
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 03 April 2025
Sec. Neurocognitive Aging and Behavior
Volume 17 - 2025 | https://doi.org/10.3389/fnagi.2025.1524474
This article is part of the Research TopicEstrogens and Neurodegeneration: a Link Between Menopause and Alzheimer’s Diseases in WomenView all articles
 Amber Watts1,2*
Amber Watts1,2* Shannon Donofry3,4
Shannon Donofry3,4 Hayley Ripperger4
Hayley Ripperger4 Nicole M. Eklund5
Nicole M. Eklund5 Lu Wan6
Lu Wan6 Chaeryon Kang7
Chaeryon Kang7 George Grove4
George Grove4 Lauren E. Oberlin6,8
Lauren E. Oberlin6,8 Swathi Gujral7
Swathi Gujral7 Eric D. Vidoni1
Eric D. Vidoni1 Jeffrey M. Burns1
Jeffrey M. Burns1 Edward McAuley9,10
Edward McAuley9,10 Charles H. Hillman11
Charles H. Hillman11 Arthur F. Kramer10,11
Arthur F. Kramer10,11 M. Ilyas Kamboh12
M. Ilyas Kamboh12 Kirk I. Erickson4,6
Kirk I. Erickson4,6Introduction: Disruptions in estrogen exposure (i.e., surgically induced menopause) have been linked to poorer cognitive aging and dementia risk. Hormone therapy use (e.g., birth control, menopausal hormone therapy) has shown mixed associations with cognitive performance, possibly due to limited cognitive test batteries. To address previous inconsistencies, we investigated baseline data from Investigating Gains in Neurocognition in an Intervention Trial of Exercise (IGNITE). We hypothesized that (1) oophorectomy prior to natural menopause would be associated with poorer cognitive performance, (2) timing and duration of birth control and menopausal hormone therapy would influence associations with cognitive performance, and (3) APOE4 carrier status would interact with oophorectomy and hormone therapy to influence cognitive performance.
Methods: In 461 post-menopausal females (M age = 69.6) we assessed oophorectomy and hormone therapy use to examine associations with the Montreal Cognitive Assessment (MoCA) and factor-analytically derived composite scores for episodic memory, processing speed, working memory, executive function/attentional control, and visuospatial processing.
Results: Hypothesis (1) We did not observe associations between oophorectomy prior to natural menopause and poorer cognitive performance. However, hormone therapy use, started on average within 2 years of oophorectomy, was associated with better episodic memory (β = 0.106, p = 0.02), working memory (β = 0.120, p = 0.005), and visuospatial processing (β = 0.095, p = 0.03). Hypothesis (2) Birth control use was associated with better performance on the MoCA (β = 0.093, p = 0.04), working memory (β = 0.102, p = 0.02), and executive function/attentional control (β = 0.103, p = 0.02). However, duration and timing of birth control and menopausal hormone therapy were not associated with cognitive performance. Hypothesis (3) We did not observe significant interactions between APOE4 status and oophorectomy or hormone therapy in their associations with cognitive performance.
Discussion: Our results suggest exposure to estrogen during adulthood, specifically birth control and hormone therapy among women undergoing pre-menopausal oophorectomy, benefits cognitive function in older adulthood. Our comprehensive cognitive battery allowed us to examine cognitive function with a high degree of granularity. Future work should evaluate causal mechanisms of associations between lifetime estrogen exposure and later life cognitive function.
Greater lifetime exposure to estrogen, a neuroprotectant, is associated with a reduced risk of age-related cognitive decline and dementia (Arevalo et al., 2015; Ryan et al., 2009). In particular, 17β-estradiol is involved in neural plasticity, adult neurogenesis, and signaling with other neuroprotective factors such as brain derived neurotrophic factor and insulin-like growth factor-1 (Arevalo et al., 2015). Assessment of brain estrogen in living humans is very rare, as it is costly and complex (Mosconi et al., 2024). A more readily measurable indicator of lifetime estrogen exposure is reported use of medications that contain hormones, particularly estradiol, including menopausal hormone therapies. Research indicates that lifetime estrogen exposure is also reflected in characteristics of reproductive and menopausal history. Several of these characteristics, including pregnancy history and reproductive surgeries have been associated with cognitive function across the lifespan, including in older adulthood (Karim et al., 2016; Rocca et al., 2021).
Drawing conclusions from the literature about associations between hormone therapy and cognitive performance is challenging for several reasons including the complexity of differing types of therapies (e.g., estrogen only, estrogen plus progesterone) and timing of hormone therapy. A recent systematic review and meta-analysis examining the effects of menopause hormone therapy on cognition in randomized controlled trials concluded that overall there were no effects on cognitive domain scores (Andy et al., 2024). However, three caveats were emphasized. First, estrogen therapy improved global cognition after surgical menopause (bilateral oophorectomy, i.e., surgical removal of both ovaries) compared to placebo. Second, the timing of estrogen therapy initiation matters. Specifically, estrogen initiated near menopause onset was associated with improved verbal memory, while later initiation had no effects. Longer durations of estrogen use were associated with worsening in some cognitive domains including visual memory. This reinforces the “critical window” hypothesis that optimal timing of estrogen therapy exposure is around the time of menopause when estrogen levels are changing, but before other age-related brain changes occur. Finally, the combination of estrogen plus progesterone had positive effects when taken in midlife, near to the age of menopause, but negative effects when taken in later life, often defined as after age 60. Long term use of hormonal contraceptives have also been associated with better global cognitive performance and verbal memory (Karim et al., 2016).
