- 1Department of Neurology, Qilu Hospital of Shandong University, Jinan, Shandong, China
- 2School of Bioengineering, Shandong Polytechnic, Jinan, Shandong, China
- 3Department of Geriatrics, Qilu Hospital of Shandong University, Jinan, Shandong, China
- 4Department of Neurology, Tianjin Medical University General Hospital, Tianjin, China
- 5Laboratory of Epidemiology, Tianjin Neurological Institute, Tianjin, China
- 6Key Laboratory of Post-Neuroinjury Neuro-Repair and Regeneration in Central Nervous System, Ministry of Education and Tianjin City, Tianjin Neurological Institute, Tianjin, China
Background: Cognitive impairment is a growing public health concern, particularly in aging populations. Obesity, as measured by various indices, has been linked to cognitive decline, but the relationship between Body Roundness Index (BRI) and cognitive impairment remains unclear. This study aims to evaluate the association between BRI and cognitive impairment in a rural, low-income, low-education population in China and to determine if BRI can be used as an independent predictor of cognitive decline.
Methods: This cross-sectional study included the participants aged 35–95 years from rural Tianjin, China. The mean age of the study population was 64.35 ± 7.58 years. Data were collected through face-to-face interviews, physical examinations, and laboratory tests. Cognitive function was assessed using the Mini-Mental State Examination (MMSE), and BRI was calculated and grouped into quartiles. Univariate and multivariate logistic regression analyses were performed to examine the relationship between BRI and cognitive impairment. Subgroup analyses were conducted to explore interactions between BRI, age, gender, and hypertension. The dose–response relationship was analyzed using restricted cubic spline models.
Results: Of the participants, 36.5% had cognitive impairment. Multivariate analysis showed that women, individuals aged 65 and over, and those with hypertension had a higher risk of cognitive impairment. Participants in the second quartile of BRI had a 31% lower risk of cognitive impairment compared to the first quartile (OR: 0.69, 95% CI: 0.51–0.94, p = 0.017). Subgroup analysis revealed that BRI was significantly associated with cognitive impairment in individuals under 65, but not in older participants. The dose–response relationship between BRI and MMSE score showed an inverted U-shaped curve, with the weakest association observed around a BRI of 4.49.
Conclusion: Body Roundness Index, in conjunction with age, gender, and hypertension, can serve as a useful predictor of cognitive impairment, particularly in younger populations. Early identification of individuals at risk through BRI may facilitate timely interventions, reducing the burden of cognitive decline on patients and healthcare systems.
1 Introduction
Cognitive impairment, including dementia, presents a significant global health challenge, with Alzheimer’s disease (AD) being the most common cause of dementia in individuals aged 65 and older. By 2024, approximately 6.9 million Americans aged 65 and above will have Alzheimer’s disease, a figure projected to rise to 13.8 million by 2060 (Tahami Monfared et al., 2022; Alzheimer’s Association, 2024). In China, the incidence of AD among the elderly reached 600.49 per 100,000 in 2019, with an annual growth rate of 1.12% (Li et al., 2024). Neurological disabilities such as AD and other dementias ranked among the top 10 contributors to disability-adjusted life years (DALYs) in 2021(GBD 2021 Nervous System Disorders Collaborators, 2024). The Global Burden of Disease study reported that in 2021, AD and dementia accounted for 1.3% of all health-related losses, resulting in 11.6 million years lost to disability (YLDs), a 45.7% increase over a decade (GBD 2021 Diseases and Injuries Collaborators, 2024). The economic impact is also staggering; it is estimated that the cost of long-term care for dementia patients aged 65 and over will reach $360 billion by 2024 (Alzheimer’s Association, 2024). Given these alarming trends, identifying and mitigating risk factors for cognitive impairment at an early stage is crucial.
Body Roundness Index is a novel anthropometric measurement that better reflects body fat percentage and visceral adipose tissue (Thomas et al., 2013). It also serves as an alternative marker for insulin resistance (IR) (Feng et al., 2019). Obesity, a global epidemic, is projected to affect nearly half of the world’s adult population by 2030 (Neto et al., 2023). China, in particular, faces the highest prevalence of overweight and obese individuals worldwide (Wang et al., 2021). Research has demonstrated that obesity and its related metabolic disturbances—such as oxidative stress, inflammation, and IR—contribute to neuronal damage, brain atrophy, and subsequent cognitive decline (Neto et al., 2023; Dye et al., 2017; Li et al., 2023). For instance, visceral fat area (VFA) has been negatively associated with delayed memory, language scores, and MMSE scores (Moh et al., 2020; Ozato et al., 2021). Additionally, VFA is linked to cognitive decline in men, while subcutaneous fat area (SFA) correlates with cognitive decline in women (Uchida et al., 2024). Intriguingly, greater fat mass and lean body mass are associated with a reduced risk of cognitive impairment in older women (Noh et al., 2017).
Despite these findings, studies specifically investigating the relationship between BRI and cognitive impairment remain scarce. A cross-sectional study in Taiwan suggested a significant inverse relationship between BRI and MMSE scores in individuals over 60 years (Huang et al., 2022).Similarly, a US population study reported a significant negative correlation between BRI levels and cognitive performance in individuals over 65 years of age (Zhang et al., 2024). However, other studies, including those conducted in rural China and Iran, found no significant association between BRI and dementia or cognitive performance (Wang et al., 2023; Ramezani Kashal et al., 2024). These conflicting results, mostly focused on individuals over 60, highlight the need for further investigation. In this context, our study aims to fill this research gap by exploring the relationship between BRI and cognitive function in a younger, low-education population aged 35–95 in rural China.
The primary objective of this study is to assess the association between BRI and cognitive impairment in a socioeconomically disadvantaged, low-education population in rural China, using a population-based cross-sectional design.
2 Methods
2.1 Study population
The cross-sectional study analyzed data from 2,577 individuals collected from 18 rural areas in Tianjin, China, between 2012 and 2020, all of whom had low income and low educational attainment. The data were obtained through the Tianjin Brain Study, an ongoing, prospective, community-based cohort study. Participants were recruited from the local community, and data collection involved face-to-face interviews and physical examinations conducted by trained researchers at local community healthcare centers.
Participants with a history of cerebrovascular diseases, including cerebral infarction, transient ischemic attack, cerebral hemorrhage, and subarachnoid hemorrhage, were excluded to investigate the relationship between BRI and non-vascular cognitive impairment, minimizing confounding effects from vascular cognitive impairment. Additionally, those lacked height and waist circumference data were excluded.
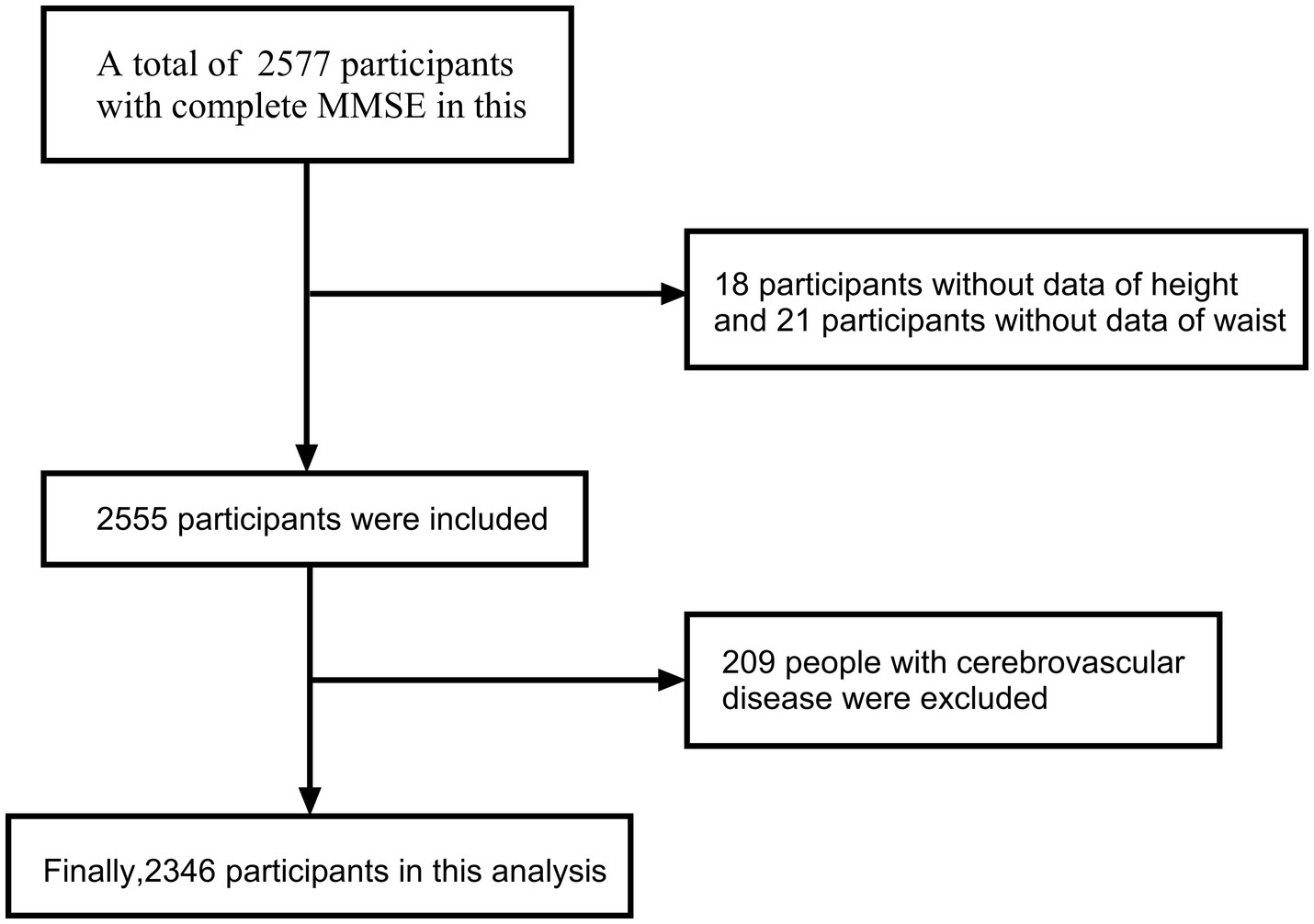
A total of 2,346 participants were included in the final analysis. The detailed selection process is illustrated in the flow chart (Figure 1). The study adhered to the principles of the Declaration of Helsinki and received approval from the Ethics Committee of Tianjin Medical University General Hospital. All participants provided written informed consent before participating in the study.
