
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 28 March 2025
Sec. Alzheimer's Disease and Related Dementias
Volume 17 - 2025 | https://doi.org/10.3389/fnagi.2025.1522498
Background: Studies indicate that butyrate can enhance memory and cognitive functions in mice by inhibiting neuroinflammation and neuronal apoptosis. Elevated fecal butyrate levels in older individuals with mild cognitive impairment correlate with reduced levels of Aβ-42, an Alzheimer’s disease biomarker. This study investigated the relationship between butyrate consumption and cognitive performance in older adults, which remains to be elucidated.
Methods: This study employed a cross-sectional, observational design to analyze data gathered from 2,078 participants enrolled in the 2011–2014 US National Health and Nutrition Examination Survey (NHANES). Butyrate intake was determined based on two 24-h dietary assessments. To evaluate cognitive function, three tests were administered: the Animal Fluency Test (AFT) to assess executive function, the Digit Symbol Substitution Test (DSST) for measuring processing speed, and the Consortium to Establish a Registry for Alzheimer’s disease (CERAD) subtest for assessing memory. Z scores were computed for each test and overall cognitive performance. Multivariate linear regression models and a generalized additive model (GAM) were used to examine the correlation between butyrate consumption and mental functions. Finally, subgroup analyses and interaction tests were used to verify the robustness of the associations.
Results: The NHANES study encompasses two surveys conducted between 2011 and 2014 that involved 2,078 participants aged 60 years or older. Higher dietary butyrate consumption had a positive correlation between superior performance on DSST, AFT, CERAD-Immediate Recall Test, and Z scores. The participants in the upper quartile of butyrate intake had significantly higher DSST (β = 1.60, 95% CI: 0.04–3.17), AFT scores (β = 0.99, 95% CI: 0.37–1.60), and Z scores (β = 0.09, 95% CI: 0.01–0.17) than individuals in the lowest quartile even after adjusting for potential confounders. Finally, no notable interactions were observed within the groupings. Finally, in subgroup analyses, BMI was found to influence the positive association between butyrate and DSST with Z score, and hypertension also influenced the association between butyrate and DSST.
Conclusion: Higher butyrate intake in individuals aged ≥60 years was linked to better cognitive functioning. This could potentially contribute to maintaining brain function during aging.
The growing trend of population aging is resulting in a surge in the prevalence of cognitive decline associated with aging (Brito et al., 2023). According to 2023 reports, 6.7 million individuals aged 65 and older in the United States have Alzheimer’s disease (AD), with projections indicating a rise to 13.8 million by 2060 (Alzheimers Dement, 2023). Cognitive impairment is increasingly recognized as a major public health challenge worldwide, and can significantly affect an individual’s daily life and independence. For example, studies have shown that individuals with subjective cognitive decline may experience significant quality of life decline and functional limitations within 6 months (Hendriksen et al., 2024). The higher prevalence of cognitive impairment among older adults may be related to a variety of factors, including chronic disease, psychosocial factors, and lifestyle. For example, studies have found that up to 76.0% of older adults living in foster care in the French West Indies have severe cognitive impairment (Boucaud-Maitre et al., 2024). In addition, cognitive decline may also be associated with specific biomarkers, such as amyloid PET scan disclosure in individuals with subjective cognitive decline can reveal the risk of future cognitive decline (Hendriksen et al., 2024). In contrast, in patients with cerebral small vessel disease, the mismatch between high white matter signal and cognitive function also suggests that cognitive decline may be associated with cerebrovascular lesions (Zeng et al., 2024). These findings emphasize the multifactorial nature and complexity of cognitive decline and the importance of early identification and intervention. However, to date, knowledge of cognitive impairment remains limited.
The role of the gut microbiome in neurodegenerative diseases is increasingly being acknowledged (Loh et al., 2024; Alpino et al., 2024). The fermentation of fiber-rich diets results in the production of short-chain fatty acids (SCFAs), primarily consisting of saturated fatty acids with 1 to 6 carbon atoms. The dosage of SCFAs, particularly butyrate, may be critical in determining their effects on psychophysiological and behavioral processes (Dalile et al., 2019). Recent studies indicate that butyrate can enhance memory and cognitive functions in mice by inhibiting microglia-mediated neuroinflammation and neuronal apoptosis (Wang et al., 2023; Wei et al., 2023). In a vascular dementia model, administration of butyrate-producing bacteria resulted in increased butyrate levels in the feces and the brain, significantly reduced cognitive deficits, and histopathological changes in the hippocampus (Liu et al., 2015). Butyrate may also exert neuroprotective effects by increasing neurotrophic factor levels and improving mitochondrial autophagy dysregulation (Guo et al., 2025). As the global population ages, cognitive decline has become an important public health issue. In recent years, more and more studies have shown that the gut microbiota and its metabolites (e.g., butyrate) play an important role in the aging process. Transplantation of gut microbes from older mice to younger mice resulted in inflammation of the gut and brain with cognitive decline, and further studies found that older gut microbes produced fewer butyrate-producing bacteria (Mishra et al., 2024). In addition, a study of older adults with mild cognitive impairment found that elevated levels of butyrate in participants’ feces were associated with lower levels of the AD biomarker Aβ-42 (Nagpal et al., 2019). Therefore, studying the relationship between butyrate intake and cognitive function in older adults is important for understanding the mechanisms of cognitive changes during aging.
