
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Aging Neurosci., 07 January 2025
Sec. Neuroinflammation and Neuropathy
Volume 16 - 2024 | https://doi.org/10.3389/fnagi.2024.1507683
Introduction: This study aimed to identify differences in the levels of inflammation-related biomarkers between patients with subcortical silent brain infarcts (SBIs) and healthy controls. We also evaluated the effect of aspirin on the subcortical SBI inflammatory processes.
Methods: Consecutive patients diagnosed with subcortical SBIs without a history of acute stroke were included. The demographic and clinical data of the 26 subjects with subcortical SBIs, such as the number and location of subcortical SBIs, were reviewed. Plasma levels of macrophage migration inhibitory factor (MIF), matrix metalloproteinase-9 (MMP-9), and visfatin were measured in patients with subcortical SBIs and ten healthy participants. These biomarkers were rechecked in patients with subcortical SBI 3 months after taking aspirin (100 mg/day).
Results: MIF and MMP-9 levels were significantly higher in patients with subcortical SBIs than in healthy control group (p = 0.031 and p = 0.026, respectively). Although MIF and MMP-9 did not show significant changes after taking aspirin for 3 months, the median plasma level of visfatin was significantly decreased from 1.00 ng/mL (range, 0.86–1.16 ng/mL) to 0.84 ng/mL (range, 0.77–0.91 ng/mL) (p = 0.002) after taking aspirin.
Discussion: Inflammation could be an essential factor in the pathogenesis of subcortical SBIs, and aspirin affects several inflammation-related biomarkers.
Subcortical silent brain infarcts (SBIs) are unrecognized cerebral infarcts in the brain subcortex that do not produce acute stroke symptoms, but are visible as focal lesions on brain computed tomography (CT) and magnetic resonance imaging (MRI) (Vermeer et al., 2007; Norrving, 2015). The annual incidence of SBI is 2 to 4%, and the prevalence ranges from 5 to 62% in population-based cohorts (Fanning et al., 2014b). Patients with subcortical SBIs have a higher risk of symptomatic stroke, depression, and dementia, and a steeper decline in cognitive function than those without such lesions (Fanning et al., 2014a). Thus, detecting subcortical SBIs to prevent secondary deteriorative events, such as symptomatic ischemic stroke and vascular dementia, has generated broad interest.
Inflammation plays a crucial role in the pathogenesis of acute symptomatic infarcts and subcortical SBIs (Wang et al., 2007). Chronic inflammation is linked to the development of atherosclerosis, which is mainly associated with plaque progression and instability in the large arteries (Jayaraj et al., 2019). One of the critical issues in the pathogenesis of SBIs is the underlying inflammatory process and its connection to specific biomarkers.
Aspirin is a well-known medication used for the prevention of secondary stroke and primary prevention in patients with comorbidities (Hart et al., 2000). Besides the anti-thrombotic effect caused by the inhibition of cyclooxygenase-1 (COX-1), which results in thromboxane synthetase, anti-inflammatory effects via the acetylation of COX-2 by aspirin reduce vascular inflammation and stabilize atherosclerotic plaques (Cyrus et al., 2002; Chiang et al., 2004). Inflammatory cytokines have been linked to the severity of chronic stable angina, and aspirin reduces these cytokines (Ikonomidis et al., 1999). Researchers suggest that aspirin also exerts a protective effect against inflammation by modulating cytokines in cerebral ischemia. However, there are no data on how aspirin affects the biomarkers associated with angiogenesis and inflammation in patients with SBI.
To demonstrate the profile of biomarkers related to inflammation in subcortical SBIs and the effect of aspirin on these factors, we selected plasma levels of migration inhibitory factor (MIF) and matrix metalloproteinase-9 (MMP-9) for analysis because MIF and MMP-9 are well-known plasma biomarkers related to inflammatory processes that disrupt blood–brain barrier permeability in acute ischemic stroke (Vandooren et al., 2014; Liu et al., 2018). We also examined plasma visfatin, a proinflammatory cytokine involved in the formation of atherosclerosis, in patients with subcortical SBIs and healthy controls (Lu et al., 2009). Biomarkers were retested after 3 months of aspirin administration to determine the effect of aspirin on the inflammatory cascade in patients with subcortical SBIs.
This study was designed as a prospective trial. We recruited 225 consecutive patients who underwent brain MRI at an outpatient clinic between January 2012 and July 2012. Patients with a history of stroke-related symptoms, signal abnormalities on brain MRI indicating acute infarction or hemorrhagic stroke, intra-and extracranial atherosclerotic stenosis (n = 187), diagnosed with an active stage of inflammatory disease (n = 8), and recent medication changes affecting inflammatory biomarkers within the last 3 months (n = 4) were excluded. Consequently, we included 26 patients who started taking 100 mg aspirin daily on the day of initial diagnosis. In addition, ten age- and sex-matched healthy subjects, confirmed via brain MRI to have no subcortical SBIs, no history of cerebrovascular accidents, or risk factors affecting vascular conditions, such as diabetes, dyslipidemia, and smoking, were recruited as controls from Hanyang University Guri Hospital. Before entering the trial, informed consent was obtained from each participant or their legal representative. This study was approved by the Institutional Review Board of Hanyang University Guri Hospital (IRB No. 2010-01-081).
