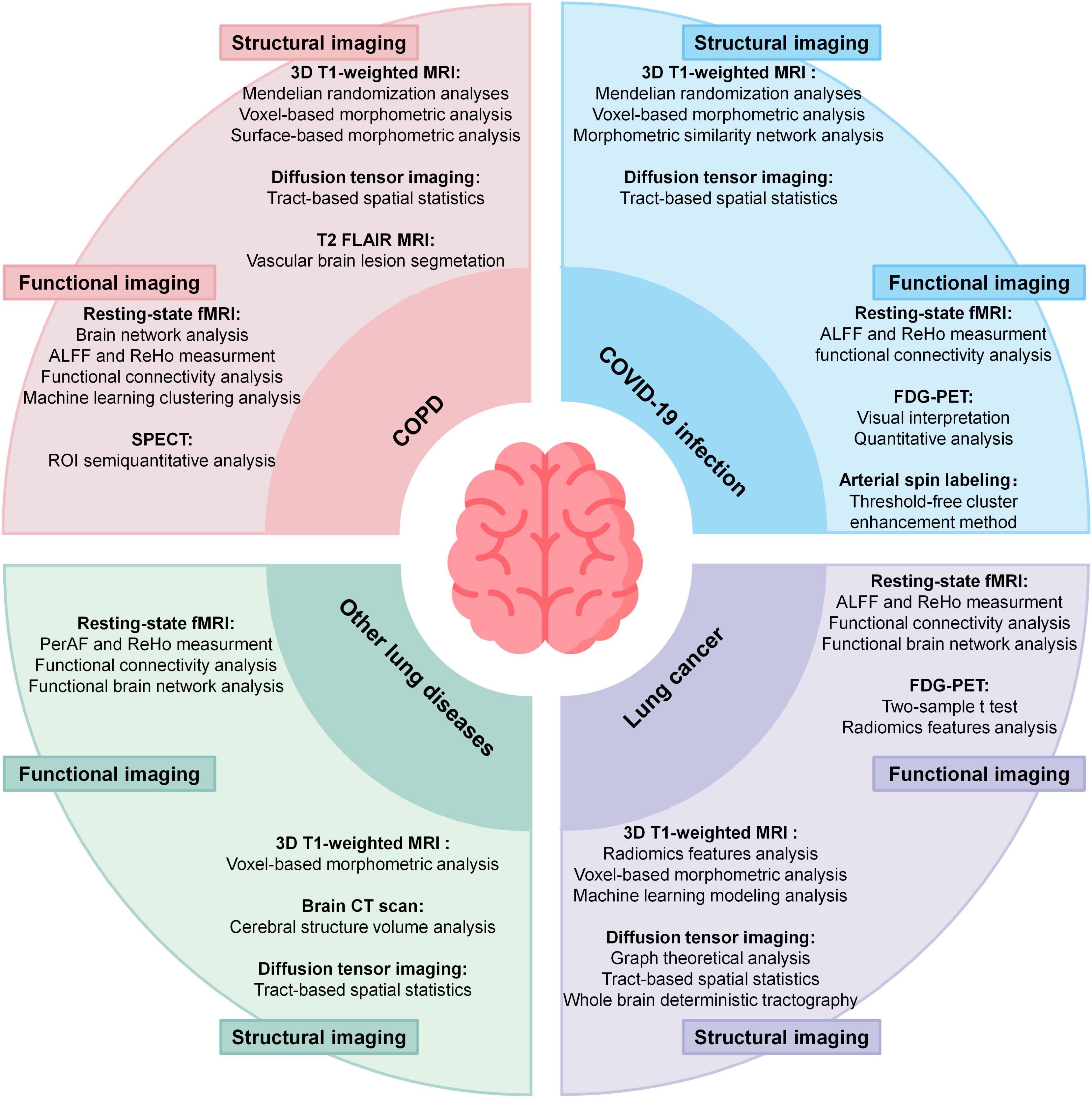
Figure 2. Summary of neuroimaging methods applied in 4 kind of lung disease and corresponding analyses methods. ALFF, amplitude of low-frequency fluctuations; ReHo, regional homogeneity; PerAF, percent amplitude of fluctuation; SPECT, single-photon emission computed tomography. 18F-FDG-PET, 18F-FDG positron emission tomograph.
The rest of this manuscript is organized as follows. Section “2 COPD and neuroimage changes” focuses on the impacts of COPD on brain structure, function, and cognition; Section “3 Neuroimaging changes due to lung cancer” discusses the effects of lung cancer on brain structure, function, and cognition; Section “4 COVID-19 and neuroimage changes” elaborates on the influence of COVID-19 on brain structure, function, and cognition; Section “5 Neuroimaging changes in other lung diseases” examines the effects of other lung diseases on brain structure and function; Section “6 Mechanisms of neuroimaging findings in lung diseases” analyze the potential mechanisms underlying neuroimaging findings in lung diseases; Section “7 Clinical significance of neuroimage research” summarize the clinical significance of neuroimaging studies; Section “8 Limitations of current research and future directions” discuss the limitations of current research and suggestions for future directions.
Growing evidence highlights the systemic impact of COPD. Chronic hypoxia and respiratory instability drive structural and functional disruptions in brain, link lung dysfunction to neurological decline.
Structural MRI studies in COPD patients reveal widespread brain abnormalities. These include reduced GM volume, WM integrity loss, WM hyperintensities (WMHs), and cortical thickness alterations. These structural changes involve multiple brain regions and are correlated with cognitive decline, motor dysfunction, and increased dementia risk (Xiao et al., 2022; Zhou L. et al., 2022). COPD-related GM atrophy predominantly affects the hippocampus, prefrontal cortex, cingulate gyrus, limbic system, and subcortical structures, along with cortical thinning in motor, parietal, prefrontal, Broca’s areas (Esser et al., 2016; Fukatsu-Chikumoto et al., 2024; Zhang et al., 2013; Zhang et al., 2012; Chen et al., 2016; Liang et al., 2024). A voxel-based meta-analysis further delineated the patterns of GM atrophy in COPD patients (Figure 3B). DTI analyses reveal decreased FA in the superior and inferior longitudinal fasciculi, corona radiata, optic radiations, and fornix, and altered diffusivity in the corticospinal tract, thalamus, and midbrain (Figure 3A; Dodd et al., 2012; Spilling et al., 2021; Lee S. et al., 2019; Zhang et al., 2012). Furthermore, COPD patients showed increased WMHs and enlarged perivascular spaces, with severity positively correlating with disease duration (Spilling et al., 2017; Qin et al., 2020). Voxel-based morphometry (VBM) and surface-based morphometry (SBM) analyses further identified GM atrophy in several brain regions. These include the anterior insula, thalamus, caudate, and para-hippocampal gyrus. Some changes correlate with cognitive performance (Montreal Cognitive Assessment, MoCA scores) and lung functions (forced expiratory volume in one second, FEV1) (Zhang et al., 2013; Yin et al., 2019; see Figure 4). Mendelian randomization studies suggest potential causal links between COPD and cortical atrophy in orbital, cuneate and inferior parietal gyrus (Fang et al., 2024). Structural damage to the corpus callosum, with reduced FA and increased mode of anisotropy, may underlie motor deficits and muscle weakness in COPD (Cabibel et al., 2020). Some studies reporting no evidence of GM atrophy in stable COPD patients. But progressive WM lesions in advanced disease stages emphasize the importance of early interventions to mitigate cognitive and neurological impacts (Spilling et al., 2017; Liang et al., 2024; Takamatsu et al., 2021; Yin et al., 2019).
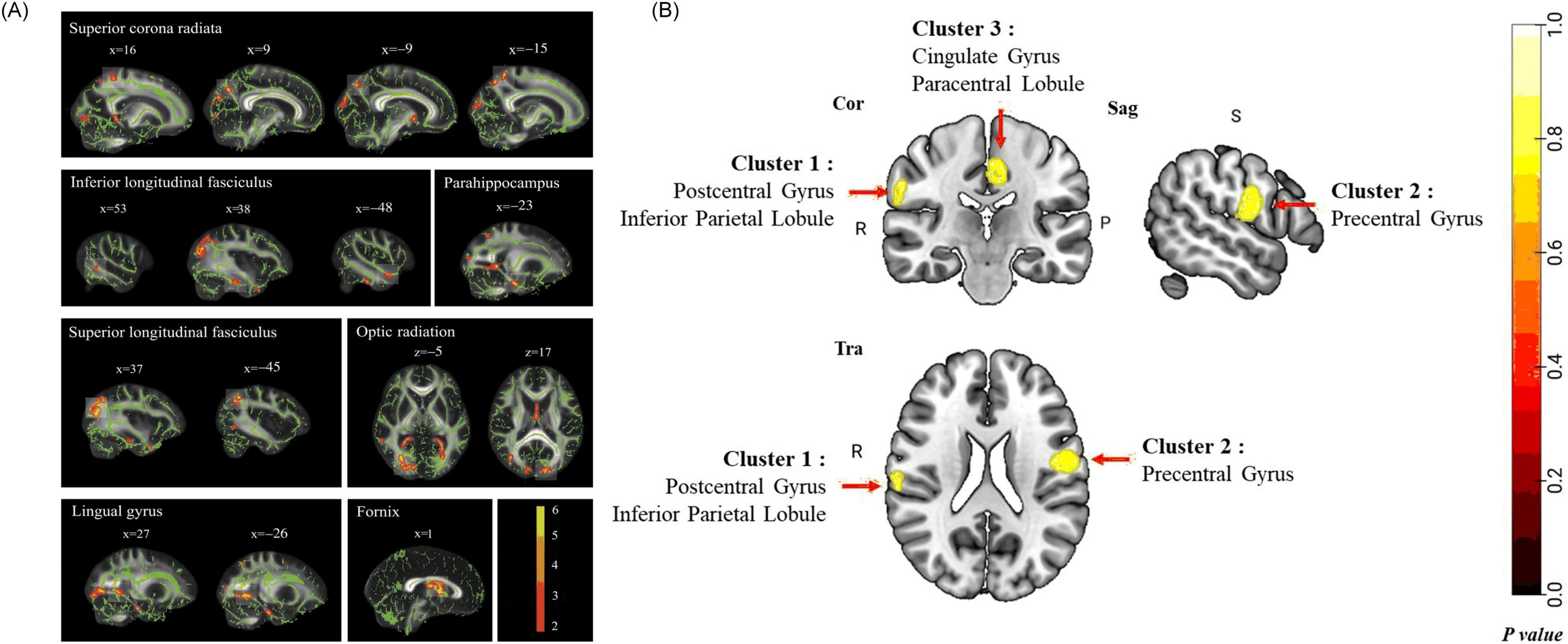
Figure 3. Cerebral structural changes on COPD patients. (A) Group comparison of fractional anisotropy (FA) value on tract-based spatial statistics (TBSS). COPD Patients show significantly lower FA value than healthy controls (p < 0.05) in superior corona radiata, inferior longitudinal fasciculus, superior longitudinal fasciculus, optic radiation, fornix, parahippocampus, and lingual gyrus (Zhang et al., 2012). (B) The pattern of GM abnormalities in COPD patients using voxel-based meta-analysis, results revealed significant GM abnormalities in the right postcentral gyrus (including inferior parietal lobule), left precentral gyrus, and left cingulate gyrus (including paracentral lobule) in COPD patients compared with HCs. Cor, coronal; Sag, sagittal; Tra, transverse; R, right; P, posterior; S, superior. Permutation test P < 0.05, FWE corrected (Liang et al., 2024).