2.2 Data collection
Demographic and clinical data were gathered through face-to-face interviews conducted by trained researchers. The information collected included participants’ name, gender, age, years of education, and medical histories such as diabetes, hypertension, cerebrovascular disease, and myocardial infarction. Laboratory data, including triglyceride (TG), total cholesterol (TC), fasting blood glucose (FBG), and creatinine levels, were also obtained. Physical measurements were conducted using standardized procedures. Weight was measured with a standard scale, height was recorded with the participant standing upright, and waist circumference (WC) was measured using a soft tape at the midpoint between the iliac crest and the lowest rib. To minimize measurement errors, all measurements were performed by the same researcher.
2.3 Definitions and grouping
Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg, or a self-reported history of hypertension and current use of antihypertensive medication (Mancia et al., 2023). Diabetes was defined according to established criteria: hemoglobin A1c (HbA1c) ≥6.5%, fasting plasma glucose (FPG) ≥126 mg/dL (7.0 mmol/L), or a 2-h plasma glucose level ≥ 200 mg/dL (11.1 mmol/L) during an oral glucose tolerance test (OGTT), or a self-reported history of diabetes or use of hypoglycemic medication (American Diabetes Association Professional Practice Committee, 2024). Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared, and participants were categorized as underweight (<18.5 kg/m2), normal weight (18.5 to <24 kg/m2), overweight (24 to <28 kg/m2), or obese (≥28 kg/m2) (Chen et al., 2023). BRI was calculated using the formula BRI = 364.2–365.5 × √(1 – [WC (cm)/2π]2/[0.5 × height (cm)]2) (Thomas et al., 2013), and participants were classified into quartiles based on BRI values (Q1, Q2, Q3, and Q4).
2.4 Cognitive function assessment
Cognitive function was evaluated using the MMSE, a widely applied tool for assessing cognitive decline (Chun et al., 2021). The MMSE assesses orientation, memory, attention and calculation, recall, and language ability, with scores ranging from 0 to 30. A lower score indicates worse cognitive function. A cohort study conducted in China has shown that MMSE scores are influenced by age, gender, and educational background. The cutoff points for cognitive impairment were set at ≤17 for illiterate participants, ≤20 for participants with 6 or fewer years of education, and ≤24 for participants with more than 6 years of education (Li et al., 2016).
2.5 Statistical analysis
Continuous variables were expressed as means with standard deviations (SD) or as medians with interquartile ranges (IQR) and were compared using Student’s t-test or the Mann–Whitney U-test, depending on the distribution of the data. Categorical variables were presented as frequencies and percentages and were compared using the chi-square test. Multivariate logistic regression analysis was employed to evaluate the association between BRI and other factors related to cognitive impairment. Participants with missing values for any variables included in the multivariate logistic regression analysis were excluded to maintain the validity and reliability of the results. To avoid collinearity, BRI and BRI quartile were included in separate multivariate logistic regression models. β values and their corresponding 95% confidence intervals (CI) were reported for all variables in the multivariate analysis.
Subgroup analyses were performed to further explore these relationships in different populations, based on variables identified in univariate analysis. In the subgroup analysis, multivariate logistic regression models were adjusted for key confounding factors, including age, gender, BMI, hypertension, and other relevant covariates such as diabetes and smoking history. The analysis aimed to explore the association between BRI and cognitive impairment within specific subgroups, controlling for these factors to assess their potential influence. The association between variables and cognitive impairment was expressed as adjusted odds ratios (OR) with 95% confidence intervals (CI). Restricted cubic spline (RCS) curves were used to investigate the dose–response relationship between BRI and cognitive impairment, with the number of spline nodes determined by the Bayesian information criterion (BIC). In the RCS plot, the bold curve represents the estimated regression coefficient, while the shaded area indicates the 95% CI. Statistical significance was defined as p < 0.05. All analyses were performed using SPSS version 27.0.1, and the flow chart and forest plots were created with GraphPad Prism version 10.2.3. R software (v.4.2) was used to generate the RCS curves.
3 Results
3.1 Demographic characteristics
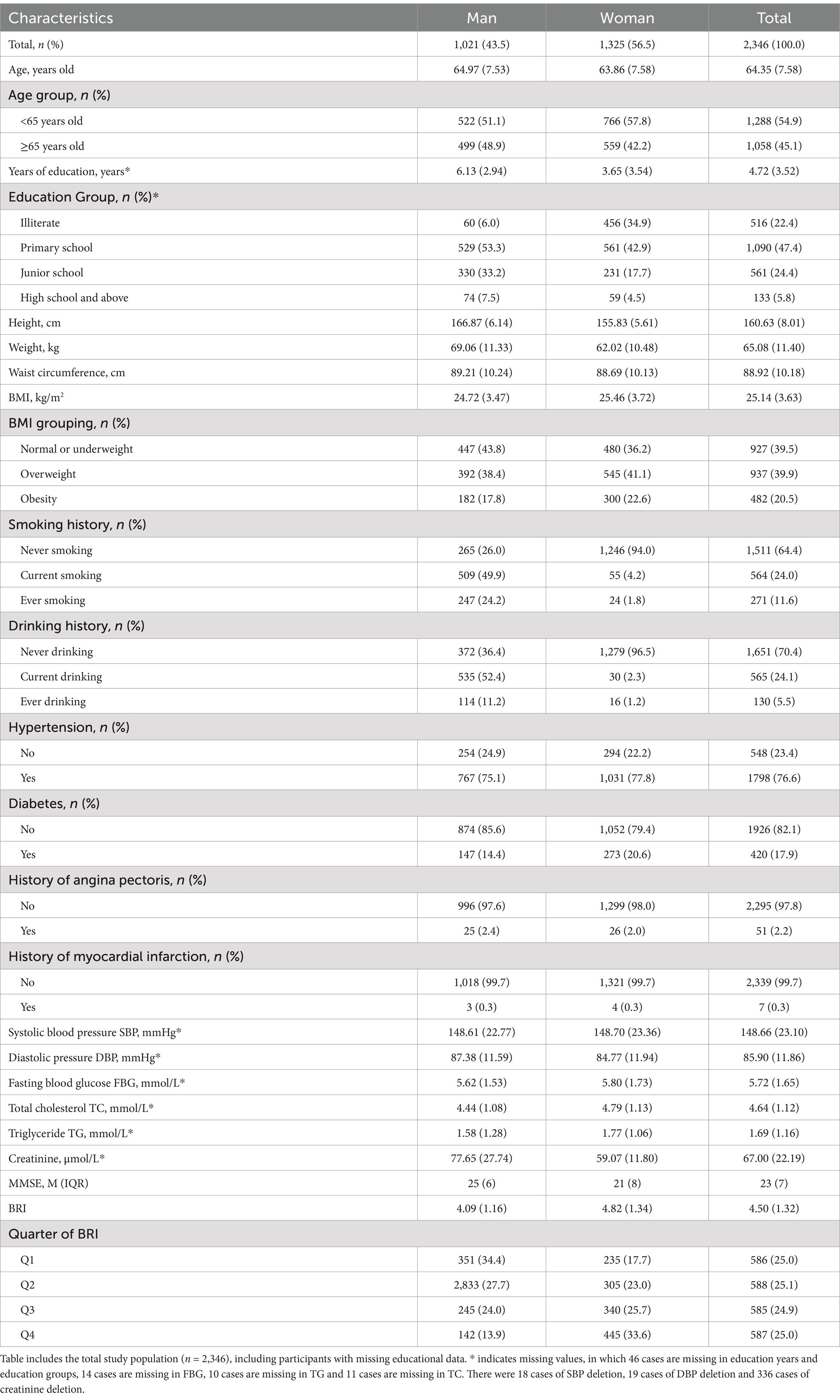
Table 1 includes the total study population (n = 2,346), including participants with missing educational data. Characteristics are presented by gender for a comprehensive overview. A total of 2,346 participants were included in this study, comprising 1,021 males (43.5%) and 1,325 females (56.5%). The mean age of participants was 64.35 ± 7.58 years, with 78% of the population being between 60 and 79 years old. The average values for height, weight, waist circumference, BMI, BRI, and MMSE scores were 160.63 ± 8.01 cm, 65.08 ± 11.40 kg, 88.92 ± 10.18 cm, 25.14 ± 3.63 kg/m2, 4.50 ± 1.32, and 23 (IQR 7), respectively (Table 1).
