- 1West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, China
- 2Hangzhou Seventh People’s Hospital, Zhejiang University School of Medicine, Zhejiang, Hangzhou, China
Background: Cognitive impairment and frailty are common issues in older adults. Understanding the co-development trajectories of these conditions can provide valuable sights for early detection and intervention in high-risk individuals.
Objectives: This study aims to identify the co-development of cognitive function and frailty and explore the associated characteristics.
Methods: We analyzed data from 8,418 individuals aged 55 years and above who participated in the China Health and Retirement Longitudinal Survey between 2011 and 2018. Group-based dual trajectory modeling and logistic regression were used to identify trajectory groups and assess associations with risk factors.
Results: Two distinct dual trajectories were identified: “Consistently Robust” group (76.12%) and “Consistently Severe” group (23.88%). Factors such as being female, older age, lower levels of education, residing in rural areas, being unmarried, and having comorbidities such as hypertension, diabetes, complete tooth loss, vision impairment, or hearing impairment were associated with a higher likelihood of being assigned to the “Consistently Severe” group.
Conclusion: Our findings suggest a co-development pattern between cognitive function and frailty in Chinese older adults aged 55 years and above. While cognitive impairment may be irreversible, frailty is a condition that can be potentially reversed. Early detecting is crucial in preventing cognitive decline, considering the shared trajectory of these conditions.
1 Introduction
China’s population is aging rapidly. By the end of 2022, the number of individuals aged 60 and above had reached 280.04 million, accounting for approximately 19.8% of the total population (Affairs MoC, 2023). With this demographic shift, the prevalence of cognitive impairment and frailty among older adults has increased, significantly affecting their quality of life and placing a considerable economic and caregiving burden on their families.
A growing body of research has explored the relationship between frailty and cognitive impairment in older adults. For example, a longitudinal study conducted in South Korea among older adults aged 65 and above found that individuals who remained frail or transitioned from non-frail to frail had higher likelihood of lower cognitive function (Nari et al., 2021). Similarly, a prospective study in Japan revealed that frailty was associated with cognitive decline in older adults over a two-year period (Chen et al., 2018). These findings are further supported by several studies demonstrating that frail older adults are at greater risk of experiencing cognitive impairment compared to their non-frail counterparts (Li et al., 2023; Kim et al., 2014). Conversely, there is evidence that cognitive decline also serves as a risk factor for developing frailty (Mulero et al., 2011; Doba et al., 2012; Yuan et al., 2021). For example, Li et al. (2023) study revealed that older adults with cognitive impairment have an estimated 19.5% higher one-year incidence of frailty compared to those without cognitive impairment (Belleville et al., 2022).
Despite substantial evidence, the causal relationship between frailty and cognitive impairment remains complex and not fully understood. A review by Robertson et al. (2013) discusses how frailty and cognitive decline interact within a cycle of age-associated decline, suggesting a bidirectional nature of this relationship. Furthermore, some researchers propose that frailty and cognitive impairment may develop simultaneously, sharing common biological causes (Buchman et al., 2007; Buchman et al., 2014). The oxidative stress theory suggests that the brain’s vulnerability to oxidative damage can lead to cognitive impairment (Mulero et al., 2011), while reactive oxygen species contribute to frailty (Kregel and Zhang, 2007). As a result, researchers are starting to investigate the co-occurrence of cognitive decline and frailty at the population level. Group-based dual trajectory modeling (GBDTM) has emerged as a powerful analytical tool for examining these parallel developments, allowing for a better understanding of how frailty and cognitive impairment influence each other over time.
GBDTM is an extension of Nagin’s Group-Based Trajectory Model (GBTM). While GBTM assumes that the population consists of multiple unobserved subgroups, each with its own trajectory, and uses finite mixture modeling to estimate these groups, GBDTM focuses on modeling the developmental trajectories of two related outcomes (Nagin and Odgers, 2010). It enhances GBTM by incorporating polynomial models of time (age) and using maximum likelihood estimation to assign individuals to subgroups (Nagin and Tremblay, 2001). Unlike GBTM, GBDTM allows for statistical associations between trajectories, enabling the exploration of their co-relationships. It employs simultaneous models to estimate the trajectories of two variables, with relationships that can vary across subgroups. These relationships can be linear or nonlinear, capturing the complex dynamics between variables. A key strength of GBDTM is its ability to identify natural subgroups within a population based on joint changes in the two variables, with these subgroups emerging from the data rather than being predefined (Nagin and Tremblay, 2001; Muthén and Muthén, 2000; Curran and Hussong, 2003).
GBDTM has proven effective in identifying dual trajectories, making it well-suited for exploring common trends in cognitive function and frailty over time. For example, a longitudinal study in the U.S. showed that 20% of older adults exhibited concurrent trajectories of “persistent frailty” and “persistent severe cognitive impairment” (Yuan et al., 2022). Similarly, another study identified four distinct trajectories of frailty and cognitive functioning, with 6.5% of older adults showing a pattern of cognitive frailty (Liu et al., 2018). In Mexico, a longitudinal study revealed that 63% of older adults with an increasing frailty index experienced rapid cognitive decline, while 68% of those with no frailty changes maintained cognitive stability (Howrey et al., 2020). In Milan, 4.3% of old adults showed continuous decline in both cognitive and physical functioning (Ferraro et al., 2021). These studies, based on GBDTM, highlight the common trajectories of frailty and cognitive impairment, though the patterns vary across countries.
While some studies have been conducted in Europe and America, our research focuses on a population that has been underrepresented in the literature. The unique cultural, lifestyle, and demographic factors in China may lead to different dual trajectories of cognitive function and frailty compared to those observed in Western populations. By exploring this specific context, we aim to provide valuable insights into how these dual trajectories manifest and to identify distinct associated factors. Our findings could help determine whether these dual trajectories are universal or context-specific, ultimately contribute to a more comprehensive understanding of cognitive and frailty dynamics.
2 Materials and methods
2.1 Data
The data for this study were obtained from the China Health and Retirement Longitudinal Study (CHARLS), which is a large-scale interdisciplinary longitudinal survey project conducted by the National Development Research Institute of Peking University and implemented by the China Social Science Survey Center of Peking University. The baseline survey was conducted in 2011, covering 150 counties and 450 communities (villages) across 28 provinces, autonomous regions, and municipalities in China, targeting individuals aged 45 and above. Subsequent nationwide follow-up surveys were conducted in 2013, 2015, 2018 and 2020.