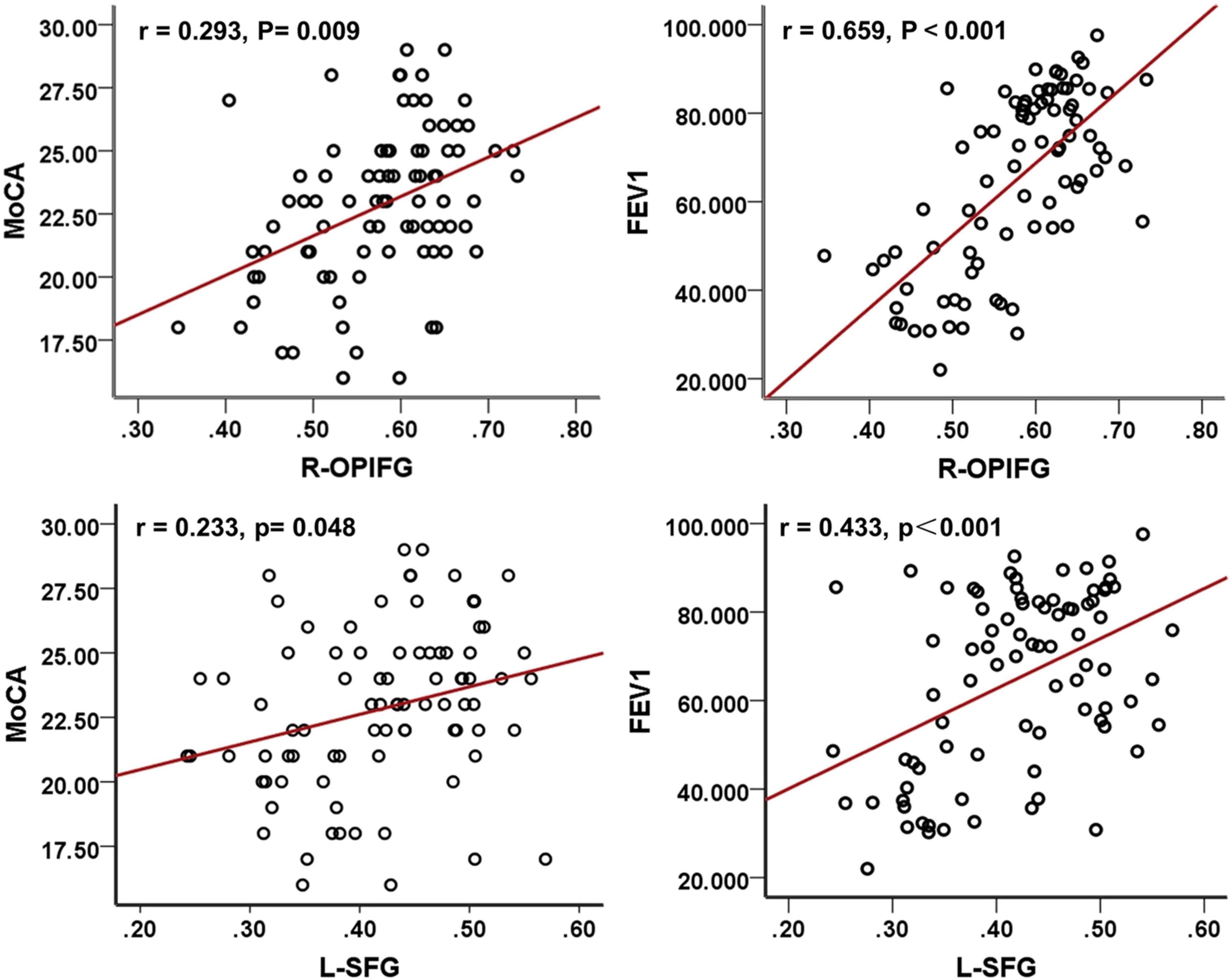
Figure 4. Correlation analyses among MoCA scores, FEV1, and GMD in COPD patients. MoCA, Montreal cognitive assessment; FEV1, forced expiratory volume in 1 s; COPD, chronic obstructive pulmonary disease; R-OPIFG, right orbital part of the inferior frontal gyrus; and L-SFG, left superior frontal gyrus (Yin et al., 2019).
COPD also alters cerebral function, largely driven by chronic hypoxia and respiratory dysfunction. Hypoxemic COPD patients exhibit greater anterior cerebral hypoperfusion and accelerated cognitive decline compared to non-hypoxemic patients (Antonelli Incalzi et al., 2003; Ortapamuk and Naldoken, 2006). Magnetic resonance spectroscopy studies indicate compensatory shifts in intracellular pH and disrupted cerebral phospholipid membranes in response to chronic hypoxia, alongside reduced metabolites in frontal and partial WM (Hamilton et al., 2003; Karakas et al., 2013). Transcranial magnetic stimulation methods indicated patients during acute exacerbation of COPD had less excitable motor cortex and higher corticospinal inhibition (Mohamed-Hussein et al., 2007; Alexandre et al., 2020). Rs-fMRI studies show widespread disturbance in intrinsic brain connectivity networks, particularly in the basal ganglia, where abnormal amplitude of low-frequency fluctuations (ALFF) correlates with partial pressure of oxygen (PaO2) (Dodd et al., 2012; Zhang J. et al., 2016; Lu et al., 2019). Network analyses reveal decreased global connectivity strength and nodal efficiency, predominantly in the visual and frontoparietal networks (Spilling et al., 2019; Wang et al., 2020). Additionally, frequency-dependent neural changes reveal ALFF abnormalities associated with arterial partial pressure of carbon dioxide (PaCO2) (Yu et al., 2021). Dynamic functional network connectivity studies show COPD patients remain in weakly connectivity longer than healthy controls, with aberrant connectivity primarily involving the default mode network, executive control network, and visual network. Correlation analysis showed dFNC indictors are correlate with FEV1, FEV1/FVC, and PaCO2 (Tang et al., 2022). Reduced motor cortical connectivity interactions with the brainstem and a shift in network dominance from the medulla to the motor cortex, potentially contributing to respiratory failure. These findings suggest therapeutic strategies to enhance respiratory muscle performance by modulating motor cortex (Yu et al., 2016). Metrics such as regional homogeneity (ReHo) and degree centrality (DC) confirm disrupted functional organization, especially in visual, motor, and default mode network (Xin et al., 2019; Li et al., 2020). ReHo values in the precuneus positively correlate with FEV1% (r = 0.465, P = 0.045), FEV1/FVC (r = 0.763, P < 0.001), while negatively correlate with PaCO2 (r = -0.611, P = 0.005) (Figure 5). The anterior insula plays a key role in modulating dyspnea perception in COPD patients. Greater activity was observed in patients with lower symptom burdens. This highlights the need for a multimodal approach to understanding dyspnea (Figure 6; Finnegan et al., 2021). COPD patients have impaired cerebrovascular reactivity (CVR) in anterior and posterior cerebral circulation. Impairment of CVR increase with the airflow limitation severity (Hlavati et al., 2019).
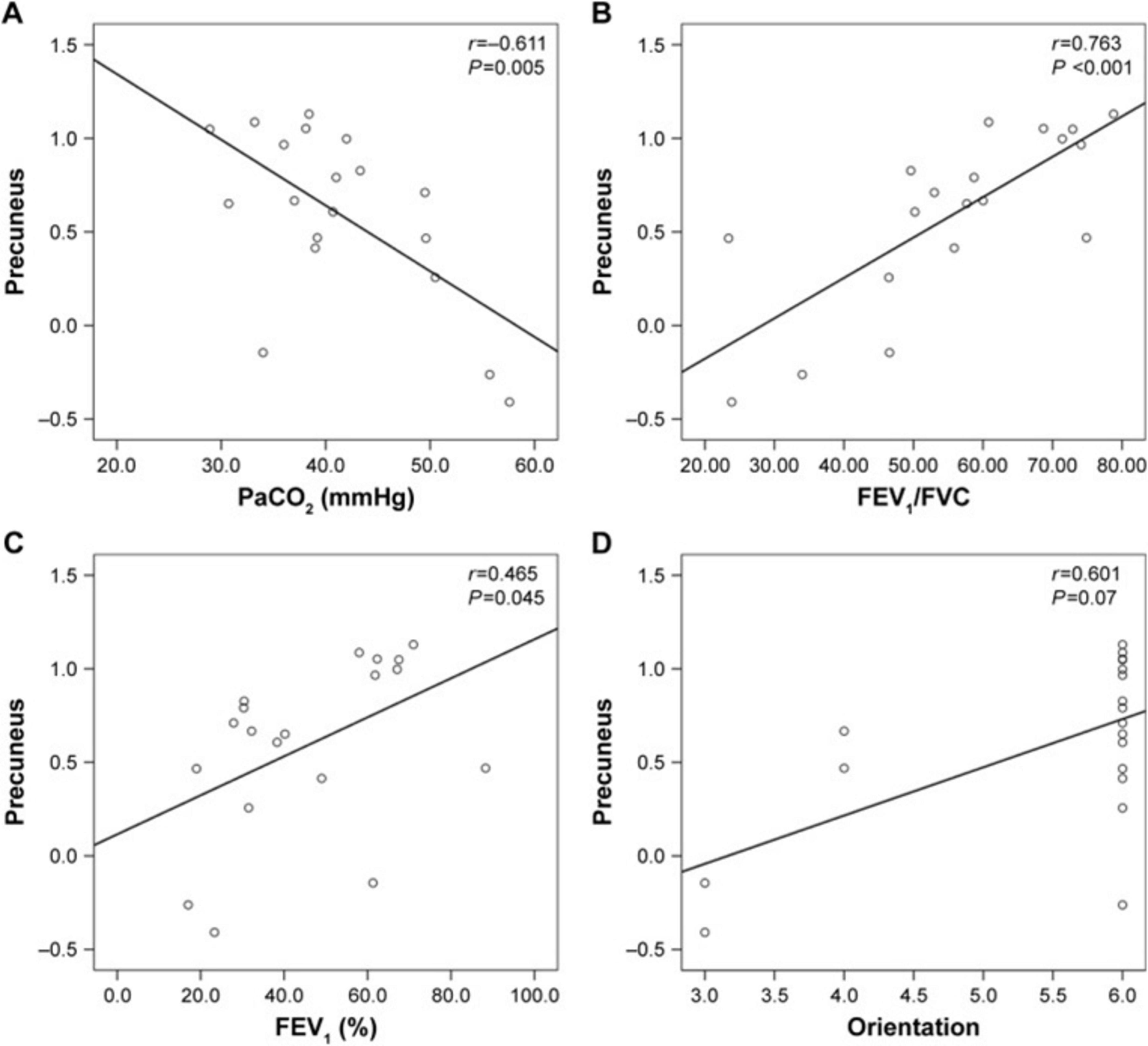
Figure 5. Correlation between the ReHo values in the precuneus and the clinical parameters (A) PaCO2 (B) FEV1/FVC (C) FEV1(%) (D) Orientation.
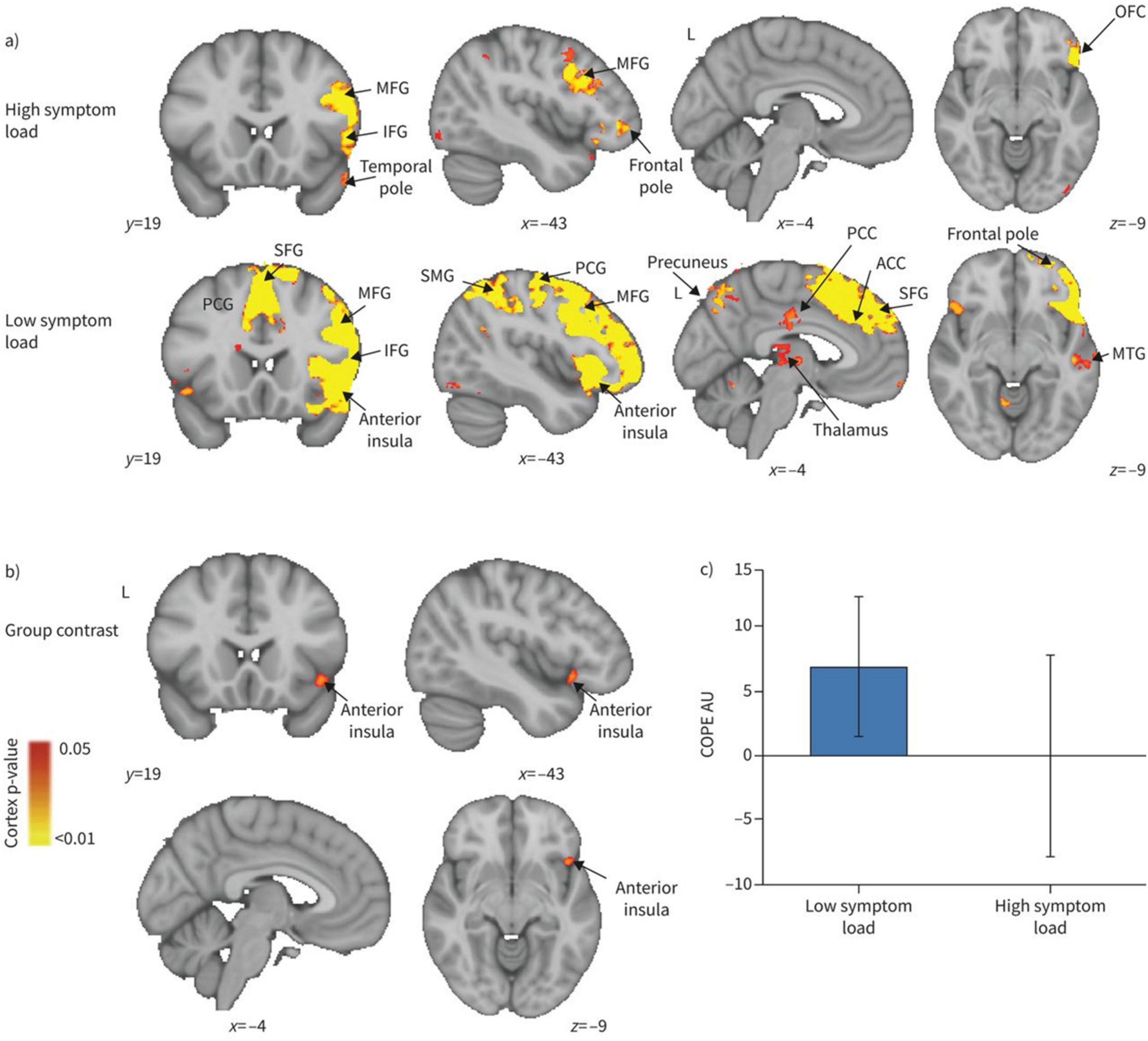
Figure 6. Blood oxygen level dependent activity in response to breathlessness-related words compared with non-words for (A) high and low symptom load groups separately, and (B) their contrast. MFG, middle frontal gyrus; IFG, inferior frontal gyrus; OFC, orbitofrontal cortex; PCG, paracingulate gyrus; SFG, superior frontal gyrus; SMG, supramarginal gyrus; PCC, posterior cingulate cortex; ACC, anterior cingulate cortex; MTG, middle temporal gyrus; COPE, contrast of parameter estimate. (A) The IFG, MFG and temporal pole demonstrated significant activity in the high symptom group, while in the low symptom group, the PCG, anterior insular, temporal pole, precuneus, PCC, ACC, SMG, thalamus SFG, MFG and IFG all demonstrated significant activity. For both high and low symptom groups, significant regions are displayed with a non-parametric threshold-free cluster enhancement p < 0.05. (B) The contrast of the two groups revealed significant regional activity in the low symptom group compared with the high symptom group in the anterior insula cortex with a non-parametric p < 0.05. (C) Mean COPE in arbitrary units (AU) for the high and low symptom load groups separately with standard deviation bars showing the variation in parameter estimates across participants (Finnegan et al., 2021).