The baseline characteristics and clinical data of the recruited subjects were collected. Demographic information included age, sex, body mass index (BMI), presence of ischemic stroke risk factors (hypertension, diabetes, hyperlipidemia, and smoking), and medication history. We recorded each subject’s height and weight to calculate BMI using the following formula: BMI = weight (kg)/height (m2). Hypertension was defined as systolic blood pressure > 140 mmHg, diastolic blood pressure > 90 mmHg, or the use of antihypertensive medication. We classified the presence of dyslipidemia as a previous diagnosis of the disease and concurrent intake of lipid-lowering agents. The presence of diabetes was characterized by taking antidiabetic drugs with a prior diagnosis of the disease or random blood glucose levels higher than 200 mg/dL.
MRI was performed on all individuals included in this study using a 1.5-T superconducting magnet (CV/I; GE Medical Systems, Seoul, Korea). Neuroradiologists initially interpreted the brain imaging results without being informed of the patient’s clinical data. Subcortical SBI was defined as a focal hyperintensity on T2-weighted images, at least 3.0 mm in size, with hypointensity on T1-weighted images and a hyperintense rim on fluid-attenuated inversion recovery images found in the brain subcortex (Vermeer et al., 2007; Zhu et al., 2011). We also collected the number, location, and size of the subcortical SBIs.
MIF, MMP-9, and visfatin levels were measured using commercially available quantitative sandwich ELISA kits following the manufacturer’s instructions (R&D Systems, Minneapolis, MN, United States). Plasma samples from patients with SBIs were collected at baseline and after 3 months. The plasma of healthy controls was collected only at the baseline. All samples were stored at −80°C.
We used R software and IBM SPSS Statistics for Windows 26.0 (Chicago, IL, United States) for statistical analyses (version 3.6.0.). A p-value less than 0.05 was defined as the threshold for significance. Pearson’s chi-square test was used to compare categorical variables between the two groups. The Shapiro–Wilk test was used to determine normal distribution of continuous variables, and data showing normal distribution were subjected to the Bartlett test to assess the homogeneity of variances. We also used the independent two-sample T-test, Welch two-sample T-test, and Mann–Whitney test to determine differences between the patient group and healthy controls. To evaluate the effect of aspirin on the biomarkers, we used the Wilcoxon signed-rank test.
The demographic and clinical data of the 26 patients with subcortical SBIs and ten healthy controls are presented in Table 1. There were no significant differences in demographic characteristics (age, sex, and BMI) between the two groups. Among the patients with subcortical SBI, eight (30.8%) had hypertension, four (15.4%) had diabetes, two (7.7%) had dyslipidemia, and one (3.8%) was a current smoker. The mean number of subcortical SBIs per patient was 1.5. The mean diameter of the lesions was 3.7 mm and ranged from 3.0 to 5.0 mm. A single SBI was detected in 17 patients, and nine subjects had two SBIs. The highest number of lesions per patient was three subcortical SBIs, which were present in three patients. All subcortical SBIs were located in the subcortical areas. The most frequent subcortical SBI regions were the basal ganglia (52.6%) and the subcortical areas (44.7%). Only one patient had SBI in the thalamus.
