- 1Affiliated Mental Health Center of Jiangnan University, Wuxi Central Rehabilitation Hospital, Wuxi, Jiangsu, China
- 2Northern Jiangsu People’s Hospital, Yangzhou, Jiangsu, China
- 3Zhongnan Hospital of Wuhan University, Wuhan, Hubei, China
- 4Jiangyin People's Hospital, Wuxi, Jiangsu, China
Background: Parkinson’s disease psychosis (PDP) is one of the most severe and disabling non-motor symptoms in the progression of Parkinson’s disease (PD), significantly impacting the prognosis of PD patients. In recent years, there has been an increase in literature on PDP. However, bibliometrics has rarely been applied to PDP research. This study provides an overview of the current state of PDP research and predicts future trends in this field.
Methods: The literature search was conducted using the Web of Science Core Collection, with the search terms (Parkinson* AND (psychotic* OR hallucination* OR illusion* OR delusion* OR misperception* OR psychosis OR psychoses)). VOSviewer and CiteSpace software were employed to perform bibliometric analysis and visual representation of the search results.
Results: A total of 603 articles were effectively included. Since 2017, there has been a significant upward trend in publications related to PDP. The United States, the United Kingdom, and Canada were the top three contributing countries in terms of publication volume, with France also having a strong influence in this field. Movement Disorders and King’s College London included and published the most articles on PDP. The paper titled “Hallucinations in Parkinson’s Disease: Prevalence, Phenomenology, and Risk Factors” received the highest number of citations and average citations. Cluster analysis results identified brain, prevalence, connectivity, and atypical antipsychotics as key hotspots in this field. High-frequency keywords were grouped into three themes: neurobiology, therapeutic strategies, and symptom research. Among them, pimavanserin, risk, and functional connectivity have been the most studied areas in the past 7 years and are likely to remain key topics in future research.
Conclusion: Research on PDP has garnered increasing attention. This study visualizes PDP research over the past 25 years to analyze global hotspots and trends. It offers researchers a valuable perspective for identifying key topics and understanding research trajectories in this expanding field.
1 Introduction
Parkinson’s disease psychosis (PDP) is a common psychiatric manifestation in the natural course of Parkinson’s disease (PD) and is one of the most complex and disabling features among the various non-motor symptoms of PD. The hallmark characteristics include a spectrum of psychotic symptoms ranging from minor hallucinations (MHs) to widespread multimodal hallucinations and delusions (Ffytche et al., 2017a). More than 40% of patients with PD experience psychosis, and these symptoms tend to worsen as the disease progresses, leading to increased hospitalization rates (Rissardo et al., 2022), morbidity, and mortality (Eichel et al., 2022). PDP is one of the primary determinants of nursing home placement and caregiver burden, further complicating the management of PD (Angelopoulou et al., 2022). It severely impacts the quality of life for patients and caregivers, posing a significant challenge in the treatment of PD.
A 12-year longitudinal study on PDP populations revealed that patients with a higher age at PD onset and the presence of rapid eye movement (REM) sleep behavior disorder (RBD) have an increased risk of developing PDP. This pattern of risk factors, along with the concurrent development of psychiatric symptoms, disability, and dementia, places PDP within a symptom complex that suggests it is indicative of a malignant progression of PD (Forsaa et al., 2010). However, the exact neurobiological mechanisms remain unclear. Multiple brain structures and pathways are involved in the onset of PDP, including cortical and neuronal loss, gliosis, and Lewy body pathology (Fischer et al., 2021). It is currently established that the neurobiological mechanisms of PDP differ from those of other psychiatric disorders and present distinct clinical features (Weintraub and Mamikonyan, 2019). Therefore, integrating clinical assessment with the evaluation of PDP symptoms and mechanisms should be the foundation of ongoing research (Mangone et al., 2024). Factors such as visual processing disorders, neurotransmitter system and structural abnormalities, genetic factors, deep brain stimulation, and sleep disturbances are believed to be associated with the development of PDP (Zahodne and Fernandez, 2008). Hallucinations and delusions may result from the hyperactivation of pyramidal neurons in the visual cortex, leading to visual hallucinations (VHs), and the hyperactivation of the mesolimbic pathway (Cummings et al., 2022). Moreover, the treatment poses significant challenges. Controlling psychiatric symptoms without exacerbating motor dysfunction is crucial in the management of PDP (Cummings et al., 2014). Additionally, the visual disturbances associated with PDP are linked to a poorer prognosis (Naumann et al., 2021) and future cognitive decline (Powell et al., 2022). Early diagnosis and the prediction of risk factors are essential for delaying the onset of PDP. Therefore, a comprehensive review of PDP research is necessary.
With the increasing public awareness, the number of PDP-related studies has also been rising. Bibliometric analysis is a tool used to study the quantitative characteristics and distribution patterns of literature and related information. It combines methods from statistics, informatics, and social sciences to systematically analyze indicators such as the number of publications, citation counts, and author’s research output, thereby revealing trends and developments in scientific research (Donthu et al., 2021). Bibliometric analysis not only focuses on the impact of individual publications but also examines the overall performance of academic journals, authors, countries, and institutions, providing a deep understanding of research hotspots, knowledge structures, and academic communication. Core methods include citation analysis, collaboration network analysis, and topic modeling. These methods assist researchers in evaluating the academic impact of research outcomes, identifying key figures and institutions in the field, and providing a basis for scientific policy-making and resource allocation (Ellegaard and Wallin, 2015).
Currently, there are no bibliometric analysis papers specifically focused on the field of PDP. Therefore, this study conducts a detailed search of the Web of Science Core Collection (WoSCC) database and performs a bibliometric and visualization analysis of various aspects such as annual publication volume, international and institutional collaborations, journals, and keywords in this field. The aim is to explore the current state of research, key topics, and trends in PDP, providing valuable references for future studies.
2 Methods
2.1 Data source and search strategy
To ensure the credibility and comprehensive coverage of the data, the WoSCC was selected as the data source, covering the period from its inception to February 7, 2024. This included the Science Citation Index Expanded (SCI-EXPANDED) and the Social Sciences Citation Index (SSCI). The search terms used were related to PDP and were developed based on the Medical Subject Headings search strategy from PubMed. The details of the data source and search strategy are presented in Table 1.
The search strategy identified papers that included these terms in the title, abstract, author keywords, or keywords plus. The data were then extracted from the selected publications, exported in the “Plain text file” format, and recorded as “Full record and cited references.” The language was restricted to English, and the publication type was limited to articles and reviews.
2.2 Data analysis
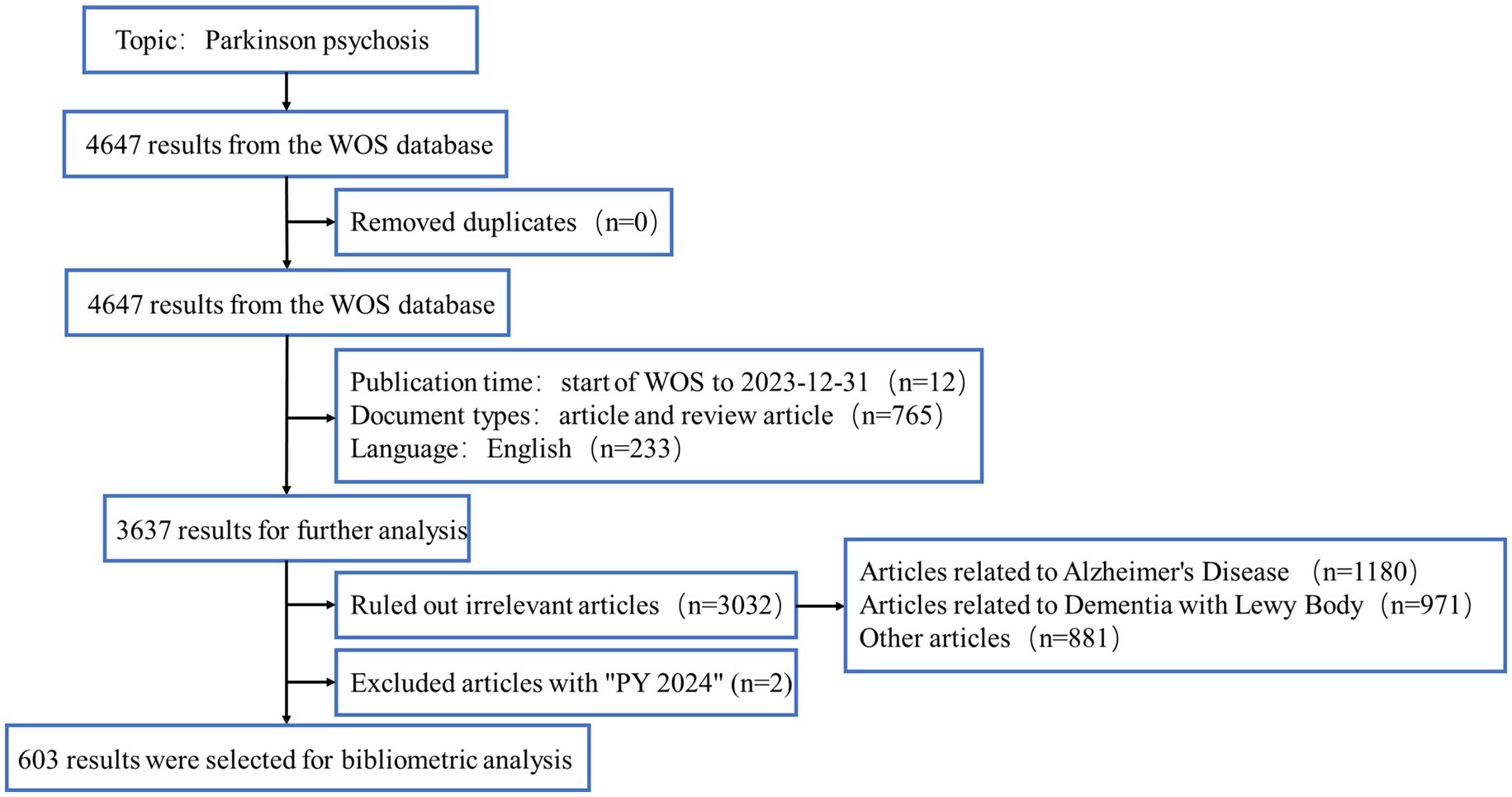
We reviewed the basic information of all documents, including titles, abstracts, and keywords, and consulted PubMed and Sci-Hub databases to complete any missing data. Articles with incomplete or irrelevant information were excluded. To ensure relevance, two authors independently reviewed and screened the papers. We excluded papers that focused primarily on other neurodegenerative diseases, such as Alzheimer’s Disease (AD) or dementia with Lewy bodies (DLB). However, studies that primarily focused on PDP but mentioned other diseases, such as AD or DLB, in a comparative or background context were retained. In cases where articles mention multiple neurodegenerative diseases, a detailed review was conducted to determine whether the focus of the study was primarily on PDP. Studies were retained if PDP was the central topic, even if other neurodegenerative diseases were discussed as part of the research or included in the discussion. Any disagreements were resolved through discussion. After the elimination process, a total of 603 documents remained, all of which were identified using CiteSpace. The flow chart of the data collection process is shown in Figure 1.
3 Results
3.1 Distribution of annual publications and citations
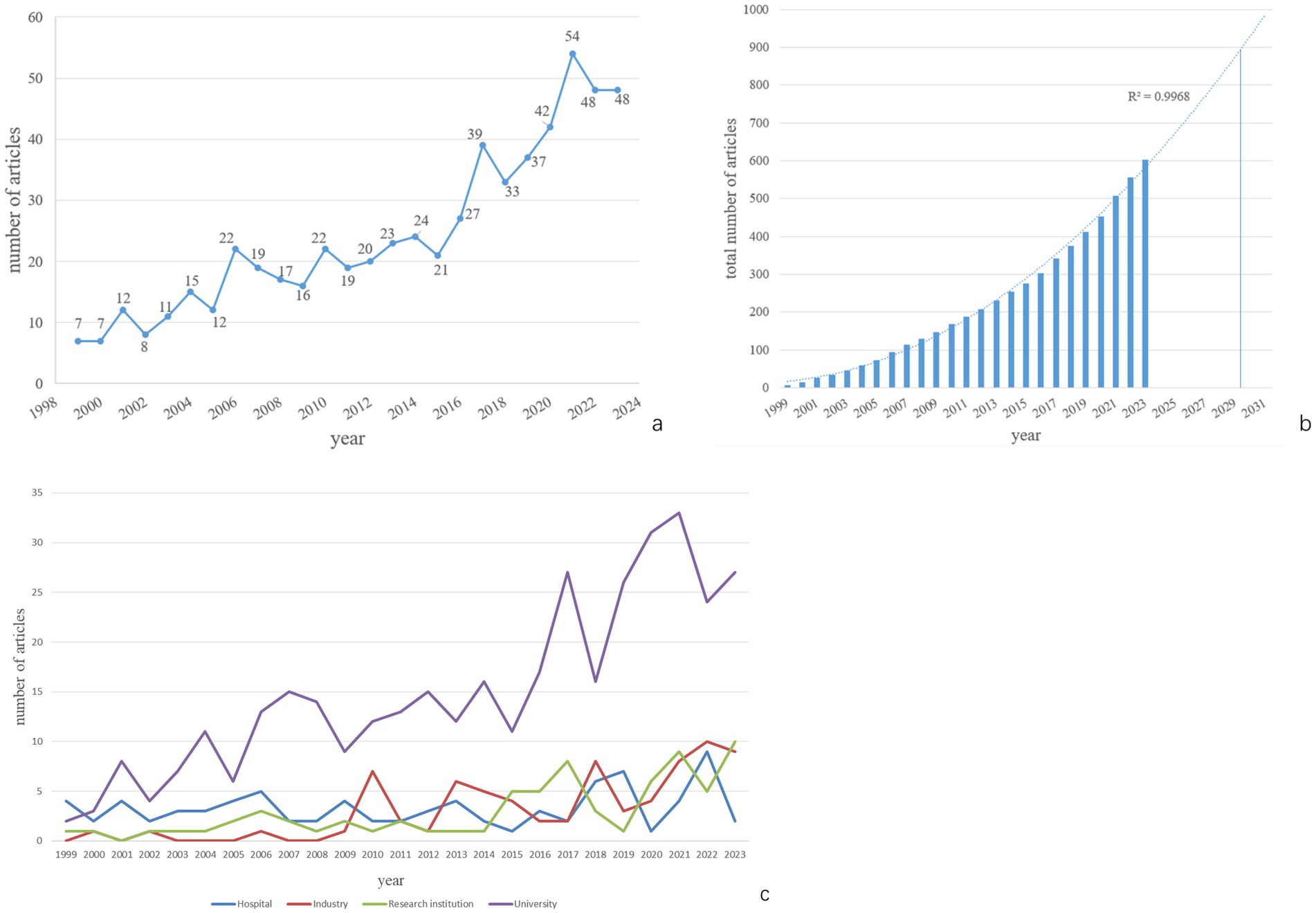
A total of 603 papers published from the inception of WoSCC to 2023 were used for data analysis. According to the trend analysis (Figure 2a), the number of publications exhibits a fluctuating upward trend. The number of published papers increased from 7 in 1999 to 48 in 2023. The growth in publications can be roughly divided into two phases: 1999–2016 and 2017–2023. From 1999 to 2016, the number of publications ranged from a few to several dozen per year. Starting in 2017, there was a dramatic increase in the number of publications, peaking in 2021. Although the second phase spans only 7 years, the number of published articles reached 301, surpassing the total from the first phase. Publications from the second phase account for 49.75% of all published literature. It is anticipated that the number of articles on PDP will increase to 900 by 2030 (Figure 2b).
By analyzing the funding sources or affiliations of the first authors, we categorized their affiliations into four groups: Hospital, Industry, Research Institution, and University. The number of publications for each year is shown in Figure 2c. It can be observed that University-affiliated publications were the most numerous overall. The growth in publication volume followed two stages similar to the overall trend. The publication volumes from Industry, Research Institutions, and Hospitals were generally comparable, with smaller fluctuations in annual output.