This study used data from the 2011 CHARLS cohort, along with newly recruited participants in 2013 as the baseline, resulting in a total of 21,131 individuals. The inclusion criteria for the study were: (1) participants aged 55 and above at baseline; and (2) participants who completed at least two follow-up waves in 2013, 2015, and 2018. The exclusion criteria included: (1) missing data for key variables. After excluding participants younger than 55 years old, 11,210 participants remained. Further exclusion of individuals with missing key variables resulted in a final sample of 8,814 older adults leading to an attrition rate of 21.37%.
2.2 Measures
2.2.1 Cognitive function
In this study, we focused on the memory dimension to ensure consistent measurement of cognitive function from 2011 to 2018. Memory was assessed using both immediate memory and delayed memory tests (Yuan et al., 2022). Immediate memory involved presenting participants with a set of 10 words, which they were then asked to recall within a two-minute period with scores ranging from 0 to 10. Delayed memory was evaluated by assessing participants’ recall of the same 10 words after testing their depression, calculation ability, and visuospatial ability. Scores for delayed memory also ranged from 0 to 10. Therefore, the combined score for immediate and delayed memory ranged from 0 to 20, with lower scores indicating poorer cognitive function.
2.2.2 Frailty
The frailty status of the individuals was assessed using the Frailty Index (FI), which evaluates the degree of frailty by calculating the cumulative number or proportion of health deficits. This is typically represented as the ratio of cumulative health deficit count to the total number of health items included, with total score ranging from 0 to 1; higher scores indicating a more severe frailty status (Liu et al., 2018). In this study, the FI included 38 health indicators, including 6 activities of daily living (ADL), five instrumental activities of daily living (IADL), nine physical function limitations, 12 chronic diseases, five mental health indicators, and one self-rated health indicator (Supplementary Table 1).
2.2.3 Covariates
We included several covariates in our analysis, including age, gender, residence, marital status, education, annual household expenditure, chronic disease, current drinking status, visual and hearing impairment, and complete tooth loss. Annual personal expenditure was categorized as “Lower than the average” and “Higher than the average” in comparison to the annual personal expenditure of Chinese residents in 2010. Vision impairment was defined as meeting one of the following criteria: self-reported poor vision, near vision impairment, or distance vision impairment (Howrey et al., 2020). Hearing impairment was defined as meeting one of the following criteria: having problems with deafness or partial deafness, wearing a hearing aid (Ferraro et al., 2021), or self-reporting poor hearing (Nagin, 1999). The coding of variables is presented in Supplementary Table 2.
2.3 Statistical analysis
We provided an overview of the participants’ baseline characteristics, as well as their cognitive function and frailty status for each wave. We then conducted trajectory analysis, which involved the following steps:
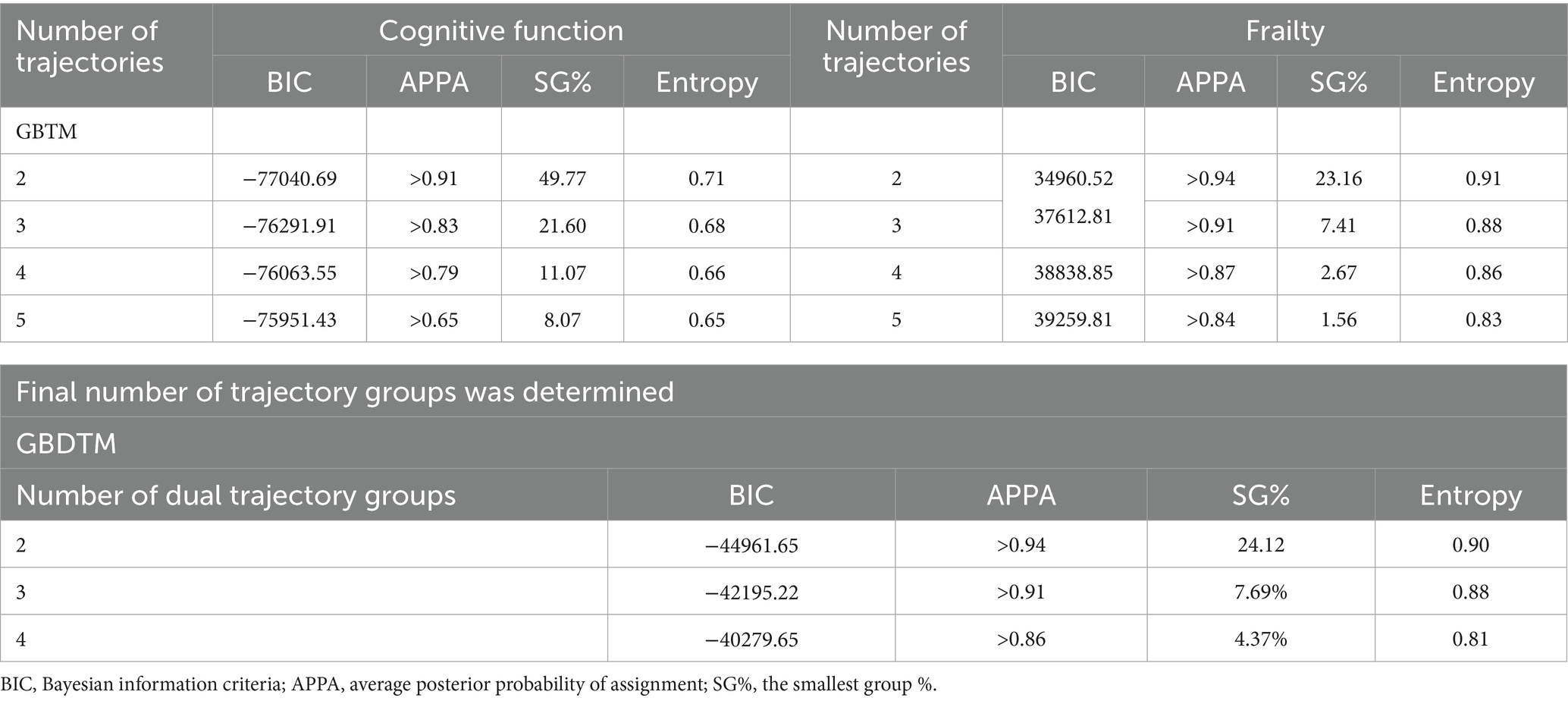
First, we performed separate group-based trajectory modeling (GBTM) for cognitive function and frailty. GBTM identifies unobserved heterogeneous subgroups within the sample population and develops trajectories based on their trends (Nagin, 1999). Individuals are assigned to the most likely subgroups based on the largest posterior probability (Huang et al., 2013).