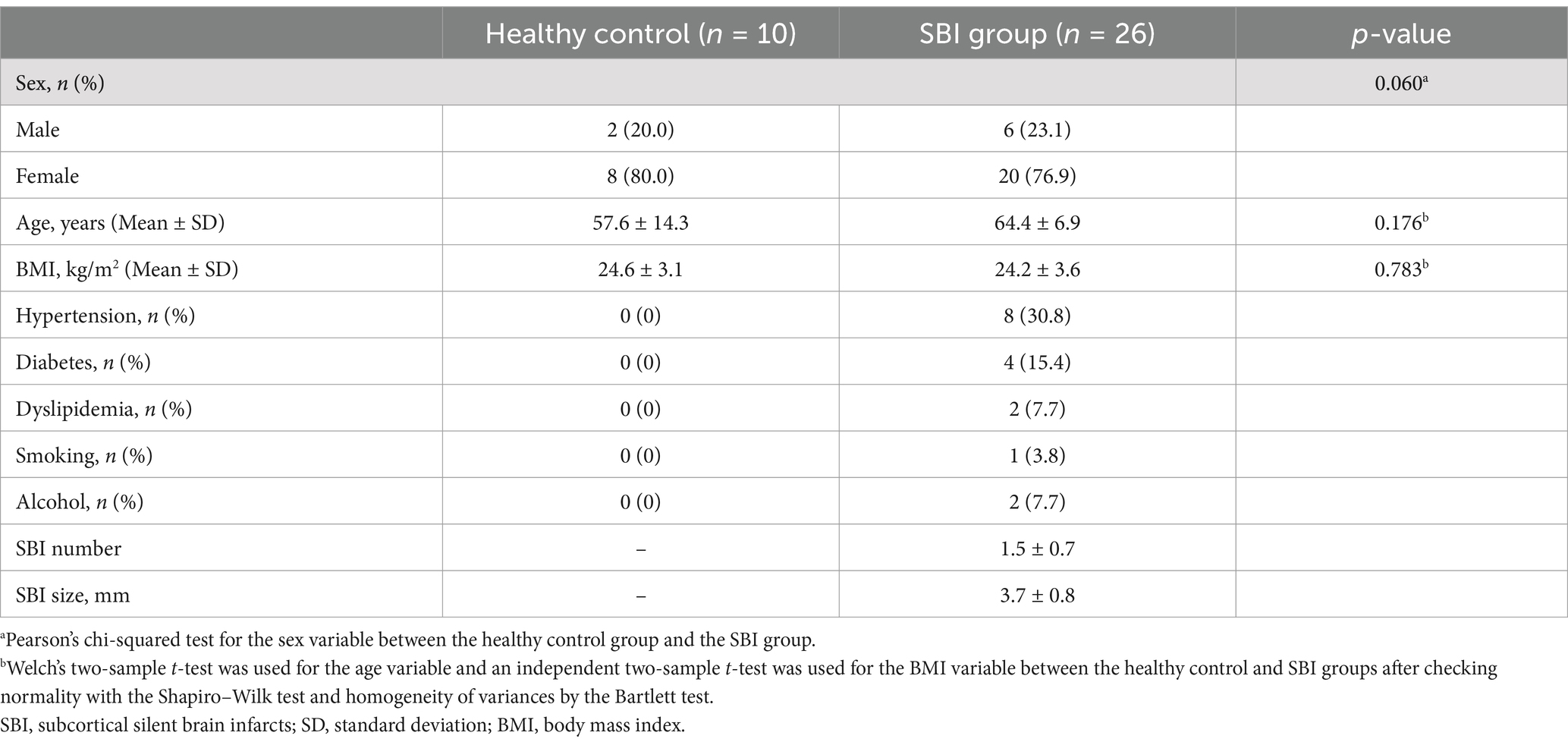
Table 1. Demographic and clinical characteristics of patients with subcortical silent brain infarcts and healthy controls.
Table 2 and figure present the biomarker levels in patients with subcortical SBIs and healthy controls. In the healthy control subjects, the median MIF level was 58.83 ng/mL (interquartile range (IQR), 44.82–78.97 ng/mL). The median MIF level in the SBI group was 76.75 ng/mL (IQR, 75.19–85.95 ng/mL), presenting a significant difference (p = 0.031). MMP-9 levels were also elevated in patients with SBIs compared to healthy subjects (28.25 ± 7.80 ng/mL vs. 38.97 ± 13.68 ng/mL, p = 0.026). However, visfatin levels did not differ significantly between the two groups were not significantly different.
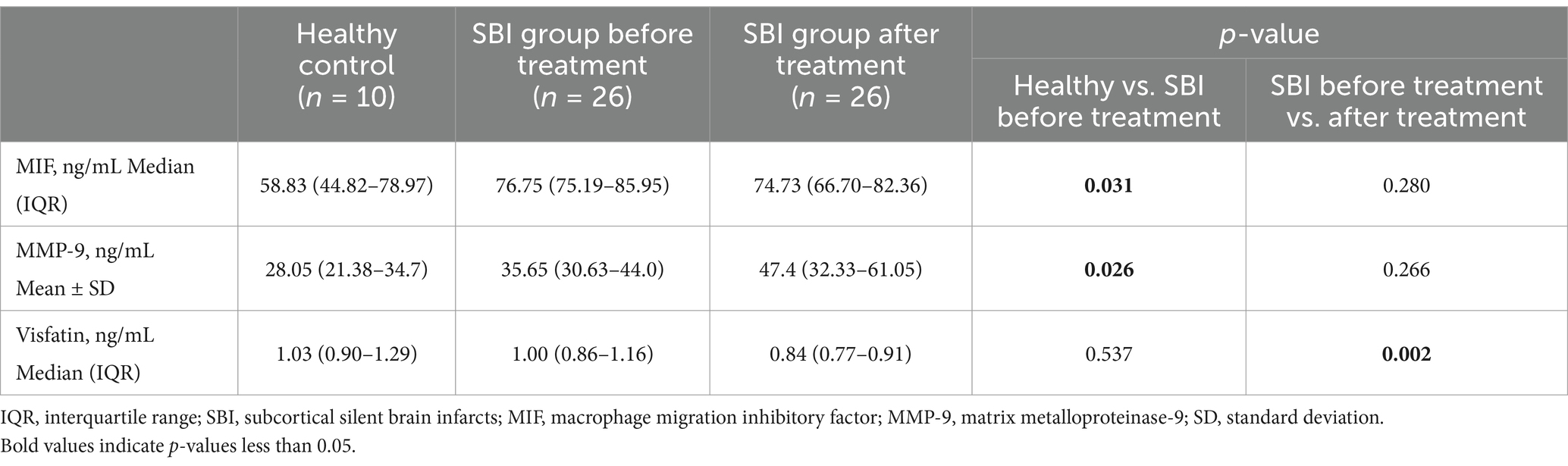
Table 2. Comparison of biomarker results in the SBI patient group before and after treatment, and the healthy control group.