COPD is associated with the cognitive impairment, either globally or in specific cognitive domains, with its severity influenced by disease severity, arterial oxygen saturation and aging (Schou et al., 2012; Cleutjens et al., 2014; Yin et al., 2016; Samareh Fekri et al., 2017; Kakkera et al., 2018). Commonly affected cognitive domains include attention, executive function, working memory, and mental speed, reflecting prefrontal deficits in information integration than storage (Areza-Fegyveres et al., 2010; Lv et al., 2020). Hypoxia-induced cognitive impairments primarily affect memory and attention allocation, with memory deficits linked to limbic system involvement and attention issues attributed to diminished resource allocation (Stuss et al., 1997). Visuospatial construction impairments correlate with cortical thinning in the fronto-parietal network, driven in part by oxygen desaturation, contributing to deficits in visual memory and drawing tasks (Chen et al., 2016). GM reductions in regions related to dyspnea processing, fear regulation, and antinociception, are partially linked to disease duration and disease-specific fears, which may exacerbate the disease trajectory (Esser et al., 2016). Increased WML volume is associated with poorer episodic memory in stable COPD patients (Spilling et al., 2017). Functional changes in the DMN highlight key roles of the left posterior cingulate cortex and left hippocampus in cognitive decline (Hu et al., 2018). Dyspnea-related cognitive interference worsens motor control and daily functioning, long-term oxygen therapy may benefit neuropsychological and neurovascular function in mildly hypoxemic patients, its effects often lack statistical significance (Hjalmarsen et al., 1999; Hoiland et al., 2018; Kozora et al., 1999; Rozenberg et al., 2024). Integrating cerebral small vessel disease burden scores with cognitive scales could improve early detection of cognitive decline (Li et al., 2024a). COPD patients tend to outperform those with mild Alzheimer disease (AD) in neuropsychological tests, potentially due to relatively preserved brain network efficiency (Spilling et al., 2019). However, COPD exacerbates cognitive and mood disturbances in AD patients, highlighting the need for multidisciplinary management (Kozora et al., 1999; Schou et al., 2012; Tondo et al., 2018; de Kort et al., 2024). Mental health impairments, including anxiety, depression, and cognitive problems, influence the disease’s trajectory. Higher values in FEV1% predicted and FVC% predicted were associated with better global cognitive function in the cross-sectional analyses (Xiao et al., 2022). Comprehensive interventions that go beyond lung rehabilitation to incorporate mental health and cognitive support is crucial (Pelgrim et al., 2019).
Lung cancer, including small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), causes notable alterations in brain structure and function. Advanced neuroimaging has revealed these alterations. These changes contribute to cognitive and emotional impairments and highlight the neuropathological impact of the disease and its treatments.
SCLC patients underwent chemotherapy and prophylactic cranial irradiation (PCI) experience notable reductions in GM volume in the right subcortical regions, bilateral insular cortex, and superior temporal gyrus. Longitudinal assessments identified additional GM reductions in the right para-hippocampal gyrus and hippocampus, accompanied by widespread WM microstructural changes, particularly in the corpus callosum (Simó et al., 2016a). VBM and tract-based spatial statistics (TBSS) analyses corroborate these findings, showing decreased GM density in the bilateral basal ganglia, thalamus, and right insula, as well as WM microstructural abnormalities in the entire corpus callosum (Simó et al., 2016b). These findings underscore the neurotoxic impact of PCI on brain integrity. In addition to PCI, chemotherapy induces substantial brain changes in SCLC patients. Significant alterations in brain volume, cortical thickness, FA values, and functional connectivity measures were observed. DTI combined with graph theory revealed the topological reorganization of hub distributions in impaired brain structural network (Mentzelopoulos et al., 2021; Mentzelopoulos et al., 2022). Extensive WM microstructural damage has been identified in lung cancer patients with cancer pain. Disruption of specific nodes along pain-related fiber bundles were noted, these alterations may serve as sensitive imaging biomarker to characterize the severity and duration of cancer pain (Ran et al., 2024).
NSCLC patients experience brain network disruptions linked to cognitive and emotional deficits. Reductions in global efficiency are particularly evident in the left inferior frontal gyrus and right Rolandic area, with diminished local efficiency in the left middle frontal gyrus and left superior temporal gyrus. These alterations highlight the disruption of topological brain network features may underlie cognitive and emotional impairment in NSCLC patients (Liu et al., 2022c). Immune checkpoint inhibitor therapy in NSCLC patients is associated with detectable brain MRI abnormalities. These include stroke, typical WM lesions, and T2 hyperintensities indicative of central nervous system vasculitis or encephalitis. Interestingly, patients with such brain changes exhibited higher clinical benefit rates, longer progression-free survival, and a trend toward improved overall survival, suggesting a potential prognostic role for these imaging findings (Ni et al., 2022).
Radiomics provides non-invasive insights into brain metastases and molecular features. Textural features on MRI images may help to discriminate brain metastases of different primary tumors and guide treatment planning (Béresová et al., 2018; Li et al., 2024b). The SVM model integrating intra-tumoral and peritumoral radiomics features was confirmed as an imaging biomarker. It predicts brain metastases (BM) in newly diagnosed lung cancer patients. This highlights its potential to impact clinical diagnosis and treatment (Yichu et al., 2024). Deep learning methods combined with multiparametric MRI can also differentiate pathological subtypes of BM in lung cancer patients (Li et al., 2024c). Deep learning model integrating brain metastasis radiomics, clinical features, and a prognostic index provided reliable multi-time-point progression-free survival predictions for patients with advanced NSCLC and brain metastases (Wang et al., 2024).
Patients with lung malignancy showed a higher right cerebellar metabolism, potentially reflecting compensatory mechanisms to maintain respiratory and immune homeostasis (Golan et al., 2009). NSCLC patients underwent 18F-FDG PET prior to oncotherapy showed hypermetabolism in visceral-to-brain signal transduction pathways, while hypometabolism in dorsal attention network and visuospatial function areas (Figure 7). These changes may be associated with visceral sympathetic activation induced by lung cancer (Zhang W. et al., 2016). Post-PCI 18F-FDG PET studies in SCLC patients demonstrated significant decreases in glucose metabolism in the basal ganglia, central regions, cingulate cortex, striatum, frontal cortex, parietal cortex, occipital cortex, precuneus, lateral temporal cortex, and cerebellum (Chammah et al., 2021). These changes, further corroborated by textural analysis, may reflect radiation-induced damage, and provide novel approaches to study cognitive impairment (Sawyer et al., 2021). Lung function indices (FVC and FEV1) correlate with decreased standardized uptake value ratios in brain regions suggest that impaired lung function may exacerbate reduction in cerebral glucose metabolism and cognitive performance (Son et al., 2023).
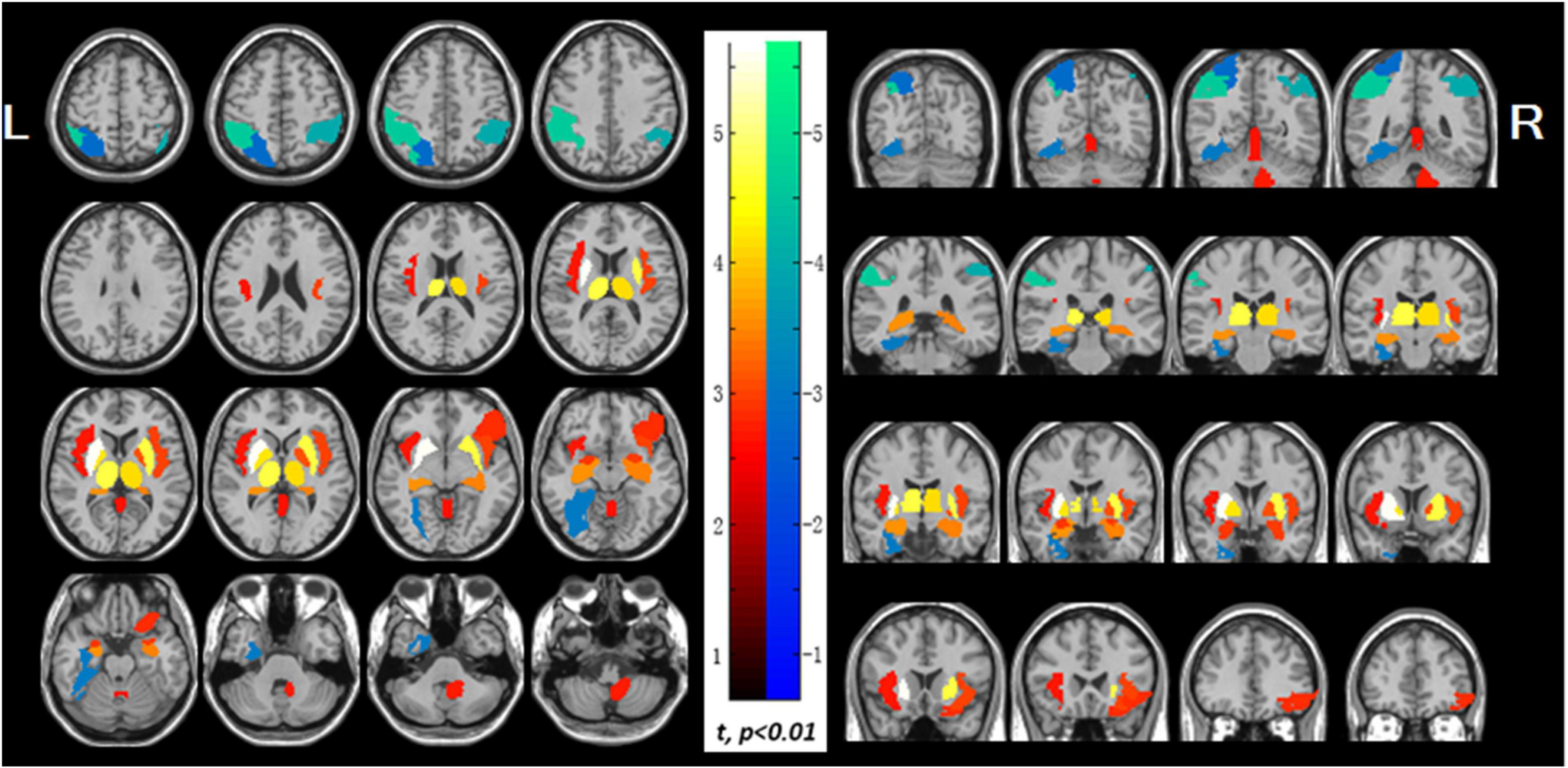
Figure 7. Abnormal glucose uptake in the non-small cell lung cancer patients. The increased regions display with red-to-white color, which include both left and right sides of the insula, putamen, pallidum, thalamus, hippocampus and amygdala, the right side of cerebellum, orbital part of right inferior frontal gyrus and vermis, while the decreased regions display with blue-to-green color, which include left superior parietal lobule, bilateral inferior parietal lobule, and left fusiform gyrus (p < 0.01). PET findings were overlaid on magnetic resonance image secondary. Color bar indicates t-values; L, left; R. right (Zhang W. et al., 2016).
Rs-fMRI analyses revealed lung cancer patients exhibited reduced rs-fMRI values in frontal and parietal regions before and after chemotherapy (You et al., 2020). Reduced dynamic activity in DMN after chemotherapy suggests its susceptibility to treatment-related damage. Decreased FC in posterior cingulate cortex (PCC) and anterior cingulate cortex (ACC) correlates with reduced MoCA, implicating DMN disruptions in chemotherapy-induced cognitive impairment (Zhang et al., 2020). Graph-theoretical analyses identify topological abnormalities in the pallidum-thalamus-cortex circuitry (see Figure 8). NSCLC patients show altered hub strength in key brain regions. Strength reduced in the inferior frontal gyrus but increased in the pallidum and thalamus. Hub mediation is also reduced in the superior occipital gyrus. These topological abnormalities in may reflect the pathological mechanisms of NSCLC and serve as potential biomarkers (Liu et al., 2022b). Dynamic functional connectivity analyses further identified deficits across sensorimotor, attention and auditory networks, emphasizing the extensive impact of chemotherapy on the brain resting-state network (Hu et al., 2022).