The present study investigated the relationship between butyrate and cognitive function in older adults by analyzing data from NHANES and a representative group of older adults to address gaps in previous studies and reveal the potential benefits of dietary modifications in preventing cognitive decline.
The National Center for Health Statistics conducts the National Health and Nutrition Examination Survey (NHANES), which gathers vital information about the nutritional status and overall health of citizens in the United States of America. This survey collects data on various aspects, including population characteristics, dietary supplements, laboratory tests, physical examinations, and questionnaire responses (Elnakib et al., 2022; Saint-Maurice et al., 2022; Kim et al., 2023). The NHANES ensures that the sample accurately represents the US civilian population by employing a stratified, multi-stage sampling method. All participants in the study provided written consent, and the Research Ethics Committee of the National Health and Demographic Survey granted its official endorsement. For further details about NHANES, you can visit http://www.cdc.gov/nchs/nhanes.htm.
Our study collected data from NHANES cycles conducted from 2011 to 2014 (n = 19,931). To ensure the validity of the findings, we selected older adults who were older than 60 years of age (n = 16,299). Participants missing complete cognitive information (n = 698) and missing information on butyrate intake (n = 410) were excluded from the study analyses. In addition, we excluded participants missing information on covariates (n = 446). Ultimately, we analyzed data from 2,078 participants aged 60 years or older (Ghaferi et al., 2021). Detailed inclusion and exclusion labeling can be found in Figure 1.
The NHANES employed a comprehensive 24-h dietary recall questionnaire to document individual food consumption throughout the previous day. Each participant completed two 24-h dietary recall interviews, and the data obtained from these interviews were used to assess their daily butyrate intake. The first interview occurred at a portable testing facility, and a further interview may be arranged via telephone within a timeframe of 3 to 10 days. Dietary butyrate intake was estimated from participants’ reported food intake. We used the U.S. Department of Agriculture (USDA) food composition database in conjunction with data on the butyrate content of foods to calculate daily butyrate intake from participant-reported food intake. This calculation is done after data collection. The results were obtained by calculating the average butyrate consumption from both interviews for each individual and subsequently classifying them according to this value.
The NHANES study incorporated cognitive assessments aimed at evaluating memory and executive functioning. The researchers assessed the ability of the study participants to learn new words using the Consortium to Establish a Registry for Alzheimer’s Disease-Immediate Recall Test (CERAD-IRT) and CERAD-Delayed Recall Test (DRT) tests, which were created by the Alzheimer’s Disease Word Study Registry Consortium. The participants were presented with a roster of 10 disparate things and instructed to recall as many terms as possible. Their scores from the initial three attempts were subsequently combined. An 8–10-min period elapsed before administering a delayed recall test. The participants of the Animal Fluency Test (AFT) were instructed to generate as many animal names as possible within a 1-min time frame. Each correctly named animal was awarded one score. This test aimed to assess participants’ linguistic and executive skills. The participants’ processing speed and executive functioning were evaluated through the use of the Digit Symbol Substitution Test (DSST). This test required the participants to rapidly associate symbols with their respective digits, utilizing a given legend. The final stage for computing Z scores was taking the mean of four standardized measures: the DSST, the AFT, the CERAD-IRT, and the CERAD-DRT (Jia et al., 2024).
Our study included individuals from several ethnic backgrounds, including non-Hispanic black, white, Mexican American, and other races. Participants’ marital statuses were divided into two groups: married, and single. There were three tiers of educational achievement: fewer than 9 years of formal education, 9 to 12 years of formal education, and 13 years of formal education or beyond. The poverty-to-income ratio (PIR) was split into three categories: low (<1.3), moderate (1.3–3.5), and high (>3.5). The participants were categorized into three groups: current smokers, former smokers (those who had quit smoking after consuming 100 cigarettes), and never smokers (those who had smoked less than 100 cigarettes in total). Similarly, individual patterns of alcohol use were categorized into three distinct groups: abstainers (those who had consumed fewer than 12 alcoholic beverages in their lifetime), current drinkers, and former drinkers.
Chi-square and one-way ANOVA tests were used to compare participant baseline characteristics. For continuous data, we employed the median and interquartile range or standard deviation as measures of central tendency and dispersion, respectively. For categorical variables, we utilized total proportions and percentages as representations. The issue of multiple comparisons was resolved by implementing the Bonferroni adjustment.
Butyrate consumption was divided into four groups, with the lowest category being used as the reference. We conducted a mixed-category multivariate linear regression analysis to examine the correlation between butyrate intake and cognitive function. Model 1 served as our basic model, lacking any adjustments. In contrast, Model 2 incorporated adjustments for age, sex, PIR, race/ethnicity, and educational level. Extending the adjustments of Model 2, Model 3 further accounted for body mass index (BMI), marital status, smoking habits, alcohol consumption, diabetes mellitus, hypertension, cardiovascular disease, stroke, cholesterol levels, creatinine, and alanine aminotransferase (ALT). The study examined the relationship between the intake of butyrate and cognitive test performance using the cubic spline method. The linear trend analysis was used to investigate the categorical components. We performed subgroup analyses by employing binary linear regression models. These models were stratified and classified based on clinical thresholds or quartiles. After that, we conducted interaction tests and carried out effect-adjusted tests for subgroup measures alongside likelihood ratio tests.