Surgically induced menopause, specifically bilateral oophorectomy, has been repeatedly associated with poorer cognitive function, especially when it occurs early relative to the typical age of menopause onset (Rocca et al., 2021). The suspected rationale for this is the sudden dramatic reduction of estrogen, particularly prior to the natural onset of menopause. This explanation is supported by studies showing that the degree of cognitive risk or decreased function is associated with age at surgical intervention, and subsequent use of estrogen therapy (Bove et al., 2014). The timing and duration of estrogen therapy appear to influence the cognitive benefits (Bove et al., 2014; Hogervorst and Bandelow, 2007). Though less well-established, some studies suggest unilateral oophorectomy and hysterectomy with conservation of ovaries are associated with lower lifetime estrogen exposure, and thus may also confer cognitive risks (Crawford, 2022; Phung et al., 2010).
The apolipoprotein E (APOE) 4 allele is the strongest known genetic risk factor for Alzheimer’s disease and evidence suggests the risk is greater in women than in men (Neu et al., 2017). This vulnerability is dependent on interactions between estrogens and APOE genotype (Valencia-Olvera et al., 2023). Specifically, APOE modulates systemic and neural outcomes of menopause and estrogen-based hormone therapy. A recent study using UK Biobank data reported that younger age at oophorectomy, particularly in APOE4 carriers, conferred higher odds of developing Alzheimer’s disease over a longitudinal follow up (Calvo et al., 2024).
Results of studies of hormone therapy and cognition as it relates to APOE genotype have been mixed, for example, suggesting hormone therapy improved cognitive function and brain volume for APOE4 carriers (Saleh et al., 2023), or conversely that hormone therapy benefitted cognition only in non-APOE4 carriers (Yaffe et al., 2000). One possible explanation for these contradictory findings is that timing of estrogen exposure matters for understanding effects on cognitive function and potential interactions with APOE genotype. In the study reporting greater benefits of estrogen therapy for APOE4 carriers (ages 50+), younger age of estrogen therapy initiation was associated with greater brain volumes cross-sectionally (Saleh et al., 2023). Whereas, in the study reporting cognitive benefits in non-APOE4 carriers, current hormone use in postmenopausal women (ages 65+) was associated with less longitudinal decline on a dementia screening tool (Yaffe et al., 2000).
Given these complexities and the inconsistent findings documented in prior literature, organizations such as The Menopause Society and the Women’s Alzheimer’s Movement have called for additional research to elucidate menopause and hormone-related mechanisms for cognitive decline and dementia. There is also broad recognition that samples with greater demographic (e.g., racial) heterogeneity are needed to maximize the generalizability of findings. Thus, the present study aimed to address some of these mixed findings in a large and racially diverse sample of cognitively well-characterized women, that includes measures of menopausal history and APOE genotype. Specifically, we evaluated the following hypotheses: (1) surgically induced menopause (i.e., oophorectomy) will be associated with poorer cognitive performance and depend on timing of surgical intervention, (2) the timing and duration of menopausal hormone therapy and hormone-based birth control will influence associations with cognitive performance, and (3) APOE4 carriers with a history of pre-menopausal oophorectomy will have poorer cognitive performance, while APOE4 carriers exposed to hormone therapy will have better cognitive performance.
The IGNITE study (Investigating Gains in Neurocognition in an Intervention Trial of Exercise: NCT02875301, R01AG053952) was a multi-center (Pittsburgh, Boston, Kansas City) randomized clinical trial that aimed to examine whether a 12 months aerobic exercise intervention would improve cognitive performance and neuroimaging markers of brain health in sedentary, cognitively unimpaired older adults in a dose-dependent manner. The present study is a secondary analysis of baseline data from the IGNITE study.
Recruitment strategies targeted community samples through newspapers, health system research registries, direct mailings, senior centers and churches, and online media, and have been previously described (Vidoni et al., 2021). Participants were enrolled on a rolling basis between 2017 and 2022 with recruitment of racially and ethnically underrepresented participants proportional to the demographic characteristics of each study site. Female participants made up 71.1% of the total study sample. The present study includes data from only female participants (N = 461). Detailed study inclusion criteria are published elsewhere (Erickson et al., 2019). Briefly, participants were required to be 65–80 years old, relatively physically inactive (i.e., exercise less than 3 days per week, and exercise less than 20 min per day on days when they do exercise), within a normal range on cognitive assessments within broad limits, and able to safely engage in moderate intensity exercise.
Female participants completed a questionnaire asking about menopause and reproductive history. This included self-reported information about whether participants experienced natural menopause, and whether they had undergone hysterectomy and/or oophorectomy. Participants reported whether they had ever used birth control or hormone therapy, and if they had used hormone therapy, whether it was used in the last 3 months. Participants reported current medications which we classified according to the Anatomical Therapeutic Chemical (ATC) system and the Defined Daily Dose (DDD) as a unit of measure according to the World Health Organization methodology. In the present study, we included medications classified as G3, indicating that they contain “sex hormones and modulators of the genital system” such as estradiol. Some participants who reported using a hormone-based medication did not report the medication type, but these were still included.
Using three pieces of information (self-reported natural menopause, hysterectomy, oophorectomy), we categorized participants into categories: natural menopause (cessation of menses for 12+ months without removal of ovaries or uterus), oophorectomy (defined in our study as removal of ovaries with or without hysterectomy), hysterectomy without oophorectomy (removal of uterus without removal of ovaries, menstrual bleeding ceased and ability to report continued ovulation absent), and unclear (no report of hysterectomy or oophorectomy, natural menopause is uncertain).
Of note, self-reports are likely based on observation of bleeding as other indicators of menstruation are more difficult to observe. There are other causes of vaginal bleeding besides menstruation (e.g., fibroids). Participants are unlikely to have experienced reproductive surgery without being able to report it, but it may occasionally be unclear what procedures occurred and for what reasons. Thus, we observed several sources of potential error related to self-report in determining menopause type which are described in Appendix 1. Lord et al. (2009) support the use of self-report measures for indicators of lifelong estrogen exposure.