Next, we compared biomarker levels at baseline and after taking aspirin for 3 months in patients with subcortical SBIs (Table 2; Figures 1, 2). The baseline median plasma visfatin level in the subcortical SBI group was 1.00 ng/mL (IQR, 0.86–1.16 ng/mL), while the median visfatin level decreased to 0.84 ng/mL (IQR, 0.77–0.91 ng/mL) after 3 months of aspirin treatment (p = 0.002). Other biomarkers did not show any significant differences.
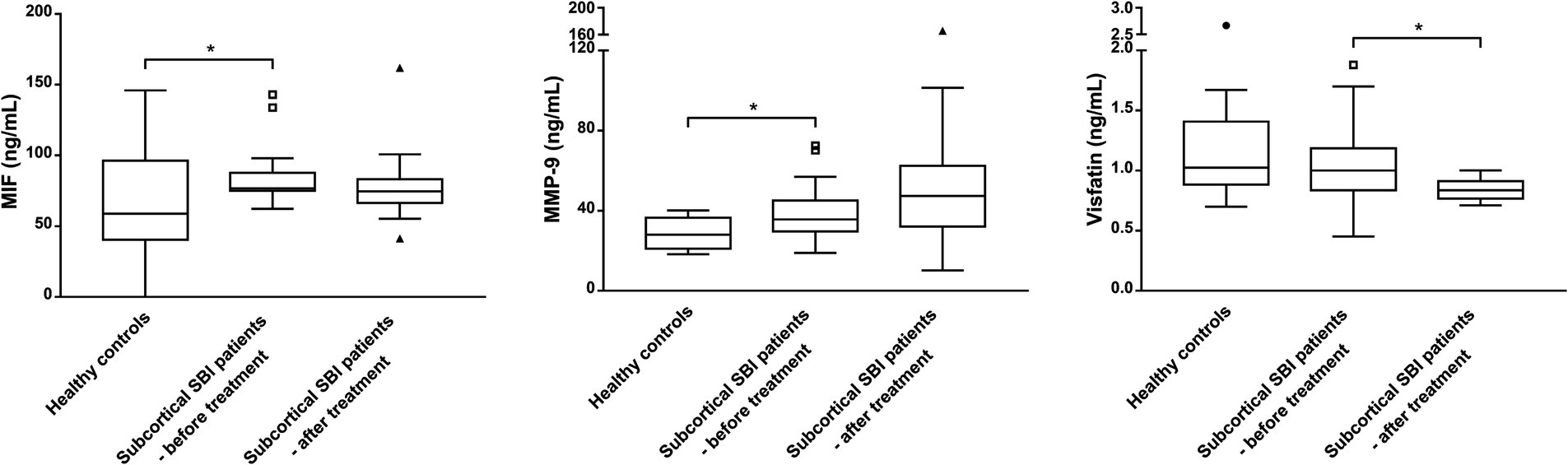
Figure 1. Biomarker levels measured after treatment with aspirin for 3 months in patients with subcortical silent brain infarcts (SBI) compared to levels in healthy controls. MIF and MMP-9 levels, which showed significant differences between healthy controls and the baseline concentrations of patients with SBIs, were not significantly different after treatment with aspirin. Comparisons were made against the control (* p < 0.05; Mann–Whitney test). Visfatin levels in patients with SBIs were significantly lower after taking aspirin for 3 months. Boxes indicate 25 and 75% percentiles, with the horizontal median line inside the boxes. Error bars represent 5 and 95% ranges, providing a clear view of the central variability in the data while reducing the impact of extreme outliers. Data points represented as triangles and circles indicate values that lie outside the error bar ranges. The conditions before and after taking aspirin were compared (* p < 0.05; Wilcoxon signed rank test).