3.2 Associated factors of cognitive impairment in univariate analysis
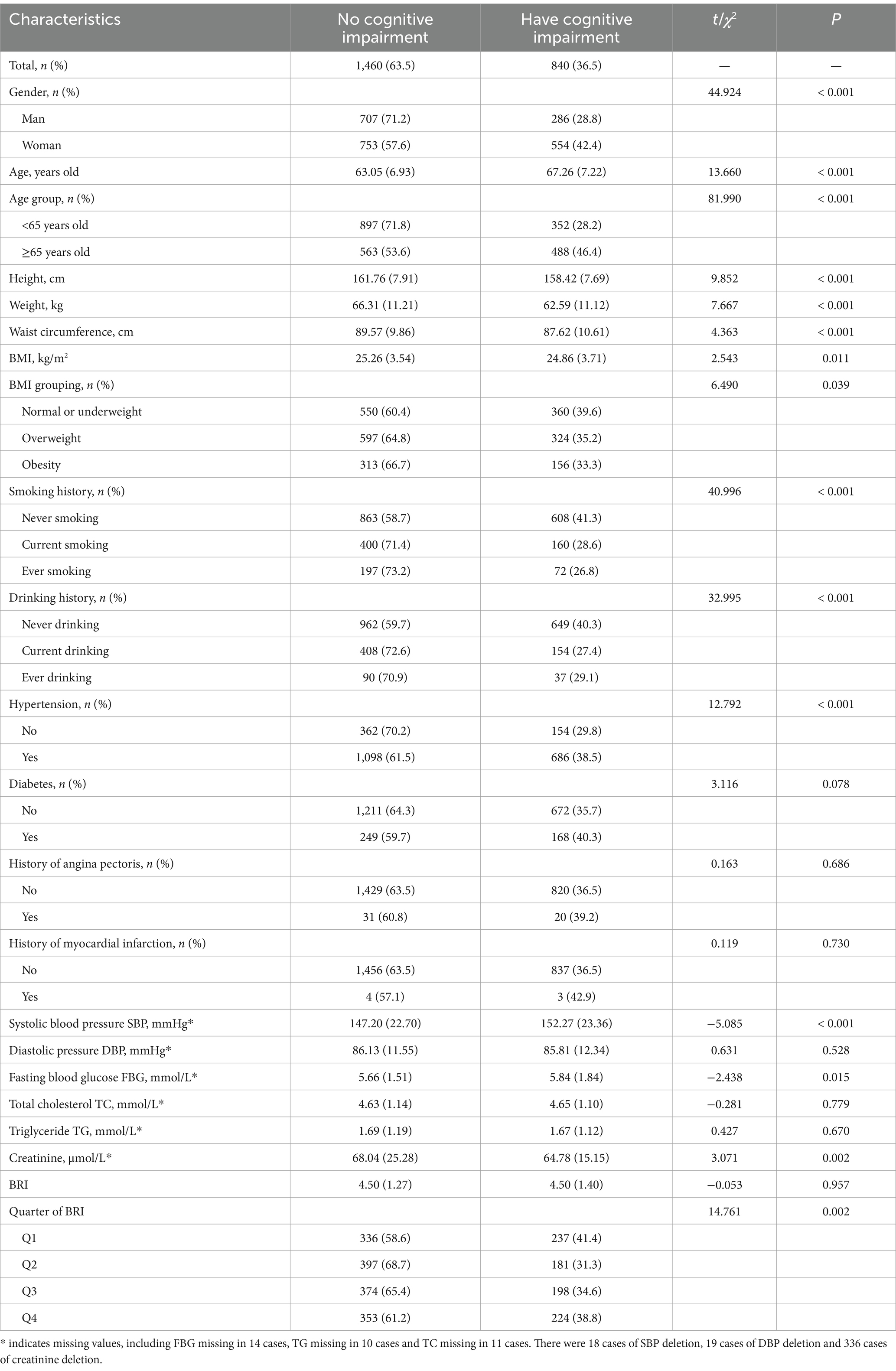
After excluding 46 individuals due to incomplete education data, 2,300 participants were included in the analysis of cognitive impairment risk. Among them, 840 participants (36.5%) were found to have cognitive impairment. Univariate analysis revealed that several factors, including age, sex, height, weight, waist circumference, BMI, smoking history, drinking history, hypertension, SBP, FBG, creatinine, and BRI quartile, were significantly associated with cognitive impairment (p < 0.05) (Table 2).
3.3 Association between BRI and cognitive impairment in multivariate analysis
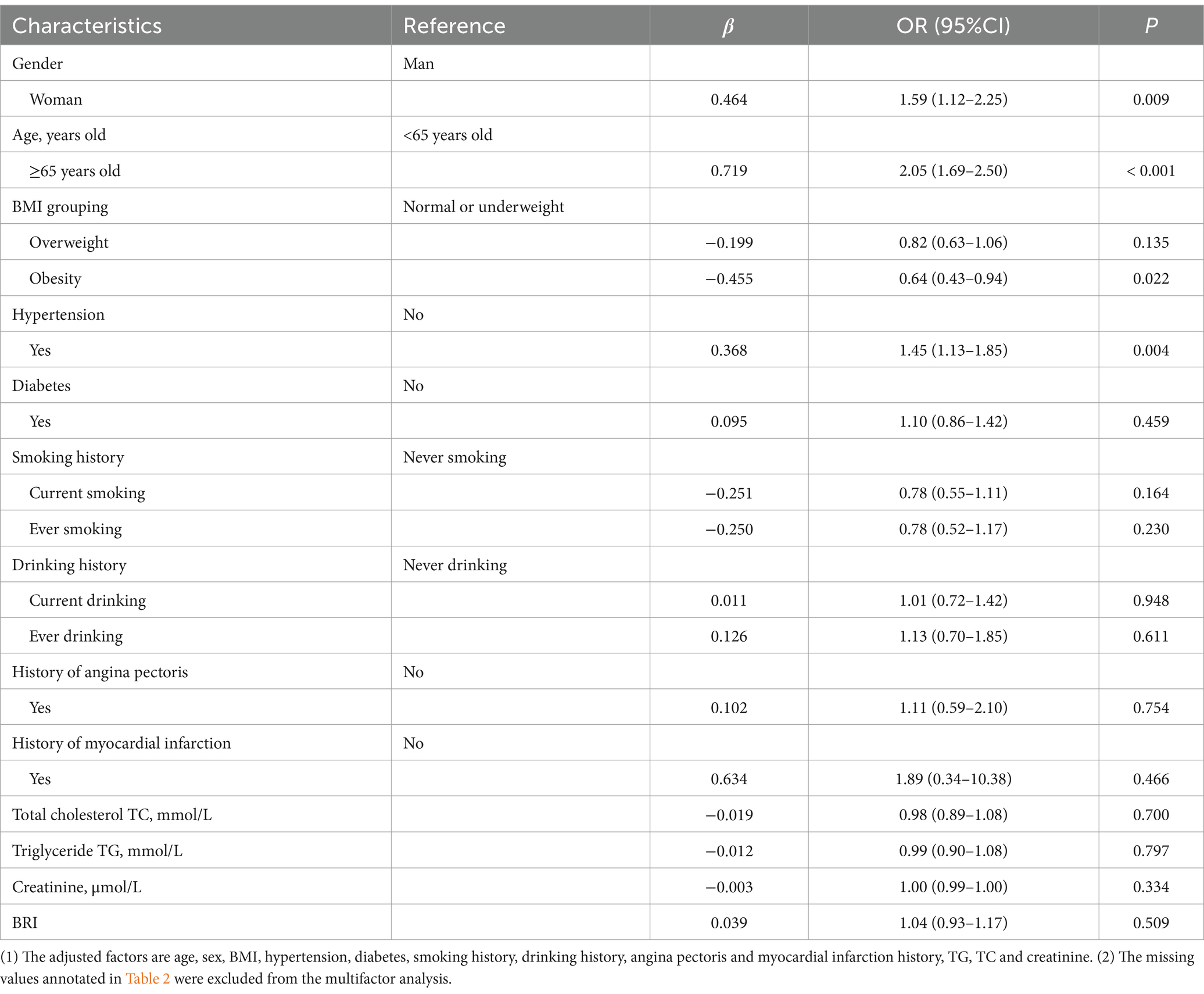
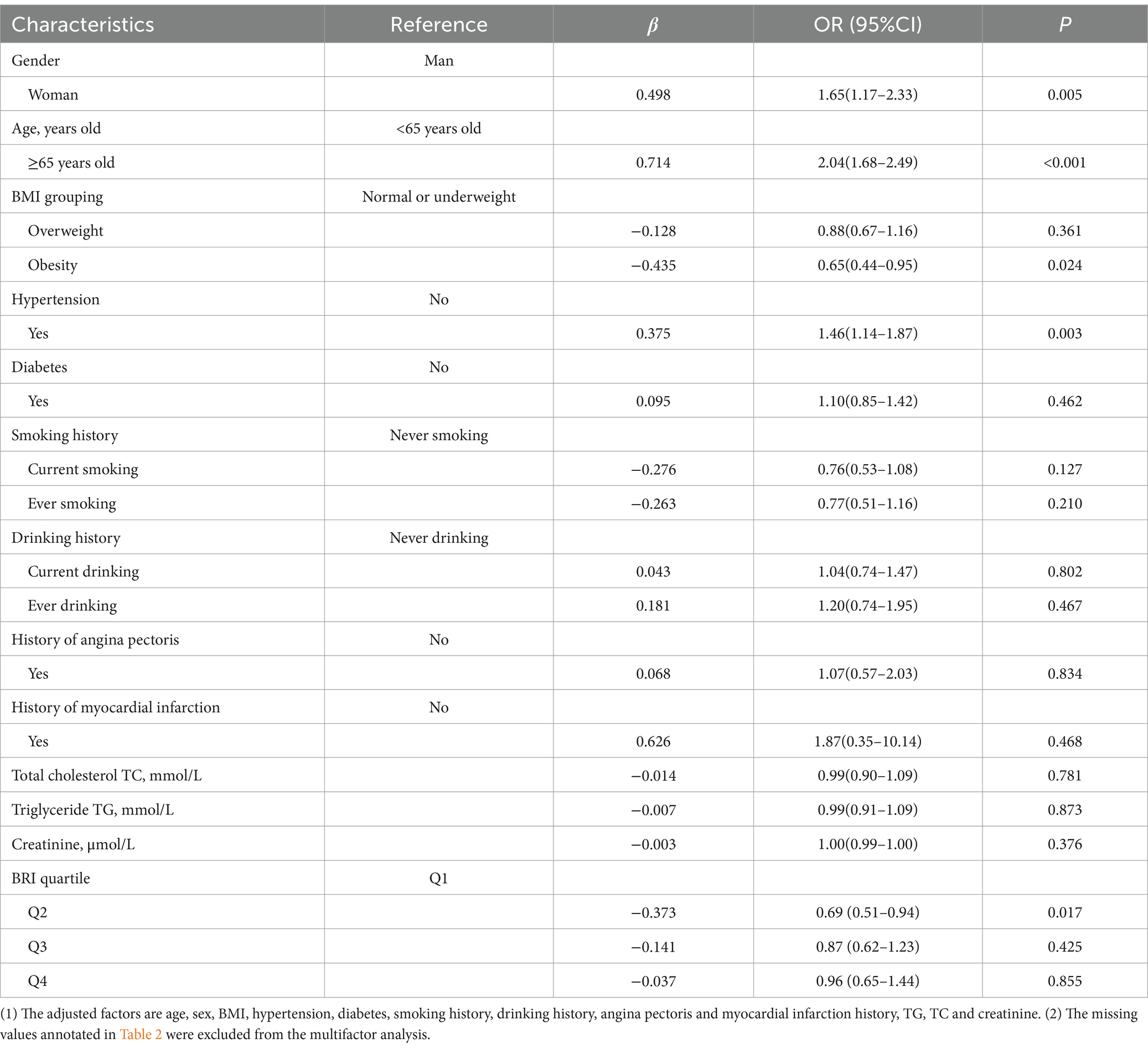
Tables 3, 4 present the results of the multivariate logistic regression analysis. To address potential collinearity between BRI and BRI quartiles, two separate models were constructed. Model 1 (Table 3) included BRI as a continuous variable, while Model 2 (Table 4) used BRI quartiles. Both models were adjusted for age, gender, BMI, hypertension, and other relevant covariates. The β values, along with 95% confidence intervals (CIs), are reported for each variable.
3.3.1 Model 1