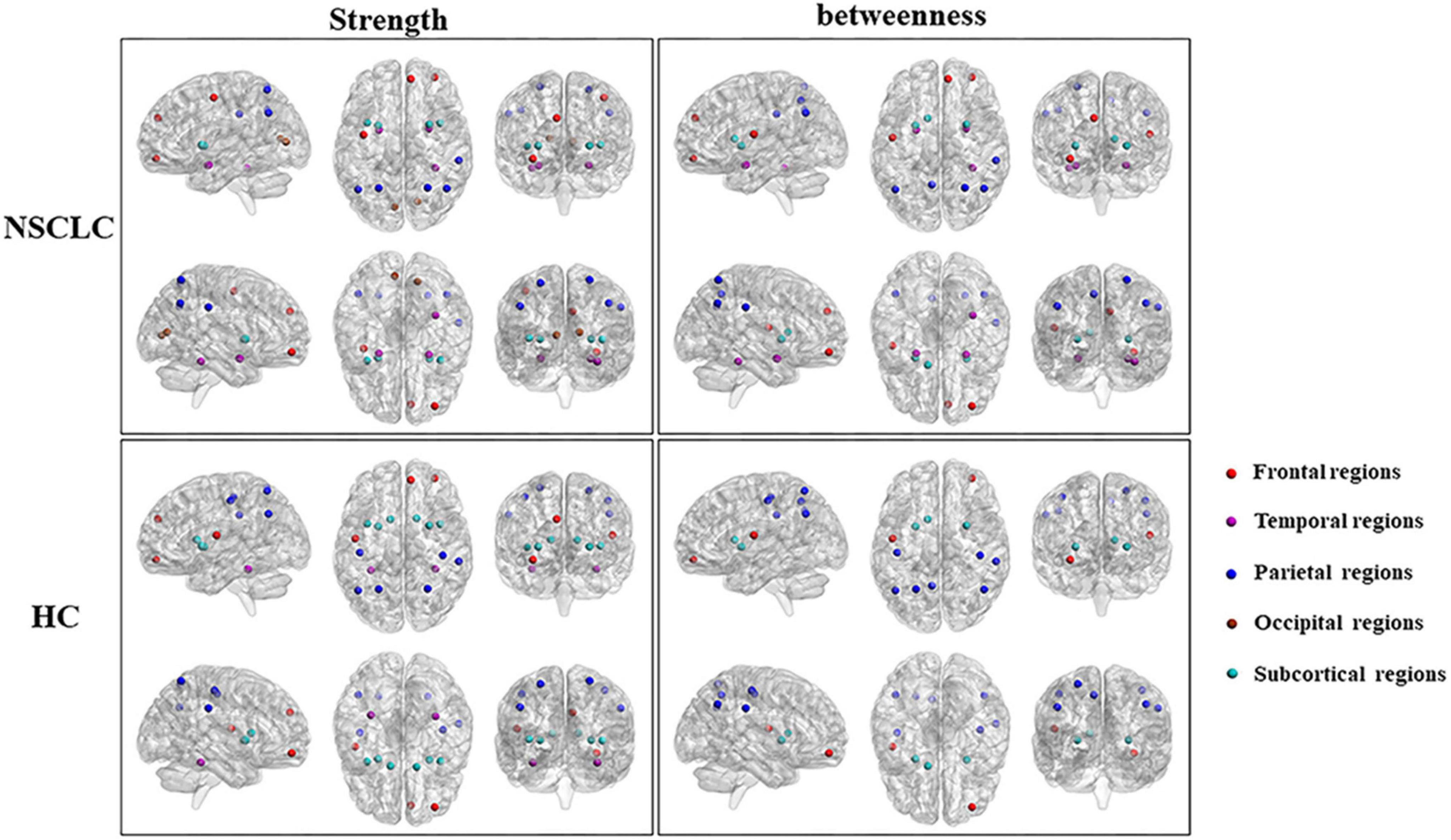
Figure 8. Differences in the distribution of hub regions between NSCLC and HC. Nodes with high nodal strength were classified as hub regions, which had intense interconnectivity with other regions in the brain and played an important in the information transfer and integration. Eleven of these regions [left and right superior parietal gyrus, putamen, and insula; left anterior cingulate gyrus; right middle frontal gyrus (orbital part), superior frontal gyrus (medial), fusiform gyrus, and supramarginal gyrus] were shared by both the NSCLC and HC groups. The NSCLC group lost hub properties in the left and right postcentral gyrus, caudate nucleus, left rolandic operculum, and fusiform gyrus, while the left and right amygdala, calcarine fissure, left precentral gyrus, and right anterior cingulate gyrus acted as new hub regions in the patient group. Nodes with high nodal betweenness were also classified as hub regions, which lied on the shortest path between two other regions and played an important role in controlling information flow. Nine of these regions (left and right anterior cingulate gyrus and putamen; left rolandic operculum, precuneus, and caudate nucleus; right middle frontal gyrus (orbital part) and supramarginal gyrus) were shared by two groups. The left and right amygdala, right superior frontal gyrus (medial), fusiform gyrus and superior parietal gyrus were defined as hub regions only in the NSCLC group. In the left and right postcentral gyrus, left superior parietal gyrus was defined as hub regions only in HC. Strength, nodal strength; betweenness, nodal betweenness; NSCLC, non-small cell lung cancer; HC, healthy control (Liu et al., 2022b).
Patients with bone metastases from lung cancer (BMP) exhibit disrupted intrinsic brain connectivity. Reduced ALFF and ReHo were observed in the prefrontal cortex, alongside increased ReHo in the bilateral thalamus and left fusiform gyrus, with decreased FC within the prefrontal cortex (Liu et al., 2022a). In patients with cancer pain (CP+), hypo-connectivity is observed across somatomotor, ventral attention, fronto-parietal control, and DMN compared to controls. However, CP+ patients uniquely demonstrate hyper-connectivity in somatomotor and visual networks, potentially reflecting neural adaptations to pain. Increased connectivity strength within and between network modules correlates strongly with pain intensity and duration, particularly involving regions such as the dorsolateral prefrontal cortex, ACC, secondary somatosensory cortex, and amygdala (Wei et al., 2024; Zhou X. et al., 2022). Altered functional activity and connectivity in the prefrontal cortex, fusiform gyrus, and thalamus may be relevant to the neuropathology of BMP and is expected to be a potential biomarker for predicting BMP patients (Liu et al., 2022a).
SCLC patients underwent chemotherapy and PCI exhibited cognitive deterioration. This included verbal fluency decline, with nearly half meeting criteria for cognitive impairment. Cognitive decline correlated with increased mean FA values in the corpus callosum (Simó et al., 2016a; Simó et al., 2016b).
The structural and functional brain changes in lung cancer patients underscore the profound impact of cancer and its treatments on neural integrity. These findings highlight the importance of incorporating advanced neuroimaging techniques into clinical practice to monitor brain health, mitigate cognitive and emotional deficits. Further research is warranted to elucidate the mechanisms underlying these changes and to explore potential protective strategies.
COVID-19 has been linked to macro- and microstructural brain changes, as demonstrated through imaging studies. These changes affect both the central and peripheral nervous systems, with radiological findings revealing a wide spectrum of vascular and inflammatory effects (Lu et al., 2023; Klironomos et al., 2020; Kiyak et al., 2024). Brain MRI has revealed unusual distribution of microbleeds predominantly in the corpus callosum, along with infractions and intracranial hemorrhages in the thalamus and corpus callosum (Fitsiori et al., 2020; Klironomos et al., 2020; Kelsch et al., 2021). DTI study revealed increased mean diffusivity (MD) and radial diffusivity (RD) alongside decreased FA in WM tracts, including the corpus callosum, coronal radiations, and superior longitudinal fasciculus (Mohammadi and Ghaderi, 2024). Network diffusion modeling suggests that pathologic changes spread across the corticospinal tracts, cerebellum, and putamen, indicating structured propagation of neurological injury (Parsons et al., 2021). Structural and functional changes are observed in the temporal lobe, orbitofrontal cortex, and cerebellum. Additional disruptions were noted in the insula and limbic system, with specific alterations in the right superior temporal gyrus showing reduced functional activity but increased GM volume (Figures 9A, B; Guo et al., 2024). However, genetic susceptibility to COVID-19 showed no causal association with structural brain changes (Ding and Xu, 2023).
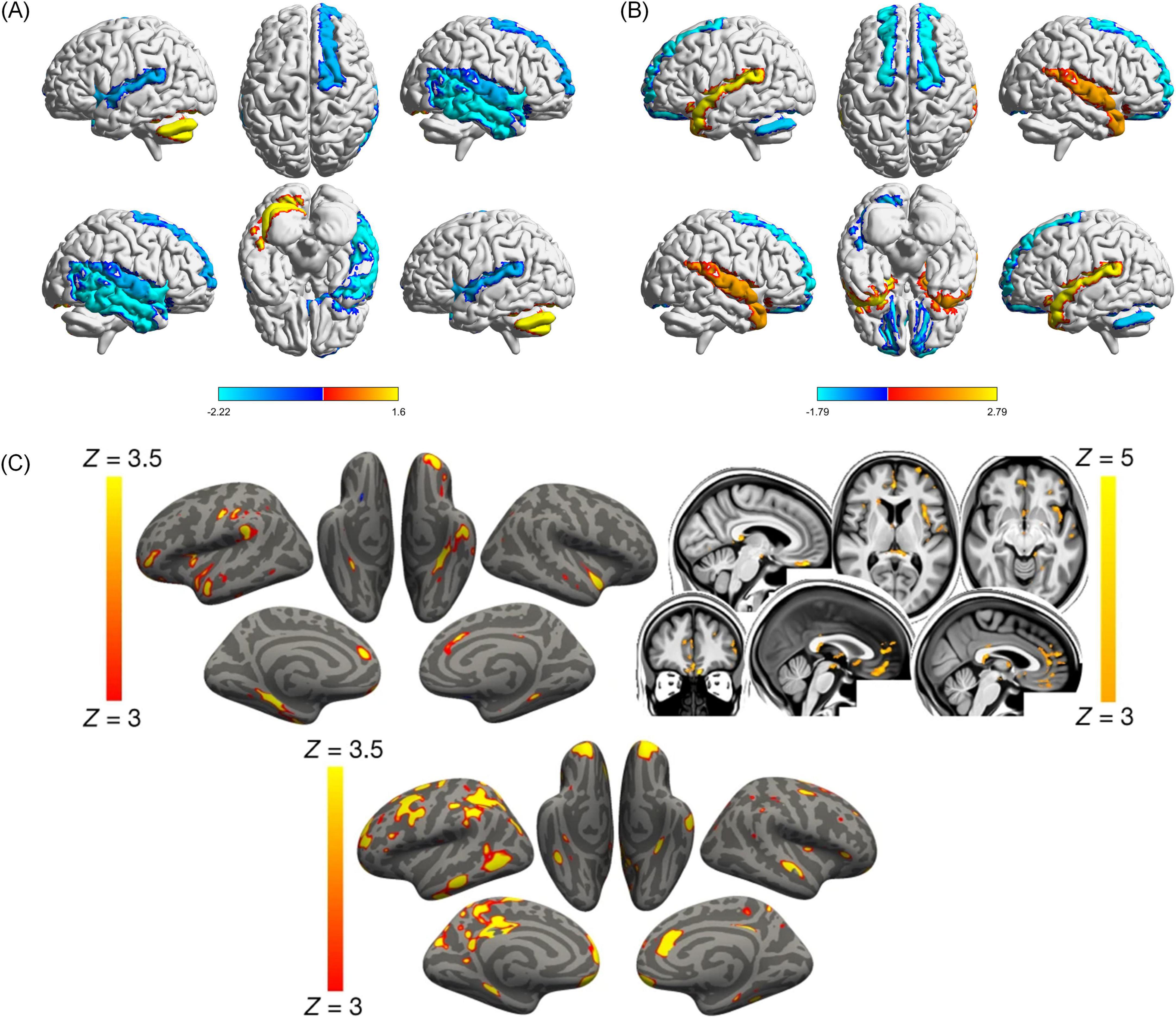
Figure 9. Cerebral structural and functional changes on COVID-19 patients. (A) COVID-19 patients displayed decreased functional activity in the right superior temporal gyrus (STG) [extending to the right middle temporal gyrus [MTG], insula, inferior temporal gyrus [ITG], and temporal pole (TP)], left insula, right orbitofrontal cortex (OFC) (extending to the right olfactory cortex), and increased functional activity in left cerebellum (Guo et al., 2024). (B) Compared with HCs, COVID-19 patients displayed decreased GMV in the bilateral anterior cingulate cortex/ medial prefrontal cortex (ACC/ mPFC) (extending to the bilateral OFC), and left cerebellum, and increased GMV in the bilateral amygdala (extending to the bilateral hippocampus, STG, TP, and MTG, and left striatum) (Guo et al., 2024). In (A,B) Red areas represent increased functional activity or GMV value, and blue areas represent decreased functional activity or GMV value. SDM-Z values are shown in color on the color bar. COVID-19, coronavirus disease 2019; HCs, healthy controls; SDM, seed-based mapping; GMV, gray matter volume (Guo et al., 2024). (C) Vertex-wise and voxel-wise longitudinal effects on COVID-19 patients. Top, the thresholded map (| Z| > 3) shows that the strongest, localized reductions in gray matter thickness in the infected participants compared with the controls are bilaterally in the parahippocampal gyrus, anterior cingulate cortex, and temporal pole, as well as in the left orbitofrontal cortex, insula and supramarginal gyrus. The strongest longitudinal differences in mean diffusivity (| Z| > 3, left is shown on the right) could be seen in the orbitofrontal cortex and anterior cingulate cortex, as well as in the left insula and amygdala (top). Bottom, the thresholded cortical thickness map (| Z| > 3) demonstrated longitudinal differences between the hospitalized and non-hospitalized COVID-19 cases in the orbitofrontal frontal cortex and parahippocampal gyrus bilaterally, right anterior cingulate cortex, as well as marked widespread differences in fronto-parietal and temporal areas, especially in the left hemisphere (Douaud et al., 2022).