All statistic computations were executed using R software (version 4.1.1, R Foundation, Vienna, Austria). A two-sided p-value <0.05 was established as the criterion for statistical significance. To ensure data integrity, we excluded all participants with missing data.
Table 1 outlines the baseline traits of the study participants according to their dietary butyrate consumption. Based on a quartile analysis of their butyrate intake, the participants were categorized into four groups: Q1 (consuming ≤0.341 g/day), Q2 (0.341 < consuming ≤0.68 g/day), Q3 (0.68 < consuming ≤1.172 g/day), and Q4 (consuming >1.172 g/day). The average age of the participants was 69.39 ± 6.73 years, 1,093 (52.60%) were women, and 1,070 (51.49%) identified as non-Hispanic white. Those with higher butyrate intake tended to be male, with higher education and economic income and lower prevalence of hypertension and diabetes (p < 0.05). Cognitive scores were significantly higher in the group with higher butyrate intake than in the group with lower intake, as assessed by DSST, AFT, CERAD-IRT, and Z scores (p < 0.01). Regarding the biochemical indicators, there were no noticeable differences across the groups.
Table 2 presents the multivariate linear regression analysis of the association between butyrate intake and cognitive function in elderly participants. Higher butyrate intake was positively associated with cognitive scores. In the crude model, participants in the highest Q4 group exhibited higher cognitive scores compared to those in the lowest Q1 group (DSST: β = 6.48, 95% CI: 4.46–8.50; AFT: β = 2.31, 95% CI: 1.65–2.96; and Z scores: β = 0.26, 95% CI: 0.16–0.35). In addition, cognitive scores increased progressively with increasing butyrate intake (P for trend <0.001). In Model 3, after adjusting for various covariates such as age, sex, race, PIR, marital status, education level, BMI, smoking, drinking, hypertension, diabetes, cardiovascular disease, ALT, AST, creatinine, and cholesterol, participants in the Q4 group had higher DSST (β = 1.60, 95% CI: 0.04–3.17), AFT scores (β = 0.99, 95% CI: 0.37–1.60), and Z scores (β = 0.09, 95% CI: 0.01–0.17), compared with those in Q1. In addition, cognitive scores including AFT and Z scores increased progressively with increasing butyrate intake (P for trend <0.05).
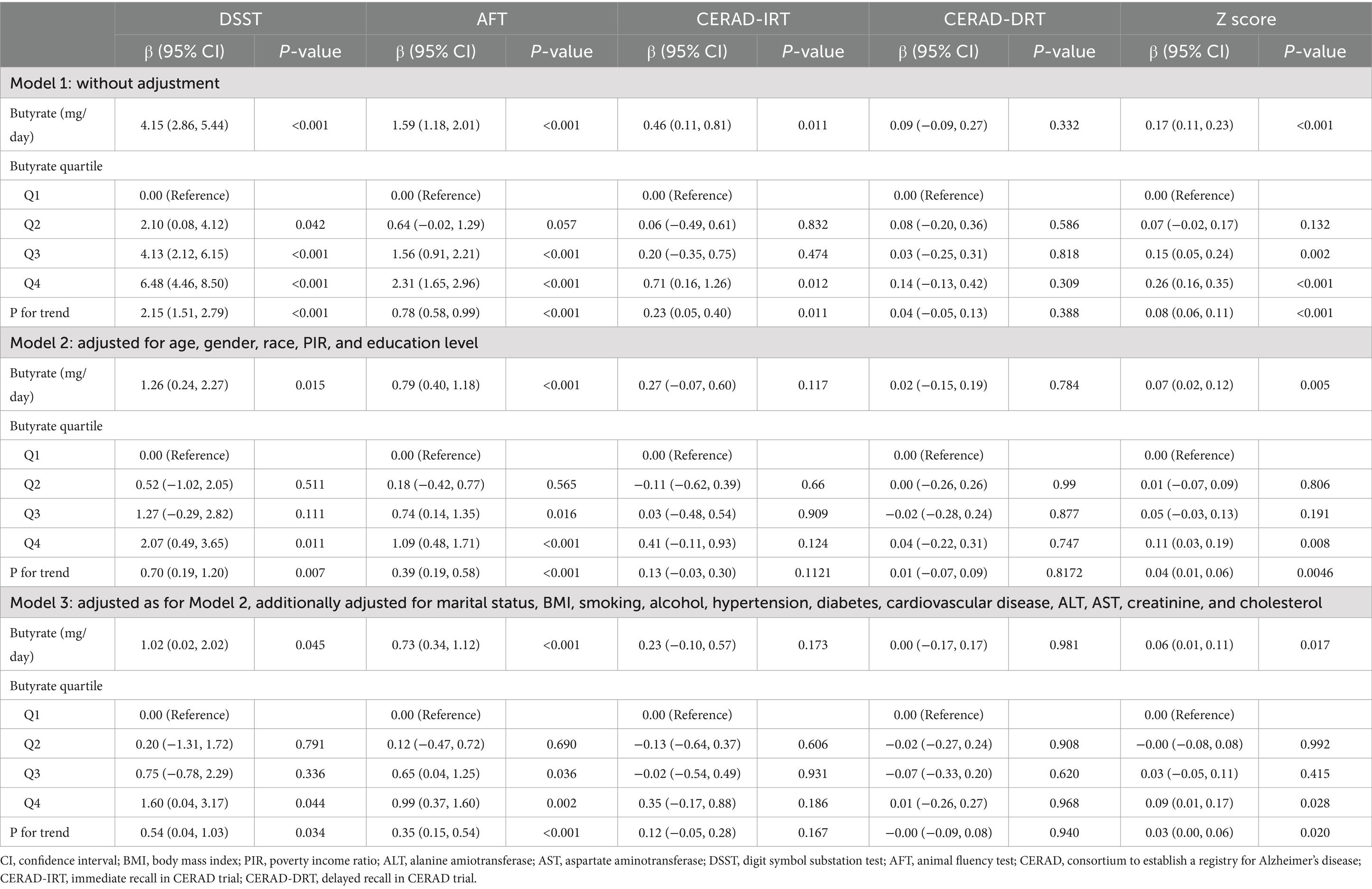
Table 2. Multivariable linear regression to assess the association of Butyrate intake with cognitive function.