After adjusting for multiple confounding variables, the analysis revealed that women had a 1.59 times higher risk of cognitive impairment compared to men (OR: 1.59, 95% CI: 1.12–2.25, p = 0.009). Participants aged 65 and older were found to have a 2.05 times greater risk of cognitive impairment compared to those under 65 (OR: 2.05, 95% CI: 1.69–2.50, p < 0.001). Interestingly, obese individuals had a 36% lower risk of cognitive impairment compared to non-obese participants (OR: 0.64, 95% CI: 0.43–0.94, p = 0.022). Additionally, hypertensive participants had a 1.45 times greater risk of cognitive impairment compared to those without hypertension (OR: 1.45, 95% CI: 1.13–1.85, p = 0.004). However, BRI as a continuous variable did not show a significant association with cognitive impairment (p = 0.509; Table 3).
3.3.2 Model 2
In contrast, when BRI was categorized into quartiles, we found that participants in the second quartile of BRI (3.5897 ≤ BRI < 4.4898) had a 31% lower risk of cognitive impairment compared to those in the first quartile (BRI < 3.5897) (OR: 0.69, 95% CI: 0.51–0.94, p = 0.017). This association suggests that there may be a non-linear relationship between BRI and cognitive impairment, with certain BRI ranges potentially offering protective effects (Table 4).