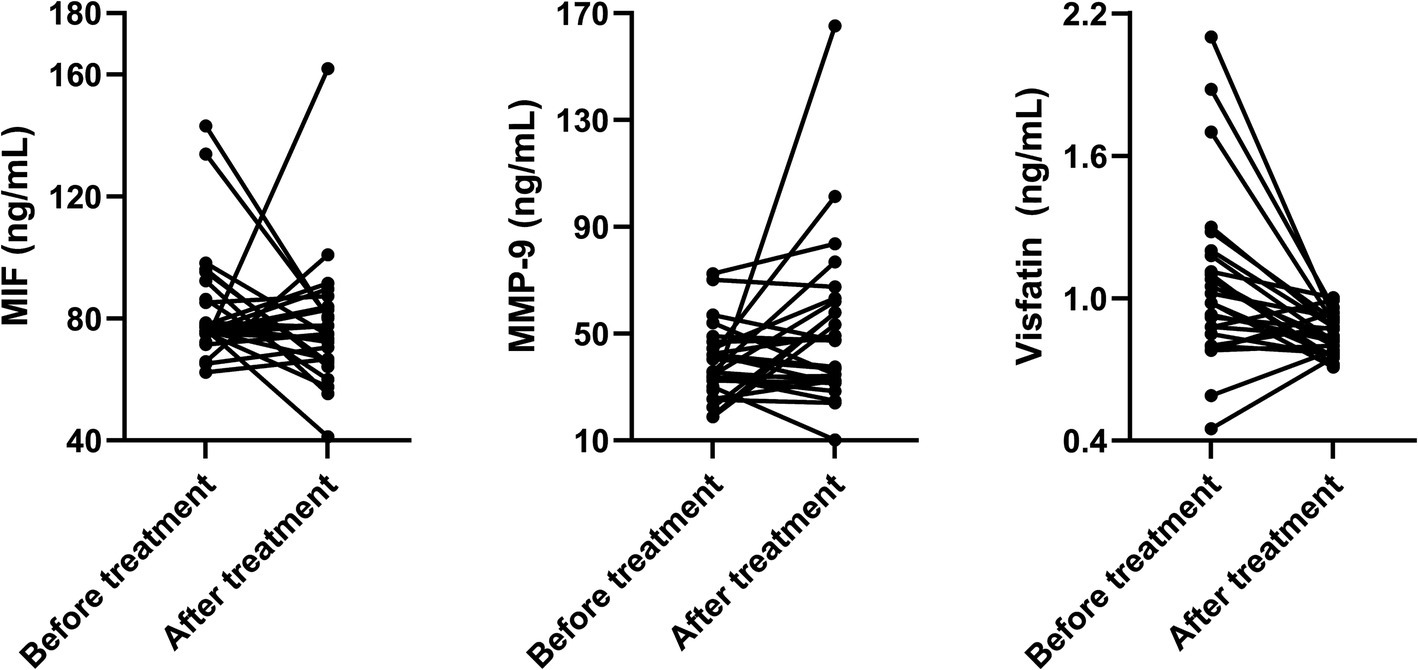
Figure 2. Paired line graphs showing individual changes in biomarkers before and after aspirin treatment in patients with subcortical silent brain infarcts.
This study investigated differences in plasma inflammatory biomarkers between patients with subcortical SBIs and healthy subjects. Higher plasma concentrations of MIF and MMP-9 were detected in patients with SBI than in healthy controls. We also determined alterations in biomarkers after 3 months of aspirin administration. A decrease in plasma visfatin levels was observed, which could be attributed to the effect of aspirin on inflammatory biomarkers.
Various inflammation-related biomarkers have been implicated in the pathophysiology of ischemic stroke. For example, MIF, a neuromodulator, particularly in neurons, promotes neuronal death and aggravates neurological deficits in an experimental stroke model (Inácio et al., 2011). Visfatin is a novel adipokine that is upregulated in ischemic stroke and promotes the expression of inflammatory cytokines and atherosclerosis through vascular smooth cell maturation (Lu et al., 2009). The neuroinflammatory response of MMP-9 in ischemic stroke has also been investigated. Elevated MMP-9 levels result in neurological deterioration in acute lacunar stroke and parenchymal hematoma formation after thrombolytic therapy (Kim et al., 2006; Castellanos et al., 2007). However, MMP-9 also participates in plasticity and recovery throughout the late phase of cerebral ischemia (Wang et al., 2007).