Participants completed a comprehensive cognitive evaluation consisting of the Montreal Cognitive Assessment (MoCA) and measures of processing speed, working memory, episodic memory, executive function/attentional control, and visuospatial processing. A full list of the tests is presented in Table 1. Performance in each domain was measured using previously established latent factors derived from a confirmatory factor analysis (Oberlin et al., 2025), with higher values reflecting better performance. Baseline assessment visits lasted approximately 4 h and were administered by annually certified psychometricians and split across 2 days of testing to prevent fatigue and frustration. The test battery included neuropsychological tests for the purposes of adjudication of cognitive status and for comprehensive assessment of cognitive function. Only cognitively unimpaired participants were included.
The common APOE polymorphism has three alleles (APOE2, APOE3, APOE4). Carriers of the APOE4 allele have an increased risk of Alzheimer’s disease, with homozygous (4/4 genotype) having the greatest elevation in risk (Yamazaki et al., 2019). Genotypes for the two APOE SNPs resulting in six genotypes was performed on DNA samples using TaqMan assays (Fan et al., 2023). Participants with at least one APOE4 allele (2/4, 3/4, 4/4) were classified as APOE4 carriers.
Age, socioeconomic status (SES), and years of education were determined by participant self-report. A composite score reflecting SES was generated from measures of income, savings, debt-adjusted savings, and financial stability from the MacArthur Socioeconomic Status Index (Seeman et al., 2004). Description of the creation of this composite score can be found in pre-print form at the following link https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5062727. Age, SES, and years of education were included as covariates in our analyses.
We conducted descriptive analysis to represent proportions of participants in subgroups (e.g., those with and without oophorectomy). We used chi-squared tests to evaluate whether differences in dichotomous outcomes (i.e., taking a medication) between groups (i.e., oophorectomy) were greater than expected due to chance. We reported the X2 value, the degrees of freedom, and the p-value. We conducted a series of multiple linear regression models to estimate the relationships between reproductive history variables (i.e., oophorectomy, birth control use, menopausal hormone therapy) and cognitive performance accounting for covariates including age, years of education, APOE4 carriage, and SES. We estimated change in R2 to indicate the proportion of variance in the outcome attributable to significant predictors of interest. We used the Benjamini-Hochberg correction with a false discovery rate (FDR) of 0.05 to correct for inflation of type 1 error associated with multiple testing (Benjamini and Hochberg, 1995). Specifically, for each hypothesis, we estimated corrected p-values for the effect of all variables in the model (i.e., predictors and covariates) across six outcome variables (five cognitive domains and the MoCA). We chose Benjamini-Hochberg as our method for correction because it has been shown to preserve statistical power substantially and be less punitive than traditional family wise error correction methods (Benjamini and Hochberg, 1995). To examine possible moderating effects, we included interaction terms in the linear regression analyses (i.e., hormone therapy use x APOE carrier status). We examined multicollinear tolerance indices in linear regression models. The hypotheses were tested according to the following multiple linear regression equations:
Hypothesis 1: Cognitive Test Score = β o + β1age1 + β2education2 + β3SES3 + β4APOE44 + β5oophorectomy5 + β6hormone therapy use6 + ε
Hypothesis 2a: Cognitive Test Score = βo + β1age1 + β2education2 + β3SES3 + β4APOE44 + β5birthcontrol5 + ε
Hypothesis 2b: Cognitive Test Score = βo + β1age1 + β2education2 + β3SES3 + β4APOE44 + β5hormone therapy5 + β6duration of hormone therapy6 + β7age started hormone therapy7 + β8menopause age minus hormone therapy start age8 + β9birth control9 + ε
Hypothesis 3a: Cognitive Test Score = βo + β1age1 + β2education2 + β3SES3 + β4APOE44 + β5oophorectomy5 + β6oophorectomy * APOE4interaction6 + ε
Hypothesis 3b: Cognitive Test Score = βo + β1age1 + β2education2 + β3SES3 + β4APOE44 + β5birth control5 + β6birth control*APOE4interaction6 + ε
Hypothesis 3c: Cognitive Test Score = βo + β1age1 + β2education2 + β3SES3 + β4APOE44 + β5hormone therapy5 + β6hormone therapy*APOE4interaction6 + ε
The present study included N = 461 female participants from the IGNITE study between the ages of 65 and 80 years. Demographic characteristics of the sample are presented in Table 2. Based on self-report data in response to survey items regarding menopause experiences, we categorized female participants into four categories of menopause type (Table 2).
We hypothesized that surgically induced menopause (i.e., oophorectomy) prior to natural menopause would be associated with poorer cognitive performance. In our sample, 107 participants (23.2% of females) reported undergoing oophorectomy, with 73 participants (15.8%) undergoing oophorectomy prior to natural menopause, at an average age of 40.18 (SD = 8.7). Of women who underwent oophorectomy prior to natural menopause, 46 (63.0%) reported using hormone therapy, including vaginal creams, at some point in their lives, with 70.2% of those starting within 2 years of the oophorectomy, and lasting an average duration of 7.8 years (SD = 12.2). The average age of staring hormone therapy for this group was 44.0 (SD = 6.5). Participants who underwent oophorectomy prior to menopause were significantly more likely to report taking a G3 class medication [X2 (df) = 9.1 (1), p = 0.003]. Adjusting for age, education, SES, APOE4 carrier status, and hormone therapy use, oophorectomy prior to natural menopause was not significantly associated with cognitive performance in any domain (Table 3). Among this group with pre-menopausal oophorectomy, hormone therapy use was associated with significantly better performance on tests of episodic memory (r2 change = 0.010), working memory (r2 change = 0.013), and visuospatial processing (r2 change = 0.006).
We hypothesized that timing and duration of birth control and menopausal hormone therapy would be associated with cognitive performance. More than three quarters of women in our sample reported a history of birth control use (n = 351, 76.6%). The average age of starting birth control was 21.7 (SD = 4.7), with an average duration of 6.4 years (SD = 7.0). These analyses were conducted in all women in the sample, regardless of oophorectomy status. Adjusting for age, education, SES, and APOE4 carrier status, (Table 4) birth control use was significantly associated with better performance on the MoCA (r2 change = 0.008), and tests of working memory (r2 change = 0.01), and executive function/attentional control (r2 change = 0.010). In follow up models of those who reported birth control use, neither duration of birth control use (p range 0.244–0.848), nor age at time of starting birth control was associated with cognitive performance in any domain (p range 0.12–0.70), adjusting for age, education, SES, and APOE4 carrier status.