Longitudinal MRI studies reported progressive reductions in GM thickness, particularly in the orbitofrontal cortex and para-hippocampal gyrus, along with global brain atrophy (Douaud et al., 2022; Figure 9C). Critically ill patients recovering from COVID-19 displayed complications during follow-up, including GM atrophy and altered WM diffusivity in cortical, limbic, and cerebellar regions, which correlated with cognitive dysfunction (Lersy et al., 2022; Díez-Cirarda et al., 2023). COVID-19 survivors exhibited cortical morphological network changes across multiple regions, this alteration reflects the extensive brain structural remodeling triggered by COVID-19 (Long et al., 2023). While longitudinal imaging data from multiple sclerosis patients suggested no acceleration of global GM atrophy after COVID-19 infection, but regional trends, such as reduced para-hippocampal gyrus volume (Rebsamen et al., 2023). Structural MRI studies in post-COVID conditions (PCCs) consistently reported gray matter volume reduction and cortical thickness in both cerebral cortical and subcortical regions. Long-term COVID-19 patients showed lower MD values in internal capsule, anterior and posterior coronal radiations, and corpus callosum, but the effect sizes were minimal. Despite reported neuropsychiatric symptoms, no significant differences in objective cognitive performance were observed (Nelson et al., 2024). These findings contribute to the understanding of the acute and chronic effects of the virus on the nervous system and potentially inform acute and long-term treatment and neurorehabilitation decisions.
Functional neuroimaging studies have revealed widespread disruptions in brain activity and connectivity among COVID-19 patients. Static amplitude of low-frequency fluctuation (sALFF) was reduced in the right lingual gyrus and left medial occipital lobe, whereas dynamic ALFF (dALFF) increased in the right rectus gyrus. Static functional connectivity (sFC) was diminished between the lingual gyrus and the right superior occipital lobe, while dynamic functional connectivity (dFC) was reduced in the anterior central gyrus. Integrating dynamic and static ALFF and FC metrics into a support vector machine (SVM) model enabled high-accuracy identification of COVID-19 patients (Han et al., 2024). Functional connectivity studies highlight extensive network disruptions, particularly in critically ill patients. These patients exhibit both low and high FC patterns, whereas those with moderate illness predominantly show high FC patterns involving networks such as DMN, sensorimotor, dorsal attention, subcortical, and cerebellar networks (Figure 10; Voruz et al., 2023). COVID-19 patients with rehabilitation syndrome present hypoconnectivity between the orbitofrontal and cerebellar regions bilaterally, altered functional brain connectivity in the para-hippocampal gyrus (Díez-Cirarda et al., 2023).
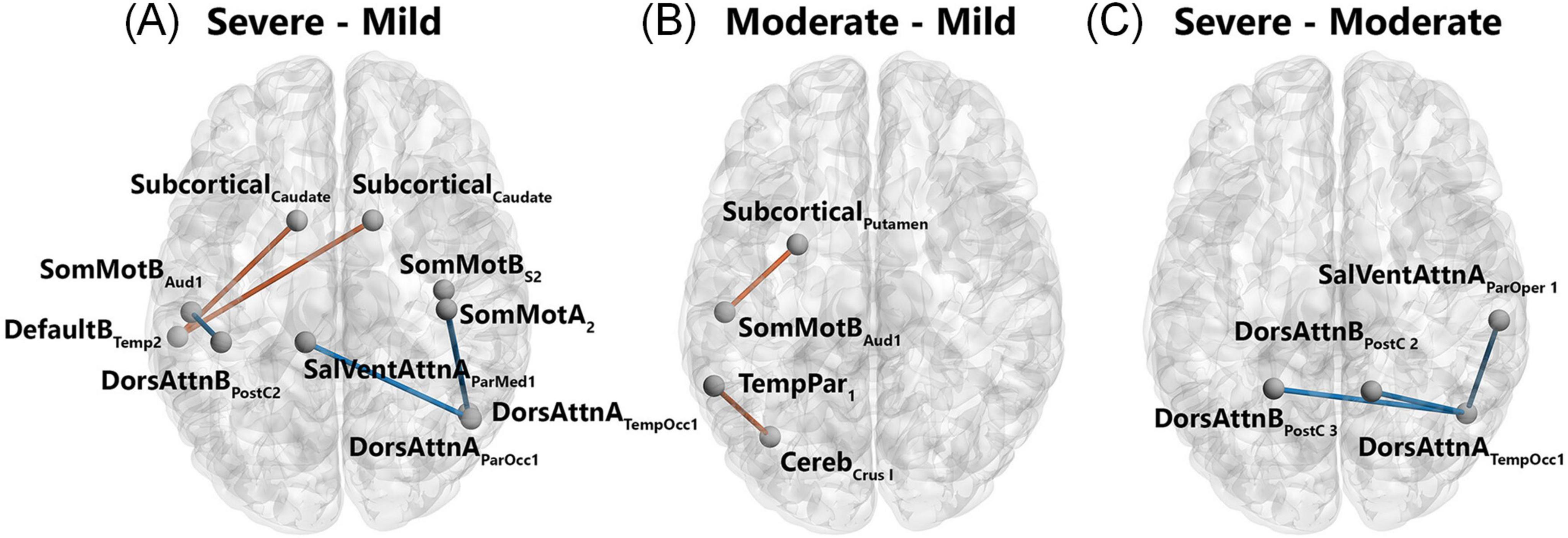
Figure 10. Patterns of significantly different functional connectivity in the COVID-19 patients. Differences in functional connectivity between brain structures shown in a network representation when comparing severe versus mild (A), moderate versus mild (B), and severe versus moderate (C). Blue lines indicate a decrease in the connectivity measurement (mean decrease = -0.3), red lines indicate an increase in the connectivity measurement (mean increase = 0.3). Statistical significance was false discovery rate (FDR)-corrected for multiple comparisons (p < 0.05 FDR). Networks: Cereb, cerebellum; DefaultB, default mode B; DorsAttnA and DorsAttnB, dorsal attention A and B; SalVentAttA, salience ventral attention A; SomMotA and SomMotB, somatosensory motor A and B; TemPar, temporoparietal; Regions: Aud, auditory cortex; ParMed, parietal medial; ParOcc, parietal occipital cortex; ParOper, parietal operculum; PostC, postcentral region; Temp, temporal region; TempOcc, temporo-occipital region (Voruz et al., 2023).
Metabolic imaging findings further corroborate these functional disruptions. FDG-PET imaging revealed hypometabolic patterns, suggesting network-level dysfunction in some long-term COVID-19 patients (Verger et al., 2022a; Verger et al., 2022b). Arterial spin labeling MRI revealed significant hypoperfusion in patients with subjective cognitive deficits, mainly affecting the prefrontal, parietal, and temporal lobes in the right hemisphere (Ajčević et al., 2023). Perfusion-weighted imaging confirmed reduced cerebral blood flow (CBF) in anterior temporal regions, thalamus, and basal ganglia among PCCs patients (Mohammadi and Ghaderi, 2024). Whole-brain and WM CBF reductions in mildly affected individuals were predictive of COVID-19 status (85.2% accuracy), suggesting that CBF patterns may be potential imaging markers (Sen et al., 2023).
Neuroimaging findings align with observed cognitive impairments in COVID-19. Cognitive and structural brain changes in hospitalized patients suggest a relationship between the severity of COVID-19 and the extent of neurocognitive impact (Díez-Cirarda et al., 2023). Critically ill patients exhibit functional deficits in verbal memory, while moderate patients show reduced mental flexibility, correlation analyses confirm specific associations between memory, executive function performance and altered FC (Voruz et al., 2023). The cognitive changes may result from a combination of neurodegenerative processes, neuroinflammation and sensory deprivation through olfactory pathway. These mechanisms likely contribute to the in vivo markers of gray matter volume (GMV) reduction in limbic system (Douaud et al., 2022).
Neuroimaging studies have also revealed brain alterations in various lung diseases, closely associated with cognitive, emotional, and functional impairments, underscoring the importance of integrating neurological assessments into lung disease management.
WM abnormalities are observed in infants receiving mechanical ventilation and chronic lung disease patients (Anjari et al., 2009), including bronchopulmonary dysplasia (Lee J. M. et al., 2019), whereas improved respiratory morbidity is associated with WH microstructure recovery (Ball et al., 2010). Survivors of acute respiratory distress syndrome (ARDS) demonstrated cognitive impairments associated with brain atrophy, ventricular enlargement, highlighting the long-term neurological sequelae of ARDS (Hopkins et al., 2006). Asthmatics exhibited enhanced functional connectivity in the DMN and salience network, correlating with higher depression, anxiety, and poor asthma control. Rs-fMRI revealed abnormalities in the ventral anterior insula linked to depression in asthma. Percent amplitude of fluctuation analysis identified altered activity in brain regions governing respiration, memory, language, and attention. In children, impairments in the superior frontal gyrus and parietal lobe were associated with attention deficits, underscoring the impact of asthma on cognitive function across age groups (Zhang et al., 2017; Wang et al., 2023; Zhu et al., 2023). Lung arterial hypertension (PAH) patients demonstrate reduced GMV in hippocampus, insula, cerebellum, and frontal, temporal, parietal, and occipital lobes. Additionally, elevated T2 relaxation values were observed in the cerebellum, hippocampus, and frontal lobe. These neuroanatomical changes may contribute to tissue damage and cognitive deficits in PAH patients (Roy et al., 2021). Genetic predispositions in idiopathic lung fibrosis patients were associated with alterations in cortical morphology and WM microstructure (Mohammadi-Nejad et al., 2022). Reduced gray matter volume in the frontal, parietal, and temporal lobes, as well as the anterior cingulate gyrus, insula, and cerebellar cortex, were observed in lung transplant patients. These changes are thought to contribute to perioperative cognitive deficits, emphasizing the need for strategies to protect brain tissue during and after transplantation (Vandiver et al., 2022). Rs-fMRI studies in chronic cough patients revealed reduced FC between the nuclear solitary tract and anterior cingulate cortex, which may contribute to cough hypersensitivity and anxiety in chronic cough patients (Wu et al., 2023).
Hypoxia and brain damage: Hypoxia reduces brain metabolism and increases oxidative stress, leading to structural and functional damage. COPD and COVID-19 show similar mechanisms of hypoxic brain damage, particularly in the prefrontal cortex, hippocampus, and subcortical structure. Hypoxia leads to volume reduction and metabolic decline in these areas, making them particularly vulnerable to cognitive decline and memory loss. Functional imaging shows that hypoxia causes decreased metabolic activity in brain regions associated with memory, mood, and executive function, particularly in COPD patients. In COVID-19 patients, hypoxia due to respiratory failure exacerbates damage in the frontal and temporal lobes, with cerebral metabolism gradually recovering but remaining below normal levels during recovery.
Neuroinflammation and cerebrovascular damage: Neuroinflammation can lead to microstructural changes in the brain. In lung cancer patients undergoing PCI and chemotherapy, cerebrovascular damage is often observed as widespread WM hyperintensities on imaging. MRI and PET studies have also revealed the extensive inflammatory response associated with COVID-19 may disrupt the blood-brain barrier, leading to substantial neural tissue injury. Additionally, growing studies highlight a strong link between COVID-19, chronic lung diseases, and cerebrovascular structural damage, manifesting as microbleeds, thrombosis, and microinfarcts. These pathologies are thought to be driven by systemic inflammation and dysregulated coagulation process associated with lung diseases.
Early identification of brain damage: Neuroimaging detects early brain changes in COPD and COVID-19 patients. These changes, often seen before cognitive decline manifests, facilitating timely intervention to minimize neurological damage (Xiao et al., 2022; Zhou L. et al., 2022; Zhang et al., 2013; Yin et al., 2019; Fitsiori et al., 2020; Klironomos et al., 2020; Kelsch et al., 2021; Mohammadi and Ghaderi, 2024; Verger et al., 2022a; Verger et al., 2022b; Ajčević et al., 2023). For the brain regional changes identified at early stage, targeted neuromodulation can be applied through techniques such as transcranial electrical stimulation (tES) or transcranial magnetic stimulation (TMS) as part of personalized intervention strategies.