Second, we used group-based dual trajectory modeling (GBDTM) for cognitive function and frailty, treated these as dependent variables with year as the time variable. A dual development trajectory was fitted to assign similar individuals to different subgroups. To determine the most appropriate number of trajectories for GBDTM, we tested separate GBTM for cognitive function and frailty with two or more trajectory groups. In GBDTM, the number of dual trajectory groups and slope parameters in each group were set according to the results from GBTM.
We evaluate model fit using the Bayesian Information Criterion (BIC), entropy, the smallest group size (SG%) including at least 5% of the sample, and an average posterior probability of assignment (APPA) >0.70.
Finally, logistic regression analyses were conducted to determine the association between the potential associated factors and the dual trajectories of cognitive function and frailty. Group differences were considered significant if p < 0.05 (two- tailed).
3 Results
3.1 Sociodemographic and health characteristics
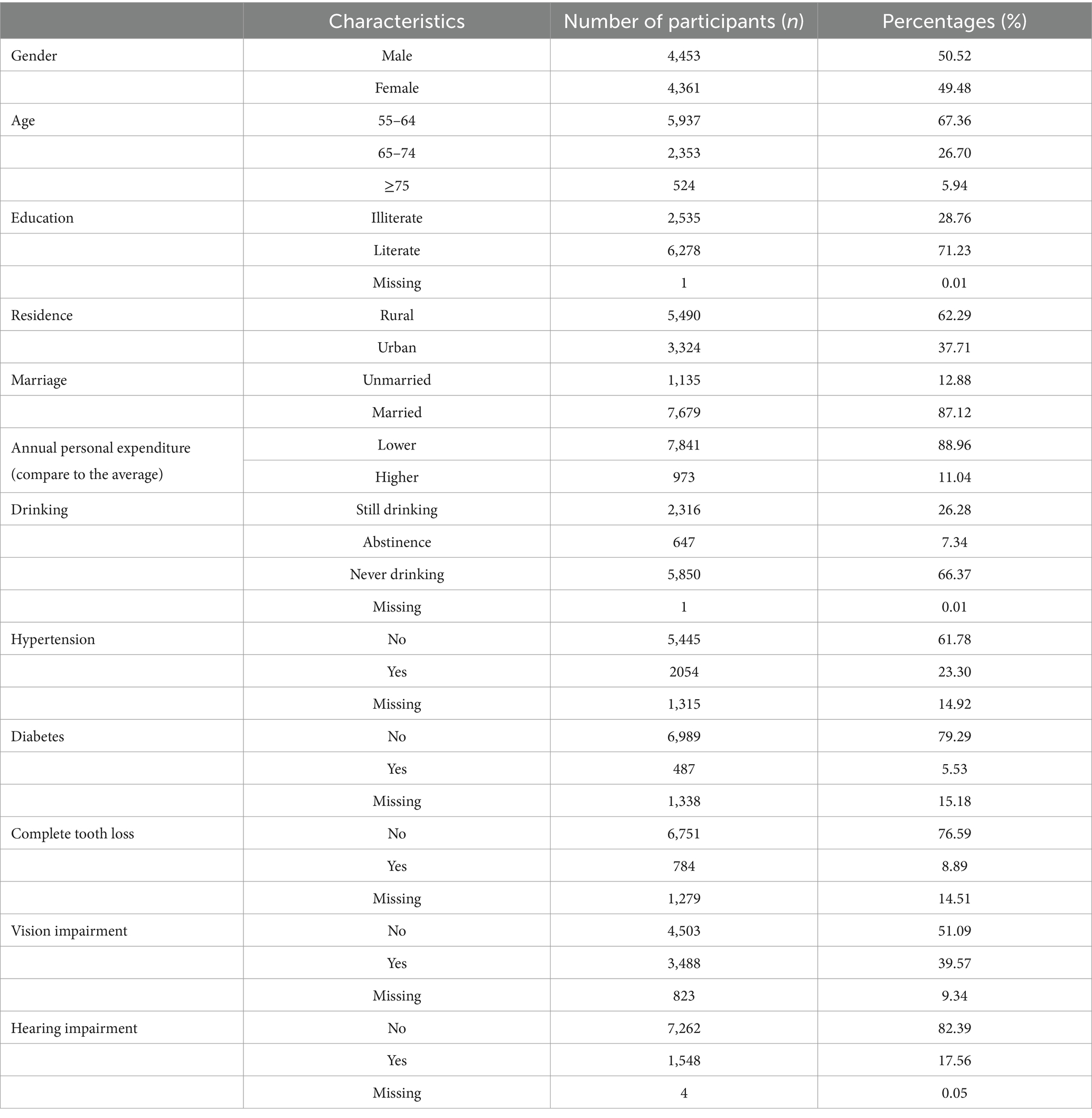
In this study, a total of 8,814 participants were included, with a mean age of 62.6 () years. Among them, males accounted for 50.52 and 49.48% for females. Most participants were literate (71.24%), residing in rural areas (62.29%), married (87.12%). Only 11.04% of the participants had annual personal expenditure higher than the average. And 66.38% of the participants reported never drinking alcohol. In terms of health conditions, with regard to missing data, at least 27.39% had hypertension, 6.51% had diabetes, 10.40% had experienced complete tooth loss, 43.65% had vision impairment, 17.57% had hearing impairment (Table 1).
3.2 Identifying dual trajectories of cognitive function and frailty among Chinese older adults
In the GBDTM models, we determine the number of dual trajectory groups and the slope parameters for each group based on the outcomes derived from the GBTM models for both cognitive function and frailty (Table 2) (Liu et al., 2018). Two dual trajectories were identified and labeled as follows (Supplementary Table 3): “Consistently Robust” group [Group 1 (G1); 76.12%] and “Consistently Severe” group [Group 2 (G2); 23.88%]. G1 demonstrated a high initial value and stable trend in cognitive function (slope = −0.29, p < 0.001), a low initial value and stable trend in frailty (slope = 0.00, p < 0.05) over time. Whereas G2 demonstrated a decline in cognitive function (slope = −0.30, p < 0.001) and an increase in frailty level (slope = 0.01, p < 0.001).
Figure 1 illustrates the levels and shapes of change of these dual trajectories. Participants in G1 exhibited better cognitive function than those in G2, despite a decline over time. By the end of the follow-up, G1 participants maintained relatively stable cognitive and were physically robust throughout the study. In contrast, participants in G2 experienced a continuous deterioration in both frailty and cognitive function, with changes occurring at a greater magnitude than in G1.
