
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Aging Neurosci., 05 March 2025
Sec. Alzheimer's Disease and Related Dementias
Volume 17 - 2025 | https://doi.org/10.3389/fnagi.2025.1541287
This article is part of the Research TopicAdvancing therapeutics for Alzheimer's disease and related dementias through multi-omics data analysis in ethnically diverse populationsView all 4 articles
Alzheimer’s disease (AD) is a progressive and debilitating neurodegenerative disorder that significantly impairs cognitive function and daily living abilities, representing a major public health challenge. Given the multifactorial nature of AD, effective therapeutic interventions targeting both cognitive and functional decline are critical. This study aimed to conduct a comprehensive comparison of the therapeutic effects of music therapy, acupuncture therapy, game therapy, cognitive training therapy, and exercise therapy on AD patients through a network meta-analysis. Randomized controlled trials (RCTs) published up until 2024 were systematically retrieved from multiple databases. Data were extracted, including the first author, publication year, country, total sample size, mean participant age, type and duration of intervention, and outcome measures such as the Mini-Mental State Examination, Activities of Daily Living, and Alzheimer’s Disease Assessment Scale-Cognitive Subscale. Statistical analyses were performed using the RevMan 5.3 and Stata 17 software. The analysis included 52 RCTs with a total of 3,409 participants, offering a strong dataset. The results indicated that game therapy produced statistically significant improvements in mental state and daily living abilities, while acupuncture therapy yielded the most pronounced improvements in cognitive function among AD patients. Notably, the comparative efficacy of these interventions suggests that game therapy may offer short-term benefits, particularly for mental health and functional abilities, whereas acupuncture therapy demonstrated superior long-term cognitive enhancements. In conclusion, tailored physical and cognitive interventions such as game therapy and acupuncture therapy may hold significant potential in optimizing treatment outcomes for AD patients, with implications for both clinical practice and future research.
Alzheimer’s disease (AD), a progressive and irreversible neurodegenerative disorder, poses a serious threat to global public health and is the fourth leading cause of death among the elderly (Villain and Michalon, 2024; Chen et al., 2021; Hall et al., 2024). The worldwide prevalence of AD is rapidly escalating due to aging populations. According to the most recent statistics from the World Health Organization (WHO), the number of people living with AD was 50 million in 2018, (Joseph et al., 2021) and this number is expected to rise to 131.5 million by 2050 (Prince et al., 2015). This increase will place substantial pressure on healthcare resources, requiring urgent attention from clinicians and policymakers. Pathologically, AD is characterized by extracellular accumulation of amyloid beta-protein (Aβ) forming senile plaques, abnormal phosphorylation of tau protein, and neuronal loss. The “Aβ cascade hypothesis” posits that the abnormal accumulation of Aβ in the extracellular space is central to the pathogenesis of AD. The increased Aβ leads to chronic neuroinflammatory responses, synaptic dysfunction, oxidative stress, and neurofibrillary tangles, all contributing to neuronal structural and functional abnormalities (Sancheti et al., 2013; Chen and Zhong, 2013). In AD, the neurons affected by neurofibrillary tangles are primarily found in specific regions of the brain, including pyramidal neurons in the CA1 area of the hippocampus, as well as neurons in the medial prefrontal cortex and temporal cortex (Zhang and McGeer, 2004). These pathological processes result in deficits in cognitive domains such as memory, executive function, language, and visuospatial abilities, as well as impairments in physiological function. As the disease advances, patients not only experience cognitive decline but also face an increased risk of psychiatric conditions such as depression, anxiety, and behavioral disturbances, further complicating their care (Fang et al., 2006).
Currently, pharmacological interventions for AD are primarily aimed at slowing the progression of the disease, as no available treatments can halt or reverse its development (Zhang, 2019). The most commonly prescribed drugs, such as cholinesterase inhibitors and NMDA receptor antagonists, offer only limited symptomatic relief and are associated with a range of adverse effects, including gastrointestinal disturbances, dizziness, and cardiovascular complications. Furthermore, the long-term efficacy of these drugs remains uncertain, with some patients showing minimal response (Connors et al., 2018; Hessmann et al., 2018). Suboptimal medication adherence is another significant challenge, as the burden of managing chronic pharmacotherapy often leads to poor compliance, which exacerbates psychological stress on patients and caregivers and limits the effectiveness of treatment (Wang et al., 2022).
In recent years, the limitations of pharmacological interventions have prompted increasing interest in non-pharmacological therapies. As a non-invasive treatment modality, physical therapy avoids the risks associated with surgical procedures and the adverse effects of long-term medication use. Physical therapy interventions have demonstrated promise in improving both physical and cognitive outcomes in AD patients by promoting neuroplasticity, enhancing cerebral perfusion, and stimulating neurogenesis (Shufen and Hongxia, 2011). These therapies encompass a wide range of approaches, including music therapy, acupuncture, game therapy, cognitive training, and exercise therapy. For instance, exercise therapy, particularly aerobic exercise, has been shown to slow cognitive decline and improve cardiovascular health, (Xu Q. et al., 2019) while cognitive training helps maintain executive function and attention by stimulating neural networks (Luo and Xu, 2017). Similarly, music therapy has been associated with improvements in mood, emotional regulation, and social interaction, providing holistic benefits that extend beyond cognitive domains (Liu et al., 2019).
Moreover, the mechanisms underlying these physical therapies suggest that they may induce long-term neuroprotective effects. Exercise, for example, increases levels of brain-derived neurotrophic factor (BDNF), which supports the survival of existing neurons and the growth of new neurons and synapses in the hippocampus, a critical region for memory (Gu and Wang, 2019). Acupuncture has been proposed to modulate neurotransmitter systems and reduce the accumulation of amyloid-beta plaques, a hallmark of AD pathology (Yang et al., 2023). Given these potential benefits, physical therapy represents a promising adjunctive treatment option for managing AD.
Existing meta-analyses have predominantly compared the effects of individual or combined interventions, often using routine care or no intervention as the control group. However, these traditional meta-analyses have limitations in that they typically only allow for pairwise comparisons between two interventions. This can leave significant gaps in our understanding of the relative efficacy of multiple treatments, particularly in complex conditions like AD where multiple therapeutic approaches may target different aspects of the disease. Network meta-analysis (NMA), on the other hand, is a more sophisticated technique that allows for the comparison of several interventions simultaneously, integrating both direct and indirect evidence to provide a more comprehensive evaluation and ranking of treatment options (Owen et al., 2020). This method enables the identification of the most effective interventions based on a statistical approach that synthesizes data from various studies.
Consequently, this study undertakes a network meta-analysis encompassing five distinct types of physical interventions—music therapy, acupuncture, game therapy, cognitive training, and exercise therapy. By employing the Surface Under the Cumulative Ranking (SUCRA) approach, the interventions are ranked according to their efficacy in improving cognitive and functional outcomes in AD patients. The findings are expected to provide valuable evidence-based guidance for clinicians in selecting the most appropriate treatment strategies, while also offering insights for future research to optimize non-pharmacological therapies for AD.
A comprehensive and systematic literature search was conducted to identify relevant publications across multiple databases, including China National Knowledge Infrastructure (CNKI), Superstar, VIP, Wanfang, PubMed, Cochrane Library, and Web of Science. The search covered all available publications from the inception of these databases until 2024. The search strategy was carefully designed to ensure inclusivity, utilizing both Medical Subject Headings (MeSH) terms and free-text keywords, to capture a wide range of studies relevant to physical therapy interventions in Alzheimer’s disease (AD). The key search terms were: (Music therapy OR acupuncture therapy OR play therapy OR cognitive training therapy OR exercise therapy) AND (Alzheimer’s disease OR dementia) AND (cognitive OR mood OR depression OR anxiety OR quality of life OR lifestyle). Additionally, backward and forward citation searches were employed to identify further relevant studies. Articles that met the inclusion criteria were obtained via direct download or interlibrary loan services.
The study design was limited to randomized controlled trials (RCTs). (1) Study population included patients diagnosed with Alzheimer’s disease, senile dementia, or cognitive impairment, irrespective of gender or nationality, who were conscious and free from severe cognitive deficits. (2) The experimental interventions comprised music therapy, acupuncture therapy, play therapy, cognitive therapy, and exercise therapy, while the control group received standard treatment, routine care, or daily activities. Detailed definitions of the physical interventions and control groups are provided in Table 1.