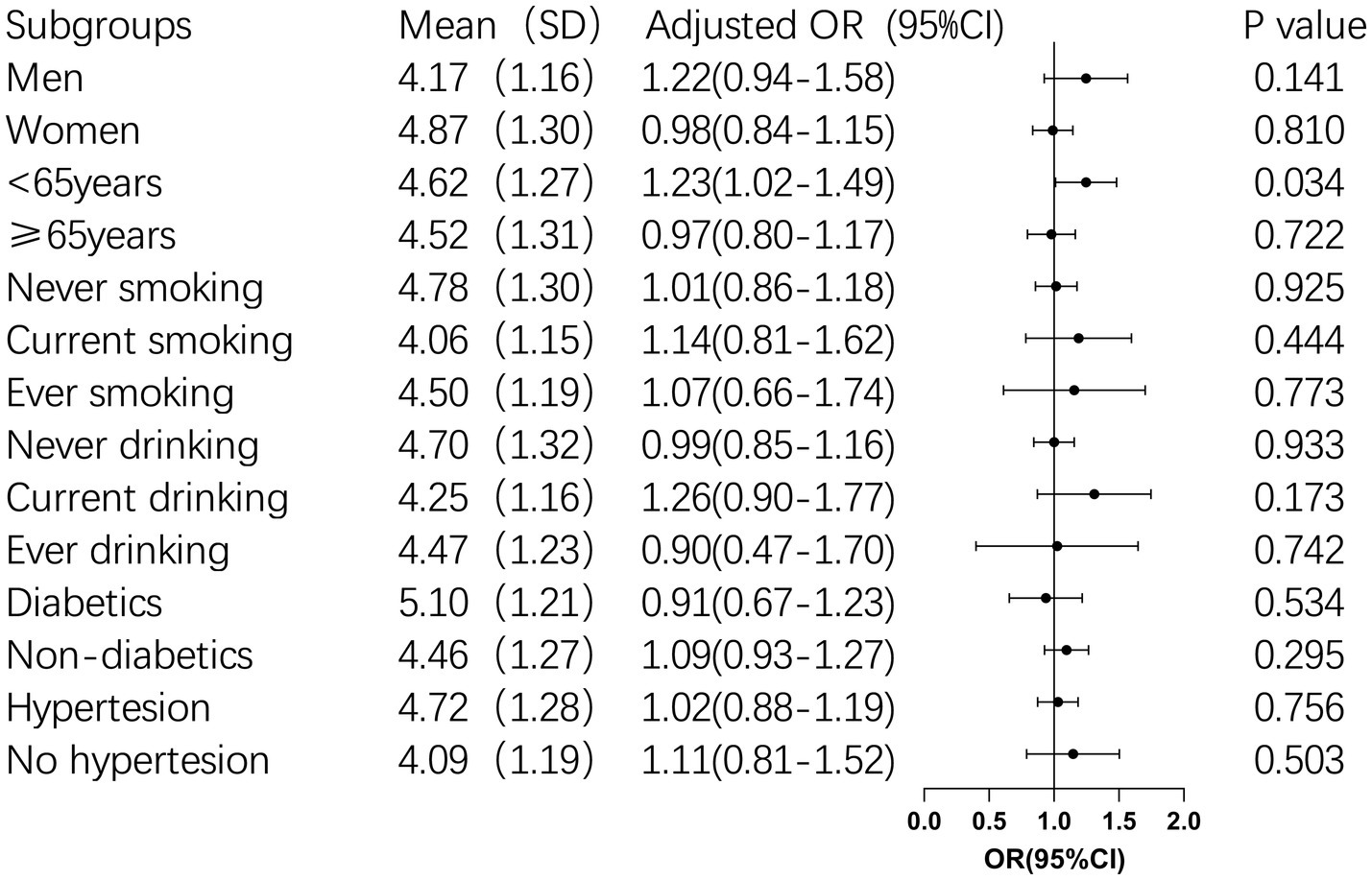
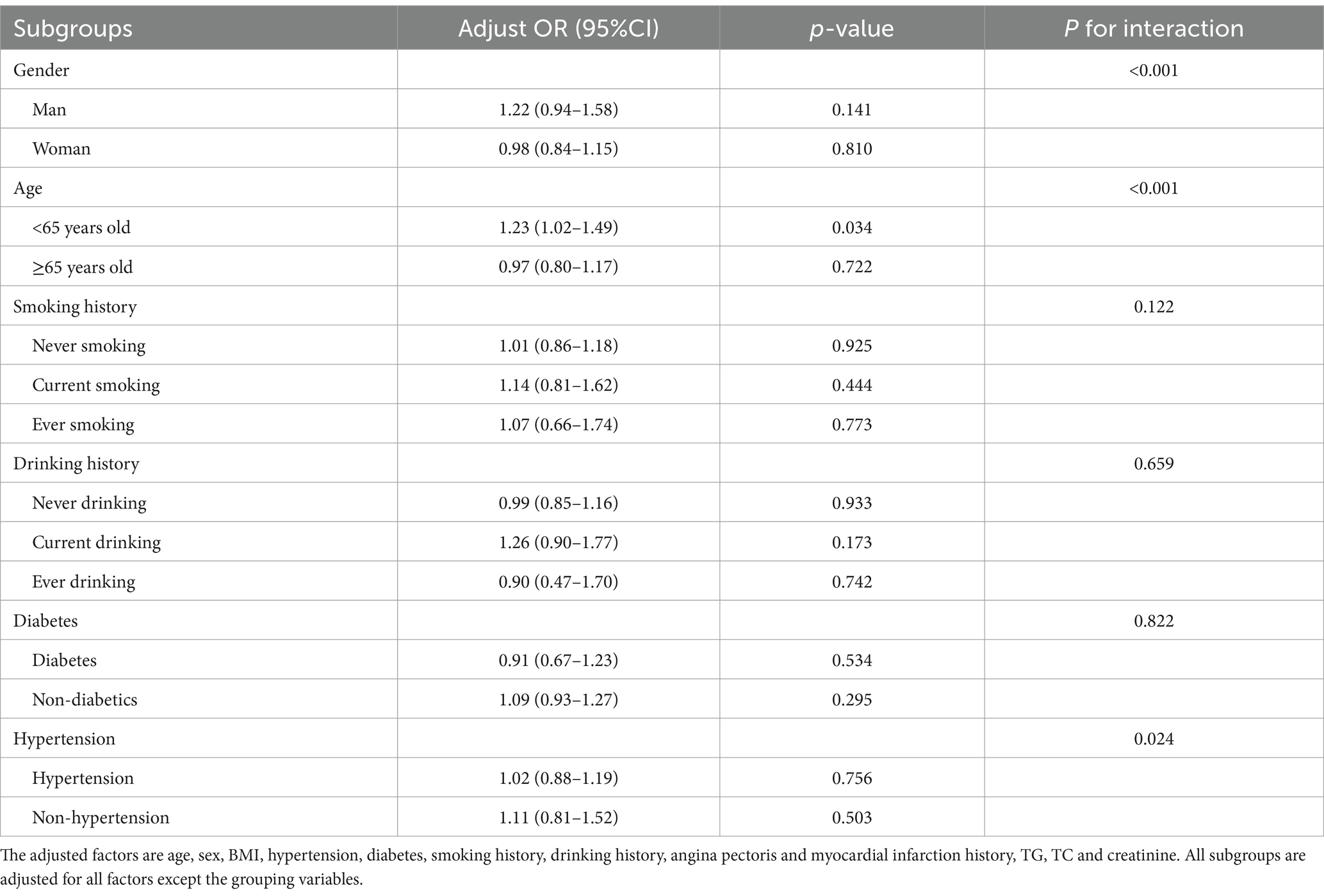
3.4 Subgroup analysis of BRI and cognitive impairment
Subgroup analysis was conducted for BRI continuous variables with insignificant results of multi-factor logistic regression to further explore their correlation. Subgroup analysis revealed that for participants younger than 65 years old, each unit increase in BRI was associated with a 23% higher risk of cognitive impairment (OR: 1.23, 95% CI: 1.02–1.49, p = 0.034). Furthermore, BRI was found to interact significantly with age, sex, and hypertension (p < 0.05), suggesting these factors jointly influence the risk of cognitive impairment (Table 4 and Figure 2).
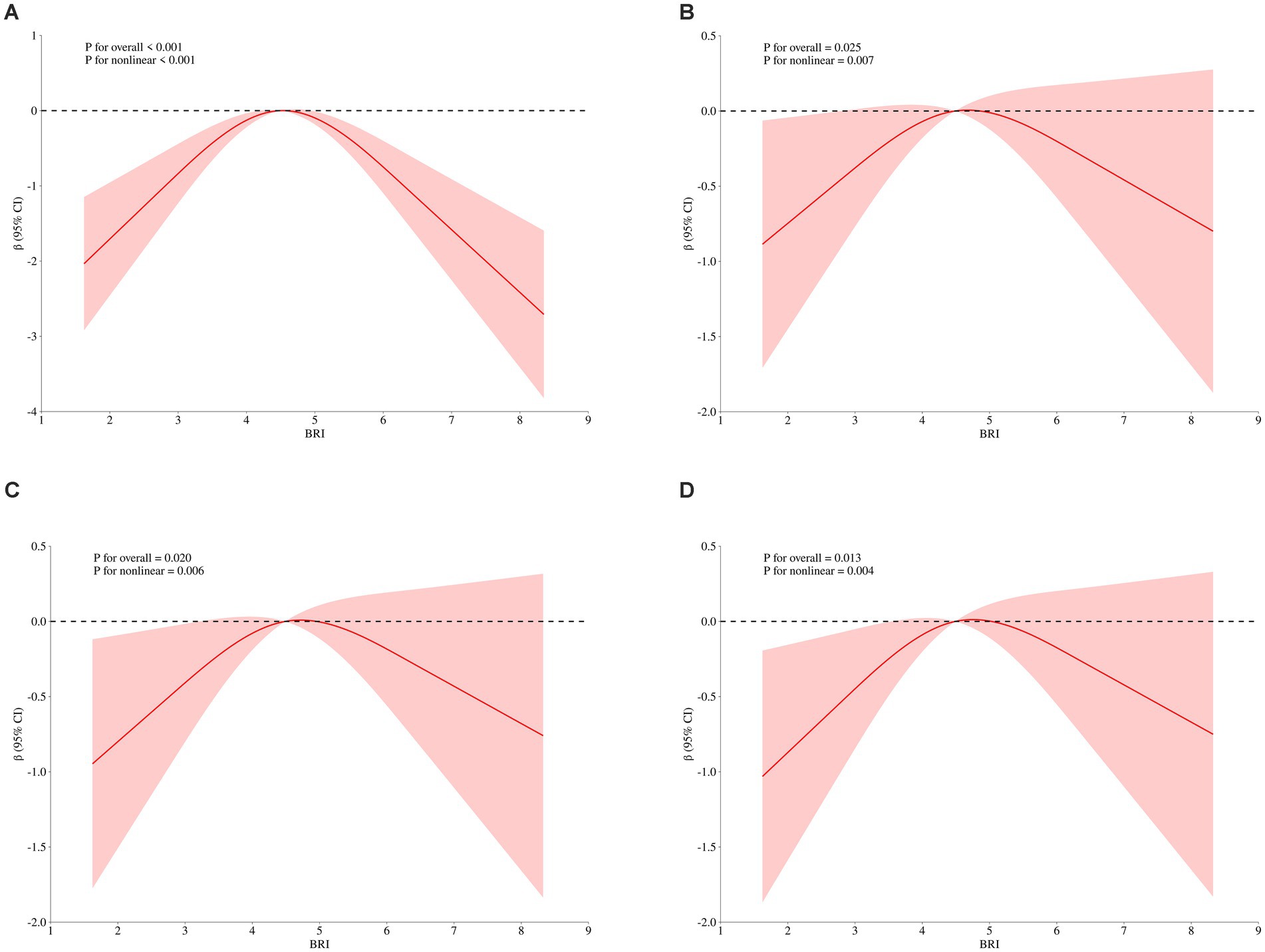
3.5 Non-linear relationship between BRI and MMSE score
To explore the dose–response relationship between BRI and cognitive function, we analyzed the non-linear relationship between BRI and MMSE score using restricted cubic splines. The analysis revealed an inverse U-shaped relationship between BRI and MMSE score (Figure 3A). After adjusting for gender, age, educational level, and BMI, the negative correlation between BRI and MMSE score increased when BRI exceeded 4.49, while below this threshold, the correlation weakened (p = 0.007; Figure 3B). These results remained significant after further adjustments for smoking, drinking, diabetes, hypertension, myocardial infarction, and angina (p = 0.006; Figure 3C). Additional adjustments for laboratory indices such as TG and TC showed that the weakest negative correlation with MMSE occurred near a BRI of 4.49 (p = 0.004; Figure 3D), confirming the inverted U-shaped relationship. We further stratified by age, and found that BRI and MMSE scores of participants under 65 years old showed a significant inverse U-shaped correlation (p < 0.05) after univariate factor (Figure 4A) and adjustment for all the above factors (Figure 4B), and the curve inflection point of multi-factor correction was 4.56. The univariate inverse U-shaped association was significant (p = 0.043; Figure 4C) in people aged 65 years and older, and the significance disappeared after adjusting for multiple factors (p = 0.082; Figure 4D), and the curve inflection point was 4.29 (Table 5).