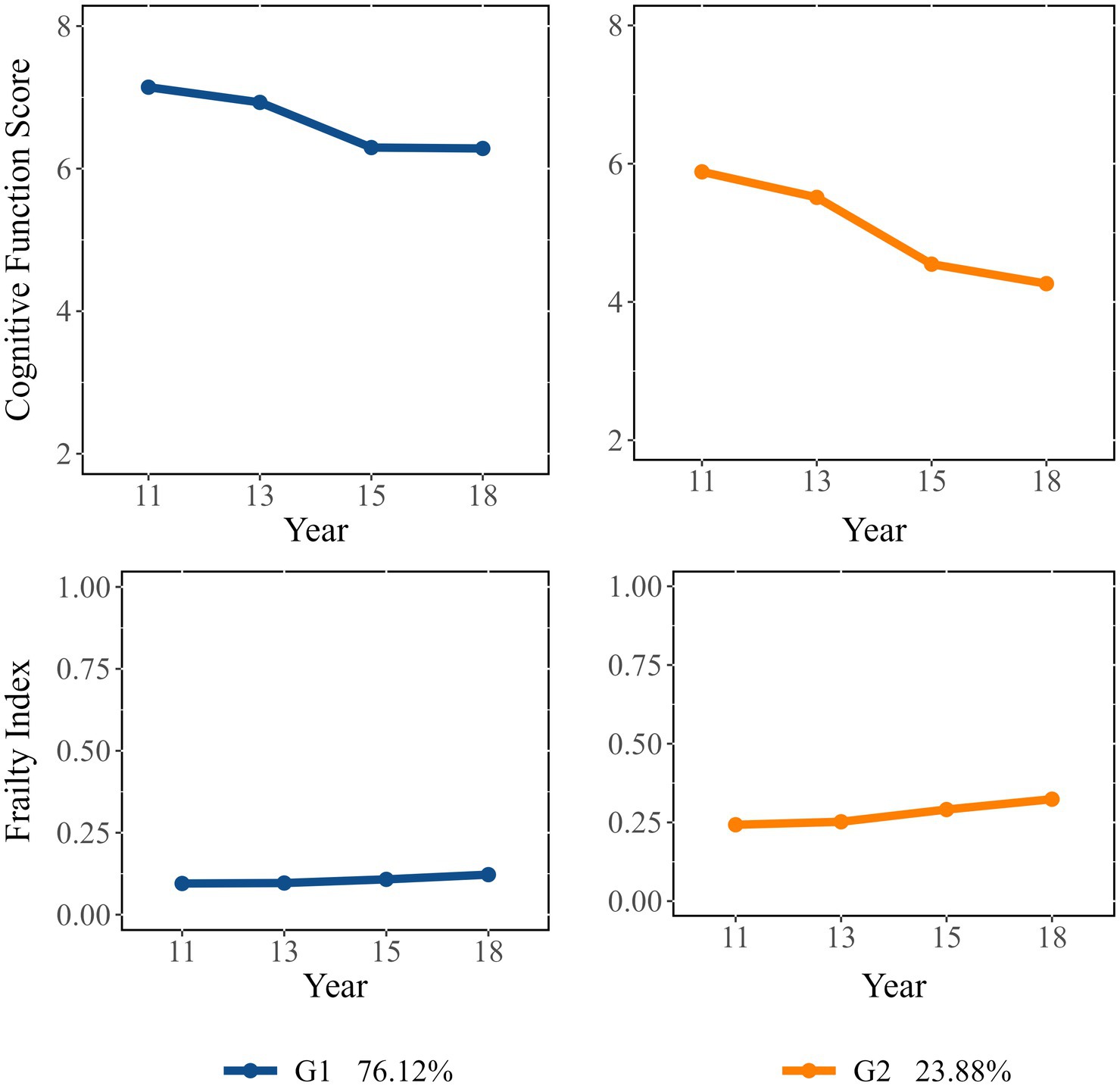
Figure 1. Dual trajectories of cognitive function (represented by cognitive function score) and frailty (represented by frailty index).
3.3 Examining associated factors of identified dual trajectories of cognitive function and frailty
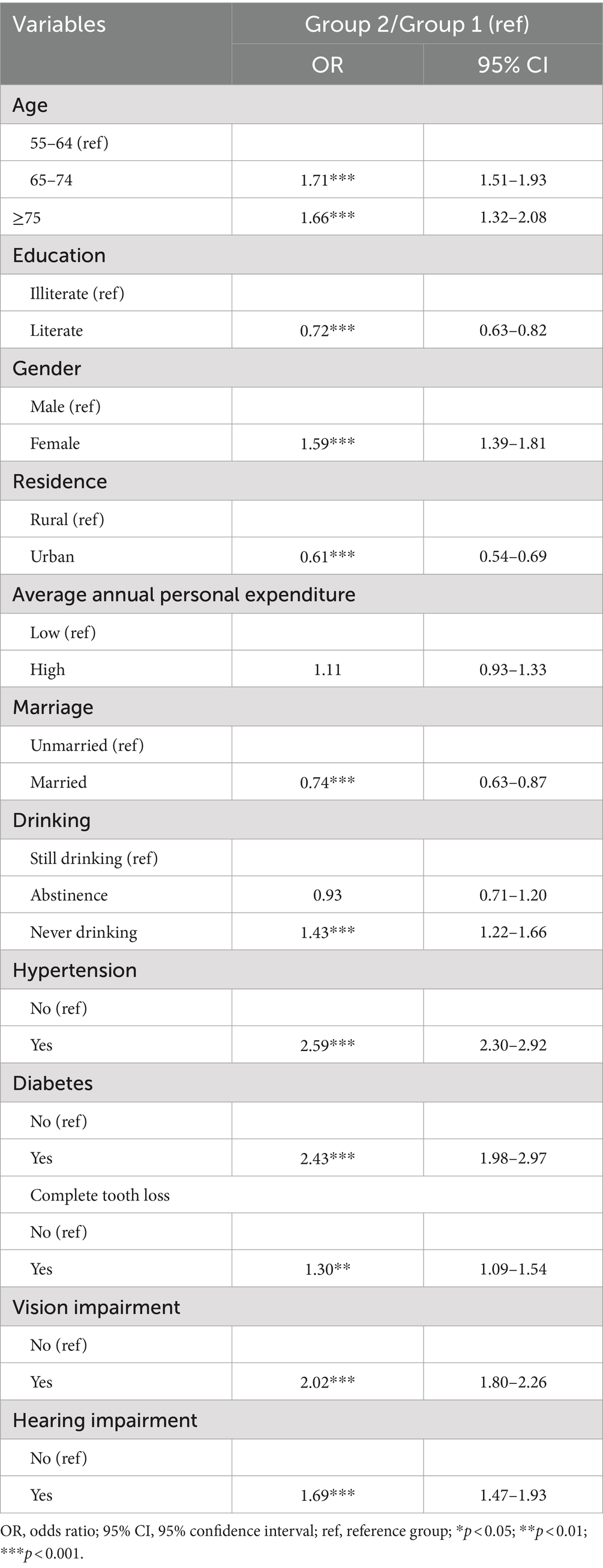
Multivariate analyses of dual trajectories indicated that participants aged 65 to 74 at baseline were 1.71 times more likely to experience deteriorating cognition and frailty [OR: 1.71; 95%CI: 1.51–1.93]. Those aged 75 and older had 1.66 times higher likelihood of falling into the “Consistently Severe” category [OR: 1.66; 95%CI: 1.32–2.08]. Women were also at increased risk, with a likelihood 1.59 times greater [OR: 1.59; 95%CI: 1.39–1.81]. Conversely, literate individuals had a lower probability of being assigned to the “Consistently Severe” group [OR: 0.72; 95%CI: 0.63–0.82]. Similarly, those living in urban areas [OR: 0.61; 95%CI: 0.54–0.69] and those who were married [OR: 0.74; 95%CI: 0.63–0.87] also showed lower likelihoods of experiencing continuous cognitive and frailty decline.