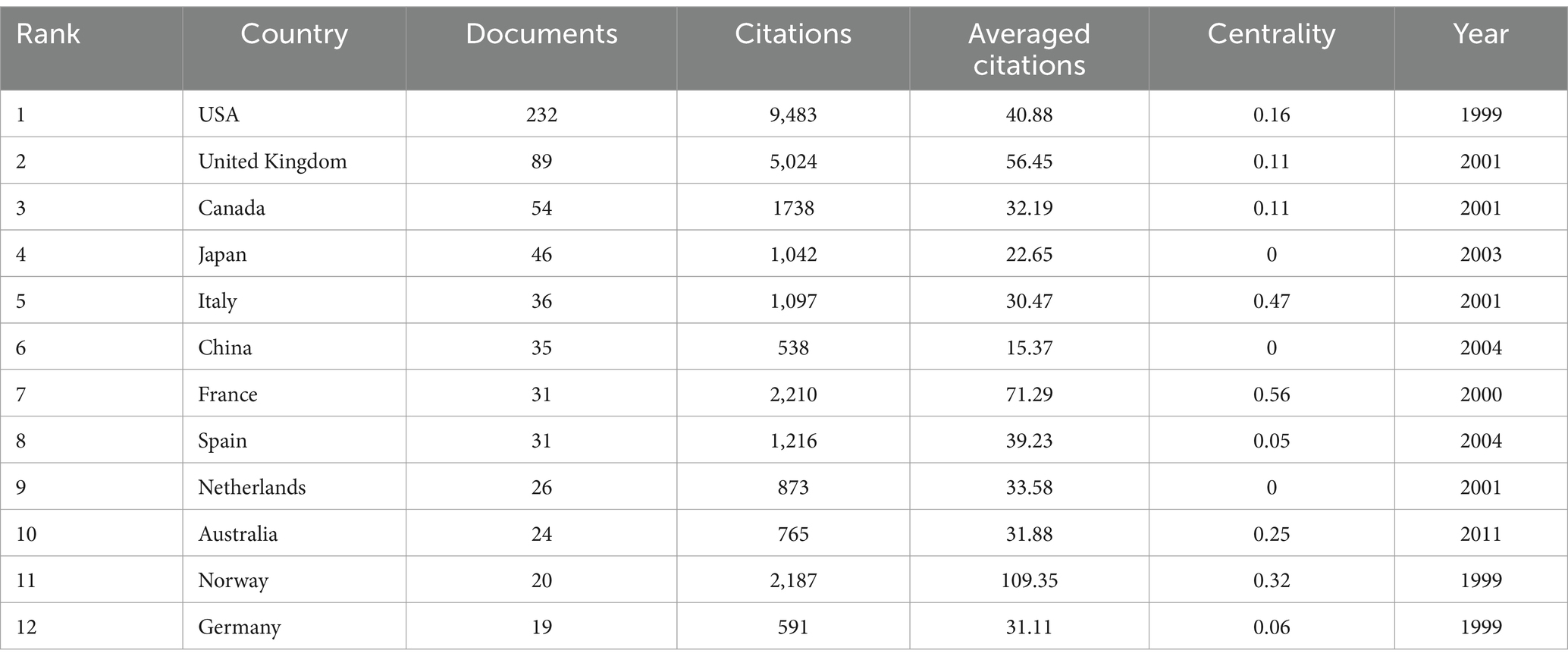
3.2 Analysis of countries
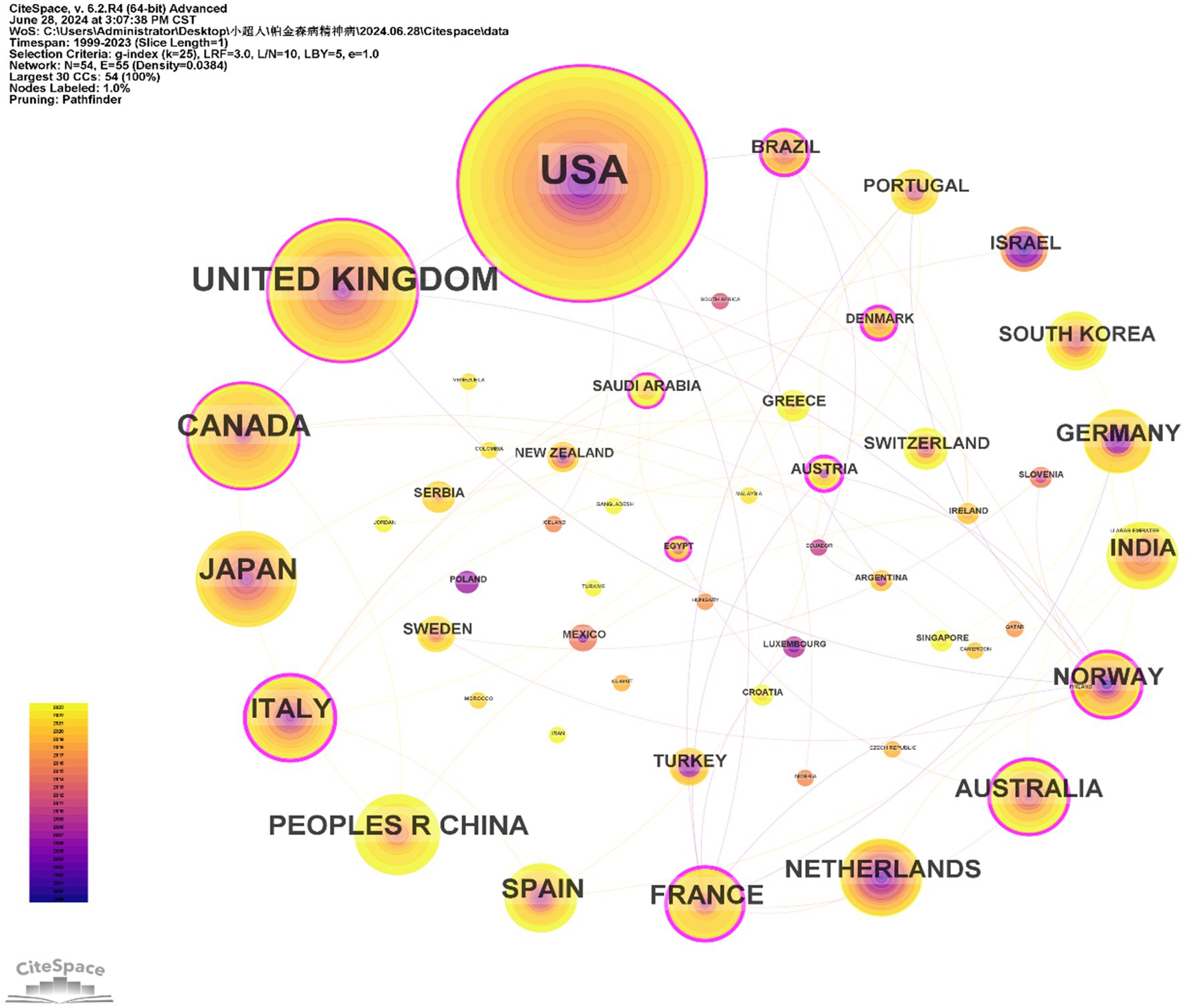
The 603 papers were contributed by researchers from 54 different countries/regions. Figure 3 displays the distribution of publications by country/region. The top 10 countries with the highest number of publications were the USA (233), the United Kingdom (89), Canada (54), Japan (46), Italy (36), China (35), France (31), Spain (31), Netherlands (26), and Australia (24). Additionally, countries such as the USA, the United Kingdom, Canada, and Italy had a broad range of research activity over time, with early contributions (Figure 3). In contrast, China, Japan, and Spain have shown increased research activity in recent years. The USA also had the highest number of citations (9,483). As a metric for measuring the frequency of international collaboration, the size of centrality reflects the influence of a country within the global academic collaboration network. France and Norway, despite not being among the top in terms of publication volume, had the highest centrality and average citation rate, respectively. However, some prolific countries such as Japan, China, and Netherlands had a centrality of less than 0.01. The figure indicates that the connections between countries are not particularly close (Table 2).
3.3 Analysis of journals
The number of publications by each journal provides an indication of its contribution to the dissemination of research on PDP. The 603 papers were published across 207 journals. The top 10 journals with the most publications on PDP are listed in Table 3. The journal Movement Disorders published the highest number of PDP-related papers (57; 9.45%; IF = 7.4), followed by Parkinsonism & Related Disorders (33; 5.47%; IF = 3.1) and Clinical Neuropharmacology (18; 2.99%; IF = 0.8). The 2023 impact factors (IF) of the top 10 journals ranged from 0.8 to 8.7, and these journals contributed 32.67% of the total publications. According to the Journal Citation Reports (JCR), 4 journals are classified as Q1, 5 as Q2, and 1 as Q4. Among the top ten journals, Movement Disorders had a significantly higher citation count (4,329), while Neurology had the highest average citation count.
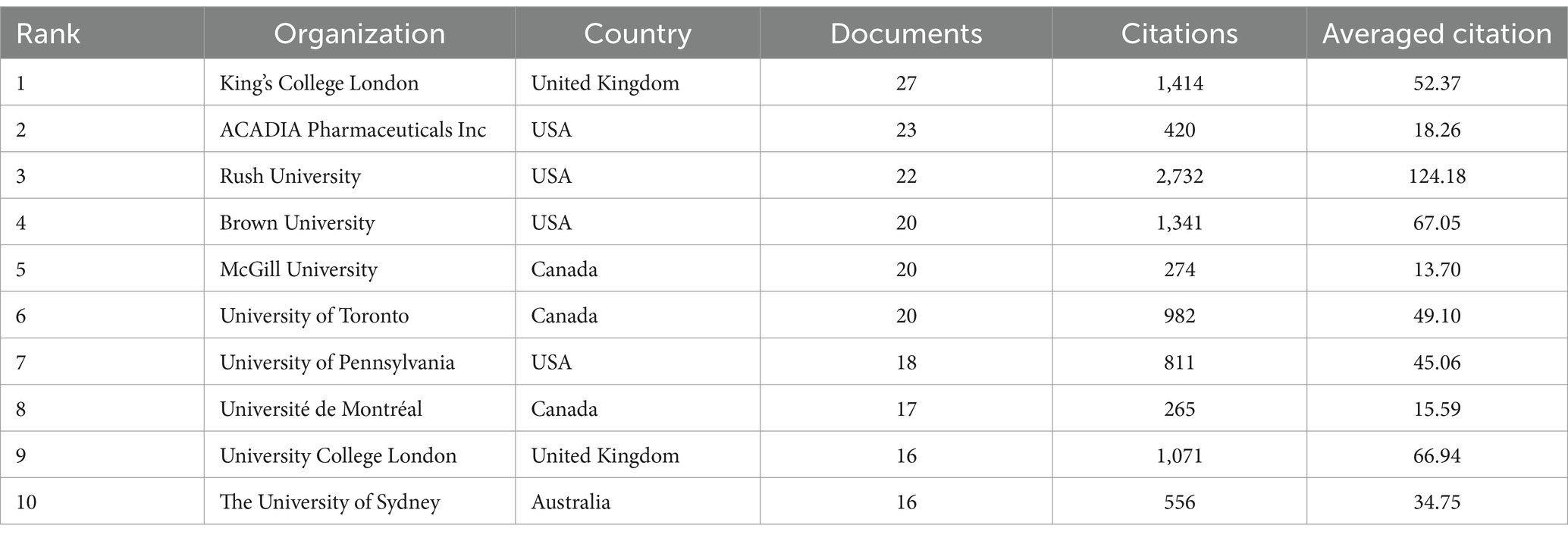
3.4 Analysis of institutions
Table 4 lists the most productive institutions in this field. The most prolific institution was King’s College London, with 27 publications, followed by ACADIA Pharmaceuticals Inc. with 23 publications, and Rush University with 22 publications. Among the top ten institutions, most are located in Canada, USA, and the United Kingdom. Rush University has the highest number of citations and average citations, indicating its significant influence in this field.
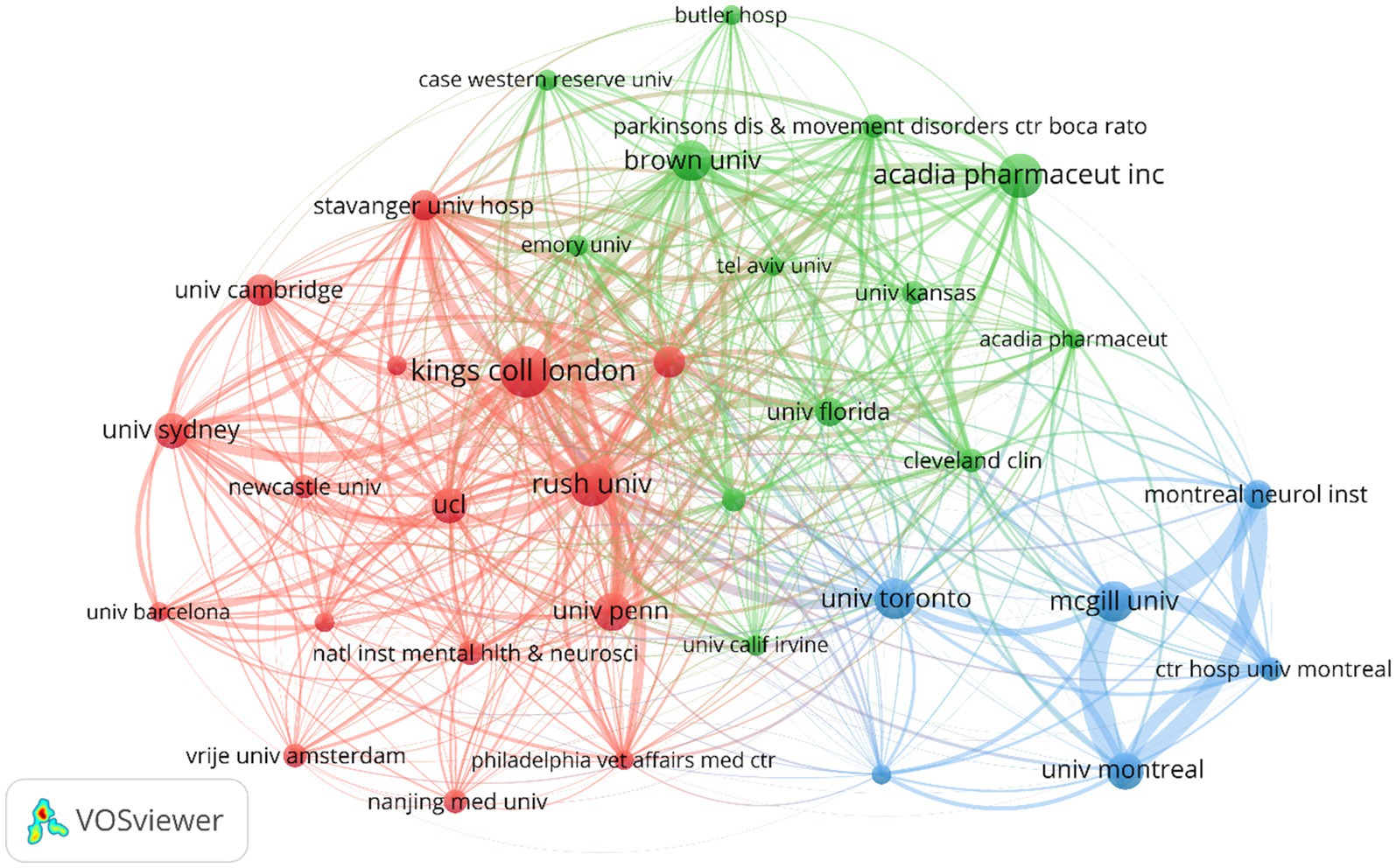
Figure 4 illustrates the partnerships between different institutions. Colors represent different clusters, nodes indicate individual institutions, and lines represent connections between institutions. The red cluster includes institutions with extensive research and collaboration on PDP symptoms and mechanisms. The green cluster involves cross-research on PDP drug therapies, including 5-HT2A inverse agonists like pimavanserin and other atypical antipsychotics (AAPs), and includes pharmaceutical companies and universities. The blue cluster shows active collaboration among Canadian institutions, particularly those in Montreal and its affiliated hospitals, the University of Toronto, and McGill University. Additionally, King’s College London, ACADIA Pharmaceuticals Inc., and McGill University play significant roles within their respective collaboration clusters. The dense connections between nodes such as the University of Pennsylvania, Rush University, and Johns Hopkins University indicate frequent collaboration among these institutions.
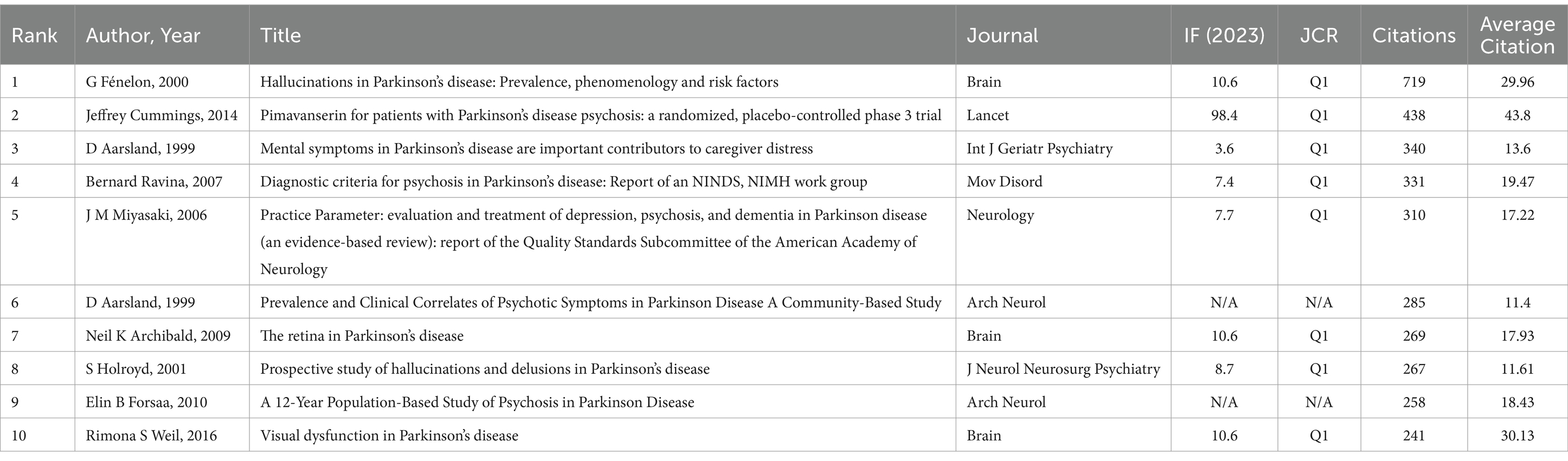
3.5 Analysis of the 10 most-cited article
The number of citations for research publications is a standard measure of academic impact. Table 5 lists the top 10 most-cited papers, with citations ranging from 241 to 719. Among these, 8 papers were published in Q1 journals. The most-cited paper was by G. Fénelon, published in 2000, titled “Hallucinations in Parkinson’s Disease: Prevalence, Phenomenology and Risk Factors” in Brain (IF = 10.6; Q1), with 719 citations.