Prediction of cognitive impairment: Structural brain changes, particularly in the prefrontal cortex and hippocampus, are strongly linked to cognitive decline in COPD patients. Brain imaging helps predict cognitive dysfunction, guide cognitive interventions, and monitor rehabilitation programs. Moreover, neuroimaging helps to assess the neurotoxic effects of PCI and chemotherapy, allowing for more tailored therapeutic approaches (Schou et al., 2012; Cleutjens et al., 2014; Yin et al., 2016; Samareh Fekri et al., 2017; Kakkera et al., 2018; Stuss et al., 1997; Simó et al., 2016a; Simó et al., 2016b).
Assessing the effects of hypoxia on brain function: Chronic hypoxia in COPD contributes to brain atrophy and cognitive decline. Neuroimaging studies reveal that hypoxia leads to metabolic and perfusion changes in the brain, further exacerbating these impairments. Thus, monitoring and intervening in hypoxic conditions is essential for preserving brain function and delaying cognitive deterioration (Antonelli Incalzi et al., 2003; Ortapamuk and Naldoken, 2006; Hamilton et al., 2003).
Optimizing treatment strategies: Neuroimaging evaluates the treatment impacts on brain structure and function. Long-term oxygen therapy has shown potential in mitigating neuropsychological decline, and brain imaging can track its effectiveness. Neuroimaging identifies early signs of brain injury in COVID-19, which can inform the development of targeted interventions, including anti-inflammatory therapies to reduce neurodegeneration (Hjalmarsen et al., 1999; Hoiland et al., 2018; Mohammadi and Ghaderi, 2024; Sen et al., 2023; Douaud et al., 2022). Combined with imaging histology and deep learning techniques, neuroimaging can predict molecular features and treatment outcomes, provide scientific basis to understand the mechanisms of neurotoxicity and adaptations in brain function associated with lung cancer treatment. This can be used to develop pharmacological or non-pharmacological neuroprotective strategies (Li et al., 2024c; Wang et al., 2024; Li et al., 2024b; Ho et al., 2019).
Support for mental health interventions: Anxiety and depression are prevalent in COPD patients and can exacerbate cognitive deficits. Neuroimaging identifies brain regions affected by these conditions, aiding interventions to improve both mental health and cognitive function (Pelgrim et al., 2019; Heck et al., 2022; Golan et al., 2009; Wei et al., 2024; Zhou X. et al., 2022).
Small sample size: many studies involve limited cohorts, compromising the reliability and stability of statistical conclusions.
Non-standardized research criteria: The research criteria for different lung diseases are not standardized. Correlation without causation: Most studies can only perform correlation analysis, which makes it difficult to determine the causal relationship between lung disease and brain changes.
Lack of longitudinal studies: Lack of follow-up studies that follow patients over time to assess chronic and progressive changes in the effects of lung disease on the brain.
Absence of reliable biomarkers: And reliable biomarkers are still needed to predict and monitor the effects of lung disease on the brain.
Large-scale, multicenter studies: recruiting more patients with multicenter studies improves the generalizability and reliability of results.
Artificial intelligence and machine learning: Leveraging machine learning to identify imaging biomarkers and explore the relationship between lung disease progression and cognitive function. For example, convolutional neural networks (CNNs) or Transformer models can be used for disease classification. Model interpretability studies can help identify biomarkers that have a positive impact on the model in disease classification. Deep unsupervised clustering models can be applied to analyze different subtypes of the same disease, providing insights into diverse disease manifestation patterns. Graph neural networks (GNNs) can be employed to analyze the causal relationships between lung diseases and brain structural changes. Causality verification: Employ longitudinal tracking and causal inference models to elucidate the bidirectional relationship between lung disease and brain changes, including whether neural abnormalities may predispose individuals to lung conditions.
Neuromodulation and autonomic nervous system: Investigating the role of brain centers in respiratory regulation and prefrontal cortex influence on the autonomic nervous system could clarify mechanisms underlying the lung-brain axis. Lung rehabilitation as a multidisciplinary strategy can improve physical function and quality of life by improving respiratory and limb motor control through neuroplasticity. Brain activity associated with breathlessness predicts improvements in symptoms. Future studies could increase imaging studies of neuromodulation and analyze whether functional changes in region of neuromodulation influence susceptibility to and progression of lung disease.
Systemic impacts of respiratory disease: understanding how autonomic nervous system dysregulation triggered by lung disease cascades into systemic effects, may reveal indirect pathways contributing to chronic disease progression.
Neuroimaging provides critical insights into the complex relationship between lung disease and brain. It enables early detection of brain structural and functional alterations, which can guide the management of cognitive impairments in COPD, lung cancer, and COVID-19 patients. Additionally, neuroimaging can identify specific brain regions affected by disease or treatment, helping to optimize therapeutic interventions. COPD primarily affects the frontal lobes, with gray matter atrophy and reduced perfusion. COVID-19 exhibits widespread microhemorrhages and neuroinflammation, particularly in regions functionally connected to the olfactory cortex. Lung cancer-related changes are often linked to the neurotoxicity of radiotherapy and chemotherapy. The affected regions are concentrated in subcortical structures, while cancer pain is associated with hyperconnectivity in motor and visual networks. The findings underscore the importance of integrating advanced neuroimaging techniques into clinical practice, offering personalized approaches for monitoring cerebral health and improving the management of lung diseases. Further research is needed to explore the mechanisms behind these brain changes and develop neuroprotective strategies to mitigate treatment-related brain damage.
MH: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review and editing. YL: Data curation, Writing – review and editing. ZG: Conceptualization, Writing – review and editing, Data curation. CL: Methodology, Writing – review and editing, Conceptualization, Supervision. ZZ: Writing – review and editing.
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abelson, J. L., Khan, S., and Giardino, N. (2010). HPA axis, respiration and the airways in stress–A review in search of intersections. Biol. Psychol. 84, 57–65. doi: 10.1016/j.biopsycho.2010.01.021
Ajčević, M., Iscra, K., Furlanis, G., Michelutti, M., Miladinović, A., Buoite Stella, A., et al. (2023). Cerebral hypoperfusion in post-COVID-19 cognitively impaired subjects revealed by arterial spin labeling MRI. Sci. Rep. 13:5808. doi: 10.1038/s41598-023-32275-3
Alexandre, F., Héraud, N., Tremey, E., Oliver, N., Bourgouin, D., and Varray, A. (2020). Specific motor cortex hypoexcitability and hypoactivation in COPD patients with peripheral muscle weakness. BMC Pulm Med. 20:1. doi: 10.1186/s12890-019-1042-0
Anjari, M., Counsell, S. J., Srinivasan, L., Allsop, J. M., Hajnal, J. V., Rutherford, M. A., et al. (2009). The association of lung disease with cerebral white matter abnormalities in preterm infants. Pediatrics 124, 268–276.
Antonelli Incalzi, R., Marra, C., Giordano, A., Calcagni, M. L., Cappa, A., Basso, S., et al. (2003). Cognitive impairment in chronic obstructive pulmonary disease–A neuropsychological and spect study. J. Neurol. 250, 325–332. doi: 10.1007/s00415-003-1005-4
Areza-Fegyveres, R., Kairalla, R. A., Carvalho, C. R. R., and Nitrini, R. (2010). Cognition and chronic hypoxia in pulmonary diseases. Dement. Neuropsychol. 4, 14–22.
Ball, G., Counsell, S. J., Anjari, M., Merchant, N., Arichi, T., Doria, V., et al. (2010). An optimised tract-based spatial statistics protocol for neonates: Applications to prematurity and chronic lung disease. Neuroimage 53, 94–102. doi: 10.1016/j.neuroimage.2010.05.055
Béresová, M., Larroza, A., Arana, E., Varga, J., Balkay, L., and Moratal, D. (2018). 2D and 3D texture analysis to differentiate brain metastases on MR images: Proceed with caution. Magma 31, 285–294. doi: 10.1007/s10334-017-0653-9
Cabibel, V., Héraud, N., Perrey, S., Oliver, N., Alexandre, F., and Varray, A. (2020). Is bilateral corticospinal connectivity impaired in patients with chronic obstructive pulmonary disease? J. Physiol. 598, 4591–4602. doi: 10.1113/JP279560
Chammah, S. E., Allenbach, G., Jumeau, R., Boughdad, S., Prior, J. O., Nicod Lalonde, M., et al. (2021). Impact of prophylactic cranial irradiation and hippocampal sparing on (18)F-FDG brain metabolism in small cell lung cancer patients. Radiother. Oncol. 158, 200–206. doi: 10.1016/j.radonc.2021.02.016
Chen, J., Lin, I. T., Zhang, H., Lin, J., Zheng, S., Fan, M., et al. (2016). Reduced cortical thickness, surface area in patients with chronic obstructive pulmonary disease: A surface-based morphometry and neuropsychological study. Brain Imaging Behav. 10, 464–476. doi: 10.1007/s11682-015-9403-7
Cleutjens, F. A., Janssen, D. J., Ponds, R. W., Dijkstra, J. B., and Wouters, E. F. (2014). COgnitive-pulmonary disease. Biomed. Res. Int. 2014:697825.
de Kort, N. O., Bischoff, E. W., Ricking, M., and Schermer, T. R. (2024). Exploring the impact of comorbid dementia on exacerbation occurrence in general practice patients with chronic obstructive pulmonary disease. Chron. Respir. Dis. 21:14799731241280283.
De Virgiliis, F., and Di Giovanni, S. (2020). Lung innervation in the eye of a cytokine storm: Neuroimmune interactions and COVID-19. Nat. Rev. Neurol. 16, 645–652. doi: 10.1038/s41582-020-0402-y
Díez-Cirarda, M., Yus, M., Gómez-Ruiz, N., Polidura, C., Gil-Martínez, L., Delgado-Alonso, C., et al. (2023). Multimodal neuroimaging in post-COVID syndrome and correlation with cognition. Brain 146, 2142–2152.
Ding, P., and Xu, R. (2023). Causal association of COVID-19 with brain structure changes: Findings from a non-overlapping 2-sample Mendelian randomization study. J. Neurol. Sci. 454:120864.
Dodd, J. W., Chung, A. W., Van den Broek, M. D., Barrick, T. R., Charlton, R. A., and Jones, P. W. (2012). Brain structure and function in chronic obstructive pulmonary disease: A multimodal cranial magnetic resonance imaging study. Am. J. Respir. Crit. Care Med. 186, 240–245. doi: 10.1164/rccm.201202-0355OC
Douaud, G., Lee, S., Alfaro-Almagro, F., Arthofer, C., Wang, C., and Mccarthy, P. (2022). SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 604, 697–707.
Esser, R. W., Stoeckel, M. C., Kirsten, A., Watz, H., Taube, K., Lehmann, K., et al. (2016). Structural brain changes in patients with COPD. Chest 149, 426–434.
Fang, C., Li, A., and Li, Y. (2024). COPD, PRISm and lung function reduction affect the brain cortical structure: A Mendelian randomization study. BMC Pulm Med. 24:341. doi: 10.1186/s12890-024-03150-2
Finnegan, S. L., Harrison, O. K., Harmer, C. J., Herigstad, M., Rahman, N. M., Reinecke, A., et al. (2021). Breathlessness in COPD: Linking symptom clusters with brain activity. Eur. Respir. J. 58:2004099. doi: 10.1183/13993003.04099-2020
Fitsiori, A., Pugin, D., Thieffry, C., Lalive, P., and Vargas, M. I. (2020). COVID-19 is associated with an unusual pattern of brain microbleeds in critically Ill patients. J. Neuroimaging 30, 593–597. doi: 10.1111/jon.12755
Fontana, I. C., Bongarzone, S., Gee, A., Souza, D. O., and Zimmer, E. R. (2020). PET imaging as a tool for assessing COVID-19 brain changes. Trends Neurosci. 43, 935–938. doi: 10.1016/j.tins.2020.10.010
Fukatsu-Chikumoto, A., Hirano, T., Takahashi, S., Ishida, T., Yasuda, K., and Donishi, T. (2024). Correlation between frailty and reduction in cortical thickness in patients with chronic obstructive pulmonary disease. Sci. Rep. 14:6106. doi: 10.1038/s41598-024-53933-0
Gandhi, L., and Johnson, B. E. (2006). Paraneoplastic syndromes associated with small cell lung cancer. J. Natl. Compr. Canc. Netw. 4, 631–638.
Golan, H., Kennedy, J. A., Frenkel, A., Parmet, Y., Feintuch, A., Levi, O., et al. (2009). Brain mapping of patients with lung cancer and controls: Inquiry into tumor-to-brain communication. J. Nucl. Med. 50, 1072–1075. doi: 10.2967/jnumed.108.061085
Guo, Z., Sun, S., Xiao, S., Chen, G., Chen, P., Yang, Z., et al. (2024). COVID-19 is associated with changes in brain function and structure: A multimodal meta-analysis of neuroimaging studies. Neurosci. Biobehav. Rev. 164:105792. doi: 10.1016/j.neubiorev.2024.105792
Hamilton, G., Mathur, R., Allsop, J. M., Forton, D. M., Dhanjal, N. S., Shaw, R. J., et al. (2003). Changes in brain intracellular pH and membrane phospholipids on oxygen therapy in hypoxic patients with chronic obstructive pulmonary disease. Metab. Brain Dis. 18, 95–109. doi: 10.1023/a:1021938926807
Han, M., He, C., Li, T., Li, Q., Chu, T., Li, J., et al. (2024). Altered dynamic and static brain activity and functional connectivity in COVID-19 patients: A preliminary study. Neuroreport 35, 306–315. doi: 10.1097/WNR.0000000000002009
Heck, D. H., Correia, B. L., Fox, M. B., Liu, Y., Allen, M., and Varga, S. (2022). Recent insights into respiratory modulation of brain activity offer new perspectives on cognition and emotion. Biol. Psychol. 170:108316. doi: 10.1016/j.biopsycho.2022.108316
Hjalmarsen, A., Waterloo, K., Dahl, A., Jorde, R., and Viitanen, M. (1999). Effect of long-term oxygen therapy on cognitive and neurological dysfunction in chronic obstructive pulmonary disease. Eur. Neurol. 42, 27–35.