Previous studies evaluated the relationship between inflammatory biomarkers and SBI. Many investigators regard subcortical SBI as sharing a core pathophysiological process with acute symptomatic ischemic stroke. For instance, Hoshi et al. reported that the levels of inflammatory biomarkers, such as high-sensitivity C-reactive protein and interleukin-6, were higher in patients with SBIs than in the healthy control group (Hoshi et al., 2005). We believe that this study strengthens the evidence that subcortical SBIs undergo hypoxic events similar to acute symptomatic cerebral ischemia by presenting higher MIF levels in patients with subcortical SBIs than in healthy controls. Although Sarchielli et al. reported that MIF levels did not differ between groups diagnosed with SBIs and healthy populations (Sarchielli et al., 2013), several additional studies have suggested that MIF expression is upregulated in ischemic conditions (Li et al., 2017; Neumaier et al., 2023). We believe that hypoxic events may cause higher MIF levels during the occurrence of subcortical SBIs. We also found that plasma MMP-9 levels were higher in patients with subcortical SBIs than in healthy subjects. MMP-9 does not exist in the central nervous system but can be detected after cerebral ischemia and breakdown of the blood–brain barrier (BBB) (Rosell et al., 2006), eventually leading to neuronal death (Rosell et al., 2006; Koh et al., 2011). We suggest that elevated MMP-9 levels in patients with subcortical SBIs contribute to the evidence that the BBB is also disrupted in subcortical SBIs, resulting in neuronal injury.
The present study also demonstrated a decrease in plasma visfatin levels after aspirin treatment. Previous studies have reported that higher visfatin levels correlate with disease severity in ischemic stroke and are independent risk factors for atherosclerosis formation (Yin et al., 2013; Kong et al., 2014). Visfatin acts as a proinflammatory cytokine and provokes chronic inflammation by stimulating the maturation of pre-B cells and the expression of inflammatory cytokines in epithelial cells and prolonging neutrophil survival (Lu et al., 2009). Aspirin may block this inflammatory cascade. By acetylating COX-2, aspirin can inhibit COX-2-mediated cell activation and proliferation, and limit the release of these cytokines into the bloodstream (Chiang et al., 2004). We can assume that aspirin’s action on visfatin is important in cerebral ischemia, including SBI, along with disturbing platelet aggregation, oxidative stress, endothelial activation/dysfunction, and other anti-inflammatory reactions (Khan and Mehta, 2005).
While the baseline plasma MIF and MMP-9 levels in patients with subcortical SBIs were significantly higher than those in healthy controls, the plasma MIF and MMP-9 levels did not show significant differences in the patient group after aspirin treatment. Although not statistically significant, plasma MIF levels decreased slightly following aspirin administration. Aspirin is well-known for its acetylation of cyclooxygenases, and previous studies have reported that it may also enhance MIF acetylation in animal models with ischemic injury (Cyrus et al., 2002; Hu et al., 2022). Based on these findings and the results of this study, we can hypothesize that aspirin may not induce a significant absolute reduction in MIF levels in SBIs but could modestly decrease MIF levels through acetylation. Further extensive studies in humans are warranted to validate this hypothesis. Regarding MMP-9, an increasing trend was observed following aspirin treatment, albeit without statistical significance. This result contrasts with prior studies reporting a reduction in MMP-9 levels after aspirin administration in in vitro models for atherosclerosis studies (Hua et al., 2009). Since MMP-9 has been implicated in plasticity and recovery during the later phases of stroke, it is plausible that the interaction between MMP-9 and aspirin in asymptomatic SBI may have distinct characteristics (Wang et al., 2007). Further research is needed to explore this interaction in in a more comprehensive manner.
This study had some limitations. First, data were collected from a single center. As a result, we only observed inflammatory biomarkers from the relatively limited sample sizes of patients and control subjects. Second, the diagnostic evaluations of other comorbidities, such as heart and other vascular diseases, which can be risk factors for SBI, were not performed. Third, we cannot explain how aspirin affects inflammatory biomarkers since this was an observational study using the plasma of patients and healthy volunteers. Investigating the molecular details of the mode of action of aspirin to obtain more definite conclusions would strengthen the findings of this study. Lastly, the relatively small sample size may limit the generalizability of the results. Future studies with larger, multicenter cohorts are essential to validate the findings of this study before broader implementation.
Despite these limitations, the present investigation revealed that inflammatory biomarkers were increased in patients with SBIs, suggesting the existence of an inflammatory process in the pathogenesis of subcortical SBI. Furthermore, our study demonstrated downregulation of inflammatory biomarkers associated with inflammation after aspirin administration. Our results also indicate that the anti-inflammatory nature of aspirin regulates inflammatory biomarker profiles in patients with subcortical SBIs. Therefore, we suggest aspirin intake in patients with comorbidities and a higher risk of symptomatic ischemic stroke.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Institutional Review Board of Hanyang University Guri Hospital (IRB No. 2010-01-081). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
WS: Writing – original draft. YK: Writing – original draft. K-YL: Investigation, Methodology, Project administration, Supervision, Writing – review & editing. J-AJ: Data curation, Validation, Writing – review & editing. HC: Formal analysis, Supervision, Validation, Writing – original draft. YL: Methodology, Project administration, Supervision, Validation, Writing – review & editing. S-HK: Data curation, Formal analysis, Supervision, Validation, Visualization, Writing – review & editing, Writing – original draft.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT (MSIT) (Grant no.: RS-2024-00431471).