Among the top 10 most-cited papers, only two were published in the past decade. The paper by Jeffrey Cummings, published in 2014, titled “Pimavanserin for Patients with Parkinson’s Disease Psychosis: A Randomized, Placebo-Controlled Phase 3 Trial” in Lancet (IF = 98.4; Q1), had the highest average annual citations in the field. These highly cited papers primarily focused on symptoms, mechanisms, prevalence, diagnosis, treatment, and the evaluation of therapeutic efficacy of PDP.
3.6 Analysis of keywords
3.6.1 Keywords co-occurrence networks
Figure 5a displays a keyword cluster analysis. Each cluster contains numerous keyword points, with the most weighted keywords serving as cluster labels. Using the Log-likelihood Ratio (LLR) algorithm, current research can be divided into 17 clusters (Q = 0.7466, S = 0.8925). After removing irrelevant topics, the clusters are defined in Table 6.

Figure 5. Network visualization map of keywords co-occurrence (a) and top 25 keywords with the strongest citation bursts (b).
These clusters represent different directions in PDP research, covering a broad range from basic science to clinical treatment. They provide a better understanding of the multifaceted nature of PDP and potential treatment approaches.
3.6.2 Keywords with the strongest citation bursts
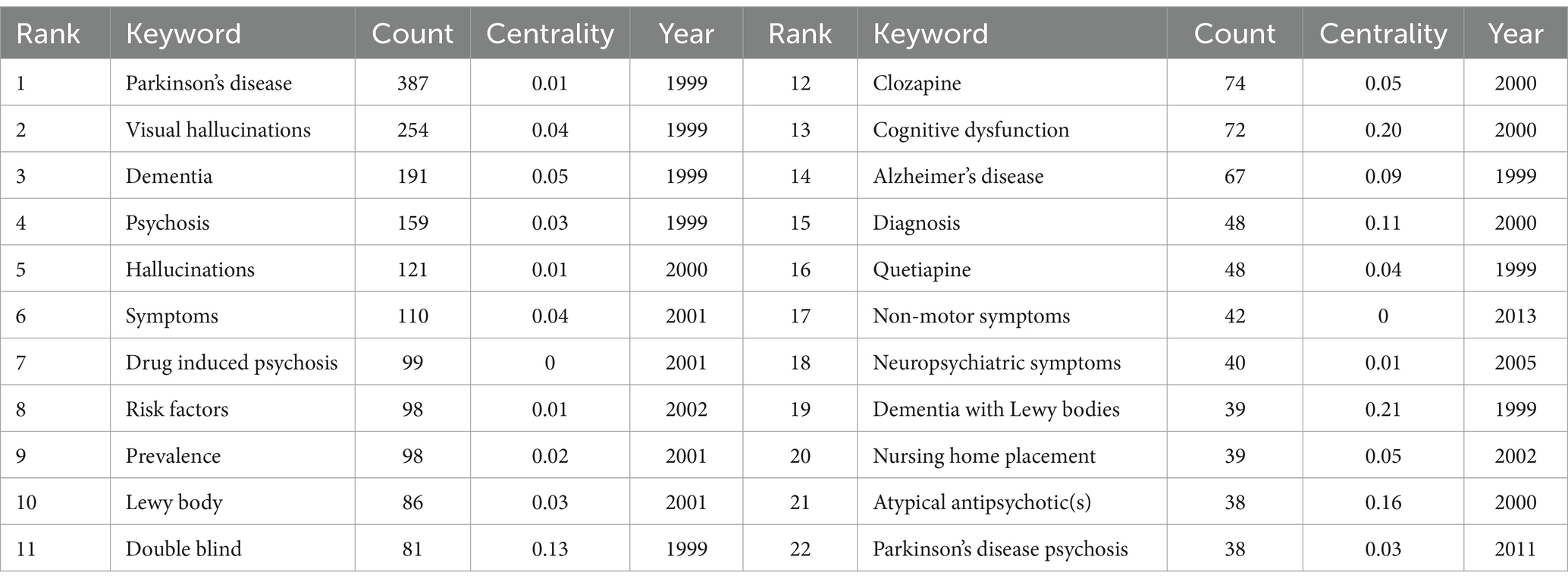
Table 7 displays the most frequently occurring keywords in this study. Besides “Parkinson’s disease” and “psychosis,” the top three keywords were “visual hallucinations” (254), “dementia” (191), and “hallucinations” (121). Figure 5b illustrates the top 25 keywords with the most significant citation bursts since 1999. The length of the red bars represents the duration of the research frontier, while the intensity of the burst reflects the magnitude of the citation increase. The keywords “dopaminergic psychosis,” “drug-induced psychosis,” and “olanzapine” experienced the strongest early bursts. After 2007, the keywords “5-HT2A inverse agonist(s),” “trial,” and “sleep behavior disorder” had the most substantial citation bursts. The latest research frontiers included “pimavanserin,” “risk,” and “functional connectivity.”
4 Discussion
4.1 General information
This study is the first comprehensive bibliometric analysis of the research trends in PDP. By analyzing 603 publications from 1999 to 2023, it reveals a rapid growth trend in PDP research, particularly over the past 7 years. This trend indicates that PDP has become an emerging and popular research topic. Key milestone articles have significantly influenced subsequent research directions. For example, the provisional diagnostic criteria for PDP established by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute of Mental Health (NIMH) in 2007 summarized specific definitions of PDP-related symptoms, marking the onset of the literature’s explosive growth (Ravina et al., 2007). Fénelon et al.’s detailed exploration of PDP-related hallucinations, particularly identifying three potential risk factors and emphasizing that simple side effects of dopaminergic treatments cannot account for all hallucinations, expanded the direction of PDP research (Fénelon et al., 2000). Additionally, Cummings et al.’s randomized, double-blind, placebo-controlled study of pimavanserin has driven the development of new mechanism-based drugs for PDP (Cummings et al., 2014). These important papers not only provide valuable insights into the various manifestations and mechanisms of PDP but also offer theoretical support for future, more in-depth research.
Under the influence of the PDP diagnostic criteria proposed by the NINDS/NIMH workgroup (Ravina et al., 2007), research findings have been published across multiple countries. The USA was the most productive country in terms of research output, but the outcomes did not fully align with centrality, indicating that the USA has a significant number of high-quality independent studies (Mack et al., 2012; Holroyd et al., 2001). Although the USA publications were highly cited domestically, international collaboration and network connections were relatively limited. France and Italy, despite having fewer publications, exhibit high centrality, suggesting that their research had significant academic impact and was widely cited and recognized internationally (Fénelon et al., 2000). Norway, due to its early contributions in epidemiological assessments and caregiver research on PDP, had filled gaps in this field and received considerable attention, reflected in the highest average citations (Forsaa et al., 2010; Aarsland et al., 1999a,b). Notably, Japan, China, and Netherlands had low centrality, which may indicate that research in these countries was primarily focused on domestic issues with limited international collaboration. These countries had distinct regional characteristics, with research concentrated in specific areas: China focused on MHs in PDP (Zhong et al., 2021a, 2022); Japan on VHs (Matsui et al., 2006) and familial gene mutations (Yoshino et al., 2017) in PDP; and the Netherlands primarily on VHs (Meppelink et al., 2009; Janzen et al., 2012).
Aside from differences in publication quantity and centrality, research on PDP exhibits significant variations in focus across different countries. These differences are likely influenced by factors such as economic development, research investment, healthcare resource allocation, and cultural background. In developed countries like USA and some European nations, PDP research was relatively mature and advanced. These countries benefit from substantial funding and state-of-the-art research facilities, enabling complex neuroimaging studies and clinical trials for new medications (Cummings et al., 2014). In contrast, some developing countries may focus more on epidemiological characteristics and clinical management strategies for PDP. These nations may lack the necessary research funding and equipment for in-depth mechanistic studies but have accumulated valuable experience in patient management and care (Zhong et al., 2021a). Additionally, some European countries, such as Norway, emphasized not only biomedical research but also the psychological well-being and social support systems for caregivers (Aarsland et al., 1999b). The fact that nearly hundreds of countries contributed to the field of PDP research indicates its broad global scope. However, future research will benefit from more extensive international collaborative efforts.
Journal analysis can assist researchers in selecting appropriate journals for their work. Different journals, based on their focus, target audience, and academic impact, exhibit unique characteristics in literature inclusion and research emphasis. High-impact journals in medicine and neuroscience, such as Movement Disorders, typically published research with significant clinical relevance and innovation. Movement Disorders frequently featured studies on PDP-related VHs (Nagano-Saito et al., 2004; Watanabe et al., 2013), neurophysiology of brain regions (Meppelink et al., 2011), brain networks (Baik et al., 2024), and molecular mechanisms (Camicioli et al., 2005), as well as articles evaluating the efficacy (Juncos et al., 2004; Mohr et al., 2000; Espay et al., 2018) and safety (Friedman et al., 2006) of drugs or treatments. The journal also emphasizes the assessment of PDP scales (Virués-Ortega et al., 2010; Voss et al., 2010) and the establishment of diagnostic criteria (Ravina et al., 2007). In contrast, Parkinsonism & Related Disorders focused more on the symptomatic features (Barrett et al., 2017; Pachi et al., 2021) and social dimensions (Wetmore et al., 2019) of PDP, including assessments of scales (Voss et al., 2013) and treatment strategies (Weintraub et al., 2024). Interdisciplinary journals, such as the Journal of Neurology and the Journal of Geriatric Psychiatry and Neurology, provided platforms for a variety of research types and methods, including basic research (Carter and Ffytche, 2015), clinical studies (Bugalho et al., 2012; Schneider et al., 2024), and review articles (Toh et al., 2023). Some journals may be more specialized in basic research, like Neurology, which often published studies on the neurobiological mechanisms of PDP (Zarkali et al., 2020a). Others, such as Clinical Neuropharmacology, focused more on clinical pharmacology and therapeutic interventions, publishing research on drug efficacy and side effects (Klein et al., 2006). The fact that fewer than one-third of the publications were distributed across the top ten most active journals indicates a broad distribution across various journals. This dispersion likely reflects the interdisciplinary nature of PDP research, which spans fields such as neurobiology, psychiatry, biochemistry, molecular biology, clinical medicine, and pharmacology.
The research differences between institutions reflect the diversity and complexity of studies in the PDP field. Institutional research focuses are often influenced by resources, team expertise, and regional needs. King’s College London and ACADIA Pharmaceuticals Inc. were pivotal nodes in the co-occurrence network, collaborating with various institutions. King’s College London has made significant contributions to understanding hallucinations of PDP, exploring its prevalence (Gibson et al., 2013), characteristics (Ffytche et al., 2017b), potential mechanisms (Whitehead et al., 2008; Pisani et al., 2023), treatment strategies (Shotbolt et al., 2009), and prognosis (Barnes and David, 2001). They emphasized that VHs in PDP patients might stem from misprocessing environmental stimuli (Barnes et al., 2003), potentially linked to abnormalities in the default mode network (DMN) (Yao et al., 2014). Their large-scale data analyses have also highlighted the epidemiological features of PDP (Gibson et al., 2013), informing public health policies. ACADIA Pharmaceuticals Inc., on the other hand, focused on pharmacological treatment strategies for PDP. In collaboration with King’s College London, they conducted a randomized, placebo-controlled Phase 3 trial on pimavanserin (Cummings et al., 2014), identifying it as a selective 5-HT2A receptor inverse agonist beneficial for PDP treatment (Hacksell et al., 2014). Compared to other AAPs, pimavanserin significantly improved PDP symptoms, such as those measured by the Scale for the Assessment of Positive Symptoms (SAPS) and the Unified Parkinson’s Disease Rating Scale (UPDRS) Parts II and III, with fewer side effects (Meltzer et al., 2010). Their collaboration with Brown University further explored drug efficacy and safety, proposing new strategies for optimizing PDP treatment. ACADIA Pharmaceuticals Inc. also highlighted pimavanserin’s lower all-cause hospitalization and psychiatric hospitalization rates (Rajagopalan et al., 2023a) and contributed to pediatric dosing studies (Darwish et al., 2023), expanding research to cover a broader age range.
The collaboration network illustrates a trend of homogeneity, reflecting close cooperation and research clustering among institutions within the same region or focusing on similar research themes. For instance, King’s College London and the University of Pennsylvania have jointly researched the spectrum of psychiatric symptoms in PDP (Ffytche et al., 2017a), including studies on the relationship between cognitive and executive deficits and quality of life deterioration in PDP patients (Pisani et al., 2024). Institutions such as Rush University have made significant contributions to epidemiological studies on PDP, including age of onset, risk factors (Forsaa et al., 2010), positive rates of scales (Fernandez et al., 2008), pathophysiology of hallucinations (Diederich et al., 2005), and treatments (Diederich et al., 2009). Another important collaborative cluster is found within Canadian institutions, including Montreal, McGill University, and the University of Toronto. These institutions have made notable contributions to PDP mechanism research, from neurobiological foundational studies to clinical treatment trials. They have played a crucial role in studying 5-HT2A receptor signaling (Ballanger et al., 2010) and evaluating clinical research drugs for treating psychosis in parkinsonian marmoset (Hamadjida et al., 2018; Frouni et al., 2019). Rush University has also established diagnostic criteria for PDP, laying the groundwork for the explosion of subsequent literature, including collaborations with Brown University (Ravina et al., 2007). Additionally, Rush University, along with the University of Toronto and the University of Pennsylvania, has published practice guidelines for PDP treatment (Miyasaki et al., 2006), providing a basis for clinical practice. Collaboration among different research institutions has been crucial in advancing PDP research, addressing resource limitations in individual countries, and facilitating cross-validation and application of research findings. However, institutions with lower centrality indicate areas where future research could benefit from enhanced collaboration between core institutions in different countries.
The most cited papers in the field reflect the most valuable and impactful discoveries, highlighting key research hotspots and trends. The article “Hallucinations in Parkinson’s disease: Prevalence, phenomenology and risk factors” published in Brain, was the most cited article (Fénelon et al., 2000). This study addressed the phenomenology, prevalence, and risk factors of PDP hallucinations, identifying three potential risk factors: severe cognitive impairment, daytime sleepiness, and the chronicity of PD. The study also emphasized that simple side effects of dopaminergic treatments are insufficient to explain all VHs and notes that the overall prevalence of PDP with MHs is significantly higher than previously reported. The second most cited paper was Cummings et al.’s 2014 study “Pimavanserin for patients with Parkinson’s disease psychosis: a randomized, placebo-controlled phase 3 trial” published in Lancet (Cummings et al., 2014). This research, a 6-week randomized, double-blind, placebo-controlled trial, found that pimavanserin could be beneficial for PDP patients. In recent years, research into PDP medications has been a hot topic, particularly focusing on 5-HT2A inverse agonists like pimavanserin. Interestingly, pimavanserin remains highly relevant in our keyword co-occurrence analysis, reflecting ongoing interest in developing treatment strategies. These highly cited works underscore significant developments in understanding PDP and advancing therapeutic approaches, revealing ongoing trends and priorities in the field.
4.2 Hotspots and frontiers
PDP has garnered significant attention in recent years as a crucial clinical feature of PD. Hotspot analysis indicates that research related to PDP has primarily focused on several key areas: neurobiology (such as brain, functional connectivity, and Lewy bodies), therapeutic strategies (including cholinesterase inhibitors, AAPs, pimavanserin, and electroconvulsive therapy (ECT)), and symptom research (such as VHs, dyskinesia, Othello syndrome, and cognitive control). Additionally, research has covered diagnosis, underlying mechanisms, and the impact on caregivers. Among these, symptom research has consistently been a focal point and a core content area in PDP studies. In recent years, there has been a shift in research focus toward identifying early risk factors for PDP (Knolle et al., 2023) and mitigating its impact on daily life. Understanding how symptoms such as depression and anxiety exacerbate hallucinations and delusions has also emerged as a significant research interest (Pachi et al., 2023). Moving forward, it is expected that more studies will concentrate on the early identification of symptoms and the development of personalized management strategies for PDP. This ongoing shift in research priorities reflects a broader trend toward enhancing the quality of life for PDP patients by addressing the complex interplay of symptoms and refining therapeutic approaches to manage the condition more effectively.