While 75.3% (n = 347) reported ever experiencing hot flashes or night sweats, only 39.5% (n = 182) reported a history of hormone therapy use. Of those reporting menopausal hormone therapy use, only 9.0% (n = 41) reported use in the last 3 months. Excluding individuals who underwent oophorectomy prior to natural menopause, the average age of reported natural menopause was 51.3 years (SD = 5.3). Among those who underwent natural menopause and reported hormone therapy use, the average starting age was 48.4 (SD = 6.9), with an average duration of 2.7 years (SD = 6.4). The average discrepancy between age at naturally occurring menopause and age at start of menopausal hormone therapy was 2.2 years prior to age at natural menopause (SD = 7.2).
Including only women who did not have oophorectomy prior to natural menopause, we estimated the effect of hormone therapy use adjusting for duration of use, age at starting hormone therapy, and distance between natural menopause and age at starting hormone therapy (Table 5). These models adjusted for age, education, SES, APOE4 carrier status, and use of birth control medications. None of the variables related to hormone therapy duration or timing were significantly associated with processing speed, working memory, or executive function/attentional control. For the MoCA and episodic memory, associations of hormone therapy use or duration between hormone therapy start and menopause, were no longer significant after adjustment for type 1 error using the False Discovery Rate procedure (Benjamini and Hochberg, 1995). For visuospatial processing, duration of hormone therapy use was no longer associated with performance in this domain after correction for type 1 error using the False Discovery Rate procedure (Benjamini and Hochberg, 1995).
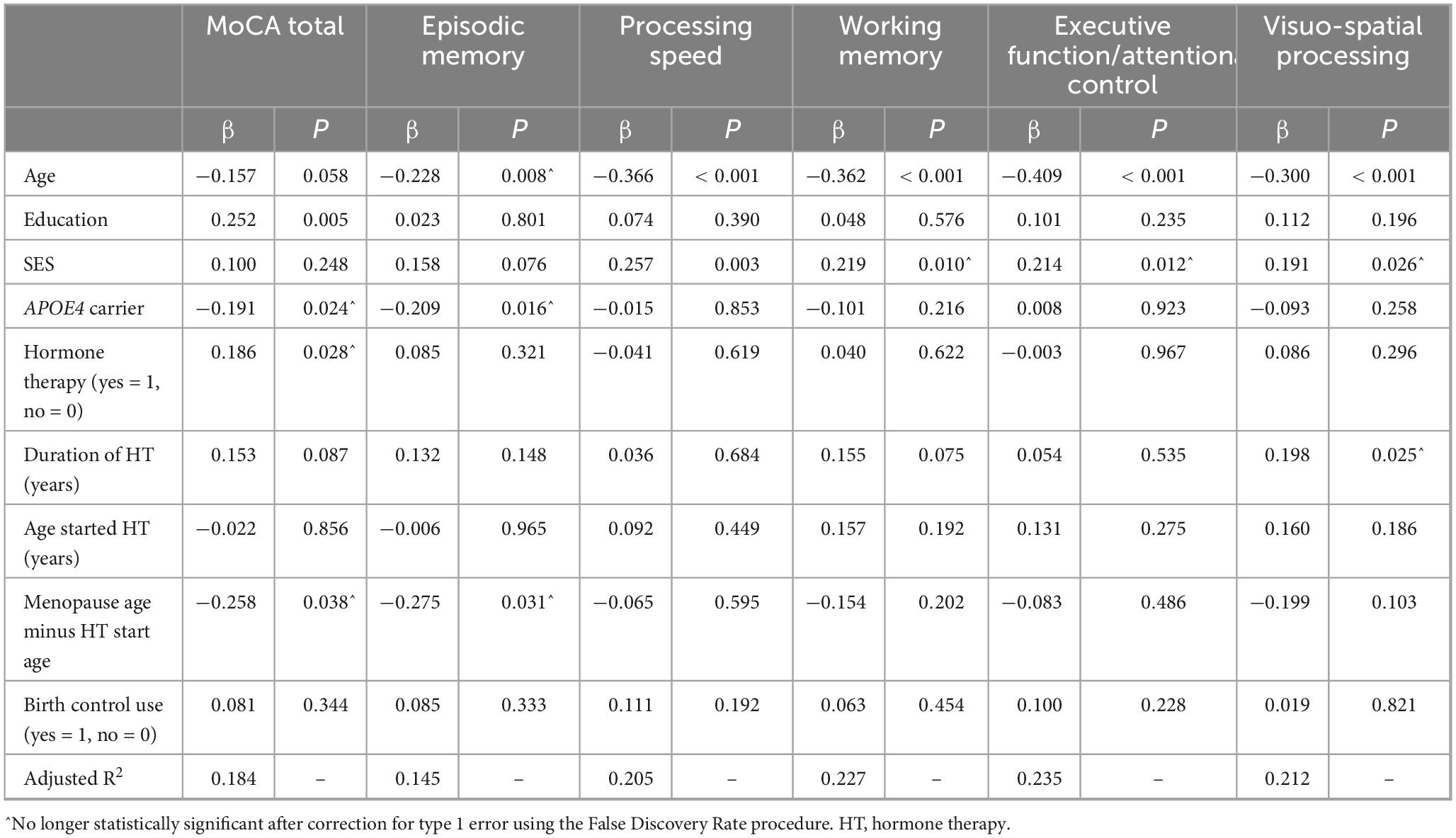
Table 5. Association of hormone therapy with cognitive performance, adjusting for covariates, excluding individuals undergoing oophorectomy prior to natural menopause.