Inclusion criteria were as follows: (1) The study design must be a randomized controlled trial. (2) Participants must meet the diagnostic criteria for Alzheimer’s disease as defined by the American Psychiatric Association for the Elderly (Connors et al., 2018). (3) The intervention must involve one or more of the following therapies: music therapy, acupuncture therapy, play therapy, cognitive therapy, or exercise therapy. The control group should receive standard treatment, standard nursing care, daily activities, or non-physical interventions. (4) Outcome indicators must include; ① Cognitive function: Mini-Mental State Examination (MMSE); ② Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-cog); ③ Activities of Daily Living Scale (ADL).
Exclusion criteria were as follows: (1) Non-randomized controlled trials. (2) Conference proceedings, master’s theses, doctoral dissertations, abstracts, and duplicate publications. (3) Studies with missing or unreported required data (e.g., means, standard deviations, etc.). (4) Inaccessibility of the full text. (5) Studies with low-quality physical interventions or lacking rigorous experimental design. (6) Studies involving subjects not diagnosed with Alzheimer’s disease or AD-type dementia. (7) Interventions that combine multiple physical therapies (e.g., exercise combined with cognitive training, music combined with exercise) versus routine nursing as a control. (8) Animal experiments. To ensure robust and high-quality data, studies were screened for methodological rigor, including appropriate randomization, blinding, and adherence to intervention protocols.
Two researchers independently screened and selected relevant literature in a double-blind manner. Data from the selected studies were extracted by both researchers, followed by mutual verification to ensure accuracy. The extracted data were then standardized using SPSS software, and any unstandardized data were processed accordingly before the mutual verification. In case of discrepancies, a third researcher would resolve the conflict through discussion and review. The extracted data included the first author, publication year, country of origin, total sample size, participant age, exercise intervention details, duration of the intervention, and outcome measures. Specifically, data extraction included recording the means, standard deviations, and sample sizes for both the experimental and control groups post-intervention.
Two researchers independently used the ROB 2.0 Risk of Bias Assessment Tool to assess the quality of the included studies (Hu et al., 2024). The assessment covered seven domains of bias: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants, (4) blinding of outcome assessors, (5) completeness of outcome data, (6) selective reporting of findings, and (7) other potential sources of bias. Each domain was rated as “low risk,” “high risk,” or “unclear risk.” The evaluations were then cross-verified by the two researchers. In case of disagreements, a third researcher mediated to reach a consensus on the study’s inclusion.
This study used Stata 17 and RevMan 5.3 software to conduct a network meta-analysis of the selected data indicators. The study strictly adhered to the guidelines set forth in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020). The effect size for continuous variables was expressed as the standardized mean difference (SMD) with the corresponding 95% confidence intervals (CIs). The heterogeneity between independent studies was assessed based on the I2 statistic and the significance level (p-value). Specifically, I2 < 50% and p > 0.1 indicated no significant heterogeneity, allowing the application of a fixed-effects model; conversely, I2 ≥ 50% and p < 0.1 indicated significant heterogeneity, necessitating sensitivity analysis. To address the issue of inconsistent data units, unit conversion was carried out using the calculator feature in RevMan 5.4. The Surface Under the Cumulative Ranking (SUCRA) values were evaluated using cumulative probability plots to facilitate the ranking and comparison of various exercise interventions, with a range of 0 ≤ SUCRA ≤100. A SUCRA value of 100 indicates the best effect, while 0 indicates the worst or no effect, with higher SUCRA values suggesting better intervention outcomes. SUCRA values for mental status, daily living abilities, and cognitive assessments were systematically evaluated across different physical therapies. Cluster stratification analysis was used to identify the most effective exercise intervention, ensuring a comprehensive evaluation and comparison of the interventions. This systematic approach enabled the identification of the most effective exercise strategies for improving outcomes in Alzheimer’s disease patients.
A comprehensive search across multiple databases, including PubMed (n = 334), Google Scholar (n = 1,356), China National Knowledge Infrastructure (n = 625), Wanfang (n = 567), Weipu (n = 598), Cochrane Library (n = 376), and Web of Science (n = 435), initially identified 4,291 articles. After removing 978 duplicates, 3,313 articles remained for title and abstract screening. A rigorous multi-stage screening process was implemented, starting with the exclusion of studies that did not meet the inclusion criteria (e.g., non-randomized studies, unrelated interventions, non-AD populations). Ultimately, 187 full-text articles were reviewed for eligibility. Following detailed evaluation, 52 RCTs comprising a total of 3,409 participants were included in the final analysis. This selection process is visually summarized in Figure 1, and the key characteristics of the included studies, such as sample size, intervention type, duration, and outcome measures, are provided in Table 2. This comprehensive screening ensures a robust evidence base for conducting a network meta-analysis and limits the risk of selection bias.
The quality assessment focused on six specific domains: random sequence generation, deviations from expected interventions, incomplete outcome data, selective reporting of results, blinding of participants and outcome assessors, and overall study quality. Random sequence generation was assessed to determine whether the studies properly randomized participants, a crucial factor in minimizing selection bias. The risk of bias for random sequence generation was unclear in 19 studies, indicating incomplete reporting of randomization procedures. The remaining studies exhibited a low risk of bias, suggesting adherence to rigorous randomization protocols.
Regarding deviations from expected interventions, 22 studies exhibited unclear risk, meaning their adherence to the study protocol was not fully reported, while 3 studies demonstrated high risk, indicating significant protocol deviations. The remaining studies showed low risk in this domain. Incomplete outcome data, often a source of attrition bias, was judged to be unclear in 15 studies due to incomplete reporting on participant follow-up. However, the majority of studies adequately reported outcome data, minimizing the risk of bias.
Selective reporting, which occurs when only favorable outcomes are reported, was unclear in 20 studies and high risk in 2 studies, suggesting potential bias in outcome reporting. Blinding was largely adequate across most studies, reducing detection and performance bias. Overall, the comprehensive risk of bias assessment revealed that 3 studies had a high risk of bias, 28 studies were classified as low risk, and 21 studies exhibited an unclear risk of bias. The evaluation of bias risk is further detailed in Figure 2, which illustrates the distribution of potential biases across all included studies. Despite some concerns regarding the reporting quality, the overall methodological rigor of the included studies supports the validity of the findings.
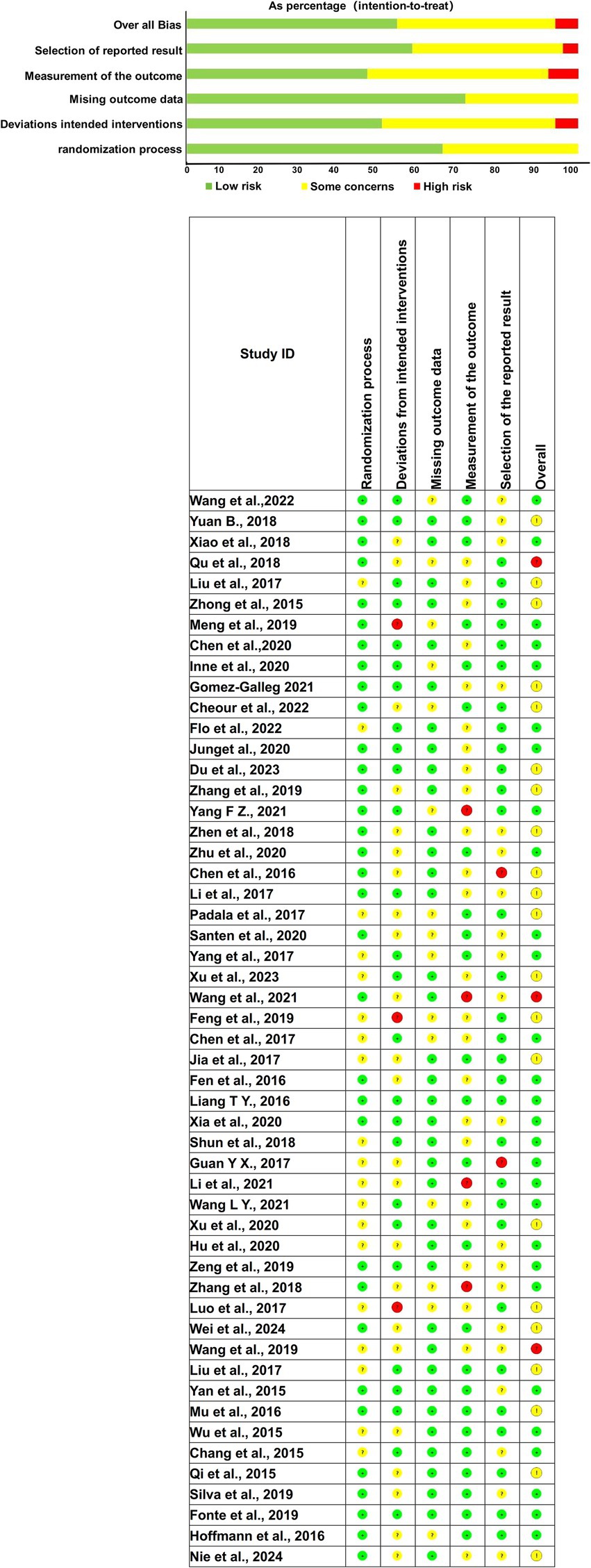
Figure 2. Quality assessment diagram and risk of bias summary. (Green, yellow, and red indicate low, moderate, and high risk levels, respectively. Columns represent: Randomization process, Deviations from intended interventions, Missing outcome data, Measurement of the outcome, Selection of the reported result, and Overall Bias).