To further explore the dose–response association between dietary butyrate intake and cognitive impairment in the elderly population, GAM analysis was used to visualize the association between butyrate intake and DSST, AFT, and Z scores (Figure 2). Following comprehensive adjustments, the results revealed a distinct association between the consumption of butyrate and cognitive function scores, showcasing a link that varies based on the dosage.
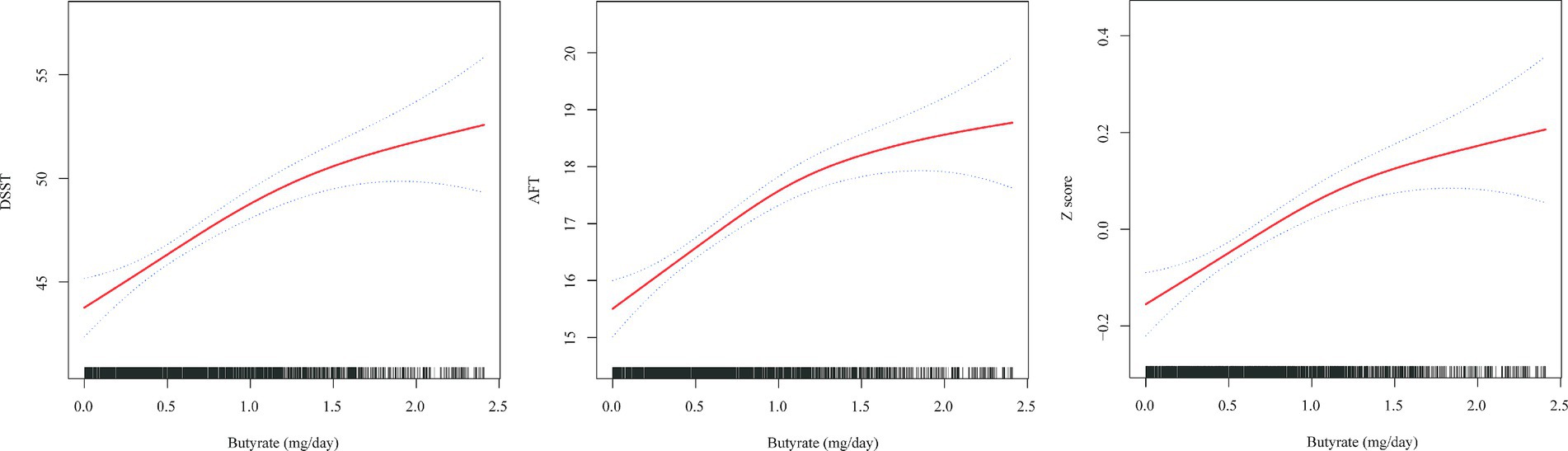
Figure 2. Association between butyrate (g/day) intake and cognitive impairment in an elderly population. The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval from the fit. All analyses were adjusted for age, gender, race, PIR, education level, marital status, BMI, smoking, alcohol, hypertension, diabetes, cardiovascular disease, stroke history, ALT, AST, creatinine, and cholesterol.
Our analyses showed that as far as the relationship between butyrate and AFT was concerned, it was positively associated across subgroups (age, gender, BMI, race, hypertension, diabetes, and cardiovascular disease) and the interactions showed that there was no significant interaction between the various factors and butyrate. (P for all interactions ≥0.05, Table 3). Furthermore, in Supplementary Tables 1, 2, positive associations between butyrate and DSST and Z score were similarly found. The interaction suggests that BMI and hypertension mediated the association between butyrate and DSST. Similarly, BMI mediated a positive association between butyrate and Z score.
This study analyzed data from a cross-sectional survey conducted between 2011 and 2014, focusing on older individuals. The findings revealed a substantial connection between butyrate intake and enhanced cognitive scores, even after accounting for potential confounding factors. Additionally, a notable dose-dependent relationship existed between butyrate intake and cognitive function in the Q1-Q4 intervals. The relationship between butyrate intake and AFT exhibited a significant nonlinear pattern, with an inflection point at approximately 1.73 g/day of butyrate intake. Before this point, AFT increased gradually with increasing butyrate intake; however, beyond the inflection point, the curve tended to decrease, suggesting that there might be an optimal range of butyrate intake for cognitive function beyond which additional intake may negatively impact cognitive function. For patients aged <80 years with hypertension without diabetes or stroke, butyrate had significant cognitive protective factors, although no significant differences were observed in the overall study population.