We hypothesized that APOE4 carriers would differ in their associations between oophorectomy, hormone therapy, and cognitive performance. In our sample, 28.4% of women carried one or more APOE4 alleles. Independent samples t-tests indicated that APOE4 carriers differed from non-APOE4 carriers on their mean performance on MoCA and the episodic memory domain [MoCA t (df) = 2.98 (453), p = 0.003, episodic memory t (df) = 2.45 (453), p = 0.015]. We did not observe mean differences in performance for any of the other cognitive domains (ps range from 0.142 to 0.419). The interaction between APOE4 carrier status and oophorectomy did not reach the level of statistical significance for any of the cognitive outcomes, adjusting for covariates (MoCA β = −0.077, p = 0.202; episodic memory β = −0.067, p = 0.275; processing speed β = −0.077, p = 0.191; working memory β = −0.085, p = 0.140; executive function/attentional control β = −0.082, p = 0.158; visuospatial β = −0.080, p = 0.168).
The interaction between APOE4 carrier status and birth control use was not significant for any of the cognitive outcomes adjusting for covariates (MoCA β = −0.167, p = 0.112; episodic memory β = −0.154, p = 0.144; processing speed β = 0.131, p = 0.196; working memory β = 0.003, p = 0.974; executive function/attentional control β = 0.123, p = 0.219; visuospatial β = −0.075, p = 0.457).
The interaction between APOE4 carrier status and hormone therapy use was not significant for any of the cognitive outcomes adjusting for covariates (MoCA β = −0.099, p = 0.130; episodic memory β = −0.122, p = 0.062; processing speed β = −0.055, p = 0.385; working memory β = −0.056, p = 0.368; executive function/attentional control β = −0.031, p = 0.617; visuospatial β = −0.062, p = 0.320).
Our study demonstrates that estrogen exposure in young adulthood, in the form of birth control, and hormone therapy in women undergoing oophorectomy before natural menopause are both associated with better cognitive function post-menopause. Further, the effects were demonstrated across multiple cognitive domains, not simply the verbal memory domain commonly assessed because of its role in detection of Alzheimer’s disease.
Our results suggest that oophorectomy prior to natural menopause was not associated with worse performance in any cognitive domain, when accounting for use of hormone therapy. Although the null finding contradicts our original hypothesis, the timing of surgery and hormone therapy relative to natural menopause onset provide a reasonable explanation that is consistent with the literature. That is, among women who underwent oophorectomy, use of hormone therapy was a significant predictor of better cognitive function in multiple domains, though effect sizes were small. In our sample, the majority of the women who underwent oophorectomy reported use of hormone therapy within 2 years before or after the surgery. Thus, any negative impact of oophorectomy on cognition may have been mitigated by hormone therapy use (Crawford, 2022; Phung et al., 2010; Rocca et al., 2014, 2021). The timing of oophorectomy relative to natural menopause has been recognized to be important for any association with cognitive outcomes. The recent meta-analysis by Andy et al. (2024) suggested that hormone therapy was most beneficial for women who had undergone surgically induced menopause, particularly at a young age. The average woman in our sample undergoing oophorectomy did so at an age relatively close to the natural age of menopause (46 vs. 51) and more than half were treated with hormone therapy at the same age. As such, our sample of individuals who underwent oophorectomy may have been at lower risk of poor cognitive effects that have been associated with this procedure compared to those who undergo oophorectomy at an earlier age, or without hormone therapy after the procedure. Because we were unable to distinguish between unilateral and bilateral oophorectomy, it is possible that some proportion of individuals in the sample underwent a unilateral oophorectomy and may therefore have continued to be exposed to estrogen produced by the remaining ovary.
Birth control medication use, with an average age of onset of 22, was associated with better performance on the MoCA, tests of working memory, and executive function/attentional control in older adulthood, though duration of use or age of onset of use were not significantly associated with performance. This is consistent with previous reports (Karim et al., 2016) associating use of hormonal contraceptives with cognitive benefits and in support of the hypothesis that greater young adult estrogen exposure has a positive effect on later life cognitive function (Matyi et al., 2019; Ryan et al., 2009). In contrast, after adjustment for covariates and correction for type 1 error, menopausal hormone therapy use was not associated with cognitive performance in our sample. This is consistent with a recent meta-analysis showing that, overall, hormone therapy is not associated with global cognitive performance (Andy et al., 2024). In contrast with prior literature, we did not find strong evidence that timing and duration of hormonal menopause therapy influenced the association with cognitive performance in our sample. We note that our ability to estimate the timing of hormone therapy use was complicated by lack of detailed participant recall of hormone formulations, dosage, routes of administration, and timing. Lord et al. (2009) warn that self-report measures of estrogen exposure are biased by poorer recall of events further in the past. Furthermore, participant reporting of natural menopause versus surgically induced menopause indicated a lack of clear understanding of the physiology of menopause (e.g., reporting both oophorectomy prior to the typical age of menopausal onset and natural menopause). Research should continue to address challenges in recall bias, perhaps by using more detailed medical record data. The Stages of Reproductive Aging Workshop (STRAW) (Harlow et al., 2012) developed guidelines for assessing reproductive staging that is recommended for standardization across studies. As a tertiary outcome in the IGNITE study, STRAW staging was not available for our sample. Anecdotal evidence from women’s health care providers suggests that current medical records documentation of menopause and its treatment is poor and agreement between physician and self-reported characterization of menopause is weak. Future studies could contribute to better understanding and utilizing medical records documentation for the purposes of studying and treating menopause symptoms.