As depicted in Figure 3, the network diagram provides a visual representation of direct and indirect comparisons across the different physical therapy interventions. Direct comparisons were particularly robust between the control group (routine care) and various therapies, notably play therapy, exercise therapy, acupuncture therapy, and cognitive therapy. However, direct head-to-head comparisons between these specific therapies were less frequent, with limited studies available for pairwise comparisons between music therapy and other interventions. This underlines the importance of integrating indirect evidence through network meta-analysis to establish a more complete comparative efficacy profile across all interventions.
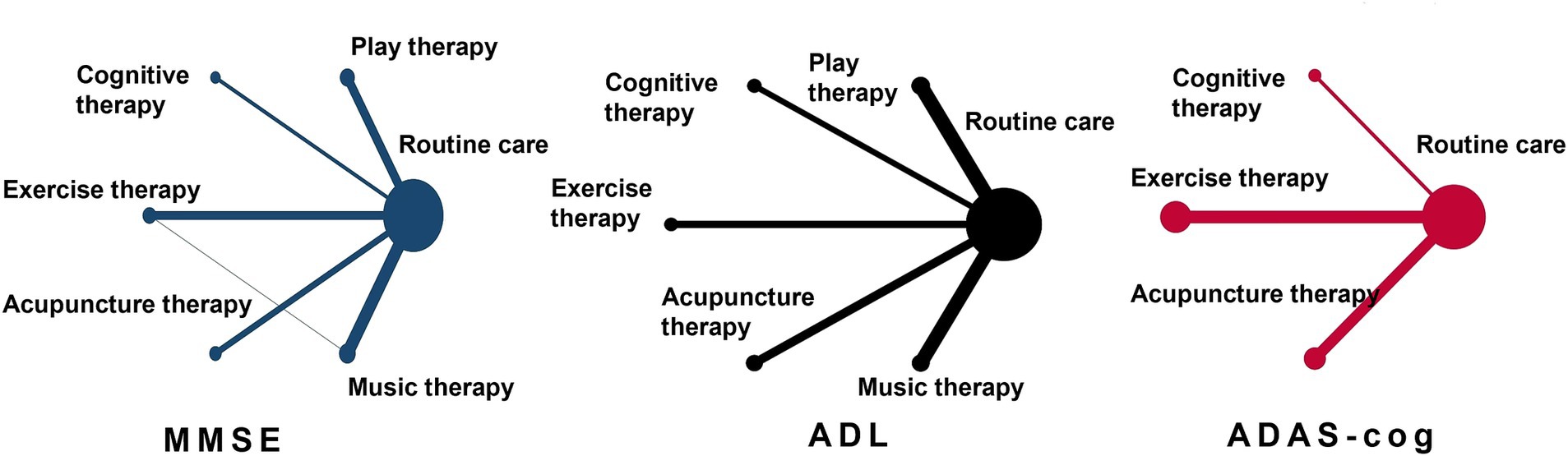
Figure 3. Evidence network diagram of network meta-analysis comparisons. The size of the nodes represents the sample size. The thickness of the lines represents the number of studies included in the comparison.
The heterogeneity across studies was evaluated using the inconsistency factor for each outcome (MMSE, ADL, ADAS-cog). Inconsistency factors ranged from 0 to 5.305, with 95% confidence intervals including 0. This suggests a high level of consistency between direct and indirect comparisons, indicating minimal heterogeneity and supporting the validity of the network model. The absence of significant inconsistency strengthens the reliability of the findings and suggests that the interventions have been evaluated in a comparable manner across different trials, allowing for meaningful comparisons.
The MMSE outcome was assessed across five physical therapy interventions: music therapy, acupuncture therapy, play therapy, cognitive therapy, and exercise therapy. The control group received standard care, routine nursing, or daily activities. Play therapy showed the most significant improvement in MMSE scores (MD = 0.03, 95% CI: 0.01–0.22, p < 0.05), reflecting its ability to engage patients cognitively in an interactive manner that may stimulate neuroplasticity. Cognitive therapy also exhibited strong efficacy (MD = 0.14, 95% CI: 0.02–1.29, p < 0.05), likely due to its focus on targeting specific cognitive deficits such as memory and executive function through structured tasks. Exercise therapy (MD = 0.13, 95% CI: 0.02–0.85, p < 0.05), acupuncture therapy (MD = 0.13, 95% CI: 0.02–1.06, p < 0.05), and music therapy (MD = 0.04, 95% CI: 0.01–0.20, p < 0.05) were also effective in enhancing mental state, but to a lesser extent compared to play and cognitive therapies. These findings suggest that interventions focusing on both physical and cognitive engagement may offer the greatest benefits in improving cognitive outcomes in AD patients.
The ADL outcome, which measures functional independence, showed that all five physical therapies—music therapy, acupuncture therapy, play therapy, cognitive therapy, and exercise therapy—were effective compared to the control group. Play therapy (MD = 0.01, 95% CI: 0.03–0.05, p < 0.05) emerged as the most effective, followed by cognitive therapy (MD = 1.19, 95% CI: 0.16–0.32, p < 0.05) and exercise therapy (MD = 0.84, 95% CI: 0.01–0.16, p < 0.05). Play therapy’s leading efficacy may be attributed to its capacity to combine physical and cognitive tasks, encouraging motor function and cognitive interaction simultaneously. Exercise therapy’s improvements in ADL reflect its ability to enhance physical strength and balance, important for maintaining independence in AD patients.
For cognitive function measured by the ADAS-cog, acupuncture therapy showed the strongest improvement (MD = 0.02, 95% CI: 0.03–0.36, p < 0.05), followed by cognitive therapy (MD = 0.03, 95% CI: 0.01–0.60, p < 0.05), and exercise therapy (MD = 0.06, 95% CI: 0.01–0.48, p < 0.05). The pronounced effect of acupuncture on cognitive improvement is consistent with its proposed neuroprotective mechanisms, including modulation of neurotransmitter levels and reduction of amyloid-beta accumulation. This suggests that acupuncture may be particularly effective for patients in earlier stages of cognitive decline. Music therapy (MD = 0.94, 95% CI: 0.32–2.73, p < 0.05) also demonstrated a notable effect, potentially due to its role in improving mood and reducing anxiety, which can positively affect cognitive outcomes.
The probabilistic ranking of the five interventions for MMSE improvement was play therapy > music therapy > acupuncture therapy > exercise therapy > cognitive therapy > routine nursing (Table 3). Play therapy’s leading rank underscores its comprehensive benefit in cognitive stimulation, potentially due to its ability to engage multiple cognitive domains simultaneously, such as problem-solving, memory, and attention.