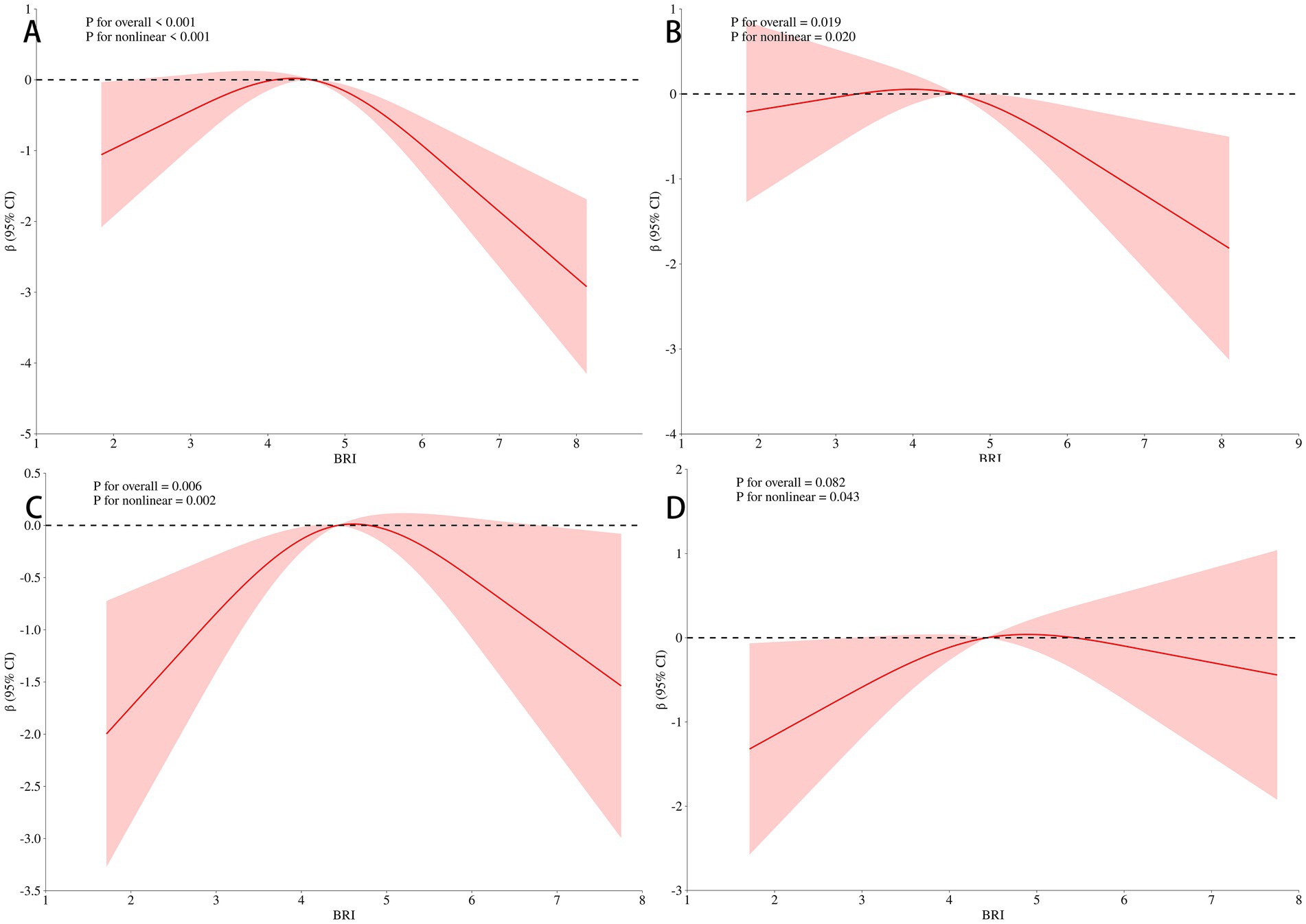
Figure 4. Association of BRI with MMSE scores in the stratified analysis. Panel (A) showed a significant inverse U-shaped correlation (P < 0.001) after univariate analysis under 65 years old. Panel (B) showed that the curve inflection point of multi-factor correction was 4.56 (P = 0.019), after adjustment for all the above factors under 65 years old. Panel (C) showed that the univariate inverse U-shaped association was significant in people aged 65 years and older (P = 0.006). Panel (D) showed the significance disappeared after adjusting for multiple factors (P = 0.082), the curve inflection point was 4.29 in people aged 65 years and older.
4 Discussion
The primary objective of this study was to investigate the relationship between BRI and cognitive impairment in a low-income, low-education population. Given the high prevalence of cognitive impairment, we aimed to explore whether BRI could serve as an independent predictor of cognitive decline. Our study is the first to assess the dose–response relationship between BRI, MMSE scores, and cognitive impairment in a rural Chinese population. The key findings reveal that women, individuals aged 65 and above, and those with hypertension are more likely to experience cognitive decline. Notably, obese participants exhibited a lower risk of cognitive impairment compared to those with normal or underweight status. Furthermore, individuals in the second BRI quartile demonstrated a lower risk of cognitive impairment than those in the first quartile. Although no direct correlation between BRI and cognitive impairment was found overall, BRI was shown to interact significantly with age, gender, and hypertension, jointly influencing cognitive outcomes.
The relationship between BRI, a measure of obesity, and cognitive function remains controversial. Obesity has been linked to cognitive impairment throughout adulthood and is known to increase the risk of dementia in later life (Leigh and Morris, 2020). In the brain, obesity may result in various types of damage, including oxidative stress, inflammation, protein aggregation, mitochondrial dysfunction, hormonal imbalances, IR, blood–brain barrier damage, and disruptions in synaptic plasticity and neurogenesis, all of which can contribute to cognitive decline and neuronal death (Neto et al., 2023). Despite this, most cross-sectional studies have not demonstrated a significant relationship between BRI and cognition. The weight-adjusted waist circumference index (WWI) is linearly related to the occurrence of AD and other dementias, but they found no association between BRI and dementia in rural China (Wang et al., 2023). Similarly, a cross-sectional study from Iran indicated that increased BMI, WC, and waist-to-hip ratio were associated with a reduced risk of cognitive decline, yet no significant correlation was observed between BRI and any cognitive test (Ramezani Kashal et al., 2024). Among non-demented multi-ethnic Asians with type 2 diabetes, while univariate analysis found a significant relationship between BRI and cognition, multivariate analysis revealed no such correlation (Moh et al., 2020). In contrast, a study conducted in a Taiwanese population over 60 years old reported a significant association between BRI and reduced MMSE scores, though it did not directly address the relationship between BRI and cognitive impairment (Huang et al., 2022). Zhang et al. (2024) also reported a significant correlation between BRI level and cognitive performance in people over 65 years of age in the United States, with higher BRI significantly associated with lower Digit Symbol Substitution Test (DSST) scores measuring cognition, and a stronger negative association in men. Our findings align with some aspects of previous research, as we did not observe a direct relationship between BRI and cognitive impairment across the entire sample. However, we did find that participants in the second quartile of BRI had a lower risk of cognitive impairment compared to those in the first quartile, though this effect was not observed in the higher quartiles. Further analysis revealed that BRI interacts significantly with age, gender, and hypertension, highlighting its varying influence on cognitive impairment across different subgroups. Specifically, the correlation between BRI and cognitive decline was stronger in women than in men, and more pronounced in participants younger than 65 years old, while the predictive power of BRI diminished in older individuals. Unlike Zhang et al. (2024), we chose MMSE, a more comprehensive scale for assessing cognitive impairment, rather than DSST, but the results may be different due to the influence of participants’ self-reported education level and the differences in age and region of the study population selected by Zhang’s team.