The relationship between AD and gut microbes has been extensively investigated in clinical studies, demonstrating a significant correlation between cognitive function and the prevalence of butyrate-producing bacteria (Verhaar et al., 2021; Hung et al., 2022). A clinical study involving 56 observers revealed a significant correlation between increased free water imaging in gray matter regions and a scarcity of butyrate-producing microorganisms in individuals belonging to the groups with AD and mild cognitive impairment (Yamashiro et al., 2024). Individuals with obesity typically exhibit imbalances in their gut microbiota, characterized by an increase in Aspergillus and a decrease in Clostridium butyrate levels. These imbalances have been strongly linked to cognitive decline. Furthermore, research indicates that oral Clostridium butyrate supplementation can effectively improve cognitive function in these individuals (Zheng et al., 2024). A prospective cohort study involving 135 patients, including those with cognitive normality, subjective cognitive decline, or mild cognitive impairment (MCI), found that patients with prodromal AD phenotype had lower levels of SCFA-producing bacteria, particularly butyrate-producing ones, compared to the control group with cognitive normality, with similar results observed in those with prior MCI (Liu et al., 2022; Liu et al., 2019). Research has demonstrated that vitamins B, D, and E possess neuroprotective qualities in the context of AD and Parkinson’s disease (Jia et al., 2024; Dysken et al., 2014; Wang et al., 2021; Samson et al., 2022; Zhou, 2023). Moreover, research on polyunsaturated fatty acids (PUFAs) has shown that increased levels of PUFAs and continuous supplementation with PUFAs over a period of time might potentially improve motor dysfunction and decelerate the advancement of cognitive decline in patients with AD (Chiu et al., 2008; Avallone et al., 2019; Chu et al., 2022). Clinical studies have shown that omega-3 PUFA supplements can enhance the presence of bacteria that are responsible for the production of butyrate and Mycobacterium anisopliae (Costantini et al., 2017; Zhuang et al., 2018; Zheng et al., 2021).
Clostridium butyrate (Cb), a member of the Clostridium genus and classified as a bacillus, is notable for its capability to produce butyrate, which has been associated with potential benefits for neurological ailments and the aging process (Stoeva et al., 2021). Research indicates that administering Cb to AD mice for 4 weeks effectively prevents Aβ accumulation, reduces microglia activation, and mitigates cognitive impairments (Sun et al., 2020). Oral administration of Cb improved motor functions impaired by MPTP, reduced dopaminergic neuron loss, mitigated synaptic dysfunction, and inhibited microglia activation in mice (Sun et al., 2021). Additionally, it significantly alleviated cognitive impairments and neuronal damage in a mouse model of sepsis-associated encephalopathy (Liu et al., 2020). Systematic transplantation and grafting of fecal microbiota from healthy wild-type mice to animals with AD improved the development of amyloid β-plaques and neurofibrillary tangles, decreased neuroglial reactions, and relieved cognitive impairment (Kim et al., 2020). Administering butyrate directly enhances cognitive function in mice with AD (Wang et al., 2022; Zhou et al., 2023). A comprehensive investigation incorporating both preclinical and clinical research discovered that a maternal diet low in fiber negatively affected cognitive function and synaptic plasticity in the offspring. Furthermore, it was determined that butyrate consumption, but not propionate, could reverse this impairment. Subsequent mechanistic studies revealed that histone deacetylase 4 is the primary mediator of butyrate-dependent neurocognitive improvement (Yu et al., 2020).
Although the exact mechanisms linking butyrate intake to cognitive increase remain unclear, our results are biologically plausible. Prior research has demonstrated a negative relationship between serum quinolinic acid levels and cognitive function and cortical gray matter reduction in human obesity. Quinolinic acid is known to harm neurons, alter intracellular signaling in dendritic spines, and decrease brain-derived neurotrophic factor (BDNF). However, butyrate, a gut microbiota metabolite, prevents quinolinic acid-induced damage by epigenetically enhancing H3K18ac on BDNF promoters, offsetting low BDNF levels and improving cognition (Ge et al., 2023). The oral administration of butyrate in mice exposed to lead improves cognitive memory by inhibiting STAT3 signaling in microglia and stimulating ACSS2 expression in hippocampal neurons. This increases acetyl-CoA levels, restoring H3K9ac deposition and BDNF expression (Li et al., 2024). Reduced butyrate production by the aged gut microbiota leads to decreased signaling of FFAR2/3, inhibiting mucin formation and increasing permeability, inflammation, and brain abnormalities (Mishra et al., 2024).
In our supplemental analyses, butyrate intake was found to be positively associated with Z-scores in participants younger than 70 years of age, whereas this relationship was no longer significant in participants older than 70 years of age. The possible reasons behind this are as follows: the composition and function of the intestinal microbiota undergoes significant changes with age, which may lead to less efficient production and absorption of butyrate (Ling et al., 2022). In addition, in older adults 70 years of age and older, chronic inflammation, oxidative stress, or other neurodegenerative pathologic changes that may be present may mask the potential benefits of butyrate (Khansari et al., 2009; Mou et al., 2022).
Employing a multifaceted approach, this study comprehensively assessed cognitive function through various tests, including CERAD-DRT, CERAD-IRT, DSST, AFT, and Z scores. To ensure a rigorous analysis, we accounted for confounders identified in prior research. To further investigate the possible relationship between butyrate consumption and cognitive performance, a dose–response study was also conducted.