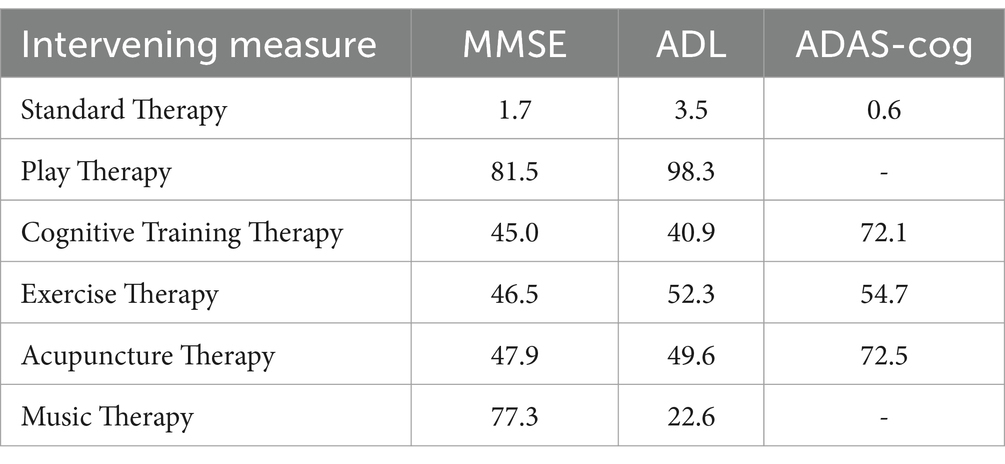
Table 3. Probability ranking results of various physiotherapy interventions for patients with AD (%).
For ADL improvement, the ranking was play therapy > exercise therapy > acupuncture therapy > cognitive therapy > music therapy > routine nursing (Table 3). This result aligns with the hypothesis that interventions combining cognitive and physical engagement, like play therapy, may provide the greatest functional benefits, particularly in maintaining or improving daily living activities.
The ranking for ADAS-cog was acupuncture therapy > cognitive therapy > exercise therapy > routine nursing (Table 3). The prominent role of acupuncture in improving cognitive function, as measured by ADAS-cog, suggests its potential as an adjunctive therapy for cognitive enhancement in AD patients, particularly in combination with other physical or cognitive interventions.
The funnel plot revealed asymmetry, indicating potential publication bias across some outcomes (Figure 4). The risk of publication bias, particularly in smaller studies with significant results, highlights the need for cautious interpretation of certain findings. Future studies should aim for pre-registration and adherence to transparent reporting standards to mitigate this issue.
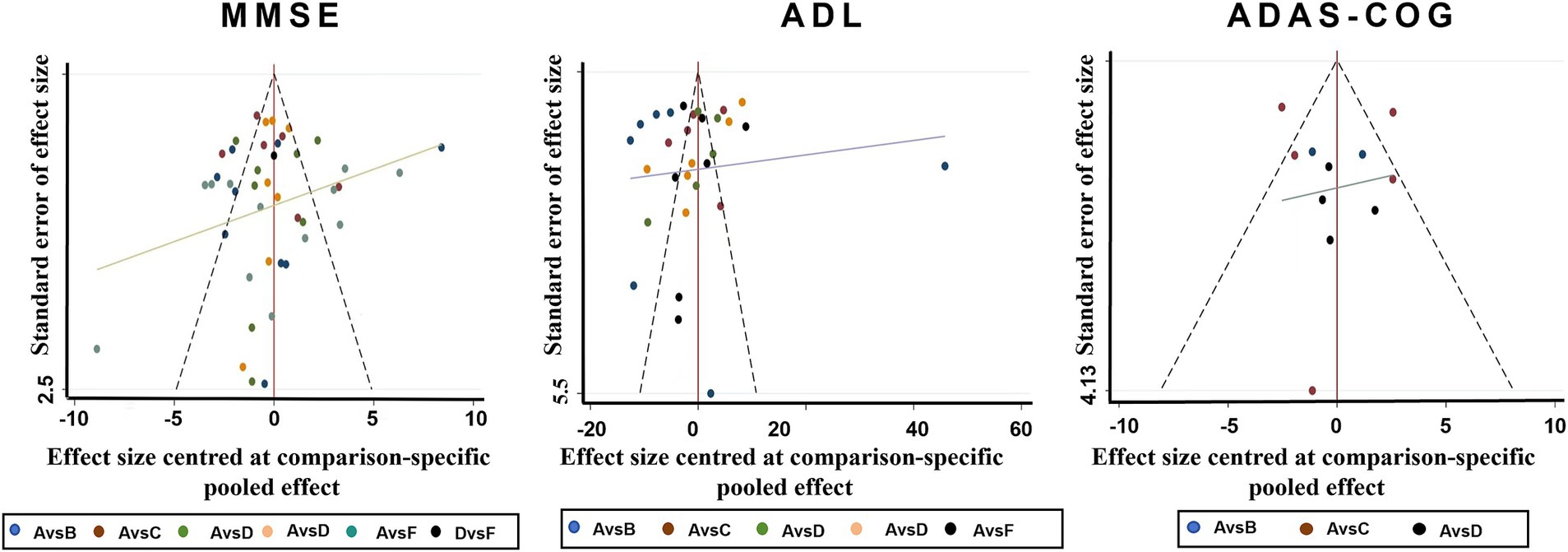
Figure 4. Funnel plots for various physiotherapy from all of the included studies. A. Routine care, B. Play therapy, C. Cognitive therapy, D. Exercise therapy, E. Acupuncture therapy, F. Music therapy.
Sensitivity analysis, performed by systematically excluding individual studies, revealed no significant changes in the effect estimates, indicating that the findings of the network meta-analysis are robust and not unduly influenced by any single study. This strengthens confidence in the consistency and reliability of the overall findings.
Subgroup analysis focusing on the duration of interventions suggested that interventions with shorter durations (<12 weeks) had more pronounced effects. However, due to the limited availability of data for longer-term studies, conclusions about the long-term efficacy of these interventions remain tentative, warranting further research with extended follow-up periods.
Play therapy with a duration of less than 12 weeks (p = 0.03) demonstrated significantly greater MMSE improvements compared to the control group. This suggests that short-term, intensive play therapy may be particularly effective in improving cognitive outcomes in AD patients.
Play therapy lasting less than 12 weeks (p < 0.00001) also showed a significant impact on ADL improvements, reinforcing the short-term benefits of this intervention for functional outcomes in AD patients. These findings support the implementation of short-term, targeted interventions in clinical settings to yield immediate functional benefits.
Acupuncture interventions of less than 12 weeks (p = 0.0001) led to significant improvements in ADAS-cog scores, highlighting the potential for acupuncture to offer meaningful cognitive benefits within a relatively short period. Future studies should explore the optimal duration and frequency of acupuncture for sustained cognitive improvements in AD patients (see Table 4).
Alzheimer’s disease, a chronic neurodegenerative condition, currently lacks specific medical treatments or interventions capable of reversing its progression (Huang and Wei, 2016). A comprehensive review of the pertinent literature indicates that while researchers have undertaken extensive clinical investigations, these studies largely focus on pharmacological and physical therapies, with no consensus regarding the most effective specific intervention. The inherent limitations of pharmacological treatments, including adverse effects, high costs, and limited long-term efficacy, underscore the growing need for non-pharmacological alternatives that are both effective and accessible. Physical therapies are distinguished by their simplicity, affordability, and safety, which positions them as attractive complementary options in AD management. Consequently, this study utilizes a network meta-analysis to evaluate the efficacy of five distinct physical therapies for patients with AD and employs the Surface Under the Cumulative Ranking (SUCRA) method for ranking. The objective is to offer more reliable, efficient, and targeted intervention options for individuals with AD. Through this method, we were able to integrate both direct and indirect comparisons across multiple interventions, thus providing a comprehensive ranking that reflects real-world clinical outcomes. The network meta-analysis allowed us to compare and rank multiple interventions simultaneously, providing a robust framework for determining the most effective therapeutic approaches. The results of cluster stratification suggest that play therapy and acupuncture therapy constitute the most effective interventions for enhancing the conditions of patients with AD.
Further subgroup analysis indicates that play therapy administered for a duration of less than 12 weeks yields a more pronounced improvement in MMSE and ADL scores among AD patients. Conversely, acupuncture therapy of the same duration demonstrates a more substantial impact on the ADAS-cog scores. These findings emphasize the importance of aligning specific interventions with the targeted outcomes of AD patients, as cognitive, functional, and emotional improvements may require different therapeutic approaches.