Finally, we hypothesized that APOE4 carrier status would interact with indicators of estrogen exposure on cognitive performance. In our sample, APOE4 carriers consistently had worse performance on the dementia screener (MoCA) and the episodic memory domain scores across analyses, which is consistent with the body of evidence tying APOE4 genotype to Alzheimer’s pathological processes and patterns of cognitive performance that are consistent with memory declines (El Haj et al., 2016). We did not find any significant interactions between APOE4 carrier status and oophorectomy, use of birth control, or hormone therapy that influenced cognitive performance scores. One possible explanation for a lack of findings is that effect sizes for genotypes on cognitive performance are notably small (Chabris et al., 2015) and our analyses were powered for medium effect sizes. This is sometimes called “the fourth law of behavior genetics” which describes that complex human behaviors are associated with many genetic variants, each of which accounts for a very small percentage of variability in the behavior. Findings of prior studies on the relationship between hormone therapy and cognition as it relates to APOE genotype have been mixed (Saleh et al., 2023; Yaffe et al., 2000) and we were unable to further elucidate these conflicting findings. Some possible explanations for this include differences in study sample and study design. Specifically, the women in the Saleh et al. study differed from our study in that they were identified as being at increased risk for dementia. The Yaffe study was longitudinal, while our study was cross-sectional.
Our study has notable limitations. The association between birth control and cognitive performance might be confounded by other unmeasured variables, for example complications of pregnancy. Pre-eclampsia during pregnancy has been associated with poorer late life cognitive outcomes (Fields et al., 2017). As the primary aims of the study were unrelated to reproductive history, we were unable to measure pregnancy history, menopause history, or hormone therapy history in greater detail. Another key limitation is the cognitively unimpaired sample who were required to be in physical condition to exercise safely and have no contraindications to MRI to be enrolled in the study. Thus, we are unable to draw conclusions about the relationships under investigation among participants at greater risk for cognitive decline or dementia. The cross-sectional, retrospective self-report of the timing of hormone therapy limits our ability to accurately evaluate potential non-linear effects of age and timing of hormone therapy in its effects on subsequent cognitive performance, which could have obscured our ability to detect effects. Furthermore, the cross-sectional nature of the study in late adulthood limits our ability to draw conclusions about causality, mechanisms occurring in midlife, or long-term impacts of earlier life hormone exposure. Finally, we did not have adequate statistical power to compare heterozygous to homozygous APOE4 carriers nor to add all possible confounding factors such as measures of diet or physical activity, though the role of these factors should be explored in future research.
Our study uniquely adds comprehensive and robust assessment of a wide array of cognitive domains rather than single tests or limited to the verbal memory domain, which is most typically assessed in prior research studies. Future directions might include creation of a risk score to combine various indicators of lifetime estrogen exposure, such as presence, duration, dosage, since each alone accounts for only a small amount of variance in the overall contribution to late life cognition, a very distal outcome. Additional investigation of contributions of lifetime estrogen exposure to change in cognition across the lifespan would add to our understanding of female-specific developmental implications related to stages including puberty, pregnancy, reproductive surgery, and menopause. It would be particularly beneficial to conduct this research in a more representative samples of women from varying racial and ethnic, sociocultural, geographic and economic backgrounds to ensure generalizability of findings. Imaging studies can help to link brain structure and function to our findings with cognitive assessment. For example, estrogen modulation of hippocampal function is likely play a role in memory consolidation (Frick et al., 2010). Innovations in the accurate assessment of reproductive and menstrual history would also greatly strengthen our ability to understand these lifelong associations. Finally, further exploration of causal mechanisms by which lifetime estrogen exposure may influence cognitive and brain function are needed and may include mitochondrial function, sarcopenia, and gut-brain axis (Jiang et al., 2019; Zhang et al., 2024).
The original contributions presented in the study are publicly available. This data can be found here: DOI 10.17605/OSF.IO/AEZJ8.
The studies involving humans were approved by Human Research Protection Office at the University of Pittsburgh. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
AW: Conceptualization, Formal Analysis, Writing – original draft, Writing – review and editing. SD: Conceptualization, Writing – review and editing. HR: Writing – review and editing. NE: Investigation, Writing – review and editing. LW: Formal Analysis, Methodology, Writing – review and editing. CK: Formal Analysis, Funding acquisition, Methodology, Writing – review and editing. GG: Writing – review and editing. LO: Formal Analysis, Writing – review and editing. SG: Writing – review and editing. EV: Writing – review and editing, Funding acquisition, Investigation, Methodology. JB: Funding acquisition, Investigation, Methodology, Writing – review and editing. EM: Funding acquisition, Investigation, Methodology, Writing – review and editing. CH: Writing – review and editing, Funding acquisition, Investigation, Methodology. AK: Writing – review and editing, Funding acquisition, Investigation, Methodology. MK: Investigation, Resources, Supervision, Writing – review and editing. KE: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review and editing.
The author(s) declare that financial support was received for the research and/or publication of this article. The IGNITE study was funded by the National Institute on Aging, R01 AG053952, R35 AG072307. Use of REDCap for the project was sponsored by the Clinical and Translational Science Institute at the University of Pittsburgh Grant Number UL1-TR-001857. AW was supported by an NIH grant from NIGMS and OD 1P20GM152280.
We are grateful for the incredible effort and engagement from the participants in the study as well as all the staff and students who helped the team to produce a successful study.
JB received research support from the NIH, research support to conduct clinical trials (paid to institution) from Eli Lilly, Amylyx, Biogen, Eisai, AbbVie, Astra-Zeneca, Roche, and Ionis. Consultant for Renew Research, Eisai, Eli Lilly, Labcorp, Roche, Renew Biotechnologies, Abbvie, Novo Nordisk. JB was also part of the Data Monitoring Committee for Intra-Cellular Therapies, Inc. KE was part of the scientific advisory board for NeoAuvra, Inc., and MedRhythms, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The reviewer CA declared a shared affiliation with the author(s) LO to the handling editor at the time of review.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Andy, C., Nerattini, M., Jett, S., Carlton, C., Zarate, C., Boneu, C., et al. (2024). Systematic review and meta-analysis of the effects of menopause hormone therapy on cognition. Front. Endocrinol. 15:1350318. doi: 10.3389/fendo.2024.1350318
Arevalo, M.-A., Azcoitia, I., and Garcia-Segura, L. M. (2015). The neuroprotective actions of oestradiol and oestrogen receptors. Nat. Rev. Neurosci. 16, 17–29. doi: 10.1038/nrn3856
Benedict, R. H. B., Schretlen, D., Groninger, L., Dobraski, M., and Shpritz, B. (1996). Revision of the brief visuospatial memory test: Studies of normal performance, reliability, and validity. Psychol. Assess. 8, 145–153. doi: 10.1037/1040-3590.8.2.145
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
Bennett, G. K., Seashore, H. G., and Wesman, A. G. (1947). Differential Aptitude Tests. Agra: Psychological Corporation.