In 2007, the concept of PDP was first introduced into international research. Prior to 2007, although the term “Parkinson’s Disease Psychosis” had not been widely adopted, symptoms such as hallucinations, delusions, and other psychotic features were already recognized in patients with Parkinson’s disease (Thanvi et al., 2005). These symptoms were often referred to as neuropsychiatric or behavioral complications associated with Parkinson’s disease, without a distinct label (Ferreri et al., 2006). Studies from the 1990s and early 2000s often focused on these psychotic symptoms, but the terminology and diagnostic criteria varied significantly across the literature (Doraiswamy et al., 1995; Williams-Gray et al., 2006). The introduction of the concept of “Parkinson’s Disease Psychosis” in 2007 helped standardize the terminology and diagnosis of these symptoms, leading to a more structured approach to research and clinical management (Ravina et al., 2007). This included unifying the previously recognized various symptoms of PDP into a framework of Parkinson’s disease psychosis spectrum symptoms. It also involved identifying a set of distinct clinical features specific to PDP, rather than shared with other psychotic syndromes (Ffytche et al., 2017a). This shift allowed for clearer criteria in identifying psychosis specifically related to Parkinson’s disease, as distinct from other neuropsychiatric conditions. Since then, the field has seen significant advancements in understanding the underlying mechanisms of PDP and the development of targeted treatment strategies. As a distinct clinical entity, research on PDP has become more focused, leading to a clearer understanding of its prevalence, risk factors, and management (Chang and Fox, 2016).
Under the new definition, studies on PDP symptoms have evolved from focusing on individual symptoms to examining a spectrum of continuous symptoms (Ffytche et al., 2017a). This spectrum includes everything from “minor” hallucinations to well-structured hallucinations and delusions. The most common and early psychiatric symptoms in PDP patients are MHs, which include visual illusions, passage hallucinations, and presence hallucinations (Ffytche et al., 2017a). These symptoms may even precede the onset of motor symptoms. Illusions are the most prevalent type of MHs, affecting more than one-third of PD patients. The most commonly reported types include complex visual illusions, kinetopsia, and pelopsia (Jucevičiūtė et al., 2024). Other associated conditions include isolated diplopia (Nebe and Ebersbach, 2007) and spatial misjudgment. MHs typically occur before the onset of well-structured VHs and are accompanied by other cumulative non-motor symptoms such as RBD (Jiang et al., 2023), cognitive impairment (Pagonabarraga et al., 2016; Pacchetti et al., 2005), and depression (Pachi et al., 2023). There are clinical differences in the characteristics of MHs between different phenotypes. For example, patients with the postural instability gait difficulty (PIGD) phenotype have a higher incidence of MHs, particularly visual illusions, a shorter latency period, earlier onset, and worse prognosis compared to those with the tremor-dominant phenotype (Wang et al., 2023).
VHs is the main clinical symptom of PDP patients, which manifest as complex images frequently involving people or animals. These visual images can be dynamic and tend to occur in low-stimulus environments, typically when the individual is alone in a quiet setting. These hallucinations may occur several times a day during the early stages of PDP, lasting from a few seconds to several minutes. The progression of cognitive decline and loss of insight often parallels the advancement of these symptoms. As the disease progresses, patients may lose the ability to recognize hallucinations as unreal, leading to the emergence of multimodal hallucinations and delusions, a condition referred to as “malignant hallucinations.” These malignant hallucinations are disabling, often accompanied by paranoid thoughts characterized by suspicion, blame, and unkemptness (Ffytche et al., 2017a). Multimodal hallucinations in PDP extend beyond visual experiences to include non-VHs such as auditory hallucinations (Clark, 1998), tactile hallucinations, and olfactory hallucinations (Bannier et al., 2012), with auditory hallucinations often occurring alongside visual ones. Once any form of hallucination appears, it tends to persist intermittently. Studies utilizing polysomnography have revealed that Parkinson’s patients experiencing hallucinations typically have poorer sleep quality and altered sleep architecture (Gu et al., 2022).
The keyword “Othello syndrome” refers to a type of delusional disorder that can occur in the later stages of PDP (Cannas et al., 2009). Delusions are irrational, unfounded, and often impossible beliefs that patients hold with conviction despite contrary evidence. In PDP, the occurrence of delusions is less frequent than hallucinations, but they tend to be more severe and disruptive, causing significant negative impacts on both patients and their families. Delusions in PDP are often accompanied by visual and auditory hallucinations, and compared to patients who experience only hallucinations, those with delusions are more likely to be younger PD patients and to exhibit aggressive and violent behaviors (Laurell et al., 2023). The delusions in PDP are predominantly of a paranoid nature, typically presenting as mixed-type delusions, with isolated delusions being less common. These delusions manifest in various forms, including guilt, grandeur, religious themes, persecution, jealousy, and theft (Factor et al., 2014), with considerable heterogeneity among cases (Warren et al., 2018). “Othello syndrome,” named after the character Othello from Shakespeare’s play Othello, describes a severe form of jealousy, often accompanied by paranoia and delusions, typically involving a strong belief in a partner’s infidelity. Research suggests that Othello syndrome may be associated with increased dopamine levels in the mesolimbic pathway (Cannas et al., 2006), particularly related to dopamine receptor hypersensitivity and neurotransmitter imbalances (Wolters, 1999).
The keyword “dementia with Lewy bodies” (DLB) refers to a disease that closely resembles the symptoms of PDP, especially when dementia symptoms manifest in PDP patients. During the literature screening process, a significant number of publications related to DLB were excluded. In the analysis of Keywords with the Strongest Citation Bursts, DLB consistently appeared with low citation burst frequency, highlighting the clinical and research challenges in differentiating between DLB and PD, as well as the diagnostic difficulties associated with both conditions. DLB is a neurodegenerative disease characterized pathologically by Lewy bodies and clinically by fluctuating progressive dementia, persistent attention deficits, visuospatial impairments, persistent complex VHs, and spontaneous parkinsonism (McKeith et al., 2017). VHs is a core feature of DLB diagnosis and is also a typical feature of PDP (Zhong et al., 2021b), making the two conditions easily confusable, especially since both exhibit parkinsonian symptoms. However, the VHs in DLB are generally more specific compared to those in PD. Most DLB patients experience true VHs that are often vivid and well-formed. The hallucinated objects are frequently familiar figures, such as people or animals, which are usually animated, speaking, or making sounds. Occasionally, these hallucinations may be distorted or grotesque. These hallucinations typically present as early symptoms and tend to respond poorly to pharmacological treatments (Ferman et al., 2011). In contrast, VHs in PDP patients often co-occur with other forms of hallucinations and typically emerge in the later stages of PD. These symptoms can be partially alleviated through medication (Cummings et al., 2014). Another distinguishing factor is that DLB generally begins with early cognitive impairment that precedes motor dysfunction, while PDP begins with motor dysfunction and later develops psychiatric spectrum symptoms, with cognitive impairment not being a primary diagnostic criterion (Jellinger and Korczyn, 2018).
Therefore, the keyword “diagnosis” highlights the critical role of diagnosing PDP in understanding and managing the condition. The diagnostic criteria established by the NINDS/NIMH have provided a vital theoretical and practical foundation for identifying PDP (Ravina et al., 2007). According to these criteria, PDP requires the presence of one or more of the following symptoms: hallucinations, illusions, false perceptions, or delusions, which must persist for more than 1 month or recur frequently, while ruling out other causes such as DLB and psychiatric disorders. Specific scales can enhance the reporting of PDP symptoms, allowing for the early detection of these symptoms in the disease’s course (Greger et al., 2022). The Movement Disorder Society (MDS) UPDRS (MDS-UPDRS) Part I is useful for assessing PDP at various stages (Goetz et al., 2008). Other scales, such as the Parkinson Psychosis Questionnaire (PPQ) (Brandstaedter et al., 2005), the SAPS for Parkinson’s Disease Psychosis (SAPS-PD) (Schubmehl and Sussman, 2018) and its modified version (Kulick et al., 2018), the Scale for Evaluation of Neuropsychiatric Disorders in Parkinson’s Disease (SEND-PD) (Martinez-Martin et al., 2012), the Parkinson Psychosis Rating Scale (PPRS) (Friedberg et al., 1998), and the Self-Administered Screening Questionnaire for Parkinson’s Disease-Associated Psychosis (SASPAP) (Koneru et al., 2023), can be specifically applied to evaluate PDP. For screening PDP, the UPDRS is first used to screen for psychiatric disorders, followed by assessments like SAPS to evaluate the severity of PDP. These scales can be combined for more comprehensive evaluation (Fernandez et al., 2008).
The clustering of keywords like “brain,” “frontal,” “connectivity,” and “Lewy body” indicates that research into the mechanisms of PDP is a major focus and is crucial for developing precise and early diagnostic methods. A Mendelian randomization study supports the inherent nature of PDP symptoms (Kim et al., 2021). PDP is associated with underlying neurodegenerative processes, where cortical and neuronal loss, gliosis, and Lewy body pathology collectively explain the neuropsychiatric symptoms (Fischer et al., 2021). PDP patients exhibit extensive grey matter volume loss, affecting at least 14 different regions (Ffytche et al., 2017a), particularly within the visual processing pathways (ventral and dorsal thalamus) (Pagonabarraga et al., 2016), pedunculopontine nucleus, and inferior frontal gyrus (Broadstock et al., 2014), as well as other related anatomical structures, including the auditory cortex (Lenka et al., 2015). Frontal lobe dysfunction is significant in PDP patients (Thota et al., 2017), with a noticeable reduction in overall hippocampal volume, hippocampal subfield atrophy, and a widening of the bilateral hippocampal fissure (Lenka et al., 2018). Neuropsychological testing has shown that the gradual loss of insight reflects the progression from frontal-striatal network dysfunction to posterior cortical damage (Wang et al., 2018). Key factors contributing to VHs include reduced fiber bundle cross section within the splenium of the corpus callosum, specific white matter tract degeneration (Zarkali et al., 2020a), and elongated visual evoked potentials latency (Matsui et al., 2005). Additionally, areas such as the parahippocampal gyrus, amygdala, frontal lobe, middle temporal gyrus, entorhinal cortex, and anterior cingulate cortex exhibit higher pathological Lewy body density (Gallagher et al., 2011), which correlates with the onset of PDP and VHs (Williams and Lees, 2005). The spectrum of PDP symptoms reflects the Braak progression of Lewy body pathology from the brainstem to the forebrain systems (Braak et al., 2003), highlighting the widespread impact of brainstem lesions on subcortical and cortical motor as well as oculomotor control networks, including the visual parietal lobe.
The keyword “visual hallucinations” highlights the critical importance of this symptom in PDP, aligning with bibliometric findings on VHs (Zhong et al., 2021b). Various models have been proposed to explain VHs, each reflecting different understandings of brain function (Collerton et al., 2023). Functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) studies indicate that brain activity patterns in PDP patients significantly differ from those in non-psychotic patients, especially regarding functional abnormalities in the prefrontal cortex and visual cortex. Some researchers suggest that hallucinations arise from altered effects within different brain networks, with changes in functional connectivity leading to reduced controllability of brain networks (Zarkali et al., 2020b). These changes in functional connectivity include abnormal connections within the DMN, between posterior DMN regions and visual processing areas, or oscillations caused by excessive synchronization (Halje et al., 2019), leading to changes in brain metastability. Other studies propose that alterations in the brainstem’s sleep–wake and dream regulation, such as RBD leading to dream imagery intruding into the waking state (Diederich et al., 2009), and abnormal cortical-striatal reward processing, may be involved in the development of VHs (Garofalo et al., 2017). PDP patients also exhibit dysfunction in the ventral striatal dopamine transporter (Jaakkola et al., 2017), and the lack of dopamine activity affects retinal function, leading to abnormal “top-down” visual processing that replaces the normal bottom-up system (Archibald et al., 2009). This results in a failure to inhibit erroneous information or the spontaneous generation of internally created images through the pontine-geniculate-occipital (PGO) system (Diederich et al., 2005). The attentional control networks hypothesis suggests that hallucinations may arise from disruptions and overloads in attention networks, where new incoming stimuli are misclassified, leading to abnormal hierarchical processing and the intrusion of false perceptions into the stream of consciousness (Collerton et al., 2005). In addition, the thalamocortical dysrhythmia (TCD) DMN decoupling hypothesis posits that TCD-induced disturbances in high-order thalamic nucleus activity leads to a loss of fine-tuning in cortico-cortical regulation and the decoupling of the DMN (Onofrj et al., 2023). Changes in the dorsal attention network (DAN), ventral attention network (VAN), and DMN play crucial roles in the occurrence of MHs and VHs (Sawczak et al., 2019), with each network contributing to the perception and processing of information, adjustment of attention, and generation of selective attention (Zhong et al., 2021b). The underactivation of the VAN and overactivation of the DAN and DMN result in the incorrect recall of perceived information, leading to VHs (Shine et al., 2011). Dysfunction in the DAN is considered a key factor and early indicator of the progression from MHs to more widespread forms of hallucinations (Baik et al., 2024), suggesting the involvement of the basal forebrain and its broader impact on cortical cholinergic projections, particularly to the ventral occipitotemporal cortex. Thus, although labeled “minor,” MHs potentially serve as early biomarkers for the progression to fully-formed hallucinations (Bejr-Kasem et al., 2019).
Cortical changes can lead to neurotransmitter imbalances (Vignando et al., 2022), and current research supports the involvement of multiple neurotransmitter dysfunctions in the development of PDP. The keyword “5-HT2A inverse agonist(s)” reflects the role of the serotonin (5-HT) system in the mechanism of PDP. The involvement of the 5-HT system in PDP is supported by evidence that 5-HT agonists can induce delirium and psychosis, while reducing 5-HT activity can alleviate psychotic symptoms (van der Mast and Fekkes, 2000). In addition, the association between grey matter volume and the local expression of 5-HT1A and 5-HT2A receptor genes suggests that serotonergic receptors play a role in PDP (Pisani et al., 2023). Overactivation of 5-HT receptors, particularly the 5-HT2A receptor, is closely linked to hallucinations and delusions in Parkinson’s patients (Huot, 2018). This may occur through increased receptor binding in the inferior lateral temporal cortex (Huot et al., 2010), the prefrontal cortex, and the ventral visual pathways (Ballanger et al., 2010). Additionally, the activation of metabotropic glutamate receptor 2 (mGlu2) has been shown to reduce psychosis-like behaviors (PLBs) in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned macaques (Nuara et al., 2021). Beyond the 5-HT and mGlu2 systems, the dopamine system also plays a crucial role in PDP. The primary pathological feature of PD is the loss of dopaminergic neurons in the nigrostriatal pathway, leading to a decrease in dopamine levels in the striatum. However, despite the overall reduction in dopamine levels in Parkinson’s patients, the emergence of psychotic symptoms is often associated with hypersensitivity and imbalance in dopamine receptors (Seeman, 2006). The hypersensitivity of dopamine2 receptors is considered a key factor in PDP (Seeman, 2006; Seeman, 2011). This multifaceted interaction between neurotransmitter systems, particularly the serotonergic and dopaminergic pathways, underscores the complexity of PDP’s pathophysiology. These insights highlight the importance of targeted pharmacological interventions, such as 5-HT2A inverse agonists, to address the specific neurochemical imbalances contributing to PDP.
We observed an evolution of keywords from “dopaminergic psychosis” (e.g., levodopa, drug-induced psychosis, dopaminergic-induced hallucinations) and atypical antipsychotics (AAPs) (e.g., olanzapine, clozapine, risperidone) to “5-HT2A inverse agonist(s),” “pimavanserin,” and “Parkinson’s disease psychosis (PDP),” reflecting a shift in research focus from AAPs to novel anti-PDP drugs. Treating PDP poses a significant challenge due to the heterogeneity of its etiology, symptoms, and underlying mechanisms. Keywords like “risperidone,” “olanzapine,” and “clozapine” were prominent before 2007, while “pimavanserin” has become a current active topic. Retrospective database analyses have shown that olanzapine and risperidone are associated with an increased risk of mortality (Abdul-Rahman et al., 2024). Despite short-term benefits in improving Brief Psychiatric Rating Scale psychosis scores (Mohr et al., 2000), risperidone worsens UPDRS scores (Ellis et al., 2000). No clear benefits were observed with olanzapine (Wilby et al., 2017), and it had a higher incidence of adverse effects (Aarsland et al., 1999c). Therefore, interest in these drugs has declined in studies after 2007. Research has found that antagonizing the 5-HT2A receptor is the pharmacological basis for most PDP treatments. This mechanism likely reduces abnormally high-frequency oscillations in prefrontal structures and abnormal synchrony between different brain regions (Stan et al., 2024). Traditional antipsychotics like clozapine have shown some efficacy in controlling PDP symptoms due to their 5-HT2A receptor antagonism (Wilby et al., 2017). However, clozapine is now mainly used for emergencies or symptoms unresponsive to other treatments due to its side effect profile (Thames and Ondo, 2023). The evidence for quetiapine is mixed (Juncos et al., 2004; Wilby et al., 2017). Meanwhile, the 5-HT2A inverse agonist pimavanserin is considered a potential first-line alternative to clozapine (Srisurapanont et al., 2024). Among the top 10 cited papers, the second most cited is a clinical study related to pimavanserin, which has the highest impact factor and average annual citations.