Additionally, older adults with chronic diseases at baseline were more likely to be in the “Consistently Severe” group. Specifically, participants with hypertension were 2.59 times more likely to experience worsening cognition and frailty [OR: 2.59; 95%CI: 2.30–2.92], those with diabetes were 2.43 times more likely [OR: 2.43; 95%CI: 1.98–2.97], and participants with vision impairment were 2.02 times more likely [OR: 2.02; 95%CI: 1.80–2.26]. Furthermore, the probability of being assigned to the “Consistently Severe” group was 30% higher [OR: 1.30; 95%CI: 1.09–1.54] for individuals with complete tooth loss compared to those without, and 69% higher [OR: 1.69; 95%CI: 1.47–1.93] for those with hearing impairment.
Above results can be found in Table 3. Additionally, the results of the logistic regression analyses conducted separately for cognitive function and frailty can be found in Supplementary Tables 4, 5.
4 Discussion
In this longitudinal study, we observed two distinct dual trajectories of cognitive function and frailty among older adults in China. One group exhibited better cognitive function and lower levels of frailty compared to the other group. This finding is consistent with previous studies conducted in both institutionalized (Yuan et al., 2022) and non-institutionalized (Howrey et al., 2020) American older adults, which also identified consistent developmental trends in cognitive function and frailty. The correlation between changes in cognitive function and frailty may contributed to common underlying mechanisms. Previous research has highlighted the influence of vascular changes, hormones, vitamin D levels, inflammation, insulin resistance, and nutrition on both cognitive function and frailty in older individuals (Halil et al., 2015). Moreover, dysregulated HPA stress response, imbalanced energy metabolism, mitochondrial dysfunction, oxidative stress, and neuroendocrine dysfunction have been proposed as shared etiological factors for the concurrent occurrence of frailty and cognitive decline (Ma and Chan, 2020). This consensus was reflected in a 2013 conference held by the International Association of Nutrition and Aging and the International Association of Gerontology and Geriatrics, which coined the term “cognitive frailty” to describe the coexistence of cognitive impairment and frailty (Kelaiditi et al., 2013). Identifying individuals at a high risk of experiencing rapid declines in cognitive function and escalating frailty is crucial, given the heightened threat of mortality and disability associated with this condition (Ma et al., 2021).
We observed that certain characteristics, such as being female, older, illiterate, residing in rural areas, and unmarried were more prevalent among individuals assigned to the “Consistently Severe” group. These individuals also displayed worse overall health compared to those in the “Consistently Robust” group, including a higher prevalence of conditions such as diabetes, hypertension, hearing impairment, vision impairment, and complete tooth loss. The association between increasing age and being assigned to the “Consistently Severe” group is likely attributable to the natural deterioration of organ functions with aging. On the other hand, married older adults were more likely to be categorized into the “Consistently Robust” group, possibly due to their engagement in social activities and greater social support. We found that older adults residing in rural areas had a higher likelihood of being assigned to the “Consistently Severe” group, which aligns with findings from other research (Liu et al., 2021). However, we did not find a significant impact of alcohol consumption on cognitive function changes in older adults. The relationship between alcohol consumption and cognition remains inconclusive. While some studies suggest a connection (Fein et al., 2006; Montejo and Rico-Villademoros, 2008; García-Marchena et al., 2020), our findings are in line with the results of Linglong et al. (2021), who found no impact of alcohol consumption on different cognitive function trajectories among adults aged 65 and above in China. To further investigate this association accurately, future research is recommended to include additional specific variables related to alcohol consumption, such as the type of alcohol consumed, dosage, and other relevant factors.
Identifying potential factors that contribute to assigning individuals into high-risk groups allows for targeted interventions aimed at delaying cognitive function decline and frailty. In our research, we identified modifiable health factors suitable for intervention, including hypertension, diabetes, visual impairment, hearing impairment and complete tooth loss. Previous studies have suggested an association between hypertension and both cognitive function and frailty (Belessiotis-Richards et al., 2021; Ma et al., 2020; Emiliano Albanese et al., 2013; Farron et al., 2020; Qiu et al., 2005). A review has indicated that higher blood pressure may increase the risk of dementia in the future, particularly in cases of untreated hypertension (Qiu et al., 2005), potentially aligning with our finding that hypertensive patients are more likely to be assigned to the “Consistently Severe” group.
Our study also found that diabetes was associated with poorer cognition and frailty, which is consistent with a review examining the impact of diabetes on brain function and structure over the past two decades (Moheet et al., 2015). However, prior research on the relationship between diabetes and cognitive function remains inconclusive. For instance, a cross-sectional study in India found that self-reported diabetes is linked to better cognitive performance (Belessiotis-Richards et al., 2021). In addition, a review emphasized that managing diabetes and reducing complications may lower the risk of cognitive impairment, with a stronger association observed in type 2 diabetes patients compared to those with type 1 diabetes (Kodl and Seaquist, 2008).