We thank the staff from the Department of Neurology, College of Medicine, Hanyang University, and the patients for participating in the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BBB, brain–blood barrier; BMI, body mass index; COX, cyclooxygenase; CT, computed tomography; IQR, interquartile range; MIF, macrophage migration inhibitory factor; MMP-9, matrix metalloproteinase-9; MRI, magnetic resonance imaging; SBI, subcortical silent brain infarcts.
Castellanos, M., Sobrino, T., Millán, M., Ma, G., Arenillas, J., Nombela, F., et al. (2007). Serum cellular fibronectin and matrix metalloproteinase-9 as screening biomarkers for the prediction of parenchymal hematoma after thrombolytic therapy in acute ischemic stroke: a multicenter confirmatory study. Stroke 38, 1855–1859. doi: 10.1161/STROKEAHA.106.481556
Chiang, N., Bermudez, E. A., Ridker, P. M., Hurwitz, S., and Serhan, C. N. (2004). Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc. Natl. Acad. Sci. USA 101, 15178–15183. doi: 10.1073/pnas.0405445101
Cyrus, T., Sung, S., Zhao, L., Funk, C. D., Tang, S., and Praticò, D. (2002). Effect of low-dose aspirin on vascular inflammation, plaque stability, and Atherogenesis in low-density lipoprotein receptor–deficient mice. Circulation 106, 1282–1287. doi: 10.1161/01.CIR.0000027816.54430.96
Fanning, J. P., Wesley, A. J., Wong, A. A., and Fraser, J. F. (2014a). Emerging spectra of silent brain infarction. Stroke 45, 3461–3471. doi: 10.1161/STROKEAHA.114.005919
Fanning, J. P., Wong, A. A., and Fraser, J. F. (2014b). The epidemiology of silent brain infarction: a systematic review of population-based cohorts. BMC Med. 12, 1–11. doi: 10.1186/s12916-014-0119-0
Hart, R. G., Halperin, J. L., McBride, R., Benavente, O., Man-Son-Hing, M., and Kronmal, R. A. (2000). Aspirin for the primary prevention of stroke and other major vascular events: meta-analysis and hypotheses. Arch. Neurol. 57, 326–332. doi: 10.1001/archneur.57.3.326
Hoshi, T., Kitagawa, K., Yamagami, H., Furukado, S., Hougaku, H., and Hori, M. (2005). Relations of serum high-sensitivity C-reactive protein and interleukin-6 levels with silent brain infarction. Stroke 36, 768–772. doi: 10.1161/01.STR.0000158915.28329.51
Hu, J.-X., Ma, W.-J., He, L.-Y., Zhang, C.-H., Zhang, C., Wang, Y., et al. (2022). Macrophage migration inhibitory factor (MIF) acetylation protects neurons from ischemic injury. Cell Death Dis. 13:466. doi: 10.1038/s41419-022-04918-2
Hua, Y., Xue, J., Sun, F., Zhu, L., and Xie, M. (2009). Aspirin inhibits MMP-2 and MMP-9 expressions and activities through upregulation of PPARα/γ and TIMP gene expressions in ox-LDL-stimulated macrophages derived from human monocytes. Pharmacology 83, 18–25. doi: 10.1159/000166183
Ikonomidis, I., Andreotti, F., Economou, E., Stefanadis, C., Toutouzas, P., and Nihoyannopoulos, P. (1999). Increased Proinflammatory cytokines in patients with chronic stable angina and their reduction by aspirin. Circulation 100, 793–798. doi: 10.1161/01.CIR.100.8.793
Inácio, A. R., Ruscher, K., Leng, L., Bucala, R., and Deierborg, T. (2011). Macrophage migration inhibitory factor promotes cell death and aggravates neurologic deficits after experimental stroke. J. Cereb. Blood Flow Metab. 31, 1093–1106. doi: 10.1038/jcbfm.2010.194
Jayaraj, R. L., Azimullah, S., Beiram, R., Jalal, F. Y., and Rosenberg, G. A. (2019). Neuroinflammation: friend and foe for ischemic stroke. J. Neuroinflammation 16, 1–24. doi: 10.1186/s12974-019-1516-2
Khan, Q., and Mehta, J. L. (2005). Relevance of platelet-independent effects of aspirin to its salutary effect in atherosclerosis-related events. J. Atheroscler. Thromb. 12, 185–190. doi: 10.5551/jat.12.185
Kim, Y. S., Lee, K.-Y., Koh, S.-H., Park, C. Y., Kim, H. Y., Lee, Y. J., et al. (2006). The role of matrix metalloproteinase 9 in early neurological worsening of acute lacunar infarction. Eur. Neurol. 55, 11–15. doi: 10.1159/000091137