Based on assessments of MMSE and ADL scores, play therapy emerged as the most effective intervention. As an innovative therapeutic approach, play therapy presents several advantages, including high entertainment value, flexibility in forms, ease of patient acceptance, cost-effectiveness, and its potential to improve family relationships. Group play activities, in particular, offer cognitive and social stimulation, helping to alleviate the social isolation commonly experienced by AD patients. Studies have shown that participation in games can enhance limb function, stimulate mental engagement, and reduce sedentary behavior in patients with AD. Group game activities provide more opportunities for social interaction, increasing communication and alleviating loneliness, effectively enhancing subjective well-being and, consequently, improving mental state and daily living abilities in AD patients. Further investigation into the physiological mechanisms reveals that play therapy can stimulate cognitive activity, expand thinking, continuously stimulate brain cells, slow down brain degeneration, accelerate cerebral blood flow, facilitate neuroplasticity and functional reorganization, promote glucose metabolism in the anterior cingulate cortex, and fully engage positive emotions, improving cognitive function and physical coordination, (Chen, 2012; Schroder et al., 2007) thereby positively influencing the mental state and daily living abilities of AD patients. Music therapy showed a secondary effect on MMSE scores. As an emerging interdisciplinary field, music therapy, based on psychological treatment theories and methods, utilizes specially designed musical activities and experiences to exert the unique physiological and psychological effects of music, aiming to eliminate psychological barriers and promote rehabilitation (Pongan et al., 2017). The effectiveness of music therapy in improving patient mood and alleviating heart and respiratory rates has been confirmed. It is widely used in settings such as operating rooms and outpatient infusion centers. Some scholars have also demonstrated that music therapy can significantly improve cognitive function and quality of life in AD patients (Palisson et al., 2015). Exercise therapy showed a secondary effect on ADL scores. Investigation into the physiological mechanisms reveals that exercise can promote blood flow to the brain, accelerate angiogenesis, enrich the brain’s microvascular network, and trigger neuroplasticity in brain regions (Lista and Sorrentino, 2010). Exercise can promote the expression of brain-derived neurotrophic factor and vascular endothelial growth factor, promoting neuronal morphological changes and enhanced synaptic plasticity, thereby improving brain structures and neural circuits involved in cognition (Pereira et al., 2007). Exercise can also inhibit excessive astrocyte activation, slowing down the abnormal accumulation of Aβ and Tau proteins, thereby improving cognitive and memory function (Zhang X. L. et al., 2019; Lu et al., 2017).
Acupuncture therapy has also been shown to positively influence the mental state and daily living abilities of patients with AD. When evaluating interventions aimed at enhancing cognitive function scores in AD patients, acupuncture therapy emerges as the most effective option. Acupuncture, grounded in traditional Chinese medicine, offers a holistic approach to managing AD by targeting underlying imbalances in the body’s energy pathways, known as meridians. Recent advances in neuroimaging techniques have also provided evidence that acupuncture can modulate specific brain networks associated with cognitive processing and emotional regulation. This further supports the therapeutic value of acupuncture beyond its traditional framework. In recent years, the progressive development of traditional Chinese medicine has garnered significant interest from researchers in the field of acupuncture. According to traditional Chinese medicine, the pathology of Alzheimer’s disease is localized in the brain, and the Governing Vessel is intimately connected to cerebral functions. In accordance with the principles of syndrome differentiation and treatment in traditional Chinese medicine, external therapeutic modalities such as electroacupuncture, meridian acupuncture, and head acupoint stimulation have demonstrated specific therapeutic efficacy in enhancing cognitive functions and decelerating disease progression in patients with AD (Guan, 2018). A thorough review of extensive literature reveals that head acupoints, including Baihui, Sishencong, Zusanli, and Xuan Zhong, are frequently selected in acupuncture treatments. The Baihui acupoint, situated at the juncture of the midline of the head and the line connecting the two ears, is intimately associated with cerebral functions. It plays a pivotal role in regulating brain activity, balancing yin and yang, and is purported to enhance cognitive alertness and sensory perception. Acupuncture at the Baihui point is believed to nourish the kidneys and marrow, thereby contributing to the improvement of brain function. The Xuan Zhong acupoint, often referred to as the “marrow meeting” point, is instrumental in nourishing the kidneys and essence, as well as replenishing the marrow. The selection of the Zusanli acupoint is effective in regulating the spleen and stomach, in addition to augmenting qi and blood. The Sishencong acupoint is noted for its calming properties (Song et al., 2019). Acupuncture treatment can facilitate the promotion of qi and blood circulation, regulation of the spirit, and enhancement of cognitive functions. Consequently, it holds potential for improving cognitive abilities and delaying the aging process, thereby exerting a beneficial impact on the cognitive function of patients with AD.
Moreover, although the precise mechanisms by which acupuncture exerts therapeutic effects on cognitive function in patients with AD are not yet fully elucidated, existing literature, particularly studies conducted on animal models (Cao et al., 2014; Jang et al., 2023), indicates several plausible pathways. Acupuncture may improve cognitive function by decreasing the levels of amyloid-beta (Aβ) protein in the brains of mice exhibiting dementia. Additionally, it appears to reduce the deposition of Aβ protein within vascular structures and to enhance the synthesis and release of acetylcholine, a critical neurotransmitter implicated in learning and memory processes. Furthermore, acupuncture has the potential to enhance the functionality of hippocampal neurons, a region integral to memory formation, and to augment the antioxidant capacity of brain tissue. These mechanisms align with the pathology of AD, wherein Aβ deposition and cholinergic deficits are major contributors to cognitive decline, thus providing a strong theoretical basis for acupuncture’s role in AD management. These findings provide theoretical insights into the mechanisms through which acupuncture may ameliorate memory and cognitive functions in patients with AD. Cognitive therapy demonstrated a secondary effect on ADAS-cog scores. Further investigation into the underlying physiological mechanisms reveals that cognitive function, particularly attention, is often impaired due to brain tissue damage and atrophy (Martinez-moreno et al., 2016). Researchers should fully leverage the plasticity of the human nervous system to implement early functional rehabilitation of the damaged nervous system. The efficacy of cognitive training for AD stems from the frequent activation of the ascending reticular activating system and the limbic system by language and behavioral stimuli (Yang et al., 2015).
However, this study has several limitations. First, the study included only published articles and journals, which may introduce potential bias or geographic limitations due to variations in patient demographics, including gender, age, region, and country. Second, some included journals had small sample sizes, potentially reducing statistical power and increasing the uncertainty of the results. This increases the risk of selection bias and reduces the reliability and interpretability of the findings. Future research requires more high-quality studies with larger sample sizes. Third, some included studies may not have implemented blinding or allocation concealment, or may not have excluded non-randomized studies, potentially introducing bias into the results. Fourth, the study included participants who were able to participate in randomized controlled trials, limiting the generalizability to older adults with limited mobility or those who are very elderly. Fifth, the model consistency checks may not have fully considered potential differences between direct and indirect evidence, potentially affecting the interpretation of the results and the credibility of the model. Finally, the analysis focused on different types of physical therapy, but the included studies lacked consistent data on the duration and frequency of different physical interventions, hindering in-depth analysis. Future research could benefit from employing more standardized data collection methods to facilitate comprehensive subgroup analyses aimed at determining optimal intervention duration.
In summary, play therapy administered for a duration of less than 12 weeks has been identified as the most effective intervention for enhancing the mental state and daily living abilities of patients with AD. Conversely, acupuncture therapy, when applied for a period of less than 12 weeks, emerges as the optimal intervention for improving cognitive function in AD patients. When formulating intervention plans, it is imperative to comprehensively consider not only the intervention period and frequency but also factors such as the type, duration, and intensity of the physical therapy, as well as the age and gender of the patients. Future personalized intervention strategies should also incorporate patient preferences, caregiver involvement, and social support structures to ensure optimal adherence and therapeutic success. This holistic, individualized approach will ensure that the physical interventions are tailored to the specific needs of AD patients, thereby optimizing treatment outcomes and enhancing their quality of life. This holistic approach will yield more reliable recommendations for the prevention and treatment of clinical Alzheimer’s Disease.
This network meta-analysis highlights the differential therapeutic effects of various non-pharmacological interventions on AD. Game therapy and acupuncture therapy emerged as the most effective treatments for improving cognitive function, mental state, and daily living abilities in AD patients. Specifically, game therapy demonstrated significant short-term benefits for mental health and functional abilities, while acupuncture therapy showed superior long-term improvements in cognitive function. These findings underscore the importance of tailored interventions that address both cognitive and physical aspects of AD. Moving forward, incorporating such personalized therapies into clinical practice may enhance treatment outcomes and improve the quality of life for AD patients. Future research should focus on further exploring the mechanisms underlying these interventions and optimizing their application for diverse AD populations.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
JW: Investigation, Writing – review & editing. YT: Methodology, Writing – original draft. YX: Formal analysis, Writing – original draft. SX: Writing – review & editing, Conceptualization. SZ: Writing – original draft.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the soft science research project of the science and technology department of Henan province, No. 242400410066.