Furthermore, our study revealed that older adults without complete tooth loss exhibited better cognitive and frailty outcomes, aligning with numerous previous research findings (Hoeksema et al., 2017; Yun et al., 2020; Zhang et al., 2022; Zhang et al., 2022). To reduce the likelihood of tooth loss, various measures can be taken, including early screening for oral health, treatment of periodontal disease, and maintenance of good oral hygiene practices. For older adults experiencing tooth loss, obtaining conventional prostheses as early as possible is advisable to prevent complications arising from the simultaneous exacerbation of cognitive function and frailty resulting from having fewer or no teeth (Yun et al., 2020).
Sensory impairments, such as hearing impairment and vision impairment, are prevalent among older adults. In our study, we found that 17.56% of older adults had hearing impairments, while and even higher proportion, 39.57%, had vision impairments. Age-related hearing loss is associated with changes in both the central and peripheral auditory systems and is characterized by difficulties in understanding words in noisy environments, which may contribute to late-life cognitive disorders (Panza et al., 2018). Similarly, hearing impairment can lead to psychosocial stress, social isolation, and the onset of depression (Johnson et al., 2015), mirroring the mechanisms observed in visual impairments (Reyes-Ortiz et al., 2005; Liljas et al., 2017). Both hearing and vision impairments have been linked to cognitive decline and dementia. Older adults with impaired vision and hearing may face challenges in accessing social support, which is crucial for preventing cognitive decline and frailty. Undetected and untreated impaired vision and hearing can have significant impacts on patients, their loved ones, and society as a whole.
Considering that cognitive decline is irreversible while frailty can be reversed (Baolin et al., 2021), and since both share common trajectories, controlling and intervening in the frailty of older adults can help prevent or slow down their cognitive decline. These findings provide valuable insights for the care of older adults.
Given that many predictors for assigning into the “Consistently Severe” group are preventable, and frailty is reversible, it is crucial to identify older adults at high risk through regular health monitoring and implement interventions as soon as possible. Early detection and intervention can significantly improve the overall health and quality of life for older adults. We recommend that community healthcare centers closely monitor the risk factors identified in the health checks of older adults and identify high-risk populations. By doing so, better management of the health status of them can be achieved, implementing appropriate intervention measures to address these factors. This will be beneficial in delaying or preventing cognitive decline and frailty.
5 Strengths and limitations
This study represents a pioneering application of GBDTM to explore the heterogeneity in co-development trajectories of cognitive function and frailty among older adults in China, using longitudinal data. Furthermore, we identified potential factors that increase the likelihood of older adults being classified into the “Consistently Severe” group, characterized by a higher rate of decline in both cognitive function and frailty.
However, it is important to acknowledge the limitations of this study. Firstly, due to data limitation, our assessment of cognitive function focused primarily on immediate and delayed memory, without considering other dimensions such as executive function or orientation function. While it is true that the memory is a highly relevant dimension in relation to dementia, the exclusion of other cognitive dimensions may limit the comprehensive understanding of cognitive function in our findings. Secondly, in our study, several items, particularly those related to chronic diseases, sensory impairment and physical activities, were self-reported by participants. The use of self-reporting introduces the potential for recall bias, as participants may not accurately remember or report their behaviors or conditions. This may impact the validity and reliability of the collected data. However, it is worth noting that self-report of medical conditions has been widely used in studies involving community-dwelling older adults. Additionally, previous research has demonstrated consistency between self-reporting and objective measurements (Yuan et al., 2022; Howrey et al., 2020; Ma et al., 2020; Reyes-Ortiz et al., 2005; Liljas et al., 2017). Despite the limitations of self-reporting, it remains a valuable and commonly employed method for gathering data in this context. Thirdly, it is important to note that while the CHARLS recruited a representative sample in China, it did not include individuals from minority groups residing in regions such as Tibet and Xinjiang. As a result, the findings of this study may not be directly applicable to minority populations. It is recommended that future research should specifically investigate the co-development trajectories of cognition and frailty among these minority populations to ensure a more comprehensive understanding of the topic.
6 Conclusion
In this longitudinal study, one group showed better cognitive function and lower levels of frailty, while the “Consistently Severe” group exhibited poorer cognitive function and higher levels of frailty. Several characteristics were associated with being in the “Consistently Severe” group, including being female, older, illiterate, residing in rural areas, and being unmarried. Additionally, individuals in this group reported worse overall health and a higher prevalence of conditions such as diabetes, hypertension, hearing impairment, vision impairment, and complete tooth loss.
While cognitive impairment may be irreversible, frailty is a reversible condition. Therefore, early detection of frailty is crucial for preventing cognitive decline, given their share trajectory. Furthermore, many predictors for being in the “Consistently Severe” group are preventable, highlighting the importance of regular health monitoring and timely interventions.
Community healthcare centers should closely monitor the risk factors identified in the annual health checks for older adults. This enables the development of targeted interventions to address these factors, which may help delay or prevent cognitive decline and frailty.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: http://charls.pku.edu.cn/en.
Author contributions
XJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YuW: Conceptualization, Data curation, Methodology, Software, Writing – original draft. ZG: Methodology, Supervision, Validation, Writing – review & editing. ZZ: Supervision, Validation, Writing – review & editing. KW: Methodology, Writing – review & editing. SY: Writing – review & editing. YaW: Supervision, Writing – review & editing. PQ: Conceptualization, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study received support from the National Natural Science Foundation of China (grant number: 72174133) and the Natural Science Foundation of Sichuan Province (grant number: 2022NSFSC0668).
Acknowledgments
Thanks to the staff of the China Health and Retirement Longitudinal Study (CHARLS) for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2024.1465914/full#supplementary-material
References
Affairs MoC. (2023). Communique on the development of the National Cause for aging in 2022. Available at: https://www.gov.cn/lianbo/bumen/202312/P020231214405906944856.pdf (Accessed November 1, 2024).