Pimavanserin is the first and only AAPs approved by the FDA for the treatment of hallucinations and delusions associated with PDP. It exhibits selective inverse agonism at the 5-HT2A receptor, with greater affinity compared to the 5-HT2C receptor, and has no significant activity at any other G protein-coupled receptors (Hacksell et al., 2014). Patients treated with pimavanserin showed greater improvements in measures including SAPS and UPDRS Parts II and III (Meltzer et al., 2010), particularly in subgroups with more pronounced cognitive impairments, with the drug demonstrating good safety and tolerability (Espay et al., 2018; Isaacson et al., 2020). Pimavanserin has also been shown to improve nighttime sleep and daytime sleepiness (Patel et al., 2018), significantly reduce the risk of psychosis relapse in Parkinson’s patients with dementia (Weintraub et al., 2024), and does not worsen motor or cognitive functions (Alva et al., 2024). Additionally, it does not increase the risk of mortality (Ballard et al., 2020) and is not associated with excess death reports in FDA Adverse Event Reporting System (Brown et al., 2021). Compared to other AAPs, pimavanserin reduces the risk of falls or fractures in PDP patients (Layton et al., 2022) but may cause a slight prolongation of the QT interval (Torres-Yaghi et al., 2021). Monotherapy with pimavanserin is associated with lower all-cause hospitalization, psychiatric-related hospitalization rates (Rajagopalan et al., 2023a), and emergency department visit rates (Rajagopalan et al., 2023b), as well as reduced likelihood of all-cause healthcare resource use (Rajagopalan et al., 2024b) and risk of long-term care (LTC) admission (LTCA) (Rajagopalan et al., 2024a). The costs related to per-patient-per-year skilled nursing facility-stay and LTCA are also lower (Rajagopalan et al., 2024c).
Some 5-HT2A inverse agonists exhibit more potent effects than pimavanserin. Pimavanserin derivative 7–16 has shown higher 5-HT2A receptor antagonist activity, inverse agonist activity, and in vivo pharmacokinetic activity compared to pimavanserin. The efficacy window between 5-HT2A and hERG activities is increased, with good safety profiles (Ma et al., 2022). In vitro competitive receptor binding and functional G protein-coupled assays have demonstrated that compounds 2, 3, and 4 exhibit higher 5-HT2A inverse agonist potency in human cortical and recombinant cells than pimavanserin (Albujuq et al., 2023). LPM6690061 is a safe and effective 5-HT2A inverse agonist that shows significantly more potent antipsychotic-like effects than pimavanserin (Zhu et al., 2023). Nelotanserin, a 5-HT2A/2C inverse agonist, is being used to treat RBD and psychosis in Parkinson’s patients with dementia (Kwan et al., 2024). The potential efficacy of ulotaront (Kuvarzin et al., 2023), SYN-120 (Huot et al., 2017), mesdopetam (IRL790) (Stan et al., 2024), and EMD-281,014 (Hamadjida et al., 2018) in PDP has also been investigated.
Considering the potential ceiling effect of 5-HT2A receptor blockade (Huot, 2018), the mGlu2 pathway has become a research focus. The burst keyword “mptp-lesioned macaques” reflects the animal models in mGlu2 pathway-based research. The 5-HT2A receptor forms heterodimers with the mGlu2 receptor, producing equivalent downstream signaling effects through 5-HT2A receptor antagonism and mGlu2 activation. Activation of the mGlu2 receptor with mGluR2/3 orthosteric agonists (OAs) like LY-404,039 (Kang et al., 2023), LY-354,740 (Kwan et al., 2021a), and mGlu2-positive allosteric modulators (PAMs) like LY-487,379 (Sid-Otmane et al., 2020), CBiPES (Frouni et al., 2021), and biphenylindanone A (Kang et al., 2024) is effective in alleviating PLBs in MPTP-lesioned marmosets, while also enhancing the therapeutic benefits of levodopa (Frouni et al., 2019). Positive allosteric modulation of the mGlu2 receptor and orthosteric stimulation of mGlu2/3 receptors may represent a novel approach for treating L-3,4-dihydroxyphenylalanine (l-DOPA)-induced PDP, potentially enhancing the anti-PLB effects of 5-HT2A antagonism (Kwan et al., 2021a). Combining 5-HT2A receptor antagonism with mGluR2 activation results in a greater reduction of L-DOPA-induced PDP. A further additive effect can be achieved when an mGlu2-OA and an mGlu2-PAM are combined with a 5-HT2A receptor antagonist, compared to adding either an mGlu2-OA or an mGlu2-PAM alone to a 5-HT2A receptor antagonist. However, the combined use of mGlu2-OA and mGlu2-PAM does not yield greater therapeutic effects (Nuara et al., 2020). Future research may focus on developing mGlu2 agonists based on 5-HT2A antagonists.
The clustering labels “5-HT3 receptor antagonists” and “cholinesterase inhibitors” represent additional research directions. Selective blockade of the 5-HT3 receptor, such as with ondansetron, has been shown to alleviate psychosis in MPTP-lesioned marmosets (Kwan et al., 2021b) and reduce VHs and BPRS scores in PDP (Kwan and Huot, 2019). In addition, the effects of the acetylcholinesterase inhibitor carbatine on psychotic symptoms are not well established (Reilly et al., 2024) and may have a smaller effect size (d'Angremont et al., 2023), but it is considered a first-line treatment for mild VHs (Powell et al., 2022). Further research is needed to explore these areas.
In addition to pharmacological treatments, non-pharmacological therapies include ECT (Ueda et al., 2010), restoration of daytime lighting and nighttime sleep, behavioral interventions, and caregiver education (Pahwa et al., 2022). ECT has been shown to be effective in treating PDP (Rojas et al., 2022), particularly in patients with early-onset PD accompanied by psychosis (Yamaoka et al., 2022) and in cases of PD refractory psychosis (Ueda et al., 2010). Although large-scale randomized controlled trials are lacking, two case series reported improvements in PD psychosis spectrum symptoms with ECT. After 5–12 sessions of ECT, SAPS scores improved within 1 week (Usui et al., 2011), with effects in some patients lasting 5–30 weeks after 6 or more sessions (Ueda et al., 2010). Additionally, unilateral anterior cingulotomy combined with bilateral STN-DBS has been shown to improve PDP (Wang et al., 2024).
The keyword “nursing home placement” highlights the significant role of LTC facilities and caregivers in the treatment strategies for PDP. The uncontrollable symptoms in PDP patients often necessitate their transfer to nursing homes, as they face increased risks, including mortality (Kang and Bronstein, 2004). Caregivers play a crucial role in the daily care and psychological support of PDP patients, but they themselves are under tremendous psychological and physical stress (Mantri et al., 2021b), including the challenge of communicating with healthcare institutions and professionals (Mantri et al., 2021a). Moreover, hallucinations in PDP patients significantly impact caregivers’ quality of life (Powell et al., 2022). Therefore, implementing proactive intervention strategies to prevent, delay, or mitigate the severity of PDP is essential for improving caregivers’ quality of life (Fredericks et al., 2017). Studies have shown that care partners prefer non-pharmacological management strategies and have a higher demand for access to medical information (Mantri et al., 2021a). Telemedicine is viewed as a means to enhance the care provided by specialists and LTC staff for PDP patients (Shaughnessy et al., 2022). The burden on caregivers not only affects their health but also directly impacts the care quality and life quality of the patients (Schaffer and Henry, 2023). Therefore, providing emotional and informational support to caregivers is crucial (Shafer et al., 2023). In recent years, support programs and interventions for caregivers, such as psychological counseling, social support networks, educational training, and caregiver support groups, have increasingly become a focus of research (Friedman and Kennedy, 2021).
The recently burst keyword “risk” highlights the risk factors associated with PDP and the challenges in its treatment. Factors such as age, disease duration, motor symptoms, sleep quality, health-related quality of life (Zhong et al., 2021a), excessive daytime sleepiness, autonomic symptom burden, and the density of Cholinergic nucleus 4 (Barrett et al., 2018) have been associated with MHs. Late-stage Hoehn-Yahr classification and frontal lobe dysfunction are considered independent risk factors for MHs (Zhang et al., 2021). Additionally, MHs (Schneider et al., 2024), current smoking status (Terravecchia et al., 2021), cognitive impairment (Lenka et al., 2023), anxiety (Factor et al., 2017), gastrointestinal autonomic dysfunction, RBD, the Aβ42/total tau ratio (Chen et al., 2024), and grey matter volume within specific structural covariance networks (Knolle et al., 2023) are independent predictors of PDP. Monitoring and managing these risk factors can aid in the early identification and delay the onset of PDP.
Studies have shown that over one-third of Parkinson’s patients discontinue antipsychotic treatment (Pham Nguyen et al., 2021). Factors such as younger age, male gender, anemia, use of anti-anxiety medication or anxiety, use of sedatives/hypnotics, bladder disease, coronary artery disease, diabetes, hypertension, and dementia are more likely associated with changes in PDP treatment in LTC settings (Rashid et al., 2022). Low doses of AAPs are commonly prescribed off-label. Therefore, for patients who cannot tolerate quetiapine or clozapine, a gradual transition to pimavanserin while maintaining adequate 5-HT2A antagonism is recommended (Black et al., 2018). Integrating clinical pharmacists into outpatient neurology clinics can improve the accessibility of pimavanserin (Livezey et al., 2021), helping maintain treatment adherence and reduce risks.
This study was the first to explore the development trends and potential frontiers of PDP over the past few decades from a bibliometric perspective. It included 603 articles across 54 countries, 207 journals, and 857 institutions. The analysis covered annual publication volumes, prolific countries, journals, institutions, as well as highly cited articles and burst keywords, providing a wealth of data. Additionally, we used CiteSpace and VOSviewer to transform quantitative literature data into visual maps and networks, making the information more accessible. However, this study has some limitations. According to CiteSpace formatting requirements, we included only review and article formats, which may lead to an incomplete representation of relevant publications. Articles published after the search date were not included, potentially affecting the timeliness of the research.
While our current analysis primarily focuses on bibliometric output, future studies could explore the relationship between research funding and publication output in the field of PDP. Understanding how funding correlates with output may reveal important insights into global research trends. For example, data from funding agencies such as the National Institutes of Health or Horizon Europe could be correlated with bibliographic data to assess the impact of financial investment on research outcomes in PDP.
5 Conclusion
Research on PDP is advancing rapidly and showing promising trends. Many countries, institutions, and authors have contributed to this field. Topics such as brain, prevalence, connectivity, and AAPs have received extensive attention. Current research hotspots include functional connectivity, pimavanserin, and risk factors. In the field of new drug development, novel 5-HT2A receptor inverse agonists and mGlu2 agonists based on 5-HT2A antagonists are being extensively studied. However, there is low connectivity between countries. Future efforts should focus on strengthening international academic collaborations around PDP research hotspots to enhance the impact of this field and increase the publication of highly cited articles.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
JW: Conceptualization, Data curation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. XJ: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft. WX: Data curation, Investigation, Resources, Writing – original draft. LL: Data curation, Funding acquisition, Writing – original draft. FW: Data curation, Investigation, Writing – original draft. LZ: Data curation, Investigation, Resources, Writing – original draft. YS: Project administration, Resources, Writing – original draft. LQ: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work is supported by the Clinical Pharmacy Research Fund Project of Chinese Medical Association (Z-2021-46-2101) and HENGRUI Foundation of the Jiangsu Pharmaceutical Association (No. H202139).
Acknowledgments
We extend our sincere gratitude to all the authors whose work has been included in this study. Their contributions to the field of Parkinson’s disease psychosis are invaluable and have significantly advanced our understanding and knowledge of this complex area.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aarsland, D., Larsen, J. P., Cummins, J. L., and Laake, K. (1999a). Prevalence and clinical correlates of psychotic symptoms in Parkinson disease: a community-based study. Arch. Neurol. 56, 595–601. doi: 10.1001/archneur.56.5.595
Aarsland, D., Larsen, J. P., Karlsen, K., Lim, N. G., and Tandberg, E. (1999b). Mental symptoms in Parkinson's disease are important contributors to caregiver distress. Int. J. Geriatr. Psychiatry 14, 866–874. doi: 10.1002/(SICI)1099-1166(199910)14:10<866::AID-GPS38>3.0.CO;2-Z
Aarsland, D., Larsen, J. P., Lim, N. G., and Tandberg, E. (1999c). Olanzapine for psychosis in patients with Parkinson's disease with and without dementia. J. Neuropsychiatry Clin. Neurosci. 11, 392–394. doi: 10.1176/jnp.11.3.392
Abdul-Rahman, T., Herrera-Calderón, R. E., Aderinto, N., Kundu, M., Wireko, A. A., Adebusoye, F. T., et al. (2024). Clearing the fog: a review of antipsychotics for Parkinson's-related hallucinations: a focus on Pimavanserin, quetiapine and clozapine. J. Integr. Neurosci. 23:80. doi: 10.31083/j.jin2304080
Albujuq, N. R., Meana, J. J., Diez-Alarcia, R., Muneta-Arrate, I., Naqvi, A., Althumayri, K., et al. (2023). Design, synthesis, molecular docking, and biological evaluation of novel Pimavanserin-based analogues as potential serotonin 5-HT(2A) receptor inverse agonists. J. Med. Chem. 66, 9057–9075. doi: 10.1021/acs.jmedchem.3c00662
Alva, G., Cubała, W. J., Berrio, A., Coate, B., Abler, V., and Pathak, S. (2024). Safety profile of Pimavanserin therapy in elderly patients with neurodegenerative disease-related neuropsychiatric symptoms: a phase 3B study. J. Alzheimers Dis. 98, 265–274. doi: 10.3233/JAD-231167
Angelopoulou, E., Bougea, A., Papageorgiou, S. G., and Villa, C. (2022). Psychosis in Parkinson's disease: a lesson from genetics. Genes 13:1099. doi: 10.3390/genes13061099