Bove, R., Secor, E., Chibnik, L. B., Barnes, L. L., Schneider, J. A., Bennett, D. A., et al. (2014). Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology 82, 222–229.
Brandt, J. (1991). The hopkins verbal learning test: Development of a new memory test with six equivalent forms. Clin. Neuropsychol. 5, 125–142. doi: 10.1080/13854049108403297
Calvo, N., McFall, G. P., Ramana, S., Galper, M., Fuller-Thomson, E., Dixon, R. A., et al. (2024). Associated risk and resilience factors of Alzheimer’s disease in women with early bilateral oophorectomy: Data from the UK Biobank. J. Alzheimer’s Dis. 102, 119–128.
Chabris, C. F., Lee, J. J., Cesarini, D., Benjamin, D. J., and Laibson, D. I. (2015). The fourth law of behavior genetics. Curr. Dir. Psychol. Sci. 24, 304–312.
Crawford, S. L. (2022). Contributions of oophorectomy and other gynecologic surgeries to cognitive decline and dementia. Menopause 29:499. doi: 10.1097/GME.0000000000001991
Drake, J. A., Jakicic, J. M., Rogers, R. J., Aghjayan, S. L., Stillman, C. M., Donofry, S. D., et al. (2022). Reduced brain activity during a working memory task in middle-aged apolipoprotein E ε4 carriers with overweight/obesity. Front. Hum. Neurosci. 16:1001229. doi: 10.3389/fnhum.2022.1001229
El Haj, M., Antoine, P., Amouyel, P., Lambert, J.-C., Pasquier, F., and Kapogiannis, D. (2016). Apolipoprotein E (APOE) ε4 and episodic memory decline in Alzheimer’s disease: A review. Ageing Res. Rev. 27, 15–22.
Erickson, K. I., Grove, G. A., Burns, J. M., Hillman, C. H., Kramer, A. F., McAuley, E., et al. (2019). Investigating gains in neurocognition in an intervention trial of exercise (IGNITE): Protocol. Contemp. Clin. Trials 85:105832.
Erickson, K. I., Voss, M. W., Prakash, R. S., Basak, C., Szabo, A., Chaddock, L., et al. (2011). Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. 108, 3017–3022.
Fan, K., Francis, L., Aslam, M. M., Bedison, M. A., Lawrence, E., Acharya, V., et al. (2023). Investigation of the independent role of a rare APOE variant (L28P; APOE* 4Pittsburgh) in late-onset Alzheimer disease. Neurobiol. Aging 122, 107–111.
Fields, J. A., Garovic, V. D., Mielke, M. M., Kantarci, K., Jayachandran, M., White, W. M., et al. (2017). Preeclampsia and cognitive impairment later in life. Am. J. Obstetr. Gynecol. 217, 74.e1–74.e11.
Frick, K. M., Fernandez, S. M., and Harburger, L. L. (2010). A new approach to understanding the molecular mechanisms through which estrogens affect cognition. Biochim. Biophys. Acta 1800, 1045–1055. doi: 10.1016/j.bbagen.2009.11.004
Harlow, S. D., Gass, M., Hall, J. E., Lobo, R., Maki, P., Rebar, R. W., et al. (2012). Executive summary of the stages of reproductive aging workshop + 10: Addressing the unfinished agenda of staging reproductive aging. Menopause 19, 387–395. doi: 10.1097/gme.0b013e31824d8f40
Hogervorst, E., and Bandelow, S. (2007). Should surgical menopausal women be treated with estrogens to decrease the risk of dementia? Neurology 69, 1070–1071. doi: 10.1212/01.wnl.0000279584.03800.3d
Jiang, Y., Greenwood-Van Meerveld, B., Johnson, A. C., and Travagli, R. A. (2019). Role of estrogen and stress on the brain-gut axis. Am. J. Physiol. Gastrointestinal Liver Physiol. 317, G203–G209. doi: 10.1152/ajpgi.00144.2019
Karim, R., Dang, H., Henderson, V. W., Hodis, H. N., St John, J., Brinton, R. D., et al. (2016). Effect of reproductive history and exogenous hormone use on cognitive function in mid- and late life. J. Am. Geriatr. Soc. 64, 2448–2456. doi: 10.1111/jgs.14658
Lord, C., Duchesne, A., Pruessner, J. C., and Lupien, S. J. (2009). Measuring indices of lifelong estrogen exposure: Self-report reliability. Climacteric 12, 387–394. doi: 10.1080/13697130802664660
Matyi, J. M., Rattinger, G. B., Schwartz, S., Buhusi, M., and Tschanz, J. T. (2019). Lifetime estrogen exposure and cognition in late life: The Cache County Study. Menopause 26, 1366–1374.
Mosconi, L., Nerattini, M., Matthews, D. C., Jett, S., Andy, C., Williams, S., et al. (2024). In vivo brain estrogen receptor density by neuroendocrine aging and relationships with cognition and symptomatology. Sci. Rep. 14:12680. doi: 10.1038/s41598-024-62820-7
Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x
Neu, S. C., Pa, J., Kukull, W., Beekly, D., Kuzma, A., Gangadharan, P., et al. (2017). Apolipoprotein E genotype and sex risk factors for Alzheimer disease: A meta-analysis. JAMA Neurol. 74, 1178–1189.