I would like to sincerely thank all the authors whose works have been referenced in this project. Their research and contributions provided invaluable insights and formed the foundation of this work. Without their dedication and scholarly efforts, this project would not have been possible.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bi, D. Y., Liu, Q., Chen, Y. X., Zou, Y. F., Ma, M. Z., Li, D., et al. (2016). Therapeutic effect of acupuncture at Zusanli and Fenglong point in the treatment of senile dementia. J. Acupunct. Tuina Sci. 14, 386–390. doi: 10.1007/s11726-016-0954-y
Cao, J., Tang, Y. S., and Li, Z. G. (2014). Research progress of electroacupuncture in the treatment of Alzheimer's disease. J. Tradit. Chin. Med. 29, 3177–3180.
Chang, C. H., Wang, W., Zhu, Y., Yang, S. Y., Li, H. Y., Wang, Q., et al. (2015). Effect of aerobic training on the treatment of Alzheimer's disease. J. Rehabil. Med. 30, 1131–1161. doi: 10.3969/j.issn.1001-1242.2015.11.007
Chen, C. F. (2012). Effect of play therapy on cognitive function and subjective well-being of patients with mild senile dementia. J. Modern Integr. Chin. Western Med. 21, 260–261. doi: 10.3969/j.issn.1008-8849.2012.03.015
Chen, X. H., Chen, P., and Li, H. (2016). Effects of brain games on quality of life in elderly patients with dementia. Chin. J. Modern Nurs. 22, 4171–4173. doi: 10.3760/cma.j.issn.1674-2907.2016.29.007
Chen, X., Li, D. M., Xu, H., and Hu, Z. (2020). Effect of traditional opera on older adults with dementia. Geriatr. Nurs. 41, 118–123. doi: 10.1016/j.gerinurse.2019.08.002
Chen, Z. Y., Schwulst, S. J., and Mentis, A. A. (2021). Correction: APOE4- mediated Alzheimer disease and "vascular"-"meningeal lymphatic" components: towards a novel therapeutic era? Mol. Psychiatry 26:5475. doi: 10.1038/s41380-021-01307-7
Chen, Y. M., Ye, P., and Tan, A. H. (2017). Clinical observation of acupuncture in the treatment of Alzheimer's disease. J. Integr. Chin. Western Med. Cardiovasc. Dis. Electr. 5, 128–130. doi: 10.3969/j.issn.2095-6681.2017.22.098
Chen, Z., and Zhong, C. (2013). Decoding Alzheimer is disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Prog. Neurobiol. 108, 21–43. doi: 10.1016/j.pneurobio.2013.06.004
Cheour, S., Cheour, C., Kilani, C., Guemri, A., Zineddine, D., Khelifa, R., et al. (2022). Salivary testosterone and cortisol levels in Tunisian elderly male patients with mild Alzheimer's disease. Implications of musical therapy and/or physical rehabilitation. Front. Physiol. 13:839099. doi: 10.3389/fphys.2022.839099
Connors, M. H., Ames, D., Woodward, M., and Brodaty, H. (2018). Psychosis and clinical outcomes in Alzheimer disease: a longitudinal study. Am. J. Geriatr. Psychiatry 26, 304–313. doi: 10.1016/j.jagp.2017.10.011
Du, J., Zhang, H. Y., and Qiao, Y. C. (2019). Effect of play therapy on cognitive ability and self-efficacy of patients with Alzheimer's disease. Chin. J. Rehabil. 38, 426–429. doi: 10.3870/zgkf.2023.07.009
Fang, Y., Kolanowski, A. M., Strumpf, N. E., and Eslinger, P. J. (2006). Improving cognition and function through exercise intervention in Alzheimer's disease. J. Nurs. Scholarship 38, 358–365. doi: 10.1111/j.1547-5069.2006.00127.x
Feng, Q., Bin, L. L., Zhai, Y. B., Xu, M., Liu, Z. S., and Peng, W. M. (2019). Long-term efficacy and safety evaluation of electroacupuncture in improving MMSE score in patients with Alzheimer's disease. Chin. J. Acupunct. Moxib. 39, 3–8. doi: 10.13703/j.0255-2930.2019.01.001
Flo, B. K., Matziorinis, A. M., Skouras, S., Sudmann, T. T., Gold, C., and Koelsch, S. (2022). Study protocol for the alzheimer and music therapy study: an RCT to compare the efficacy of music therapy and physical activity on brain plasticity, depressive symptoms, and cognitive decline, in a population with and at risk for Alzheimer's disease. PLoS One 17:e0270682. doi: 10.1371/journal.pone.0270682
Fonte, C., Smania, N., Pedrinolla, A., Munari, D., Gandolfi, M., Picelli, A., et al. (2019). Comparison between physical and cognitive treatment in patients with MCI and Alzheimer’s disease. Aging (Albany NY) 11, 3138–3155. doi: 10.18632/aging.101970
Gomez-gallego, M., Gomez-gallego, J. C., Gallego-mellado, M., and Garcia-garcia, J. (2021). Comparative efficacy of active group music intervention versus group music listening in Alzheimer's disease. Int. J. Environ. Res. Public Health 18:8067. doi: 10.3390/ijerph18158067
Gu, X. Y., and Wang, Q. Y. (2019). Effect of exercise on brain aging induced Alzheimer's disease. Chin. J. Gerontol. 44, 1006–1010. doi: 10.3969/j.issn.1005-9202.2024.04.060
Guan, Y. X. (2017). Clinical study of combined therapy for Alzheimer's disease. Asian Pac. Trad. Med. 13, 113–114. doi: 10.11954/ytctyy.201706046
Guan, Q. Y. (2018). Research progress of acupuncture in the treatment of Alzheimer's disease. China Prescript. Drugs 16, 16–17. doi: 10.3969/j.issn.1671-945X.2018.02.012
Hall, A., Barbera, M., Lehtisalo, J., Antikainen, R., Huque, H., Laatikainen, T., et al. (2024). The Australian National University Alzheimer's disease risk index (ANU- ADRI) score as a predictor for cognitive decline and potential surrogate outcome in the FINGER lifestyle randomized controlled trial. Eur. J. Neurol. 31:e16238. doi: 10.1111/ene.16238
Hessmann, P., Dodel, R., Baum, E., Muller, M. J., Paschke, G., Kis, B., et al. (2018). Antidepressant medication in a German cohort of patients with Alzheimer's disease. Int. J. Clin. Pharmacol. Ther. 56, 101–112. doi: 10.5414/CP203121
Hoffmann, K., Sobol, N. A., Frederiksen, K. S., Beyer, N., Vogel, A., Vestergaard, K., et al. (2016). Moderate-to-high intensity physical exercise in patients with Alzheimer’s disease: a randomized controlled trial. J. Alzheimers Dis. 50, 443–453. doi: 10.3233/JAD-150817
Hu, G. D., Xiang, M., Zhang, X. P., Deng, C. X., and Shen, A. D. (2019). Effect evaluation of comprehensive cognitive rehabilitation training in patients with Alzheimer's disease. Chin. Modern Doctor 58, 98–101.
Hu, Y. P., Zhu, T., Deng, X. J., Zeng, W., Chen, J. L., Tang, Y., et al. (2024). Analysis of evidence deletion bias risk assessment tool ROB-MEN in mesh meta-analysis. Chin. J. Evid. Based Med. 24, 451–458.
Huang, B., and Wei, H. L. (2016). Progress in nursing of patients with Alzheimer's disease. Nurs. Res. 30, 1422–1424.
Innes, K. E., Montgomery, C., Selfe, T. K., Wen, S., Khalsa, D. S., and Flick, M. (2021). Incorporating a usual care comparator into a study of meditation and music listening for older adults with subjective cognitive decline: a randomized feasibility trial. J. Alzheimerrs Dis. Rep. 5, 187–206. doi: 10.3233/ADR-200249
Jang, H., Oh, H., Shin, S., and Lee, E. (2023). Acupuncture for spontaneous intracerebral hemorrhage; a systematic review and Meta-analysis. Eur. J. Neurol. 30, 457–458.