Hlavati, M., Buljan, K., Tomić, S., Horvat, M., and Butković-Soldo, S. (2019). Impaired cerebrovascular reactivity in chronic obstructive pulmonary disease. Acta Neurol. Belg. 119, 567–575.
Ho, K. C., Toh, C. H., Li, S. H., Liu, C. Y., Yang, C. T., Lu, Y. J., et al. (2019). Prognostic impact of combining whole-body PET/CT and brain PET/MR in patients with lung adenocarcinoma and brain metastases. Eur. J. Nucl. Med. Mol. Imaging 46, 467–477. doi: 10.1007/s00259-018-4210-1
Hoiland, R. L., Mladinov, S., Barak, O. F., Willie, C. K., Mijacika, T., Stembridge, M., et al. (2018). Oxygen therapy improves cerebral oxygen delivery and neurovascular function in hypoxaemic chronic obstructive pulmonary disease patients. Exp. Physiol. 103, 1170–1177. doi: 10.1113/EP086994
Hopkins, R. O., Gale, S. D., and Weaver, L. K. (2006). Brain atrophy and cognitive impairment in survivors of acute respiratory distress syndrome. Brain Inj. 20, 263–271.
Hosang, L., Canals, R. C., Van der Flier, F. J., Hollensteiner, J., Daniel, R., Flügel, A., et al. (2022). The lung microbiome regulates brain autoimmunity. Nature 603, 138–144.
Hu, L., Ding, S., Zhang, Y., You, J., Shang, S., Wang, P., et al. (2022). Dynamic functional network connectivity reveals the brain functional alterations in lung cancer patients after chemotherapy. Brain Imaging Behav. 16, 1040–1048. doi: 10.1007/s11682-021-00575-9
Hu, X., Wang, H., Tu, Y., Fei, M., Yin, M., Fei, G., et al. (2018). Alterations of the default mode network and cognitive impairments in patients with chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon Dis. 13, 519–528. doi: 10.2147/COPD.S146870
Kakkera, K., Padala, K. P., Kodali, M., and Padala, P. R. (2018). Association of chronic obstructive pulmonary disease with mild cognitive impairment and dementia. Curr. Opin. Pulm. Med. 24, 173–178.
Karakas, E., Yildizhan, M., Karakas, O., Boyaci, F. N., Cullu, N., Cece, H., et al. (2013). Examining cerebral metabolic abnormalities in chronic obstructive pulmonary disease (COPD) patients by localized proton magnetic resonance spectroscopy (MRS). Clin. Ter. 164, e179–e182. doi: 10.7417/CT.2013.1565
Kelsch, R. D., Silbergleit, R., and Krishnan, A. (2021). Neuroimaging in the first 6 weeks of the COVID-19 pandemic in an 8-Hospital campus: Observations and patterns in the brain, head and neck, and Spine. J. Comput. Assist. Tomogr. 45, 592–599. doi: 10.1097/RCT.0000000000001179
Kiyak, C., Ijezie, O. A., Ackah, J. A., Armstrong, M., Cowen, J., Cetinkaya, D., et al. (2024). Topographical Distribution of neuroanatomical abnormalities following COVID-19 invasion : A systematic literature review. Clin. Neuroradiol. 34, 13–31. doi: 10.1007/s00062-023-01344-5
Klironomos, S., Tzortzakakis, A., Kits, A., Öhberg, C., Kollia, E., Ahoromazdae, A., et al. (2020). Nervous system involvement in coronavirus disease 2019: Results from a retrospective consecutive neuroimaging cohort. Radiology 297, E324–E334. doi: 10.1148/radiol.2020202791
Kozora, E., Filley, C. M., Julian, L. J., and Cullum, C. M. (1999). Cognitive functioning in patients with chronic obstructive pulmonary disease and mild hypoxemia compared with patients with mild Alzheimer disease and normal controls. Neuropsychiatry Neuropsychol. Behav. Neurol. 12, 178–183.
Lee, J. M., Choi, Y. H., Hong, J., Kim, N. Y., Kim, E. B., Lim, J. S., et al. (2019). Bronchopulmonary dysplasia is associated with altered brain volumes and white matter microstructure in preterm infants. Neonatology 116, 163–170.
Lee, S., Pyun, S. B., and Tae, W. S. (2019). Reduced axial diffusivity and increased mode and T2 signals in cerebral white matter of chronic obstructive pulmonary disease using tract-based spatial statistics. Neuroradiology 61, 795–801. doi: 10.1007/s00234-019-02178-0
Lersy, F., Bund, C., Anheim, M., Mondino, M., Noblet, V., Lazzara, S., et al. (2022). Evolution of neuroimaging findings in severe COVID-19 patients with initial neurological impairment: An observational study. Viruses 14:949. doi: 10.3390/v14050949
Li, C., Chen, W., Lin, F., Li, W., Wang, P., Liao, G., et al. (2023). Functional two-way crosstalk between brain and lung: The brain-lung axis. Cell Mol. Neurobiol. 43, 991–1003. doi: 10.1007/s10571-022-01238-z
Li, G., Ji, Z., and Sun, Q. (2024a). Deep multi-instance conv-transformer frameworks for landmark-based brain MRI classification. Electronics 13:980.
Li, H., Xin, H., Yu, J., Yu, H., Zhang, J., Wang, W., et al. (2020). Abnormal intrinsic functional hubs and connectivity in stable patients with COPD: A resting-state MRI study. Brain Imaging Behav. 14, 573–585. doi: 10.1007/s11682-019-00130-7
Li, Y., Jin, Y., Wang, Y., Liu, W., Jia, W., and Wang, J. (2024b). MR-based radiomics predictive modelling of EGFR mutation and HER2 overexpression in metastatic brain adenocarcinoma: A two-centre study. Cancer Imaging 24:65. doi: 10.1186/s40644-024-00709-4
Li, Y., Yu, R., Chang, H., Yan, W., Wang, D., Li, F., et al. (2024c). Identifying pathological subtypes of brain metastasis from lung cancer using MRI-based deep learning approach: A multicenter study. J. Imaging Inform. Med. 37, 976–987. doi: 10.1007/s10278-024-00988-0
Liang, J., Yu, Q., Chen, L., Li, Z., Liu, Y., Qiu, Y., et al. (2024). Gray matter and cognitive alteration related to chronic obstructive pulmonary disease patients: Combining ALE meta-analysis and MACM analysis. Brain Imaging Behav. 1–14. doi: 10.1007/s11682-024-00946-y
Liu, D., Zhou, X., Tan, Y., Yu, H., Cao, Y., Tian, L., et al. (2022a). Altered brain functional activity and connectivity in bone metastasis pain of lung cancer patients: A preliminary resting-state fMRI study. Front. Neurol. 13:936012. doi: 10.3389/fneur.2022.936012
Liu, S., Yin, N., Li, C., Li, X., Ni, J., Pan, X., et al. (2022b). Topological abnormalities of pallido-thalamo-cortical circuit in functional brain network of patients with nonchemotherapy with non-small cell lung cancer. Front. Neurol. 13:821470. doi: 10.3389/fneur.2022.821470
Liu, S., Yin, N., Ma, R., Cao, H., Jing, C., Zhang, Y., et al. (2022c). Abnormal topological characteristics of brain white matter network relate to cognitive and emotional deficits of non-small cell lung cancer (NSCLC) patients prior to chemotherapy. Int. J. Neurosci. 132, 328–337. doi: 10.1080/00207454.2020.1813130
Long, J., Li, J., Xie, B., Jiao, Z., Shen, G., Liao, W., et al. (2023). Morphometric similarity network alterations in COVID-19 survivors correlate with behavioral features and transcriptional signatures. Neuroimage Clin. 39:103498. doi: 10.1016/j.nicl.2023.103498
Lu, C. Q., Xu, W., Zeng, C. H., Ge, L. Y., Wang, Y. C., Meng, X. P., et al. (2019). Altered amplitude of low-frequency fluctuation in basal ganglia correlates to pulmonary ventilation function in COPD patients: A resting-state fMRI study. Brain Behav. 9:e01336. doi: 10.1002/brb3.1336
Lu, J., Huang, R., Peng, Y., Zhang, J., Liang, K., Wang, Y., et al. (2023). Mendelian randomization analyses accounting for causal effect of COVID-19 on brain imaging-derived phenotypes. J. Alzheimers Dis. 96, 1059–1070. doi: 10.3233/JAD-230626
Lv, Z., Hu, P., Jiang, Y., Yang, W., Wang, R., Wang, K., et al. (2020). Changes in spatial working memory in stable chronic obstructive pulmonary disease: A retrospective study. Biomed. Res. Int. 2020:7363712. doi: 10.1155/2020/7363712
Mentzelopoulos, A., Gkiatis, K., Karanasiou, I., Karavasilis, E., Papathanasiou, M., Efstathopoulos, E., et al. (2021). Chemotherapy-induced brain effects in small-cell lung cancer patients: A multimodal MRI study. Brain Topogr. 34, 167–181. doi: 10.1007/s10548-020-00811-3
Mentzelopoulos, A., Karanasiou, I., Papathanasiou, M., Kelekis, N., Kouloulias, V., and Matsopoulos, G. K. (2022). A comparative analysis of white matter structural networks on SCLC patients after chemotherapy. Brain Topogr. 35, 352–362.
Mohamed-Hussein, A. A., Hamed, S. A., and Abdel-Hakim, N. (2007). Cerebral cortical dysfunction in chronic obstructive pulmonary disease: Role of transcranial magnetic stimulation. Int. J. Tuberc. Lung Dis. 11, 515–521.
Mohammadi, S., and Ghaderi, S. (2024). Post-COVID-19 conditions: A systematic review on advanced magnetic resonance neuroimaging findings. Neurol. Sci. 45, 1815–1833.
Mohammadi-Nejad, A. R., Allen, R. J., Kraven, L. M., Leavy, O. C., Jenkins, R. G., Wain, L. V., et al. (2022). Mapping brain endophenotypes associated with idiopathic pulmonary fibrosis genetic risk. EBioMedicine 86:104356. doi: 10.1016/j.ebiom.2022.104356
Nelson, B. K., Farah, L. N., Grier, A., Su, W., Chen, J., Sossi, V., et al. (2024). Differences in brain structure and cognitive performance between patients with long-COVID and those with normal recovery. Neuroimage 300: 120859.
Ni, J., Zhou, Y., Wang, S., Guo, T., Hu, J., Chu, Q., et al. (2022). A brief report on incidence, radiographic feature and prognostic significance of brain MRI changes after anti-PD-1/PD-L1 therapy in advanced non-small cell lung cancer. Cancer Immunol. Immunother. 71, 1275–1280. doi: 10.1007/s00262-021-03070-8
Ortapamuk, H., and Naldoken, S. (2006). Brain perfusion abnormalities in chronic obstructive pulmonary disease: Comparison with cognitive impairment. Ann. Nucl. Med. 20, 99–106.
Parsons, N., Outsikas, A., Parish, A., Clohesy, R., D’aprano, F., Toomey, F., et al. (2021). Modelling the anatomic distribution of neurologic events in patients with COVID-19: A systematic review of MRI findings. AJNR Am. J. Neuroradiol. 42, 1190–1195.
Pelgrim, C. E., Peterson, J. D., Gosker, H. R., Schols, A., van Helvoort, A., Garssen, J., et al. (2019). Psychological co-morbidities in COPD: Targeting systemic inflammation, a benefit for both? Eur. J. Pharmacol. 842, 99–110. doi: 10.1016/j.ejphar.2018.10.001
Qin, W., Yin, J., Yang, L., Yang, S., Li, Y., Li, X., et al. (2020). The relationship between chronic obstructive pulmonary disease and cerebral small vessel disease assessed by magnetic resonance imaging: A case-control study from a single center in Beijing. China. Med. Sci. Monit. 26:e925703. doi: 10.12659/MSM.925703
Ran, L., Liu, J., Lan, X., Zhou, X., Tan, Y., Zhang, J., et al. (2024). White matter microstructure damage measured by automated fiber quantification correlates with pain symptoms in lung cancer patients. Brain Imaging Behav. 18, 1524–1535. doi: 10.1007/s11682-024-00942-2
Rebsamen, M., Friedli, C., Radojewski, P., Diem, L., Chan, A., Wiest, R., et al. (2023). Multiple sclerosis as a model to investigate SARS-CoV-2 effect on brain atrophy. CNS Neurosci. Ther. 29, 538–543. doi: 10.1111/cns.14050
Roy, B., Vacas, S., Ehlert, L., Mccloy, K., Saggar, R., and Kumar, R. (2021). Brain structural changes in patients with pulmonary arterial hypertension. J. Neuroimaging 31, 524–531.