Nonetheless, it is crucial to recognize the study’s limitations. First, given its cross-sectional design, determining the precise temporal relationship between butyrate intake and cognitive function remains elusive. Randomized controlled trials are essential for further validating our findings. Second, although efforts were made to account for potential confounding factors, it was not possible to completely remove the chance of reverse causation or residual confounding. Third, our reliance on self-reported 24-h dietary recalls for butyrate intake data introduced the risk of measurement and recall inaccuracies. Fourth, due to the limitations of the retrospective study, we did not collect information on whether participants additionally ingested butyrate supplements; we should take this factor into account in future studies and should be mindful of the scope of application in terms of interpretation of results. Lastly, further research is needed to determine if our findings extend beyond the specific US adult population studied.
In summary, our study found an association between butyrate consumption and cognitive performance among a nationally representative cohort of older adults in the United States of America. The findings hint at the possibility of a causal relationship, which warrants further investigation through large-scale prospective studies. Our results indicated that elevating butyrate levels, whether through dietary means or otherwise, could potentially contribute to maintaining brain function during aging.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Research Ethics Committee of the National Health. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
JT: Writing – original draft. JZ: Writing – original draft. GC: Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors thank the participants of the NHANES databases.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2025.1522498/full#supplementary-material
AD, Alzheimer’s disease; AFT, Animal Fluency Test; ALT, alanine aminotransferase; BDNF, brain-derived neurotrophic factor; BMI, body mass index; Cb, clostridium butyrate; CI, confidence interval; DSST, Digit Symbol Substitution Test; MCI, mild cognitive impairment; NHANES, National Health and Nutrition Examination Survey; PIR, poverty to income ratio; PUFA, polyunsaturated fatty acid; SCFAs, short-chain fatty acids; WHO, World Health Organization.
Alpino, G. C. A., Pereira-Sol, G. A., Dias, M. M. E., Aguiar, A. S., and Peluzio, M. (2024). Beneficial effects of butyrate on brain functions: a view of epigenetic. Crit. Rev. Food Sci. Nutr. 64, 3961–3970. doi: 10.1080/10408398.2022.2137776
Alzheimers Dement (2023). 2023 Alzheimer's disease facts and figures. Alzheimers Dement. 19, 1598–1695. doi: 10.1002/alz.13016
Avallone, R., Vitale, G., and Bertolotti, M. (2019). Omega-3 fatty acids and neurodegenerative diseases: new evidence in clinical trials. Int. J. Mol. Sci. 20:256. doi: 10.3390/ijms20174256
Boucaud-Maitre, D., Villeneuve, R., Rambhojan, C., Simo-Tabue, N., Thibault, N., Rinaldo, L., et al. (2024). Clinical characteristics of older adults living in Foster families in the French West Indies: baseline screening of the KArukera study of aging in Foster families (KASAF) cohort. Innov. Aging 8:igae063. doi: 10.1093/geroni/igae063
Brito, D. V. C., Esteves, F., Rajado, A. T., Silva, N., Consortium, A. S., Araujo, I., et al. (2023). Assessing cognitive decline in the aging brain: lessons from rodent and human studies. NPJ Aging 9:23. doi: 10.1038/s41514-023-00120-6
Chiu, C. C., Su, K. P., Cheng, T. C., Liu, H. C., Chang, C. J., Dewey, M. E., et al. (2008). The effects of omega-3 fatty acids monotherapy in Alzheimer's disease and mild cognitive impairment: a preliminary randomized double-blind placebo-controlled study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 32, 1538–1544. doi: 10.1016/j.pnpbp.2008.05.015
Chu, C. S., Hung, C. F., Ponnusamy, V. K., Chen, K. C., and Chen, N. C. (2022). Higher serum DHA and slower cognitive decline in patients with Alzheimer's disease: two-year follow-up. Nutrients 14:159. doi: 10.3390/nu14061159
Costantini, L., Molinari, R., Farinon, B., and Merendino, N. (2017). Impact of Omega-3 fatty acids on the gut microbiota. Int. J. Mol. Sci. 18:645. doi: 10.3390/ijms18122645
Dalile, B., Van Oudenhove, L., Vervliet, B., and Verbeke, K. (2019). The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478. doi: 10.1038/s41575-019-0157-3
Dysken, M. W., Sano, M., Asthana, S., Vertrees, J. E., Pallaki, M., Llorente, M., et al. (2014). Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 311, 33–44. doi: 10.1001/jama.2013.282834