Jia, Y. J., Meng, D., Sun, M. L., Shi, J. W., Liu, X. X., Yu, T., et al. (2017). A randomized controlled clinical study of three-jiao acupuncture in the treatment of mild to moderate Alzheimer's disease. Liaoning J. Trad. Chin. Med. 44, 1911–1914. doi: 10.13192/j.issn.1000-1719.2017.09.043
Joseph, G., Bryan, J., Tricia, J., Jessica, R., and Jennifer, W. (2021). Alzheimer’s disease facts and figures. Alzheimers Dement. 17, 327–406. doi: 10.1002/alz.12328
Jung, Y. H., Lee, S., Kim, W. J., Lee, J. H., Kim, M. J., and Han, H. J. (2020). Effect of integrated cognitive intervention therapy in patients with mild to moderate Alzheimer's disease. Dement. Neurocogn. Disord. 19, 86–95. doi: 10.12779/dnd.2020.19.3.86
Li, C. X., Fang, Y. U., Jiang, N., and Kong, L. (2019). Study on cognitive improvement of patients with mild to moderate Alzheimer's disease by adaptive cognitive training. J. Hunan Univ. Sci. Technol. 34, 43–46. doi: 10.3969/j.issn.1672-5298.2021.04.010
Li, D. M., and Li, X. X. (2017). The effect of folk recreation program in improving symptoms: a study of Chinese elder dementia patients. Int. J. Geriatr. Psychiatry 32, 901–908. doi: 10.1002/gps.4543
Liang, T. Y. (2016). 37 cases of senile dementia treated by warm acupuncture. Res. Trad. Chin. Med. 29, 57–59.
Lista, I., and Sorrentino, G. (2010). Biological mechanisms of physical activity in preventing cognitive decline. Cell. Mol. Neurobiol. 30, 493–503. doi: 10.1007/s10571-009-9488-x
Liu, X. R., Li, S. S., and Wang, L. F. (2019). Research progress of music therapy for Alzheimer's disease. Nurs. Res. 38, 2881–2884. doi: 10.12102/j.issn.1009-6493.2024.16.012
Liu, L. C., and Liu, Y. (2017). Effect of music therapy on patients with Alzheimer's disease. Chin. J. Gerontol. 37, 1215–1216. doi: 10.3969/j.issn.1005-9202.2017.05.083
Liu, Y., Wang, T., Zhu, Y., Wang, W., Yin, M. M., Zhang, W. R., et al. (2017). Study on improving cognitive function of patients with Alzheimer's disease with moderate intensity aerobic exercise. China Rehabil. 32, 386–389. doi: 10.3870/zgkf.2017.05.010
Lu, Y. J., Dong, Y., Tucker, D., Wang, R. M., Ahmed, M. E., Brann, D., et al. (2017). Treadmill exercise exerts neuroprotection and regulates microglial polarization and oxidative stress in a streptozotocin-induced rat model of sporadic Alzheimer's disease. J. Alzheimers Dis. 56, 1469–1484. doi: 10.3233/JAD-160869
Luo, C. X., and Xu, T. (2017). Effect of cognitive training on rehabilitation of patients with Alzheimer's disease. Primary Med. Forum 21, 3633–3634. doi: 10.19435/j.1672-1721.2017.27.001
Martinez-moreno, M., Cerulla, N., Chico, G., Quintana, M., and Garolera, M. (2016). Comparison of neuropsychological and functional outcomes in Alzheimer's disease patients with good or bad response to a cognitive stimulation treatment: a retrospective analysis. Int. Psychogeriatr. 28, 1821–1833. doi: 10.1017/S104161021600123X
Meng, S., Qi, X., and Wen, C. Y. (2019). Comparison of different types of music in the treatment of Alzheimer's disease patients. Chin. J. Gerontol. 39, 4510–4513. doi: 10.3969/j.issn.1005-9202.2019.18.045
Mu, H. Y., Lv, J. H., Hao, Z. H., Li, W. J., and Li, M. (2016). Effects of aerobic exercise on life ability, cognitive function and psychiatric symptoms in patients with mild to moderate Alzheimer's disease. Chin. J. Geriatr. Multiple Organ Dis. 15, 451–454.
Nie, Y., Song, S. H., and Dong, Y. Y. (2019). Effects of audiovisual combined finger activity training on cognitive function of elderly patients with dementia. Bull. Sports Sci. Technol. Lit. 32, 157–182. doi: 10.19379/j.cnki.issn.1005-0256.2024.01.041
Owen, P. J., Miller, C. T., Mundell, N. L., Verswijveren, S. J. J. M., Tagliaferri, S. D., Brisby, H., et al. (2020). Which specific modes of exercise training are most effective for treating low back pain? Network meta-analysis. Br. J. Sports Med. 54, 1279–1287. doi: 10.1136/bjsports-2019-100886
Padala, K. P., Padala, P. R., Malloy, T. R., Geske, J. A., Dubbert, P. M., Dennis, R. A., et al. (2012). Wii-fit for improving gait and balance in an assisted living facility: a pilot study. J. Aging Res. 2012, 1–6. doi: 10.1155/2012/597573
Palisson, J., Roussel-Baclet, C., Maillet, D., Belin, C., Ankri, J., and Narme, P. (2015). Music enhances verbal episodic memory in Alzheimer's disease. J. Clin. Exp. Neuropsychol. 37, 503–517. doi: 10.1080/13803395.2015.1026802
Pereira, A. C., Huddleston, D. E., Brickman, A. M., Sosunov, A. A., Hen, R., Mcjhann, G. M., et al. (2007). An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA 104, 5638–5643. doi: 10.1073/pnas.0611721104
Pongan, E., Tillmann, B., Leveque, Y., Trombert, B., Getenet, J. C., Auguste, N., et al. (2017). Can musical or painting interventions improve chronic pain, mood, quality of life, and cognition in patients with mild Alzheimer's disease? Evidence from a randomized controlled trial. J. Alzheimers Dis. 60, 663–677. doi: 10.3233/JAD-170410
Prince, M., Wimo, A., Guerhet, M., Ali, C., Prina, M., and Wu, Y. T. (2015). World Alzheimer report 2015. The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends. [Research Report] Alzheimer’s Disease International. Available at: https://unilim.hal.science/hal-03495438/document
Qi, M., and Zhang, L. (2015). Efficacy of aerobic training based on resting state fMRI in patients with Alzheimer's disease. Chin. J. Clin. Imaging 26, 761–767.
Qu, X. N., Ning, Q., Huang, T. L., Peng, Z. J., Li, X. Y., and Zhang, J. (2018). Effect of music training intervention on functional magnetic resonance imaging and anxiety and depression in patients with Alzheimer's disease. Anhui Med. J. 39, 1400–1404. doi: 10.3969/j.issn.1000-0399.2018.11.029
Sancheti, H., Akopian, G., Yin, F., Brinton, R. D., Walsh, J. P., and Cadenas, E. (2013). Age-dependent modulation of synaptic plasticity and insulin mimetic effect of lipoic acid on a mouse model of Alzheimer is disease. PLoS One 8:e69830. doi: 10.1371/journal.pone.0069830
Santen, J. V., Dröes, R. M., Twisk, J. W. R., Henkemans, O. A. B., Straten, A. V., and Meiland, F. J. M. (2020). Effects of exergaming on cognitive and social functioning of people with dementia: a randomized controlled trial. J. Am. Med. Dir. Assoc. 21, 1958–1967.e5. doi: 10.1016/j.jamda.2020.04.018
Schroder, S., Liepert, J., Remppis, J., and Greten, J. H. (2007). Acupuncture treatment improves nerve conduction in peripheral neuropathy. Eur. J. Neurol. 14, 276–281. doi: 10.1111/j.1468-1331.2006.01632.x
Shufen, W., and Hongxia, S. (2011). Physical therapy in combination with strengthening nursing intervention of the clinical observation of Alzheimer's disease. Chin. J. Clin. Ratnl. Drug Use 4, 90–91. doi: 10.3969/j.issn.1674-3296.2011.25.073
Silva, F. D. E., Ferreira, J. V., Placido, J., Anna, P. S., Araujo, J., Marinho, V., et al. (2019). Three months of multi⁃modal training contributes to mobility and executive function in eld⁃ erly individuals with mild cognitive impairment, but not in those with Alzheimer’ s disease: a randomized controlled trial. Maturitas 126, 28–33. doi: 10.1016/j.maturitas.2019.04.217
Song, R. F., Zhu, C. F., Zhang, J. Y., Wang, C., and Jia, Y. M. (2019). Systematic review and meta-analysis of acupuncture treatment for Alzheimer's disease. J. Tradit. Chin. Med. 35, 1529–1536. doi: 10.16448/j.cjtcm.2023.0819
Sun, H. N., Zhu, M. D., and Zhang, W. (2018). Effect of acupuncture at Baihui and Yongquan points on plasma acetylcholine and β-amyloid protein in the treatment of Alzheimer's disease. World Chin. Med. 13:2861.