A key difference between our study and previous research (Huang et al., 2022), is the recognition that the relationship between BRI and MMSE scores is not strictly linear. Using a RCS model, we demonstrated that the association between BRI and cognitive decline is more complex, exhibiting an inverted U-shaped correlation. The weakest relationship between BRI and MMSE scores occurred when BRI was around 4.49, suggesting that both low and high BRI values may have differing impacts on cognitive outcomes. Unlike earlier studies, we included a wider age range. At the same time, the age stratification results still showed an inverted U-shaped curve, and it was obvious in people under 65 years old, which allowed us to identify that BRI may serve as a better predictor of cognitive function among younger individuals.
The relationship between cognitive impairment and the traditional obesity measure was well-established. A cross-sectional study found that being overweight was associated with a reduced risk of cognitive impairment among the elderly in China (Hou et al., 2019). Similarly, as BMI increased, the risk of cognitive decline decreased among elderly individuals in Iran (Ramezani Kashal et al., 2024). Most prospective studies support the view that being underweight is linked to cognitive decline (Ren et al., 2021; Zhang et al., 2022; Wu et al., 2021; Dong et al., 2023), whereas being overweight or obese is often considered protective against cognitive impairment (Zhang et al., 2022; Wu et al., 2021; Dong et al., 2023; Liang et al., 2022). A study highlighted the age-related effects of BMI on cognition, noting that before the age of 65, a higher BMI was associated with lower cognitive ability, but after 65, the relationship reversed, with higher BMI correlating with better cognitive outcomes (Crane et al., 2023). In line with these studies, we found that individuals with cognitive impairment had a lower BMI compared to those without cognitive impairment, and obese individuals had a lower risk of cognitive impairment compared to those with normal or underweight status. These variations may be explained by the complex interplay between BMI, age, and gender. The impact of BMI on cognition appears to shift depending on the demographic characteristics of the study population, which may account for the differing conclusions across studies. Our study is the first to report that the association between BRI and cognition is more significant in middle-aged and young adults, while the relationship is weakened in older adults.
In addition to BMI, the risk of cognitive impairment is also influenced by gender, age, and hypertension. Numerous cross-sectional studies have shown that the prevalence of cognitive impairment is significantly higher in women compared to men, particularly among middle-aged and elderly populations (Miyawaki and Liu, 2019; Roh et al., 2021; Han et al., 2022; Wang et al., 2020). Furthermore, a meta-analysis demonstrated that individuals with hypertension have a notably high prevalence of cognitive impairment (Qin et al., 2021). Cross-sectional studies further support the role of hypertension as a significant risk factor for cognitive impairment (Chen et al., 2022), making it a valuable predictor of cognitive decline in rural populations (Bao et al., 2022). Our findings was similar to these previous studies, as we observed that the risk of cognitive impairment increases with age, and women are more likely to experience cognitive impairment compared to men. Additionally, there is a strong correlation between hypertension and cognitive impairment, with hypertensive individuals facing a greater risk of cognitive decline. Notably, our study is the first to identify that BRI interacts with age, gender, and hypertension, jointly influencing the risk of cognitive impairment. This interaction enhances BRI’s utility as an independent predictor of cognitive decline.
This study has several limitations. First, the sample population was drawn exclusively from rural areas of Tianjin, China, which may limit the generalizability of the findings to urban populations or those in other regions. Future studies should include more diverse populations to validate these results. Second, as a cross-sectional study, we were unable to establish a causal relationship between BRI and cognitive impairment. Longitudinal studies would be necessary to explore the temporal relationship and potential causality. Third, cognitive impairment was assessed using the MMSE, which, while widely used, may not capture all aspects of cognitive function. Incorporating more comprehensive cognitive assessments in future studies could provide a more complete evaluation. Fourth, lifestyle factors such as diet, exercise, and medication history were not considered, which may have influenced the relationship between BRI and cognition. Future research should account for these confounding factors to strengthen the reliability of the findings. Finally, education years were self-reported, which may introduce reporting bias. Using objective data from medical or educational records in future studies could reduce this potential bias.
5 Conclusion
This study highlights the complex relationship between BRI and cognitive impairment, demonstrating that BRI, when considered alongside factors such as age, gender, and hypertension, can provide valuable insights into cognitive risk. The findings suggest that BRI may serve as an independent predictor of cognitive decline, particularly in younger individuals and women, offering clinicians a potential tool for early identification of those at risk. Patients can slow cognitive decline by early detection of cognitive impairment through easy-to-measure indicators such as BRI and timely intervention. Healthcare providers can incorporate BRI into routine assessments to enhance screening practices, especially in high-risk populations. Early intervention and risk stratification based on BRI may reduce the economic burden associated with long-term care for dementia, thereby improving public health outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Tianjin Medical University General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DG: Investigation, Writing – original draft. TL: Investigation, Writing – original draft. QY: Investigation, Writing – original draft. CY: Investigation, Writing – original draft. YY: Investigation, Writing – original draft. FL: Investigation, Writing – original draft. JM: Funding acquisition, Investigation, Writing – original draft. JT: Investigation, Writing – original draft. XN: Formal analysis, Writing – original draft. JW: Formal analysis, Writing – original draft. CS: Conceptualization, Writing – review & editing. YL: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported partly by National Natural Science Foundation of China (No. 82171245) and Shandong Provincial Natural Science Foundation (No. ZR2020QH101).