Archibald, N. K., Clarke, M. P., Mosimann, U. P., and Burn, D. J. (2009). The retina in Parkinson's disease. Brain 132, 1128–1145. doi: 10.1093/brain/awp068
Baik, K., Kim, Y. J., Park, M., Chung, S. J., Sohn, Y. H., Jeong, Y., et al. (2024). Functional brain networks of minor and well-structured major hallucinations in Parkinson's disease. Mov. Disord. 39, 318–327. doi: 10.1002/mds.29681
Ballanger, B., Strafella, A. P., van Eimeren, T., Zurowski, M., Rusjan, P. M., Houle, S., et al. (2010). Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch. Neurol. 67, 416–421. doi: 10.1001/archneurol.2010.35
Ballard, C. G., Kreitzman, D. L., Isaacson, S., Liu, I. Y., Norton, J. C., Demos, G., et al. (2020). Long-term evaluation of open-label pimavanserin safety and tolerability in Parkinson's disease psychosis. Parkinsonism Relat. Disord. 77, 100–106. doi: 10.1016/j.parkreldis.2020.06.026
Bannier, S., Berdagué, J. L., Rieu, I., de Chazeron, I., Marques, A., Derost, P., et al. (2012). Prevalence and phenomenology of olfactory hallucinations in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 83, 1019–1021. doi: 10.1136/jnnp-2012-302414
Barnes, J., Boubert, L., Harris, J., Lee, A., and David, A. S. (2003). Reality monitoring and visual hallucinations in Parkinson's disease. Neuropsychologia 41, 565–574. doi: 10.1016/S0028-3932(02)00182-3
Barnes, J., and David, A. S. (2001). Visual hallucinations in Parkinson's disease: a review and phenomenological survey. J. Neurol. Neurosurg. Psychiatry 70, 727–733. doi: 10.1136/jnnp.70.6.727
Barrett, M. J., Blair, J. C., Sperling, S. A., Smolkin, M. E., and Druzgal, T. J. (2018). Baseline symptoms and basal forebrain volume predict future psychosis in early Parkinson disease. Neurology 90, e1618–e1626. doi: 10.1212/WNL.0000000000005421
Barrett, M. J., Smolkin, M. E., Flanigan, J. L., Shah, B. B., Harrison, M. B., and Sperling, S. A. (2017). Characteristics, correlates, and assessment of psychosis in Parkinson disease without dementia. Parkinsonism Relat. Disord. 43, 56–60. doi: 10.1016/j.parkreldis.2017.07.011
Bejr-Kasem, H., Pagonabarraga, J., Martínez-Horta, S., Sampedro, F., Marín-Lahoz, J., Horta-Barba, A., et al. (2019). Disruption of the default mode network and its intrinsic functional connectivity underlies minor hallucinations in Parkinson's disease. Mov. Disord. 34, 78–86. doi: 10.1002/mds.27557
Black, K. J., Nasrallah, H., Isaacson, S., Stacy, M., Pahwa, R., Adler, C. H., et al. (2018). Guidance for switching from off-label antipsychotics to pimavanserin for Parkinson's disease psychosis: an expert consensus. CNS Spectr. 23, 402–413. doi: 10.1017/S1092852918001359
Braak, H., Del Tredici, K., Rüb, U., de Vos, R. A., Jansen Steur, E. N., and Braak, E. (2003). Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging 24, 197–211. doi: 10.1016/S0197-4580(02)00065-9
Brandstaedter, D., Spieker, S., Ulm, G., Siebert, U., Eichhorn, T. E., Krieg, J. C., et al. (2005). Development and evaluation of the Parkinson psychosis questionnaire a screening-instrument for the early diagnosis of drug-induced psychosis in Parkinson's disease. J. Neurol. 252, 1060–1066. doi: 10.1007/s00415-005-0816-x
Broadstock, M., Ballard, C., and Corbett, A. (2014). Novel pharmaceuticals in the treatment of psychosis in Parkinson's disease. Expert. Rev. Clin. Pharmacol. 7, 779–786. doi: 10.1586/17512433.2014.966814
Brown, J. D., Cicali, B., Henriksen, C., Malaty, I., Okun, M. S., and Armstrong, M. J. (2021). Comparative pharmacovigilance assessment of mortality with pimavanserin in Parkinson disease-related psychosis. J. Manag. Care Spec. Pharm. 27, 785–790. doi: 10.18553/jmcp.2021.27.6.785
Bugalho, P., da Silva, J. A., Cargaleiro, I., Serra, M., and Neto, B. (2012). Psychiatric symptoms screening in the early stages of Parkinson's disease. J. Neurol. 259, 124–131. doi: 10.1007/s00415-011-6140-8
Camicioli, R., Rajput, A., Rajput, M., Reece, C., Payami, H., Hao, C., et al. (2005). Apolipoprotein E epsilon4 and catechol-O-methyltransferase alleles in autopsy-proven Parkinson's disease: relationship to dementia and hallucinations. Mov. Disord. 20, 989–994. doi: 10.1002/mds.20481
Cannas, A., Solla, P., Floris, G., Tacconi, P., Loi, D., Marcia, E., et al. (2006). Hypersexual behaviour, frotteurism and delusional jealousy in a young parkinsonian patient during dopaminergic therapy with pergolide: a rare case of iatrogenic paraphilia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 30, 1539–1541. doi: 10.1016/j.pnpbp.2006.05.012
Cannas, A., Solla, P., Floris, G., Tacconi, P., Marrosu, F., and Marrosu, M. G. (2009). Othello syndrome in Parkinson disease patients without dementia. Neurologist 15, 34–36. doi: 10.1097/NRL.0b013e3181883dd4
Carter, R., and Ffytche, D. H. (2015). On visual hallucinations and cortical networks: a trans-diagnostic review. J. Neurol. 262, 1780–1790. doi: 10.1007/s00415-015-7687-6
Chang, A., and Fox, S. H. (2016). Psychosis in Parkinson's disease: epidemiology, pathophysiology, and management. Drugs 76, 1093–1118. doi: 10.1007/s40265-016-0600-5
Chen, J., Chen, B., Zhao, D., Feng, X., Wang, Q., Li, Y., et al. (2024). Predictors for early-onset psychotic symptoms in patients newly diagnosed with Parkinson's disease without psychosis at baseline: a 5-year cohort study. CNS Neurosci. Ther. 30:e14651. doi: 10.1111/cns.14651
Clark, J. (1998). Case history of a patient with musical hallucinations and Parkinson's disease. Int. J. Geriatr. Psychiatry 13, 886–887. doi: 10.1002/(SICI)1099-1166(1998120)13:12<886::AID-GPS883>3.0.CO;2-G
Collerton, D., Barnes, J., Diederich, N. J., Dudley, R., Ffytche, D., Friston, K., et al. (2023). Understanding visual hallucinations: a new synthesis. Neurosci. Biobehav. Rev. 150:105208. doi: 10.1016/j.neubiorev.2023.105208
Collerton, D., Perry, E., and McKeith, I. (2005). Why people see things that are not there: a novel perception and attention deficit model for recurrent complex visual hallucinations. Behav. Brain Sci. 28, 737–757. doi: 10.1017/S0140525X05000130
Cummings, J. L., Devanand, D. P., and Stahl, S. M. (2022). Dementia-related psychosis and the potential role for pimavanserin. CNS Spectr. 27, 7–15. doi: 10.1017/S1092852920001765
Cummings, J., Isaacson, S., Mills, R., Williams, H., Chi-Burris, K., Corbett, A., et al. (2014). Pimavanserin for patients with Parkinson's disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet 383, 533–540. doi: 10.1016/S0140-6736(13)62106-6
d'Angremont, E., Begemann, M. J. H., van Laar, T., and Sommer, I. E. C. (2023). Cholinesterase inhibitors for treatment of psychotic symptoms in Alzheimer disease and Parkinson disease: a Meta-analysis. JAMA Neurol. 80, 813–823. doi: 10.1001/jamaneurol.2023.1835
Darwish, M., Bugarski-Kirola, D., Jaworowicz, D., Owen, J., Al Qaraghuli, F., Barry, A., et al. (2023). Population pharmacokinetic modeling and stochastic simulations to support pediatric dose selection of Pimavanserin. J. Clin. Pharmacol. 63, 1408–1416. doi: 10.1002/jcph.2315
Diederich, N. J., Fénelon, G., Stebbins, G., and Goetz, C. G. (2009). Hallucinations in Parkinson disease. Nat. Rev. Neurol. 5, 331–342. doi: 10.1038/nrneurol.2009.62
Diederich, N. J., Goetz, C. G., and Stebbins, G. T. (2005). Repeated visual hallucinations in Parkinson's disease as disturbed external/internal perceptions: focused review and a new integrative model. Mov. Disord. 20, 130–140. doi: 10.1002/mds.20308
Donthu, N., Kumar, S., Mukherjee, D., Pandey, N., and Lim, W. M. (2021). How to conduct a bibliometric analysis: an overview and guidelines. J. Bus. Res. 133, 285–296. doi: 10.1016/j.jbusres.2021.04.070
Doraiswamy, M., Martin, W., Metz, A., and Deveaugh-Geiss, J. (1995). Psychosis in Parkinson's disease: diagnosis and treatment. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 19, 835–846. doi: 10.1016/0278-5846(95)00114-B
Eichel, H. V., Heine, J., Wegner, F., Rogozinski, S., Stiel, S., Groh, A., et al. (2022). Neuropsychiatric symptoms in Parkinson's disease patients are associated with reduced health-related quality of life and increased caregiver burden. Brain Sci. 12:89. doi: 10.3390/brainsci12010089
Ellegaard, O., and Wallin, J. A. (2015). The bibliometric analysis of scholarly production: how great is the impact? Scientometrics 105, 1809–1831. doi: 10.1007/s11192-015-1645-z
Ellis, T., Cudkowicz, M. E., Sexton, P. M., and Growdon, J. H. (2000). Clozapine and risperidone treatment of psychosis in Parkinson's disease. J. Neuropsychiatry Clin. Neurosci. 12, 364–369. doi: 10.1176/jnp.12.3.364
Espay, A. J., Guskey, M. T., Norton, J. C., Coate, B., Vizcarra, J. A., Ballard, C., et al. (2018). Pimavanserin for Parkinson's disease psychosis: effects stratified by baseline cognition and use of cognitive-enhancing medications. Mov. Disord. 33, 1769–1776. doi: 10.1002/mds.27488
Factor, S. A., Scullin, M. K., Freeman, A., Bliwise, D. L., McDonald, W. M., and Goldstein, F. C. (2017). Affective correlates of psychosis in Parkinson's disease. Mov. Disord. Clin. Pract. 4, 225–230. doi: 10.1002/mdc3.12381
Factor, S. A., Scullin, M. K., Sollinger, A. B., Land, J. O., Wood-Siverio, C., Zanders, L., et al. (2014). Cognitive correlates of hallucinations and delusions in Parkinson's disease. J. Neurol. Sci. 347, 316–321. doi: 10.1016/j.jns.2014.10.033
Fénelon, G., Mahieux, F., Huon, R., and Ziégler, M. (2000). Hallucinations in Parkinson's disease: prevalence, phenomenology and risk factors. Brain 123, 733–745. doi: 10.1093/brain/123.4.733
Ferman, T. J., Boeve, B. F., Smith, G. E., Lin, S. C., Silber, M. H., Pedraza, O., et al. (2011). Inclusion of RBD improves the diagnostic classification of dementia with Lewy bodies. Neurology 77, 875–882. doi: 10.1212/WNL.0b013e31822c9148
Fernandez, H. H., Aarsland, D., Fénelon, G., Friedman, J. H., Marsh, L., Tröster, A. I., et al. (2008). Scales to assess psychosis in Parkinson's disease: critique and recommendations. Mov. Disord. 23, 484–500. doi: 10.1002/mds.21875
Ferreri, F., Agbokou, C., and Gauthier, S. (2006). Recognition and management of neuropsychiatric complications in Parkinson's disease. CMAJ 175, 1545–1552. doi: 10.1503/cmaj.060542
Ffytche, D. H., Creese, B., Politis, M., Chaudhuri, K. R., Weintraub, D., Ballard, C., et al. (2017a). The psychosis spectrum in Parkinson disease. Nat. Rev. Neurol. 13, 81–95. doi: 10.1038/nrneurol.2016.200
Ffytche, D. H., Pereira, J. B., Ballard, C., Chaudhuri, K. R., Weintraub, D., and Aarsland, D. (2017b). Risk factors for early psychosis in PD: insights from the Parkinson's progression markers initiative. J. Neurol. Neurosurg. Psychiatry 88, 325–331. doi: 10.1136/jnnp-2016-314832
Fischer, N. M., Hinkle, J. T., Perepezko, K., Bakker, C. C., Morris, M., Broen, M. P. G., et al. (2021). Brainstem pathologies correlate with depression and psychosis in Parkinson's disease. Am. J. Geriatr. Psychiatry 29, 958–968. doi: 10.1016/j.jagp.2020.12.009
Forsaa, E. B., Larsen, J. P., Wentzel-Larsen, T., Goetz, C. G., Stebbins, G. T., Aarsland, D., et al. (2010). A 12-year population-based study of psychosis in Parkinson disease. Arch. Neurol. 67, 996–1001. doi: 10.1001/archneurol.2010.166
Fredericks, D., Norton, J. C., Atchison, C., Schoenhaus, R., and Pill, M. W. (2017). Parkinson's disease and Parkinson's disease psychosis: a perspective on the challenges, treatments, and economic burden. Am. J. Manag. Care 23, S83–s92
Friedberg, G., Zoldan, J., Weizman, A., and Melamed, E. (1998). Parkinson psychosis rating scale: a practical instrument for grading psychosis in Parkinson's disease. Clin. Neuropharmacol. 21, 280–284
Friedman, J. H., Berman, R. M., Goetz, C. G., Factor, S. A., Ondo, W. G., Wojcieszek, J., et al. (2006). Open-label flexible-dose pilot study to evaluate the safety and tolerability of aripiprazole in patients with psychosis associated with Parkinson's disease. Mov. Disord. 21, 2078–2081. doi: 10.1002/mds.21091
Friedman, E. M., and Kennedy, D. P. (2021). Typologies of dementia caregiver support networks: a pilot study. The Gerontologist 61, 1221–1230. doi: 10.1093/geront/gnab013
Frouni, I., Hamadjida, A., Kwan, C., Bédard, D., Nafade, V., Gaudette, F., et al. (2019). Activation of mGlu(2/3) receptors, a novel therapeutic approach to alleviate dyskinesia and psychosis in experimental parkinsonism. Neuropharmacology 158:107725. doi: 10.1016/j.neuropharm.2019.107725
Frouni, I., Kwan, C., Nuara, S. G., Belliveau, S., Kang, W., Hamadjida, A., et al. (2021). Effect of the mGlu(2) positive allosteric modulator CBiPES on dyskinesia, psychosis-like behaviours and parkinsonism in the MPTP-lesioned marmoset. J. Neural Transm. (Vienna) 128, 73–81. doi: 10.1007/s00702-020-02287-8
Gallagher, D. A., Parkkinen, L., O'Sullivan, S. S., Spratt, A., Shah, A., Davey, C. C., et al. (2011). Testing an aetiological model of visual hallucinations in Parkinson's disease. Brain 134, 3299–3309. doi: 10.1093/brain/awr225
Garofalo, S., Justicia, A., Arrondo, G., Ermakova, A. O., Ramachandra, P., Tudor-Sfetea, C., et al. (2017). Cortical and striatal reward processing in Parkinson's disease psychosis. Front. Neurol. 8:156. doi: 10.3389/fneur.2017.00156
Gibson, G., Mottram, P. G., Burn, D. J., Hindle, J. V., Landau, S., Samuel, M., et al. (2013). Frequency, prevalence, incidence and risk factors associated with visual hallucinations in a sample of patients with Parkinson's disease: a longitudinal 4-year study. Int. J. Geriatr. Psychiatry 28, 626–631. doi: 10.1002/gps.3869
Goetz, C. G., Tilley, B. C., Shaftman, S. R., Stebbins, G. T., Fahn, S., Martinez-Martin, P., et al. (2008). Movement Disorder Society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170. doi: 10.1002/mds.22340
Greger, J., Aladeen, T., Rainka, M., Kale, A., and Capote, H. (2022). Evaluating rates of reporting symptoms of Parkinson's disease psychosis: provider versus targeted questionnaire. Int. J. Neurosci. 132, 459–465. doi: 10.1080/00207454.2020.1821678
Gu, R., Zhu, J., Zhong, M., Jiang, Y., Zhu, S., Wang, Y., et al. (2022). Characteristics of sleep structure in Parkinson's disease patients with hallucinations based on polysomnography. Front. Neurol. 13:929569. doi: 10.3389/fneur.2022.929569
Hacksell, U., Burstein, E. S., McFarland, K., Mills, R. G., and Williams, H. (2014). On the discovery and development of pimavanserin: a novel drug candidate for Parkinson's psychosis. Neurochem. Res. 39, 2008–2017. doi: 10.1007/s11064-014-1293-3
Halje, P., Brys, I., Mariman, J. J., da Cunha, C., Fuentes, R., and Petersson, P. (2019). Oscillations in cortico-basal ganglia circuits: implications for Parkinson's disease and other neurologic and psychiatric conditions. J. Neurophysiol. 122, 203–231. doi: 10.1152/jn.00590.2018
Hamadjida, A., Nuara, S. G., Bédard, D., Gaudette, F., Beaudry, F., Gourdon, J. C., et al. (2018). The highly selective 5-HT(2A) antagonist EMD-281,014 reduces dyskinesia and psychosis in the l-DOPA-treated parkinsonian marmoset. Neuropharmacology 139, 61–67. doi: 10.1016/j.neuropharm.2018.06.038