Baolin, L., Zebin, L., Senyun, C., Xiaoqi, X., Yan, X., and Chujun, S. (2021). Interventions to prevent, delay or reverse frailty in the elderly: evidence summary. J. Nurs. Sci. 36, 32–37. doi: 10.3870/j.issn.1001-4152.2021.14.032
Belessiotis-Richards, C., Livingston, G., Marston, L., and Mukadam, N. (2021). A cross-sectional study of potentially modifiable risk factors for dementia and cognitive function in India: a secondary analysis of 10/66, LASI, and SAGE data. Int. J. Geriatr. Psych. 37:5661. doi: 10.1002/gps.5661
Belleville, S., Cuesta, M., Bieler-Aeschlimann, M., Giacomino, K., Widmer, A., Mittaz Hager, A. G., et al. (2022). Pre-frail older adults show improved cognition with StayFitLonger computerized home–based training: a randomized controlled trial. GeroScience. 45, 811–822. doi: 10.1007/s11357-022-00674-5
Buchman, A. S., Boyle, P. A., Wilson, R. S., Tang, Y., and Bennett, D. A. (2007). Frailty is associated with incident Alzheimer’s disease and cognitive decline in the elderly. Psychosom. Med. 69, 483–489. doi: 10.1097/psy.0b013e318068de1d
Buchman, A. S., Yu, L., Wilson, R. S., Boyle, P. A., Schneider, J. A., Bennett, D. A., et al. (2014). Brain pathology contributes to simultaneous change in physical frailty and cognition in old age. J. Gerontol. Series A. 69, 1536–1544. doi: 10.1093/gerona/glu117
Chen, S., Honda, T., Narazaki, K., Chen, T., Kishimoto, H., Haeuchi, Y., et al. (2018). Physical frailty is associated with longitudinal decline in global cognitive function in non-demented older adults: a prospective study. J. Nutr. Health Aging 22, 82–88. doi: 10.1007/s12603-017-0924-1
Curran, P. J., and Hussong, A. M. (2003). The use of latent trajectory models in psychopathology research. J. Abnorm. Psychol. 112, 526–544. doi: 10.1037/0021-843X.112.4.526
Doba, N., Tokuda, Y., Goldstein, N. E., Kushiro, T., and Hinohara, S. (2012). A pilot trial to predict frailty syndrome: the Japanese Health Research volunteer study. Exp. Gerontol. 47, 638–643. doi: 10.1016/j.exger.2012.05.016
Emiliano Albanese, F. L. L., Prince, M. J., and Stewart, R. (2013). Dementia and lower blood pressure in Latin America, India, and China: a 10/66 cross-cohort study. Am. Acad. Neurol. 81, 228–235. doi: 10.1212/WNL.0b013e31829bfe66
Farron, M. R., Kabeto, M. U., Dey, A. B., Banerjee, J., Levine, D. A., and Langa, K. M. (2020). Hypertension and cognitive health among older adults in India. J. Am. Geriatr. Soc. 68:16741. doi: 10.1111/jgs.16741
Fein, G., Torres, J., Price, L. J., and Di Sclafani, V. (2006). Cognitive performance in long-term abstinent alcoholic individuals. Alcohol. Clin. Exp. Res. 30, 1538–1544. doi: 10.1111/j.1530-0277.2006.00185.x
Ferraro, O. E., Guaita, A., and Villani, S. (2021). Cognitive, physical and disability trajectories in community-dwelling elderly people. Aging Clin. Exp. Res. 33, 2671–2677. doi: 10.1007/s40520-021-01804-3
García-Marchena, N., Pizarro, N., Pavón, F. J., Martínez-Huélamo, M., Flores-López, M., Requena-Ocaña, N., et al. (2020). Potential association of plasma lysophosphatidic acid (LPA) species with cognitive impairment in abstinent alcohol use disorders outpatients. Sci. Rep. 10:17163. doi: 10.1038/s41598-020-74155-0
Halil, M., Kizilarslanoglu, M. C., Kuyumcu, M. E., Yesil, Y., and Jentoft, A. J. C. (2015). Cognitive aspects of frailty: mechanisms behind the link between frailty and cognitive impairment. J. Nutr. Health Aging 19, 276–283. doi: 10.1007/s12603-014-0535-z
Hoeksema, A. R., Spoorenberg, S. L. W., Peters, L. L., Meijer, H. J. A., Raghoebar, G. M., Vissink, A., et al. (2017). Elderly with remaining teeth report less frailty and better quality of life than edentulous elderly: a cross-sectional study. Oral Dis. 23, 526–536. doi: 10.1111/odi.12644
Howrey, B. T., Al Snih, S., Middleton, J. A., and Ottenbacher, K. J. (2020). Trajectories of frailty and cognitive decline among older Mexican Americans. J. Gerontol. Series A. 75, 1551–1557. doi: 10.1093/gerona/glz295
Huang, D. Y. C., Lanza, H. I., and Anglin, M. D. (2013). Association between adolescent substance use and obesity in young adulthood: a group-based dual trajectory analysis. Addict. Behav. 38, 2653–2660. doi: 10.1016/j.addbeh.2013.06.024
Johnson, B., Dawes, P., Emsley, R., Cruickshanks, K. J., Moore, D. R., Fortnum, H., et al. (2015). Hearing loss and cognition: the role of hearing aids, social isolation and depression. PLoS One 10:9616. doi: 10.1371/journal.pone.0119616
Kelaiditi, E., Cesari, M., Canevelli, M., Van Kan, G. A., Ousset, P. J., Gillette-Guyonnet, S., et al. (2013). Cognitive frailty: rational and definition from an (IANA/IAGG) international consensus group. J. Nutr. Health Aging 17, 726–734. doi: 10.1007/s12603-013-0367-2
Kim, S., Park, J. L., Hwang, H. S., and Kim, Y. P. (2014). Correlation between frailty and cognitive function in non-demented community dwelling older Koreans. Korean journal of. Fam. Med. 35:309. doi: 10.4082/kjfm.2014.35.6.309
Kodl, C. T., and Seaquist, E. R. (2008). Cognitive dysfunction and diabetes mellitus. Endocr. Rev. 29, 494–511. doi: 10.1210/er.2007-0034
Kregel, K. C., and Zhang, H. J. (2007). An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am. J. Phys. Regul. Integr. Comp. Phys. 292, R18–R36. doi: 10.1152/ajpregu.00327.2006
Li, F., Yan, Y., Zheng, L., Wang, C., Guan, X., Hong, S., et al. (2023). Frailty and its combined effects with lifestyle factors on cognitive function: a cross-sectional study. BMC Geriatr. 23:79. doi: 10.1186/s12877-023-03761-0
Liljas, A. E. M., Carvalho, L. A., Papachristou, E., De Oliveira, C., Wannamethee, S. G., Ramsay, S. E., et al. (2017). Self-reported vision impairment and incident prefrailty and frailty in English community-dwelling older adults: findings from a 4-year follow-up study. J. Epidemiol. Community Health 71, 1053–1058. doi: 10.1136/jech-2017-209207
Linglong, Y., Lei, Q., and Ben-Chang, S. (2021). Heterogeneous growth trajectories of cognitive function influencing factors for elderly adults. Chin. J. Health Stat. 38, 183–187.