Elnakib, S., Vecino-Ortiz, A. I., Gibson, D. G., Agarwal, S., Trujillo, A. J., Zhu, Y., et al. (2022). A novel score for mHealth apps to predict and prevent mortality: further validation and adaptation to the US population using the US National Health and nutrition examination survey data set. J. Med. Internet Res. 24:e36787. doi: 10.2196/36787
Ge, X., Zheng, M., Hu, M., Fang, X., Geng, D., Liu, S., et al. (2023). Butyrate ameliorates quinolinic acid-induced cognitive decline in obesity models. J. Clin. Invest. 133:612. doi: 10.1172/JCI154612
Ghaferi, A. A., Schwartz, T. A., and Pawlik, T. M. (2021). STROBE reporting guidelines for observational studies. JAMA Surg. 156, 577–578. doi: 10.1001/jamasurg.2021.0528
Guo, B., Zhang, J., Zhang, W., Chen, F., and Liu, B. (2025). Gut microbiota-derived short chain fatty acids act as mediators of the gut-brain axis targeting age-related neurodegenerative disorders: a narrative review. Crit. Rev. Food Sci. Nutr. 65, 265–286. doi: 10.1080/10408398.2023.2272769
Hendriksen, H. M. A., de Rijke, T. J., Fruijtier, A., van de Giessen, E., van Harten, A. C., van Leeuwenstijn-Koopman, M., et al. (2024). Amyloid PET disclosure in subjective cognitive decline: patient experiences over time. Alzheimers Dement. 20, 6556–6565. doi: 10.1002/alz.14148
Hung, C. C., Chang, C. C., Huang, C. W., Nouchi, R., and Cheng, C. H. (2022). Gut microbiota in patients with Alzheimer's disease spectrum: a systematic review and meta-analysis. Aging 14, 477–496. doi: 10.18632/aging.203826
Jia, W., Wang, H., Li, C., Shi, J., Yong, F., and Jia, H. (2024). Association between dietary vitamin B1 intake and cognitive function among older adults: a cross-sectional study. J. Transl. Med. 22:165. doi: 10.1186/s12967-024-04969-3
Khansari, N., Shakiba, Y., and Mahmoudi, M. (2009). Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Patents Inflamm. Allergy Drug Discov. 3, 73–80. doi: 10.2174/187221309787158371
Kim, S., Cho, J., Shin, D. W., Jeong, S. M., and Kang, D. (2023). Racial differences in long-term social, physical, and psychological health among adolescent and young adult cancer survivors. BMC Med. 21:289. doi: 10.1186/s12916-023-03005-3
Kim, M. S., Kim, Y., Choi, H., Kim, W., Park, S., Lee, D., et al. (2020). Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer's disease animal model. Gut 69, 283–294. doi: 10.1136/gutjnl-2018-317431
Li, Y., Liu, A., Chen, K., Li, L., Zhang, X., Zou, F., et al. (2024). Sodium butyrate alleviates lead-induced neuroinflammation and improves cognitive and memory impairment through the ACSS2/H3K9ac/BDNF pathway. Environ. Int. 184:108479. doi: 10.1016/j.envint.2024.108479
Ling, Z., Liu, X., Cheng, Y., Yan, X., and Wu, S. (2022). Gut microbiota and aging. Crit. Rev. Food Sci. Nutr. 62, 3509–3534. doi: 10.1080/10408398.2020.1867054
Liu, F., Duan, M., Fu, H., Zhao, G., Han, Y., Lan, F., et al. (2022). Orthopedic surgery causes gut microbiome Dysbiosis and intestinal barrier dysfunction in prodromal Alzheimer disease patients: a prospective observational cohort study. Ann. Surg. 276, 270–280. doi: 10.1097/SLA.0000000000005489
Liu, J., Jin, Y., Li, H., Yu, J., Gong, T., Gao, X., et al. (2020). Probiotics exert protective effect against Sepsis-induced cognitive impairment by reversing gut microbiota abnormalities. J. Agric. Food Chem. 68, 14874–14883. doi: 10.1021/acs.jafc.0c06332
Liu, J., Sun, J., Wang, F., Yu, X., Ling, Z., Li, H., et al. (2015). Neuroprotective effects of Clostridium butyricum against vascular dementia in mice via metabolic butyrate. Biomed. Res. Int. 2015:412946. doi: 10.1155/2015/412946
Liu, P., Wu, L., Peng, G., Han, Y., Tang, R., Ge, J., et al. (2019). Altered microbiomes distinguish Alzheimer's disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 80, 633–643. doi: 10.1016/j.bbi.2019.05.008
Loh, J. S., Mak, W. Q., Tan, L. K. S., Ng, C. X., Chan, H. H., Yeow, S. H., et al. (2024). Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 9:37. doi: 10.1038/s41392-024-01743-1
Mishra, S. P., Jain, S., Wang, B., Wang, S., Miller, B. C., Lee, J. Y., et al. (2024). Abnormalities in microbiota/butyrate/FFAR3 signaling in aging gut impair brain function. JCI Insight 9:443. doi: 10.1172/jci.insight.168443
Mou, Y., Du, Y., Zhou, L., Yue, J., Hu, X., Liu, Y., et al. (2022). Gut microbiota interact with the brain through systemic chronic inflammation: implications on Neuroinflammation, neurodegeneration, and aging. Front. Immunol. 13:796288. doi: 10.3389/fimmu.2022.796288
Nagpal, R., Neth, B. J., Wang, S., Craft, S., and Yadav, H. (2019). Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer's disease markers in subjects with mild cognitive impairment. EBioMedicine 47, 529–542. doi: 10.1016/j.ebiom.2019.08.032
Saint-Maurice, P. F., Graubard, B. I., Troiano, R. P., Berrigan, D., Galuska, D. A., Fulton, J. E., et al. (2022). Estimated number of deaths prevented through increased physical activity among US adults. JAMA Intern. Med. 182, 349–352. doi: 10.1001/jamainternmed.2021.7755