Holroyd, S., Currie, L., and Wooten, G. F. (2001). Prospective study of hallucinations and delusions in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 70, 734–738. doi: 10.1136/jnnp.70.6.734
Huot, P. (2018). 5-HT(2A) receptors and Parkinson's disease psychosis: a pharmacological discussion. Neurodegener. Dis. Manag. 8, 363–365. doi: 10.2217/nmt-2018-0039
Huot, P., Johnston, T. H., Darr, T., Hazrati, L. N., Visanji, N. P., Pires, D., et al. (2010). Increased 5-HT2A receptors in the temporal cortex of parkinsonian patients with visual hallucinations. Mov. Disord. 25, 1399–1408. doi: 10.1002/mds.23083
Huot, P., Sgambato-Faure, V., Fox, S. H., and McCreary, A. C. (2017). Serotonergic approaches in Parkinson's disease: translational perspectives, an update. ACS Chem. Neurosci. 8, 973–986. doi: 10.1021/acschemneuro.6b00440
Isaacson, S. H., Coate, B., Norton, J., and Stankovic, S. (2020). Blinded SAPS-PD assessment after 10 weeks of Pimavanserin treatment for Parkinson's disease psychosis. J. Parkinsons Dis. 10, 1389–1396. doi: 10.3233/JPD-202047
Jaakkola, E., Joutsa, J., Mäkinen, E., Johansson, J., and Kaasinen, V. (2017). Ventral striatal dopaminergic defect is associated with hallucinations in Parkinson's disease. Eur. J. Neurol. 24, 1341–1347. doi: 10.1111/ene.13390
Janzen, J., van ‘t Ent, D., Lemstra, A. W., Berendse, H. W., Barkhof, F., and Foncke, E. M. J. (2012). The pedunculopontine nucleus is related to visual hallucinations in Parkinson's disease: preliminary results of a voxel-based morphometry study. J. Neurol. 259, 147–154. doi: 10.1007/s00415-011-6149-z
Jellinger, K. A., and Korczyn, A. D. (2018). Are dementia with Lewy bodies and Parkinson's disease dementia the same disease? BMC Med. 16:34. doi: 10.1186/s12916-018-1016-8
Jiang, Y., Zhu, J., Zhao, Y., Li, D., Chen, Y., Wang, Y., et al. (2023). Minor hallucinations in Parkinson's disease with probable rapid eye movement sleep behavior disorder. Front. Neurosci. 17:1205439. doi: 10.3389/fnins.2023.1205439
Jucevičiūtė, N., Balnytė, R., and Laucius, O. (2024). Exploring the Spectrum of visual illusions and other minor hallucinations in patients with Parkinson's disease in Lithuania. Medicina (Kaunas) 60:606. doi: 10.3390/medicina60040606
Juncos, J. L., Roberts, V. J., Evatt, M. L., Jewart, R. D., Wood, C. D., Potter, L. S., et al. (2004). Quetiapine improves psychotic symptoms and cognition in Parkinson's disease. Mov. Disord. 19, 29–35. doi: 10.1002/mds.10620
Kang, G. A., and Bronstein, J. M. (2004). Psychosis in nursing home patients with Parkinson's disease. J. Am. Med. Dir. Assoc. 5, 167–173. doi: 10.1016/S1525-8610(04)70110-3
Kang, W., Frouni, I., Bédard, D., Kwan, C., Hamadjida, A., Nuara, S. G., et al. (2024). Positive allosteric mGluR(2) modulation with BINA alleviates dyskinesia and psychosis-like behaviours in the MPTP-lesioned marmoset. Naunyn Schmiedeberg's Arch. Pharmacol. 397, 8917–8924. doi: 10.1007/s00210-024-03215-3
Kang, W., Nuara, S. G., Bédard, D., Frouni, I., Kwan, C., Hamadjida, A., et al. (2023). The mGluR(2/3) orthosteric agonist LY-404,039 reduces dyskinesia, psychosis-like behaviours and parkinsonism in the MPTP-lesioned marmoset. Naunyn Schmiedeberg's Arch. Pharmacol. 396, 2347–2355. doi: 10.1007/s00210-023-02587-2
Kim, K., Kim, S., Myung, W., Shim, I., Lee, H., Kim, B., et al. (2021). Shared genetic background between Parkinson's disease and schizophrenia: a two-sample Mendelian randomization study. Brain Sci. 11:1042. doi: 10.3390/brainsci11081042
Klein, C., Prokhorov, T., Miniovich, A., Dobronevsky, E., and Rabey, J. M. (2006). Long-term follow-up (24 months) of quetiapine treatment in drug-induced Parkinson disease psychosis. Clin. Neuropharmacol. 29, 215–219. doi: 10.1097/01.WNF.0000228176.98582.93
Knolle, F., Arumugham, S. S., Barker, R. A., Chee, M. W. L., Justicia, A., Kamble, N., et al. (2023). A multicentre study on grey matter morphometric biomarkers for classifying early schizophrenia and parkinson's disease psychosis. NPJ Parkinsons Dis. 9:87. doi: 10.1038/s41531-023-00522-z
Koneru, V., Espay, A. J., Cole, A. J., Weintraub, D., Crist, K., Pascual, M. B., et al. (2023). The self-administered screening questionnaire for Parkinson's disease-associated psychosis (SASPAP). Mov. Disord. 38, 1982–1987. doi: 10.1002/mds.29627
Kulick, C. V., Montgomery, K. M., and Nirenberg, M. J. (2018). Comprehensive identification of delusions and olfactory, tactile, gustatory, and minor hallucinations in Parkinson's disease psychosis. Parkinsonism Relat. Disord. 54, 40–45. doi: 10.1016/j.parkreldis.2018.04.008
Kuvarzin, S. R., Sukhanov, I., Onokhin, K., Zakharov, K., and Gainetdinov, R. R. (2023). Unlocking the therapeutic potential of Ulotaront as a trace amine-associated receptor 1 agonist for neuropsychiatric disorders. Biomedicines 11:1977. doi: 10.3390/biomedicines11071977
Kwan, C., Frouni, I., Bédard, D., Hamadjida, A., Nuara, S. G., Gourdon, J. C., et al. (2024). The 5-HT(2A/2C) inverse agonist nelotanserin alleviates L-DOPA-induced dyskinesia in the MPTP-lesioned marmoset. Eur. J. Neurosci. 59, 1169–1176. doi: 10.1111/ejn.16104
Kwan, C., Frouni, I., Nuara, S. G., Belliveau, S., Kang, W., Hamadjida, A., et al. (2021a). Combined 5-HT(2A) and mGlu(2) modulation for the treatment of dyskinesia and psychosis in Parkinson's disease. Neuropharmacology 186:108465. doi: 10.1016/j.neuropharm.2021.108465
Kwan, C., and Huot, P. (2019). 5-HT(3) receptors in Parkinson's disease psychosis: a forgotten target? Neurodegener. Dis. Manag. 9, 251–253. doi: 10.2217/nmt-2019-0014
Kwan, C., Nuara, S. G., Bédard, D., Gaudette, F., Gourdon, J. C., Beaudry, F., et al. (2021b). Selective blockade of the 5-HT(3) receptor acutely alleviates dyskinesia and psychosis in the parkinsonian marmoset. Neuropharmacology 182:108386. doi: 10.1016/j.neuropharm.2020.108386
Laurell, A. A. S., Watson, E., Hatfield, C. F., and Dudas, R. B. (2023). Violence and delusional jealousy in Parkinson's disease. BMJ Case Rep. 16:e256682. doi: 10.1136/bcr-2023-256682
Layton, J. B., Forns, J., Turner, M. E., Dempsey, C., Bartsch, J. L., Anthony, M. S., et al. (2022). Falls and fractures in patients with Parkinson's disease-related psychosis treated with Pimavanserin vs atypical antipsychotics: a cohort study. Drugs 9, 9–22. doi: 10.1007/s40801-021-00284-1
Lenka, A., Hegde, S., Arumugham, S. S., Singh, P., Yadav, R., and Pal, P. K. (2023). Cognitive correlates of visual and minor hallucinations in Parkinson's disease. Can. J. Neurol. Sci. 50, 44–48. doi: 10.1017/cjn.2021.507
Lenka, A., Ingalhalikar, M., Shah, A., Saini, J., Arumugham, S. S., Hegde, S., et al. (2018). Hippocampal subfield atrophy in patients with Parkinson's disease and psychosis. J. Neural Transm. (Vienna) 125, 1361–1372. doi: 10.1007/s00702-018-1891-3
Lenka, A., Jhunjhunwala, K. R., Saini, J., and Pal, P. K. (2015). Structural and functional neuroimaging in patients with Parkinson's disease and visual hallucinations: a critical review. Parkinsonism Relat. Disord. 21, 683–691. doi: 10.1016/j.parkreldis.2015.04.005
Livezey, S., Shah, N. B., McCormick, R., DeClercq, J., Choi, L., and Zuckerman, A. D. (2021). Specialty pharmacist integration into an outpatient neurology clinic improves pimavanserin access. Ment. Health Clin. 11, 187–193. doi: 10.9740/mhc.2021.05.187
Ma, M., Yang, Y., Du, G., Dai, Y., Zhu, X., Wang, W., et al. (2022). Improving the treatment of Parkinson's disease: structure-based development of novel 5-HT(2A) receptor antagonists/inverse agonists. Eur. J. Med. Chem. 234:114246. doi: 10.1016/j.ejmech.2022.114246
Mack, J., Rabins, P., Anderson, K., Goldstein, S., Grill, S., Hirsch, E. S., et al. (2012). Prevalence of psychotic symptoms in a community-based Parkinson disease sample. Am. J. Geriatr. Psychiatry 20, 123–132. doi: 10.1097/JGP.0b013e31821f1b41
Mangone, G., Tosin, M. H. S., Goetz, C. G., Stebbins, G. T., and Mestre, T. A. (2024). Unveiling assessment gaps in Parkinson's disease psychosis: a scoping review. Mov. Disord. 39, 560–570. doi: 10.1002/mds.29710
Mantri, S., Edison, B., Alzyoud, L., Albert, S. M., Daeschler, M., Kopil, C., et al. (2021a). Knowledge, responsibilities, and peer advice from Care Partners of Patients with Parkinson Disease Psychosis. Front. Neurol. 12:633645. doi: 10.3389/fneur.2021.633645
Mantri, S., Klawson, E., Albert, S., Rapoport, R., Precht, C., Glancey, S., et al. (2021b). The experience of care partners of patients with Parkinson's disease psychosis. PLoS One 16:e0248968. doi: 10.1371/journal.pone.0248968
Martinez-Martin, P., Frades-Payo, B., Agüera-Ortiz, L., and Ayuga-Martinez, A. (2012). A short scale for evaluation of neuropsychiatric disorders in Parkinson's disease: first psychometric approach. J. Neurol. 259, 2299–2308. doi: 10.1007/s00415-012-6490-x
Matsui, H., Udaka, F., Tamura, A., Oda, M., Kubori, T., Nishinaka, K., et al. (2005). The relation between visual hallucinations and visual evoked potential in Parkinson disease. Clin. Neuropharmacol. 28, 79–82. doi: 10.1097/01.wnf.0000157066.50948.65
Matsui, H., Udaka, F., Tamura, A., Oda, M., Kubori, T., Nishinaka, K., et al. (2006). Impaired visual acuity as a risk factor for visual hallucinations in Parkinson's disease. J. Geriatr. Psychiatry Neurol. 19, 36–40. doi: 10.1177/0891988705284739
McKeith, I. G., Boeve, B. F., Dickson, D. W., Halliday, G., Taylor, J. P., Weintraub, D., et al. (2017). Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology 89, 88–100. doi: 10.1212/WNL.0000000000004058
Meltzer, H. Y., Mills, R., Revell, S., Williams, H., Johnson, A., Bahr, D., et al. (2010). Pimavanserin, a serotonin(2A) receptor inverse agonist, for the treatment of parkinson's disease psychosis. Neuropsychopharmacology 35, 881–892. doi: 10.1038/npp.2009.176
Meppelink, A. M., de Jong, B. M., Renken, R., Leenders, K. L., Cornelissen, F. W., and van Laar, T. (2009). Impaired visual processing preceding image recognition in Parkinson's disease patients with visual hallucinations. Brain 132, 2980–2993. doi: 10.1093/brain/awp223
Meppelink, A. M., de Jong, B. M., Teune, L. K., and van Laar, T. (2011). Regional cortical grey matter loss in Parkinson's disease without dementia is independent from visual hallucinations. Mov. Disord. 26, 142–147. doi: 10.1002/mds.23375
Miyasaki, J. M., Shannon, K., Voon, V., Ravina, B., Kleiner-Fisman, G., Anderson, K., et al. (2006). Practice parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson disease (an evidence-based review): report of the quality standards Subcommittee of the American Academy of neurology. Neurology 66, 996–1002. doi: 10.1212/01.wnl.0000215428.46057.3d
Mohr, E., Mendis, T., Hildebrand, K., and Deyn, D. (2000). Risperidone in the treatment of dopamine-induced psychosis in Parkinson's disease: an open pilot trial. Mov. Disord. 15, 1230–1237. doi: 10.1002/1531-8257(200011)15:6<1230::AID-MDS1026>3.0.CO;2-9
Nagano-Saito, A., Washimi, Y., Arahata, Y., Iwai, K., Kawatsu, S., Ito, K., et al. (2004). Visual hallucination in Parkinson's disease with FDG PET. Mov. Disord. 19, 801–806. doi: 10.1002/mds.20129
Naumann, W., Gogarten, J., Schönfeld, S., Klostermann, F., Marzinzik, F., and Schindlbeck, K. A. (2021). Diplopia in Parkinson's disease: indication of a cortical phenotype with cognitive dysfunction? Acta Neurol. Scand. 144, 440–449. doi: 10.1111/ane.13479
Nebe, A., and Ebersbach, G. (2007). Selective diplopia in Parkinson's disease: a special subtype of visual hallucination? Mov. Disord. 22, 1175–1178. doi: 10.1002/mds.21298
Nuara, S. G., Gourdon, J. C., Maddaford, S., and Huot, P. (2021). Additive effects of mGluR(2) positive allosteric modulation, mGluR(2) orthosteric stimulation and 5-HT(2A)R antagonism on dyskinesia and psychosis-like behaviours in the MPTP-lesioned marmoset. Naunyn Schmiedeberg's Arch. Pharmacol. 394, 2381–2388. doi: 10.1007/s00210-021-02162-7
Nuara, S. G., Hamadjida, A., Kwan, C., Bédard, D., Frouni, I., Gourdon, J. C., et al. (2020). Combined mGlu(2) orthosteric stimulation and positive allosteric modulation alleviates L-DOPA-induced psychosis-like behaviours and dyskinesia in the parkinsonian marmoset. J. Neural Transm. (Vienna) 127, 1023–1029. doi: 10.1007/s00702-020-02185-z
Onofrj, M., Russo, M., Delli Pizzi, S., De Gregorio, D., Inserra, A., Gobbi, G., et al. (2023). The central role of the thalamus in psychosis, lessons from neurodegenerative diseases and psychedelics. Transl. Psychiatry 13:384. doi: 10.1038/s41398-023-02691-0
Pacchetti, C., Manni, R., Zangaglia, R., Mancini, F., Marchioni, E., Tassorelli, C., et al. (2005). Relationship between hallucinations, delusions, and rapid eye movement sleep behavior disorder in Parkinson's disease. Mov. Disord. 20, 1439–1448. doi: 10.1002/mds.20582
Pachi, I., Maraki, M. I., Giagkou, N., Kosmidis, M. H., Yannakoulia, M., Dardiotis, E., et al. (2021). Late life psychotic features in prodromal Parkinson's disease. Parkinsonism Relat. Disord. 86, 67–73. doi: 10.1016/j.parkreldis.2021.04.001
Pachi, I., Papadopoulos, V., Koros, C., Simitsi, A. M., Bougea, A., Bozi, M., et al. (2023). Comprehensive evaluation of psychotic features and their clinical correlates in early Parkinson's disease. J. Parkinsons Dis. 13, 1185–1197. doi: 10.3233/JPD-230056
Pagonabarraga, J., Martinez-Horta, S., Fernández de Bobadilla, R., Pérez, J., Ribosa-Nogué, R., Marín, J., et al. (2016). Minor hallucinations occur in drug-naive Parkinson's disease patients, even from the premotor phase. Mov. Disord. 31, 45–52. doi: 10.1002/mds.26432
Pahwa, R., Isaacson, S. H., Small, G. W., Torres-Yaghi, Y., Pagan, F., and Sabbagh, M. (2022). Screening, diagnosis, and Management of Parkinson's disease psychosis: recommendations from an expert panel. Neurol. Ther. 11, 1571–1582. doi: 10.1007/s40120-022-00388-y
Patel, N., LeWitt, P., Neikrug, A. B., Kesslak, P., Coate, B., and Ancoli-Israel, S. (2018). Nighttime sleep and daytime sleepiness improved with Pimavanserin during treatment of Parkinson's disease psychosis. Clin. Neuropharmacol. 41, 210–215. doi: 10.1097/WNF.0000000000000307
Pham Nguyen, T. P., Abraham, D. S., Thibault, D., Weintraub, D., and Willis, A. W. (2021). Low continuation of antipsychotic therapy in Parkinson disease – intolerance, ineffectiveness, or inertia? BMC Neurol. 21:240. doi: 10.1186/s12883-021-02265-x
Pisani, S., Gosse, L., Wieretilo, R., Ffytche, D., Velayudhan, L., and Bhattacharyya, S. (2024). Cognitive and executive impairments in Parkinson's disease psychosis: a Bayesian meta-analysis. J. Neurol. Neurosurg. Psychiatry 95, 277–287. doi: 10.1136/jnnp-2022-331028
Pisani, S., Gunasekera, B., Lu, Y., Vignando, M., Ffytche, D., Aarsland, D., et al. (2023). Grey matter volume loss in Parkinson's disease psychosis and its relationship with serotonergic gene expression: a meta-analysis. Neurosci. Biobehav. Rev. 147:105081. doi: 10.1016/j.neubiorev.2023.105081
Powell, A., Matar, E., and Lewis, S. J. G. (2022). Treating hallucinations in Parkinson’s disease. Expert. Rev. Neurother. 22, 455–468. doi: 10.1080/14737175.2021.1851198