Koh, S. H., Park, C., Kim, M., Lee, K. Y., Kim, J., Chang, D. I., et al. (2011). Microbleeds and free active MMP-9 are independent risk factors for neurological deterioration in acute lacunar stroke. Eur. J. Neurol. 18, 158–164. doi: 10.1111/j.1468-1331.2010.03100.x
Kong, Q., Xia, M., Liang, R., Li, L., Cu, X., Sun, Z., et al. (2014). Increased serum visfatin as a risk factor for atherosclerosis in patients with ischaemic cerebrovascular disease. Singapore Med. J. 55, 383–387. doi: 10.11622/smedj.2014091
Li, Y.-S., Chen, W., Liu, S., Zhang, Y.-Y., and Li, X.-H. (2017). Serum macrophage migration inhibitory factor levels are associated with infarct volumes and long-term outcomes in patients with acute ischemic stroke. Int. J. Neurosci. 127, 539–546. doi: 10.1080/00207454.2016.1211648
Liu, Y.-C., Tsai, Y.-H., Tang, S.-C., Liou, H.-C., Kang, K.-H., Liou, H.-H., et al. (2018). Cytokine MIF enhances blood-brain barrier permeability: impact for therapy in ischemic stroke. Sci. Rep. 8:743. doi: 10.1038/s41598-017-16927-9
Lu, L.-F., Yang, S.-S., Wang, C.-P., Hung, W.-C., Yu, T.-H., Chiu, C.-A., et al. (2009). Elevated visfatin/pre-B-cell colony-enhancing factor plasma concentration in ischemic stroke. J. Stroke Cerebrovasc. Dis. 18, 354–359. doi: 10.1016/j.jstrokecerebrovasdis.2009.01.003
Neumaier, F., Stoppe, C., Stoykova, A., Weiss, M., Veldeman, M., Höllig, A., et al. (2023). Elevated concentrations of macrophage migration inhibitory factor in serum and cerebral microdialysate are associated with delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Front. Neurol. 13:1066724. doi: 10.3389/fneur.2022.1066724
Norrving, B. (2015). Evolving concept of small vessel disease through advanced brain imaging. J. Stroke 17, 94–100. doi: 10.5853/jos.2015.17.2.94
Rosell, A., Ortega-Aznar, A., Alvarez-Sabín, J., Fernández-Cadenas, I., Ribó, M., Molina, C. A., et al. (2006). Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke 37, 1399–1406. doi: 10.1161/01.STR.0000223001.06264.af
Sarchielli, P., Nardi, K., Chiasserini, D., Eusebi, P., Tantucci, M., Di Piero, V., et al. (2013). Immunological profile of silent brain infarction and lacunar stroke. PLoS One 8:e68428. doi: 10.1371/journal.pone.0068428
Vandooren, J., Van Damme, J., and Opdenakker, G. (2014). On the structure and functions of gelatinase B/matrix metalloproteinase-9 in neuroinflammation. Prog. Brain Res. 214, 193–206. doi: 10.1016/B978-0-444-63486-3.00009-8
Vermeer, S. E., Longstreth, W. T. Jr., and Koudstaal, P. J. (2007). Silent brain infarcts: a systematic review. Lancet Neurol. 6, 611–619. doi: 10.1016/S1474-4422(07)70170-9
Wang, Q., Tang, X. N., and Yenari, M. A. (2007). The inflammatory response in stroke. J. Neuroimmunol. 184, 53–68. doi: 10.1016/j.jneuroim.2006.11.014
Yin, C.-G., Jiang, L., Tang, B., Zhang, H., Qian, Q., and Niu, G.-Z. (2013). Prognostic significance of plasma visfatin levels in patients with ischemic stroke. Peptides 42, 101–104. doi: 10.1016/j.peptides.2013.01.005
Keywords: subcortical silent brain infarct, aspirin, inflammation, macrophage migration inhibitory factor, matrix metalloproteinase-9, visfatin
Citation: Sung W, Kim YS, Lee K-Y, Jung J-A, Choi H, Lee YJ and Koh S-H (2025) Aspirin modulates inflammatory biomarkers in patients with subcortical silent brain infarcts. Front. Aging Neurosci. 16:1507683. doi: 10.3389/fnagi.2024.1507683
Received: 08 October 2024; Accepted: 16 December 2024;
Published: 07 January 2025.
Edited by:
Ke Zhang, China Medical University, ChinaReviewed by:
Dongwei Sun, University of California, Riverside, United StatesCopyright © 2025 Sung, Kim, Lee, Jung, Choi, Lee and Koh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seong-Ho Koh, a3NoMjEzQGhhbnlhbmcuYWMua3I=
†ORCID: Wonjae Sung, https://orcid.org/0000-0002-4637-5890
Young Seo Kim, https://orcid.org/0000-0002-7050-3426
Kyu-Yong Lee, https://orcid.org/0000-0001-8855-7513
Hojin Choi, https://orcid.org/0000-0002-9637-4423
Young Joo Lee, https://orcid.org/0000-0002-7531-9011
Seong-Ho Koh, https://orcid.org/0000-0001-5419-5761
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.