Oberlin, L., Wan, L., Kang, C., Romano, A., Aghjayan, S. L., and Erickson, K. I. (2025). Cardiorespiratory fitness is associated with cognitive function in late adulthood: Baseline findings from the IGNITE study. Br. J. Sports Med. 59, 167–176. doi: 10.1136/bjsports-2024-108257
Phung, T. K. T., Waltoft, B. L., Laursen, T. M., Settnes, A., Kessing, L. V., Mortensen, P. B., et al. (2010). Hysterectomy, oophorectomy and risk of dementia: A nationwide historical cohort study. Dement. Geriatr. Cogn. Disord. 30, 43–50. doi: 10.1159/000314681
Reitan, R. M. (1958). Validity of the trail making test as an indicator of organic brain damage. Perceptual Motor Skills 8, 271–276. doi: 10.2466/PMS.8.7.271-276
Rocca, W. A., Grossardt, B. R., and Shuster, L. T. (2014). Oophorectomy, estrogen, and dementia: A 2014 update. Mol. Cell. Endocrinol. 389, 7–12.
Rocca, W. A., Lohse, C. M., Smith, C. Y., Fields, J. A., Machulda, M. M., and Mielke, M. M. (2021). Association of premenopausal bilateral oophorectomy with cognitive performance and risk of mild cognitive impairment. JAMA Netw. Open 4:e2131448. doi: 10.1001/jamanetworkopen.2021.31448
Ryan, J., Carrière, I., Scali, J., Ritchie, K., and Ancelin, M.-L. (2009). Life-time estrogen exposure and cognitive functioning in later life. Psychoneuroendocrinology 34, 287–298.
Saleh, R. N. M., Hornberger, M., Ritchie, C. W., and Minihane, A. M. (2023). Hormone replacement therapy is associated with improved cognition and larger brain volumes in at-risk APOE4 women: Results from the European Prevention of Alzheimer’s Disease (EPAD) cohort. Alzheimer’s Res. Therapy 15:10. doi: 10.1186/s13195-022-01121-5
Salthouse, T. A., and Babcock, R. L. (1991). Decomposing adult age differences in working memory. Dev. Psychol. 27, 763–776.
Salthouse, T. A., Fristoe, N., and Rhee, S. H. (1996). How localized are age-related effects on neuropsychological measures? Neuropsychology 10, 272–285.
Seeman, T. E., Crimmins, E., Huang, M.-H., Singer, B., Bucur, A., Gruenewald, T., et al. (2004). Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Soc. Sci. Med. 58, 1985–1997.
Stroop, J. R. (1935). Studies of interference in serial verbal reactions. J. Exp. Psychol. 18, 643–662.
Tulsky, D. S., Chiaravalloti, N. D., Palmer, B. W., and Chelune, G. J. (2003). “The wechsler memory scale: A new perspective,” in Clinical interpretation of the WAIS-III and WMS-III, ed. D. S. Tulsky (Amsterdam: Elsevier), 93–139.
Valencia-Olvera, A. C., Maldonado Weng, J., Christensen, A., LaDu, M. J., and Pike, C. J. (2023). Role of estrogen in women’s Alzheimer’s disease risk as modified by APOE. J. Neuroendocrinol. 35:e13209. doi: 10.1111/jne.13209
Vidoni, E. D., Morris, J. K., Watts, A., Perry, M., Clutton, J., Van Sciver, A., et al. (2021). Effect of aerobic exercise on amyloid accumulation in preclinical Alzheimer’s: A 1-year randomized controlled trial. PLoS One 16:e0244893. doi: 10.1371/journal.pone.0244893
Wechsler, D. (2008). Wechsler Adult Intelligence Scale—Fourth Edition Administration and Scoring Manual, 4th Edn. London: Pearrson.
Yaffe, K., Haan, M., Byers, A., Tangen, C., and Kuller, L. (2000). Estrogen use, APOE, and cognitive decline. Neurology 54, 1949–1954. doi: 10.1212/WNL.54.10.1949
Yamazaki, Y., Zhao, N., Caulfield, T. R., Liu, C.-C., and Bu, G. (2019). Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat. Rev. Neurol. 15, 501–518.
Zelazo, P. D., Anderson, J. E., Richler, J., Wallner-Allen, K., Beaumont, J. L., and Weintraub, S. (2013). II Nih toolbox cognition battery (cb): Measuring executive function and attention. Monogr. Soc. Res. Child Dev. 78, 16–33. doi: 10.1111/mono.12032
Zhang, C., Feng, X., Zhang, X., Chen, Y., Kong, J., and Lou, Y. (2024). Research progress on the correlation between estrogen and estrogen receptor on postmenopausal sarcopenia. Front. Endocrinol. 15:1494972. doi: 10.3389/fendo.2024.1494972
Keywords: cognition, aging, women, estrogen, oophorectomy, menopause
Citation: Watts A, Donofry S, Ripperger H, Eklund NM, Wan L, Kang C, Grove G, Oberlin LE, Gujral S, Vidoni ED, Burns JM, McAuley E, Hillman CH, Kramer AF, Kamboh MI and Erickson KI (2025) Lifetime estrogen exposure and domain-specific cognitive performance: results from the IGNITE study. Front. Aging Neurosci. 17:1524474. doi: 10.3389/fnagi.2025.1524474
Received: 07 November 2024; Accepted: 06 March 2025;
Published: 03 April 2025.
Edited by:
Manuela Leri, University of Florence, ItalyReviewed by:
Hande Karahan, Indiana University Bloomington, United StatesCopyright © 2025 Watts, Donofry, Ripperger, Eklund, Wan, Kang, Grove, Oberlin, Gujral, Vidoni, Burns, McAuley, Hillman, Kramer, Kamboh and Erickson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amber Watts, YW1iZXJ3YXR0c0BrdS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.