Villain, N., and Michalon, R. (2024). What is Alzheimer's disease? An analysis of nosological perspectives from the 20th and 21st centuries. Eur. J. Neurol. 31:e16302. doi: 10.1111/ene.16302
Wang, L. X. (2021). Effect and influence of computer-assisted cognitive training on mild Alzheimer's disease : Zhengzhou University.
Wang, W., Wang, T., Sha, L. J., Zhang, R., Liu, R., Xie, Y., et al. (2019). Changes of low-frequency amplitude fMRI and cognitive function in patients with Alzheimer's disease before and after aerobic exercise. J. Rehabil. Med. 34, 371–377. doi: 10.3969/j.issn.1001-1242.2019.04.002
Wang, Y. J., Zhang, H. H., Chen, M. M., Yang, J. N., Wang, Y. Y., Yu, J. X., et al. (2022). Effect of family coordinated continuous care model combined with music therapy on quality of life of patients with Alzheimer's disease. Chin. J. Appl. Neurol. Dis. 25, 1270–1274. doi: 10.12083/SYSJ.220736
Wang, X. W., Zhu, C. F., Ge, H. H., and Wu, Y. Y. (2021). Clinical study of warming Yang and invigorating kidney moxibustion combined with acupuncture in treatment of senile dementia. Clin. J. Acupunct. Moxib. 37, 10–14. doi: 10.19917/j.cnki.1005-0779.021236
Wei, Y. H., Jian, H. L., Huang, X. X., Ye, J. R., Yang, H., Lu, X. L., et al. (2018). Effects of aerobic exercise based on Baduanjin on cognitive function and quality of life in patients with mild to moderate Alzheimer's disease. Evid. Based Nurs. 10, 335–338. doi: 10.12102/j.issn.2095-8668.2024.02.027
Wu, D., and Huang, K. (2015). Effects of walking on cognitive function and quality of life in patients with Alzheimer's disease. Chin. J. Modern Med. 25, 94–97.
Xia, K. P., Pang, J., Li, S. L., Zhang, M., Li, H. L., and Wang, Y. J. (2020). Effects of doumai on learning and memory ability and serum APP and Aβ-1 in patients with Alzheimer's disease. Chin. J. Acupunct. Moxib. 40, 375–378.
Xiao, J. P., Xie, L., Liang, L. Y., Jiang, G. Q., and Wu, H. H. (2018). Effects of music therapy on sleep quality, quality of life, cognitive function and agitated behavior in patients with Alzheimer's disease. Adv. Modern Biomed. 18, 3896–3900. doi: 10.13241/j.cnki.pmb.2018.20.021
Xu, L. F., Dong, Q. P., and Li, H. (2019). Effect of Tiaoshen acupuncture on the treatment of Alzheimer's disease and its influence on serum superoxide dismutase level. Chin. Contemp. Med. 30, 92–95. doi: 10.3969/j.issn.1674-4721.2023.16.022
Xu, Q., Liu, X. W., Li, P. P., Deng, Z. A., and Ji, P. (2020). Effect of tablet cognitive training on mild to moderate Alzheimer's disease. Chin. J. Gerontol. 40, 2813–2814. doi: 10.3969/j.issn.1005-9202.2020.13.041
Xu, Q., Zhao, J. L., and Wang, X. (2019). Effect of aerobic exercise on cognitive function in patients with Alzheimer's disease: a meta-analysis. Chin. J. Rehabil. Med. 34, 824–830. doi: 10.3969/j.issn.1001-1242.2019.07.014
Yan, L. Y., Wang, W., Shen, F. F., Yang, S. Y., Wang, W., Wang, Q., et al. (2015). Clinical study of aerobic exercise with different training time in intervention of mild to moderate Alzheimer disease. J. Rehabil. Med. 30, 771–776. doi: 10.3969/j.issn.1001-1242.2015.08.004
Yang, F. Z. (2019). Influence of motion-sensing interactive games on senile dementia patients. J. Chin. For. Med. Res. 19, 126–129. doi: 10.14033/j.cnki.cfmr.2021.14.042
Yang, S. Q., Fan, D. H., Luo, M. X., Zhong, Y. H., Yue, T., and Yang, J. M. (2023). Effects of abdominal response on cognitive function and serum 5-HT, BDNF and IGF-1 in patients with mild to moderate Alzheimer's disease. Shanghai J. Acumox 5, 485–490. doi: 10.13460/j.issn.1005-0957.2023.05.0485
Yang, Y., and Kwak, Y. T. (2017). Improvement of cognitive function after computer-based cognitive training in early stage of Alzheimer's dementia. Dement. Neurocogn. Disord. 16, 7–11. doi: 10.12779/dnd.2017.16.1.7
Yang, S. Y., Shan, C. L., Qing, H., Wang, W., Zhu, Y., Yin, M. M., et al. (2015). The effects of aerobic exercise on cognitive function of Alzheimer's disease patients. CNS Neurol. Disord. Drug Targets 14, 1292–1297. doi: 10.2174/1871527315666151111123319
Yuan, B. (2018). The clinical effect of music therapy on mild Alzheimer's disease. Chin. Natnl. Health Med. 30, 25–26. doi: 10.3969/j.issn.1672-0369.2018.22.012
Zeng, T. T., Huang, L. Y., and Lin, Q. Y. (2019). Effect of rehabilitation cognitive training on quality of life of patients with Alzheimer's disease. Fujian J. Med. 41, 153–154. doi: 10.3969/j.issn.1002-2600.2019.04.058
Zhang, L. (2019). Clinical application and development of new drugs for Alzheimer's disease. Chin. J. Pharmacol. Toxicol. 33:407.
Zhang, X. L., He, Q., Huang, T., Zhao, N., Liang, F., Xu, B., et al. (2019). Treadmill exercise decreases Aβ deposition and counteracts cognitive decline in APP/PS1 mice, possibly via hippocampal microglia modifications. Front. Aging Neurosci. 11:78. doi: 10.3389/fnagi.2019.00078
Zhang, X. J., and McGeer, P. L. (2004). Relationship between senile plaques, neurofibrillary tangles and complement receptor type 2 in hippocampal and temporal lobe brain tissues of Alzheimer's disease patients. J. Clin. Rehabil. 28, 6212–6214. doi: 10.3321/j.issn:1673-8225.2004.28.031
Zhang, Y., Qiao, Y. C., Wang, J. M., Sun, J., and Chang, H. (2018). Effects of home cognitive training on cognitive function of patients with mild cognitive impairment. Nurs. Res. 32, 3236–3239. doi: 10.12102/j.issn.1009-6493.2018.20.018
Zhang, X., Wu, G., and Ke, Y. (2019). Study on the application of educational rehabilitation nursing to Alzheimer's patients with mild to moderate cognitive impairment. J. Qiqihar Med. Coll. 40, 2488–2489. doi: 10.3969/j.issn.1002-1256.2019.19.042
Zheng, J. Y., and Chen, X. P. (2018). Application of motion-sensing interactive games in elderly patients with dementia. J. Nurs. Sci. 33, 5–9. doi: 10.3870/j.issn.1001-4152.2018.09.005
Zhong, W. A., Cao, L. Q., and Gao, W. (2015). Effect of group music therapy on patients with senile dementia. Chin. Natnl. Health Med. 27:85. doi: 10.3969/j.issn.1672-0369.2015.03.044
Keywords: Alzheimer’s disease patients, mesh meta-analysis, physical intervention, music therapy, intervention effect
Citation: Wu J, Teng Y, Xie Y, Xing S and Zhi S (2025) Comparing the efficacy of physical therapy interventions in Alzheimer’s disease: a network meta-analysis. Front. Aging Neurosci. 17:1541287. doi: 10.3389/fnagi.2025.1541287
Received: 07 December 2024; Accepted: 10 February 2025;
Published: 05 March 2025.
Edited by:
Ravindra Kumar, Washington University in St. Louis, United StatesReviewed by:
Ajeet Kumar, Washington University in St. Louis, United StatesCopyright © 2025 Wu, Teng, Xie, Xing and Zhi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiawen Wu, d3VqaWF3ZW5AaHR1LmVkdS5jbg==; Shuangtao Xing, eGluZ3NodWFuZ3Rhb0BodHUuY24=; Songsong Zhi, MTkwMzE4MzA1N0BzdHUuaHR1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.