Acknowledgments
We thank all participants of the Tianjin Brain Study, and local medical care professionals for their valuable contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alzheimer’s Association (2024). 2024 Alzheimer’s disease facts and figures. Alzheimers Dement. 20, 3708–3821. doi: 10.1002/alz.13809
American Diabetes Association Professional Practice Committee (2024). 2. Diagnosis and classification of diabetes: standards of Care in Diabetes-2024. Diabetes Care 47, S20–S42. doi: 10.2337/dc24-S002
Bao, J., Liu, J., Li, Z., Zhang, Z., Su, X., Sun, J., et al. (2022). Relationship between hypertension and cognitive function in an elderly population: a population-based study in rural northern China. Front. Neurol. 13:885598. doi: 10.3389/fneur.2022.885598
Chen, N., Cao, J., Zhang, W., Chen, Y., and Xu, L. (2022). Gender differences in the correlation between body mass index and cognitive impairment among the community-dwelling oldest-old in China: a cross-sectional study. BMJ Open 12:e065125. doi: 10.1136/bmjopen-2022-065125
Chen, K., Shen, Z., Gu, W., Lyu, Z., Qi, X., Mu, Y., et al. (2023). Prevalence of obesity and associated complications in China: a cross-sectional, real-world study in 15.8 million adults. Diabetes Obes. Metab. 25, 3390–3399. doi: 10.1111/dom.15238
Chun, C. T., Seward, K., Patterson, A., Melton, A., and MacDonald-Wicks, L. (2021). Evaluation of available cognitive tools used to measure mild cognitive decline: a scoping review. Nutrients 13:3974. doi: 10.3390/nu13113974
Crane, B. M., Nichols, E., Carlson, M. C., Deal, J. A., and Gross, A. L. (2023). Body mass index and cognition: associations across mid- to late life and gender differences. J. Gerontol. A Biol. Sci. Med. Sci. 78, 988–996. doi: 10.1093/gerona/glad015
Dong, W., Kan, L., Zhang, X., Li, M., Wang, M., and Cao, Y. (2023). Association between body mass index and cognitive impairment in Chinese older adults. Front. Public Health 11:1255101. doi: 10.3389/fpubh.2023.1255101
Dye, L., Boyle, N. B., Champ, C., and Lawton, C. (2017). The relationship between obesity and cognitive health and decline. Proc. Nutr. Soc. 76, 443–454. doi: 10.1017/S0029665117002014
Feng, J., He, S., and Chen, X. (2019). Body adiposity index and body roundness index in identifying insulin resistance among adults without diabetes. Am J Med Sci 357, 116–123. doi: 10.1016/j.amjms.2018.11.006
GBD 2021 Diseases and Injuries Collaborators (2024). Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet 403, 2133–2161. doi: 10.1016/S0140-6736(24)00757-8
GBD 2021 Nervous System Disorders Collaborators (2024). Global, regional, and national burden of disorders affecting the nervous system, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet Neurol. 23, 344–381. doi: 10.1016/S1474-4422(24)00038-3
Han, F., Luo, C., Lv, D., Tian, L., and Qu, C. (2022). Risk factors affecting cognitive impairment of the elderly aged 65 and over: a cross-sectional study. Front. Aging Neurosci. 14:903794. doi: 10.3389/fnagi.2022.903794
Hou, Q., Guan, Y., Yu, W., Liu, X., Wu, L., Xiao, M., et al. (2019). Associations between obesity and cognitive impairment in the Chinese elderly: an observational study. Clin. Interv. Aging 14, 367–373. doi: 10.2147/CIA.S192050
Huang, S. H., Chen, S. C., Geng, J. H., Wu, D. W., and Li, C. H. (2022). Metabolic syndrome and high-obesity-related indices are associated with poor cognitive function in a large Taiwanese population study older than 60 years. Nutrients 14:1535. doi: 10.3390/nu14081535
Leigh, S. J., and Morris, M. J. (2020). Diet, inflammation and the gut microbiome: mechanisms for obesity-associated cognitive impairment. Biochim. Biophys. Acta Mol. basis Dis. 1866:165767. doi: 10.1016/j.bbadis.2020.165767
Li, H., Jia, J., and Yang, Z. (2016). Mini-mental state examination in elderly Chinese: a population-based normative study. J. Alzheimers Dis. 53, 487–496. doi: 10.3233/JAD-160119
Li, H., Ren, J., Li, Y., Wu, Q., and Wei, J. (2023). Oxidative stress: the nexus of obesity and cognitive dysfunction in diabetes. Front Endocrinol 14:1134025. doi: 10.3389/fendo.2023.1134025
Li, L., Wei, H., Yang, X., Liu, X., Li, Q., Han, S., et al. (2024). Age-period-cohort analysis of the burden of Alzheimer's disease in the elderly population in China. Modern Prevent Med 51, 1754–1759. doi: 10.20043/j.cnki.MPM.202401100
Liang, F., Fu, J., Moore, J. B., Zhang, X., Xu, Y., Qiu, N., et al. (2022). Body mass index, waist circumference, and cognitive decline among Chinese older adults: a Nationwide retrospective cohort study. Front. Aging Neurosci. 14:737532. doi: 10.3389/fnagi.2022.737532
Mancia, G., Kreutz, R., Brunström, M., Burnier, M., Grassi, G., Januszewicz, A., et al. (2023). 2023 ESH guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the European Society of Hypertension: endorsed by the International Society of Hypertension (ISH) and the European renal association (ERA). J. Hypertens. 41, 1874–2071. doi: 10.1097/HJH.0000000000003480
Miyawaki, C. E., and Liu, M. (2019). Gender differences in cognitive impairment among the old and the oldest-old in China. Geriatr Gerontol Int 19, 586–592. doi: 10.1111/ggi.13666
Moh, M. C., Low, S., Ng, T. P., Wang, J., Ang, S. F., Tan, C., et al. (2020). Association of traditional and novel measures of central obesity with cognitive performance in older multi-ethnic Asians with type 2 diabetes. Clin Obes. 10:e12352. doi: 10.1111/cob.12352
Neto, A., Fernandes, A., and Barateiro, A. (2023). The complex relationship between obesity and neurodegenerative diseases: an updated review. Front. Cell. Neurosci. 17:1294420. doi: 10.3389/fncel.2023.1294420
Noh, H. M., Oh, S., Song, H. J., Lee, E. Y., Jeong, J. Y., Ryu, O. H., et al. (2017). Relationships between cognitive function and body composition among community-dwelling older adults: a cross-sectional study. BMC Geriatr. 17:259. doi: 10.1186/s12877-017-0651-9
Ozato, N., Saitou, S., Yamaguchi, T., Katashima, M., Misawa, M., Jung, S., et al. (2021). Association between visceral fat and brain structural changes or cognitive function. Brain Sci. 11:1036. doi: 10.3390/brainsci11081036
Qin, J., He, Z., Wu, L., Wang, W., Lin, Q., Lin, Y., et al. (2021). Prevalence of mild cognitive impairment in patients with hypertension: a systematic review and meta-analysis. Hypertens. Res. 44, 1251–1260. doi: 10.1038/s41440-021-00704-3
Ramezani Kashal, F., Nouredini, G., Hezaveh, Z. S., Fakhrzadeh, H., Moodi, M., Khorashadizadeh, M., et al. (2024). The association between cognitive impairment and anthropometric indices among the elderly: Birjand longitudinal aging study. J. Diabetes Metab. Disord. 23, 1173–1182. doi: 10.1007/s40200-024-01404-8
Ren, Z., Li, Y., Li, X., Shi, H., Zhao, H., He, M., et al. (2021). Associations of body mass index, waist circumference and waist-to-height ratio with cognitive impairment among Chinese older adults: based on the CLHLS. J. Affect. Disord. 295, 463–470. doi: 10.1016/j.jad.2021.08.093
Roh, M., Dan, H., and Kim, O. (2021). Influencing factors of subjective cognitive impairment in middle-aged and older adults. Int. J. Environ. Res. Public Health 18:11488. doi: 10.3390/ijerph182111488
Tahami Monfared, A. A., Byrnes, M. J., White, L. A., and Zhang, Q. (2022). Alzheimer's disease: epidemiology and clinical progression. Neurol Ther. 11, 553–569. doi: 10.1007/s40120-022-00338-8
Thomas, D. M., Bredlau, C., Bosy-Westphal, A., Mueller, M., Shen, W., Gallagher, D., et al. (2013). Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity 21, 2264–2271. doi: 10.1002/oby.20408
Uchida, K., Sugimoto, T., Tange, C., Nishita, Y., Shimokata, H., Saji, N., et al. (2024). Association between abdominal adiposity and cognitive decline in older adults: a 10-year community-based study. J. Nutr. Health Aging 28:100175. doi: 10.1016/j.jnha.2024.100175
Wang, J., Xiao, L. D., Wang, K., Luo, Y., and Li, X. (2020). Gender differences in cognitive impairment among rural elderly in China. Int. J. Environ. Res. Public Health 17:3724. doi: 10.3390/ijerph17103724
Wang, S., Zhang, Q., Hou, T., Wang, Y., Han, X., Song, L., et al. (2023). Differential associations of 6 adiposity indices with dementia in older adults: the MIND-China study. J. Am. Med. Dir. Assoc. 24, 1412–1419.e4. doi: 10.1016/j.jamda.2023.06.029
Wang, Y., Zhao, L., Gao, L., Pan, A., and Xue, H. (2021). Health policy and public health implications of obesity in China. Lancet Diabetes Endocrinol. 9, 446–461. doi: 10.1016/S2213-8587(21)00118-2
Wu, S., Lv, X., Shen, J., Chen, H., Ma, Y., Jin, X., et al. (2021). Association between body mass index, its change and cognitive impairment among Chinese older adults: a community-based, 9-year prospective cohort study. Eur. J. Epidemiol. 36, 1043–1054. doi: 10.1007/s10654-021-00792-y
Zhang, W., Chen, Y., and Chen, N. (2022). Body mass index and trajectories of the cognition among Chinese middle and old-aged adults. BMC Geriatr. 22:613. doi: 10.1186/s12877-022-03301-2
Keywords: Body Roundness Index, cognitive impairment, obesity, rural population, cross-sectional study
Citation: Guo D, Li T, Yang Q, Yang C, Yang Y, Liu F, Ma J, Tu J, Ning X, Wang J, Song C and Liu Y (2025) Relationship between Body Roundness Index and cognitive impairment in middle-aged and older adults: a population-based cross-sectional study. Front. Aging Neurosci. 17:1522989. doi: 10.3389/fnagi.2025.1522989
Edited by:
Simone Varrasi, University of Catania, ItalyReviewed by:
Xing Ge, Xuzhou Medical University, ChinaNurtami Soedarsono, University of Indonesia, Indonesia
Nezire Ince, Eastern Mediterranean University, Türkiye
Copyright © 2025 Guo, Li, Yang, Yang, Yang, Liu, Ma, Tu, Ning, Wang, Song and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiming Liu, bGl1eW1Ac2R1LmVkdS5jbg==; Chengyuan Song, ZGNzY2hlbmd5dWFuQDE2My5jb20=
 Dandan Guo1
Dandan Guo1 Fuchen Liu
Fuchen Liu Xianjia Ning
Xianjia Ning Jinghua Wang
Jinghua Wang Chengyuan Song
Chengyuan Song Yiming Liu
Yiming Liu