Rozenberg, D., Reid, W. D., Camp, P., Campos, J. L., Dechman, G., Davenport, P. W., et al. (2024). Translating the interplay of cognition and physical performance in COPD and interstitial lung disease: Meeting report and literature review. Chest 166, 721–732. doi: 10.1016/j.chest.2024.05.027
Samareh Fekri, M., Hashemi-Bajgani, S. M., Naghibzadeh-Tahami, A., and Arabnejad, F. (2017). Cognitive impairment among patients with chronic obstructive pulmonary disease compared to normal individuals. Tanaffos 16, 34–39.
Sawyer, D. M., Sawyer, T. W., Eshghi, N., Hsu, C., Hamilton, R. J., Garland, L. L., et al. (2021). Pilot study: Texture analysis of PET imaging demonstrates changes in (18)F-FDG uptake of the brain after prophylactic cranial irradiation. J. Nucl. Med. Technol. 49, 34–38. doi: 10.2967/jnmt.120.248393
Schou, L., Østergaard, B., Rasmussen, L. S., Rydahl-Hansen, S., and Phanareth, K. (2012). Cognitive dysfunction in patients with chronic obstructive pulmonary disease–A systematic review. Respir. Med. 106, 1071–1081.
Sen, S., Newman-Norlund, R., Riccardi, N., Rorden, C., Newman-Norlund, S., Sayers, S., et al. (2023). Cerebral blood flow in patients recovered from mild COVID-19. J. Neuroimaging 33, 764–772.
Simó, M., Vaquero, L., Ripollés, P., Gurtubay-Antolin, A., Jové, J., and Navarro, A. (2016a). Longitudinal brain changes associated with prophylactic cranial irradiation in lung cancer. J. Thorac. Oncol. 11, 475–486. doi: 10.1016/j.jtho.2015.12.110
Simó, M., Vaquero, L., Ripollés, P., Jové, J., Fuentes, R., Cardenal, F., et al. (2016b). Brain damage following prophylactic cranial irradiation in lung cancer survivors. Brain Imaging Behav. 10, 283–295.
Son, S. H., Ahn, J. H., Shin, K. C., Kim, H. W., and Kong, E. (2023). Brain FDG PET for visualizing the relation between impaired lung function and cognitive decline in lung cancer: A preliminary study. Nucl. Med. Commun. 44, 488–494. doi: 10.1097/MNM.0000000000001686
Spilling, C. A., Dhillon, M. K., Burrage, D. R., Ruickbie, S., Baker, E. H., Barrick, T. R., et al. (2021). Factors affecting brain structure in smoking-related diseases: Chronic obstructive pulmonary disease (COPD) and coronary artery disease. PLoS One 16:e0259375. doi: 10.1371/journal.pone.0259375
Spilling, C. A., Jones, P. W., Dodd, J. W., and Barrick, T. R. (2017). White matter lesions characterise brain involvement in moderate to severe chronic obstructive pulmonary disease, but cerebral atrophy does not. BMC Pulm. Med. 17:92. doi: 10.1186/s12890-017-0435-1
Spilling, C. A., Jones, P. W., Dodd, J. W., and Barrick, T. R. (2019). Disruption of white matter connectivity in chronic obstructive pulmonary disease. PLoS One 14:e0223297. doi: 10.1371/journal.pone.0223297
Stuss, D. T., Peterkin, I., Guzman, D. A., Guzman, C., and Troyer, A. K. (1997). Chronic obstructive pulmonary disease: Effects of hypoxia on neurological and neuropsychological measures. J. Clin. Exp. Neuropsychol. 19, 515–524.
Takamatsu, K., Park, K., and Yokoyama, A. (2021). Association between airflow limitation and leukoaraiosis of the brain. Respir. Investig. 59, 320–326. doi: 10.1016/j.resinv.2020.11.004
Tang, F., Li, L., Peng, D., Yu, J., Xin, H., Tang, X., et al. (2022). Abnormal static and dynamic functional network connectivity in stable chronic obstructive pulmonary disease. Front. Aging Neurosci. 14:1009232. doi: 10.3389/fnagi.2022.1009232
Tondo, G., de Marchi, F., Terazzi, E., Prandi, P., Sacchetti, M., Comi, C., et al. (2018). Chronic obstructive pulmonary disease may complicate Alzheimer’s disease: A comorbidity problem. Neurol. Sci. 39, 1585–1589.
van der Knaap, N., Ariës, M. J. H., van der Horst, I. C. C., and Jansen, J. F. A. (2024). On the merits and potential of advanced neuroimaging techniques in COVID-19: A scoping review. Neuroimage Clin. 42:103589. doi: 10.1016/j.nicl.2024.103589
Vandiver, M. S., Roy, B., Mahmud, F., Lavretsky, H., and Kumar, R. (2022). Functional comorbidities and brain tissue changes before and after lung transplant in adults. Front. Cell Neurosci. 16:1015568. doi: 10.3389/fncel.2022.1015568
Verger, A., Barthel, H., Tolboom, N., Fraioli, F., Cecchin, D., Albert, N. L., et al. (2022a). 2-[(18)F]-FDG PET for imaging brain involvement in patients with long COVID: Perspective of the EANM neuroimaging committee. Eur. J. Nucl. Med. Mol. Imaging 49, 3599–3606. doi: 10.1007/s00259-022-05913-7
Verger, A., Kas, A., Dudouet, P., Goehringer, F., Salmon-Ceron, D., and Guedj, E. (2022b). Visual interpretation of brain hypometabolism related to neurological long COVID: A French multicentric experience. Eur. J. Nucl. Med. Mol. Imaging 49, 3197–3202. doi: 10.1007/s00259-022-05753-5
Voruz, P., Cionca, A., Jacot, de Alcântara, I., Nuber-Champier, A., Allali, G., et al. (2023). Brain functional connectivity alterations associated with neuropsychological performance 6-9 months following SARS-CoV-2 infection. Hum. Brain Mapp. 44, 1629–1646. doi: 10.1002/hbm.26163
Wang, T. W., Chao, H. S., Chiu, H. Y., Lu, C. F., Liao, C. Y., Lee, Y., et al. (2024). Radiomics of metastatic brain tumor as a predictive image biomarker of progression-free survival in patients with non-small-cell lung cancer with brain metastasis receiving tyrosine kinase inhibitors. Transl. Oncol. 39:101826. doi: 10.1016/j.tranon.2023.101826
Wang, T., Huang, X., Dai, L. X., Zhan, K. M., and Wang, J. (2023). Investigation of altered spontaneous brain activity in patients with bronchial asthma using the percent amplitude of fluctuation method: A resting-state functional MRI study. Front. Hum. Neurosci. 17:1228541. doi: 10.3389/fnhum.2023.1228541
Wang, W., Wang, P., Li, Q., Peng, Z., Wang, X., Wang, G., et al. (2020). Alterations of grey matter volumes and network-level functions in patients with stable chronic obstructive pulmonary disease. Neurosci. Lett. 720:134748. doi: 10.1016/j.neulet.2020.134748
Wei, X., Lai, Y., Lan, X., Tan, Y., Zhang, J., Liu, J., et al. (2024). Uncovering brain functional connectivity disruption patterns of lung cancer-related pain. Brain Imaging Behav. 18, 576–587. doi: 10.1007/s11682-023-00836-9
Wu, M. S., Chen, Z. W., Chen, X., Wang, G. X., Xu, C. S., Zhu, Y. F., et al. (2023). Altered functional connectivity of the nucleus tractus solitarii in patients with chronic cough after lung surgery: An rs-fMRI study. Thorac. Cancer 14, 3202–3207. doi: 10.1111/1759-7714.15110
Xiao, T., Wijnant, S. R. A., van der Velpen, I., Terzikhan, N., Lahousse, L., Ikram, M. K., et al. (2022). Lung function impairment in relation to cognition and vascular brain lesions: The Rotterdam Study. J. Neurol. 269, 4141–4153. doi: 10.1007/s00415-022-11027-9
Xin, H., Li, H., Yu, H., Yu, J., Zhang, J., Wang, W., et al. (2019). Disrupted resting-state spontaneous neural activity in stable COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 14, 499–508. doi: 10.2147/COPD.S190671
Yichu, S., Fei, L., Ying, L., and Youyou, X. (2024). Potential of radiomics analysis and machine learning for predicting brain metastasis in newly diagnosed lung cancer patients. Clin. Radiol. 79, e807–e816. doi: 10.1016/j.crad.2024.01.038
Yin, M., Wang, H., Hu, X., Li, X., Fei, G., and Yu, Y. (2019). Patterns of brain structural alteration in COPD with different levels of pulmonary function impairment and its association with cognitive deficits. BMC Pulm. Med. 19:203. doi: 10.1186/s12890-019-0955-y
Yin, P., Ma, Q., Wang, L., Lin, P., Zhang, M., Qi, S., et al. (2016). Chronic obstructive pulmonary disease and cognitive impairment in the Chinese elderly population: A large national survey. Int. J. Chron. Obstruct. Pulmon. Dis. 11, 399–406. doi: 10.2147/COPD.S96237
You, J., Hu, L., Zhang, Y., Chen, F., Yin, X., Jin, M., et al. (2020). Altered dynamic neural activity in the default mode network in lung cancer patients after chemotherapy. Med. Sci. Monit. 26:e921700. doi: 10.12659/MSM.921700
Yu, J., Wang, W., Peng, D., Luo, J., Xin, H., Yu, H., et al. (2021). Intrinsic low-frequency oscillation changes in multiple-frequency bands in stable patients with chronic obstructive pulmonary disease. Brain Imaging Behav. 15, 1922–1933. doi: 10.1007/s11682-020-00385-5
Yu, L., de Mazancourt, M., Hess, A., Ashadi, F. R., Klein, I., Mal, H., et al. (2016). Functional connectivity and information flow of the respiratory neural network in chronic obstructive pulmonary disease. Hum. Brain Mapp. 37, 2736–2754. doi: 10.1002/hbm.23205
Zhang, H., Wang, X., Lin, J., Sun, Y., Huang, Y., Yang, T., et al. (2012). Grey and white matter abnormalities in chronic obstructive pulmonary disease: A case-control study. BMJ Open 2:e000844.
Zhang, H., Wang, X., Lin, J., Sun, Y., Huang, Y., Yang, T., et al. (2013). Reduced regional gray matter volume in patients with chronic obstructive pulmonary disease: A voxel-based morphometry study. AJNR Am. J. Neuroradiol. 34, 334–339.
Zhang, J., Chen, J., Yu, Q., Fan, C., Zhang, R., Lin, J., et al. (2016). Alteration of spontaneous brain activity in COPD patients. Int. J. Chron. Obstruct. Pulmon. Dis. 11, 1713–1719.
Zhang, W., Ning, N., Li, X., Niu, G., Bai, L., Guo, Y., et al. (2016). Changes of brain glucose metabolism in the pretreatment patients with non-small cell lung cancer: A retrospective PET/CT study. PLoS One 11:e0161325. doi: 10.1371/journal.pone.0161325
Zhang, Y., Chen, Y. C., Hu, L., You, J., Gu, W., Li, Q., et al. (2020). Chemotherapy-induced functional changes of the default mode network in patients with lung cancer. Brain Imaging Behav. 14, 847–856.
Zhang, Y., Yang, Y., Bian, R., Yin, Y., Hou, Z., Yue, Y., et al. (2017). Abnormal functional connectivity of ventral anterior insula in asthmatic patients with depression. Neural Plast. 2017:7838035. doi: 10.1155/2017/7838035
Zhou, L., Yang, H., Zhang, Y., Li, H., Zhang, S., Li, D., et al. (2022). Association of impaired lung function with dementia, and brain magnetic resonance imaging indices: A large population-based longitudinal study. Age Ageing 51:afac269.
Zhou, X., Tan, Y., Chen, J., Wang, C., Tang, Y., Liu, J., et al. (2022). Altered functional connectivity in pain-related brain regions and its correlation with pain duration in bone metastasis with cancer pain. Dis. Markers 2022:3044186. doi: 10.1155/2022/3044186
Keywords: lung-brain axis, neuroimaging, lung disease, MRI-based brain changes, neurological impact of lung disease
Citation: He M, Liu Y, Guan Z, Li C and Zhang Z (2025) Neuroimaging insights into lung disease-related brain changes: from structure to function. Front. Aging Neurosci. 17:1550319. doi: 10.3389/fnagi.2025.1550319
Received: 23 December 2024; Accepted: 07 February 2025;
Published: 20 February 2025.
Edited by:
Qiong Wu, Suzhou University of Science and Technology, ChinaReviewed by:
Bin Wang, Taiyuan University of Technology, ChinaCopyright © 2025 He, Liu, Guan, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunlin Li, bGljaHVubGluMTk4MUAxNjMuY29t; Zhixi Zhang, emhhbmd6aGl4aUBhaXJjYXMuYWMuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.