Samson, M. E., Yeung, L. F., Rose, C. E., Qi, Y. P., Taylor, C. A., and Crider, K. S. (2022). Vitamin B-12 malabsorption and renal function are critical considerations in studies of folate and vitamin B-12 interactions in cognitive performance: NHANES 2011-2014. Am. J. Clin. Nutr. 116, 74–85. doi: 10.1093/ajcn/nqac065
Stoeva, M. K., Garcia-So, J., Justice, N., Myers, J., Tyagi, S., Nemchek, M., et al. (2021). Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 13, 1–28. doi: 10.1080/19490976.2021.1907272
Sun, J., Li, H., Jin, Y., Yu, J., Mao, S., Su, K. P., et al. (2021). Probiotic Clostridium butyricum ameliorated motor deficits in a mouse model of Parkinson's disease via gut microbiota-GLP-1 pathway. Brain Behav. Immun. 91, 703–715. doi: 10.1016/j.bbi.2020.10.014
Sun, J., Xu, J., Yang, B., Chen, K., Kong, Y., Fang, N., et al. (2020). Effect of Clostridium butyricum against microglia-mediated Neuroinflammation in Alzheimer's disease via regulating gut microbiota and metabolites butyrate. Mol. Nutr. Food Res. 64:e1900636. doi: 10.1002/mnfr.201900636
Verhaar, B. J. H., Hendriksen, H. M. A., de Leeuw, F. A., Doorduijn, A. S., van Leeuwenstijn, M., Teunissen, C. E., et al. (2021). Gut microbiota composition is related to AD pathology. Front. Immunol. 12:794519. doi: 10.3389/fimmu.2021.794519
Wang, X., Wang, Z., Cao, J., Dong, Y., and Chen, Y. (2023). Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome 11:17. doi: 10.1186/s40168-022-01452-3
Wang, R., Wang, W., Hu, P., Zhang, R., Dong, X., and Zhang, D. (2021). Association of Dietary Vitamin D Intake, serum 25(OH)D(3), 25(OH)D(2) with cognitive performance in the elderly. Nutrients 13:89. doi: 10.3390/nu13093089
Wang, H., Zhang, M., Li, J., Liang, J., Yang, M., Xia, G., et al. (2022). Gut microbiota is causally associated with poststroke cognitive impairment through lipopolysaccharide and butyrate. J. Neuroinflammation 19:76. doi: 10.1186/s12974-022-02435-9
Wei, H., Yu, C., Zhang, C., Ren, Y., Guo, L., Wang, T., et al. (2023). Butyrate ameliorates chronic alcoholic central nervous damage by suppressing microglia-mediated neuroinflammation and modulating the microbiome-gut-brain axis. Biomed. Pharmacother. 160:114308. doi: 10.1016/j.biopha.2023.114308
Yamashiro, K., Takabayashi, K., Kamagata, K., Nishimoto, Y., Togashi, Y., Yamauchi, Y., et al. (2024). Free water in gray matter linked to gut microbiota changes with decreased butyrate producers in Alzheimer's disease and mild cognitive impairment. Neurobiol. Dis. 193:106464. doi: 10.1016/j.nbd.2024.106464
Yu, L., Zhong, X., He, Y., and Shi, Y. (2020). Butyrate, but not propionate, reverses maternal diet-induced neurocognitive deficits in offspring. Pharmacol. Res. 160:105082. doi: 10.1016/j.phrs.2020.105082
Zeng, S., Ma, L., Mao, H., Shi, Y., Xu, M., Gao, Q., et al. (2024). Dynamic functional network connectivity in patients with a mismatch between white matter hyperintensity and cognitive function. Front. Aging Neurosci. 16:1418173. doi: 10.3389/fnagi.2024.1418173
Zheng, S. Y., Li, H. X., Xu, R. C., Miao, W. T., Dai, M. Y., Ding, S. T., et al. (2021). Potential roles of gut microbiota and microbial metabolites in Parkinson's disease. Ageing Res. Rev. 69:101347. doi: 10.1016/j.arr.2021.101347
Zheng, M., Ye, H., Yang, X., Shen, L., Dang, X., Liu, X., et al. (2024). Probiotic Clostridium butyricum ameliorates cognitive impairment in obesity via the microbiota-gut-brain axis. Brain Behav. Immun. 115, 565–587. doi: 10.1016/j.bbi.2023.11.016
Zhou, L. (2023). Association of vitamin B2 intake with cognitive performance in older adults: a cross-sectional study. J. Transl. Med. 21:870. doi: 10.1186/s12967-023-04749-5
Zhou, Y., Xie, L., Schroder, J., Schuster, I. S., Nakai, M., Sun, G., et al. (2023). Dietary Fiber and microbiota metabolite receptors enhance cognition and alleviate disease in the 5xFAD mouse model of Alzheimer's disease. J. Neurosci. 43, 6460–6475. doi: 10.1523/JNEUROSCI.0724-23.2023
Keywords: cognitive function, butyrate intake, short-chain fat acids, NHANES, cross-sectional study
Citation: Tu J, Zhang J and Chen G (2025) Higher dietary butyrate intake is associated with better cognitive function in older adults: evidence from a cross-sectional study. Front. Aging Neurosci. 17:1522498. doi: 10.3389/fnagi.2025.1522498
Received: 04 November 2024; Accepted: 13 March 2025;
Published: 28 March 2025.
Edited by:
Vijay Karkal Hegde, Texas Tech University, United StatesReviewed by:
Sarah Holden, Oregon Health and Science University, United StatesCopyright © 2025 Tu, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Chen, Y2hlbmdhbmcxMjBAemp1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.