Liu, Z., Han, L., Gahbauer, E. A., Allore, H. G., and Gill, T. M. (2018). Joint trajectories of cognition and frailty and associated burden of patient-reported outcomes. J. Am. Med. Dir. Assoc. 19, 304–309. doi: 10.1016/j.jamda.2017.10.010
Liu, D., Li, L., An, L., Cheng, G., Chen, C., Zou, M., et al. (2021). Urban-rural disparities in mild cognitive impairment and its functional subtypes among community-dwelling older residents in Central China. Gen. Psychiatr. 34:100564. doi: 10.1136/gpsych-2021-100564
Ma, L., and Chan, P. (2020). Understanding the physiological links between physical frailty and cognitive decline. Aging Dis. 11, 405–418. doi: 10.14336/AD.2019.0521
Ma, L., Chhetri, J. K., Liu, P., Ji, T., Zhang, L., and Tang, Z. (2020). Epidemiological characteristics and related factors of frailty in older Chinese adults with hypertension: a population-based study. J. Hypertens. 38, 2192–2197. doi: 10.1097/HJH.0000000000002650
Ma, Y., Li, X., Pan, Y., Zhao, R., Wang, X., Jiang, X., et al. (2021). Cognitive frailty predicting death and disability in Chinese elderly. Neurol. Res. 43, 815–822. doi: 10.1080/01616412.2021.1939235
Moheet, A., Mangia, S., and Seaquist, E. R. (2015). Impact of diabetes on cognitive function and brain structure. Ann. N. Y. Acad. Sci. 1353, 60–71. doi: 10.1111/nyas.12807
Montejo, Á. L., and Rico-Villademoros, F. (2008). Psychometric properties of the psychotropic-related sexual dysfunction questionnaire (PRSexDQ-SALSEX) in patients with schizophrenia and other psychotic disorders. J. Sex Marital Ther. 34, 227–239. doi: 10.1080/00926230701866125
Mulero, J., Zafrilla, P., and Martinez-Cacha, A. (2011). Oxidative stress, frailty and cognitive decline. J Nutr. Health Aging 15, 756–760. doi: 10.1007/s12603-011-0130-5
Muthén, B., and Muthén, L. K. (2000). Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcoholism Clin. Exp. Res. 24, 882–891. doi: 10.1111/j.1530-0277.2000.tb02070.x
Nagin, D. S. (1999). Analyzing developmental trajectories: a semiparametric, group-based approach. Psychol. Methods 4, 139–157. doi: 10.1037/1082-989X.4.2.139
Nagin, D. S., and Odgers, C. L. (2010). Group-based trajectory modeling in clinical research. Annu. Rev. Clin. Psychol. 6, 109–138. doi: 10.1146/annurev.clinpsy.121208.131413
Nagin, D. S., and Tremblay, R. E. (2001). Analyzing developmental trajectories of distinct but related behaviors: a group-based method. Psychol. Methods 6, 18–34. doi: 10.1037/1082-989X.6.1.18
Nari, F., Jang, B. N., Youn, H. M., Jeong, W., Jang, S.-I., and Park, E.-C. (2021). Frailty transitions and cognitive function among South Korean older adults. Sci. Rep. 11:10658. doi: 10.1038/s41598-021-90125-6
Panza, F., Lozupone, M., Sardone, R., Battista, P., Piccininni, M., Dibello, V., et al. (2018). Sensorial frailty: age-related hearing loss and the risk of cognitive impairment and dementia in later life. Ther. Adv. Chronic Dis. 10:1000. doi: 10.1177/2040622318811000
Qiu, C., Winblad, B., and Fratiglioni, L. (2005). The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 4, 487–499. doi: 10.1016/S1474-4422(05)70141-1
Reyes-Ortiz, C. A., Kuo, Y. F., DiNuzzo, A. R., Ray, L. A., Raji, M. A., and Markides, K. S. (2005). Near vision impairment predicts cognitive decline: data from the Hispanic established populations for epidemiologic studies of the elderly. J. Am. Geriatr. Soc. 53, 681–686. doi: 10.1111/j.1532-5415.2005.53219.x
Robertson, D. A., Savva, G. M., and Kenny, R. A. (2013). Frailty and cognitive impairment—a review of the evidence and causal mechanisms. Ageing Res. Rev. 12, 840–851. doi: 10.1016/j.arr.2013.06.004
Yuan, Y., Lapane, K. L., Tjia, J., Baek, J., Liu, S.-H., and Ulbricht, C. M. (2022). Trajectories of physical frailty and cognitive impairment in older adults in United States nursing homes. BMC Geriatr. 22:3012. doi: 10.1186/s12877-022-03012-8
Yuan, L., Zhang, X., Guo, N., Li, Z., Lv, D., Wang, H., et al. (2021). Prevalence of cognitive impairment in Chinese older inpatients and its relationship with 1-year adverse health outcomes: a multi-center cohort study. BMC Geriatr. 21:595. doi: 10.1186/s12877-021-02556-5
Yun, J.-h., Ki, S.-k., Kim, J., Chon, D., Shin, S.-y., and Lee, Y. (2020). Relationships between cognitive function and frailty in older Korean adults: the moderating effect of the number of teeth. Arch. Gerontol. Geriatr. 91:104213. doi: 10.1016/j.archger.2020.104213
Zhang, X.-M., Jiao, J., Cao, J., and Wu, X. (2022). The association between the number of teeth and frailty among older nursing home residents: a cross-sectional study of the CLHLS survey. BMC Geriatr. 22:1007. doi: 10.1186/s12877-022-03688-y
Keywords: cognitive function, frailty, dual trajectories, older adults, aging
Citation: Ji X, Wu Y, Gu Z, Zhong Z, Wang K, Ye S, Wan Y and Qiu P (2024) Trajectories of cognitive function and frailty in older adults in China: a longitudinal study. Front. Aging Neurosci. 16:1465914. doi: 10.3389/fnagi.2024.1465914
Edited by:
Takao Yamasaki, Minkodo Minohara Hospital, JapanReviewed by:
Gabriela Cabett Cipolli, State University of Campinas, BrazilMohammad Saiful Islam, Bangladesh Livestock Research Institue, Bangladesh
Copyright © 2024 Ji, Wu, Gu, Zhong, Wang, Ye, Wan and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Wan, d2FueWFuZzIwMTRAc2N1LmVkdS5jbg==; Peiyuan Qiu, cWl1cGVpeXVhbkBzY3UuZWR1LmNu
 Xiaoyi Ji
Xiaoyi Ji Yue Wu
Yue Wu Zijie Gu
Zijie Gu Zhujun Zhong
Zhujun Zhong Kerui Wang
Kerui Wang Suni Ye
Suni Ye Yang Wan
Yang Wan Peiyuan Qiu
Peiyuan Qiu