Rajagopalan, K., Rashid, N., and Doshi, D. (2023a). Patients treated with pimavanserin or quetiapine for Parkinson's disease psychosis: analysis of health resource utilization patterns among Medicare beneficiaries. J. Med. Econ. 26, 769–776. doi: 10.1080/13696998.2023.2220597
Rajagopalan, K., Rashid, N., and Doshi, D. (2024a). Risk of long-term care admissions among Medicare beneficiaries treated with pimavanserin or quetiapine for Parkinson's disease psychosis in USA: a retrospective administrative claims database analysis. J. Comp. Eff. Res. 13:e230114. doi: 10.57264/cer-2023-0114
Rajagopalan, K., Rashid, N., Gopal, D., and Doshi, D. (2024b). Healthcare resource utilization among nursing home residents with Parkinson's disease psychosis: an analysis of Medicare beneficiaries treated with pimavanserin or other-atypical antipsychotics. J. Comp. Eff. Res. 13:e240038. doi: 10.57264/cer-2024-0038
Rajagopalan, K., Rashid, N., Kumar, S., and Doshi, D. (2023b). Health care resource utilization patterns among patients with Parkinson's disease psychosis: analysis of Medicare beneficiaries treated with pimavanserin or other-atypical antipsychotics. J. Med. Econ. 26, 34–42. doi: 10.1080/13696998.2022.2152600
Rajagopalan, K., Rashid, N., Yakkala, V., and Doshi, D. (2024c). Analysis of Medicare patients treated with Pimavanserin versus other atypical antipsychotics: a cost-offset model evaluating skilled nursing facility stays and long-term care admissions in Parkinson's disease psychosis. Clinicoecon. Outcomes Res. 16, 149–159. doi: 10.2147/CEOR.S452162
Rashid, N., Shim, A., Andes, S., Quale, S., and Abler, V. (2022). Treatment patterns with antipsychotics in long-term care patients with Parkinson's disease psychosis. J. Appl. Gerontol. 41, 198–206. doi: 10.1177/0733464820987032
Ravina, B., Marder, K., Fernandez, H. H., Friedman, J. H., McDonald, W., Murphy, D., et al. (2007). Diagnostic criteria for psychosis in Parkinson's disease: report of an NINDS, NIMH work group. Mov. Disord. 22, 1061–1068. doi: 10.1002/mds.21382
Reilly, S., Dhaliwal, S., Arshad, U., Macerollo, A., Husain, N., and Costa, A. D. (2024). The effects of rivastigmine on neuropsychiatric symptoms in the early stages of Parkinson's disease: a systematic review. Eur. J. Neurol. 31:e16142. doi: 10.1111/ene.16142
Rissardo, J. P., Durante, Í., Sharon, I., and Fornari Caprara, A. L. (2022). Pimavanserin and Parkinson's disease psychosis: a narrative review. Brain Sci. 12:1286. doi: 10.3390/brainsci12101286
Rojas, M., Ariza, D., Ortega, Á., Riaño-Garzón, M. E., Chávez-Castillo, M., Pérez, J. L., et al. (2022). Electroconvulsive therapy in psychiatric disorders: a narrative review exploring neuroendocrine-immune therapeutic mechanisms and clinical implications. Int. J. Mol. Sci. 23:6918. doi: 10.3390/ijms23136918
Sawczak, C. M., Barnett, A. J., and Cohn, M. (2019). Increased cortical thickness in attentional networks in Parkinson's disease with minor hallucinations. Parkinsons Dis. 2019, 1–6. doi: 10.1155/2019/5351749
Schaffer, K. M., and Henry, M. L. (2023). Implementing a telehealth-delivered psychoeducational support group for care partners of individuals with primary progressive aphasia. Aphasiology 37, 1087–1111. doi: 10.1080/02687038.2022.2076281
Schneider, R. B., Auinger, P., Dobkin, R. D., Mills, K. A., Kulick-Soper, C. V., Myers, T. L., et al. (2024). Minor phenomena in Parkinson's disease-prevalence, associations, and risk of developing psychosis. J. Geriatr. Psychiatry Neurol. 37, 134–145. doi: 10.1177/08919887231195220
Schubmehl, S., and Sussman, J. (2018). Perspective on Pimavanserin and the SAPS-PD: novel scale development as a means to FDA approval. Am. J. Geriatr. Psychiatry 26, 1007–1011. doi: 10.1016/j.jagp.2018.06.001
Seeman, P. (2006). Targeting the dopamine D2 receptor in schizophrenia. Expert Opin. Ther. Targets 10, 515–531. doi: 10.1517/14728222.10.4.515
Seeman, P. (2011). All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2(high) receptors. CNS Neurosci. Ther. 17, 118–132. doi: 10.1111/j.1755-5949.2010.00162.x
Shafer, J. S., Haley, K. L., and Jacks, A. (2023). Barriers to informational support for Care Partners of People with Aphasia after Stroke. Am. J. Speech Lang. Pathol. 32, 2211–2231. doi: 10.1044/2023_AJSLP-22-00391
Shaughnessy, L., Brunton, S., Chepke, C., Farmer, J. G., Rosenzweig, A. S., and Grossberg, G. (2022). Using telemedicine to assess and manage psychosis in neurodegenerative diseases in long-term care. J. Am. Med. Dir. Assoc. 23, 1145–1152. doi: 10.1016/j.jamda.2021.12.033
Shine, J. M., Halliday, G. M., Naismith, S. L., and Lewis, S. J. (2011). Visual misperceptions and hallucinations in Parkinson's disease: dysfunction of attentional control networks? Mov. Disord. 26, 2154–2159. doi: 10.1002/mds.23896
Shotbolt, P., Samuel, M., Fox, C., and David, A. S. (2009). A randomized controlled trial of quetiapine for psychosis in Parkinson's disease. Neuropsychiatr. Dis. Treat. 5, 327–332. doi: 10.2147/NDT.S5335
Sid-Otmane, L., Hamadjida, A., Nuara, S. G., Bédard, D., Gaudette, F., Gourdon, J. C., et al. (2020). Selective metabotropic glutamate receptor 2 positive allosteric modulation alleviates L-DOPA-induced psychosis-like behaviours and dyskinesia in the MPTP-lesioned marmoset. Eur. J. Pharmacol. 873:172957. doi: 10.1016/j.ejphar.2020.172957
Srisurapanont, M., Suradom, C., Suttajit, S., Kongsaengdao, S., and Maneeton, B. (2024). Second-generation antipsychotics for Parkinson's disease psychosis: a systematic review and network meta-analysis. Gen. Hosp. Psychiatry 87, 124–133. doi: 10.1016/j.genhosppsych.2024.02.008
Stan, T. L., Ronaghi, A., Barrientos, S. A., Halje, P., Censoni, L., Garro-Martínez, E., et al. (2024). Neurophysiological treatment effects of mesdopetam, pimavanserin and clozapine in a rodent model of Parkinson's disease psychosis. Neurotherapeutics 21:e00334. doi: 10.1016/j.neurot.2024.e00334
Terravecchia, C., Mostile, G., Rascunà, C., Arabia, G., Barone, P., Marconi, R., et al. (2021). Does an association between cigarette smoking and Parkinson's disease-related psychosis exist? Insights from a large non-demented cohort. J. Neurol. Sci. 427:117509. doi: 10.1016/j.jns.2021.117509
Thames, B. H., and Ondo, W. G. (2023). Clozapine: efficacy for Parkinson disease psychosis in patients refractory to pimavanserin. Parkinsonism Relat. Disord. 109:105356. doi: 10.1016/j.parkreldis.2023.105356
Thanvi, B. R., Lo, T. C., and Harsh, D. P. (2005). Psychosis in Parkinson's disease. Postgrad. Med. J. 81, 644–646. doi: 10.1136/pgmj.2004.032029
Thota, N., Lenka, A., George, L., Hegde, S., Arumugham, S. S., Prasad, S., et al. (2017). Impaired frontal lobe functions in patients with Parkinson's disease and psychosis. Asian J. Psychiatr. 30, 192–195. doi: 10.1016/j.ajp.2017.10.013
Toh, W. L., Yolland, C., Gurvich, C., Barnes, J., and Rossell, S. L. (2023). Non-visual hallucinations in Parkinson's disease: a systematic review. J. Neurol. 270, 2857–2889. doi: 10.1007/s00415-022-11545-6
Torres-Yaghi, Y., Carwin, A., Carolan, J., Nakano, S., Amjad, F., and Pagan, F. (2021). QTc interval prolongation with therapies used to treat patients with Parkinson's disease psychosis: a narrative review. Neuropsychiatr. Dis. Treat. 17, 3791–3818. doi: 10.2147/NDT.S324145
Ueda, S., Koyama, K., and Okubo, Y. (2010). Marked improvement of psychotic symptoms after electroconvulsive therapy in Parkinson disease. J. ECT 26, 111–115. doi: 10.1097/YCT.0b013e3181c18a3d
Usui, C., Hatta, K., Doi, N., Kubo, S., Kamigaichi, R., Nakanishi, A., et al. (2011). Improvements in both psychosis and motor signs in Parkinson's disease, and changes in regional cerebral blood flow after electroconvulsive therapy. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 35, 1704–1708. doi: 10.1016/j.pnpbp.2011.05.003
van der Mast, R. C., and Fekkes, D. (2000). Serotonin and amino acids: partners in delirium pathophysiology? Semin. Clin. Neuropsychiatry 5, 125–131. doi: 10.153/SCNP00500125
Vignando, M., Ffytche, D., Lewis, S. J. G., Lee, P. H., Chung, S. J., Weil, R. S., et al. (2022). Mapping brain structural differences and neuroreceptor correlates in Parkinson's disease visual hallucinations. Nat. Commun. 13:519. doi: 10.1038/s41467-022-28087-0
Virués-Ortega, J., Rodríguez-Blázquez, C., Micheli, F., Carod-Artal, F. J., Serrano-Dueñas, M., and Martínez-Martín, P. (2010). Cross-cultural evaluation of the modified Parkinson psychosis rating scale across disease stages. Mov. Disord. 25, 1391–1398. doi: 10.1002/mds.23081
Voss, T., Bahr, D., Cummings, J., Mills, R., Ravina, B., and Williams, H. (2013). Performance of a shortened scale for assessment of positive symptoms for Parkinson's disease psychosis. Parkinsonism Relat. Disord. 19, 295–299. doi: 10.1016/j.parkreldis.2012.10.022
Voss, T. S., Brocht, A. F., and Ravina, B. (2010). Performance of the scale for assessment of positive symptoms in Parkinson's disease psychosis. Mov. Disord. 25, 124–125. doi: 10.1002/mds.22575
Wang, F., Dai, L., Pan, Y., Huang, P., Zhang, C., Sun, B., et al. (2024). Unilateral anterior capsulotomy combined with deep brain stimulation for Parkinson's disease psychosis and motor dysfunctions. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 128:110865. doi: 10.1016/j.pnpbp.2023.110865
Wang, Y., Li, D., Chen, Y., Zhu, S., Jiang, X., Jiang, Y., et al. (2023). Clinical features of minor hallucinations in different phenotypes of Parkinson's disease: a cross-sectional study. Front. Neurol. 14:1158188. doi: 10.3389/fneur.2023.1158188
Warren, N., O'Gorman, C., Hume, Z., Kisely, S., and Siskind, D. (2018). Delusions in Parkinson's disease: a systematic review of published cases. Neuropsychol. Rev. 28, 310–316. doi: 10.1007/s11065-018-9379-3
Watanabe, H., Senda, J., Kato, S., Ito, M., Atsuta, N., Hara, K., et al. (2013). Cortical and subcortical brain atrophy in Parkinson's disease with visual hallucination. Mov. Disord. 28, 1732–1736. doi: 10.1002/mds.25641
Weintraub, D., Espay, A. J., Sharma, V. D., Tariot, P. N., Abler, V., Pathak, S., et al. (2024). Pimavanserin for psychosis in Parkinson's disease dementia: subgroup analysis of the HARMONY trial. Parkinsonism Relat. Disord. 119:105951. doi: 10.1016/j.parkreldis.2023.105951
Weintraub, D., and Mamikonyan, E. (2019). The neuropsychiatry of Parkinson disease: a perfect storm. Am. J. Geriatr. Psychiatry 27, 998–1018. doi: 10.1016/j.jagp.2019.03.002
Wetmore, J. B., Li, S., Yan, H., Irfan, M., Rashid, N., Peng, Y., et al. (2019). Increases in institutionalization, healthcare resource utilization, and mortality risk associated with Parkinson disease psychosis: retrospective cohort study. Parkinsonism Relat. Disord. 68, 95–101. doi: 10.1016/j.parkreldis.2019.10.018
Whitehead, D. L., Davies, A. D., Playfer, J. R., and Turnbull, C. J. (2008). Circadian rest-activity rhythm is altered in Parkinson's disease patients with hallucinations. Mov. Disord. 23, 1137–1145. doi: 10.1002/mds.22057
Wilby, K. J., Johnson, E. G., Johnson, H. E., and Ensom, M. H. H. (2017). Evidence-based review of pharmacotherapy used for Parkinson's disease psychosis. Ann. Pharmacother. 51, 682–695. doi: 10.1177/1060028017703992
Williams, D. R., and Lees, A. J. (2005). Visual hallucinations in the diagnosis of idiopathic Parkinson's disease: a retrospective autopsy study. Lancet Neurol. 4, 605–610. doi: 10.1016/S1474-4422(05)70146-0
Williams-Gray, C. H., Foltynie, T., Lewis, S. J., and Barker, R. A. (2006). Cognitive deficits and psychosis in Parkinson's disease: a review of pathophysiology and therapeutic options. CNS Drugs 20, 477–505. doi: 10.2165/00023210-200620060-00004
Wolters, E. C. (1999). Dopaminomimetic psychosis in Parkinson's disease patients: diagnosis and treatment. Neurology 52, S10–S13
Yamaoka, K., Masuda, Y., and Wada, K. (2022). Effectiveness of electroconvulsive therapy for apathy accompanied by psychosis in early-onset Parkinson's disease. Psychiatry Clin. Neurosci. 76, 344–345. doi: 10.1111/pcn.13365
Yao, N., Shek-Kwan Chang, R., Cheung, C., Pang, S., Lau, K. K., Suckling, J., et al. (2014). The default mode network is disrupted in Parkinson's disease with visual hallucinations. Hum. Brain Mapp. 35, 5658–5666. doi: 10.1002/hbm.22577
Yoshino, H., Hirano, M., Stoessl, A. J., Imamichi, Y., Ikeda, A., Li, Y., et al. (2017). Homozygous alpha-synuclein p.A53V in familial Parkinson's disease. Neurobiol. Aging 57, 248.e7–248.e12. doi: 10.1016/j.neurobiolaging.2017.05.022
Zahodne, L. B., and Fernandez, H. H. (2008). Pathophysiology and treatment of psychosis in Parkinson's disease: a review. Drugs Aging 25, 665–682. doi: 10.2165/00002512-200825080-00004
Zarkali, A., McColgan, P., Leyland, L. A., Lees, A. J., Rees, G., and Weil, R. S. (2020a). Fiber-specific white matter reductions in Parkinson hallucinations and visual dysfunction. Neurology 94, e1525–e1538. doi: 10.1212/WNL.0000000000009014
Zarkali, A., McColgan, P., Ryten, M., Reynolds, R., Leyland, L. A., Lees, A. J., et al. (2020b). Differences in network controllability and regional gene expression underlie hallucinations in Parkinson's disease. Brain 143, 3435–3448. doi: 10.1093/brain/awaa270
Zhang, Y., Zhang, G. Y., Zhu, X. B., Zhang, Z. E., Gan, J., and Liu, Z. G. (2021). Clinical characteristics of minor hallucinations in Chinese Parkinson's disease patients. Front. Aging Neurosci. 13:723405. doi: 10.3389/fnagi.2021.723405
Zhong, M., Gu, R., Zhu, S., Bai, Y., Wu, Z., Jiang, X., et al. (2021a). Prevalence and risk factors for minor hallucinations in patients with Parkinson's disease. Behav. Neurol. 2021, 1–10. doi: 10.1155/2021/3469706
Zhong, M., Wu, Z., Jiang, X., Shen, B., Zhu, J., and Zhang, L. (2021b). Knowledge domain and emerging trends in visual hallucination research: a scientometric analysis. World J. Psychiatry 11, 491–506. doi: 10.5498/wjp.v11.i8.491
Zhong, M., Zhu, S., Gu, R., Wang, Y., Jiang, Y., Bai, Y., et al. (2022). Elevation of plasma homocysteine and minor hallucinations in Parkinson's disease: a cross-sectional study. Behav. Neurol. 2022, 1–10. doi: 10.1155/2022/4797861
Keywords: Parkinson’s disease psychosis, PDP, bibliometric analysis, hotspots, global trends
Citation: Wu J, Jin X, Xie W, Liu L, Wang F, Zhu L, Shen Y and Qiu L (2024) Global research trends and hotspots in Parkinson’s disease psychosis: a 25-year bibliometric and visual analysis. Front. Aging Neurosci. 16:1480234. doi: 10.3389/fnagi.2024.1480234
Edited by:
Anastasia Bougea, National and Kapodistrian University of Athens, GreeceReviewed by:
Harrison Tudor Evans, New York University, United StatesHoracio Capote, Dent Neurologic Institute, United States
Copyright © 2024 Wu, Jin, Xie, Liu, Wang, Zhu, Shen and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Shen, OTU4MzAwNzIxQHFxLmNvbQ==; Linghe Qiu, MTAzNzA3MDM0OEBxcS5jb20=
†These authors have contributed equally to this work
 Jianhong Wu
Jianhong Wu Xin Jin2†
Xin Jin2† Linghe Qiu
Linghe Qiu